Klinisch beleid bij uitzakken van de navelstreng
Uitgangsvraag
Wat is het optimale klinische beleid bij uitzakken van de navelstreng?
Aanbeveling
Overweeg bij uitzakken van de navelstreng om de vrouw in knie-elleboog houding te brengen of het voorliggend deel omhoog te drukken, door retrograde vulling van de blaas of manueel, om compressie van de navelstreng en als gevolg foetale nood te voorkomen, en bespoedig de geboorte van de baby.
Overwegingen
Considerations – evidence to decision
Pros and cons of the intervention and quality of the evidence
The level of evidence for interventions in women with umbilical cord prolapse is very low, since only relatively small retrospective studies were found with considerable risk of bias. Several interventions were investigated, and it seems that in general these interventions were effective compared to no intervention, as is obvious considering the pathophysiology. The information is not sufficient to draw any conclusions regarding the relative size of the effect of the various interventions.
No adverse effects are described of the interventions. Catheterization and vaginal examinations are known to increase the risk of cystitis and intra-uterine infections, respectively. In case the cord prolapse occurs in the home setting, maintaining manual elevation of the presenting part or knee-chest position can be difficult or unsafe during transport to the hospital.
Values and preferences of patients (and their caregivers)
In the literature no information was found about women’s experiences. From the women’s perspective it can be reasoned that they want to give birth to their child in the best possible health. Manually elevating the presenting part can be a more burdensome intervention compared to filling the bladder and knee-chest position. Both manually elevating the presenting part, as well as filling the bladder can increase the risk of infections. This has to be explained before and during these procedures. These risks and discomfort may outweigh the benefits for the baby who is in serious danger. Reasoned from the invasiveness of the other interventions, the knee-chest position might be the preferred intervention by women in case of cord prolapse.
As for all interventions that have to be instituted in an emergency setting, it is of utmost importance to inform women and their partners before they give informed consent. The woman and her partner have to make the final decision for a particular intervention. Of course, this may include the urgent advice from a healthcare professional. Retrospectively, this should be documented in the clinical charts.
Costs
Because of the very low level of evidence, it is difficult to state to what extent the interventions would give positive effects. However, knee-chest position does not give extra costs, filling the bladder and manually elevating the presenting part gives extra (personnel) costs but these are minimal. Reduced risk of perinatal mortality and some factors of perinatal morbidity outweigh the minimal extra costs of filling the bladder and manually elevating the presenting part.
Acceptability, feasibility and implementation
The interventions are of low impact and might have an effect on perinatal mortality, the risk of an Apgar score <7 and the risk of umbilical artery pH <7.10. For healthcare professionals like midwives, emergency doctors, and obstetricians all three interventions are taught during emergency drills. For filling the bladder, equipment is necessary and should be readily available. It is necessary to determine whether healthcare professionals who work in an out-of-hospital setting have this equipment and know the procedures.
Differences between countries
It appeared that regional differences exist within and between the countries in their education for preferred intervention in case of umbilical cord prolapse.
This is a brief overview of education and preferred management according to the knowledge of the guideline authors in order to describe the differences between countries. Irrespective of which country, all interventions should be performed after informed consent has been given.
In Germany manually elevating the presenting part is preferred, followed by knee-chest position. Filling the bladder is rarely taught as intervention for this obstetric emergency.
In contrast with Germany, in the Netherlands filling the bladder is the intervention of first choice, followed by manually elevating the presenting part. Knee-chest position is not taught. This difference might be explained by the fact that in the Netherlands at onset of labour around 50% of the women are in primary midwifery care, and home births are still common. Because of this, there is an increased risk that women need to be transported in case of an umbilical cord prolapse, which appears to be easier when the bladder is filled compared to knee-chest position.
In the UK, the RCOG states “to prevent cord compression, it is recommended that the presenting part be elevated either manually or by filling the urinary bladder” (RCOG Green-top Guideline No. 50, November 2014).
In Belgium, the national policy is to manually elevate the presenting part, although in regional protocols knee-chest position and filling the bladder is also described.
Recommendation
|
In case of umbilical cord prolapse, consider knee-chest position or elevation of the presenting part, by filling the bladder or manually, in order to prevent cord compression and secondary fetal compromise, and expedite the birth of the baby. |
Onderbouwing
Achtergrond
Introduction
If during labour the obstetrician or midwife detects an umbilical cord prolapse, immediate preparations need to be made for an emergency caesarean birth or, if possible, vaginal birth. There is no consensus about the optimal preventive actions that need to be taken to protect the unborn baby during the waiting time until the caesarean birth or vaginal birth can be performed. Possible interventions are manually elevating the presenting part, filling the bladder and knee-chest position. This guideline aims to explore whether these interventions are effective and if recommendations for practice can be made.
Conclusies / Summary of Findings
|
Manual elevation may reduce the risk of perinatal mortality in women with umbilical cord prolapse, but the evidence is very uncertain.
Sources: (Bako, 2009.) |
|
Very low GRADE |
Knee-chest position may reduce the risk of perinatal mortality in women with umbilical cord prolapse, but the evidence is very uncertain.
Sources: (Bako, 2009; Wasswa, 2014.) |
|
Very low GRADE |
Bladder filling and subsequent caesarean birth may reduce the risk of perinatal mortality compared to rapid vaginal birth in women with umbilical cord prolapse, but the evidence is very uncertain.
Sources: (Caspi, 1983.) |
|
Very low GRADE |
The evidence is very uncertain about the effect of manual elevation plus bladder filling compared to manual elevation only in women with umbilical cord prolapse on the risk of 5 minutes’ Apgar score <7.
Sources: (Bord, 2011.) |
|
Very low GRADE |
Bladder filling and subsequent caesarean section may reduce the risk of 5 minutes’ Apgar score <7 compared to rapid vaginal birth in women with umbilical cord prolapse, but the evidence is very uncertain.
Sources: (Caspi, 1983.) |
|
Very low GRADE |
The evidence is very uncertain about the effect of manual elevation plus bladder filling compared to manual elevation only in women with umbilical cord prolapse on the risk of umbilical artery pH <7.10.
Sources: (Bord, 2011.) |
|
Very low GRADE |
The evidence is very uncertain about the effect of manual elevation plus bladder filling compared to manual elevation only in women with umbilical cord prolapse on time to delivery.
Sources: (Bord, 2011.) |
|
- |
No conclusions could be drawn for the following outcome measures: 5 minutes’ Apgar score <4, neonatal seizures, NICU admission and maternal parameters. |
Samenvatting literatuur
Summary of literature
Description of studies
Four historical cohort studies were included, two from Israel (Bord, 2011; Caspi, 1983), one from Nigeria (Bako, 2009) and one from Uganda (Wasswa, 2014). Different comparisons were described, as shown in the table below: manual elevation (n=13) vs no intervention (n=27) (Bako 2009), knee-chest position (n=26 (Bako, 2009) and n=348 (Wasswa, 2014)) vs no intervention (n=27 (Bako, 2009) and n=90 (Wasswa, 2014)), manual elevation plus bladder filling (n=15) vs manual elevation only (n=29) Bord (2011), and bladder filling and subsequent caesarean birth (n=88) vs rapid vaginal birth (n=39) Caspi (1983).
|
|
Bako, 2009 |
Wasswa, 2014 |
Bord, 2011 |
Caspi, 1983 |
|
no intervention |
27 |
90 |
|
|
|
manual elevation |
13 |
|
29 |
|
|
knee-chest position |
26 |
348 |
|
|
|
manual elevation plus bladder filling |
|
|
15 |
|
|
bladder filling and subsequent caesarean birth |
|
|
|
88 |
|
rapid vaginal birth |
|
|
|
39 |
In all studies confounding by indication (the decision about the intervention by the clinicians was probably influenced by the clinical status of the patient) was very likely, except in the study by Bord (2011), because they reported that the women in the control group were included after the procedure of bladder filling was stopped. This subsequent inclusion of the intervention and control groups may also have induced bias, since it is possible that other clinical care variables were different during the different time periods. In the study by Caspi (1983) the intervention groups were not comparable at baseline, since bladder instillation and subsequent caesarean birth was only applied in women in the first stage of labour, and rapid vaginal birth was applied in the second stage of labour when “the obstetrical conditions were considered suitable for rapid vaginal delivery” Caspi (1983). The only study reporting statistical adjustment for confounding factors was the study by Wasswa. However, the description of the statistical analysis was insufficient and not informative. In all four studies the risk of bias is considered to be moderate to high.
Results
For all reported comparisons the results for the outcome measures perinatal mortality and 5 minutes’ Apgar score <7 were in favour of the intervention, though not all statistically significant. The outcome measures umbilical artery pH<7.10 and time to delivery were only reported by Bord (2011), with equivocal results.
1. Perinatal mortality
This outcome measure (only defined in the study report of Bako as mortality within 24 hours of birth) was reported for the comparison manual elevation vs no intervention (Bako, 2009), knee-chest position vs no intervention (Bako, 2009; Wasswa, 2014), and bladder filling and subsequent caesarean birth (CB) vs rapid vaginal delivery (Caspi, 1983).
- Comparing manual elevation vs no intervention the unadjusted RR (95% CI) for perinatal mortality was 0.35 (0.09 to 1.33) favoring manual elevation (Bako, 2009).
- Comparing knee-chest position vs no intervention the unadjusted RR (95% CI) for perinatal mortality was 0.35 (0.13 to 0.94) (Bako, 2009) and 0.56 (0.39 to 0.79) (Wasswa, 2014). The adjusted RR (95% CI) (not clear for what variables) was 0.81 (0.70 to 0.95) (Wasswa, 2014), all favoring knee-chest position.
- Comparing bladder filling and subsequent CB vs rapid vaginal delivery the unadjusted RR (95% CI) for perinatal mortality was 0.064 (0.003 to 1.214) favoring bladder filling and subsequent CB (Caspi, 1983).
2. 5 minutes’ Apgar score <7
This outcome measure was reported by Bord (2011) for the comparison manual elevation plus bladder filling vs manual elevation only and by Caspi (1983) for the comparison bladder filling and subsequent CB vs rapid vaginal birth In the study by Bord (2011) a 5 minutes’ Apgar score of 7 was included.
- Comparing manual elevation plus bladder filling vs manual elevation only the RR (95% CI) for 5 minutes’ Apgar score ≤7 was 0.63 (0.03 to 14.5) favoring manual elevation plus bladder filling (Bord, 2011).
- Comparing bladder filling and subsequent CB vs rapid vaginal birth the RR (95% CI) for 5 minutes’ Apgar score <7 was 0.148 (0.042 to 0.516) favoring bladder filling and subsequent CB (Caspi, 1983).
3. Umbilical artery pH<7.10
Only the outcome umbilical artery pH<7 was reported, which is somewhat more severe than pH<7.10. Bord (2011) reported that 0/6 neonates in the manual support and bladder filling group and and 0/21 in the manual support only group had umbilical artery pH <7.
4. Time to delivery
Comparing manual support and bladder filling vs manual support only Bord (2011) reported a time to delivery of 19.8 ± 9.4 and 21.6 ± 11.9 minutes, respectively, p=0.57.
The other outcomes that were considered important (5 minutes’ Apgar score <4, neonatal seizures, NICU admission and maternal parameters) were not reported in the included articles.
Level of evidence of the literature
1. Perinatal mortality
1a. Comparison manual elevation vs no intervention
The level of evidence for the comparison manual elevation vs no intervention regarding the outcome measure perinatal mortality started low and was downgraded to very low GRADE because of study limitations (risk of bias) and number of included women (imprecision).
1b. Comparison knee-chest position vs no intervention
The level of evidence for the comparison knee-chest position vs no intervention regarding the outcome measure perinatal mortality started low and was downgraded to very low GRADE because of study limitations (risk of bias) and number of included patients (imprecision).
1c. bladder filling and subsequent CB vs rapid vaginal birth
The level of evidence for the comparison bladder filling and subsequent CB vs rapid vaginal delivery regarding the outcome measure perinatal mortality started low and was downgraded to very low GRADE because of study limitations (risk of bias) and number of included women (imprecision).
2. 5 minutes’ Apgar score <7
2a. Comparison manual elevation plus bladder filling vs manual elevation only
The level of evidence for the comparison manual elevation plus bladder filling vs manual elevation only regarding the outcome measure 5 minutes’ Apgar score <7 started low and was downgraded to very low GRADE because of study limitations (risk of bias) and number of included women (imprecision).
2b. Comparison bladder filling and subsequent caesarean birth vs rapid vaginal birth
The level of evidence for the comparison bladder filling and subsequent caesarean birth vs rapid vaginal birth regarding the outcome measure 5 minutes’ Apgar score <7 started low and was downgraded to very low GRADE because of study limitations (risk of bias) and number of included patients (imprecision).
3. Umbilical artery pH<7.10
Comparison manual elevation plus bladder filling vs manual elevation only
The level of evidence for the comparison manual elevation plus bladder filling vs manual elevation only regarding the outcome measure umbilical artery pH<7.10 started low and was downgraded to very low GRADE because of study limitations (risk of bias) and number of included women (imprecision).
4. Time to delivery
Comparison manual elevation plus bladder filling vs manual elevation only.
The level of evidence for the comparison manual elevation plus bladder filling vs manual elevation only regarding the outcome measure time to delivery started low and was downgraded to very low GRADE because of study limitations (risk of bias) and number of included women (imprecision).
Zoeken en selecteren
Search and select
A systematic review of the literature was performed to answer the following question:
What is the effect of manually elevating the presenting part or filling the bladder or knee-chest position compared to no intervention in women with an umbilical cord prolapse after rupture of membranes on maternal and fetal outcomes?
P: term pregnant women with ruptured membranes and umbilical cord prolapse
I: manual elevation of the presenting part, filling the bladder, knee-chest position
C: no intervention
O: maternal and fetal outcomes: umbilical cord blood pH, Apgar score at 5 minutes, neonatal cooling, admission to neonatal unit or Neonatal Intensive Care Unit (NICU), perinatal mortality, time to delivery, patient satisfaction, pain, perineal trauma, psychological trauma, mother-child bonding, breast feeding
Relevant outcome measures
The guideline development group considered perinatal mortality as a crucial outcome measure for decision making, and 5 minutes’ Apgar scores <7 and <4, umbilical artery pH <7.10, neonatal seizures, NICU admission, time to delivery and maternal parameters as important outcome measures for decision making.
A priori, the guideline development group did not define the outcome measures listed above but used the definitions used in the studies.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until January 10th, 2020. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 205 hits. Studies were selected based on the following criteria: prospective or retrospective studies comparing two or more clinical management interventions in women in labour with umbilical cord prolapse. Fourteen studies were initially selected based on title and abstract screening. After reading the full text, ten studies were excluded (see the table with reasons for exclusion under the tab Methods), and four studies were included.
Results
Four studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence table (attached documents). The assessment of the risk of bias is summarized in the risk of bias table (attached documents).
Referenties
- Bako, B., Chama, C., & Audu, B. M. (2009). Emergency obstetrics care in a Nigerian tertiary hospital: a 20 year review of umblical cord prolapse. Nigerian journal of clinical practice, 12(3).
- Bord, I., Gemer, O., Anteby, E. Y., & Shenhav, S. (2011). The value of bladder filling in addition to manual elevation of presenting fetal part in cases of cord prolapse. Archives of gynecology and obstetrics, 283(5), 989-991.
- Caspi, E., Lotan, Y., & Schreyer, P. (1983). Prolapse of the cord: reduction of perinatal mortality by bladder instillation and cesarean section. Israel journal of medical sciences, 19(6), 541.
- Wasswa, E. W., Nakubulwa, S., & Mutyaba, T. (2014). Fetal demise and associated factors following umbilical cord prolapse in Mulago hospital, Uganda: a retrospective study. Reproductive health, 11(1), 12.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Bako, 2009 |
Type of study: historical cohort study
Setting and country: University hospital Nigeria, January 1986 – December 2005
Funding and conflicts of interest: not reported |
Inclusion criteria: all women who presented with umbilical cord prolapse from January 1986 to December 2005
Exclusion criteria: none reported
N total at baseline: 66 Intervention: manual elevation: 13, Knee-chest position: 26 Control: 27
Missing values: 9 out of 75 records not available (12%)
Important prognostic factors2: not reported per intervention group
Groups comparable at baseline? unclear
|
Manual elevation OR Knee-chest position
|
No intervention
|
Length of follow-up: 24 hours after delivery(?)
Loss-to-follow-up: not reported
Incomplete outcome data: not reported
|
Perinatal death
Comparison 1. Manual elevation vs. no intervention:
2/13 vs 12/27 RR [95% CI] = 0.35 [0.09; 1.33]
Comparison 2. Knee-chest position vs. no intervention:
4/26 vs 12/27 RR [95% CI] = 0.35 [0.13; 0.94]
|
No adjustment for confounding factors;
Raw data extracted from article; calculation of RR [95% CI] in MedCalc |
|
Bord, 2011 |
Type of study: historical cohort study
Setting and country: Medical center Israel
Funding and conflicts of interest: not reported |
Inclusion criteria: all deliveries complicated by cord prolapse between 1988 and 2009; singleton pregnancies of at least 24 weeks’ gestation; cord prolapse occurring after admission to the delivery room
Exclusion criteria: women diagnosed with a cord prolapsed upon admission
N total at baseline: 44 Intervention: 15 Control: 29
Important prognostic factors2:
Groups comparable at baseline? No statistically significant differences. However, “the procedure of bladder filling was abandoned more than 10 years ago.”
|
Manual elevation plus bladder filling
|
Manual elevation only
|
Length of follow-up: 5 minutes after delivery(?)
Loss-to-follow-up: not reported
Incomplete outcome data: not reported
|
1. 5 minutes’ Apgar score ≤ 7
0/15 vs 1/29 RR [95% CI] = 0.63 [0.03; 14.5]
2. umbilical artery pH<7 0/6 vs 0/21
3. Time to delivery 19.8 ± 9.4 and 21.6 ± 11.9 minutes(?), p=0.57 |
No adjustment for confounding factors;
Raw data extracted from article; calculation of RR [95% CI] in MedCalc |
|
Caspi 1983 |
Type of study: historical cohort study
Setting and country: University Medical center, Israel
Funding and conflicts of interest: not reported |
Inclusion criteria: 1970 – 1979 umbilical cord prolapse, >28 wk of gestation
Exclusion criteria: occult prolapse and cord presentation, intrauterine fetal death
N total at baseline: 127 Intervention: 88 Control: 39 (9 spontaneous, 9 vacuum extraction, 3 forceps, 18 breech extraction)
Important prognostic factors2: not reported per group
Groups comparable at baseline? No
|
(in first stage of labor) bladder instillation and subsequent CS
|
(in second stage of labor) rapid vaginal delivery
|
Length of follow-up: 5 minutes after delivery(?)
Loss-to-follow-up: not reported
Incomplete outcome data: not reported
|
Outcome 1. Perinatal mortality
0/88 vs 3/39 RR [95% CI] = 0.064 [0.003; 1.214]
Outcome 2. 5-min Apgar score < 7
3/88 vs 9/39 RR [95% CI] = 0.148 [0.042; 0.516] |
No adjustment for confounding factors;
Raw data extracted from article; calculation of RR [95% CI] in MedCalc |
|
Wasswa, 2014 |
Type of study: historical cohort study
Setting and country: University Medical Center, Uganda
Funding: Belgium Technical Cooperation (BTC), Uganda
Conflicts of interest: none declared |
Inclusion criteria: mothers who had UCP and delivered between 1st January 2000 to 31st December 2009; ≥28 weeks of gestation; live fetus
Exclusion criteria: inadequate data like those missing information on time of diagnosis of cord prolapse, time of delivery of the baby and fetal outcome
N total at baseline: 438 (random sample of 661 eligible files) Intervention: 348 Control: 90
Important prognostic factors2: not reported per group
Groups comparable at baseline? unclear
|
Knee-chest position
|
No knee-chest position
|
Length of follow-up: 5 minutes after delivery(?)
Loss-to-follow-up: not reported
Incomplete outcome data: not reported
|
Perinatal death
reported adjusted RR [95% CI] = 0.81 [0.70-0.95]
raw data: 69/348 vs 32/90 unadjusted RR [95% CI] = 0.56 [0.39; 0.79]
|
Reported statistical analysis compared variables in women with and without perinatal death. Not clear what confounders were adjusted for.
|
Risk of bias table
|
Study reference
(first author, year of publication) |
Bias due to a non-representative or ill-defined sample of patients?
(unlikely/likely/unclear) |
Bias due to insufficiently long, or incomplete follow-up, or differences in follow-up between treatment groups?
(unlikely/likely/unclear)
|
Bias due to ill-defined or inadequately measured outcome ?
(unlikely/likely/unclear) |
Bias due to inadequate adjustment for all important prognostic factors?
(unlikely/likely/unclear) |
|
Bako, 2009 |
unclear records of 9/75 women were missing |
unlikely
|
unlikely |
likely no adjustment |
|
Bord, 2011 |
unlikely |
unlikely |
unlikely |
likely no adjustment |
|
Caspi 1983 |
unclear confounding by indication is obvious |
unlikely |
unlikely |
likely no adjustment |
|
Wasswa, 2014 |
unclear not clear how random sample was obtained |
unlikely |
unlikely |
unclear adjustment for confounding was performed, but not clear for what variables. |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 30-12-2022
Beoordeeld op geldigheid : 30-12-2022
Validity period
The Board of the Dutch Society of Obstetrics and Gynaecology (NVOG) will assess whether these guidelines are still up-to-date in 2026 at the latest. If necessary, a new working group will be appointed to revise the guideline. The guideline’s validity may lapse earlier if new developments demand revision at an earlier date.
As the holder of this guideline, the NVOG is chiefly responsible for keeping the guideline up to date. Other scientific organizations participating in the guideline or users of the guideline share the responsibility to inform the chiefly responsible party about relevant developments within their fields.
Algemene gegevens
Er is meegelezen vanuit de Nederlandse Vereniging voor Kindergeneeskunde (NVK). De NVK heeft de richtlijn niet geautoriseerd, maar heeft geen bezwaar tegen publicatie.
De Koninklijke Nederlandse Organisatie van Verloskundigen (KNOV) is betrokken geweest bij de ontwikkeling van de richtlijn.
De Patiëntenfederatie Nederland heeft de richtlijn goedgekeurd.
De Vlaamse Vereniging voor Obstetrie en Gynaecologie (VVOG), Royal College of Obstetrics and Gynaecology (RCOG) en Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (DGGG) zijn betrokken geweest bij de ontwikkeling van de richtlijn.
Regiehouder: NVOG
Samenstelling werkgroep
Composition guideline development panel
An international panel for the development of the guidelines was formed in 2019. The panel consisted of representatives from all relevant medical disciplines that are involved in medical care for pregnant women.
All panel members have been officially delegated for participation in the guideline development panel by their (scientific) societies. The panel developed the guidelines in the period from May 2019 until March 2021.
The guideline development panel is responsible for the entire text of this guideline.
All panel members have been officially delegated for participation in the guideline development panel by their scientific societies. The guideline development panel is responsible for the entire text of this guideline.
Guideline development panel
- J.J. Duvekot, obstetrician, Consultant Obstetrics and Gynaecology, Erasmus Medical Centre, Rotterdam, the Netherlands (chair)
- I. Dehaene, obstetrician, Consultant Obstetrics and Gynaecology, Ghent University Hospital Belgium
- S. Galjaard, obstetrician, Consultant Obstetrics and Gynaecology, Erasmus Medical Centre, Rotterdam, the Netherlands
- A. Hamza, obstetrician, Consultant Obstetrics and Gynaecology, University Medical Center of Saarland, Homburg an der Saar, Germany
- S.V. Koenen, obstetrician, Consultant Obstetrics and Gynaecology, ETZ, locatie Elisabeth Ziekenhuis Tilburg, the Netherlands
- M. Kunze, obstetrician, Consultant Obstetrics and Gynaecology, Department of Gynecology& Obstetrics University of Freiburg, Germany
- M.A. Ledingham, obstetrician, Consultant Obstetrics and Gynaecology, the Queen Elizabeth Hospital Glasgow, UK
- B. Magowan, obstetrician, Consultant Obstetrics and Gynaecology, and Co-Chair UK RCOG Guidelines Committee, NHS Borders, Scotland, UK
- G. Page, obstetrician, Consultant Obstetrics and Gynaecology, Jan Yperman Hospital, Ypres, Belgium
- S.J. Stock, Reader and Consultant in Maternal and Fetal Medicine, University of Edinburgh Usher Institute and NHS Lothian, Edinburgh, Scotland, UK
- A.J. Thomson, obstetrician, Consultant Obstetrics and Gynaecology, Royal Alexandra Hospital (NHS Greater Glasgow and Clyde), UK
- G. Verhulst, obstetrician, Consultant Obstetrics and Gynaecology, ASZ Aalst/Geraardsbergen/Wetteren, Belgium
- D.C. Zondag, midwife/practice owner verloskundige praktijk De Toekomst-Geldermalsen, the Netherlands
Methodological support
- E. den Breejen, senior advisor, Knowledge Institute of the Dutch Association of Medical Specialists (until June 2019)
- J.H. van der Lee, senior advisor, Knowledge Institute of the Dutch Association of Medical Specialists (since May 2019)
- Y. Labeur, junior advisor, Knowledge Institute of the Dutch Association of Medical Specialists
Belangenverklaringen
The Code for the prevention of improper influence due to conflicts of interest was followed (https://storage.knaw.nl/2022-08/Code-for-the-prevention-of-improper-influence-due-to-conflicts-of-interest.pdf).
The working group members have provided written statements about (financially supported) relations with commercial companies, organisations or institutions related to the subject matter of the guideline during the past three years. Furthermore, inquiries have been made regarding personal financial interests, interests due to personal relationships, interests related to reputation management, interest related to externally financed research and interests related to knowledge valorisation. The chair of the guideline development panel is informed about changes in interests during the development process. The declarations of interests are reconfirmed during the commentary phase. The declarations of interests can be requested at the administrative office of the Knowledge Institute of the Dutch Association of Medical Specialists and are summarised below.
|
Last name |
Principal position |
Ancillary position(s) |
Declared interests |
Action |
|
Duvekot (chair) |
Consultant Obstetrics and Gynaecology, Erasmus MC, Rotterdam |
Director Medisch Advies en Expertise Bureau Duvekot, Ridderkerk |
none |
none |
|
Dehaene |
Consultant Obstetrics and Gynaecology, Ghent University Hospital |
none |
none |
none |
|
Galjaard |
Consultant Obstetrics and Gynaecology, Erasmus MC, Rotterdam |
Associated member of Diabetes in Pregnancy Group (DPSG) |
none |
none |
|
Hamza |
Consultant Obstetrics and Gynaecology, University Medical Center of Saarland, Homburg |
part of the advisory board of clinical innovations, which produces Kiwi-Vacuum Extractors® and Ebb Balloon Catheter®;
|
gave ultrasound courses sponsored by ultrasound producing companies: Samsung Germany and Matramed |
Recommendations do not involve either vacuum extractor or Ebb catheter (which is used for postpartum hemorrhage); therefore no actions |
|
Koenen |
Consultant Obstetrics and Gynaecology, ETZ, locatie Elisabeth Ziekenhuis Tilburg |
Chairman 'Koepel Kwaliteit' NVOG |
none |
none |
|
Kunze |
Divison Chief, Maternal-Fetal Medicine and Obstetrics, Departement of Gynecology & Obstetrics, University of Freiburg |
none |
none |
none |
|
Ledingham |
Consultant in Maternal and Fetal Medicine, Queen Elizabeth Hospital, Glasgow |
Co-chair RCOG Guidelines committee, Guideline developer for sign (scottisch intercollegiate guidelines group) |
none |
none |
|
Magowan |
Consultant Obstetrics and Gynaecology, and Co-Chair UK RCOG Guidelines Committee, NHS Borders, Scotland |
Co-chair RCOG Guidelines committee |
none |
none |
|
Page |
Consultant Obstetrics and Gynaecology, Jan Yperman Hospital, Ypres |
none |
none |
none |
|
Stock |
Reader and Consultant in Maternal and Fetal Medicine, University of Edinburgh and NHS Lothian, Edinburgh, Scotland, UK |
Consultant Obstetrician and Subspecialist Maternal and Fetal Medicine, member of the NIHR HTA General committee (grant funding board) and Chair of the RCOG Stillbirth Clinical Studies Group |
Research grants paid to the institution for research into pregnancy problems from National Institute of Healthcare Research (NIHR) Health Technology Assessment (HTA), NIHR Global Research Fund, Wellcome Trust, Medical Research Council, Tommy's Baby Charity, Cheif Scientist Office Scotland. Some of this work focuses on improving risk prediction of preterm labour and researching the benefits and harms of antenatal corticosteroids. Non-financial support from HOLOGIC, non-financial support from PARSAGEN, non-financial support from MEDIX BIOCHEMICA during the conduct of an NIHR HTA study in the form of provision of reduced cost assay kits to participating sites and blinded test assay analysers |
none |
|
Thomson |
Consultant Obstetrics and Gynaecology, Royal Alexandra Hospital (NHS Greater Glasgow and Clyde) |
Guideline developer for the RCOG |
none |
none |
|
Verhulst |
Head of Department of Gynaecology and Obstetrics, ASZ Aalst/Geraardsbergen/Wetteren |
none |
none |
none |
|
Zondag |
Midwife/practice owner verloskundige praktijk De Toekomst-Geldermalsen |
Policy adviser at the Dutch association of midwives (KNOV). Teacher at PA clinical midwives - Hogeschool Rotterdam |
none |
none |
Inbreng patiëntenperspectief
Representation of the patient perspective
Involvement of patient representatives from all four participating countries was challenging. Representatives of patient organisations from three countries (UK, Belgium, the Netherlands) commented on the draft guideline texts and discussed these during an online meeting. They represented the RCOG Women’s Network, the Flemish organisation for people with fertility problems ‘De verdwaalde ooievaar’, the Netherlands Patient Federation, and the Dutch association for people with fertility problems ‘Freya’. The comments were discussed and where relevant incorporated by the guideline development panel.
Methode ontwikkeling
Evidence based
Implementatie
Implementation
Guideline implementation and practical applicability of the recommendations was taken into consideration during various stages of guideline development. Factors that may promote or hinder implementation of the guideline in daily practice were given specific attention.
The guideline is distributed digitally among all relevant professional groups. The guideline can also be downloaded from the following websites: www.nvog.nl, www.vvog.be, www.rcog.org.uk, www.dggg.de,and the Dutch guideline website: www.richtlijnendatabase.nl.
Werkwijze
Method
AGREE
This guideline has been developed conforming to the requirements of the report of Guidelines for Medical Specialists 2.0 by the advisory committee of the Quality Counsel. This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (www.agreetrust.org)(Brouwers, 2010), a broadly accepted instrument in the international community and on the national quality standards for guidelines: “Guidelines for guidelines” (www.zorginstituutnederland.nl).
Identification of subject matter
During the initial phase of the guideline development the chairman, guideline development panel and the advisor inventoried the relevant subject matter for the guideline. Since this was a pilot project, the content of the questions and the support base in clinical practice was considered of less importance than the process of international collaboration and learning from each other. Key questions were selected in such a way that:
-
-
- they were relevant for obstetric practice in all collaborating countries;
- it was expected that the amount of literature identified for each question would be reasonable, i.e. some literature was expected, but not much;
- the recommendations were expected not to lead to extensive discussion among working group members because no major controversy was expected;
- there were no recent guidelines available for these particular topics in any of the four countries.
-
Clinical questions and outcomes
The guideline development panel then formulated definitive clinical questions and defined relevant outcome measures (both beneficial and harmful effects). The working group rated the outcome measures as critical, important and not important. Furthermore, where applicable, the working group defined relevant clinical differences.
Strategy for search and selection of literature
For the separate clinical questions, specific search terms were formulated and published scientific articles were searched for in (several) electronic databases. Furthermore, studies were scrutinized by cross-referencing for other included studies. The studies with potentially the highest quality of research were searched for first. The panel members selected literature in pairs (independently of each other) based on title and abstract. A second selection was performed based on full text. The databases, search terms and selection criteria are described in the modules containing the clinical questions.
Quality assessment of individual studies
Individual studies were systematically assessed, based on methodological quality criteria that were determined prior to the search, so that risk of bias could be estimated. This is described in the “risk of bias” tables.
Summary of literature
The relevant research findings of all selected articles are shown in evidence tables. The most important findings in literature are described in literature summaries. In case there were enough similarities between studies, the study data were pooled.
Grading quality of evidence and strength of recommendations
The strength of the conclusions of the scientific publications was determined using the GRADE-method. GRADE stands for Grading Recommendations Assessment, Development and Evaluation (see http://www.gradeworkinggroup.org/).
GRADE defines four gradations for the quality of scientific evidence: high, moderate, low or very low. These gradations provide information about the amount of certainty about the literature conclusions (http://www.guidelinedevelopment.org/handbook/).
The basic principles of the GRADE method are: formulating and prioritising clinical (patient) relevant outcome measures, a systematic review for each outcome measure, and appraisal of the evidence for each outcome measure based on the eight GRADE domains (domains for downgrading: risk of bias, inconsistency, indirectness, imprecision, and publication bias; domains for upgrading: dose-effect association, large effect, and residual plausible confounding).
GRADE distinguishes four levels for the quality of the scientific evidence: high, moderate, low and very low. These levels refer to the amount of certainty about the conclusion based on the literature, in particular the amount of certainty that the conclusion based on the literature adequately supports the recommendation (Schünemann, 2013; Hultcrantz, 2017).
|
Definition |
|
|
High |
|
|
Moderate |
|
|
Low |
|
|
Very low |
|
For the wording of the conclusions we used the statements suggested by the GRADE working group (Santesso, 2020), as shown below.
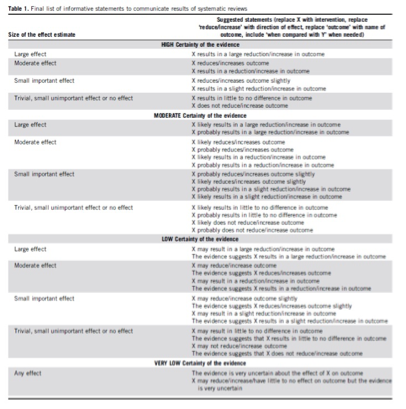
Source: Santesso (2020)
The limits of clinical decision making are very important in grading the evidence in guideline development according to the GRADE methodology (Hultcrantz, 2017). Exceedance of these limits would give rise to adaptation of the recommendation. All relevant outcome measures and considerations need to be taken into account to define the limits of clinical decision making. Therefore, the limits of clinical decision making are not one to one comparable to the minimal clinically relevant difference. In particular for interventions of low costs and without important drawbacks the limit of clinical decision making regarding the effectiveness of the intervention may be lower (i.e. closer to no effect) than the Minimal Clinically Important Difference (MCID) (Hultcrantz, 2017).
Considerations (evidence to decision)
Aspects such as expertise of working group members, patient preferences, costs, availability of facilities, and organisation of healthcare aspects are important to consider when formulating a recommendation. For each clinical question, these aspects are discussed in the paragraph Considerations, using a structured format based on the evidence-to-decision framework of the international GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). The evidence-to-decision framework is an integral part of the GRADE methodology.
Recommendations provide an answer to the primary question, and are based on the best scientific evidence available and the most important considerations. The level of scientific evidence and the importance given to considerations by the working group jointly determine the strength of the recommendation. In accordance with the GRADE method, a low level of evidence for conclusions in the systematic literature review does not rule out a strong recommendation, while a high level of evidence may be accompanied by weak recommendations. The strength of the recommendation is always determined by weighing all relevant arguments.
Knowledge gaps
During the development of this guideline, systematic searches were conducted for research contributing to answering the primary questions. For each primary question, the working group determined whether (additional) scientific research is desirable.
Commentary and authorisation phase
The concept guideline was subjected to commentaries by the scientific societies and patient organisations involved. The draft guideline was also submitted to the following organisations for comment: RCOG Guideline Committee and RCOG Patient Information Committee, German Neonatology and Peaediatric Intensive Care Association (Gesellschaft für Neonatologie und pädiatrische Intensivmedzin e.V.), German Midwives Society (Deutscher Hebammenverband), Flemish Midwives Society (VBOV), Belgian Federal Knowledge Centre for Health Care (KCE), Flemish College of Maternity and Neonatal Medicine (College Moeder Kind), Flemish patient organization for fertility problems (De Verdwaalde Ooievaar), Dutch Pediatric Society (NVK), Dutch College of General Practitioners (NHG), Healthcare Insurers Netherlands (ZN), The Dutch Healthcare Authority (NZA), the Health Care Inspectorate (IGJ), Netherlands Care Institute (ZIN), Dutch Organisation of Midwives (KNOV), Hospital organization (NVZ), Patient organisations Dutch Patient Federation and Freya. The comments were collected and discussed with the working group. The feedback was used to improve the guideline; afterwards the working group made the guideline definitive. The final version of the guideline was offered for authorization to the involved scientific societies and patient organisations and was authorized or approved, respectively.
Legal standing of guidelines
Guidelines are not legal prescriptions but contain evidence-based insights and recommendations that care providers should meet in order to provide high quality care. As these recommendations are primarily based on ‘general evidence for optimal care for the average patient’, care providers may deviate from the guideline based on their professional autonomy when they deem it necessary for individual cases. Deviating from the guideline may even be necessary in some situations. If care providers choose to deviate from the guideline, this should be done in consultation with the patient, where relevant. Deviation from the guideline should always be justified and documented.
Zoekverantwoording
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Anuwutnavin, 2019 |
no information about procedures for cord prolapse |
|
Kalu, 2011 |
no comparison |
|
Katz, 1982 |
no comparison |
|
Katz, 1988 |
no comparison |
|
Kaymak, 2015 |
no comparison |
|
Portz, 2016 |
only one retrospective study in which one umbilical cord prolapse occured described. No information on procedures. |
|
Smit, 2014 |
case series, no comparison |
|
Tan, 2003 |
no information about procedures for cord prolapse |
|
Usta, 1999 |
no information about procedures for cord prolapse |
|
White, 1964 |
no comparison |
Literature search strategy
|
Database |
Search elements |
Total |
|
Medline (OVID)
10-1-2020 |
1 ((exp Prolapse/ or prolapse.ti,ab,kf.) and (exp Umbilical Cord/ or cord.ti,ab,kf. or umbilical.ti,ab,kf.)) or ucp.ti,ab,kf. (2893) 2 ((exp Urinary Bladder/ or bladder.ti,ab,kf.) and (filling or distentsion or instillation).ti,ab,kf.) or 'manual elevation'.ti,ab,kf. or 'funic reduction'.ti,ab,kf. or management.ti,ab,kf. (1084174) 3 1 and 2 (167) 4 limit 3 to english language (145) 5 (meta-analysis/ or meta-analysis as topic/ or (meta adj analy$).tw. or ((systematic* or literature) adj2 review$1).tw. or (systematic adj overview$1).tw. or exp "Review Literature as Topic"/ or cochrane.ab. or cochrane.jw. or embase.ab. or medline.ab. or (psychlit or psyclit).ab. or (cinahl or cinhal).ab. or cancerlit.ab. or ((selection criteria or data extraction).ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (427547) 6 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (1936702) 7 Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or (cohort adj (study or studies)).tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] (3341438) 8 4 and 5 (7) 9 (4 and 6) not 8 (13) 10 (4 and 7) not (8 or 9) (36) 11 8 or 9 or 10 (56)
7 SRs + 13 RCTs + 36 observational studies = 56 total (24 unqiue) |
205 |
