Overige medicatie bij longaanval astma
Uitgangsvraag
Welke medicatie heeft gunstige effecten bij patiënten met een longaanval astma, naast de standaardbehandeling? Onder standaardbehandeling wordt verneveling of dosisaerosol met een bronchodilatator en systemische toediening van steroïden verstaan.
1. In de acute fase bij opname/presentatie op de SEH
- magnesiumsulfaat i.v./parentaal
- salbutamol i.v./parentaal
- theophylline (verwijzing naar module Fosfodiësterase-3 en -4 remmersaerosos bij longaanval astma)
2. Als onderhoud gedurende de opname
- Biologicals met indicatie ernstig astma
- Leukotriene receptor antagonisten (montelukast; in montelukast naïeve patiënten)
Aanbeveling
Geef alleen magnesiumsulfaat iv op een SEH of ICU als poging om mechanische ventilatie te voorkomen of om duur van mechanische ventilatie te verkorten.
Geef alleen salbutamol iv op een SEH of ICU als poging om mechanische ventilatie te voorkomen of om duur van mechanische ventilatie te verkorten. Hierbij moet het continue vernevelen met salbutamol worden aangepast.
Geef geen montelukast als toegevoegde behandeling bij een longaanval astma.
Geef geen biologicals met indicatie ernstig astma als toegevoegde behandeling bij een longaanval astma.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
1. Magnesiumsulfaat i.v./parentaal
Er zijn geen studies gevonden die het effect van magnesiumsulfaat bovenop standaardzorg op de duur van beademing hebben onderzocht bij patiënten op de IC. De uitkomstmaat mortaliteit werd gerapporteerd in 2 studies (Goodacre, 2004; Porter, 2001), maar de patiëntenaantallen waren zo laag dat de bewijslast niet bepaald kon worden.
Goodacre (2014) rapporteerde ook over de uitkomstmaten IC opname, duur op de IC en de noodzaak tot beademing. Magnesiumsulfaat lijkt geen effect te hebben op deze uitkomstmaten, maar er is een grote onzekerheid van de resultaten door het lage aantal patiënten in de studie. Door deze imprecisie is de bewijslast gegradeerd als laag.
Toediening van magnesiumsulfaat bovenop standaardzorg lijkt ook geen effect te hebben op de noodzaak tot ziekenhuisopname. In totaal waren er 9 studies die rapporteerden over ziekenhuisopname, maar de resultaten waren imprecies en indirect. Dit is te wijten aan het lage aantal patiënten in de geïncludeerde studies, en het positieve en negatieve effect van toediening van magnesiumsulfaat in de individuele studies. Hierdoor werd de bewijslast gegradeerd tot zeer laag.
Ook ten aanzien van het risico op bijwerkingen lijkt magnesiumsulfaat geen effect te hebben. De bewijslast was gegradeerd als laag, door het lage aantal patiënten in de studie.
Magnesiumsulfaat geeft bronchodilatatie door inhibitie van calciumkanalen en remming parasympatische tonus, en werkt mogelijk ook anti-inflammatoir (Schivo, 2015). Hiermee zou een beperkte rol bij onvoldoende effect van de standaard farmacotherapeutische behandeling gerechtvaardigd zijn bij een longaanval astma met respiratoir falen. Het betreft dan een off-label toepassing. Magnesiumsulfaat i.v. mag vanwege het bijwerkingenprofiel alleen op een afdeling gegeven worden waar monitoring aanwezig is, dit zal in de praktijk betekenen alleen op een SEH of IC.
2. Salbutamol i.v./parentaal
Er werden geen studies gevonden die over het effect van salbutamol i.v. bovenop standaardzorg en de uitkomstmaten mortaliteit, IC opname, duur op de IC, noodzaak tot beademing, duur van de beademing en ziekenhuisopname rapporteerden.
Het effect van salbutamol i.v. bovenop standaardzorg op het ontstaan van bijwerkingen werd gerapporteerd door één studie (Cheong, 1998). Echter waren de patiëntenaantallen zo laag dat de kwaliteit van het bewijs niet bepaald kon worden.
Salbutamol i.v. geeft bronchodilatatie door relaxatie van de gladde bronchuswandspieren via de beta2-receptor. Als bronchusdilatatoren per verneveling tekortschieten kan overwogen om over te gaan op intraveneuze toediening en het vernevelen van continue naar zo nodig te zetten. Bij het opstarten van salbutamol iv is het belangrijk om de bijwerkingen die kunnen ontstaan mee te laten wegen, tachycardie en hypokaliaemie. De voorkeur is om deze behandeling alleen op een SEH of IC in te zetten en onder controle van kalium.
3. Biologicals met indicatie ernstig astma
Er zijn geen studies gevonden die het effect van biologicals bovenop standaardzorg op de gedefinieerde uitkomstmaten hebben onderzocht in patiënten met een longaanval astma. Door gebrek aan bewijs kon de GRADE methodiek niet toegepast worden en kunnen er geen conclusies gedaan worden op basis van beschikbare wetenschappelijke literatuur.
Biologicals met indicatie ernstig astma zijn geregistreerd voor ernstig astma dat niet goed onder controle is en waarmee longaanvallen astma kunnen worden voorkomen. Tijdens een longaanval astma zijn verschillende inflammatoire patronen aangetoond. De eosinofiele inflammatie in de luchtwegen lijkt op dat moment zeker niet altijd de belangrijkste component. Hiermee lijkt er geen theoretische gronden voor een behandeling met biologicals met indicatie ernstig astma tijdens een longaanval astma. Bij een opname ten gevolge van een longaanval astma behoort deze eventuele onderhoudsbehandeling wel voortgezet te worden.
4. Leukotriene receptor antagonisten
Er zijn geen studies gevonden die over het effect van leukotriene receptor antagonisten bovenop standaardzorg en de uitkomstmaten mortaliteit, IC opname, duur op de IC, noodzaak tot beademing en duur van beademing rapporteerden.
Het effect van leukotriene receptor antagonisten bovenop standaardzorg op de ziekenhuisopname was gerapporteerd door 1 studie (Silverman, 2014). In deze studie werd een groep patiënten behandeld met een lage dosering zafirlukast, een hoge dosering zafirlukast of placebo. Het is onduidelijk wat het effect is van deze behandeling op de ziekenhuisopname. De onduidelijkheid van de resultaten is te wijten aan de zeer ruime onzekerheidsmarges in de resultaten, die het gevolg zijn van een klein aantal deelnemers aan de geïncludeerde studies, en het gebruik van zowel een lage als hoge dosering zafirlukast. Dit zorgt ervoor dat zowel de imprecisie, als indirectheid van de resultaten leidde tot afwaardering binnen de GRADE-methodiek, met drie levels tot een zeer lage bewijskracht.
Drie (3) studies rapporteerden bijwerkingen (Camargo, 2010; Çýllý, 2003; Silverman, 2004). Er was veel heterogeniteit in de wijze van rapporteren, zo rapporteerde Camargo (2010) de incidentie van bijwerkingen in de interventie- en controlegroep, maar Silverman (2004) rapporteerde alleen hoeveel patiënten last had van hoofdpijn. Door deze heterogeniteit kon de bewijslast van de resultaten niet gegradeerd worden.
Leukotriene receptor antagonist heeft geen meerwaarde op basis van het werkingsmechanisme, geringe bronchodilatatie, en kan alleen per os gegeven worden.
Concluderend kan gesteld dat de vaak gebruikte aanvullende farmacotherapeutische behandeling magnesiumsulfaat iv en salbutamol iv als ook leukotriene receptor antagonist bij een longaanval astma op de SEH geen toegevoegde waarde hebben in de behandeling van een longaanval astma voor de door ons opgestelde cruciale parameters, naast de standaardbehandeling. Dit geldt voor magnesium sulfaat i.v. en salbutamol i.v. als ook voor leukotriene receptor antagonist.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Patiënten met een longaanval met respiratoir falen zullen zo snel mogelijk afname van hun klachten willen hebben en willen dat intubatie en mechanische ventilatie voorkomen kan worden. Dit maakt dat ze uitgebreide behandeling sneller accepteren. Voor de zorgverleners is het belangrijk om het bijwerkingenprofiel van deze medicatie goed te monitoren, zodat deze instabiele patiënt niet nog meer medische problemen zal krijgen.
Kosten (middelenbeslag)
Er zal geen verandering in kosten zijn, omdat de beschreven middelen in de praktijk al ingezet worden.
Aanvaardbaarheid, haalbaarheid en implementatie
Er zullen geen problemen zijn met aanvaardbarheid, haalbaarheid en implementatie, omdat de beschreven middelen in de praktijk al ingezet worden.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Magnesiumsulfaat iv heeft op grond van het werkingsmechanisme een geringe rol als aanvullende behandeling van een longaanval astma met respiratoir falen. Salbutamol iv heeft op grond van het werkingsmechanisme een geringe rol als aanvullende behandeling van een longaanval astma met respiratoir falen.
Onderbouwing
Achtergrond
Er is praktijkvariatie met betrekking tot de gebruikte medicatie tijdens een longaanval astma bij volwassenen. Magnesium i.v. wordt vaak toegepast (o.a. laag in kosten, weinig bijwerkingen) maar hoe sterk het bewijs ten aanzien van de werkzaamheid is, is de vraag.
Conclusies / Summary of Findings
- Magnesium sulphate i.v./parenterally
|
No GRADE |
Due to lack of data, it is not possible to draw a conclusion about the effect of magnesium sulphate in addition to usual care on mortality in patients with an acute asthma exacerbation |
|
Low GRADE |
The evidence suggests that magnesium sulphate in addition to usual care does not reduce or increase ICU admission of patients with acute asthma exacerbation
Source: Goodacre (2014) |
|
Low GRADE |
The evidence suggests that magnesium sulphate in addition to usual care does not reduce or increase the length of stay at the ICU of patients with acute asthma exacerbation
Source: Goodacre (2014) |
|
Low GRADE |
The evidence suggests that magnesium sulphate in addition to usual care does not reduce or increase the need for mechanical ventilation in patients with acute asthma exacerbation
Source: Goodacre (2014) |
|
No GRADE |
No evidence was found regarding the effect of magnesium sulphate in addition to usual care on the duration of mechanical ventilation in patients with an acute asthma exacerbation |
|
Very low GRADE |
The evidence is very uncertain regarding the effect of magnesium sulphate in addition to usual care on admission rates of patients with acute asthma exacerbation
Source: Bloch (1995), Boonyavorakul (2000), Bradshaw (2007), Goodacre (2014), Green (1992), Porter (2001), Silverman (2002), Singh (2008) and Skobeloff (1989) |
|
Low GRADE |
The evidence suggests that magnesium sulphate in addition to usual care does not reduce or increase the occurrence of adverse events in patients with acute asthma exacerbation
Source: Bradshaw (2007), Goodacre (2017) and Porter (2001) |
- Salbutamol i.v./parenterally
|
No GRADE |
No evidence was found regarding the effect of salbutamol in addition to usual care on:
in patients with an acute asthma exacerbation |
|
No GRADE |
Due to lack of data, it is not possible to draw a conclusion about the effect of salbutamol i.v. in addition to usual care on the occurrence of adverse events in patients with an acute asthma exacerbation |
- Biologics (benralizumab/mepolizumab, reslizumab; only in ‘biological-naive patients)
|
No GRADE |
No evidence was found regarding the effect of biologics in addition to usual care on:
in patients with an acute asthma exacerbation |
- Leukotriene receptor antagonists (montelukast, only in montulekast-naive patients)
|
No GRADE |
No evidence was found regarding the effect of leukotriene receptor antagonists in addition to usual care on:
in patients with an acute asthma exacerbation |
|
Very low GRADE |
The evidence is very uncertain regarding the effect of leukotriene receptor antagonists in addition to usual care on admission rates of patients with acute asthma exacerbation
Source: Silverman (2004) |
|
No GRADE |
Due to lack of data, it is not possible to draw a conclusion about the effect of leukotriene receptor antagonists in addition to usual care on the occurrence of adverse events in patients with an acute asthma exacerbation |
Samenvatting literatuur
1. Magnesium sulphate i.v./parenterally
Description of studies
Bloch (1995) performed a randomized controlled trial to compare whether i.v. magnesium sulphate in addition to usual care can improve pulmonary function and decrease admission rate in patients presenting to the ED with exacerbations of asthma. A total of 135 patients were included. All patients received usual care upon presentation. Thirty (30) minutes after entry, patients either received 2 g of magnesium sulphate i.v. (n=67) or placebo (n=68). Subgroup analysis was conducted in patients with either severe asthma, or moderate asthma, based on their initial FEV1. More details about the medication and asthma severity are shown in Table 1. Relevant outcomes were admission rates (stratified per asthma severity) and the occurrence of adverse events (overall).
Boonyavorakul (2000) performed a randomized controlled trial with the aim to assess whether i.v. magnesium sulphate in addition to usual care improves admission rate or severity score in acute severe asthma patients. Thirty-three (33) patients who presented themselves to the ED were included and randomized to either receive 2 g of i.v. magnesium sulphate (n=17) or placebo (n=16). Refer to Table 1 for more information on the medication and asthma severity. Relevant outcome was the admission rate.
Bradshaw (2008) conducted a randomized controlled trial to investigate whether the addition of magnesium sulphate to usual care improves the outcome in acute asthma. A total of 120 patients were included in the study and randomized to receive either 1.2 g of magnesium sulphate (n=62) or placebo (n=67). Subgroup analysis were conducted for patients with life-threatening asthma, severe asthma or moderate asthma. More details about the medication and asthma severity are shown in Table 1. Relevant outcomes were admission rates (stratified per asthma severity) and the occurrence of adverse events (overall).
Goodacre (2014) performed a randomized controlled trial to determine whether magnesium sulphate in addition to usual care improves symptoms of breathlessness and reduces the need for hospital admission in adults with acute severe asthma. Patients with acute severe asthma were included in the study, but patients with life-threatening features were excluded. Patients were randomized to receive 2 g magnesium sulphate (n=394) or placebo (n=358) (refer to Table 1 for more information about the medication and asthma severity). Relevant outcomes were mortality, admission rate, ICU admission, length of stay at the ICU, need for mechanical ventilation and the occurrence of (serious) adverse events.
Green (1992) conducted a prospective randomized clinical trial to evaluate the efficacy of magnesium sulphate to usual care in patients with acute asthma. A total of 120 patients with acute asthma and who were unresponsive to a single albuterol treatment and randomized to receive 2 g magnesium sulphate (n=58) or placebo (n=62). More details about the medication and asthma severity are shown in Table 1. Relevant outcome was admission rate.
Porter (2001) performed a randomized controlled trial to evaluate the effect of magnesium sulphate in addition to usual care in adult patients with a moderate to severe exacerbation. A total of 44 patients presenting themselves at the ED were included and randomized to receive either 2 g of magnesium sulphate (n=18) or placebo (n=22). More details about the medication and asthma severity are shown in Table 1. Relevant outcomes were mortality, hospital admission rate and the occurrence of adverse events.
Silverman (2002) conducted a randomized controlled trial to test the hypothesis that administration of magnesium sulphate in addition to usual care improves pulmonary function in patients with acute severe asthma. Patients with acute severe asthma who presented themselves at the ED (n=248) were included in the study. Thirty (30) minutes after arrival at the ED, patients either received 2 g of magnesium sulphate (n=122) or placebo (n=126). Refer to Table 1 for more information on the medication and asthma severity. Relevant outcome was hospital admission rate.
Singh (2008) performed a randomized controlled trial to evaluate whether the addition of magnesium sulphate to usual care has beneficial effects in patients with severe exacerbations of asthma. Patients who presented themselves at the ED were included and randomized to receive either 2 g of magnesium sulphate or placebo 30 minutes after arrival at the ED (see Table 1 for more information on the medication and asthma severity). Relevant outcomes were hospital admission rate and the occurrence of adverse events.
Skobeloff (1989) conducted a randomized controlled trial to evaluate the effect of magnesium sulphate in addition to usual care on asthma in moderate to severe asthma patients who may be characterized as poor responders. Patients suffering from acute moderate to severe asthma exacerbations were treated at the ED with conventional β-agonist therapy. A total of 38 patients who failed to produce significant improvement in peak expiratory flow rate were randomized to receive 1.2 g of magnesium sulphate (n=19) or placebo (n=19). Refer to Table 1 for more information on the medication and asthma severity. Relevant outcome was hospital admission rate.
Table 1. Medication details and asthma severity – Magnesium sulphate
|
Author, year |
Usual care |
Magnesium sulphate regimen |
Control regimen |
Asthma severity |
|
Bloch, 1995 |
Albuterol (2.5 mg in 2.5 mL saline solution by high-flow nebulizer). Patients with FEV1<40% predicted, or who received oral steroids within the past 6 months: 125 mg of methylprednisolone i.v. within 30 min of presentation |
2 g MgSO4 i.v. over 20 minutes
|
Placebo in 50 mL saline i.v. over 20 minutes |
Moderate to severe |
|
Boonyavorakul, 2000 |
5 mg i.v. dexamethasone 2.5 mg nebulized salbutamol at 0, 20, 40, 60 min Oxygen mask if necessary |
2 g MgSO4 in 50 mL of 0.9% saline
|
2 mL of sterile water in 50 mL of 0.9% saline |
Severe |
|
Bradshaw, 2008 |
35% of oxygen 5 mg nebulised salbutamol 500 mcg nebulised ipratropium 200 mg i.v. hydrocortisone |
1.2 g MgSO4 in 50 mL saline i.v. over 15 minutes |
50 mL saline i.v. over 15 minutes |
Life-threatening, severe and moderate |
|
Goodacre, 2014 |
Oxygen Nebulised salbutamol Nebulised ipratropium bromide Oral prednisolone Followed by up to 5 mg salbutamol added to each trial nebuliser. Three 7.5-mL vials of 0.9% saline nebulised at 20-minutes intervals |
i.v. MgSO4 2 gram (8 mmol) in 100 mL saline given over 20 minutes
|
i.v. 100 mL saline given over 20 minutes
|
Severe |
|
Green, 1992 |
Treatment with oxygen and inhaled albuterol, administered either as a nebulized aerosol (0.5 mL in 2.5 mL saline) or through supervised inhalations of a metred-dose inhaler with spacer titrated to a therapeutic effective dose. If no improvement was observed, patient received 125 mg i.v. methylprednisolone |
2 g MgSO4 diluted in 50 mL D5W i.v. over 20 minutes
|
No MgSO4 |
Acute asthma (severity not reported) |
|
Porter, 2001 |
2.5 mg albuterol sulphate via nebulizer 125 mg methylprednisolone i.v. |
2 g MgSO4 in 50 mL saline i.v. over 20 minutes |
50 mL saline i.v. over 20 minutes
|
Moderate to severe |
|
Silverman, 2002 |
0.5 mL of 0.5% albuterol (2.5 mg) administered via wet nebulizer with 100% oxygen 125 mg of i.v. methylprednisolone. Albuterol was readministered 30, 60, 120 and 180 min after ED arrival |
2 g MgSO4 in 50 mL saline i.v. over 10-15 minutes
|
Placebo in 50 mL saline i.v. over 10-15 minutes
|
Severe |
|
Singh, 2008 |
Nebulising solution consisting of 1 mL of 2.5% salbutamol (2.5 mg) 250 mg of 1.5 mL of ipratropium bromide administered in 2.5 mL of saline aerosolized via a wet nebuliser with 100% oxygen at 0, 20, 40 minutes |
2 g MgSO4 in 250 mL saline i.v. over 20 minutes
|
Placebo in 250 mL saline i.v. over 20 minutes
|
Severe |
|
Skobeloff, 1989 |
Nebulized treatment of 0.3 mL metaproterenol sulphate in 3.0 mL saline, or 0.5 mL albuterol sulphate in 2.5 mL saline 125 mg methylprednisolone sodium succinate i.v.. If necessary, theophylline was given i.v.. |
1.2 g MgSO4 in 50 mL saline i.v. over 20 minutes
|
50 mL saline i.v. over 20 minutes
|
Moderate to severe |
Results
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care - mortality
Goodacre, 2014 reported the percentage of patients who died, which was 1 patient in both the intervention group and control group. The risk ratio was 0.91 (95% CI 0.06 to 14.47), the confidence interval includes the minimal clinical important difference, as well as the null effect. Porter, 2001 also reported mortality rates, which was 0% in both groups.
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care – ICU admission
Goodacre, 2014 reported the percentage of patients who were admitted to the ICU. In the intervention group, 3% (11/394). In the control group, 1% (5/358) was admitted to the ICU. The risk ratio was 2.00 (95% CI 0.70 to 5.70), the confidence interval includes the minimal clinical important difference, as well as the null effect.
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care –
length of stay at the ICU
Goodacre, 2014 reported the mean number of days that patients, who were admitted to the ICU, stayed at the ICU. In the intervention group, patients spend on average 3.1 (standard deviation 5.0) days at the ICU, while patients in the control group stayed on average 2.9 (standard deviation 4.6) days at the ICU. The mean difference was 0.20 (95% CI -0.49 to 0.89), the confidence interval includes the minimal clinical important difference, as well as the null effect.
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care –need for mechanical ventilation
Goodacre, 2014 reported the percentage of patients who required mechanical ventilation. In the intervention and control group, respectively 2% (6/394) and 1% (4/358) patients required mechanical ventilation. The risk ratio was 1.36 (95% CI 0.39 to 4.79), the confidence interval includes the minimal clinical important difference, as well as the null effect.
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care – duration of mechanical ventilation
No studies reported on the outcome measure duration of mechanical ventilation.
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care – admission rate
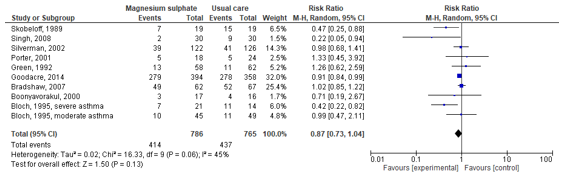
Bloch (1995), Boonyavorakul (2000), Bradshaw (2007), Goodacre (2014), Green (1992), Porter (2001), Silverman (2002), Singh (2008) and Skobeloff (1989) reported admission rates. The study by Bloch (1995) stratified the admission rates for severe and moderate asthma. These studies were used to calculate a pooled risk ratio (see Figure 1). The pooled risk ratio was 0.87 (95% CI: 0.73 to 1.04). The confidence interval includes the minimal clinical important difference, as well as the null effect.
Figure 1. Forest plot to compare the effect of magnesium sulfate in addition to usual care versus usual care alone on the admission rate
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care – adverse events
Bloch (1995) reported on adverse events in patients treated with magnesium sulphate. There were no life-threatening side effects, and minor side effects were observed in 58% of patients.
Singh (2008) reported on the occurrence of specific adverse events. Patients treated with magnesium sulphate suffered from anxiety (intervention: 8/30; placebo: 10/30), palpitations (intervention: 4/30; placebo: 6/30), tremors (intervention: 15/30; placebo: 14/30), headache (intervention: 6/30; placebo: 5/30), dry mouth (intervention: 16/30; placebo: 17/30), and non-specific nausea (intervention: 2/30; placebo: 1/30). There was no statistically significant difference in any of these side effects.
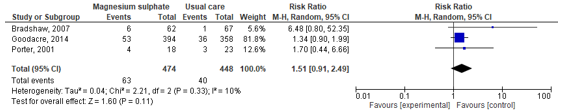
Bradshaw (2007), Goodacre (2017) and Porter (2001) reported on the occurrence of adverse events in both the intervention and placebo groups. These studies were used to calculate the pooled risk ratio. The pooled risk ratio was 1.51 (95% CI: 0.91 to 2.49). The confidence interval includes the minimal clinical important difference, as well as the null effect.
Figure 2. Forest plot to compare the effect of magnesium sulphate in addition to usual care versus usual care alone on the occurrence of adverse events
Level of evidence of the literature
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care - mortality
There were insufficient cases to assess the level of evidence of the literature. Therefore, the level of evidence regarding the outcome mortality was ungraded due to lack of data.
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care – ICU admission
The level of evidence regarding the outcome measure ICU admission started as High (randomized controlled trial) and was downgraded by two levels to Low because of the imprecision (confidence intervals crossed clinically important difference and low numbers of patients).
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care –
length of stay at the ICU
The level of evidence regarding the outcome measure length of stay at the ICU started as High (randomized controlled trial) and was downgraded by two levels to Low because of the imprecision (confidence intervals crossed clinically important difference on detrimental as well as beneficial effect directions).
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care –need for mechanical ventilation
The level of evidence regarding the outcome measure need for mechanical ventilation started as High (randomized controlled trial) and was downgraded by two levels to Low because of the imprecision (confidence intervals crossed clinically important difference and low numbers of patients).
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care – duration of mechanical ventilation
The level of evidence regarding the outcome duration of mechanical ventilation was ungraded due to lack of data.
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care – admission rate
The level of evidence regarding the outcome measure admission rate started as High (randomized clinical trial) and was downgraded by three levels to Very low because of the imprecision (two levels: confidence intervals crossed clinically important difference and low numbers of patients) and the inconsistency (one level: effects of individual studies are both in the detrimental as well as beneficial effect directions).
The (un)beneficial effects of administrating magnesium sulphate in addition to usual care – adverse events
The level of evidence regarding the outcome measure occurrence of adverse events started as High (randomized controlled trial) and was downgraded by two levels to Low because of the imprecision (confidence intervals crossed clinically important difference and low numbers of patients).
2. Salbutamol i.v./parenterally
Description of studies
Cheong (1998) performed a randomized trial to investigate whether salbutamol is more effective in treating severe asthma when given intravenously or by inhalation. A total of 76 patients were included. All patients received usual care upon admission. Thirty (30) minutes after admission, patients received either 12.5 µg/min salbutamol i.v. (n=37) or 5 mg nebulized salbutamol (n=34). More details about the medication and asthma severity are shown in Table 2. Relevant outcomes were occurrence of adverse outcomes (tachycardia).
Table 2. Medication details and asthma severity – Salbutamol i.v.
|
Author, year |
Usual care |
Salbutamol i.v. regimen |
Control regimen |
Asthma severity |
|
Cheong, 1998 |
At admission, 5 mg nebulised salbutamol with a Hudson nebuliser driven by oxygen at 6 l/min and an intravenous bolus of 200 mg hydrocortisone. Patients with a peak flow rate below half of the predicted value 30 minutes after nebulised treatment were randomised. No other bronchodilator was used. All were given continuous 35% inspired oxygen.
|
Intravenous salbutamol as a continuous infusion in a dose of 12.5 µg/min starting 30 minutes after admission and lasting for a further four hours. 2g potassium was added to the infusion (because of the occurrence of hypokalemia). |
5 mg nebulized salbutamol 30 minutes after the first treatment on admission and again two hours later. |
Acute severe asthma (peak expiratory flow rate <50% of predicted). |
Results
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care - mortality
No studies reported on the outcome measure mortality.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care – ICU admission
No studies reported on the outcome measure ICU admission.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care –
length of stay at the ICU
No studies reported on the outcome measure length of stay at the ICU.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care –need for mechanical ventilation
No studies reported on the outcome measure need for mechanical ventilation.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care – duration of mechanical ventilation
No studies reported on the outcome measure duration of mechanical ventilation.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care – admission rate
No studies reported on the outcome measure admission rate.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care – adverse events
Cheong, 1998 reported the percentage of patients who were withdrawn due to tachycardia, which was 5% (n=2) patients in the intervention group. They also reported that tachycardia was prominent in the intervention group.
Level of evidence of the literature
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care - mortality
The level of evidence regarding the outcome mortality was ungraded due to lack of data.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care – ICU admission
The level of evidence regarding the outcome ICU admission was ungraded due to lack of data.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care – length of stay at the ICU
The level of evidence regarding the outcome length of stay at the ICU was ungraded due to lack of data.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care –need for mechanical ventilation
The level of evidence regarding the outcome need for mechanical ventilation was ungraded due to lack of data.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care – duration of mechanical ventilation
The level of evidence regarding the outcome duration of mechanical ventilation was ungraded due to lack of data.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care – admission rate
The level of evidence regarding the outcome admission rate was ungraded due to lack of data.
The (un)beneficial effects of administrating salbutamol i.v. in addition to usual care – adverse events
There were insufficient cases to assess the level of evidence of the literature. Therefore, the level of evidence regarding the outcome adverse events was ungraded due to lack of data.
3. Biologics (benralizumab/mepolizumab, reslizumab; only in ‘biological-naive patients)
No studies were found that reported on the use of biologics in addition to usual care in patients with acute asthma exacerbation.
4. Leukotriene receptor antagonists (montulekast, only in montulekast-naïve patients)
Description of studies
Camargo, 2010 performed a randomized controlled trial to evaluate whether montulekast treatment may complement usual care in patients with acute asthma exacerbation. A total of 583 patients were randomized to receive montulekast (n=291) or placebo (292) in addition to usual care (refer to Table 3 for more details on medication and asthma severity). Relevant outcome was the occurrence of adverse events.
Çýllý, 2003 performed a randomized controlled trial to compare the effects of oral montulekast in addition to standard care in patients with asthma exacerbations. Patients were randomized to montulekast in addition to usual care (intervention; n=23), usual care only (control; n=22) or usual care without steroids (n=25). Only the first 2 study groups were deemed relevant for this analysis. Refer to Table 3 for more information about the medication and asthma severity. Relevant outcome was the occurrence of adverse events.
Silverman, 2004 conducted a randomized controlled trial to assess whether adding zafirlukast therapy to usual care further improves asthma in patients with acute asthma in the ED. A total of 641 patients were randomized to receive 160 mg zafirlukast (treatment 1; n=162)), 20 mg zafirlukast (treatment 2; n=158) or placebo (n=321). See Table 3 for more information on the medication and asthma severity. Relevant outcomes were admission rate (defined as extended care) and the occurrence of adverse events.
Table 3. Medication details and asthma severity – Leukotriene receptor antagonists
|
Author, year |
Usual care |
Leukotriene receptor antagonist regimen |
Control regimen |
Asthma severity |
|
Camargo, 2010 |
Oxygen Inhaled short-acting β-agonist (2.5-5mg in 3 mL saline every 20 min) as needed Inhaled ipratropium or nebulized ipratropium as needed After intervention or control treatment: Systemic corticosteroids (60 mg prednisone or 50 mg prednisolone orally) Continued baseline medication |
i.v. administration (manual bolus over 2-5 min) of 7 mg montulekast (within 60 min after initiation of standard treatment).
|
i.v. administration (manual bolus over 2-5 min) of placebo.
|
Acute asthma (severity not reported) |
|
Çýllý, 2003 |
1 mg/kg prednisolone i.v. 3 aerosol treatments of 0.5 mg terbutaline sulphate as dry powder inhaler separated by 20 min intervals |
10 mg tablet oral montulekast
|
No additional treatment/ placebo |
Acute asthma (severity not reported) |
|
Silverman, 2004 |
ED entry: nebulized albuterol (2.5 mg). 25 min after ED entry: patients with FEV1<70% predicted were randomized. 60 mg po dose of prednisone, nebulized albuterol. Additional albuterol at 60, 120 and 180 min after ED entry. |
1: 160 mg zafirlukast 2: 20 mg zafirlukast
|
Placebo |
Acute asthma (severity not reported) |
Results
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care - mortality
No studies reported on the outcome measure mortality.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care – ICU admission
No studies reported on the outcome measure ICU admission.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care –
length of stay at the ICU
No studies reported on the outcome measure length of stay at the ICU.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care –need for mechanical ventilation
No studies reported on the outcome measure need for mechanical ventilation.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care – duration of mechanical ventilation
No studies reported on the outcome measure duration of mechanical ventilation.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care – admission rate
Silverman (2014) reported the admission rates (defined as extended care by the authors) for both interventions and the control group. Admission rate was 9.9% (16/162) in intervention 1 group (160 mg of zafirlukast), 16.5% (26/158) in intervention 2 group (20 mg of zafirlukast) and 15% (48/321) in the control group. The risk ratio for intervention 1 vs control was 0.66 (95% CI 0.39 to 1.13) and for intervention 2 vs control 1.10 (95% CI 0.71 to 1.70). The overall risk ratio for both interventions combined vs control was 0.87 (95% CI 0.53 to 1.44), and the confidence interval includes the minimal clinical important difference, as well as the null effect.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care – adverse events
Camargo (2010), Çýllý (2003) and Silverman (2004) reported on the occurrence of adverse events. Camargo, 2010, reported that in 19.6% of patients in the intervention group, and 20.2% of patients in the control group, 1 or more adverse events occurred. Serious adverse events occurred in 9.6% and 8.9% of patients in the intervention and control group, respectively. Drug-related adverse events were not observed, and adverse events leading to discontinuation from the study was only observed in 0.3% of patients in the control group.
Çýllý, 2003 did not observe any severe adverse events. The most frequent adverse events included malaise (8%) and headache (6%), which occurred in the intervention group.
Silverman, 2004 reported on the occurrence of headaches, which was 1% (2/162) in intervention group 1, 1% (2/158) in intervention group 2 and 2% (5/321) in the control group.
Level of evidence of the literature
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care - mortality
The level of evidence regarding the outcome mortality was ungraded due to lack of data.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care – ICU admission
The level of evidence regarding the outcome ICU admission was ungraded due to lack of data.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care – length of stay at the ICU
The level of evidence regarding the outcome length of stay at the ICU was ungraded due to lack of data.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care –need for mechanical ventilation
The level of evidence regarding the outcome need for mechanical ventilation was ungraded due to lack of data.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care – duration of mechanical ventilation
The level of evidence regarding the outcome duration of mechanical ventilation was ungraded due to lack of data.
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care – admission rate
The level of evidence regarding the outcome measure admission rate started as High (randomized controlled trial) and was downgraded by three levels to Very low because of the imprecision (two levels: confidence intervals crossed clinically important difference and low numbers of patients) and indirectness (pooled data of a very high dose and low dose of zafirlukast).
The (un)beneficial effects of administrating leukotriene receptor antagonists in addition to usual care – adverse events
The level of evidence regarding the outcome adverse events was ungraded due to lack of data.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
Which medication has beneficial effects in addition to standard treatment in patients with acute asthma exacerbation?
P (Patients) = (young) adults (above 16 years of age) with an asthma exacerbation that requires presentation at the ED
I (Intervention) = usual care (refer to C) + add on:
- Magnesium sulphate i.v./parenterally
- Salbutamol i.v./parenterally
- Biologics (benralizumab/mepolizumab, reslizumab (only in ‘biological-naive patients))
- Leukotriene receptor antagonists (montulekast; only in montulekast-naïve patients)
Time frame of intervention and effect of intervention: 72 hours
C (Comparison) = usual care (nebulized treatment or dose aerosol with a bronchodilator and systemic administration of steroids)
O (Outcomes) = mortality (during admission and after 30 days), ICU admission, length of stay at the ICU, need for mechanical ventilation, duration of mechanical ventilation, admission rate, adverse events
Relevant outcome measures
A priori, the working group did not define the outcome measures listed above but used the definitions used in the studies.
The working group defined the following minimally clinically important differences:
Mortality: reduction of 10%
ICU admission: reduction of 10%
Length of stay at the ICU: reduction of 0.5 days
Need for mechanical ventilation: reduction of 10%
Adverse events: reduction of 25%
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until August 31, 2022. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 37 hits. Studies were selected based on the following criteria: systematic review of randomised controlled trials, randomised controlled trial, relevant to PICO, article in English or Dutch, no conference abstract. Fifteen (15) studies were initially selected based on title and abstract screening. After reading the full text, 3 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 12 studies were included.
Results
Twelve (12) studies were included in the analysis of the literature. Of these studies, 9 studies reported on the use of magnesium sulphate and 3 studies reported on the use of leukotriene receptor antagonists. No studies were found that reported on the use of biologicals or salbutamol i.v.. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Bloch, H., Silverman, R., Mancherje, N., Grant, S., Jagminas, L., & Scharf, S. M. (1995). Intravenous magnesium sulfate as an adjunct in the treatment of acute asthma. Chest, 107(6), 1576-1581. https://doi.org/10.1378/chest.107.6.1576
- Boonyavorakul, C., Thakkinstian, A., & Charoenpan, P. (2000). Intravenous magnesium sulfate in acute severe asthma. Respirology (Carlton, Vic.), 5(3), 221-225. https://doi.org/10.1046/j.1440-1843.2000.00252.x
- Bradshaw, T. A., Matusiewicz, S. P., Crompton, G. K., Innes, J. A., & Greening, A. P. (2008). Intravenous magnesium sulphate provides no additive benefit to standard management in acute asthma. Respiratory medicine, 102(1), 143-149. https://doi.org/10.1016/j.rmed.2007.07.022
- Camargo, C. A., Jr, Gurner, D. M., Smithline, H. A., Chapela, R., Fabbri, L. M., Green, S. A., Malice, M. P., Legrand, C., Dass, S. B., Knorr, B. A., & Reiss, T. F. (2010). A randomized placebo-controlled study of intravenous montelukast for the treatment of acute asthma. The Journal of allergy and clinical immunology, 125(2), 374-380. https://doi.org/10.1016/j.jaci.2009.11.015
- Cheong, B., Reynolds, S. R., Rajan, G., & Ward, M. J. (1988). Intravenous beta agonist in severe acute asthma. BMJ (Clinical research ed.), 297(6646), 448-450. https://doi-org.saz.idm.oclc.org/10.1136/bmj.297.6646.448
- Cýllý, A., Kara, A., Ozdemir, T., O?ü?, C., & Gülkesen, K. H. (2003). Effects of oral montelukast on airway function in acute asthma. Respiratory medicine, 97(5), 533-536. https://doi.org/10.1053/rmed.2003.1479
- Goodacre, S., Cohen, J., Bradburn, M., Stevens, J., Gray, A., Benger, J., Coats, T., & 3Mg Research Team (2014). The 3Mg trial: a randomised controlled trial of intravenous or nebulised magnesium sulphate versus placebo in adults with acute severe asthma. Health technology assessment (Winchester, England), 18(22), 1-168. https://doi.org/10.3310/hta18220
- Green, S. M., & Rothrock, S. G. (1992). Intravenous magnesium for acute asthma: failure to decrease emergency treatment duration or need for hospitalization. Annals of emergency medicine, 21(3), 260-265. https://doi.org/10.1016/s0196-0644(05)80885-6
- Porter, R. S., Nester, Braitman, L. E., Geary, U., & Dalsey, W. C. (2001). Intravenous magnesium is ineffective in adult asthma, a randomized trial. European journal of emergency medicine : official journal of the European Society for Emergency Medicine, 8(1), 9-15. https://doi.org/10.1097/00063110-200103000-00003
- Schivo M, Phan C, Louie S, Harper RW. Critical asthma syndrome in the ICU. Clin Rev Allergy Immunol. 2015 Feb;48(1):31-44. PMID: 25759905.
- Silverman, R. A., Osborn, H., Runge, J., Gallagher, E. J., Chiang, W., Feldman, J., Gaeta, T., Freeman, K., Levin, B., Mancherje, N., Scharf, S., & Acute Asthma/Magnesium Study Group (2002). IV magnesium sulfate in the treatment of acute severe asthma: a multicenter randomized controlled trial. Chest, 122(2), 489-497. https://doi.org/10.1378/chest.122.2.489
- Silverman, R. A., Nowak, R. M., Korenblat, P. E., Skobeloff, E., Chen, Y., Bonuccelli, C. M., Miller, C. J., & Simonson, S. G. (2004). Zafirlukast treatment for acute asthma: evaluation in a randomized, double-blind, multicenter trial. Chest, 126(5), 1480-1489. https://doi.org/10.1378/chest.126.5.1480
- Singh, A. K., Gaur, S., & Kumar, R. (2008). A randomized controlled trial of intravenous magnesium sulphate as an adjunct to standard therapy in acute severe asthma. Iranian journal of allergy, asthma, and immunology, 7(4), 221-229.
- Skobeloff, E. M., Spivey, W. H., McNamara, R. M., & Greenspon, L. (1989). Intravenous magnesium sulfate for the treatment of acute asthma in the emergency department. JAMA, 262(9), 1210-1213.
Evidence tabellen
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])
Research question: Which medication has beneficial effects in addition to standard treatment in patients with acute asthma exacerbation?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Magnesium sulphate |
|||||||
|
Bloch, 1995 |
Type of study: RCT
Setting and country: USA
Funding: Reported
Conflicts of interest: Not reported |
Inclusion criteria: -Aged 18-65 years -Presenting with acute asthma at the ED
Exclusion criteria: -History of congestive heart failure -Suffering from diabetes mellitus, angina, chronic renal insufficiency, temperature >38°C, pneumonia or pregnant -Patients requiring intubation -Unable to perform spirometry -Unable to give informed consent -Patients with forced expired volume in 1 s (FEV1) was greater or equal to 75% predicted on ED presentation or after a single albuterol treatment
N total at baseline: Intervention: 67 (severe asthma: n=21) Control: 68 (severe asthma: n=14)
Important prognostic factors2: Age ± SD: Intervention: 36 ± 12.7 (severe: 34 ± 13.4) Control: 38.6 ± 13.2 (severe: 42 ± 14)
Sex: Intervention: 27% M (severe: 19% M) Control: 29% M (severe: 43% M)
Groups comparable at baseline? Yes |
Baseline medication: Inhaled albuterol treatment (2.5 mg in 2.5 mL saline solution by high-flow nebulizer). Patients with FEV1 < 40% predicted, or who received oral steroids within the past 6 months, received 125 mg of methylprednisolone IV within 30 minutes of presentation
Intervention: 2 g MgSO4 in IV over 20 minutes
|
Baseline medication: Inhaled albuterol treatment (2.5 mg in 2.5 mL saline solution by high-flow nebulizer). Patients with FEV1 < 40% predicted, or who received oral steroids within the past 6 months, received 125 mg of methylprednisolone IV within 30 minutes of presentation
Control: Placebo in 50 mL saline IV over 20 minutes |
Length of follow-up: 1 week
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: 149 patients were originally randomized (distribution unknown). 12 patients were enrolled more than once (first visit was used for analysis), 1 patient had irreversible airway disease, 1 patient had congestive heart failure, 4 patients were included as ITT because the study protocol was violated, in 6 patients baseline FEV1 was unavailable.
|
Hospital admission - severe asthma: Intervention: 33.3% (7/21) Control: 78.6% (11/14) P=0.009
Hospital admission - moderate asthma: Intervention: 22.2 (10/45) Control: 22.4% (11/49) P=0.98
Adverse effects: Intervention: 58% of patients suffered from mild side effects: flushing, mild fatigue, burning at the IV stie and transient urticaria in the upper extremities Control: no side effects reported |
No comments |
|
Boonyavorakul, 2000 |
Type of study: RCT
Setting and country: Thailand
Funding and conflicts of interest: Not reported |
Inclusion criteria: -Aged 15-65 years -Diagnosed as acute severe asthma
Exclusion criteria: -History of heart disease, hypertension, diabetes mellitus, chronic renal disease, infection, (suspected) pregnancy and FISCHL index of 4 or more
N total at baseline: Intervention: 17 Control: 16
Important prognostic factors2: Age ± SD: Intervention: 42.88 ± 2.99 Control: 35 ± 3.03
Sex: Intervention: 11.76% M Control: 12.50% M
Groups comparable at baseline? Yes |
Baseline medication: 5 mg IV dexamethasone, 2.5 mg nebulized salbutamol at 0, 20, 40, 60 min, and oxygen mask if necessary
Intervention: 2 g MgSO4 in 50 mL of 0.9% saline
|
Baseline medication: 5 mg IV dexamethasone, 2.5 mg nebulized salbutamol at 0, 20, 40, 60 min, and oxygen mask if necessary
Control: 2 mL of sterile water in 50 mL of 0.9% saline |
Length of follow-up: 240 min after receipt of treatment
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: Not applicable
|
Hospital admission: Intervention: 17.65% (3/17) Control: 25% (4/16) RR: 0.71 95% CI [0.19-2.67]
|
No comments |
|
Bradshaw, 2007 |
Type of study: RCT
Setting and country: UK
Funding and conflicts of interest: Not reported |
Inclusion criteria: -Aged >16 years -Past diagnosis of asthma -PEF<75% predicted indicating a moderate to severe attack
Exclusion criteria: -COPD or other chronic lung disease, pneumonia, congestive cardiac failure, coronary artery disease, renal insufficiency, hypertension treated with medication, pregnancy -Inability to perform peak flow measurements
N total at baseline: Intervention: Overall (62), life-threatening (12), severe (30), moderate (20) Control: Overall (67), life-threatening (17), severe (31), moderate (19)
Important prognostic factors2: Age ± SD: Intervention: Overall (36, 18-73), life-threatening (38.1 ± 16.8), severe (34.8 ± 11.2), moderate (35.2 ± 14.3) Control: : Overall (38.8, 17-73), life-threatening (34.5 ± 14.1), severe (42.8 ± 17.1), moderate (36.2 ± 13.1)
Sex: Intervention: Overall (39% M), life-threatening (33% M), severe (43% M), moderate (35% M) Control: Overall (45% M), life-threatening (29% M), severe (61% M), moderate (32% M)
Groups comparable at baseline? Yes |
Baseline medication: 35% of oxygen, 5 mg nebulised salbutamol, 500 mcg nebulised ipratropium and 200 mg IV hydrocortisone
Intervention: 1.2 g MgSO4 in 50 mL saline IV over 15 minutes
|
Baseline medication: 35% of oxygen, 5 mg nebulised salbutamol, 500 mcg nebulised ipratropium and 200 mg IV hydrocortisone
Control: 50 mL saline IV over 15 minutes |
Length of follow-up: 60 min after receipt of treatment
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: 150 patients were recruited into the study and 129 were included in the final analysis. Patients were excluded due to subsequent recognition of PEF >75% predicted or missing record data.
|
Hospital admission - overall: Intervention: 79% (49/62) Control: 78% (52/67) P=0.98
Hospital admission – life-threatening: Intervention: 100% (12/12) Control: 88% (15/17) P=0.50
Hospital admission - severe: Intervention: 70% (21/30) Control: 84% (26/31) P=0.32
Hospital admission - moderate: Intervention: 80% (16/20) Control: 58% (11/19) P=0.18
Adverse events – overall: Intervention: 5/62 patients suffered from minor side effects (headache, flushing and dizziness) Control: one patient suffered from flushing |
No comments |
|
Goodacre, 2014 |
Type of study: RCT
Setting and country: UK
Funding and conflicts of interest: Both reported |
Inclusion criteria: -Aged >16 years -Presenting at the ED with acute severe asthma (PEFR<50% best or predicted, >25 breaths per minute, heart rate > 110 bpm or inability to complete sentences in one breath
Exclusion criteria: -Patients with life-threatening features -Patients with a contraindication to either nebulised or IV magnesium sulphate (pregnancy, hepatic or renal failure, heart block or known hypermagnesemia) -Unable to provide written or oral consent -Previous participants in the 3Mg trial -Patients who had received IV or nebulised MgSO4 in the 24 hours prior to attendance at the ED
N total at baseline: Intervention: 394 Control: 358
Important prognostic factors2: Age ± SD: Intervention: 35.6 ± 13.1 Control: 36.4 ± 14.1
Sex: Intervention: 29% M Control: 30% M
Groups comparable at baseline? Yes |
Baseline medication: Oxygen, nebulised salbutamol, nebulised ipratropium bromide and oral prednisolone administered during recruitment, followed by up to 5 mg salbutamol added to each trial nebuliser. Three 7.5-mL vials of 0.9% saline nebulised at 20-minutes intervals
Intervention: IV MgSO4 2 gram (8 mmol) in 100 mL saline given over 20 minutes
|
Baseline medication: Oxygen, nebulised salbutamol, nebulised ipratropium bromide and oral prednisolone administered during recruitment, followed by up to 5 mg salbutamol added to each trial nebuliser. Three 7.5-mL vials of 0.9% saline nebulised at 20-minutes intervals
Control: IV 100 mL saline given over 20 minutes
|
Length of follow-up: 30 days
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: 1109 patients were recruited, of which 25 either withdrew or were recruited in error (protocol violations).
|
Mortality: Intervention: 0.3% (1/394) Control: 0.3% (1/358)
Hospital admission: Intervention: 71% (279/394) Control: 78% (278/358)
ICU admission: Intervention: 3% (11/394) Control: 1% (5/358)
Days on ICU: Intervention: 3.1 ± 5.0 Control: 2.9 ± 3.9
Mechanical ventilation: Intervention: 2% (6/394) Control: 1% (4/358)
Adverse events: Intervention: 13.4% (53/394) Control: 10.1% (36/358)
Serious adverse events: Intervention: 11.4% (45/394) Control: 7.8% (28/358) |
No comments |
|
Green, 1992 |
Type of study: RCT
Setting and country: USA
Funding and conflicts of interest: Not reported
|
Inclusion criteria: -Aged 18-65 years -Presenting at the ED with acute asthma
Exclusion criteria: -Patients with atherosclerotic heart disease, angina, chest pain, uncontrolled hypertension, congestive heart failure, heart block, metastatic cancer, renal disease, temperature above 38.3°C, systolic blood pressure below 120 mm Hg or pregnancy -Patients with radiographic evidence of confounding pulmonary disease -Patients requiring mechanical ventilation at any point during their ED visit or subsequent hospitalization
N total at baseline: Intervention: 58 Control: 62
Important prognostic factors2: Age ± SD: Intervention: 40 Control: 39.8
Sex: Intervention: 21% M Control: 16% M
Groups comparable at baseline? Yes |
Baseline medication: Treatment with oxygen and inhaled albuterol. Albuterol was administered either as a nebulized aerosol (0.5 mL in 2.5 mL saline) or through supervised inhalations of a metred-dose inhaler with spacer titrated to a therapeutic effective dose. If no improvement was observed, patient received 125 mg IV methylprednisolone
Intervention: 2 g MgSO4 diluted in 50 mL D5W IV over 20 minutes
|
Baseline medication: Treatment with oxygen and inhaled albuterol. Albuterol was administered either as a nebulized aerosol (0.5 mL in 2.5 mL saline) or through supervised inhalations of a metred-dose inhaler with spacer titrated to a therapeutic effective dose. If no improvement was observed, patient received 125 mg IV methylprednisolone
Control: No MgSO4 |
Length of follow-up: Not reported
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: Not applicable
|
Hospital admission: Intervention: 22% (13/58) Control: 18% (11/62)
|
No comments |
|
Porter, 2001 |
Type of study: RCT
Setting and country: USA
Funding and conflicts of interest: Not reported
|
Inclusion criteria: -Aged 18-55 years -History of asthma -PEF≤100 l/min or ≤25% predicted (moderate to severe asthma) -Ability to provide informed consent
Exclusion criteria: -Possible non-asthmatic causes of wheezing -Patients who on arrival appeared to require endotracheal intubation -Pregnant patients
N total at baseline: Intervention: 18 Control: 24
Important prognostic factors2: Age ± SD: Intervention: 32 ± 13 Control: 38 ± 15
Sex: Intervention: 50% M Control: 25% M
Groups comparable at baseline? Yes |
Baseline medication: 2.5 mg albuterol sulphate via nebulizer, 125 mg methylprednisolone IV
Intervention: 2 g MgSO4 in 50 mL saline IV over 20 minutes
|
Baseline medication: 2.5 mg albuterol sulphate via nebulizer, 125 mg methylprednisolone IV
Control: 50 mL saline IV over 20 minutes
|
Length of follow-up: 60 minutes
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: There were 55 asthma episodes meeting inclusion criteria. These episodes represented visits by 42 different patients, with all consenting to participate on all episodes. Patients completed the study protocol in each of the 55 episodes. The following results refer to the first asthma episodes in the 42 study patients |
Mortality: Intervention: 0% (0/18) Control: 0% (0/24)
Hospital admission: Intervention: 28% (5/18) Control: 21% (5/24)
Adverse events: Intervention: 22% (4/18) Control: 13% (3/23) |
No comments |
|
Silverman, 2002 |
Type of study: RCT
Setting and country: USA
Funding and conflicts of interest: Not reported
|
Inclusion criteria: -Aged 18-60 years -Presenting with acute severe asthma at the ED -Diagnosed with asthma in the past by a clinician and using asthma medication in the previous 6 months -FEV1≤30% predicted -Willing to remain in the ED for 4 hours -Ability to provide informed consent
Exclusion criteria: -History of COPD or other chronic lung disease, congestive heart failure, coronary artery disease, diabetes mellitus, renal insufficiency or hypertension treated with medication -Temperature above 38.9°C -Suspected of having pneumonia -Pregnant patients -Patients requiring intubation or unable to perform spirometry
N total at baseline: Intervention: 122 Control: 126
Important prognostic factors2: Age ± SD: Intervention: 36.4 ± 11.1 Control: 36.5 ± 11.4
Sex: Intervention: 45% M Control: 51% M
Groups comparable at baseline? Yes |
Baseline medication: 0.5 mL of 0.5% albuterol (2.5 mg) administered via wet nebulizer with 100% oxygen, 125 mg of IV methylprednisolone. Albuterol was readministered 30, 60, 120 and 180 min after ED arrival
Intervention: 2 g MgSO4 in 50 mL saline IV over 10-15 minutes
|
Baseline medication: 0.5 mL of 0.5% albuterol (2.5 mg) administered via wet nebulizer with 100% oxygen, 125 mg of IV methylprednisolone. Albuterol was readministered 30, 60, 120 and 180 min after ED arrival
Control: Placebo in 50 mL saline IV over 10-15 minutes
|
Length of follow-up: 7 days
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: 254 patients were randomized, but 6 patients were inadvertently enrolled twice. 248 patients formed the ITT population
|
Hospital admission: Intervention: 32% (39/122) Control: 32% (41/126)
|
No comments |
|
Singh, 2008 |
Type of study: RCT
Setting and country: India
Funding and conflicts of interest: Not reported
|
Inclusion criteria: -Aged 18-60 years -Presenting with acute severe asthma at the ED -Diagnosed with asthma in the past by a clinician with spirometry records showing a reversibility of 12%, and using asthma medication in the previous 6 months -FEV1<30% predicted -Willing to remain in the ED for 3 hours
Exclusion criteria: -History of COPD or other chronic lung disease, and other known cardiac, renal and hepatic dysfunctions -Pregnant or lactating patients -Patients requiring intubation or unable to perform spirometry
N total at baseline: Intervention: 30 Control: 30
Important prognostic factors2: Age ± SD: Intervention: 34.79 ± 8.05 Control: 35.9 ± 8.76
Sex: Intervention: 46.7% M Control: 50% M
Groups comparable at baseline? Yes |
Baseline medication: Nebulising solution consisting of 1 mL of 2.5% salbutamol (2.5 mg), 250 mg of 1.5 mL of ipratropium bromide administered in 2.5 mL of saline aerosolized via a wet nebuliser with 100% oxygen at 0, 20, 40 minutes
Intervention: 2 g MgSO4 in 250 mL saline IV over 20 minutes
|
Baseline medication: Nebulising solution consisting of 1 mL of 2.5% salbutamol (2.5 mg), 250 mg of 1.5 mL of ipratropium bromide administered in 2.5 mL of saline aerosolized via a wet nebuliser with 100% oxygen at 0, 20, 40 minutes
Control: Placebo in 250 mL saline IV over 20 minutes
|
Length of follow-up: Not reported
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: 75 patients were screened, 5 patients were excluded before randomization, 10 patients randomized had protocol violations (in both groups: n=5)
|
Hospital admission: Intervention: 6.7% (2/30) Control: 30% (9/30)
|
No comments |
|
Skobeloff, 1989 |
Type of study: RCT
Setting and country: USA
Funding and conflicts of interest: Not reported
|
Inclusion criteria: -Aged 18-70 years -Presenting with acute moderate to severe asthma at the ED -Patients met the diagnostic criteria for asthma as set forth by the American Thoracic Society
Exclusion criteria: -Rectal temperature >38°C -Systolic blood pressure below 120 mm Hg -History of kidney disease, purulent sputum, infiltrate on a chest roentgenogram, pregnancy
N total at baseline: Intervention: 19 Control: 19
Important prognostic factors2: Age ± SD: Intervention: 39.9 ± 12.4 Control: 36.2 ± 11.5
Sex: Not reported
Groups comparable at baseline? Yes |
Baseline medication: Nebulized treatment of 0.3 mL metaproterenol sulfate in 3.0 mL saline, or 0.5 mL albuterol sulfate in 2.5 mL saline, 125 mg methylprednisolone sodium succinate IV. If necessary, theophylline was given IV.
Intervention: 1.2 g MgSO4 in 50 mL saline IV over 20 minutes
|
Baseline medication: Nebulising solution consisting of 1 mL of 2.5% salbutamol (2.5 mg), 250 mg of 1.5 mL of ipratropium bromide administered in 2.5 mL of saline aerosolized via a wet nebuliser with 100% oxygen at 0, 20, 40 minutes
Control: 50 mL saline IV over 20 minutes
|
Length of follow-up: Not reported
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: 40 patients entered the study, 2 patients were excluded due to protocol violation or entering the study twice (only the first entry was used). |
Hospital admission: Intervention: 36.8% (7/19) Control: 78.9% (15/19) P<0.01
|
Only patients who did not to the baseline treatment (β-agonist non-responders) were included in the study and randomized to either intervention or control |
|
Leukotriene antagonists |
|||||||
|
Camargo, 2010 |
Type of study: RCT
Setting and country: USA and 15 other countries
Funding and conflicts of interest: Reported
|
Inclusion criteria: -Aged ≥15 years -≥1 year history of physician-diagnosed asthma -Presenting with acute asthma at the ED -Patients >54 years were included if they also had FEV1 reversibility ≥15% predicted after β-agonist treatment during the current episode or within the past 5 years or if their lifetime tobacco exposure was ≤10 pack-years
Exclusion criteria: -Clinically significant, active comorbid disease -Body mass index >35 kg/m2 -Smoking history of >15 pack-years
N total at baseline: Intervention: 292 Control: 291
Important prognostic factors2: Age ± SD: Intervention: 41.1 ± 15.0 Control: 41.0 ± 15.3
Sex: Intervention: 47% M Control: 40% M
Groups comparable at baseline? Yes |
Baseline medication: Oxygen, inhaled short-acting β-agonist (2.5-5mg in 3 mL saline every 20 min) as needed, and inhaled ipratropium or nebulized ipratropium as needed
Intervention: IV administration (manual bolus over 2-5 min) of 7 mg montulekast (within 60 min after initiation of standard treatment). Systemic corticosteroids (60 mg prednisone or 50 mg prednisolone orally) Continued baseline medication
|
Baseline medication: Oxygen, inhaled short-acting β-agonist (2.5-5mg in 3 mL saline every 20 min) as needed, and inhaled ipratropium or nebulized ipratropium as needed
Control: IV administration (manual bolus over 2-5 min) of placebo. Systemic corticosteroids (60 mg prednisone or 50 mg prednisolone orally) Continued baseline medication
|
Length of follow-up: 14 days
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: Intervention: n=291 enrolled, 4 patients discontinued or had no baseline FEV1 measurement Placebo: n=292 enrolled, 8 patients discontinued or had no baseline FEV1 measurement. |
1 or more AEs Intervention: 19.6% Control: 20.2%
Drug-related AEs Intervention: 0% Control: 0%
Serious AEs Intervention: 9.6% Control: 8.9%
AEs leading to discontinuation from the study Intervention: 0% Control: 0.3%
|
No comments |
|
Çýllý, 2003 |
Type of study: RCT
Setting and country: Turkey
Funding and conflicts of interest: Not reported
|
Inclusion criteria: -History of at least 1 year of chronic asthma -FEV1 40-80% predicted and with a ≥15% improvement in FEV1 after inhaled β-agonist -non-smokers -Had not taken oral, intravenous or intramuscular corticosteroids during the month prior to the start of the study
Exclusion criteria: -Patients taking long acting antihistamines within 2 weeks, short acting antihistamines during the 48h before the visit or theophylline, oral or long-acting inhaled β-agonists, cromolyn sodium or nedocromil, or inhaled anticholinergics within 1 week
N total at baseline: Intervention: 23 Control: 22
Important prognostic factors2: Age ± SD: Intervention: 64.0 ± 7.7 Control: 64.6 ± 7.6
Sex: Intervention: 30.4% M Control: 31.8% M
Groups comparable at baseline? Yes |
Baseline medication: 1 mg/kg prednisolone IV, 3 aerosol treatments of 0.5 mg terbutaline sulphate as dry powder inhaler separated by 20 min intervals
Interventions: 10 mg tablet oral montulekast
|
Baseline medication: 1 mg/kg prednisolone IV, 3 aerosol treatments of 0.5 mg terbutaline sulphate as dry powder inhaler separated by 20 min intervals
Control: No additional treatment/placebo
|
Length of follow-up: 24 hours
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: Not applicable. |
Adverse events – No severe adverse events
Most frequent adverse events included malaise (8%) and headache (6%) in the intervention group
|
No comments |
|
Silverman, 2004 |
Type of study: RCT
Setting and country: USA
Funding and conflicts of interest: Not reported
|
Inclusion criteria: -Aged 12-65 years -Presenting with acute asthma at the ED -History of asthma -FEV1<70% predicted both at ED entry and 25 min after receiving a single aerosol treatment with 2.5 mg albuterol
Exclusion criteria: -History of smoking of >10 pack-years -Positive pregnancy test result -Recent history of oral corticosteroid use (≥5 days) or treatment with a leukotriene-modifying drug within 2 weeks of ED entry -Need for intubation before randomization -Pneumonia or elevated temperature (>38.9°C) -Chronic lung disease other than asthma, diabetes mellitus or any other clinically significant medical condition that could affect the required conditions -Willingness to stay in the ED for at least 4 hours and participation in a 28-day outpatient treatment program
N total at baseline: Intervention 1: 162 Intervention 2: 158 Control: 321
Important prognostic factors2: Age ± SD: Intervention 1: 32.3 ± 12.6 Intervention 2: 32.5 ± 11.4 Control: 32.6 ± 11.7
Sex: Intervention 1: 54.3% M Intervention 2: 36.7% M Control: 40.2% M
Groups comparable at baseline? Yes |
Baseline medication: At ED entry: nebulized albuterol (2.5 mg). 25 min after ED entry: patients with FEV1<70% predicted were randomized. 60 mg po dose of prednisone, nebulized albuterol, additional albuterol at 60, 120 and 180 min after ED entry.
Interventions: 1: 160 mg zafirlukast 2: 20 mg zafirlukast
|
Baseline medication: At ED entry: nebulized albuterol (2.5 mg). 25 min after ED entry: patients with FEV1<70% predicted were randomized. 60 mg po dose of prednisone, nebulized albuterol, additional albuterol at 60, 120 and 180 min after ED entry.
Control: Placebo
|
Length of follow-up: 42 days
Loss-to-follow-up: No loss-to-follow-up in intervention or control group.
Incomplete outcome data: Not applicable. |
Hospital admission: Intervention 1: 9.9% (16/162) Intervention 2: 16.5% (26/158) Control: 15% (48/321)
Adverse events – headache ED period: Intervention 1: 1% (2/162) Intervention 2: 1% (2/158) Control: 2% (5/321)
|
No comments |
|
Salbutamol IV |
|||||||
|
Cheong, 1998 |
Type of study: RCT
Setting and country: District general hospital (secondary care centre).
Funding and conflicts of interest: Not reported.
|
Inclusion criteria: -16 and 70 years -Admitted to hospital with severe acute asthma
Exclusion criteria: -History of cardiovascular disease -Corticosteroid or intravenous bronchodilator treatment before admission
N total at baseline: Intervention: 37 Control: 34
Important prognostic factors2: Age (range): Intervention: 37 (16-69) Control: 35 (16-66)
Sex: Intervention: 70% M Control: 68% M
Groups comparable at baseline? Yes |
Baseline medication: At admission, 5 mg nebulised salbutamol with a Hudson nebuliser driven by oxygen at 6 l/min and an intravenous bolus of 200 mg hydrocortisone. Patients with a peak flow rate below half of the predicted value 30 minutes after nebulised treatment were randomised. No other bronchodilator was used. All were given continuous 35% inspired oxygen.
Intervention: Intravenous salbutamol as a continuous infusion in a dose of 12.5 µg/min starting 30 minutes after admission and lasting for a further four hours. 2g potassium was added to the infusion (because of the occurrence of hypokalemia). |
Baseline medication: At admission, 5 mg nebulised salbutamol with a Hudson nebuliser driven by oxygen at 6 l/min and an intravenous bolus of 200 mg hydrocortisone. Patients with a peak flow rate below half of the predicted value 30 minutes after nebulised treatment were randomised. No other bronchodilator was used. All were given continuous 35% inspired oxygen.
Control: 5 mg nebulized salbutamol 30 minutes after the first treatment on admission and again two hours later.
|
Length of follow-up: Not reported.
Loss-to-follow-up: Not reported.
Incomplete outcome data: Five patients were withdrawn because of either adverse effects or non-response to treatment. |
Adverse events: 5% (n=2) of patients in IG withdrawn because of tachycardia.
Tachycardia was prominent in IG.
|
No comments. |
Risk of bias tabellen
Research question: Which medication has beneficial effects in addition to standard treatment in patients with acute asthma exacerbation? Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Magnesium sulphate |
|||||||
|
Bloch, 1995 |
Definitely yes;
Reason: Randomization using computer-generated tables |
Definitely yes;
Reason: Physicians were blinded, randomization by the pharmacy |
Probably yes;
Reason: Patients, health care providers and outcome assessors blinded (blinding of data collectors and analysts not reported) |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: The authors did not include all enrolled patients in the statistical analysis. 135/149 enrolled patients (<10% of patients were excluded) were included in the statistical analysis. 12 patients were enrolled more than once (first visit was used for analysis), 1 patient had irreversible airway disease, 1 patient had congestive heart failure, 4 patients were included as ITT because the study protocol was violated, in 6 patients baseline FEV1 was unavailable. |
LOW |
|
Boonyavorakul, 2000 |
Definitely yes;
Reason: Randomization using computer-generated random lists |
Probably yes;
Reason: Study investigators were blinded, method unknown |
Probably yes;
Reason: Patients, health care providers and outcome assessors blinded (blinding of data collectors and analysts not reported) |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Definitely yes;
Reason: No other problems noted |
LOW |
|
Bradshaw, 2008 |
Probably yes;
Reason: Generation of allocation sequence not described |
Probably yes;
Reason: Study investigators were blinded, method unknown |
Probably yes;
Reason: Patients, health care providers and outcome assessors blinded (blinding of data collectors and analysts not reported) |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably no;
Reason: The authors did not include all enrolled patients in the statistical analysis. 129/150 recruited patients (>10% of patients were excluded were included in the final analysis. Patients were excluded due to subsequent recognition of PEF >75% predicted or missing record data. |
SOME CONCERNS Hospital admission |
|
Goodacre, 2014 |
Definitely yes;
Reason: The recruiting clinician accessed a web-based randomisation system or automated telephone hotline and participants were allocated to a numbered treatment pack kept in the ED. |
Definitely yes;
Reason: The randomisation system only revealed the allocated pack number. Each treatment pack contained either magnesium or placebo solution. A 24-hour unblinding service was available via the randomisation system. Tamper stickers were checked regularly to ensure that envelopes had not been opened and were returned, still sealed, to the central study team. If an envelope was opened, it was reported and recorded as a participant unblinding |
Probably yes;
Reason: Patients, health care providers and outcome assessors blinded (blinding of data collectors and analysts not reported) |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. Adequate imputation methods (multiple imputation) were used. |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: The authors did not include all enrolled patients in the statistical analysis. 1109 patients were recruited, of which 25 either withdrew or were recruited in error (protocol violations)(<10% of patients were excluded). |
LOW |
|
Green, 1992 |
Definitely no;
Reason: Patients on odd days were given 2 mg of magnesium sulphate |
Definitely no;
Reason: Due to the allocation method, investigators were not blinded. |
Probably no;
Reason: Patients and respiratory therapists were unaware that a study was being performed. Physicians were not blinded. |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Definitely yes;
Reason: No other problems noted |
HIGH |
|
Porter, 2001 |
Definitely yes;
Reason: Allocation according to a random number generator
|
Definitely yes;
Reason: Enrolment packets containing data recording sheets and the study solutions in random order were kept in the ED and assigned to new patients as they entered the study. The placebo/magnesium solutions were identical in appearance. The pharmacy had the only copy of the code, which was not broken until the study was completed and data analysis begun |
Probably yes;
Reason: Patients, health care providers and outcome assessors blinded. Data analysist were not blinded |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: The authors did not include all enrolled patients in the statistical analysis. There were 55 asthma episodes meeting inclusion criteria. These episodes represented visits by 42 different patients, with all consenting to participate on all episodes. Patients completed the study protocol in each of the 55 episodes. The following results refer to the first asthma episodes in the 42 study patients (<10% of episodes were excluded). |
LOW |
|
Silverman, 2002 |
Definitely yes;
Reason: Allocation according to a 1:1 ratio randomization table unique for each center |
Definitely yes;
Reason: The study pharmacist at each site placed drug or placebo in identically appearing vials, with only the study identification number printed on the label |
Probably yes;
Reason: Patients, health care providers and outcome assessors blinded (blinding of data collectors and analysts not reported) |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: The authors did not include all enrolled patients in the statistical analysis. 254 patients were randomized, but 6 patients were inadvertently enrolled twice (<10% of patients were excluded). |
LOW |
|
Singh, 2008 |
Definitely yes;
Reason: Allocation according to a 1:1 ratio randomization table |
Definitely yes;
Reason: The individual random numbers were kept in separate envelopes so that concealment could be maintained until the patient was included in the assigned group |
Probably yes;
Reason: Patients, health care providers and outcome assessors blinded (blinding of data collectors and analysts not reported) |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: The authors did not include all enrolled patients in the statistical analysis. 75 patients were screened, 5 patients were excluded before randomization, 10 patients randomized had protocol violations (in both groups: n=5) (<10% of patients were excluded). |
LOW |
|
Skobeloff, 1989 |
Definitely yes;
Reason: Allocation according to a randomization list |
Definitely yes;
Reason: The placebo/magnesium solutions were pre-packaged in identical vials by the pharmacy and coded from a randomization list |
Probably yes;
Reason: Patients, health care providers and outcome assessors blinded (blinding of data collectors and analysts not reported) |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: The authors did not include all enrolled patients in the statistical analysis. 40 patients entered the study, 2 patients were excluded due to protocol violation or entering the study twice (only the first entry was used) (<10% of patients were excluded). |
LOW |
|
Montulekast |
|||||||
|
Camargo, 2010 |
Definitely yes;
Reason: Allocation according to a computer-generated randomization schedule with a blocking factor of4 provided by the study sponsor |
Definitely yes;
Reason: The treatment solution was prepared in a foil-wrapped syringe by a qualified person who was no directly associated with the care of the patients |
Probably yes;
Reason: Patients and health care providers blinded (blinding of outcome assessors, data collectors and analysts not reported) |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: The authors did not include all enrolled patients in the statistical analysis. 573/583 randomized patients were included in the statistical analysis (<10%). Excluded from intervention: n=8, control: n=3 |
LOW |
|
Çýllý, 2003 |
Probably no;
Reason: Allocation method not described |
Definitely no;
Reason: single blinded randomized trial, investigators were not blinded |
Probably no;
Reason: Only patients were blinded. |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Definitely yes;
Reason: No other problems noted |
HIGH |
|
Silverman, 2004 |
Definitely yes;
Reason: Allocation according to a computer-generated randomization schedule, specific for each site |
Probably yes;
Reason: concealment of allocation not described |
Probably yes;
Reason: Patients, health care providers and study investigators were blinded |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. |
Definitely yes;
Reason: All relevant outcomes were reported |
Definitely yes;
Reason: No other problems noted |
LOW |
|
Salbutamol IV |
|
|
|
|
|
|
|
|
Cheong, 1998 |
Probably yes;
Reason: Generation of allocation sequence not described |
Probably yes;
Reason: sealed envelope system was used, but method not further described. |
Probably no;
Reason: blinding not described, but study compared iv salbutamol with nebulised salbutamol. A double dummy design was not specified. |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. No description of the use of adequate imputation methods |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: The authors did not include all enrolled patients in the statistical analysis. 71/76 enrolled patients (<10% of patients were excluded) were included in the statistical analysis. Patients were excluded due to adverse effects or non-response to treatment. |
SOME CONCERNS |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Lin AT, Moore-Clingenpeel M, Karsies TJ. Comparison of two continuous nebulized albuterol doses in critically ill children with status asthmaticus. J Asthma. 2020 Sep;57(9):980-986. doi: 10.1080/02770903.2019.1623249. Epub 2019 Jun 12. PMID: 31119958. |
no use of systemic corticosteroids |
|
Travers AH, Milan SJ, Jones AP, Camargo CA Jr, Rowe BH. Addition of intravenous beta(2)-agonists to inhaled beta(2)-agonists for acute asthma. Cochrane Database Syst Rev. 2012 Dec 12;12:CD010179. doi: 10.1002/14651858.CD010179. PMID: 23235685. |
3 studies included (2 on children, 1 on bedoradrine in adults): does not fit PICO |
|
Travers AH, Rowe BH, Barker S, Jones A, Camargo CA Jr. The effectiveness of IV beta-agonists in treating patients with acute asthma in the emergency department: a meta-analysis. Chest. 2002 Oct;122(4):1200-7. doi: 10.1378/chest.122.4.1200. PMID: 12377842. |
heterogenic group (medication and population), only 1 outcome measure of PICO reported, poor methodologic quality |
|
Manning M, Garin M, Jiang B, Sun SX. Impact of Reslizumab on Duration of Exacerbations in Patients with Severe Eosinophilic Asthma. J Allergy Clin Immunol Pract. 2018 Feb;141(2):supp. doi: 10.1016/j.jaci.2017.12.053 |
abstract only |
|
High-dose zafirlukast in emergency department provides small benefit in acute asthma. J Fam Pract. 2005 Apr;54(4):304-6. PMID: 15833217. |
summary of publication of Silverman |
|
Adachi M, Taniguchi H, Tohda Y, Sano Y, Ishine T, Smugar SS, Hisada S. The efficacy and tolerability of intravenous montelukast in acute asthma exacerbations in Japanese patients. J Asthma. 2012 Aug;49(6):649-56. doi: 10.3109/02770903.2012.690479. Epub 2012 Jun 28. PMID: 22742205. |
corticosteroids were not usual care |
|
Camargo CA Jr, Smithline HA, Malice MP, Green SA, Reiss TF. A randomized controlled trial of intravenous montelukast in acute asthma. Am J Respir Crit Care Med. 2003 Feb 15;167(4):528-33. doi: 10.1164/rccm.200208-802OC. Epub 2002 Nov 27. PMID: 12456380. |
corticosteroids were given as needed |
|
Chaudhury A, Gaude GS, Hattiholi J. Effects of oral montelukast on airway function in acute asthma: A randomized trial. Lung India. 2017 Jul-Aug;34(4):349-354. doi: 10.4103/0970-2113.209234. PMID: 28671166; PMCID: PMC5504892. |
effect of oral montelukast on lung function in first 4 weeks after exacerbation |
|
Ferreira MB, Santos AS, Pregal AL, Michelena T, Alonso E, de Sousa AV, Pereira E, Palma-Carlos AG. Leukotriene receptor antagonists (Montelukast) in the treatment of asthma crisis: preliminary results of a double-blind placebo controlled randomized study. Allerg Immunol (Paris). 2001 Oct;33(8):315-8. PMID: 11763721. |
no relevant outcome measures |
|
Motamed H, Verki MM, Barzegari H, Shariat S, Hesam, S. Montelukast efficacy for improvement of adult acute phase mild-moderate asthma attack: A clinical trial study. Curr Respir Med Rev. 2020. 16(4): 246-251(6). |
no relevant outcome measures |
|
Ramsay CF, Pearson D, Mildenhall S, Wilson AM. Oral montelukast in acute asthma exacerbations: a randomised, double-blind, placebo-controlled trial. Thorax. 2011 Jan;66(1):7-11. doi: 10.1136/thx.2010.135038. Epub 2010 Oct 18. PMID: 20956393. |
effect of oral montelukast on lung function in first 4 weeks after exacerbation |
|
Watts K, Chavasse RJ. Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children. Cochrane Database Syst Rev. 2012 May 16;2012(5):CD006100. doi: 10.1002/14651858.CD006100.pub2. PMID: 22592708; PMCID: PMC7387678. |
RCTs that fit PICO are separately included |
|
Zhang HP, Jia CE, Lv Y, Gibson PG, Wang G. Montelukast for prevention and treatment of asthma exacerbations in adults: Systematic review and meta-analysis. Allergy Asthma Proc. 2014 Jul-Aug;35(4):278-87. doi: 10.2500/aap.2014.35.3745. PMID: 24992547. |
does not fit PICO |
|
Green RH. Asthma in adults (acute): magnesium sulfate treatment. BMJ Clin Evid. 2016 Jan 13;2016:1513. PMID: 26761432; PMCID: PMC4711892. |
onvolledige GRADE beoordeling, onduidelijk welke studies geincludeerd en of behandeling met steroiden |
|
Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA Jr. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: a systematic review of the literature. Ann Emerg Med. 2000 Sep;36(3):181-90. doi: 10.1067/mem.2000.105659. PMID: 10969218. |
onduidelijk welke studies geincludeerd en of behandeling met steroïden |
|
Rodrigo G, Rodrigo C, Burschtin O. Efficacy of magnesium sulfate in acute adult asthma: a meta-analysis of randomized trials. Am J Emerg Med. 2000 Mar;18(2):216-21. doi: 10.1016/s0735-6757(00)90024-x. PMID: 10750936. |
oud, matige kwaliteit, |
|
Dennis RJ, Solarte I, Fitzgerald JM. Asthma in adults. BMJ Clin Evid. 2007 Aug 15;2007:1501. PMID: 19454105. |
chronic asthma |
|
Conway J, Friedman B. Intravenous Magnesium Sulfate for Acute Asthma Exacerbation in Adults. Acad Emerg Med. 2020 Oct;27(10):1061-1063. doi: 10.1111/acem.14066. Epub 2020 Aug 14. PMID: 32574392. |
No systematic review, unclear study selection |
|
Rodrigo G. Asthma in adults (acute). BMJ Clin Evid. 2011 Apr 4;2011:1513. PMID: 21463536; PMCID: PMC3661228. |
No systematic review, unclear study selection |
|
Mohammed S, Goodacre S. Intravenous and nebulised magnesium sulphate for acute asthma: systematic review and meta-analysis. Emerg Med J. 2007 Dec;24(12):823-30. doi: 10.1136/emj.2007.052050. PMID: 18029512; PMCID: PMC2658351. |
Most studies also found in our search, low quality SR |
|
Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA Jr. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;(2):CD001490. doi: 10.1002/14651858.CD001490. PMID: 10796650. |
old, 7 studies, no differentiation adults and children |
|
Shan Z, Rong Y, Yang W, Wang D, Yao P, Xie J, Liu L. Intravenous and nebulized magnesium sulfate for treating acute asthma in adults and children: a systematic review and meta-analysis. Respir Med. 2013 Mar;107(3):321-30. doi: 10.1016/j.rmed.2012.12.001. Epub 2013 Jan 3. PMID: 23290189. |
Most studies also found in our search, low quality SR |
|
Cheong B, Reynolds SR, Rajan G, Ward MJ. Intravenous beta agonist in severe acute asthma. BMJ. 1988 Aug 13;297(6646):448-50. doi: 10.1136/bmj.297.6646.448. PMID: 3139138; PMCID: PMC1833883. |
No relevant outcomes |
|
Zubairi AB, Salahuddin N, Khawaja A, Awan S, Shah AA, Haque AS, Husain SJ, Rao N, Khan JA. A randomized, double-blind, placebo-controlled trial of oral montelukast in acute asthma exacerbation. BMC Pulm Med. 2013 Mar 28;13:20. doi: 10.1186/1471-2466-13-20. PMID: 23537391; PMCID: PMC3616955. |
No relevant outcomes |
|
Hirashima J, Yamana H, Matsui H, Fushimi K, Yasunaga H. Effect of intravenous magnesium sulfate on mortality in patients with severe acute asthma. Respirology. 2016 May;21(4):668-73. doi: 10.1111/resp.12733. Epub 2016 Jan 18. PMID: 26781339. |
No RCT |
|
Self TH, Abou-Shala N, Burns R, Stewart CF, Ellis RF, Tsiu SJ, Kellermann AL. Inhaled albuterol and oral prednisone therapy in hospitalized adult asthmatics. Does aminophylline add any benefit? Chest. 1990 Dec;98(6):1317-21. doi: 10.1378/chest.98.6.1317. PMID: 2245667. |
Does no fit PICO (wrong intervention) |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 28-05-2024
Beoordeeld op geldigheid : 19-02-2024
Algemene gegevens
De ontwikkeling van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2020 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg rondom de longaanval astma.
Samenstelling werkgroep
- Dr. E.J.M. (Els) Weersink, longarts, Amsterdam UMC te Amsterdam, NVALT (voorzitter)
- Drs. A. (Annelies) Beukert, longarts, Martini Ziekenhuis te Groningen, NVALT
- Dr. G.J. (Gert-Jan) Braunstahl, longarts, Franciscus Gasthuis & Vlietland te Rotterdam, NVALT
- Drs. R.C. (Rachel) Numan, AIOS longgeneeskunde, HagaZiekenhuis te ’s Gravenhage, NVALT
- Drs. L.C. (Louise) Urlings-Strop, intensivist, Reinier de Graaf Ziekenhuis, Delft, NVIC
- Drs. F.E.C. Geijsel, SEH-artsKNMG, OLVG te Amsterdam, NVSHA
- Dr. E.C. (Erwin) Vasbinder, ziekenhuisapotheker, Franciscus Gasthuis & Vlietland te Rotterdam, NVZA
- Prof. Dr. J.W.M. (Jean) Muris, huisarts en hoogleraar huisartsgeneeskunde, Maastricht UMC+ te Maastricht, NHG
- M.H.A. (Mariëtte) Scholma MSc, verpleegkundig specialist longziekten, Wilhelmina Ziekenhuis Assen te Assen, V&VN
- L.A.M. (Betty) Frankemölle, patiëntvertegenwoordiger, Longfonds
- M.A.P. (Marjo) Poulissen, patiëntvertegenwoordiger, Longfonds
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Weersink* |
Longarts, afdeling longziekten Academisch Medisch Centrum, Amsterdam |
voorzitter RL ernstig astma (afgerond) |
In 2017 voor meerdere farmaceutische bedrijven een betaald adviseurschap. (GSK, Novartis, TEVA, Chiesi Boehringer). In 2019-2020 nog wel adviseurschap niet meer tegen betaling. 2019: dienstverlening, bijdrage aan sympsoium van Astra Zeneca, Novartis, Genzyme BV 2019: vergoeding gastvrijheid Genzyme BV.
Bestuurslid stichting RAPSODI, de stichting die de database voor ernstig astma beheerd en mede gefinancierd wordt door ZONMW, Novartis, GSK, TEVA, Astra Zeneca en Sanofi. Hier is inmiddels een governance vastgelegd welke rol de farmaceuten hierbij hebben. |
Geen actie |
|
Urlings |
Intensivist (longarts) – Reinier de Graaf Gasthuis |
Waarneming diverse ziekenhuizen Intensive Care – betaald |
Geen |
Geen actie |
|
Vasbinder |
ziekenhuisapotheker |
Redacteur van medisch-farmaceutisch handboek “Praktische Farmacotherapie bij Longaandoeningen”, betaald door uitgever Lannoo Campus |
Betrokken bij meerdere onderzoeken bij patiënten met moeilijk behandelbaar/ernstig astma dat financieel wordt ondersteund door: * diverse zorgverzekeraars * TEVA (farmaceutische industrie) * Astra Zeneca (hoofdonderzoeker)
Geneesmiddeleninkoop voor het ziekenhuis, waaronder biologicals, verwachting dat deze binnen het kader van deze RL niet relevant zijn |
Geen actie |
|
Muris |
Universiteit Maastricht 1.0 fte Huisartspraktijk Geulle 17 werkdagen spreekuur / jaar |
Geen |
Webinar over orale corticosteroïden bij astma (GSK) |
Geen actie |
|
Geijsel |
SEH arts KNMG bij OLVG, tevens plaatsvervangend opleider en fellow opleider (95%) SEH arts bij MyEmergencyDoctor, Australische werkgever, telehealth (5%) |
EM-masterclass ontwikkelaar en faculty (betaald) |
Geen |
Geen actie |
|
Poulissen |
Projectleider zorg Longfonds full time (met detachering voor 12 uur naar astmaVereniging Nederland en Davos). |
Geen |
Geen |
Geen actie |
|
Beukert |
longarts te Martini Ziekenhuis Groningen tot 1-8-2021 Longarts te Deventer Ziekenhuis, Deventer, vanaf 23-8-2021 |
Secretaris Sectie Astma en Allergie (SAA) van de NVALT, onbetaald Lid werkgroep binnen SAA over biologicals, onbetaald |
Enkele keer deelname aan een betaalde adviesraadbijeenkomst (laatste in 2021, over biologicals) bij farmaceut maar dat houdt m.i. geen relatie met de inhoud van deze richtlijn |
Geen actie |
|
Numan |
AIOS longgeneeskunde HAGA ziekenhuis |
Geen |
Geen |
Geen actie |
|
Frankemölle |
Vrijwilligster bij het Longfonds, lid van de Longfonds Ervaringsdeskundigengroep. astmaVereniging Nederland en Davos Vrijwilligster European Lung Foundation, lid van SHARP |
Ik neem deel aan diverse werkgroepen maar geen van allen heeft als hoofdthema astma-aanval. |
Geen |
Geen actie |
|
Braunstahl |
Longarts, Franciscus Gasthuis & Vlietland Rotterdam |
Null-aanstelling ErasmusMC voor onderzoek: onbetaald. Deelname RL ernstig astma en KNO-RL, obesitas |
Vergoeding: Presentaties en incidenteel advieswerk voor Boehringer Ingelheim, Sanofi, Novartis, GSK, AstraZeneca, ALK, MEDA en Chiesi. (wrsch speelt deze longmedicatie geen rol in deze richtlijn)
Deelname richtlijn ernstig astma Deelname klankbordgroep van het project ‘Obesitas volwassenen’
Webinar over orale corticosteroïden bij astma (GSK)
Geen vergoeding: Redactie NTvAAKI Bestuur RoLeX astma/COPD nascholingen Bestuur Rapsodi, ernstig astma database NL Voorzitter astmasectie NVALT Wetenschappelijke adviescommissie Longfonds |
Geen actie |
|
Scholma |
Verpleegkundig specialist longziekten Wilhelmina Ziekenhuis Assen |
vrijwilliger longfonds, expertgroep zorgaanpak COPD (chiesi). Geen relatie met longaanval astma voorzitter kwaliteitsteam Assen van de HZD |
Geen |
Geen actie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntperspectief door afgevaardigde patiëntenvereniging Longfonds in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan het Longfonds en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
|
Module |
Uitkomst raming |
Toelichting |
|
Module Overige medicatie bij longaanval astma |
Geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling(en) niet breed toepasbaar zijn (<5.000 patiënten) en zal daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met een longaanval astma.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodi
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. Doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. Doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. Doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. Doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. Doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. Doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy
Algemene informatie
|
Richtlijn: NVALT longaanval astma |
|
|
Uitgangsvraag: UV6 Welke medicatie heeft tijdens een longaanval astma bij volwassenen aantoonbare meerwaarde t.o.v. standaardbehandeling = vernevelen/DA met bronchusverwijding en systemische steroïden)? |
|
|
Database(s): Ovid/Medline, Embase |
Datum: 31-8-2022 |
|
Periode: 2010- |
Talen: nvt |
|
Literatuurspecialist: Ingeborg van Dusseldorp |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: Voor deze vraag is gezocht met de volgende concepten: Astma aanval EN (magnesium, salbutamol, benralizumab, mepolizumab, reslizumab, anti-il5) EN ‘volwassenen’ Van de 20 sleutelartikelen worden er 5 niet gevonden vanwege het studiedesign |
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase en Ovid/Medline is op 31-8-2022 met relevante zoektermen gezocht vanaf 2010 naar systematische reviews en RCTs over welke medicatie aantoonbare meerwaarde heeft tijdens een longaanval astma-t.o.v. standaardbehandeling. De literatuurzoekactie leverde 1259 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
243 |
128 |
341 |
|
RCTs |
579 |
495 |
918 |
|
Observationele studies |
|
|
|
|
Overig |
|
|
|
|
Totaal |
|
|
1259 |
Zoekstrategie
Embase
|
No. |
Query |
Results |
|
#37 |
#10 NOT #9 RCT |
579 |
|
#36 |
#11 NOT #9 |
1517 |
|
#35 |
#33 NOT #34 sleutelartikelen niet gevonden |
5 |
|
#34 |
#12 AND #33 |
16 |
|
#33 |
#13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 |
21 |
|
#32 |
the AND inflammatory AND profile AND of AND exacerbations AND in AND patients AND with AND severe AND refractory AND eosinophilic AND asthma AND receiving AND mepolizumab |
1 |
|
#31 |
acute AND effect AND of AND benralizumab AND on AND asthma AND exacerbation AND without AND concomitant AND corticosteroid AND use |
1 |
|
#30 |
compassionate AND use AND single AND dose AND of AND benralizumab AND in AND a AND 'near fatal' AND asthma AND exacerbation |
1 |
|
#29 |
'anti interleukin 5' AND therapy AND mepolizumab AND in AND 'life threatening' AND asthma AND attack |
1 |
|
#28 |
leukotriene AND receptor AND antagonists AND addition AND to AND usual AND care AND for AND acute AND asthma AND in AND adults AND children AND watts |
1 |
|
#27 |
oral AND montelukast AND in AND acute AND asthma AND exacerbations AND a AND randomised, AND 'double blind,' AND 'placebo controlled' AND trial AND ramsay |
1 |
|
#26 |
a AND randomized, AND 'double blind,' AND 'placebo controlled' AND trial AND of AND oral AND montelukast AND in AND acute AND asthma AND exacerbation AND zubairi |
1 |
|
#25 |
effects AND of AND oral AND montelukast AND on AND airway AND function AND in AND acute AND asthma AND a AND randomized AND trial AND chaudhury |
1 |
|
#24 |
'anti interleukin 5' AND therapy AND mepolizumab AND in AND 'life threatening' AND asthma AND attack AND a AND 'case based' AND discussion AND tello |
1 |
|
#23 |
compassionate AND use AND single AND dose AND of AND benralizumab AND in AND a AND 'near fatal' AND asthma AND exacerbation AND llano |
1 |
|
#22 |
use AND benralizumab AND in AND the AND treatment AND of AND 'near fatal' AND asthma AND a AND new AND approach AND ramakrishnan |
1 |
|
#21 |
intravenous AND infusion AND of AND salbutamol AND in AND severe AND acute AND asthma AND johnson |
1 |
|
#20 |
intravenous AND salbutamol AND tobin AND 2005 |
1 |
|
#19 |
(intravenous OR nebulised) AND magnesium AND sulphate AND versus AND standard AND therapy AND for AND severe AND acute AND asthma AND goodacre AND 2013 NOT comparative:ti |
1 |
|
#18 |
intravenous AND magnesium AND sulfate AND for AND treating AND adults AND kayleigh AND 2014 |
2 |
|
#17 |
magnesium AND sulfate AND for AND treating AND exacerbations AND of AND acute AND asthma AND in AND the AND emergency AND department AND rowe AND 2000 |
3 |
|
#16 |
efficacy AND magnesium AND sulfate AND in AND acute AND adult AND asthma AND a AND 'meta analysis' AND of AND randomized AND trials AND rodrigo AND 2000 |
1 |
|
#15 |
intravenous AND magnesium AND sulfate AND as AND an AND adjunct AND in AND the AND treatment AND of AND acute AND asthma AND bloch AND 1995 |
1 |
|
#14 |
inhaled AND magnesium AND sulfate AND in AND the AND treatment AND of AND acute AND asthma AND knightly AND 2017 |
1 |
|
#13 |
magnesium AND sulphate AND versus AND nebulized AND salbutamol AND in AND acute AND bronchial AND asthma |
1 |
|
#12 |
#9 OR #10 OR #11 |
1780 |
|
#11 |
#4 AND (#7 OR #8) |
1663 |
|
#10 |
#4 AND #6 |
704 |
|
#9 |
#4 AND #5 SR |
243 |
|
#8 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
12939487 |
|
#7 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6767914 |
|
#6 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1839814 |
|
#5 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#4 |
#3 NOT (('child'/exp OR child*:ti,ab,kw OR schoolchild*:ti,ab,kw OR infant*:ti,ab,kw OR girl*:ti,ab,kw OR boy*:ti,ab,kw OR teen:ti,ab,kw OR teens:ti,ab,kw OR teenager*:ti,ab,kw OR youth*:ti,ab,kw OR pediatr*:ti,ab,kw OR paediatr*:ti,ab,kw OR puber*:ti,ab,kw) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp OR adult*:ti,ab,kw OR man:ti,ab,kw OR men:ti,ab,kw OR woman:ti,ab,kw OR women:ti,ab,kw)) NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
3162 |
|
#3 |
#1 AND #2 |
5199 |
|
#2 |
'leukotriene receptor blocking agent'/exp OR 'benralizumab'/exp OR 'magnesium sulfate'/exp OR 'montelukast'/exp OR 'salbutamol'/exp OR 'mepolizumab'/exp OR 'reslizumab'/exp OR 'interleukin 5 antibody'/exp OR ((leu?otriene NEAR/3 (antagonist* OR block* OR inhibitor*)):ti,ab,kw) OR 'epsom salt':ti,ab,kw OR 'magnesium sulfate':ti,ab,kw OR 'mg longoral':ti,ab,kw OR 'sulfamag':ti,ab,kw OR 'sulmetin':ti,ab,kw OR 'magnesium sulphate':ti,ab,kw OR 'aero clenil':ti,ab,kw OR 'aeroclenil':ti,ab,kw OR 'ah 3365':ti,ab,kw OR 'ah3365':ti,ab,kw OR 'albuterol':ti,ab,kw OR 'almotex':ti,ab,kw OR 'asmacaire':ti,ab,kw OR 'asmadil':ti,ab,kw OR 'asmalin':ti,ab,kw OR 'asmasal':ti,ab,kw OR 'asmatol':ti,ab,kw OR 'asmaven':ti,ab,kw OR 'asmavent':ti,ab,kw OR 'asmidon':ti,ab,kw OR 'asmol':ti,ab,kw OR 'broncho spray':ti,ab,kw OR 'broncho-spray':ti,ab,kw OR 'bronter':ti,ab,kw OR 'brytolin':ti,ab,kw OR 'butahale':ti,ab,kw OR 'buto-asma':ti,ab,kw OR 'butomix':ti,ab,kw OR 'butotal':ti,ab,kw OR 'butovent':ti,ab,kw OR 'buventol':ti,ab,kw OR 'cibutamol':ti,ab,kw OR 'cletal':ti,ab,kw OR 'cybutol':ti,ab,kw OR 'dilatamol':ti,ab,kw OR 'ecovent':ti,ab,kw OR 'emplusal':ti,ab,kw OR 'exafil':ti,ab,kw OR 'farcolin':ti,ab,kw OR 'frespire':ti,ab,kw OR 'glisend':ti,ab,kw OR 'grafalin':ti,ab,kw OR 'krosalburol':ti,ab,kw OR 'libretin':ti,ab,kw OR 'loftan':ti,ab,kw OR 'mozal':ti,ab,kw OR 'novosalmol':ti,ab,kw OR 'parasma':ti,ab,kw OR 'proventil':ti,ab,kw OR 'prox-s':ti,ab,kw OR 'pulmol s':ti,ab,kw OR 'repetabs':ti,ab,kw OR 'respolin':ti,ab,kw OR 'salamol':ti,ab,kw OR 'salbuair':ti,ab,kw OR 'salbulin':ti,ab,kw OR 'salbumol fort':ti,ab,kw OR 'salbupart':ti,ab,kw OR 'salbutamol':ti,ab,kw OR 'salbuvent':ti,ab,kw OR 'salden':ti,ab,kw OR 'salgem':ti,ab,kw OR 'salmol':ti,ab,kw OR 'saltos':ti,ab,kw OR 'solbutamol':ti,ab,kw OR 'spacehaler':ti,ab,kw OR 'sultanol':ti,ab,kw OR 'venetlin':ti,ab,kw OR 'ventilan':ti,ab,kw OR 'ventodisk':ti,ab,kw OR 'ventodisks':ti,ab,kw OR 'ventol':ti,ab,kw OR 'volmac':ti,ab,kw OR 'benralizumab':ti,ab,kw OR 'fasenra':ti,ab,kw OR 'medi 563':ti,ab,kw OR 'medi563':ti,ab,kw OR 'bosatria':ti,ab,kw OR 'mepolizumab':ti,ab,kw OR 'nucala':ti,ab,kw OR 'sb 240563':ti,ab,kw OR 'sb-240563':ti,ab,kw OR 'sb240563':ti,ab,kw OR 'cinqaero':ti,ab,kw OR 'cinqair':ti,ab,kw OR 'reslizumab':ti,ab,kw OR 'sch 55700':ti,ab,kw OR 'sch55700':ti,ab,kw OR 'actamone':ti,ab,kw OR 'airathon':ti,ab,kw OR 'airing':ti,ab,kw OR 'alvokast':ti,ab,kw OR 'apilone':ti,ab,kw OR 'ascafi':ti,ab,kw OR 'ascolin':ti,ab,kw OR 'asmenol':ti,ab,kw OR 'asprevent':ti,ab,kw OR 'astecon':ti,ab,kw OR 'asthator':ti,ab,kw OR 'asthmasan':ti,ab,kw OR 'asthmont':ti,ab,kw OR 'astmirex':ti,ab,kw OR 'astmodil':ti,ab,kw OR 'atentus':ti,ab,kw OR 'atlabiclo':ti,ab,kw OR 'belokast':ti,ab,kw OR 'brolyt':ti,ab,kw OR 'castispir':ti,ab,kw OR 'chesmon':ti,ab,kw OR 'deprive':ti,ab,kw OR 'elukan':ti,ab,kw OR 'elunkast':ti,ab,kw OR 'eonic':ti,ab,kw OR 'filkast':ti,ab,kw OR 'fulmont':ti,ab,kw OR 'imvlo':ti,ab,kw OR 'ispyrra':ti,ab,kw OR 'jepafex':ti,ab,kw OR 'kipres':ti,ab,kw OR 'l 706631':ti,ab,kw OR 'l706631':ti,ab,kw OR 'lanair':ti,ab,kw OR 'leukast':ti,ab,kw OR 'lukair':ti,ab,kw OR 'lukanof':ti,ab,kw OR 'lukas aiwa':ti,ab,kw OR 'lukasm':ti,ab,kw OR 'lukastang':ti,ab,kw OR 'lukavent':ti,ab,kw OR 'melarth':ti,ab,kw OR 'metigrenul':ti,ab,kw OR 'milukante':ti,ab,kw OR 'mintalos':ti,ab,kw OR 'miralust':ti,ab,kw OR 'mk 0476':ti,ab,kw OR 'mk 476':ti,ab,kw OR 'mk0476':ti,ab,kw OR 'mk476':ti,ab,kw OR 'modrian':ti,ab,kw OR 'modulair':ti,ab,kw OR 'mofenstra':ti,ab,kw OR 'mokast':ti,ab,kw OR 'molucar':ti,ab,kw OR 'monalux':ti,ab,kw OR 'monart':ti,ab,kw OR 'monast':ti,ab,kw OR 'moncas':ti,ab,kw OR 'mondeo':ti,ab,kw OR 'monkasta':ti,ab,kw OR 'monlast':ti,ab,kw OR 'monlucare':ti,ab,kw OR 'monspes':ti,ab,kw OR 'monstonol':ti,ab,kw OR 'montair':ti,ab,kw OR 'montast':ti,ab,kw OR 'montecell':ti,ab,kw OR 'montecon':ti,ab,kw OR 'montefar':ti,ab,kw OR 'montegen':ti,ab,kw OR 'montelair':ti,ab,kw OR 'montelak':ti,ab,kw OR 'montelar':ti,ab,kw OR 'montelex':ti,ab,kw OR 'montelubronch':ti,ab,kw OR 'montelucaste':ti,ab,kw OR 'montelukast*':ti,ab,kw OR 'montelux':ti,ab,kw OR 'montemyl':ti,ab,kw OR 'montep':ti,ab,kw OR 'monterast':ti,ab,kw OR 'monteresp':ti,ab,kw OR 'montespir':ti,ab,kw OR 'montewin':ti,ab,kw OR 'montexal':ti,ab,kw OR 'monthan':ti,ab,kw OR 'montol':ti,ab,kw OR 'montus':ti,ab,kw OR 'moolpas':ti,ab,kw OR 'nal 6336':ti,ab,kw OR 'nal6336':ti,ab,kw OR 'orilukast':ti,ab,kw OR 'otelus':ti,ab,kw OR 'pentafeno':ti,ab,kw OR 'perasm':ti,ab,kw OR 'pluralais':ti,ab,kw OR 'pneumo-kast':ti,ab,kw OR 'promonta':ti,ab,kw OR 'rasec':ti,ab,kw OR 'relukas':ti,ab,kw OR 'respilukas':ti,ab,kw OR 'romilast':ti,ab,kw OR 'saslong':ti,ab,kw OR 'singodem':ti,ab,kw OR 'singulair':ti,ab,kw OR 'singulair allergy':ti,ab,kw OR 'singulair ar':ti,ab,kw OR 'singulair chew':ti,ab,kw OR 'singulair mini':ti,ab,kw OR 'singulergy':ti,ab,kw OR 'solok':ti,ab,kw OR 'spirokast':ti,ab,kw OR 'spiromon':ti,ab,kw OR 'surfair':ti,ab,kw OR 'symlukast':ti,ab,kw OR 'telelux':ti,ab,kw OR 'telukast':ti,ab,kw OR 'teluki':ti,ab,kw OR 'tevalukast':ti,ab,kw OR 'thordel':ti,ab,kw OR 'valnuen':ti,ab,kw OR 'velukast':ti,ab,kw OR 'xaira':ti,ab,kw OR 'yekast':ti,ab,kw OR 'zakomoxit':ti,ab,kw OR 'interleukin 5 antibody':ti,ab,kw |
87777 |
|
#1 |
'asthma attack'/exp OR 'asthma exacerbation'/exp OR 'asthmatic state'/exp OR 'acute severe asthma':ti,ab,kw OR 'severe acute asthma':ti,ab,kw OR 'status asthmaticus':ti,ab,kw OR ((asthma* NEAR/3 (cris?s OR shock OR state OR attack* OR exacerbation*)):ti,ab,kw) OR (('asthma'/exp OR asthma:ti,ab,kw) AND ('respiratory failure'/exp OR 'fatal attack*':ti,ab,kw OR 'near fatal':ti,ab,kw OR 'respiratory failure':ti,ab,kw)) OR 'acute asthma':ti,ab,kw |
30801 |
Ovid/Medline
|
# |
Searches |
Results |
|
9 |
8 not 7 RCT |
495 |
|
8 |
4 and 6 |
589 |
|
7 |
4 and 5 SR |
128 |
|
6 |
exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1542296 |
|
5 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
615236 |
|
4 |
3 not ((Adolescent/ or Child/ or Infant/ or adolescen*.ti,ab,kf. or child*.ti,ab,kf. or schoolchild*.ti,ab,kf. or infant*.ti,ab,kf. or girl*.ti,ab,kf. or boy*.ti,ab,kf. or teen.ti,ab,kf. or teens.ti,ab,kf. or teenager*.ti,ab,kf. or youth*.ti,ab,kf. or pediatr*.ti,ab,kf. or paediatr*.ti,ab,kf. or puber*.ti,ab,kf.) not (Adult/ or adult*.ti,ab,kf. or man.ti,ab,kf. or men.ti,ab,kf. or woman.ti,ab,kf. or women.ti,ab,kf.)) |
1692 |
|
3 |
1 and 2 |
2470 |
|
2 |
exp Leukotriene Antagonists/ or Magnesium Sulfate/ or exp Albuterol/ or exp Interleukin-5/ or (leu?otriene adj3 (antagonist* or block* or inhibitor*)).ti,ab,kf. or epsom salt.ti,ab,kf. or magnesium sulfate.ti,ab,kf. or mg longoral.ti,ab,kf. or sulfamag.ti,ab,kf. or sulmetin.ti,ab,kf. or magnesium sulphate.ti,ab,kf. or aero clenil.ti,ab,kf. or aeroclenil.ti,ab,kf. or ah 3365.ti,ab,kf. or ah3365.ti,ab,kf. or albuterol.ti,ab,kf. or almotex.ti,ab,kf. or asmacaire.ti,ab,kf. or asmadil.ti,ab,kf. or asmalin.ti,ab,kf. or asmasal.ti,ab,kf. or asmatol.ti,ab,kf. or asmaven.ti,ab,kf. or asmavent.ti,ab,kf. or asmidon.ti,ab,kf. or asmol.ti,ab,kf. or broncho spray.ti,ab,kf. or broncho-spray.ti,ab,kf. or bronter.ti,ab,kf. or brytolin.ti,ab,kf. or butahale.ti,ab,kf. or buto-asma.ti,ab,kf. or butomix.ti,ab,kf. or butotal.ti,ab,kf. or butovent.ti,ab,kf. or buventol.ti,ab,kf. or cibutamol.ti,ab,kf. or cletal.ti,ab,kf. or cybutol.ti,ab,kf. or dilatamol.ti,ab,kf. or ecovent.ti,ab,kf. or emplusal.ti,ab,kf. or exafil.ti,ab,kf. or farcolin.ti,ab,kf. or frespire.ti,ab,kf. or glisend.ti,ab,kf. or grafalin.ti,ab,kf. or krosalburol.ti,ab,kf. or libretin.ti,ab,kf. or loftan.ti,ab,kf. or mozal.ti,ab,kf. or novosalmol.ti,ab,kf. or parasma.ti,ab,kf. or proventil.ti,ab,kf. or prox-s.ti,ab,kf. or pulmol s.ti,ab,kf. or repetabs.ti,ab,kf. or respolin.ti,ab,kf. or salamol.ti,ab,kf. or salbuair.ti,ab,kf. or salbulin.ti,ab,kf. or salbumol fort.ti,ab,kf. or salbupart.ti,ab,kf. or salbutamol.ti,ab,kf. or salbuvent.ti,ab,kf. or salden.ti,ab,kf. or salgem.ti,ab,kf. or salmol.ti,ab,kf. or saltos.ti,ab,kf. or solbutamol.ti,ab,kf. or spacehaler.ti,ab,kf. or sultanol.ti,ab,kf. or venetlin.ti,ab,kf. or ventilan.ti,ab,kf. or ventodisk.ti,ab,kf. or ventodisks.ti,ab,kf. or ventol.ti,ab,kf. or volmac.ti,ab,kf. or benralizumab.ti,ab,kf. or fasenra.ti,ab,kf. or medi 563.ti,ab,kf. or medi563.ti,ab,kf. or bosatria.ti,ab,kf. or mepolizumab.ti,ab,kf. or nucala.ti,ab,kf. or sb 240563.ti,ab,kf. or sb-240563.ti,ab,kf. or sb240563.ti,ab,kf. or cinqaero.ti,ab,kf. or cinqair.ti,ab,kf. or reslizumab.ti,ab,kf. or sch 55700.ti,ab,kf. or sch55700.ti,ab,kf. or actamone.ti,ab,kf. or airathon.ti,ab,kf. or airing.ti,ab,kf. or alvokast.ti,ab,kf. or apilone.ti,ab,kf. or ascafi.ti,ab,kf. or ascolin.ti,ab,kf. or asmenol.ti,ab,kf. or asprevent.ti,ab,kf. or astecon.ti,ab,kf. or asthator.ti,ab,kf. or asthmasan.ti,ab,kf. or asthmont.ti,ab,kf. or astmirex.ti,ab,kf. or astmodil.ti,ab,kf. or atentus.ti,ab,kf. or atlabiclo.ti,ab,kf. or belokast.ti,ab,kf. or brolyt.ti,ab,kf. or castispir.ti,ab,kf. or chesmon.ti,ab,kf. or deprive.ti,ab,kf. or elukan.ti,ab,kf. or elunkast.ti,ab,kf. or eonic.ti,ab,kf. or filkast.ti,ab,kf. or fulmont.ti,ab,kf. or imvlo.ti,ab,kf. or ispyrra.ti,ab,kf. or jepafex.ti,ab,kf. or kipres.ti,ab,kf. or l 706631.ti,ab,kf. or l706631.ti,ab,kf. or lanair.ti,ab,kf. or leukast.ti,ab,kf. or lukair.ti,ab,kf. or lukanof.ti,ab,kf. or lukas aiwa.ti,ab,kf. or lukasm.ti,ab,kf. or lukastang.ti,ab,kf. or lukavent.ti,ab,kf. or melarth.ti,ab,kf. or metigrenul.ti,ab,kf. or milukante.ti,ab,kf. or mintalos.ti,ab,kf. or miralust.ti,ab,kf. or "mk 0476".ti,ab,kf. or mk 476.ti,ab,kf. or mk0476.ti,ab,kf. or mk476.ti,ab,kf. or modrian.ti,ab,kf. or modulair.ti,ab,kf. or mofenstra.ti,ab,kf. or mokast.ti,ab,kf. or molucar.ti,ab,kf. or monalux.ti,ab,kf. or monart.ti,ab,kf. or monast.ti,ab,kf. or moncas.ti,ab,kf. or mondeo.ti,ab,kf. or monkasta.ti,ab,kf. or monlast.ti,ab,kf. or monlucare.ti,ab,kf. or monspes.ti,ab,kf. or monstonol.ti,ab,kf. or montair.ti,ab,kf. or montast.ti,ab,kf. or montecell.ti,ab,kf. or montecon.ti,ab,kf. or montefar.ti,ab,kf. or montegen.ti,ab,kf. or montelair.ti,ab,kf. or montelak.ti,ab,kf. or montelar.ti,ab,kf. or montelex.ti,ab,kf. or montelubronch.ti,ab,kf. or montelucaste.ti,ab,kf. or montelukast*.ti,ab,kf. or montelux.ti,ab,kf. or montemyl.ti,ab,kf. or montep.ti,ab,kf. or monterast.ti,ab,kf. or monteresp.ti,ab,kf. or montespir.ti,ab,kf. or montewin.ti,ab,kf. or montexal.ti,ab,kf. or monthan.ti,ab,kf. or montol.ti,ab,kf. or montus.ti,ab,kf. or moolpas.ti,ab,kf. or nal 6336.ti,ab,kf. or nal6336.ti,ab,kf. or orilukast.ti,ab,kf. or otelus.ti,ab,kf. or pentafeno.ti,ab,kf. or perasm.ti,ab,kf. or pluralais.ti,ab,kf. or pneumo-kast.ti,ab,kf. or promonta.ti,ab,kf. or rasec.ti,ab,kf. or relukas.ti,ab,kf. or respilukas.ti,ab,kf. or romilast.ti,ab,kf. or saslong.ti,ab,kf. or singodem.ti,ab,kf. or singulair.ti,ab,kf. or singulair allergy.ti,ab,kf. or singulair ar.ti,ab,kf. or singulair chew.ti,ab,kf. or singulair mini.ti,ab,kf. or singulergy.ti,ab,kf. or solok.ti,ab,kf. or spirokast.ti,ab,kf. or spiromon.ti,ab,kf. or surfair.ti,ab,kf. or symlukast.ti,ab,kf. or telelux.ti,ab,kf. or telukast.ti,ab,kf. or teluki.ti,ab,kf. or tevalukast.ti,ab,kf. or thordel.ti,ab,kf. or valnuen.ti,ab,kf. or velukast.ti,ab,kf. or xaira.ti,ab,kf. or yekast.ti,ab,kf. or zakomoxit.ti,ab,kf. or interleukin 5 antibody.ti,ab,kf. |
37209 |
|
1 |
Status Asthmaticus/ or acute severe asthma.ti,ab,kf. or severe acute asthma.ti,ab,kf. or status asthmaticus.ti,ab,kf. or (asthma* adj3 (cris?s or shock or state or attack* or exacerbat*)).ti,ab,kf. or ((exp Asthma/ or asthma.ti,ab,kf.) and (Airway Obstruction/ or fatal attack*.ti,ab,kf. or near fatal.ti,ab,kf. or respiratory failure.ti,ab,kf.)) or acute asthma.ti,ab,kf. |
20455 |