Matten bij liesbreuk volwassenen
Uitgangsvraag
Welke mat is het beste voor liesbreukchirurgie?
De uitgangsvraag omvat de volgende deelvragen:
- Welke mat is het beste voor TEP (en TAPP): “lichtgewicht” of “zwaargewicht”?
- Welke mat is het beste voor Lichtenstein: “lichtgewicht” of “zwaargewicht”?
- Is een zelfklevende mat (Progrip) bij Lichtenstein beter dan een niet- zelfklevende mat?
Aanbeveling
Het wordt chirurgen aanbevolen zich te verdiepen in de verschillende typen matten en de daarbij horende eigenschappen inclusief kosten, gezien de grote variaties hierin. Gebruik bij voorkeur een mat die is onderzocht en beschreven in de literatuur.
Overweeg geen lichtgewicht mat te gebruiken voor een TEP (of TAPP) liesbreukoperatie: er is geen bewezen voordeel ten aanzien van chronische pijn en de kans op een recidief (bij grotere, mediale defecten) is hoger.
Hoewel het verschil in chronische pijn klein is, kan overwogen worden een lichtgewicht mat te gebruiken voor een liesbreukoperatie volgens Lichtenstein. Men moet zich daarbij bewust zijn van een mogelijk beperkt verhoogde kans op een recidief, met name bij patiënten met een groot defect.
Gebruik geen ProGrip zelfklevende mat voor een operatie volgens Lichtenstein aangezien er geen voordeel is ten aanzien van chronische pijn of recidieven vergeleken met een niet zelfklevende mat.
Overwegingen
TEP
Na analyse van de huidige literatuur is er voldoende bewijs dat het gebruik van een “lichtgewicht” mat de kans op een recidief na een TEP verhoogt.
Het is echter opvallend dat de meeste studies beschrijven dat de kans op een recidief met name verhoogd is bij “grote” mediale defecten. Er bestaat geen duidelijke definitie over wat “groot” is. Verschillende studies nemen als afkap punt >3 cm. Het is onduidelijk hoe onderzoekers de grootte van het defect meten. Het berust vaak op een schatting.
De werkgroep heeft een subgroep analyse kunnen verrichten van de studie van Burgmans en Roos. Deze laat zien dat het aantal recidieven significant verhoogd is na het gebruik van een “lichtgewicht” mat bij mediale breuken. Er is geen verschil bij laterale breuken. Van de andere studies van de meta-analyse is het niet mogelijk een subgroep analyse te verrichten. Het aantal recidieven per studie is laag en veel studies zijn te klein, maar er is vaak wel een trend. Om een recidief na TEP te voorkomen, is het gebruik van een “zwaargewicht” mat aan te bevelen.
De vraag blijft dan, of een zwaargewicht mat niet meer kans geeft op chronische pijn. Uit de analyse blijkt dat het gebruik van een “lichtgewicht” mat bij de TEP de kans op chronische pijn mogelijk verlaagt. Het verschil is echter niet significant en de bewijskracht is beperkt. Studies hanteren verschillende definities van pijn (een bepaalde NRS of VAS-score, het verschil tussen geen pijn of wel pijn of het verschil tussen niet klinisch relevante en klinisch relevante pijn). Daarnaast is de follow-up duur niet altijd gelijk. De meest recente RCT met de grootste aantallen en een goede opzet, laat zelf een iets verhoogde kans zien op chronische pijn na het gebruik van een “lichtgewicht” (Ultrapro) mat.
De kosten van een “zwaargewicht” mat zijn lager dan van een “lichtgewicht” mat. Ook is een “zwaargewicht” mat gemakkelijker te hanteren en te positioneren in vergelijking met een “lichtgewicht” mat. Het is belangrijk om dit mee te laten wegen in de keuze voor een mat bij een TEP.
Op basis van de analyse en bovenstaande overwegingen, adviseert de werkgroep in ieder geval een “zwaargewicht” mat te gebruiken bij patiënten met (grotere) mediale defecten. Voor kleinere, laterale defecten zou een “lichtgewicht” mat overwogen kunnen worden.
Het is tot op heden onduidelijk of naast het gebruik van een “zwaargewicht” mat het fixeren van de mat bij grote defecten bijdraagt om de kans op een recidief (en bulging) te verkleinen. Hiervoor verwijzen wij naar module 11: matfixatie. Ook is er discussie over het eventueel verkleinen van de holte van een groot mediaal defect door het reven en sluiten van de uitpuilende fascia transversalis. Deze laatste techniek lijkt de kans op seroom te verkleinen, maar het is niet aangetoond dat de kans op een recidief hiermee ook lager wordt.
Lichtenstein
Na analyse van de huidige literatuur lijkt er een lichte voorkeur voor het gebruik van een “lichtgewicht” mat bij een Lichtenstein plastiek omdat de kans op chronische pijn lager lijkt. De bewijskracht hiervoor is echter beperkt. Daarnaast zijn er zorgen over het aantal recidieven dat na het gebruik van een “lichtgewicht” mat hoger lijkt. Een harde aanbeveling is dus niet te geven.
Bij de keuze tussen een “lichtgewicht” en een “zwaargewicht” mat, zullen meerdere factoren van belang zijn die nauwelijks beschreven zijn in de literatuur. Zo kunnen andere factoren (leeftijd, gewicht, beroep, collageenziekten, type breuk en grootte van het defect) meespelen bij deze keuze. Een lichtgewicht mat is een goede keuze. Als de chirurg de kans op een recidief aanzienlijk groter inschat, is de keuze voor een zwaargewicht mat wellicht beter.
Een recente review van Molegraaf laat zien dat er geen voordeel is voor het plaatsen van een zelf-klevende ProGrip mat ten opzichte van een niet klevende mat. De kans op chronische pijn is niet lager en het aantal recidieven is ook niet minder.
Onderbouwing
Achtergrond
Er bestaat een enorm aanbod van verschillende type matten voor liesbreukchirurgie, variërend in materiaal, gewicht, porie-grootte, vorm en kosten. Het doel van het plaatsen van een mat is het voorkomen van een recidief zonder dat de mat bijwerkingen (chronische pijn of discomfort door een hinderlijk, stijf gevoel) geeft. Daarom moet een mat voldoen aan verschillende eigenschappen zoals elasticiteit met behoud van voldoende kracht (om herhaalde mechanische druk te weerstaan en een recidief te voorkomen) en een goede bio compatibiliteit.
Er bestaan zorgen over het optreden van eventuele bijwerkingen veroorzaakt door de geplaatste mat. Men moet zich evenwel realiseren dat naast de mat er vele andere factoren zijn (type operatie, complicaties, ervaring van de chirurg, patiënt gerelateerde factoren) die kunnen bijdragen aan deze bijwerkingen.
Door de diversiteit van alle typen matten die beschikbaar zijn voor liesbreukchirurgie en door de grote variatie van operatie- en patiënt factoren, is het onmogelijk de ideale mat te beschrijven en aan te bevelen.
Voor een uitgebreid overzicht met verschillende uitgangsvragen aangaande matten, verwijzen wij naar module 10 van de internationale richtlijn (HerniaSurge Group, 2018).
Voor de huidige Nederlandse richtlijn hebben wij de moeilijke discussie over wat de ideale mat zou zijn voor een bepaalde techniek gepoogd te vereenvoudigen door deze toe te schrijven naar de meest voorkomende technieken voor liesbreuk correctie in Nederland: de Lichtenstein plastiek en de TEP en als uitgangsmaat te kijken naar chronische pijn (vanaf 3 maanden) en recidieven (geen termijn). In de analyse hebben wij 3D matten uitgesloten, omdat deze nauwelijks onderzocht zijn voor TEP en niet gebruikt worden voor Lichtenstein. Specifiek voor de operatie volgens Lichtenstein, worden in Nederland ook zelf-fixerende matten gebruikt. Deze zijn vergeleken met matten die met hechtingen in de patiënt gefixeerd zijn.
Omdat de meeste RCT’s onderscheid maken tussen een (grove poriën) “lichtgewicht mat”, (waarbij Ultrapro het meest is onderzocht) en een kleinere poriën “zwaargewicht” mat (polypropyleen), en deze matten ook veel in Nederland worden gebruikt, hebben wij de uitkomsten van deze typen matten opnieuw geanalyseerd. Tot op heden is er geen duidelijke definitie van een “lichtgewicht” en een “zwaargewicht” mat. Voor deze uitgangsvraag is daarom gekozen voor het meest gebruikelijke afkappunt ≤ 50 g/m2 voor “lichtgewicht” en >70 g/m2 voor “zwaargewicht”.
Conclusies / Summary of Findings
|
Redelijk GRADE |
Voor patiënten die een TEP (of TAPP) correctie ondergaan lijkt er geen verschil in het risico op chronische pijn met het gebruik van een “lichtgewicht” mat lager dan met een “zwaargewicht” mat. |
|
Hoog GRADE |
Voor patiënten die een TEP (of TAPP) correctie ondergaan is het risico op een recidief hoger na het gebruik van een “lichtgewicht” mat in vergelijking met een “zwaargewicht” mat. Dit risico lijkt met name verhoogd bij patiënten met een (grote) mediale breuk. |
|
Redelijk GRADE |
Voor patiënten die een Lichtenstein correctie ondergaan is het risico op chronische pijn lager na het gebruik van een “lichtgewicht” mat in vergelijking met een “zwaargewicht” mat. |
|
Redelijk GRADE |
Voor patiënten die een Lichtenstein correctie ondergaan lijkt het risico op een recidief hoger na het gebruik van een “lichtgewicht” mat in vergelijking met een “zwaargewicht” mat. |
|
Redelijk GRADE |
De kans op chronische pijn met het gebruik van een zelfklevende ProGrip mat bij patiënten die een Lichtenstein correctie ondergaan is niet lager vergeleken met een niet zelfklevende mat. |
|
Redelijk GRADE |
De kans op een recidief met het gebruik van een zelfklevende ProGrip mat bij patiënten die een Lichtenstein correctie ondergaan is niet lager vergeleken met een niet zelfklevende mat. |
Samenvatting literatuur
Description of the studies on endoscopic inguinal hernia repair
Sajid (2013) systematically searched relevant databases till June 2011 and analysed the RCTs on the use of lightweight mesh (LWM) versus heavyweight mesh (HWM) in patients undergoing laparoscopic inguinal hernia repair (LIHR) by both trans-abdominal pre-peritoneal (TAPP) and total extraperitoneal (TEP) approach. Sajid (2013) included the RCTs on patients of any age, any gender, on both elective and emergency LIHR for every indication i.e. groin lump, groin pain, inguinoscrotal lump, unilateral, bilateral, or recurrent hernia. They included eleven RCTs (Agarwal, 2009; Bittner, 2011; Bittner, 2011*; Bringman, 2005; Champault, 2007; Chowbey, 2010; Chui, 2010; Heikkinen, 2006; Langenbach, 2006; Langenbach, 2008; Peeters, 2010) encompassing 2189 patients.
Sajid (2013) concluded that there were significant limitations in the methodological quality of few included RCTs analysed in this review. Methodological limitations included not adequately (reporting of) blinding of the trial participants and outcome assessors, lack of intention-to-treat analysis and potential Risk of Bias in industry-sponsored trials.
Two mistakes in the review by Sajid (2013) were detected. Sajid included the mesh of 55 g/m2 in the study by Bittner (2011)* as a LWM. Moreover, chronic pain in the study of Bringman (2005) was assessed although follow-up was only 2 months. For current analysis both mistakes were adjusted for and the mesh and study were not included in the meta-analyses.
Burgmans(2015 and 2016)/ Roos (2018) conducted a prospective double-blinded RCT in 949 patients. This is the largest sample size in the field up to date. They included adult, male patients with a primary, reducible, unilateral inguinal hernia and no contraindications for TEP repair. All patients underwent TEP repair without the use of any fixation, sealant, adjunct, or glue. Outcomes of chronic pain were assessed up to 2 years and recurrences up to 5 years postoperatively.
Risk of Bias in this study was low.
Prakash (2016) randomised 140 adult patients with uncomplicated inguinal hernia into a HW mesh group or LW mesh group. All the patients underwent LIHR by either TAPP or TEP method. No mesh fixation was used in either group. A total of 131 patients completed a minimum of 3 months follow-up period, 66 in HW mesh group and 65 in LW mesh group. Outcomes were assessed up to 12 months postoperatively.
Risk of Bias in this study was low, the only potential Risk of Bias was due to inadequate blinding of care providers and outcome assessors to treatment allocation.
Kalra (2017) conducted a multi-center RCT comparing TAPP repair with placement of either a LWM or a HWM. Tackers were used for the fixation of the mesh. Patients were 15 to 60 years, with a unilateral, reducible inguinal hernia. Sixty patients were included with a follow-up of 3 months.
Risk of Bias in this study was high due to underreporting. Any form of blinding and loss to follow-up was not reported.
Wong (2017) performed a double-blind RCT in 85 patients with a mean follow-up of 20.3 months (range 12 to 34 months). All patients underwent TEP repair. No device or agent was used to anchor the mesh. Inclusion criteria were age 18 to 81 years and a primary uncomplicated unilateral or bilateral inguinal hernia. The recurrence rate was assessed.
Risk of Bias in this study was low, only the randomization technique was not described.
Results
1. Chronic pain
The meta-analysis from Sajid (2013) could be updated with data from Burgmans (2016). Prakash (2016) and Kalra (2017) presented chronic pain outcome as the mean VAS ± SD. We were unable to include these studies in the meta-analysis. The forest plot is shown in figure 1. Overall, the risk of chronic groin pain was estimated to be reduced by 34% when using a LWM compared with a HWM (RR 0.66, 95%CI: 0.38 to 1.16), but the confidence interval was wide and enclosed 1, indicating a not statistically significant and imprecise effect estimate.
There was substantial heterogeneity, possibly due to variation in the duration of follow-up (chi-square = 13.66, df = 6, P = 0.03, I² = 57%) among the RCTs.
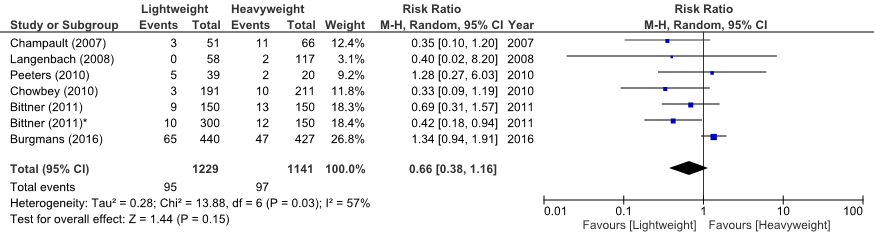
Figure 1 Meta-analysis of trials comparing LWM versus HWM in patients undergoing endoscopic surgery for hernia repair (Outcome chronic groin pain)
2. Recurrence
The meta-analysis from Sajid 2013 could be updated with data from Prakash 2016; Wong, 2017 and Roos, 2018. The pooled analysis showed that the risk of recurrences was increased by a factor 2.83 when a LWM was used compared to a HWM (RR 2.83, 95%CI 1.41 to 5.70).
There was no statistical heterogeneity among the RCTs (chi-square = 2.44, df = 7, P = 0.93, I² = 0%).
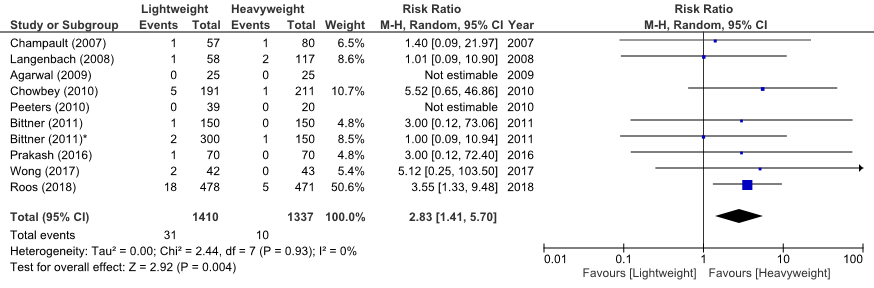
Figure 2 Meta-analysis of trials comparing LWM versus HWM in patients undergoing endoscopic surgery for hernia repair (Outcome recurrence)
Recurrences after direct or indirect hernia repair:
The study by Burgmans (2016) and Roos (2018) showed significantly more recurrences for LWM after direct hernia repair (LWM n=12, HWM n=1, p=0.003), but no significance for recurrences after indirect hernia repair (LWM n=6, HWM n=4, p=0.545). The study by Wong (2017) does not report on the matter for the 2 recurrences. Prakash 2016 showed 1 recurrence in the LWM-group after an indirect inguinal hernia repair. Bittner (2011)* reported all 3 recurrences (2 LWM, 1 HWM) after direct hernia repair. Bittner 2011 found 1 recurrence after direct hernia repair. Chowbey 2010 found out of five recurrences in the LWM-group, 3 patients had large indirect hernias and 2 patients had large direct hernias. Langenbach (2008) found all 3 recurrences were after direct hernia repair. Champault (2007) does not report on the matter for 2 recurrences.
Quality of the evidence
Evidence originated from RCTs and the level of the quality of the evidence comparing LWM versus HWM started therefore at ‘High’. However, the quality of the evidence regarding chronic pain was downgraded with one level to ‘Moderate’ for imprecision (the confidence interval crosses 1). We did not downgrade further for limitations in the methodological quality of the included RCTs (Risk of Bias).
The quality of the evidence regarding recurrence was not downgraded. The lower boundary of the confidence interval was considered clinically relevant.
Description of the studies on Lichtenstein inguinal hernia repair
Sajid (2012) performed a systematic review with a meta-analysis of the literature on lightweight mesh (LWM) compared with heavyweight mesh (HWM) in open inguinal hernia repair. Relevant databases were searched till May 2011. Any trials that compared LMW with HWM were included. A total of 9 RCT were found and described (Bringman, 2006; Champault, 2007; Koch, 2008; Nikkolo, 2010; O’Dwyer, 2005; Paajanen, 2007; Post, 2004; Smietanski, 2008); Smietanski, 2011; Torcivia, 2011) encompassing 1156 patients with a LWM and 1154 patients with a HWM. In all trials the HWM consisted of polypropylene. In all trials all patients underwent a Lichtenstein technique. All trials scored highly enough to suggest good quality of the included trials.
Sajid (2012( concluded that the duration of operation, postoperative pain, postoperative complications, hernia recurrence rate, risk of testicular atrophy and time to return to work were comparable between LWM and HWM. LWM was associated with a reduced risk of developing chronic groin pain and other groin symptoms.
For the current analysis the short-term results published by Paajanen (2007), Nikkolo (2010) and Smietanski (2008) were replaced with the long-term results published by Paajanen (2012), Nikkolo (2012) and Bury (2012), respectively.
A mistake in the review by Sajid was detected. The review wrongly presented Smietanski (2011) as 5 year follow-up of Smietanski (2008). Smietanski (2011) is an original study, with no previously published short-term results, that investigates a different LWM compared to Smietanski (2008). Bury (2012) is the correct 5 year follow-up of Smietanski (2008). In our analysis we adjusted for this mistake.
Apart from the long-term follow-up studies, in 9 more studies after the latest review and meta-analysis by Sajid another 978 patients with a LWM and 964 patients with a HWM were studied. In all studies the groups were comparable at baseline. In all studies patients were aged over 18 years and most studies included primary unilateral inguinal hernias. Only Pielaciński (2013) and Bona (2018) included bilateral inguinal hernias. Pielaciński (2013) was the only study that included recurrent hernias. In all studies a Lichtenstein technique was performed. Most studies used sutures for fixation of the mesh. In four studies (Paradowski, 2009; Sadowski, 2011, Rutegård, 2017), Bona 92018) mesh fixation was not reported. In the study by Canonico (2012) human fibrin glue was used for fixation of the mesh. Follow-up of the studies ranged from 3 to 60 months. Canonico (2012) and Rutegård (2017) were the only two studies that did not report chronic pain as an outcome. Sadowski (2011) was the only study that did not have recurrence as an outcome.
Risk of Bias in half of the studies was low (see: Risk of Bias table). Risk of Bias was high in the following studies: Sadowski (2011), Canonico (2012) and Pielaciński (2013) did not report on the randomization technique and concealment of allocation, and bias due to loss to follow-up was likely or unclear. Moreover in the study of Sadowski (2011) and Pielaciński (2013) bias due to blinding was likely or unclear and Canonico (2012) performed selective outcome reporting because the 6 months results were not described. In the study of Nikkolo (2012) blinding was not reported and loss to follow-up was high. Lee (2017) did not report on the randomization technique, concealment of allocation and blinding of outcome assessors. Bona (2018) did not blind the outcome assessors, loss to follow-up was high and bias due to selective outcome reporting was likely because the 12 months results were not described.
Results
1. Chronic pain
The data from the review by Sajid (2012) could be updated with the long-term follow-up results of Paajanen (2012), Nikkolo (2012) and Bury (2012), and with 6 additional studies (Paradowski, 2009; Sadowski, 2011; Yazdankhah Kenary, 2013; Demetrashvili, 2014; Lee, 2017; Rutegård, 2017; Bona, 2018).
Therefore, 15 trials (n=3394) reported applicable data for meta-analysis on the outcome chronic groin pain. In the LWM-group 234 of 1669 patients reported chronic pain, compared to 325 of 1725 patients in the HWM-group. The risk of having chronic groin pain after a hernioplasty with a LWM was estimated to be 23% lower than with a HWM. The pooled risk ratio estimate was 0.77 (95%CI: 0.61 to 0.96). Inapplicable for meta-analysis, Lee 2017 reported chronic pain as a mean VAS (SD) of 0.7 (1.1) for the LWM and 0.8 (1.4) for the HWM.
There was low statistical heterogeneity among the RCTs (chi-square = 19.11, df = 14, P = 0.16, I² = 27%).
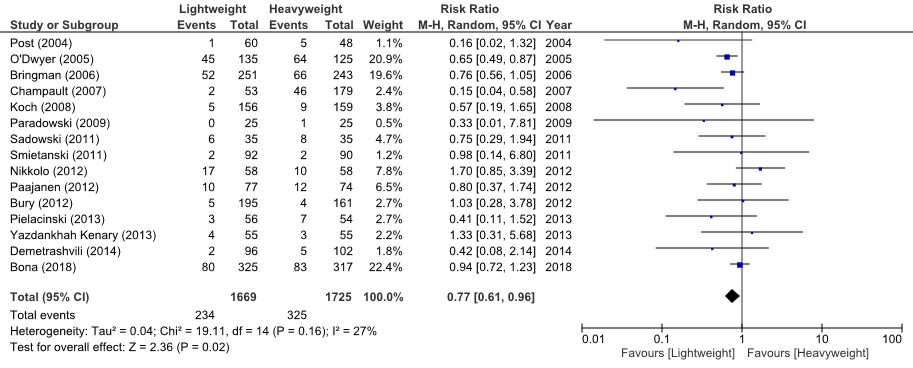
Figure 3 Meta-analysis of trials comparing LWM versus HWM in patients undergoing open surgery for hernia repair (Outcome chronic groin pain)
1. Recurrence
The data from the review by Sajid (2012) could be updated with the long-term follow-up results of Paajanen (2012), Nikkolo (2012) and Bury (2012), and with 8 additional studies (Paradowski, 2009; Canonico, 2012; Pielaciński, 2013; Yazdankhah Kenary, 2013; Demetrashvili, 2014; Lee, 2017; Rutegård, 2017; Bona, 2018).
Seventeen trials (n=3953) reported data on the outcome recurrences. In the combined results 40 patients who received a LWM had a recurrence, while 27 patients with a HWM had a recurrence. The risk for recurrence was therefore increased with the use of LWM with a pooled risk ratio estimate of 1.48 (95%CI: 0.86 to 2.52).
There was no heterogeneity among the RCTs (chi-square = 8.53, df = 12, P = 0.74, I² = 0%).
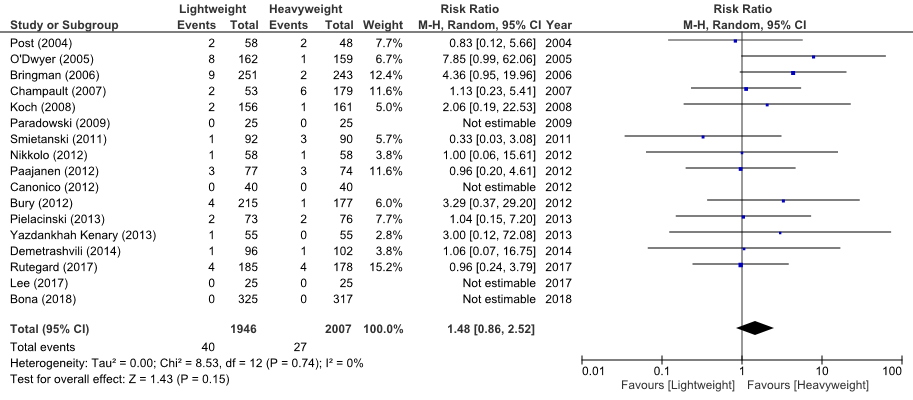
Figure 4 Meta-analysis of trials comparing LWM versus HWM in patients undergoing open surgery for hernia repair (Outcome recurrence).
Quality of the evidence
Evidence originated from RCTs and the level of the quality of the evidence comparing LWM versus HWM started therefore at ‘High’. However, the quality of the evidence regarding chronic pain was downgraded with one level to ‘Moderate’ for imprecision, as the upper boundary of the confidence is very close to 1 and we therefore cannot rule out that the difference might not be clinically relevant.
The quality of the evidence regarding recurrence was downgraded with one level to ‘Moderate’ for imprecision (the confidence interval crosses 1). We did not downgrade further for limitations in the methodological quality of the included RCTs (Risk of Bias).
Description of the SR on Lichtenstein inguinal hernia repair with ProGrip
The 10 RCTs that were included in the SR of Molegraaf 2018 described 2,541 patients (n = 1,216 self-gripping mesh group, n = 1,245 sutured mesh group). The duration of follow-up ranged from 6 to 72 months.
The quality assessment of the studies by Molegraaf 2018 showed that the quality of 2 trials was poor due the absence of an adequate randomization technique or no information about it, absence of blinding, no power calculations, and no baseline score. The other trials were of moderate or good quality, despite the common absence of blinding and poor reporting of the definition or assessment method of chronic pain. In four studies a baseline pain score was lacking, although preoperative pain is a well-known risk factor for chronic pain. Furthermore, some trials compared different types of meshes in the 2 study groups instead of only changing the method of mesh fixation (polypropylene and polyester, and heavy and low weight).
Results
Chronic pain (critical outcome)
Chronic pain was assessed in all trials and 9 of them reported the incidence of chronic pain according to the definition used in their study protocol. Incidence rates were analysed separately for the different moments of follow-up (3, 6 to 12, 24, 36, and 72 months).
At all follow-up time points, there was no significant difference in the incidence of CPIP between the self-gripping mesh and sutured mesh group (3 months OR = 0.89, 95% CI 0.48 to 1.64 (n=425); 6 to 12 months OR = 1.00; 95% CI 0.75 to 1.34 (n=1517); 24 months OR = 1.00; 95% CI 0.39 to 2.61 (n=372); 36 to 72 months OR = 0.77; 95% CI 0.38 to 1.58 (n=464)).
Recurrence (important outcome)
All trials reported recurrence rates after 12 months of follow-up. Two studies also provided recurrence rates after 24 months, 2 after 36 months and 1 after 72 months. The difference in recurrence rate between the self-gripping mesh group and the sutured mesh group was not significant at 12 months (OR = 1.19, 95% CI 0.61 to 2.31 (n=2435)), 24 months (OR = 1.06, 95% CI, 0.27 to 4.17(n=372)), or 36 months (OR = 0.95, 95% CI, 0.18 to 5.14 (n=450) (Molegraaf 2018).
Quality of the evidence
Evidence comparing open inguinal hernia repair with a self-gripping ProGrip mesh and a conventional Lichtenstein hernioplasty originated from RCTs and the level of the quality of the evidence regarding chronic pain and recurrence after inguinal hernia repair with self-gripping meshes started therefore at ‘High’. However, the quality of the evidence was downgraded one level for substantial methodological limitations of the studies to ‘Moderate’. We did not downgrade for the aforementioned clinical heterogeneity between studies, because the subgroup analyses accounting for mesh weight and including only studies that used a light weighted mesh in both the study and control group also showed no difference in CPIP rates between the self-gripping mesh and sutured mesh (Molegraaf, 2018).
Zoeken en selecteren
In order to answer the clinical ‘question’, a systematic literature search was done for the following research questions:
Which mesh is recommended for endoscopic and open inguinal hernia repair?
PICO 1
P: patients with an inguinal hernia undergoing endoscopic surgery;
I: mesh (Lightweight; ≤50 g/m2);
C: mesh (Heavyweight; >70 g/m2)
O: chronic pain (>3 months), recurrence.
PICO 2
P: patients with an inguinal hernia undergoing Lichtenstein inguinal hernia repair;
I: mesh (Lightweight; ≤50 g/m2);
C: mMesh (Heavyweight; >70 g/m2);
O: chronic pain (>3 months), recurrence.
PICO 3
P: patients with an inguinal hernia undergoing Lichtenstein inguinal hernia repair;
I: self-fixing/self-gripping mesh (ProGrip);
C: mesh with fixation by sutures;
O: chronic pain (>3 months), recurrence.
Relevant outcome measures
The working group decided that chronic pain and recurrences were crucial outcome measures for decision-making. Recurrences after inguinal hernia repair have dropped dramatically with the introduction of tension-free mesh repair and endoscopic preperitoneal approaches. Still, recurrences and chronic pain are the most common long-term complications after inguinal hernia repair. According to the International Association for the Study of Pain, chronic pain is defined as (inguinal) pain lasting a minimum of 3 months. Any level of pain was considered relevant. For the detection of recurrences any time-period was considered relevant for comparison. A distinction between recurrences after direct hernia repairs or indirect hernia repairs was sought for in included endoscopic repair studies.
Searching and selecting (Methods)
A literature search, for open and for endoscopic inguinal hernia repair, was conducted in MEDLINE, EMBASE and the Cochrane Library at June 6th 2018. The search was not limited by publication date or language. The search details can be found in the tab Acknowledgement. Subsequently, a filter for identifying RCTs was used to filter out nonrandomized trials. All duplicates were removed. Literature experts excluded non-relevant studies based on title and abstract and/or full-text screening.
For the comparison of lightweight versus heavyweight meshes after endoscopic inguinal hernia repair the working group selected 19 studies based on title-abstract that could possibly answer the research questions. After reading full text, 2 studies were excluded (see exclusion table in the tab Acknowledgement). Finally, the studies from the latest review and meta-analysis for endoscopic mesh comparison by Sajid 2013 and 6 additional original studies that were not included in Sajid (2013) were included for analysis (Burgmans, 2015; Burgmans, 2016; Prakash, 2016; Kalra, 2017; Wong, 2017 and Roos, 2018).
For the comparison of lightweight versus heavyweight meshes after open inguinal hernia repair the working group selected 25 studies based on title-abstract, that could possibly answer the research questions. After reading full text, 2 studies were excluded (see exclusion table in the tab Acknowledgement). Finally, the studies from the latest review and meta-analysis for open mesh comparison by Sajid (2012) and 12 additional original studies that were not included in Sajid 2012 were included for analysis (Paradowski, 2009; Sadowski, 2011; Bury, 2012; Nikkolo, 2012; Canonico, 2013; Paajanen, 2013; Pielaciński, 2013; Yazdankhah Kenary, 2013; Demetrashvili, 2014; Lee, 2017; Rutegård, 2017; Bona, 2018).
The 2 SRs with meta-analyses (Sajid, 2012 and Sajid, 2013), containing 20 RCTs were included in the literature analysis. These SRs were updated with the recent RCTS.
During the preparation and writing of the guideline text of this module, a relevant systematic review was published that answered PICO 3 (Molegraaf, 2018). Molegraaf (2018) performed a systematic review of the literature to identify RCTs comparing open inguinal hernia repair with a self-gripping ProGrip mesh and a conventional Lichtenstein hernioplasty. The working group decided that this SR and meta-analysis provided the most up-to-date overview of RCTs regarding self-gripping (ProGrip) mesh.
Data extraction and analysis
The most important study characteristics and results were extracted from the SRs or original studies (also, in case of missing information in the review). The most important study characteristics and relevant results are shown in the evidence tables. The judgement of the individual study quality (Risk of Bias) is shown in the Risk of Bias tables.
It was agreed that the blinding of the care providers (operating surgeon) was impossible. The Risk of Bias however was rated as low, since the type of mesh used is an unlikely influence on the success of surgery execution. In the case of not reported intention-to-treat analysis it was considered an unlikely Risk of Bias due to the inability to cross-over. The lack of an adequate randomization technique (or not reported), inadequate blinding (or not reported) and a high or non-proportionally divided loss to follow-up was considered a high Risk of Bias.
The summary of included studies, study characteristics and quality of the SRs by Sajid are presented in the evidence table for SRs and the quality assessment table for SRs.
Relevant pooled and/or standardised effect measures were, if useful, calculated using Review Manager 5.3 (Cochrane Collaboration, Oxford, United Kingdom). If pooling results was not possible, the outcomes and results of the original study were used as reported by the authors.
The working group did not define clinical (patient) relevant differences for the outcome measures. Therefore, we used the following boundaries for clinical relevance, if applicable: for continue outcome measures: RR <0.75 or >1.25 (GRADE recommendation) or Standardized mean difference (SMD=0.2 (little); SMD 0.5 (reasonable); SMD=0.8 (large). These boundaries were compared with the results of our analysis. The interpretation of dichotomous outcome measures is strongly related to context; therefore, no clinical relevant boundaries were set beforehand. For dichotomous outcome measures, the absolute effect was calculated (Number Needed to Treat (NNT) or Number Needed to Harm (NNH)).
Referenties
- Bona S, Rosati R, Opocher E, et al. SUPERMAT Study Group. (2018) Pain and quality of life after inguinal hernia surgery: a multicenter randomized controlled trial comparing lightweight vs heavyweight mesh (Supermesh Study). Updates Surg 70(1):77-83.
- Burgmans JP, Voorbrood CE, Schouten N, et al. (2015) Three-month results of the effect of Ultrapro or Prolene mesh on post-operative pain and well-being following endoscopic totally extraperitoneal hernia repair (TULP trial). Surg Endosc 29(11):3171-3178.
- Burgmans JP, Voorbrood CE, Simmermacher RK, et al. (2016) Long-term Results of a Randomized Double-blinded Prospective Trial of a Lightweight (Ultrapro) Versus a Heavyweight mesh (Prolene) in Laparoscopic Total Extraperitoneal Inguinal Hernia Repair (TULP-trial). Ann Surg 263(5):862-866.
- Bury K, Smietanski M, Polish Hernia Study Group. (2012) Five-year results of a randomized clinical trial comparing a polypropylene mesh with a poliglecaprone and polypropylene composite mesh for inguinal hernioplasty. Hernia 16(5):549-553.
- Canonico S, Benevento R, Perna G, et al. (2013) Sutureless fixation with fibrin glue of lightweight mesh in open inguinal hernia repair: effect on postoperative pain: a double-blind, randomized trial versus standard heavyweight mesh. Surgery 153(1):126-130.
- Demetrashvili Z, Khutsishvili K, Pipia I, et al. (2014) Standard polypropylene mesh vs lightweight mesh for Lichtenstein repair of primary inguinal hernia: a randomized controlled trial. Int J Surg 12(12):1380-1384.
- Kalra T, Soni RK, Sinha A. (2017) Comparing Early Outcomes using Non Absorbable Polypropylene mesh and Partially Absorbable Composite mesh through Laparoscopic Transabdominal Preperitoneal Repair of Inguinal Hernia. J Clin Diagn Res 11(8):PC13-PC16.
- Lee SD, Son T, Lee JB, et al. (2017) Comparison of partially-absorbable lightweight mesh with heavyweight mesh for inguinal hernia repair: multicenter randomized study. Ann Surg Treat Res 93(6):322-330.
- Molegraaf M, Kaufmann R, Lange J. (2018) Comparison of self-gripping mesh and sutured mesh in open inguinal hernia repair: A meta-analysis of long-term results. Surgery 163 351360.
- Nikkolo C, Murruste M, Vaasna T, et al. (2012) Three-year results of randomised clinical trial comparing lightweight mesh with heavyweight mesh for inguinal hernioplasty. Hernia 16(5):555-559.
- Paajanen H, Ronka K, Laurema A. (2013) A single-surgeon randomized trial comparing three meshes in lichtenstein hernia repair: 2- and 5-year outcome of recurrences and chronic pain. Int J Surg 11(1):81-84.
- Paradowski T, Olejarz A, Kontny T, et al. (2009) Polypropylene vs. ePTFE vs. WN mesh for Lichtenstein inguinal hernia repair - A prospective randomized, double blind pilotstudy of one-year follow-up. Wideochir Inne Tech Ma?oinwazyjne 4(1):6-9.
- Pielacinski K, Szczepanik AB, Wroblewski T. (2013) Effect of mesh type, surgeon and selected patients' characteristics on the treatment of inguinal hernia with the Lichtenstein technique. Randomized trial. Wideochir Inne Tech Maloinwazyjne 8(2):99-106.
- Prakash P, Bansal VK, Misra MC, et al. (2016) A prospective randomised controlled trial comparing chronic groin pain and quality of life in lightweight versus heavyweight polypropylene mesh in laparoscopic inguinal hernia repair. J Minim Access Surg 12(2):154-161.
- Roos M, Bakker WJ, Schouten N, et al. (2018) Higher Recurrence Rate After Endoscopic Totally Extraperitoneal (TEP) Inguinal Hernia Repair With Ultrapro Lightweight mesh: 5-Year Results of a Randomized Controlled Trial (TULP-trial). Ann Surg 268(2):241-246.
- Rutegard M, Gumuscu R, Stylianidis G, et al. (2018) Chronic pain, discomfort, quality of life and impact on sex life after open inguinal hernia mesh repair: an expertise-based randomized clinical trial comparing lightweight and heavyweight mesh. Hernia 22(3):411-418.
- Sadowski B, Rodriguez J, Symmonds R, et al. (2011) Comparison of polypropylene versus polyester mesh in the Lichtenstein hernia repair with respect to chronic pain and discomfort. Hernia 15(6):643-654.
- Sajid MS, Leaver C, Baig MK, et al. (2012) Systematic review and meta-analysis of the use of lightweight versus heavyweight mesh in open inguinal hernia repair. Br J Surg. 99(1):29-37. doi: 10.1002/bjs.7718. Epub 2011 Oct 31. Review. PubMed PMID: 22038579.
- Sajid MS, Kalra L, Parampalli U, et al. (2013) A systematic review and meta-analysis evaluating the effectiveness of lightweight mesh against heavyweight mesh in influencing the incidence of chronic groin pain following laparoscopic inguinal hernia repair. Am J Surg 205(6):726-736. PubMed PMID: 23561639.
- Wong JC, Yang GP, Cheung TP, et al. (2018) Prospective randomized controlled trial comparing partially absorbable lightweight mesh and multifilament polyester anatomical mesh in laparoscopic inguinal hernia repair. Asian J Endosc Surg 11(2):146-150.
- Yazdankhah Kenary A, Afshin SN, Ahmadi Amoli H, et al. (2013) Randomized clinical trial comparing lightweight mesh with heavyweight mesh for primary inguinal hernia repair. Hernia 17(4):471-477.
Evidence tabellen
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question: Which mesh is recommended for endoscopic and open inguinal hernia repair?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Open inguinal hernia repair |
|||||||
|
Sajid 2012
|
SR and meta-analysis of RCTs
Literature search up to May 2011
A: Bringman (2006) B: Champault (2007) C: Koch (2008) D: Nikkolo (2010) E: O’Dwyer (2005) F: Paajanen (2007) G: Post (2004) H: Smietanski (2008) I: Smietanski (2011) J: Torcivia (2011)
Study design: All RCTs
Setting and Country: A: Sweden and Finland B: France C: Sweden D: Estonia E: UK and Germany F: Finland G: Germany H: Poland I: Poland J: France
Source of funding: Not reported for the included trials or review
|
Inclusion criteria SR:
10 studies included
Important patient characteristics at baseline:
N, age (y) A: 494 patients LWM: 55 HWM: 55 B: 232 patients, 54 C: 317 patients LWM: 56 HWM: 57 D: 135 patients LWM: 59 HWM: 57 E: 321 patients LWM: 55 HWM: 57 F: 233 patients LWM: 56 HWM: 59 G: 108 patients LWM: 60 HWM: 62 H: 392 patients LWM: 56 HWM: 56 I: 182 patients LWM: 55 HWM: 58 J: 47 patients LWM: 54 HWM: 54
Sex: A: All male B: Mixed C: All male D: Mixed E: Mixed F: Mixed G: Mixed H: Mixed I: Mixed J: Mixed
|
Open inguinal hernia repair + Lightweight mesh (LWM)
LWM was defined as surgical mesh with a tensile strength of 16 N/cm, elasticity of 20–35 per cent at a tensile strength of 16 N/cm, pore size more than 1 mm, and containing woven lightweight polymers of biomaterial usually weighing less than 50 g/m2. |
Open inguinal hernia repair + Heavyweight mesh (HWM)
In all trials a polypropylene mesh was used. |
End-point of follow-up:
A: 37 months B: 24 months C: 12 months D: 6 months E: 12 months F: 24 months G: 6 months H: 12 months I: 60 months J: 30 days
For how many participants were no complete outcome data available? Not reported
|
Outcome measure-1 Defined as chronic pain, measured as groin pain
Effect measure: RR, [95% CI]: A: 0.76 (0.56-1.05) B: 0.15 (0.04-0.58) C: 0.57 (0.19-1.65) D: 0.57 (0.27-1.22) E: 0.65 (0.49-0.87) F: 3.02 (0.37-24.64) G: 0.16 (0.02-1.32) H: Not reported, due to mistake (see: comments) I: 0.98 (0.14-6.80) J: 0.38 (0.18-0.82)
Pooled effect (fixed effects model): RR 0.61 [95% CI 0.50 to 0.74] favoring LWM. Heterogeneity (I2): 31%
Outcome measure-2 Defined as recurrence
Effect measure: RR, [95% CI]: A: 4.36 (0.95-19.96) B: 1.13 (0.23-5.41) C: 2.06 (0.19-22.53) D: Not estimable E: 7.85 (0.99-62.06) F: 0.75 (0.13-4.42) G: 0.83 (0.12-5.66) H: Not reported, due to mistake (see: comments) I: 0.33 (0.03-3.08)
Pooled effect (fixed effects model): RR 1.82 [95% CI 0.97 to 3.42] favoring HWM. Heterogeneity (I2): 19%
|
Mistake in review detected: Smietanski (2011) is not 5 year follow-up of Smietanski (2008) as wrongly interpreted by Sajid. Smietanski (2011) is original, not previously published, data with a different LWM. Bury (2012) is 5 year follow-up of Smietanski (2008).
Study J was not included in current meta-analyses: J: Only 1 months follow-up
|
|
Endoscopic inguinal hernia repair |
|||||||
|
Sajid 2013
|
SR and meta-analysis of RCTs
Literature search up to June 2011
A: Agarwal (2009) B: Bittner (2011) C: Bittner (2011)* D: Bringman (2005) E: Champault (2007) F: Chowbey (2010) G: Chui (2010) H: Heikkinen (2006) I: Langenbach (2006) J: Langenbach (2008) K: Peeters (2010)
Study design: All RCTs
Setting and Country: A: India B: Germany C: Germany D: Sweden and Finland E: France F: India G: China H: Sweden I: Germany J: Germany K: Belgium
Source of funding: Not reported for the included trials or review
|
Inclusion criteria SR: RCTs comparing the effectiveness of LWM versus HWM (both short-term and long-term outcomes) in patients undergoing LIHR with TEP or TAPP approach. Irrespective of language, country of origin, hospital of origin, blinding, sample size, or publication status.
11 studies included
Important patient characteristics at baseline:
N, age (y) A: 50 patients, 62 B: 300 patients LWM: 53 HWM 52 C: 600 patients LWM: 59 HWM 57 D: 139 patients LWM: 55 HWM 55 E: 137 patients, 54 F: 402 patients LWM: 53 HWM: 52 G: 100 patients, 61 H: 137 patients LWM: 60 HWM: 59 I: 90 patients LWM: 64 HWM: 65 J: 175 patients LWM: 62 HWM: 63 K: 59 patients LWM: 43 HWM: 34
Sex: A: Mixed B: Mixed C: Mixed D: All male E: All male F: Mixed G: Mixed H: All male I: All male J: All male K: All male |
Describe intervention:
A: TEP + 12 X 15-cm polypropylene mesh Weight: lightweight B: TAPP + 10 X 15-cm titanium coated polypropylene Weight: 16 g/m2 C: TAPP + 10 X 15-cm - Titanium coated polypropylene Weight: 35 g/m2 - Polypropylene Weight: 55 g/m2 - Polypropylene-poliglecaprone Weight: 28 g/m2 D: TEP + 12 X 15-cm Vypro II Weight: lightweight E: TEP + 7.5 X 15-cm Polypropylene-beta-glucan Weight: 50 g/m2 F: TEP + 12 X 15-cm Polypropylene-poliglecaprone Weight: 28 g/m2 G: TEP + Polypropylene-polyvinylidene fluoride Weight: 60 g/m2 H: TEP + 12 X 15-cm Polypropylene -Polyglactin mesh (VYPRO II) I: TAPP + 12 X 15-cm Polypropylene -Polyglactin mesh (VYPRO II) Weight: 55 g/m2 J: TAPP + 10 X 15-cm Polypropylene -Polyglactin mesh Weight: 35 g/m2 K: TEP + 13 X 15-cm Polypropylene -Polyglactin mesh Weight: 30 g/m2 TiMesh Weight: 35 g/m2
|
Describe control:
A: TEP + 12 X 15-cm polypropylene (HW) mesh Weight: Heavyweight B: TAPP + 10 X 15-cm polypropylene (HW) mesh Weight: 90 g/m2 C: TAPP + 10 X 15-cm polypropylene (HW) mesh Weight: 90 g/m2 D: TEP + 12 X 15-cm polypropylene mesh Weight: heavyweight E: TEP + 7.5 X 15-cm polypropylene mesh Weight: 105 g/m2 F: TEP + 2 X 15-cm polypropylene mesh Weight: 105 g/m2 G: TEP + polypropylene mesh Weight: heavyweight (>50 g/m2) H: TEP + 12 x 15-cm polypropylene mesh I: TAPP + 12 x 15-cm polypropylene double-filament mesh Weight: 108 g/m2 polypropylene multifilament mesh Weight: 116 g/m2 J: TAPP + 10 X 15-cm polypropylene double-filament mesh Weight: 108 g/m2 polypropylene multifilament mesh Weight: 116 g/m2 K: TEP + 13 X 15-cm polypropylene mesh Weight: 95 g/m2
|
End-point of follow-up: A: 16 months B: 12 months C: 12 months D: 2 months E: 24 months F: 12 months G: 12 months H: 2 months I: 3 months J: 60 months K: 12 months
For how many participants were no complete outcome data available? Not stated
|
Outcome measure-1 Defined as chronic pain, measured as groin pain
Effect measure: RR [95% CI]: B: 0.69 (0.31-1.57) C: 0.39 (0.18-0.82) D: 0.34 (0.01-8.16) E: 0.35 (0.10-1.20) F: 0.33 (0.09-1.19) J: 0.40 (0.02-8.20) K: 1.28 (0.27-6.03)
Pooled effect (fixed effects model): 0.48 [95% CI 0.31 to 0.75] favoring LWM Heterogeneity (I2): 0%
Outcome measure-2 Defined as recurrence.
Effect measure: RR, RD, mean difference [95% CI]: A: Not estimable B: 3.00 (0.12-73.06) D: Not estimable E: 1.40 (0.09-21.97) F: 5.52 (0.65-46.86) J: 1.01 (0.09-10.90) K: Not estimable
Pooled effect (fixed effects model): 2.01 [95% CI 0.71 to 5.67] favoring HWM Heterogeneity (I2): 0%
|
Mistake in review detected: C: Sajid included the mesh of 55 g/m2 as a LWM. For current analysis this was adjusted for and the mesh was not included in meta-analyses.
Studies G, H and I not included in meta-analyses of Sajid: G: No LWM H: Only 2 months follow-up I: No LWM
In current analysis in addition study D was excluded from meta-analysis because follow-up was only 2 months.
|
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies (cohort studies, case-control studies, case series))1
Research question: Which mesh is recommended for endoscopic inguinal hernia repair?
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4
|
Comments |
|
Burgmans 2015 |
Type of study: Double-blind RCT
Setting: Single-center
Country: Netherlands
Source of funding: Research grant Johnson & Johnson |
Inclusion criteria: >18 years. Primary unilateral inguinal hernia. No contra-indications TEP-repair.
Exclusion criteria: Collagen or connective tissue disorders. Unlikely cooperation.
N total at baseline: Intervention: 478 Control: 471
Important prognostic factors2: Median age (range): I: 55 (19-88) C:55 (18-94)
Sex: I: 100% M C: 100% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
TEP repair + LWM Polypropylene (Ultrapro, 28 g/m2)
Fixation: None.
|
Describe control (treatment/procedure/test):
TEP repair + HWM Polypropylene (Prolene, 80 g/m2)
Fixation: None.
|
Length of follow-up: 3 months
Loss-to-follow-up: Intervention: N=15 (3,2%) Reasons (describe): 1 wrongly randomized 8 no respons 4 complications 1 died 1 time consuming
Control: N=17 (3,6%) Reasons (describe): 3 wrongly randomized 5 no respons 2 complications 2 died 1 time consuming 3 not satisfied 1 leukemia |
Outcome measures and effect size (include 95%CI and p-value if available):
Any pain (NRS1-10): I: n=86 (18.6%) C: n=89 (19.6%) p-value: 0.65
Foreign body feeling: I: n=93 (20.0%) C: n=80 (17.6%) p-value: 0.56
Recurrences: I: n=2 (0.4%) C: n=2 (0.4%) p-value: 1.000
|
|
|
Burgmans 2016 |
Type of study: Double-blind RCT
Setting: Single-center. Country: Netherlands
Source of funding: Research grant Johnson & Johnson |
Inclusion criteria: >18 years. Primary unilateral inguinal hernia. No contra-indications TEP-repair.
Exclusion criteria: Collagen or connective tissue disorders. Unlikely cooperation.
N total at baseline: Intervention: 478 Control: 471
Important prognostic factors2: Median age (range): I: 55 (19-88) C:55 (18-94)
Sex: I: 100% M C: 100% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
TEP repair + LWM Polypropylene (Ultrapro, 28 g/m2)
Fixation: None.
|
Describe control (treatment/procedure/test):
TEP repair + HWM Polypropylene (Prolene, 80 g/m2)
Fixation: None.
|
Length of follow-up: 24 months
Loss-to-follow-up: Intervention: N=38 (7,9%) Reasons (describe): Not reported.
Control: N=44 (9,3%) Reasons (describe): Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Any pain 1 year (NRS1-10): I: n=52 (11.5%) C: n=48 (10.9%)
Any pain 2 year (NRS1-10): I: n=65 (14.8%) C: n=47 (11.0%)
Foreign body feeling 1 year: I: n= 60 (13.8%) C: n=54 (12.2%) p-value: 0.49
Foreign body feeling 2 years: Percentages unchanged.
Recurrences 2 years: I: n=13 (2.7%) C: n=4 (0.8%) p-value: 0.03
|
2 year follow-up of previous study by Burgmans et al (2015). |
|
Prakash 2016 |
Type of study: Single-blind RCT
Setting: Single-center
Country: India
Source of funding: No funding. |
Inclusion criteria: Age 18-60. ASA I/II. Unilateral or bilateral primary inguinal hernia.
Exclusion criteria: Previous inguinoscrotal surgery. Significant co-morbidities. Obstructed/strangulated inguinal hernia.
N total at baseline: Intervention: 70 Control: 70
Important prognostic factors2: Mean age ± SD: I: 47.1 ± 15.4 C: 43.2 ± 17.3 p-value: 0.24
TEP/TAPP (%): I: 34 (51.5) / 30 (45.5) C: 35 (53.8) / 29 (44.6) p-value: 0.75
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Laparoscopic inguinal hernia repair (TEP/TAPP) + LWM Polypropylene (Prolene soft, 30-45 g/m2) Fixation: None.
|
Describe control (treatment/procedure/test):
Laparoscopic inguinal hernia repair (TEP/TAPP) + HWM Polypropylene (3D max large, 80-85 g/m2)
Fixation: None.
|
Length of follow-up (mean ± SD): I: 9.8 ± 3.8 months C: 10.1 ± 3.8 months
Loss-to-follow-up: Intervention: N=5 (7,1%) Control: N=4 (5.7%) Reasons: Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Chronic relevant pain (VAS 4-10): I: n=2 (3%) C: n=2 (3%)
Any pain 1 year: 2.3% overall.
Mean ± SD pain 1 year (1-10): I: 0.07 ± 0.2 C: 0.03 ± 0.2 p-value: 0.54
Foreign body feeling 1 year: I: n= 10 (15.4%) C: n=14 (21.2%) p-value: 0.49
Recurrences 1 year: I: n=1 (1.5%) C: n=0 (0.0%) |
|
|
Kalra 2017 |
Type of study: RCT
Setting: Multi-center
Country: India
Source of funding: No funding.
|
Inclusion criteria: Age 15-60 years. Unilateral, reducible inguinal hernia.
Exclusion criteria: Emergency setting. Strangulated, recurrent hernia. Contraindications to endoscopic surgery.
N total at baseline: Intervention: 30 Control: 30
Important prognostic factors2: Mean age ± SD: I: 39.6 ± 11.3 C: 44.2 ± 10.5
Sex: 95% male.
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
TAPP + LWM Polypropylene (Ultrapro, 28 g/m2)
Fixation: Tacks. |
Describe control (treatment/procedure/test):
TAPP + HWM Polypropylene (3D BARD Max Mesh, 80-100 g/m2)
Fixation: Tacks. |
Length of follow-up: 3 months
Loss-to-follow-up: Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Pain (mean VAS score ± SD): I: 2.73 ± 0.944 C: 3.53 ± 0.973 p-value: 0.003
Foreign body sensation 3 months: I: n=5 (16.7%) C: n=11 (36.6%) |
|
|
Wong 2017
|
Type of study: Double-blind RCT
Setting: Single-center
Country: China
Source of funding: No financial ties. |
Inclusion criteria: Age 18-81. Primary uncomplicated unilateral or bilateral inguinal hernia.
Exclusion criteria: Inguino-scrotal hernia. Sliding hernia. Lower abdominal scar. Using anti-coagulant. Bleeding diathesis. Chronic liver disease with ascites or portal hypertension.
N total at baseline: Intervention: 42 Control: 43
Important prognostic factors2: age median (range): I: 62 (27-81) C: 58 (35-78)
Sex: I: 92.3% M C: 100% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
TEP + LWM Polypropylene-poliglecaprone (Ultrapro, 28 g/m2)
Fixation: None.
|
Describe control (treatment/procedure/test):
TEP + HWM Polyester multifilament (Parietex, 116 g/m2)
Fixation: None.
|
Length of follow-up: Mean 20.3 months
Loss-to-follow-up: Intervention: N=4 (9.3%) Control: N=4 (9.5%) Reasons (describe): Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Recurrences: I: n=2 (5.1%) C: n=0 (0.0%) p-value: 0.157
Foreign body sensation while resting 1 year: I: n=2 (5.1%) C: n=0 (0%) p-value: 0.16 |
|
|
Roos 2018 |
Type of study: Double-blind RCT
Setting: Single-center
Country: Netherlands
Source of funding: Research grant Johnson & Johnson |
Inclusion criteria: >18 years. Primary unilateral inguinal hernia. No contra-indications TEP-repair.
Exclusion criteria: Collagen or connective tissue disorders. Unlikely cooperation.
N total at baseline: Intervention: 478 Control: 471
Important prognostic factors2: Median age (IQR): I: 55 (45-64) C: 56 (44-64)
Sex: I: 100% M C: 100% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
TEP repair + LWM Polypropylene (Ultrapro, 28 g/m2)
Fixation: None.
|
Describe control (treatment/procedure/test):
TEP repair + HWM Polypropylene (Prolene, 80 g/m2)
Fixation: None.
|
Length of follow-up: 60 months
Loss-to-follow-up: Intervention: N=76 (15.9%) Reasons (describe): 1 Wrongly randomized 13 Death 4 Complications 3 Disease unrelated to hernia repair 6 Withdrawal consent 49 Unresponsiveness.
Control: N=83 (17.6%) Reasons (describe): 3 Wrongly randomized 13 Death 2 Complications 2 Disease unrelated to hernia repair 11 Withdrawal consent 52 Unresponsiveness.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Recurrences after 5 years: I: n=18 (3.8%) C: n=5 (1.1%) p-value: 0.01
|
5 year follow-up of previous studies by Burgmans et al (2015/2016). |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures.
- Provide data per treatment group on the most important prognostic factors ((potential) confounders).
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls.
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders.
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies (cohort studies, case-control studies, case series))1
Research question: Which mesh is recommended for open inguinal hernia repair?
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|||||
|
Paradowski 2009 |
Type of study: Double-blind RCT
Setting: Single-center
Country: Poland
Source of funding: Not reported. |
Inclusion criteria: >18 years. Primary inguinal hernia.
Exclusion criteria: Femoral hernia. Emergency operation. Skin infection groin.
N total at baseline: Intervention: 25 Control: 25
Important prognostic factors2: Age, mean ± SD: I: 59. C: 53.9
Sex: I: 100% M C: 84% M
Groups comparable at baseline? Yes, although minimally reported. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM polyporpylene (Surgimesh WN, 43 g/m2)
Fixation: Not reported. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM Polypropylene (Surgipro, 80 g/m2)
Fixation: Not reported. |
Length of follow-up: 12 months
Loss-to-follow-up: Overall: 3 months: 100% 12 months: 96% Reasons (describe) Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
VAS score 3 months (mean ± SD): I: 0.46 ± 1.22 C: 0.56 ± 1.13 p-value: 0.673
Any pain 3 months(VAS 1-10) I: n=1 (4%) C: n=4 (16%)
Any pain 12 months(VAS 1-10) I: n=0 (0%) C: n=1 (4%)
Recurrences 12 months: I: 0 C: 0
|
|
|||||
|
Sadowski 2011 |
Type of study: Single-blind RCT
Setting: Single-center
Country: USA
Source of funding: Not reported. |
Inclusion criteria: >18 years. Primary inguinal hernia.
Exclusion criteria: Pregnancy. Previous inguinal surgery. Cognitive disorder.
N total at baseline: Intervention: 39 Control: 39
Important prognostic factors2: Age, mean ± SD: I: 54 ± 17. C: 56 ± 16.4
Sex: I: 97% M C: 97% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM Polyester (±40 g/m2)
Fixation: Not reported. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM Polypropylene (80-100 g/m2)
Fixation: Not reported. |
Length of follow-up: 3 months
Loss-to-follow-up: Intervention: N=4 (10.3%) Control: N=4 (10.3%) Reasons (describe) Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
VAS score 3 months (mean ± SD): I: 0.46 ± 1.22 C: 0.56 ± 1.13 p-value: 0.673
Any pain 3 months(VAS 1-10) I: n=6 (17.65%) C: n=8 (23.53%) p-value: 0.5486 |
3 months publication of 2 years of planned follow-up (never published). |
|||||
|
Bury 2012 |
Type of study: RCT
Setting: Multi-center
Country: Poland
Source of funding: Minor grand Ethicon Poland. |
Inclusion criteria: 20-75 years. Primary, unilateral inguinal hernia.
Exclusion criteria: Active treatment for serious comorbidity. Trombocytopenia. Mental illness. Pregnancy.
N total at baseline: Intervention: 215 Control: 177
Important prognostic factors2: Age, median (range): I: 56 (18-80) C: 56 (23-87)
Sex: I: 98.6% M C: 97.7% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM Polypropylene (Ultrapro, 28 g/m2)
Fixation: Three suturing modifications necessary due to mesh specifications. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM Polypropylene (Prolene, 80 g/m2)
Fixation: Sutures. |
Length of follow-up: 60 months
Loss-to-follow-up: Intervention: N=20 (9.3%) Reasons (describe) 13 death 4 recurrences
Control: N=16 (9.0%) Reasons (describe) 11 death 1 recurrences
|
Outcome measures and effect size (include 95%CI and p-value if available):
Recurrences 5 years: I: 1.86% C: 0.57% p-value: 0.493
Pain 5 years: I: n=5 C: n=4
Mean VAS: I: 2.25 (range 2-3) C: 2.4 (range 2-3) Not significant |
5 year follow-up of Smietanski (2008) (see: review Sajid (2012)) |
|||||
|
Nikkolo 2012 |
Type of study: RCT
Setting: Single-center
Country: Estonia
Source of funding: Not reported. |
Inclusion criteria: >18 years. Primary, unilateral inguinal hernia.
Exclusion criteria: Irreducible, strangulated hernia.
N total at baseline: Intervention: 69 Control: 66
Important prognostic factors2: Mean age ± SD: I: 59.2 C: 59.7
Sex: I: 91.0% M C: 93.8% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM Polypropylene (Optilene, 36 g/m2)
Fixation: Sutures. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM Polypropylene (Premilene, 82 g/m2)
Fixation: Sutures. |
Length of follow-up: 36 months
Loss-to-follow-up: Intervention: N= 11 (15.9%) Reasons (describe) 5 did not attend follow-up. 5 death 1 recurrence
Control: N= 8 (12.1%) Reasons (describe) 4 did not attend follow-up. 3 death 1 recurrence
|
Outcome measures and effect size (include 95%CI and p-value if available):
Any pain 6 months: I: 47.8% C: 59.4%
Any pain 3 years: I: n=17 (29.3%) C: n=10 (17.2%) p-value: 0.1323
Median VAS (0-100) 3 years: I: 30.0 C: 30.5
Foreign body feeling: I: 20.7% C: 27.6% p-value: 0.3967
Recurrences 3 years: I: n=1 (1.7%) C: n=1 (1.7%) |
3 years follow-up of Nikkolo et al. (2010) (see: review Sajid (2012)) |
|||||
|
Canonico 2012 |
Type of study: Double-blind RCT
Setting: Single-center
Country: Italy
Source of funding: Not reported. |
Inclusion criteria: >18 years. BMI <35 kg/m2. Uncomplicated primary, unilateral inguinal hernia.
Exclusion criteria: Large hernias (M3 or L3). Concurrent diabetes, immunologic or psychiatric disorders, hypertensitivity to aprotinin. Patients taking anti-inflammatory drugs.
N total at baseline: Intervention: 40 Control: 40
Important prognostic factors2: Mean age ± SD: I: 63 ± 12 C: 66 ± 10 p-value: >0.99
Sex: I: 90% M C: 80 % M p-value: 0.34
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM Polypropylene (Evolution P3EM, 48 g/m2).
Fixation: Human fibrin glue. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM Polypropylene (Prolene, 80 g/m2).
Fixation: Human fibrin glue. |
Length of follow-up: 6 months
Loss-to-follow-up: Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Recurrences 6 months: I: n=0 C: n=0
|
|
|||||
|
Paajanen 2012 |
Type of study: Double-blind RCT
Setting: Multi-center
Country: Finland
Source of funding: None declared. |
Inclusion criteria: Uni- or bilateral primary or recurrent inguinal hernias.
Exclusion criteria: Recurrent hernias: no mesh repair.
N total at baseline: Intervention: 104 Control: 101
Important prognostic factors2: Mean age ± SD: I: 58 ± 14 C: 61 ± 15
Sex: I: 94.2% M C: 92.1% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM partly absorbable polypropylene-polyglactin (Vypro II, 50 g/m2)
Lichtenstein + LWM polypropylene (Premilene Mesh LP, 55 g/m2)
Fixation: Sutures. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM polypropylene (Premilene, 82 g/m2)
Fixation: Sutures. |
Length of follow-up: 56 months
Loss-to-follow-up: Intervention: N=27 (21.2%) Reasons (describe) 11 death 16 not reached
Control: N=27 (26.7%) Reasons (describe) Not given.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Pain 5 years: I: n=10 (12.9%) C: n=12 (16.2%)
Recurrences 5 years: I: n=3 (3.9%) C: n=3 (4.1%) p-value: 0.8209 |
5 year follow-up of Paajanen et al (2007). (see: review Sajid (2012))
Mesh of 55 g/m2 not applicable for meta-analysis. |
|||||
|
Pielaciński 2013 |
Type of study: RCT
Setting: Not reported.
Country: Poland
Source of funding: Not reported. |
Inclusion criteria: >18 years. Uni- or bilateral, primary or recurrent inguinal hernia.
Exclusion criteria: Other changes in groin or scrotum.
N total at baseline: Intervention: 73 Control: 76
Important prognostic factors2: Median age (range): I:58 (24-87) C:59 (20-89)
Sex: I: 100% M C: 100% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM partially absorbable composite; polypropylene-polyglactin (35 g/m2)
Fixation: Sutures. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM non-absorbable polypropylene (100 g/m2)
Fixation: Sutures. |
Length of follow-up: 6 months
Loss-to-follow-up: Intervention: N=17 (23%) Reasons (describe) (does not add up): 5 Intra-operative damage of anatomical structures 9 Post-operative complications or worsening of general condition. 2 recurrence 3 did not show
Control: N=22 (29%) Reasons (describe) (does not add up): 4 Intra-operative damage of anatomical structures 7 Post-operative complications or worsening of general condition. 2 recurrence 7 did not show |
Outcome measures and effect size (include 95%CI and p-value if available):
Recurrences 6 months: I: n=2 (2.94%) C: n=2 (2.81%) OR: 0.97 (0.132-7.093) p-value: 0.977
Pain 6 months (VAS 2-4): I: n=3 (5.4%) C: n=7 (13%)
Foreign body feeling 6 months: I: 17 (30%) C: 23 (43%)
|
Pielaciński (2011) was also encountered in search. After full-text screening this study was interpreted as preliminary data of Pielaciński (2013). |
|||||
|
Yazdankhah Kenary 2013 |
Type of study: Double-blind RCT
Setting: Single-center
Country: Iran
Source of funding: Not reported. |
Inclusion criteria: >18 years. Primary unilateral inguinal hernia.
Exclusion criteria: Inability to walk >500m. Probable loss to follow-up. Irreducible or strangled hernia.
N total at baseline: Intervention: 55 Control: 55
Important prognostic factors2: Mean age ± SD: I: 50.4 ± 16.1 C: 44.0 ± 15.9
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM Polypropylene (Dynamesh, 36 g/m2)
Fixation: Sutures. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM Polypropylene (Dynamesh, 72 g/m2)
Fixation: Sutures. |
Length of follow-up: 12 months
Loss-to-follow-up: No loss to follow-up.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Foreign body feeling 1 year: I: n=0 (0%) C: n=7 (12.7%) p-value: 0.007
Recurrences 1 year: I: n=1 (1.9%) C: n=0 (0%) p-value: 0.3
Pain 6 month: I: n=9 (6.7%) C: n=12 (21.8%) (mean VAS ± SD): I: 2.2 ± 1.2 C: 2.2 ± 0.8 p-value: 0.9
Pain 1 year: I: n=4 (7.4%) C: n=3 (5.5%) (mean VAS ± SD): I: 1.7 ± 0.5 C: 2.2 ± 1.2 p-value: 0.5 |
|
|||||
|
Demetrashvili 2014) |
Type of study: Single-blind RCT
Setting: Single-center
Country: Georgia
Source of funding: None. |
Inclusion criteria: >18 years. Primary unilateral inguinal hernia.
Exclusion criteria: Irreducible or strangulated hernia.
N total at baseline: Intervention: 113 Control: 113
Important prognostic factors2: Mean age ± SD: I: 54.7 ± 14.3 C: 51.3 ± 17.5 p-value: 0.14
Sex: I: 93.8% M C: 90.2% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM Polypropylene (Ultrapro, 28 g/m2)
Fixation: Sutures. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM Polypropylene (Prolene, 82 g/m2)
Fixation: Sutures. |
Length of follow-up: 36 months
Loss-to-follow-up: Intervention: N=17 (15%) Reasons (describe) 4 death 12 absence of examination 1 recurrence
Control: N=11 (9.7%) Reasons (describe) 3 death 7 absence of examination 1 recurrence
|
Outcome measures and effect size (include 95%CI and p-value if available):
Foreign body feeling 3 years: I: n=1 (1.1%) C: n=9 (9.4%) p-value: 0.02
Recurrences 3 years: I: n=1 (1.04%) C: n=1 (0.98%) p-value: 0.99
Pain 3 month: I: n=10 (10.4%) C: n=12 (11.8%) p-value: 0.82 (mean VAS ± SD): I: 2.5 ± 1.4 C: 2.4 ± 1.3 p-value: 0.82
Pain 1 year: I: n=6 (6.3%) C: n=9 (8.8%) p-value: 0.60 (mean VAS ± SD): I: 2.2 ± 1.2 C: 2.4 ± 1.2 p-value: 0.70
Pain 3 years: I: n=2 (2.1%) C: n=5 (4.9%) p-value: 0.45 (mean VAS ± SD): I: 2.0 ± 1.4 C: 2.4 ± 1.1 p-value: 0.71 |
|
|||||
|
Lee 2017 |
Type of study: Single blind RCT
Setting: Multicenter
Country: Korea
Source of funding: None |
Inclusion criteria: Male. Age 20-85 years. Primary unilateral inguinal hernia.
Exclusion criteria: Incarcerated or strangulated hernia. Previous urologic or inguinal surgery. Immune disease. Thromboembolic disease or treatment. Hepatic or renal disease. Malignancy.
N total at baseline: Intervention: 25 Control: 25
Important prognostic factors2: Median age (range): I: 64 (24-83) C: 64 (30-76)
Sex: I: 100% M C: 100% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM Partially absorbable lightweight polypropylene-poligecaprone (Proflex, 28-29 g/m2)
Fixation: Sutures. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM Polypropylene (Marlex, 100 g/m2)
Fixation: Sutures. |
Length of follow-up: 4 months
Loss-to-follow-up: Intervention: N=1 (4%) Reasons (describe) 1 Adverse event
Control: N=2 (8%) Reasons (describe) 1 withdrawal consent 1 loss to follow-up
|
Outcome measures and effect size (include 95%CI and p-value if available):
Recurrences 4 months: I: n=0 C: n=0
Foreign body feeling 4 months: I: n=1 (4.2%) C: n=7 (30.4%) p-value: 0.023
Pain (mean VAS ± SD) 4 month: I: 0.7 ± 1.1 C: 0.8 ± 1.4 p-value: 0.746 |
|
|||||
|
Rutegård 2017 |
Type of study: Not-blinded RCT
Setting: Multi-center
Country: Sweden
Source of funding: Västerbotten County Council, VISARE NORR Fund, Northern Country Councils Regional Federation. |
Inclusion criteria: Male. >25 years. Unilateral inguinal hernia.
Exclusion criteria: Bleeding disorders. Use of anti-coagulantia.
N total at baseline: Intervention: 197 Control: 194
Important prognostic factors2: Mean age ± SD: I: 59.1 ± 12.7 C: 58.4 ± 13.0
Sex: I: 100% M C: 100% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM Polypropylene (Ultrapro, 28 g/m2)
Fixation: Not reported. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM Polypropylene (Bard Flatmesh, 90 g/m2)
Fixation: Not reported. |
Length of follow-up: 12 months
Loss-to-follow-up: Intervention: N=9 (4.6%) Reasons (describe) 4 Declined follow-up 5 Excluded from analysis
Control: N=19 (9.6%) Reasons (describe) 13 Declined follow-up 2 Operation non-participating hospital. 4 Excluded from analysis
Incomplete outcome data: Intervention: N 36-52 (19.5-28.1%) Reasons (describe) Not reported.
Control: N 34-52 (19.1-29.2%) Reasons (describe) Not reported. |
Outcome measures and effect size (include 95%CI and p-value if available):
Recurrences 1 year: I: 4 (2.2%) C: 4 (2.2%)
Foreign body feeling 1 year: I: 34 (23.6%) C: 21 (14.1%) p-value: 0.051
|
Pain presented on a “worse, no change, better”-scale. Therefore, not applicable for meta-analysis. |
|||||
|
Bona 2018
|
Type of study: Single-blind RCT
Setting: Multi-center
Country: Italy
Source of funding: Not reported. |
Inclusion criteria: >18 years. Primary reducible uni- or bilateral inguinal hernia. No contraindications open inguinal hernia repair.
Exclusion criteria: Emergency surgery.
N total at baseline: Intervention: 411 Control: 397
Important prognostic factors2: Mean age (IQR I-III): I: 59 (47-69) C: 61 (50-70)
Sex: I: 95% M C: 95% M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Lichtenstein + LWM Polypropylene (Ultrapro, 28 g/m2)
Fixation: Not reported. |
Describe control (treatment/procedure/test):
Lichtenstein + HWM Polypropylene (80-100 g/m2)
Fixation: Not reported. |
Length of follow-up: 12 months
Loss-to-follow-up 6 months (not further reported): Intervention: N=86 (20.9%) Control: N=80 (20.2%) Reasons (describe) Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Pain 6 months: I: n=80 (24.6%) C: n=83 (26.2%) p-value: 0.76 p-value adjusted for baseline characteristics: 0.04
Recurrences 6 months: I: n=0 C: n=0
|
|
|||||
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures.
- Provide data per treatment group on the most important prognostic factors ((potential) confounders).
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls.
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders.
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea, 2007; BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009; PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Sajid 2012 |
Yes |
Yes |
Yes |
Yes |
NA |
Yes |
Yes |
No |
No |
|
Sajid 2013 |
Yes |
Yes |
Yes |
Yes |
NA |
Yes |
Yes |
No |
No |
- Research question (PICO) and inclusion criteria should be appropriate and predefined.
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched.
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons.
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported.
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs).
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table et cetera).
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (For example. Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (For example funnel plot, other available tests) and/or statistical tests (For example Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias table for intervention studies (randomized controlled trials)
Research question: Which mesh is recommended for endoscopic inguinal hernia repair?
|
Study reference
(first author, publication year) |
Describe method of randomisation1 |
Bias due to inadequate concealment of allocation?2
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?4
(unlikely/likely/unclear) |
Bias due to loss to follow-up?5
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?6
(unlikely/likely/unclear) |
|
Burgmans 2015 |
Computer generated. |
Unlikely |
Unlikely; patients were blinded for allocation of mesh type. |
Likely; blinding of care providers was not possible. |
Unlikely; researchers were blinded for allocation of mesh type. |
Unlikely |
Unlikely; 3.2%/3.6% in 3 months of follow-up. |
Unlikely; all data were analysed on an intention-to-treat basis. |
|
Burgmans 2016 |
Computer generated. |
Unlikely |
Unlikely; patients were blinded for allocation of mesh type. |
Likely; blinding of care providers was not possible. |
Unlikely; researchers were blinded for allocation of mesh type. |
Unlikely |
Unlikely; 7.9%/9.3% in 2 years of follow-up. |
Unlikely; all data were analysed on an intention-to-treat basis. |
|
Prakash 2016 |
Computer generated. |
Unlikely |
Unlikely; patients were blinded for allocation of mesh type. |
Likely; blinding of care providers was not possible. |
Likely; single-blinded study only for patients. |
Unlikely |
Unlikely; 7.1%/5.7% in 1 year of follow-up.
|
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Kalra 2017 |
Sealed envelope system. |
Unlikely |
Unclear; not reported. |
Likely; blinding of care providers was not possible. |
Unclear; not reported. |
Unlikely |
Unclear; not reported. |
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Wong 2017 |
Not reported. |
Unlikely |
Unclear; double-blinded, yet no further report on the method used. |
Likely; blinding of care providers was not possible. |
Unlikely; surgeons assigned to do postoperative assessment were blind to the type of mesh used. |
Unlikely |
Likely; 9.3%/9.5% in 20 months of follow-up. |
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Roos 2018 |
Computer generated. |
Unlikely |
Unlikely: Patients were blinded for allocation of mesh type. |
Likely; blinding of care providers was not possible. |
Unlikely; Telephonic follow-up was performed by 5 independent researchers who were blinded for allocation of mesh type. |
Unlikely |
Likely; 15.9%/17.6% in 5 years of follow-up. |
Unlikely; all data were analysed on an intention-to-treat basis. |
- Randomisation: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomisation process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomisation (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomisation procedures or open allocation schedules.
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the risk of bias is unclear.
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the risk of bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Risk of bias table for intervention studies (randomized controlled trials)
Research question: Which mesh is recommended for open inguinal hernia repair?
|
Study reference
(first author, publication year) |
Describe method of randomisation1 |
Bias due to inadequate concealment of allocation?2
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?4
(unlikely/likely/unclear) |
Bias due to loss to follow-up?5
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?6
(unlikely/likely/unclear) |
|
Paradowski 2009
|
Not reported; during operation after preparation of the hernia sac. |
Unclear. |
Unlikely; patients were blinded to the mesh they received. |
Likely; blinding of care providers was not possible. |
Unlikely; examiners of follow-up were blinded to the received mesh. |
Unlikely. |
Unlikely; 4% in 1 year of follow-up.
|
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Sadowski 2011 |
Not reported. |
Unclear. |
Unlikely; patients were blinded to the mesh they received and remained blinded throughout the follow-up period. |
Likely; blinding of care providers was not possible. |
Likely; not reported, although it states single-blinded for patients. |
Unlikely. |
Likely; 10.3% in 3 months of follow-up. |
Unlikely; ITT analysis. |
|
Bury 2012 |
Wichmann-Hill pseudorandom number generator (modified by McLeod). |
Unlikely. |
Unclear; not reported. |
Likely; blinding of care providers was not possible. |
Unlikely; surgeons were blinded for allocation of mesh type for follow-up visits. |
Unlikely. |
Likely; 9.3%/9.0% in 5 years of follow-up. |
Unlikely; ITT analysis. |
|
Nikkolo 2012 |
Blind envelope system. |
Unlikely. |
Unclear; not reported. |
Likely; blinding of care providers was not possible. |
Unclear; not reported. |
Unlikely. |
Likely; 15.9%/12.1% in 3 years of follow-up. |
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Canonico 2012 |
Not reported. |
Unclear. |
Unlikely; patients were blinded to the mesh they received. |
Likely; blinding of care providers was not possible. |
Unlikely; observers of outcomes were blinded to the received mesh. |
Likely; 6 months follow-up results not described. |
Unclear; not reported. |
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Paajanen 2012 |
Sealed and numbered envelopes. |
Unlikely. |
Unlikely; patients were unaware of the treatment allocation. |
Likely; blinding of care providers was not possible. |
Unlikely; dedicated research nurse blinded to the type of mesh. |
Unlikely. |
Likely; 21.2%/26.7% in 5 years of follow-up. |
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Pielaciński 2013 |
Not reported. |
Unclear. |
Unclear; not reported. |
Likely; blinding of care providers was not possible. |
Unclear; not reported. |
Unlikely. |
Likely; 23%/29% in 6 months of follow-up. |
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Yazdankhah Kenary 2013
|
Computer generated random numbers. |
Unlikely. |
Unlikely; patients were blinded to the mesh they received. |
Likely; blinding of care providers was not possible. |
Unlikely; examiners of follow-up were blinded to the received mesh. |
Unlikely. |
Unlikely; no loss to follow-up.
|
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Demetrashvili 2014 |
Simple random sampling. |
Unlikely. |
Unlikely; patients were blinded to the mesh they received. |
Likely; blinding of care providers was not possible. |
Unlikely; the examinations were performed by surgeons who had not been participating previously in the study. |
Unlikely. |
Likely; 15%/9.7% in 3 years of follow-up. |
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Lee 2017 |
Not reported. |
Unclear. |
Unlikely; patients were blinded to the mesh they received. |
Likely; blinding of care providers was not possible. |
Unclear; not reported. |
Unlikely. |
Unlikely; 4%/8% in 4 months of follow-up. |
Unlikely; per protocol analysis (PP). The last observation carried forward method was used to handle missing values in the PP population. |
|
Rutegård 2017 |
Concealed allocation sequence was computer generated by statistician with a random number technique and stratified at the hospital level. |
Unlikely. |
Likely; surgeons were divided into two groups according to mesh preference. Patients were not blinded to the group of surgeons that performed the operation. |
Likely; blinding of care providers was not possible. |
Unlikely; examiners of follow-up were blinded to the received mesh. |
Unlikely. |
Unlikely. 4.6%/9.6% in 1 year of follow-up. |
Unlikely; not reported, however unlikely due to inability to cross-over. |
|
Bona 2018 |
Centralized. Balanced every 10 patients within centre, using for this purpose the algorithm of Pocock and Simon. |
Unlikely. |
Unlikely; single-blind. |
Likely; blinding of care providers was not possible. |
Likely; mesh type was mentioned in operative report. |
Likely; 12 months follow-up results not described. |
Likely; 20,9% and 20.2% in 1 year of follow-up. |
Unlikely; not reported, however unlikely due to inability to cross-over. |
- Randomisation: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomisation process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomisation (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomisation procedures or open allocation schedules.
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the risk of bias is unclear.
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the risk of bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 05-04-2019
Beoordeeld op geldigheid : 11-12-2020
Voor het beoordelen van de actualiteit van deze richtlijn is de werkgroep in stand gehouden. Uiterlijk in 2022 bepaalt het bestuur van de NVvH of de modules van deze richtlijn nog actueel zijn. Op modulair niveau is een onderhoudsplan beschreven. Bij het opstellen van de richtlijn heeft de werkgroep per module een inschatting gemaakt over de maximale termijn waarop herbeoordeling moet plaatsvinden en eventuele aandachtspunten geformuleerd die van belang zijn bij een toekomstige herziening (update). De geldigheid van de richtlijn komt eerder te vervallen indien nieuwe ontwikkelingen aanleiding zijn een herzieningstraject te starten.
De NVvH is regiehouder van deze richtlijn(modules) en eerstverantwoordelijke op het gebied van de actualiteitsbeoordeling van de richtlijn(modules). De andere aan deze richtlijn deelnemende wetenschappelijke verenigingen of gebruikers van de richtlijn delen de verantwoordelijkheid en informeren de regiehouder over relevante ontwikkelingen binnen hun vakgebied.
Algemene gegevens
De richtlijnontwikkeling werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). Patiëntenparticipatie bij deze richtlijn werd medegefinancierd uit de Kwaliteitsgelden Patiënten Consumenten (SKPC) binnen het programma KIDZ.
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijn.
Doel en doelgroep
Doel
Het hoofddoel van de richtlijn is de patiëntresultaten te verbeteren en de meest voorkomende problemen na een liesbreukoperatie te verminderen, met name recidivering en chronische pijn.
Doelgroep
De richtlijn wordt geschreven voor de medisch specialisten die betrokken zijn bij de zorg voor patiënten met liesbreuk.
Samenstelling werkgroep
Werkgroep
- Dr. B. (Baukje) van den Heuvel, chirurg, Pantein Zorggroep, Radboudumc, Nijmegen, NVvH (voorzitter)
- Dr. M.P. (Maarten) Simons, chirurg, OLVG, Amsterdam, NVvH
- Dr. Th.J. (Theo) Aufenacker, chirurg, Rijnstate, Arnhem, NVvH
- Dr. J.P.J. (Ine) Burgmans, chirurg, Diakonessenhuis Utrecht, Utrecht, NVvH
- Mw. R. (Rinie) Lammers, beleidsadviseur, Patiëntenfederatie Nederland, Utrecht
- Dr. M.J.A. (Maarten) Loos, chirurg, Maxima Medisch Centrum, Veldhoven, NVvH
- Dr. M. (Marijn) Poelman, chirurg, Sint Franciscus Vlietland Groep, Rotterdam, NVvH
- Dr. G.H. (Gabriëlle) van Ramshorst, chirurg, NKI-Antoni van Leeuwenhoek Ziekenhuis/VU Medisch Centrum, Amsterdam, NVvH
- Drs. J.W.L.C. (Ronald) Schapendonk, anesthesioloog-pijnspecialist, Diakonessenhuis Utrecht, Utrecht, NVA
- Dr. E.J.P. (Ernst) Schoenmaeckers, chirurg, Meander MC, Amersfoort, NVvH
- Dr. N. (Nelleke) Schouten, AIOS heelkunde regio Maastricht, NVvH
- Dr. R.K.J. (Rogier) Simmermacher, chirurg, UMC Utrecht, Utrecht, NVvH
Met medewerking van
- Drs. W. (Wouter) Bakker, arts-onderzoeker heelkunde, Diakonessenhuis Utrecht, Utrecht
Met ondersteuning van
- Dr. J.S. (Julitta) Boschman, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. W.A. (Annefloor) van Enst, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. Ing. R. (Rene) Spijker, Informatie Specialist, Cochrane Netherlands
- Dr. C. (Claudia) Orelio, Cochrane Netherlands
- Drs. P. (Pauline) Heus, Cochrane Netherlands
- Prof. dr. R. (Rob) Scholten, Cochrane Netherlands
- Dr. L. (Lotty) Hooft, Cochrane Netherlands
- D.P. (Diana) Gutierrez, projectsecretaresse, Kennisinstituut van de Federatie Medisch Specialisten
- J. (Jill) Heij, junior projectsecretaresse, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De KNMG-code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement, kennisvalorisatie) hebben gehad. Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
|
Van den Heuvel |
Chirurg |
Onbetaald: - Advies-commissie Kwaliteit EHS (European Hernia Society) - Dutch Hernia Society - International Guidelines Groin Hernia Management (ontwikkeling gesponsord door BARD en Johnson&Johnson - |
geen (03/03/2017) |
niet van toepassing |
|
|
Simons |
Chirurg |
Onbetaald: - Bestuur EHS (European Hernia Society) - Dutch Hernia Society - International Guidelines Groin Hernia Management (ontwikkeling gesponsord door BARD en Johnson&Johnson |
Lid Board van de European Hernia Society (20/7/2017) |
geen |
|
|
Aufenacker |
Chirurg |
Penningmeester DHS (Dutch Hernia Society), onbetaald |
Prevent Studie, (Preventieve matplaatsing bij aanleggen colostoma) ZonMW gesponsord (19/3/2018) |
geen |
|
|
Bakker |
Chirurg io |
- |
- |
niet van toepassing |
|
|
Burgmans |
Chirurg |
Lid bestuur Dutch Hernia Society |
geen (19/3/2018) |
niet van toepassing |
|
|
Lammers |
Beleidsadviseur |
- |
geen (19/6/2017) |
|
|
|
Loos |
Chirurg |
- |
geen (13/6/2017) |
niet van toepassing |
|
|
Poelman |
Chirurg |
Lid bestuur Dutch Hernia Society |
geen (11/7/2017) |
geen |
|
|
van Ramshorst |
Fellow Chirurgie |
|
Sponsor van mijn fellowship, KWF, heeft geen belangen bij deze richtlijn. Publicaties waar ik auteur van ben zouden gebruikt kunnen worden als referentie. (16/4/2018) |
geen |
|
|
Schapendonk |
Anesthesioloog-pijnspecialist |
|
Niet persoonlijk, maar mijn instelling heeft deelgenomen/ neemt deel aan wetenschappelijk onderzoek gesponsord door Medtronic, Spinal Modulaton of St. Jude Medical thans Abbott. Fee ontvangen van St. Jude Medical voor voordracht op scholing pijnverpleegkundigen inzake DRG stimulatie. Daarnaast in 2015 congres bezocht op kosten Spinal Modulation. (3/5/2017) |
geen |
|
|
Schoenmaeckers |
Chirurg |
|
geen (20/6/2017) |
niet van toepassing |
|
|
Schouten |
AIOS Heelkunde |
- |
geen (6/6/2017) |
niet van toepassing |
|
|
Simmermacher |
Chirurg |
- |
geen (21/5/2017 |
niet van toepassing |
|
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door inbreng van: 1) patiëntenvereniging Meshed-up tijdens de invitational conference; 2) door de deelname van mevrouw. Lammers (Patiëntenfederatie Nederland) in de werkgroep en 3) door het raadplegen van volwassenen behandeld voor liesbreuk via een door de Patiëntenfederatie uitgezette enquête. De reacties naar aanleiding van deze invitational en enquête (zie aanverwante producten) zijn besproken in de werkgroep en de belangrijkste knelpunten zijn verwerkt in de richtlijn.
Methode ontwikkeling
Evidence based
Implementatie
In de verschillende fasen van de richtlijnontwikkeling is rekening gehouden met de implementatie van de richtlijn (module) en de praktische uitvoerbaarheid van de aanbevelingen. Daarbij is uitdrukkelijk gelet op factoren die de invoering van de richtlijn in de praktijk kunnen bevorderen of belemmeren. Het implementatieplan is te vinden bij de aanverwante producten. De werkgroep heeft geen interne kwaliteitsindicatoren ontwikkeld om het toepassen van de richtlijn in de praktijk te volgen en te versterken (zie Indicatorontwikkeling).
Werkwijze
AGREE
Deze richtlijn is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010), dat een internationaal breed geaccepteerd instrument is. Voor een stap-voor-stap beschrijving hoe een evidence-based richtlijn tot stand komt wordt verwezen naar het stappenplan Ontwikkeling van Medisch Specialistische Richtlijnen van het Kennisinstituut van de Federatie Medisch Specialisten.
Knelpuntenanalyse
Tijdens de voorbereidende fase inventariseerden de voorzitter van de werkgroep en de adviseur de knelpunten en onderwerpen beschreven in de internationale richtlijn (HerniaSurge Group 2018) die in aanmerking kwamen voor de Nederlandse adaptatie en update. De aanwezigen tijdens de invitational conference bevestigden deze knelpunten en onderwerpen. Een verslag hiervan is opgenomen onder aanverwante producten.
Uitgangsvragen en uitkomstmaten
De knelpunten en onderwerpen beschreven in de internationale richtlijn (HerniaSurge Group, 2018) die in aanmerking kwamen voor de Nederlandse adaptatie en update zijn met de werkgroep besproken. Daarna heeft de werkgroep de definitieve uitgangsvragen en modules vastgesteld en vastgesteld welke modules volledig zouden worden geupdate met ondersteuning van het Kennisinstituut en welke modules uit de internationale richtlijn zouden worden geadapteerd door de werkgroep. Voor alle modules, ook de modules die geadapteerd zijn, heeft de werkgroep de recente en relevante literatuur doorgenomen. In de geadapteerde modules zijn nieuwe studies verwerkt bij het formuleren van overwegingen en aanbevelingen. In de volledig geupdate modules zijn nieuwe studies geïntegreerd in de literatuuranalyse, risk of bias assessment en gradering. Hieronder is per module aangegeven of de module volledig is ge-update of geadapteerd:
- Risicofactoren (geadapteerd)
- Diagnostiek (geadapteerd)
- Indicatie behandeling asymptomatische liesbreuken (volledig geupdate)
- Chirurgische behandeling unilaterale liesbreuk (volledig geupdate)
- Mat of Shouldice (volledig geupdate)
- Lichtenstein of een andere open anterieure techniek (geadapteerd)
- Lichtenstein of een open pre-peritoneale techniek (geadapteerd)
- Endoscopische techniek (geadapteerd)
- Lichtenstein of een laparo-endoscopische techniek (geadapteerd)
- Een open posterieure techniek of laparo-endoscopisch (geadapteerd)
- Geïndividualiseerde behandeling (geadapteerd)
- Matten (volledig geupdate)
- Matfixatie (volledig geupdate)
- Open anterieure benadering
- TEP/TAPP
- Liesbreuken bij vrouwen (geadapteerd)
- Femoraalbreuken (geadapteerd)
- Antibioticaprofylaxe (volledig geupdate)
- Anesthesie (volledig geupdate)
- Postoperatieve pijn (geadapteerd)
- Chronische pijn
- Definitie, risicofactoren en preventie (geadapteerd)
- Reductie incidentie CPIP (volledig geupdate)
- Behandeling CPIP (volledig geupdate)
- Behandeling van recidief liesbreuk (geadapteerd)
- Na een anterieure benadering
- Na een posterieure benadering
- Na een anterieure en posterieure benadering
- Acute liesbreukchirurgie (geadapteerd)
- Organisatie van zorg (nieuw)
Vervolgens inventariseerde de werkgroep voor de uitgangsvragen van de modules die waren geselecteerd voor een volledige update welke uitkomstmaten voor de patiënt relevant zijn. Er werd zowel naar gewenste als ongewenste effecten gekeken. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal, belangrijk (maar niet cruciaal) en onbelangrijk. Tevens poogde de werkgroep tenminste voor de cruciale uitkomstmaten te definiëren welke verschillen zij klinisch (patiënt) relevant vonden.
Strategie voor zoeken en selecteren van literatuur
De informatiespecialist van Cochrane Nederland doorzocht Medline en Embase (op 11 april 2017) en het Cochrane Register (op 12 april 2017) naar artikelen over de diagnostiek of behandeling van volwassenen met liesbreuk zonder beperkingen op de publicatiedatum. Dit betrof een herhaling van de searches uitgevoerd voor de 2013 European Hernia Society Guidelines on the treatment of inguinal hernia in adult patients en de 2018 International Guidelines for Groin Hernia Management: The HerniaSurge Group (literatuursearch tot 1 januari 2015 en 1 juli 2015 voor level 1 publicaties (RCTs). De literatuurzoekactie leverde voor reviews 583 unieke treffers (waarvan 339 reviews reeds gescreend voor de vorige richtlijnen) op (Medline n=419; Embase n=378; en de Cochrane Library n=12) en voor RCTs 2174 unieke treffers (Medline n=1376; Embase n=1537; en de Cochrane Library n=160).
De werkgroepleden selecteerden per uitgangsvraag in duplo de via de zoekactie gevonden artikelen op basis van vooraf opgestelde selectiecriteria en in eerste instantie de studies met de hoogste bewijskracht. De gehanteerde selectiecriteria zijn te vinden in de module met desbetreffende uitgangsvraag.
Kwaliteitsbeoordeling individuele studies
Voor de modules die volledig werden ge-update, zijn de geselecteerde artikelen systematisch beoordeeld, op basis van op voorhand opgestelde methodologische kwaliteitscriteria, om zo het risico op vertekende studieresultaten (Risk of Bias) te kunnen inschatten. Deze beoordelingen kunt u vinden in de Risk of Bias (RoB) tabellen. De gebruikte RoB instrumenten zijn gevalideerde instrumenten die worden aanbevolen door de Cochrane Collaboration: AMSTAR – voor systematische reviews; Cochrane – voor gerandomiseerd gecontroleerd onderzoek; ACROBAT-NRS – voor observationeel onderzoek; QUADAS II – voor diagnostisch onderzoek.
Samenvatten van de literatuur
Voor de modules die volledig werden ge-update, zijn de geselecteerde artikelen toegevoegd aan de set relevante artikelen genoemd in de internationale richtlijn. De relevante onderzoeksgegevens van alle geselecteerde artikelen werden overzichtelijk weergegeven in evidence-tabellen. De belangrijkste bevindingen uit de literatuur werden beschreven in de Engelstalige samenvatting van de literatuur. Bij een voldoende aantal studies en overeenkomstigheid (homogeniteit) tussen de studies werden de gegevens ook kwantitatief samengevat (meta-analyse) met behulp van Review Manager 5.
Beoordelen van de kracht van het wetenschappelijke bewijs
A) Voor interventievragen (vragen over therapie of screening)
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie (Schünemann 2013).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
B) Voor vragen over diagnostische tests, schade of bijwerkingen, etiologie en prognose
De kracht van het wetenschappelijke bewijs werd eveneens bepaald volgens de GRADE-methode: GRADE-diagnostiek voor diagnostische vragen (Schünemann, 2008), en een generieke GRADE-methode voor vragen over schade of bijwerkingen, etiologie en prognose. In de gehanteerde generieke GRADE-methode werden de basisprincipes van de GRADE-methodiek toegepast: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van bewijskracht op basis van de vijf GRADE-criteria (startpunt hoog; downgraden voor Risk of Bias, inconsistentie, indirectheid, imprecisie, en publicatiebias).
Formuleren van de conclusies
Voor elke relevante uitkomstmaat werd het wetenschappelijk bewijs samengevat in een of meerdere Nederlandstalige literatuurconclusies waarbij het niveau van bewijs werd bepaald volgens de GRADE-methodiek. De werkgroepleden maakten de balans op van elke interventie (overall conclusie). Bij het opmaken van de balans werden de gunstige en ongunstige effecten voor de patiënt afgewogen. De overall bewijskracht wordt bepaald door de laagste bewijskracht gevonden bij een van de cruciale uitkomstmaten. Bij complexe besluitvorming waarin naast de conclusies uit de systematische literatuuranalyse vele aanvullende argumenten (overwegingen) een rol spelen, werd afgezien van een overall conclusie. In dat geval werden de gunstige en ongunstige effecten van de interventies samen met alle aanvullende argumenten gewogen onder het kopje Overwegingen.
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals de expertise van de werkgroepleden, de waarden en voorkeuren van de patiënt (patient values and preferences), kosten, beschikbaarheid van voorzieningen en organisatorische zaken. Deze aspecten worden, voor zover geen onderdeel van de literatuursamenvatting, vermeld en beoordeeld (gewogen) onder het kopje Overwegingen.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk. De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen.
Randvoorwaarden (Organisatie van zorg)
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijn is expliciet rekening gehouden met de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, menskracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van een specifieke uitgangsvraag maken onderdeel uit van de overwegingen bij de bewuste uitgangsvraag. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van Zorg.
Indicatorontwikkeling
Er werden geen interne kwaliteitsindicatoren ontwikkeld. In de module Organisatie van Zorg is een suggestie opgenomen voor een toekomstige registratie.
Kennislacunes
Tijdens de ontwikkeling van deze richtlijn is systematisch gezocht naar onderzoek waarvan de resultaten bijdragen aan een antwoord op de uitgangsvragen. Bij elke uitgangsvraag is door de werkgroep nagegaan of er (aanvullend) wetenschappelijk onderzoek gewenst is om de uitgangsvraag te kunnen beantwoorden. Een overzicht van de onderwerpen waarvoor (aanvullend) wetenschappelijk van belang wordt geacht, is als aanbeveling in de Kennislacunes beschreven .
Commentaar- en autorisatiefase
De conceptrichtlijn werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijn aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijn werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Brouwers MC, Kho ME, Browman GP, et al. (2010) AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. https://richtlijnendatabase.nl/over_deze_site/richtlijnontwikkeling.html.
Ontwikkeling van Medisch Specialistische Richtlijnen: stappenplan. Kennisinstituut van Medisch Specialisten.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008;336(7654). doi: 10.1136/bmj.a139. PubMed PMID: 18483053.
Zoekverantwoording
Endoscopisch:
“inguinal hernia” OR “groin hernia”
AND
“laparoscopic” OR “endoscopic” OR “total extraperitoneal” OR “trans-abdominal pre-peritoneal repair”
AND
“lightweight mesh” OR “heavyweight mesh” OR “lightweight” OR “heavyweight” OR “polypropylene mesh” OR “composite mesh” OR “partially absorbable mesh” OR “titanium coated mesh” OR “polyglactin mesh” OR “poliglecaprone mesh” OR “Prolene mesh” OR “VYPRO II mesh” OR “3D mesh”
Ofwel:
(“inguinal hernia” OR “groin hernia”) AND ((“laparoscopic” OR “endoscopic” OR “total extraperitoneal” OR “trans-abdominal pre-peritoneal repair”) AND (“lightweight mesh” OR “heavyweight mesh” OR “lightweight” OR “heavyweight” OR “polypropylene mesh” OR “composite mesh” OR “partially absorbable mesh” OR “titanium coated mesh” OR “polyglactin mesh” OR “poliglecaprone mesh” OR “Prolene mesh” OR “VYPRO II mesh” OR “3D mesh”))
In Medline leverde dit 216 hits of, waarvan 44 clinical trials
In Embase 340 hits, waarvan 41 RCT’s.
Cochrane levered geen aanvullende artikelen op.
Na abstract, titel en zo nodig full-text screening bleven 6 nieuwe artikelen sinds de laatste review van Sajid over.
Open:
“inguinal hernia” OR “groin hernia”
AND
“open repair” OR “open” OR “Lichtenstein” OR “anterior repair” OR “anterior”
AND
“lightweight mesh” OR “heavyweight mesh” OR “lightweight” OR “heavyweight” OR “polypropylene mesh” OR “composite mesh” OR “partially absorbable mesh” OR “titanium coated mesh” OR “polyglactin mesh” OR “poliglecaprone mesh” OR “Prolene mesh” OR “VYPRO II mesh” OR “3D mesh”
Ofwel:
(“inguinal hernia” OR “groin hernia”) AND ((“open repair” OR “open” OR “Lichtenstein” OR “anterior repair” OR “anterior”) AND (“lightweight mesh” OR “heavyweight mesh” OR “lightweight” OR “heavyweight” OR “polypropylene mesh” OR “composite mesh” OR “partially absorbable mesh” OR “titanium coated mesh” OR “polyglactin mesh” OR “poliglecaprone mesh” OR “Prolene mesh” OR “VYPRO II mesh” OR “3D mesh”))
In Medline leverde dit 243 hits of, waarvan 72 clinical trials
In Embase 696 hits, waarvan 122 RCT’s.
Cochrane leverde geen aanvullende artikelen op.
Na abstract, titel en zo nodig full-text screening bleven 11 nieuwe artikelen sinds de laatste review van Sajid over.
Tabel Exclusie na het lezen van het volledige artikel
|
Auteur en jaartal |
Redenen van exclusie |
|
Schopf et al 2010 |
Endoscopisch: Vergelijking LWM met extra-LWH |
|
Horstmann et al 2006 |
Endoscopisch: Geen RCT |
|
Magnusson et al 2016 |
Open: Geen zuivere mat vergelijking, maar vergelijking tussen fixatie/operatie technieken |
|
Pieridis et al 2011 |
Open: Geen zuivere mat vergelijking, maar vergelijking tussen fixatie/operatie technieken |
