Chirurgische behandeling liesbreuk bij volwassenen
Uitgangsvraag
Welke methode heeft voor de operatieve behandeling van een primaire liesbreuk bij mannen de voorkeur?
- Operatie met een mat of weefselplastiek?
- Operatie volgens Lichtenstein of een andere open flat mesh of gepreformeerde mesh gadgets via een anterieure benadering?
- Operatie volgens Lichtenstein of een open pre-peritoneale benadering?
- Indien laparo-endoscopisch: TEP of TAPP techniek?
- Operatie volgens Lichtenstein of laparo-endoscopische techniek?
- Open posterieure benadering of laparo-endoscopische techniek?
Aanbeveling
Maak de keuze voor een bepaald type liesbreukoperatie op basis van de expertise van de chirurg (leercurves van de technieken verschillen), het type breuk en patiëntgerelateerde factoren (module Risico) en kosten. Er is toenemend aanleiding voor het individualiseren van de techniek keuze (zie module Individualisatie).
Gebruik bij voorkeur voor de operatieve behandeling van een liesbreuk een techniek met mat:
Bespreek met de patient de voor- en nadelen van de verschillende opties.
- Overweeg een laparo-endoscopische operatie volgens TEP of TAPP, omdat er minder postoperatieve en minder chronische pijn bij voorkomt, mits het chirurgisch team goed getraind is. Baseer de keuze voor TAPP of TEP op de ervaring van het chirurgische team.
- Overweeg de operatie volgens Lichtenstein in het bijzonder bij specifieke patiënt- en breuk-karakteristieken:
- Indien er geen laparo-endoscopische expertise aanwezig is (overweeg verwijzing).
- Bij contra-indicaties voor algehele anesthesie.
- Bij contra-indicaties voor laparo-endoscopische operatie.
- Bij wens of noodzaak voor regionale of lokale anesthesie.
- Bij niet reponibele scrotaalbreuken.
- Het gebruik van TIPP, PHS of Plug (and Patch) wordt niet aanbevolen.
- De TREPP is nog onvoldoende onderzocht om een aanbeveling te kunnen geven. Voer deze operatie bij voorkeur uit in onderzoeksverband.
Kies voor een Shouldice:
- wanneer de patiënt voorkeur heeft voor een weefselplastiek. De patient dient zich wel te realiseren dat er een verhoogd risico is op recidief zonder bewezen verminderd risico op (chronische) pijn;
- onder bijzondere omstandigheden, zoals bij infectie.
Draag zorg voor adequate training bij de leercurve voor een laparo-endoscopische operatie. De leercurve is namelijk langer en kent met name aan de start zeldzame, maar ernstige complicaties.
Overwegingen
Algemeen
Wat vinden patiënten: patiëntenvoorkeur en –perspectief?
Uit zowel de inbreng tijdens de invitational conference als de respons op de enquete kwam naar voren dat patiënten het belangrijk vinden om informatie te krijgen over de keuzemogelijkheden (zie ook de module Individualisatie) en gezamenlijk met de arts tot een behandelbesluit te komen. Het is onbekend of patiënten een specifieke voorkeur voor een bepaald type operatie hebben. Er is bij sommige patiënten een zorg betreffende de veiligheid van matten. Indien de patiënt geen mat wenst, dan kan na bespreking van de voor- en nadelen, een Shouldice verricht worden.
1. Welke methode heeft voor de operatieve behandeling van een liesbreuk de voorkeur? Operatie met mat of weefselplastiek?
Balans tussen voor- en nadelen
De internationale richtlijn adviseert in alle electieve gevallen een mat te gebruiken. Enige uitzondering is er bij patiënten die een mat weigeren en onder infectieuze omstandigheden. Er is maatschappelijke onrust betreffende het gebruik van matten en er is twijfel bij chirurgen (met name ‘Hernia specialisten”) of er niet bepaalde patiënten categorieën zijn die beter af zijn zonder mat. Bijvoorbeeld bij jonge mannen met een indirecte breuk. Dit is niet goed onderzocht en het schaarse laag-gradige bewijs dat er wel is wijst op verhoogde recidiefkans als er geen mat gebruikt wordt. Er zijn ook specialistische klinieken (bijvoorbeeld Shouldice Hospital) die hele goede resultaten rapporteren met recidief percentages onder de 2%. Dit betreft helaas alleen retrospectief cohortonderzoek zonder deugdelijk uitgevoerde follow-up. Uit alle evidence (ook uit registeronderzoek met data over meerdere jaren) blijkt dat een mat een lager recidief risico heeft en een vergelijkbaar of lager risico op postoperatieve pijn en chronische pijn. Het niveau van onderzoek is over de hele linie laag en vaak ook heel laag. In studies naar recidief bleek bij analyse van de grootste studie dat in de mat groep een groot deel van de recidieven veroorzaakt werd door 1 chirurg. Indien deze uit de analyse bleef, bleek er een evident significant voordeel van mat versus Shouldice. Bijkomend probleem is dat de Shouldice een technisch moeilijke operatie is en nauwelijks tot niet meer onderwezen of gebruikt wordt in Nederland.
Samenvattend adviseert de Werkgoep een mat te gebruiken bij de operatieve behandeling van liesbreuken. Hier kan van afgeweken worden na overleg met een patiënt en documentatie van de redenen van het afwijken van de richtlijn.
Hoewel er zeer vele RCT’s uitgevoerd zijn blijkt de bewijskracht Laag of Zeer laag (volgens Grade). Dit ligt aan onvoldoende gebruik van de juiste randomisatietechnieken, patiënten selectie, gebrek aan definities van pijn en recidief, te korte follow-up en onzekerheid over het gebruiken van een (gouden) standaard techniek. Er is nog steeds behoefte aan goede RCT’s betreffende jonge mannen met indirecte breuk en de resultaten van gespecialiseerde klinieken.
Kosten
Er moet onderscheid gemaakt worden tussen ziekenhuiskosten en maatschappelijke kosten. Open technieken met mat zijn mogelijk duurder (door de prijs van een matje) dan zonder mat. Laparo-endoscopische operaties zijn door het gebruik van materialen duurder dan open technieken met of zonder mat. hoewel een laparo-endoscopische techniek uitgevoerd kan worden met minimaal gebruik van disposable materialen kan het gebruik van herbruikbare instrumenten de ziekenhuiskosten verlagen. De HerniaSurge Group (2018) concludeerde dat mits de kortere hersteltijd en het eerder terugkeren in het arbeidsproces erbij betrokken wordt waarschijnlijk een laparo-endoscopische techniek het meest kosteneffectief is. Hierbij moet in aanmerking genomen worden dat er een langere leercurve is en er zeldzame ernstige complicaties kunnen voorkomen die bij een open techniek onwaarschijnlijk zijn.
Wat vinden artsen: professioneel perspectief
In Nederland worden 98% van de liesbreukoperaties uitgevoerd met gebruik van een mat. In 2018 werd 53% van de patiënten met een laparo-endoscopische techniek geopereerd. De werkgroep adviseert de internationale richtlijn te volgen. In Nederland worden de meeste patiënten door middel van een Lichtenstein of TEP geopereerd. Een klein aantal ziekenhuizen prefereert een andere techniek zoals TAPP, TREPP, TIPP, Plug en PHS. De laatste twee worden afgeraden door de internationale richtlijn. TREPP is onvoldoende onderzocht om een goed advies te geven.
2. Welke methode heeft voor de operatieve behandeling van een liesbreuk de voorkeur?
Operatie volgens Lichtenstein of andere open vlakke mat en gepreformeerde matten via een anterieure benadering?
Balans tussen voor- en nadelen
De TIPP techniek lijkt in studies met korte follow-up een klein voordeel te geven in hersteltijd en chronische pijn ten opzichte van de Lichtenstein-techniek. Een nadeel van deze techniek is echter dat er door twee vlakken geopereerd wordt (anterieure benadering en plaatsen mat in pre-peritoneale vlak) en de hogere kosten van de mat die hierbij noodzakelijk is. Bij recidief en pijnklachten zijn reoperaties moeilijker. Casuistisch wordt vaak beschreven dat de zogenaamde memory ring verwijderd moet worden ivm pijn en/of verkleving aan darmen. De experts van de internationale richtlijn adviseren de TIPP niet als eerste keus operatie te kiezen (lage evidence).
De PHS heeft als extra nadeel dat er een mat geplaatst worden in beide vlakken en de experts van de internationale richtlijn concluderen dat er een te grote hoeveelheid mesh gebruikt wordt zonder dat er bewijs is dat deze techniek beter is dan een operatie volgens Lichtenstein. Beide bovengenoemde technieken maken gebruik van een veel duurdere mat.
De werkgroepleden, en de HerniaSurge Group, raden het gebruik van plugs af, omdat deze regelmatig verwijderd moet worden in verband met pijn en/of recidief en/of migratie, hoewel dit niet uit de studies met korte follow-up blijkt. Ook bij de plug wordt prothesemateriaal achtergelaten in beide anatomische vlakken en spelen hogere kosten een rol.
De internationale richtlijn adviseert als eerste keus te kiezen uit een Lichtenstein en TEP of TAPP. TREPP moet verder onderzocht en TIPP, PHS en Plugs worden (met lage evidence) afgeraden.
Kwaliteit van het bewijs
De kwaliteit van dit onderzoek is laag. De aanbevelingen zijn door de experts van de interntionale richtlijn door consensus ge-upgrade op basis van anatomische argumenten, klinische expertise en kosten.
Kosten
Wanneer er vergeleken wordt tussen de open technieken dan zijn de kosten van het matje en de operatieduur bepalend. Een Lichtenstein kan met een “simpel” plat matje uitgevoerd worden versus hogere kosten van speciale matjes die nodig zijn bij TREPP, TIPP, PHS en Plug.
Wat vinden artsen: professioneel perspectief
De werkgroep adviseert de internationale richtlijn te volgen. De eerste keus techniek voor een primaire breuk zijn: Lichtenstein, TEP of TAPP. TREPP zou bij voorkeur beter onderzocht moeten worden, bij voorkeur in gerandomiseerde studies vergelijkend met eerder genoemde technieken. Het wordt met lage evidente aangeraden geen TIPP, PHS of Plug techniek uit te voeren.
3. Wat is de voorkeurstechniek: Operatie volgens Lichtenstein of een open preperitoneale benadering?
De update van de literatuur over de periode 2015-2018 heeft geen goede studies met voldoende follow up opgeleverd.
Derhalve blijven de conclusies van de internationale richtlijn overeind.
Op basis van het in de internationale richtlijn gepresenteerde bewijs kan geconcludeerd worden dat de open pre-peritoneale benadering, bij een korte follow up van 1 jaar, even effectief lijkt als de operatie volgens Lichtenstein ten aanzien van recidief en mogelijk minder postoperatieve pijn en een sneller herstel geeft. Echter dit is met name gebaseerd op studies met de TIPP (trans-inguinaal pre peritoneaal) en de volledig posterieure techniek beschreven als Kugel. Overige technieken zijn onvoldoende (lang) onderzocht.
Er is geen studie die preperitoneale technieken met elkaar heeft vergeleken. Hierdoor kan er niet worden gesproken van de optimale open preperitoneale techniek.
Ten aanzien van deze technieken bestaan ook nieuwe zorgen met name ten aanzien van de kosten en de lange termijn veiligheid. Bij de Kugel-techniek werd een ruime hoeveelheid vreemd lichaam geïmplanteerd en waren er problemen met de geheugenring die leiden tot complicaties als pijn en darmperforaties. (de laatste versie heeft een oplosbare geheugenring).
Het wordt vooralsnog aanbevolen om de open preperitoneale technieken in onderzoeksvorm uit te zetten tegen de tot op heden gouden standaard (Lichtenstein-techniek) waarbij met name een lange follow up duur interessante bevindingen zou kunnen leveren. Hierbij dient men zich overigens wel te realiseren dat een trans-inguinale benadering van het preperitoneale vlak zowel het anterieure als posterieure anatomische vlak gebruik waarbij het een theoretisch nadeel is om via 1 van deze benadering een recidief te herstellen.
4. Welke laparo-endoscopische techniek heeft de voorkeur TEP of TAPP? Balans tussen voor- en nadelen
TAPP en TEP zijn beide goede technieken voor de behandeling van liesbreuken. Beide technieken hebben vergelijkbare resultaten wat betreft acute postoperatieve pijn, chronische pijn, recidiefpercentages, postoperatief herstel en kosten. Het risico op serieuze complicaties is zeer laag, met een hogere kans op darmletsel bij TAPP en op vaatletsel bij TEP. Literatuur gepubliceerd na de internationale richtlijn (HerniaSurge Group, 2018) heeft niet geleid tot wijziging van de aanbevelingen. De keuze voor een van de technieken zou gebaseerd moeten zijn op de ervaring van het chirurgische team.
Kwaliteit van het bewijs
De kwaliteit van het bewijs is hoog en gebaseerd op meerdere meta-analyses en systematic reviews.
Kosten
De kosten van TAPP en TEP zijn vergelijkbaar.
Wat vinden artsen: professioneel perspectief
De belangrijkste factor in de beslissing van de uitvoer van TAPP of TEP is de ervaring van het chirurgische team. Beide technieken zijn geschikt voor de behandeling van liesbreuken.
Haalbaarheid
De TAPP-techniek heeft als voordeel dat de aanwezigheid van een contralaterale liesbreuk vastgesteld kan worden voorafgaand aan de chirurgische exploratie. Bij TEP blijft de chirurg in het extraperitoneale vlak. Factoren zoals twijfel over de aanwezigheid van een contralaterale breuk of voorgeschiedenis van abdominale chirurgie kunnen doorslaggevend zijn voor de keuze voor één van de technieken, mits voldoende expertise aanwezig is bij het chirurgische team.
5. Als recidief, pijn, leercurve, postoperatief herstel en kosten belangrijke uitkomsten zijn, welke techniek heeft dan de voorkeur voor primaire liesbreuken bij mannen: Operatie volgens Lichtenstein of een laparo-endoscopische techniek?
Bij het wegen van de punten postoperatief herstel, postoperatieve pijn en chronische pijn is er steeds meer bewijs dat er winst is voor het gebruik van een laparo-endoscopische techniek. De voorwaarde hiervoor is echter wel dat de chirurg voldoende ervaren is in de techniek. De vele studies gaan echter mank op definitie van pijn, chirurgische ervaring en caseload per chirurg. Hierdoor kan de optimale indicatie voor deze technieken niet bepaald worden. Hierbij dient ook te worden meegewogen dat de leercurve en de (initiële) kosten van deze technieken langer respectievelijk hoger zijn dan bij de operatie volgens Lichtenstein. De leercurve voor de laparo-endoscopische technieken, met name de TEP, is geschat tussen de 50 en 100 procedures waarbij de 1e 30 tot 50 het meest kritisch zijn.
Vanuit het perspectief van het ziekenhuis is een Lichtenstein techniek het meest kosteneffectief. Vanuit een macroeconomisch kosten perspectief inclusief kwaliteit van leven is de laparo-endoscopische techniek gunstiger gezien de lagere incidentie van chronisch pijn en een sneller herstel.
In de 2014 update van de EHS guidelines werd een nieuwe meta-analyse gedaan bij patiënten met een follow up van meer dan 48 maanden. Hier was een niet significant verschil in chronische pijn (p=0.12) en in recidief, mits 1 studie met maar liefst 32% recidief, waarschijnlijk te beschouwen al technisch falen, in de endoscopie groep werd uitgesloten.
Een grotere RCT met een goede externe validiteit en of grote database studies zijn nodig om de laparo-endoscopie en de Lichtenstein techniek in primaire unilatere hernia bij mannen te kunnen vergelijken. Hierbij dient het niveau van de betrokken chirurgen goed gekwantificeerd te worden.
Het wordt aanbevolen om de laparo-endoscopische en Lichtenstein-technieken via een gestructureerd trainingprogramma en gedegen supervisie gedurende de leercurve te onderwijzen.
6. Open posterieure benadering (TIPP/TREPP) of laparo-endoscopische techniek?
Balans tussen voor- en nadelen
Open posterieure technieken worden in enkele ziekenhuizen in Nederland aangeboden en betreffen de TREPP, TIPP, plug en PHS. De laatste twee worden beargumenteerd afgeraden door de internationale richtlijn.
TREPP en TIPP zijn onvoldoende onderzocht om een goed advies te geven. Er zijn op dit moment nog geen resultaten bekend van vergelijkende studies tussen bijvoorbeeld de TREPP-techniek en de laparo-endoscopische technieken. Voor de TIPP geldt dat er in studies met korte follow-up een klein voordeel lijkt te zijn in hersteltijd en chronische pijn ten opzichte van de Lichtenstein-techniek. Een transinguinale benadering van het preperitoneale vlak via zowel het anterieure als posterieure anatomische vlak is een theoretisch nadeel voor herstel van een recidief via één van deze benadering. Ook zijn de kosten voor de benodigde mat hoger dan voor de operatie volgens Lichtenstein. De laparo-endoscopische technieken (TEP, TAPP) zijn beide geschikt voor liesbreukoperatie.
De preperitoneale benadering kan gepaard gaan met blaas- of vaatletsel. Ervaring van de chirurg in het preperitoneale vlak is daarom van belang.
Kwaliteit van het bewijs
De kwaliteit van het bewijs is beperkt, onder andere door het ontbreken van een risk bias assessment in de enige beschikbare metaanalyse.
Kosten
Er is geen informatie over kosteneffectiviteit. Voor het gebruik van laparo-endoscopische technieken is aanvullende laparo-endoscopisch apparatuur nodig en worden kosten gemaakt voor eventuele extra materialen.
Wat vinden artsen: professioneel perspectief
Eerste keus technieken zijn Lichtenstein, TEP en TAPP, mits voldoende ervaring aanwezig is in het chirurgische team. TREPP zou beter onderzocht moeten worden, bij voorkeur in gerandomiseerde studies vergelijkend met eerder genoemde technieken. Het wordt met lage bewijskracht aangeraden om geen TIPP, PHS of Plug repair aan te bieden.
Onderbouwing
Achtergrond
Zoals in de internationale richtlijn goed beschreven is, bestaan er veel technieken om een liesbreuk operatief te behandelen. Deze varieren van “non-mesh” oftewel “weefselplastiek” tot variaties van “open mesh” (open mat technieken) en “laparo-endoscopische mat” (TEP/TAPP) technieken. Teneinde de beste techniek te identificeren zou gekeken moeten worden naar de volgende variabelen: leercurve (complexiteit), veiligheid (complicaties), risico’s op chronische pijn en recidief, postoperatief herstel en kosten. Een specifieke techniek voor alle liesbreuktypes bestaat niet. De internationale richtlijn beveelt aan om te individualiseren op grond van chirurg gerelateerde factoren (expertise, ervaring), patiëntgerelateerde factoren (geslacht, risicofactoren voor complicaties, recidief, pijn, anesthesie opties), liesbreuktype (recidief, scrotaal, reponibel, eerdere operaties) en aanwezige middelen (mogelijkheid tot scopische operaties bijvoorbeeld). De Werkgoep is nagegaan welke technieken geschikt zijn onder de voorspelbare omstandigheden van de primaire liesbreuk. Het is de vraag of er gestandardiseerd kan worden naar één of twee technieken die voldoen voor de verschillende typen liesbreuk. Ook is kritisch gekeken of het soort benadering en het type matje gestandardiseerd kunnen worden. Dit zou potentieel kostenverlagend kunnen werken. Dankzij een enquête onder chirurgen in 2018 is het bekend dat 98% een matje gebruikt, 53% van de patiënten middels een laparo-endoscopische techniek geopereerd wordt en er is een meerderheid die als eerste keus opties een Lichtenstein (60%) of TEP (85%) adviseert. Voorts bleken TREPP, TIPP, Plug en PHS in respectievelijk 6, 2, 2 en 1 ziekenhuis aangeboden te worden. Een Shouldice werd in 1 ziekenhuis aangeboden als een eerste keus optie. Meerdere eerste keus opties konden aangegeven worden. Dit wijst op een grote variatie waarbij ook naar voren komt dat chirurgen binnen een ziekenhuis meerdere technieken adviseren.
Conclusies / Summary of Findings
1. Which is the preferred repair method for inguinal hernias: mesh or Shouldice?
Conclusies operatie met mat of weefselplastiek
|
Laag GRADE |
Er lijkt geen verschil in het aantal patiënten met chronische postoperatieve pijn 3 jaar of langer na een liesbreukoperatie met mat vergeleken met een operatie volgens Shouldice.
Bronnen: (Lockhart, 2015 – unpublished results; Wamalwa, 2015; Miserez, 2014) |
|
Laag GRADE |
Een operatie volgens Shouldice lijkt een bijna twee keer zo grote kans op recidief 3 jaar of langer na een liesbreukoperatie te geven vergeleken met een liesbreukoperatie met mat.
Bronnen: (Lockhart, 2015 - unpublished results; Wamalwa, 2015, Miserez, 2014) |
|
Zeer laag GRADE |
Een verschil in postoperatieve pijn (herstelfase <3 maanden) tussen een liesbreukoperatie met mat versus een operatie volgens Shouldice kon niet worden aangetoond, noch verworpen.
Wel lijken er aanwijzingen te zijn dat postoperatieve pijn na een laparo-endoscopische operatie lager is dan na een operatie volgens Shouldice.
Bronnen: (Lockhart, 2015 - unpublished results; Wamalwa, 2015) |
2. Which is the preferred open mesh technique for inguinal hernias: Lichtenstein or other open flat mesh and gadgets via an anterior approach?
Conclusies Operatie volgens Lichtenstein versus andere open flat mesh en gepreformeerde mesh gadgets via een anterieure benadering
Conform de International Guidelines wordt ondanks vergelijkbare resultaten het gebruik van de plug en patch en de PHS niet aanbevolen vanwege de ruime hoeveelheid vreemdlichaam dat geïmplanteerd wordt, het gebruik van zowel het anterieure als het posterieure vlak en de extra kosten voor de mat.
|
Conclusie internationale richtlijn: Recidief kans en postoperatieve pijn zijn wellicht vergelijkbaar tussen plug-and-patch/PHS en een operatie volgens Lichtenstein. Ondanks de vergelijkbare resultaten worden de plug-and-patch/PHS niet aanbevolen vanwege de ruime hoeveelheid vreemdlichaam, de kosten en het gebruik van het anterieure en posterieure vlak in dezelfde procedure.
Bron: (HerniaSurge Group, 2018) |
|
Chronische pijn (>3 maanden): Aanvullend bewijs met lage bewijskracht lijkt geen verschil te tonen in chronische post-operatieve pijn tussen de operatie volgens Lichtenstein, de PHS en de UHS techniek en ook niet tussen de TIPP en Progrip techniek.
Bronnen: (Magnusson, 2016; Čadanová, 2016) |
|
Recidief: Aanvullend bewijs met lage bewijskracht lijkt geen verschil te tonen in recidief kans na 1 jaar tussen Progrip en de TIPP techniek en ook niet tussen de Lichtenstein, PHS en de Rutkow-Robins techniek.
Bronnen: (Čadanová, 2016; Karaca, 2013) |
|
Complicaties: Aanvullend bewijs met lage bewijskracht lijkt meer complicaties te tonen na een Progrip techniek vergeleken met de TIPP techniek 2 weken na operatie. Of er verschil is tussen de operatie volgens Lichtenstein versus andere open anterieure technieken is onzeker.
Bronnen: (Čadanová, 2016; Karaca, 2013) |
3. Which is preferred open mesh technique: Lichtenstein versus open pre-peritoneal?
Conclusies operatie volgens Lichtenstein versus open pre-peritoneale benadering
Bij een open Liesbreukoperatie is er conform de internationale richtlijn geen bewijs om een open preperitoneale benadering aan te bevelen boven een Lichtenstein plastiek. Het gebruik van het anterieure en posterieure anatomische vlak in één technique is op theoretische gronden een reden voor terughoudendheid.
|
Conclusies internationale richtlijn: Er is onzeker bewijs dat open preperitoneale technieken resulteren in een korte termijn winst (na 1 jaar) met betrekking tot direct posteratieve pijn, chronische pijn en postoperatief herstel. Hierbij dient in ogenschouw genomen te worden dat een aantal technieken gelijktijdig het anterieure en posterieure anatomische vlak gebruiken tijdens één procedure hetgeen niet de voorkeur verdient.
Het gebruik van speciale matten ten behoeve van matplaatsing resulteert in verhoogde kosten voor de mat. Tevens is er onzeker bewijs dat er problemen zijn met de geheugenring van sommige matten.
Bron: (HerniaSurge Group, 2018) |
|
Recidief: Aanvullend bewijs met lage bewijskracht (2015 tot 2017) ten aanzien van recidief staat geen nieuwe conclusies toe.
Bronnen: (Azeem, 2015; Andresen, 2017; Mahmoudvand, 2017) |
|
Chronische pijn: Aanvullend bewijs met lage bewijskracht (2015 tot 2017) ten aanzien van chronische pijn staat geen nieuwe conclusies toe.
Bronnen: (Andresen, 2015; Azeem, 2015 Mahmoudvand, 2017) |
|
Postoperatief herstel: Pijn: Aanvullend bewijs met lage bewijskracht (2015 tot 2017) ten aanzien van postoperatieve pijn staat geen nieuwe conclusies toe.
Bronnen: (Andresen, 2015; Mahmoudvand, 2017) |
|
Complicaties: Aanvullend bewijs met lage bewijskracht (2015 tot 2017) en ten aanzien van complicaties staat geen nieuwe conclusies toe.
Bronnen: (Azeem, 2015; Andresen, 2015 en 2017; Mahmoudvand, 2017) |
|
Mortaliteit:
Er is geen nieuw (2015 tot 2017) bewijs ten aazien van de uitkomstmaat ‘mortaliteit’.
Bronnen: - |
4. Which endoscopic technique is preferred in terms of postoperative outcome?
Conclusies TEP versus TAPP
Conform de internationale richtlijn hebben TEP en TAPP vergelijkbare uitkomsten. De keuze voor de techniek kan het beste gebaseerd worden op patiëntfactoren en de vaardigheiden c.q. ervaring van de chirurg.
|
Conclusies internationale richtlijn: Er lijkt geen verschil tussen TAPP en TEP met betrekking tot operatietijden, totaal aantal complicaties, postoperatieve acute en chronisch pijn en recidief percentages.
Hoewel de complicatie zeer zeldzaam is lijkt er een trend naar meer darmletsels bij TAPP.
Hoewel de complicatie zeer zeldzaam is lijkt er een trend naar meer vaatletsels bij TAPP.
Hoewel de complicatie zeldzaam is lijken trocart hernia’s vaker voor te komen bij TAPP. Hoewel het fenomeen zeldzaam is lijkt een conversie bij TEP vaker voor te komen.
Er lijkt geen verschil in kosten tussen TAPP en TEP
Er zijn aanwijzingen dat de leercurve bij TEP langer is dan bij TAPP.
Bron: (HerniaSurge Group, 2018) |
|
Recidief (Follow up > 3 jaar): Er is geen aanvullend bewijs voor de uitkomst recidief (2015 tot 2017).
Bronnen: - |
|
Acute postoperatieve pijn: Het aanvullende bewijs voor de uitkomst acute postoperatieve pijn is van erg lage kwaliteit (2015 en 2017).
Bronnen: (Wei, 2015) |
|
Chronische pijn (>6 maanden) Aanvullend bewijs voor de uitkomst chronische pijn na 6 maanden toonde geen significant verschil tussen TAPP en TEP (2015 tot 2017).
Bronnen: (Bansal, 2017) |
|
Herstel: Aanvullend bewijs voor de uitkomst hersteltijd (tijd tot hervatting van normale activiteiten) is van erg lage kwaliteit (2015 tot 2017).
Bronnen: (Wei, 2015) |
|
Kosten: Aanvullend bewijs betreffende kosteneffectiviteit is van erg lage kwaliteit (2015 tot 2017).
Bronnen: (Wei, 2015) |
5. When considering recurrence, pain, learning curve, postoperative recovery and costs which is preferred technique for inguinal hernias: Best open mesh (Lichtenstein) or a laparo-endoscopic (TEP and TAPP) technique?
Conclusies operatie volgens Lichtenstein versus laparo-endoscopische techniek
Conform de internationale richtlijn wordt bij mannen met een primaire unilaterale liesbreuk aanbevolen om de liesbreuk met een laparo-endoscopische techniek te behandelen in verband met een lagere postoperatieve incidentie van vroege en late postoperatieve pijn. Er zijn echter patient en hernia karakteristieken waarbij de Lichtenstein de 1e keuze is.
|
Conclusies internationale richtlijn: Het lijkt aannemelijk dat een chirurg met voldoende ervaring in de laparo-endoscopische techniek een vergelijkbaar recidief percentage kan halen in vergelijking met de operatie volgens Lichtenstein.
Er lijkt een voordeel aanwezig voor een laparo-endoscopische procedure door een ervaren chirurg in vergelijking met de operatie volgens Lichtenstein ten aanzien van vroege postoperatieve pijn (in rust en bij beweging) en chronische pijn
Er lijkt geen verschil in operatieduur tussen de laparo-endoscopische techniek en de operatie volgens Lichtenstein in handen van een ervaren chirurg. Er lijkt geen verschil tussen percentage reoperaties na laparo-endopische of Lichtenstein operaties in ervaren handen.
Er lijkt een toename in directe operatieve kosten bij het verrichten van laparo-endoscopische operaties. De impact van deze kosten lijkt af te nemen als de macroeconomische kosten worden meegewogen en als de chirurg voldoende ervaring heeft.
Er zijn aanwijzingen dat de leercurve voor laparo-endoscopische operaties (met name TEP) langer is dan die voor de operatie volgens Lichtenstein. Er zijn zeldzame doch ernstige complicaties die vooral optreden aan het begin van de leercurve. Het is dan ook zeer verstandig dat laparo-endoscopische operaties met afdoende supervisie worden onderwezen.
Bron: (HerniaSurge Group, 2018) |
|
Recidief: Er lijkt geen verschil in het aantal patiënten met een recidief tussen Lichtenstein en TEP (data 2015 tot 2017)
Bronnen: (Westin, 2015; Gürbulak 2016) |
|
Vroege en late postoperatieve pijn: Aanvullende data (2015 tot 2017) suggereert meteen laag bewijsniveau een afname van de vroege en late postoperatieve pijn bij laparo-endoscopie in vergelijking met de operatie volgens Lichtenstein.
Bronnen: (Salma, 2015; Westin, 2015; Waris, 2016) |
|
Kosten: Geen aanvullende data (2015 tot 2017) gevonden.
Bronnen: - |
|
Complicaties: Aanvullende data (2015 tot 2017) toonde geen verschil tussen laparo-endoscopische operaties en de operatie volgens Lichtenstein.
Bronnen: (Gürbulak, 2015) |
|
Leercurve: Geen aanvullende data (2015 tot 2017) gevonden.
Bronnen: - |
6. Open posterior or laparoscopic technique?
Conclusies Open posterieure benadering (TIPP, TREPP) of laparo-endoscopische techniek
Conform de internationale richtlijn is er een gebrek aan bewijs door insufficiënte en heterogene data. Een conclusie over deze vraag zal derhalve uitblijven.
|
Conclusie internationale richtlijn: Er is gebrek aan bewijs om een preperitoneale mat te prefereren boven de operatie volgens Lichtenstein. Open pre-peritoneale technieken worden bij voorkeur in onderzoeks verband verricht zodat verdere uitkomsten kunnen worden verzameld. Het is te overwegen om niet tijdens één operatie het anterieure en posterieure anatomische vlak te gebruiken.
Bron: (HerniaSurge Group, 2018) |
|
Recidief: Aanvullend bewijs toonde geen verschil in recidiefkans tussen laparo-endoscopische technieken en open preperitoneale technieken (2015 tot 2017).
Bronnen: (Sajid, 2015) |
|
Potoperatief herstel (complicaties): Aanvullend bewijs toonde dat de kans op postoperatieve complicaties niet toe- of afneemt bij laparo-endoscopische technieken vergeleken met open preperitoneale technieken (2015 tot 2017).
Bronnen: - |
|
Mortaliteit: Er is geen aanvullend bewijs voor de uitkomst mortaliteit (2015 tot 2017) .
Bronnen: - |
Samenvatting literatuur
1. Which is the preferred repair method for inguinal hernias: mesh or Shouldice?
Description of studies
A protocol for a Cochrane systematic review comparing mesh versus non-mesh for inguinal and femoral hernia repair was found (Lockhart, 2015), which will update a previous Cochrane review about the topic (Scott 2001). Authors of the protocol were contacted and they were willing to share their preliminary results. Furthermore, a second relevant Cochrane review that compared Shouldice technique versus other open techniques for inguinal hernia repair was identified (Amato, 2012). In additionan, six randomized, controlled studies (RCTs) were included (Bhatti, 2015; Olasehinde, 2016; Palermo, 2015; Szopinski, 2012; Wamalwa, 2015; Youssef, 2015).
For their update of the Cochrane review of Scott 2001, Lockhart and colleagues searched the following databases up to January 8 2017: Cochrane Colorectal Cancer Group Specialized Register, Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE and Web of Science. Trial registers were also searched and references of included studies checked. The review compared mesh with non-mesh (both open and laparoscopic techniques) in adult patients (> 18 years) with inguinal hernia. Twenty-five RCTs were included with a total of 6293 participants. Primary outcome measures in this review were recurrences, complications and mortality. Secondary outcomes were duration of operation, duration of hospital admission, time needed to take up daily life activities and number of operations in which a laparoscopic technique was switched to an open technique.
Sixteen studies comparing Shouldice technique to other open techniques for inguinalhernia repair were included in the Cochrane review by Amato and colleagues (Amato, 2012). Eight of the included studies compared Shouldice to mesh techniques. MEDLINE, EMBASE, and The Cochrane Central Register of Controlled Trials (CENTRAL) were searched and the review is assessed as up to date until February 2012. Recurrence of hernia was the primary outcome measure and secondary outcomes were postoperative hospital stays, chronic pain, postoperative satisfaction, complication rate, and duration of operation. The main characteristics and results of this systematic review are included in the evidence table (see Appendix 1). Assessment of the quality of the review is presented in Appendix 2.
Of the six additionally identified RCTs, three compared mesh with the Desarda method (Bhatti, 2015; Szopinski, 2012; Youssef, 2015), one compared mesh with the Bassini method (Palermo. 2015), one mesh with the darning technique (Olasehinde, 2016) and one mesh with Shouldice (Wamalwa 2015). In all six RCTs, the mesh operation consisted of a Lichtenstein technique. The size of the study populations varied from 50 to 263. With the exception of the RCT of Bhatti (2015), all RCTs examined the outcomes of pain and recurrences. All RCTs also looked at one or more complications.
The randomization method was clearly described in all RCTs and was considered to be adequate, with the exception of one RCT in which an alternating sequence was used, as a result of which the allocation of the intervention was not concealed (Olasehinde, 2016). For another RCT, in which the randomization sequence with a table was assigned, it was unclear whether this allocation was also concealed (Bhatti, 2015). Blinding of the intervention for patients was unlikely, with the exception of one RCT in which patients were not informed about the chosen surgical technique (Szopinski, 2012). Blinding of caregivers was not possible. The outcome measurement was blinded in one RCT (Szopinski, 2012) for the other RCTs the chance of bias with regard to the outcome measurement was unclear, because it was unclear who, in addition to the results reported by the (unblinded) patients, judged the other outcomes and whether this happened blinded . A study protocol was available for two RCTs and there seemed to be no selective reporting (Szopinski, 2012; Youssef, 2015), for the other RCTs there was no protocol, but the results were reported for all outcomes described in the methods. The probability of bias due to incomplete follow-up or violation of the 'intention-to-treat' principle was not plausible in any of the RCTs. The main study characteristics and results are included in the evidence table (see Appendix 1) and the assessment of the individual study design (Risk of Bias) is included in the risk or bias table (Appendix 2).
Results
In total, 36 primary studies comparing mesh with no mesh were identified (Overview table in Appendix 1). Looking at the type of non-mesh techniques that were used, the following subgroups can be distinghuised:
- Shouldice
- Bassini
- Desarda
- Darning techniques
- Marcy repair
- using multiple non-mesh techniques ('mixed')
Since Shouldice is the most relevant non-mesh technique for the Dutch situation, in presenting the results and grading of the evidence (GRADE), the focus is on the comparison mesh versus Shouldice. In total, 19 studies addressed this comparison (9 studies comparing Lichtenstein and Shouldice, 3 studies comparing TEP or TAPP with Shouldice, and 7 studies comparing multitple or other mesh techniques such as mesh-plug, with Shouldice) (Overview table in Appendix 1). Risk of Bias of these studies is summarized in Figure 1, in Appendix 2.
Chronic pain (crucial outcome)
Sixteen RCTs that compared postoperative (chronic) pain between mesh and Shouldice techniques are listed in Table 1 (Barth, 1998; Arvidsson, 2005/Berndsen, 2007; Danielsson, 1999; Hauters, 1996; Kux, 1994; Leibl, 2000; Lermite, 2012; McGillicuddy, 1998; Miedema, 2004; Nordin, 2002; Pokorny, 2008; Prieto-Díaz-Chávez, 2009; Schmitz, 1997; Tschudi, 2001; Wamalwa, 2015; Zieren, 1998). The ways and times at which postoperative pain was measured varied greatly between the studies.
Eight RCTs (n = 2447 participants) reported results for chronic pain (critical outcome; defined as presence of pain measured 3 months or longer after surgery) (Arvidsson, 2005/Berndsen, 2007; Leibl, 2000, McGillicuddy, 1998; Miedema, 2004; Nordin, 2002; Prieto-Díaz-Chávez, 2009; Tschudi, 2001; Wamalwa, 2015). Seven of them are displayed in a forest plot (Figure 1). Due to heterogeneity in measurement methods and follow-up duration, the results were not pooled. With the exception of one study that measured chronic pain 2 years postoperatively (RR 0.54, 95% CI 0.39 to 0.77 in favor of mesh), no differences were found between the study groups treated with mesh and Shouldice for the other time points. The eighth study, with a follow-up of 24 months, reported a significantly lower monthly score on a visual analogue scale (VAS) for mesh, however, it is unclear how such a monthly VAS score should be interpreted (Prieto-Díaz-Chávez, 2009).
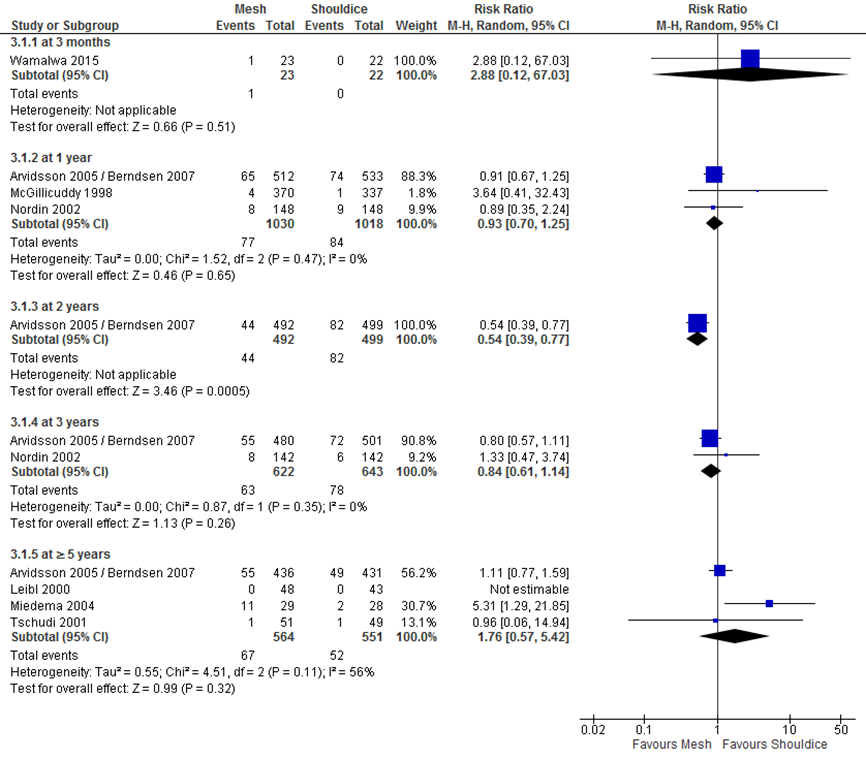
Figure 1 Forest plot mesh versus Shouldice for chronic postoperative pain (per follow-up duration) (n=7)
Postoperative pain
The results in 14 RCTs (n = 3106 participants) that examined postoperative pain up to 3 months postoperatively (important outcome) ranged between a benefit for mesh and no difference between mesh and Shouldice.
A benefit for mesh was found in the 7 studies comparing TAPP (Berndsen, 2007; Tschudi, 2001), mesh-plug (Lermite, 2012; Prieto-Diaz-Chavez, 2009), mesh-plug or TAPP (Zieren, 1998), Lichtenstein (Kux, 1994) and a not-specified mesh technique (Hauters, 1996).
No difference between mesh and Shouldice was found in 5 studies comparing Lichtenstein and Shouldice (Barth, 1998; Danielsson, 1999; Miedema, 2004; Nordin, 2002; Wamalwa, 2015), one study comparing another mesh technique with Shouldice (Schmitz, 1997) and one study comparing Lichtenstein, TEP and TAPP with Shouldice (Pokorny, 2008) (Table 1).
Table 1 Overview of reported outcomes regarding postoperative (chronic) pain for mesh versus Shouldice (n=16 RCTs).
|
Study |
Outcome |
Time points |
Measurement tool |
Results |
|
Arvidsson 2005 /Berndsen 2007 |
Postoperative pain |
1 week postoperatively |
Self-report (VAS) |
Median (min-max) VAS (mm) 98 (range 0-405) versus 165 (range 0-450); p <0.001 |
|
|
Chronic post-operative pain or discomfort |
1, 2, 3 and 5 years postoperatively |
Description by patient, classified by two independent observers as mild (occasional pain or discomfort without affecting daily activities), moderate (pain or discomfort that occasionally affects daily activities) or severe (daily pain or discomfort affecting daily activities) . |
1 year 65/512 versus 74/533 - Mild: 48 versus 56 - Moderate: 16 versus 18 - Severe: 1 versus 0
2 years 55/492 versus 82/499 - Mild: 44 versus 67 - Moderate: 11 versus 14 - Severe: 0 versus 1
3 years 55/480 versus 82/501 - Mild: 44 versus 53 - Moderate: 11 versus 19 - Severe: 0 versus 0
5 years 55/436 versus 49/431 - Mild: 24 versus 34 - Moderate: 12 versus 12 - Severe: 1 versus 3 |
|
Barth 1998 |
Postoperative pain at rest |
daily up to 4 weeks postoperatively |
Self-report with respect to 5-point pain score (0 = none, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe) |
Average postoperative day (IQR) Mild pain: 1 (0-3) versus 2 (0-3); NS Very mild pain: 3 (2-7) versus 4 (1-7); NS
Postoperative day on which 50% patients without pain: 9 versus 9 |
|
|
Postoperative pain during walking |
daily up to 4 weeks postoperatively |
Self-report with respect to 5-point pain score (0 = none, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe) |
Average postoperative day (IQR) Mild pain: 2 (1-5) versus 3 (1-5); NS Very mild pain: 6 versus 6; NS
Postoperative day (IQR) in which 50% of patients without pain: 14 (7-22) versus 12 (8-20) |
|
|
Use of painkillers |
n.a. |
Self-report |
Median number of days 3 versus 4; NS (Within 15 days, everyone stopped taking pain relief) |
|
Danielsson 1999 |
Postoperative pain |
1, 2, and 3 days postoperatively |
VAS (0-100) |
Day 1 postoperative, mean ± SD 48 ± 25 versus 45 ± 25
Day 2 postoperative, mean ± SD 43 ± 23 versus 43 ± 24
Day 3 postoperative, mean ± SD 36 ± 23 versus 35 ± 25 |
|
|
First day without appreciable pain |
Not specified. |
Self-report |
mean ± SD 10 ± 4.9 versus 10 ± 5.2 |
|
Hauters 1996 |
Postoperative pain |
1 day and 3 days postoperatively |
VAS (0-10) |
Day 1 postoperative, mean ± SD 3.4 ± 1.5 versus 5.3 ± 1.9; p <0.001
Day 3 postoperative, mean ± SD 1.3 ± 1.4 versus 2.8 ± 1.8; p <0.005 |
|
Kux 1994 |
Use of painkillers |
1 day postoperatively |
Not reported |
% of people who do not use painkillers on day 1 postoperatively: 29.4 versus ? *
% of people who did not use painkillers at all: 9.8 versus ? *
"The amount of local anesthetic and postoperative pain medication was significantly reduced in the Lichtenstein group"
* Data for Shouldice group not reported.
|
|
Leibl 2000 |
Chronic pain (unspecified) |
Median follow-up duration of 70 months |
Not reported |
0/48 versus 0/43 “Neither of the two therapeutic options led to a chronic pain syndrome” |
|
Lermite 2012 |
Postoperative pain (intensity) |
1 day and 2 days postoperatively |
VAS |
Day 1 postoperative 22.1 versus 27.4; p = 0.003
Day 2 postoperative 13.2 versus 21.4; p <0.0001 |
|
McGillicuddy 1998 |
Chronic pain
|
>1 jaar postoperatively |
Focused physical examination |
4/370 versus 1/337; NS |
|
Miedema 2004 |
Postoperative pain |
30 days postoperative |
Self-report of number of tablets used |
Median number of tablets used 6 versus 7, p=NS |
|
|
Chronic groin pain |
up to 6-9 years follow-up |
Self-report of groin pain, categorized as none, mild, moderate, or severe. |
Number of patients reporting chronic groin pain: 11/29 (38%) versus 2/28 (7%), p<0.05
Severity of groin pain Mild: 3 versus 1 Moderate: 3 versus 0 Severe: 5 versus 1 |
|
Nordin 2002 |
Postoperative pain |
direct postoperative, 8 weeks postoperatively |
VAS (0-10) |
Direct postoperatively, average VAS (?) Lichtenstein (n = 149) versus Shouldice (n = 148) 3.6 versus 3.8
8 weeks postoperatively 1.7 versus 1.7 |
|
|
Persistent pain (not specified) |
8 weeks postoperatively |
VAS |
% of patients with persistent pain 4.0 versus 3.4 |
|
|
Chronic postoperative pain |
1 and 3 years postoperatively |
Questionnaire (1 year postoperative), examination by surgeon who is not aware of the used surgical technique (3 years postoperatively) |
1 year postoperatively % of patients with persistent pain 5.4 versus 6.1
3 years postoperatively % of patients with persistent pain Mild: 4.9 versus 0.7 Moderate: 0.7 versus 2.1 Neuralgia: 0 versus 1.4 |
|
Pokorny 2008 |
Use of painkillers |
2-4 weeks postoperatively |
Not specified |
Number of participants reporting the use of painkillers 11/182 (6%) versus 8/64 (13%); RR 0.48, 95% CI 0.20 to 1.15 |
|
Prieto-Díaz-Chávez 2009 |
(Chronic) postoperative pain |
7 days, 8 weeks, 12 and 24 months postoperatively |
VAS |
VAS (monthly)* 1.75 versus 2.80; p<0.05 * Unclear how to interpret this score |
|
|
Use of painkillers |
Not reported |
Self-report |
No results reported |
|
Schmitz 1997 |
Postoperative pijn while lying down, getting up and walking |
Daily for 6 days postoperatively |
VAS |
"Während am 1. postoperativen Tag im Liegen die Mittelwerte in beiden Gruppen bei 30% des stärksten vorstellbaren Schmerzes lagen, befanden sich die Mittelwerte beim Aufrichten bei 50–60%; beim Gehen lagen sie etwa bei 43%. Die nur geringfügig geringere Schmerzintensität bei den spannungsfrei operierten Patienten ergab keinen signifikanten Unterschied zur Shouldice-Gruppe. Dieser Trend setzt sich bis zum Entlassungstag fort, so daß zu keinem Zeitpunkt ein signifikanter Unterschied entsprechend der Studienhypothese auftrat. Beide Patientengruppen waren nach dem 5. Tag beschwerdefrei." |
|
|
Use of painkillers |
Daily for 6 days postoperatively |
Not reported |
"Beide Gruppen unterschieden sich nicht signifikant voneinander, denn ab dem 2. postoperativen Tag betrug der mittlere Analgeticaverbrauch nur noch 1 Tablette (0,5) täglich. Lediglich am 1. postoperativen Tag lag der mittlere Verbrauch in der Shouldice-Gruppe um 50% über dem der TF-Gruppe. Bei jedoch großer Schwankungsbreite (sx) läßt die relativ kleine Probandenzahl die Aussage nach einer Signifikanzdifferenz allein für den 1. Tag nicht zu, was allerdings auch nicht Gegenstand der Studienfrage war." |
|
Tschudi 2001 |
Postoperative pain |
1 day postoperatively |
VAS (0-100) |
Mean pain score ± SD 24.51 ± 18.37 versus 32.96 ± 21.96, p=0.053 |
|
|
Use of paracetamol and narcotic analgesics (morphine-equivalent dose) |
1 day postoperatively |
Not reported |
Paracetamol in mg, mean ± SD 2549.02 ± 4360.34 versus 4577.55 ± 6441.31, p=0.048
Narcotic analgesics in mg, mean ± SD 6.52 ± 11.21 versus 18.96 ± 23.73, p=0.0005 |
|
Wamalwa 2015 |
Postoperative pain |
24 hours, 2 weeks, 3 months postoperatively |
Asked for the presence of pain. |
24 hours postoperatively 4/23 (17%) versus 3/22 (14%); p-value not reported
2 weeks postoperatively 3/23 (13%) versus 1/22 (5%); p-value not reported
3 months postoperatively 1/23 (4%) versus 0/22 (0%); p-value not reported
“One patient had persistent chronic pain after Lichtenstein repair. This was classified as neuralgia on account of its radiation along the inguinal nerve.” |
|
|
Use of painkillers > 1 week postoperatively |
2 weeks |
Not reported |
Not reported |
|
Zieren 1998 |
Postoperative pain |
3 times a day for a postoperative period of 2 months |
VAS, self-report |
"Mean pain analogue scores and analgesia requirements were comparable between mesh (TAPP and Plug) but both were significantly lower compared with Shouldice (SH)" |
|
|
Chronic postoperative pain (not specified) |
Average follow-up duration of 25 months |
Unclear (VAS?) |
Tapp (n=80) versus Plug (n=80) versus Shouldice (n=80); n (%): 3 (4) versus 2 (3) versus 4 (5); p=NS |
|
|
Use of painkillers |
Unclear (2 months postoperative?) |
Not reported |
Tapp (n=80) versus Plug (n=80) versus Shouldice (n=80); Mean (sd) days 2 (4) versus 3 (7) versus 10 (6); p<0.05 (Shouldice significantly different from Tapp and Plug)
"Mean pain analogue scores and analgesia requirements were comparable between TAPP and PP but both were significantly lower compared with SH" |
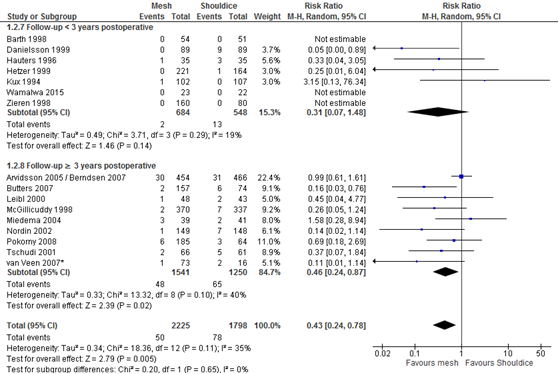
Figure 2 Forest plot mesh versus Shouldice for the outcome 'recurrences' (n = 16)
* In the study of Van Veen patients were randomized to either mesh (Lichtenstein) or non-mesh (surgeon’s choice). Shouldice was among the non-mesh techniques and was chosen by the surgeon in 20% of the patients. As allocation to Shouldice is not completely at random, a sensitivity analysis was performed excluding the study of Van Veen (2007) (resulting overall RR of 0.47, 95% CI 0.26 to 0.85; RR for subgroup ‘≥3 years postoperative’ was 0.52, 95% CI 0.28 to 0.96).
Recurrences (critical outcome) >3jaar
Thirty-one RCTs reported the outcome 'recurrences' for the comparison mesh versus non-mesh (Figure B1 in Appendix 3). In 10 of these there were no recurrences in both study groups. The pooled result of the remaining 21 RCTs shows fewer recurrences after a mesh technique (RR 0.46, 95% CI 0.30 to 0.68).
For the comparison of mesh versus Shouldice 16 RCTs reported the outcome 'recurrences' and these are shown in Figure 2, grouped per follow-up (either shorter or longer than three years postoperatively). There were three RCTs without recurrences in both study groups. The pooled result of the other 13 RCTs, showed a difference in favour of mesh (RR 0.43, 95% CI 0,24 to 0.87). As in the study of Van Veen (2007) allocation to Shouldice was not completely at random, a sensitivity analysis was done excluding this study. The resulting overall pooled RR remained in favour of mesh (RR 0.47, 95% CI 0.26 to 0.85; RR for subgroup ‘≥3 years postoperative’ was 0.52, 95% CI 0.28 to 0.96).
Postoperative complications
An overview of the following postoperative complications is given in Table 2 for the comparison of mesh versus non-mesh and also for the subgroup mesh versus Shouldice: neurovascular or visceral complications, wound infection, hematoma, seroma, postoperative swelling of the wound, wound dehiscence, testicular complications and urinary retention. A graphic representation can be found in Appendix 3.
For both comparisons, no differences in post-operative complications were reported between the study groups, with the exception of neurovascular or visceral complications and seroma. Neurovascular or visceral complications occurred less frequently after mesh compared to non-mesh (RR 0.60; 95% CI 0.49 to 0.75) or Shouldice (RR 0.58; 95% CI 0.47 to 0.73). Seroma occurred more frequently after mesh compared to non-mesh (RR 1.75; 95% CI 1.15 to 2.66) or Shouldice (RR 4.10; 95% CI 1.21 to 1.97).
Table 2 Overview of postoperative complications
|
Complication |
mesh versus non-mesh |
mesh versus Shouldice |
||||
|
|
Number of RCTs |
Number of RCTs pooled* |
Pooled RR (95%CI) |
Number of RCTs |
Number of RCTs pooled* |
Pooled RR (95%CI) |
|
Neurovascular or visceral complications |
24 |
8 |
0.60 (0.49-0.75) |
11 |
4 |
0.58 (0.47- 0.73) |
|
Wound infection |
29 |
24 |
1.15 (0.81-1.61) |
16 |
10 |
1.23 (0.66-2.30) |
|
Hematoma |
22 |
20 |
0.89 (0.70-1.13) |
11 |
10 |
0.99 (0.73-1.34) |
|
Seroma |
21 |
19 |
1.75 (1.15-2.66) |
7 |
5 |
4.10 (1.21-13.97) |
|
Swelling of the wound |
2 |
3 |
3.51 (0.45-27.29) |
1 |
1 |
0.99 (0.06-15.73) |
|
Wound dehiscence |
2 |
2 |
0.63 (0.06-6.58) |
0 |
n.a |
n.a. |
|
Testicular complications |
18 |
16 |
1.12 (0.78-1.60) |
8 |
7 |
1.16 (0.69-1.95) |
|
Urinary retention |
12 |
12 |
0.69 (0.40-1.20) |
8 |
8 |
0.74 (0.34-1.63) |
* Results of RCTs with 0 complications in both study groups were not pooled
Evidence of literature
The GRADE profile for the comparison mesh versus Shouldice can be found in Appendix 4. Given limitations in the research design and imprecision, the strength of the evidence for the outcome measure recurrences has been reduced by two levels.
For the outcome chronic pain, the strength of the evidence was reduced by three levels given limitations in the research design (Risk of Bias), the variety of measurements of the outcome (indirectness), and imprecision.
The weight of evidence for the outcome postoperative pain was reduced by three levels, due to limitations in the research designs, the variety of measurements of the outcome, and imprecision. For the conflicting results (inconsistency) was not downgraded as the type of mesh technique (i.e. Lichtenstein or lapara-endoscopic) seems to be related to presence of postoperative pain.
2. Which is the preferred open mesh technique for inguinal hernias: Lichtenstein or other open flat mesh and gadgets via an anterior approach?
The search using PICO 2 criteria identified 3 RCTs (Magnusson, 2016; Čadanová, 2016; Karaca, 2013) that were not included in the literature summary of the International Guidelines (HerniaSurge Group, 2018) comparing open mesh techniques for inguinal hernias. The Progrip mesh results will be discussed in module discussing mesh types.
Magnusson (2016) conducted an RCT with three arms in Sweden to compare long-term pain outcomes of the Lichtenstein’s repair (with a with a standard polypropylene mesh (the Prolene Hernia System, PHS), and the UltraPro Hernia System (UHS). The Lichtenstein-group (n=108) had a median age of 60 years (IQR: 49 to 64) and a median BMI of 24.6 (IQR: 23 to 26). A total of 28 participants (26%) had an occupation involving a high physical workload. The PHS-group (n=99) had a median age of 58 (IQR: 48 to 63) and a median BMI of 24.8 (IQR: 23.2 to 26.3). In the PHS group there were 37 persons (37%) with high physical workload in their occupation. Participants in the UHS-group (n=102) had a median age of 59 (IQR: 46 to 66) and a median BMI of 25 (IQR: 23 to 26.5). There were 25 participants (25%) in the PHS-group with high physical workload in their occupation. All participants were male. Pain during rest and activity was measured with a Visual Analogue Scale (VAS, score range: 0 to 10) at 12, 24 and 36 months. At 12 months missing data for VAS-scores among the three groups ranged from 4 to 7%, at 24 months from 12 to 21%, and at 36 months from 4 to 15%. At 36 months there were 27 participants (8%) lost to follow up, however it was unclear to what groups those participants were allocated originally.
Čadanová (2016) compared the ProGrip repair with the transinguinal preperitoneal (TIPP, with a PolySoft mesh) repair on recurrence, complications and number of participants in pain during an RCT in the Netherlands with a follow-up at 2 weeks, 3 months, and 1 year. Participants in the ProGrip-group (n=116, 94.8% males) had a mean age of 57.2 years (range: 54.5 to 59.9) and a mean BMI of 25.1 (range: 24.5 to 25.7). Participants had an ASA status of I (55.2% of the participants), II (38.8% of the participants, or III (6% of the participants). The TIPP-group (n=122, 93.4% male) had a mean age of 59 years (range: 56.5 to 61.4) and a mean BMI of 25.7 (range:25.1 to 26.3). In this group the participants had an ASA status of I (53.3% of the participants), II (41% of the participants), or III (5.7% of the participants). Twenty participants were excluded from analyses because they did not show up for the first follow-up visit, did not receive the assigned treatment, or declined surgery. Follow-up participation rate among the participants declined from 99.6% at 2 weeks to 38.4% at 1 year. Pain was measured with the VAS, the Verbal Descriptor Scale (VDS, a 7-point Likert-scale describing terms to express pain), and the Leeds Assessment of Neuropathic Symptoms and Signs (LARSS).
An RCT conducted by Karaca (2013) compared the Lichtenstein’s repair, the Rutkow-Robbins repair, and the Gilbert double layer repair (Prolene Hernia System, PHS) for complications and recurrence. Randomization and allocation of participants into three groups was performed by throwing heads or tail (presumably with a coin). The Lichtenstein-group (n=50, 86% male) had a mean age of 53.06 years (SD: 13.03) and a mean BMI of 26.45 (SD: 4.94). Participants in de Rutkow-Robbins-group (n=50, 92% male) had a mean age of 51.69 years (SD: 14.66) and a mean BMI of 24.22 (SD: 2.71), while the PHS-group (n=50, 86% male) had a mean age of 48.06 years (SD: 17.27) and a mean BMI of 25.06 (SD: 3.71). Early and late complications were measured, however no timeframe was defined for early and late complications.
Results
Chronic pain (>3 months)
Two RCTs reported pain outcomes after 3 months. Magnussen 2016 found no statistically significant differences between the Lichtenstein’s repair, the PHS repair, and the UHS repair for pain in rest and pain during activity at 12, 24, and 36 months as measured with the VAS (no p-values reported).
At 12, 24, and 26 months the Lichtenstein-group had a median VAS score for pain in rest of 0.2 (IQR: 0 to 0.6), 0.3 (IQR: 0.1 to 0.7), and 0.4 (IQR: 0.2 to 1.7), while for pain during activity the median VAS-score was 0.5 (IQR: 0.2 to 3.1), 0.7 (IQR: 0.4 to 1.5), and 0.6 (IQR: 0.201.7), respectively. For the PHS-group the median VAS score for pain in activity at 12, 24, and 36 months was 0.2 (IQR: 0 to 1.9), 0.3 (IQR: 0.1 to 0.8), and 0.2 (IQR: 0.1 to 2.3), while for pain during activity this was 0.5 (IQR: 0.3 to 3), 0.9 (IQR: 0.4 to 2.1), and 0.4 (IQR: 0.2 to 2.3), respectively. The UHS group had a median VAS score for pain in rest at 12, 24, and 36 months of 1 (IQR: 0.2-2.5), 0.8 (IQR: 0.4 to 1.3), and 1.6 (IQR: 0.7 to 4.6), while they had a median VAS-score of 2.3 (IQR: 0.7 to 2.9), 1.2 (IQR: 0.4 to 2.6), and 1.2 (IQR: 0.4 to 2.6) for pain during activity, respectively. No statistically significant differences between groups were found in the number of participants having pain at 12, 24, and 36 months (no p-value reported). For the Lichtenstein-group at 12, 24, and 36 months there were 15 (15%), 7 (8%), and 6 (6%) participants having pain, while in the PHS-group there were 12 (12%), 12 (14%), and 6 (7%) participants having pain, respectively. For the UHS-group there were 13 participants (13%) in pain at 12 months, 12 participants (15%) having pain at 24 months, and 9 participants (9%) in pain at 36 months.
Čadanová (2016) reported pain at rest, pain in daily activity, and pain in sports or heavy lifting at 3 months and at 1 year for the ProGrip repair compared to the TIPP repair (using a PolySoft mesh). At three months there were statistically significant differences between groups for the number of persons having chronic pain with a VAS-score of 3 or more for pain in rest (ProGrip: 12 participants (13.9%), TIPP: 4 participants (4.3%), p=0.031), for pain in daily activities (ProGrip: 15 participants (16.5%), TIPP: 4 participants (4.3%), p=0.006), and for pain during sports or heavy lifting (ProGrip: 19 participants (20.9%), TIPP: 3 participants (3.2%), p<0.001). At 1 year Čadanová (2016) found no statistically significant differences for the number of participants having chronic pain with a VAS-score of more than 3 for pain in rest (ProGrip: 3 participants (4.3%), TIPP: 5 participants (6.7%), p=0.504), for pain during daily activities (ProGrip: 3 participants (4.3%), TIPP: 6 participants (8%), p=0.332), and for pain during sports or heavy lifting (ProGrip: 3 participants (4.3%), TIPP: 6 participants (8%), p=0.332). No statistically significant differences in proportions between groups were found when pain was categorized as no pain (VAS 0), mild pain (VAS 1 to 3), moderate pain (VAS 4 to 6), and severe pain (VAS ≥7) for pain in rest (p=0.624), daily activity (p=0.494), and during sports or heavy lifting (p=0.570) at 1 year. No statistically significant differences in proportion of participants with neuropathic pain was found between groups at 1 year (p=0.37). In the ProGrip-group there were 11 participants (15.5%) having neuropathic pain measured with the LARSS, while in the TIPP-group there were 9 participants (10.5%) with neuropathic pain. Čadanová (2016) also measured pain with the Verbal Descriptor Scale (VDS) to describe te proportion of participants in both groups having moderate pain or worse. No statistically significant differences were found between groups for moderate pain or worse at 3 months (ProGrip: 8 participants (8.8%), TIPP: 5 participants (5.3%), p=0.356) and at 1 year (ProGrip: 4 participants (4.6%), TIPP: 4 participants (5.4%), p=0.953).
Factors influencing the quality of evidence
Evidence originated from RCTs and the quality of the evidence comparing Lichtenstein versus preperitoneal techniquees is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the studies (Risk of Bias due to the lack of blinding of care providers (Magnusson, 2016; Čadanová, 2016). Other bias may arise due to a decline in participation rate (38.4%, unclear if loss-to follow-up or missing data) and violation of intention to treat analysis in one RCT (Čadanová, 2016). Furthermore, both RCTs have a relative small sample. The certainty of evidence due to these factors is reflected in the conclusions.
Recurrence
No statistically significant differences were found in recurrences at 2 weeks, 3 months, and 1 year in Čadanová (2016), comparing the ProGrip repair with the TIPP repair (using a PolySoft mesh). Over the period of 1 year there were a cumulative number of 3 recurrences in both the ProGrip and the TIPP-group. At 2 weeks not a single recurrence was reported in in the ProGrip-group, while there was 1 recurrence (0.8%) in the TIPP-group (p=0.331). Both the ProGrip-group and the TIPP-group had 1 new recurrence (1.1% and 1%, respectively) at 3 months (p=0.976). At the 1-year follow-up the ProGrip-group had 2 new recurrences (2.7%) and the TIPP-group had 1 new recurrence (1.3%, p=0.536).
Karaca (2013) did not find any recurrences when comparing the Lichtenstein repair, the PHS repair, and the Rutkow-Robbins repair over the course of 1 year (0 recurrences overall, p-value not reported).
Factors influencing the quality of evidence
Evidence originated from RCTs and the quality of the evidence comparing Lichtenstein versus preperitoneal techniques is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the studies (Risk of Bias due to the lack of blinding of care providers (Čadanová, 2016; Karaca, 2013), Risk of Bias due to the randomization method and its allocation concealment (Karaca, 2013)). Other bias may arise due to a decline in participation rate (38.4%, unclear if loss-to follow-up or missing data) and violation of intention to treat analysis in one RCT (Čadanová, 2016). Furthermore, both RCTs add a small number of events to the body of evidence. The certainty of evidence due to these factors is reflected in the conclusions.
Complications
Čadanová (2016) presented self-reported complications at 2 weeks, 3 months, and 1 year comparing the ProGrip repair with the TIPP repair (using a PolySoft mesh). Recorded complications were seromas, bleeding, wound infection, hypersensitivity, scrotal pain, testis atrophia, and other (undefined). After 2 weeks a statistically significant difference (p=0.005) was found between groups where 57 participants (50.4%) in the ProGrip-group had at least 1 complication, while the TIPP-group had 39 participants (32.5%) having at least 1 complication. At 3 months there were no significant differences between groups (p=0.140. The ProGrip-group had 19 participants (20.9%) with at leat 1 complication at 3 months and the TIPP-group had 12 participants (12.8%) with at least one complication. No statistically significant differences were found at 1 year (p=0.893), where the ProGrip-group and the TIPP-group had 19 and 12 participants (13.9% and 14.7%) having a complication, respectively.
Karaca (2013) reported early and late complication when comparing the Lichtenstein’s repair, the Rutkow-Robbins repair, and the PHS repair, however the timeframe of early and late complications was not defined. A statistically significant difference (p=0.033) was found between groups for early complications in the Lichtenstein-group (1 participant (2%)), the Rutkow-Robbins-group (5 participants (10%)), and the PHS-group (3 participants (6%)). No post-hoc analyses were conducted to show which groups significantly differed from each other. There were no statistically significant differences (p=0.924) in late complications found between the Lichtenstein-group (5 paricipants (10%)), the Rutkow-Robbins-group (4 participants (8%)), or the PHS-group (5 participants (10.4%)).
Factors influencing the quality of evidence
Evidence originated from RCTs and the quality of the evidence comparing Lichtenstein versus preperitoneal techniques is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the studies (Risk of Bias due to the lack of blinding of care providers (Čadanová, 2016, Karaca, 2013), Risk of Bias due to the randomization method and its allocation concealment (Karaca 2013)). Other bias may arise due to a decline in participation rate (38.4%, unclear if loss-to follow-up or missing data) and violation of intention to treat analysis in one RCT (Čadanová, 2016). Furthermore, both RCTs add a small number of events to the body of evidence. The certainty of evidence due to these factors is reflected in the conclusions.
3. Which is preferred open mesh technique: Lichtenstein versus open pre-peritoneal?
The search using PICO 3 criteria identified 4 publications (Andresen, 2015 (short-term results); Andresen, 2017 (long-term results); Mahmoudvand, 2017; Azeem, 2015 2x) that were published after the studies included in the International Guidelines for groin hernia management (HerniaSurge Group, 2018). The publications reported on two RCTs relevant for the subject of preferred open mesh technique: Lichtenstein versus open preperitoneal.
Mahmoudvand (2017) compared Lichtenstein with an open preperitoneal method (flatmesh transinguinal preperitoneal “TIPP” combined with bassini) in a total of 150 patients with a direct inguinal hernia using a follow-up of 6 to 12 months in Iran. The intervention group (n=75, 64% males) received an inguinal hernia repair using the Lichtenstein’s technique after reinforcement of the posterior wall, while the control group (n=75, 54.7% males) received an inguinal hernia repair using a preperitoneal method.
The other RCT in Denmark (Andresen, 2015; Andresen, 2017) compared the Lichtenstein’s technique in the intervention group with the Onstep technique in the control group. A total of 290 patients with a primary inguinal hernia were recruited in this study and were followed for 10 and 30 days (Andresen 2015) and 6 and 12 months (Andresen, 2017). The intervention group (n=144) had a mean age of 53.8 years (SD: 14.6) and had a mean BMI of 24.8 kg/m2 (SD: 2.6). In this group 38 participants (26.4%) were smokers. The control group (n=146) had a mean age of 54.5 years (SD: 15.2) and had a mean BMI of 24.7 kg/m2 (SD:2.9). The control group had 32 participants (21.9%) who were smokers.
Azeem (2015) used an RCT in Pakistan to compare the Lichtenstein’s repair with the modified Kugel repair. Follow-up was at 2 months, 4 months, and 2 years postoperatively. The intervention group (n=87) received the Lichtenstein’s repair (fixation with tackers) and the control group (n=89) received the modified Kugel repair (flat mesh in TEP position with two point fixation as done in TEPP through Kugel open approach. The mean age in the intervention group was 50.01 years (SD: 13.22) and consisted of men and women. The control group had a mean age of 49.4 years (SD: 12.06) and consisted of men and woman. In both study arms one participant was lost-to-follow-up (no reasons presented) and were excluded from analyses. Overall, the methodological quality and reporting of this study was poor.
Andresen (2017) Surgery Reported on the previous onstep group also the data of 259 patients from the above mentioned study (129 Lichtenstein, 130 Onstep) 6 months after surgery regarding pain during sexual activity.
Results
Recurrence (critical outcome)
Recurrence rates were reported in all three RCTs. Mahmoudvand (2017) (n=150 patients) reported five times more recurrences in the Lichtenstein group (n=10) compared to the preperitoneal group (n=2) (RR 5.00 (95% CI 1.13 to 22.05; p=0.016). Andresen (2015 and2017) (n=290 patients) did not find statistically significant differences between the Lichtenstein and the Onstep repairs within 30 days (Lichtenstein: 1 recurrence , Onstep: 3 recurrences, p=0.30) and within 12 months (Lichtenstein: 5 recurrences (4%), Onstep: 6 recurrences (4.8%), p=0.78). Since there were 4 recurrences (1.4%) within 10 days after surgery and 4.4% after one year one might wonder if the surgery was of acceptable/representative quality.
Azzeem (2015) observed a statistically significant difference between the Lichtenstein’s repair and the modified Kugel repair. In the Lichtenstein’s repair group 3 recurrences within 2 years were reported, while none were observed for the modified Kugel repair group (p<0.05).
Factors affecting the quality of the evidence
Evidence originated from RCTs and the quality of the evidence comparing Lichtenstein versus preperitoneal techniques is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the studies (Risk of Bias due to unclear randomisation, allocation concealment and blinding in two RCTs (Mahmoudvand, 2015; Azeem, 2015) and violation of intention to treat analysis in the other RCT (Andresen, 2015 and 2017). Furthermore, these RCTs showed inconsistent results and add a low number of events to the body of evidence. Also follow up ranged between 10 days and 12 months. Ìn the Mahmoedvand study the surgical techniques are difficult to compare with the standard techniques of the techniques. The certainty of evidence due to these factors is reflected in the conclusions.
Chronic pain (critical outcome)
Andresen (2017) (n=290 patients) did not find differences in chronic pain scores (Visual Analogue Scale (VAS)) between the Lichtenstein and the Onstep techniques at 6 months (Lichtenstein: VAS=0 (IQR: 0 to 2), Onstep: VAS=0 (IQR: 0 to 2), p=0.67) and at 12 months (Lichtenstein: VAS=0 (IQR: 0 to 1), Onstep: VAS=0 (IQR: 0 to 2), p=0.1) after surgery.
Azeem (2015) (n=86 patients) compared Lichtenstein with modified Kugel. Both techniques included tacking of the mesh. Chronic pain was defined as any vas above zero. 3 months after surgery mean VAS was 0,3 for the modified kugel and 0,9 for the Lichtenstein repair (p=0.002).
Factors affecting the quality of the evidence
Evidence originated from two RCT and the quality of the evidence is therefore ‘high’. However, the quality of the evidence is reduced due to violation of the intention to treat analysis (Andresen). For the Azeem study there are methodogical limitations (randomization) and especially technical limitations since the mesh was tacked in place in Lichtenstein repair which does not resemble a standard fixation. Therefore results regarding pain in the Azeem study are not suitable for extrapolation into routine daily practice. Therefore, results originated from only one RCT. The certainty of evidence due to these factors is reflected in the conclusions.
Postoperative recovery: pain (critical outcome)
Pain was reported in all three RCTs. Mahmoudvand (2017) (n=150 patients) reported more patients with pain after surgery in the Lichtenstein group (n=21, 28%) compared to the preperitoneal group (n=9, 12%) (RR 2.33 (95% CI 1.14 to 4.76), p=0.014). However, it is unclear how pain was defined and when it measured in the sample.
The effect of the Lichtenstein’s repair compared to the modified Kugel repair on pain at 4 months was not deducible from Azeem (2015).
Factors affecting the quality of the evidence
Evidence originated from RCTs and the quality of the evidence comparing Lichtenstein versus preperitoneal techniques is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the studies (Risk of Bias due to unclear randomisation, allocation concealment, blinding and selective reporting and surgical techniques are difficult to compare with the standard techniques of the techniques in one RCT (Andresen, 2015 and 2017). Furthermore, these RCTs showed inconsistent results. The certainty of evidence due to these factors is reflected in the conclusions.
Complications after surgery
Mahmoudvand (2017), Andresen (2015) and Azeem (2015) reported complications after surgery. In the RCT of Mahmoudvand 2017 (n=150 patients) 7 patients in the Lichtenstein group versus 9 patients in the preperitoneal group had a postoperative hematoma (RR 0.78 (95% CI 0.31 to 1.98)) and respectively 8 and 1 patients had a postoperative seroma (RR 8.00 (95% CI 1.03 to 62.40)). Andresen (2015) (n=290 patients) classified complications according to the Clavien-Dindo classification (I, II, IIIa, IIIb) and did not find differences in incidence of complications between the groups. Thirteen patients in the Lichtenstein group reported level I or II classification versus 10 in the Onset group.
Azeem (2015) reported significant differences between observed complications in the Lichtenstein’s repair group and the modified Kugel repair group (p<0.005) at an unclear follow-up period. In the Lichtenstein’s repair group 21 participants (24%) with a complication were observed, while 5 participants (6%) with complications were observed in the modified Kugel repair group.
Andresen (2017) demonstrated pain during sexual activity (6 months postop) in 17 onstep patients (13.1%) compared to 30 lichtenstein patients (23%), p=0.034. In both groups about half of the patients had this pain already before surgery, the others started having pain after surgery. So for inducing new pain the difference is 7/74 (9%) onstep versus Lichtenstein 14/70 (20%), p=0.073 and therefore no relevant conclusions can be drawn.
Factors affecting the quality of the evidence
Evidence originated from RCTs and the quality of the evidence comparing Lichtenstein versus preperitoneal techniques is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the studies (Risk of Bias due to unclear randomisation, unclear allocation concealment and blinding in two RCT (Mahmoudvand, 2015; Azeem, 2015) and violation of intention to treat analysis in the other RCT (Andresen 2015 and 2017). Furthermore, these RCTs showeded inconsistent results. These RCTs add a low number of events to the body of evidence. The certainty of evidence due to these factors is reflected in the conclusions.
Mortality
The outcome measure mortality was not reported in the RCT’s. The RCT of Mahmoudvand (2017) however had no lost-to-follow-ups or incomplete outcome data suggesting that all the participants survived. The RCT of Andresen (2015 and 2017) mentioned reasons for incomplete outcome data and did not report a deceased participant.
In 2017 a systematic review (Andresen, 2017 AJS) was published describing a total of 67 artikels ranging from retrospective cohort to RCT on open preperitoneal repairs. They concluded that although the techniques seem promising a meta-analysis could not be performed and more RCT with proper length of follow up are needed.
4. Which endoscopic technique is preferred in terms of postoperative outcome?
The HerniaSurge Group (2018) recommended that both TAPP and TEP repair are suitable techniques for the treatment of inguinal hernia. Eight meta-analyses and systematic reviews concluded that there is insufficient evidence to recommend the use of one technique over the other. (Memon, BJS, 2003; McCormack Hernia, 2005; Tolver, Acta Anaesthesiol Scand, 2012; O’Reilly, Ann Surg, 2012; Bracale, Surg Endosc, 2012; Antoniou, Surg, 2013; Wei, Surg Lap Endosc Percutan Techn, 2015; McCormack, Health Technol Assess, 2005)
Both techniques have very low risks of serious complications, with a higher risk of visceral injury in TAPP and of vascular injury in TEPP. These results have been found in randomized controlled trials as well as in ‘real life’ registries such as the German Herniamed registry and the Swiss hernia registry. Minor complications such as seroma, scrotal edema, testicular atrophy, and bladder injury are similar for both techniques.
Early comparative studies between TAPP and TEP were often conducted in the surgeons’ learning curves, were biased and underpowered. Studies failed to report on important technical aspects such as mesh size and type of fixation. The most recently published meta-analysis of ten RCTs failed to show any significant differences in operative times, total complication rates, hospital length of stay, recovery time, pain, recurrence rates or costs between TAPP and TEP. (Weister, Viszeralchirurgie, 2000).
Update of the literature
Wei (2015) performed a systematic review and meta-analyses of RCTs comparing the transabdominal preperitoneal (TAPP) repair for inguinal hernias with the totally extraperitoneal repair (TEP) for unguinal hernias. Pubmed, Embase, the Cochrane library, and Web of Science were searched and yielded 333 hits in March 2014. Inclusion criteria were: participants with unilateral or bilateral inguinal hernia; aged below 71; TAPP and TEP were perfomed; the study reported the outcomes of interest. Exclusion criteria were: the study included patients with previous gastric surgery, chemotherapy, radiotherapy, deep venous thrombosis, or cardiovascular, respiratory, hepatic, or renal disease. Excluded studies were not referenced. After screening 10 RCTs remained for inclusion, originating from Austria, Greece, USA, China, Egypt, or India. The selected RCTs included unilateral, bilateral, recurrent, non-recurrent, direct, and/or indirect inguinal hernias. One study also included femoral hernias. Follow-up in the included studies was heterogenous, ranging from one week to 38 months (with unclear follow-up periods in 2 studies). All studies were reported to contain more than 80% follow-up data. Sample characteristics such as age and gender were not reported.
Bansal 2017 conducted in India an RCT to compare TAPP with TEP for inguinal hernia repairs. The intervention group received a TAPP repair, while the control group received a TEP repair. Pain was measured at follow-ups, up till 6 months. A vast majority of the participants (88.7%) completed the follow-up at 6 months. It is likely that the participants, surgeon(s), and outcome assessor(s) were not blinded and there was no mention of blinding in the study’s manuscript. The intervention group (n=80) had a mean age of 40.9 years (SD: 12.3) and a mean BMI of 24.1 (SD: 2). In this group, 58 participants (72.5%) had a unilateral inguinal hernia. The contol group (n=80) had a mean age of 40 years (SD: 12.5) and a mean BMI of 24 (SD: 2). Unilateral hernias were diagnosed in 46 participants (57.5%) of the control group. All study participants were male.
Results
Recurrence (>3 years follow-up) (critical outcome)
None of the included study reported and/or compared the recurrence after a follow-up of three years or longer. Wei (2015) reported the meta-analysis of six RCTs that reported recurrence rates after shorter periods of follow-up. In a fixed-effects model, there was no statistically significant difference of postoperative hernia recurrence between TAPP and TEP (RR=1.18; 95% CI, 0.47 to 2.98).
Chronic pain (>6 months) (critical outcome)
Bansal 2017 reported no significant differences in pain at 6 months (p=0.86), using the Visual Analogue Scale (VAS). The group who received a TAPP inguinal hernia repair had a mean VAS-score of 0.87 (SD: 0.6), while the group who received a TEP inguinal hernia repair reported a mean VAS-score of 0.86 (SD: 0.7).
Factors affecting the quality of the evidence
Evidence on chronic pain originated from an RCT comparing TAPP repair with TEP repair, and is therefore ‘high’. However, the quality of evidence is reduced due to methodological limitations (patients, care provider(s), and outcome assessors were likely not to be blinded) and a limited sample size. The certainty of evidence due to these factors is reflected in the conclusions.
Postoperative recovery: acute postoperative pain (critical outcome)
Wei (2015) pooled two studies (n=414) that reported pain at 1 hour postoperatively by using the SMD as an effect measure and a random effects model. The pooled effect was SMD=1.03 (95%CI: -0.75 to 2.81). The point estimate suggests a large clinical relevant difference in favour of TEP. The confidence interval however, encloses zero and indicates that the real effect may either be in favour of TEP or TAPP. Heterogeneity was high (I2 = 97.8%).
Two studies (n=414) reported pain at 6 hours postoperatively and were pooled in Wei (2015) by using the SMD as an effect measure and a random effects model. The pooled effect was SMD=-0.58 (95%CI: -2.08 to 0.92), suggesting a small to reasonable clinical relevant difference in favour of TEP. The confidence interval however, encloses zero and indicates that the effect may either be in favour of TEP or TAPP. Heterogeneity was reported using the I2 statistic (I2 = 97.4%).
Pain at one day postoperatively was reported in 4 of the included studies (n=564 participants) in Wei (2015). All 4 studies were pooled by using the SMD as an effect measure and a random effects model. The pooled effect was SMD=0.63 (95%CI: -0.20 to 1.45), suggesting a reasonable clinical relevant difference in favour of TEP. The confidence interval however, encloses zero and indicates that the effect may either be in favour of TEP or TAPP. Statistical heterogeneity was reported using the I2 statistic and was high (I2 = 94.5%).
Wei (2015) pooled two studies (n=200) that reported pain 1 week postoperatively by using the SMD as an effect measure and a random effects model. The pooled effect was 0.61 (95%CI: -0.59 to 1.81), suggesting a reasonable clinical relevant difference in favour of TEP. The confidence interval however, encloses zero and indicates that the effect may either be in favour of TEP or TAPP. Heterogeneity was high (I2 = 94.2%).
In Wei (2015), there were two studies which had an unclear follow-up. Those two studies (n=208 participants) were pooled by using the SMD as an effect measure and a random effects model. The pooled effect was SMD=0.72 (95%CI: -0.21 to 1.48), suggesting a reasonable clinical relevant difference in favour of TEP. The confidence interval however, encloses zero and indicates that the effect may either be in favour of TEP or TAPP. Statistical heterogeneity was high (I2 = 85.6%).
Factors affecting the quality of the evidence
Evidence originated from meta-analyses containing RCTs comparing the TAPP repair with the TEP repair for inguinal hernias and is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the meta-analysis (potential risk of publication bias for pain outcomes, the quality appraisel might be an appraisel of reporting-completeness rather than an appraisel of potential bias, and the characteristics of the included samples were not reported), inconsistency in the pooled analyses (considerable heterogeneity), and a limited sample size in the meta-analyses. The certainty of evidence due to these factors is reflected in the conclusions.
Postoperative recovery: return to usual activities (critical outcome)
Four studies (n=514) reporting the time to return to usual activities were pooled in Wei (2015) by using the SMD as an effect measure and a random effect model. The pooled effect was SMD=-0.08 (95%CI: -0.33 to 0.16), indicating no clinical relevant difference. Statistical heterogeneity was substantial (I2 = 33.8%).
Factors affecting the quality of the evidence
Evidence originated from meta-analyses containing RCTs comparing the TAPP repair with the TEP repair for inguinal hernias and is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the meta-analysis (potential risk of publication bias for return to usual activities, and the quality appraisel might be an appraisel of reporting-completeness rather than an appraisel of potential bias, and the characteristics of the included samples were not reported) and a limited sample size in the meta-analyses. The certainty of evidence due to these factors is reflected in the conclusions.
Costs
Wei (2015) found that 4 of its included studies reported the costs of the TAPP repair and the TEP repair for inguinal hernias. None of the four studies found significant differences in costs between both techniques. The studies reporting and comparing the costs were conducted in China and India and therefore considered as not relevant for the Dutch guideline.
Factors affecting the quality of the evidence
Evidence originated from RCTs comparing the TAPP repair with the TEP repair for inguinal hernias and is therefore ‘high’. However, costs from the Chinese or Indian healthcare system can not be extrapolated to the Dutch healthcare system. The certainty of evidence due to these factors is reflected in the conclusions.
5. When considering recurrence, pain, learning curve, postoperative recovery and costs which is preferred technique for inguinal hernias: Best open mesh (Lichtenstein) or a laparo-endoscopic (TEP and TAPP) technique?
Salma (2015) conducted an RCT in Pakistan comparing the effects of the classic Lichtenstein’s repair with TAPP repair for inguinal hernia in an all male sample. Total follow-up time was unclear, however postoperative pain was measured 4 hours postoperatively. The intervention group (n=30) received the Lichtenstein’s repair, while the control group (n=30) received TAPP. The sample’s mean age (61.46 years (SD: 7)) was not described per study arm, but as a whole group. Other sample characteristics were not described. Patients received a diclofenic sodium injection (75mg I/M) immediately after and 6 hours after surgery.
Westin (2015) conducted an RCT in Sweden to compare the effects of the Lichtenstein’s repair with the TEP repair in an all male sample. A long-term follow-up of 1 year was used. The intervention group (n=195) received the Lichtenstein’s repair and the control group (n=194) received the TEP repair. The intervention group had a mean age of 53.2 years (range: 27 to 77) and a mean BMI of 24.9 (no measure of dispersion reported). In this group 134 patients (71.7%) had an ASA classification of I, 51 participants (27.3%) of II, and 2 participants (1.1%) of III. Diagnoses of the hernia among the intervention group were indirect inguinal hernia (n=109 (58.2%)), direct inguinal hernia (n=57 (30.5%)), combined hernia (n=20 (10.7%)), or other types (n=1 (0.5%)). The control group had a mean age of 52.9 years (range: 23 to 79) with a mean BMI of 26.5 (no measure of dispersion reported). ASA classification among the control group was in 124 patients (66%) I, in 62 patients (33%) II, and in 2 patients (1.1%) III. The control group was diagnosed with indirect inguinal hernia (n=124 (66%)), direct inguinal hernia (n=56 (29.8%)), femoral hernia (n=1 (0.5%)), combined hernia (n=7 (3.8%)), or other types (n=1 (0.5%)). After one year, 4 participants were lost-to-follow-up in the intervention group (reasons: no response or declined further participation) and 5 participants in the control group (reasons: deceased, no response, or declined further participation). Furthermore, 5 participants (n=4 from the intervention group, n=1 from the control group) were excluded from analyses due to protocol violations before surgery was performed.
Waris (2016) compared the effect of Lichtenstein’s repair with TEP repair in Pakistan conducting a study with an RCT design. The length of follow up was not reported, however pain was measured on the first and second day postoperatively. No loss-to-follow-up or incomplete data were reported. The intervention group (n=200) received the Lichtenstein’s repair, while the control group (n=200) received the TEP repair. No sample characteristics per study arm were reported, however the mean age of both groups combined was 33.89 years (SD. 89). In total, 304 male participants and 96 female participants were recruited. No other characteristics were reported. Participants received surgery under general or spinal anesthesia, however a technique concering postoperative analgesics was not described.
Gürbulak (2015) used an RCT in Turkey to compare the effects of Lichtenstein’s repair with TEP repair in an all male sample. Participants had a follow-up at 10 days, 3 months, and 6 months after discharge form the hospital (mean hospital stay: 1 day). Participants in the intervention group (n=70) received the Lichtenstein’s repair, while participants in the control group (n=64) received the TEP repair. The intervention group had a mean age of 51.4 years (range: 18 to 70) and the control group had a mean age of 48.2 (range: 18 to 70). No other sample characteristics were described. A total of 14 participants were lost-to-follow-up because they did not show up for follow-up after surgery. It was not described to which intervention the lost participants were allocated, however they were excluded from analyses.
Results
Recurrence (critical outcome)
Westin (2015) observed recurrence within 1 year postoperatively in 4 participants who received the Lichtenstein’s repair. In the group who received TEP repairs 2 participants had a recurrence. No significant differences were found, however the p-value was not reported.
Gürbulak (2015) reported that there was no difference in recurrence between the groups which received either the Lichtenstein’s repair or the TEP repair (p=0.89, follow-up period unclear). In both groups there was 1 recurrence each.
Factors affecting the quality of the evidence
Evidence originated from RCTs comparing the Lichtenstein’s repair with the TEP repair for inguinal hernias and is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the studies (one study did not conceal the allocation and in both studies patients, care providers and outcome assessors were likely not to be blinded) and the studies add a low number of events to the body of evidence. The certainty of evidence due to these factors is reflected in the conclusions.
Postoperative recovery: Early and late postoperative pain (critical outcome)
Pain was recorded 4 hours postoperatively in Salma (2015) by using the Visual Analogue Scale (VAS, scale: 0 to 10). Patients receiving the Lichtenstein’s repair reported a mean VAS-score of 6.23 (SD: 1.87), while patients receiving a TAPP repair reported a mean VAS-score of 4.43 (SD: 1.59). This was reported as a significant difference between groups (p=0.005).
The number of participants having postoperative pain on the first or second day was reported to be statistically different between Lichtenstein’s repair and TEP in the RCT conducted by Waris 2016. In the group which received the Lichtenstein’s repair 20 participants (10%) had postoperative pain, compared to 8 (4%) participants in the TEP repair group (p=0.031).
Chronic pain (critical outcome)
Westin (2015) observed that at 1 year postoperatively, a significant difference existed in the proportion of participants who reported persistent pain when the Lichtenstein’s repair was compared to TEP repair (p=0.007). In the group which received the Lichtenstein’s repair 62 participants (33.2%) reported persistent pain, compared to 39 participants (20.7%) in the group which received TEP. Significant differences in proportions of participants were also found using the inguinal pain questionnaire (IPQ) on the items ‘no pain’ (Lichtenstein: 65%, TEP: 77%; p=0.007) and ‘pain that can easily be ignored’ (Lichtenstein: 22%, TEP: 14%; p=0.011). No significant differences were reported fort he IPQ items ‘pain that cannot be ignored but does not affect activities’ (Lichtenstein: 7%, TEP: 5%; p-value not reported), ‘pain affects concentration and activities’ (Lichtenstein: 2%, TEP: 1%; p-value not reported), and pain affects most activities (Lichtenstein: <1%, TEP: <1%; p-value not reported). Percentages from the IPQ items were estimated from a bar chart.
Factors affecting the quality of the evidence
Evidence originated from RCTs comparing pain after the Lichtenstein’s repair with the TAPP or TEP repair for inguinal hernias and is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the studies (unclear allocation concealment in 2 studies and a non-concealed allocation in 1 study, in all studies patients, care providers and outcome assessors were likely not to be blinded). The certainty of evidence due to these factors is reflected in the conclusions.
Costs
No included study reported and/or compared the costs of the repair techniques.
Complications
Gürbulak (2015) found no difference in intraoperative complications between the Lichtenstein’s repair and totally extraperitoneal repair (p=0.92). In the Lichtenstein’s repair group 1 participant had an intraoperative complication, compared to 2 participants in the TEP group. There was no difference reported by Gürbalak (2015) in postoperative complications in both groups (p=0.24, follow-up duration unclear). A total of 10 participants who received the Lichtenstein’s repair were observed to have postoperative complication, compared to 3 participants who received the TEP repair.
Factors affecting the quality of the evidence
Evidence originated from an RCT comparing the Lichtenstein’s repair with the TEP repair for inguinal hernias and is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the study (unclear allocation concealment technique and patients, care providers and outcome assessors were likely not to be blinded) and a limited sample size. The certainty of evidence due to these factors is reflected in the conclusions.
Learning curve
No included study reported and/or compared the learning curve of the repair techniques.
In 2017 Scheuermann (BMC surgery) reported on a systematic review and meta-analysis comparing TAPP and Lichtenstein for primary inguinal hernia repair. They reported on 8 studies (including Salma, 2015) demonstrating non significance in the areas of neuralgia, recurrence and time to return to work. There was however a significant difference in favour of the TAPP regarding chronic pain OR 0.42 (95%CI 0.23 to 0.78). They also concluded that because of moderate methodological quality and low number of patients further multicenter trials are needed.
6. Open posterior or laparoscopic technique?
The literature included for this comparison for the International Guidelines was limited both in terms of data and quality of evidence. HerniaSurge could not give any recommendation on the choice of laparoscopic versus open pre-peritoneal mesh placement for the treatment of inguinal hernia.
Sajid (2015) performed a systematic review and meta-analyses comparing open preperitoneal techniques with laparoscopic preperitoneal techniques. In both groups several different techniques were included. The Cochrane Library (Cochrane Colorectal Cancer Group controlled trial register and the Cochrane Central Register of Controlled Trials), MEDLINE, Embase and the SCI Expanded were searched and yielded 42 hits in June 2014. Study inclusion criteria were: placement of a mesh in the preperitoneal space through trans-inguinal incision or any other open technique. Exclusion criteria were not reported in the text, although the study selection flow-diagram showed the excluded studies with reasons. Excluded studies were not referenced. After screening, 10 RCTs were eligible for inclusion. Included studies originated from Finland, Turkey, Egypt, Sweden, Austria, the Netherlands, and India. Studies included unilateral, bilateral, primary, and/or recurrent inguinal hernias. One study also included femoral hernias. The open repair technique varied among included studies and techniques included were trans-inguinal preperitoneal repair (6 studies), Nyhus repair (3 studies), and Ugahary repair (1 study). Laparoscopic repairs were either TAPP or TEP repairs. The used follow-up in the included studies ranged from 1 week to 96 months (1 week: 1 study, 12 months: 3 studies, 18 months: 2 studies, 24 months: 2 studies, 96 months: 1 study, not reported: 2 studies), however there was no information provided on missing data or loss-to-follow-up. Included sample sizes varied from n=41 to n=400.
Andresen (2017) performed a systematic review of open preperitoneal techniques for inguinal hernia repair including 67 articles describing nine methods of preperitoneal inguinal hernia repair: Onstep, TIPP, Kugel, TREPP, Nyhus, Ugahary, Horton/Florence, Stoppa and Read. Pooled group sizes varied from 157 patients (Horton/Florence) to 4781 patients (Kugel). Many of the included studies originated from specialized centers, describing patient cohorts. Two randomized controlled studies were included, none of which compared open preperitoneal techniques with laparoscopic techniques.
Akgül (2017) conducted an RCT in Turkey to compare the effects of Stoppa repairs with TEP repairs. The intervention group (n=25) received the Stoppa repair and the control group received the TEP repair. The RCT had a follow-up range of 4 to 12 months. The intervention group had a mean age of 45.1 (range: 22 to 64) and consisted of 25 men. Mean operation time was 55 minutes (no measure of dispersion reported) in the intervention group. The control group had a mean age of 44.1 (range: 19 to 64) and consisted of 22 men and 3 women. This group had a mean operation time of 70 minutes (no measure of dispersion reported). No other characteristics were reported. No loss-to-follow-up and/or incomplete data were described.
Results
Recurrence (critical outcome)
Andresen (2017) found recurrence rates varying between 1.1% (TREPP) to 6.3% (Ugahary).
Seven studies (n=1003, 16 events in the open repair group and 14 events in the laparoscopic repair group) reporting recurrence were pooled in Sajid 2015 by using the odds ratio as an effect measure and a random effects model. In the random effects model (OR 1.21; 95% CI 0.52 to 2.83; z=0.45, p=0.65) the risk of developing recurrence was similar after open or laparoscopic repair. There was no statistical heterogeneity (I2 0%).
Factors affecting the quality of the evidence
Evidence originated from a meta-analysis containing RCTs and is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the meta-analysis (risk of publication bias was not assessed) and the limited number of events in the meta-analysis. Caution is advised when assessing the heterogeneity in the meta-analysis (CIs overlap each other and OR=1, however OR estimates from individual studies lie on both sides of OR=1 (3 studies <OR=1, 2 studies > OR=1). The certainty of evidence due to these factors is reflected in the conclusions.
Postoperative recovery: Complications
Sajid (2015) pooled 9 studies (n=1245, 90 events in the open repair group and 111 events in the laparoscopic repair group) reporting complications by using the odds ratios as an effect measure and a random effects model. The pooled effect was OR=1.00 (95%CI: 0.60 to 2.03), favouring neither open nor laparoscopic repairs. Heterogeneity was moderate (I2 = 40%).
Akgül (2016) observed 6 (25%) participants with complications in the Stoppa repair group versus 3 (12%) participants with complications in the TEP repair group. No statistical test was reported to test for differences between groups.
Factors affecting the quality of the evidence
Evidence originated from a meta-analysis containing RCTs and from a single RCT and is therefore ‘high’. However, the quality of the evidence is reduced due to methodological limitations of the meta-analysis (risk of publication bias was not assessed) and the limited number of events in the meta-analysis. Caution is advised when assessing the heterogeneity in the meta-analysis (CIs overlap eachother; CIs overlap OR=1; OR estimates from individual studies lie on both sides of OR=1 (4 studies <OR=1, 5 studies >OR=1)). The quality of evidence from the individual RCT is reduced due to methodological limitations (Risk of Bias due to the lack of blinding for the patient, care provider, and outcome assessor is likely) and the limited sample size.
Mortality
None of the included studies reported results for mortality.
Zoeken en selecteren
In order to answer the clinical ‘question’, a systematic literature search was done for the following research question(s):
Which is the preferred repair method for inguinal hernias: mesh or non-mesh?
PICO 1
P: Adult patients with an inguinal hernia;
I: mesh;
C: non-mesh (i.e. Shouldice);
O: recurrence, chronic pain (>3 months), recovery (including post-operative pain).
Which is the preferred open mesh technique for inguinal hernias: Lichtenstein or other open flat mesh and gadgets via an anterior approach?
PICO 2
P: adult patients with an inguinal hernia;
I: flat mesh;
C: progrip in open;
O: recurrence, chronic pain (>3 months), recovery (including post-operatieve pain).
Which is preferred open mesh technique: Lichtenstein versus open preperitoneal?
PICO 3
P: adult patients with an inguinal hernia;
I: Lichtenstein repair;
C: any open preperitoneal technique, TREPP;
O: recurrence, chronic pain (>3 months), complications, mortaliteit.
Which endoscopic technique is preferred in terms of postoperative outcome?
PICO 4
P: adult patients with an primairy unilateral inguinal hernia;
I: TAPP;
C: TEP;
O: acute postoperative pain, chronic pain (>6 months), recurrence after >3 years, recovery, costs.
When considering recurrence, pain, learning curve, postoperative recovery and costs which is preferred technique for inguinal hernias: Best open mesh (Lichtenstein) or a laparo-endoscopic (TEP and TAPP) technique?
PICO 5
P: adult patients with an primairy unilateral inguinal hernia;
I: Lichtenstein;
C: TEP/TAPP;
O: recurrence, early and late postoperative pain, costs, complications, learning curve.
Open posterior or laparoscopic?
PICO 6
P: adult patients with an primairy unilateral inguinal hernia;
I: laparoscopic repair (TEP and TAPP);
C: any open preperitoneal technique, TREPP;
O: morbidity, mortality, recurrence.
Relevant outcome measures
The working group decided that chronic pain, persisting chronic pain (≥3 years) and recurrence were crucial outcome measures for decision-making. Other important outcomes for each PICO are listed above. The working group defined chronic pain as the presence of pain after a follow-up period of at least 3 months. The working group did not define the other outcome measures beforehand, however, they used the definitions described in the studies. The working group did not define clinical (patient) relevant differences.
Searching and selecting (Methods)
The information specialist from the Cochrane Centre in the Netherlands searched Medline and Embase on April 11th 2017 and the Cochrane Register on April 12th 2017 for systematic reviews (SRs) and randomized controlled trials (RCTs) about inguinal hernias, without restrictions on publication date. All duplicates (including duplicates from the former search on the 30th of June 2015) were removed. The search details can be found in the tab Acknowledgement.
Literature experts excluded studies that were clearly not relevant for answering clinical questions about inguinal hernias. Therefore, 66 SRs and 241 RCTs remained to be judged by the working group.
The working group selected studies based on title-abstract, that could possibly answer the research questions. After reading the full text, studies were excluded (see exclusion table in the tab Acknowledgement); and finally, the number of studies stated below were included for analysis:
PICO 1: 8 studies selected, 7 included
PICO 2: 5 studies selected, 3 included
PICO 3: 6 studies selected, 4 included
PICO 4: 4 studies selected, 2 included
PICO 5: 13 studies selected, 4 included
PICO 6: 5 studies selected, 2included
Data extraction and analysis
The most important study characteristics and results were extracted from the SRs or original studies (in case of missing information in the review). The most important study characteristics and results are shown in the evidence tables. The judgement of the individual study quality (Risk of Bias) is shown in the Risk of Bias tables.
Relevant pooled and/or standardised effect measures were, if useful, calculated using Review Manager 5.3 (Cochrane Collaboration, Oxford, United Kingdom). If pooling results was not possible, the outcomes and results of the original study were used as reported by the authors.
The working group did not define clinical (patient) relevant differences for the outcome measures. Therefore, we used the following boundaries for clinical relevance, if applicable: for continue outcome measures: RR<0.75 or >1.25 (GRADE recommendation) or Standardized mean difference (SMD=0.2 (little); SMD 0.5 (reasonable); SMD=0.8 (large). These boundaries were compared with the results of our analysis. The interpretation of dichotomous outcome measures is strongly related to context; therefore, no clinical relevant boundaries were set beforehand. For dichotomous outcome measures, the absolute effect was calculated (Number Needed to Treat (NNT) or Number Needed to Harm (NNH)).
Referenties
- Akgül N, Yaprak M, Dogru V, et al. (2017) Quantitative assessment of the impacts of stoppa repair and total extraperitoneal repair on the lower extremity muscular functions in cases of unilateral inguinal hernia: a randomized controlled study. Hernia 21(3):377-382. doi: 10.1007/s10029-016-1559-6. Epub 2016 Dec 10. PubMed PMID: 27942876.
- Andresen K, Burcharth J, Fonnes S, et al. (2015) Long-term outcome after Onstep versus Lichtenstein technique for inguinal herniarepair: results from a randomized clinical trial. Hernia 19(6):871-7.doi: 10.1007/s10029-015-1428-8. Epub 2015 Oct 7. PubMed PMID: 26445862.
- Andresen K, Burcharth J, Fonnes S, et al. (2017) Chronic pain after inguinal hernia repair with the ONSTEP versus the Lichtenstein technique, results of a double-blinded multicenter randomized clinical trial. Langenbecks Arch Surg 402(2):213-218. doi: 10.1007/s00423-016-1532-y.Epub 2016 Nov 11. PubMed PMID: 27837273.
- Bansal VK, Krishna A, Manek P, et al. (2017) Prospective randomized comparison of testicular functions, sexual functions and quality of life following laparoscopic totally extra-peritoneal (TEP) and trans-abdominal pre-peritoneal (TAPP) inguinal hernia repairs. Surg Endosc 31(3):1478-1486. doi: 10.1007/s00464-016-5142-0. Epub 2016 Aug 5. PubMed PMID: 27495344.
- Barth RJ Jr., Burchard KW, Tosteson A, et al. (1998) Colacchio TA, Henriques HF, et al. Short-term outcome after mesh or shouldice herniorrhaphy: a randomized, prospective study. Surgery 123(2):121-6.
- Berndsen FH, Petersson U, Arvidsson D, et al. (2007) Discomfort five years after laparoscopic and Shouldice inguinal hernia repair: a randomised trial with 867 patients. A report from the SMIL study group. Hernia 11(4):307-13.
- Bhatti I, Ishaqu H, Ahmad Z, et al. (2015) Desarda's versus lichtenstein technique of hernia repair. Pakistan Journal of Medical and Health Sciences 9(4): 1331-1333.
- Cadanová D, van Dijk JP, Mollen RMHG. (2017) The transinguinal preperitoneal technique (TIPP) in inguinal hernia repair does not cause less chronic pain in relation to the ProGrip technique: a prospective double-blind randomized clinical trial comparing the TIPP technique, using the PolySoft mesh, with the ProGrip self-fixing semi-resorbable mesh. Hernia 21(1):17-27. doi: 10.1007/s10029-016-1522-6. Epub 2016 Aug 18. PubMed PMID: 27539079.
- Gürbulak EK, Gürbulak B, Akgün IE, et al. (2015) Effects of totally extraperitoneal (TEP) and Lichtenstein hernia repair on testicular blood flow and volume. Surgery 158(5):1297-303. doi: 10.1016/j.surg.2015.03.028. Epub 2015 Apr 30. PubMed PMID: 25937159.
- Hauters P, Meunier D, Urgyan S, et al. (1996) Prospective controlled study comparing laparoscopy and the Shouldice technique in the treatment of unilateral inguinal hernia. Annales de Chirurgie 50(9):776-81.
- Karaca AS, Ersoy OF, Ozkan N, et al. (2015) Comparison of inguinal hernia repairs performed with lichtenstein, rutkow-robbins, and gilbert double layer graft methods. Indian J Surg 77(1):28-33. doi: 10.1007/s12262-013-0809-4. Epub 2013 Jan 16. PubMed PMID: 25829708; PubMed Central PMCID: PMC4376828.
- Kux M, Fuchsjager N, Feichter A. (1994) Lichtenstein patch versus Shouldice technique in primary inguinal hernia with a high risk of recurrence. Der Chirurg Zeitschrift fur alle Gebiete der operativen Medizen 65(1):59-62; discussion 3.
- Leibl BJ, Daubler P, Schmedt CG, et al. (2000) Long-term results of a randomized clinical trial between laparoscopic hernioplasty and shouldice repair. The British journal of surgery 87(6):780-3.
- Lermite E, Arnaud JP. (2012) Prospective randomized study comparing quality of life after shoudice or mesh plug repair for inguinal hernia: short-term results. Surgical technology international 22:101-6.
- Lockhart K, Teo E, Teo S, et al. (2015) Mesh versus non-mesh for inguinal and femoral hernia repair. CochraneDatabase of Systematic Reviews, Issue 2. Art. No.: CD011517. DOI: 10.1002/14651858.CD011517.
- Magnusson J, Nygren J, Gustafsson UO, et al. (2016) UltraPro Hernia System, Prolene Hernia System and Lichtenstein for primary inguinal hernia repair: 3-year outcomes of a prospective randomized controlled trial. Hernia 20(5):641-8. doi: 10.1007/s10029-016-1507-5. Epub 2016 May 18. PubMed PMID: 27194437.
- Mahmoudvand H, Forutani S, Nadri S. (2017) Comparison of Treatment Outcomes of Surgical Repair in Inguinal Hernia with Classic versus Preperitoneal Methods onReduction of Postoperative Complications. Biomed Res Int 3785302. doi:10.1155/2017/3785302. Epub 2017 Jan 23. PubMed PMID: 28232939; PubMed CentralPMCID: PMC5292369.
- McGillicuddy JE. (1998) Prospective randomized comparison of the Shouldice and Lichtenstein hernia repair procedures. Archives of surgery (Chicago, Ill : 1960) 133(9):974-8.
- Nordin P, Bartelmess P, Jansson C, et al. (2002) Randomized trial of Lichtenstein versus Shouldice hernia repair in general surgical practice. The British journal of surgery 89(1):45-9.
- Olasehinde O, Lawal O, Agbakwuru E, et al. (2016) Comparing Lichtenstein with darning for inguinal hernia repair in an African population. Hernia 20(5): 667-674.
- Palermo M, Acquafresca P, Bruno M, et al. (2015) Hernioplasty with and without mesh: analysis of the immediate complications in a randomized controlled clinical trial. ABCD, Arquivos Brasileiros de Cirurgia Digestiva 28 (3): 157-160.
- Prieto-Diaz-Chavez E, Medina-Chavez JL, Anaya-Prado R. (2009) A cost-effectiveness analysis of tension-free versus shouldice inguinal hernia repair: a randomized double-blind clinical trial. Hernia 13(3):233-8.
- Sajid MS, Caswell J, Singh KK. (2015) Laparoscopic Versus Open Preperitoneal mesh Repair of Inguinal Hernia: an Integrated Systematic Review and Meta-analysis of Published Randomized Controlled Trials. Indian J Surg 77(Suppl 3):1258-69. doi: 10.1007/s12262-015-1271-2. Epub 2015 Apr 28. PubMed PMID: 27011548; PubMed Central PMCID: PMC4775580.
- Salma U, Ahmed I, Ishtiaq S. (2015) A comparison of post operative pain and hospital stay between Lichtenstein's repair and Laparoscopic Transabdominal Preperitoneal (TAPP) repair of inguinal hernia: A randomized controlled trial. Pak J Med Sci 31(5):1062-6. doi: 10.12669/pjms.315.4811. PubMed PMID: 26648987; PubMed Central PMCID: PMC4641256.
- Schmitz R, Treckmann J, Shah S, et al. (1997) Tension-free-Technik bei offener LeistenhernienreparationEine prospektive, randomisierte Studie zur postoperativen Schmerzperception (Tension-free-Rekonstruktion vs. Shouldice-Technik). Der Chirurg 68(3):259-63.
- Szopinski J, Dabrowiecki S, Pierscinski S, et al. (2012) Desarda Versus Lichtenstein Technique for Primary Inguinal Hernia Treatment: 3-Year Results of a Randomized Clinical Trial. World J Surg 36: 984992.
- Waris A, Abid KJ, Ishaque S, et al. (2016) Totally Extra-Peritoneal (TEP) Versus Tension Free mesh Repair for Inguinal Hernia. Pakistan journal of medical & health sciences 10(1):186-9.
- Wei FX, Zhang YC, Han W, et al. (2015) Transabdominal Preperitoneal (TAPP) Versus Totally Extraperitoneal (TEP) for Laparoscopic Hernia Repair: A Meta-Analysis. Surg Laparosc Endosc Percutan Tech 25(5):375-83. doi: 10.1097/SLE.0000000000000123. PubMed PMID: 25654182.
- Westin L, Wollert S, Ljungdahl M, et al. (2016) Less Pain 1 Year After Total Extra-peritoneal Repair Compared With Lichtenstein Using Local Anesthesia: Data From a Randomized Controlled Clinical Trial. Ann Surg 263(2):240-3. doi: 10.1097/SLA.0000000000001289. PubMed PMID: 26079901.
- Wamalwa A, Siwo E, Mohamed M. (2015) Shouldice versus lichtenstein hernia repair techniques: A prospective randomized study. Annals of African Surgery 12(1):22-26.
- Youssef T, El-Alfy K, Farid M. (2015) Randomized clinical trial of Desarda versus Lichtenstein repair for treatment of primary inguinal hernia. International Journal Of Surgery 20:28-34.
- Zieren J, Zieren HU, Jacobi CA, et al. (1998) Prospective randomized study comparing laparoscopic and open tension-free inguinal hernia repair with Shouldice's operation. American journal of surgery 175(4):330-3.
Evidence tabellen
Evidence-table (PICO 1)
Research question: Which is the preferred repair method for inguinal hernias: mesh or non-mesh?
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3 |
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
|
Amato 2012
|
SR and meta-analysis of RCTs
Literature search up to 27 February 2012
n=16 included RCTs of which the following compared Shouldice to mesh techniques for hernia repair: A: Barth 1998 B: Butters 2007 C: Danielsson 1999 D: Hetzer 1999 E: McGillicuddy 1998 F: Miedema 2004 G: Nordin 2002 H: Zieren 1998
The remaining RCTs compared Shouldice to non-mesh techniques; these are not addressed in this evidence table. |
Inclusion criteria SR: randomised or quasi-randomised controlled trials (RCT) on the surgical treatment (non-laparoscopic techniques) of primary inguinal hernia in adults (>18 years)
Exclusion criteria SR: patients referred for femoral hernias
n=16 studies included, of which n=8 addressed the comparison mesh versus Shouldice.
N (patients); mean age (I/C) A: 105; 53/51 yrs B: 186; 56/51 yrs C: 200; 58/56 yrs D: 385; 58/53 yrs E: 672; not reported F: 101 hernias; 63/62 G: 300; age range 25-75 H: 160; 47/46
Sex: A: 99% Male B: 100% Male C: 100% Male D: 100% Male E: 100% Male F: 100% Male G: 100% Male H: 92% Male |
Describe intervention: non-laparoscopic techniques, a.o. mesh:
A: Lichtenstein standard (n=54). Polypropylene mesh. Polypropylene 2/0 B: Lichtenstein standard (n=93). No details. C: Lichtenstein standard (n=89). Polypropylene mesh D: Lichtenstein (n=239 hernias) Polypropylene meshes. Polypropylene 2/0 E: Lichtenstein standard (n=371 hernias). Polypropylene mesh. F: Lichtenstein standard (n=39). No details. G: Lichtenstein (n=149 hernias). Marlex meshes. Polypropylene 2/0. H: Plug and Patch (n=80). Polypropylene meshes and plugs.
|
Describe control: Shouldice
A: Shouldice standard (n=51) performing 4 layers. Polypropylene 2/0. B: Shouldice (n=93). No details. C: Shouldice standard (n=89) performing 4 layers. Non-absorbable monofilament D: Shouldice Standard (n=171 hernias). 4 layers. PDS 2/0. E: Shouldice standard (n=337 hernias) performing 4 layers. Non-absorbable monofilament. F: Shouldice modified (n=41). 4 layers. Polypropylene. G: Shouldice Standard (n=148 hernias). 4 layers. Polypropylene 2/0. H: Shouldice modified (n=80). 4 layers. Unabsorbable monofilament.
|
End-point of follow-up:
A: 7 days B: 52 months C: 12 months D: 3 months E: 5 years F: 7 years G: 6 years H: 25 months
For how many participants were no complete outcome data available? A: none B: 36 C: 8 D: none E: 251 F: 29 G: 9 H: none
|
N.B. Results are presented for Shouldice versus mesh (control versus intervention)!
Recurrences Defined as clinically manifest bulge or protrusion exacerbated by a Valsalva manoeuvre in the operated groin. The assessor could be a surgeon, a physician or the patient himself. Only studies with at least one year follow-up were considered.
Pooled effect (fixed effects model); 5 studies, n=1415 patients: Peto OR 3.65 (95% CI 1.79 to 7.47) favoring mesh. Heterogeneity (I2): 43.6%
Chronic pain Defined as pain persisting for more than three months (only presence or absence)
Pooled effect (fixed effects model); 5 studies, n=1371 patients: Peto OR 0.87 (95% CI 0.55 to 1.39). Heterogeneity (I2): 59.6%
Postoperative complications
Wound infections Pooled effect (fixed effects model); 7 studies, n=1938 patients Peto OR 0.74 (95% CI 0.37 to 1.49). Heterogeneity (I2): 0%
Seroma Pooled effect (fixed effects model); 3 studies, n=1165 patients Peto OR 0.96 (95% CI 0.37 to 2.50). Heterogeneity (I2): 0%
Testicular atrophy Pooled effect (fixed effects model); 3 studies, n=1155 patients Peto OR 1.05 (95% CI 0.047 to 27.38). Heterogeneity (I2): 56%
Haematoma Pooled effect (fixed effects model); 3 studies, n=562 patients Peto OR 0.64 (95% CI 0.25 to 1.64). Heterogeneity (I2): 0% |
Authors’ conclusion Shouldice herniorrhaphy is a good technique for inguinal hernia repair compared to other non-mesh techniques, giving better results in terms of recurrence. When comparing Shouldice to mesh, the use of mesh is associated with a lower rate of recurrence. The quality of included studies, assessed with jaded scale, was low. Reasons for lost to follow-up were often not reported and the length of follow-up varied broadly among studies.
|
|
|
Bhatti 2015 |
Type of study: RCT N=200
Setting: multicentre study
Country: Pakistan
Source of funding: not specified |
Inclusion criteria: Unilateral, primary, reducible inguinal hernia.
Exclusion criteria: Patients with renal failure (serum creatinine more than 2mg/dl) and diabetes were excluded.
N total at baseline: Intervention: 100 Control: 100
Important prognostic factors2: Age ± SD: 53.25±6.768 (range 32-60)
Sex: M 60.5% F 39.5%
Groups comparable at baseline? Not specified.
|
Inguinal hernias were repaired by standard Lichtenstein (mesh) method (group L)
|
Inguinal hernias were repaired by Desarda’s (non-mesh) method (group D) |
Length of follow-up: 30 days post-operation
Loss-to-follow-up: I: N=0 (0%) C: N=0 (0%)
Incomplete outcome data: I: N=0 (0%) C: N=0 (0%)
|
Outcome measures and effect size (include 95%CI and p-value if available):
Chronic pain: Not reported
Recurrences: Not reported
Seroma formation: I: 10/100 (10.0%) C: 6/100 (6.0%) p>0.05
|
|
|
|
Olasehinde 2016 |
Type of study: quasi randomised N=67
Setting: single center, outpatient clinic
Country: Nigeria
Source of funding: not specified |
Inclusion criteria: inguinal or inguinoscrotal hernia. >18 years
Exclusion criteria: recurrent, obstructed or strangulated hernias, patients who had bilateral hernias with dissimilar types on each side (inguinal/inguinoscrotal), immunosuppression (diabetes mellitus, malnutrition, patients on long- term steroids, known retroviral positive patients), chronic debilitating illnesses (chronic renal or liver failure, congestive cardiac failure) and urinary bladder outlet obstruction
N total at baseline: Intervention: 33 Control: 34
Important prognostic factors2: Age ± SD: I: 48.1 ± 20.3 years C: 48.8 ± 17.9 years
Sex: I: M 90.9 % C: M 79.4 %
Groups comparable at baseline? Unclear Participants were separated into two blocks based on the type of hernia (inguinal hernia / inguinoscrotal hernia). This was aimed at achieving a balance in the type of hernias allocated to the two treatment groups. |
Inguinal hernia repair with Lichtenstein (mesh) method
|
Inguinal hernia repair with darning (non-mesh) method
|
Length of follow-up: Mean duration of follow-up was 7.5 months
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Chronic groin pain: I: 1/33 (3.0%) C: 1/34 (2.9%)
Post-operative pain (4h): All patients had varying degrees of pain, but was found to be worse in those who had darning repair. (p = 0.004).
Post-operative pain (10d): I: 8/33 (24.2%) C:15/34 (47.1 %)
Post-operative pain (1mo): I: 0/33 (0%) C: 3/34 (8.8 %) Pain was assessed as present or absent and pain scores when present were categorized as ‘none’, ‘mild’, ‘moderate’, or ‘severe’. Zero score on the scale being regarded as ‘no pain’, 1–3 as ‘mild’ pain, 4–6 as ‘moderate’ and 7–10 as ‘severe’.
Recurrences (6mo): There was no recurrence in any of the groups.
Complications: Overall: I:12/33 (36.4%) C: 13/34 (38.2%)
Surgical site infection: I:2/33 (6.1%) C: 1/34 (2.9%) P > 0.05
Wound haematoma: I: 5/33 (15.2%) C: 9/34 (25.5%) P>0.05
Scrotal oedema: I: 6/33 (18.2%) C:5/34 (14.7 %) P>0.05
Acute urinary retention (4h): I: 1/33 (3.0%) C: 1/34 (2.9%)
Orchitis: I:0/33 (0%) C:1/34 (2.9%) |
|
|
|
Palermo 2015 |
Type of study: RCT N=263
Setting: single center
Country: Brasil
Source of funding: none |
Inclusion criteria: Nonsurgical risk or liable to compensation; primary inguinal hernia unilateral or bilateral; non complicated hernia; patients without coagulation disorder.
Exclusion criteria: elevated surgical risk; high blood pressure that not respond to treatment; obstructed hernias; strangulated hernias; recurrent hernias; coagulation disorders.
N total at baseline: Intervention: 135 Control: 128
Important prognostic factors2: Age ± SD: I: 55.5 ±16.1 C: 54.4±17.0
Sex: I: M 88.2% C: M 82.8%
Groups comparable at baseline? Yes
|
Lichtenstein (mesh) inguinal hernia repair
|
Bassini (non-mesh) inguinal hernia repair
|
Length of follow-up: 6 month
Loss-to-follow-up: I: N=0 (0%) C: N=0 (0%)
Incomplete outcome data: I: N=0 (0%) C: N=0 (0%)
|
Outcome measures and effect size (include 95%CI and p-value if available):
Postoperative pain: (analgesia at day 7) I: 74/135 =54.8% C: 112/128 =87.5% P<0.0001 Analgesia at day 15 and 1 month also reported. Pain is reported as EVA scale outcome.
Recurrence: I: 1/135 (0.7%) C: 3/128 (2.3%) p=0.36
Seroma (day7): I: 10/135 =7.4% C: 8/128 = 6.2% p=0.71 Seroma at day 15 also reported.
Seroma (day30): I: 1/135 =0.7% C: 2/128 = 1.6% p=0.61
Hematoma: Amount not reported. No significant difference between groups
Infection: I: 2/135 =1.5% C: 9/128 =7.0% p=0.031
|
Sample size needed was recalculated and reduced after finding benefit for mesh in an interim analysis. |
|
|
Szopinski 2012 |
Type of study: RCT N=208
Setting: multicenter
Country: Poland
Source of funding: grant from the Nicolaus Copernicus University of Torun, Collegium Medicum in Bydgoszcz, Poland (grant 19/2006) and the Foundation for Clinical Surgery in Bydgoszcz, Poland.
|
Inclusion criteria: adult male Caucasian patients with primary inguinal hernias, assessment of the condition of the external oblique aponeurosis.
Exclusion criteria: with exclusion of patients with an aponeurosis that was divided, tiny, and/or weak. Patients with recurrent or strangulated hernias or mental disorders, those participating in other clinical trials, ASA> 3, history of a forced hernia reduction with subsequent hospitalization, a history of infection, or the presence of any scar in the inguinal area.
N total at baseline: Intervention: 103 Control: 105
Important prognostic factors2: Age ± SD: I: 54.1 ±15.3 C: 50.2 ±17.5
Sex: I: M 100% C: M 100%
Groups comparable at baseline? Yes |
Primary inguinal hernia repair with Lichtenstein (mesh) method
|
Primary inguinal hernia repair with Desarda method (non-mesh)
|
Length of follow-up: at 7, 30 days, and 6, 12, 24, 36 months
Loss-to-follow-up: Given for 1yr, 2yr and 3yr I: baseline N=103 assessed at 1yr N=92 assessed at 2yr N=91 assessed at 3yr N=87 (16%) 5 no reason given 3 refused 7 no data 1 death (heart infarct)
C: baseline N=105; assessed at 1yr N=96 assessed at 2yr N=91 assessed at 3yr N=83 (21%) 6 no reason given 3 refused 12 no data 1 death (heart infarct)
Incomplete outcome data: I: Analyzed after 3 y n = 87 Excluded from analysis n = 0
C: Analyzed after 3 y n = 83 Excluded from analysis n = 0
|
Outcome measures and effect size (include 95%CI and p-value if available):
Chronic pain (>6mo after surgery): I: 3/103 (2.9%) C: 5/105 (4.8%) p=0.464 Pain is measured using a visual analog scale (VAS)
Recurrence (>1 year): I: 2/103 (1.9%) C: 2/105 (1.9%) p=1.00
Seroma (7d): I: 6/103 (5.8%) C: 4/105 (3.8%) p=0.508
Seroma (30d): I: 8/103 (7.8%) C: 0/105 p=0.004
SSSI (infection): I: 2/103 (1.9%) C: 1/105 (0.9%) p=1.0
Hematoma: I: 7/ 103 (6.8%) C: 8/ 105 (7.6%)
Testicular edema (30d): I: 6/103 (5.8%) C: 6/105 (5.7%)
|
|
|
|
Wamalwa 2015 |
Type of study: RCT N=50; 5 did not show up for surgery
Setting: single center
Country: Kenya
Source of funding: not reported |
Inclusion criteria: Primary unilateral inguinal hernia, men, age 18-80,
Exclusion criteria: irreducibility, femoral hernia, coagulation abnormalities, anticoagulant treatment, and patients for whom anaesthesia was considered unsuitable
N total at baseline: Intervention: 23 Control: 22
Important prognostic factors2: Median age (range): I: 60 (22-80) C: 63.5 (25-76)
Sex: I: M 100% C: M 100%
Types of hernia: I: direct n=5 indirect n=17 combined n=1 all types n=23
C: direct n=3 indirect n=18 combined n=1 all types n=22
Groups comparable at baseline? yes
|
Inguinal hernia repair with the tension-free Lichtenstein (mesh) method
|
Inguinal hernia repair with 4-layer tissue repair Shouldice (no-mesh) method
|
Length of follow-up: 24hrs, 2 weeks, 3 months and yearly for two years.
Loss-to-follow-up: I: N=0 (0%) C: N=0 (0%)
Incomplete outcome data: I: N=0 (0%) C: N=0 (0%)
|
Outcome measures and effect size (include 95%CI and p-value if available):
Chronic pain “One patient had persistent chronic pain after Lichtenstein repair. This was classified as neuralgia on account of its radiation along the inguinal nerve.”
Post-operative pain (>3mo): I: 1/23 (4.3%)) C: 0/22 Also reported after 24hours and after 2 weeks Not reported how pain was assessed
Recurrences: None in both groups
Urinary retention: I: 1/23 (4.3%) C: 0/22
Major complications: There were no major complications related to the operation and no reoperations.
Postoperative complications (24hrs): I: 4/23 (17.4%) C: 3/22 (13.6%)
Postoperative complications (2wks): I: 3/23 (13.0%) C: 1/22 (4.5%) |
|
|
|
Youssef 2015 |
Type of study: RCT N=168
Setting: Single center
Country: Egypt
Source of funding: none
The study was registered at the clinical trials registry of the National Institute of Health (NCT02329938). |
Inclusion criteria: All patient with inguinal or inguinoscrotal hernia
Exclusion criteria: patients under 18 years , patients with scar in inguinal region, patients with strangulated, recurrent or giant inguinoscrotal hernia, history of forced hernia reduction with subsequent hospitalization, patients with poorly controlled DM, chronic cough, those on TB treatment, severe hypertension, chronic obstructive pulmonary disease, obstructive uropathy, and ASA > 3, major psychological instability and drug abuse, patients found to have thin, weak or divided external oblique aponeurosis intraoperatively
N total at baseline: Intervention: 83 Control: 85
Important prognostic factors2: Age ± SD: I: 44±10 C: 46±11
Sex: I: M 96% C: M 97%
Groups comparable at baseline? Yes The age gender, BMI, and comorbidities were comparable in both groups. No significant difference in the characters of hernia was detected in both groups |
Lichtenstein (mesh) hernia repair method for inguinal hernia
|
Desarda (no mesh) hernia repair method for inguinal hernia |
Length of follow-up: 3, 6, 12, and 24 months Hernia recurrence and postoperative complications were assessed by physical and instrumental examinations
Loss-to-follow-up: I: 11/83 (13.3%) C: 14/85 (16.5%) Reasons not described
Incomplete outcome data: The analyzed patients were 72 patients in Lichtenstein group and 71 patients in Desarda repair group.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Chronic pain (6mo): I: 3/72 (4.2%) C: 4/71 (5.6%) P=0.68 pain was classified as moderate pain (VAS 3-5.5).
Post-operative pain at (7d): mean±SD (VAS score) I: 1.4 ± 1.2 C: 1.5 ± 1.3 P=0.65 Also pain at 3hr, 24hr, 48hr, day 14 reported Pain was assessed using visual analogue scale (VAS)
Recurrence (2yrs): I: 1/72 (1.4%) C: 1/71 (1.4%) P=0.99 There were no early recurrences (<1 year).
Hematoma (at 14d): I: 0/72 C: 1/71 (1.4)
SSSI (infection) (7d) I: 2/72=2.7% C: 1/71= 1.4% P=0.55
Wound infection (14d) I: 0/72 C: 0/71
Seroma (7d): I: 1/72=1.4% C: 0/71= 0% P=0.32
|
|
|
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures.
- Provide data per treatment group on the most important prognostic factors ((potential) confounders).
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls .
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders.
Overview of primary studies relevant to PICO 1 (n=36)
|
Primary study |
Mesh technique |
Non-mesh technique |
|
Barth 1998 |
Lichtenstein |
Shouldice |
|
Berndsen 2007 |
Tapp |
Shouldice (modified) |
|
Butters 2007 |
Tapp and Lichtenstein |
Shouldice |
|
Danielsson 1999 |
Lichtenstein |
Shouldice |
|
Hauters 1996 |
not specified |
Shouldice |
|
Hetzer 1999 |
Lichtenstein |
Shouldice |
|
Kux 1994 |
Lichtenstein |
Shouldice |
|
Leibl 1995 |
Tapp |
Shouldice |
|
Lermite 2012 |
mesh Plug |
Shouldice |
|
McGillicuddy 1998 |
Lichtenstein |
Shouldice (slightly modified) |
|
Miedema 2004 |
Lichtenstein |
Shouldice |
|
Nordin 2002 |
Lichtenstein |
Shouldice |
|
Pokorny 2008 |
Lichtenstein/Tapp/Tep |
Bassini/Shouldice |
|
Prieto-Diaz-Chavez 2009 |
mesh-plug tension-free (MPTF) |
Shouldice |
|
Schmitz 1997 |
Tension-free surgical treatment |
Shouldice |
|
Tschudi 2001 |
Tapp |
Shouldice |
|
Wamalwa 2015 |
Lichtenstein |
Shouldice |
|
Zieren 1998 |
mesh plug/Tapp |
Shouldice |
|
Abd El Maksoud 2014 |
Lichtenstein |
Darning - Modified darn |
|
Bhatti 2015 |
Lichtenstein |
Desarda |
|
Chakraborty 2007 |
not specified |
Darning - Abraham's darn |
|
Elsebae 2008 |
Lichtenstein |
Bassini |
|
Kaynak 2007 |
Lichtenstein |
Darning - Moloney darn |
|
Kucuk 2010 |
Lichtenstein |
Darning - Moloney darn |
|
Manyilirah 2012 |
Lichtenstein |
Desarda |
|
Nakagawa 2013 |
Prolene hernia system |
Marcy |
|
Naveen 2014 |
Lichtenstein |
Bassini (modified) |
|
Olasehinde |
Lichtenstein |
Darning technique |
|
Palermo 2015 |
Lichtenstein |
Bassini |
|
Panda 2012 |
Lichtenstein |
Bassini (modified) |
|
Prior 1998 |
Lichtenstein |
Bassini (modified) |
|
Shi 2010 |
Tension-free |
Bassini |
|
Szopinski 2012 |
Lichtenstein |
Desarda |
|
van Veen 2007 |
Lichtenstein |
According to surgeon's method of choice (applied were Bassini–McVay (in 51% of patients), Bassini (25%), Shouldice (20%), and McVay (4%)) |
|
Witkowski 2000 |
mesh plug |
Bassini |
|
Youssef 2015 |
Lichtenstein |
Desarda |
Risk of Bias tables PICO 1
Table of quality assessment for systematic reviews of RCTs
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Amato 2012 |
Yes |
Yes |
Yes |
Yes |
Not applicable |
Yes |
Yes |
No |
Yes |
- Research question (PICO) and inclusion criteria should be appropriate and predefined.
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched.
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons.
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported.
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs).
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, Risk of Bias table et cetera).
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (e.g. Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (e.g., funnel plot, other available tests) and/or statistical tests (e.g., Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of Bias table for randomized controlled trials
|
Study reference
(first author, publication year) |
Describe method of randomisation1 |
Bias due to inadequate concealment of allocation?2
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?4
(unlikely/likely/unclear) |
Bias due to loss to follow-up?5
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?6
(unlikely/likely/unclear) |
|
Bhatti 2015 |
Random table
“Included patients were randomly divided into two groups using random table …” |
Unclear
Insufficient information. “200 patients admitted for unilateral, primary, reducible inguinal hernia were evaluated by consultant, and the patients fulfilling the inclusion criteria were included in the study after taking the informed consent” |
Likely
No blinding. |
Likely
Blinding is not possible.
|
Unclear
Not specified who were outcome assessors and whether they were blinded. |
Unlikely
Study aimed at looking at seroma and duration of operation and did not report key outcomes that would be expected for such a study (i.e. recurrence, wound infection, pain etc.) |
Unlikely
No loss-to-follow-up reported. |
Unlikely
No study flow presented, however patients seem to be analyzed as randomized. |
|
Olasehinde 2016 |
“Patients were allocated into two treatment groups (mesh repair, Group A and Darning repair Group B). Participants were separated into two blocks based on the type of hernia.(…) Patients within each block were alternated between the treatment groups as they presented.” |
Likely
“Patients within each block were alternated between the treatment groups as they presented” |
Likely
No blinding. |
Likely
Blinding is not possible. |
Unclear
Pain was self-reported and patients were unlikely to be blinded. Unclear whether staff that performed the physical examination was aware of the operation technique. |
Unclear
No protocol available, however, outcomes reported in methods were reported results for. |
Unlikely
No participants lost to follow up. |
Unlikely
No study flow presented, however patients seem to be analyzed in the group they were randomly allocated to. |
|
Palermo 2015 |
Randomisation table, sealed envelopes
NB. Sample size needed was recalculated and reduced after finding benefit for mesh in an interim analysis. |
Unlikely
“The surgical technique to be performed was contained in sequentially numbered opaque envelopes, using a table of random numbers to produce the series of interventions. The envelopes were in an inviolable dispenser that could only be extracted per unit in a sequentially way, and its extraction was performed before the surgery, when the patient was in the pre-anesthesia room” |
Likely
No blinding. |
Likely
Blinding is not possible.
|
Unclear Pain was self-reported and patients were unlikely to be blinded. Not specified who were other outcome assessors and whether they were blinded.
|
Unclear
No protocol available, however, results were reported for all outcomes listed in the methods section. |
Unlikely
No loss-to-follow-up reported. |
Unlikely
All patients were treated as randomized |
|
Szopinski 2012 |
“Computer-generated allotments that were disclosed to the surgeon via sealed envelope”
|
Unlikely
“The envelope was not opened until after the dissection and assessment of the external oblique aponeurosis had been performed because the condition of the aponeurosis was the final inclusion criterion.” |
Unlikely
“They (patients) each agreed to not be informed about the technique used until 2 years following the date of surgery,” |
Likely
Blinding is not possible.
|
Unlikely
“Both the patients and controlling investigators were blinded to the hernia surgery method used.” |
Unlikely
Trial register record available: all outcomes listed were reported results for. |
Unlikely
Loss-to-follow-up (including reasons) is reported and is balanced between intervention and control group. |
Unlikely
All patients were treated as randomized according to flow chart. |
|
Wamalwa 2015 |
Cards in sealed opaque envelopes |
Unlikely
Cards in sealed opaque envelopes |
Likely
No blinding. |
Likely
Blinding is not possible.
|
Unclear
Pain was self-reported and patients were unlikely to be blinded. Outcomes were assessed by the 2 recruiting physicians who were unaware of the allocation. |
Unclear
No protocol available, however, results were reported for all outcomes listed in the methods section. |
Unlikely
No loss-to-follow-up reported |
Unlikely
All patients were treated as randomized. |
|
Youssef 2015 |
Sealed envelopes
“Randomization was simple and achieved using sealed envelopes” |
Unlikely
All patients were subjected to preoperative evaluation including careful history taking, clinical examination and basic laboratory investigation. Patients were randomized into two study groups undergo one of two repairs: Desarda tissue-based repair (group I) or Lichtenstein mesh-based repair (group II). Randomization was simple and achieved using sealed envelopes. |
Likely
No blinding. |
Likely
Blinding is not possible.
|
Unclear
Patients were followed for 2 years postoperatively. The first follow-up assessments were performed at 1st and 2nd postoperative weeks by examining the patient in the outpatient clinic and data were collected during interview by an independent observer unaware of the surgical details. However, pain was self-reported and patients were unlikely to be blinded. |
Unlikely
The study was registered at the clinical trials registry of the National Institute of Health (NCT02329938). Authors report additional outcomes compared to the registered outcomes, however, all were logical outcomes to report. |
Unclear
Loss-to-follow-up is balanced between intervention and control group, but reasons for loss-in follow up are not reported. |
Unlikely
All patients were treated as randomized according to flow chart. |
- Randomisation: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomisation process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomisation (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomisation procedures or open allocation schedules.
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the Risk of Bias is unclear.
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the Risk of Bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
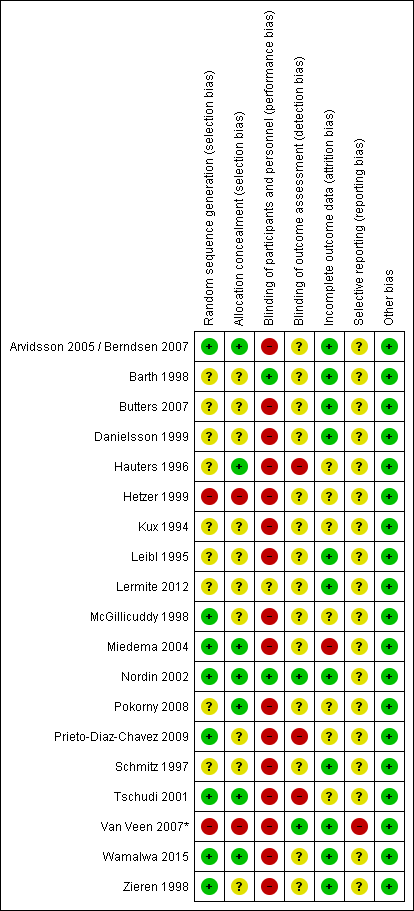
Risk of Bias in RCTs addressing the comparison mesh versus Shouldice (n=19).
For studies included in one of the systematic reviews (Scott, 2001; Lockhart, 2015; Amato, 2012) Risk of Bias judgements of the review authors were copied, however, we made some adjustmens for the sake of consistency (mainly judgements regarding performance bias and reporting bias).
* In the study of Van Veen (2007) patients were randomized to either mesh (Lichtenstein) or non-mesh (surgeon’s choice). Shouldice was among the non-mesh techniques chosen by the surgeon, and was used in 20% of the patients). Although randomisation procedures were adequate (leading to a low Risk of Bias for mesh versus no mesh), we judged Risk of Bias to be high for the comparison mesh versus Shouldice, as the choice for Shouldice is not completely at random.
Forest plots PICO 1
Figure 1. Forest plot mesh versus non-mesh for the outcome 'recurrences' (n = 31). All lengths of follow up combined (from 7 days until 7 years)
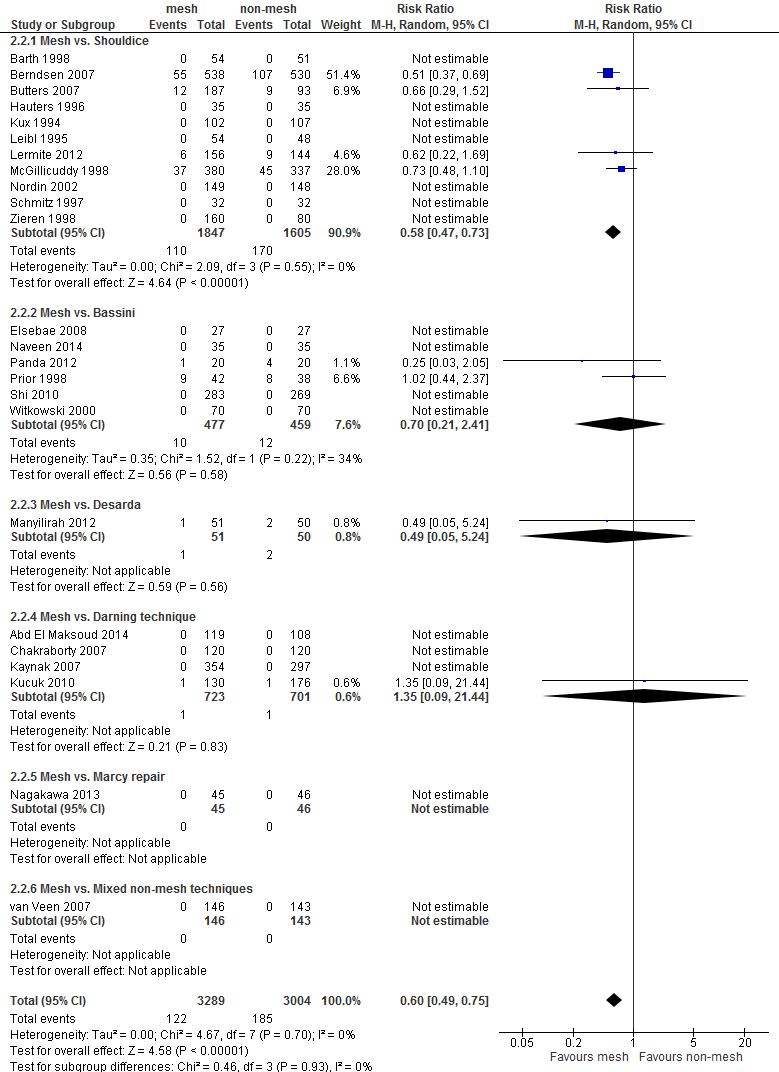
Figure 2. Forest plot mesh versus non-mesh for the outcome 'neurovascular or visceral complications' (n = 24)
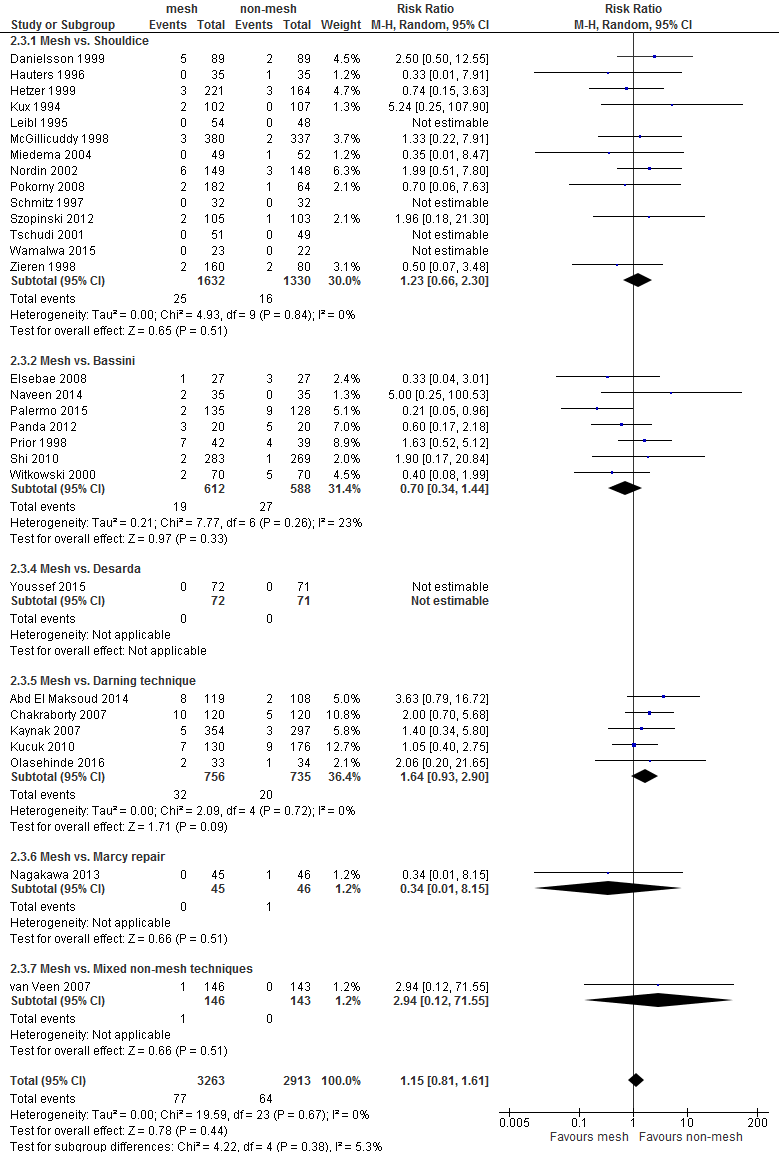
Figure 3. Forest plot mesh versus non-mesh for the outcome 'wound infections' (n = 29)
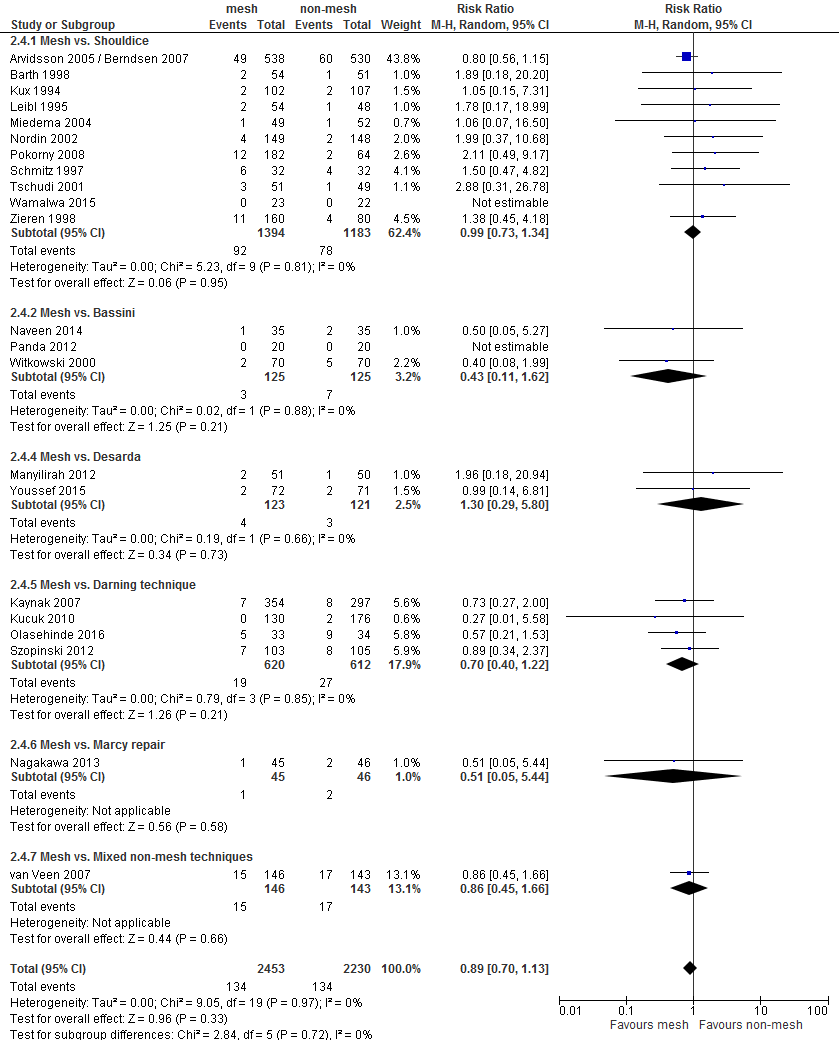
Figure 4. Forest plot mesh versus non-mesh for the outcome 'hematoma' (n = 22)
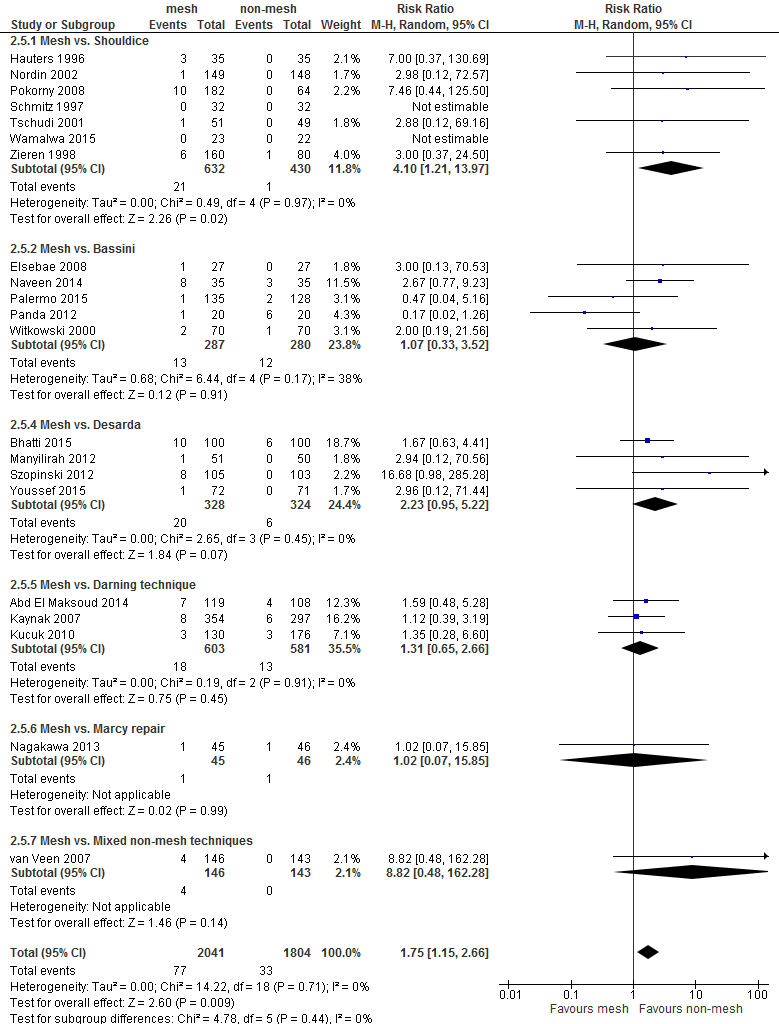
Figure 5. Forest plot mesh versus non-mesh for the outcome 'seroma' (n = 21)
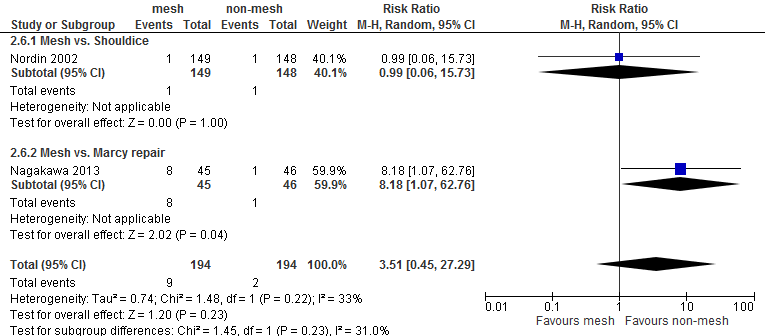
Figure 6. Forest plot mesh versus non-mesh for the outcome 'postoperative swelling of the wound' (n = 2)
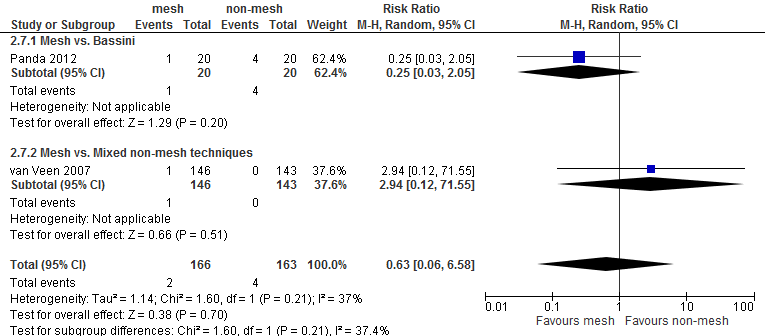
Figure 7. Forest plot mesh versus non-mesh for the outcome ‘rupture of scar tissue’ (n = 2)
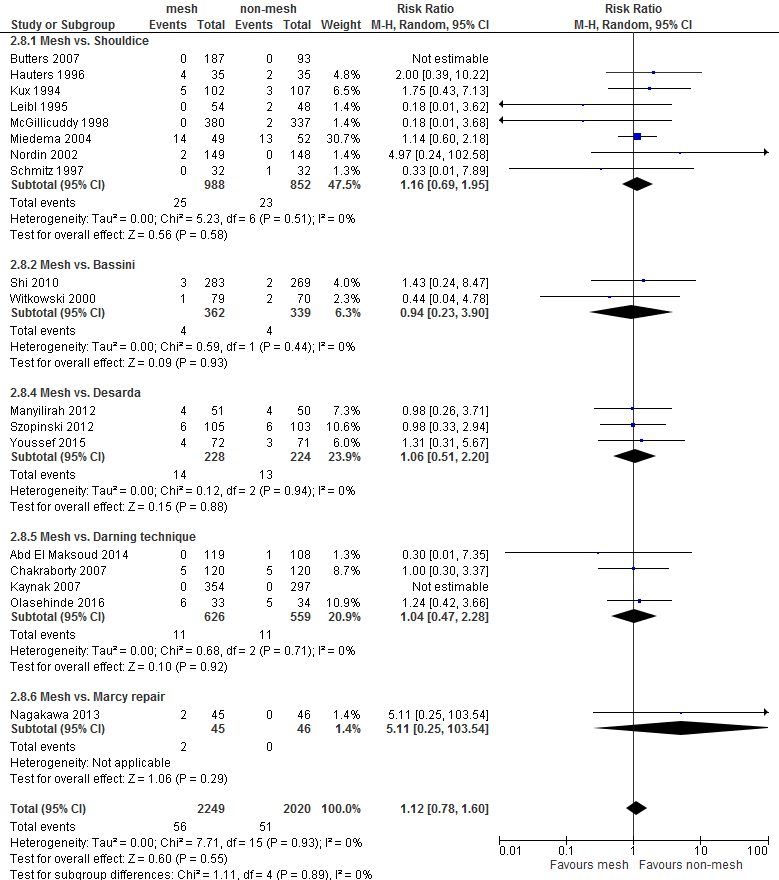
Figure 8. Forest plot mesh versus non-mesh for the outcome 'testicular complications' (n = 19)
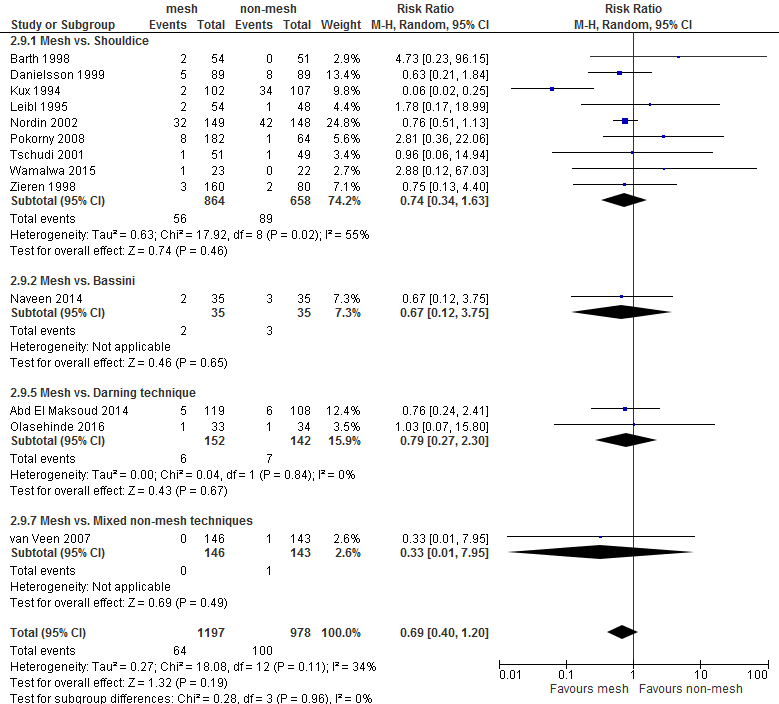
Figure 9. Forest plot mesh verus non-mesh for the outcome 'urinary retention' (n = 12)
GRADE profile
Question: Mesh compared to Shouldice for inguinal hernias; Bibliography: Amato 2012; Lockhart (draft update Cochrane SR of Scott, 2001); Pokorny, 2008; Tschudi, 2001; Wamalwa, 2015
|
Certainty assessment |
№ of patients |
Effect |
Certainty |
Importance |
||||||||
|
№ of studies |
Study design |
Risk of Bias |
Inconsistency |
Indirectness |
Imprecision |
Other considerations |
mesh |
Shouldice |
Relative |
Absolute |
||
|
Recurrence (follow up: range 4 weeks to over 5 years; assessed with: various methods) |
||||||||||||
|
16 |
randomised trials |
serious a |
not serious |
not serious |
serious b |
none |
50/2225 (2.2%) |
78/1798 (4.3%) |
RR 0.43 |
25 fewer per 1.000 |
⨁⨁◯◯ |
CRITICAL |
|
Chronic pain (follow up: range 3 months to over 5 years; assessed with: various methods) |
||||||||||||
|
8 |
randomised trials |
serious a |
not serious |
not serious c |
serious d |
none |
No differences were found between mesh and Shouldice for any follow-up term, except for outcome measurement at 2 years, for which 2 studies (n=1097) reported significantly less pain for mesh compared to Shouldice. |
⨁⨁◯◯ |
CRITICAL |
|||
|
Postoperative pain (assessed with: various methods) |
||||||||||||
|
14 |
randomised trials |
serious a |
serious e |
serious f |
serious b |
none |
In seven RCTs less postoperative pain was found for mesh compared to Shouldice. In the remaining RCTs no differences were found between mesh and Shouldice. |
⨁◯◯◯ |
IMPORTANT |
|||
CI: Confidence interval; RR: Risk ratio
Explanations
- Unclear methods of randomization in half of the RCTs; unclear or high risk of detection bias in almost all RCTs; in addition, unclear risk of attrition bias and reporting bias in several RCTs
- Optimal information size not reached.
- Various methods of outcome measurement used (selfreport and/or physical examination), no downgrading.
- Optimal information size not reached and confidence intervals include both benefit and harm for most follow-up terms.
- Heterogeneity in results: less postoperative pain for mesh in seven RCTs, and no difference between mesh and Shouldice in seven remaining RCTs.
- Heterogeneity in outcome measurement methods and timepoints.
Evidence-tables PICO 2-6
Research questions PICO 2-6
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Magnusson 2016
(PICO2) |
Type of study: RCT
Setting: Hospital/free living
Country: Sweden
Source of funding: Swedish Research Counsil, The Erling-Persson Family Foundation Sweden. Authors declare that there are no conflicts of interest. |
Inclusion criteria: Male, age 18-75, scheduled for daycare surgery under local anesthesia
Exclusion criteria: Impaired cognitive function, substance abuse, limited mobility, limited capacity to communicate in Swedish
N total at baseline: L: 108 PHS: 99 UHS: 102
Important prognostic factors2: Sex: All male sample
Number in pain at baseline, n (%): L: 67/102 (62%) PHS: 65/99 (66%) UHS: 65/102 (64%)
Median age (IQR): L: 60 (49-64) PHS: 58 (48-63) UHS: 59 (46-66)
Median BMI (IQR): L: 24.6 (23-26) PHS: 24.8 (23.2-26.3) UHS: 25 (23-26.5)
Heavy workload in occupation, n (%): L: 28/109 (26%) PHS: 37/99 (37%) UHS: 25/102 (25%)
Groups comparable at baseline? Yes, however larger proportion heavy work in occupation in the PHS group. |
Describe intervention (treatment/procedure/test):
Prolene Hernia System (PHS) under local anesthesia
Or,
UltraPro Hernia System (UHS) under local anesthesia
|
Describe control (treatment/procedure/test):
Lichtenstein with a standard polypropylene mesh under local anesthesia. |
Length of follow-up: 12/24/36 months
Loss-to-follow-up: At 3 years: n=27 (8%)
Incomplete outcome data: Lichtenstein: At 3 months: N=8 (8%); At 6 months: N=13 (13%); At 12 months: N=5 (6%); At 24 months: N=22 (21%); At 36 months: N=15 (15%)
PHS: At 3 months: N=12 (12%); At 6 months: N=11 (11%); At 12 months: N=4 (4%); At 24 months: N=12 (12%); At 36 months: N=7 (8%)
UHS: At 3 months: N=12 (12%); At 6 months: N=12 (12%); At 12 months: N=7 (7%); At 24 months: N=21 (21%); At 36 months: N=4 (4%)
No reasons for incomplete data provided. |
Outcome measures and effect size (include 95%CI and p-value if available):
Participants in pain at 12/24/36 months, n(%): L: 15 (15%) / 7 (8%) / 6 (6%) PHS: 12 (12%) / 12 (14%) / 6 (7%) UHS: 13 (13%) / 12 (15%) / 9 (9%) No sign. differences between groups (no p-value reported)
Median pain at 12 months in rest, VAS-score (IQR): L: 0.2 (0-0.6) PHS: 0.2 (0-1.9) UHS: 1 (0.2-2.5) No significant difference between groups reported, No p-value reported
Median pain at 24 months in rest, VAS-score (IQR): L: 0.3 (0.1-0.7) PHS: 0.3 (0.1-0.8) UHS: 0.8 (0.4-1.3) No significant difference between groups reported, No p-value reported/
Median pain at 36 months in rest, VAS-score (IQR): L: 0.4 (0.2-1.7) PHS: 0.2 (0.1-2.3) UHS: 1.6 (0.7-4.6) No significant difference between groups reported, No p-value reported/
Median pain at 12 months in activity, VAS-score (IQR): L: 0.5 (0.2-3.1) PHS: 0.5 (0.3-3) UHS: 2.3 (0.7-2.9) No significant difference between groups reported, No p-value reported/
Median pain at 24 months in activity, VAS-score (IQR): L: 0.7 (0.4-1.5) PHS: 0.9 (0.4-2.1) UHS: 1.2 (0.4-2.6) No significant difference between groups reported, No p-value reported/
Median pain at 36 months in activity, VAS-score (IQR): L: 0.6 (0.2-1.7) PHS: 0.4 (0.2-2.3) UHS: 2 (1.4-3) No significant difference between groups reported, No p-value reported |
L= Lichtenstein PHS= Prolene Hernia System UHS= UltraPro Hernia System
Sample characteristics from: Magnusson J, Nygren J, Thorell A (2012) Lichtenstein, prolene hernia system, and UltraPro Hernia System for primary inguinal hernia repair: one-year outcome of a prospective randomized controlled trial. Hernia 16(3):277–285 |
|
Čadanová 2016
(PICO2) |
Type of study: RCT
Setting: Hospital/outpatient clinic/free living
Country: Netherlands
Source of funding: Not reported in the manuscript.
|
Inclusion criteria: ASA status I-III, adults, unilateral primary hernia, signed informed consent
Exclusion criteria: Incarcerated hernia, recurrent hernia, local inguinal inflammation, ASA≥4, previous inguinal or preperitoneal surgery, inadequacy for follow-up
N total at baseline: Intervention: 116 Control: 122
Important prognostic factors2: age ± range: I: 57.2 (54.5-59.9) C: 59 (56.5-61.4)
Sex: I: 110/116 (94.8% M) C: 114/122 (93.4% M)
Mean BMI (range): I: 25.1 (24.5-25.7) C: 25.7 (25.1-26.3)
ASA status: I: I: 55.2%; II: 38.8%; III: 6% C: I: 53.3%; II: 41%; III: 5.7%
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
Inguinal hernia repair using the ProGrip technique
|
Describe control (treatment/procedure/test):
Inguinal hernia repair using the transinguinal preperitoneal technique with a PolySoft mesh |
Length of follow-up: 14 days/ 3 months / 1 year
Loss-to-follow-up: N=20 were excluded for analyses: declined surgery, did not show up for first follow-up visit, did not receive the assigned treatment. Not mentioned to what groups the excluded cases were allocated. Follow-up participation rate declined from 99.6% (2 weeks) to 62.6% (1 year). Loss to follow-up of 38.4% over the course of a year.
Incomplete outcome data: Recurrence at 3mo: I: 24/116 (20.6%) C: 26/122 (21.3%) No reasons given
Recurrence at 1y: I: 43/116 (37%) C: 46/122 (37.7%) No reasons given
Complications unclear.
VAS pain 3mo: I: 25/116 (21.5%) C: 28/122 (22.9%) No reason given
VAS pain 1y: I: 44/116 (37.9%) C: 47/122 (38.5%) No reasons given
LANSS at 3mo: I: 25/116 (21.5%) C: 26/122 (21.3%) No reasons given
LANSS at 1y: I: 45/116 (38.7%) C: 46/122 (37.7%) No reasons given |
Outcome measures and effect size (include 95%CI and p-value if available):
Recurrence at 2 weeks: I: 0/115 (0%) C: 1/121 (0.8%) No sign. differences between groups (p=0.331)
Recurrence at 3 months: I: 1/92 (1.1%) C: 1/96 (1%) No sign. differences between groups (p=0.976)
Recurrence at 1 year: I: 2/73 (2.7%) C: 1/76 (1.3%) No sign. differences between groups (p=0.536)
Complications according to patients at 2 weeks, n (%): I: 57/113 (50.4%) C: 39/120 (32.5%) Sign. differences between groups (p=0.005)
Complications according to patients at 3 months, n (%): I: 19/91 (20.9%) C: 12/94 (12.8%) No sign. differences between groups (p=0.140)
Complications according to patients at 1 year, n (%): I: 10/72 (13.9%) C: 11/75 (14.7%) No sign. differences between groups (p=0.893)
Pain in rest at 3 months (>VAS3), n(%): I: 12/91 (13.2%) C: 4/94 (4.3%) Sign. difference (p=0.031)
Pain in rest at 1 year (>VAS3), n(%): I: 3/72 (4.3%) C: 5/75 (6.7%) No sign. difference (p=0.504)
Pain in ADL at 3 months (>VAS3), n(%): I: 15/91 (16.5%) C: 4/94 (4.3%) Sign. difference (p=0.006)
Pain in ADL at 1 year (>VAS3), n(%): I: 3/72 (4.2%) C: 6/75 (8%) No sign. difference (p=0.332)
Pain in sport/heavy lifting at 3 months (>VAS3), n(%): I: 19/91 (20.9%) C: 3/94 (3.2%) Sign. difference (p<0.001)
Pain in sport/heavy lifting at 1 year (>VAS3), n(%): I: 3/72 (4.2%) C: 6/75 (8%) No sign. difference (p=0.332)
Number of participants in pain at rest at 1 year, n (%): I: no pain (VAS 0): 57/72 (79%); mild pain (VAS 1-3): 13/72 (18.1%), moderate pain (VAS 4-6): 2/72 (2.8%), severe pain (VAS >7): 0 (0%) C: no pain (VAS 0): 60 (80%); mild pain (VAS 1-3): 12/75 (16%), moderate pain (VAS 4-6): 3/75 (4%), severe pain (VAS >7): 0 (0%) No sign. differences between groups (p=0.624)
Number of participants in pain in ADL at 1 year, n (%): I: no pain (VAS 0): 62 (83.3%); mild pain (VAS 1-3): 10/72 (13.9%), moderate pain (VAS 4-6): 2/72 (2.8%), severe pain (VAS >7): 0 (0%) C: no pain (VAS 0): 60 (80%%); mild pain (VAS 1-3): 13/75 (17.3%), moderate pain (VAS 4-6): 1/75 (1.3%), severe pain (VAS >7): 1 (1.3%) No sign. differences between groups (p=0.494)
Number of participants in pain in sport/ heavy lifting at 1 year, n (%): I: no pain (VAS 0): 55/75 (73.3%); mild pain (VAS 1-3): 14/75 (18.6%), moderate pain (VAS 4-6): 3/75 (4%), severe pain (VAS >7): 0 (0%) C: no pain (VAS 0): 59 (78.7%); mild pain (VAS 1-3): 12/75 (16%), moderate pain (VAS 4-6): 3/75 (4%), severe pain (VAS >7): 1 (1.3%) No sign. differences between groups (p=0.494)
LANSS neuropathic pain at 3 months, n (%): I: not neuropathic (<12): 72/91 (79.1%); neuropathic (>12): 19/91 (20.9%) C: not neuropathic (<12): 94/96 (97.9%); neuropathic (>12): 2/96 (2.1%) Sign. differences between groups (p<0.001)
VDS pain at 3 months: I: <moderate pain: 83/91 (91.2%); ≥ moderate pain: 8/91 (8.8%) C: <moderate pain: 89/94 (94.7%); ≥ moderate pain: 5/94 (5.3%) No sign. differences between groups (p=0.356)
VDS pain at 1 year: I: <moderate pain: 68/72 (94.4%); ≥ moderate pain: 4/72 (5.6%) C: <moderate pain: 71/75 (94.6%); ≥ moderate pain: 4/75 (5.4%) No sign. differences (p=0.953)
LANSS neuropathic pain at 1 year, n (%): I: not neuropathic (<12): 60/71 (84.5%); neuropathic (>12): 11/71 (15.5%) C: not neuropathic (<12): 68/76 (89.5%); neuropathic (>12): 8/76 (10.5%) No sign. differences between groups (p=0.37) |
|
|
Karaca 2013
(PICO2) |
Type of study: RCT
Setting: Hospital/free living
Country: Turkey
Source of funding: Not stated in manuscript |
Inclusion criteria:
Exclusion criteria: Systemic disease, Gilbert type 7 and 8 hernia
N total at baseline: L: 50 RR: 50 PHS: 50
Important prognostic factors2: age ± SD: L: 53.06 (13.03) RR: 51.69 (14.66) PHS: 48.06 (17.27)
Sex: L: 86% M RR: 92% M PHS: 86% M
Mean BMI (SD): L: 26.45 (4.94) RR: 24.22 (2.71) PHS: 25.06 (3.71)
Groups comparable at baseline? Differences in age. |
Describe intervention (treatment/procedure/test):
Rutkow-Robbins onlay method using a premade Rutkow plug hernia sac.
Or,
Gilbert double layer graft (Prolene Hernia System, PHS)
|
Describe control (treatment/procedure/test):
Lichtenstein procedure with polypropylene mesh (6x11 cm) |
Length of follow-up: Patients were followed 4-9 years
Loss-to-follow-up: Not described
Incomplete outcome data: Late complications, PHS group: n=2 (4%), no reasons given.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Early complications, n (%) L: 1/50 (2%) RR: 5/50 (10%) PHS: 3/50 (6%) Sign. difference between groups (p=0.033)
Late complications, n (%): L: 5/50 (10%) RR: 4/50 (8%) PHS: 5/48 (10.4%) No sign. difference between groups (p=0.924)
1y recurrence L: 0 RR: 0 PHS: 0 No difference between groups (p-value not reported) |
Randomization in 3 groups by throwing heads/tail.
Definition of time in early and late complications is unclear.
Pain scores not extraced because the follow-up for pain was shorter than 3 months (i.e. 1/7/30 days).
L= Lichtenstein RR= Rutkow-Robbins PHS = Prolene hernia system |
|
Mahmoudvand 2017
(PICO3) |
Type of study: parallel RCT
Setting: Lorestan University of Medical Sciences, Khorramabad, Iran
Country: Iran
Source of funding: not reported |
Inclusion criteria: having direct hernia with defects in the posterior wall, being a candidate for classic herniorrhaphy, being a candidate for preperitoneal herniorrhaphy, and satisfaction to enter the study
Exclusion criteria: diabetes, bleeding disorders, and aspirin and corticosteroid consumption
N total at baseline: Intervention: 75 Control: 75
Important prognostic factors2:
Sex: I: 64% M C: 54.7% M
Groups comparable at baseline? Unclear, only sex was described |
Describe intervention (treatment/procedure/test):
Lichtenstein Patients underwent a surgical repair in inguinal hernia with classic method (Lichtenstein) under spinal anesthesia.
The surgeon incised the skin and subcutaneous tissue of the lower part of the abdomen and then the fascia of Scarpa and the roof of the inguinal canal. After reinforcement of the posterior wall of the inguinal canal, the Mersilene mesh (7.5 × 10 cm) was placed and fixed using Round nylon stitch 3/0 to the edges of the defect or weakness in the posterior wall.
|
Describe control (treatment/procedure/test):
Preperitoneal method Patients underwent a surgical repair in inguinal hernia with preperitoneal method under spinal anesthesia.
The surgeon incised the skin and subcutaneous tissue of the lower part of the abdomen and then the fascia of Scarpa and the roof of the inguinal canal. after acquiring the posterior wall of the inguinal canal, the Mersilene mesh (7.5 × 10 cm) was placed and fixed using Round nylon stitch 3/0 under the posteriorwall and then was rehabilitated based on modified Bassini repair method.
|
Length of follow-up: 6-12 months
Loss-to-follow-up: Intervention: 0 Control: 0
Incomplete outcome data: Intervention: 0 Control: 0
|
Outcome measures and effect size (include 95%CI and p-value if available):
Morbidity Pain after surgery I: 21 C: 9 P=0.014
Postoperative hematoma I: 7 C: 9 P=0.597
Postoperative seroma I: 8 C: 1 P=0.034
Mortality Not reported.
Recurrence I: 10 C: 2 P=0.016 |
Author’s conclusion It seems that the preperitoneal method is a more suitable method for inguinal herniorrhaphy than the classic one because of fewer complications, according to the findings of this study. It should be noted that the determination of the type of operation needs a lot of benchmarks, and medical staffs should perform the most appropriate procedure according to all aspects to treat the patients. |
|
Andresen 2015
(PICO3) |
Type of study: parallel RCT
Setting: five surgical departments in Denmark. The participating centers had to have one or two designated surgeons that were experienced with the Lichtenstein technique ((40 procedures) and had performed a minimum of 10 Onstep procedures following a standard training program with hands-on proctoring.
Country: Denmark
Source of funding: not reported |
Inclusion criteria: Participants had to be adult, male patients,>18 years, diagnosed with a primary inguinal hernia that required surgical intervention and with no contraindications against general anesthesia.
Exclusion criteria: not being able to understand Danish, requiring an emergency procedure, having recurrent inguinal hernia, having an ASA score of more than 3, suffering from incarcerated or irreducible hernia, suffering from local (site of surgery) or systemic infection, having a contralateral hernia that had to be operated at the same time or planned operated during follow-up (1 year), suffering from other abdominal hernias being operated at the same time or planned operated during the follow-up period, having had previous surgery that had impaired the sensation in the groin area, having a body mass index (BMI) more than 40 kg/m2 or lower than 20 kg/m2, having a daily intake of alcohol larger than five units (1 unit = 12 g pure alcohol), having a disease that impaired central or peripheral nerve function, suffering from concurrent malignant disease, being diagnosed with cognitive functional impairment (e.g., dementia), suffering from chronic pain that required daily pain medication, or suffering from mental disorder that required medication.
N total at baseline: Intervention: 144 Control: 146
Important prognostic factors2:
age ± SD: I: 53.8 (14.6) C:54.5 (15.2)
BMI ± SD: I: 24.8 (2.6) C: 24.7 (2.9)
Current smoker: I: 38 (26.4%) C: 32 (21.9%)
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
Lichtenstein Lichtenstein repair of inguinal hernia where a Softmesh (Bard, Davol Inc., Warwick RI) was used. The Lichtenstein repair had to be performed according to the guidelines published by the Danish Hernia Database.
|
Describe control (treatment/procedure/test):
Onstep This procedure is an open procedure where access to the inguinal canal is made through a 4-cm horizontal incision lateral to the rectus muscle and cranial to the pubic bone. A Polysoft mesh (Bard, Davol Inc., Warwick RI) is then inserted with the medial part of the mesh in the preperitoneal space and the lateral part surrounding the spermatic cord and placed between the oblique muscle layers. Three sutures are used to close the mesh around the cord, but no sutures are placed in the tissue to fixate the mesh.
|
Length of follow-up: 10 and 30 days
Loss-to-follow-up: Intervention: 0 Control: 0
Incomplete outcome data: Intervention: 3 2 (%) Reasons: 1 recurrence, 1 did not respond to phone call, 1 because of pain
Control: 7 (4.7%) Reasons: 3 recurrence, 1 did not respond to phone call, 1 withdrew due to personal reasons, 1 did not want to continue due to back pain, 2 did not want to continue in study after conversion to Lichtenstein.
Analysed at 30 days: I:141 C: 139
|
Outcome measures and effect size (include 95%CI and p-value if available):
Morbidity
Complications classified according to the Clavien-Dindo classification (I/ II/ IIIa/ IIIb) I: 5/ 7/ 1/ 1 (out of the 148) C: 2/ 8/ 0/ 1 (out of the 142)
Pain at rest at 1st postoperative day, median (range) I: 18 (0–100) C: 16 (0–100) p = 0.778
Pain at rest at day 30 I: 0 (0-4) C: 0 (0-6) P=0.38
Mortality Not reported.
Recurrence within 30 days I: 1 C: 3 P=0.30 |
Author’s conclusion The Onstep technique for inguinal hernia repair was safe and with comparable results to the Lichtenstein repair regarding short-term pain and postoperative complications. Further follow-up of the patients will report levels of chronic pain and sexual dysfunction. |
|
Andresen 2017
(PICO3) |
See Andresen 2015 (above) |
See Andresen 2015 (above) |
See Andresen 2015 (above)
|
See Andresen 2015 (above)
|
Length of follow-up: 6 and 12 months
Loss-to-follow-up: Intervention: 0 Control: 1 (0.7%) Reason coordinating center did not receive info.
Incomplete outcome data: Intervention: 27 (18.8%) Reasons: recurrence before day 30 (n=1), 6 months questionnaire not received (n=13), excluded few days after surgery due to pain (n=1), recurrence (n=4), 12 months questionnaire not received (n=6), disabling chronic pain at 6 months (n=2)
Control: 26 (17.8%) Reasons (describe) withdrew after surgery due to back pain (n=1), withdrew after converted Lichtenstein (n=2), withdrew, personal reasons (n=1), - Recurrence (n=2), 12 months questionnaire not received (n=9)
Analysed at 6 months: I:129 C: 130
Analysed at 12 months: I:117 C: 119 |
Outcome measures and effect size (include 95%CI and p-value if available):
Morbidity Pain at 6 months at rest, median (IQR) I: 0 (0-2) C: 0 (0-2) P=0.67
Pain at 12 months at rest, median (IQR) I: 0 (0-1) C: 0 (0-2) P=0.10
Mortality Not reported.
Recurrence within 12 months I: 5/124 C: 6/125 P=0.78
|
Author’s conclusion This study found no difference between the ONSTEP and the Lichtenstein repairs of primary inguinal hernia in men regarding chronic pain. Both the number of patients with impairment of daily function measured by AAS and comparison of VAS for pain during rest and movement showed no differences between groups. Two patients from the Lichtenstein group and none in the ONSTEP group were diagnosed with disabling chronic pain. |
|
Azeem 2015 (PICO3) |
Type of study: RCT
Setting: Hospital
Country: Pakistan
Source of funding: not reported
|
Inclusion criteria: Inguinal hernia
Exclusion criteria: Recurrent hernia, BMI >40, type IV hernia
N total at baseline: Intervention: 87 Control: 89
Important prognostic factors2: age ± SD: I: 50.01 (13.22) C: 49.4 (12.06)
Sex (M/F): I: 85/2 C: 88/1
Mean BMI (SD): I: 25.01 (2.9) C: 24.9 (2.02)
ASA classification, n: I: I: 57, II: 23, III: 7 C: I: 61, II: 19, III: 9
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
Lichtenstein’s repair for inguinal hernia |
Describe control (treatment/procedure/test):
Modified Kugel repair for inguinal hernia |
Length of follow-up: 2 and 4 months (pain), 2 year (recurrence rate)
Loss-to-follow-up: Intervention: N=1 (1.1%) Reason: not provided
Control: N=1 (1.1%) Reason: not provided
N=2 who were lost to follow up were excluded from analyses.
Incomplete outcome data: Not described other than loss-to follow-up.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Chronic pain at 4 months: not clearly deducible from description or figures in manuscript.
Complications at unclear follow-up, n: I: 23 C: 5 Significant difference between groups (p<0.005)
Recurrence at 2 years, n I: 3 C: 0 Significant difference between groups (p<0.05) |
|
|
Bansal 2016
(PICO4) |
Type of study: RCT
Setting: hospital
Country: India
Source of funding: not reported in manuscript
|
Inclusion criteria: Age 18-60, primary uncomplicated uni/bilateral inguinal hernia
Exclusion criteria: Female, previous surgery in inguino-scrotal region, recurrent or complicated inguinal hernia, orchiectomy, co-morbidities: coronary artery disease / uncontrolled hypertension and DM / chronic bronchitis / renal and hepatic failure, unfir for general anaesthesia, uncontrolled coagulopathy, not giving consent
N total at baseline: Intervention: 80 Control: 80
Important prognostic factors2: age ± SD: I: 40.9 (12.3) C: 40 (12.5)
Sex: All male sample
BMI, kg/m2 (SD): I: 24.1 (2) C: 24 (2)
Unilateral hernia, n (%): I: 58 (72.5%) C: 46 (57.5%)
Groups comparable at baseline? |
Describe intervention (treatment/procedure/test):
Trans-abdominal preperitoneal repair for inguinal hernia (TAPP) |
Describe control (treatment/procedure/test):
Totally extraperitoneal repair for inguinal hernia (TEP) |
Length of follow-up: 3 and 6 months
Loss-to-follow-up: 11.3% of the participants did not complete the 6-months follow-up and were lost.
Incomplete outcome data: None reported other than known to low-to-folow-up
|
Outcome measures and effect size (include 95%CI and p-value if available):
Mean chronic pain at 6 months, VAS (SD): I: 0.87 (0.6) C: 0.86 (0.7) No sign. difference between groups (p=0.86). Measure of dispersion not described. |
|
|
Salma 2015 (PICO5) |
Type of study: RCT
Setting: Hospital
Country: Pakistan
Source of funding: Not stated in manuscript
|
Inclusion criteria: Male, age >30,direct hernia, ASA I or II
Exclusion criteria: Contraindications to pelvic laparoscopy, history of repair with mesh, recurrent hernia, previous pelvic surgery, transvesical prostatectomy, hepatitis B or C
N total at baseline: Intervention: 30 Control: 30
Important prognostic factors2: No baseline characteristics per group were presented. age ± SD: n=60: 61.46 (7)
Sex: All male sample
Groups comparable at baseline? unclear |
Describe intervention (treatment/procedure/test):
Lichtenstein’s repair (classic)
|
Describe control (treatment/procedure/test):
Trans-abdominal preperitoneal repair (TAPP), 3-port technique |
Length of follow-up: Total follow-up is unclear. Pain measured 4 hours postoperatively.
Loss-to-follow-up: None described
|
Outcome measures and effect size (include 95%CI and p-value if available):
Pain at 4h PO, VAS (SD): I: 6.23 (1.87) C: 4.43 (1.59) Sign. difference (p=0.005).
|
Patients received an analgesic (diclofenic sodium, 75mg I/M) immediately after surgery and 6h after surgery. No preoperative or further postoperative analgesics were provided. |
|
Westin 2015 (PICO5) |
Type of study: RCT
Setting: Hospital
Country: Sweden
Source of funding: Uppsala-Örebro Regional Research council, Stockholm County council, Swedish Society of Medicine, Olle Engqvist Research Foundation.
|
Inclusion criteria: Age 20-80, primary unilateral hernia
Exclusion criteria: Female, <20 years, >80 years, ASA ≥3, bilateral / scrotal / recurrent hernia, previous surgery in lower abdomen (except appendectomy)
N total at baseline: Intervention: 195 Control: 194
Important prognostic factors2: Mean age (range): I: 53.2 (27-77) C: 52.9 (23-79)
Sex: All male sample
BMI, kg/m2: I: 24.9 C: 26.5
ASA classification, n (%): I: I: 134 (71.7%), II: 51 (27.3%), III: 2 (1.1%) C: I: 124 (66%), II: 62 (33%), III: 2 (1.1%)
Anatomy, n (%): I: indirect: 109 (58.2%), direct 57 (30.5%), femoral: 0, combined: 20 (10.7%), other: 1 (0.5%) C: indirect: 124 (66%), direct 56 (29.8%), femoral: 1 (0.5%), combined: 7 (3.8%), other: 1 (0.5%)
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
Lichtenstein’s procedure for inguinal hernia repair |
Describe control (treatment/procedure/test):
Totally extraperitoneal procedure for inguinal hernia repair (TEP) |
Length of follow-up: 1 year
Loss-to-follow-up: Intervention: N=4 Reasons: no response (n=2), declined further participation (n=2)
Control: N=5 Reasons: deceased (n=1), no response (n=3), declined further participation (n=1)
Incomplete outcome data: Intervention: N=4 Reasons: excluded from analysis, protocol violation before surgery
Control: N=1 Reasons: excluded from analyses, protocol violation before surgery |
Outcome measures and effect size (include 95%CI and p-value if available):
Pain after 1 year (% of participants): Persistent pain, n (%): I: 62 (33.2%) C: 39 (20.7%) Sign. difference (p=0.007)
IPQ no pain (% estimated from figure) I: 65% C: 77% Sign difference (p=0.007)
IPQ ignorable pain (% estimated from figure) I: 22% C: 14% Sign difference (p=0.011)
IPQ non-ignorable pain (% estimated from figure) I: 7% C: 5% No sign difference (p-value not reported
IPQ pain affecting concentration and activities (% estimated from figure) I: 2% C: 1% No sign difference (p-value not reported
IPQ pain affecting most activities (% estimated from figure) I: <1% C: <1% No sign difference (p-value not reported
Recurrence, n: I: 4 C: 2 No sign. differences (p-value not reported) |
Complications not reported, although this was an outcome measure reported in the methods. |
|
Waris 2016 (PICO5) |
Type of study:RCT
Setting: Hospital
Country: Pakistan
Source of funding: Not reported in manuscript
|
Inclusion criteria: Inguinal hernia
Exclusion criteria: Obstructed hernia
N total at baseline: Intervention: 200 Control: 200
Important prognostic factors2: Not reported for groups separately
Mean age: 33.89 (SD: 13.45)
Sex (M/F): 304/96
Groups comparable at baseline? Unclear |
Describe intervention (treatment/procedure/test):
Lichtenstein’s procedure
|
Describe control (treatment/procedure/test):
Totally Extraperitoneal repair (TEP) |
Length of follow-up: Unclear, pain measurement on 1st and 2nd day postoperatively
Loss-to-follow-up: None described
Incomplete outcome data: None described
|
Outcome measures and effect size (include 95%CI and p-value if available):
Postoperative pain on day 1 and 2, n(%): I: 20 (10%) C: 8 (4%) Sign difference (p=0.031) |
|
|
Gürbulak 2015 (PICO5) |
Type of study: RCT
Setting: Hospital
Country: Turkey
Source of funding: not reported in the manuscript
|
Inclusion criteria: Age 18-70, unilateral hernia Nyhus type 1/2/3a/3b, ASA I/II
Exclusion criteria: Age <18, age >70, female, femoral / recurrent /bilateral hernia, previous pelvic trauma or surgery, benign prostate hypertrophy, COPD, ASA III or IV, previous incarcerated hernia
N total at baseline: Intervention: 70 Control: 64
Important prognostic factors2: age (range): I: 51.4 (18-70) C: 48.2 (18-70)
Sex: All male sample
Groups comparable at baseline? Unclear |
Describe intervention (treatment/procedure/test):
Lichtenstein’s procedure
|
Describe control (treatment/procedure/test):
Totally extraperitoneal repair (TEP) |
Length of follow-up: Follow-up at 10 days, 3 months and 6 months after discharge (mean hospital stay 1.1 (TEP) and 1.2 (Lichtenstein) days
Loss-to-follow-up: N=14 lost: did not show up for follow-up after surgery. Excluded from all analyses. Unclear what study arm they were allocated to.
Incomplete outcome data: None other than reported as loss-to-follow-up
|
Outcome measures and effect size (include 95%CI and p-value if available):
Intraoperative complications, n: I: 1 C: 2 No sign. differences (p=0.92)
Postoperative complications, n: I: 10 C: 3 No sign. difference (p=0.24)
Recurrence, n: I: 1 C: 1 No sign. difference between groups (p=0.89)
|
|
|
Akgül 2016
(PICO6) |
Type of study: RCT
Setting: Hospital
Country: Turkey
Source of funding: sponsored by Akdeniz University (authors declare no financial interest)
|
Inclusion criteria: Age 18-65, presented between March 2012 and February 2013
Exclusion criteria: Bilateral hernia, recurrent hernia, contralateral hernia, comorbidities (heart failure/ rheumatoid arthritis /severe hypertension, systemic or neurologic diseases altering lower extremity function)
N total at baseline: Intervention: 25 Control:25
Important prognostic factors2: age (range): I: 45.1 (22-64) C: 44.1 (19-64)
Sex: I: 25 M C: 22 M / 3 F
Mean operation time (no measure of dispersion): I: 55 min C: 70 min
Groups comparable at baseline? Unclear, only operation time, age and gender are reported. |
Describe intervention (treatment/procedure/test):
Stoppa repair for inguinal hernia by using a 6cm Pfannenstiel incision (modifiel Stoppa) |
Describe control (treatment/procedure/test):
Totally extraperitoneal repair for inguinal hernia (TEP) |
Length of follow-up: Range: 4-15 months
Loss-to-follow-up: Not described
Incomplete outcome data: Not described
|
Outcome measures and effect size (include 95%CI and p-value if available):
Morbidity (complications), n (%): I:6/25 C: 3/25 Not reported/tested if this was a significant difference between groups |
|
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures.
- Provide data per treatment group on the most important prognostic factors ((potential) confounders).
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls.
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders.
Risk of Bias Table PICO 2-6
|
Study reference
(first author, publication year) |
Describe method of randomisation1 |
Bias due to inadequate concealment of allocation?2
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?4
(unlikely/likely/unclear) |
Bias due to loss to follow-up?5
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?6
(unlikely/likely/unclear) |
|
Magnusson 2016 (PICO2) |
Computer generated information for group allocation was produced. When the patient was under local anaesthesia a sealed envelope was opened containing the group allocation. |
Unlikely
Reason: Sealed envelopes were used, however there was no description of who performed the randomization procedure (i.e. who generated the list and sealed the envelopes) and that the envelopes were opaque. |
Unlikely
Reason: It was stated that the patients did not receive information about which technique was used until after the study. |
Likely
Reason: Surgeons performing the hernia repair can not be blinded for the procedure. |
Unlikely
Reason: It was stated that a designated nurse collected all data and was blinded for the treatment allocation. However, it is unclear if the nurse could tell what procedure was used if they observed the wound/scar tissue from surgery. |
Unlikely
Reason: Outcomes of interest were reported both in the methods and in the results |
Unlikely
Reason: Loss-to-follow-up was low (8%), however it was unclear how the loss-to-follow-up was distributed among the groups. |
Unlikely
Reason: No ITT was performed, however missing data and the loss-to-follow-up were considered small which was deemed unlikely to introduce bias. |
|
Cadanova 2016 (PICO2) |
Patients were randomized by computer generated allocation sequence and stratified by gender, age, BMI, and surgeon. |
Unclear
Reason: no information provided on how the allocation was concealed. |
Unlikely
Reasons: It was stated that the patients remained unaware of the used procedure, although it is not stated whether the procedure was disclosed at a later time in follow-up. |
Likely
Reasons: Surgeons were not blinded for the procedure they conducted. |
Unlikely
Reason: Participants were assessed in the outpatient department by a resident who did not have access to the surgery reports. However, it is unclear if the residents could tell what procedure was used if they observed the wound/scar tissue from surgery. |
Unlikely
Reasons: Outcomes in interest were reported in the methods and results. |
Likely
Reasons: Loss of participation rate (unclear if missing data or loss to follow-up) of 38.4% over the course of 1 year is high. |
Likely
Reason: Loss to follow-up and missing data is large while ITT was not followed. |
|
Karaca 2013 (PICO2) |
Randomization in three groups was performed by throwing heads or tail. |
Likely
Reason: It is not described who performed the randomization. When throwing heads or tail (when done with a coin) the allocation can be predicted while randomizing if there is a consistent method, introducing selection bias. |
Unclear
Reasons: No information provided on blinding |
Likely
Reasons: Surgeons performing a procedure cannot be blinded. |
Unclear
Reason: It is not described who assessed the participants. |
Unlikely
Reasons: Outcomes in interest were reported in the methods and results. |
Unlikely
Reason: no loss to follow-up described |
Unlikely
Reason: No mention of ITT, however no loss-to-follow-up was described and missing data (4%) was seen in only 1 outcome. |
|
Mahmoudvand 2017 (PICO3) |
Not described |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear* |
Unlikely |
Unlikely |
|
Andresen 2015 (PICO3) |
Randomization was done on participant level in blocks of six. Each center received a unique batch of CRFs to stratify on center level. Randomization sequence was generated by an online sequence generator |
Unlikely |
Unlikely |
Likely |
Unlikely |
Unlikely |
Unlikely |
Likely |
|
Andresen 2017 (PICO3) |
Randomization was done on participant level in blocks of six. Each center received a unique batch of CRFs to stratify on center level. Randomization sequence was generated by an online sequence generator |
Unlikely |
Unlikely |
Likely |
Unlikely |
Unlikely |
Unlikely |
Likely |
|
Azeem 2017 (PICO3) |
Prospectively randomized; unclear randomization procedure |
Unclear
Reason: no allocation concealment procedure reported. |
Likely
Reason: blinding is not mentioned in the manuscript. It is likely that patients cannot be blinded for the surgical procedure |
Likely
Reason: blinding is not mentioned in the manuscript. Surgeons cannot be blinded for the surgical procedure |
Likely
Reason: blinding is not mentioned in the manuscript. It is likely that the surgeons were the outcome assessors and were not blinded for the surgical procedure |
Unlikely:
Reason: No mention of a trial protocol, however outcomes of interest were mentioned in methods and reported in the results. Complications were reported in the results only. |
Unlikely
Reason: loss of n=2 (1 in each study arm) |
Unlikely
Reason: Although ITT was not followed, no indication of protocol deviations and loss-to-follow-up was n=2. |
|
Bansal 2017 (PICO4) |
Block randomisation was done using computer generated random numbers (size of the blocks were not reported). Allocation was concealed by using sealed opaque envelopes. |
Unlikely
Reason: sealed opaque envelopes were used |
Likely
Reason: although both procedures are endoscopic, it is likely that the patients were aware which procedure was/would be used. |
Likely
Reason: no mention of blinding in the manuscript. It is likely the surgeon could not be blinded for the procedure. |
Likely
Reason: no mention of blinding in the manuscript. It is likely that the surgeon and/or healthcare team were outcome assessors. The surgeon could not be blinded for the procedure. |
Unlikely
Reason: No mention of a trial protocol, however outcomes of interest were mentioned in methods and reported in the results. |
Unclear
Reason: although 11.3% loss is relatively low, it was not described in which arm these participants dropped out or whether the drop-out was the same among the both arms. |
Unlikely
Reason: stated that 160 (80/80) participants were analysed |
|
Salma 2015 (PICO5) |
Simple computer generated randomization |
Unclear
Reason: no mention of allocation concealment or its procedure. |
Likely
Reason: Authors state that blinding was not possible |
Likely
Reason: Authors state that blinding was not possible |
Likely
Reason: Authors state that blinding was not possible |
Unlikely
Reason: No mention of a trial protocol, however outcomes of interest were mentioned in methods and reported in the results. |
Unlikely (pain post-op)
Reason: stated that all 60 patients were analysed for pain (4h after operation). |
Unlikely (pain post-op)
Reason: stated that all 60 patients were analysed for pain (4h after operation). |
|
Westin 2016 (PICO5) |
Block randomization (size 20) in a 1:1 ratio. Consecutively numbered sealed envelopes were used. Method of sequence generation is unclear. |
Likely
Reason: Reported that allocation was not concealed. |
Likely
Reason: No mention of blinding in the manuscript. Patients were likely to know which procedure was/would be conducted |
Likely
Reason: Surgeons are aware of the procedure they are conducting |
Likely
Reasons: Outcome assessors were most likely the surgeons (at 1y follow-up). |
Unclear
Reason: postoperative complications are mentioned in the methods as a secondary outcome measure, but were not reported in the results. (could be described in the short-term paper) |
Unlikely
Reason: loss to follow up is small and similar in both groups |
Unlikely
Reason: although ITT was not followed, protocol deviations were excluded from analyses. This accounted for a small number participants (n=4 in intervention group, n=1 in control group) |
|
Waris 2016 (PICO5) |
Randomly allocated in two groups after matching for confounders |
Unclear
Reason: allocation concealment procedure not described |
Likely
Reason: No mention of blinding in the manuscript. Patients were likely to know which procedure was/would be conducted |
Likely
Reason: Surgeons are aware of the procedure they are conducting |
Likely
Reasons: Outcome assessors were most likely the surgeons |
Likely
Reason: no predefined outcomes were given in the methods. No mention of a research protocol. |
Unlikely
Reason: no loss to follow-up reported |
Unlikely
Reason: no protocol deviations and loss-to-follow-up reported. |
|
Gürbulak 2015 (PICO5) |
Randomly divided in groups using a random number table. |
Unclear
Reason: allocation concealment procedure not described |
Likely
Reason: No mention of blinding in the manuscript. Patients were likely to know which procedure was/would be conducted |
Likely
Reason: Surgeons are aware of the procedure they are conducting |
Likely
Reasons: Outcome assessors were most likely the surgeons |
Unlikely
Reason: No mention of a trial protocol, however outcomes of interest were mentioned in methods and reported in the results. |
Unclear
Reason: although n=14 is relatively small, the drop-out distribution among groups was not described. |
Unclear
Reason: ITT was not followed and it was not described to which groups the n=14 were allocated. No conversions from laparoscopic to open procedure were made. |
|
Akgül 2016 (PICO6) |
Randomization was performed at the presentation of the inguinal hernia. Patients were randomized 1:1 by the primary researcher using a box with opaque sealed envelopes. |
Unlikely
Reason: Opaque sealed envelopes were used |
Likely
Reason: surgery was either Stoppa or laparoscopic. |
Likely
Reason: The primary researcher was the operating surgeon (which also performed the randomization). It was stated that the entire health care team was informed about the procedure. |
Likely
Reason: it was stated that the patients were followed up by the primary researcher / operating surgeon to assess complications and recurrence |
Unlikely
Reason: variables of interest were stated in the protocol and reported in the manuscript. |
Unclear
Reason: there was a variable follow-up time (4-12 month). No information on loss-to-follow-up was provided. |
Unlikely
Reason: Only one potential protocol deviation, which is unlikely to bias the results. When describing the complications, probably all participants were considered. |
*No protocol or registration in trial registry available.
- Randomisation: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomisation process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomisation (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomisation procedures or open allocation schedules.
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the Risk of Bias is unclear.
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the Risk of Bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Evidence-tables PICO 2-6
Systematic reviews
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Wei 2015
Study characteristics and results are extracted from the SR (unless stated otherwise)
(PICO4) |
SR and meta-analysis of RCTs
Literature search up to March 2014
A: Schrenk 1996 B: Dedemadi 2006 C: Butler 2007 D: Pokomy 2008 E: Zhu 2009 F: Hamza 2010 G: Gong 2011 H: Krishna 2012 I: Wang 2013 J: Bansal 2013
Study design: All RCTs
Setting and Country: A: Austria B: Greece C: USA D: Austria E: China F: Egypt G: China H: India I: China J: India
Source of funding: Not reported
|
Inclusion criteria SR: uni- or bilateral inguinal hernia, age<71 years, TAPP and TEP performed, reported outcomes: recurrence / pain / complications / operation time / time to return to activities / hospital stay
Exclusion criteria SR: patients with gastric surgery / previous chemo/radiationtherapy, DVT, cardiovascular / respiratory / hepatic / renal disease
10 studies included
Important patient characteristics at baseline:
N, mean age Age not reported A: 28/24 B: 24/26 C: 22/22 D: 93/36 E: 20/20 F: 25/25 G: 50/50 H: 47/53 I: 84/84 J: 154/160
Sex: Not reported
Hernia type: A: unilateral indirect, direct, recurrent B: uni- and bilateral recurrent C: unilateral D: unilateral E: unilateral nonrecurrent F: unclear indirect G: uni- and bilateral indirect, direct, mixed H: uni- and bilateral, indirect, direct I: uni- and bilateral, indirect, direct, femoral J: uni- and bilateral nonrecurrent
Groups comparable at baseline? Heterogeneity in type of inguinal hernias between studies |
Describe intervention:
Transabdominal preperitoneal repair (TAPP)
|
Describe control:
Totally extraperitoneal repair (TEP) |
End-point of follow-up:
A: Unclear B: Mean: 1087 days ±588 C: Range: 7-14 days D: Range: 2-3 weeks; 3 months; Range: 1-3 years E: Unclear F: Range: 2, 12, 24 weeks G: Mean: 15.6 months ±9.9 / 15.6 months ±7.7 H: Range: 1, 6 weeks; 3, 6, 12, 18, 24, 38 months I: Mean: 16 months, range: 3-32 months J: Mean: 30.1 months ±14.3 / 30.9 months ±13.2
For how many participants were no complete outcome data available? All studies were reported to have more than 80% follow-up data
|
Postoperative pain Pain at 1-h PO: Effect measure: Standardized mean difference (95% CI): H: 1.95 (1.47-2.43) J: 0.14 (-0.09-0.36)
Pooled effect (random effects model): 1.03 (95% CI -0.75 to 2.81) favoring TEP Heterogeneity (I2): 97.8%
Pain at 6-h PO: Effect measure: Standardized mean difference (95% CI): H: -1.36 (-1.79- -0.92) J: 0.18 (-0.04-0.40)
Pooled effect (random effects model): -0.58 (95% CI -2.08 to 0.92) favoring TAPP Heterogeneity (I2): 97.4%
Pain at 1-d PO: Effect measure: Standardized mean difference (95% CI): F: 0.50 (-0.06-1.07) G: -0.14 (-0.53-0.25) H: 2.02 (1.54-2.50) J: 0.18 (-0.04-1.45)
Pooled effect (random effects model): 0.63 (95% CI -0.20 to 1.45) favoring TEP Heterogeneity (I2): 94.5%
Pain at 1-w PO: Effect measure: Standardized mean difference (95% CI): G: 0.00 (-0.39-0.39) H: 1.22 (0.80-1.65)
Pooled effect (random effects model): 0.61 (95% CI -0.59 to1.81) favoring TEP Heterogeneity (I2): 94.2%
Pain at unclear follow-up: Effect measure: Standardized mean difference (95% CI): E: 0.21 (-41, 0,83) I: 1.15 (0.82-1.48)
Pooled effect (random effects model): 0.72 (95% CI -0.21 to 1.48) favoring TEP Heterogeneity (I2): 85.6%
Recurrence No included study for pooling the recurrence had a mean follow- up of >3 years.
Time to return to activities Effect measure: Standardized mean difference (95% CI): B: 0.12 (-44-0.67) F: 0.20 (-0.36-0.75) G: 0.00 (-0.39-0.39) J: -0.29 (-0.51- -0.07)
Pooled effect (random effects model): -0.08 (95% CI -0.33 to 0.16) favoring TAPP Heterogeneity (I2): 33.8%
Costs: G, H, I, J compared the costs of TAPP vs TEP, however found no significant differences (no p-values reported).
|
Facultative:
Brief description of author’s conclusion
Personal remarks on study quality, conclusions, and other issues (potentially) relevant to the research question
Level of evidence: GRADE (per comparison and outcome measure) including reasons for down/upgrading
Sensitivity analyses (excluding small studies; excluding studies with short follow-up; excluding low quality studies; relevant subgroup-analyses); mention only analyses which are of potential importance to the research question
Heterogeneity: clinical and statistical heterogeneity; explained versus unexplained (subgroupanalysis)
|
|
Sajid 2015
Study characteristics and results are extracted from the SR (unless stated otherwise)
(PICO6) |
SR and meta-analysis of RCTs
Literature search up to June 2014
A: Aitola 1998 B: Bostanci 1998 C: Günal 2007 D: Hamza 2010 E: Johanson 1999 F: Kawji 1999 G: Ozmen 2004 H: Simmermacher 2000 I: Sinha 2006 J: Vatansev 2002
Study design: All RCTs
Setting and Country: A: Finland B: Turkey C: Turkey D: Egypt E: Sweden F: Austria G: Turkey H: Netherlands I: India J: Turkey
Source of funding: No funding
|
Inclusion criteria SR: Placement of a mesh in the preperitoneal space through trans-inguinal incision or any other open approach
Exclusion criteria SR: Not reported
10 studies included
Important patient characteristics at baseline:
N (n male), mean age Age not reported A: 49 (48), 55/53 B: 64 (63), 31/25 C: 118 (118), 23/25/22 D: 75 (75), 35/36/34 E: 400 (400), 56/55 F: 56 (unclear), 65/48 G: 80 (75), 41/51 H: 162 (unclear), 54 I: 241 (241), unclear J: 41 (36), 50.7/54.6
Hernia type: A: bilateral, primary, recurrent B: primary C: primary D: primary E: primary, recurrent F: primary G: primary, recurrent H: primary I: recurrent, unilateral, bilateral J: primary, femoral
Groups comparable at baseline? Heterogeneity in type of inguinal hernias between studies |
Describe intervention:
Open preperitoneal hernia repair
A: Trans-inguinal preperitoneal + Marlex mesh + suture mesh fixation B: TIPP + Prolene mesh + suture mesh fixation C: Nyhus + Prolene mesh + fixation not reported D: TIPP + mesh and fixation not reported E: TIPP + Prolene mesh +suture fixation F: Wantz TIPP + mesh and fixation not reported G: Nyhus + prolene mesh + stitch fixation H: Ugahary (gridiron) + Prolene mesh + no fixation I: TIPP + Prolene mesh + no fixation J: Nyhus + mesh and fixation not reported
|
Describe control:
Laparoscopic preperitoneal hernia repair
A: TAPP + Marlex mesh + staple mesh fixation B: TEP + Prolene esh + no fixation reported C: TAPP and TEP + Prolene mesh + staple fixation D: TAPP and TEP + mesh and fixation not reported E: 3-port TAPP + Prolene mesh + staple fixation F: TAPP + mesh and fixation not reported G: TEP + Prolene mesh + tacker fixation H: TEP + Prolene mesh + no fixation I: TEP + Prolene mesh + tacker fixation J: TEP + mesh and fixation not reported
|
End-point of follow-up:
A: 18 months B: 24 months C: 96 months D: 12 months E: 12 months F: 18 months G: NR H: NR I: 12 months J: 1 week
For how many participants were no complete outcome data available? Not reported
|
Recurrence Effect measure: Odds ratio (95% CI): A: 0.61 (0.09-4.01) C: 2.05 (0.12-33.71) D: 0.38 (0.02-8.23) E: 2.85 (0.89-9.11) I: 0.49 (0.09-2.71)
Pooled effect (random effects model): 1.21 (95% CI 0.52 to 2.83) favoring laparoscopic Heterogeneity (I2): 8% N=1003 (Events in I: 16, events in C: 14)
Morbidity (complications) Effect measure: Odds ratio (95% CI): A: 0.33 (0.06-1.90) B: 2.14 (0.36-12.63) C: 2.07 (0.76-5.61) D: 2.09 (0.28-15.76) E: 0.66 (0.43-1.02) F: 5.67 (0.55-58.48) G: 1.79 (0.61-5.22) H: 0.56 (0.22-1.43) I: 0.39 (0.07-2.03)
Pooled effect (random effects model): 1.00 (95% CI 0.60 to 2.03) favoring none Heterogeneity (I2): 40% N=1245 (Events in I: 90, events in C: 111) |
Facultative:
Brief description of author’s conclusion
Personal remarks on study quality, conclusions, and other issues (potentially) relevant to the research question
Level of evidence: GRADE (per comparison and outcome measure) including reasons for down/upgrading
Sensitivity analyses (excluding small studies; excluding studies with short follow-up; excluding low quality studies; relevant subgroup-analyses); mention only analyses which are of potential importance to the research question
Heterogeneity: clinical and statistical heterogeneity; explained versus unexplained (subgroupanalysis)
|
Risk of Bias table PICO 2-6 Systematic reviews
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Wei 2015 (PICO4) |
Yes, however outcomes could be specified more precisely |
Yes, however no complete search strategy is provided. Multiple sources were searched.Date of last search was stated. |
Yes, not referenced but exact number and reasons were provided. |
No, studies were minimally described and no sample description was included or which group received TEP or TAPP. |
NA |
No, there is a “quality assessment table” includeed but the authors seem to indicate only whether information on the criteria was reported and not whether there was a (un)likely Risk of Bias. |
Unclear, because no information was provided on the sample characteristics. Heterogeneity (I2) is large in most analyses. |
Yes, Egger’s test was performed and reported for recurrence, operation time, and total complication.However, no Funnel plots were presented. |
No, no mention of conflicts of interest for the manuscript or its included studies. |
|
No, Egger’s test and funnelplots were not presented for pain and time to return to activities. |
|||||||||
|
Sajid 2015 (PICO6) |
Yes, however complications is not named as an outcome in the research aim. |
Yes, however no complete search strategy is provided. Multiple sources were searched.Date of last search was stated.Unclear how many hits from each database. Number of hits is limited. |
Yes, not referenced but exact number and reasons were provided (however no exclusion criteria were given in the text) |
Yes, minimally described |
NA |
Yes, a quality table (Jadad) and GRADE table are presented. |
Unclear, minimal information on the sample characteristics were given and in both groups several different techniques were combined for meta-analysis (for laparoscopic some subgroup analyses were performed, but not fort he open technique group) |
No, publication bias was not considered |
Unclear, the authors declase that they have no conflict of interest, however this was not checked for the included studies. |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 05-04-2019
Beoordeeld op geldigheid : 11-12-2020
Voor het beoordelen van de actualiteit van deze richtlijn is de werkgroep in stand gehouden. Uiterlijk in 2022 bepaalt het bestuur van de NVvH of de modules van deze richtlijn nog actueel zijn. Op modulair niveau is een onderhoudsplan beschreven. Bij het opstellen van de richtlijn heeft de werkgroep per module een inschatting gemaakt over de maximale termijn waarop herbeoordeling moet plaatsvinden en eventuele aandachtspunten geformuleerd die van belang zijn bij een toekomstige herziening (update). De geldigheid van de richtlijn komt eerder te vervallen indien nieuwe ontwikkelingen aanleiding zijn een herzieningstraject te starten.
De NVvH is regiehouder van deze richtlijn(modules) en eerstverantwoordelijke op het gebied van de actualiteitsbeoordeling van de richtlijn(modules). De andere aan deze richtlijn deelnemende wetenschappelijke verenigingen of gebruikers van de richtlijn delen de verantwoordelijkheid en informeren de regiehouder over relevante ontwikkelingen binnen hun vakgebied.
Algemene gegevens
De richtlijnontwikkeling werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). Patiëntenparticipatie bij deze richtlijn werd medegefinancierd uit de Kwaliteitsgelden Patiënten Consumenten (SKPC) binnen het programma KIDZ.
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijn.
Doel en doelgroep
Doel
Het hoofddoel van de richtlijn is de patiëntresultaten te verbeteren en de meest voorkomende problemen na een liesbreukoperatie te verminderen, met name recidivering en chronische pijn.
Doelgroep
De richtlijn wordt geschreven voor de medisch specialisten die betrokken zijn bij de zorg voor patiënten met liesbreuk.
Samenstelling werkgroep
Werkgroep
- Dr. B. (Baukje) van den Heuvel, chirurg, Pantein Zorggroep, Radboudumc, Nijmegen, NVvH (voorzitter)
- Dr. M.P. (Maarten) Simons, chirurg, OLVG, Amsterdam, NVvH
- Dr. Th.J. (Theo) Aufenacker, chirurg, Rijnstate, Arnhem, NVvH
- Dr. J.P.J. (Ine) Burgmans, chirurg, Diakonessenhuis Utrecht, Utrecht, NVvH
- Mw. R. (Rinie) Lammers, beleidsadviseur, Patiëntenfederatie Nederland, Utrecht
- Dr. M.J.A. (Maarten) Loos, chirurg, Maxima Medisch Centrum, Veldhoven, NVvH
- Dr. M. (Marijn) Poelman, chirurg, Sint Franciscus Vlietland Groep, Rotterdam, NVvH
- Dr. G.H. (Gabriëlle) van Ramshorst, chirurg, NKI-Antoni van Leeuwenhoek Ziekenhuis/VU Medisch Centrum, Amsterdam, NVvH
- Drs. J.W.L.C. (Ronald) Schapendonk, anesthesioloog-pijnspecialist, Diakonessenhuis Utrecht, Utrecht, NVA
- Dr. E.J.P. (Ernst) Schoenmaeckers, chirurg, Meander MC, Amersfoort, NVvH
- Dr. N. (Nelleke) Schouten, AIOS heelkunde regio Maastricht, NVvH
- Dr. R.K.J. (Rogier) Simmermacher, chirurg, UMC Utrecht, Utrecht, NVvH
Met medewerking van
- Drs. W. (Wouter) Bakker, arts-onderzoeker heelkunde, Diakonessenhuis Utrecht, Utrecht
Met ondersteuning van
- Dr. J.S. (Julitta) Boschman, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. W.A. (Annefloor) van Enst, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. Ing. R. (Rene) Spijker, Informatie Specialist, Cochrane Netherlands
- Dr. C. (Claudia) Orelio, Cochrane Netherlands
- Drs. P. (Pauline) Heus, Cochrane Netherlands
- Prof. dr. R. (Rob) Scholten, Cochrane Netherlands
- Dr. L. (Lotty) Hooft, Cochrane Netherlands
- D.P. (Diana) Gutierrez, projectsecretaresse, Kennisinstituut van de Federatie Medisch Specialisten
- J. (Jill) Heij, junior projectsecretaresse, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De KNMG-code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement, kennisvalorisatie) hebben gehad. Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
|
Van den Heuvel |
Chirurg |
Onbetaald: - Advies-commissie Kwaliteit EHS (European Hernia Society) - Dutch Hernia Society - International Guidelines Groin Hernia Management (ontwikkeling gesponsord door BARD en Johnson&Johnson - |
geen (03/03/2017) |
niet van toepassing |
|
|
Simons |
Chirurg |
Onbetaald: - Bestuur EHS (European Hernia Society) - Dutch Hernia Society - International Guidelines Groin Hernia Management (ontwikkeling gesponsord door BARD en Johnson&Johnson |
Lid Board van de European Hernia Society (20/7/2017) |
geen |
|
|
Aufenacker |
Chirurg |
Penningmeester DHS (Dutch Hernia Society), onbetaald |
Prevent Studie, (Preventieve matplaatsing bij aanleggen colostoma) ZonMW gesponsord (19/3/2018) |
geen |
|
|
Bakker |
Chirurg io |
- |
- |
niet van toepassing |
|
|
Burgmans |
Chirurg |
Lid bestuur Dutch Hernia Society |
geen (19/3/2018) |
niet van toepassing |
|
|
Lammers |
Beleidsadviseur |
- |
geen (19/6/2017) |
|
|
|
Loos |
Chirurg |
- |
geen (13/6/2017) |
niet van toepassing |
|
|
Poelman |
Chirurg |
Lid bestuur Dutch Hernia Society |
geen (11/7/2017) |
geen |
|
|
van Ramshorst |
Fellow Chirurgie |
|
Sponsor van mijn fellowship, KWF, heeft geen belangen bij deze richtlijn. Publicaties waar ik auteur van ben zouden gebruikt kunnen worden als referentie. (16/4/2018) |
geen |
|
|
Schapendonk |
Anesthesioloog-pijnspecialist |
|
Niet persoonlijk, maar mijn instelling heeft deelgenomen/ neemt deel aan wetenschappelijk onderzoek gesponsord door Medtronic, Spinal Modulaton of St. Jude Medical thans Abbott. Fee ontvangen van St. Jude Medical voor voordracht op scholing pijnverpleegkundigen inzake DRG stimulatie. Daarnaast in 2015 congres bezocht op kosten Spinal Modulation. (3/5/2017) |
geen |
|
|
Schoenmaeckers |
Chirurg |
|
geen (20/6/2017) |
niet van toepassing |
|
|
Schouten |
AIOS Heelkunde |
- |
geen (6/6/2017) |
niet van toepassing |
|
|
Simmermacher |
Chirurg |
- |
geen (21/5/2017 |
niet van toepassing |
|
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door inbreng van: 1) patiëntenvereniging Meshed-up tijdens de invitational conference; 2) door de deelname van mevrouw. Lammers (Patiëntenfederatie Nederland) in de werkgroep en 3) door het raadplegen van volwassenen behandeld voor liesbreuk via een door de Patiëntenfederatie uitgezette enquête. De reacties naar aanleiding van deze invitational en enquête (zie aanverwante producten) zijn besproken in de werkgroep en de belangrijkste knelpunten zijn verwerkt in de richtlijn.
Methode ontwikkeling
Evidence based
Implementatie
In de verschillende fasen van de richtlijnontwikkeling is rekening gehouden met de implementatie van de richtlijn (module) en de praktische uitvoerbaarheid van de aanbevelingen. Daarbij is uitdrukkelijk gelet op factoren die de invoering van de richtlijn in de praktijk kunnen bevorderen of belemmeren. Het implementatieplan is te vinden bij de aanverwante producten. De werkgroep heeft geen interne kwaliteitsindicatoren ontwikkeld om het toepassen van de richtlijn in de praktijk te volgen en te versterken (zie Indicatorontwikkeling).
Werkwijze
AGREE
Deze richtlijn is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010), dat een internationaal breed geaccepteerd instrument is. Voor een stap-voor-stap beschrijving hoe een evidence-based richtlijn tot stand komt wordt verwezen naar het stappenplan Ontwikkeling van Medisch Specialistische Richtlijnen van het Kennisinstituut van de Federatie Medisch Specialisten.
Knelpuntenanalyse
Tijdens de voorbereidende fase inventariseerden de voorzitter van de werkgroep en de adviseur de knelpunten en onderwerpen beschreven in de internationale richtlijn (HerniaSurge Group 2018) die in aanmerking kwamen voor de Nederlandse adaptatie en update. De aanwezigen tijdens de invitational conference bevestigden deze knelpunten en onderwerpen. Een verslag hiervan is opgenomen onder aanverwante producten.
Uitgangsvragen en uitkomstmaten
De knelpunten en onderwerpen beschreven in de internationale richtlijn (HerniaSurge Group, 2018) die in aanmerking kwamen voor de Nederlandse adaptatie en update zijn met de werkgroep besproken. Daarna heeft de werkgroep de definitieve uitgangsvragen en modules vastgesteld en vastgesteld welke modules volledig zouden worden geupdate met ondersteuning van het Kennisinstituut en welke modules uit de internationale richtlijn zouden worden geadapteerd door de werkgroep. Voor alle modules, ook de modules die geadapteerd zijn, heeft de werkgroep de recente en relevante literatuur doorgenomen. In de geadapteerde modules zijn nieuwe studies verwerkt bij het formuleren van overwegingen en aanbevelingen. In de volledig geupdate modules zijn nieuwe studies geïntegreerd in de literatuuranalyse, risk of bias assessment en gradering. Hieronder is per module aangegeven of de module volledig is ge-update of geadapteerd:
- Risicofactoren (geadapteerd)
- Diagnostiek (geadapteerd)
- Indicatie behandeling asymptomatische liesbreuken (volledig geupdate)
- Chirurgische behandeling unilaterale liesbreuk (volledig geupdate)
- Mat of Shouldice (volledig geupdate)
- Lichtenstein of een andere open anterieure techniek (geadapteerd)
- Lichtenstein of een open pre-peritoneale techniek (geadapteerd)
- Endoscopische techniek (geadapteerd)
- Lichtenstein of een laparo-endoscopische techniek (geadapteerd)
- Een open posterieure techniek of laparo-endoscopisch (geadapteerd)
- Geïndividualiseerde behandeling (geadapteerd)
- Matten (volledig geupdate)
- Matfixatie (volledig geupdate)
- Open anterieure benadering
- TEP/TAPP
- Liesbreuken bij vrouwen (geadapteerd)
- Femoraalbreuken (geadapteerd)
- Antibioticaprofylaxe (volledig geupdate)
- Anesthesie (volledig geupdate)
- Postoperatieve pijn (geadapteerd)
- Chronische pijn
- Definitie, risicofactoren en preventie (geadapteerd)
- Reductie incidentie CPIP (volledig geupdate)
- Behandeling CPIP (volledig geupdate)
- Behandeling van recidief liesbreuk (geadapteerd)
- Na een anterieure benadering
- Na een posterieure benadering
- Na een anterieure en posterieure benadering
- Acute liesbreukchirurgie (geadapteerd)
- Organisatie van zorg (nieuw)
Vervolgens inventariseerde de werkgroep voor de uitgangsvragen van de modules die waren geselecteerd voor een volledige update welke uitkomstmaten voor de patiënt relevant zijn. Er werd zowel naar gewenste als ongewenste effecten gekeken. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal, belangrijk (maar niet cruciaal) en onbelangrijk. Tevens poogde de werkgroep tenminste voor de cruciale uitkomstmaten te definiëren welke verschillen zij klinisch (patiënt) relevant vonden.
Strategie voor zoeken en selecteren van literatuur
De informatiespecialist van Cochrane Nederland doorzocht Medline en Embase (op 11 april 2017) en het Cochrane Register (op 12 april 2017) naar artikelen over de diagnostiek of behandeling van volwassenen met liesbreuk zonder beperkingen op de publicatiedatum. Dit betrof een herhaling van de searches uitgevoerd voor de 2013 European Hernia Society Guidelines on the treatment of inguinal hernia in adult patients en de 2018 International Guidelines for Groin Hernia Management: The HerniaSurge Group (literatuursearch tot 1 januari 2015 en 1 juli 2015 voor level 1 publicaties (RCTs). De literatuurzoekactie leverde voor reviews 583 unieke treffers (waarvan 339 reviews reeds gescreend voor de vorige richtlijnen) op (Medline n=419; Embase n=378; en de Cochrane Library n=12) en voor RCTs 2174 unieke treffers (Medline n=1376; Embase n=1537; en de Cochrane Library n=160).
De werkgroepleden selecteerden per uitgangsvraag in duplo de via de zoekactie gevonden artikelen op basis van vooraf opgestelde selectiecriteria en in eerste instantie de studies met de hoogste bewijskracht. De gehanteerde selectiecriteria zijn te vinden in de module met desbetreffende uitgangsvraag.
Kwaliteitsbeoordeling individuele studies
Voor de modules die volledig werden ge-update, zijn de geselecteerde artikelen systematisch beoordeeld, op basis van op voorhand opgestelde methodologische kwaliteitscriteria, om zo het risico op vertekende studieresultaten (Risk of Bias) te kunnen inschatten. Deze beoordelingen kunt u vinden in de Risk of Bias (RoB) tabellen. De gebruikte RoB instrumenten zijn gevalideerde instrumenten die worden aanbevolen door de Cochrane Collaboration: AMSTAR – voor systematische reviews; Cochrane – voor gerandomiseerd gecontroleerd onderzoek; ACROBAT-NRS – voor observationeel onderzoek; QUADAS II – voor diagnostisch onderzoek.
Samenvatten van de literatuur
Voor de modules die volledig werden ge-update, zijn de geselecteerde artikelen toegevoegd aan de set relevante artikelen genoemd in de internationale richtlijn. De relevante onderzoeksgegevens van alle geselecteerde artikelen werden overzichtelijk weergegeven in evidence-tabellen. De belangrijkste bevindingen uit de literatuur werden beschreven in de Engelstalige samenvatting van de literatuur. Bij een voldoende aantal studies en overeenkomstigheid (homogeniteit) tussen de studies werden de gegevens ook kwantitatief samengevat (meta-analyse) met behulp van Review Manager 5.
Beoordelen van de kracht van het wetenschappelijke bewijs
A) Voor interventievragen (vragen over therapie of screening)
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie (Schünemann 2013).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
B) Voor vragen over diagnostische tests, schade of bijwerkingen, etiologie en prognose
De kracht van het wetenschappelijke bewijs werd eveneens bepaald volgens de GRADE-methode: GRADE-diagnostiek voor diagnostische vragen (Schünemann, 2008), en een generieke GRADE-methode voor vragen over schade of bijwerkingen, etiologie en prognose. In de gehanteerde generieke GRADE-methode werden de basisprincipes van de GRADE-methodiek toegepast: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van bewijskracht op basis van de vijf GRADE-criteria (startpunt hoog; downgraden voor Risk of Bias, inconsistentie, indirectheid, imprecisie, en publicatiebias).
Formuleren van de conclusies
Voor elke relevante uitkomstmaat werd het wetenschappelijk bewijs samengevat in een of meerdere Nederlandstalige literatuurconclusies waarbij het niveau van bewijs werd bepaald volgens de GRADE-methodiek. De werkgroepleden maakten de balans op van elke interventie (overall conclusie). Bij het opmaken van de balans werden de gunstige en ongunstige effecten voor de patiënt afgewogen. De overall bewijskracht wordt bepaald door de laagste bewijskracht gevonden bij een van de cruciale uitkomstmaten. Bij complexe besluitvorming waarin naast de conclusies uit de systematische literatuuranalyse vele aanvullende argumenten (overwegingen) een rol spelen, werd afgezien van een overall conclusie. In dat geval werden de gunstige en ongunstige effecten van de interventies samen met alle aanvullende argumenten gewogen onder het kopje Overwegingen.
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals de expertise van de werkgroepleden, de waarden en voorkeuren van de patiënt (patient values and preferences), kosten, beschikbaarheid van voorzieningen en organisatorische zaken. Deze aspecten worden, voor zover geen onderdeel van de literatuursamenvatting, vermeld en beoordeeld (gewogen) onder het kopje Overwegingen.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk. De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen.
Randvoorwaarden (Organisatie van zorg)
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijn is expliciet rekening gehouden met de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, menskracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van een specifieke uitgangsvraag maken onderdeel uit van de overwegingen bij de bewuste uitgangsvraag. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van Zorg.
Indicatorontwikkeling
Er werden geen interne kwaliteitsindicatoren ontwikkeld. In de module Organisatie van Zorg is een suggestie opgenomen voor een toekomstige registratie.
Kennislacunes
Tijdens de ontwikkeling van deze richtlijn is systematisch gezocht naar onderzoek waarvan de resultaten bijdragen aan een antwoord op de uitgangsvragen. Bij elke uitgangsvraag is door de werkgroep nagegaan of er (aanvullend) wetenschappelijk onderzoek gewenst is om de uitgangsvraag te kunnen beantwoorden. Een overzicht van de onderwerpen waarvoor (aanvullend) wetenschappelijk van belang wordt geacht, is als aanbeveling in de Kennislacunes beschreven .
Commentaar- en autorisatiefase
De conceptrichtlijn werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijn aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijn werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Brouwers MC, Kho ME, Browman GP, et al. (2010) AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. https://richtlijnendatabase.nl/over_deze_site/richtlijnontwikkeling.html.
Ontwikkeling van Medisch Specialistische Richtlijnen: stappenplan. Kennisinstituut van Medisch Specialisten.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008;336(7654). doi: 10.1136/bmj.a139. PubMed PMID: 18483053.
Zoekverantwoording
Tabel Exclusie na het lezen van het volledige artikel
|
Auteur en jaartal (PICO2) |
Redenen van exclusie |
|
Zhou 2016 |
Vergelijkt een techniek die niet in Nederland gebruikt wordt |
|
Okinaga 2016 |
Vergelijkt een techniek die niet in Nederland gebruikt wordt |
|
Auteur en jaartal (PICO3) |
Redenen van exclusie |
|
Yan 2014 |
Artikel in Chinees |
|
Jia 2014 |
Conference/poster abstract |
|
Auteur en jaartal (PICO4) |
Redenen van exclusie |
|
Ghosh 2014 |
Komt een modified TAPP overeen met TEP? Pico gaat over TAPP versusTEP |
|
Ayyaz 2015 |
niet de juiste comparison: mesh fixatie vs non-fixatie bij een TEP procedure |
|
Auteur en jaartal (PICO5) |
Redenen van exclusie |
|
Li 2014 |
Includeert geen studies die Lichtenstein uitvoeren met een publicatiedatum na 2014. De studies die in Li 2014 zijn meegenomen zitten daarom al in de wereldrichtlijn. O'Reily includeert ook andere technieken (Sttoppa, Bassini, mesh plug, giant prosthetic reinforcement of the visceral sac)) en heeft geen subanalyses voor Lichtenstein, includeert naast RCTs ook prospectieve en retrospectieve onderzoeken voor de meta-analyse. |
|
Bowling 2016 |
Meeting abstract, evt voor overwegingen |
|
Kao 2015 |
Vergelijkt wel vs geen ligation en niet Lichtenstein vs TEP/TAPP, 4/5 gebruiken open techiek, 1/5 gebruikt laparo-endoscopische techniek |
|
O'Reily 2012 |
Includeert geen studies die Lichtenstein technique uitvoeren met een publicatiedatum na 2014. De studies die in O'Reily 2012 zijn meegenomen zitten daarom al in de wereldrichtlijn. O'Reily includeert ook andere technieken (Shouldice, Bassini) en heeft geen subanalyses voor Lichtenstein. |
|
Turculet 2016 |
Poster abstract (P044), evt voor overwegingen |
|
Neumayer 2005 |
Letter to the editor |
|
Kockerling 2016 |
non-randomized uit data registratie. Bespreekt wel pijn, complicaties en recurrence bij 1 jaar follow-up |
|
Beuran 2016 |
Oral presentation abstract (O017) |
|
OReily 2012 |
Erratum van O'Reily 2012 SR |
|
Auteur en jaartal (PICO6) |
Redenen van exclusie |
|
Andresen 2015 protocol |
Onderzoeksprotocol |
|
Andresen 2015 short-term |
Vergelijkt Onstep met Lichtenstein |
|
Sgourakis 2013 |
Geen interventie onderzoek, gaat over recurrent inguinal hernia |
