Eerstelijnsbehandeling incurabel NSCLC met EGFR exon 19/21 mutatie
Uitgangsvraag
Welke behandeling heeft de voorkeur in de eerste lijn bij patiënten met een incurabel NSCLC met EGFR exon 19/21 mutatie?
Aanbeveling
Behandel patiënten met een incurabel NSCLC (niet-curatief behandelbaar stadium III-IV) met een exon(19)del of L858R-activerende EGFR mutatie in de eerste lijn met een EGFR TKI.
Overweeg behandeling met osimertinib of erlotinib-ramucirumab in de eerste lijn bij patiënten met een incurabel NSCLC met een exon(19)del of L858R-activerende EGFR mutatie. Betrek hierin de aan-/afwezigheid van hersenmetastasen of co-mutaties (bijv. TP53).
Bespreek de voor- en nadelen met de patiënt en maak samen met de patiënt een keuze.
Overweeg behandeling met de volgende opties als osimertinib of erlotinib-ramucirumab
niet verdragen wordt:
- erlotinib-bevacizumab;
- tweede generatie TKI;
- eerste generatie TKI.
Overleg bij progressie met een gespecialiseerd centrum (NVALT centrum voor zeldzame driver mutaties).
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Na de presentatie van de FLAURA studie, en voornamelijk na de update van de overall survival data, is osimertinib in de praktijk de meest gebruikte (monotherapie) TKI geworden. De overwegingen hiervoor waren:
- Osimertinib was de eerste EGFR-TKI die een klinisch relevante verbetering van OS gaf t.o.v. de eerstegeneratie-EGFR-TKI in de gehele (common) EGFR-gemuteerde populatie. Eerder werd voor afatinib (in de LUX-Lung 7 studie) geen overlevingsvoordeel ten opzichte van gefitinib in de eerstelijnsbehandeling aangetoond, evenwel, de powerberekening van deze studie was o.b.v. PFS en niet o.b.v. OS. Voor dacomitinib werd een statistisch en klinisch significant overlevingsvoordeel van ruim 7 maanden gezien ten opzichte van gefitinib, echter, dit werd alleen gezien in de eerstelijnsbehandeling bij patiënten die geen hersenmetastasen hadden bij aanvang van de behandeling. Voor osimertinib werd een OS voordeel gezien met een mediane OS van 38,6 maanden (95%BI, 34,5 tot 41,8) in de osimertinib-groep en 31,8 maanden (95%BI, 26,6 tot 36,0) in de controle arm (HR = 0,80; 95,05% BI, 0,64 tot 1,00; P = 0,046) (Ramalingam, 2020).
- De PFS in de eerstelijnsbehandeling was significant hoger voor afatinib, dacomitinib en osimertinib vergeleken met eerstegeneratie EGFR-TKI’s. In absolute aantallen werd de hoogste PFS gezien met osimertinib, namelijk 18.9 maanden. Het verschil met gefitinib was 8.7 maanden, dit verschil is niet eerder gezien met andere TKI’s.
- De ORR was vergelijkbaar tussen eerstegeneratie-EGFR-TKI’s en dacomitinib en osimertinib. Alleen afatinib liet een hogere tumorrespons zien ten opzichte van gefitinib.
- In meer dan de helft van de patiënten die behandeld worden met eerste- en tweedegeneratie EGFR-TKI’s ontstaat de secundaire resistentiemutatie T790M, die verantwoordelijk is voor ziekteprogressie. Deze vorm van resistentieontwikkeling wordt niet gezien met osimertinib omdat osimertinib juist ontwikkeld is om T790M te blokkeren. Dit roept de vraag op of osimertinib monotherapie beter is dan de sequentiële combinatie van een eerste- of tweedegeneratie EGFR-TKI gevolgd door osimertinib in geselecteerde patiëntengroepen. Op basis van de huidige onderzoeken kunnen geen conclusies getrokken worden over welke volgorde van TKI’s de grootste overlevingswinst zal opleveren.
- Er werd in de beoordeelde studies geen significante toename gezien van ernstige toxiciteit (graad 3 of hoger) tussen de eerste- en tweedegeneratie EGFR-TKI’s. Evenwel lijken de huid- en darmklachten van de tweedegeneratiemiddelen ernstiger in de praktijk. Dit zou ermee te maken kunnen hebben dat de tweedegeneratie TKI’s zijn ontworpen om irreversibel te binden aan EGFR, maar ook aan meerdere andere receptoren van de ERB-familie (waartoe EGFR behoort). Het niet-gemuteerde EGFR wordt daarbij ook gebonden, weliswaar aan een lagere affiniteit. Dit zou ertoe kunnen leiden dat de bijwerkingen van afatinib en dacomitinib voor de aanbevolen dosis in het algemeen meer zijn dan van de eerstegeneratie EGFR-TKI’s. De tweedegeneratie EGFR-TKI’s adviseren dosisreductie om ernstige bijwerkingen tegen te gaan. Hiervoor zijn er formuleringen met lagere doseringen beschikbaar. Voor zowel afatinib als dacomitinib zijn er data die hun effectiviteit ondersteunen ondanks dosisreductie. Daarentegen is osimertinib ontworpen om alleen het gemuteerde EGFR en T790M te binden, het zou daarom niet binden aan het wild-type EGFR. Als gevolg daarvan laat osimertinib een gunstiger bijwerkingenprofiel zien dan de eerste generatie EGFR-TKI’s.
- Kwaliteit van leven werd onderzocht in de studies met afatinib en dacomitinib, hierbij werden geen significante verschillen gemeten t.o.v. eerstegeneratie EGFR-TKI’s. Voor osimertinib werden er wel aanwijzingen gezien voor een beter emotioneel, sociaal en cognitief functioneren.
Recente studies, zoals de RELAY-studie, onderzoeken of de toevoeging van antivasculaire middelen zoals bevacizumab of ramucirumab aan eerstegeneratie EGFR-TKI's de progressievrije overleving (PFS) bij niet-kleincellige longkanker (NSCLC) kan verbeteren. Hoewel osimertinib nog niet is vergeleken met dergelijke combinaties, is er momenteel geen duidelijke voorkeur aan te geven welke benadering effectiever zou zijn. Bij patiënten met hersenmetastasen kan osimertinib mogelijk een betere keuze zijn, gezien de doeltreffendheid en CNS-penetratie. Tevens lijkt het erop dat co-mutaties in het tumorgenoom relevant kunnen zijn voor de keuze van eerstelijnsbehandeling bij EGFR-gemuteerd NSCLC. In de RELAY-studie werd het effect van TP53 comutaties op de behandeling van niet-behandelde, gemetastaseerde EGFR-gemuteerde NSCLC onderzocht. Hier bleek dat TP53 mutaties, vooral op exon 8, geassocieerd waren met slechtere PFS uitkomsten met erlotinib, maar niet met de combinatie van ramucirumab met erlotinib.
In de praktijk geldt osimertinib als de EGFR-TKI van keuze, en daarom worden studies opgezet met osimertinib monotherapie als controle-arm. Recentelijk werden de resultaten gepresenteerd van twee grote fase-III studies die osimertinib als controle-arm-behandeling hebben, echter deze data zijn niet meegenomen in de huidige richtlijn-aanbevelingen, aangezien ze zijn gepubliceerd na de formulering van de zoekvraag:
- FLAURA2 is een gerandomiseerde fase-III studie die de werkzaamheid en veiligheid van eerstelijns osimertinib + chemotherapie versus osimertinib monotherapie voor gevorderde EGFR-gemuteerde NSCLC onderzocht heeft (Janne, 2023). In totaal werden 557 patiënten gerandomiseerd in de osimertinib + chemotherapie arm (n=279) of de osimertinib monotherapie-arm (n=278). De chemotherapie bestond uit pemetrexed en cisplatine of carboplatine voor vier cycli, gevolgd door osimertinib + pemetrexed. Het primaire eindpunt PFS toonde een significante verbetering in de combinatietherapie-arm vergeleken met monotherapie (HR 0.62; 95% CI 0.49, 0.79; p<0.0001). De mediane PFS verbeterde met 8,8 maanden; BICR PFS beoordeling toonde zelfs een verbetering van 9,5 maanden. De graad ≥3 AEs waren 64% in de combinatietherapie-arm en 27% in de monotherapie-arm. OS is nog niet matuur.
- De MARIPOSA-studie onderzocht de combinatie van amivantamab (AMI), een EGFR-MET bispecifiek antilichaam, en lazertinib (LAZ), een derdegeneratie EGFR-TKI, versus osimertinib (OSI) in de eerstelijnsbehandeling (Cho, 2023). In deze fase 3-studie werden patiënten 2:2:1 gerandomiseerd in de AMI+LAZ, OSI, of alleen LAZ-arm. Het primaire eindpunt was PFS van AMI+LAZ versus OSI. In totaal werden 1074 patiënten gerandomiseerd (AMI+LAZ 429; OSI 429; LAZ 216). Bij een mediane follow-up van 22,0 maanden toonde AMI+LAZ een 30% afname in het risico op progressie vergeleken met OSI (HR, 0.70; 95% CI, 0.58–0.85; P<0.001), met een mediane PFS van 23,7 maanden (95% CI, 19.1–27.7) versus 16,6 maanden (95% CI, 14.8–18.5) voor OSI. ORR was 86% (95% CI, 83–89) voor AMI+LAZ versus 85% (95% CI, 81–88) voor OSI. Bij de interim OS-analyse was er een gunstige trend voor AMI+LAZ over OSI (HR, 0.80; 95% CI, 0.61 tot 1.05; P=0.1). EGFR- en MET-gerelateerde bijwerkingen waren hoger voor AMI+LAZ, behalve diarree, die hoger was voor OSI. Een opmerkelijke bijwerking in de AMI+LAZ arm waren de trombo-veneuze events, meestal graad 1-2, die vroeg tijdens de behandeling optraden, echter goed konden behandeld worden met anticoagulantia.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Bij het afwegen van behandelopties voor patiënten met EGFR-gemuteerd niet-kleincellig longcarcinoom, is het belangrijk om de specifieke voor- en nadelen van osimertinib versus de combinatie van erlotinib met ramucirumab te overwegen. Osimertinib, als monotherapie, staat bekend om zijn ‘gebruiksgemak’ door orale toediening en heeft een relatief gunstig bijwerkingenprofiel met minder ziekenhuisbezoeken en mogelijk een betere kwaliteit van leven. Daarentegen kan de combinatie van erlotinib en ramucirumab in bepaalde subgroepen effectiever zijn, maar behoeft regelmatige ‘infusen’ en leidt waarschijnlijk tot een verhoogde toxiciteit. Deze afweging vereist een ernstige afweging van de klinische effectiviteit en persoonlijke voorkeuren van de patiënt. Vooral omdat de studiedata onzekerheid biedt over de superioriteit van de ene behandeling over de andere, wordt de subjectieve ervaring van de patiënt met betrekking tot toxiciteit en bijwerkingen belangrijk. Zorgverleners dienen patiënten actief te betrekken bij de besluitvorming, waarbij zowel de fysieke behoeften als de emotionele en sociale aspecten worden meegewogen, om tot een behandeling te komen die het beste aansluit bij de individuele situatie en voorkeuren van de patiënt.
Kosten (middelenbeslag)
Bij het beoordelen of de voordelen van een behandeloptie opwegen tegen de kosten, is het belangrijk om rekening te houden met zowel de directe als indirecte uitgaven. Volgens het Farmakotherapeutisch Kompas is osimertinib monotherapie ongeveer vier keer zo duur als erlotinib monotherapie. De combinatie van erlotinib met ramucirumab is 50% duurder dan osimertinib monotherapie. Deze kosten omvatten echter niet de bijkomende uitgaven van ramucirumab voor ziekenhuisbezoeken, zorgpersoneel en de dagbehandelingsunit, evenals de indirecte kosten zoals vervoer, parkeren, en tijdverlies voor de patiënt en begeleiders. Deze besluitvorming vraagt om een ‘holistische’ benadering, waarbij de klinische voordelen worden gebalanceerd met de financiële lasten, om zo de best mogelijke zorg te waarborgen binnen de context van de beschikbare gezondheidszorgmiddelen.
Aanvaardbaarheid, haalbaarheid en implementatie
Ziekenhuizen kunnen terughoudend zijn om ramucirumab te gebruiken vanwege zorgen over de kosten, complexiteit en de vereiste capaciteit voor dagbehandeling. Dergelijke drempels kunnen leiden tot ongelijkheden in de beschikbaarheid van de ramucirumab-behandeloptie. De drempels die de implementatie belemmeren omvatten echter meer dan alleen de behoefte aan gespecialiseerd zorgpersoneel en de directe kosten; uitdagingen op het gebied van patiënttherapietrouw spelen ook een rol. Om de implementatie te verbeteren, kan coördinatie tussen lokale zorginstellingen en gespecialiseerde regionale TKI-centra, waarheen patiënten verwezen kunnen worden, uitkomst bieden. Daarnaast is er speciale aandacht nodig voor bepaalde patiëntengroepen, zoals ouderen of mensen met comorbiditeiten, vanwege hun unieke behoeften en omstandigheden.
Onderbouwing
Achtergrond
EGFR-Tyrosine Kinase inhibitors (TKIs) are the mainstay of first line treatment of metastasized EGFR-mutated non-small cell lung carcinoma (NSCLC). With the development of second and third generation EGFR-TKIs, several treatment options became available. This module aims to compare TKIs in terms of overall survival (OS), progression free survival (PFS), objective response rate (ORR), quality of life (QoL) and adverse events (AE) and to aid decision making in first line treatment.
Conclusies / Summary of Findings
|
Moderate GRADE |
First line treatment with second or third generation TKIs (dacomitinib, osimertinib, afatinib) may increase overall survival (mean absolute difference 7.6%) when compared with first generation TKIs (gefitinib or erlotinib) in patients with non–small-cell lung cancer stage IIIB/IV and an EGFR exon 19 deletion or exon 21 L858R mutation.
Source: Mok, 2021; Ramalingam, 2020; Paz-Ares, 2017 |
|
Low GRADE |
First line treatment with first generation TKI (gefitinib or erlotinib) + other treatment (carboplatin/pemetrexed or bevacizumab) may increase overall survival (mean absolute difference 5%) when compared with first generation TKIs (gefitinib or erlotinib) in patients with non–small-cell lung cancer stage IIIB/IV and an EGFR exon 19 deletion or exon 21 L858R mutation.
Source: Miyauchi, 2022; Piccirillo, 2022 |
|
Low GRADE |
First line treatment with second or third generation TKIs (dacomitinib, osimertinib, afatinib) may increase progression free survival when compared with first generation TKIs (gefitinib or erlotinib) in patients with non–small-cell lung cancer stage IIIB/IV and an EGFR exon 19 deletion or exon 21 L858R mutation.
Source: Wu, 2017; Soria, 2018; Park, 2016 |
|
Low GRADE |
First line treatment with first generation TKI (gefitinib or erlotinib) + other treatment (ramucirumab, carboplatin/pemetrexed or bevacizumab) may increase progression free survival when compared with first generation TKIs (gefitinib or erlotinib) in patients with non–small-cell lung cancer stage IIIB/IV and an EGFR exon 19 deletion or exon 21 L858R mutation.
Source: Nakagawa, 2019; Miyauchi, 2022; Saito, 2019; Zhou, 2021, Piccirillo, 2022 |
|
Low GRADE |
First line treatment with second or third generation TKIs (dacomitinib, osimertinib, afatinib) may result in little to no difference in objective response rate when compared with first generation TKIs (gefitinib or erlotinib) in patients with non–small-cell lung cancer stage IIIB/IV and an EGFR exon 19 deletion or exon 21 L858R mutation.
Source: Wu, 2017; Ramalingam, 2020; Paz-Ares, 2017 |
|
Low GRADE |
First line treatment with first generation TKI (gefitinib or erlotinib) + other treatment (ramucirumab, carboplatin/pemetrexed or bevacizumab) may result in little to no difference in objective response rate when compared with first generation TKIs (gefitinib or erlotinib) in patients with non–small-cell lung cancer stage IIIB/IV and an EGFR exon 19 deletion or exon 21 L858R mutation.
Source: Nakagawa, 2019; Hosomi 2020; Saito, 2019; Zhou, 2021, Piccirillo, 2022 |
|
Very Low GRADE |
In general, when pooled together, little to no difference could be seen regarding the effect of first line treatment with second or third generation TKIs (dacomitinib, osimertinib, afatinib) on adverse events when compared with first generation TKIs (gefitinib or erlotinib) in patients with non–small-cell lung cancer stage IIIB/IV and an EGFR exon 19 deletion or exon 21 L858R mutation.
However, differences in the effect on adverse events might exist between second and third generation TKIs and between specific TKIs.
Source: Mok, 2021; Ramalingam, 2020; Paz-Ares, 2017 |
|
Very Low GRADE |
First line treatment with first generation TKI (gefitinib or erlotinib) + other treatment (ramucirumab, carboplatin/pemetrexed or bevacizumab) may increase adverse events when compared with first generation TKIs (gefitinib or erlotinib) in patients with non–small-cell lung cancer stage IIIB/IV and an EGFR exon 19 deletion or exon 21 L858R mutation, but the evidence is very uncertain.
Source: Nakagawa, 2019; Miyauchi, 2022; Saito, 2019; Zhou, 2021, Piccirillo, 2022 |
|
Low GRADE |
First line treatment with second or third generation TKIs (dacomitinib, osimertinib, afatinib) may result in little to no difference in quality of life when compared with first generation TKIs (gefitinib or erlotinib) in patients with non–small-cell lung cancer stage IIIB/IV and an EGFR exon 19 deletion or exon 21 L858R mutation.
Source: Paty, 2021; Park 2016 |
|
Low GRADE |
First line treatment with first generation TKI (gefitinib or erlotinib) + other treatment (ramucirumab, carboplatin/pemetrexed or bevacizumab) may result in little to no difference in quality of life when compared with first generation TKIs (gefitinib or erlotinib) in patients with non–small-cell lung cancer stage IIIB/IV and an EGFR exon 19 deletion or exon 21 L858R mutation.
Source: Hosomi 2020; Zhou, 2021; Piccirillo, 2022, Yoh, 2020 |
Samenvatting literatuur
Description of studies
The study characteristics of the eight included trials are summarized in Table 1.
Table 1. Study characteristics of the included studies
|
Study (Author, Year) |
Study design |
Patients |
Intervention |
Control |
Reported outcomes |
Overall risk of bias |
|
2nd/3rd generation TKI versus 1st generation TKI |
||||||
|
ARCHER 1050 (Wu, 2017 Mok, 2018 Paty, 2021 Mok, 2021) |
Randomized, open-label, phase III study |
Newly diagnosed advanced or recurrent NSCLC (min. 12 months disease-free) and an exon 19 deletion or L858R, no brain metastases included. |
Dacomitinib 45 mg daily. n=227
|
Gefitinib 250 mg daily. n=225 |
|
Some concerns |
|
FLAURA trial (Ramalingam, 2020 Soria, 2018 Leighl, 2020) |
Double-blind, phase 3 trial |
Treatment naïve patients with locally advanced or metastatic NSCLC and an exon 19 deletion or L858R. 21% had CNS metastases. |
Osimertinib 80 mg daily. n=279
|
C1: Gefitinib 250 mg daily n=183
C2: Erlotinib 150 mg daily. n=94 |
|
Some concerns |
|
LUX-Lung 7 (Park, 2016 Paz-Ares, 2017) |
Phase 2B, open-label, randomised controlled trial |
Treatment naïve patients with stage IIIB or IV adenocarcinoma and an exon 19 deletion or L858R. 15% had brain metastases. |
Afatinib 40 mg daily. n=160
|
Gefitinib 250 mg daily. n=159
|
|
Some concerns |
|
1st generation TKI + other treatment versus 1st generation TKI |
||||||
|
RELAY study (Nakagawa, 2019, Yoh, 2020, Nadal, 2022) |
Double-blind, placebo-controlled, phase 3 trial |
Untreated, metastatic, EGFR mutated NSCLC patients.
|
Erlotinib 150 mg/day AND Ramucirumab 10 mg/kg once every 2 weeks n=224 |
Placebo once every 2 weeks AND erlotinib 150 mg/day n= 225 |
|
Some concerns |
|
NEJ009 study (Hosomi, 2020 Miyauchi, 2022) |
Randomised, phase 3 study |
Chemotherapy naïve, stage IIIB or IV or relapsed nonsquamous NSCLC with EGFR mutations (exon 19 deletion, L858R, G719A, G719C, G719S, and L861Q) |
Gefitinib 250 mg daily combined with carboplatin area under the curve 5 and pemetrexed 500 mg/m2 in a 3-week cycle for up to six cycles, followed by concurrent gefitinib and pemetrexed maintenance. n=172 |
Gefitinib 250 mg daily. n=173 |
|
Low |
|
NEJ026 (Saito, 2019) |
A randomised, open-label, multicentre, phase 3 study |
Non-squamous NSCLC with EGFR-positive status (exon 19 deletion or exon 21 Leu858Arg point mutation); stage IIIB–IV disease or recurrent disease. |
Erlotinib 150 mg daily and bevacizumab 15 mg/kg every 21 days. n=114 |
Erlotinib 150 mg daily. n=114 |
|
Some concerns |
|
ARTEMIS-CTONG1509 (Zhou, 2021)
|
A randomized, open-label, controlled, multicenter phase 3 study.
|
Inoperable, locally advanced, metastatic, or recurrent non squamous NSCLC with ex19del or ex21 L858R mutation in EGFR. |
Erlotinib 150 mg/day with bevacizumab 15 mg/kg once every 3 weeks n=157 |
Erlotinib 150 mg/day n=154
|
|
High |
|
The BEVERLY study (Piccirillo, 2022) |
Multicenter, randomized, phase 3 trial |
Metastatic/locally advanced nonsquamous NSCLC harboring an activating EGFR mutation |
Erlotinib 150 mg daily + bevacizumab, 15 mg/kg every 21 days. n=80 |
Erlotinib 150 mg daily. n=80 |
|
Low |
OS: Overall Survival; PFS: Progression free survival; ORR: Objective response rate; AEs: Adverse events; QoL: Quality of Life
Wu (2017) / Mok (2018) / Paty (2021) / Mok (2021) - ARCHER 1050 is a randomized, open-label, phase III study. Wu (2017) reported on progression free survival (PFS), objective response rate (ORR) and number of patients with serious adverse events (AEs). At that time, the median duration of treatment was 15.3 months in the dacomitinib group and 12.0 months in the gefitinib group. The median duration of follow-up was 22.1 months. Mok (2018) reported the results of overall survival (OS) after a median follow-up period of 31.3 months. Mok (2021) reported the updated OS after a median follow-up period of 47.9 months. Paty (2021) reported the results of PRO questionnaires.
Soria (2018) / Ramalingam (2020) / Leighl (2020) – FLAURA is a double-blind, phase 3 trial. Soria (2018) reported the primary end point PFS after a median duration of treatment of 16.2 months (range 0.1 to 27.4) for patients receiving osimertinib and 11.5 months (range, 0 to 26.2) for those receiving a standard EGFR-TKI. According to protocol, central collection of progression events was stopped after data cutoff (June 12, 2017). Ramalingam (2020) reported the results of the secondary end point, OS (data cutoff June 25, 2019). The median duration of total treatment exposure was 20.7 months (range 0.1 to 49.8) for patients receiving osimertinib and 11.5 months (range 0.0 to 50.6) for those receiving a standard EGFR-TKI. The median follow-up for OS was 35.8 months for patients receiving osimertinib and 27.0 months for those receiving a standard EGFR-TKI. Leighl (2020) reported the results of PRO questionnaires.
Park (2016) / Paz-Ares (2017) - LUX-Lung 7 is Phase 2B, open-label, randomised controlled trial. Park (2016) reported the result of the primary analysis after a median duration of follow-up for PFS of 27.3 months (IQR 15.3 to 33.9). The reported outcome measures were PFS, ORR, severe AEs (grade ≥3), and QoL (measured with the Euroqol questionnaire EQ-5D). The OS data were immature at that time. Paz-Ares (2017) reported the OS data of the LUX-Lung 7 trial after a median follow-up of 42.6 months. The median duration of treatment was 13.7 months (range 0 to 6.4) with afatinib and 11.5 months (range 0.5 to 8.7) with gefitinib.
Nakagawa (2019) / Nadal (2021) / Yoh (2020) - RELAY is a double-blind, placebo-controlled, phase 3 trial, performed in 100 hospitals, clinics, and medical centres in 13 countries. Nakagawa (2019) reported the PFS after a median follow-up of 20·7 months (IQR: 15·8–27·2). Nadal (2021) reported adverse events. Yoh (2020) reported the results of PRO questionnaires.
Hosomi (2020)/ Miyauchi (2022) - NEJ009 is a randomised, phase 3 study. Hosomi (2020) reported the OS, PFS, ORR, AEs, and QoL after a median follow-up duration of 45 months (data cutoff: September 3, 2018). Miyauchi (2022) reported the updated results (data cutoff: May 22, 2020) after a median follow-up duration of 84 months.
Saito (2019) - NEJ026 is a randomised, open-label, multicentre, phase 3 study. Median follow up for PFS was 12.4 months (IQR 7·0–15·7). The independent data monitoring committee held a meeting on Jan 23, 2018, and recommended early termination of the study based on the results of the interim analysis. However, the authors considered that the study had to be continued to obtain data for other endpoints in addition to PFS.
Zhou (2021) - ARTEMIS-CTONG1509 is a randomized, open-label, controlled, multicentre phase 3 study. Median follow-up was not reported.
Piccirillo (2022) - The BEVERLY study is a multicentre, randomized, phase 3 trial. The median duration of follow-up was 36.3 months (95% CI: 30.7–40.9).
Results
Overall survival - Critical outcome
Seven of the eight included studies reported on overall survival.
Three studies (ARCHER 1050, FLAURA, and LUX-Lung-7) reported the effect of second or third generation TKI versus first generation TKI on OS. Treatment with second or third generation TKI resulted in a longer OS compared to treatment with first generation TKI. The absolute differences in OS between the intervention and control group was 7.4% in the ARCHER 1050 trial after 42 months, 10% in the FLAURA trial after 36 months, and 5.5% after a median follow-up of 42.6 months in the LUX-Lung 7 study. The pooled Hazard Ratio (HR) is 0.80 (95%CI 0.69; 0.92) favoring treatment with second or third generation TKI. This difference was not considered clinically relevant.
Two studies (NEJ009 and the Beverly study) reported the effect of first generation TKI + other treatment versus first generation TKI on OS. Treatment with first generation TKI + other treatment resulted in a longer OS compared to treatment with first generation TKI. The absolute differences in OS between the intervention and control group was 5% in the NEJ009 trial after 5 years and 5% in the Beverly study after a median follow up of 36.3 months. The pooled HR is 0.79 (95%CI 0.64; 0.99) favoring treatment with first generation TKI+other treatment. This difference was not considered clinically relevant.
In the ARTEMIS-CTONG1509 study data for OS remained immature, with 55% (172/311) of the events recorded. Median OS was 36.2 months (95%CI 32.5–42.4) in the intervention group versus 31.6 months (95%CI 27.2–40.0) in the control group, with a HR of 0.92 (95% CI, 0.69–1.23). Data for OS were immature at data cutoff In the RELAY study (Nakagawa 2019). Median interim OS was not reached in either group. A final analysis is planned when 300 events have occurred.
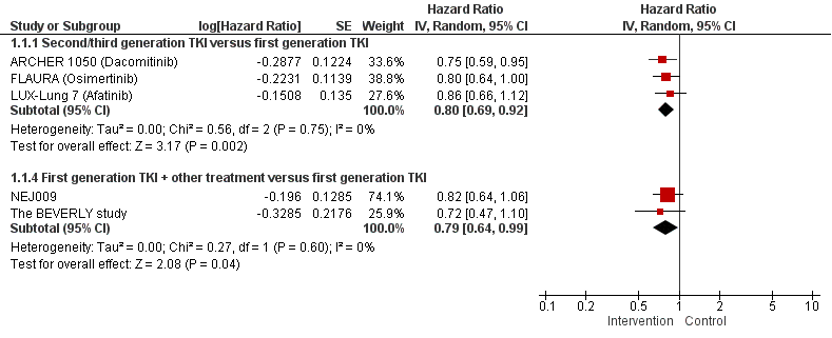
Figure 1. Forest plot of OS for first line treatment with second or third generation TKI versus first generation TKI and first generation TKI + other treatment versus first generation TKI
Progression free survival (PFS) - Important outcome
Eight of the eight included studies reported on PFS.
Three studies (ARCHER 1050, FLAURA, and LUX-Lung-7) reported the effect of second or third generation TKI versus first generation TKI on PFS. Treatment with second or third generation TKI resulted in a longer PFS compared to treatment with first generation TKI. The pooled HR is 0.58 (95%CI 0.45; 0.75) favoring treatment with second or third generation TKI. This difference exceeds the minimal clinically (patient) important difference of HR <0.7.
Five studies (RELAY, NEJ009, ARTEMIS-CTONG1509, NEJ026, and the Beverly study) reported the effect of first generation TKI + other treatment versus first generation TKI on PFS. Treatment with first generation TKI + other treatment resulted in a longer PFS compared to treatment with first generation TKI. The pooled HR is 0.65 (95%CI 0.57; 0.74) favoring treatment with first generation TKI + other treatment. This difference exceeds the minimal clinically (patient) important difference of HR <0.7.
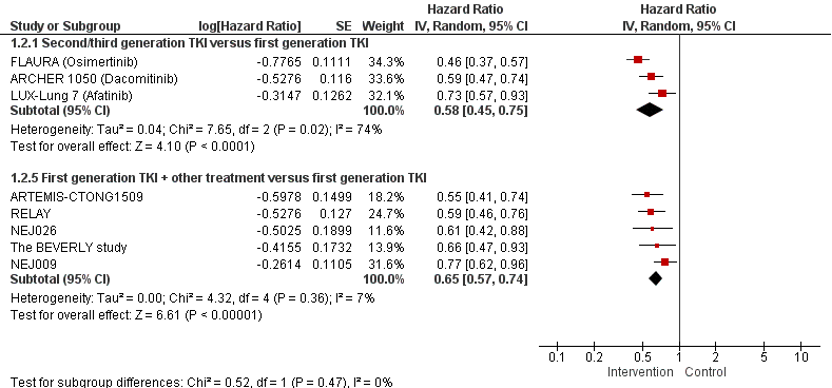
Figure 2. Forest plot of progression free survival for first line treatment with second or third generation TKI OR first generation TKI + other treatment versus first generation TKI
Objective response rate (ORR) - Important outcome
Overall, the percentage of patients who responded on first line treatment ranged from 56% to 84%.
Three studies (ARCHER 1050, FLAURA, and LUX-Lung-7) reported the effect of second or third generation TKI versus first generation TKI on the ORR. In patients treated with second or third generation TKI 77% responded versus 70% in patients treated with first generation TKI (absolute difference 0.07, 95%CI -0.00 to 0.15, NNT=14).
Five studies (RELAY, NEJ009, ARTEMIS-CTONG1509, NEJ026, and the Beverly study) reported the effect of first generation TKI + other treatment versus first generation TKI on the ORR. In patients treated with first generation TKI + other treatment the ORR was 79% versus 71% in patients treated with first generation TKI (absolute difference 0.09; 95%CI 0.01 to 0.16; NNT=11).
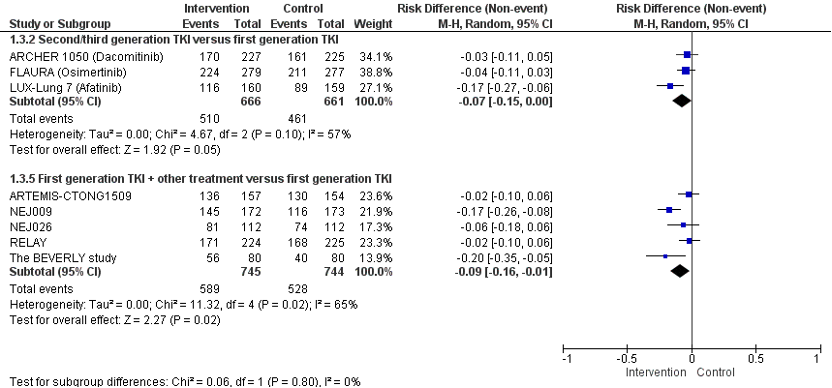
Figure 3. Forest plot of the objective response rate of first line treatment with second or third generation TKI OR first generation TKI + other treatment versus first generation TKI
Adverse events (AEs) grade ≥ 3 - Important outcome
The percentages of severe adverse events ranged from 34% to 57%.
Three studies (ARCHER 1050, FLAURA, and LUX-Lung-7) reported the effect of second or third generation TKI versus first generation TKI on AEs. No statistically significant differences in the risk of adverse events grade ≥ 3 were found between second or third generation TKI versus first generation TKI. The pooled risk difference is 0.01 (95%CI -0.06; 0.07; NNH= 100) favoring treatment with first generation TKI. This difference is not considered clinically relevant.
Five studies (RELAY, NEJ009, ARTEMIS-CTONG1509, NEJ026, and the Beverly study) reported the effect of first generation TKI + other treatment versus first generation TKI on AEs. A lower risk of AEs grade≥ 3 was observed in patients treated with first generation TKI as compared to patients treated with first generation TKI + other treatment. The pooled risk difference is 0.27 (95%CI 0.16; 0.37; NNH= 4) favoring treatment with first generation TKI. This difference is considered clinically relevant.
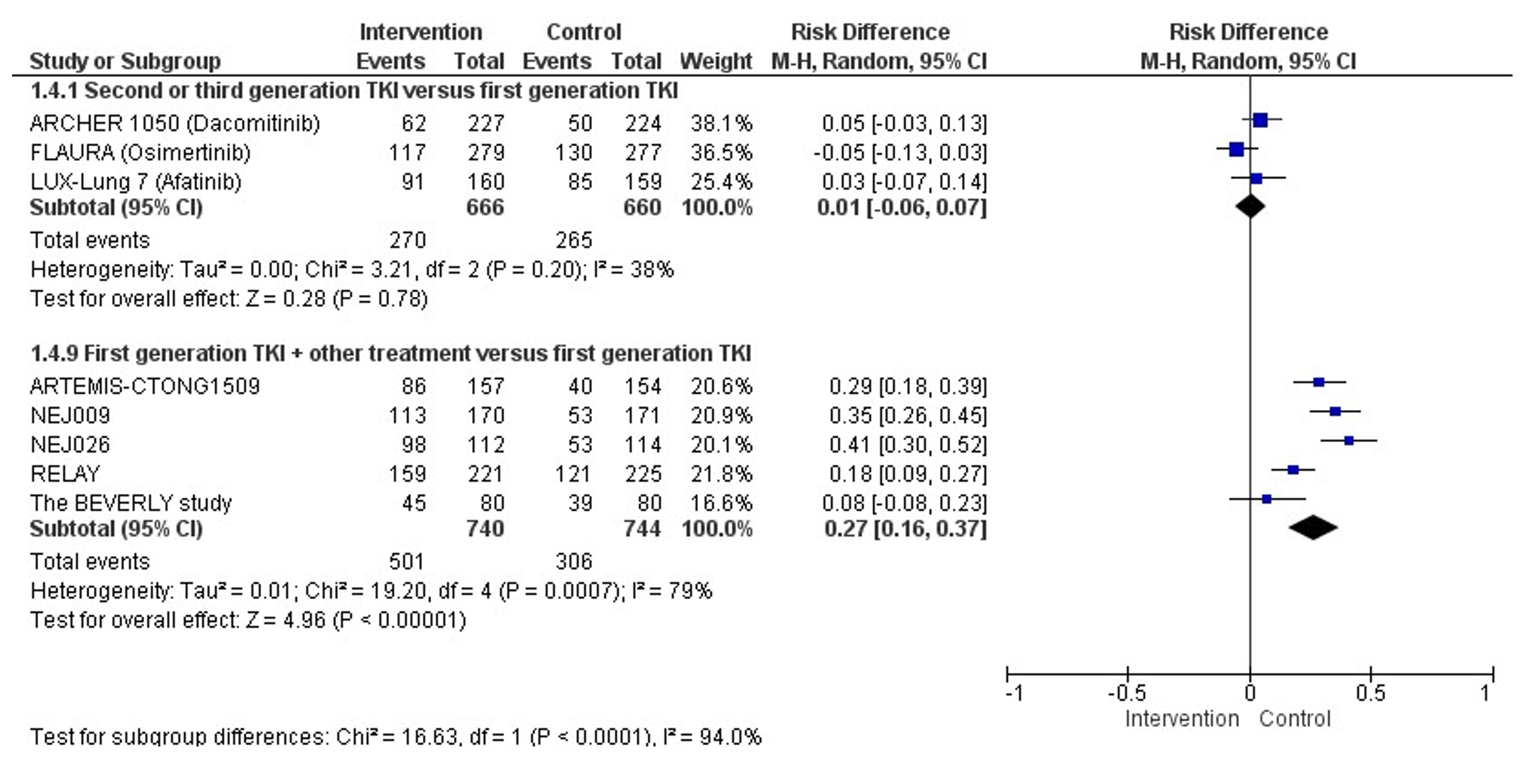
Figure 4. Forest plot of Severe adverse events grade ≥3 after first line treatment with second or third generation TKI OR first generation TKI + other treatment versus first generation TKI
Quality of life (QoL) - Important outcome
Three studies (ARCHER 1050, FLAURA and LUX-Lung-7) reported the effect of second or third generation TKI versus first generation TKI on QoL. In the Archer 1050 study, transformed Global Health Status(GHS)/QoL scores were calculated on a scale ranging to 100, where 100 represents excellent health. The transformed GHS/QoL scores ranged between 61.5 (cycle 30) and 69.8 (cycle 28) after first line treatment with dacomitinib. Transformed GHS/QoL scores ranged between 68.5 (cycle 17) and 73.1 (cycle 28) in the gefitinib group. This difference was not considered clinically relevant (difference less than 10 points). The authors state that patients treated with dacomitinib who received dose reductions reported improvements in GHS/QoL and physical functioning after dose reductions compared with scores prior to the dose reduction. In the FLAURA study, patients completed the EORTC QLQ-LC13 and the EORTC QLQ-C30. Global health status/QoL and functional scores were assessed using a mixed-effects model for repeated measures analysis. Both treatment arms showed improvements from baseline to randomized treatment discontinuation. A statistically significant greater improvement was observed after first line treatment with osimertinib, compared to treatment with erlotinib/gefitinib for emotional functioning (8.79 vs 4.91; p=0.004) and social functioning (7.66 vs 1.74; p < 0.001). After first line treatment with osimertinib, cognitive functioning remained stable, but deteriorated in the erlotinib/gefitinib arm (0.03 vs -3.91; p=0.005). These differences were not considered clinically relevant (difference less than 10 points). In the Lux-Lung-7 study, the post-baseline adjusted mean score for quality of life measured with the EQ-5D was not different between patients treated with afatinib and patients treated with gefitinib in the first line. The mean post-baseline score was 0.77 (SE=0.01) in patients treated with afatinib and 0.80 (SE=0.01) in patients treated with gefitinib (p=0.14).
Four studies (NEJ009, ARTEMIS-CTONG1509, the Beverly study, and the RELAY study) reported the effect of first generation TKI + other treatment versus first generation TKI on QoL. In the NEJ009 study, the authors reported global QOL scores ranging from 2 points (worst) to 14 points (best). At 6 months or later, no differences in global QoL scores were observed between the intervention and control group. These results suggest that addition of pemetrexed to gefitinib did not impair global QOL. In the ARTEMIS-CTONG1509 study, QoL scores were measured with the EQ-5D. QoL scores were comparable between the intervention and control group, with no change from baseline for either group. This might indicate that the addition of bevacizumab to erlotinib does not affect the patients’ overall QoL. The RELAY study, the Lung Cancer Symptom Scale (LCSS) was used to measure disease-related symptoms and their impact. Mean changes from baseline indicated no significant differences between treatment arms except for blood in sputum (HR: 1.987; 95% CI: 1.206–3.275) showing a significant deterioration in the ramucirumab/erlotinib group. The EuroQol 5-dimension 5-level questionnaire (EQ-5D-5L) was used to measure the impact of treatment on patient-reported general health status. No difference in mean changes from baseline health status was observed between treatment arms (Q-5D index score: least square mean= -0.01, SE = 0.01, p=0.94; and visual analogue scale: least square mean= 1.00, SE = 1.21, p=0.95). The Beverly study reported on the QoL measured with the European Organisation for Research and Treatment of Cancer C30 and LC13 questionnaires. The authors state that time-to-deterioration of functional and symptom’s scales was similar in the two arms. They reported a statistically significant difference favoring the standard arm in coughing (p = 0.02) and sore mouth (p = 0.04). In the RELAY study the Lung Cancer Symptom Scale (LCSS) and EQ-5D questionnaires were conducted at baseline and every other cycle. The article reports that time-to-deterioration did not differ between treatment arms for LCSS Total Score (HR = 0.962, 95% CI 0.690–1.343), Average Symptom Burden Index (HR = 1.012, 95% CI 0.732–1.400), and the following individual LCSS items: appetite loss, fatigue, cough, shortness of breath, pain, symptom distress, difficulties with daily activities, quality of life. The intervention group (ramucirumab/erlotinib) reported worse blood in sputum (HR = 1.99, 95% CI 1.21–3.28) than the control group. There were no differences observed between treatment arms considering mean changes from baseline in EQ-5D index score (p=.94) and visual analogue scale (p=.95).
In one study (NEJ026) no data was available on patients’ quality of life after treatment.
Level of evidence of the literature
There are four levels of evidence: high, moderate, low, and very low. RCTs start at a high level of evidence.
OS - second or third generation TKIs
The level of evidence regarding the outcome measure OS was downgraded by one level because of imprecision (confidence interval encloses the threshold for a clinically relevant effect and no clinically relevant effect). Therefore, the level of evidence was graded as moderate.
OS - first generation TKI + other treatment
The level of evidence regarding the outcome measure OS was downgraded by two levels because of imprecision (confidence interval encloses the threshold for a clinically relevant effect and no clinically relevant effect). Therefore, the level of evidence was graded as low.
Progression free survival
The level of evidence regarding the outcome measure progression free survival was downgraded by two levels because of study limitations (risk of bias: no blinding, role of the sponsor); imprecision (confidence interval encloses both the threshold for a clinically relevant effect and no clinically relevant effect). Therefore, the level of evidence was graded as low.
Objective response rate
The level of evidence regarding the outcome measure objective response rate was downgraded by two levels because of study limitations (risk of bias: no blinding, role of the sponsor) and inconsistency (wide variance of point estimates). Therefore, the level of evidence was graded as low.
Adverse events
The level of evidence regarding the outcome measure adverse events was downgraded by three levels because of study limitations (risk of bias: no blinding, role of the sponsor), inconsistency (wide variance of point estimates), and imprecision (confidence interval crosses threshold for clinical relevance). Therefore, the level of evidence was graded as very low.
Quality of life
The level of evidence regarding the outcome measure quality of life was downgraded by two levels because of study limitations (risk of bias: no blinding, role of the sponsor) and imprecision (OIS not reached). Therefore, the level of evidence was graded as low.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the effect of first line treatment with second or third generation tyrosine kinase inhibitors (afatinib, dacomitinib, osimertinib) (with/without other treatment) OR first generation tyrosine kinase inhibitors (gefitinib of erlotinib) + other treatment compared to first generation TKIs (gefitinib, erlotinib) in patients with incurable stage III/IV non–small-cell lung cancer with an EGFR exon 19 deletion or exon 21 L858R mutation?
P: patients with non–small-cell lung cancer incurable stage III/IV and an EGFR exon 19 deletion or exon 21 L858R mutation;
I: first line treatment with:
1) Second or third generation tyrosine kinase inhibitors (afatinib, dacomitinib,
osimertinib) with or without other treatment;
2) First generation TKI (gefitinib or erlotinib) + other treatment (such as bevacizumab).
C: first generation tyrosine kinase inhibitors (gefitinib or erlotinib);
O: overall survival, progression free survival, objective response rate, adverse events,
and quality of life.
Relevant outcome measures
The guideline development group considered overall survival as a critical outcome measure for decision making; and progression free survival, objective response rate, adverse events, and quality of life as an important outcome measures for decision making.
The working group defined clinically relevant differences based on the PASKWIL criteria (https://www.nvmo.org):
- Overall survival: >16 weeks and HR<0.7
- Progression-free survival: >16 weeks and HR<0.7
- Objective response rate: absolute difference >10%
- Adverse events: absolute difference <5% for lethal complications, or <25% for serious complications
- Quality of life: A minimal clinically important difference of 10 points on the quality-of-life instrument EORTC QLQ-C30 or a difference of a similar magnitude on other quality of life instruments
Search and select (Methods)
Previous search
A previous search for an earlier version of this guideline in the databases Medline (via OVID) and Embase (via Embase.com) was updated. The previous search covered the period from 1st of January 2008 up to 20th of June 2018 using relevant search terms for systematic reviews (SRs), randomized controlled trials (RCTs) and observational studies (OBS). The systematic literature search resulted in 637 hits. A total of seven publications that examined the clinical outcome after second or third generation TKIs in NSCLC patient with a EGFR mutation were included in the previous literature analysis (Ramalingam, 2012; Ramalingam, 2016; Wu, 2017; Mok, 2018; Soria, 2018; Park, 2016; Paz-Ares, 2017). The publications by Ramalingam (2012 and 2016) were focused on second line treatment and therefore excluded for this literature analysis.
Updated search
On the 18th of April 2023, an update of the previous systematic search was performed in the databases Embase.com and Ovid/Medline for systematic reviews and RCTs about tyrosine kinase inhibitors and non-small cell lung cancer and EGFR mutation. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 1378 unique hits. Screening of the systematic reviews yielded a review published by Chen (2022), of which the search was used for the selection of the literature up to and including 2020.
The review by Chen (2022) included RCTs discussing first-line therapeutic strategies for patients with advanced NSCLC with EGFR Leu858Arg or EGFR 19del mutations, with progression free survival as primary outcome and published in English. The detailed search strategy is depicted in the article published by Chen (2022). The systematic literature search performed by Chen (2022) resulted in 1419 hits. Based on title and abstract screening, 58 studies were initially selected. After reading the full text and thorough assessment of the studies, 37 studies were excluded, and 21 RCTs were included in the review.
For this literature analysis, 13 from the 21 included studies by Chen (2022) were excluded (reasons: comparator not first generation TKI, wrong intervention, wrong study design). Eight articles were included (Wu, 2017; Nakagawa, 2019; Park, 2016; Soria, 2018; Piccirillo, 2021; Saito, 2019, Zhou, 2021; Hosomi, 2020). For the FLAURA study (Soria, 2018) two additional articles with updated results were published (Ramalingam, 2020 and Leighl, 2020), and for the RELAY study (Nakagawa, 2019) patient reported outcomes were published in a separate publication (Yoh, 2020). These three articles were also included in this literature analysis.
For this literature analysis, RCTs published after 2020 were screened by the working group based on title and abstract. Studies were selected based on the following criteria:
- Randomized controlled trials phase III;
- Patients with non–small-cell lung cancer stage IIIB/IV and an EGFR mutation;
- Studies about first line treatment;
- English language.
37 studies were initially selected based on title and abstract screening. After reading the full text, 31 studies were excluded (see the table with reasons for exclusion under the tab Methods), and six articles were included (Zhou, 2021; Piccirillo, 2021, Paty, 2021; Mok, 2021; Miyauchi, 2022; Nadal, 2022).
Results
Seventeen articles (yielded by the previous search and updated search) describing the results of eight trials were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- 1 - Cho, B. C., Felip, E., Spira, A. I., Girard, N., Lee, J. S., Lee, S. H., ... & Lu, S. (2023). LBA14 Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): Primary results from MARIPOSA, a phase III, global, randomized, controlled trial. Annals of Oncology, 34, S1306.
- 2 - Chen C, Zhang C, Lin H, Liu Q, Wu L, Zhou C, Zhang J. First-line therapeutic strategy for patients with advanced non-small cell lung cancer with Leu858Arg epidermal growth factor receptor mutations: a Bayesian network meta-analysis. Ther Adv Chronic Dis. 2022 Oct 17;13:20406223221125706. doi: 10.1177/20406223221125706. PMID: 36274751; PMCID: PMC9580106.
- 3 - Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, Minato K, Takamura K, Hagiwara K, Kobayashi K, Nukiwa T, Inoue A; North-East Japan Study Group. Gefitinib Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer With Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J Clin Oncol. 2020 Jan 10;38(2):115-123. doi: 10.1200/JCO.19.01488. Epub 2019 Nov 4. PMID: 31682542.
- 4 - Janne, P., Planchard, D., Cheng, Y., Yang, J. H., Yanagitani, N., Kim, S. W., ... & Kobayashi, K. (2023). PL03. 13 osimertinib with/without platinum-based chemotherapy as first-line treatment in patients with EGFRm advanced NSCLC (FLAURA2). Journal of Thoracic Oncology, 18(11), S36-S37.
- 5 - Leighl NB, Karaseva N, Nakagawa K, Cho BC, Gray JE, Hovey T, Walding A, Rydén A, Novello S. Patient-reported outcomes from FLAURA: Osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer. 2020 Jan;125:49-57. doi: 10.1016/j.ejca.2019.11.006. Epub 2019 Dec 12. PMID: 31838405.
- 6 - Miyauchi E, Morita S, Nakamura A, Hosomi Y, Watanabe K, Ikeda S, Seike M, Fujita Y, Minato K, Ko R, Harada T, Hagiwara K, Kobayashi K, Nukiwa T, Inoue A; North-East Japan Study Group. Updated Analysis of NEJ009: Gefitinib-Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer With Mutated EGFR. J Clin Oncol. 2022 Nov 1;40(31):3587-3592. doi: 10.1200/JCO.21.02911. Epub 2022 Aug 12. PMID: 35960896; PMCID: PMC9622660.
- 7 - Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Chawla A, Rosell R, Corral J, Migliorino MR, Pluzanski A, Noonan K, Tang Y, Pastel M, Wilner KD, Wu YL. Updated Overall Survival in a Randomized Study Comparing Dacomitinib with Gefitinib as First-Line Treatment in Patients with Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. Drugs. 2021 Feb;81(2):257-266. doi: 10.1007/s40265-020-01441-6. PMID: 33331989; PMCID: PMC7932969.
- 8 - Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Lee M, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Wu YL. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol. 2018 Aug 1;36(22):2244-2250. doi: 10.1200/JCO.2018.78.7994. Epub 2018 Jun 4. Erratum in: J Clin Oncol. 2020 Nov 1;38(31):3725. PMID: 29864379.
- 9 - Nadal E, Horinouchi H, Shih JY, Nakagawa K, Reck M, Garon EB, Wei YF, Kollmeier J, Frimodt-Moller B, Barrett E, Lipkovich O, Visseren-Grul C, Novello S. RELAY, Ramucirumab Plus Erlotinib Versus Placebo Plus Erlotinib in Patients with Untreated, Epidermal Growth Factor Receptor Mutation-Positive, Metastatic Non-Small-Cell Lung Cancer: Safety Profile and Manageability. Drug Saf. 2022 Jan;45(1):45-64. doi: 10.1007/s40264-021-01127-2. Epub 2021 Dec 20. PMID: 34928484; PMCID: PMC8763844.
- 10 - Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, Chiu CH, Park K, Novello S, Nadal E, Imamura F, Yoh K, Shih JY, Au KH, Moro-Sibilot D, Enatsu S, Zimmermann A, Frimodt-Moller B, Visseren-Grul C, Reck M; RELAY Study Investigators. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019 Dec;20(12):1655-1669. doi: 10.1016/S1470-2045(19)30634-5. Epub 2019 Oct 4. PMID: 31591063.
- 11 - Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, Shi Y, Kim SW, Laskin J, Kim DW, Arvis CD, Kölbeck K, Laurie SA, Tsai CM, Shahidi M, Kim M, Massey D, Zazulina V, Paz-Ares L. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016 May;17(5):577-89. doi: 10.1016/S1470-2045(16)30033-X. Epub 2016 Apr 12. PMID: 27083334.
- 12 - Paty J, Sandin R, Reisman A, Wu YL, Migliorino MR, Zhou X, Cheng Y, Lee KH, Nakagawa K, Niho S, Corral J, Płużański A, Linke R, Meyers O, Mok TS. The patient's perspective on treatment with dacomitinib: patient-reported outcomes from the Phase III trial ARCHER 1050. Future Oncol. 2021 Mar;17(7):783-794. doi: 10.2217/fon-2020-0888. Epub 2020 Nov 9. PMID: 33164569.
- 13 - Paz-Ares L, Tan EH, O'Byrne K, Zhang L, Hirsh V, Boyer M, Yang JC, Mok T, Lee KH, Lu S, Shi Y, Lee DH, Laskin J, Kim DW, Laurie SA, Kölbeck K, Fan J, Dodd N, Märten A, Park K. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017 Feb 1;28(2):270-277. doi: 10.1093/annonc/mdw611. PMID: 28426106; PMCID: PMC5391700.
- 14 - Piccirillo MC, Bonanno L, Garassino MC, Esposito G, Dazzi C, Cavanna L, Burgio MA, Rosetti F, Rizzato S, Morgillo F, Cinieri S, Veccia A, Papi M, Tonini G, Gebbia V, Ricciardi S, Pozzessere D, Ferro A, Proto C, Costanzo R, D'Arcangelo M, Proietto M, Gargiulo P, Di Liello R, Arenare L, De Marinis F, Crinò L, Ciardiello F, Normanno N, Gallo C, Perrone F, Gridelli C, Morabito A. Addition of Bevacizumab to Erlotinib as First-Line Treatment of Patients With EGFR-Mutated Advanced Nonsquamous NSCLC: The BEVERLY Multicenter Randomized Phase 3 Trial. J Thorac Oncol. 2022 Sep;17(9):1086-1097. doi: 10.1016/j.jtho.2022.05.008. Epub 2022 Jun 1. PMID: 35659580.
- 15 - Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Shah R, Cobo M, Lee KH, Cheema P, Tiseo M, John T, Lin MC, Imamura F, Kurata T, Todd A, Hodge R, Saggese M, Rukazenkov Y, Soria JC; FLAURA Investigators. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020 Jan 2;382(1):41-50. doi: 10.1056/NEJMoa1913662. Epub 2019 Nov 21. PMID: 31751012.
- 16 - Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, Nakachi I, Gemma A, Azuma K, Kurimoto F, Tsubata Y, Fujita Y, Nagashima H, Asai G, Watanabe S, Miyazaki M, Hagiwara K, Nukiwa T, Morita S, Kobayashi K, Maemondo M. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019 May;20(5):625-635. doi: 10.1016/S1470-2045(19)30035-X. Epub 2019 Apr 8. PMID: 30975627.
- 17 - Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS; FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018 Jan 11;378(2):113-125. doi: 10.1056/NEJMoa1713137. Epub 2017 Nov 18. PMID: 29151359.
- 18 - Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Nadanaciva S, Sandin R, Mok TS. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017 Nov;18(11):1454-1466. doi: 10.1016/S1470-2045(17)30608-3. Epub 2017 Sep 25. PMID: 28958502.
- 19 - Yoh K, Atagi S, Reck M, Garon EB, Ponce Aix S, Moro-Sibilot D, Winfree KB, Frimodt-Moller B, Zimmermann A, Visseren-Grul C, Nakagawa K; RELAY investigators. Patient-reported outcomes in RELAY, a phase 3 trial of ramucirumab plus erlotinib versus placebo plus erlotinib in untreated EGFR-mutated metastatic non-small-cell lung cancer. Curr Med Res Opin. 2020 Oct;36(10):1667-1675. doi: 10.1080/03007995.2020.1808781. Epub 2020 Aug 28. PMID: 32780643.
- 20 - Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY, Cui JW, Yang N, Song Y, Li XL, Lu S, Zhou JY, Ma ZY, Yu SY, Huang C, Shu YQ, Wang Z, Yang JJ, Tu HY, Zhong WZ, Wu YL. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell. 2021 Sep 13;39(9):1279-1291.e3. doi: 10.1016/j.ccell.2021.07.005. Epub 2021 Aug 12. PMID: 34388377.
Evidence tabellen
Evidence tables
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Wu, 2017 Mok, 2018 Paty, 2021 Mok, 2021
ARCHER 1050
NCT01774721 |
International multicentre, randomised, open-label, phase 3 study.
71 academic medical centres and university hospitals in seven countries (China, Hong Kong, Japan, South Korea, Poland, Italy, and Spain)
Patient enrolment between: May 9, 2013 - March 20, 2015.
Funding and conflicts of interest:
|
Inclusion criteria:
Exclusion criteria:
Age, years Median (range) I: 62 (53–68) C: 61 (54–68)
Female I: 64% C: 56 %
ECOG PS: 0 – I: 33.0% 0 – C: 27.6 % 1 – I: 67.0% 1 – C: 72.4%
Exon 19 deletion: I: 134 (59%) C: 133 (59%)
Exon 21 L858R mutation: I: 93 (41%) C: 92 (41%)
Groups were comparable at baseline. |
Oral dacomitinib 45 mg once daily in 28-day cycles.
Dose reductions for a maximum of two dose levels were permitted for treatment-related toxicity in the case of ≥ grade 3 toxicity, or prolonged grade 2 AEs.
n=227 |
Oral gefitinib 250 mg once daily in 28-day cycles. Gefitinib was only available as a 250 mg dose. If treatment was interrupted for grade 3, grade 4, or intolerable grade 2 toxicity, gefitinib was resumed at a daily or every-other-day dosing at the investigator’s discretion.
n=225 |
Mok 2021 data cut off 3, May 2019: Median duration of follow-up for OS: 47.9 months
Still on treatment, n (%): I: 11 (5%) C: 0
Median duration of treatment, months (range): I: 15.4 (0.07–60.5) C: 12.0 (0.07–48.1)
Discontinuation due to treatment related AEs, n (%): I: 23 (10.1%) C: 15 (6.7%)
Mok 2018 data cut off 2, Feb 2017: Each patient was observed for survival status and subsequent cancer therapies for up to 48 months from the date of first dosing.
Still on treatment, n (%): I: 49 (21.6%) C: 18 (8.0%)
Median duration of follow-up for OS, months (IQR): I: 31.3 C: 31.4
Wu 2017 data cutoff 1, Jul 2016: Median duration of treatment, months (IQR) I: 15·3 (6·9–20·9) C: 12·0 (7·3–18·4)
Still on treatment: I: 66 (29%) C: 38 (17%)
Median duration of follow-up for PFS months (IQR): ITT:22·1 (20·3–23·9; I: 22·1 (20·2–23·9) C: 23·0 (20·3–25·8). |
Mok 2021: Deaths I: 133 [58.6%] C: 152 [67.6%]
Median OS, months (95% CI) I: 34.1 (29.5–39.8) C: 27.0 (24.4–31.6) HR: 0.75; 95% CI 0.59–0.95; two-sided P = 0.016
12 month OS, months (95% CI): I: 85.7% (80.4–89.7) C: 86.0% (80.7–89.9)
42 month OS, months (95% CI): I: 41.0% (34.3–47.6) C: 33.6% (27.2–40.0)
OS for Exon 19 del, HR (95% CI): HR= 0.85 (0.62–1.16) two-sided P = 0.30) OS L858R mutation, HR (95% CI): HR= 0.67 (0.47–0.94) two-sided P = 0.02)
AEs were consistent with those reported in the primary analysis.
Serious AEs, n (%): I: 69 (30.4%) C: 53 (23.7%)
Treatment related serious AEs, n (%): I: 22 (9.7%) C: 10 (4.5%)
Deaths reported as AEs, n (%): I: 24 (10.6%) C: 22 (9.8%)
Paty 2021: At baseline, PRO questionnaires were completed: I: 226 C: 222 More than 90% of patients answering all questions for almost all cycles in both treatment groups.
Global QoL scores: Transformed GHS/QoL scores, range: I: Between 61.5 (cycle 30) - 69.8 (cycle 28) C: Between 68.5 (cycle 17) - 73.1 (cycle 28) [against a possible total score of 100, where 100 represents excellent health]
Physical function transformed scores, range: I: Between 81.7 (cycle 4) - 86.9 (cycle 13) C: Between 82.4 (cycle 17) - 88.6 (cycle 5)
Dacomitinib-treated patients who received dose reductions (DR) reported improvement in GHS/QoL and physical functioning after DR compared with scores prior to the DR.
Mok 2018: Deaths I: 103/227 (45.4%) C: 117/225 (52.0%)
Median OS, months (95% CI) I: 34.1 (29.5 to 37.7) C: 26.8 (23.7 to 32.1) HR, 0.76; 95% CI, 0.58 to 0.99; P = .044).
HR= 0.88 (0.61 - 1.26) OS for L858R HR (95% CI): HR= 0.71 (0.48 - 1.05)
Wu 2017: Median PFS by masked IRC review, months (95% CI) I: 14·7 (11·1–16·6) C: 9·2 (9·1–11·0) HR 0·59 (95% CI 0·47–0·74); p<0·0001
ORR: I: n= 170 (75%; 69–80) C: n=161 (72%; 65–77)
Serious AEs any cause, n (%) I: 62 (27%) C: 50 (22%)
Treatment related serious AEs, n (%) I: 21 (9%) C: 10 (4%)
Deaths AEs related, n (%) (according to investigators) I: 22 (10%) C: 20 (9%)
I: 0.20 C: 4·94 p=0·0002 |
I: 130 (57.3%) C: 146 (64.9%)
Authors conclusions: Mok 2021: In conclusion, the OS benefit from first-line treatment with dacomitinib versus gefitinib was maintained after extended follow-up in patients with advanced NSCLC with EGFR-activating mutations and persisted in patients who had a dose reduction. Improvement of OS was observed in most of the predefined subgroups, including the Asian population and those with exon 21 L858R substitution mutation.
Paty 2021: Dacomitinib was previously shown to improve PFS and OS versus gefitinib. Longer treatment duration, enabled by DR, allowed patients on dacomitinib to improve disease-related symptoms and maintain functioning and overall QoL for longer than patients on gefitinib.
Mok 2018: In conclusion, dacomitinib is the first EGFR TKI to show a significant improvement in OS in a randomized, head-to-head comparison with another EGFR TKI for the first-line treatment of patients with locally advanced or metastatic NSCLC with EGFR activating mutations. Therefore, dacomitinib should be considered one of the standard treatment options for this population.
Wu 2017: In conclusion, dacomitinib treatment was superior to gefitinib with respect to progression-free survival and duration of response in the first-line treatment of patients with EGFR-mutation-positive NSCLC and should be considered as a new treatment option for this population.
|
|
Ramalingam, 2020 Soria, 2018
FLAURA study
NCT02296125 |
Double-blind, phase 3 trial
Performed at 132 sites in 29 countries
Patients were enrolled between: From December 2014 through March 2016
Funding and conflicts of interest:
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. |
Main inclusion criteria:
Main Exclusion criteria:
For more information on in-/exclusion see the trial protocol, available at NEJM.org.
Median age (range) I: 64 yrs (26-85) C: 64 yrs (35-93)
Sex: I: Female: 64% C: Female 62%
Mutation type at randomisation: Exon 19 del: 63% L858R: 37%
CNS metastases: n=116/556=21%
Asian: I: 62% C: 62%
|
Osimertinib 80 mg orally once daily
Randomized: N=279
|
A standard of Care EGFR-TKI (gefitinib at a dose of 250 mg orally once daily or erlotinib at a dose of 150 mg orally once daily)
Randomized: N=277 (n=183: gefitinib;
|
After the analysis of the primary end point of PFS had been performed central collection of progression events was stopped.
At June 12, 2017 the median duration of total treatment exposure, months (range): I: 16.2 (0.1 - 27.4) C: 11.5 (0 - 26.2)
Continued to receive trial treatment: I: N=141 (51%) C: N=64 (23%)
At June 25, 2019 the median duration of total treatment exposure, months (range): I: 20.7 (0.1 - 49.8) C: 11.5 (0.0 - 50.6)
Continued to receive trial treatment: I: N=61 (22%) C: N=13 (5%)
Length of follow-up for overall survival (median): I: 35.8 months C: 27.0 months |
Ramalingam 2020, data cutoff June 2019: Death: N=321 (58% maturity)
Median OS, months (95% CI): I: 38.6 (34.5 - 41.8) C: 31.8 (26.6 - 36.0)
12 month OS, months (95% CI): I: 89 % (85–92) C: 83 % (77–87)
24 month OS, months (95% CI): I: 74 % (69–79) C: 59 % (53–65)
36 month OS, months (95% CI): I: 54 %(48–60) C: 44 % (38–50)
Objective response rate (95% CI): I: 80% (75 - 85) C: 76% (70 - 81) (OR, 1.27; 0.85 - 1.90; P = 0.24)
Safety: AEs grade ≥3 I: 42% C: 47%
Serious AEs: I: 27% C: 27%
Fatal adverse events I: 9 (3%) C: 10 (4%) Possibly related to standard EGFR-TKIs: N=2
For more information on AEs see results section of the article and the Appendix.
Soria 2018 (data cutoff: June 2017): Median PFS (investigator assessed), months (95% CI) I: 18.9 ( 15.2 to 21.4) C: 10.2 (9.6 to 11.1) HR=0.46 (95% CI, 0.37–0.57); P<0.001
Exon 19 deletion: HR=0.43 (0.32–0.56) L858R: HR= 0.51 (0.36–0.71) Median overall survival, I: NC (NC–NC) C: NC (NC–NC) (NC=could not be calculated)
Percent of patients alive at 18 months (95% CI) I: 83 (78–87) C: 71 (65–76)
ORR (95% CI): I: 80% (75 to 85) C: 76% ( 70 to 81) Odds ratio, 1.27; 95% CI, 0.85 to 1.90; P = 0.24)
AEs grade ≥3 I: 95/279=34% C: 125/277=45%
Fatal adverse events I: 6/ 279 patients (2%) C: 10/277 patients (4%)
None of the fatal adverse events were considered to be possibly related to osimertinib, and one fatal adverse event (of diarrhea) was considered to be possibly related to standard EGFR-TKIs. |
Authors conclusion: Ramalingam 2020:
|
|
Park, 2016 Paz-Ares, 2017
NCT01466660
|
A multicentre, international, randomised, open-label phase IIb trial. Performed at 64 sites; in 13 countries.
Patient enrolment between: Dec 13, 2011 - Aug 8, 2013.
Funding and conflicts of interest:
|
pathologically confirmed stage IIIB (ineligible for curative intent surgery or local radiotherapy) or IV (recurrent or metastatic) adenocarcinoma of the lung
according to RECIST V.1.1 - adequate organ function
Main exclusion criteria:
advanced disease
malignancies at other sites
disease
gastrointestinal disorders
Median age (range) I: 63 (30–86) C: 63 (32–89)
Sex: I: Female: 91 (57%) C: Female: 106 (67%)
ECOG PS: 0 – I: 51 (32%) 0 – C: 47 (30%) 1 – I: 109 (68%) 1 – C: 112 (70%)
Adenocarcinoma I: 159 (99%) C: 158 (99%)
Mutation type: Exon 19 del: I: 93 (58%) C: 93 (58%)
Leu858Arg: I: 67 (42%) C: 66 (42%)
Clinical stage: IIIB: I: 8 (5%) C: 3 (2%) IV: C: 156 (98%)
Groups were comparable at baseline. |
Afatinib 40 mg orally once daily.
Dose escalation to 50 mg was allowed after 4 weeks of treatment for patients who did not experience drug-related AE of grade >1.
N=160 |
Gefitinib daily dose of 250 mg.
Modifications were allowed according to the summary of product characteristics or prescribing information or institutional guidelines.
N=159 |
Paz-Ares, 2017 All randomised patients were included in the primary assessment of OS, and updated analysis of PFS.
Safety analysis included all patients who received at least one dose of study drug.
Median follow-up: 42.6 months
Median duration of treatment, months (range): Afatinib: 13.7 ( 0–46.4) Gefitinib: 11.5 (0.5–48.7)
Remained on treatment: I: 14 (8.8%) C: 8 (5.0%)
Park 2016: Tumours were assessed by CT (preferred) or MRI scan after 4 and 8 weeks of treatment, then every 8 weeks until week 64 and every 12 weeks thereafter until permanent discontinuation of study treatment.
Median follow-up for PFS, months (IQR): 27·3 (15·3–33·9).
|
OS events at date cut-off : I: 109 (68.1%) C: 117 (73.6%)
Median OS, months I: 27.9 C: 24.5 HR: 0.86; 95% CI 0.66–1.12; P=0.26
Exon 19 deletions, months: I: 30.7 C: 26.4 HR: 0.83, 95% CI 0.58–1.17, P=0.28
L858R mutation, months: I: 25.0 C: 21.2 HR: 0.91, 95%CI 0.62–1.36, P=0.66
ORR I: 116/160=72.5% C: 89/159=56.0% Odds ratio 2.12 (95% CI 1.32–3.40)
All cause grade ≥3 AEs I: 56.9% C: 53.5%
Treatment related grade ≥3 AEs: I: 50/161=31.3% C: 31/159= 19.5% Fatal AE related with gefitinib (Hepatic and renal failure): N=1
Park: Overall survival data were immature at the time of this primary analysis.
Median overall survival, months (95% CI) I: 27·9 (25·1–32·2) C: 25·0 (20·6–29·3) HR= 0·87 (95% CI 0·66–1·15); p=0·33.
Median PFS by blinded independent review I: 11.0 (10.6–12.9) C: 10.9 (9.1–11.5) HR 0.73, 95% CI 0.57–0.95
Median PFS Subgroup Leu858Arg I: 10·9 (8·1–12·9) C: 10·8 (7·2–12·8) HR= 0·71 (0·48–1·06)
Median PFS Subgroup Exon 19 deletion I: 12·7 (10·6–14·7) C: 11·0 (9·1–12·7) HR= 0·76 (0·55–1·06)
Objective response rate (ORR) I: 112/160 (70%) C: 89/159 (56%) Odds ratio 1·87 [95% CI 1·18–2·99]; p=0·0083.
AEs grade ≥3: I: n= 91 (57%) C: n=83 (52%)
QoL, EQ 5D Post-baseline adjusted mean (SE) I: 0·77 (0·01) C: 0·80 (0·01) P=0.14 mean score up to median follow-up of 56 weeks. |
Data were collected by the investigators and were analysed jointly with the funder.
Authors conclusion: Paz-Ares: In LUX-Lung 7, there was no significant difference in OS with afatinib versus gefitinib. Updated PFS (independent review), TTF and ORR data were significantly improved with afatinib.
Park: of data reported herein indicates that afatinib might offer improved efficacy compared with gefitinib, while conferring a predictable tolerability profile. Our findings suggest that first-generation and second-generation EGFR targeted drugs might not be interchangeable. We believe that these data provide additional evidence to help to inform decision making when choosing a first-line treatment for patients with EGFR mutation positive NSCLC. |
|
Nakagawa, 2019 Nadal, 2022, Yoh 2020
RELAY study
NCT02411448 |
Double-blind, placebo-controlled, phase 3 trial
Performed in 100 hospitals, clinics, medical centres in 13 countries (UK, South Korea, Hong Kong, USA, Japan, Taiwan, Canada, France, Italy, Germany, Spain, Romania, Turkey).
Patients were enrolled between: Jan 28, 2016 - Feb 1, 2018.
Funding and conflicts of interest:
|
Main inclusion criteria:
Main exclusion criteria:
For more details on in-/exclusion see the appendix of the article.
N total at baseline: I: N=224 C: N=225
Age, Median (IQR): I: 65 (57-71) C: 64 (56-70)
Sex: I: 63% Female C: 63% Female
East Asia: I: 74% C: 76%
|
Intravenous ramucirumab 10 mg/kg once every 2 weeks and oral erlotinib 150 mg/day.
Study treatment continued until radiographic progression as assessed by the investigator according to RECIST V. 1.1, or unacceptable toxicity or withdrawal of consent, non-compliance, or investigator decision.
|
Intravenous placebo once every 2 weeks and oral erlotinib 150 mg/day.
Study treatment continued until radiographic progression as assessed by the investigator according to RECIST V.1.1, or unacceptable toxicity or withdrawal of consent, non-compliance, or investigator decision.
|
Length of follow-up in months, median (IQR): 20·7 (15·8–27·2)
Patients who discontinued study treatment were followed up for survival until study completion.
Did not receive allocated treatment: I: 3 (1.3%)
C: 0
Discontinued study treatment: I: 157 (70.1 %) Reasons:
C: 182 (80.9%) Reasons:
At data cutoff still on treatment: I: 64/224=29% C: 43/225=19% |
OS: Data were immature at data cutoff. Median interim OS was not reached in either group. A final analysis is planned when at least 300 OS events have occurred.
Median PFS, months (95% CI): I: 19·4 (15·4–21·6) C: 12·4 (11·0–13·5) HR for progression/ death: 0·59 [95% CI 0·46–0·76], p<0·0001
1-year PFS (95% CI) I: 71·9% (65·1–77·6) C: 50·7% (43·7–57·3)
PFS in subgroups:
I: 64/123 C: 84/120 Unstratified HR: 0·65 (0·47–0·90)
I: 58/99 C: 74/105 Unstratified HR: 0·62 (0·44–0·87)
AEs grade ≥3: I: 159/221 (72%) C: 121/225 (54%)
Overall response (95% CI): I: 76% (71–82) C: 75% (69–80) Stratified p value: 0·74
Nadal (2022): I: n=6 C: n=0
Death related to study drug: I: n=1 (hemothorax)
For a detailed list of AEs see appendix of the article Table S6 and separate publication by Nadal (2022).
|
Authors conclusion: Nakagawa (2019): In conclusion, ramucirumab plus erlotinib provided superior progression-free survival versus placebo plus erlotinib in first-line metastatic EGFR-mutated NSCLC. Safety was consistent with the established safety profiles of the individual compounds and a metastatic NSCLC population. The RELAY regimen is therefore a viable new treatment option for the initial treatment of patients with metastatic EGFR-mutated NSCLC.
Yoh, 2020: Patients’ overall quality of life and symptom burden did not differ with the addition of ramucirumab to erlotinib compared to placebo/erlotinib. These data support the clinical benefit of ramucirumab/erlotinib in untreated EGFR-mutated metastatic NSCLC.
|
|
Hosomi, 2020 Miyauchi, 2022
NEJ009 study
UMIN000006340 |
Randomised, phase 3 study
Performed in 47 institutions in Japan.
Patients were enrolled between: October 2011 and September 2015.
Funding and conflicts of interest: Supported by grant-in-aids from the Japan Society for Promotion of Science and Japanese Foundation for the Multidisciplinary Treatment of Cancer. Also supported by the North-East Japan Study Group. Disclosures by the authors are online available with the article. |
Main inclusion criteria:
Main exclusion criteria:
N randomly assigned: I: N=172 C: N=173
Age, Mean (SD, range): I: 64.8 (7.8, 34-75) C: 64 (8.4, 37-75)
Sex: I: 67.1 % Female C: 62.8 % Female
ECOG PS: 0 – I: 98 (57.6) 0 – C: 107 (62.2) 1 – I: 72 (42.4) 1 – C: 65 (37.8)
Adenocarcinoma I: 168 (98.8) C: 170 (98.8)
Mutation type: Exon 19 del: I: 93 (54.7) C: 95 (55.2) L858R: I: 69 (40.6) C: 67 (39.0) Other: I: 8 (4.7) C: 10 (5.8)
Clinical stage: IIIA I: 0 (0.0) C: 1 (0.6) IIIB: I: 6 (3.5) C: 4 (2.3) IV: I: 139 (81.8) C: 137 (79.7) Postoperative relapse: I: 24 (14.7) C: 30 (17.4)
CNS metastases: I: 50 (29.4) C: 38 (22.1) Groups were comparable at baseline. |
GCP regimen: Gefitinib 250 mg orally once every day combined with carboplatin area under the curve 5 and pemetrexed 500 mg/m2 in a 3-week cycle for up to six cycles, followed by concurrent gefitinib and pemetrexed maintenance.
The regimens administered after the protocol treatment are summarized in Appendix. |
Gefitinib 250 mg orally once every day.
The regimens administered after the protocol treatment are summarized in Appendix. |
Miyauchi 2022, with updated results: Median follow-up duration on May 22, 2020: 84 months.
Hosomi 2020: Median follow-up time at September 3, 2018: (with 195 events) 45 months.
Median duration of gefitinib treatment, months (range) I: 22.4 (0.5 – 59.8) C: 11.6 (1.0 – 70.8
Median duration of pemetrexed maintenance therapy, months (range) I: 11.9 (0 – 57.9)
|
Miyauchi 2022, with updated results: Deaths, n (%): 243 (71%)
Mean or median survival time (unclear: text and figure not in concordance): I: 49.0 months C: 38.5 months HR: 0.822; 95% CI, 0.639 to 1.058; P = 0.127
2-year survival rate: I: 77.1%, C: 69%,
5-year survival rate I: 39% C: 34%
Subgroup analyses: Larger numerical between-group differences in the HRs for OS were observed between male and female patients.
5-year restricted mean survival time (RMST): I: 43.6 months C: 38.6 months P = 0.017
7-year RMST: I: 51.6 months C: 45.3 months P = 0.037
Updated median corrected PFS2, months (95% CI): I: 20.9 (18.0 – 24.0) C: 18.0 (16.3 – 20.7) HR: 0.77; 95% CI 0.62 – 0.97; P= 0.027
Updated median PFS2 with the same definition, months (95% CI): I: 32.5 (29.0 – 36.6) C: 20.7 (17.9 – 24.6) HR: 0.58; 95% CI, 0.46 – 0.73; P <0.001.
Updated result treatment related AEs: Grade ≥3: I: 66.5% C: 31.0% Odds ratio: 0.23; 95% CI, 0.15 – 0.36; P <0.001.
Hosomi 2020: Overall survival, median (95% CI): I: 50.9 (41.8 – 62.5) C: 38.8 (31.1 – 47.3) HR for death: 0.72 (0.55 – 0.95) P = 0.021
Deaths, n (%): 195 (57%)
PFS in months, median (95% CI): I: 20.9 (17.9 – 24.2) C: 11.2 (9.0 – 13.4) HR, 0.49; 95% CI, 0.39-0.62; P<0.001.
PFS2 in months, median (95% CI): I: 20.9 (18.0 – 24.0) C: 20.7 (15.7 –21.2) HR: 0.99; P = 0.90
Corrected PFS2, months: I: 20.9 C: 18.0 HR: 0.82 (95% CI, 0.65 to 1.03); P = 0.09
PFS2 with same definition, months I: 32.5 (29.0 – 36.6) C: 20.7 (17.9 – 24.9) HR: 0.59; P<.001
ORR (95% CI): I: 84% (79 – 90) C: 67% (60 – 74) P < 0.001
Treatment related AEs I: 95.9% C: 98.2%
Treatment related AEs grade ≥3 I: 65.3% C: 31.0%
Fatal AEs (severe infection) I: n=1 C: n=0
Treatment discontinued due to AES: I: 10.7% C: 9.9% [For more information about the observed AEs see the article]
QOL:
After 24 months, more patients in the GCP group completed the questionnaire than those in the gefitinib group which indicates that informative censoring occurred in the gefitinib group more because of poorer prognosis. |
Authors conclusion:
|
|
Saito, 2019
UMIN000017069 |
A randomised, open-label, multicentre, phase 3 study
Performed in 69 centres across Japan
Patients were enrolled between: June 3, 2015, and Aug 31, 2016
Funding and conflicts of interest: The funder Chugai Pharmaceutical approved the study design, and was involved in the provision of information. Funder had no role in data collection, data analysis, data interpretation, or writing of the report. Declaration of interests is disclosed. |
Main inclusion criteria:
Main Exclusion criteria:
For a full list of in-/exclusion, and eligibility criteria regarding pretreatments and washout periods before entry, see the appendix.
Median age (IQR) I: 67 (61–73) C: 68 (62–73)
Sex: I: Female: 71 (63%) C: Female: 73 (65%)
ECOG PS: 0 – I: 64 (57%) 0 – C: 68 (61%) 1 – I: 48 (43%) 1 – C: 42 (38%) 2 – I: 0 2 – C: 2 (2%)
Adenocarcinoma I: 110 (98%) C:112 (100%)
Mutation type: Exon 19 del: I: 56 (50%) C: 55 (49%)
Postoperative recurrence I: 22 (20%) C: 20 (18%)
CNS metastases: I: 36 (32%) C: 36 (32%)
Groups were comparable at baseline. |
Oral erlotinib 150 mg once daily and intravenous bevacizumab 15 mg/kg once every 21 days.
Randomized: n=114 Modified intention-to-treat analysis: n=112 Safety analysis: n=112
Dose reductions:
fulfilled.
Patients remained on treatment until disease progression or intolerable toxicity.
|
Oral erlotinib 150 mg once daily.
Randomized: n=114 Modified intention-to-treat analysis: n=112 Safety analysis: n=114
Dose reductions:
fulfilled.
Patients remained on treatment until disease progression or intolerable toxicity. |
At Sept 21, 2017 median follow up was: 12·4 months (IQR 7·0–15·7).
Median duration of treatment, days (range) Erlotinib I: 405 (5–807) C: 364 (43–736) Bevacizumab I: 350 (21–736)
|
Median PFS, months (95% CI) I: 16·9 (14·2–21·0) C: 13·3 (11·1–15·3) HR= 0·605 (95% CI 0·417–0·877; p=0·016)
L858R subgroup PFS, months (95% CI): I: 17·4 (12·6–not estimable) C: 13·7 (8·8–15·5)
Objective response (OR) (95% CI): I: 72% (63·1–80·4) C: 66% (56·5–74·7) p=0·31
Leu858Arg OR (95% CI) I: 68% C: 65%
Exon 19 deletion OR (95% CI) I: 77%
AEs grade ≥3 I: 88% C: 46%
Serious AEs: I: 8% C: 4% Bevacizumab discontinuation due to AEs: 29%
Erlotinib discontinuation due to AEs: I:19% C: 15%
For more information on AEs see results section of the article and the Appendix. |
Authors conclusion: In conclusion, NEJ026 met the primary endpoint at the preplanned interim analysis, showing that patients with EGFR-positive NSCLC treated with a combination of bevacizumab and erlotinib had longer progression-free survival than patients treated with erlotinib alone. These results suggest that combination therapy with bevacizumab and erlotinib has the potential to become a standard treatment for patients with EGFR-positive NSCLC if the overall survival data and quality of life analyses are favourable. |
|
Zhou, 2021
ARTEMIS-CTONG1509
NCT02759614 |
A randomized, open-label, controlled, multicenter phase 3 study.
Conducted at 14 centers in China.
Patient enrolment: From May 2016 to July 2017.
Funding and conflicts of interest: Funded by the National Key R&D Program of China (grant no. 2016YFC1303800), the Chinese Thoracic Oncology Group (CTONG), and the High-level Hospital Construction Project (grant no. DFJH201810 to Q.Z.). Editorial support was funded by CTONG. Declaration of interests is disclosed.
|
Main inclusion criteria:
adjuvant chemotherapy in the previous 6 months since the final administration date;
Main exclusion criteria: Mixed adenosquamous carcinomas with squamous component and evidence of CNS metastases, except for patients without any symptoms or patients with symptoms but who had stable disease for at least 28 days after treatment of CNS metastases.
Age, Median (range): I: 57 (33-78) C: 59 (27-77)
Female, n (%): I: 97 (61.8) C: 96 (62.3)
ECOG PS: 0 – I: 25 (15.9) 0 – C: 17 (11.0) 1 – I: 132 (84.1) 1 – C: 137 (89.0)
Adenocarcinoma I: 157 (100) C: 154 (100)
Mutation type: Exon 19 del: I: 82 (52.2) C: 79 (51.3)
CNS metastases at baseline: I: 44 (28.0) C: 47 (30.5)
Clinical stage: IIIB: I: 4 (2.6) C: 6 (3.9) IV: I: 141 (89.8) C: 133 (86.4) Recurrence: I: 12 (7.6) C: 15 (9.7)
Groups were comparable at baseline. |
Bevacizumab plus erlotinib
Oral erlotinib 150 mg/day with intravenous bevacizumab 15 mg/kg once every 3 weeks and study treatment continued until disease progression, intolerable toxicity, or withdrawal of patient consent.
n=157
|
Erlotinib only
Oral erlotinib 150 mg/day and study treatment continued until disease progression, intolerable toxicity, or withdrawal of patient consent.
n=154
|
At January 18, 2019 Treatment discontinuation: I: 109 (69.4%) with a median time-to-treatment failure (TTF) of 18.2 months C: 126 (81.8%) with a median TTF of 12.4 months
Median exposure to erlotinib, days(IQR): I: 544.5 (20–928) C: 377.0 (6–890)
Median exposure bevacizumab cycles (IQR): I: 22 (1–45)
|
The independent review committee (IRC)-assessed PFS in months, median (95% CI): I: 17.9 (15.2–19.9) C: 11.2 (9.7–13.8) (HR for progression/ death: 0·55 [95% CI 0.41–0.73], p<0·001)
IRC-assessed PFS in months per mutation type (95% CI):
I: 7.7 13.8–19.5 C: 12.5 (11.1–16.6) HR = 0.62; 95% CI, 0.42–0.93; p = 0.017
I: 19.5 (15.3–22.2) C: 9.7 (9.6–12.4) HR = 0.50; 95% CI, 0.32–0.77; p = 0.001
IRC-assessed PFS in months in patients with brain metastases at baseline (95% CI): I: 17.9 (15.2–20.7) C: 11.1 (9.7–12.5) HR = 0.48; 95% CI, 0.27–0.84; p=0.008
OS: At January 8, 2021: OS data remained immature with 55% (172/311) of the events recorded.
Median OS, months (95% CI): I: 36.2 (32.5–42.4) C: 31.6 (27.2–40.0) p=0.581
2- year OS, months (95% CI): I: 70.1% (61.9–76.8) C: 64.6% (56.1–71.8) p=0.317
3- year OS, months (95% CI): I: 51.1% (42.5–59.1) C: 46.3% (37.8–54.4) p=0.424
Median OS in months (95% CI):
I: 31.6 (23.0–44.3) C: 26.8 (19.5–32.6) HR = 0.62; 95% CI, 0.38–1.01; p = 0.052 - without brain metastases at baseline: I: 37.9 (32.7–43.7) C: 41.1 (27.9–not evaluable) HR = 1.09; 95% CI, 0.76–1.58; p=0.625
ORR (95% CI): I: 86.8% (80.4–91.8) C: 84.7% (77.9–90.0) p=0.624
AEs grade ≥3: I: 54.8% C: 26.1% [For more information about AEs see the article Table 4 and the appendix Table S2].
Erlotinib discontinuation due to AEs: I: 7.0% C: 3.3%
Bevacizumab discontinuation due to AEs: I: 24.2 %
Death due to AEs: I: n=1 C: n=1
QoL: QoL scores were comparable, with no change from baseline reported for either group (Figure S6). Our results demonstrate the addition of bevacizumab to erlotinib does not affect the patients’ overall QoL. |
Authors conclusion: This study shows that bevacizumab plus erlotinib can provide superior PFS over erlotinib alone in Chinese patients with untreated
|
|
Piccirillo, 2022
The BEVERLY study
NCT02633189
EudraCT 2015-002235-17 |
Multicenter, randomized, phase 3 trial (open label; however, central radiologic revision and statistical analyses were conducted with blinded arms)
Performed in 43 centers in Italy.
Patients were enrolled between: Between April 11, 2016 - February 27, 2019
Funding and conflicts of interest: Hoffmann–La Roche provided partial funding and experimental drugs. Hoffmann–La Roche had no role in the study design, protocol writing, data collection, data analysis, data interpretation, and writing of the report. Declaration of interests is reported with the article. |
Main inclusion criteria:
[See protocol for full list of inclusion criteria]
Main exclusion criteria:
Age, Median (IQR): I: 65.9 (57.9–71.8) C: 67.7 (60.7–73.6)
Age >65 years, n (%) I: 42 (52.5) C: 49 (61.3)
Sex: I: 65.0 Female C: 62.5 Female
Smoking status, n (%) Never I: 46 (57.5) C: 37 (46.3) Former/Current I: 34 (42.5) C: 43 (53.8)
ECOG PS: 0 – I: 52 (65.0) 0 – C: 47 (58.8) 1 – I: 26 (32.5) 1 – C: 29 (36.3) 2 – I: 2 (2.5) 2 – C: 4 (5.0)
Exon 19 del: I: 44 (55.0) C: 44 (55.0) L858R: I: 34 (42.5) C: 32 (40.0) Other: I: 2 (2.5) C: 4 (5.0)
Clinical stage IIIB: I: 3 (3.8) C: 5 (6.3) Clinical stage IV: I: 77 (96.3) C: 75 (93.8) Groups were comparable at baseline. |
Erlotinib 150 mg orally once daily + bevacizumab, 15 mg/kg intravenously, every 21 days.
Treatment continued until disease progression, unacceptable toxicity or patient’s or physician’s motivated decision.
Randomly assigned: N=80
|
Erlotinib 150 mg orally once daily.
Treatment continued until disease progression or unacceptable toxicity or patient’s or physician’s motivated decision.
Randomly assigned: N=80
|
Median follow-up: 36.3 months (95% CI: 30.7–40.9) With 140 PFS events (87.5%) reported: I: 68 (85.0%) C: 72 (90.0%)
Median duration of erlotinib treatment, months (IQR): I: 14.4 (8.8–24.2) C: 9.3 (4.6–14.7)
Median duration of bevacizumab, months (IQR): I: 14.2 (5.8–22.3)
Continued to receive trial treatment: I: N=9 (11.3%) C: N=1 (1.3%) n.
Discontinuation of erlotinib, n (%): I: 11 patients (13.8%) C: 13 patients (16.5%)
Discontinuation of bevacizumab for toxicity, n (%): I: 24 (30.0%) |
Deaths, n (%): I: 41 (51.3%) C: 49 (61.3%)
Overall survival, median (95% CI): I: 33.3 (24.3–45.1) C: 22.8 (18.3–33.0) HR: 0.72 [95% CI: 0.47–1.10], p = 0.132
Median investigator-assessed (IA)-PFS, months (95% CI): I: 15.4 (12.2–18.6) C: 9.6 (8.2–10.6) HR = 0.66 [95% CI: 0.47–0.92], p = 0.015.
Subgroup analysis: OS for former or current smokers, months (95% CI): I: 35.3 (25.6–not estimated) C: 19.7 (12.5–23.4)
Interaction effect of smoking with treatment for OS: p = 0.0077
IA-PFS for former or current smokers, months (95% CI): I: 16.9 (10.2–21.8] C: 8.8 (5.6–9.6)
Interaction effect of smoking with treatment for IA-PFS: p = 0.0323
ORR (complete or partial response ) (%; 95% CI): I: 56 (70%; 60% – 80%) C: 40 (50%; 39% – 61%) P= 0.010
Serious AEs, n: I: 45 C: 30
Likely or certainly treatment related AEs I: 9 of 45 (20.0%) C: 1 of 30 (3.3%)
Fatal AEs: I: 4 (5.0%) C: 4 (5.0%)
Death due to intracranial hemorrhage related to the study treatment I: 1 C: 0
AEs grade ≥3 I: 45 (56%) C: 39 (49%) [For more info on AEs see Table 2 and sup. Table 7]
Completed QoL questionnaires baseline: I: 74 (92.5%) C: 76 (95.0%)
|
- Dose reduction for adverse events was allowed for erlotinib. - Dose reduction of bevacizumab was not planned, but drug could be temporarily or permanently suspended in case of hypertension, proteinuria, thrombosis/embolism, hemorrhage, cardiac heart failure, or wound healing complications in addition to any other grade 3 or 4 bevacizumab-related toxicity.
Authors conclusion: In conclusion, bevacizumab plus erlotinib might be considered among the first-line therapeutic option in patients who cannot receive osimertinib and the strategy of combining an antiangiogenic drug with a TKI is worth of further investigation.
|
Risk of bias tables
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
ARCHER 1050 (Wu, 2017 NCT01774721 |
Definitely yes;
Reason: Using a computer-generated random code that was assigned by a central interactive web response system (IWRS). The IWRS was managed by a vendor who had no clinical involvement with the trial. The allocation sequence, based on a randomisation-requirement specification form (prepared by the IWRS vendor in accordance with the requirements of the study sponsor), was generated by the IWRS. |
Probably yes;
Reason: variables), and assigned each patient to a treatment group on the basis of the IWRS output. |
Definitely no;
Reason: Patients were not masked to treatment assignment. Sites were unmasked to study drug but the vendors and sponsor remained masked.
Tumour assessment by independent review was masked. Central imaging was masked to the reviewers by BioClinica.
Investigators were not masked to treatment assignment. Study sponsor personnel were unmasked at database lock for the primary analysis of progression-free survival. |
Probably yes;
Reason: I: assigned to dacomitinib N=227 C: assigned to gefitinib N=225 Discontinued treatment: I: Wu (2017) N=161 I: Mok (2018) N=178 C: Wu (2017) N=186 Mok (2018) N=206
Included in the intention to treat analysis: I: N=227 C: N=225
Mature OS analysis for the ITT population is presented.
WU (2017): Efficacy analysis, PFS and time to treatment failure was calculated in the ITT population. Duration of response among the objective responders in the ITT population.
Patients in the ITT population who received at least one dose of study drug were included in the safety analysis. PROs were evaluated in the ITT population who also had a baseline assessment and at least one post-baseline assessment. |
Probably yes;
Reason: Secondary outcomes not reported:
|
Definitely no;
Reason: |
Some concerns (no blinding, role of the funder)
OS, PFS and ORR: Low QoL some concerns |
|
FLAURA trial (Ramalingam, 2020 Soria, 2018) |
Definitely yes;
Reason: In study protocol: A unique 7-digit enrolment number obtained by the investigator through the Interactive Voice Response System /Interactive Web Response System. |
Probably yes;
Reason: Eligible patients will be centrally randomised in a 1:1 ratio using the IVRS/IWRS system. The actual EGFR TKI has to be selected at a site/country level according to country’s marketing authorisation prior to the site initiation.
The IVRS/IWRS will assign the bottles of study material to be dispensed to each patient. |
Probably yes;
Reason: Not explicitly described.
The investigational product will be labelled using a unique material pack code, which is linked to the randomisation code. (…) The active and placebo tablets will be identical and presented in the same packaging to ensure blinding of the medication.
A protocol amendment allowed patients who had been assigned to a standard EGFR-TKI to cross over to open-label osimertinib after confirmation of objective disease progression by blinded independent central review and post-progression documentation of T790M-positive mutation status |
Definitely no;
Reason: Randomized: N=556
Continued to receive trial treatment at time of data cut off: Loss to follow-up was more frequent in the comparator group.
|
Probably yes;
Reason: All relevant outcomes were reported. Secondary outcomes not reported:
|
Definitely no;
Reason:
|
Some concerns |
|
LUX-Lung 7 (Park, 2016 Paz-Ares, 2017) |
Definitely yes;
Reason: Permuted blocks of size four were created with a validated random number generating system at Boehringer Ingelheim verified by trial-independent statistician, and implemented centrally via an interactive voice or web-based response system. |
Probably yes;
Reason: Randomisation was implemented centrally via an interactive voice or web-based response system. |
Probably no;
Reason: Patients and clinicians were not masked to treatment allocation.
Individuals directly involved in the conduct and analysis of the trial did not have access to the randomisation schedule.
Independent review of tumour response was done in a blinded manner.
|
Probably yes;
Reason: I: assigned to afatinib N=160 C: assigned to gefitinib N=159
Discontinued treatment: I: Park (2016): N=140 I: Paz-Ares (2017): N=146 C: Park (2016): N=149 C: Paz-Ares: N=151
Included in the ITT analysis: I: N=160 C: N=159
Park (2016): Efficacy analyses were done in the intention-to-treat population and safety analyses were done in patients who received at least one dose of study drug.
Paz-Ares (2020): All randomised patients were included in the primary assessment of OS, and updated analysis of PFS and TTF. Safety analysis included all patients who received at least one dose of study drug. |
Definitely yes;
Reason: All relevant outcomes were reported. |
Definitely no;
The funder designed the trial in collaboration with the steering committee. Data were collected by the investigators and were analysed jointly with the funder. The funder and all authors were responsible for data interpretation and the development of the Article, and approved the final version. The Article was written by the corresponding author in collaboration with the coauthors, with independent medical writing assistance, supported financially by the funder. The steering committee had access to the raw data.
|
Some concerns (role of the funder)
For OS, PFS, ORR Low For QoL some concerns |
|
RELAY study (Nakagawa, 2019 |
Definitely yes;
Reason: Patients were randomly allocated (1:1) to treatment with ramucirumab plus erlotinib or placebo plus erlotinib via an interactive web-response system with a computer generated random sequence.
|
Probably yes;
Reason: The IWRS registration consists of assigning the patient a unique study identification number for all patients (Parts A and B) and patients in Part B will be randomized into 1 of the 2 treatment arms on a 1:1 basis.
Patient-level unblinded data will not be shared with sites until the study is completed. Treatment assignment will be scrambled in the reporting database until the database lock for data analysis. This will ensure that unblinded aggregate efficacy and safety results are not available until the time of final data analysis. |
Probably yes;
Reason: Physicians, patients, and all clinical study personnel were masked to assigned treatment.
For the primary analysis, some sponsor personnel were unmasked to collate, analyse, and communicate data to authors.
|
Definitely no;
Reason: Randomized: N=449
Continued to receive trial treatment at time of data cut off: Loss to follow-up was more frequent in the comparator group.
|
Definitely yes;
Secondary patient-reported outcomes (on the Lung Cancer Symptom Scale and EuroQol 5-dimension, 5-level questionnaire) will be reported elsewhere. |
Definitely no;
Reason: The funder was involved in study design, data collection, data analysis, data interpretation, and writing of the report.
|
Some concerns |
|
NEJ026 |
Probably yes.
Patients were randomly assigned (1:1).
Randomisation was done by minimisation, stratified by sex, smoking status, clinical stage, and EGFR mutation subtype. |
Probably yes
Central randomisation was done at a data centre (AC Medical, Tokyo, Japan) using patient enrolment sheets provided by the investigators. The data centre notified investigators of treatment allocation for each patient. |
Definitely no.
All patients and investigators were unmasked to treatment allocation. |
Definitely yes. |
Probably yes.
(Secondary endpoints will be reported once follow-up is completed.
|
Probably no
The sample size was small. The study was not powered for subgroup analysis of the primary endpoint.
The independent data monitoring committee held a meeting on Jan 23, 2018, and recommended early termination of the study based on the results of the interim analysis. However, the authors considered that the study had to be continued to obtain data for other endpoints in addition to PFS. |
Some concerns |
|
ARTEMIS-CTONG1509
|
Probably yes.
Randomly allocated (1:1) to treatment with erlotinib or using a dynamic randomization process. |
Probably no.
Not described. |
All patients and investigators were unblinded to treatment allocation. |
Probably yes |
Definitely yes;
Reason: All relevant outcomes were reported. |
Probably yes.
|
High |
|
NEJ009 study |
Definitely yes.
Eligible patients were randomly assigned. |
Definitely yes.
Protocol: TCOG Enrollment Center: the patient is numbered and anonymized and entered in the intra-institution communication chart. (…). The Assignment Results Report is faxed to the attending physician and Tohoku University’s Trial office. |
Probably no.
Not reported |
Definitely yes. |
Definitely yes;
Reason: All relevant outcomes were reported. |
Probably yes.
|
Low |
|
The BEVERLY study (Piccirillo, 2022) |
Probably yes.
The participants were randomly assigned (1:1).
Registration was web based. |
Definitely yes.
Registration, randomization, and data collection were web based at Clinical Trials Unit of Istituto Nazionale Tumori, Istituto di Ricovero e Cura a Carattere Scientifico, Fondazione G. Pascale (Naples, Italy). |
Probably no.
The trial was open label; however, central radiologic revision and statistical analyses were conducted with blinded arms. |
Definitely yes. |
Definitely yes
Reason: All relevant outcomes were reported. |
Probably yes.
|
Low |
Table of excluded studies
Exclusietabel
Tabel Exclusie na het lezen van het volledige artikel
Previous search
|
Auteur en jaartal |
Redenen van exclusie |
|
Aguiar 2018 |
a decision-analytic model |
|
Beau-Faller 2014 |
No comparison |
|
Belani 2013 |
Other intervention (Combination therapy) |
|
Chouaid 2017 |
Costeffectiveness analysis, model based (no new data collection; Data from LUX-Lung 7) |
|
Ding 2017 |
Other research question |
|
Fan 2014 |
Specific population: patients with brain metastases |
|
Greenhalgh 2016 |
Other research question |
|
Heymach 2014 |
Not relevant for PICO |
|
Isla 2017 |
Other research question |
|
Remon 2017 |
PROTOCOL |
|
Reck 2018 |
Other intervention (Combination therapy) |
|
Zhang 2016 |
Other intervention |
|
Zhang 2017 |
Relevant individual studies were included in our review |
Updated search
|
Nr. |
Reference |
Reason for exclusion |
|
1 |
Zhao H, Yao W, Min X, Gu K, Yu G, Zhang Z, Cui J, Miao L, Zhang L, Yuan X, Fang Y, Fu X, Hu C, Zhu X, Fan Y, Yu Q, Wu G, Jiang O, Du X, Liu J, Gu W, Hou Z, Wang Q, Zheng R, Zhou X, Zhang L. Apatinib Plus Gefitinib as First-Line Treatment in Advanced EGFR-Mutant NSCLC: The Phase III ACTIVE Study (CTONG1706). J Thorac Oncol. 2021 Sep;16(9):1533-1546. doi: 10.1016/j.jtho.2021.05.006. Epub 2021 May 24. PMID: 34033974. |
Wrong intervention |
|
2 |
Lu S, Dong X, Jian H, Chen J, Chen G, Sun Y, Ji Y, Wang Z, Shi J, Lu J, Chen S, Lv D, Zhang G, Liu C, Li J, Yu X, Lin Z, Yu Z, Wang Z, Cui J, Xu X, Fang J, Feng J, Xu Z, Ma R, Hu J, Yang N, Zhou X, Wu X, Hu C, Zhang Z, Lu Y, Hu Y, Jiang L, Wang Q, Guo R, Zhou J, Li B, Hu C, Tong W, Zhang H, Ma L, Chen Y, Jie Z, Yao Y, Zhang L, Jie W, Li W, Xiong J, Ye X, Duan J, Yang H, Sun M, Sun C, Wei H, Li C, Ali SM, Miller VA, Wu Q. AENEAS: A Randomized Phase III Trial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or MetastaticNon-Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations. J Clin Oncol. 2022 Sep 20;40(27):3162-3171. doi: 10.1200/JCO.21.02641. Epub 2022 May 17. PMID: 35580297; PMCID: PMC9509093. |
Wrong intervention |
|
3 |
Cheng Y, Mok TS, Zhou X, Lu S, Zhou Q, Zhou J, Du Y, Yu P, Liu X, Hu C, Lu Y, Zhang Y, Lee KH, Nakagawa K, Linke R, Wong CH, Tang Y, Zhu F, Wilner KD, Wu YL. Safety and efficacy of first-line dacomitinib in Asian patients with EGFR mutation-positive non-small cell lung cancer: Results from a randomized, open-label, phase 3 trial (ARCHER 1050). Lung Cancer. 2021 Apr;154:176-185. doi: 10.1016/j.lungcan.2021.02.025. Epub 2021 Feb 23. PMID: 33721611. |
wrong population |
|
4 |
Le X, Nilsson MB, Robichaux JP, Heymach JV. ARTEMIS highlights VEGF inhibitors as effective partners for EGFR TKIs in EGFR mutant NSCLC. Cancer Cell. 2021 Sep 13;39(9):1178-1180. doi: 10.1016/j.ccell.2021.07.017. Epub 2021 Aug 12. PMID: 34388379. |
wrong study design |
|
5 |
Ito K, Morise M, Wakuda K, Hataji O, Shimokawaji T, Takahashi K, Furuya N, Takeyama Y, Goto Y, Abe T, Kato T, Ozone S, Ikeda S, Kogure Y, Yokoyama T, Kimura M, Yoshioka H, Murotani K, Kondo M, Saka H. A multicenter cohort study of osimertinib compared with afatinib as first-line treatment for EGFR-mutated non-small-cell lung cancer from practical dataset: CJLSG1903. ESMO Open. 2021 Jun;6(3):100115. doi: 10.1016/j.esmoop.2021.100115. Epub 2021 May 10. PMID: 33984681; PMCID: PMC8134659. |
wrong study design |
|
6 |
Planchard D, Feng PH, Karaseva N, Kim SW, Kim TM, Lee CK, Poltoratskiy A, Yanagitani N, Marshall R, Huang X, Howarth P, Jänne PA, Kobayashi K. Osimertinib plus platinum-pemetrexed in newly diagnosed epidermal growth factor receptor mutation-positive advanced/metastatic non-small-cell lung cancer: safety run-in results from the FLAURA2 study. ESMO Open. 2021 Oct;6(5):100271. doi: 10.1016/j.esmoop.2021.100271. Epub 2021 Sep 17. PMID: 34543864; PMCID: PMC8453202. |
wrong comparator |
|
7 |
Cheng Y, He Y, Li W, Zhang HL, Zhou Q, Wang B, Liu C, Walding A, Saggese M, Huang X, Fan M, Wang J, Ramalingam SS. Osimertinib Versus Comparator EGFR TKI as First-Line Treatment for EGFR-Mutated Advanced NSCLC: FLAURA China, A Randomized Study. Target Oncol. 2021 Mar;16(2):165-176. doi: 10.1007/s11523-021-00794-6. Epub 2021 Feb 5. PMID: 33544337; PMCID: PMC7935816. |
wrong population |
|
8 |
Li A, Chen HJ, Yang JJ. Design and Rationale for a Phase II, Randomized, Open-Label, Two-Cohort Multicenter Interventional Study of Osimertinib with or Without Savolitinib in De Novo MET Aberrant, EGFR-Mutant Patients with Advanced Non-Small-Cell Lung Cancer: The FLOWERS Trial. Clin Lung Cancer. 2023 Jan;24(1):82-88. doi: 10.1016/j.cllc.2022.09.009. Epub 2022 Sep 30. PMID: 36333268. |
wrong study design |
|
9 |
Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y, Liu C, Zhu S, Zhang X, Li Y, Liu J, Cao L, Cheng Y, Zhao H, Zhang S, Zang A, Cui J, Feng J, Yang N, Liu F, Jiang Y, Gu C. Central Nervous System Efficacy of Furmonertinib (AST2818) Versus Gefitinib as First-Line Treatment for EGFR-Mutated NSCLC: Results From the FURLONG Study. J Thorac Oncol. 2022 Nov;17(11):1297-1305. doi: 10.1016/j.jtho.2022.07.1143. Epub 2022 Aug 3. PMID: 35932953. |
Wrong intervention |
|
10 |
Hou X, Li M, Wu G, Feng W, Su J, Jiang H, Jiang G, Chen J, Zhang B, You Z, Liu Q, Chen L. Gefitinib Plus Chemotherapy vs Gefitinib Alone in Untreated EGFR-Mutant Non-Small Cell Lung Cancer in Patients With Brain Metastases: The GAP BRAIN Open-Label, Randomized, Multicenter, Phase 3 Study. JAMA Netw Open. 2023 Feb 1;6(2):e2255050. doi: 10.1001/jamanetworkopen.2022.55050. PMID: 36753281; PMCID: PMC9909498. |
wrong population |
|
11 |
Cortot AB, Madroszyk A, Giroux-Leprieur E, Molinier O, Quoix E, Bérard H, Otto J, Rault I, Moro-Sibilot D, Raimbourg J, Amour E, Morin F, Hureaux J, Moreau L, Debieuvre D, Morel H, Renault A, Pichon E, Huret B, Charpentier S, Denis MG, Cadranel J. First-Line Afatinib plus Cetuximab for EGFR-Mutant Non-Small Cell Lung Cancer: Results from the Randomized Phase II IFCT-1503 ACE-Lung Study. Clin Cancer Res. 2021 Aug 1;27(15):4168-4176. doi: 10.1158/1078-0432.CCR-20-4604. Epub 2021 May 24. PMID: 34031056. |
wrong study design |
|
12 |
Yamamoto N, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, Yamanaka T, Tanaka M, Takahashi K, Fukuoka M. Erlotinib plus bevacizumab vs erlotinib monotherapy as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer: Survival follow-up results of the randomized JO25567 study. Lung Cancer. 2021 Jan;151:20-24. doi: 10.1016/j.lungcan.2020.11.020. Epub 2020 Nov 20. PMID: 33279874. |
wrong study design |
|
13 |
Hosomi Y, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, Tajima K, Kajihara Y, Yamamoto N. Erlotinib with or without bevacizumab as a first-line therapy for patients with advanced nonsquamous epidermal growth factor receptor-positive non-small cell lung cancer: Exploratory subgroup analyses from the phase II JO25567 study. Thorac Cancer. 2022 Aug;13(15):2192-2200. doi: 10.1111/1759-7714.14541. Epub 2022 Jun 29. PMID: 35768976; PMCID: PMC9346191. |
wrong study design |
|
14 |
Goss GD, Cobo M, Lu S, Syrigos K, Lee KH, Göker E, Georgoulias V, Isla D, Morabito A, Min YJ, Ardizzoni A, Bender S, Cseh A, Felip E. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung: Final analysis of the randomised phase 3 LUX-Lung 8 trial. EClinicalMedicine. 2021 Jun 18;37:100940. doi: 10.1016/j.eclinm.2021.100940. PMID: 34195574; PMCID: PMC8225678. |
Wrong intervention |
|
15 |
Kawashima Y, Fukuhara T, Saito H, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, Nakachi I, Seike M, Azuma K, Kurimoto F, Tsubata Y, Fujita Y, Nagashima H, Asai G, Watanabe S, Miyazaki M, Hagiwara K, Nukiwa T, Morita S, Kobayashi K, Maemondo M. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir Med. 2022 Jan;10(1):72-82. doi: 10.1016/S2213-2600(21)00166-1. Epub 2021 Aug 26. PMID: 34454653. |
Wrong intervention |
|
16 |
Ponce Aix S, Novello S, Garon EB, Nakagawa K, Nadal E, Moro-Sibilot D, Alonso Garcia M, Fabre E, Frimodt-Moller B, Zimmermann AH, Visseren-Grul CM, Reck M; RELAY investigators. RELAY, ramucirumab plus erlotinib versus placebo plus erlotinib in patients with untreated, EGFR-mutated, metastatic non-small cell lung cancer: Europe/United States subset analysis. Cancer Treat Res Commun. 2021;27:100378. doi: 10.1016/j.ctarc.2021.100378. Epub 2021 Apr 19. PMID: 33905962. |
wrong population |
|
17 |
Nakagawa K, Nadal E, Garon EB, Nishio M, Seto T, Yamamoto N, Park K, Shih JY, Paz-Ares L, Frimodt-Moller B, Zimmermann AH, Wijayawardana S, Visseren-Grul C, Reck M. RELAY Subgroup Analyses by EGFR Ex19del and Ex21L858R Mutations for Ramucirumab Plus Erlotinib in Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res. 2021 Oct 1;27(19):5258-5271. doi: 10.1158/1078-0432.CCR-21-0273. PMID: 34301751; PMCID: PMC9662911. |
wrong population |
|
18 |
Nakagawa K, Garon EB, Gao L, Callies S, Zimmermann A, Walgren R, Visseren-Grul C, Reck M. RELAY, ramucirumab plus erlotinib versus placebo plus erlotinib in untreated EGFR-mutated metastatic non-small cell lung cancer: exposure-response relationship. Cancer Chemother Pharmacol. 2022 Aug;90(2):137-148. doi: 10.1007/s00280-022-04447-x. Epub 2022 Jul 16. PMID: 35841410; PMCID: PMC9360106. |
Wrong outcome |
|
19 |
Nishio K, Seto T, Nishio M, Reck M, Garon EB, Sakai K, Goto K, Kato T, Nakanishi Y, Takahashi T, Yamamoto N, Kiura K, Ohe Y, Tamura T, Visseren-Grul C, Frimodt-Moller B, Hozak RR, Wijayawardana SR, Zimmermann A, Homma G, Enatsu S, Nakagawa K. Ramucirumab Plus Erlotinib Versus Placebo Plus Erlotinib in Patients With Untreated Metastatic EGFR-Mutated NSCLC: RELAY Japanese Subset. JTO Clin Res Rep. 2021 Apr 16;2(6):100171. doi: 10.1016/j.jtocrr.2021.100171. PMID: 34590023; PMCID: PMC8474372. |
wrong population |
|
20 |
Haratake N, Hayashi H, Shimokawa M, Nakano Y, Azuma K, Oki M, Ota K, Yoshioka H, Sakamoto T, Yamamoto N, Nakagawa K, Seto T. Phase III Clinical Trial for the Combination of Erlotinib Plus Ramucirumab Compared With Osimertinib in Previously Untreated Advanced or Recurrent Non-Small Cell Lung Cancer Positive for the L858R Mutation of EGFR: REVOL858R (WJOG14420L). Clin Lung Cancer. 2022 May;23(3):e257-e263. doi: 10.1016/j.cllc.2021.10.007. Epub 2021 Oct 24. PMID: 34887192. |
No results yet |
|
21 |
Saito R, Sugawara S, Ko R, Azuma K, Morita R, Maemondo M, Oizumi S, Takahashi K, Kagamu H, Tsubata Y, Seike M, Kikuchi T, Okamoto I, Satoshi M, Asahina H, Tanaka K, Sugio K, Kobayashi K. Phase 2 study of osimertinib in combination with platinum and pemetrexed in patients with previously untreated EGFR-mutated advanced non-squamous non-small cell lung cancer: The OPAL Study. Eur J Cancer. 2023 May;185:83-93. doi: 10.1016/j.ejca.2023.02.023. Epub 2023 Mar 2. PMID: 36966696. |
wrong study design |
|
22 |
Asahina H, Tanaka K, Morita S, Maemondo M, Seike M, Okamoto I, Oizumi S, Kagamu H, Takahashi K, Kikuchi T, Isobe T, Sugio K, Kobayashi K. A Phase II Study of Osimertinib Combined With Platinum Plus Pemetrexed in Patients With EGFR-Mutated Advanced Non-Small-cell Lung Cancer: The OPAL Study (NEJ032C/LOGIK1801). Clin Lung Cancer. 2021 Mar;22(2):147-151. doi: 10.1016/j.cllc.2020.09.023. Epub 2020 Oct 16. PMID: 33199228. |
wrong study design |
|
23 |
Kenmotsu H, Wakuda K, Mori K, Kato T, Sugawara S, Kirita K, Yoneshima Y, Azuma K, Nishino K, Teraoka S, Shukuya T, Masuda K, Hayashi H, Toyozawa R, Miura S, Fujimoto D, Nakagawa K, Yamamoto N, Takahashi T. Randomized Phase 2 Study of Osimertinib Plus Bevacizumab Versus Osimertinib for Untreated Patients With Nonsquamous NSCLC Harboring EGFR Mutations: WJOG9717L Study. J Thorac Oncol. 2022 Sep;17(9):1098-1108. doi: 10.1016/j.jtho.2022.05.006. Epub 2022 May 27. PMID: 35636696. |
wrong study design |
|
24 |
Passaro A, de Marinis F, Tu HY, Laktionov KK, Feng J, Poltoratskiy A, Zhao J, Tan EH, Gottfried M, Lee V, Kowalski D, Yang CT, Srinivasa BJ, Clementi L, Jalikop T, Huang DCL, Cseh A, Park K, Wu YL. Afatinib in EGFR TKI-Naïve Patients with Locally Advanced or Metastatic EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Pooled Analysis of Three Phase IIIb Studies. Front Oncol. 2021 Jul 9;11:709877. doi: 10.3389/fonc.2021.709877. PMID: 34307179; PMCID: PMC8298067. |
no comparator |
|
25 |
Shi Y, Zhou J, Zhao Y, Zhu B, Zhang L, Li X, Fang J, Shi J, Zhuang Z, Yang S, Wang D, Yu H, Zhang L, Zheng R, Greco M, Wang T. Results of the phase IIa study to evaluate the efficacy and safety of rezivertinib (BPI-7711) for the first-line treatment of locally advanced or metastatic/recurrent NSCLC patients with EGFR mutation from a phase I/IIa study. BMC Med. 2023 Jan 8;21(1):11. doi: 10.1186/s12916-022-02692-8. PMID: 36617560; PMCID: PMC9827694. |
wrong study design |
|
26 |
Kaneda H, Sawa K, Daga H, Okada A, Nakatani Y, Atagi S, Okishio K, Tani Y, Matsumoto Y, Ogawa K, Nakahama K, Izumi M, Mitsuoka S, Kawaguchi T. Phase 1b study of ramucirumab in combination with erlotinib or osimertinib for untreated EGFR-mutated non-small cell lung cancer patients with asymptomatic brain metastases. Invest New Drugs. 2021 Dec;39(6):1598-1603. doi: 10.1007/s10637-021-01147-w. Epub 2021 Jul 2. PMID: 34215931. |
wrong study design |
|
27 |
Lee Y, Kim HR, Hong MH, Lee KH, Park KU, Lee GK, Kim HY, Lee SH, Lim KY, Yoon SJ, Cho BC, Han JY. A randomized Phase 2 study to compare erlotinib with or without bevacizumab in previously untreated patients with advanced non-small cell lung cancer with EGFR mutation. Cancer. 2023 Feb 1;129(3):405-414. doi: 10.1002/cncr.34553. Epub 2022 Nov 30. PMID: 36451343; PMCID: PMC10100207. |
wrong study design |
|
28 |
Gijtenbeek RGP, van der Noort V, Aerts JGJV, Staal-van den Brekel JA, Smit EF, Krouwels FH, Wilschut FA, Hiltermann TJN, Timens W, Schuuring E, Janssen JDJ, Goosens M, van den Berg PM, de Langen AJ, Stigt JA, van den Borne BEEM, Groen HJM, van Geffen WH, van der Wekken AJ. Randomised controlled trial of first-line tyrosine-kinase inhibitor (TKI) versus intercalated TKI with chemotherapy for EGFR-mutated nonsmall cell lung cancer. ERJ Open Res. 2022 Oct 17;8(4):00239-2022. doi: 10.1183/23120541.00239-2022. PMID: 36267895; PMCID: PMC9574558. |
The study was stopped due to slow accrual. Small sample size. |
|
29 |
Gu W, Zhang H, Lu Y, Li M, Yang S, Liang J, Ye Z, Li Z, He M, Shi X, Wang F, You D, Gu W, Feng W. EGFR-TKI Combined with Pemetrexed versus EGFR-TKI Monotherapy in Advanced EGFR-mutated NSCLC: A Prospective, Randomized, Exploratory Study. Cancer Res Treat. 2023 Jul;55(3):841-850. doi: 10.4143/crt.2022.1438. Epub 2023 Feb 13. PMID: 36791768; PMCID: PMC10372607. |
Wrong study design |
|
30 |
Yu YF, Luan L, Zhu FF, Dong P, Ma LH, Li LT, Gao L, Lu S. Modelled Economic Analysis for Dacomitinib-A Cost Effectiveness Analysis in Treating Patients With EGFR-Mutation-Positive Non-Small Cell Lung Cancer in China. Front Oncol. 2021 Dec 14;11:564234. doi: 10.3389/fonc.2021.564234. PMID: 34970476; PMCID: PMC8712321. |
Wrong outcomes |
|
31 |
Xu X, Fang N, Li H, Liu Y, Yang F, Li X. Cost-effectiveness analysis of dacomitinib versus gefitinib for the first-line therapy of patients with EGFR mutation-positive non-small-cell lung cancer in the United States and China. Ann Transl Med. 2021 May;9(9):760. doi: 10.21037/atm-20-6992. PMID: 34268373; PMCID: PMC8246172. |
Wrong outcomes |
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 01-01-2023
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodules werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodules.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodules is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met niet kleincellig longcarcinoom. Deze werkgroep is ingesteld in het kader van het cluster longoncologie.
Werkgroep
• dr. A. (Annemarie) Becker (voorzitter), Longarts, Amsterdam UMC, NVALT
• dr. A.J. (Anthonie) van der Wekken, Longarts, UMCG, NVALT
• dr. A. (Annemarieke) Bartels – Rutten, Radioloog, AVL, NVvR
• prof. dr. V. (Volkher) Scharnhorst, Klinisch chemicus, Catharina Ziekenhuis, NVKC
• prof. dr. E.F.I. (Emile) Comans, Nucleaire geneeskundige, Amsterdam UMC, NVNG
• prof. dr. J.J.C. (Joost) Verhoeff, Radiotherapeut-Oncoloog, Amsterdam UMC, NVRO
• prof. dr. J. (Jerry) Braun, Hoogleraar Cardio-thoracale chirurgie, LUMC, NVT
• dr. K.J. (Koen) Hartemink, Chirurg, AVL, NVvH
• dr. R.A.M. (Ronald) Damhuis, Arts-onderzoeker, IKNL
• drs. L.A. (Lidia) Barberio, Directeur Longkanker Nederland
• dr. B.J.M. (Bas) Peters, Ziekenhuisapotheker, St. Antonius Ziekenhuis, NVZA
• dr. R. (Rob) ter Heine, Ziekenhuisapotheker- klinisch farmacoloog, Radboudumc, NVZA
• drs. D.C.M. (Desirée) Verheijen, Klinisch geriater, ZGV, NVKG
• dr. J.H. (Jan) von der Thüsen, Patholoog, Erasmus MC, NVVP
• dr. W. (Wouter) van Geffen, Longarts, MCL, NVALT
• dr. L.E.L. (Lizza) Hendriks, Longarts, MUMC+, NVALT
• dr. A. (Arifa) Moons-Pasic, Longarts, OLVG, NVALT
• dr. I. (Idris) Bahce, Longarts, Amsterdam UMC, NVALT
• prof. dr. E. (Ed) Schuuring, Hoogleraar in de Moleculaire Oncologische Pathologie, UMCG, NVVP
• dr. J.M.J. (Josephine) Stoffels, Internist Ouderengeneeskunde, NIV
• dr. J.W. (Joost) van den Berg, Internist Ouderengeneeskunde i.o., NIV
In het kader van het cluster longoncologie
Met ondersteuning van
• dr. J.S. (Julitta) Boschman, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten (tot december 2023)
• M.L. (Miriam) te Lintel Hekkert, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
• dr. L.C. (Lisanne) Verbruggen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
• dr. D. (Dagmar) Nieboer, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
• A. (Alies) Oost, medisch informatiespecialist, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de volgende clustercyclus.
De NVALT heeft vastgesteld dat het niet mogelijk was werkgroepleden af te vaardigen met voldoende expertise zonder potentiële belangenverstrengeling. Het gaat daarbij met name om werkgroepleden die deelnemen aan adviesraden/kennisuitwisselingsbijeenkomsten met de farmaceutische industrie. Gedurende de ontwikkeling van de modules heeft daarom afstemming plaatsgevonden tussen de werkgroepvoorzitter, de belangencommissie van het Kennisinstituut van de Federatie Medisch Specialisten en de NVALT over passende acties naar aanleiding van de gemelde belangen.
Restricties voor de modules over onderwerpen (medicamenteuze behandeling) waar de adviesraden betrekking op hebben:
• Werkgroeplid werkt niet als enige inhoudsdeskundige aan de module;
• Werkgroeplid werkt tenminste samen met een werkgroeplid met een vergelijkbare expertise in alle fasen (zoeken, studieselectie, data-extractie, evidence synthese, Evidence-to-decision, aanbevelingen formuleren) van het ontwikkelproces. Indien nodig worden werkgroepleden toegevoegd aan de werkgroep;
• In alle fasen van het ontwikkelproces is een onafhankelijk methodoloog betrokken;
• Overwegingen en aanbevelingen worden besproken en vastgesteld tijdens een werkgroepvergadering onder leiding van een onafhankelijk voorzitter (zonder gemelde belangen).
Aansluitend op de reguliere commentaarronde bij de achterban van de bij de richtlijn betrokken wetenschappelijke verenigingen, hebben (een aantal) leden van de richtlijn- en kwaliteitscommissie van de NVALT en een methodoloog van het Kennisinstituut die niet betrokken waren bij ontwikkeling van de modules, aanvullend beoordeeld of de aanbevelingen logischerwijs aansluiten bij het gevonden bewijs en de overwegingen, om de onafhankelijkheid van de richtlijn te waarborgen.
Wellicht ten overvloede willen wij erop wijzen dat medisch specialistische richtlijnen niet worden vastgesteld door de betreffende richtlijnwerkgroep maar door de besturen/ledenvergadering van de betrokken verenigingen.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenwerkzaamheden |
Gemelde belangen |
Ondernomen actie |
|
Becker |
Longarts Amsterdam UMC |
Geen |
Extern gefinancierd onderzoek: SYMPRO studie Project over beeldvorming in de follow-up na behandeling voor longkanker gaat nog starten (gefinancierd door ZIN) |
geen |
|
Bartels-Rutten |
Radioloog, NKI-AVL (betaald) |
Geen |
Geen |
geen |
|
van der Wekken |
UMCG - longarts |
Geen |
Extern gefinancierd onderzoek: Lid bestuur Sectie oncologie NVALT Lid dure geneesmiddelen commissie NVALT Lid Cie Geneesmiddelen FMS Specialisten panel Longkanker Nederland Consortium partner 3D modeling in MTB Adviesraden voor UMCG bij Lilly, Astra Zeneca, Roche, Janssen |
restricties |
|
Comans |
Nucleair geneeskundige HMC Den Haag (0.6 FTE) |
Hoogleraar nucleaire geneeskunde Amsterdam UMC lokatie VUmc (0.4 FTE). |
Geen |
geen |
|
Braun |
Hoogleraar Cardio-thoracale Chirurgie, Leids Universitair Medisch Centrum en Amsterdams Universitair Medisch Centrum. |
Lid bestuur Nederlandse Vereniging voor Thoraxchirurgie (onbetaald). |
Geen |
geen |
|
Verhoeff |
Radiation oncologist and professor of radiotherapy at the Amsterdam UMC |
Niet van toepassing |
Extern gefinancierd onderzoek: 1. STOPSTORM H2020, bestraling van hartritmestoornissen |
geen |
|
Hartemink |
Chirurg NKI-AVL, Amsterdam |
Onbetaald: |
KWF-grant ontvangen (2021) voor het doen van onderzoek naar radiotherapie en chirurgie bij het vroeg-stadium NSCLC. |
geen |
|
Scharnhorst |
Klinisch chemicus, Catharina Ziekenhuis Eindhoven |
Deeltijdhoogleraar klinische chemie TU/e |
Extern gefinancierd onderzoek: |
geen |
|
Moons -Pasic |
Longarts OLVG Amsterdam |
Niet van toepassing |
Extern gefinancierd onderzoek: |
geen |
|
Peters |
Ziekenhuisapotheker |
Niet van toepassing |
Extern gefinancierd onderzoek: * Abbvie - Systematic evaluation of the efficacy-effectiveness gap of systemic treatmens in extensive disease - Geen projectleider |
geen |
|
Verheijen |
Klinisch geriater, voor 0,8 fte. |
Geen |
Geen |
geen |
|
Schuuring |
Senior staflid en lid van MT (Management Team) van de afdeling Pathologie van het Universitair Medisch Centrum Groningen. |
Adviseur/KMBP voor Moleculaire Pathologie voor de Stichting Pathologie Friesland in Leeuwarden, voor Martini Ziekenhuis te Groningen, voor Treant Zorggroep Bethesda ziekenhuis in Hoogeveen en voor ADCNV to Curacao (allen onbetaald) |
Adviseur/consultant m.b.t. (moleculaire) diagnostiek voor firma's (2019-2023): MSD/Merck, AstraZeneca, Astellas Pharma, Roche, Novartis, Bayer, BMS, Lilly, Amgen, Illumina, Agena Bioscience, Janssen Cilag (Johnson&Johnson), GSK, Diaceutics, CC Diagnostics (honoraria op UMCG rekening) Extern gefinancierd onderzoek: In UMCG-team heb ik financiele ondersteuning voor onderzoek ontvangen van: |
restricties |
|
Bahce |
Commissielid |
Geen |
Geen dienstverband, eigendom, patent of anderszins Extern gefinancierd onderzoek: Diverse investigator-initiated onderzoeken op de longafdeling van het Amsterdam worden gefinancierd door de bedrijven Boehringer Ingelheim, AstraZeneca en BMS |
restricties |
|
von der Thüsen |
Patholoog |
Geen |
Honoraria en consulting fees van: Extern gefinancierd onderzoek: |
restricties |
|
Barberio |
Directeur patiëntenorganisatie Longkanker Nederland (betaald) |
lid RvT Stichting Agora, leven tot het einde (betaald) |
Extern gefinancierd onderzoek: |
geen |
|
Hendriks |
Longarts |
mede-auteur geweest van ESMO richtlijn SCLC |
Betaling voor webinars Benecke, MedTalks, VJOncology Extern gefinancierd onderzoek: chair metastatic NSCLC systemic therapy EORTC secretaris stichting NVALT studies adviesraden (betaald aan instituut niet aan mij): Amgen, BMS, Boehringer Ingelheim, Janssen, Lilly, Merck, MSD, Novartis, Pfizer, Roche and Takeda betaling voor educational webinars (betaald aan instituut niet aan mij) Janssen, podcasts Takeda, invited speaker AstraZeneca, Bayer, high5oncology, Lilly en Merck Sharp & Dohme (MSD), interview sessies Roche betaling aan instituut voor lokale PI farma studies van AbbVie, AstraZeneca, Blueprint Medicines, Gilead, GlaxoSmithKline (GSK), Merck Serono, Mirati, MSD, Novartis, Roche en Takeda |
restricties |
|
ter Heine |
Ziekenhuisapotheker-klinisch farmacoloog, Radboudumc |
Geen |
Ik heb een onkostenvergoeding ontvangen voor het geven van een scholing over de klinische farmacologie van sotorasib, een nieuw middel in de behandeling van longkanker, van de firma AMGEN. Extern gefinancierd onderzoek: |
geen |
|
Damhuis |
Onderzoeker IKNL (betaald) |
Lid wetenschappelijke commissie DLCA-S (onbetaald) |
Extern gefinancierd onderzoek: |
geen |
|
van Geffen |
Medisch Centrum Leeuwarden |
NVALT bestuur |
Extern gefinancierd onderzoek: * Projectleider |
restricties |
|
Stoffels |
Internist ouderengeneeskunde Amsterdam UMC |
freelance nieuwsschrijver Nederlands Tijdschrift voor Geneeskunde
|
Amsterdam UMC innovatiefonds |
geen |
|
van den Berg |
Arts assistent Inwendige Geneeskunde Amsterdam UMC |
Betaalde nevenfunctie: * Post-doctoraal onderzoek Professional Performance and Compassionate Care-onderzoeksgroep AmsterdamUMC (niet extern gefinancierd) |
Geen |
geen |
Inbreng patiëntenperspectief
Longkanker Nederland heeft bijgedragen aan de schriftelijke knelpunteninventarisatie voor het cluster Longoncologie. Daarnaast werd er aandacht besteed aan het patiëntenperspectief door de afvaardiging van Longkanker Nederland in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptmodule werden tevens voor commentaar voorgelegd bij Longkanker Nederland (in afstemming met de Nederlandse Federatie van Kankerpatiëntenorganisaties).
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
|
Module |
Uitkomst raming |
Toelichting |
|
Module ‘Eerstelijnsbehandeling incurabel NSCLC met EGFR exon 19/21 mutatie’ |
Geen financiële gevolgen |
Hoewel het niet duidelijk is of de aanbevelingen breed toepasbaar zijn, volgt uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de schriftelijke knelpuntenanalyse werden verschillende partijen uitgenodigd om input te geven op een concept-raamwerk voor de richtlijnen niet kleincellig longcarcinoom en kleincellig longcarcinoom. Deze richtlijnen zijn dermate verouderd, dat een grotere herziening noodzakelijk is om uiteindelijk goed mee te kunnen draaien in het modulair onderhoud zoals in de Koploperprojecten wordt beoogd.
Het doel van deze stakeholderraadpleging was om te inventariseren welke knelpunten men ervaart rondom de te herziene richtlijnen. De benoemde knelpunten werden vervolgens door de richtlijnwerkgroep geprioriteerd en vertaald in uitgangsvragen.
De volgende vijf modules kregen prioriteit bij het uitvoeren van onderhoud aan de richtlijn Niet kleincellig longcarcinoom:
- (Neo)adjuvante immuuntherapie
- Dubbele immuuntherapie
- Behandeling na (chemo-)immuuntherapie
- Eerstelijnsbehandeling incurabel NSCLC met EGFR exon 19/21 mutatie
- Behandeling incurabel NSCLC met (zeldzame) mutaties
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model (Review Manager 5.4). De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule wordt aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren worden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren wordt de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule wordt aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
