Adjuvante behandeling Niercelcarcinoom
Uitgangsvraag
Wat is de plaats van adjuvante therapie in de behandeling van patiënten met niercelcarcinoom zonder aanwijzing voor ziekte na operatie?
Aanbeveling
Aanbeveling-1
Overweeg en bespreek met patiënten de voor- en nadelen van adjuvante therapie met pembrolizumab bij patiënten met heldercellig niercelcarcinoom en een TNM stadium die voldoen aan de inclusiecriteria van de Keynote 564 studie:
- gemiddeld tot hoog (tumorstadium T2 met nucleaire graad 4 of sarcomatoïde kenmerken, of tumorstadium T3; geen regionale lymfeklieren of metastasen op afstand aanwezig);
- hoog (tumorstadium T4 zonder regionale lymfeklieren of metastasen op afstand, of elk tumorstadium met aanwezigheid van betrokkenheid van regionale lymfeklieren); of
- stadium M1 NED (geen bewijs van ziekte).
Aanbeveling-2
Gebruik gevalideerde risico scores (bijvoorbeeld Leibovich) bij patiënten met een heldercellig niercelcarcinoom pT3a ISUP/WHO graad 1 N0 M0 om hen bij laag of intermediair risico te wijzen op de gevaren van overbehandeling.
Aanbeveling-3
Overweeg bij patiënten met een heldercellig niercelcarcinoom met een solitaire metachrone metastasering binnen 1 jaar na de nefrectomie beeldvorming na een interval van 3 maanden te herhalen alvorens tot metastasectomie over te gaan.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Voor de tyrosinekinase inhibitors is er een systematische review met 5 RCT’s gepubliceerd die een hoge mate van bewijskracht levert. Samengevat leidt adjuvante therapie met VEGFR-TKI niet tot een betere overall of klinisch relevant verschil in ziektevrije overleving vergeleken met geen (neo) adjuvante therapie bij patiënten met niet gemetastaseerd niercelcarcinoom. Wel hebben de patiënten die tyrosinekinase inhibitors kregen meer kans op bijwerkingen, met name ernstige bijwerkingen ≥graad 3. De kwaliteit van leven is vergelijkbaar bij patiënten die wel en geen tyrosinekinase inhibitors kregen.
Voor de immuncheckpoint inhibitoren zijn 3 studies gepubliceerd tijdens de literatuursearch. Voor atezolizumab, de tot nu toe enige PD-L1 inhibitor, is er een RCT gepubliceerd met overall een lage bewijskracht, gezien de lage patiëntaantallen. Het gebruik van atezolizumab leidt niet tot een betere overall of ziekte specifieke overleving vergeleken met geen (neo) adjuvante therapie bij patiënten met niet gemetastaseerd niercelcarcinoom. Wel hebben de patiënten die atezolizumab kregen meer kans op bijwerkingen, met name ernstige bijwerkingen ≥graad 3. De kwaliteit van leven is niet gerapporteerd bij patiënten die wel en geen atezolizumab kregen en er kan geen vergelijking worden gemaakt.
Daarnaast is er een RCT gepubliceerd voor de combinatie nivolumab en ipilimumab, een PD-1 inhibitor met anti-CTLA4 incombinatie. Deze RCT heeft overall een lage bewijskracht, gezien de lage patiëntaantallen. Het gebruik van nivolumab en ipilimumab leidt niet tot een betere overall of ziekte specifieke overleving vergeleken met geen (neo) adjuvante therapie bij patiënten met niet gemetastaseerd niercelcarcinoom. Inmiddels is, na de zoekdatum van de literatuuranalyse, ook deel B van deze RCT gepubliceerd, waarin alleen nivolumab met placebo wordt vergeleken (Motzer, 2024). Ook het gebruik van nivolumab alleen leidt niet tot een betere overall of ziekte specifieke overleving vergeleken met geen (neo) adjuvante therapie bij patiënten met niet gemetastaseerd niercelcarcinoom (Motzer, 2024). Wel hebben de patiënten die nivolumab en ipilimumab kregen meer kans op bijwerkingen, met name ernstige bijwerkingen ≥graad 3. De kwaliteit van leven is niet gerapporteerd bij patiënten die wel en geen nivolumab en ipilimumab kregen en er kan geen vergelijking worden gemaakt.
Wat betreft pembrolizumab is de Keynote studie gepubliceerd: een RCT met overall een lage bewijskracht, gezien de lage patiëntaantallen (Chioueri, 2021; Powqles, 2022). Het gebruik van pembrolizumab kan leiden tot een betere overall overleving en ziektevrije overleving vergeleken met placebo bij patiënten met niet gemetastaseerd niercelcarcinoom in de intermediate of hoge risico groep of M1 NED (no evidence of disease). Wel hebben de patiënten die pembrolizumab krijgen mogelijk meer risico op ernstige (graad 3 of hoger) bijwerkingen, terwijl de kwaliteit van leven vergelijkbaar is met de patiënten die placebo kregen.
Na de literatuursearch datum is er een update gepubliceerd van de Keynote studie (Choueiri, april 2024), waarin de uitkomsten bij een mediane follow-up van 57.2 maanden werden gerapporteerd. Bij de langere follow-up lijkt het effect van pembrolizumab op overleving nog gunstiger te worden. Met pembrolizumab werd een significante verbetering van de overall overleving waargenomen vergeleken met placebo (hazard ratio voor overlijden, 0,62; 95% BI, 0,44 tot 0,87). De geschatte totale overleving na 48 maanden was 91,2% in de pembrolizumab-groep, vergeleken met 86,0% in de placebogroep. Het gebruik van pembrolizumab ging gepaard met een hogere incidentie van ernstige bijwerkingen (20,7%, versus 11,5% met placebo) en van graad 3 of 4 bijwerkingen gerelateerd aan pembrolizumab of placebo (18,6% vs. 1,2%).
Hieruit valt af te leiden dat de Number Needed to treat om 1 sterfgeval binnen 57 maanden te voorkomen is in dit geval 17 (95% CI: 9.5 – 53.7). Voor de ziektevrije overleving is de NNT: 11 (95% CI: 6.3 – 26). Wat betreft het risico op bijwerkingen van graad 3 of 4 is de Number Needed to Harm: 6 (95% 4.8 – 7.3).
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Verschillende patientenorganisaties hebben op het gevaar van overbehandeling gewezen en het gebrek aan biomarkers die kunnen bepalen wie een recidief ontwikkeld en bij wie pembrolizumab een recidief kan voorkomen en eisen verder onderzoek (Apolo, 2022). Ook bestaat bij patientenorganisaties onzekerheid over het feit dat andere studie met middelen die effectief blijken te zijn in de gemetastaseerde situatie niet werkzaam bleken in adjuvante studies.
Kosten (middelenbeslag)
Er zijn geen kosten-effectiviteitsstudies naar de effecten van adjuvante therapie bij patiënten met niercelcarcinoom die te vertalen zijn naar de Nederlandse situatie. Pembrolizumab is nog tot 2028 in patent en de te verwachten kosten van deze behandeling zijn EUR 100 000 per patiënt indien deze volgens protocol gedurende 1 jaar word gegeven, waarbij in Nederland minimaal 400 patienten per jaar in aanmerking komen. Hier tegenover staat dat bij ruim 30% een recidief (tijdelijk) word voorkomen. Hierdoor kunnen de kosten voor de duurdere combinatietherapie die bovendien vaak gedurende meerdere jaren word gegeven (tijdelijk) worden bespaard.
Aanvaardbaarheid, haalbaarheid en implementatie
De werkgroep voorziet geen problemen met aanvaardbaarheid, haalbaarheid of implementatie van de aanbevelingen.
Rationale van aanbeveling-1: weging van argumenten voor en tegen de interventies
Opmerkelijk is dat noch de dubbel blind gerandomiseerde fase 3 studie CheckMate 914 met de combinatie ipilimumab/nivolumab noch de Immotion010 met atezolizumab een DFS of OS voordeel heeft laten zien (references Pal & MotzeR). Alhoewel de studies niet direct vergelijkbaar zijn (atezolizumab is een PD-L1 inhibitor en nivolumab en ipilimumab werden gedurende 6 maanden gegeven) heeft dat de interpretatie van de toegevoegde waarde van pembrolizumab complexer gemaakt. Het is onder andere aanleiding geweest om niet op basis van 1 studie met alleen DFS voordeel over te gaan tot implementatie na het beschikbaar komen van het voorlopig positieve CieBOM advies. Nu er overlevingsvoordeel is aangetoond, moet deze beslissing worden herzien. Het overlevingsvoordeel dat is aangetoond is een 38% lager risico op overlijden bij patiënten behandeld met pembrolizumab ten opzichte van placebo. Tevens waren er na 24, 36 en 48 maanden meer patiënten in leven in de pembrolizumab groep dan in de placebo groep (2.4, 4.4 en 5.2 % respectievelijk). Niet iedereen waarbij in de placebo groep een recidief werd gevonden heeft alsnog een PD(L)1 remmer gehad. Dat is echter wel goed verklaarbaar. Immers, patiënten in de Keynote-564 hebben een veel intensiever radiologisch en laboratorium follow-up schema gehad. Hierdoor zijn er eerder aanwijzingen voor recidief ziekteactiviteit naar voren gekomen. Een aanzienlijk deel van deze groep zal vallen in de categorie favourable risk conform de IMDC risico classificatie en zal conform eerder richtlijnadvies met tyrosinekinase inhibitoren zijn behandeld. Tevens zal er een aanzienlijk deel bij het debuut van recidief ziekte slechts een beperkte tumorload hebben gehad waardoor zowel actieve surveillance als metastase gericht lokale therapie een optie zal zijn geweest.
Tegenover dit gunstige effect op de overleving moet worden afgewogen dat de events wat de overleving betreft ook na 4 jaar laag zijn (14%) en er een hoog risico van overbehandeling bestaat bij patienten met een laaggradige pT3a N0 M0 niertumor (zie NNT). Ook moet rekening worden gehouden met de NNH. Er is een toename in bijwerkingen gerapporteerd ten opzichte van placebo (20.7 vs 11.5 %) echter het verschil in graad 3-4 toxiciteit is aanzienlijk groter (18.6 vs 1.2%) In de Keynote-564 is geen letale toxiciteit gerapporteerd, maar de kans daarop zal niet onvermeld mogen blijven bij de counseling. Voorts zullen permanente bijwerkingen zoals hypothyreoidie en bijnierschorsinsufficiëntie (met de bijbehorende risico’s) besproken moeten worden, waarvan de frequentie van die laatste weliswaar laag is (1.2%), maar niet verwaarloosbaar. Verder is er onzekerheid over de vervolgtherapie bij patiënten die ook na pembrolizumab binnen 6 maanden een recidief
ontwikkelen omdat deze patiënten niet meer voor combinatietherapie in aanmerking komen.
In de Keynote-564 studie mochten patiënten deelnemen met een heldercellig niercelcarcinoom met stadium pT2N0M0graad4; en de volgende categorieën ongeacht gradering van de tumor: pT3N0M0 en pT4N0M0, pT 1-4N+M0 of patiënten met M1 NED die zich presenteerden met gemetastaseerde ziekte waarbij zowel primaire tumor als metastasen verwijderd zijn. Vanuit de subgroepen in de KN-564 is helaas geen duidelijk onderscheid te maken bij welke patiënten categorie er een groter dan wel kleiner voordeel is dan het gemiddelde. Op basis van de studie gegevens is het daarom te adviseren om met deze patiënten na in opzet curatieve strategie de adjuvante behandeling met 17 cycli pembrolizumab 3-wekelijks 200 mg intraveneus te bespreken.
Rationale van aanbeveling-2: weging van argumenten voor en tegen de interventie
Het is belangrijk om in de selectie en bij de counseling van de patiënt een goede risico schatting te maken. Dit kan met name een uitdaging zijn in de groep patiënten met een pT3aN0M0 en lage gradering. Vaak gaat het om patiënten met een upstaging na minimaal invasieve chirurgie bij vermeende cT1b tumoren. Deze pT3a graad 1 tumoren hebben in verschillende retrospectieve studies een 5-jaar ziektevrije overleving van 79 t/m 82.1 % (Patel, 2020; Yim, 2021). Patiënt zal alvorens een besluit te kunnen nemen in het proces van gezamenlijke besluitvorming, op de hoogte gebracht moeten worden zowel over het risico op blijvende bijwerkingen, alsover de kans dat er weer recidief ziekteactiviteit op kan treden.
Een hulpmiddel hiervoor kunnen web- based algoritmes zijn zoals bijvoorbeeld https://cancernomograms.com/nomograms/492. Dergelijke algoritmes zijn momenteel nog niet uitgerust om het specifieke overlevingsvoordeel weer te geven, maar kunnen wel een handvat bieden voor de risico inschatting voor bespreken van de kans op terugkeer ziekte activiteit van de maligniteit. Een vaak gebruikte risico score, de Leibovich score, werd bijvoorbeeld meerdere keren gevalideerd en geupdate en kan pT3a graad 1 tumoren op basis van necrose en omvang verder onderscheiden in laag-intermediar en intermediar risico.
Rationale van aanbeveling-3: weging van de argumenten voor en tegen de interventie
voor M1 NED
In de Keynote-564patiënten studie werden ook patiënten met M1 NED geincludeerd. Dit waren voornamelijk patiënten die zich of presenteerden met primair (synchroon) gemetastaseerde ziekte of waarbij de metastase binnen 1 jaar na de nefrectomie was opgetreden en verwijderd (metachrone metastasering). In meerdere nomogrammen voor de beslissing omtrend metastasectomie is een tijd tot recidief van minder dan 1 jaar een ongunstige factor (Tosco, 2013). Met name de metachroon gemetastaseerde subgroep binnen een jaar na nefrectomie heeft een hoog risico voor snelle ziekteprogressie. Het valt derhalve te overwegen beeldvorming na een interval van 3 maanden te herhalen om verdere ziekteprogressie uit te sluiten alvorens tot metastasectomie en mogelijk adjuvante therapie met pembrolizumab over te gaan.
Onderbouwing
Achtergrond
Van meerdere studies met immuuntherapie heeft slechts een studie met adjuvant pembrolizumab een verbetering van DFS en OS laten zien en is inmiddels door de EMA geregistreerd. In het TKI-tijdperk heeft van meerdere studies alleen een studie met sunitinib een verbetering van DFS getoond maar werd niet door de EMA geregistreerd.
Conclusies / Summary of Findings
Adjuvant Tyrosine kinase inhibitors versus placebo
Overall survival
|
High GRADE |
Adjuvant therapy with VEGFR-TKI results in little to no difference in overall survival when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Riaz, 2021 |
Disease-free survival
|
High GRADE |
Adjuvant therapy with VEGFR-TKI results in little to no difference in disease-free survival when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Riaz, 2021 |
All-cause adverse event (AE) of any grade
|
High GRADE |
Adjuvant therapy with VEGFR-TKI results in little to no difference in all-cause adverse event (AE) of any grade when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Riaz, 2021 |
All-cause adverse event (AE) of grade ≥3
|
High GRADE |
Adjuvant therapy with VEGFR-TKI increases the risk of all-cause adverse event (AE) of grade ³3 when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Riaz, 2021 |
Treatment-related adverse events (trAEs) of any grade
|
Moderate GRADE |
Adjuvant therapy with VEGFR-TKI likely increases risk of treatment-related adverse events (trAEs) of any grade when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Riaz, 2021 |
Treatment-related adverse events (trAEs) of grade ≥3
|
High GRADE |
Adjuvant therapy with VEGFR-TKI increases the risk of treatment-related adverse events (trAEs) of grade ≥3 of grade ≥3 when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Riaz, 2021 |
Quality of life
|
Moderate GRADE |
Adjuvant therapy with VEGFR-TKI likely results in little to no difference in quality of life when compared with no (neo)adjuvant in patients with non-metastatic renal cell carcinoma.
Source: Riaz, 2021 |
Nivolumab with or without + ipilimumab versus placebo
Overall survival
|
Low GRADE |
Nivolumab with or without ipilimumab therapy may result in little to no difference in overall survival when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Motzer, 2023 |
Disease-free survival
|
Low GRADE |
Nivolumab with or without ipilimumab therapy may result in little to no difference in disease-free survival when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Motzer, 2023 |
All-cause adverse event (AE) of any grade
|
Low GRADE |
Nivolumab plus ipilimumab therapy may increase the risk of all-cause adverse event (AE) of any grade when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Motzer, 2023 |
All-cause adverse event (AE) of grade ≥3
|
Low GRADE |
Nivolumab plus ipilimumab therapy may increase the risk of all-cause adverse event (AE) of grade ≥3 when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Motzer, 2023 |
Treatment-related adverse events (trAEs) of any grade
|
Low GRADE |
Nivolumab plus ipilumanb therapy may increase the risk of treatment-related adverse events (trAEs) of any grade when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Motzer, 2023 |
Treatment-related adverse events (trAEs) of grade ≥3
|
Low GRADE |
Nivolumab plus ipilumanb therapy may increase the risk of treatment-related adverse events (trAEs) of grade ≥3 when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: Motzer, 2023 |
Quality of life
|
No GRADE |
No evidence was found on nivolumab plus ipilumab regarding the quality of life when compared with no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma.
Source: - |
Pembrolizumab versus placebo
Overall survival
|
Low GRADE |
Adjuvant pembrolizumab therapy may result in a better overall survival when compared with placebo in patients with renal cell carcinoma and the following risk profile:
Sources: Chioueri, 2021; Powles, 2022 |
Disease-free survival
|
Low GRADE |
Adjuvant pembrolizumab therapy may result in a better in disease-free survival when compared with placebo in patients with renal cell carcinoma and the following risk profile:
Sources: Chioueri, 2021; Powles, 2022 |
All-cause adverse event (AE) of any grade
|
Low GRADE |
Adjuvant pembrolizumab therapy may result in little to no difference in the risk of all-cause adverse event (AE) of any grade when compared with placebo in patients with renal cell carcinoma and the following risk profile:
Sources: Chioueri, 2021; Powles, 2022 |
All-cause adverse event (AE) of grade ≥3
|
Low GRADE |
Adjuvant pembrolizumab therapy may increase the risk of all-cause adverse event (AE) of grade ≥3 when compared with placebo in patients with renal cell carcinoma and the following risk profile:
Sources: Chioueri, 2021; Powles, 2022 |
Treatment-related adverse events (trAEs) of any grade
|
Low GRADE |
Adjuvant pembrolizumab therapy may increase the risk of treatment-related adverse events (trAEs) of any grade when compared with placebo in patients with renal cell carcinoma and the following risk profile:
Sources: Chioueri, 2021; Powles, 2022 |
Treatment-related adverse events (trAEs) of grade ≥3
|
Low GRADE |
Adjuvant pembrolizumab therapy may increase the risk of treatment-related adverse events (trAEs) of grade ≥3 when compared with placebo in patients with renal cell carcinoma and the following risk profile:
Sources: Chioueri, 2021; Powles, 2022 |
Quality of life
|
Low GRADE |
Adjuvant pembrolizumab therapy may result in little to no difference in health-related quality of life when compared with placebo in patients with renal cell carcinoma and the following risk profile:
Source: Chioueri, 2024 |
Atezolizumab versus placebo
Overall survival
|
Low GRADE |
Adjuvant atezolizumab therapy may result in little to no difference in overall survival when compared with placebo in patients with renal cell carcinoma.
Source: Pal, 2022 |
Disease-free survival
|
Low GRADE |
Adjuvant atezolizumab therapy may result in little to no difference in disease-free survival when compared with placebo in patients with renal cell carcinoma.
Source: Pal, 2022 |
All-cause adverse event (AE) of any grade
|
Low GRADE |
Adjuvant atezolizumab therapy may result in little to no difference in the risk of all-cause adverse event (AE) of any grade when compared with placbo in patients with renal cell carcinoma.
Source: Pal, 2022 |
All-cause adverse event (AE) of grade ≥3
|
Low GRADE |
Adjuvant atezolizumab therapy may increase the risk of all-cause adverse events (AE) of grade ≥3 when compared with placebo in patients with renal cell carcinoma.
Source: Pal, 2022 |
Treatment-related adverse events (trAEs) of any grade
|
Low GRADE |
Adjuvant atezolizumab therapy may increase the risk of treatment-related adverse events (trAEs) of any grade when compared with placebo in patients with renal cell carcinoma.
Source: Pal, 2022 |
Treatment-related adverse events (trAEs) of grade ≥3
|
Low GRADE |
Adjuvant atezolizumab therapy may increase the risk of treatment-related adverse events (trAEs) of grade ≥3 when compared with placebo in patients with renal cell carcinoma.
Source: Pal, 2022 |
Quality of life
|
No GRADE |
No evidence was found on atezolizumab regarding the quality of life when compared with placebo in patients with renal cell carcinoma.
Source: - |
Samenvatting literatuur
Adjuvant Tyrosine kinase inhibitors versus placebo
Description of studies
Riaz, 2021 performed a systematic review and meta-analysis of randomized controlled trials (RCTs) evaluating risk-benefit for adjuvant postoperative treatments in high-risk renal cell carcinoma by assessing reported disease-free survival (DFS), overall survival (OS), toxicity, and quality of life. A literature search was performed in PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials to identify relevant RCTs (from database inception through May 15, 2018). The results of the ATLAS trial were published while writing this manuscript, and the manuscript was updated accordingly. Four phase 3 clinical trials with a total of 4820 patients were included in qualitative analysis and meta-analysis.
Results
Both disease free survival (DFS) and overall survival (OS) did not benefit from adjuvant tyrosine kinase inhibitors (TKIs). The highest-risk patients demonstrated no benefit in DFS in the subgroup analysis of either risk category. However the definition of highest risk varied across trials.
Therapy with TKI resulted in a higher risk of all-cause adverse event (AE) of any grade and all-cause adverse event (AE) of grade ³3 compared to the placebo.
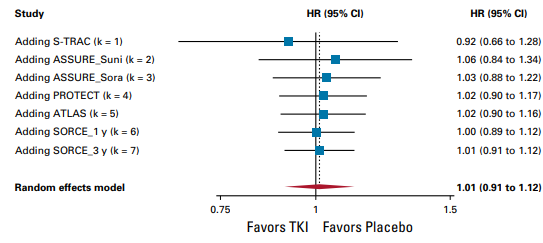
1. Overall survival
TKI monotherapy offers no benefit in Overall survival (OS) (hazard ratio, 1.01; 95% CI, 0.91 to 1.12, high certainty) and is not considered clinically relevant.
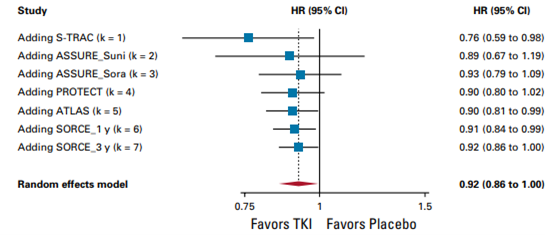
2. Disease-free survival
TKI monotherapy offers benefit in disease-free survival (DFS) (hazard ratio, 0.92; 95% CI, 0.86 to 1.00, high certainty) but this difference is not considered clinically relevant.
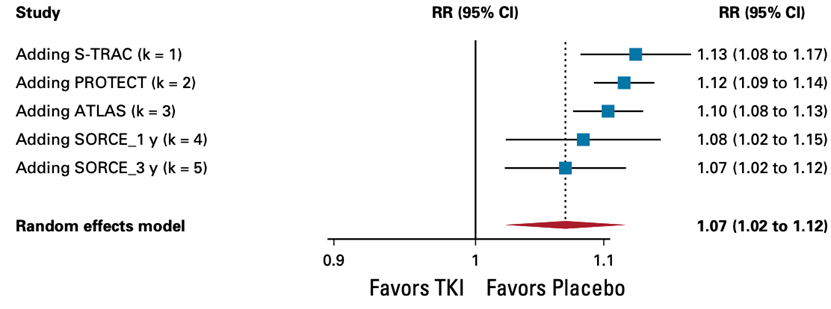
3. All-cause adverse events
TKI monotherapy offers no benefit in all-cause adverse event (AE) of any grade (hazard ratio, 1.07; 95% CI, 1.02 to 1.12, high certainty) and is not considered clinically relevant.
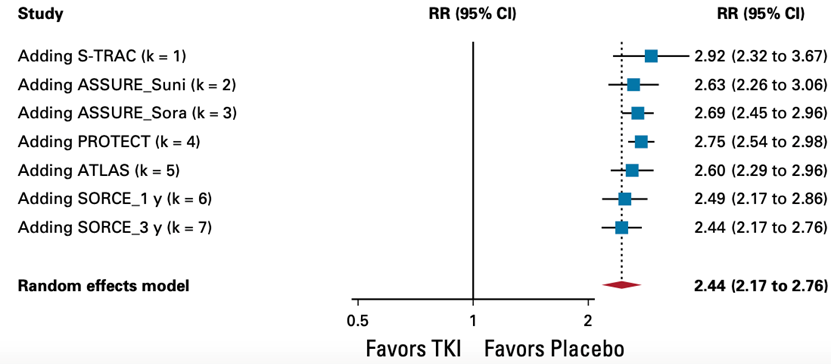
4. All-cause adverse event (AE) of grade ≥3
TKI monotherapy offers no benefit in all-cause adverse event (AE) of grade ≥3 (hazard ratio, 2.44; 95% CI, 2.17 to 2.76, high certainty) and is considered clinically relevant.
5. Treatment-related adverse events TKI monotherapy offers no benefit in treatment-related adverse events (trAEs) of any grade
(hazard ratio, 1.45; 95% CI, 1.15 to 1.83, high certainty) and is considered clinically relevant.
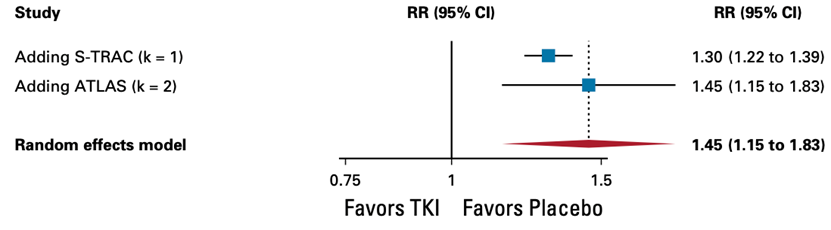
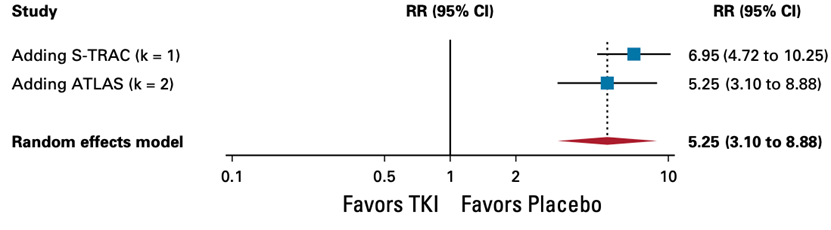
6. Treatment-related adverse events (trAEs) of grade ≥3treatment-related adverse events (trAEs) of grade ≥3 TKI monotherapy offers no benefit in treatment-related adverse events (trAEs) of grade ≥3
(hazard ratio, 5.25; 95% CI, 3.10 to 8.88, high certainty) and is considered clinically relevant.
7. Quality of life
The PROTECT, S-TRAC, and ASSURE trials included some measure of QoL as a study outcome. The PROTECT trial used the Functional Assessment of Cancer Therapy-Kidney Symptom Index 19 (FKSI-19) questionnaire to assess QoL, and although significantly greater deterioration in QoL from baseline was seen in the TKI group compared with placebo (adjusted mean change in total FKSI-19 score at 1 year: −4.49 v −0.47, P , .01), this was not considered clinically relevant and QoL scores in both groups returned to baseline after treatment cessation. Similarly, in S-TRAC, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) scores favored placebo, but the difference was not clinically meaningful. The ASSURE trial collected patient-reported data on a quality-of-life (QoL) assessment for fatigue, called PROMIS Fatigue-SF1, but these results are yet to be published.
Level of evidence of the literature
Overall survival (OS)
The level of evidence regarding the outcome measure Overall Survival started at high (RCT) and was not downgraded.
Disease-free survival (DFS)
The level of evidence regarding the outcome measure Disease-free survival started at high (RCT) and was not downgraded.
All-cause adverse event (AE) of any grade
The level of evidence regarding the outcome measure all-cause adverse event (AE) of any grade started at high (RCT) and was not downgraded.
All-cause adverse event (AE) of grade ≥3
The level of evidence regarding the outcome measure all-cause adverse event (AE) of grade ≥3 started at high (RCT) and was not downgraded.
Treatment-related adverse events (trAEs) of any grade
The level of evidence regarding the outcome measure Treatment-related adverse events (trAEs) of any grade started at high (RCT) and was downgraded by 1 level to moderate because of number of included patients (imprecision, overlap of boundary of clinical relevance).
Treatment-related adverse events (trAEs) of grade ≥3
The level of evidence regarding the outcome measure treatment-related adverse events (trAEs) of grade ≥3 started at high (RCT) and was not downgraded.
Quality of life
The level of evidence regarding the outcome measure quality of life started at high (RCT) and was downgraded by 1 level to moderate because of applicability (bias due to indirectness: different measurement tools are used thus pooling of results is not possible).
Nivolumab with or without + ipilimumab versus placebo
Description of studies
Motzer, 2013 reported a double-blind, randomised, trial (CheckMate 914 ). Patients with localised clear cell renal cell carcinoma who were at high risk of relapse after radical or partial nephrectomy between 4–12 weeks before random assignment were included.Patients were randomly assigned (1:1) to nivolumab (240 mg) intravenously every 2 weeks for 12 doses plus ipilimumab (1 mg/kg) intravenously every 6 weeks for four doses, or matching placebo, via an interactive response technology system. The expected treatment period was 2 weeks, and treatment could be continued until week 36, allowing for treatment delays. 816 patients were randomly assigned to receive either adjuvant nivolumab plus ipilimumab (405 patients) or placebo (411 patients). 580 (71%) of 816 patients were male and 236 (29%) patients were female.
Results
1. Overall survival
The number of events required for the planned overall survival interim analysis was not reached at the time of the data cutoff, and only 61 events occurred (33 in the nivolumab plus ipilimumab group and 28 in the placebo group).
2. Disease-free survival
With a median follow-up of 37·0 months (IQR 31·3–43·7), median disease-free survival was not reached in the nivolumab plus ipilimumab group and was 50·7 months (95% CI 48·1 to not estimable) in the placebo group (hazard ratio 0·92, 95% CI 0·71–1·19; p=0·53).
3. All-cause adverse events
In the all-treated population, 392 (97%) of 404 patients who received nivolumab plus ipilimumab and 361 (89%) of 407 patients who received placebo had at least one adverse event of any grade and of any cause. All-cause adverse events of any grade led to discontinuation of nivolumab plus ipilimumab in 129 (32%) of 404 treated patients and of placebo in nine (2%) of 407 treated patients. Four deaths were attributed to treatment with nivolumab plus ipilimumab and no deaths were attributed to treatment with placebo.
4. All-cause adverse event (AE) of grade ≥3
In total, 154 (38%) of 404 patients who received nivolumab plus ipilimumab and 42 (10%) of 407 patients who received placebo had an adverse event of grade 3–5. The most common adverse events of any cause in the two arms were pruritus (32% with nivolumab plus ipilimumab and 17% with placebo), fatigue (30% with nivolumab plus ipilimumab and 27% with placebo), and diarrhoea (27% with nivolumab plus ipilimumab and 21% with placebo).
5. Treatment related adverse events
A total of 359 (89%) of 404 patients treated with nivolumab plus ipilimumab and 231 (57%) of 407 patients treated with placebo had at least one treatment-related adverse event of any grade. Treatment-related adverse events of any grade led to the discontinuation of nivolumab plus ipilimumab in 117 (29%) of 404 treated patients and of placebo in four (1%) of 407 treated patients
6. Treatment-related adverse events (trAEs) of grade ³3treatment-related adverse events (trAEs) of grade ≥3
28% of patients treated with nivolumab plus ipilimumab experienced a treatment-related adverse event of grade 3 or 4 as compared to 2% of patients treated with placebo.
7. Quality of life
Not reported.
Level of evidence of the literature
Overall survival (OS)
The level of evidence regarding the outcome measure Overall Survival started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
Disease-free survival (DFS)
The level of evidence regarding the outcome measure Disease-free survival started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
All-cause adverse event (AE) of any grade
The level of evidence regarding the outcome measure all-cause adverse event (AE) of any grade started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
All-cause adverse event (AE) of grade ≥3
The level of evidence regarding the outcome measure all-cause adverse event (AE) of grade ³3 started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
Treatment-related adverse events (trAEs) of any grade
The level of evidence regarding the outcome measure Treatment-related adverse events (trAEs) of any grade started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
Treatment-related adverse events (trAEs) of grade ≥3
The level of evidence regarding the outcome measure treatment-related adverse events (trAEs) of grade ³3 started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
Quality of life
This outcome was not reported and could not be graded.
Pembrolizumab versus placebo
Description of studies
A total of three publications, describing one study reported a double-blind, randomised, trial (Keynote 564): Choueiri, 2021; Choueiri, 2024 and Powles 2022. A fourth publication updating overall survival was published after the literature search and shall be discussed in “Overwegingen – van bewijs tot aanbeveling” at the end of this chapter to support the recommendation. Patients with localised clear cell renal cell carcinoma with an increased risk of recurrence were included. Eligible participants had an Eastern Cooperative Oncology Group performance status of 0 or 1, had undergone nephrectomy 12 weeks or less before randomisation, and had not received previous systemic therapy for advanced renal cell carcinoma. The risk of disease recurrence was classified according to protocol-defined criteria as intermediate to high (tumor stage T2 with nuclear grade 4 or sarcomatoid features, or tumor stage T3; no regional lymph node or distant metas tasis present), high (tumor stage T4 with no regional lymph node or distant metastasis, or any tumor stage with the presence of regional lymph-node involvement), or stage M1 NED (no evidence of disease). Patients were randomly assigned to pembrolizumab 200 mg (n=496) or placebo (n=498) intravenously every 3 weeks for up to 17 cycles or until a new malignancy or any progression or recurrence of the malignancy under study occurred, the participant or physician decided to discontinue treatment, or any occurrence of pregnancy, intercurrent illness, or recurrent grade 2 or worse pneumonitis. Dose modifications for pembrolizumab were not permitted. Some patients had metastases and randomisation was stratified by metastatic disease status (M0 vs M1), and the M0 group was further stratified by ECOG performance status and geographical region.
Choueiri, 2021 reported the outcomes at the median time from randomization to the data-cutoff date of 24.1 months.
Powles reported long-term follow-up outcomes. The median follow-up (time from randomisation to the data cutoff date of June 14, 2021) was 30·1 months (IQR 25·7−36·7). The median number of treatment cycles administered was 17 (IQR 9–17) in the pembrolizumab group and 17 (16–17) in the placebo group. Among participants who received at least one dose of study treatment, 298 (61%) of 488 in the pembrolizumab group and 366 (74%) of 496 in the placebo group completed all 17 planned cycles of treatment.
Choueiri, 2024 reported the health-related quality of life (HRQoL) compared between treatment and placebo group. This was assessed by the European Organization for Research and Treatment of Cancer core quality of life questionnaire, (EORTC QLQ-C3)0 global health status/quality of life (GHS/QoL) and physical functioning scale scores and Functional Assessment of Cancer Therapy Kidney Cancer Symptom Index—Disease-Related Symptoms (FKSI-DRS).
Results
1. Overall survival
Chioueri, 2021 reports a total of 51 deaths (18 in the pembrolizumab group and 33 in the placebo group). The median overall survival was not reached in either group (hazard ratio for death, 0.54; 95% CI, 0.30 to 0.96).
Powles, 2022 reports similar results: overall survival was better with pembrolizumab compared with placebo (HR 0.52 ( 95% CI 0.31–0.86). At 30 months follow up also median overall survival was not reached in either group. At 30 months, the estimated proportion of participants who were alive was 95.7% (95% CI 93.3–97.2) in the pembrolizumab group and 91.4% (88.3–93.7) in the placebo group.
2. Disease-free survival
Chioueri, 2021 reports that as of the data-cutoff date, 260 events of disease recurrence or death had occurred (109 events in the pembrolizumab group and 151 in the placebo group). The median disease-free survival was not reached in either group. The risk of disease recurrence or death was 32% lower with adjuvant pembrolizumab therapy than with placebo (hazard ratio for recurrence or death, 0.68; 95% confidence interval [CI], 0.53 to 0.87; P=0.002 [two-sided]).
Powles, 2022 reports similar results: disease-free survival was better with pembrolizumab compared with placebo (HR 0·63 [95% CI 0·50–0·80]) in the intention-to-treat population. Median disease-free survival was not reached in either group.
3. All-cause adverse events
Chioueri, 2021 reports that in the as-treated population, 96.3% of the patients who received pembrolizumab and 91.1% of those who received placebo had at least one adverse event of any grade and of any cause.
Powles, 2021 reports that the adverse event profile of pembrolizumab was in line with those reported for this study previously, with no new safety signals.
4. All-cause adverse event (AE) of grade ≥3
Chioueri, 2021, describes that in total, 32.4% of the patients who received pembrolizumab and 17.7% of those who received placebo had an adverse event of grade 3 to 5. There were two deaths in the pembrolizumab group, which were due to multiple organ dysfunction syndrome and pneumonia (in one patient each), and one death in the placebo group, which was due to intracranial hemorrhage,Powles, 2021 states that Grade 3 or worse adverse events of any cause were reported in 157 (32%) of 488 participants in the pembrolizumab group and 88 (18%) of 496 participants in the placebo group. The most common grade 3 or worse adverse events of any cause were hypertension (in 14 [3%] participants) and increased alanine aminotransferase (11 [2%] participants) in the pembrolizumab group and hypertension (13 [3%] participants) in the placebo group.
5. Treatment related adverse events
Chioueri repoerted that a total of 386 patients (79.1%) who received pembrolizumab and 265 (53.4%) who received placebo had at least one adverse event of any grade that was attributed to pembrolizumab or placebo by the investigator, including an event of grade 3 or 4 in 18.9% of the patients who received pembrolizumab and 1.2% of those who received placebo.
Powles, 2022 did not report treatment related adverse events specifically.
6. Treatment-related adverse events (trAEs) of grade ³3treatment-related adverse events (trAEs) of grade ≥3
Chioueri, 2021 describes that no deaths that were attributed to either pembrolizumab or placebo occurred. At least one treatment-related serious adverse event occurred in 12.1% of the patients who received pembrolizumab and in 0.2% of those who received placebo.
Powles, 2022 similarly states that no deaths from treatment-related adverse events occurred in either study group. Serious adverse events attributed to study treatment occurred in 59 (12%) participants in the pembrolizumab group and one (<1%) participant in the placebo group. The most common serious treatment-related adverse events (≥1% incidence) in the pembrolizumab group were adrenal insufficiency (six [1%] participants), colitis (six [1%]), and diabetic ketoacidosis (five [1%])
7. Quality of life
Chioueri, 2024 reported that adjuvant treatment with pembrolizumab did not result in deterioration of HRQoL. No clinically meaningful difference in least squares mean scores for pembrolizumab versus placebo were observed at week 52 for EORTC QLQ-C30 GHS/QoL (–2.5; 95% CI –5.2 to 0.1), EORTC QLQ-C30 physical functioning (–0.87; 95% CI –2.7 to 1.0), and FKSI-DRS (–0.7; 95% CI –1.2 to –0.1). Most PRO scores remained stable or improved for the EORTC QLQ-C30 GHS/QoL (pembrolizumab, 54.3%; placebo, 67.5%), EORTC QLQ-C30 physical functioning (pembrolizumab, 64.7%; placebo, 68.8%), and FKSI-DRS (pembrolizumab, 58.2%; placebo, 66.3%).
Level of evidence of the literature
Overall survival (OS)
The level of evidence regarding the outcome measure Overall Survival started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers, confidence interval crossing border for clinical relevance).
Disease-free survival (DFS)
The level of evidence regarding the outcome measure Disease-free survival started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers, confidence interval crossing border for clinical relevance).
All-cause adverse event (AE) of any grade
The level of evidence regarding the outcome measure all-cause adverse event (AE) of any grade started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers) and heterogeneity.
All-cause adverse event (AE) of grade ≥3
The level of evidence regarding the outcome measure all-cause adverse event (AE) of grade ≥3 started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
Treatment-related adverse events (trAEs) of any grade
The level of evidence regarding the outcome measure Treatment-related adverse events (trAEs) of any grade started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers) and heterogeneity.
Treatment-related adverse events (trAEs) of grade ≥3
The level of evidence regarding the outcome measure treatment-related adverse events (trAEs) of grade ≥3 started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers) and heterogeneity.
Quality of life
The level of evidence regarding the outcome measure Overall Survival started at high (RCT) and was downgraded by two levels to low due to imprecision (wide confidence intervals).
Atezolizumab versus placebo
Description of studies
Pal, 2022 reported a double-blind, randomised, trial (IMmotion010 ). Eligible patients were patients aged 18 years or older with renal cell carcinoma with a clear cell or sarcomatoid component and increased risk of recurrence. After nephrectomy with or without metastasectomy, patients were randomly assigned (1:1) to receive atezolizumab (1200 mg) or placebo (both intravenous) once every 3 weeks for 16 cycles or 1 year. Patients with metastases were eligible for inclusion. Stratification factors were disease stage (T2 or T3a vs T3b–c or T4 or N+ vs M1 no evidence of disease), geographical region (north America [excluding Mexico] vs rest of the world), and PD-L1 status on tumour-infiltrating immune cells. 778 patients were enrolled; 390 (50%) were assigned to the atezolizumab group and 388 (50%) to the placebo group. The median follow-up duration was 44.7 months (IQR 39·1–51·0).
Results
1. Overall survival
At data cutoff, the overall survival was immature and patients alive were censored. There were 107 deaths, 54 (14%) in the atezolizumab group and 53 (14%) in the placebo group. There was no evidence of a reduced risk of death from any cause with adjuvant atezolizumab compared with placebo (HR 0.97, 95% CI 0.67–1.42). The 3-year overall survival rate was 90.3% (95% CI 87.3–93.3) in the atezolizumab group and 89.8% (86.6–92.9) in the placebo group.
2. Disease-free survival
332 events of investigator-assessed disease-free survival occurred (164 [42%] events in the atezolizumab group and 168 [43%] in the placebo group. Median disease-free survival (investigator assessment) was 57·2 months (95% CI 44.6 to not estimable) in the atezolizumab group and 49.5 months (47.4 to not estimable) in the placebo group (HR 0.93, 95% CI 0.75–1.15; p=0·50). 3-year disease-free survival was 59.4% (95% CI 54.4–64.5) in the atezolizumab group and 59.0% (54.0–64.0) in the placebo group. The corresponding percentages at 1 year were 77.4% (73.2–81.6; atezolizumab) and 74.1% (69.7–78.5; placebo), and 67.3% (62.6–72.1; atezolizumab) and 65.0% (60.2–69.9; placebo) at 2 years.
3. All-cause adverse events
Adverse events of any grade were reported in 373 (96%) patients who received atezolizumab and 341 (89%) patients who received placebo. Adverse events that occurred in at least 15% of patients who received atezolizumab were fatigue in 109 (28%) patients, diarrhoea in 87 (22%), arthralgia in 78 (20%), and pruritus in 74 (19%), whereas those occurring in at least 15% of patients who received placebo were fatigue in 93 (24%) and diarrhoea in 79 (21%).
4. All-cause adverse event (AE) of grade ≥3
Grade 3–4 adverse events occurred in 106 (27%) patients who received atezolizumab and 81 (21%) patients who received placebo.
There were four deaths due to adverse events in the study, one among patients who received atezolizumab and three among patients who received placebo. One patient who received atezolizumab died due to acute myeloid leukaemia. Among patients who received placebo, deaths due to adverse events resulted from respiratory failure, sepsis, and unknown cause.
5. Treatment related adverse events
296 patients (76%) who received atezolizumab and 203 (53%) who received placebo experienced at least one adverse event deemed by investigators as related to atezolizumab or placebo. Adverse events related to treatment that occurred in more than 10% of patients in the atezolizumab group were fatigue in 77 (20%) patients, pruritus in 56 (14%), hypothyroidism in 52 (13%), and diarrhoea in 45 (12%); those occurring in more than 10% of patients in the placebo group were fatigue in 69 (18%) patients, pruritus in 40 (10%), and diarrhoea in 39 (10%).
6. Treatment-related adverse events (trAEs) of grade ³3treatment-related adverse events (trAEs) of grade ≥3
Grade 3–4 adverse events related to treatment occurred in 55 (14%) patients who received atezolizumab and 18 (5%) who received placebo. The most common grade 3–4 adverse events were hypertension (seven [2%] patients who received atezolizumab versus 15 [4%] patients who received placebo), hyperglycaemia (ten [3%] vs six [2%]), and diarrhoea (two [1%] vs seven [2%]). There were no deaths attributed to treatment.
7. Quality of life
Not reported.
Level of evidence of the literature
Overall survival (OS)
The level of evidence regarding the outcome measure Overall Survival started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
Disease-free survival (DFS)
The level of evidence regarding the outcome measure Disease-free survival started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
All-cause adverse event (AE) of any grade
The level of evidence regarding the outcome measure all-cause adverse event (AE) of any grade started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
All-cause adverse event (AE) of grade ≥3
The level of evidence regarding the outcome measure all-cause adverse event (AE) of grade ≥3 started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
Treatment-related adverse events (trAEs) of any grade
The level of evidence regarding the outcome measure Treatment-related adverse events (trAEs) of any grade started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
Treatment-related adverse events (trAEs) of grade ≥3
The level of evidence regarding the outcome measure treatment-related adverse events (trAEs) of grade ³3 started at high (RCT) and was downgraded by two levels to low due to imprecision (low event numbers).
Quality of life
This outcome was not reported and could not be graded.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the (un)desirable effects of adjuvant therapy compared to no adjuvant therapy in patients with non-metastatic renal cell carcinoma?
| P: |
Patients with renal cell carcinoma after surgery with no evidence of disease |
| I: |
Adjuvant therapy (sunitinib, cabozantinib, pazopanib, pembrolizumab, avelumab, ipilimumab, nivolumab) |
| C: |
No adjuvant therapy (placebo or observation) |
| O: |
Disease-free survival, overall survival, quality of life, adverse events (toxicity) |
Relevant outcome measures
The guideline development group considered survival and progression-free survival a critical outcome measure for decision making; and quality of life as an important outcome measure for decision making.
The working group followed the definitions of the outcome measures as described in the included literature.
The working group used the GRADE default boundaries for clinical relevance (0.80-1.25) for all outcome measures as minimal clinically (patient) important differences.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 25-04-2022. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 37 hits. Studies were selected based on the following criteria: systematic reviews, RCT’s and observational studies. Studies were initially selected based on title and abstract screening. After reading the full text, 36 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 1 study was included. The search was updated on the 22nd of December 2023. The updated literature search yielded another 529 hits. After reading the full text, out of 14 studies a total of 10 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 4 additional studies were included in the literature analysis.
Thus the literature analysis contains 5 studies in total.
Results
5 of studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- 1 - Apolo, Andrea B., et al. "Evolving role of adjuvant systemic therapy for kidney and urothelial cancers." American Society of Clinical Oncology Educational Book 42 (2022): 311-326.
- 2 - Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, Hajek J, Symeonides SN, Lee JL, Sarwar N, Thiery-Vuillemin A. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. New England Journal of Medicine. 2021 Aug 19;385(8):683-94.
- 3 - Choueiri TK, Tomczak P, Park SH, Venugopal B, Symeonides S, Hajek J, Ferguson T, Chang YH, Lee JL, Haas N, Sawrycki P. Patient-reported outcomes in KEYNOTE-564: adjuvant pembrolizumab versus placebo for renal cell carcinoma. The Oncologist. 2024 Feb 1;29(2):142-50.
- 4 - Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, Hajek J, Chang YH, Lee JL, Sarwar N, Haas NB. Overall survival with adjuvant pembrolizumab in renal-cell carcinoma. New England Journal of Medicine. 2024 Apr 18;390(15):1359-71.
- 5 - Motzer RJ, Russo P, Grünwald V, Tomita Y, Zurawski B, Parikh O, Buti S, Barthélémy P, Goh JC, Ye D, Lingua A. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. The Lancet. 2023 Mar 11;401(10379):821-32.
- 6 - Motzer, Robert J., et al. "Adjuvant Nivolumab for Localized Renal Cell Carcinoma at High Risk of Recurrence After Nephrectomy: Part B of the Randomized, Placebo-Controlled, Phase III CheckMate 914 Trial." Journal of Clinical Oncology (2024): JCO-24.
- 7 - Pal SK, Uzzo R, Karam JA, Master VA, Donskov F, Suarez C, Albiges L, Rini B, Tomita Y, Kann AG, Procopio G. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increase risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. The Lancet. 2022 Oct 1;400(10358):1103-16.
- 8 - Patel SH, Uzzo RG, Larcher A, et al. Oncologic and Functional Outcomes of Radical and Partial Nephrectomy in pT3a Pathologically Upstaged Renal Cell Carcinoma: A Multi-institutional Analysis. Clin Genitourin Cancer. 2020;18(6):e723-e729.
- 9 - Powles T, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, Hajek J, Gurney H, Chang YH, Lee JL, Sarwar N. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The lancet oncology. 2022 Sep 1;23(9):1133-44.
- 10 - Riaz IB, Siddiqi R, Islam M, He H, Riaz A, Asghar N, Naqvi SA, Warner JL, Murad MH, Kohli M. Adjuvant tyrosine kinase inhibitors in renal cell carcinoma: a concluded living systematic review and meta-analysis. JCO Clinical Cancer Informatics. 2021 May;5:588-99.
- 11 - Tosco L, Van Poppel H, Frea B, Gregoraci G, Joniau S. Survival and impact of clinical prognostic factors in surgically treated metastatic renal cell carcinoma. Eur Urol. 2013;63(4):646-652.
- 12 - Yim K, Aron M, Rha KH, et al. Outcomes of Robot-assisted Partial Nephrectomy for Clinical T3a Renal Masses: A Multicenter Analysis. Eur Urol Focus. 2021;7(5):1107-1114.
Evidence tabellen
Risk of bias table for intervention studies
|
Study reference (first author, year) |
Was the allocation sequence adequately generated? |
Was the allocation adequately concealed? |
Blinding: |
Was loss to follow-up (missing outcome data) infrequent? |
Are reports of the study free of selective outcome reporting? |
Was the study apparently free of other problems that could put it at a risk of bias? |
Overall risk of bias |
|
Motzer, 2013 |
Definitely yes
Reason: Authors used a computer-generated randomization schedule. |
Definitely yes
Reason: Authors used an interactive response technology system. Permuted blocks within each stratum. |
Definitely yes
Reason: Blinding of patients, physicians, physicians’ staff (except pharmacist who prepared study drugs), and the study sponsor. |
Definitely yes
Reason: Most incomplete outcome data due to other reasons. |
Definitely yes
Reason: All outcomes reported in the trial registration are reported. |
Probably yes
Reason: Registered trial, industry sponsored trial (sponsor had a role in study design, data, analysis, interpretation and manuscript preparation, not in data collection). |
Low risk of bias
Reason: Correct randomization and blinding, registered RCT, but involvement of sponsoring industry. |
|
Choueiri, 2021; Choueiri, 2024 and Powles, 2022 |
Definitely yes
Reason: Authors used central permuted block randomization with an interactive voice-response system and interactive web-response system. |
Definitely yes
Reason: Authors used an interactive response technology system. |
Definitely yes
Reason: Blinding of patients and investigators (except pharmacist who prepared study drugs). |
Definitely yes
Reason: Most incomplete outcome data due to other reasons. |
Definitely yes
Reason: All outcomes reported in the trial registration are reported. |
Probably yes
Reason: Registered trial, industry sponsored trial (sponsor had a role in study design, data collection, analysis, interpretation and manuscript preparation). |
Low risk of bias
Reason: Correct randomization and blinding, registered RCT, but involvement of sponsoring industry. |
|
Pal, 2022 |
Definitely yes
Reason: Authors used an interactive voice-web response system. |
Definitely yes
Reason: Statistician created a predetermined randomization schedule. |
Definitely yes
Reason: Blinding of patients, study site personnel (except pharmacist and injecting physician), and the study sponsor. |
Definitely yes
Reason: Most incomplete outcome data due to other reasons. |
Definitely yes
Reason: All outcomes reported in the trial registration are reported. |
Probably yes
Reason: Registered trial, industry sponsored trial (sponsor had a role in study design, data collection, analysis, interpretation and manuscript preparation). |
Low risk of bias
Reason: Correct randomization and blinding, registered RCT, but involvement of sponsoring industry. |
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
|
Appropriate and clearly focused question?1
|
Comprehensive and systematic literature search?2
|
Description of included and excluded studies?3
|
Description of relevant characteristics of included studies?4
|
Appropriate adjustment for potential confounders in observational studies?5 |
Assessment of scientific quality of included studies?6
|
Enough similarities between studies to make combining them reasonable?7 |
Potential risk of publication bias taken into account?8 |
Potential conflicts of interest reported?9 |
|
Riaz, 2021 |
Yes. Research question is described. |
Yes. PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials were searched, and search period and strategy were reported.
|
Yes the included studies are described. |
Yes. Characteristics were presented. |
Not applicable. |
Yes using MAGIC app. |
Yes, clinical and statistical heterogeneity were considered. |
Unclear. Less than 10 studies (5 in total) are included in the review. Therefore risk of publication bias cannot be adequately assessed. |
Yes of all the authors but not the included studies. |
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question: What are the (un)desirable effects of (neo)adjuvant therapy compared to no (neo)adjuvant therapy in patients with non-metastatic renal cell carcinoma?
P: patients with non-metastatic renal cell carcinoma
I: (neo)adjuvant therapy (sunitinib, cabozantinib, pazopanib, pembrolizumab, avelumab, ipilimumab, nivolumab)
C: no (neo)adjuvant therapy (placebo or observation)
O: disease-free survival, overall survival, quality of life, adverse events (toxicity)
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Riaz, 2021
[individual study characteristics deduced from [1st author, year of publication ]]
PS., study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis
Literature search up to February, 2021
6 23-25 A: Eisen, 2020 B: Motzer, 2018 C: Staehler, 2018 D: Motzer, 2021
Study design: RCT
Setting and Country: A: United Kingdom B: USA C: USA D: USA
Source of funding and conflicts of interest: [non-commercial]
|
Inclusion criteria SR: Reports in peer-reviewed journals, abstracts, FDA documents, company summary information. Reports in any language. All RCTs comparing VEGF TKIs with placebo that reported DFS, OS, and safety data were included.
Exclusion criteria SR:
4 studies included
Important patient characteristics at baseline:
Number of patients; characteristics important to the research question and/or for statistical adjustment (confounding in cohort studies); for example, age, sex, bmi, ...
N, mean age S-TRAC: n=615, median age= 58 ASSURE: n=1943, median age= 56 PROTECT: n=1538, median age= 59 ATLAS: n=724, median age = 58 SORCE: n=1711, median age = 58
Sex: S-TRAC= 74% male ASSURE= 67% male PROTECT= 72% male ATLAS= 73% male SORCE= 71% male
Groups comparable at baseline? Yes |
Describe intervention:
S-TRAC: Sunitinib 50 mg once daily ASSURE: arm 1: Sunitinib 50 mg once daily, arm 2 Sorafenib 400 mg twice daily PROTECT: Pazopanib 800 mg (amended to 600 mg) once daily ATLAS: Axitinib 5 mg twice daily SORCE: arm 1: Sorafenib 400 mg twice daily for 1 year, followed by placebo for 2 years, arm 2: Sorafenib 400 mg twice daily for 3 years
|
Describe control:
S-TRAC: placebo once daily ASSURE: arm 1: placebo once daily, arm 2: placebo twice daily PROTECT: placebo once daily ATLAS: placebo twice daily SCORCE: arm 1: placebo twice daily.
|
End-point of follow-up:
S-TRAC: 12.4 months ASSURE: approximately 12 months PROTECT: 10.4 months ATLAS: NA SCORCE: arm 1: 11.7 months, arm 2: 10.6 months
For how many participants were no complete outcome data available? (intervention/control) S-TRAC: control: 30, treatment: 44 ASSURE: control: 29, arm 1: 52, arm 2: 50 PROTECT: control: 27, treatment: 51 ATLAS: control: 28, treatment: 31 SCORCE: control: 26, arm 1: 49, arm 2: 50
|
Outcome measure-1 Overall survival (OS)
Random effects model: HR [95% CI] S-TRAC: 0.92 [0.66 to 1.28] ASSURE_Suni: 1.06 [0.84 to 1.34] ASSURE_Sora: 1.03 [0.88 to 1.22] PROTECT: 1.02 [0.90 to 1.17] ATLAS: 1.02 [0.90 to 1.16] SCORCE_1 y: 1.00 [0.89 to 1.12] SCORCE_3 y: 1.01 [0.91 to 1.12]
Outcome measure-2 Defined as disease-free survival (DFS)
Random effects model: HR [95% CI] S-TRAC: 0.76 [0.59 to 0.98] ASSURE_Suni: 0.89 [0.67 to 1.19] ASSURE_Sora: 0.93 [0.79 to 1.09] PROTECT: 0.90 [0.80 to 1.02] ATLAS: 0.90 [0.81 to 0.99] SCORCE_1 y: 0.91 [0.84 to 0.99] SCORCE_3 y: 0.92 [0.86 to 1.00]
Outcome measure-3 all-cause adverse event (AE) of any grade
Random effects model: RR [95% CI] S-TRAC: 1.13 [1.08 to 1.17] ASSURE_Suni: - ASSURE_Sora: - PROTECT: 1.12 [1.09 to 1.14] ATLAS: 1.10 [1.08 to 1.13] SCORCE_1 y: 1.08 [1.02 to 1.15] SCORCE_3 y: 1.07 [1.02 to 1.12]
Outcome measure-4 all-cause adverse event (AE) of grade ³3
Random effects model: RR [95% CI] S-TRAC: 2.92 [2.32 to 3.67] ASSURE_Suni: 2.63 [2.26 to 3.06] ASSURE_Sora: 2.69 [2.45 to 2.96] PROTECT: 2.75 [2.54 to 2.98] ATLAS: 2.60 [2.29 to 2.96] SCORCE_1 y: 2.49 [2.17 to 2.86] SCORCE_3 y: 2.44 [2.17 to 2.76]
Outcome measure-5 treatment-related adverse events (trAEs) of any grade
Random effects model: RR [95% CI] S-TRAC: 1.30 [1.22 to 1.39] ASSURE_Suni: - ASSURE_Sora: - PROTECT: - ATLAS: 1.45 [1.15 to 1.83] SCORCE_1 y: - SCORCE_3 y: -
Outcome measure-5 treatment-related adverse events (trAEs) of grade ³3
Random effects model: RR [95% CI] S-TRAC: 6.95 [4.72 to 10.25] ASSURE_Suni: - ASSURE_Sora: - PROTECT: - ATLAS: 5.25 [3.10 to 8.88] SCORCE_1 y: - SCORCE_3 y: -
|
Risk of bias (high, some concerns or low): See figure below.
Facultative: Brief description of author’s conclusion There is no guidance on when to stop maintaining a living review. In this example we used trial sequential analysis and high certainty of evidence (future clinical trials unlikely to change current conclusions) as a benchmark to conclude a living review in view of convincing evidence. Overall these results are consistent with previous analyses.
Personal remarks on study quality, conclusions, and other issues (potentially) relevant to the research question
Level of evidence: GRADE (per comparison and outcome measure) including reasons for down/upgrading
Sensitivity analyses (excluding small studies; excluding studies with short follow-up; excluding low quality studies; relevant subgroup-analyses); mention only analyses which are of potential importance to the research question
Heterogeneity: clinical and statistical heterogeneity; explained versus unexplained (subgroupanalysis) |
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])1
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy – otherwise the evidence table for studies of diagnostic test accuracy should be used.
Research question:
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3 |
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Motzer, 2013 |
Type of study: Multicentre, randomised, double-blind, phase 3 trial.
Setting and country:
Funding: Bristol Myers Squibb and Ono Pharmaceutical.
Conflicts of interest: Many |
In-/ exclusion criteria - age ≥ 18 years - localized renal cell carcinoma with a predominantly clear cell histology - high risk of relapse - partial or radical nephrectomy - negative surgical margins with no clinical or radiological evidence of macroscopic residual disease or distant metastases after nephrectomy - ECOG performance status of 0 or 1 - TNM stage pT2aN0M0, pT2b,N0M0, pT3N0M0, pT4N0M0, or pT4N1M0 - no previous systemic therapy for renal cell carcinoma - no malignancy ≤3 years ago (except locally cured cancer) - other exclusion criteria related to a suppressed immune status
N total at baseline: 816 Intervention: 405 Control:411
Important prognostic factors2: Age, median (IQR): I: 58 (51 – 65) C: 57 (50 – 65)
Sex: I: 71% male C: 72% male
Disease risk category: I: 56% high risk, 43% moderate risk, <1% other C: 57% high risk, 43% moderate risk, <1% other |
Nivolumab (240 mg) intravenously every 2 weeks for 12 doses plus ipilimumab (1 mg/kg) intravenously every 6 weeks for four doses. |
Placebo intravenously every 2 weeks for 12 doses plus placebo intravenously every 6 weeks for four doses. |
Length of follow-up: Median 37.0 months (IQR 31.3 – 43.7)
Incomplete outcome data: Intervention: 173 (42.7%) - 132 study drug toxicity - 11 disease recurrence - 8 adverse event - 8 patients’ decision - 3 consent withdrawal - 1 death - 1 poor / no compliance - 9 COVID
Control: 46 (11.2%) - 5 study drug toxicity - 20 disease recurrence - 4 adverse event - 4 patients’ decision - 4 consent withdrawal - 1 lost to follow-up - 1 pregnancy - 7 other
|
Median time of disease-free survival: I: not reached C: 50.7 months (48.1 to not estimable) HR 0.92 (95% CI 0.71 - 1.19, p=0.53)
Overall survival: Not reached. I: 33 events C: 28 events |
Name of study: CheckMate 914 |
|
Choueiri, 2021; Choueiri, 2024 and Powles, 2022 |
Type of study: Multicentre, randomised, double-blind, phase 3 trial.
Setting and country: 213 sites in 21 countries.
Funding: Merck Sharp and Dohme, a subsidiary of Merck.
Conflicts of interest: Many |
In-/ exclusion criteria - age ≥ 18 years - renal cell carcinoma with a clear cell component - intermediate or high risk of recurrence - partial or radical nephrectomy or metastectomy with negative surgical margins ≤12 weeks of randomization - ECOG performance status of 0 or 1 - no evidence of disease at screening - no previous systemic therapy for renal cell carcinoma
N total at baseline: 994 Intervention: 496 Control:498
Important prognostic factors2: Age, median (range): I: 60.0 (27 – 81) C: 60.0 (25 – 84)
Sex: I: 70% male C: 72.1% male
Disease risk category: I: 86.1% M0 intermediate-to-high risk, 8.1% M0 high risk, 5.8% M1. C: 86.9% M0 intermediate-to-high risk, 7.2% M0 high risk, 5.8% M1.
PD-L1 immune cell expression: I: 25% <1%, 73.6% ≥1% C: 22.7% <1%, 76.9% ≥1% |
Pembrolizumab 200 mg intravenously every 3 weeks for up to 17 cycles or until a new malignancy or any progression or recurrence of the malignancy under study occurred, the participant or physician decided to discontinue treatment, or any occurrence of pregnancy, intercurrent illness, or recurrent grade 2 or worse pneumonitis. |
Placebo intravenously every 3 weeks for up to 17 cycles or until a new malignancy or any progression or recurrence of the malignancy under study occurred, the participant or physician decided to discontinue treatment, or any occurrence of pregnancy, intercurrent illness, or recurrent grade 2 or worse pneumonitis. |
Length of follow-up: Choueiri 2021: 24.1 months Powles 2022: 30.1 months (IQR 25.7 - 36.7).
Incomplete outcome data: Intervention: 38.9% - 21.3% adverse event - 10.5% disease recurrence - other not reported
Control: 26.2% - 20.4% disease recurrence - other not reported |
Disease-free survival at data-cutoff: I: 109 events C: 151 events
Risk of disease recurrence or death: Choueiri 2021: HR 0.68 (95% CI 0.53 – 0.87, p=0.002) Powles 2022: HR 0.63 (95% CI 0.50 – 0.80)
Overall survival: Choueiri 2021: I: 18 events C: 33 events HR 0.54 (95% CI 0.30 – 0.96)
Powles 2022: HR 0.52 (95% CI 0.31 – 0.86)
30-month survival rate: I: 95.7% (95% CI 93.3 - 97.2) C: 91.4% (95% CI 88.3 - 93.7) |
Name of study: KEYNOTE-564
Median disease-free survival and overall survival were not reached.
Data update of 2024 is presented in ‘Overwegingen’ |
|
Pal, 2022 |
Type of study: Multicentre, randomised, double-blind, phase 3 trial.
Setting and country: 215 centres in 28 countries or regions.
Funding: F Hoffmann-La Roche and Genentech, a member of the Roche group.
Conflicts of interest: Many |
In-/ exclusion criteria: - age ≥ 18 years - renal cell carcinoma with a clear cell or sarcomatoid component - increased risk of recurrence - nephrectomy with or without metastectomy ≤12 weeks of randomization - ECOG performance status of 0 or 1 - no evidence of disease at screening - no previous anticancer therapy for renal cell carcinoma
N total at baseline: 778 Intervention: 390 Control:388
Important prognostic factors2: Age, median (IQR): I: 60.5 (52.0 – 69.0) C: 60.0 (52.8 – 68.0)
Sex: I: 74% male C: 72% male
Disease stage: I: 65% T2 or T3a, 21% T3-c or T4 or N+, 14% no evidence of disease, 15% metastasis. C: 64% T2 or T3a, 23% T3-c or T4 or N+, 13% no evidence of disease, 13% metastasis.
PD-L1 immune cell expression: I: 41% <1%, 59% ≥1% C: 39% <1%, 61% ≥1% |
Atezolizumab 1200 mg intravenously once every 3 weeks for 16 cycles or 1 year (whichever occurred first), or until disease recurrence, unacceptable toxicity, intercurrent illness that might affect patient safety with continued treatment, pregnancy, withdrawal of consent, or study termination by the sponsor. |
Placebo intravenously every 3 weeks for 16 cycles or 1 year (whichever occurred first), or until disease recurrence, unacceptable toxicity, intercurrent illness that might affect patient safety with continued treatment, pregnancy, withdrawal of consent, or study termination by the sponsor. |
Length of follow-up: Median: 44.7 months (IQR 39.1 – 51.0)
Incomplete outcome data: Intervention: 135 (34.6%) - 51 disease relapse - 45 adverse events - 20 consent withdrawal - 16 other reasons - 2 physician decision - 1 death
Control: 109 (28.1%) - 60 disease relapse - 10 adverse events - 12 consent withdrawal - 25 other reasons - 1 physician decision - 1 death |
Disease-free survival assessed by investigators: I: 42%, 164 events C: 43%, 168 events
Median time of disease-free survival: I: 57.2 months (44.6 to not estimable) C: 49.5 months (47.4 to not estimable) HR 0.93 (95% CI 0.75 - 1.15, p=0.50)
Overall survival: I: 14%, 54 events C: 14%, 53 events
3-year survival: I: 90.3% (95% CI 87.3 - 93.3) C: 89.8% (95% CI 86.6 - 92.9)
|
Name of study: Mmotion010
The primary endpoint of the study was not met. |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors [(potential) confounders]
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 30-09-2025
Beoordeeld op geldigheid : 13-05-2025
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met nierkanker en een vertegenwoordiger namens de patiëntenvereniging.
Werkgroep
- Dr. A. (Axel) Bex, uroloog, Antoni van Leeuwenhoek, Amsterdam, NVU (voorzitter)
- Dr. R.F.M. (Rob) Bevers, uroloog, Leids Universitair Medisch Centrum, Leiden, NVU
- Dr. J.F. (Hans) Langenhuijsen, uroloog, Radoudumc, Nijmegen, NVU
- Dr. A.P. (Paul) Hamberg, internist-oncoloog, Franciscus Gasthuis en Vlietland Ziekenhuis, Rotterdam, NIV/NVMO
- Dr. J.V. (Hans) van Thienen, internist-oncoloog, Antoni van Leeuwenhoek, Amsterdam, NIV/NVMO
- J. (Jolanda) Bloos-van der Hulst, verpleegkundig specialist, Antoni van Leeuwenhoek, Amsterdam, V&VN
- Dr. A.M.E. (Anna) Bruynzeel, radiotherapeut-oncoloog, Amsterdam Medisch Centrum, NVRO
- Dr. L. (Linda) Kerkmeijer, radiotherapeut-oncoloog, Radboudumc, Nijmegen, NVRO
- Drs. E. (Else) Wolak, belangenbehartiger Kwaliteit van zorg, Patiëntenvereniging Blaas- of Nierkanker
- Dr. M.A.J. (Mark) Meier, interventieradioloog, Isala Ziekenhuis, Zwolle, NVvR / NVIR
Met ondersteuning van
- Drs. D.A.M. (Danique) Middelhuis, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. M.H.D. (Majke) van Bommel, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. M. (Mohammadreza) Abdollahi, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. J.H. (Hanneke) van der Lee, senior-adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. I.M. (Irina) Mostovaya, senior-adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Linda Niesink, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
- Esther van der Bijl, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Bex (voorzitter) |
Uroloog AVL Amsterdam. Afdelingshoofd in het Specialist Centre of Kidney Cancer, Royal Free London NHS Foundation Trust, Professor of Urology, UCL Division of Surgery and Investigational Science. |
Alle nevenfuncties zijn onbetaald: |
- Restricted educational grant van Pfizer tbv een neoadjuvante studie (sponsor is het NKI-AvL). - Steering committee op twee internationale adjuvante fase 3 studies van BMS en Roche. - Lid van medical steering committee van twee patientenorganisaties (Kidney Cancer Association en IKCC). - Financier BMS: randomised phase 3 trial of adjuvant nivolumab plus ipilimumab versus placebo in high risk RCC. - Financier Roche: randomised phase 3 trial of adjuvant atezolizumab versus placebo in high risk RCC. - Financier Pfizer: single-arm phase 2 trial of neoadjuvant avelumab plus axitinib in high risk RCC (funded by restricted educational grant) |
Geen restricties |
|
Bevers |
Uroloog LUMC Leiden |
Geen |
Geen |
Geen restricties |
|
Langenhuijsen |
Uroloog Radboudumc Nijmegen |
Invited speaker Update Urology (Astra Zeneca), vergoeding+reiskosten |
Financier: ZonMW Voorbereidende studie Doelmatigheidsonderzoek, Pentixafor PET vs veneuze sampling bij primair hyperaldosteronisme - Financier: PentixaPharm GmbH, CASTUS trial. |
Geen restricties |
|
Hamberg |
Oncoloog Franciscus Gasthuis en Vlietland Rotterdam |
voorzitter WIN-O nier (onbetaald) bestuurslid Pro RCC (onbetaald) |
- Adviesraden meerdere farmaceutische bedrijven actief binnen RCC zorg - lokale hoofonderzoeker van aantal adjuvante nierkanker studies (Farma sponsored). Tevens ook van studies (farma sponsored) naar medicamenteuze interventies bij gemetastaseerde ziekte (oa RCC) |
Geen restricties |
|
Van Thienen |
Internist-oncoloog NKI-AvL Amsterdam |
Alle nevenfuncties zjn onbetaald: - Inhoudelijk/vice voorzitter Medisch Inhoudelijke Standpunten (MIS) groep van DRCG - Lid wetenschappelijke adviesraad Stichting PRO-RCC |
Pfizer Neoadjuvant axitinib en avelumab bij niercelcarcinoom (projectleider); BMS Checkmate 914 Adjuvant immunotherapy in high-risk renal cancer (onderzoeker); Eisai CLEAR study: levantinib and everolimus or pembrolizumab vs sunitinib in mRCC (onderzoeker); Goethe University Frankfurt am Main Sunniforecast (nivolumab+ipilimumab vs sunitinib in non-clear cell mRCC)(onderzoeker); Roche Adjuvant atezolizumab in high risk renal cancer (onderzoeker) |
Geen restricties |
|
Bloos-van der Hulst |
Verpleegkundig specialist uro-oncologie AVL Amsterdam |
Geen |
Geen |
Geen restricties |
|
Kerkmeijer |
Radiotherapeut-oncoloog, Radboudumc Nijmegen. Plaatsvervangend keteneigenaar Urologische Oncologie Radboudumc Nijmegen |
Alle nevenfuncties zjn onbetaald: - DUOS bestuurslid - Raad van Advies Tie Ribbon - Associate Editor Frontiers in oncology - Radiotherapeut-oncoloog UMC Utrecht (gastaanstelling) |
KWF subsidie FLAME studie prostaatcarcinoom |
Geen restricties |
|
Wolak |
Belangenbehartiger kwaliteit van zorg Patiëntenvereniging blaas- of nierkanker (PBNK) |
Patiëntenvereniging blaas- of nierkanker |
Werkzaam bij patiëntenorganisatie Leven met blaas- of nierkanker, geen boegbeeldfunctie |
Geen restricties |
|
Meier |
Interventieradioloog Isala Zwolle Voorzitter RVE Medische Beeldvorming, Isala Zwolle |
|
Geen |
Geen restricties |
|
Bruynzeel |
Radiotherapeut-oncoloog, Amsterdam UMC |
Geen |
ViewRay Inc: Een onderzoek naar hoge en precieze bestraling (stereotactische ablatieve radiotherapie) bij patiënt |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door uitnodigen van de Patiëntenvereniging blaas- of nierkanker (PBNK) voor de schriftelijke knelpunteninventarisatie en afvaardiging namens PBNK in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen (zie per module ook “Waarden en voorkeuren van patiënten”). De conceptrichtlijn is tevens voor commentaar voorgelegd aan Patiëntenvereniging blaas- of nierkanker (PBNK) en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Module |
Uitkomst raming |
Toelichting |
|
Module Adjuvante behandeling |
geen financiële gevolgen |
Uitkomst 1 |
De kwalitatieve raming volgt na de commentaarfase.
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 3.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met nierkanker. Tevens zijn er knelpunten aangedragen door de NVU (Nederlandse Vereniging voor Urologie) en NVRO, NVVR en Patiëntenvereniging blaas- of nierkanker (PBNK) via een schriftelijke knelpuntenanalyse.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekstrategie
Embase
|
No. |
Query |
Results |
|
#1 |
('renal cell carcinoma'/exp/mj OR 'non clear cell renal cell carcinoma'/exp/mj OR ((('kidney cell*' OR 'renal cell*' OR nephroid OR hypernephroid OR 'collecting duct') NEAR/3 (cancer* OR carcinoma* OR adenocarcinoma* OR neoplasm* OR tumor* OR tumour* OR mass*)):ti,ab,kw) OR ((grawitz* NEAR/3 (tumor* OR tumour*)):ti,ab,kw) OR hypernephroma*:ti,ab,kw OR 'hyper nephroma*':ti,ab,kw OR rcc:ti,kw OR nccrcc:ti,kw OR ncrcc:ti,kw) NOT ((metastatic:ti OR advanced:ti) NOT ('non metastatic':ti OR nonmetastatic:ti)) |
63472 |
|
#2 |
'sunitinib'/exp/mj OR 'cabozantinib'/exp OR 'pazopanib'/exp OR 'nivolumab'/exp OR 'pembrolizumab'/exp OR 'ipilimumab'/exp OR 'avelumab'/exp OR sunitinib:ti,ab,kw OR cabozantinib:ti,ab,kw OR pazopanib:ti,ab,kw OR nivolumab:ti,ab,kw OR pembrolizumab:ti,ab,kw OR ipilimumab:ti,ab,kw OR avelumab:ti,ab,kw OR adjuvant:ti,kw |
134562 |
|
#3 |
#1 AND #2 AND ([english]/lim OR [dutch]/lim) AND [2019-2022]/py NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) |
1053 |
|
#5 |
'meta analysis'/de OR 'meta analysis (topic)'/exp OR cochrane:ab OR embase:ab OR psycinfo:ab OR cinahl:ab OR medline:ab OR ((systematic NEAR/1 (review OR overview)):ab,ti) OR ((meta NEAR/1 analy*):ab,ti) OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de |
606845 |
|
#6 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1902965 |
|
#7 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
12774067 |
|
#8 |
#3 AND #5 – SR’s |
67 |
|
#9 |
#3 AND #6 NOT #8 – RCT’s |
71 |
|
#10 |
#3 AND #7 NOT (#8 OR #9) – observationele studies |
379 |
|
#11 |
#8 OR #9 OR #10 |
517
|
Medline (OVID)
|
Zoektermen |
|
1 (exp Carcinoma, Renal Cell/ or exp Kidney Neoplasms/ or (('kidney' or 'renal' or nephroid or hypernephroid or 'collecting duct') adj3 (cancer* or carcinoma* or adenocarcinoma* or neoplasm* or tumor* or tumour* or mass*)).ti,ab,kf. or (grawitz* adj3 (tumor* or tumour*)).ti,ab,kf. or hypernephroma*.ti,ab,kf. or (RCC or MRCC or nccrcc or ncrcc).ti,ab,kf.) not ((metastatic.ti. or advanced.ti.) not (non-metastatic or nonmetastatic).ti.) (104585) 2 exp *Chemotherapy, Adjuvant/ or exp *Neoadjuvant Therapy/ or exp *Sunitinib/ or exp *Nivolumab/ or exp *Ipilimumab/ or adjuvant.ti,kf. or neoadjuvant.ti,kf. or sunitinib.ti,ab,kf. or pazopanib.ti,ab,kf. or nivolumab.ti,ab,kf. or (ipilimumab or pembrolizumab or avelumab or cabozantinib).ti,ab,kf. (85291) 3 1 and 2 (2834) 5 limit 3 to ((english or dutch) and yr="2019 -Current") (901) 6 5 not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) (845) 7 (meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.) (588379) 8 (exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf.) not (animals/ not humans/) (1370304) 9 Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) (5140446) 10 6 and 7 (48) – SRs 11 (6 and 8) not 10 (60) - RCTs 12 (6 and 9) not (10 or 11) (157) – observationele studies 13 10 or 11 or 12 (265) |

