Preventie van navelstrengprolaps
Deze module bestaat uit de volgende submodules:
- Preventie van navelstrengprolaps - ballonkatheter
- Preventie van navelstrengprolaps – vliezen breken
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 30-12-2022
Beoordeeld op geldigheid : 30-12-2022
Validity period
The Board of the Dutch Society of Obstetrics and Gynaecology (NVOG) will assess whether these guidelines are still up-to-date in 2026 at the latest. If necessary, a new working group will be appointed to revise the guideline. The guideline’s validity may lapse earlier if new developments demand revision at an earlier date.
As the holder of this guideline, the NVOG is chiefly responsible for keeping the guideline up to date. Other scientific organizations participating in the guideline or users of the guideline share the responsibility to inform the chiefly responsible party about relevant developments within their fields.
Algemene gegevens
Er is meegelezen vanuit de Nederlandse Vereniging voor Kindergeneeskunde (NVK). De NVK heeft de richtlijn niet geautoriseerd, maar heeft geen bezwaar tegen publicatie.
De Koninklijke Nederlandse Organisatie van Verloskundigen (KNOV) is betrokken geweest bij de ontwikkeling van de richtlijn.
De Patiëntenfederatie Nederland heeft de richtlijn goedgekeurd.
De Vlaamse Vereniging voor Obstetrie en Gynaecologie (VVOG), Royal College of Obstetrics and Gynaecology (RCOG) en Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (DGGG) zijn betrokken geweest bij de ontwikkeling van de richtlijn.
Regiehouder: NVOG
Samenstelling werkgroep
Composition guideline development panel
An international panel for the development of the guidelines was formed in 2019. The panel consisted of representatives from all relevant medical disciplines that are involved in medical care for pregnant women.
All panel members have been officially delegated for participation in the guideline development panel by their (scientific) societies. The panel developed the guidelines in the period from May 2019 until March 2021.
The guideline development panel is responsible for the entire text of this guideline.
All panel members have been officially delegated for participation in the guideline development panel by their scientific societies. The guideline development panel is responsible for the entire text of this guideline.
Guideline development panel
- J.J. Duvekot, obstetrician, Consultant Obstetrics and Gynaecology, Erasmus Medical Centre, Rotterdam, the Netherlands (chair)
- I. Dehaene, obstetrician, Consultant Obstetrics and Gynaecology, Ghent University Hospital Belgium
- S. Galjaard, obstetrician, Consultant Obstetrics and Gynaecology, Erasmus Medical Centre, Rotterdam, the Netherlands
- A. Hamza, obstetrician, Consultant Obstetrics and Gynaecology, University Medical Center of Saarland, Homburg an der Saar, Germany
- S.V. Koenen, obstetrician, Consultant Obstetrics and Gynaecology, ETZ, locatie Elisabeth Ziekenhuis Tilburg, the Netherlands
- M. Kunze, obstetrician, Consultant Obstetrics and Gynaecology, Department of Gynecology& Obstetrics University of Freiburg, Germany
- M.A. Ledingham, obstetrician, Consultant Obstetrics and Gynaecology, the Queen Elizabeth Hospital Glasgow, UK
- B. Magowan, obstetrician, Consultant Obstetrics and Gynaecology, and Co-Chair UK RCOG Guidelines Committee, NHS Borders, Scotland, UK
- G. Page, obstetrician, Consultant Obstetrics and Gynaecology, Jan Yperman Hospital, Ypres, Belgium
- S.J. Stock, Reader and Consultant in Maternal and Fetal Medicine, University of Edinburgh Usher Institute and NHS Lothian, Edinburgh, Scotland, UK
- A.J. Thomson, obstetrician, Consultant Obstetrics and Gynaecology, Royal Alexandra Hospital (NHS Greater Glasgow and Clyde), UK
- G. Verhulst, obstetrician, Consultant Obstetrics and Gynaecology, ASZ Aalst/Geraardsbergen/Wetteren, Belgium
- D.C. Zondag, midwife/practice owner verloskundige praktijk De Toekomst-Geldermalsen, the Netherlands
Methodological support
- E. den Breejen, senior advisor, Knowledge Institute of the Dutch Association of Medical Specialists (until June 2019)
- J.H. van der Lee, senior advisor, Knowledge Institute of the Dutch Association of Medical Specialists (since May 2019)
- Y. Labeur, junior advisor, Knowledge Institute of the Dutch Association of Medical Specialists
Belangenverklaringen
The Code for the prevention of improper influence due to conflicts of interest was followed (https://storage.knaw.nl/2022-08/Code-for-the-prevention-of-improper-influence-due-to-conflicts-of-interest.pdf).
The working group members have provided written statements about (financially supported) relations with commercial companies, organisations or institutions related to the subject matter of the guideline during the past three years. Furthermore, inquiries have been made regarding personal financial interests, interests due to personal relationships, interests related to reputation management, interest related to externally financed research and interests related to knowledge valorisation. The chair of the guideline development panel is informed about changes in interests during the development process. The declarations of interests are reconfirmed during the commentary phase. The declarations of interests can be requested at the administrative office of the Knowledge Institute of the Dutch Association of Medical Specialists and are summarised below.
|
Last name |
Principal position |
Ancillary position(s) |
Declared interests |
Action |
|
Duvekot (chair) |
Consultant Obstetrics and Gynaecology, Erasmus MC, Rotterdam |
Director Medisch Advies en Expertise Bureau Duvekot, Ridderkerk |
none |
none |
|
Dehaene |
Consultant Obstetrics and Gynaecology, Ghent University Hospital |
none |
none |
none |
|
Galjaard |
Consultant Obstetrics and Gynaecology, Erasmus MC, Rotterdam |
Associated member of Diabetes in Pregnancy Group (DPSG) |
none |
none |
|
Hamza |
Consultant Obstetrics and Gynaecology, University Medical Center of Saarland, Homburg |
part of the advisory board of clinical innovations, which produces Kiwi-Vacuum Extractors® and Ebb Balloon Catheter®;
|
gave ultrasound courses sponsored by ultrasound producing companies: Samsung Germany and Matramed |
Recommendations do not involve either vacuum extractor or Ebb catheter (which is used for postpartum hemorrhage); therefore no actions |
|
Koenen |
Consultant Obstetrics and Gynaecology, ETZ, locatie Elisabeth Ziekenhuis Tilburg |
Chairman 'Koepel Kwaliteit' NVOG |
none |
none |
|
Kunze |
Divison Chief, Maternal-Fetal Medicine and Obstetrics, Departement of Gynecology & Obstetrics, University of Freiburg |
none |
none |
none |
|
Ledingham |
Consultant in Maternal and Fetal Medicine, Queen Elizabeth Hospital, Glasgow |
Co-chair RCOG Guidelines committee, Guideline developer for sign (scottisch intercollegiate guidelines group) |
none |
none |
|
Magowan |
Consultant Obstetrics and Gynaecology, and Co-Chair UK RCOG Guidelines Committee, NHS Borders, Scotland |
Co-chair RCOG Guidelines committee |
none |
none |
|
Page |
Consultant Obstetrics and Gynaecology, Jan Yperman Hospital, Ypres |
none |
none |
none |
|
Stock |
Reader and Consultant in Maternal and Fetal Medicine, University of Edinburgh and NHS Lothian, Edinburgh, Scotland, UK |
Consultant Obstetrician and Subspecialist Maternal and Fetal Medicine, member of the NIHR HTA General committee (grant funding board) and Chair of the RCOG Stillbirth Clinical Studies Group |
Research grants paid to the institution for research into pregnancy problems from National Institute of Healthcare Research (NIHR) Health Technology Assessment (HTA), NIHR Global Research Fund, Wellcome Trust, Medical Research Council, Tommy's Baby Charity, Cheif Scientist Office Scotland. Some of this work focuses on improving risk prediction of preterm labour and researching the benefits and harms of antenatal corticosteroids. Non-financial support from HOLOGIC, non-financial support from PARSAGEN, non-financial support from MEDIX BIOCHEMICA during the conduct of an NIHR HTA study in the form of provision of reduced cost assay kits to participating sites and blinded test assay analysers |
none |
|
Thomson |
Consultant Obstetrics and Gynaecology, Royal Alexandra Hospital (NHS Greater Glasgow and Clyde) |
Guideline developer for the RCOG |
none |
none |
|
Verhulst |
Head of Department of Gynaecology and Obstetrics, ASZ Aalst/Geraardsbergen/Wetteren |
none |
none |
none |
|
Zondag |
Midwife/practice owner verloskundige praktijk De Toekomst-Geldermalsen |
Policy adviser at the Dutch association of midwives (KNOV). Teacher at PA clinical midwives - Hogeschool Rotterdam |
none |
none |
Inbreng patiëntenperspectief
Representation of the patient perspective
Involvement of patient representatives from all four participating countries was challenging. Representatives of patient organisations from three countries (UK, Belgium, the Netherlands) commented on the draft guideline texts and discussed these during an online meeting. They represented the RCOG Women’s Network, the Flemish organisation for people with fertility problems ‘De verdwaalde ooievaar’, the Netherlands Patient Federation, and the Dutch association for people with fertility problems ‘Freya’. The comments were discussed and where relevant incorporated by the guideline development panel.
Methode ontwikkeling
Evidence based
Implementatie
Implementation
Guideline implementation and practical applicability of the recommendations was taken into consideration during various stages of guideline development. Factors that may promote or hinder implementation of the guideline in daily practice were given specific attention.
The guideline is distributed digitally among all relevant professional groups. The guideline can also be downloaded from the following websites: www.nvog.nl, www.vvog.be, www.rcog.org.uk, www.dggg.de,and the Dutch guideline website: www.richtlijnendatabase.nl.
Werkwijze
Method
AGREE
This guideline has been developed conforming to the requirements of the report of Guidelines for Medical Specialists 2.0 by the advisory committee of the Quality Counsel. This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (www.agreetrust.org)(Brouwers, 2010), a broadly accepted instrument in the international community and on the national quality standards for guidelines: “Guidelines for guidelines” (www.zorginstituutnederland.nl).
Identification of subject matter
During the initial phase of the guideline development the chairman, guideline development panel and the advisor inventoried the relevant subject matter for the guideline. Since this was a pilot project, the content of the questions and the support base in clinical practice was considered of less importance than the process of international collaboration and learning from each other. Key questions were selected in such a way that:
-
-
- they were relevant for obstetric practice in all collaborating countries;
- it was expected that the amount of literature identified for each question would be reasonable, i.e. some literature was expected, but not much;
- the recommendations were expected not to lead to extensive discussion among working group members because no major controversy was expected;
- there were no recent guidelines available for these particular topics in any of the four countries.
-
Clinical questions and outcomes
The guideline development panel then formulated definitive clinical questions and defined relevant outcome measures (both beneficial and harmful effects). The working group rated the outcome measures as critical, important and not important. Furthermore, where applicable, the working group defined relevant clinical differences.
Strategy for search and selection of literature
For the separate clinical questions, specific search terms were formulated and published scientific articles were searched for in (several) electronic databases. Furthermore, studies were scrutinized by cross-referencing for other included studies. The studies with potentially the highest quality of research were searched for first. The panel members selected literature in pairs (independently of each other) based on title and abstract. A second selection was performed based on full text. The databases, search terms and selection criteria are described in the modules containing the clinical questions.
Quality assessment of individual studies
Individual studies were systematically assessed, based on methodological quality criteria that were determined prior to the search, so that risk of bias could be estimated. This is described in the “risk of bias” tables.
Summary of literature
The relevant research findings of all selected articles are shown in evidence tables. The most important findings in literature are described in literature summaries. In case there were enough similarities between studies, the study data were pooled.
Grading quality of evidence and strength of recommendations
The strength of the conclusions of the scientific publications was determined using the GRADE-method. GRADE stands for Grading Recommendations Assessment, Development and Evaluation (see http://www.gradeworkinggroup.org/).
GRADE defines four gradations for the quality of scientific evidence: high, moderate, low or very low. These gradations provide information about the amount of certainty about the literature conclusions (http://www.guidelinedevelopment.org/handbook/).
The basic principles of the GRADE method are: formulating and prioritising clinical (patient) relevant outcome measures, a systematic review for each outcome measure, and appraisal of the evidence for each outcome measure based on the eight GRADE domains (domains for downgrading: risk of bias, inconsistency, indirectness, imprecision, and publication bias; domains for upgrading: dose-effect association, large effect, and residual plausible confounding).
GRADE distinguishes four levels for the quality of the scientific evidence: high, moderate, low and very low. These levels refer to the amount of certainty about the conclusion based on the literature, in particular the amount of certainty that the conclusion based on the literature adequately supports the recommendation (Schünemann, 2013; Hultcrantz, 2017).
|
Definition |
|
|
High |
|
|
Moderate |
|
|
Low |
|
|
Very low |
|
For the wording of the conclusions we used the statements suggested by the GRADE working group (Santesso, 2020), as shown below.
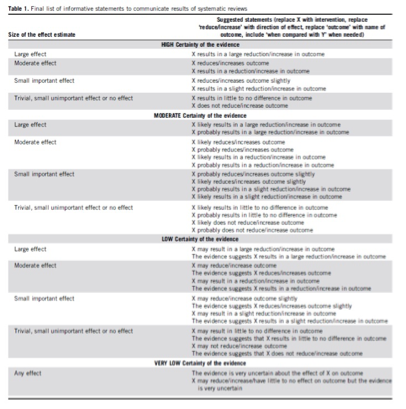
Source: Santesso (2020)
The limits of clinical decision making are very important in grading the evidence in guideline development according to the GRADE methodology (Hultcrantz, 2017). Exceedance of these limits would give rise to adaptation of the recommendation. All relevant outcome measures and considerations need to be taken into account to define the limits of clinical decision making. Therefore, the limits of clinical decision making are not one to one comparable to the minimal clinically relevant difference. In particular for interventions of low costs and without important drawbacks the limit of clinical decision making regarding the effectiveness of the intervention may be lower (i.e. closer to no effect) than the Minimal Clinically Important Difference (MCID) (Hultcrantz, 2017).
Considerations (evidence to decision)
Aspects such as expertise of working group members, patient preferences, costs, availability of facilities, and organisation of healthcare aspects are important to consider when formulating a recommendation. For each clinical question, these aspects are discussed in the paragraph Considerations, using a structured format based on the evidence-to-decision framework of the international GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). The evidence-to-decision framework is an integral part of the GRADE methodology.
Recommendations provide an answer to the primary question, and are based on the best scientific evidence available and the most important considerations. The level of scientific evidence and the importance given to considerations by the working group jointly determine the strength of the recommendation. In accordance with the GRADE method, a low level of evidence for conclusions in the systematic literature review does not rule out a strong recommendation, while a high level of evidence may be accompanied by weak recommendations. The strength of the recommendation is always determined by weighing all relevant arguments.
Knowledge gaps
During the development of this guideline, systematic searches were conducted for research contributing to answering the primary questions. For each primary question, the working group determined whether (additional) scientific research is desirable.
Commentary and authorisation phase
The concept guideline was subjected to commentaries by the scientific societies and patient organisations involved. The draft guideline was also submitted to the following organisations for comment: RCOG Guideline Committee and RCOG Patient Information Committee, German Neonatology and Peaediatric Intensive Care Association (Gesellschaft für Neonatologie und pädiatrische Intensivmedzin e.V.), German Midwives Society (Deutscher Hebammenverband), Flemish Midwives Society (VBOV), Belgian Federal Knowledge Centre for Health Care (KCE), Flemish College of Maternity and Neonatal Medicine (College Moeder Kind), Flemish patient organization for fertility problems (De Verdwaalde Ooievaar), Dutch Pediatric Society (NVK), Dutch College of General Practitioners (NHG), Healthcare Insurers Netherlands (ZN), The Dutch Healthcare Authority (NZA), the Health Care Inspectorate (IGJ), Netherlands Care Institute (ZIN), Dutch Organisation of Midwives (KNOV), Hospital organization (NVZ), Patient organisations Dutch Patient Federation and Freya. The comments were collected and discussed with the working group. The feedback was used to improve the guideline; afterwards the working group made the guideline definitive. The final version of the guideline was offered for authorization to the involved scientific societies and patient organisations and was authorized or approved, respectively.
Legal standing of guidelines
Guidelines are not legal prescriptions but contain evidence-based insights and recommendations that care providers should meet in order to provide high quality care. As these recommendations are primarily based on ‘general evidence for optimal care for the average patient’, care providers may deviate from the guideline based on their professional autonomy when they deem it necessary for individual cases. Deviating from the guideline may even be necessary in some situations. If care providers choose to deviate from the guideline, this should be done in consultation with the patient, where relevant. Deviation from the guideline should always be justified and documented.
