Immunosuppression and immunomodulation in IIM
Uitgangsvraag
What is the treatment strategy for patients with idiopathic inflammatory myopathies (IIMs)?
Aanbeveling
Recommendations: initial treatment
Start bij patiënten met een idiopathische inflammatoire myopathie (IIM, uitgezonderd IBM) met corticosteroïden 1 mg/kg lichaamsgewicht tot een maximum van 80 mg per dag, of dexamethasonstootkuren (40 mg 1dd1 gedurende 4 dagen elke 28 dagen)
Overweeg bij ernstig aangedane patiënten methylprednisolon i.v. (1g per dag, gedurende 3 dagen gevolgd door 40-60 mg prednison 1dd1).
Overweeg bij patiënten met geringe beperkingen (nauwelijks spierzwakte, of alleen spierpijn, of uitsluitend huidafwijkingen) een lagere dosering prednison (bijv. 0.5 mg/kg).
Overleg bij tegelijkertijd vastgestelde IIM en systemische sclerose met een expertisecentrum voor een multidisciplinair advies (zie https://myositisexpertisecentrum.nl/).
Overweeg elke nieuw gediagnosticeerde patiënt (het liefst al in het diagnostisch traject) te (laten) informeren door een expertisecentrum over eventuele deelname aan een medicijnstudie.
Recommendations: steroid sparing treatment and steroid tapering
Overweeg direct te starten met additionele immunosuppressieve therapie met een voorkeur voor methotrexaat (opbouw tot 25 mg/week) of azathioprine (2mg/kg ;1dd). Mycophenolaat mofetil/myfortic (bijv. bij ILD) en tacrolimus zijn redelijke alternatieven.
Vermijd langdurige behandeling met corticosteroïden; bouw prednison af met 10mg elke 4 weken tot aan 20 mg 1dd1; bouw hierna trager af, bijvoorbeeld 2.5 mg elke 4 weken tot 10 mg 1dd1; hierna afhankelijk van kliniek, ervaring en patiëntvoorkeur.
Recommendations: treatment of severe IIM
Overweeg combinatietherapie (corticosteroïden en IVIg 2g/kg elke 4 weken) bij patiënten met zeer ernstige of snel progressieve myositis. Dit dient snel (of direct) na de diagnose te worden overwogen, met name bij subtype IMNM.
Overweeg Rituximab (1000 mg i.v.; herhaald na 2 weken, gevolgd door 500 (of 1000) mg i.v. na 6 maanden) of IVIg (2g/kg, elke 4 weken) bij patiënten met (matig) ernstige IIM, die niet, of slechts gering verbeteren op initiële behandeling (refractaire patiënten).
Recommendations: ILD
-
Bespreek de diagnose en behandeling van interstitiële longziekte bij IIM (IIM-ILD) multidisciplinair in aanwezigheid van een longarts met ILD expertise en neuroloog/immunoloog/reumatoloog met myositis expertise.
-
Overweeg bij IIM-ILD met milde tot matige ernst combinatietherapie met glucocorticoïden en een steroidsparend middel.
-
Overweeg bij ernstige of snel progressieve IIM-ILD, of als tweedelijnsmedicatie, combinatietherapie met hoge dosis glucocorticoïden en cyclofosfamide i.v. of Rituximab (Figuur 5).
-
Progressieve ILD met anti-MDA5 antistoffen dient vroeg besproken te worden met de longarts en een ILD expertisecentrum voor behandelingsopties en eventuele longtransplantatiemogelijkheid.
Overwegingen
Pros and cons of the intervention and the quality of the evidence
The treatment of IIM relies largely on empirical approaches due to the limited availability of high-quality controlled clinical studies. A Cochrane review from 2012 examined a total of 10 studies on the treatment of dermatomyositis/polymyositis (DM/PM). An updated Cochrane review (expected 2024) has additionally included more recent studies (n=17 in review on targeted treatments; n=16 studies in review on non-targeted treatments), allowing for aggregated data analysis. From the analysis, the following came forward:
- For non-targeted therapies, only intravenous immunoglobulin treatment (IVIg) showed a greater improvement in refractory dermatomyositis with a moderate level of evidence. For all other therapies and assessed outcomes, the level of the evidence was low or very low.
- For targeted therapies for all of the investigated immunosuppressive treatments the evidence was low or very low and, in most comparisons, no clear effect was observed.
The working group combined these indicative results (yet with low level of evidence) with clinical experience to provide an overview of how stepwise treatment can be administered, based on expert opinion.
Consider contacting an expertise center for every newly diagnosed IIM patient (preferably during the diagnostic process), to determine whether there is a possibility of study participation; the limited evidence of the literature review illustrates this need and the organization of care for IIM in the Netherlands increasingly facilitates participation in trials (the working group refers to https://myositisexpertisecentrum.nl/ for further information).
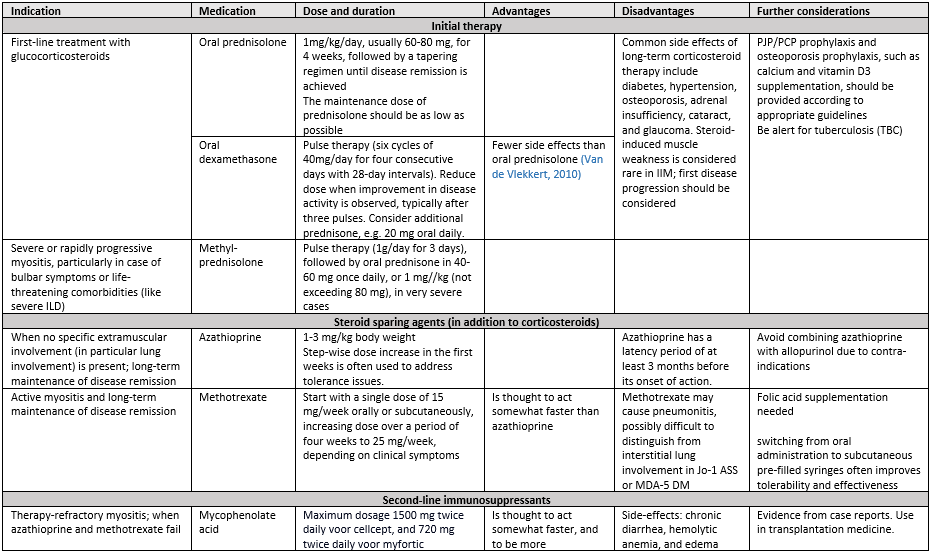
Initial Therapy
Initial therapy is defined as therapy initiated within the first three months after the diagnosis with the aim to induce significant reduction of disease activity. High-dose glucocorticoids are the initial treatment choice for all inflammatory myopathies except inclusion body myositis (IBM – see module Immunomodulation and immunosuppression in IBM). The optimal dosage has not been studied; a widely used dosage is 1 mg/kg generally not exceeding 80 mg once daily (Sevim, 2023). Oral dexamethasone pulse therapy is considered an alternative because (non-serious) side effects do not appear to be more frequent (based on one study with 62 IIM patients; low level of evidence (van de Vlekkert, 2010)).
In patients with mild IIM – mild muscle weakness not interfering with Activities of Daily Living (ADL), or only muscle complaints without weakness – a lower prednisone dosage can be considered (e.g. 0.5 mg/kg body weight with a maximum of 30 to 40 mg once daily).
Long-term glucocorticoid therapy should be avoided due to significant side effects. Benefits and harms of different steroid tapering schemes have not been studied in IIM; tapering should be guided by clinical improvement. A tapering scheme for prednisone is to taper the daily dose of prednisone by 10mg every 4 weeks until 20 mg/day; then by 5 mg every 4 weeks until 10 mg/day. If possible, taper by 1 to 2.5 mg every 4 weeks thereafter. Relapses often occur between daily dosages between 20 and 10 mg.
Steroid sparing agents: disease-modifying antirheumatic drugs (DMARDS)
While most patients initially respond well to high dose glucocorticoids (with the exception of patients with IIM and concomitant systemic sclerosis, for whom a different treatment should be considered), administration of other immunomodulatory agents is required in many cases to reduce glucocorticoid dose and related side effects.
Evidence from rheumatic disorders (other than IIM) has shown a steroid-sparing effect of disease-modifying antirheumatic drugs (DMARDS; e.g. methotrexate) (Mahr, 2007). These agents may also have a corticosteroid sparing effect in IIMs, yet evidence is very weak to support this. Methotrexate or azathioprine are often prescribed as initial steroid sparing therapy and are considered first line therapy together with glucocorticoids.
Within the initial treatment phase, decisions on therapy are guided by the severity of the disease:
- For patients without severe or rapidly progressive myositis or severe extramuscular organ involvement, steroid sparing therapy typically includes methotrexate or azathioprine. These agents can be prescribed promptly (simultaneously with prescription of glucocorticoid treatment or when side effects of two agents need to be assessed separately, two weeks apart). Currently, there is insufficient evidence to specifically recommend any of the two immunosuppressants. Based on experience in the committee there is a slight preference for MTX. In a meta-analysis and cohorts with 5-12 years follow-up in rheumatoid arthritis, methotrexate was less often discontinued than other DMARDs, except for hydroxychloroquine (Salliot, 2009; Doran, 2002). For the latter drug, the committee felt that this should not be considered as immunosuppressant therapy for IIMs. Mycophenolate acid and tacrolimus are reasonable alternatives in case of side effects, toxicity or inefficacy and considered second line therapy. Prescription and monitoring of steroid sparing agents should follow existing age-appropriate local guidelines (e.g. guideline for indication and dosing during pregnancy, and dosing recommendations in Smolen, 2023 or at farmacotherapeutisch kompas).
The initial therapy phase may necessitate treatment intensification including “second line therapy” from the start of treatment or early after.
- For patients with severe or rapidly progressive myositis or severe extramuscular organ involvement, early treatment intensification should be considered (Allenbach, 2018). Severe myositis should be interpreted as moderate to severe dysphagia or not being able to walk unaided for more than 10 meters. Rapid progression should be interpreted as progression which is noted on a weekly basis. In these patients – including those with dropped head, or severe extramuscular organ involvement (e.g. interstitial lung disease (ILD) or myocarditis) – methylprednisolone (MPS) pulse therapy (e.g. 1g/day for 3 days followed by 40-60 mg once daily)) is recommended instead of oral corticosteroids.
- IVIg in combination with MPS, should be considered in these patients, based on the fast mode of action (expert opinion).
- Mycophenolate acid should be considered in IIM with ILD (for treatment options based on severity of ILD, see section ILD further down in text).
- Although sometimes mentioned in international literature, ciclosporin has no place in the IIM treatment strategy in the Netherlands, as there is little experience with, nor evidence for effectiveness for, ciclosporin in IIM.
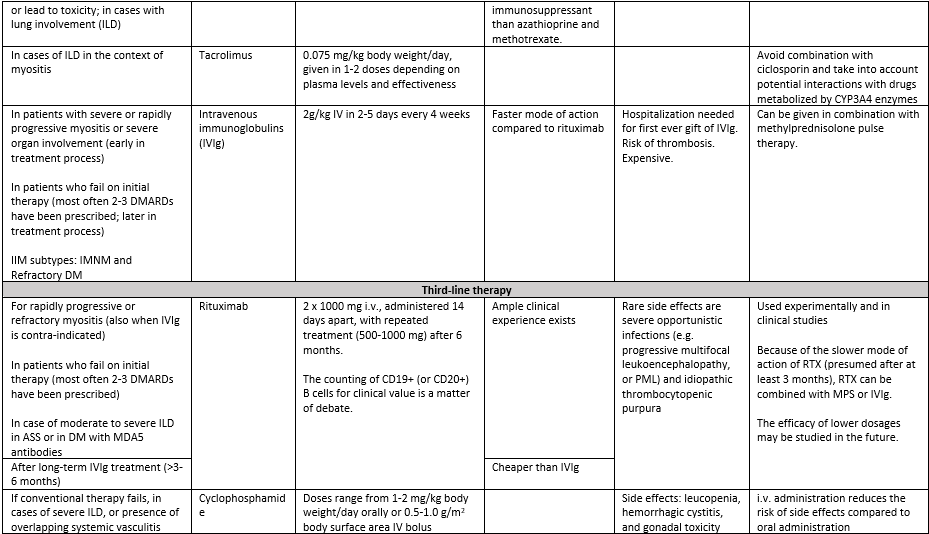
Third line therapy: refractory disease
In patients with or without severe disease, who fail on initial therapy (most often two or three DMARDs are subsequently prescribed in non-severe patients), third line immunosuppressant or immunomodulatory therapy should be considered. The therapy regimen is mainly based on the subtype of IIM and the presence of ILD. Here, we focus on IVIg, rituximab and cyclophosphamide.
- Disease subtype: IMNM: In particular patients with the subtype immune-mediated necrotizing myopathy (IMNM) may show insufficient response to glucocorticoids and DMARDs (Allenbach, 2018). Based on the faster mode of action of IVIg as compared to rituximab, IVIg is preferred. Although the efficacy of IVIg in IMNM has not been demonstrated in RCTs, (small) cohort studies have shown efficacy and suggested safety of IVIg (Lim, 2021).
- Disease subtype: DM (refractory): A study has demonstrated the efficacy and safety of IVIg in refractory dermatomyositis (DM), with positive results regarding disability (Health Assessment Questionnaire), muscle strength, and skin disease activity (Aggarwal, 2022 “ProDERM").
- Disease subtype: Juvenile IIM: In juvenile IIM a systematic review reported a complete response following rituximab in 10/26 (38%) patients (Marrani, 2022).
- All subtypes: Rituximab (RTX) is considered for refractory myositis (all subtypes, in particular when MSAs are present) and in severe or rapidly progressive myositis when IVIg is contraindicated. In these cases, because of the slower mode of action of RTX (presumed after at least 2 months), RTX can be combined with MPS or IVIg. Although a paucity of evidence on the efficacy of RTX exists (only one RCT: Oddis, 2023, “RIM-study”), ample clinical experience is at hand for clinical response. Presence of MSAs with presumed pathogenicity: For IIMs with MSAs with presumed pathogenicity (anti-SRP and anti-HMGCR), plasmapheresis can be considered (expert opinion) (Arouche-Delaperche, 2017; Allenbach, 2017). One RCT on plasmapheresis (as compared to leucopheresis and sham-pheresis) showed no effect in 39 patients (insufficient data for GRADE analysis) (Miller, 1992); plasmapheresis in MDA5 DM-ILD is discussed below.
- In case of long term IVIg therapy: For adult IIM patients on long term IVIg therapy (>3-6 months), a switch to rituximab can be considered, predominantly in the non-IMNM patients, because of a large difference in costs and presumed equal efficacy, although this has not been examined.
- Presence of ILD: For considerations regarding RTX in case of clinically relevant concomitant ILD we refer to the section ILD further down in text.
- Presence of overlapping systemic vasculitis or patients refractory to multiple second- or other third-line agents: Cyclophosphamide is generally reserved for patients with severe IIM. Cyclophosphamide can be administered orally or intravenously, but its use is limited due to toxic effects.
It is important to note that except for IVIg in refractory DM, most therapies used in myositis lack specific approval studies and are used off-label. We have listed indications, dosage and further considerations for initial treatment, second, and third-line treatment options as described above in table 11.
The experience of the committee with JAK-inhibitors is limited to severe cases, the majority of whom had concomitant ILD (with MDA5 antibodies); all patients were treated with tofacitinib. There is insufficient evidence to guide decisions on the initiation of JAK-inhibitors in IIM. Based on case reports (Hornung, 2014), cohort studies (Paik, 2021), pathophysiological considerations (Ladislau, 2018) and insights from blood-based biomarkers (Graf, 2021; Lerkvaleekul, 2022), a JAK-inhibitor may be considered in children and adults with treatment refractory DM, and to a lesser extent in ASS and OM. Treatment of juvenile and adult IIM patients with JAK-inhibitors should be carried out in selected centers with expertise, preferably within the context of a clinical study. Contra-indications for JAK-inhibitors (cardiovascular risk, thrombosis) should be taken into account (Ytterberg, 2022).
For complement inhibitors, TNF-alpha blockers and other targeted therapies, current evidence is too weak to be considered in IIM.
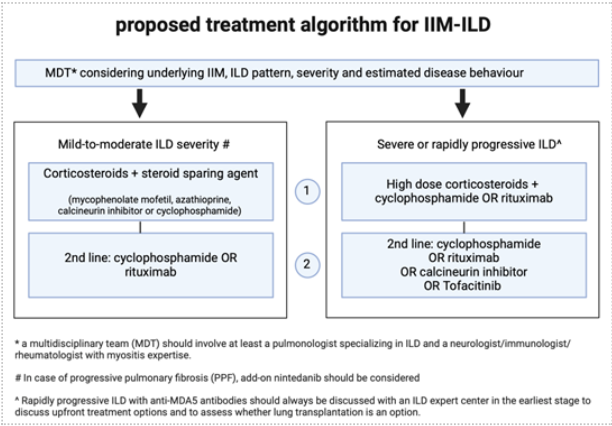
Interstitial lung disease (ILD)
ILD is a serious manifestation of IIM and associated with high morbidity and increased mortality. The committee did not perform a systemic review on the best treatment for ILD. Several authors have proposed treatment algorithms in published literature, but there is insufficient evidence for any particular regimen and the optimal treatment for ILD in the context of IIM (IIM-ILD) remains uncertain.
It is important to mention the heterogeneity of IIM-ILD patients, both in radiological pattern, clinical phenotype and progression over time. The underlying autoantibody profile is associated with clinical phenotype and risk of progression. Patients may present with indolent or reversible lung involvement, slowly progressive fibrotic ILD or rapidly progressive ILD (RP-ILD) associated with high mortality. Therefore, the diagnosis and treatment plan should be discussed and evaluated, at regular intervals during the disease course, in a multidisciplinary team (MDT), involving a pulmonologist specializing in ILD and a neurologist/immunologist/rheumatologist with sufficient myositis expertise.
The treatment approach is based on IIM subtype and autoantibody profile, ILD pattern, severity of lung involvement and an estimation of disease progression.
Corticosteroids have historically been the mainstay of treatment for IIM-ILD. We suggest these should always be accompanied by a steroid-sparing agent from the start, such as mycophenolate mofetil, azathioprine, a calcineurin inhibitor or cyclophosphamide. There is a paucity of literature and insufficient evidence for the treatment of ILD with methotrexate. In the current treatment paradigm, rituximab or cyclophosphamide are often considered treatments for severe IIM-ILD or treatment-refractory IIM-ILD. A minority of IIM-ILD patients will demonstrate progressive pulmonary fibrosis for which add-on antifibrotic treatment with nintedanib should be considered, again, in a (regional) ILD center (Raghu, 2022). Treatment in, or access to ILD centers can be discussed with the local pulmonologist. There is currently very limited evidence on the role of IVIg for ILD treatment. The scheme provided in figure 5 can be helpful for the MDT discussion and should be considered as a proposed algorithm, not a guideline.
Anti-MDA5 antibodies are associated with DM with rapid progressive (RP-) ILD and an independent predictor of mortality in IIM-ILD (Sato, 2018). The treatment of RP-ILD with anti-MDA5 antibodies can be challenging and high-quality evidence is lacking. The suggested initial approach requires double or triple immunosuppressive combination therapy with high dose glucocorticoids and calcineurin inhibitors, with or without cyclophophamide (Romero-Bueno, 2020) (or rituximab; expert opinion) with significant risks (Selva, 2021). Based on its working mechanism and recent reports, the JAK inhibitor tofacitinib may also be considered in refractory cases of RP-ILD and anti-MDA5 antibodies. Plasmapheresis may be used as rescue option (Romero-Bueno, 2020). We suggest that RP-ILD in IIM with anti-MDA5 antibodies should always be discussed at with an ILD (expert) center in the earliest stage to discuss treatment options, usually triple immunosuppressive combination therapy, and to assess whether lung transplantation is an option.
Figure 5. Proposed algorithm for IIM-ILD (adapted from Cottin, 2019)
Other considerations:
- For osteoporosis prophylaxis, Pneumocystis jirovecii pneumonie (PCP/PJP) prophylaxis, for screening of TBC and pregnancy related issues concerning prescription of immunosuppressants, we refer to relevant guidelines (available at https://richtlijnendatabase.nl)
- Outpatient clinic follow-up should be frequent in the first 6 months, depending on clinical course; for example: clinical evaluation after 1-, 3- and 6-months including check of relevant laboratory parameters. For the examination of other biomarkers than CK (i.e. interferon markers) evidence of the literature is insufficient to guide decisions in adult IIM, but can be considered based on recent literature.
Values and preferences of patients
Medication side effects are ranked as the fourth most important domain by patients with IIM and health care professionals. In particular side effects of chronic use of prednisone negatively impact quality of life of patients (Mecoli, 2020). A large variability between patients regarding susceptibility to side effects, and the related unpredictable nature of their presence and intensity makes that the treatment of IIM, on an individual basis is also trial and error, in terms of learning each patient’s side effect profile.
From the patient’s perspective dosing medication at the easiest way (once daily), preferably via the oral route and administered at home is highly valued. Physicians and patients should decide together on the most fitting treatment option for the patient’s context and treatment goals.
Costs
For most of the medication described as first and second line therapy for IIM, the efficacy seems to outweigh the costs, as the costs are low to very low and administration route is oral (e.g.: prednisone » 1 cent/mg; methotrexate » 8 cents/mg). IVIg (monthly) is far more costly than RTX and therefore should not be prescribed chronically, when RTX can be considered. For chronic use of IVIg, the question remains whether efficacy justifies its costs.
Acceptability, feasibility and implementation
The proposed treatment strategy seems acceptable and feasible as it reflects the standard of care. More use of RTX in the chronic phase of IIM leads to more hospital admissions (day-care) but the low prevalence of IIMs makes this hardly relevant in terms of feasibility or implementation.
Onderbouwing
Achtergrond
IIMs are a group of diseases marked by auto‐immune mediated inflammation of skeletal muscles. For this module we considered dermatomyositis (DM), immune‐mediated necrotizing myopathy (IMNM), anti‐synthetase syndrome (ASS), overlap‐myositis (OM) and polymyositis (PM). Although IIMs account for the most common cause of acquired muscle diseases in adults, they are uncommon in general practice. Optimal immunosuppressive or immunomodulatory therapy has not been established. Treatment consists of induction therapy (usually with high-dosed glucocorticoids) and maintenance therapy (usually with steroid sparing treatment). Challenges in the context of treatment include: 1) the intensity of immunosuppressive treatment immediately after diagnosis; 2) the lack of evidence complicating the choice of newer potentially (more) effective immunosuppressive compounds.
Conclusies / Summary of Findings
For readability, only conclusions with Low, Moderate or High grade are presented. Comparisons with Very low or no Grade can be found in the level of evidence assessment in the “Summary of literature” section.
1. Immunoglobulin versus placebo, no treatment or standard care
|
1c. Improvement |
|
|
Moderate GRADE |
The evidence indicates a beneficial effect of immunoglobulin treatment reflected by a higher proportion with improvement, compared to placebo, in patients with DM.
Source: Aggarwal (2022) |
|
1d. Skin symptoms |
|
|
Low GRADE |
The evidence suggests a minimal clinically relevant improvement of skin symptoms after immunoglobulin treatment, compared to placebo, in patients with IIM.
Source: Aggarwal (2022) |
2. Azathioprine versus placebo, no treatment or standard care
For all outcome measures Very Low GRADE (muscle strength and serious adverse events) or no GRADE (function, improvement, and skin symptoms).
3. Methotrexate versus placebo, no treatment or standard care
For all outcome measures Very Low GRADE (function, muscle strength, improvement, and serious adverse events) or no GRADE (skin symptoms).
4. Other comparisons (in non-targeted therapies)
For all outcome measures Very Low GRADE (function, muscle strength, and serious adverse events) or no GRADE (improvement, skin symptoms).
5. Rituximab versus placebo, no treatment or standard care
|
5c. Improvement |
|
|
Low GRADE |
The evidence suggests a negative effect of rituximab on (proportion of) improvement, compared to placebo, in patients with IIM.
Source: Oddis (2013) |
6. Abatacept versus placebo, no treatment or standard care
|
6a. Function |
|
|
Low GRADE |
The evidence suggests no difference in the effect of abatacept treatment compared to placebo, on function or disability, in patients with IIM.
Source: NCT-683, Tjärnlund (2018) |
7. Complement inhibitors versus placebo, no treatment or standard care
For all outcome measures Very Low GRADE (function, muscle strength, and improvement) or no GRADE (skin symptoms and serious adverse events).
8. Other comparisons (in targeted therapies)
|
8b. Muscle strength |
|
|
Low GRADE |
The evidence suggests no difference in the effect of Siponimod compared to placebo on muscle strength, in patients with IIM.
Source: NCT-917; NCT-274 |
|
8d. Skin symptoms |
|
|
Low GRADE |
The evidence suggests no difference in the effect of bazlitoran or lenabasum compared to placebo on skin symptoms, in patients with IIM.
Source: EUCT-10 “DETERMINE”; NCT-243, NCT-857 |
Samenvatting literatuur
Description of studies
One Cochrane review [expected 2024] investigated targeted treatments (e.g. biologicals, Janus kinase inhibitors, complement inhibitors), the other review [expected 2024] investigated non-targeted treatments (corticosteroids, intravenous immunoglobulins, plasmapheresis and conventional steroid sparing agents).
In both reviews, a search was performed until February 3rd, 2023 in the following databases: Cochrane Neuromuscular Specialised Register (via CRS‐Web), Cochrane Central Register of Controlled Trials (via the Cochrane Library), Embase (via Ovid SP), US National Institutes of Health Ongoing Trials Register (via ClinicalTrials.gov) and World Health Organization International Clinical Trials Registry Platform.
Studies were selected based on the following criteria:
-
Study design: Randomized controlled trials (RCTs) or quasi-RCTs
-
Participants: adults and children with probable or definite DM, (including JDM), IMNM, ASS, OM and PM
-
Intervention: treatment with immunosuppressant or immunomodulatory treatments used at any dosage, by any route, in any regimen and for any duration.
No restrictions on type of outcome measures reported or language were applied in the selection process. Risk of bias of included studies was assessed using the Cochrane Risk of Bias tool.
Non-targeted therapies
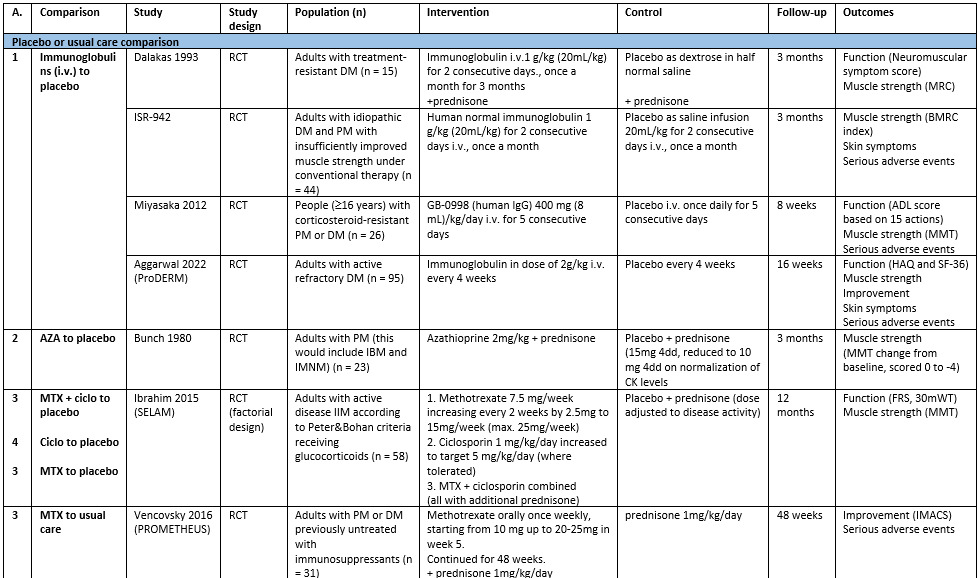
In the review for the non-targeted therapies, 16 trials were included. Eleven reported relevant outcomes for this guideline module, and two studies reported outcomes in JDM (see module Immunosuppression and immunomodulation in JDM). Characteristics of the 11 included studies can be found in table 1.
Targeted therapies
In the review for targeted therapies, 17 trials were included. Fifteen reported relevant outcomes for this guideline module, of which one also for patients with JDM. Characteristics for the 15 included studies can be found in table 2.
Table 1. Characteristics of included studies reporting on non-targeted therapies for IIM. (zie ook evidence tabellen)
Abbreviations: A.: analysis (corresponds to number below in analysis), ADL: activities of daily living, AZA: azathioprine, ciclo: ciclosporin, CK: creatinine kinase, DM: dermatomyositis, FRS: Functional Rating Scale (as mentioned in The Amyothrophic Lateral Sclerosis Functional Rating Scale, 1996), i.v.: intravenously, MMT: manual muscle test, MRC: Medical Research Council (scale of muscle strength), MTX: methotrexate, PM: polymyositis, RCT: randomized controlled trial, 30mWT: 30 meter walking time.
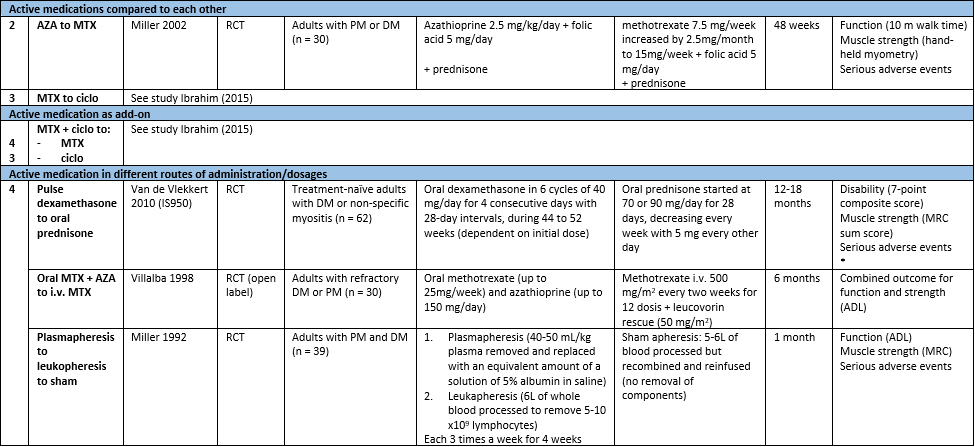
*Outcomes used for this guideline module; in this individual study, more outcomes have been assessed: time to remission and time to relapse.
Abbreviations: A.: analysis (corresponds to number below in analysis), AZA: azathioprine, CDASI: Cutaneous Dermatomyositis Disease Area and Severity Index, DM: dermatomyositis, IMACS: International Myositis Assessment and Clinical Studies Group, i.v.: intravenously, HAQ: Health Assessment Questionnaire, MDAAT: Myositis Disease Activity Assessment Tool, MMT: manual muscle test, MTX: methotrexate, PM: polymyositis, PROMIS-29: Patient-Reported Outcomes Measurement Information System-29,RCT: randomized controlled trial, s.c.: subcutaneously, S1P: sphingosine-1-phosphate, TIS: Total improvement score, 3TUG: triple Timed Up and Go test, 6MWD: 6-minute walking distance. TNF = TLR = AZA =
Results
Non-targeted therapies
-
Immunoglobulin versus placebo, no treatment or standard care
Four studies were included for this comparison. All studies included patients with DM; Miyasaka (2012) included patients with DM and PM.
1a. Function
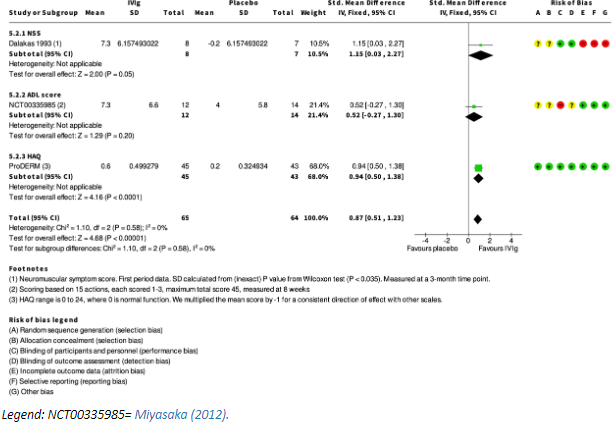
Three studies reported on change in disability: Dalakas (1993) after 3 months through the Neuromuscular Symptom Score (NSS, score 0 to 60 with maximum score being normal function); Miyasaka (2012) through the change in activities of daily living (ADL) after 8 weeks, and Aggarwal (2022, ‘ProDERM’) the mean change in HAQ (ranging from 0 to 24, where 0 is normal function) after 16 weeks. The results are combined through a standardized mean difference (see figure 1).
Figure 1. Functional outcomes after IVIg treatment, compared with placebo, in patients with IIM (squares represent standardized mean difference per study, of which the size is relative to the study population. Diamonds represent the pooled (sub)total standardized mean difference).
1b. Muscle strength
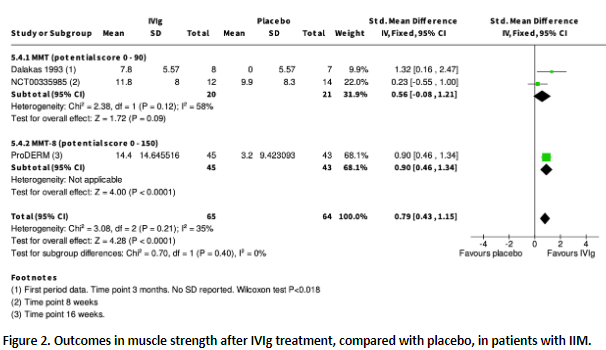
ISR-942 reported the achievement of a meaningful improvement in muscle strength (percentage of patients). In the IVIg group, 8 patients (out of 22, 36.4%) achieved this outcome compared to 6 patients (out of 22, 27.3%) in the placebo group; resulting in an RR of 1.33 (95% CI 0.55 to 3.21).
Dalakas (1993), Miyasaka (2012) and Aggarwal (2022, ‘ProDERM’) reported change in muscle strength as continuous data. The results are shown in figure 2
Figure 2. Outcomes in muscle strength after IVIg treatment, compared with placebo, in patients with IIM. (squares represent standardized mean difference per study, of which the size is relative to the study population. Diamonds represent the pooled (sub)total standardized mean difference).
1c. Improvement
Aggarwal (2022, ‘ProDERM’) reported the proportion of patients with at least moderate improvement as per the ACR/EULAR Myositis Response Criteria (based on the Total Improvement Score, TIS), defined as a score of ³40, and without deterioration (on a scale from 0 to 100, higher scores are better). In the IVIg group, 37 patients (out of 47, 78.7%) had at least moderate improvement, compared to 21 patients (out of 48, 43.8%) in the placebo group; resulting in an RR of 1.80 (95% CI 1.26 to 2.56).
1d. Skin symptoms
ISR-942 measured the severity of cutaneous signs according to a 3-point scale, but did not provide data.
Aggarwal (2022, ‘ProDERM’) reported the mean change in a modified CDASI after 16 weeks of treatment, as total activity (0-100) and total damage scores (0-32) with higher scores indicating higher activity or damage. The mean difference (MD) for activity was -8.2 (95% CI -11.91 to -4.49) and for damage the MD was -0.68 (95% CI -1.26 to -0.10), both favouring IVIg.
1e. Serious adverse events
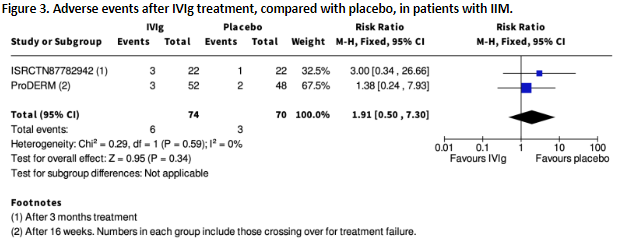
ISR-942 and Aggarwal (2022, ‘ProDERM’) reported serious adverse events after IVIg compared to placebo, at 3 months and 16 weeks, respectively. Results are shown in figure 3.
Figure 3. Adverse events after IVIg treatment, compared with placebo, in patients with IIM (squares represent the risk ratio per study, of which the size is relative to the study population. Diamonds represent the pooled (sub)total risk ratio).
Mayasaka (2012) is not included in the figure as it is unclear to which group the 4 serious AEs can be assigned as they occurred during the 20-week cross-over period and can possibly be ascribed to IVIg but also to other previous medications.
1. Level of evidence of the literature
The level of evidence regarding the outcome measures was downgraded by according to the table below.
|
Outcome measure |
Domains |
Level of evidence |
|
Function |
-1 risk of bias of included studies; -2 imprecision (heterogeneity in outcome measures and broad confidence interval) |
VERY LOW |
|
Muscle strength |
-1 risk of bias of included studies; -2 imprecision (heterogeneity in assessment measure and CI crossing border of clinical relevance) |
VERY LOW |
|
Improvement |
-1 imprecision (results of single study) |
MODERATE |
|
Skin symptoms |
-2 imprecision (confidence interval crossing border of clinical relevance and results of single study) |
LOW |
|
Serious adverse events |
-1 risk of bias of included studies; -2 imprecision (broad confidence intervals crossing border of clinical relevance) |
VERY LOW |
-
Azathioprine versus placebo, no treatment or standard care
Two studies were included for this comparison.
2a. Function
Miller (2002) (comparison azathioprine to methotrexate) reported the average improved walk time (%) without standard deviations. The azathioprine-treated group had a 16% improvement, and the methotrexate-treated group 30%.
2b. Muscle strength
Bunch (1980) reported muscle strength both as improvement of 15% or more (dichotomous), and as the change in mean muscle strength score from baseline to 3 months (MMT-score ranging from 0 to -136, sum of 18 muscles scoring 0 = normal strength to -4 = no movement). Miller (2002) reported the proportion of patients with a 30% improvement in hand-held myometry. Results are shown in table 3.
Table 3. Outcomes in muscle strength after treatment with azathioprine, compared to placebo (Bunch, 1980) or methotrexate (Miller, 2002), in patients with IIM.
|
Study |
n |
Outcome measure |
Measurement timepoint |
Result (95% CI) |
|
Bunch (1980) |
16 |
Muscle strength improvement of ³15% |
3 months |
RR 1.33 (0.43 to 4.13) |
|
Mean change in muscle strength score |
MD -5.4 (-13.08 to 23.88) |
|||
|
Miller (2002) |
28 |
Improvement of ³30% in hand-held myometry |
1 year |
RR 0.95 (0.40 to 2.27) |
2c. Improvement and 6d. Skin symptoms
None of the included studies reported on improvement through the IMACS criteria or the effect on skin symptoms (CDASI).
2e. Serious adverse events
Miller (2002) (comparison azathioprine to methotrexate) reported WHO grade 3 or 4 toxicity: 4/12 participants in the azathioprine-treated group and in 0/16 participant in the methotrexate-treated group. This results in an RR of 11.77 (95% CI 0.69 to 199.65).
2. Level of evidence of the literature
The level of evidence regarding the outcome measures was downgraded by according to the table below.
|
Outcome measure |
Domains |
Level of evidence |
|
Function |
No GRADE assessment (insufficient data) |
- |
|
Muscle strength |
-1 risk of bias in included studies, -2 imprecision (heterogeneity in outcomes measured, broad confidence intervals crossing the borders of clinical relevance, low number of participants) |
VERY LOW |
|
Improvement |
No GRADE assessment |
- |
|
Skin symptoms |
No GRADE assessment |
- |
|
Serious adverse events |
-1 risk of bias in included studies, -3 imprecision (extremely broad confidence interval crossing borders of clinical relevance, single study with low number of participants) |
VERY LOW |
-
Methotrexate versus placebo, no treatment, other treatment or standard care
Two studies were included for this comparison. The study by Ibrahim (2015, ‘SELAM’) had a factorial design with 4 study arms, resulting in the following comparisons for methotrexate:
-
Methotrexate versus placebo
-
Methotrexate + ciclosporin versus placebo
-
Methotrexate versus ciclosporin
-
Methotrexate + ciclosporin versus ciclosporin alone
3a. Function
Ibrahim (2015) measured functional ability using two assessments: the change in disability through the Functional Rating Scale (score 0-40 and higher is better), and the change in 30 meter walking time in seconds. These mean differences between the change in these scores, for the different comparisons regarding methotrexate, are shown in table 4.
Table 4. Mean differences in functional outcomes for different comparisons with methotrexate, in patients with IIM.
|
Comparison |
Functional Rating scale |
30 meter walking time (seconds) |
||
|
|
Mean difference (95% CI) |
Interpretation |
Mean difference (95% CI) |
Interpretation |
|
MTX to placebo |
1.24 (-1.60 to 4.08) |
Favouring MTX |
1.13 (-9.85 to 12.11) |
Favouring placebo |
|
MTX + ciclo to placebo |
2.36 (-1.14 to 5.86) |
Favouring MTX + ciclo |
-0.57 (-11.47 to 10.33) |
Favouring MTX + ciclo |
|
MTX to ciclo |
0.12 (-3.12 to 3.36) |
Favouring MTX |
0.90 (-9.85 to 11.65) |
Favouring ciclo |
|
MTX+ ciclo to ciclo (MTX add-on) |
1.24 (-2.59 to 5.07) |
Favouring MTX add-on |
-0.80 (-11.46 to 9.86) |
Favouring MTX add-on |
3b. Muscle strength
Muscle strength was assessed in the study of Ibrahim (2015) through manual muscle testing (MMT). In none of the comparisons, a significant or clinically relevant difference was observed:
-
Methotrexate versus placebo: MD -5.68 (95% CI -12.94 to 1.58) favouring placebo
-
Methotrexate + ciclosporin versus placebo: MD -4.82 (95% CI-11.68 to 2.04) favouring placebo
-
Compared to ciclosporin: MD 1.80 (95% CI -6.42 to 10.02) favouring methotrexate
-
As add-on to ciclosporin compared to ciclosporin alone: MD 2.66 (-5.22 to 10.54) favouring methotrexate add-on
Vencovsky (2016, “PROMETHEUS”) reported the proportion of patients achieving the IMACS definitions of improvement at 48 weeks. In the methotrexate group, 86% of patients achieved improvement (12 out of 14), compared to 85% in the usual care group (11 out of 13), resulting in an RR of 1.01 (95% CI 0.74 to 1.39).
3d. Skin symptoms
None of the included studies reported on the effect on skin symptoms (CDASI).
3e. Serious adverse events
In the study by Vencovsky (2016, “PROMETHEUS”), 5 serious adverse events occurred in the methotrexate group (out of 15, 33%), compared to 4 in the usual care group (out of 16, 25%). This results in an RR of 1.33 (0.44 to 4.05).
3. Level of evidence of the literature
The level of evidence regarding the outcome measures was downgraded by according to the table below.
|
Outcome measure |
Domains |
Level of evidence |
|
Function |
-1 risk of bias in included study, -2 imprecision (broad confidence intervals crossing the borders of clinical relevance, low number of participants) |
VERY LOW |
|
Muscle strength |
-1 risk of bias in included study, -2 imprecision (broad confidence intervals crossing the borders of clinical relevance, low number of participants) |
VERY LOW |
|
Improvement |
-1 risk of bias in included study, -2 imprecision (broad confidence intervals crossing both borders of clinical relevance, low number of participants) |
VERY LOW |
|
Skin symptoms |
No GRADE assessment |
- |
|
Serious adverse events |
-1 risk of bias in included study, -2 imprecision (broad confidence intervals crossing both borders of clinical relevance, low number of participants) |
VERY LOW |
-
Other comparisons
Four studies were included for this section. Other comparisons of non-targeted therapies for IIM included ciclosporin (ciclosporin versus placebo and ciclosporin as add-on to methotrexate), and the comparison of different administration routes or dosing (see table 2).
4a. Function
Ciclosporin
Ibrahim (2015) investigated in a factorial trial ciclosporin, in comparison to placebo and as add-on to methotrexate (compared to methotrexate only). Function was measured through the change in Functional Rating Score (0-40, higher is better) and through 30 meter walking time. Mean differences for these outcomes are shown in table 5; none were statistically significant, nor clinically relevant.
Table 5. Functional outcomes after treatment with ciclosporin, in patients with IIM.
|
Comparison |
Functional Rating scale |
30 meter walking time (seconds) |
||
|
|
Mean difference (95% CI) |
Interpretation |
Mean difference (95% CI) |
Interpretation |
|
Ciclo to placebo |
1.12 (-2.17 to 4.41) |
Favouring ciclo |
0.23 (-9.49 to 9.95) |
Favouring placebo |
|
Ciclo + MTX to MTX (add-on) |
1.12 (-2.33 to 4.57) |
Favouring ciclo add-on |
-1.70 (-13.53 to 10.13) |
Favouring ciclo add-on |
Administration routes or dosing
Van de Vlekkert (2010) compared oral dexamethasone pulse therapy versus daily prednisone and measured a seven-point composite score (not validated) for disability. A total of 5/30 patients (16.7%) in the dexamethasone pulse therapy group had a favourable composite score at 18 months, compared to 9/32 patients (28.1%) in the oral prednisone group (RR 0.59; 95% CI 0.22 to 1.57).
Miller (1992) compared plasmapheresis to leukopheresis to sham apheresis. After one month follow-up, three of the 13 participants in each intervention group demonstrated improvement defined by strength and functional grade.
Villalba (1998) compared weekly oral methotrexate with daily azathioprine to i.v. methotrexate every two weeks. The primary outcome was a combined evaluation of function and strength (Activities of daily living, ADL), in which improvement was defined as an increase of at least one grade of strength in at least two involved muscle groups using standard MMT, and an increase of at least one functional level in one or more involved areas of function on a Convery Assessment scale modified for myositis. After 6 months, 8 out of 15 participants in the methotrexate + azathioprine group showed improvement, compared to 4 out of 15 participants in the i.v. methotrexate group (RR 2.00; 95% CI 0.76 to 5.24).
4b. Muscle strength
Ciclosporin
Ibrahim (2015) measured muscle strength using the MMT (score 0-80, higher score is better). The mean change in MMT after 56 weeks was measured. An MD of -7.48 (95% CI -15.00 to 0.04) was found for the comparison between ciclosporin and placebo (favouring placebo), and MD 0.86 (95% CI -6.77 to 8.49) for the comparison between methotrexate with ciclosporin compared to methotrexate alone (favouring ciclosporin add-on). These differences were not statistically significant, nor clinically relevant.
Administration routes or dosing
Data for muscle strength, measured with the MRC sum score (maximum of 140), from Van de Vlekkert (2010) showed a mean score of 136 (SD 5) after 18 months for the dexamethasone group, compared to mean 135 (SD 6) for the prednisolone group (MD 1.00; 95% CI -1.92 to 3.92).
Miller (1992) only reported no significant differences in final muscle strength among the three treatment groups. Villalba (1998) measured ADL, a combined outcome of function and strength (see above).
4c. Improvement and 8d. Skin symptoms
None of the included studies reported on improvement through the IMACS criteria or the effect on skin symptoms (CDASI).
4e. Serious adverse events
Administration routes or dosing
Van de Vlekkert (2010) did not explicitly categorise adverse effects into serious and nonserious, yet serious side effects that necessitated discontinuation of the study were described as serious: 3/30 in the dexamethasone group (10%), and 5/32 in the prednisolone group (15.6%) (RR 0.67, 95% CI 0.17 to 2.60).
Miller (1992) reported that “no major toxicities were seen”, but did not provide the proportion of participants with adverse events in each intervention group. Villalba (1998) reported adverse effects narratively and it was not possible to separate serious and non-serious events.
4. Level of evidence of the literature
The level of evidence regarding the outcome measures was downgraded according to the table below.
|
Outcome measure |
Domains |
Level of evidence |
|
Function |
Ciclosporin: -1 risk of bias in included study, -2 imprecision (broad confidence intervals crossing the borders of clinical relevance, low number of participants) Administration route/dosage: -2 risk of bias in included studies, -2 imprecision (heterogeneity in outcomes measured, confidence intervals crossing the borders of clinical relevance, low number of participants) |
VERY LOW
VERY LOW |
|
Muscle strength |
Ciclosporin: -1 risk of bias in included study, -2 imprecision (broad confidence intervals crossing the borders of clinical relevance, low number of participants) Administration route/dosage: -2 risk of bias in included study, -1 imprecision (low number of participants) |
VERY LOW
VERY LOW |
|
Improvement |
No GRADE assessment |
- |
|
Skin symptoms |
No GRADE assessment |
- |
|
Serious adverse events |
Administration route/dosage: -1 risk of bias in included study, -2 imprecision (broad confidence intervals crossing the borders of clinical relevance, low number of participants) |
VERY LOW |
Targeted therapies
-
Rituximab versus placebo, no treatment or standard care
One study was used for this comparison (Oddis, 2013; “RIM study”). In order to compare results, only the results before the initiation of rituximab in the delayed start group are analyzed (first 8 weeks).
5a. Function
The included study did not report on function through the HAQ or other measurements.
5b. Muscle strength
The included study did not report on (improvement in) muscle strength after 8 weeks.
5c. Improvement
Oddis (2013, “RIM study”) reported the proportion of patients achieving the IMACS definitions of improvement (based on six core set measures among five domains) at 8 weeks. In the rituximab group 14 participants improved versus 21 in the placebo group (RR 0.72, 95% CI 0.39 to 1.34; favouring placebo).
5d. Skin symptoms
Oddis (2013, “RIM study”) reported the proportion of patients with 20% improvement in rashes. After 8 weeks, approximately 60% of patients, compared to approximately 40% in the placebo group. Exact numbers are not provided.
5e. Serious adverse events
The included study did not report on serious adverse events.
5. Level of evidence of the literature
|
Outcome measure |
Domains |
Level of evidence |
|
Function |
No GRADE assessment |
- |
|
Muscle strength |
No GRADE assessment (insufficient data) |
- |
|
Improvement |
-2 imprecision (confidence interval crossing both borders of clinical relevance)) |
LOW |
|
Skin symptoms |
Insufficient data available |
- |
|
Serious adverse events |
No GRADE assessment |
- |
-
Abatacept versus placebo, no treatment or standard care
Two studies were included for this comparison (NCT-683; Tjärnlund, 2018).
NCT-683 compared abatacept to placebo. Tjärnlund (2018, “ARTEMIS”) compared abatacept to delayed initiation of abatacept (after 3 months); in order to compare results, only the results before the initiation of abatacept in the delayed start group are analyzed.
6a. Function
Both studies reported the change in HAQ disability index (8 items scored from 0 (= no difficulties) to 3 (= unable to do), summed, and then divided by 8). A pooled mean difference (MD) of -0.14 (95% CI -0.29 to 0.02), favouring abatacept.
6b. Muscle strength
Both studies reported muscle strength through a change in MMT8 (score ranging from 0 to 80, higher scores are better). At 3 months, Tjärnlund (2018, “ARTEMIS”) reported a mean difference of 7.4 (95% CI 0.78 to 14.02) favouring abatacept.
At 6 months, NCT-683 reported a mean difference of 1.8 (95% CI -2.7 to 6.4) favouring abatacept; however the relevant number of participants was not reported.
6c. Improvement
Both studies (Tjärnlund, 2018; NCT-683) reported the proportion of patients achieving the IMACS definitions of improvement (based on six core set measures among five domains) and a total improvement score (TIS). Results are shown in table 6.
Table 6. Improvement outcomes of different studies on abatacept compared to placebo in patients with IIM.
|
Study |
n |
Outcome measure |
Measurement timepoint |
Result (95% CI) |
|
Tjärnlund (2018) |
19 |
IMACS definitions of improvement |
3 months |
RR 4.50 (0.64 to 31.60) |
|
Achievement of minimal improvement (-20 points) in TIS score |
RR 2.70 (0.72 to 10.14) |
|||
|
NCT-683 |
120 |
IMACS definitions of improvement |
6 months |
RR 1.32 (0.94 to 1.84) |
|
Achievement of myositis response criteria (moderate improvement) |
RR 1.12 (0.81 to 1.57) |
6d. Skin symptoms
The included studies did not report on skin symptoms.
6e. Serious adverse events
No serious adverse events occurred in the study by Tjärnlund (2018). In NCT-683, 4 patients (out of 73) in the abatacept group, and 4 patients (out of 75) in the placebo group had serious adverse events.
6. Level of evidence of the literature
|
Outcome measure |
Domains |
Level of evidence |
|
Function |
-1 risk of bias of included studies; -1 inconsistency due to heterogeneity in timing of outcome measurement |
LOW |
|
Muscle strength |
-1 risk of bias of included studies; -2 imprecision (broad confidence interval and unclear number of patients assessed for this outcome) |
VERY LOW |
|
Improvement |
-1 risk of bias of included studies, -2 imprecision (heterogeneity in outcome measures and confidence interval crossing border of clinical relevance) |
VERY LOW |
|
Skin symptoms |
No GRADE assessment |
- |
|
Serious adverse events |
-1 risk of bias of included studies; -2 imprecision (broad confidence interval crossing both borders of clinical relevance) |
VERY LOW |
-
Complement inhibitors versus placebo, no treatment or standard care
One study was included for this comparison, on zilucoplan (Mammen, 2023). Zilucoplan is an inhibitor of complement component C5.
7a. Function
Mammen (2023) NCT-632 measured function at 8 weeks through the HAQ and the Triple Timed Up and Go test (3TUG: seconds it takes to rise from a seated position, walk 3 meters, turning back and sitting down again, thrice, and then divided by three). Results were not clinically relevant.
-
The mean difference in HAQ score was -0.15 (95% CI -0.61 to 0.32)
-
The mean difference in change in 3TUG was -0.69 (-2.87 to 1.50)
Mammen (2023) assessed muscle strength using proximal manual muscle testing in seven muscle groups (range 0 to 140, higher scores are better). At week 8 a mean difference in muscle strength was found of 3.89 (95% CI -6.17 to 13.95).
7c. Improvement
Mammen (2023) reported the total improvement score at 8 weeks. A threshold for minimal improvement was ³20. Six patients (out of 11, 55%) in the zilucoplan group met this threshold, compared to 7 patients (out of 14, 50%) in the placebo group (RR 1.09, 95% CI 0.51 to 2.31).
7d. Skin symptoms
Skin symptoms were not reported in Mammen (2023).
7e. Serious adverse events
Mammen (2023) reported three serious adverse events in the placebo group, none in the zilucoplan group.
7. Level of evidence of the literature
|
Outcome measure |
Domains |
Level of evidence |
|
Function |
-2 risk of bias of included study; -1 imprecision (inclusion of single study with low number of patients) |
VERY LOW |
|
Muscle strength |
-2 risk of bias of included study; -1 imprecision (inclusion of single study with low number of patients) |
VERY LOW |
|
Improvement |
-2 risk of bias of included study, -2 imprecision (confidence interval crossing both borders of clinical relevance) |
VERY LOW |
|
Skin symptoms |
No GRADE assessment |
- |
|
Serious adverse events |
No GRADE assessment (insufficient data) |
- |
-
Other comparisons
8A. Anti-TNF-alpha inhibitors
Two studies were included for this comparison: one on etanercept with a duration of 24 weeks (Muscle Study Group, 2011) and one on infliximab (Schiffenbauer, 2018). The study on infliximab continued after 16 weeks, with the placebo group receiving infliximab as well. Only the results from the first 16 weeks are included in the analysis.
8A.a. Function
Etanercept
The Muscle Study Group (2011) reported change in disability through the HAQ (scoring from 0 to 3, higher scores indicate more disability). After 24 weeks, a mean difference of -0.10 (95% CI -0.78 to 0.58) was found, favouring etanercept.
Infliximab
Schiffenbauer (2018) reported the number of participants achieving ³ 20% improvement at 16 weeks. Three (out of 6) patients in the infliximab group and 4 (out of 6) patients in the placebo group achieved improvement (RR 0.75, 95% CI 0.28 to 2.00, favouring placebo).
8A.b. Muscle strength
The results for the achievement of ³ 15% increase in muscle strength for both studies, are shown in table 7.
Despite a relative risk of 2.05, little to no difference in mean MMT score was found after 24 weeks for etanercept.
Table 7. achievement of ³ 15% increase in muscle strength, in patients with IIM receiving anti-TNF-alpha inhibitors compared to placebo.
|
Study |
n |
Measurement tool |
Timepoint |
Intervention |
Control |
Result (95% CI) |
|
Muscle Study Group, 2011 |
16 |
MMT (26 muscle groups scored 0-5, max. score 130) |
24 weeks |
Etanercept 9/11 patients |
Placebo 2/5 patients |
RR 2.05 (0.67 to 6.20) |
|
Schiffenbauer, 2018 |
12 |
MMT8 (score from 0 to 80, higher scores are better) |
16 weeks |
Infliximab 1/6 patients |
Placebo 0/6 patients |
RR not calculated (1 and 0 cases) |
8A.c. Improvement
The proportion of patients achieving the IMACS definitions of improvement (based on six core set measures among five domains) for both studies, are shown in table 8.
Table 8. Proportion of patients achieving IMACS definitions of improvement, in patients with IIM receiving anti-TNF-alpha inhibitors compared to placebo.
|
Study |
n |
Timepoint |
Intervention |
Control |
Result (95% CI) |
Favouring |
|
Muscle Study Group, 2011 |
16 |
24 weeks |
Etanercept 9/11 patients |
Placebo 2/5 patients |
RR 2.05 (0.67 to 6.20) |
Etanercept |
|
Schiffenbauer, 2018 |
12 |
16 weeks |
Infliximab 3/6 patients |
Placebo 2/6 patients |
RR 1.50 (0.38 to 6.00) |
Infliximab |
8A.d. Skin symptoms
Etanercept
The Muscle Study Group (2011)reported change in modified CDASI using 13 anatomical sites (maximum score not defined, but higher scores indicate worse disease). A mean difference of -6.60 (95% CI -10.62 to -2.58) was found, favouring etanercept.
Infliximab
Skin symptoms were not reported by Schiffenbauer (2018).
8A.e. Serious adverse events
For etanercept, in Muscle Study Group (2011), 6 participants (55%) experienced adverse events in the etanercept group, compared to 3 participant (60%) in the placebo group (RR 0.91; 95% CI 0.37 to 2.23). At 16 weeks, no serious adverse events were reported for infliximab (Schiffenbauer, 2018).
8B. Gevokizumab
One study was used for this comparison (EUCT-34), which was stopped early “due to strategic and business reasons unrelated to safety”. Gevokizumab is an IL-1-b inhibitor, aiming to reduce inflammation.
8B.a. Function
Function or disability was not reported in EUCT-34.
8B.b. Muscle strength
EUCT-34 reported an achievement of ³ 15% increase in muscle strength using the MMT8 (score ranging from 0 to 80, higher scores are better), at 24 weeks. In the gevokizumab group, 5 (out of 14, 36%) achieved improvement, compared to 7 (out of 13, 54%) in the placebo group (RR 0.67, 95% CI 0.30 to 1.51; favouring placebo).
8B.c. Improvement
Improvement was not reported in EUCT-34.
8B.d. Skin symptoms
Skin symptoms were not reported in EUCT-34.
8B.e. Serious adverse events
Serious adverse events occurred in 3 patients (21%) in the gevokizumab group, and in 1 patient (8%) in the placebo group (RR 2.79, 95% CI 0.33 to 23.5; favouring placebo).
8C. Bazlitoran
One study was used for bazlitoran (NCT-857). Bazlitoran is an oligonucleotide antagonist of toll-like receptor 7/8/9; therefore blocking type I interferon signaling.
8C.a. Function
Function or disability was not reported in NCT-857.
8C.b. Muscle strength
NCT-857 reported on muscle strength through the MMT8 (score ranging from 0 to 80, higher scores are better). After 28 weeks, the mean change from baseline in the low-dose group was 1.8; in the high-dose group 2.8, and in the placebo group 3.8. No standard deviations were provided to calculate mean differences.
8C.c. Improvement
Improvement was not reported in NCT-857.
8C.d. Skin symptoms
NCT-857 used a modified CDASI to assess skin symptoms. A higher score indicates worse disease. A decrease in activity score was observed in all groups; resulting in a mean difference of -0.70 (95% CI -5.39 to 3.99) in the low-dose group, and a mean difference of -3.20 (-7.70 to 1.30) in the high-dose group.
8C.e. Serious adverse events
NCT-857 reported one serious adverse event in the high-dose bazlitoran group.
8D. Lenabasum
Two studies were included for this comparison (EUCT-10 “DETERMINE”; NCT-243). Lenabasum is a cannabinoid agonist, selectively binding to the CB2 receptors, activating signaling pathways to reduce inflammation and promote tissue healing.
8D.a. Function
NCT-243 reported physical function through the Patient-Reported Outcomes Measurement Information System-29 (PROMIS-29). The range is not described in the study; therefore no clinical interpretation could be given.
8D.b. Muscle strength
Muscle strength outcomes were not reported in the included studies (EUCT-10 “DETERMINE”; NCT-243).
8D.c. Improvement
EUCT-10 “DETERMINE” reported the change in Total Improvement Score (TIS) from IMAC core set measures (scores 0 to 100, higher scores indicate better improvement), but the reported scores differed between text and figures, and data could not be analysed.
8D.d. Skin symptoms
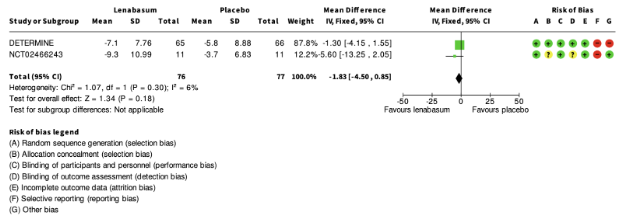
Both studies reported change in CDASI (Scale ranging from 0 to 100, with higher scores indicating greater disease severity) (EUCT-10 “DETERMINE”; NCT-243). Pooled data from both trials (20 mg dose) can be found in figure 4.
Figure 4. Change in CDASI score for patients with IIM treated with lenabasum 20 mg or placebo (squares represent mean difference per study, of which the size is relative to the study population. Diamonds represent the pooled (sub)total mean difference).
In addition, NCT-243 reported the achievement of a clinically meaningful improvement in CDASI (mean reduction of ³5 points) after 28 weeks. In the lenabasum group 7 patients (out of 11, 64%) and in the placebo group 5 patients (out of 11, 45%) achieved this outcome (RR 1.40, 95% CI 0.64 to 3.07, favouring lenabasum).
8D.e. Serious adverse events
In NCT-243, no serious events occurred. In EUCT-10 “DETERMINE”, 8 patients (out of 69, 11.6%) treated with lenabasum 20 mg had serious adverse events, 3 patients (out of 35, 8.6%) treated with lenabasum 5 mg, and 3 patients (out of 71, 4.2%) treated with placebo.
8E. Sifalimumab
One study was used for the comparison of sifalimumab to placebo (NCT-091). Sifalimumab is a monoclonal antibody inhibiting interferon-a.
8E.a. Function
Function or disability was not reported in NCT-091.
8E.b. Muscle strength
Muscle strength outcomes were not reported in NCT-091.
8E.c. Improvement
Improvement was not reported in NCT-091.
8E.d. Skin symptoms
Skin symptoms were not reported in NCT-091.
8E.e. Serious adverse events
At 14 weeks, NCT-091 reported 4 serious adverse events in 2 patients treated with sifalimumab (out of 39, 5%): coagulopathy, increased INR, and musculoskeletal chest pain twice. In the placebo group, 2 events occurred in 1 patient (out of 12, 8%): atrioventricular block and hyponatremia. This results in an RR of 0.31 (95% CI 0.05 to 1.96, favouring sifalimumab).
8F. Siponimod
Three studies about siponimod were included (NCT-810; NCT-917; NCT-274). All were terminated early, due to “interim analysis for futility”, due to “new data available”, and due to “overall slow recruitment and no evidence for efficacy in parallel study”.
Siponimod is a sphingosine-1-phosphate (S1P) receptor modulator; by selectively binding to these receptors it prevents lymphocytes from exiting the lymph nodes and entering the blood stream, therefore reducing inflammation. It is most commonly used in individuals with Multiple Sclerosis (MS).
8F.a. Function
NCT-917 and NCT-274 reported 6-minute walking distance (6MWD), change from baseline after 12 weeks and 6 months, respectively. The results are shown in table 9.
Table 9. Change in baseline 6MWD, in patients with IIM receiving siponimod compared to placebo.
|
Study |
n |
Timepoint |
Siponimod 0.5mg (mean, SD) |
Siponimod 2mg (mean, SD) |
Siponimod 10mg (mean, SD) |
Placebo (mean change, SD) |
|
NCT-917 |
14 |
12 weeks |
- |
(n = 6) 46.8 (65.6) |
(n = 1) 23 (0) |
(n = 4) -6.4 (22.0) |
|
NCT-274 |
17 |
6 months |
(n = 3) 26.8 (104.2) |
(n = 4) 22.2 (81.6) |
(n = 3) 7.7 (66.7) |
(n = 3) 4.1 (72.2) |
8F.b. Muscle strength
NCT-917 and NCT-274 measured muscle strength through the MMT24 (range 0 to 240, with higher scores indicating better outcomes), both after 3 months. The results are shown in table 10.
Table 10. Change in baseline MMT24 after 12 weeks, in patients with IIM receiving siponimod compared to placebo.
|
Study |
n |
Timepoint |
Siponimod 0.5mg (mean, SD) |
Siponimod 2mg (mean, SD) |
Siponimod 10mg (mean, SD) |
Placebo (mean change, SD) |
|
NCT-917 |
14 |
12 weeks |
- |
(n = 7) 11.2 (12.7) |
(n = 2) 39 (24.3) |
(n = 5) 9.1 (45.8) |
|
NCT-274 |
17 |
12 weeks |
(n = 4) 26.1 (7.1) |
(n = 4) 21.8 (7.1) |
(n = 4) 4.0 (7.1) |
(n = 4) 32.3 (7.1) |
8F.c. Improvement
NCT-810 reported the proportion of patients achieving the IMACS definitions of improvement (based on six core set measures among five domains): 4 patients (out of 8; 50%) in the Siponimod group, compared to 1 patient (out of 8, 12.5%) in the placebo group. This results in an RR of 4.00 (95% CI 0.56 to 28.40).
8F.d. Skin symptoms
Skin symptoms were not reported in the included studies (NCT-810; NCT-917; NCT-274).
8F.e. Serious adverse events
All included studies reported adverse events: 0 (out of 4) for Siponimod 0.5 mg (NCT-274); 1 (out of 11) for Siponimod 2mg (NCT-274, NCT-917), 1 (out of 14) for Siponimod 10mg; and 5 (out of 20) for placebo (NCT-810; NCT-917; NCT-274).
8G. Tocilizumab
One study was used for the comparison of tocilizumab to placebo (Oddis, 2022; “TIM”). Tocilizumab is an interleukin-6 (IL-6) receptor antagonist. It blocks the activity of this cytokine, therefore reducing the inflammatory response.
8G.a. Function
As the number of assessed patients for HAQ at 24 weeks was unclear in Oddis (2022, “TIM”), it is not included in this literature analysis.
8G.b. Muscle strength
As the number of assessed patients for MMT-8 score at 24 weeks was unclear in Oddis (2022, “TIM”), it is not included in this literature analysis.
8G.c. Improvement
Oddis (2022, “TIM”) reported the achievement of TIS (according to ACR/EULAR criteria) of 20 (minimal improvement), 40 (moderate improvement), 60 or more (major improvement) at the last visit of each patient, i.e. 24 weeks or earlier. In the tocilizumab group, 10 (out of 18 patients) and in the placebo group 15 (out of 18 patients) achieved at least minimal improvement (RR 0.67, 95% CI 0.42 to 1.06, favouring placebo).
8G.d. Skin symptoms
Skin symptoms were not reported in Oddis (2022, “TIM”).
8G.e. Serious adverse events
After 24 weeks, serious adverse events occurred in 1/18 participants in the tocilizumab group (bilateral sub-massive pulmonary embolism) and 0/18 in the placebo group (Oddis, 2022; “TIM”).
8. Level of evidence of the literature
|
Outcome measure |
Domains |
Level of evidence |
|
Function |
8A: -1 risk of bias of included studies; -2 imprecision (heterogeneity in outcome measures and broad confidence interval crossing both borders of clinical relevance) |
VERY LOW
|
|
8B: No GRADE assessment |
- |
|
|
8C: No GRADE assessment |
- |
|
|
8D: insufficient data available |
- |
|
|
8E: No GRADE assessment |
- |
|
|
8F: -1 risk of bias of included studies; -3 imprecision (heterogeneity in timepoint measured and very broad confidence interval crossing both borders of clinical relevance) |
VERY LOW |
|
|
8G: No GRADE assessment |
- |
|
|
Muscle strength |
8A: -1 risk of bias of included studies; -2 imprecision (broad confidence interval crossing both borders of clinical relevance) |
VERY LOW
|
|
8B: -1 risk of bias of included studies; -2 imprecision (broad confidence interval crossing both borders of clinical relevance) |
VERY LOW
|
|
|
8C: insufficient data available |
- |
|
|
8D: No GRADE assessment |
- |
|
|
8E: No GRADE assessment |
- |
|
|
8F: -1 risk of bias of included studies; -1 imprecision (broad confidence interval crossing the border of clinical relevance) |
LOW |
|
|
8G: No GRADE assessment |
- |
|
|
Improvement |
8A: -1 risk of bias of included studies -2 imprecision (broad confidence interval crossing both borders of clinical relevance) |
VERY LOW |
|
8B: No GRADE assessment |
- |
|
|
8C: No GRADE assessment |
- |
|
|
8D: No GRADE assessment |
- |
|
|
8E: No GRADE assessment |
- |
|
|
8F: -1 risk of bias of included studies; -3 imprecision (extremely broad confidence interval crossing both borders of clinical relevance) |
VERY LOW |
|
|
8F: No GRADE assessment |
|
|
|
Skin symptoms |
8A: 1- risk of bias of included studies; -2 imprecision (confidence interval crossing border of clinical relevance and results of single study with low number of patients) |
VERY LOW |
|
8B: No GRADE assessment |
- |
|
|
8C: -1 risk of bias in included study, -1 imprecision (inclusion of single study with low number of patients |
LOW |
|
|
8D: -1 risk of bias in included studies; -1 imprecision (heterogeneity in timepoint of measurement) |
LOW |
|
|
8E: No GRADE assessment |
- |
|
|
8F: No GRADE assessment |
- |
|
|
8G: No GRADE assessment |
- |
|
|
Serious adverse events |
8A: -1 risk of bias in included studies; -2 imprecision (broad confidence intervals crossing both borders of clinical relevance) |
VERY LOW |
|
8B: -1 risk of bias in included studies; -3 imprecision (very broad confidence interval crossing both borders of clinical relevance) |
VERY LOW |
|
|
8C: insufficient data available |
- |
|
|
8D: -1 risk of bias in included studies; -2 imprecision (broad confidence interval crossing both borders of clinical relevance) |
VERY LOW |
|
|
8E: -1 risk of bias in included study; -2 imprecision (broad confidence interval crossing both borders of clinical relevance and low number of events) |
VERY LOW |
|
|
8F: -1 risk of bias of included studies; -1 imprecision (broad confidence interval crossing both borders of clinical relevance) |
VERY LOW |
|
|
8G: -1 risk of bias of included studies; -3 imprecision (extremely broad confidence interval crossing both borders of clinical relevance, low number of events) |
VERY LOW |
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the effects (benefits and harms) of immunosuppressant and immunomodulatory treatments for IIM?
| P: | Patients with IIMs |
| I: |
Immunosuppressant and/or immunomodulatory medication
|
| C: | Placebo or usual care (usually glucocorticoids with or without a DMARD) |
| O: | Function or disability, muscle strength, improvement, skin symptoms, serious adverse events |
Relevant outcome measures
The guideline development group considered function/disability and muscle strength as critical outcome measures for decision making; and improvement, skin symptoms and serious adverse events as important outcome measures for decision making.
Search and select (Methods)
No literature search was performed because of the publication of recent Cochrane reviews with an identical PICO to answer what the effects (benefits and harms) of immunosuppressant and immunomodulatory treatments are for IIM.
Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
The working group used similar outcomes and preferred outcome measures - with their respective minimal clinically important difference, when available – as compared to the Cochrane review. These outcomes are based on expert opinion and include the following:
-
Function or disability: improvement on a validated function or disability scale; preferred is the (C-)HAQ ((Childhood-)Health Assessment Questionnaire). Clinical relevance is based on the minimal clinical meaningful improvement for the scale used.
-
Muscle strength: improvement compared to baseline, preferably measured by the MMT-8 score (Manual Muscle Test-8) or another validated score. Meaningful improvement has been defined, and as such used in studies, as both ³15% or ³20% increase of the sum-score (Rider, 2003; Oddis, 2005; Rider, 2004).
-
Improvement: proportion of patients reaching improvement measured through 6 core set measures from the International Myositis Assessment and Clinical Studies Group (IMACS) definitions of improvement: if three of any six core set measures improve by ≥ 20%, with no more than two worsening by ≥ 25%.
-
Skin symptoms: change in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI; the activity subscale of this index (0-100, higher scores indicate more severity) is most often used with a clinically relevant change set at 5 points (Anyanwu, 2015)); or another validated score for DM.
-
Serious adverse events: any untoward medical occurrence that at any dose results in death, is life‐threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability/incapacity or is a congenital anomaly/birth defect.
Referenties
- Algemeen
- Allenbach Y, Mammen AL, Benveniste O, Stenzel W; Immune-Mediated Necrotizing Myopathies Working Group. 224th ENMC International Workshop:: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14-16 October 2016. Neuromuscul Disord. 2018 Jan;28(1):87-99. doi: 10.1016/j.nmd.2017.09.016. Epub 2017 Oct 23. PMID: 29221629.
- Anyanwu CO, Fiorentino DF, Chung L, Dzuong C, Wang Y, Okawa J, Carr K, Propert KJ, Werth VP. Validation of the Cutaneous Dermatomyositis Disease Area and Severity Index: characterizing disease severity and assessing responsiveness to clinical change. Br J Dermatol. 2015 Oct;173(4):969-74. doi: 10.1111/bjd.13915. Epub 2015 Aug 11. PMID: 25994337; PMCID: PMC4878996.
- Arouche-Delaperche L, Allenbach Y, Amelin D, Preusse C, Mouly V, Mauhin W, Tchoupou GD, Drouot L, Boyer O, Stenzel W, Butler-Browne G, Benveniste O. Pathogenic role of anti-signal recognition protein and anti-3-Hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: Myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann Neurol. 2017 Apr;81(4):538-548. doi: 10.1002/ana.24902. PMID: 28224701.
- Cottin V, Barba T, Mainbourg S, Nasser M, Valenzuela C, Lega JC. Pulmonary involvement in inflammatory myopathies. In: Pulmonary Manifestations of Systemic Diseases. 2019. 86: 68-89. DOI:?10.1183/2312508X.10014119
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002 Sep;46(9):2294-300. doi: 10.1002/art.10529. PMID: 12355476.
- Goswami RP, Haldar SN, Chatterjee M, Vij P, van der Kooi AJ, Lim J, Raaphorst J, Bhadu D, Gelardi C, Danieli MG, Kumar U. Efficacy and safety of intravenous and subcutaneous immunoglobulin therapy in idiopathic inflammatory myopathy: A systematic review and meta-analysis. Autoimmun Rev. 2022 Feb;21(2):102997. doi: 10.1016/j.autrev.2021.102997. Epub 2021 Nov 17. PMID: 34800685.
- Graf M, von Stuckrad SL, Uruha A, Klotsche J, Zorn-Pauly L, Unterwalder N, Buttgereit T, Krusche M, Meisel C, Burmester GR, Hiepe F, Biesen R, Kallinich T, Stenzel W, Schneider U, Rose T. SIGLEC1 enables straightforward assessment of type I interferon activity in idiopathic inflammatory myopathies. RMD Open. 2022 Feb;8(1):e001934. doi: 10.1136/rmdopen-2021-001934. PMID: 35177553; PMCID: PMC8860073.
- Hornung T, Janzen V, Heidgen FJ, Wolf D, Bieber T, Wenzel J. Remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med. 2014 Dec 25;371(26):2537-8. doi: 10.1056/NEJMc1412997. PMID: 25539124.
- Ladislau L, Suárez-Calvet X, Toquet S, Landon-Cardinal O, Amelin D, Depp M, Rodero MP, Hathazi D, Duffy D, Bondet V, Preusse C, Bienvenu B, Rozenberg F, Roos A, Benjamim CF, Gallardo E, Illa I, Mouly V, Stenzel W, Butler-Browne G, Benveniste O, Allenbach Y. JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain. 2018 Jun 1;141(6):1609-1621. doi: 10.1093/brain/awy105. PMID: 29741608.
- Lerkvaleekul B, Veldkamp SR, van der Wal MM, Schatorjé EJH, Kamphuis SSM, van den Berg JM, Hissink Muller PCE, Armbrust W, Vastert SJ, Wienke J, Jansen MHA, van Royen-Kerkhof A, van Wijk F. Siglec-1 expression on monocytes is associated with the interferon signature in juvenile dermatomyositis and can predict treatment response. Rheumatology (Oxford). 2022 May 5;61(5):2144-2155. doi: 10.1093/rheumatology/keab601. PMID: 34387304; PMCID: PMC9071568.
- Lim J, Eftimov F, Verhamme C, Brusse E, Hoogendijk JE, Saris CGJ, Raaphorst J, De Haan RJ, van Schaik IN, Aronica E, de Visser M, van der Kooi AJ. Intravenous immunoglobulins as first-line treatment in idiopathic inflammatory myopathies: a pilot study. Rheumatology (Oxford). 2021 Apr 6;60(4):1784-1792. doi: 10.1093/rheumatology/keaa459. PMID: 33099648; PMCID: PMC8023983.
- Mahr AD, Jover JA, Spiera RF, Hernández-García C, Fernández-Gutiérrez B, Lavalley MP, Merkel PA. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007 Aug;56(8):2789-97. doi: 10.1002/art.22754. PMID: 17665429.
- Marrani E, Abu-Rumeileh S, Mastrolia MV, Maccora I, Pagnini I, Simonini G. A systematic review on biological therapies in juvenile idiopathic inflammatory myopathies: an evidence gap in precision medicine. Clin Exp Rheumatol. 2022 Feb;40(2):457-470. doi: 10.55563/clinexprheumatol/ltrj4l. Epub 2021 Dec 14. PMID: 34905479.
- Mecoli CA, Park JK, Alexanderson H, Regardt M, Needham M, de Groot I, Sarver C, Lundberg IE, Shea B, de Visser M, Song YW, Bingham CO 3rd, Christopher-Stine L. Perceptions of Patients, Caregivers, and Healthcare Providers of Idiopathic Inflammatory Myopathies: An International OMERACT Study. J Rheumatol. 2019 Jan;46(1):106-111. doi: 10.3899/jrheum.180353. Epub 2018 Sep 15. PMID: 30219767; PMCID: PMC7497902.
- Miller FW, Leitman SF, Cronin ME, Hicks JE, Leff RL, Wesley R, Fraser DD, Dalakas M, Plotz PH. Controlled trial of plasma exchange and leukapheresis in polymyositis and dermatomyositis. N Engl J Med. 1992 May 21;326(21):1380-4. doi: 10.1056/NEJM199205213262102. PMID: 1472183.
- Mithoowani S, Gregory-Miller K, Goy J, Miller MC, Wang G, Noroozi N, Kelton JG, Arnold DM. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2016 Oct;3(10):e489-e496. doi: 10.1016/S2352-3026(16)30109-0. Epub 2016 Sep 16. PMID: 27658982.
- Oddis CV, Rider LG, Reed AM, Ruperto N, Brunner HI, Koneru B, Feldman BM, Giannini EH, Miller FW; International Myositis Assessment and Clinical Studies Group. International consensus guidelines for trials of therapies in the idiopathic inflammatory myopathies. Arthritis Rheum. 2005 Sep;52(9):2607-15. doi: 10.1002/art.21291. PMID: 16142757.
- Paik JJ, Casciola-Rosen L, Shin JY, Albayda J, Tiniakou E, Leung DG, Gutierrez-Alamillo L, Perin J, Florea L, Antonescu C, Leung SG, Purwin G, Koenig A, Christopher-Stine L. Study of Tofacitinib in Refractory Dermatomyositis: An Open-Label Pilot Study of Ten Patients. Arthritis Rheumatol. 2021 May;73(5):858-865. doi: 10.1002/art.41602. Epub 2021 Mar 24. PMID: 33258553; PMCID: PMC8084900.
- Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, Kreuter M, Lynch DA, Maher TM, Martinez FJ, Molina-Molina M, Myers JL, Nicholson AG, Ryerson CJ, Strek ME, Troy LK, Wijsenbeek M, Mammen MJ, Hossain T, Bissell BD, Herman DD, Hon SM, Kheir F, Khor YH, Macrea M, Antoniou KM, Bouros D, Buendia-Roldan I, Caro F, Crestani B, Ho L, Morisset J, Olson AL, Podolanczuk A, Poletti V, Selman M, Ewing T, Jones S, Knight SL, Ghazipura M, Wilson KC. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2022 May 1;205(9):e18-e47. doi: 10.1164/rccm.202202-0399ST. PMID: 35486072; PMCID: PMC9851481.
- Rider LG, Giannini EH, Brunner HI, Ruperto N, James-Newton L, Reed AM, Lachenbruch PA, Miller FW; International Myositis Assessment and Clinical Studies Group. International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis Rheum. 2004 Jul;50(7):2281-90. doi: 10.1002/art.20349. PMID: 15248228.
- Rider LG, Giannini EH, Harris-Love M, Joe G, Isenberg D, Pilkington C, Lachenbruch PA, Miller FW; International Myositis Assesment and Clinical Studies Group. Defining Clinical Improvement in Adult and Juvenile Myositis. J Rheumatol. 2003 Mar;30(3):603-17. PMID: 12610824.
- Romero-Bueno F, Diaz Del Campo P, Trallero-Araguás E, Ruiz-Rodríguez JC, Castellvi I, Rodriguez-Nieto MJ, Martínez-Becerra MJ, Sanchez-Pernaute O, Pinal-Fernandez I, Solanich X, Gono T, Gonzalez-Gay MA, Plana MN, Selva-O'Callaghan A; MEDRA5 (Spanish MDA5 Register) group (listed contributors at the end of the article). Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin Arthritis Rheum. 2020 Aug;50(4):776-790. doi: 10.1016/j.semarthrit.2020.03.007. Epub 2020 Jun 1. PMID: 32534273.
- Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009 Jul;68(7):1100-4. doi: 10.1136/ard.2008.093690. Epub 2008 Dec 5. PMID: 19060002; PMCID: PMC2689525.
- Sato S, Masui K, Nishina N, Kawaguchi Y, Kawakami A, Tamura M, Ikeda K, Nunokawa T, Tanino Y, Asakawa K, Kaneko Y, Gono T, Ukichi T, Kaieda S, Naniwa T, Kuwana M; JAMI investigators. Initial predictors of poor survival in myositis-associated interstitial lung disease: a multicentre cohort of 497 patients. Rheumatology (Oxford). 2018 Jul 1;57(7):1212-1221. doi: 10.1093/rheumatology/key060. PMID: 29596687.
- Selva-O'Callaghan A, Romero-Bueno F, Trallero-Araguás E, Gil-Vila A, Ruiz-Rodríguez JC, Sánchez-Pernaute O, Pinal-Fernández I. Pharmacologic Treatment of Anti-MDA5 Rapidly Progressive Interstitial Lung Disease. Curr Treatm Opt Rheumatol. 2021;7(4):319-333. doi: 10.1007/s40674-021-00186-x. Epub 2021 Sep 28. PMID: 34603940; PMCID: PMC8476986.
- Sevim E, Kobrin D, Casal-Dominguez M, Pinal-Fernandez I. A comprehensive review of dermatomyositis treatments - from rediscovered classics to promising horizons. Expert Rev Clin Immunol. 2023 Oct 16:1-13. doi: 10.1080/1744666X.2023.2270737. Epub ahead of print. PMID: 37842905.
- Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, Caporali R, Edwards CJ, Hyrich KL, Pope JE, de Souza S, Stamm TA, Takeuchi T, Verschueren P, Winthrop KL, Balsa A, Bathon JM, Buch MH, Burmester GR, Buttgereit F, Cardiel MH, Chatzidionysiou K, Codreanu C, Cutolo M, den Broeder AA, El Aoufy K, Finckh A, Fonseca JE, Gottenberg JE, Haavardsholm EA, Iagnocco A, Lauper K, Li Z, McInnes IB, Mysler EF, Nash P, Poor G, Ristic GG, Rivellese F, Rubbert-Roth A, Schulze-Koops H, Stoilov N, Strangfeld A, van der Helm-van Mil A, van Duuren E, Vliet Vlieland TPM, Westhovens R, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023 Jan;82(1):3-18. doi: 10.1136/ard-2022-223356. Epub 2022 Nov 10. Erratum in: Ann Rheum Dis. 2023 Mar;82(3):e76. PMID: 36357155.
- Assessment of activities of daily living in patients with amyotrophic lateral sclerosis. The ALS CNTF treatment study (ACTS) phase I-II Study Group. Arch Neurol. 1996 Feb;53(2):141-7. PMID: 8639063.
- van de Vlekkert J, Hoogendijk JE, de Haan RJ, Algra A, van der Tweel I, van der Pol WL, Uijtendaal EV, de Visser M; Dexa Myositis Trial. Oral dexamethasone pulse therapy versus daily prednisolone in sub-acute onset myositis, a randomised clinical trial. Neuromuscul Disord. 2010 Jun;20(6):382-9. doi: 10.1016/j.nmd.2010.03.011. Epub 2010 Apr 25. PMID: 20423755.
- Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, Germino R, Menon S, Sun Y, Wang C, Shapiro AB, Kanik KS, Connell CA; ORAL Surveillance Investigators. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022 Jan 27;386(4):316-326. doi: 10.1056/NEJMoa2109927. PMID: 35081280.
- Non-targeted therapies
- Aggarwal R, Charles-Schoeman C, Schessl J, Bata-Csörg? Z, Dimachkie MM, Griger Z, Moiseev S, Oddis C, Schiopu E, Vencovský J, Beckmann I, Clodi E, Bugrova O, Dankó K, Ernste F, Goyal NA, Heuer M, Hudson M, Hussain YM, Karam C, Magnolo N, Nelson R, Pozur N, Prystupa L, Sárdy M, Valenzuela G, van der Kooi AJ, Vu T, Worm M, Levine T; ProDERM Trial Group. Trial of Intravenous Immune Globulin in Dermatomyositis. N Engl J Med. 2022 Oct 6;387(14):1264-1278. doi: 10.1056/NEJMoa2117912. PMID: 36198179. (PRODERM)
- Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med. 1980 Mar;92(3):365-9. doi: 10.7326/0003-4819-92-3-365. PMID: 6986827.
- Dalakas MC, Illa I, Dambrosia JM, Soueidan SA, Stein DP, Otero C, Dinsmore ST, McCrosky S. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993 Dec 30;329(27):1993-2000. doi: 10.1056/NEJM199312303292704. PMID: 8247075.
- Ibrahim F, Choy E, Gordon P, Doré CJ, Hakim A, Kitas G, Isenberg D, Griffiths B, Lecky B, Chakravarty K, Winer J, Danko K, Cooper RG, White-Alao B, Scott DL. Second-line agents in myositis (SELAM): 1-year factorial trial of additional immunosuppression in patients who have partially responded to steroids. Rheumatology (Oxford). 2015 Jun;54(6):1050-5. doi: 10.1093/rheumatology/keu442. Epub 2014 Nov 27. PMID: 25433040; PMCID: PMC4476843.
- ISRCTN87782942 (ISR-942). Efficacy and safety of L0133 in the treatment of dermatomyositis and polymyositis: prospective, randomised, double-blind, placebo-controlled study. https://trialsearch.who.int/Trial2.aspx? TrialID=ISRCTN87782942 2006.
- Miller J, Walsh Y, Saminaden S, Lecky BRF, Winer JB. Randomised double blind controlled trial of methotrexate and steroids compared with azathioprine and steroids in the treatment of idiopathic inflammatory myopathy. Journal of the Neurological Sciences 2002;199:S53.
- Miller FW, Leitman SF, Cronin ME, Hicks JE, Leff RL, Wesley R, Fraser DD, Dalakas M, Plotz PH. Controlled trial of plasma exchange and leukapheresis in polymyositis and dermatomyositis. N Engl J Med. 1992 May 21;326(21):1380-4. doi: 10.1056/NEJM199205213262102. PMID: 1472183.
- Miyasaka N, Hara M, Koike T, Saito E, Yamada M, Tanaka Y; GB-0998 Study Group. Effects of intravenous immunoglobulin therapy in Japanese patients with polymyositis and dermatomyositis resistant to corticosteroids: a randomized double-blind placebo-controlled trial. Mod Rheumatol. 2012 Jun;22(3):382-93. doi: 10.1007/s10165-011-0534-4. Epub 2011 Oct 5. PMID: 21971943; PMCID: PMC3375426.
- Vencovsky J. A prospective, randomised, assessor-blind, multicenter study of efficacy and safety of combined treatment of methotrexate + glucocorticoids versus glucocorticoids alone in patients with polymyositis and dermatomyositis. - PROMETHEUS. 2016. https://trialsearch.who.int/Trial2.aspx? TrialID=EUCTR2007-004410-13-SE 2008.
- Villalba L, Hicks JE, Adams EM, Sherman JB, Gourley MF, Leff RL, Thornton BC, Burgess SH, Plotz PH, Miller FW. Treatment of refractory myositis: a randomized crossover study of two new cytotoxic regimens. Arthritis Rheum. 1998 Mar;41(3):392-9. doi: 10.1002/1529-0131(199803)41:3<392::AID-ART3>3.0.CO;2-X. PMID: 9506565.
- van de Vlekkert J, Hoogendijk JE, de Haan RJ, Algra A, van der Tweel I, van der Pol WL, Uijtendaal EV, de Visser M; Dexa Myositis Trial. Oral dexamethasone pulse therapy versus daily prednisolone in sub-acute onset myositis, a randomised clinical trial. Neuromuscul Disord. 2010 Jun;20(6):382-9. doi: 10.1016/j.nmd.2010.03.011. Epub 2010 Apr 25. PMID: 20423755.
- Targeted therapies
- EUCTR2012-005772-34-DE (EUCT-34). A placebo-controlled, proof-of concept study of the efficacy of gevokizumab subcutaneously over 24 weeks in the treatment of patients with polymyositis, dermatomyositis or necrotizing autoimmune myopathy disease. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2012-005772- 34-DE 2013.
- EUCTR2018-003273-10-DE (EUCT-10, DETERMINE). A study of safety and efficacy of lenabasum in dermatomyositis patients. https://clinicaltrialsregister.eu/ctr-search/trial/2018-003273-10/DE 2018.
- Mammen AL, Amato AA, Dimachkie MM, Chinoy H, Hussain Y, Lilleker JB, Pinal-Fernandez I, Allenbach Y, Boroojerdi B, Vanderkelen M, Delicha EM, Koendgen H, Farzaneh-Far R, Duda PW, Sayegh C, Benveniste O. Zilucoplan in immune-mediated necrotising myopathy: a phase 2, randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2023 Feb;5(2):e67-e76. doi: 10.1016/s2665-9913(23)00003-6. Epub 2023 Jan 24. PMID: 36923454; PMCID: PMC10009502.
- Muscle Study Group. A randomized, pilot trial of etanercept in dermatomyositis. Ann Neurol. 2011 Sep;70(3):427-36. doi: 10.1002/ana.22477. Epub 2011 Jun 17. PMID: 21688301; PMCID: PMC3170432.
- NCT00533091 (NCT-091). A Study to Evaluate Safety of Multi-Dose MEDI-545 in Adult Patients With Dermatomyositis or Polymyositis. https://clinicaltrials.gov/ct2/show/NCT00533091 2008.
- NCT01148810 (NCT-810). Efficacy and Tolerability of BAF312 in Patients With Polymyositis and Dermatomyositis. https://clinicaltrials.gov/ct2/show/NCT01148810 2010.
- NCT01801917 (NCT-917). Efficacy and Tolerability of BAF312 in Patients With Polymyositis. https://clinicaltrials.gov/ct2/show/NCT01801917 2013.
- NCT02029274 (NCT-274). Safety and Efficacy of BAF312 in Dermatomyositis. https://clinicaltrials.gov/ct2/show/NCT02029274 2013.
- NCT02466243 (NCT-243). Safety, Tolerability, and Efficacy of JBT-101 in Subjects With Dermatomyositis. https://clinicaltrials.gov/ct2/show/NCT02466243 2015.
- NCT02612857 (NCT-857). Trial of IMO-8400 in Adult Patients With Dermatomyositis. https://clinicaltrials.gov/ct2/show/NCT02612857 2015.
- NCT02971683 (NCT-683). Trial to Evaluate the Efficacy and Safety of Abatacept in Combination With Standard Therapy Compared to Standard Therapy Alone in Improving Disease Activity in Adults With Active Idiopathic Inflammatory Myopathy. https://clinicaltrials.gov/ct2/show/NCT02971683 2017
- Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC, Barohn RJ, Feldman BM, Harris-Love MO, Koontz DC, Fertig N, Kelley SS, Pryber SL, Miller FW, Rockette HE; RIM Study Group. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013 Feb;65(2):314-24. doi: 10.1002/art.37754. PMID: 23124935; PMCID: PMC3558563. (RIM)
- Oddis CV, Rockette HE, Zhu L, Koontz DC, Lacomis D, Venturupalli S, Moghadam-Kia S, Ascherman DP, Crofford L, Dimachkie MM, Ernste F, Gazeley D, Marder G, Aggarwal R. Randomized Trial of Tocilizumab in the Treatment of Refractory Adult Polymyositis and Dermatomyositis. ACR Open Rheumatol. 2022 Nov;4(11):983-990. doi: 10.1002/acr2.11493. Epub 2022 Sep 20. PMID: 36128663; PMCID: PMC9661830. (TIM)
- Schiffenbauer A, Garg M, Castro C, Pokrovnichka A, Joe G, Shrader J, Cabalar IV, Faghihi-Kashani S, Harris-Love MO, Plotz PH, Miller FW, Gourley M. A randomized, double-blind, placebo-controlled trial of infliximab in refractory polymyositis and dermatomyositis. Semin Arthritis Rheum. 2018 Jun;47(6):858-864. doi: 10.1016/j.semarthrit.2017.10.010. Epub 2017 Oct 16. PMID: 29174792; PMCID: PMC6208161.
- Tjärnlund A, Tang Q, Wick C, Dastmalchi M, Mann H, Tomasová Studýnková J, Chura R, Gullick NJ, Salerno R, Rönnelid J, Alexanderson H, Lindroos E, Aggarwal R, Gordon P, Vencovsky J, Lundberg IE. Abatacept in the treatment of adult dermatomyositis and polymyositis: a randomised, phase IIb treatment delayed-start trial. Ann Rheum Dis. 2018 Jan;77(1):55-62. doi: 10.1136/annrheumdis-2017-211751. Epub 2017 Oct 9. PMID: 28993346. (ARTEMIS)
Evidence tabellen
See table 1 and 2 in this module, and the individual characteristics of studies in the Cochrane review for study characteristics and risk of bias assessment. The list of excluded studies can be found in the Cochrane review.
Table 1. Characteristics of included studies reporting on non-targeted therapies for IIM.
Abbreviations: A.: analysis (corresponds to number below in analysis), ADL: activities of daily living, AZA: azathioprine, ciclo: ciclosporin, CK: creatinine kinase, DM: dermatomyositis, FRS: Functional Rating Scale (as mentioned in The Amyothrophic Lateral Sclerosis Functional Rating Scale, 1996), i.v.: intravenously, MMT: manual muscle test, MRC: Medical Research Council (scale of muscle strength), MTX: methotrexate, PM: polymyositis, RCT: randomized controlled trial, 30mWT: 30 meter walking time.
|
A. |
Comparison |
Study |
Study design |
Population (n) |
Intervention |
Control |
Follow-up |
Outcomes |
|
Placebo or usual care comparison |
||||||||
|
1 |
Immunoglobulins (i.v.) to placebo |
Dalakas 1993 |
RCT |
Adults with treatment-resistant DM (n = 15) |
Immunoglobulin i.v.1 g/kg (20mL/kg) for 2 consecutive days., once a month for 3 months +prednisone |
Placebo as dextrose in half normal saline
+ prednisone |
3 months |
Function (Neuromuscular symptom score) Muscle strength (MRC) |
|
ISR-942 |
RCT |
Adults with idiopathic DM and PM with insufficiently improved muscle strength under conventional therapy (n = 44) |
Human normal immunoglobulin 1 g/kg (20mL/kg) for 2 consecutive days i.v., once a month |
Placebo as saline infusion 20mL/kg for 2 consecutive days i.v., once a month |
3 months |
Muscle strength (BMRC index) Skin symptoms Serious adverse events |
||
|
Miyasaka 2012 |
RCT |
People (³16 years) with corticosteroid-resistant PM or DM (n = 26) |
GB-0998 (human IgG) 400 mg (8 mL)/kg/day i.v. for 5 consecutive days |
Placebo i.v. once daily for 5 consecutive days |
8 weeks |
Function (ADL score based on 15 actions) Muscle strength (MMT) Serious adverse events |
||
|
Aggarwal 2022 (ProDERM) |
RCT |
Adults with active refractory DM (n = 95) |
Immunoglobulin in dose of 2g/kg i.v. every 4 weeks |
Placebo every 4 weeks |
16 weeks |
Function (HAQ and SF-36) Muscle strength Improvement Skin symptoms Serious adverse events |
||
|
2 |
AZA to placebo |
Bunch 1980 |
RCT |
Adults with PM (this would include IBM and IMNM) (n = 23) |
Azathioprine 2mg/kg + prednisone |
Placebo + prednisone (15mg 4dd, reduced to 10 mg 4dd on normalization of CK levels |
3 months |
Muscle strength (MMT change from baseline, scored 0 to -4) |
|
3
4
3 |
MTX + ciclo to placebo
Ciclo to placebo
MTX to placebo
|
Ibrahim 2015 (SELAM) |
RCT (factorial design) |
Adults with active disease IIM according to Peter&Bohan criteria receiving glucocorticoids (n = 58) |
1. Methotrexate 7.5 mg/week increasing every 2 weeks by 2.5mg to 15mg/week (max. 25mg/week) 2. Ciclosporin 1 mg/kg/day increased to target 5 mg/kg/day (where tolerated) 3. MTX + ciclosporin combined (all with additional prednisone) |
Placebo + prednisone (dose adjusted to disease activity) |
12 months |
Function (FRS, 30mWT) Muscle strength (MMT) |
|
3 |
MTX to usual care |
Vencovsky 2016 (PROMETHEUS) |
RCT |
Adults with PM or DM previously untreated with immunosuppressants (n = 31) |
Methotrexate orally once weekly, starting from 10 mg up to 20-25mg in week 5. Continued for 48 weeks. + prednisone 1mg/kg/day |
prednisone 1mg/kg/day |
48 weeks |
Improvement (IMACS) Serious adverse events |
|
Active medications compared to each other |
||||||||
|
2 |
AZA to MTX |
Miller 2002 |
RCT |
Adults with PM or DM (n = 30) |
Azathioprine 2.5 mg/kg/day + folic acid 5 mg/day
+ prednisone |
methotrexate 7.5 mg/week increased by 2.5mg/month to 15mg/week + folic acid 5 mg/day + prednisone |
48 weeks |
Function (10 m walk time) Muscle strength (hand-held myometry) Serious adverse events |
|
3 |
MTX to ciclo |
See study Ibrahim (2015) |
||||||
|
Active medication as add-on |
||||||||
|
4 3 |
MTX + ciclo to:
|
See study Ibrahim (2015) |
||||||
|
Active medication in different routes of administration/dosages |
||||||||
|
4 |
Pulse dexamethasone to oral prednisone |
Van de Vlekkert 2010 (IS950) |
RCT |
Treatment-naïve adults with DM or non-specific myositis (n = 62) |
Oral dexamethasone in 6 cycles of 40 mg/day for 4 consecutive days with 28-day intervals |
Oral prednisone started at 70 or 90 mg/day for 28 days, decreasing every week with 5 mg every other day |
12-18 months |
Disability (7-point composite score) Muscle strength (MRC sum score) Serious adverse events |
|
Oral MTX + AZA to i.v. MTX |
Villalba 1998 |
RCT (open label) |
Adults with refractory DM or PM (n = 30) |
Oral methotrexate (up to 25mg/week) and azathioprine (up to 150 mg/day) |
Methotrexate i.v. 500 mg/m2 every two weeks for 12 dosis + leucovorin rescue (50 mg/m2) |
6 months |
Combined outcome for function and strength (ADL) |
|
|
Plasmapheresis to leukopheresis to sham |
Miller 1992 |
RCT |
Adults with PM and DM (n = 39) |
Each 3 times a week for 4 weeks |
Sham apheresis: 5-6L of blood processed but recombined and reinfused (no removal of components) |
1 month |
Function (ADL) Muscle strength (MRC) Serious adverse events |
|
Table 2. Characteristics of included studies reporting on targeted therapies for IIM.
Abbreviations: A.: analysis (corresponds to number below in analysis), AZA: azathioprine, CDASI: Cutaneous Dermatomyositis Disease Area and Severity Index, DM: dermatomyositis, IMACS: International Myositis Assessment and Clinical Studies Group, i.v.: intravenously, HAQ: Health Assessment Questionnaire, MDAAT: Myositis Disease Activity Assessment Tool, MMT: manual muscle test, MTX: methotrexate, PM: polymyositis, PROMIS-29: Patient-Reported Outcomes Measurement Information System-29,RCT: randomized controlled trial, s.c.: subcutaneously, S1P: sphingosine-1-phosphate, TIS: Total improvement score, 3TUG: triple Timed Up and Go test, 6MWD: 6-minute walking distance. TNF = TLR = AZA =
|
A. |
Medication |
Study |
Study design |
Population (n) |
Intervention |
Control |
Follow-up |
Outcomes |
|
5 |
Rituximab |
Oddis 2013 (RIM study) |
RCT |
Adults and children >5 years with definite or probable refractory DM or PM (n = 200) |
Rituximab infusions in week 0 and 1, dosing based on patient’s body surface area (750mg-1g/m2 per infusion). Placebo infusions at week 8 and 9 |
Placebo infusions at week 0 and 1 (not further specified). Rituximab at week 8 and 9 |
44 weeks (results 8 weeks used for analysis) |
Improvement (IMACS) Skin symptoms (MDAAT) |
|
6 |
Abatacept |
NCT-683 |
RCT |
Adults with DM or PM (n = 148) |
Abatacept 125 mg weekly s.c. for 24 weeks In combination with standard treatment |
Placebo to match abatacept s.c. In combination with standard treatment |
24 weeks |
Function (HAQ) Muscle strength (MMT8) Improvement (IMACS) Serious adverse events |
|
Tjärnlund 2018 (ARTEMIS) |
RCT |
Adults with refractory DM or PM (n = 20) |
Abatacept 500-1000mg i.v. (depending on body weight), for 7 infusions over 6 months (week 0, 2, 4, 8, 12, 16, 20) |
Abatacept 500-1000mg i.v.; initiation after 12 weeks. 7 infusions (week 12, 14, 16, 20, 24, 28, 32) |
6 months (results 3 months used for analysis) |
Function (HAQ) Muscle strength (MMT8) Improvement (IMACS) Serious adverse events |
||
|
7 |
Zilucoplan (Complement 5 inhibitor) |
(NCT-632) |
RCT |
Adults with immune-mediated necrotizing myopathy (n = 27) |
Zilucoplan 0.3 mg/kg/day s.c. for 8 weeks |
Matching placebo doses s.c. for 8 weeks |
8 weeks |
Function (HAQ, 3TUG) Muscle strength Improvement (TIS) |
|
8A |
Anti-TNF-alpha inhibitors |
Muscle study group 2011 |
RCT |
Adults with DM, newly diagnosed or refractory (n = 16) |
Etanercept 50mg s.c. weekly |
Placebo prefilled liquid syringes consisting of 25mM Na phosphate, 25mM L-arginine-HCI, 100mM. NaCI, 1% sucrose per syringe |
24 and 52 weeks |
Function (HAQ) Muscle strength (MMT) Improvement (IMACS) Skin symptoms (CDASI) Serious adverse events |
|
Schiffenbauer 2018 |
RCT |
Adults with DM and PM, using corticosteroids with MTX or AZA (n = 13) |
Infliximab 4 infusions of 5mg/kg at week 0, 2, 6, and 14 |
Placebo 4 infusions at week 0, 2, 6 and 14 |
16 weeks |
Function (HAQ) Muscle strength (MMT8) Improvement (IMACS) Serious adverse events |
||
|
8B |
Gevokizumab (IL-1-b inhibitor) |
EUCT-34 |
RCT |
Adults with PM, DM, or necrotizing autoimmune myopathy (n = 27) |
Gevokizumab 60 mg s.c. every 4 weeks, for 24 weeks |
Placebo s.c. every 4 weeks, for 24 weeks |
24 weeks |
Muscle strength (MMT8) Serious adverse events |
|
8C |
Bazlitoran (TLR 7/8/9 inibitor) |
NCT-857 |
Three-arm RCT |
Adults with DM (n = 30) |
1. Bazlitoran 0.6 mg/kg s.c. injections once weekly for 24 weeks 2. Bazlitoran 1.8 mg/kg s.c. injections once weekly for 24 weeks Concomitant prednisone or DMARD was allowed |
Placebo in the form of saline injections s.c. for 24 weeks Concomitant prednisone or DMARD was allowed |
28 weeks |
Muscle strength (MMT8) Skin symptoms (modified CDASI) Serious adverse events |
|
8D |
Lenabasum (cannabinoid agonist) |
EUCT-10 (DETERMINE) |
RCT |
Adults with DM (n = 175) |
1. Lenabasum 20 mg orally, twice daily as hard capsule, for 52 weeks 2. Lenabasum 5 mg orally, twice daily as hard capsule, for 52 weeks |
Placebo s a powder-in-capsule containing microcrystalline cellulose and magnesium stearate |
28 and 52 weeks |
Improvement (IMACS) Skin symptoms (CDASI) Serious adverse events |
|
NCT-243 |
RCT |
Adults with DM (classic or amyopathic) (n = 22) |
Lenabasum 20 mg once daily on days 1-28, then twice daily on days 29-84 |
Placebo once daily on days 1-28, then twice daily on days 29-84 |
16 weeks |
Function (PROMIS-29) Skin symptoms (CDASI) Serious adverse events |
||
|
8E |
Sifalimumab (anti-IFN-a) |
NCT-091 |
RCT |
Adults with DM or PM (n = 51) |
Sifalimumab for 6 months dosed at 0.3 mg/kg, 1 mg/kg, 3 mg/kg or 10 mg/kg (4 treatment arms), dosing every other week (14 doses total) |
Placebo for 3 months, then switched to sifalimumab for 3 months |
52 weeks |
Adverse events (reported after 14 weeks) |
|
8F |
Siponimod (S1P receptor modulator) |
NCT-274 |
RCT |
Adults with DM (n = 17) |
1. 0.5 mg siponimod daily 2. 2 mg siponimod daily 3. 10 mg siponimod daily For 24 weeks |
Matching placebo for 24 weeks |
48 weeks |
Function (6MWD) Muscle strength (MMT24) Serious adverse events |
|
NCT-810 |
RCT |
Adults with DM or PM (n = 18) |
Siponimod 10mg once daily (2 tablets of 5 mg) for 12 weeks With 10-day dose up-titration |
Placebo 2 tablets once daily |
12 weeks |
Improvement (IMACS) Serious adverse events |
||
|
NCT-917 |
RCT |
Adults with PM (n = 14) |
1. 2 mg siponimod daily (1 tablet + 4 tablets placebo) 2. 10 mg siponimod daily (5 tablets) |
Placebo (5 tablets) |
12 weeks |
Function (6MWD) Muscle strength (MMT24) Serious adverse events |
||
|
8G |
Tocilizumab (IL-6 inhibitor) |
Oddis 2022 (TIM) |
RCT |
Adults with refractory DM or PM (n = 36) |
Tocilizumab 8mg/kg i.v. every 4 weeks for 24 weeks |
Placebo i.v. infusions every 4 weeks for 24 weeks |
24 weeks |
Function (HAQ) Muscle strength (MMT8) Improvement (TIS) Serious adverse events |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 17-09-2024
Beoordeeld op geldigheid : 13-09-2024
Algemene gegevens
Elucidation:
The consultation on this chapter has already taken place in the autumn of 2023. The chapter has now been added for information purposes but does not need to be commented on.
The development of this guideline module was supported by the Knowledge Institute of the Federation of Medical Specialists (www.demedischspecialist.nl/ kennisinstituut) and was financed from the Quality Funds for Medical Specialists (SKMS). The financier has had no influence whatsoever on the content of the guideline module.
Reason for revising the guideline
Idiopathic inflammatory myopathy (IIM, “myositis”) comprises the most commonly acquired myopathies in adults with an estimated incidence of 6-10 per million persons per year and a prevalence of 12 per 100.000 persons (Deenen, 2016). The disease can present at all ages and has a bimodal distribution with peaks in childhood/adolescence and around the 5th decade. The disease is more common in women (F:M = 3:2), with the exception of inclusion body myositis (IBM) (M:F = 2:1). The previous guideline was published in 2005 (NVN, 2005) and since then many developments have taken place that warrant revision.
Doel en doelgroep
Aim of the guideline
The intended effect of the revised guideline is to clarify the diagnostic process of IIM, adding the new topic 'antibodies', and to describe new developments in the field of treatment. This should result in a modular revised guideline in accordance with the current requirements for the development of guidelines for medical specialists.
Scope of the guideline
Which group of patients is described?
The idiopathic inflammatory myopathies (IIM) (also called myositis) are a heterogeneous group of disorders with striated muscle inflammation that usually lead to loss of strength. Almost all forms of IIM cause subacute, symmetrical proximal muscle weakness. The weakness starts in the hip and thigh regions with difficulties in running, climbing stairs or walking longer distances. Weakness of the shoulder and upper arm muscles often occur. Neck muscle weakness, respiratory problems and dysphagia are less frequent but may be the initial presentation. Muscle pain and subcutaneous edema may be present. The distribution of muscle weakness and disease progression in IBM differs from the other forms of IIM. Systemic or so-called extra-muscular disease activity can occur in various IIM: skin lesions (especially in dermatomyositis), calcinosis, arthritis, Raynaud's phenomenon (in overlap myositis), malignancies, interstitial lung disease (ILD) and/or peri/myocarditis, which can lead to arrhythmias and/or heart failure. These disease manifestations make a multidisciplinary approach essential for this group of patients.
This guideline is limited to four of the most common IIM: dermatomyositis (DM), non-specific/overlap myositis (which also includes anti-synthetase syndrome (ASS), immune-mediated necrotizing myopathy (IMNM), and IBM.
What are the possible interventions/therapies or (diagnostic) tests?
A definitive diagnosis is usually made with histopathological examination of a muscle biopsy. Serum CK activity, EMG, muscle imaging with ultrasound or MRI and myositis antibody assessment can make an important contribution to the diagnosis and together provide detailed information about expected extra-muscular manifestations including malignancies, therapy response and prognosis.
The drug treatment of IIM is mainly based on expert opinion/consensus; for IBM effective drug treatment is currently lacking. The cornerstone of immunosuppressive treatment of IIM (excluding IBM) is still high dosed glucocortiocoids; methotrexate, azathioprine or mycophenolate mofetil (MMF), are usually prescribed as steroid sparing agents without solid evidence to guide decisions (Gordon, 2012).
In the case of rapidly progressive or (expected) refractory disease, or in case of severe ILD, intravenous immunoglobulins (IVIg), rituximab cyclophosphamide, and/or tacrolimus or ciclosporin can be added to the treatment. The hope is that targeted (biological) therapy will greatly improve outcomes in IIM in the future.
Early diagnosis of IIM prevents irreversible muscle fiber damage and permanent physical limitations. Despite treatment, more than 2/3 of IIM patients have a polyphasic or chronic disease course and a comparable proportion of patients have residual limitations such as reduced mobility (van de Vlekkert, 2014).
What are the most important outcome measures relevant to the patient?
Screening for extra-muscular disease activity, especially ILD and malignancies, is relevant because these are important for morbidity and determine mortality in IIM. Pain and fatigue appear to be two of the most important outcome measures in the field of quality of life (QoL) in international research among patients and IIM care providers (Mecoli, 2019; de Groot, 2019). Other important determinants of QoL are degree of physical activity, muscle complaints, lung complaints, joint complaints and skin complaints.
Users of the guideline
This guideline is written for all who provide care for patients with IIM. Users of the guideline include neurologists, rheumatologists, rehabilitation physicians, dermatologists, pulmonologists, paediatricians, pathologists and internists.
Abbreviations and terms
Umbrella term myositis
Within the guideline, myositis and IIM are used as umbrella terms. This includes all autoimmune-mediated forms of myositis.
Myositis is a collective name for a number of diseases. Myositis comes from the Greek word myos (muscle). The ending -itis means inflammation. Myositis is inflammation of skeletal muscles. The muscle inflammation can sometimes be caused by a bacterial or viral infection or a reaction to medication. But usually the cause is unknown (idiopathic); these conditions are therefore also referred to as idiopathic inflammatory myopathies - IIM. There are also dermatological components, without muscle inflammation (yet).
Inflammatory connective tissue diseases: These diseases used to be referred to as 'connective tissue diseases'. They are associated with predominantly lymphocytic inflammation of various organs, including the striated muscles. There is some evidence that they are due to derangements of the immune system. They are also referred to as 'systemic autoimmune diseases'.
Dermatomyositis: this type of IIM is characterized by heliotrope rash and the pathognomonic Gottron’s papules. Muscle weakness can be absent, which is termed amyopathic dermatomyositis when no or insufficient evidence of an inflammatory myopathy is found. Dermatomyositis can be a classic paraneoplastic syndrome; there is an association with cancer.
Juvenile (dermato)myositis: Juvenile dermatomyositis (JDM) (up to 18 years) is distinguished from adult DM because of severe and extensive vasculitis of skin and organs, involvement of joints and oral mucosa, higher incidence of calcinosis, and lack of an association with malignancies. So-called overlap myositis, especially in the context of mixed connective tissue disease, and immune-mediated necrotizing myopathy can also occur in children.
Immune-mediated necrotizing myopathy (IMNM) (earlier necrotizing auto-immune myopathy (NAM): the muscle weakness is usually severe and rapidly progressive. Anti HMGCR IMNM is statin-associated in about 50%. IMNM may be associated with cancer.
‘Inclusion body’-myositis: Inclusion body myositis (IBM) is a slowly progressive striated muscle disease of unknown origin, occurring mainly in the second half of life, with predominantly lymphocytic inflammation in striated muscles and characteristic structural abnormalities in muscle fibers.
Non-specific or overlap myositis (OM): a residual category without the obvious clinical, pathological, or serological features of the other myositis subtypes. Extra-muscular symptoms are common and may be the presenting symptom of a systemic connective tissue disorder such as systemic sclerosis, Sjögren's disease, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), or mixed connective tissue disease (MCTD).
Anti-synthetase syndrome (ASS): this syndrome is characterized by a combination of myositis, Raynaud's phenomena, "mechanic's hands", non-erosive polyarthritis and ILD. Not all of these symptoms need to be present.
Polymyositis: Is a controversial entity. It is the rarest form of myositis and is considered a diagnosis by exclusion after all other forms have been ruled out.
Disease activity: The concept of activity is used to indicate the dynamics, the severity of a disease process.
Remission: Disease activity is no longer present.
Relaps: Recurrence or increase of disease activity after a period of low disease activity or remission
Disease damage: Irreversible structural changes in tissue (mostly muscle)
|
Afkorting |
Toelichting |
|
ASS |
Anti-synthetase syndrome |
|
DM |
Dermatomyositis |
|
IBM |
inclusion-body myositis |
|
IIM |
Idiopathic inflammatory myopathies |
|
ILD |
Interstitial lung diseases |
|
IMNM |
Immune-mediated necrotizing myopathy (formerly known as NAM: necrotizing autoimmune myopathy) |
|
Juvenile (dermato)myositis |
|
|
OM |
Non-specific or overlap myositis |
|
PM |
Polymyositis |
|
RA |
Rheumatoid arthritis |
|
SLE |
Systemic lupus erythematosus |
Literatuur
Deenen JC, van Doorn PA, Faber CG, van der Kooi AJ, Kuks JB, Notermans NC, Visser LH, Horlings CG, Verschuuren JJ, Verbeek AL, van Engelen BG. The epidemiology of neuromuscular disorders: Age at onset and gender in the Netherlands. Neuromuscul Disord. 2016 Jul;26(7):447-52. doi: 10.1016/j.nmd.2016.04.011. Epub 2016 Apr 21. PMID: 27212207.
De Groot I, van der Lubbe PAHM, Huisman AM. OMERACT Special Interest Group Myositis: met patiënten op zoek naar patiëntgerapporteerde uitkomstmaten. Nederlands Tijdschrift voor Reumatologie. 2. 2019
Gordon PA, Winer JB, Hoogendijk JE, Choy EH. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD003643. doi: 10.1002/14651858.CD003643.pub4. PMID: 22895935; PMCID: PMC7144740.
Mecoli CA, Park JK, Alexanderson H, Regardt M, Needham M, de Groot I, Sarver C, Lundberg IE, Shea B, de Visser M, Song YW, Bingham CO 3rd, Christopher-Stine L. Perceptions of Patients, Caregivers, and Healthcare Providers of Idiopathic Inflammatory Myopathies: An International OMERACT Study. J Rheumatol. 2019 Jan;46(1):106-111. doi: 10.3899/jrheum.180353. Epub 2018 Sep 15. PMID: 30219767; PMCID: PMC7497902.
Nederlandse Vereniging voor Neurologie. Dermatomyositis, polymyositis en sporadische ‘inclusion body’-myositis. 2005
van de Vlekkert J, Hoogendijk JE, de Visser M. Long-term follow-up of 62 patients with myositis. J Neurol. 2014 May;261(5):992-8. doi: 10.1007/s00415-014-7313-z. PMID: 24658663.
Samenstelling werkgroep
A multidisciplinary working group was set up in 2020 for the development of the guideline module, consisting of representatives of all relevant specialisms and patient organisations (see the Composition of the working group) involved in the care of patients with IIM/myositis.
Working group
- Dr. A.J. van der Kooi, neurologist, Amsterdam UMC, location AMC. Nederlandse Vereniging voor Neurologie (chair)
- Dr. U.A. Badrising, neurologist, LUMC. Nederlandse Vereniging voor Neurologie
- Dr. C.G.J. Saris, neurologist, Radboudumc. Nederlandse Vereniging voor Neurologie
- Dr. S. Lassche, neurologist, Zuyderland MC. Nederlandse Vereniging voor Neurologie
- Dr. J. Raaphorst, neurologist, Amsterdam UMC, locatie AMC. Nederlandse Vereniging voor Neurologie
- Dr. J.E. Hoogendijk, neurologist, UMC Utrecht. Nederlandse Vereniging voor Neurologie
- Drs. T.B.G. Olde Dubbelink, neurologist, Rijnstate, Nederlandse Vereniging voor Neurologie
- Dr. I.L. Meek, rheumatologist, Radboudumc. Nederlandse Vereniging voor Reumatologie
- Dr. R.C. Padmos, rheumatologist, Erasmus MC. Nederlandse Vereniging voor Reumatologie
- Prof. dr. E.M.G.J. de Jong, dermatologist, werkzaam in het Radboudumc. Nederlandse Vereniging voor Dermatologie en Venereologie
- Drs. W.R. Veldkamp, dermatologist, Ziekenhuis Gelderse Vallei. Nederlandse Vereniging voor Dermatologie en Venereologie
- Dr. J.M. van den Berg, pediatrician, Amsterdam UMC, locatie AMC. Nederlandse Vereniging voor Kindergeneeskunde
- Dr. M.H.A. Jansen, pediatrician, UMC Utrecht. Nederlandse Vereniging voor Kindergeneeskunde
- Dr. A.C. van Groenestijn, rehabilitation physician, Amsterdam UMC, locatie AMC. Nederlandse Vereniging van Revalidatieartsen
- Dr. B. Küsters, pathologist, Radboudumc. Nederlandse Vereniging voor Pathologie
- Dr. V.A.S.H. Dalm, internist, Erasmus MC. Nederlandse Internisten Vereniging
- Drs. J.R. Miedema, pulmonologist, Erasmus MC. Nederlandse Vereniging van Artsen voor Longziekten en Tuberculose
- I. de Groot, patient representatieve. Spierziekten Nederland
Advisory board
- Prof. dr. E. Aronica, pathologist, Amsterdam UMC, locatie AMC. External expert.
- Prof. dr. D. Hamann, Laboratory specialist medical immunology, UMC Utrecht. External expert.
- Drs. R.N.P.M. Rinkel, ENT physician, Amsterdam UMC, locatie VUmc. Vereniging voor Keel-Neus-Oorheelkunde en Heelkunde van het Hoofd-Halsgebied
- dr. A.S. Amin, cardiologist, werkzaam in werkzaam in het Amsterdam UMC, locatie AMC. Nederlandse Vereniging voor Cardiologie
- dr. A. van Royen-Kerkhof, pediatrician, UMC Utrecht. External expert.
- dr. L.W.J. Baijens, ENT physician, Maastricht UMC+. External expert.
- Em. Prof. Dr. M. de Visser, neurologist, Amsterdam UMC. External expert.
Methodological support
- Drs. T. Lamberts, senior advisor, Knowledge institute of the Federation of Medical Specialists
- Drs. M. Griekspoor, advisor, Knowledge institute of the Federation of Medical Specialists
- Dr. M. M. J. van Rooijen, advisor, Knowledge institute of the Federation of Medical Specialists
Belangenverklaringen
The ‘Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling’ has been followed. All working group members have declared in writing whether they have had direct financial interests (attribution with a commercial company, personal financial interests, research funding) or indirect interests (personal relationships, reputation management) in the past three years. During the development or revision of a module, changes in interests are communicated to the chairperson. The declaration of interest is reconfirmed during the comment phase.
An overview of the interests of working group members and the opinion on how to deal with any interests can be found in the table below. The signed declarations of interest can be requested from the secretariat of the Knowledge Institute of the Federation of Medical Specialists.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
van der Kooi |
Neuroloog, Amsterdam UMC |
|
Immediate studie (investigator initiated, IVIg behandeling bij therapie naive patienten). --> Financiering via Behring. Studie januari 2019 afgerond |
Geen restricties (middel bij advisory board is geen onderdeel van rcihtlijn) |
|
Miedema |
Longarts, Erasmus MC |
Geen. |
|
Geen restricties |
|
Meek |
Afdelingshoofd a.i. afdeling reumatische ziekten, Radboudumc |
Commissaris kwaliteit bestuur Nederlandse Vereniging voor Reumatologie (onkostenvergoeding) |
Medisch adviseur myositis werkgroep spierziekten Nederland |
Geen restricties |
|
Veldkamp |
AIOS dermatologie Radboudumc Nijmegen |
|
Geen. |
Geen restricties |
|
Padmos |
Reumatoloog, Erasmus MC |
Docent Breederode Hogeschool (afdeling reumatologie EMC wordt hiervoor betaald) |
Geen. |
Geen restricties |
|
Dalm |
Internist-klinisch immunoloog Erasmus MC |
Geen. |
Geen. |
Geen restricties |
|
Olde Dubbelink |
Neuroloog in opleiding Canisius-Wilhelmina Ziekenhuis, Nijmegen |
Promotie onderzoek naar diagnostiek en outcome van het carpaletunnelsyndroom (onbetaald) |
Geen. |
Geen restricties |
|
van Groenestijn |
Revalidatiearts AmsterdamUMC, locatie AMC |
Geen. |
Lokale onderzoeker voor de I'M FINE studie (multicentre, leiding door afdeling Revalidatie Amsterdam UMC, samen met UMC Utrecht, Sint Maartenskliniek, Klimmendaal en Merem. Evaluatie van geïndividualiseerd beweegprogramma o.b.v. combinatie van aerobe training en coaching bij mensen met neuromusculaire aandoeningen, NMA). Activiteiten: screening NMA-patiënten die willen participeren aan deze studie. Subsidie van het Prinses Beatrix Spierfonds. |
Geen restricties |
|
Lassche |
Neuroloog, Zuyderland Medisch Centrum, Heerlen en Sittard-Geleen |
Geen. |
Geen. |
Geen restricties |
|
de Jong |
Dermatoloog, afdelingshoofd Dermatologie Radboudumc Nijmegen |
Geen. |
All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud university medical centre Nijmegen, the Netherlands |
Geen restricties |
|
Hoogendijk |
Neuroloog Universitair Medisch Centrum Utrecht (0,4) Neuroloog Sionsberg, Dokkum (0,6) |
beide onbetaald |
Geen. |
Geen restricties |
|
Badrising |
Neuroloog Leids Universitair Medisch Centrum |
(U.A.Badrising Neuroloog b.v.: hoofdbestuurder; betreft een vrijwel slapende b.v. als overblijfsel van mijn eerdere praktijk in de maatschap neurologie Dirksland, Het van Weel-Bethesda Ziekenhuis) |
Medisch adviseur myositis werkgroep spierziekten Nederland |
Geen restricties |
|
van den Berg |
Kinderarts-reumatoloog/-immunoloog Emma kinderziekenhuis/ Amsterdam UMC |
Geen. |
Geen. |
Geen restricties |
|
de Groot |
Patiënt vertegenwoordiger/ ervaringsdeskundige: voorzitter diagnosewerkgroep myositis bij Spierziekten Nederland in deze commissie patiënt(vertegenwoordiger) |
|
Ik ben als voorzitter van de Nederlandse diagnose werkgroep Myositis (vallend onder Spierziekten Nederland) en lid van onder andere het Myositis Netwerk Nederland (als patiënten vertegenwoordiger) een soort van 'bekend myositis patiënt' in het kleine myositis wereldje. Datzelfde geldt voor een paar internationale projecten. |
Geen restricties |
|
Küsters |
Patholoog, Radboud UMC |
Geen. |
Geen. |
Geen restricties |
|
Saris |
Neuroloog/ klinisch neurofysioloog, Radboudumc |
Geen. |
Geen. |
Geen restricties |
|
Raaphorst |
Neuroloog, Amsterdam UMC |
Geen. |
|
Restricties m.b.t. opstellen aanbevelingen IvIg behandeling. |
|
Jansen |
Kinderarts-immunoloog-reumatoloog, WKZ UMC Utrecht |
Docent bij Mijs-instituut (betaald) |
Onderzoek biomakers in juveniele dermatomyositis. Geen belang bij uitkomst richtlijn. |
Geen restricties |
Inbreng patiëntenperspectief
Attention was paid to the patient's perspective by offering the Vereniging Spierziekten Nederland to take part in the working group. Vereniging Spierziekten Nederland has made use of this offer, the Dutch Artritis Society has waived it. In addition, an invitational conference was held to which the Vereniging Spierziekten Nederland, the Dutch Artritis Society nd Patiëntenfederatie Nederland were invited and the patient's perspective was discussed. The report of this meeting was discussed in the working group. The input obtained was included in the formulation of the clinical questions, the choice of outcome measures and the considerations. The draft guideline was also submitted for comment to the Vereniging Spierziekten Nederland, the Dutch Artritis Society and Patiëntenfederatie Nederland, and any comments submitted were reviewed and processed.
Qualitative estimate of possible financial consequences in the context of the Wkkgz
In accordance with the Healthcare Quality, Complaints and Disputes Act (Wet Kwaliteit, klachten en geschillen Zorg, Wkkgz), a qualitative estimate has been made for the guideline as to whether the recommendations may lead to substantial financial consequences. In conducting this assessment, guideline modules were tested in various domains (see the flowchart on the Guideline Database).
The qualitative estimate shows that there are probably no substantial financial consequences, see table below.
|
Module |
Estimate |
Explanation |
|
Immunosuppression and immunomodulation in IIM |
No substantial financial consequences |
Outcome 1 No financial consequences. The recommendations are not widely applicable (<5,000 patients) and are therefore not expected to have any substantial financial consequences on collective expenditures. |
Implementatie
|
Aanbeveling |
Tijdspad voor implementatie: 1 tot 3 jaar of > 3 jaar |
Verwacht effect op kosten |
Randvoorwaarden voor implementatie (binnen aangegeven tijdspad) |
Mogelijke barrières voor implementatie1 |
Te ondernemen acties voor implementatie2 |
Verantwoordelijken voor acties3 |
Overige opmerkingen |
|
Aanbevelingen initial treatment |
<1 jaar |
Nihil |
Beschikbaarheid en bekendheid met middelen |
Bereidheid om expertisecentra te betrekken |
- |
Wetenschappelijke verenigingen, betrokken zorgprofessionals |
|
|
Aanbevelingen steroid sparing treatment and steroid tapering |
< 1 jaar |
Nihil |
Beschikbaarheid en bekendheid met middelen |
- |
- |
Wetenschappelijke verenigingen, betrokken zorgprofessionals |
|
|
Aanbevelingen treatment of severe IIM |
<1 jaar |
Nihil |
Beschikbaarheid en bekendheid met middelen |
Reeds conform huidige praktijk |
- |
Wetenschappelijke verenigingen, betrokken zorgprofessionals |
|
|
Aanbevelingen ILD |
< 1 jaar |
Nihil |
Mogelijkheid tot multidisciplinair overleg |
Bereidheid om expertisecentra te betrekken |
- |
Wetenschappelijke verenigingen, betrokken zorgprofessionals |
|
1 Barriëres kunnen zich bevinden op het niveau van de professional, op het niveau van de organisatie (het ziekenhuis) of op het niveau van het systeem (buiten het ziekenhuis). Denk bijvoorbeeld aan onenigheid in het land met betrekking tot de aanbeveling, onvoldoende motivatie of kennis bij de specialist, onvoldoende faciliteiten of personeel, nodige concentratie van zorg, kosten, slechte samenwerking tussen disciplines, nodige taakherschikking, etc.
2 Denk aan acties die noodzakelijk zijn voor implementatie, maar ook acties die mogelijk zijn om de implementatie te bevorderen. Denk bijvoorbeeld aan controleren aanbeveling tijdens kwaliteitsvisitatie, publicatie van de richtlijn, ontwikkelen van implementatietools, informeren van ziekenhuisbestuurders, regelen van goede vergoeding voor een bepaald type behandeling, maken van samenwerkingsafspraken.
3 Wie de verantwoordelijkheden draagt voor implementatie van de aanbevelingen, zal tevens afhankelijk zijn van het niveau waarop zich barrières bevinden. Barrières op het niveau van de professional zullen vaak opgelost moeten worden door de beroepsvereniging. Barrières op het niveau van de organisatie zullen vaak onder verantwoordelijkheid van de ziekenhuisbestuurders vallen. Bij het oplossen van barrières op het niveau van het systeem zijn ook andere partijen, zoals de NZA en zorgverzekeraars, van belang.
Werkwijze
AGREE
This guideline module has been drawn up in accordance with the requirements stated in the Medisch Specialistische Richtlijnen 2.0 report of the Advisory Committee on Guidelines of the Quality Council. This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Clinical questions
During the preparatory phase, the working group inventoried the bottlenecks in the care of patients with IIM. Bottlenecks were also put forward by the parties involved via an invitational conference. A report of this is included under related products.
Based on the results of the bottleneck analysis, the working group drew up and finalized draft basic questions.
Outcome measures
After formulating the search question associated with the clinical question, the working group inventoried which outcome measures are relevant to the patient, looking at both desired and undesired effects. A maximum of eight outcome measures were used. The working group rated these outcome measures according to their relative importance in decision-making regarding recommendations, as critical (critical to decision-making), important (but not critical), and unimportant. The working group also defined at least for the crucial outcome measures which differences they considered clinically (patient) relevant.
Methods used in the literature analyses
A detailed description of the literature search and selection strategy and the assessment of the risk-of-bias of the individual studies can be found under 'Search and selection' under Substantiation. The assessment of the strength of the scientific evidence is explained below.
Assessment of the level of scientific evidence
The strength of the scientific evidence was determined according to the GRADE method. GRADE stands for Grading Recommendations Assessment, Development and Evaluation (see http://www.gradeworkinggroup.org/). The basic principles of the GRADE methodology are: naming and prioritizing the clinically (patient) relevant outcome measures, a systematic review per outcome measure, and an assessment of the strength of evidence per outcome measure based on the eight GRADE domains (downgrading domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias; domains for upgrading: dose-effect relationship, large effect, and residual plausible confounding).
GRADE distinguishes four grades for the quality of scientific evidence: high, fair, low and very low. These degrees refer to the degree of certainty that exists about the literature conclusion, in particular the degree of certainty that the literature conclusion adequately supports the recommendation (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
High |
|
|
Moderate |
|
|
Low |
|
|
Very low |
|
When assessing (grading) the strength of the scientific evidence in guidelines according to the GRADE methodology, limits for clinical decision-making play an important role (Hultcrantz, 2017). These are the limits that, if exceeded, would lead to an adjustment of the recommendation. To set limits for clinical decision-making, all relevant outcome measures and considerations should be considered. The boundaries for clinical decision-making are therefore not directly comparable with the minimal clinically important difference (MCID). Particularly in situations where an intervention has no significant drawbacks and the costs are relatively low, the threshold for clinical decision-making regarding the effectiveness of the intervention may lie at a lower value (closer to zero effect) than the MCID (Hultcrantz, 2017).
Considerations
In addition to (the quality of) the scientific evidence, other aspects are also important in arriving at a recommendation and are taken into account, such as additional arguments from, for example, biomechanics or physiology, values and preferences of patients, costs (resource requirements), acceptability, feasibility and implementation. These aspects are systematically listed and assessed (weighted) under the heading 'Considerations' and may be (partly) based on expert opinion. A structured format based on the evidence-to-decision framework of the international GRADE Working Group was used (Alonso-Coello, 2016a; Alonso-Coello 2016b). This evidence-to-decision framework is an integral part of the GRADE methodology.
Formulation of conclusions
The recommendations answer the clinical question and are based on the available scientific evidence, the most important considerations, and a weighting of the favorable and unfavorable effects of the relevant interventions. The strength of the scientific evidence and the weight assigned to the considerations by the working group together determine the strength of the recommendation. In accordance with the GRADE method, a low evidential value of conclusions in the systematic literature analysis does not preclude a strong recommendation a priori, and weak recommendations are also possible with a high evidential value (Agoritsas, 2017; Neumann, 2016). The strength of the recommendation is always determined by weighing all relevant arguments together. The working group has included with each recommendation how they arrived at the direction and strength of the recommendation.
The GRADE methodology distinguishes between strong and weak (or conditional) recommendations. The strength of a recommendation refers to the degree of certainty that the benefits of the intervention outweigh the harms (or vice versa) across the spectrum of patients targeted by the recommendation. The strength of a recommendation has clear implications for patients, practitioners and policy makers (see table below). A recommendation is not a dictate, even a strong recommendation based on high quality evidence (GRADE grading HIGH) will not always apply, under all possible circumstances and for each individual patient.
|
Implications of strong and weak recommendations for guideline users |
||
|
|
||
|
|
Strong recommendation |
Weak recommendations |
|
For patients |
Most patients would choose the recommended intervention or approach and only a small number would not. |
A significant proportion of patients would choose the recommended intervention or approach, but many patients would not. |
|
For practitioners |
Most patients should receive the recommended intervention or approach. |
There are several suitable interventions or approaches. The patient should be supported in choosing the intervention or approach that best reflects his or her values and preferences. |
|
For policy makers |
The recommended intervention or approach can be seen as standard policy. |
Policy-making requires extensive discussion involving many stakeholders. There is a greater likelihood of local policy differences. |
Organization of care
In the bottleneck analysis and in the development of the guideline module, explicit attention was paid to the organization of care: all aspects that are preconditions for providing care (such as coordination, communication, (financial) resources, manpower and infrastructure). Preconditions that are relevant for answering this specific initial question are mentioned in the considerations. More general, overarching or additional aspects of the organization of care are dealt with in the module Organization of care.
Commentary and authtorisation phase
The draft guideline module was submitted to the involved (scientific) associations and (patient) organizations for comment. The comments were collected and discussed with the working group. In response to the comments, the draft guideline module was modified and finalized by the working group. The final guideline module was submitted to the participating (scientific) associations and (patient) organizations for authorization and authorized or approved by them.
References
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwaliteit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, . GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy
See appendices from research protocol for Immunosuppressive and immunomodulatory therapies for idiopathic inflammatory myopathies, available via: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD014510/appendices/es#CD014510-sec2-0010