Echogeleid aanprikken
Uitgangsvraag
Wat is de waarde van echogeleid aanprikken van een centraal veneuze of perifeer veneuze lijn bij volwassen patiënten?
Aanbeveling
Centraal veneuze katheter
Prik de vena femoralis, vena jugularis interna en vena subclavia voor het plaatsen van een centrale lijn echogeleid aan.
Perifere lijnen
Overweeg een perifere lijn echogeleid te prikken in geval van:
- Niet zichtbare en niet palpabele venen; of
- Een verleden van meer dan twee pogingen voorafgaand aan het succesvol plaatsen van de perifere lijn.
PICC-lijnen
Prik een vene in de bovenarm voor een perifeer ingebrachte centrale katheter echogeleid aan.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek uitgevoerd naar de waarde van echogeleid aanprikken van een centraal veneuze of perifere lijn. De uitgangsvraag werd gevangen in drie PICO’s. PICO 1 vergeleek echogeleid aanprikken met niet-echogeleid aanprikken (ofwel aanprikken op basis van landmarks) bij patiënten die een centraal veneuze katheter kregen. PICO 2 en 3 vergeleken dezelfde interventies, waarbij PICO 2 zich richtte op patiënten die lastig te prikken zijn voor het aanbrengen van een perifere lijn en PICO 3 richtte zich op patiënten die een PICC-lijn kregen. Het succesvol inbrengen van de lijn/katheter en complicaties werden als cruciale uitkomstmaten gedefinieerd. Kwaliteit van leven, patiënttevredenheid, katheter-gerelateerde interventies, duur van inbrengen, mortaliteit en tromboflebitis werden als belangrijke uitkomstmaten gedefinieerd.
Er werden in totaal zeventien studies geïncludeerd in de literatuuranalyse. Vijftien gerandomiseerde studies werden geïncludeerd voor PICO 1, één systematische review en één gerandomiseerde studie voor PICO 2. Voor PICC-lijnen (PICO 3) werden geen geschikte studies gevonden.
Centraal veneuze katheters
Er werden vijftien studies geïncludeerd voor PICO 1. Bij het plaatsen van centraal veneuze katheters is de locatie van aanprikken van belang. Binnen deze PICO werden drie aanpriklocaties beschreven, namelijk in de vena jugularis interna, de vena subclavia en vena femoralis. De resultaten werden per aanpriklocatie uitgesplitst. Er werd een klinisch relevant verschil gevonden in het succesvol aanprikken in het voordeel van echogeleid aanprikken in de vena femoralis en succesvol aanprikken in één enkele poging in het voordeel van echogeleid aanprikken van de vena subclavia. Daarnaast werd een klinisch relevant verschil gevonden in het aantal complicaties wanneer er gebruik werd gemaakt van echogeleid aanprikken in de vena jugularis interna, vena subclavia en vena femoralis. Geen van de studies rapporteerde mortaliteitscijfers, kwaliteit van leven, patiënttevredenheid, katheter-gerelateerde interventies of tromboflebitis.
Perifere intraveneuze katheters
Er werden twee systematische reviews gevonden die voldeden aan de inclusiecriteria van PICO 2. In deze PICO werd gekozen voor een vergelijking bij patiënten die bekend waren met het feit dat er sprake was van een moeilijk te verkrijgen intraveneuze toegang. Dit werd gedefinieerd als het niet zichtbaar en palpabel zijn van venen of meer dan twee pogingen nodig om succesvol een perifere lijn te plaatsen of door een anesthesioloog beoordeeld als moeilijk te verkrijgen intraveneuze toegang. De systematische review van Tada (2022) includeerde zestien studies, waarvan er acht geschikt waren om op te nemen in deze richtlijnmodule. Deze acht studies richtten zich alleen op patiënten waarbij een moeilijke procedure werd verwacht. Geen van de studies rapporteerde mortaliteitscijfers, informatie over kwaliteit van leven of tromboflebitis. Er werden klinisch relevante verschillen gevonden voor succesvol aanprikken na één enkele poging en complicaties in het voordeel van echogeleid aanprikken.
PICC lijnen
Er is geen literatuur die de besluitvorming kan ondersteunen. De werkgroep is van mening dat PICC-lijnen in de huidige praktijk bij voorkeur altijd echogeleid geplaatst worden. De venen die het meest gebruikt worden voor het plaatsen van een PICC-lijn, de vena basilica, vena brachialis, zijn doorgaans niet of moeilijk zichtbaar wat motiveert tot het gebruik van een echo om de vene aan te prikken.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Voor patiënten is het belangrijk dat het inbrengen van een centrale of perifere lijn gepaard gaat met zo min mogelijk ongemak. Het in een keer correct inbrengen gaat gepaard met zo min mogelijk pijn, weke delenletsel/blauwe plekken en kans op complicaties. Het gebruik van de echo zorgt voor zichtbaarheid van aan te prikken vaten. Patiënten die bekend zijn met een moeilijke intraveneuze toegang hebben een voorkeur voor het gebruik van de echo bij het plaatsen van de centrale of perifere lijn.
Kosten (middelenbeslag)
Er is geen bewijs dat het gebruik van een echo voor het aanprikken van een vene resulteert in significant meer of minder kosten. Er is wel bewijs voor het verlagen van het aantal pogingen en het aantal complicaties bij gebruik van een echo, dit leidt tot het besparen van kosten op materiaal en interventies. Het gebruik van een echoapparaat brengt kosten met zich mee, maar vaak kan er met enkele apparaten volstaan worden voor een heel ziekenhuis voor het inbrengen van centrale en perifere lijnen.
Aanvaardbaarheid, haalbaarheid en implementatie
Het gebruik van een echo leidt tot een afname in het aantal puncties tot succesvolle toegang en tot een afname in complicaties. Het aanschaffen van een echoapparaat lijkt haalbaar gezien het gebruik breed geïmplementeerd is in de Nederlandse gezondheidszorg. Het leren gebruiken van een echoapparaat is haalbaar, er zijn diverse interne en externe mogelijkheden tot scholing.
Doordat diverse specialismen centrale en perifere lijnen plaatsen waar het gebruik van een echo ook voor andere doeleinden wordt gebruikt zal daar de implementatie eenvoudig zijn.
Bij het inbrengen van perifere lijnen lijkt het gebruik van een echo wel waardevol bij moeilijk te prikken patiënten. Bij perifere lijnen die veelal door verpleegkundigen worden ingebracht zal dit meer inzet en scholing nodig hebben.
Centraal veneuze katheter
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Het gebruik van een echo verhoogt de kans op het succesvol in één keer aanprikken en verlaagt waarschijnlijk de kans op complicaties bij het aanprikken van de vena jugularis interna, vena subclavia en vena femoralis voor centrale lijnen. Er zijn geen argumenten tegen echogeleid aanprikken.
Perifere lijnen
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Het gebruik van een echo lijkt de kans op het succesvol in één keer aanprikken te verhogen en leidt waarschijnlijk tot grotere patiënttevredenheid. Het effect op complicaties is onduidelijk door de zeer lage bewijskracht. Echogeleid aanprikken van iedere perifere lijn is niet bijdragend, niet haalbaar en niet wenselijk. Bij moeilijk te prikken vaten is het te overwegen echogeleid de perifere lijn te plaatsen.
PICC-lijnen
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Er is geen literatuur die de besluitvorming kan ondersteunen. De werkgroep adviseert het gebruik van echo gezien de locatie, diepte en onzichtbaarheid van de venen in de bovenarm.
Onderbouwing
Achtergrond
In de huidige praktijk worden perifere lijnen veelal geprikt zonder gebruik te maken van echografie of infrarood technologie. Als het niet lukt zonder deze hulpmiddelen wordt er gebruik gemaakt van echografie of infrarood technologie. Voor het inbrengen van centrale lijnen, perifeer of centraal, wordt in de praktijk veelvuldig gebruik gemaakt van echografie, zowel echogeleid inbrengen als de inzet van de echo voor bepaling van aanprik lokalisatie. Dit is echter niet vastgelegd als voorkeurstechniek. Het gebruik van echografie bij de plaatsing van perifere en centrale lijnen biedt voordelen die ondersteund worden door wetenschappelijk onderzoek. Studies tonen aan dat echogeleide technieken het risico op complicaties, zoals pneumothorax of arteriële puncties bij centrale lijnen, significant verminderen in vergelijking met de traditionele methode (blind prikken) (Hind, 2003). Daarnaast kan echografie de kans op succesvolle plaatsing mogelijk verhogen bij de eerste poging. Een nadeel van echografie is dat de apparatuur aanzienlijke investeringskosten met zich meebrengt en dat zorgverleners specifieke scholing en training nodig hebben om deze techniek effectief toe te passen. Ondanks de voordelen is er sprake van een discrepantie tussen het beschikbare bewijsmateriaal en de implementatie in de klinische praktijk. Deze kloof wordt onder meer toegeschreven aan organisatorische barrières, beperkte toegang tot apparatuur, en onvoldoende training van zorgverleners.
Voor patiënten op de intensive care wordt verwezen naar de Richtlijn Centraal veneuze lijn van de NVIC. De aanleg van een vaattoegang voor hemodialyse valt buiten de afbakening van deze richtlijn en wordt beschreven in de richtlijn ‘Vaattoegang voor hemodialyse’.
Conclusies / Summary of Findings
PICO 1
1. Success rate (critical)
1.1. Overall success rate
|
High GRADE |
Ultrasound-guided placement of a central venous catheter in the internal jugular vein, subclavian vein, or femoral vein results in little to no difference in overall success rate when compared with landmark-based placement.
Sources: Airapetian, 2013; Benali, 2022; Ethesham, 2020; Faithi, 2016; Nazari, 2015; Oh, 2014; Palkhiwala, 2020; Rando, 2014; Riaz, 2015; Srinivasan, 2017; Subramony, 2022; Vinayagamurugan, 2021; Wang, 2020; Zhang, 2023 |
1.2. First attempt success rate
|
Moderate GRADE |
Ultrasound-guided placement of a central venous catheter in the internal jugular vein, subclavian vein, or femoral vein likely results in a higher first attempt success rate of the central venous catheter when compared with landmark-based placement.
Sources: Benali, 2022; Faithi, 2016; Nazari, 2015; Palkhiwala, 2020; Riaz, 2015; Srinivasan, 2017; Vinayagamurugan, 2021; Wang, 2020; Zhang, 2023 |
2. Complications (critical)
|
Moderate GRADE |
Ultrasound-guided placement of a central venous catheter in the internal jugular vein, subclavian vein, or femoral vein likely results in a large reduction of complications when compared with landmark-based placement.
Sources: Airapetian, 2013; Benali, 2022; Dolu, 2015; Ethesham, 2020; Palkhiwala, 2020; Rando, 2014; Riaz, 2015; Srinivasan, 2017; Vinayagamurugan, 2021; Wang, 2020; Zhang, 2023 |
3. Number of attempts (important)
No conclusions were formulated due to the absence of a definition for the minimally important difference.
4. Duration of the procedure
No conclusions were formulated due to the absence of a definition for the minimally important difference.
5. Mortality (important)
|
No GRADE |
None of the included studies reported mortality rates after central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein. Therefore, a GRADE-conclusion could not be determined.
Source(s): - |
6. Quality of life (important)
|
No GRADE |
None of the included studies reported patients’ quality of life after central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein. Therefore, a GRADE-conclusion could not be determined.
Source(s): - |
7. Patients’ satisfaction (important)
|
No GRADE |
None of the included studies reported patients’ satisfaction after central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein. Therefore, a GRADE-conclusion could not be determined.
Source(s): - |
8. Catheter-related interventions (important)
|
No GRADE |
None of the included studies reported catheter-related intervention rates after central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein. Therefore, a GRADE-conclusion could not be determined.
Source(s): - |
9. Thrombophlebitis (important)
|
No GRADE |
None of the included studies reported thrombophlebitis rates after central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein. Therefore, a GRADE-conclusion could not be determined.
Source(s): - |
PICO 2
1. Success rate (critical)
1.1 Overall success rate
|
Low GRADE |
Ultrasound-guided placement of a peripheral intravenous catheter may result in little to no difference in overall successful placement when compared with landmark-based placement in patients with difficult vascular access.
Sources: Tada, 2022; Aponte, 2007; Bridey, 2018; Kerforne, 2012; Niishizawa, 2020; River, 2009; Stein, 2009; Weiner, 2013 |
1.2 First attempt success rate
|
Low GRADE |
Ultrasound-guided placement of a peripheral intravenous catheter may result in a higher first attempt success rate when compared with landmark-based placement in patients with difficult vascular access.
Sources: Tada, 2022; Aponte, 2007; Bahl, 2016; Bridey, 2018; Kerforne, 2012; McCarthy, 2016a; Nishizawa, 2020; Stein, 2009; Weiner, 2013; Yalcinli, 2022 |
2. Complications (critical)
|
Very low GRADE |
The evidence is very uncertain about the number of complications between of ultrasound-guided placement of a peripheral intravenous catheter when compared with landmark-based placement in patients with difficult vascular access.
Sources: Tada, 2022; Stein, 2009; McCarthy, 2016a; Nishizama, 2020 |
3. Number of attempts (important)
No conclusions were formulated due to the absence of a definition for the minimally important difference.
4. Duration of the procedure
No conclusions were formulated due to the absence of a definition for the minimally important difference.
5. Mortality (important)
|
No GRADE |
None of the included studies reported mortality rates after peripheral intravenous catheter placement in patients with difficult vascular access. Therefore, a GRADE-conclusion could not be determined.
Source(s): - |
6. Quality of life (important)
|
No GRADE |
None of the included studies reported patients’ quality of life after peripheral intravenous catheter placement in patients with difficult vascular access. Therefore, a GRADE-conclusion could not be determined.
Source(s): - |
7. Patients’ satisfaction (important)
|
Moderate GRADE |
Ultrasound-guided placement of a peripheral intravenous catheter likely results in higher patient satisfaction when compared with landmark-based placement in patients with difficult vascular access.
Sources: Tada, 2022; Bridey, 2018; River, 2009; Stein, 2009; Weiner, 2013 |
8. Catheter-related interventions (important)
|
Low GRADE |
Ultrasound-guided placement of a peripheral intravenous catheter may result in little to no difference in catheter-related interventions when compared with landmark-based placement in patients with difficult vascular access.
Source: Yalcinli, 2022 |
9. Thrombophlebitis (important)
|
No GRADE |
None of the included studies reported thrombophlebitis rates after peripheral intravenous catheter placement in patients with difficult vascular access. Therefore, a GRADE-conclusion could not be determined.
Source(s): - |
Samenvatting literatuur
Description of studies
PICO 1: Central venous catheters (CVC)
The randomized controlled trial of Airapetian (2013) compared ultrasound-guided placement of central venous catheters with landmark-based placement of central venous catheters. Adult patients who needed jugular or femoral central venous catheter placement were included. In total, 118 patients were included. Thirty-six patients underwent ultrasound-guided central venous catheter placement. Thirty-eight patients underwent landmark-based central venous catheter placement. Twenty-one patients in the ultrasound-guidance group had the central venous catheter placed in the jugular vein, while the other fifteen patients had the central venous catheter placed in the femoral vein. Twenty-eight out of the 38 patients in the landmark-based cannulation group had the central venous catheter placed in the jugular vein. The ten other patients had the central venous catheter placed in the femoral vein. The reported outcomes in Airepetian (2013) were the success rate, complications, and the number of attempts.
The randomized controlled trial of Benali (2022) compared ultrasound-guided cannulation with landmark-based cannulation for central venous catheters. Adult patients requiring elective central venous catheters were included. In total, 70 patients were included. Thirty-five patients underwent ultrasound-guided central venous catheter placement and 35 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the subclavian vein. The reported outcomes in Benali (2022) were the success rate, complications, and the number of attempts.
The randomized controlled trial of Dolu (2015) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients requiring elective cardiovascular surgery were included. In total, 100 patients were included. Fifty patients underwent ultrasound-guided central venous catheter placement and 50 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the internal jugular vein. The reported outcomes in Dolu (2015) were the success rate, complications, and the number of attempts.
The randomized controlled trial of Ethesham (2020) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients who underwent major surgeries under general anaesthesia requiring central venous pressure monitoring, rapid infusion of fluids for major surgery, drug administration, and inadequate peripheral access were included. In total, 90 patients were included. Forty-five patients underwent ultrasound-guided central venous catheter placement and 45 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the internal jugular vein. The reported outcomes in Ethesham (2020) were the success rate and complications.
The randomized controlled trial of Faithi (2016) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients who were scheduled for cardiac surgery in the surgical ward of a general hospital were included. In total, 321 patients were included. One hundred and seventy patients underwent ultrasound-guided central venous catheter placement and 151 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the internal jugular vein. The reported outcome in Faithi (2016) was the success rate.
The randomized controlled trial of Nazari (2015) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Critically ill and hemodialysis patients who had indications for central venous catheter placement were included. In total, 336 patients were included. One hundred and sixty-eight patients underwent ultrasound-guided central venous catheter placement and 168 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the jugular vein. The reported outcomes in Nazari (2015) were the success rate, complications, and the number of attempts.
The randomized controlled trial of Oh (2014) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients who required subclavian venous catheterization for neurosurgery were included. In total, 66 patients were included. Thirty-three patients underwent ultrasound-guided central venous catheter placement and 33 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the subclavian vein. The reported outcomes in Oh (2014) were the success rate and complications.
The randomized controlled trial of Palkhiwala (2020) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients with ASA Grade II and III posted for major surgeries were included. In total, 60 patients were included. Thirty patients underwent ultrasound-guided central venous catheter placement and 30 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the internal jugular vein. The reported outcomes in Palkhiwala (2020) were the success rate, complications, and the number of attempts.
The randomized controlled trial of Rando (2014) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Critically ill patients or those that required surgery and a CVL were included. In total, 257 patients were included. One hundred and twenty-three patients underwent ultrasound-guided central venous catheter placement and 134 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the internal jugular vein. The reported outcomes in Rando (2014) were the success rate and complications.
The randomized controlled trial of Riaz (2015) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients who required internal jugular vein catheterization were included. In total, 200 patients were included. One hundred patients underwent ultrasound-guided central venous catheter placement and 100 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the internal jugular vein. The reported outcomes in Riaz (2015) were the success rate and complications.
The randomized controlled trial of Srinivasan (2017) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients who required central venous lines were included. In total, 170 patients were included. Eighty patients underwent ultrasound-guided central venous catheter placement and 90 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the internal jugular vein. The reported outcomes in Srinivasan (2017) were the success rate and complications.
The randomized controlled trial of Subramony (2022) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients who required assessment of administration of vasoactive drugs or large volume fluid resuscitation or patients in which there was failure to obtain the necessary peripheral venous access were included. In total, 85 patients were included. Forty-four patients underwent ultrasound-guided central venous catheter placement and 41 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the subclavian vein. The reported outcome in Subramony (2022) was the success rate.
The randomized controlled trial of Vinayagamurugan (2021) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients undergoing elective or emergency surgery under general anesthesia were included. In total, 188 patients were included. Ninety-four patients underwent ultrasound-guided central venous catheter placement and 94 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the internal jugular vein. The reported outcomes in Vinayagamurugan (2021) were the success rate and complications.
The randomized controlled trial of Wang (2020) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients, ICU inpatient, and requiring subclavian vein puncture were included. In total, 200 patients were included. One hundred patients underwent ultrasound-guided central venous catheter placement and 100 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the subclavian vein. The reported outcomes in Wang (2020) were the success rate, complications, and the number of attempts.
The randomized controlled trial of Zhang (2023) compared ultrasound-guided placement of central venous catheters with landmark-based placement for central venous catheters. Adult patients with right subclavian vein catheterization were included. In total, 60 patients were included. Thirty patients underwent ultrasound-guided central venous catheter placement and 30 patients underwent landmark-based central venous catheter placement. In both groups, the central venous catheter was placed in the subclavian vein. The reported outcomes in Zhang (2023) were the success rate and complications.
PICO 2: Peripheral intravenous catheters
The systematic (Cochrane) review of Tada (2022) compared ultrasound-guided placement of peripheral venous catheters with landmark-based placement in adult patients. Tada (2022) searched the electronic databases of the Cochrane Vascular Specialised Register, CENTRAL, Medline, Embase, CINAHL, and LILACS with the most recent searches carried out on the 29th of November 2021. In total, Tada (2022) included sixteen randomized controlled trials. For this guideline, we were only interested in studies investigating patients with difficult intravenous access. Therefore, only eight out of the sixteen studies from the systematic review of Tada (2022) were eligible for inclusion in this guideline (Aponte, 2007; Bahl, 2016; Bridey, 2018; Keforne, 2012; McCarthy, 2016; Nishizawa, 2020; Stein, 2009, Weiner, 2013). Tada (2022) used the definition of the difficulty of peripheral intravenous cannulation adopted by original studies. The reported outcomes in Tada (2022) were the success rate, complications, number of attempts, patients’ satisfaction
The randomized controlled trial of Yalcinli (2022) compared ultrasound-guided placement of peripheral venous catheters with landmark-based placement in adult patients with difficult vascular access (DVA). In total, 270 patients were included. For this guideline, we excluded patients allocated to near-infrared based placement (N=90) and only included patients allocated to ultrasound-guided placement (N=90) and landmark-based placement (N=90). Difficult vascular access criteria of patients were more than two attempts on previous visits, invisible veins, or anticipated difficulties. The reported outcomes in Yalcinli (2022) were the success rate and number of attempts.
PICO 3: Peripherally inserted central catheter (PICC)
No studies were included for PICO 3.
Table 1. Study characteristics of the included studies
|
Author |
Setting |
Type |
Location |
Difficult accessible patients yes/no? |
PICO |
|
Airapetian (2013) |
Intensive care unit |
Central venous catheter |
Jugular and femoral vein |
No information |
1 |
|
Benali (2022) |
Intensive care unit |
Central venous catheter |
Subclavian vein |
Benali (2022) makes a distinction in outcomes for patients with a BMI higher and lower than 30. Benali (2022) also reports outcomes for both groups combined. |
1 |
|
Dolu (2015) |
Not reported |
Central venous catheter |
Jugular vein |
Overweight patients (mean BMI is 26.6 kg/m2 vs. 25.7 kg/m2. |
1 |
|
Ehtesham (2020) |
Not reported |
Central venous catheter |
Jugular vein |
No information |
1 |
|
Faithi (2016) |
Surgical ward of a general hospital |
Central venous catheter |
Jugular vein |
No information |
1 |
|
Nazari (2015) |
Not reported |
Central venous catheter |
Jugular vein |
No information |
1 |
|
Palkhiwala (2020) |
Not reported |
Central venous catheter |
Jugular vein |
No information |
1 |
|
Rando (2014) |
Intensive care unit |
Central venous catheter |
Jugular vein |
46/123 patients undergoing US guidance placement had DIVA compared to 37/134 patients undergoing landmark-based placement. |
1 |
|
Riaz (2015) |
Not reported |
Central venous catheter |
Jugular vein |
No information |
1 |
|
Srinivasan (2017) |
Intensive care unit |
Central venous catheter |
Jugular vein |
No information |
1 |
|
Subramony (2022) |
Urban tertiary teaching hospital |
Central venous catheter |
Subclavian vein |
No information |
1 |
|
Vinayagamurugan (2021) |
Tertiary care university hospital |
Central venous catheter |
Jugular vein |
No information |
1 |
|
Wang (2020) |
Intensive care unit |
Central venous catheter |
Subclavian vein |
No information |
1 |
|
Oh (2014) |
Not reported |
Central venous catheter |
Subclavian vein |
No information |
1 |
|
Zhang (2023) |
Not reported |
Central venous catheter |
Subclavian vein |
No information |
1 |
|
Yalcinli (2022) |
Tertiary care hospital |
Peripheral intravenous catheter |
No information |
Yalcinli (2022) investigated patients with difficult intravenous access, defined as: history (>two trial histories during vascular access on a previous visit), with no visible or palpable veins on the upper extremity, and who were assessed to have a difficult procedure by the senior nurse. |
2 |
|
Tada (2022) |
Aponte (2007): Operating room
Bahl (2016): Emergency department
Bridey (2018): Intensive care unit
Keforne (2012): Intensive care unit
McCarthy (2016): Emergency department
Nishizawa (2020): Intensive care unit
Stein (2009): Emergency department
Weiner (2013): Emergency department |
Peripheral intravenous catheter |
No information |
Ten RCTs from Tada (2022) investigated patients with difficult intravenous access, defined as:
|
2 |
LM: landmark-based approach
Results
PICO 1. Central venous catheters (CVC)
1. Success rate (critical)
1.1. Overall success rate internal jugular vein placement
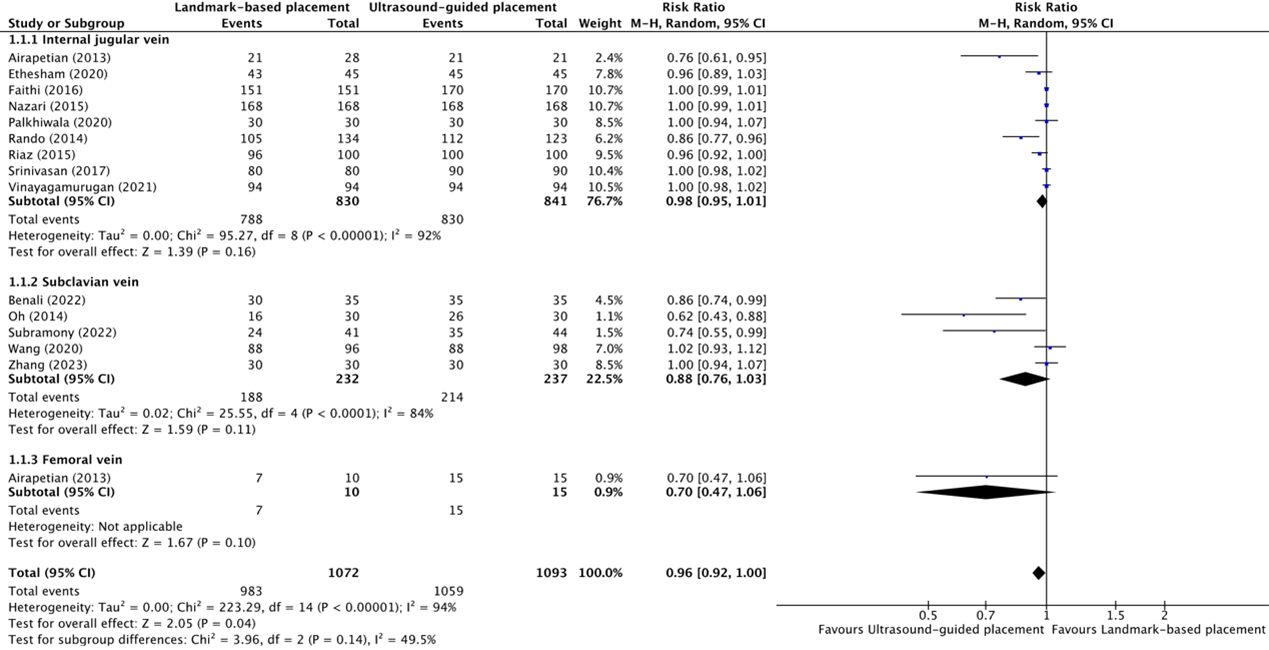
The overall success rate of placement of central venous catheters in the internal jugular vein between ultrasound-guided placement and landmark-based placement was reported in nine trials (Airapetian, 2013; Ethesham, 2020; Faithi, 2016; Nazari, 2015; Palkhiwala, 2020; Rando, 2014; Riaz, 2015; Srinivasan, 2017; Vinayagamurugan, 2021). The results were pooled in a meta-analysis. The pooled success rate in the ultrasound-guided placement group was 830/841 (98.7%), compared to 788/830 (94.9%) in the landmark-based placement group. This resulted in a pooled risk ratio (RR) of 0.98 (95% CI 0.95 to 1.01), in favor of ultrasound-guided placement (figure 1.1.1). This was not considered clinically relevant.
1.2 Overall success rate subclavian vein placement
The overall success rate of placement of central venous catheters in the subclavian vein between ultrasound-guided placement and landmark-based placement was reported in five trials (Benali, 2022; Oh, 2014; Subramony, 2022; Wang, 2020; Zhang, 2023). The results were pooled in a meta-analysis. The pooled success rate in the ultrasound-guided placement group was 214/237 (90.3%), compared to 188/232 (81.0%) in the landmark-based placement group. This resulted in a pooled RR of 0.88 (95% CI 0.76 to 1.03), in favor of ultrasound-guided placement (figure 1.1.2). This was not considered clinically relevant.
1.3 Overall success rate femoral vein placement
The overall success rate of placement of central venous catheters in the femoral vein between ultrasound-guided placement and landmark-based placement was reported in one trial (Airapetian, 2013). The success rate in the ultrasound-guided placement group was 15/15 (100%), compared to 7/10 (70.0%) in the landmark-based placement group. This resulted in a pooled RR of 0.70 (95% CI 0.47 to 1.06), in favor of ultrasound-guided placement (figure 1.1.3). This was considered clinically relevant.
Figure 1.1.1, 1.1.2, and 1.1.3 Forest plot showing the comparison between ultrasound-guided placement and landmark-based placement for the overall success rate of central venous catheter placement in the internal jugular vein, subclavian vein, and femoral vein
Pooled risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
1.4 First attempt success rate internal jugular vein placement
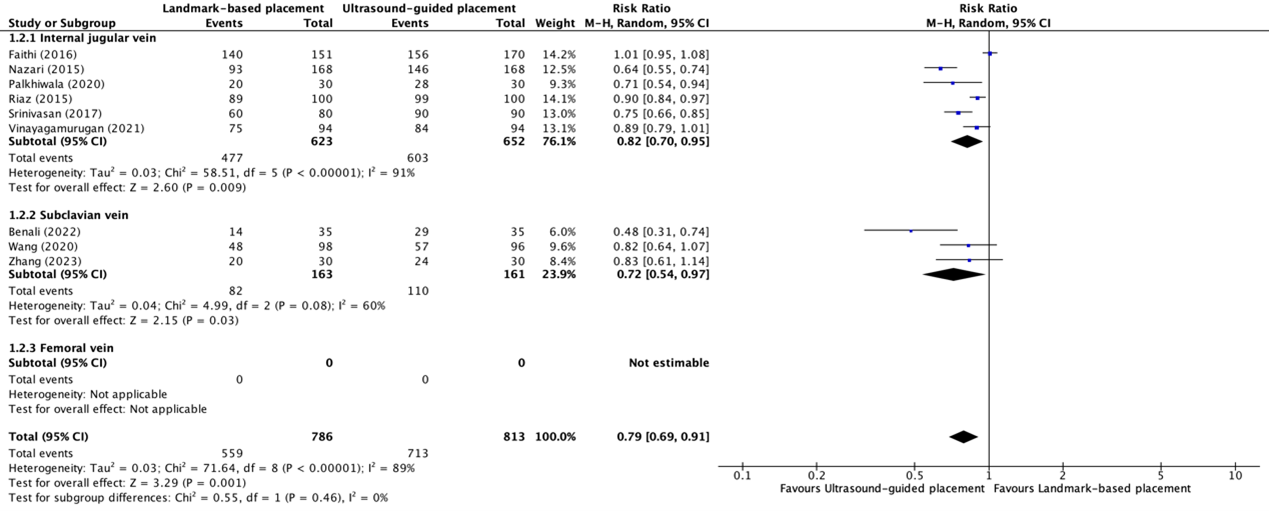
The first attempt success rate of placement of central venous catheters in the internal jugular vein between ultrasound-guided placement and landmark-based placement was reported in six trials (Faithi, 2016; Nazari, 2015; Palkhiwala, 2020; Riaz, 2015; Srinivasan, 2017; Vinayagamurugan, 2021). The results were pooled in a meta-analysis. The pooled success rate in the ultrasound-guided placement group was 603/652 (92.5%), compared to 477/623 (76.6%) in the landmark-based placement group. This resulted in a pooled RR of 0.82 (95% CI 0.70 to 0.95), in favor of ultrasound-guided placement (see figure 1.2.1). This was not considered clinically relevant.
1.5 First attempt success rate subclavian vein placement
The first attempt success rate of placement of central venous catheters in the subclavian vein between ultrasound-guided placement and landmark-based placement was reported in three trials (Benali, 2022; Wang, 2020; Zhang, 2023). The results were pooled in a meta-analysis. The pooled success rate in the ultrasound-guided placement group was 110/161 (68.3%), compared to 82/163 (50.3%) in the landmark-based placement group. This resulted in a pooled RR of 0.72 (95% CI 0.54 to 0.97), in favor of ultrasound-guided placement (see figure 1.2.2). This was considered clinically relevant.
1.6 First attempt success rate femoral vein
None of the included studies reported success rates on the first attempt of central venous catheter placement in the femoral vein.
Figure 1.2.1, 1.2.2, and 1.2.3 Forest plot showing the comparison between ultrasound-guided placement and landmark-based placement for the first attempt success rate of central venous catheter placement in the internal jugular vein, subclavian vein, and femoral vein
Pooled risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
2. Complications (critical)
2.1 Complications internal jugular vein placement
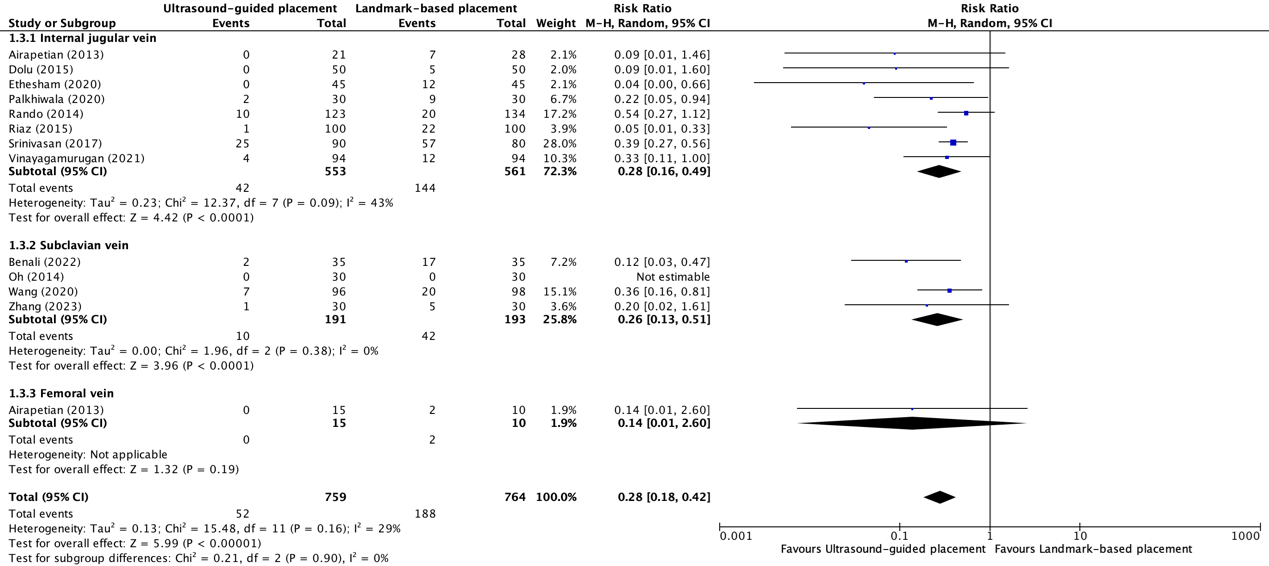
Complications* due to central venous catheter placement in the internal jugular vein between ultrasound-guided placement and landmark-based placement were reported in eight trials (Airapetian, 2013; Dolu, 2015; Ethesham, 2020; Palkhiwala, 2020; Rando, 2014; Riaz, 2015; Srinivasan, 2017; Vinayagamurugan, 2021). The results were pooled in a meta-analysis. The pooled number of complications in the ultrasound-guided placement group was 42/553 (7.6%), compared to 144/561 (25.7%) in the landmark-based placement group. This resulted in a pooled RR of 0.28 (95% CI 0.16 to 0.49), in favor of ultrasound-guided placement (see figure 1.3.1). This was considered as clinically relevant.
2.2 Complications subclavian vein placement
Complications* due to central venous catheter placement in the subclavian vein between ultrasound-guided placement and landmark-based placement were reported in four trials (Benali, 2022; Oh, 2014; Wang, 2020; Zhang, 2023). The results were pooled in a meta-analysis. The pooled number of complications in the ultrasound-guided placement group was 10/191 (5.2%), compared to 42/193 (21.8%) in the landmark-based placement group. This resulted in a pooled RR of 0.26 (95% CI 0.13 to 0.51), in favor of ultrasound-guided placement (see figure 1.3.2). This was considered as clinically relevant.
2.3 Complications femoral vein placement
Complications* because of central venous catheter placement in the femoral vein between ultrasound-guided placement and landmark-based placement were reported in one trial (Airapetian, 2013). The number of complications in the ultrasound-guided placement group was 0/15 (0%), compared to 2/10 (20.0%) in the landmark-based placement group. This resulted in a pooled RR of 0.14 (95% CI 0.01 to 2.60), in favor of ultrasound-guided placement (see figure 1.3.3). This was considered as clinically relevant.
*Reported complications were mechanical complications, hematomas, arterial punctures, pneumothoraxes, malposition, carotid artery punctures, double wall punctures, and irritation of the brachial plexus.
Figure 1.3.1, 1.3.2, and 1.3.3 Forest plot showing the comparison between ultrasound-guided placement and landmark-based placement for the overall complication rates of central venous catheter placement in the internal jugular vein, subclavian vein, and femoral vein
Pooled risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
3. Number of attempts (important)
3.1 Number of attempts in the internal jugular vein
The number of attempts for placement of a central venous catheter in the internal jugular vein was reported in three trials (Dolu, 2015; Nazari, 2015; Palkhiwala, 2020). Dolu (2015) reported the mean (SD) number of needles passes. Nazari (2015) reported the number of patients who required more than one attempt. Palkhiwala (2015) reported the mean (SD) number of attempts.
The mean (SD) number of needles passes for placement of a central venous catheter in the internal jugular vein in the study of Dolu (2015) in the ultrasound-guided placement group was 1.1 (0.5) needles, compared to 2.2 (1.6) needles in the landmark-based placement group. This resulted in a MD of -1.10 (95% CI -1.56 to -0.64), in favor of ultrasound-guided placement.
The mean (SD) number of patients who required more than one attempt of placement of a central venous catheter in the internal jugular vein in the study of Nazari (2015) in the ultrasound-guided placement group was 22/168 (13.1%), compared to 75/168 (44.6%). This resulted in a RR of 0.29 (95% CI 0.19 to 0.45), in favor of ultrasound-guided placement.
The mean (SD) number of attempts of placement of a central venous catheter in the internal jugular vein in the study of Palkhiwala (2020) in the ultrasound-guided placement group was 1.06 (0.24) compared to 1.43 (0.66). This resulted in a MD of -0.37 (95% CI -0.62 to -0.12) attempts, in favor of ultrasound-guided placement.
3.2 Number of attempts in the subclavian vein
The number of attempts for placement of a central venous catheter in the subclavian vein was reported in two trials (Benali, 2022; Wang, 2020). Benali (2022) reported the median (IQR) number of attempts. Wang (2020) reported the mean (SD) number of punctures.
The median (IQR) number of attempts for placement of a central venous catheter in the subclavian vein in the study of Benali (2022) in the ultrasound-guided placement group was 1.0 (IQR: 1.0 to 1.0), compared to 2.0 (IQR: 1.0 to 4.0) in the landmark-based placement group.
The mean (SD) number of attempts of placement of a central venous catheter in the subclavian vein in the study of Wang (2020) in the ultrasound-guided placement group (N = 96) was 1.6 (1.0) compared to 1.5 (0.7) in the landmark-based placement group (N=98). This resulted in a MD of 0.10 (95% CI -0.14 to 0.34), in favor of landmark-based placement.
3.3 Number of attempts in the femoral vein
None of the included studies reported the number of attempts for central venous catheter placement in the femoral vein.
4. Duration of the procedure (important)
The duration of the procedures was reported in eight trials (Dolu, 2015; Ethasham, 2020; Faithi, 2016; Nazari, 2015; Palkhiwala, 2020; Riaz, 2015; Vinayagamurugan, 2021; Wang, 2020; Zhang, 2023). Ethasham (2020) reported the mean duration of the procedure in minutes. Dolu (2015), Faithi (2016), Palkhiwala (2020), Riaz (2015), Vinayagamurugan (2021), and Wang (2020) reported the mean (SD) duration of the procedure in seconds and were also pooled in a second meta-analysis. Zhang (2023) only reported the mean duration of the procedure in minutes without reporting a standard deviation and was therefore reported descriptively.
4.1 Duration of the procedure in the internal jugular vein (in minutes)
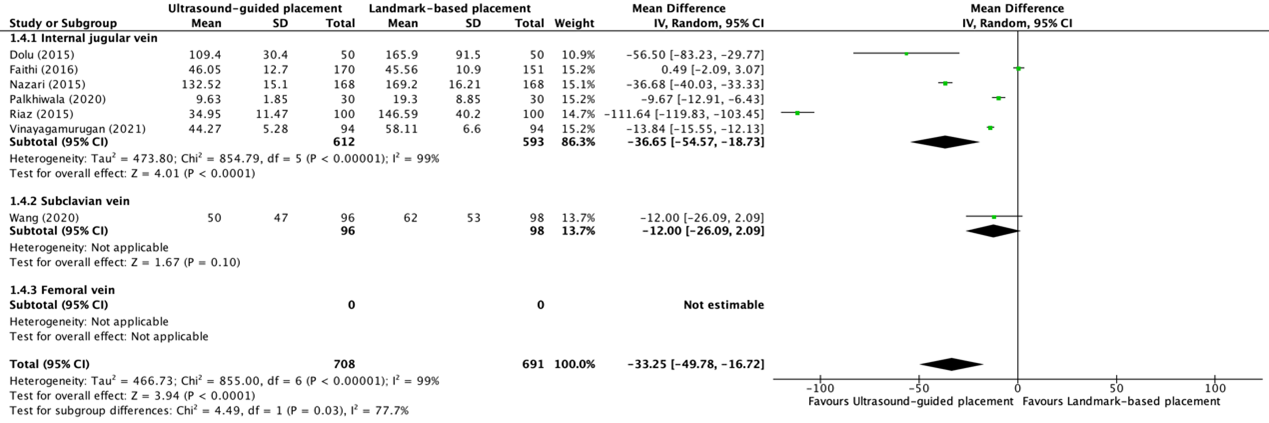
One trial reported duration of the procedure for placement of a central venous catheter in minutes in the internal jugular vein (Ethesham, 2020). The mean (SD) duration of placement of a central venous catheter in the internal jugular vein in the ultrasound-guided placement group (N=45) was 4.2 (0.4) minutes, compared to 4.7 (0.8) minutes in the landmark-based placement group (N=45). This resulted in a mean difference (MD) of -0.50 (95% CI -0.76 to -0.24) minutes.
4.2 Duration of the procedure in the Internal jugular vein (in seconds)
Six trials reported duration of the procedure for placement of a central venous catheter in minutes between ultrasound-guided placement (N=612) and landmark-based placement (N=593) in the internal jugular vein (Dolu, 2015; Faithi, 2016; Nazari, 2015; Palkhiwala, 2020; Riaz, 2015; Vinayagamurugan, 2021). The results were pooled in a meta-analysis. The pooled mean difference (MD) for placement of a central venous catheter in the internal jugular vein was -36.65 (95% CI -54.57 to -18.73) seconds (see figure 1.4.1), in favor of ultrasound-guided placement.
4.3 Duration of the procedure in the subclavian vein (in seconds)
One trial reported duration of the procedure for placement of a central venous catheter in seconds in the subclavian vein (Wang, 2020). The mean (SD) duration of placement of a central venous catheter in the subclavian vein in the ultrasound-guided placement group (N=96) was 50 (47) seconds, compared to 62 (53) seconds in the landmark-based placement group (N=98). This resulted in a mean difference (MD) of -12.00 (95% CI -26.09 to 2.09) seconds (see figure 1.4.2).
Zhang (2023) only reported the mean duration of placement of central venous catheters in the subclavian vein between ultrasound-guided placement and landmark-based placement without a standard deviation and/or confidence interval. Duration of the procedure in the ultrasound-guided placement group was 6.3333 minutes, compared to 11.3667 minutes in the landmark-based placement group.
Figure 1.4.1, 1.4.2, and 1.4.3 Forest plot showing the comparison between ultrasound-guided placement and landmark-based placement for the duration of the procedure of central venous catheter placement in the internal jugular vein, subclavian vein, and femoral vein
Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
4.4 Duration of the procedure in the femoral vein
None of the included studies reported the duration of the procedure for placement of central venous catheter placement in the femoral vein.
5 Mortality (important)
None of the included studies reported mortality rates for central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein.
6. Quality of life (important)
None of the included studies reported information regarding quality of life for central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein.
7. Patients’ satisfaction (important)
None of the included studies reported information regarding patient’s satisfaction for central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein.
8. Catheter-related interventions (important)
None of the included studies reported catheter-related intervention rates for central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein.
9. Thrombophlebitis (important)
None of the included studies reported thrombophlebitis rates for central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein.
Level of evidence of the literature
1. Success rate (critical)
1.1. Overall success rate
The level of evidence regarding the outcome overall success rate of central venous catheter placement in the internal jugular vein, subclavian vein, and femoral vein was derived from randomized controlled trials and therefore started high. The level of evidence was not downgraded. The level of evidence was considered as high.
1.2. First attempt success rate
The level of evidence regarding the outcome first attempt success rate of central venous catheter placement in the internal jugular vein, subclavian vein, and femoral vein was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by one level because of the wide confidence interval crossing the lower threshold of clinical relevance (imprecision, -1). The level of evidence was considered as moderate.
2. Complications (critical)
The level of evidence regarding the outcome complications because of central venous catheter placement in the internal jugular vein was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by one level because of heterogeneity in the definition of the outcome measure complications between the included studies (inconsistency, -1). The level of evidence was considered as moderate.
3. Number of attempts (important)
The evidence could not be graded due to the absence of a definition for the minimally important difference.
4. Duration of the procedure (important)
The evidence could not be graded due to the absence of a definition for the minimally important difference.
5. Mortality (important)
None of the included studies reported mortality rates of central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein. Therefore, the level of evidence could not be determined.
6. Quality of life (important)
None of the included studies reported patients’ quality of life after central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein. Therefore, the level of evidence could not be determined.
7. Patients’ satisfaction (important)
None of the included studies reported patients’ satisfaction after central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein. Therefore, the level of evidence could not be determined.
8. Catheter-related interventions (important)
None of the included studies reported catheter-related intervention rates after central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein. Therefore, the level of evidence could not be determined.
9. Thrombophlebitis (important)
None of the included studies reported thrombophlebitis rates of central venous catheter placement in the internal jugular vein, subclavian vein, or femoral vein. Therefore, the level of evidence could not be determined.
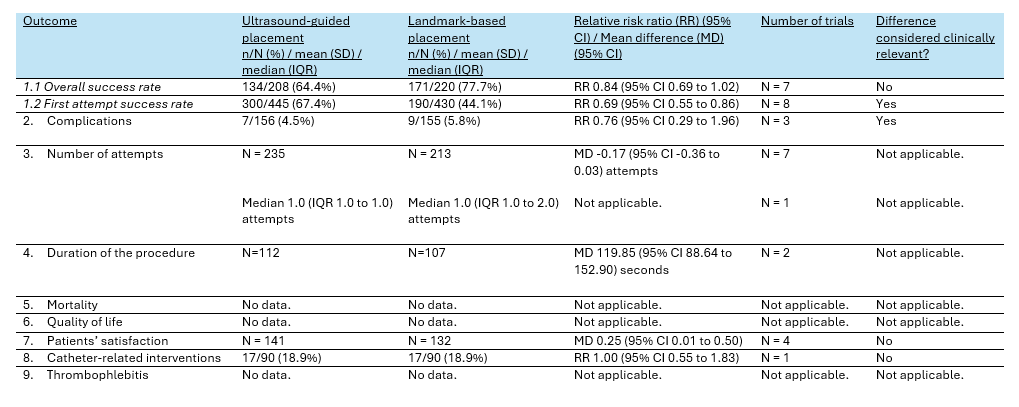
Table 2. Summary of findings PICO 1
|
Outcome |
Ultrasound-guided placement n/N (%) / mean (SD) / median (IQR) |
Landmark-based placement n/N (%) / mean (SD) / median (IQR) |
Relative risk ratio (RR) (95% CI) / Mean difference (MD) (95% CI) |
Number of trials |
Difference considered clinically relevant? |
|
1.1 Overall success rate internal jugular vein placement |
830/841 (98.7%) |
788/830 (94.9%) |
RR 0.98 (95% CI 0.95 to 1.01) |
N = 9 |
No |
|
1.2 Overall success rate internal subclavian vein placement |
214/237 (90.3%) |
188/232 (81.0%) |
RR 0.88 (95% CI 0.76 to 1.03) |
N = 5 |
No |
|
1.3 Overall success rate femoral vein placement |
15/15 (100%) |
7/10 (70.0%) |
RR 0.70 (95% CI 0.47 to 1.06) |
N = 1 |
Yes |
|
1.4 First attempt success rate internal jugular vein placement |
603/652 (92.5%) |
477/623 (76.6%) |
RR 0.82 (95% CI 0.70 to 0.95) |
N = 6 |
No |
|
1.5 First attempt success rate subclavian vein placement |
110/161 (68.3%) |
82/163 (50.3%) |
RR 0.72 (95% CI 0.54 to 0.97) |
N = 3 |
Yes |
|
1.6 First attempt success rate femoral vein placement |
No data. |
No data. |
Not applicable. |
Not applicable. |
Not applicable. |
|
2.1 Complications internal jugular vein placement |
42/553 (7.6%) |
144/561 (25.7%) |
RR 0.28 (95% CI 0.16 to 0.49) |
N = 8 |
Yes |
|
2.2 Complications subclavian vein placement |
10/192 (5.2%) |
42/193 (21.8%) |
RR 0.26 (95% CI 0.13 to 0.51 |
N = 4 |
Yes |
|
2.3 Complications femoral vein placement |
0/15 (0%) |
2/10 (20.0%) |
RR 0.14 (95% CI 0.01 to 2.60) |
N = 1 |
Yes |
|
3.1 Number of attempts in the internal jugular vein
|
22/168 (13.1%) who required more than one attempt
1.1 (0.5) needles passes for placement of a central venous catheter
1.06 (0.24) attempts
|
75/168 (44.6%) who required more than one attempts
2.2 (1.6) needles passes for placement of a central venous catheter
1.43 (0.66) attempts |
RR 0.29 (95% CI 0.19 to 0.45)
MD -1.10 (95% CI -1.56 to -0.64)
MD 0.37 (95% CI -0.62 to -0.12) |
N = 1
N = 1
N =1 |
Yes
Not applicable.
Not applicable. |
|
3.2 Number of attempts in the subclavian vein |
Median 1.0 (IQR 1.0 to 1.0)
1.6 (1.0) attempts |
Median 2.0 (IQR 1.0 to 4.0)
1.5 (0.7) attempts |
Not applicable.
MD -0.10 (95% CI -0.14 to 0.34) |
N = 1
N = 1 |
Not applicable.
No |
|
3.3 Number of attempts in the femoral vein |
No data. |
No data. |
Not applicable. |
Not applicable. |
Not applicable. |
|
4.1 Duration of the procedure in the internal jugular vein |
4.2 (0.4) minutes |
4.7 (0.8) minutes |
MD -0.50 (95% CI -0.76 to -0.24)
Pooled MD -36.65 (95% CI -54.47 to -18.73) seconds |
N = 1
N = 6 |
Not applicable.
Not applicable. |
|
4.2 Duration of the procedure in the subclavian vein |
50 (47) seconds |
62 (53) seconds |
MD -12.00 (95% CI -26.09 to 2.09) |
N = 1 |
Not applicable. |
|
4.3 Duration of the procedure in the femoral vein |
No data. |
No data. |
Not applicable. |
Not applicable. |
Not applicable. |
|
No data. |
No data. |
Not applicable. |
Not applicable. |
Not applicable. |
|
No data. |
No data. |
Not applicable. |
Not applicable. |
Not applicable. |
|
No data. |
No data. |
Not applicable. |
Not applicable. |
Not applicable. |
|
No data. |
No data. |
Not applicable. |
Not applicable. |
Not applicable. |
|
No data. |
No data. |
Not applicable. |
Not applicable. |
Not applicable. |
PICO 2: Peripheral intravenous catheters
1. Success rate (critical)
1.1 Overall success rate
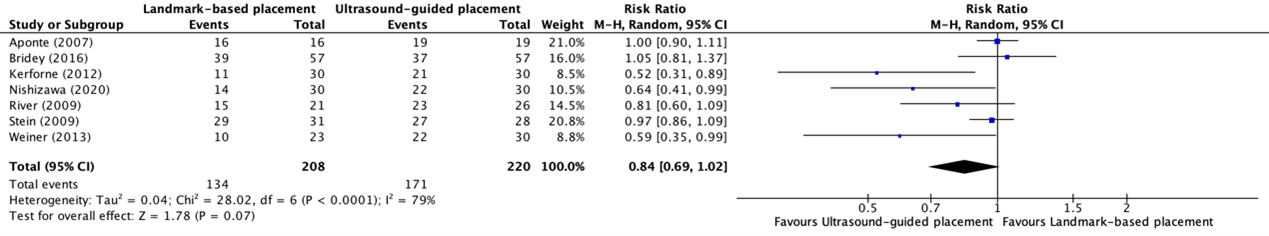
The overall success rate of placement of peripheral intravenous catheter placement between ultrasound-guided placement and landmark-based placement in patients with difficult vascular access was reported in seven trials included in the systematic review of Tada (2022) (Aponte, 2007; Bridey, 2018; Kerforne, 2012; Nishizawa, 2020; River, 2009; Stein, 2009; Weiner, 2013). The results were pooled in a meta-analysis. The pooled overall success rate in the ultrasound-guided placement group was 171/220 (77.7%), compared to 134/208 (64.4%) the landmark-based placement group. This resulted in a pooled RR of 0.84 (95% CI 0.69 to 1.02), in favor of ultrasound-guided placement (see figure 2). This was not considered clinically relevant.
Figure 2. Forest plot showing the comparison between ultrasound-guided placement and landmark-based placement for the overall success rate of peripheral intravenous catheter placement in patients with difficult vascular access
Pooled risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
1.2 First attempt success rate
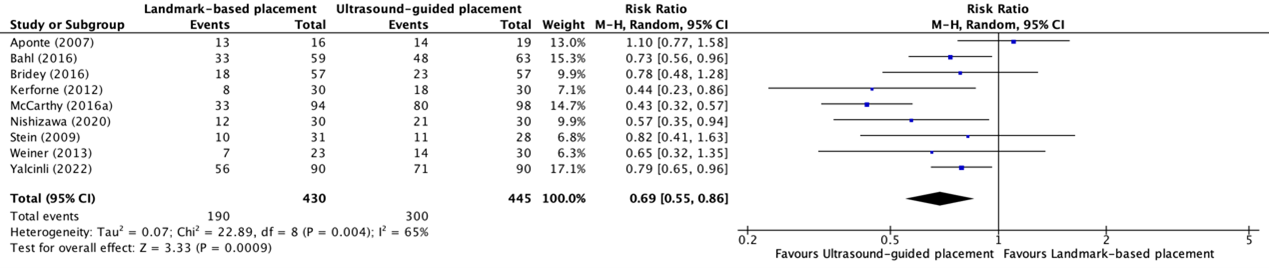
The first attempt success rate of placement of peripheral intravenous catheter in patients with difficult vascular access between ultrasound-guided placement and landmark-based placement was reported in eight trials included in the systematic review of Tada (2022) (Aponte, 2007; Bahl, 2016; Bridey, 2018; Kerforne, 2012; McCarthy, 2016a; Nishizawa, 2020; Stein, 2009; Weiner, 2013) and in one additional trial (Yalcinli, 2022). The results were pooled in a meta-analysis. The pooled first attempt success rate in the ultrasound-guided placement group was 300/445 (67.4%), compared to 190/430 (44.1%) in the landmark-based placement group. This resulted in a pooled RR of 0.69 (95% CI 0.55 to 0.86), in favor of ultrasound-guided placement (see figure 3). This was considered clinically relevant.
Figure 3. Forest plot showing the comparison between ultrasound-guided placement and landmark-based placement for the first attempt success rate of peripheral intravenous catheter placement in patients with difficult vascular access
Pooled risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
2. Complications (critical)
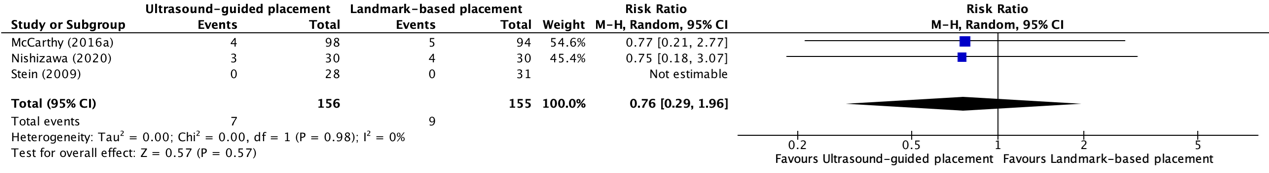
Complications because of peripheral intravenous catheter placement in patients with difficult vascular access between ultrasound-guided placement and landmark-based placement were reported in three trials included in the systematic review of Tada (2022) (Stein, 2009; McCarthy, 2016a; Nishizawa, 2020). The results were pooled in a meta-analysis. The pooled number of complications in the ultrasound-guided placement group was 7/156 (4.5%), compared to 9/155 (5.8%) in the landmark-based placement group. This resulted in a pooled RR of 0.76 (95% CI 0.29 to 1.96), in favor of ultrasound-guided placement (see figure 4). This was considered as clinically relevant. Complications were not further specified.
Figure 4. Forest plot showing the comparison between ultrasound-guided placement and landmark-based placement for complication rate of peripheral intravenous catheter placement in patients with difficult vascular access
Pooled risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
3. Number of attempts (important)
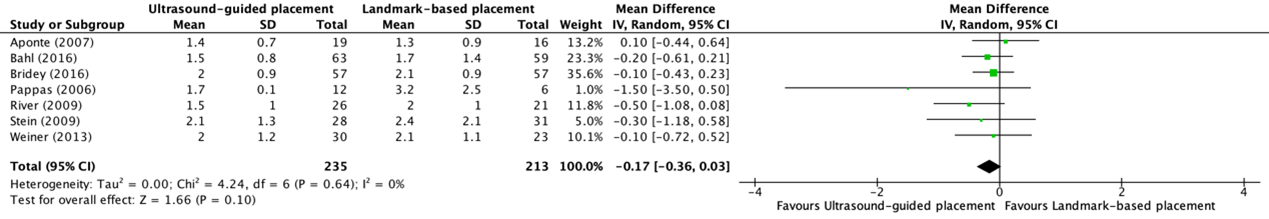
The number of attempts for placement of a peripheral intravenous catheter in patients with difficult vascular access was reported in seven trials included in the systematic review of Tada (2022) (Aponte, 2007; Bahl, 2016; Bridey, 2018; Pappas, 2006; River, 2009; Stein, 2009; Weiner, 2013) and in one additional trial (Yalcinli, 2022). The results of these studies, except for Yalcinli (2022), were pooled in a meta-analysis. The pooled mean difference (MD) in number of attempts between ultrasound-guided (N=235) and landmark-based placement (N=213) of a peripheral intravenous catheter was -0.17 (95% CI -0.36 to 0.03) attempts (see figure 5).
Figure 5. Forest plot showing the comparison between ultrasound-guided placement and landmark-based placement for number of attempts of peripheral intravenous catheter placement in patients with difficult vascular access
Pooled mean difference, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
Yalcinli (2022) reported the median (IQR) number of attempts and could therefore not be pooled in the meta-analysis. The median (IQR) number of attempts of peripheral intravenous catheter placement in the ultrasound-guided placement group (N=90) was 1.0 (1.0 to 1.0) attempts, compared to 1.0 (IQR 1.0 to 2.0) seconds in the landmark-based placement group.
4. Duration of the procedure (important)
The duration of the procedures was reported in two trials (Aponte, 2007; McCarthy, 2016a). The results were pooled in a meta-analysis. The pooled MD between ultrasound-guided placement (N=112) and landmark-based placement (N=107) of a peripheral intravenous catheter was 119.85 (95% CI 88.64 to 151.05) seconds.
Figure 6. Forest plot showing the comparison between ultrasound-guided placement and landmark-based placement for duration of the procedure of peripheral intravenous catheter placement in patients with difficult vascular access
Pooled mean difference, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
5. Mortality (important)
None of the included studies reported mortality rates for peripheral intravenous catheter placement in patients with difficult intravenous access.
6. Quality of life (important)
None of the included studies reported information regarding quality of life for peripheral intravenous catheter placement in patients with difficult vascular access.
7. Patient satisfaction (important)
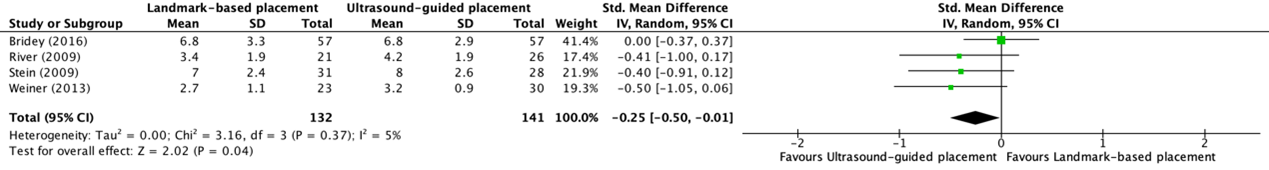
Patient satisfaction was reported in four trials from the systematic review of Tada (2022) (Bridey, 2018; River, 2009; Stein, 2009; Weiner, 2013). Patient satisfaction was measured on a numeric rating scale from zero to ten or a four-step Likert scale. The higher the score, the higher the level of satisfaction. The results were pooled in a meta-analysis. The pooled standardized MD in patient satisfaction between ultrasound-guided (N=141) and landmark-based placement (N=132) of peripheral intravenous catheters was -0.25 (95% CI -0.50 to -0.01), in favor of ultrasound-guided placement (see figure 7). This was not considered as clinically relevant.
Figure 7. Forest plot showing the comparison between ultrasound-guided placement and landmark-based placement for patient’s satisfaction of peripheral intravenous catheter placement in patients with difficult vascular access
Pooled mean difference, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
8. Catheter-related interventions (important)
Catheter-related interventions rates due to ultrasound-guided or landmark-based placement of peripheral intravenous catheters in patients with difficult vascular access were reported in one trial (Yalcinli, 2022). Yalcinli (2022) defined catheter-related interventions as ‘need for rescue methods’. The number of patients who required catheter-related interventions in the ultrasound-guided placement group was 17/90 (18.9%), compared to 17/90 (18.9%) in the landmark-based placement group. This resulted in a RR of 1.00 (95% CI 0.55 to 1.83), not in favor of one of the groups. This was not considered as clinically relevant.
9. Thrombophlebitis (important)
None of the included studies reported thrombophlebitis rates for peripheral intravenous catheter placement in patients with difficult vascular access.
Level of evidence of the literature
1. Success rate (critical)
1.1 Overall success rate
The level of evidence regarding the outcome overall success rate of peripheral intravenous catheter placement in patients with difficult vascular access was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by one level because of the wide confidence interval crossing the lower threshold of clinical relevance (imprecision, -1). The level of evidence was considered as moderate.
1.2 First attempt success rate
The level of evidence regarding the outcome first attempt success rate of peripheral intravenous catheter placement in patients with difficult vascular access was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by two levels because of the wide confidence interval crossing the lower threshold of clinical relevance (imprecision, -1). The level of evidence was considered as moderate.
2. Complications (critical)
The level of evidence regarding the outcome complications of peripheral intravenous catheter placement in patients with difficult vascular access was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of the wide confidence interval crossing both thresholds of clinical relevance (imprecision, -3). The level of evidence was considered as very low.
3. Number of attempts (important)
The evidence could not be graded due to the absence of a definition for the minimally important difference.
4. Duration of the procedure (important)
The evidence could not be graded due to the absence of a definition for the minimally important difference.
5. Mortality (important)
None of the included studies reported mortality rates of peripheral intravenous catheter placement in patients with difficult vascular access. Therefore, the level of evidence could not be determined.
6. Quality of life (important)
None of the included studies reported patient’s quality of life after peripheral intravenous catheter placement in patients with difficult vascular access. Therefore, the level of evidence could not be determined.
7. Patients’ satisfaction (important)
The level of evidence regarding the outcome first attempt success rate of peripheral intravenous catheter placement in patients with difficult vascular access was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by two levels because of the small number of included patients (imprecision, -1). The level of evidence was considered as moderate.
8. Catheter-related interventions (important)
The level of evidence regarding the outcome first attempt success rate of peripheral intravenous catheter placement in patients with difficult vascular access was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by two levels because of the wide confidence interval crossing both thresholds of clinical relevance (imprecision, -2). The level of evidence was considered as low.
9. Thrombophlebitis (important)
None of the included studies reported thrombophlebitis rates of peripheral intravenous catheter placement in patients with difficult vascular access. Therefore, the level of evidence could not be determined.
Table 3. Summary of findings PICO 2
PICO 3. Peripherally inserted central catheter (PICC)
No studies were included that reported outcomes between ultrasound-guided placement and landmark-guided placement for peripherally inserted central catheters.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following questions:
- What are the benefits and harms of ultrasound-guided placement in comparison with landmark-based placement in adult patients receiving a central venous catheter?
- What are the benefits and harms of ultrasound-guided placement in comparison with landmark-based placement in adult patients receiving a peripheral intravenous catheter?
- What are the benefits and harms of ultrasound-guided placement in comparison with landmark-based placement in adult patients receiving a peripherally inserted central catheter?
PICO 1. Central venous catheters (CVC)
| P: | Adult patients receiving a central venous catheter |
| I: | Ultrasound-guided placement |
| C: | Landmark-based placement |
| O: | Success rate, procedure duration, complications, quality of life, patient satisfaction, catheter-related interventions per time, mortality, thrombophlebitis |
PICO 2. Peripheral intravenous catheters
| P: |
Adult patients with difficult venous access receiving a peripheral intravenous catheter |
| I: |
Ultrasound-guided placement |
| C: |
Landmark-based placement |
| O: | Success rate, procedure duration, complications, quality of life, patient satisfaction, catheter-related interventions per time, mortality, thrombophlebitis |
PICO 3: Peripherally inserted central catheter (PICC)
| P: |
Adult patients receiving a peripherally inserted central catheter |
| I: |
Ultrasound-guided placement |
| C: |
Landmark-based placement |
| O: | Success rate, procedure duration, complications, quality of life, patient satisfaction, catheter-related interventions per time, mortality, thrombophlebitis |
Relevant outcome measures
The guideline development group considered complications and success rate as critical outcomes for decision making; and procedure duration, quality of life, patient satisfaction, catheter-related interventions, mortality, and thrombophlebitis as important outcomes for decision making.
The working group defined a threshold of 10% for continuous outcomes, a relative risk (RR) for dichotomous outcomes of <0.80 and >1.25, or a difference of 0.5 standard deviations for standardized mean differences (SMD) as minimal clinically (patient) important differences. For procedure duration and number of attempts, no minimal important differences were defined.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until the 7th of February 2024. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 721 hits. Studies were selected based on the following criteria: Systematic reviews and randomized controlled trials on ultrasound-guided puncturing of a central venous or peripheral line. Twenty-nine studies were initially selected based on title and abstract screening. After reading the full text, twelve studies were excluded (see the table with reasons for exclusion under the tab Methods) and seventeen studies were included.
Results
Seventeen studies were included in the analysis of the literature. Fifteen studies for PICO 1 and two studies for PICO 2. No studies were included for PICO 3. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Airapetian N, Maizel J, Langelle F, Modeliar SS, Karakitsos D, Dupont H, Slama M. Ultrasound-guided central venous cannulation is superior to quick-look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013 Nov;39(11):1938-44. doi: 10.1007/s00134-013-3072-z. Epub 2013 Sep 12. PMID: 24026296.
- Benali M, Trabelsi B, Abdouli H, Yedes A, Elhadj Kacem H, Fki M. Ultrasound guidance versus anatomical landmarks for subclavian vein catheterization: a prospective study. Tunis Med. 2022 juillet;100(7):520-524. PMID: 36571740; PMCID: PMC9703904.
- Dolu H, Goksu S, Sahin L, Ozen O, Eken L. Comparison of an ultrasound-guided technique versus a landmark-guided technique for internal jugular vein cannulation. J Clin Monit Comput. 2015 Feb;29(1):177-82. doi: 10.1007/s10877-014-9585-3. Epub 2014 May 18. PMID: 24838550.
- Ehtesham, Atiyah & Patkar, Charushila & Phalgune, Deepak. (2020). Study between Ultrasound Guided Technique and Conventional Landmark Technique for Internal Jugular Vein Cannulation: A Randomised Controlled Trial. JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH. 14. 10.7860/JCDR/2020/43578.13597.
- Fathi M, Izanloo A, Jahanbakhsh S, Taghavi Gilani M, Majidzadeh A, Sabri Benhangi A, Paravi N. Central Venous Cannulation of the Internal Jugular Vein Using Ultrasound-Guided and Anatomical Landmark Techniques. Anesth Pain Med. 2016 May 9;6(3):e35803. doi: 10.5812/aapm.35803. PMID: 27642580; PMCID: PMC5018146.
- Nazari I, Musavi M, Alavi M. Comparing Outcomes and Complication of Central Venous Cannulation Using Both Conventional and Ultrasound Guide. Biosci Biotechnol Res Asia 2015;12(3).
- Oh AY, Jeon YT, Choi EJ, Ryu JH, Hwang JW, Park HP, Do SH. The influence of the direction of J-tip on the placement of a subclavian catheter: real time ultrasound-guided cannulation versus landmark method, a randomized controlled trial. BMC Anesthesiol. 2014 Feb 28;14:11. doi: 10.1186/1471-2253-14-11. PMID: 24581318; PMCID: PMC3975933.
- Rando K, Castelli J, Pratt JP, Scavino M, Rey G, Rocca ME, Zunini G. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessel. 2014;6(1):13-23. PMID: 24800194; PMCID: PMC4009593.
- Riaz A, Shan Khan RA, Salim F. Ultrasound guided internal jugular venous cannulation: comparison with land-mark technique. J Coll Physicians Surg Pak. 2015 May;25(5):315-9. PMID: 26008653.
- Srinivasan S, Govil D, Gupta S, Patel S, Jagadeesh KN, Tomar DS. Incidence of posterior wall penetration during internal jugular vein cannulation: A comparison of two techniques using real-time ultrasound. Indian J Anaesth. 2017 Mar;61(3):240-244. doi: 10.4103/ija.IJA_632_16. PMID: 28405038; PMCID: PMC5372405.
- Subramony R, Spann R, Medak A, Campbell C. Ultrasound-Guided vs. Landmark Method for Subclavian Vein Catheterization in an Academic Emergency Department. J Emerg Med. 2022 Jun;62(6):760-768. doi: 10.1016/j.jemermed.2021.11.002. Epub 2022 May 11. PMID: 35562246.
- Tada M, Yamada N, Matsumoto T, Takeda C, Furukawa TA, Watanabe N. Ultrasound guidance versus landmark method for peripheral venous cannulation in adults. Cochrane Database Syst Rev. 2022 Dec 12;12(12):CD013434. doi: 10.1002/14651858.CD013434.pub2. PMID: 36507736; PMCID: PMC9744071.
- Vinayagamurugan A, Badhe AS, Jha AK. Comparison of external jugular vein-based surface landmark approach and ultrasound-guided approach for internal jugular venous cannulation: A randomised crossover clinical trial. Int J Clin Pract. 2021 Mar;75(3):e13783. doi: 10.1111/ijcp.13783. Epub 2020 Nov 13. PMID: 33095965.
- Wang Q, Cai J, Lu Z, Zhao Q, Yang Y, Sun L, He Q, Xu S. Static Ultrasound Guidance VS. Anatomical Landmarks for Subclavian Vein Puncture in the Intensive Care Unit: A Pilot Randomized Controlled Study. J Emerg Med. 2020 Dec;59(6):918-926. doi: 10.1016/j.jemermed.2020.07.039. Epub 2020 Sep 22. PMID: 32978029.
- Yalçınlı S, Karbek Akarca F, Can Ö, Uz İ, Konakçı G. Comparison of Standard Technique, Ultrasonography, and Near-Infrared Light in Difficult Peripheral Vascular Access: A Randomized Controlled Trial. Prehosp Disaster Med. 2022 Feb;37(1):65-70. doi: 10.1017/S1049023X21001217. Epub 2021 Dec 6. PMID: 34865664.
- Zhang YS, Zhang SL, Guo WM, Liu T, Ma YJ. Clinical Effect of Modified Ultrasound-Guided Subclavian Vein Puncture. Int J Clin Pract. 2023 Jul 6;2023:5534451. doi: 10.1155/2023/5534451. PMID: 37457808; PMCID: PMC10344633.
Evidence tabellen
Systematic review(s) PICO 1
Niet van toepassing.
Systematic review(s) PICO 2
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Tada (2022) |
SR and meta-analysis of RCTs
Literature search up to the 29th of November 2021.
A: Aponte (2007) B: Bahl (2016) C: Bridey (2018) D: Costantino (2005) E: Glasin (2020) F: Ismailoglu (2015) I: McCarthy (2016B) K: Nishizama (2020) L: Pappas (2006) M: River (2009) N: Skulec (2019) O: Stein (2009) P: Weiner (2013)
Study design: A: RCT B: RCT C: RCT D: RCT E: RCT F: RCT H: RCT I: RCT J: RCT K: RCT L: RCT M: RCT N: RCT O: RCT P: RCT
Setting and Country: A: Operating room B: Emergency department C: ICU D: Emergency department E: Emergency department F: Emergency department H: Emergency department I: Emergency department J: Emergency department K: ICU L: Operating room M: Emergency department N: Prehospital O: Emergency department P: Emergency department
Source of funding and conflicts of interest: MT: declared that his institute received research grants from Nakatani Foundation (ongoing multicentre prospective cohort study for myocardial infarction in the emergency department) and Radiometer America, Inc. (ongoing multicentre prospective cohort study of myocardial infarction in the emergency department). MT declared that he has received royalties from Japan Medical Journal as he coauthored a textbook about ultrasound-guided peripheral intravenous cannulation in emergency medicine. The textbook is about the technical issues of the review intervention. It explains the review intervention as one of various options and is not intended to promote the review intervention. Japan Medical Journal has no role in this Cochrane Review and meta-analysis. NY: none known TM: none known CT: none known TF: has received financial paymentfor speaker's fees (Mitsubishi Tanabe Pharma Corporation), clinicaltrial consultancy (Mitsubishi Tanabe Pharma Corporation, Sony Electronics), scientific advisory board (Kyoto University Original), grant (Shionogi) and declares intellectual properties and patent-pending (2020-548587) for smartphone CBT apps (Mitsubishi Tanabe Pharma Corporation). NW: his institution has received research funds from the Japanese Ministry of Health Labor and Welfare and the Japanese Ministry of Education, Science, and Technology.He has also received royalties from Sogensha and Akatsuki for writing a book and developing soNware about interventions for insomnia. This review is completely independent from the intention of these grants.
|
Inclusion criteria SR:
Exclusion criteria
16 studies included
Important patient characteristics at baseline:
N A: 35 B: 122 C: 114 D: 60 E: 90 F: 60 H: 192 I: 401 J: 596 K: 60 L: 18 M: 47 N: 300 O: 59 P: 53
Groups comparable at baseline? Yes. |
Describe intervention:
A: Ultrasound guidance. B: Ultrasound guidance. C: Ultrasound guidance. D: Ultrasound guidance. E: Ultrasound guidance. F: Ultrasound guidance. H: Ultrasound guidance. I: Ultrasound guidance. J: Ultrasound guidance. K: Ultrasound guidance. L: Ultrasound guidance. M: Ultrasound guidance. N: Ultrasound guidance. O: Ultrasound guidance. P: Ultrasound guidance.
|
Describe control:
A: Landmark technique. B: Landmark technique. C: Landmark technique. D: Landmark technique. E: Landmark technique. F: Landmark technique. H: Landmark technique. I: Landmark technique. J: Landmark technique. K: Landmark technique. L: Landmark technique. M: Landmark technique. N: Landmark technique. O: Landmark technique. P: Landmark technique.
|
End-point of follow-up:
A: No information. B: No information. C: No information. D: No information. E: No information. F: No information. H: No information. I: No information. J: No information. K: No information. L: No information. M: No information. N: No information. O: No information. P: No information.
For how many participants were no complete outcome data available? (intervention/control) A: None. B: None. C: None. D: None. E: None. F: None. H: None. I: None. J: None. K: None. L: None. M: None. N: None. O: None. P: None.
|
Results See original publication.
|
Author’s conclusion There is very low- and low-certainty evidence that, compared to the landmark method, ultrasound guidance may benefit difficult participants for increased first-pass and overall success of cannulation, with no difference detected in pain. There is moderate- and low certainty evidence that, compared to the landmark method, ultrasound guidance may benefit moderately difficult participants due to a small increased first-pass success of cannulation with no difference detected in pain. There is moderate- and high-certainty evidence that, compared to the landmark method, ultrasound guidance does not benefit easy participants: ultrasound guidance decreased the first-pass success of cannulation with no difference detected in overall success of cannulation and increased pain. |
Systematic review(s) PICO 3
Not applicable.
Randomized controlled trial(s) PICO 1
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Airapetian (2013) |
Type of study: Randomized controlled trial.
Setting and country: Eight-bed medical ICU of a university hospital.
Funding and conflicts of interest: Authors have no conflict of interest. No information regarding funding.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Jugular vein I: N = 21 C: N = 21
Femoral vein I: N = 15 C: N = 10
Important prognostic factors2: age ± SD: I: 63 (15) years. C: 67 (16) years.
Sex: I: Ratio 2.6 C: Ratio 1.9
BMI: I: 25 (6) kg/m2 C: 28 (6) kg/m2
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound-guided technique.
|
Describe control (treatment/procedure/test):
Landmark technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate Jugular vein I: 21/21 (100%) C: 21/28 (75%)
Femoral vein I: 15/15 (100%) C: 7/10 (70%)
All sites I: 36/36 (100%) C: 28/38 (74.0%)
Complications Jugular vein I: 0/21 (0%) C: 7/28 (25.0%)
Femoral vein I: 0/15 (0%) C: 2/10 (20.0%)
Number of attempts I: 1 times C: 3 (1) times
Access time I: 4 (2) minutes C: 8 (7) minutes
Catheter colonization I: 9/36 (25.0%) C: 7/28 (18.0%) |
Author’s conclusion: Ultrasound-guided cannulation of the internal jugular or femoral vein by inexperienced residents appears to be more reliable than the LM or UM methods and was associated with a lower mechanical complication rate among ICU patients. |
|
Benali (2022) |
Type of study: Randomized controlled trial.
Setting and country: No information.
Funding and conflicts of interest: No information.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Subclavian vein Intervention: N = 35 Control: N = 35
Important prognostic factors2: age ± SD: I: 48 (20) years. C: 44 (18) years.
BMI >30 I: 7/35 (20%) C: 4/35 (11.4%)
BMI <30 I: 28/35 (80.0%) C: 31/35 (88.6%)
Groups comparable at baseline? |
Describe intervention (treatment/procedure/test):
Ultrasound guidance
|
Describe control (treatment/procedure/test):
Landmark technique |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate (overall) I: 35/35 (100%) C: 30/35 (85.7%)
Success rate (obesitas) I: 7/7 (100%) C: 1/4 (25.0%)
First attempt success rate (overall) I: 29/35 (82.9%) C: 14/35 (40.0%)
Number of attempts (overall) I: 1 (range 1 to 1) C: 2 (range 1 to 4)
Complications (overall) Arterial puncture I: 0/35 (0%) C: 5/35 (14.3%)
Hematoma I: 0/35 (0%) C: 9/35 (25.7%)
Pneumothorax I: 0/35 (0%) C: 2/35 (5.7%)
Malposition I: 2/35 (5.7%) C: 1/35 (2.9%) |
Author’s conclusion: According to our study, US guidance for SCV catheterization seems to be an interesting alternative to anatomical landmarks approaches. |
|
Dolu (2015) |
Type of study: Randomized controlled trial.
Setting and country: Medical faculty of Gaziantep University.
Funding and conflicts of interest: The authors declare that they have no conflict of interest.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Jugularis vein Intervention: N = 50 Control: N = 50
Important prognostic factors2: age ± SD: I: 53.6 (5.8) years. C: 53.2 (9.10) years.
BMI: I: 25.7 (2.6) kg/m2 C: 26.6 (3.7) kg/m2
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound guidance.
|
Describe control (treatment/procedure/test):
Landmark technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Number of needles passes I: 1.1 (0.5) needles C: 2.2 (1.6) needles
Duration of procedure in seconds I: 109.4 (30.4) seconds C: 165.9 (91.5) seconds
Complications Arterial puncture I: 0/50 (0%) C: 4/50 (8.0%)
Hematoma I:(1/50 (2.0%) C: 1/50 (2.0%)
Total I: 1/50 (2.0%) C: 5/50 (10.0%) |
Author’s conclusion: The findings of this study indicate that internal jugular vein catheterization guided by real-time ultrasound results in a lower access time and a lower rate of attempts. |
|
Ethesham (2020) |
Type of study: Randomized controlled trial.
Setting and country: Poona Hospital and Research Centre, Pune, India
Funding and conflicts of interest: None declared.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Internal jugular vein Intervention: N = 45 Control: N = 45
Important prognostic factors2: age ± SD: I: 47.3 (13.6) years. C: 46.4 (14.2) years.
Sex: I: 22/45 (48.9%) M C: 28/45 (62.2%) M
BMI around 22 kg/m2 in both groups.
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound-guidance. |
Describe control (treatment/procedure/test):
Landmark-based technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate I: 45/45 (100%) C: 43/45 (95.6%)
Complications Nil I: 45/45 (100%) C: 33/45 (73.3%)
Hematoma I: 0/45 (0%) C: 8/45 (17.8%)
Carotid artery puncture I: 0/45 (0%) C: 4/45 (8.9%)
Mean time required for the procedure in minutes I: 4.2 (0.4) minutes C: 4.7 (0.8) minutes |
Author’s conclusion: USG guided cannulation of IJV decreases access time, reduces attempts, and complication rates. USG guided technique may be preferred for cannulation of IJV. |
|
Faithi (2016) |
Type of study: Randomized controlled trial.
Setting and country: No information.
Funding and conflicts of interest: This study was supported by the research deputy of Mashhad at the University of Medical Sciences
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Internal jugular vein Intervention: N = 170 Control: N = 151
Important prognostic factors2: age ± SD: I: 64.45 (11.29) years. C: 62.15 (9.76) years.
Sex: I: 103/170 M C: 102/151 M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound guidance.
|
Describe control (treatment/procedure/test):
Landmark-based technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate First attempt I: 156/170 (91.8%) C: 140/151 (92.7%)
Second attempt I: 8/170 (4.2%) C: 6/151 (4.0%)
Third attempt I: 6/170 (3.6%) C: 5/151 (3.3%)
Time required for cannulation I: 46.05 (12.7) seconds C: 45.56 (10.9) seconds |
Author’s conclusion: In our conditions, the use of an anatomical landmark-guided procedure was the preferred treatment method due to limited resources and a lack of adequate training. |
|
Nazari (2015) |
Type of study: Randomized controlled trial.
Setting and country: No information.
Funding and conflicts of interest: No information. |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Internal jugular vein Intervention: N = 168 Control: N = 168
Important prognostic factors2: age ± SD: I: 54.8 (7.6) years. C: 49.9 (10.6) years.
Sex: I: 90/168 (53.5%) M C: 87/168 (51.7%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound guidance.
|
Describe control (treatment/procedure/test):
Landmark-based technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate From skin prep to successful aspiration (in seconds) I: 132.52 (15.1) seconds. C: 169.2 (16.21)
Number of patients required more than one attempt I: 22/168 (13.09%) C: 75/168 (44.6%)
Successful cannulation Mean number of attempts I: 1.4 (0.42) C: 1.98 (0.61) |
Author’s conclusion: The results of our study showed that USG approach took lesser time, required lesser attempts, and had lower incidence of complications for cannulation of the internal jugular vein. |
|
Oh (2014) |
Type of study: Randomized controlled trial.
Setting and country: Seoul National University Bundang Hospital.
Funding and conflicts of interest: The authors declare that they have no competing interests
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Subclavian Intervention: N = 30 Control: N = 30
Important prognostic factors2: age ± SD: I: 51 (14) years. C: 50 (16) years.
Sex: I: 15/30 (50%) M C: 16/30 (53.3%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound guidance.
|
Describe control (treatment/procedure/test):
Landmark-based technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate I: 26/30 (86.7%) C: 16/30 (53.3%)
Complications I: 0/30 (0%) C: 0/30 (0%) |
Author’s conclusion: The proper placement of guidewire was less influenced by the direction of the guidewire J-tip with ultrasound-guided subclavian venous cannulation than with the landmark approach. |
|
Palkhiwala (2020) |
Type of study: Randomized controlled trial.
Setting and country: No information.
Funding and conflicts of interest: No information.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Jugularis Intervention: N = 30 Control: N = 30
Important prognostic factors2: age ± SD: I: 53.1 (15.05) years. C: 50.1 (15.70) years.
Sex: I: 20/30 (66.7%) M C: 22/30 (73.3%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound guidance.
|
Describe control (treatment/procedure/test):
Landmark-based technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate First attempt I: 28/30 (93.33%) C: 20.30 (66.67%)
Second attempt I: 2/30 (6.67%) C: 7/30 (23.34%)
Third attempt I: 0/30 (0%) C: 3/30 (10.0%)
Complications Total I: 2/30 (6.67%) C: 9/30 (30.0%)
Hematoma I: 1/30 (3.33%) C: 2/30 (6.67%)
Carotid artery puncture I: 0/30 (0%) C: 3/30 (10.0%)
Pneumothorax I: 0/30 (0%) C: 1/30 (3.3%)
Double wall puncture I: 1/30 (3.3%) C: 3/30 (10.0%)
Number of attempts I: 1.06 (0.24) C: 1.43 (0.66)
Access time I: 9.63 (1.85) seconds C: 19.30 (8.85) seconds |
Author’s conclusion: We conclude that use of ultrasound makes cannulation of the IJV a much safer technique, especially in high-risk patients, and leaves almost none to minimal chances of any complications. With experience, expertise and under real-time vision, the contra-indications to a central line insertion are almost nullified |
|
Rando (2014) |
Type of study: Randomized controlled trial.
Setting and country: Intensive care unit and operating rooms of the military hospital in motevideo, Uruguay.
Funding and conflicts of interest: None declared.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Jugular vein Intervention: N = 123 Control: N = 134
Important prognostic factors2: age ± SD: Ranging from a mean of 55 to 62 years.
BMI: Ranging from 27.5 to 27.8 kg/m2
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound guidance.
|
Describe control (treatment/procedure/test):
Landmark technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate (overall) I: 112/123 (91.0%) C: 105/134 (78.0%)
Success rate (difficult neck) I: 42/46 (93.0%) C: 24/37 (65.0%)
Complications (overall) I: 10/123 (8.1%) C: 20/134 (15.0%)
Multiple puncture (overall) I: 48/123 (39.0%) C: 54/134 (40.0%)
|
Author’s conclusion: Ultrasound reduces the incidence of complications when placement is performed by inexperienced operators. Centers with residents should emphasize the necessity of ultrasound for central line catheterization. Training in ultrasound might be of paramount importance in the effectiveness of the technique. |
|
Riaz (2015) |
Type of study: Randomized controlled trial.
Setting and country: Anaesthesia department.
Funding and conflicts of interest: No information.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Jugularis Intervention: N = 100 Control: N = 100
Important prognostic factors2: age ± SD: I: 44.25 (14.43) years. C: 48.59 (14.57) years.
Sex: I: 74/100 (74.0%) M C: 81/100 (81.0%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound guidance.
|
Describe control (treatment/procedure/test):
Landmark-based technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate First attempt I: 99/100 (99.0%) C: 89/100 (89.0%)
Second attempt I: 1/100 (1.0%) C: 7/100 (7.0%)
Complications Carotid artery puncture I: 1/100 (1.0%) C: 9/100 (9.0%)
Irritation of brachial plexus I: 0/100 (0%) C: 6/100 (6.0%)
Hematoma I: 0/100 (0%) C: 7/100 (07.0%)
Hemothorax I: 0/100 (0%) C: 0/100 (0%)
Pneumothorax I: 0/100 (0%) C: 0/100 (0%)
Access time (in seconds) I: 34.95 (11.47) seconds C: 146.59 (40.20) seconds |
Author’s conclusion: Access time, failure rate and procedure related complications are reduced when real-time ultrasonography is used to cannulate internal Jugular vein. |
|
Srinivasan (2017) |
Type of study: Randomized controlled trial.
Setting and country: Adult Gastroenterology and Liver Intensive Care Unit.
Funding and conflicts of interest: There are no conflicts of interest. No funding.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Jugularis Intervention: N = 90 Control: N = 80
Important prognostic factors2: age ± SD: I: 52.1 (14.2) C: 48.6 (15.7)
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound guidance.
|
Describe control (treatment/procedure/test):
Landmark-based technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate First attempt I: 90//90 (100.0%) C: 60/80 (75.0%)
Second attempt I: 0/90 (0%) C: 18/80 (22.5%)
Third attempt I: 0/90 (0%) C: 2/80 (2.5%)
Complications Posterior wall puncture of internal jugular vein I: 19/90 (21.0%) C: 37/80 (46.0%)
Inadvertent arterial puncture I: 5/90 (5.5%) C: 8/80 (10.0%)
Hematoma I: 1/90 (1.1%) C: 11/80 (13.8%)
Pneumothorax I: 0/90 (0%) C: 1/80 (1.3%)
|
Author’s conclusion: Real-time ultrasound-guided IJV cannulation significantly reduces but does not wholly eliminate the incidence of posterior venous wall penetrations. It also significantly reduces the incidence of inadvertent arterial punctures and number of attempts for successful cannulation. |
|
Subramony (2022) |
Type of study: Randomized controlled trial.
Setting and country: Urban tertiary care teaching hospital.
Funding and conflicts of interest:
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Subclavian vein Intervention: N = 44 Control: N = 41
Important prognostic factors2: age ± SD: No information
Sex: No information
Groups comparable at baseline? No information. |
Describe intervention (treatment/procedure/test):
Ultrasound-guidance.
|
Describe control (treatment/procedure/test):
Landmark technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate I: 35/44 (79.5%) C: 24/41 (58.5%)
|
Author’s conclusion: Ultrasound-guided subclavian vein catheterization was found to be associated with a higher overall success rate compared with the landmark method with no significant difference with respect to complication rate in an ED setting. |
|
Vinayagamurugan (2021) |
Type of study: Randomized cross-over clinical trial.
Setting and country: Tertiary care University hospital.
Funding and conflicts of interest: None.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Jugularis Intervention: N = 94 Control: N = 94
Important prognostic factors2: age ± SD: I: 45.9 (14.32) years. C: 45.01 (15.35) years.
Sex: I: 54/94/ (57.44%) M C: 61/94 (64.89%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound guidance.
|
Describe control (treatment/procedure/test):
Landmark-based technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate (overall) I: 94/94 (100%) C: 94/94 (100%)
Success rate (first attempt) I: 84/94 (89.34%) C: 75/94 (79.78%)
Complications Total I: 4/94 (4.25%) C: 12/94 (12.76%)
Carotid artery puncture I: 2/94 (2.12%) C: 2/94 (2.12%)
Hematoma I: 2/94 (2.12%) C: 4/94 (4.25%)
Mechanical complications (pneumothorax, hemothorax, hemomediastinum, cardic tamponade, nerve injury, catheter malposition) I: 0/94 (0%) C: 0/94 (0%)
Cannulation time I: 44.27 (5.28) seconds C: 58.11 (6.6) seconds. |
Author’s conclusion: In patients with non-distorted neck anatomy and a visible EJV, IJV catheterization using the EJV-based LM approach and standard US-guided technique yielded similar first attempt and overall success rates. Cannulation time was longer and complications occurred more frequently in the EJV-based LM compared to the standard US-guided technique. |
|
Wang (2020) |
Type of study: Pilot RCT.
Setting and country: ICU hospital.
Funding and conflicts of interest: This study was funded by the grants from the Science and Technology Bureau of Jiaxing city, Zhejiang, China (No.2017AY33034 to J.M. Cai; and No.2020AD30082 to Q.Y. Wang).
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Subclavian Intervention: N = 96 Control: N = 98
Important prognostic factors2: age ± SD: I: 21.6 (8.7) C: 22.5 (8.3)
Sex: I: 23/96 (24.0%) F C: 34/98 (34.7%) F
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound guidance.
|
Describe control (treatment/procedure/test):
Landmark-based technique. |
Length of follow-up:
Loss-to-follow-up:
|
Success rate I: 88/96 (91.7%) C: 76/98 (77.6%)
First attempt success I: 57/96 (59.4%) C: 48/98 (49.0%)
Complications Total I: 7/96 (7.3%) C: 20/98 (20.4%)
Mispuncture of artery I: 2/96 (2.1%) C: 14/98 (14.3%)
Hematoma I: 0/96 (0%) C: 1/98 (1.0%)
Pneumothorax I: 0/96 (0%) C: 2/98 (2.0%)
Number of punctures I: 1.6 (1.0) C: 1.5 (0.7)
Puncture time I: 50 (47) seconds C: 62 (53) seconds
|
Author’s conclusion: Static ultrasoundguided subclavian vein puncture is superior to the traditional landmark-guided approach for critically ill patients in the ICU. It is suggested that static ultrasound-guided puncture techniques should be considered for subclavian vein puncture in the ICU. |
|
Zhang (2023) |
Type of study: Randomized controlled trial.
Setting and country: No information.
Funding and conflicts of interest: The authors declare that they have no conflicts of interest.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Subclavian vein Intervention: N = 30 Control: N = 30
Important prognostic factors2: age ± SD: I: <60 N = 4 / >60 N = 26 C: <60 N =5 / >60 N = 25
Sex: I: 27/30 M C: 22/30 M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound-guidance.
|
Describe control (treatment/procedure/test):
Landmark technique. |
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Success rate First attempt I: 24/30 C: 20/30
Total I: 30/30 C: 30/30
Complications Pneumothorax I: 1/30 C: 5/30
Hematoncus I: 2/30 C: 6/30
Intubation time I: 6.3333 minutes C: 11.3667 minutes |
Author’s conclusion: Te improved ultrasound-guided subclavian vein catheterization technique can greatly reduce the catheterization time and improve the success rate of puncture and catheterization. It can also reduce the occurrence of complications and damage to adjacent tissues. Te operation is simple, fast, and easy to master, and it has a high popularization clinical value. |
Randomized controlled trial(s) PICO 2
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Yalcinli (2022) |
Type of study: Randomized controlled trial.
Setting and country: An emergency department of an academic tertiary care hospital
Funding and conflicts of interest: No conflicts of interest declared. This article received no specific grant from any funding agency.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 90 Control: N = 90
Important prognostic factors2: age (median. IQR): I: 64 (49 to 77) C: 68.5 (51 to 76)
Sex: I: 39/90 male C: 34/90 male
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
Ultrasound-guided placement.
|
Describe control (treatment/procedure/test):
Landmark-based technique. |
Length of follow-up: No information.
Loss-to-follow-up: None. |
First attempt success I: 71/90 (78.9%) C: 56/90 (62.2%)
Catheter-related interventions I: 17/90 (18.9%) C: 17/90 (18.9%)
Duration of the procedure (Median, IQR, 95% CI) I: 107 (IQR 69 to 228, 95% CI 140 to 209) seconds C: 72 (IQR 47 to 134, 95% 86 to 128) seconds
Number of total attempts (Median, IQR, 95% CI) I: 1.0 (IQR 1.0 to 1.0, 1.25 to 1.64) C: 1.0 (IQR 1.0 to 2.0, 95% CI 1.35 to 1.74) |
Author’s conclusion: It was found that USG increases the success of the first attempt compared with the standard method and NIR in patients with DVA. |
Risk of bias tabel
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients/healthcare providers/data collectors/assessors/data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
PICO 1: Central venous catheters
|
|||||||
|
Airapetian (2013) |
Definitely yes.
Reason: patients were randomly assigned to the three groups |
No information.
Reason: - |
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported. |
Probably yes.
Reason: No other bias reported. |
Low. |
|
Benali (2022) |
Definitely yes.
Reason: Patients were randomly divided according to computer generated randomized table into two groups.
|
Definitely yes.
Reason: Computer generated. |
No information.
Reason: - |
No loss to follow-up reported.
Reason: -
|
Definitely yes.
Reason: All predefined outcomes were reported. |
Probably yes.
Reason: No other bias reported. |
Low. |
|
Dolu (2015) |
Definitely yes.
Reason: patients were randomly assigned to the groups.
|
No information.
Reason: - |
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Ethesham (2020) |
Definitely yes.
Reason: Randomly divided into two groups. |
No information.
Reason: - |
Partly yes.
Reason: The patients were blind (single blind study) to the study.
|
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Faithi (2016) |
Definitely yes.
Reason: patients were randomly assigned to either the control (anatomical landmark-guided) or experimental (ultrasound-guided) groups
|
Probably yes.
Reason: based upon the order of their entrance to the operating room. |
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Nazari (2015) |
Definitely yes.
Reason: From patients 340 cases were selected based on inclusion and exclusion criteria, 4 of them not participate to the study and other 336 cases allocated with a simple random sampling method in 2 groups of intervention and control (168 cases in each groups).
|
No information.
Reason: - |
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Oh (2014) |
Definitely yes.
Reason: Patients were randomly divided into a landmark group (n = 30) or an ultrasound group (n = 30).
|
No information.
Reason: - |
Definitely no.
Reason: this in- vestigation was not-blinded because the catheterization could not be disguised; therefore, there was a possibility of a bias.
|
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Palkhiwala (2020) |
Definitely yes.
Reason: Randomly divided into two groups. |
No information.
Reason: - |
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Rando (2014) |
Definitely yes.
Reason: Randomization was performed through a computer random number generator |
Definitely yes.
Reason: placing the results in sheets inside closed enve- lopes, which were opened right before CVL placement.
|
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Riaz (2015) |
Definitely yes.
Reason: patients who required internal jugular vein cannulation were randomly assigned.
|
No information.
Reason: - |
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Srinivasan (2017) |
Definitely yes.
Reason: Randomly divided into two groups. |
Definitely yes.
Reason: The allocation was concealed in an opaque-sealed envelope. The envelope was opened just before the central line (IJV line) placement |
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Subramony (2022) |
Definitely yes.
Reason: Patients were randomized according to an odd or even numbering system found on the inside of the data packet. Odd numbers were randomized to the traditional method while even numbers were assigned to the ultrasoundguided group.
|
No information.
Reason: - |
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Vinayagamurugan (2021) |
Definitely yes.
Reason: Randomly divided into two groups. |
Reason: |
Partly yes.
Reason: Resident physician who performed the procedures were not blinded to study. However, patients and postoperative assessors of mechanical complications were blinded to study.
|
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Wang (2020) |
Definitely yes.
Reason: Randomly divided into two groups. |
Definitely yes.
Reason: The allocation sequence, which was based on a random number table, was prepared by a third-party biostatistician in sequentially numbered sealed opaque envelopes.
|
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
Zhang (2023) |
Definitely yes.
Reason: Randomly divided into two groups. |
No information.
Reason: - |
No information.
Reason: - |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
PICO 2: Peripheral intravenous catheters
|
|||||||
|
Yalcinli (2022) |
Reason: |
Reason: |
Reason: |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
|
PICO 3: Peripherally inserted central catheters
|
|||||||
|
Wang (2016) |
Reason: |
Reason: |
Reason: |
No loss to follow-up reported.
Reason: - |
Definitely yes.
Reason: All predefined outcomes were reported.
|
Probably yes.
Reason: No other bias reported. |
Low. |
Exclusie tabel
|
Author and year |
Reason for exclusion |
|
Anderssen (2022) |
The included RCTs in this systematic review are already included in a more recent published systematic review which is included in this guideline. |
|
Berlanga-Macis (2022) |
Wrong study design. |
|
Hansel (2023) |
Wrong study population. |
|
McCarthy (2016) |
The RCT is already included in the systematic review of Tada (2022) which is included in this guideline. |
|
Misiołek (2012) |
The full-text version of this study is not available. |
|
Oleti (2019) |
Wrong study population. |
|
Poulsen (2023) |
The studies regarding adults are already included in the systematic review of Tada (2022). Poulsen (2023) includes three more studies about children, but children are beyond the scope of this review. |
|
Sazdov (2017) |
Study did not report results for the jugular vein, subclavian vein, and femoral vein separately. |
|
Tran (2021) |
The included RCTs in this systematic review are already included in a more recent published systematic review which is included in this guideline. |
|
Wang (2016) |
Wrong comparison for PICO 3. |
|
Xia (2014) |
The full-text version of this study is not available. |
|
Xu (2013) |
The full-text version of this study is not available. |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 25-06-2025
Beoordeeld op geldigheid : 25-06-2025
Nog in afwachting autorisatie van de volgende partijen:
- Vereniging voor Hygiëne en Infectiepreventie in de Gezondheidszorg
- Patiëntenfederatie Nederland
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg rondom vaattoegangswegchirurgie.
Werkgroep
- dr. C.G. (Niels) Vos (voorzitter), Nederlandse Vereniging voor Heelkunde
- dr. M.E. (Marianne) Sitsen (vice-voorzitter), Nederlandse Vereniging voor Anesthesiologie
- dr. M.J. (Marijke) Molegraaf, Nederlandse Vereniging voor Heelkunde
- dr. M.G.J. (Maarten) Snoeijs, Nederlandse Vereniging voor Heelkunde
- dr. M. (Mahir) Uslu, Nederlandse Vereniging voor Anesthesiologie
- dr. M. (Michelle) Gompelman, Nederlandse Internisten Vereniging
- dr. E.R. (Eric) van der Vorm, Nederlandse Vereniging voor Medische Microbiologie / Samenwerkingsverband Richtlijnen Infectiepreventie
- drs. Ir. P.A.A. (Pum) Le Haen, Nederlandse Vereniging voor Radiologie
Klankbordgroep
- dr. H. (Hanneke) Buter, Nederlandse Vereniging voor Intensive Care
- Werkgroep SRI-richtlijn Veneuze en arteriële katheters
Met ondersteuning van
- dr. M.S. (Matthijs) Ruiter, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- M. (Mitchel) Griekspoor, MSc, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Hoofdfunctie |
Project Tekstveld |
Nevenwerkzaamheden |
Persoonlijke Financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intell. Belangen en reputatie |
Overige belangen |
Ondernomen actie |
|
Niels Vos (vz.) |
Vaatchirurg |
Richtlijn Centraal Veneuze Toegang |
Voorzitter huidige richtlijncommissie. |
Geen. |
Geen. |
1. Studie naar functioneren van Artivion (Jotec) EVAR prothese. Gefinancierd door Artivion. Multicenter, ik ben lokale (martini ziekenhuis) hoofdonderzoeker. Het ziekenhuis krijgt een onkosten vergoeding van een paar 100 euro. Persoonlijk krijg ik er niets voor. Het is een reguliere verkrijgbare stent waar de leverancier wilde kijken hoe deze presteert (technisch/klinisch en QoL).
2.Door ZonMW gefinancierde multicenter trial naar antistolling na pta (CLEARPATH trial, RCT, clopidogrel / placebo vs clopidogrel / ascal na dotter). Hier is een onkostenvergoeding per patiënt voor die naar het ziekenhuis (wetenschappelijk instituut) gaat. Ik ben ook hier lokale hoofdonderzoeker.
|
Geen. |
Geen. |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek van de richtlijn. |
|
Eric van der Vorm |
Arts-microbioloog |
Richtlijn Centraal Veneuze Toegang |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties. |
|
Maarten Snoeijs |
Vaatchirurg |
Centraal Veneuze Toegang & Cluster expertisegroep Arteriële en Veneuze Pathologie. |
Geen. |
Geen. |
Geen. |
1. OASIS Zorgevaluatie (Zorgevaluatie Nederland) 2. FLOW Zorgevaluatie (ZonMw) 3. Personalised hemodynamic modeling of arteriovenous grafts for prediction of vascular access stenosis and thrombosis (Vascular Access Society research grant) 4. ShuntSimulationStudy (Nierstichting).
|
Leider expertisecentrum met topreferente zorgfunctie voor vaattoegangchirurgie in MUMC+ |
Geen. |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek van de richtlijn. |
|
Mahir Uslu |
Anesthesioloog |
Richtlijn Centraal Veneuze Toegang
|
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties. |
|
Marianne Elisabeth Sitsen |
Anesthesioloog en medisch manager OK centrum |
Richtlijn Centraal Veneuze Toegang |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties. |
|
Marijke Molegraaf |
Vaatchirurg |
Richtlijn Centraal Veneuze Toegang
|
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties. |
|
Michelle Gompelman |
Internist-infectioloog, Arts-onderzoeker |
Richtlijn Centraal Veneuze Toegang |
Hoofdredactieraad lid Tijdschrift voor Infectieziekten – onbetaald. |
Geen. |
Geen. |
CARRIER-trial (ZonMW), Staphylococcus aureus decolonization in patients on home parenteral nutrition, geen PI. |
Geen. |
Geen. |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek van de richtlijn.
|
|
Pum le Haen |
Interventieradioloog |
Richtlijn Centraal Veneuze Toegang |
Geen. |
Geen. |
Geen. |
Lokale P.I. voor onderzoek naar de verbeterde/snellere toegang tot de AFS bij antegrade punctie van de AFC met de Speedwire. Multi center internationale studie onder P.I-schap van D. van den Heuvel, interventieradioloog St Antonius Ziekenhuis in Nieuwegein. Voor de inclusie van een patiënt in het bovengenoemde onderzoek wordt een onkostenvergoeding van 100 euro betaald. Deze vergoeding loopt via het ziekenhuis en de specialisten coöperatie en wordt betaald door Speedwire. Gaat om in totaal 20-25 patiënten in de looptijd van het onderzoek. |
Geen. |
Geen. |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek van de richtlijn. |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door uitnodigen van de Patiëntenfederatie Nederland voor de invitational conference. Het verslag hiervan is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de deelnemers van de invitational conference en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz (Volgt na de commentaarfase)
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Module |
Uitkomst raming |
Toelichting |
|
Echogeleid aanprikken |
Geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen mogelijk een kostenbesparend effect hebben doordat bij gebruik van echo bij het aanprikken van een vene het aantal pogingen en het aantal complicaties afneemt. Dit leidt tot het besparen van kosten op materiaal en interventies. Het gebruik van een echoapparaat brengt kosten met zich mee, maar met enkele apparaten kan worden volstaan om een heel ziekenhuis te ondervangen voor het inbrengen van centrale en perifere lijnen. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met die een centraal veneuze lijn krijgen. Tevens zijn er knelpunten aangedragen via een invitational conference. Een verslag hiervan is opgenomen onder aanverwante producten. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. [Review Manager 5.4] werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Algemene informatie
|
Cluster/richtlijn: NVvH Centraal veneuze toegang |
|
|
Uitgangsvraag/modules: UV2 Wat is de waarde van echogeleid aanprikken van een centraal veneuze of perifere lijn? |
|
|
Database(s): Embase.com, Ovid/Medline |
Datum: 7 februari 2024 |
|
Periode: vanaf 2012 |
Talen: geen restrictie |
|
Literatuurspecialist: Alies Oost |
Rayyan review: https://rayyan.ai/reviews/923533 |
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Deduplication: voor het ontdubbelen is gebruik gemaakt van http://dedupendnote.nl/ |
|
|
Toelichting: Voor deze vraag is gezocht op de elementen:
De sleutelartikelen worden gevonden met deze search (PMID 23249991, 30032874 en 25656255) |
|
|
Te gebruiken voor richtlijntekst: In de databases Embase.com en Ovid/Medline is op 7 februari 2024 systematisch gezocht naar systematische reviews en RCTs over echogeleid aanprikken van een centraal veneuze of perifere lijn. De literatuurzoekactie leverde 752 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR |
103 |
88 |
125 |
|
RCT |
495 |
340 |
627 |
|
Totaal |
598 |
428 |
752* |
*in Rayyan
Zoekstrategie
Embase.com
|
No. |
Query |
Results |
|
#1 |
'central venous catheter'/exp OR 'central venous catheterization'/exp OR (((central* OR peripheral*) NEAR/3 (venous OR vein OR intravenous OR vascular) NEAR/3 (catheter* OR access OR line* OR device* OR cannulation)):ti,ab,kw) OR cvc:ti,ab,kw OR cvcs:ti,ab,kw OR pivc:ti,ab,kw OR pivcs:ti,ab,kw OR ((central NEAR/3 (cath* OR line*)):ti,ab,kw) OR 'peripherally inserted central venous catheter'/exp OR 'peripheral venous catheter'/exp OR ((peripheral* NEAR/3 (insert* OR catheter* OR line* OR infusion OR access OR cannulation)):ti,ab,kw) OR picc*:ti,ab,kw OR 'port a cath':ti,ab,kw OR portacath:ti,ab,kw OR ((('venous access' OR 'central venous' OR cv OR implant* OR catheter*) NEAR/2 port*):ti,ab,kw) OR 'implantable port system'/exp OR ((implant* NEAR/2 venous NEAR/2 (device* OR port*)):ti,ab,kw) OR tivad*:ti,ab,kw OR tivap*:ti,ab,kw OR hickman*:ti,ab,kw OR (((tunnel* OR cuffed) NEAR/3 (catheter* OR 'central catheter*' OR line* OR cvad*)):ti,ab,kw) |
82913 |
|
#2 |
(((ultrasound OR ultrasonograph* OR ultrasonic OR echo* OR sonograph*) NEAR/3 (guidance OR guided)):ti,ab,kw) OR 'point of care ultrasound*':ti,ab,kw OR pocus:ti,ab,kw |
87255 |
|
#3 |
('ultrasound'/exp/mj OR 'echography'/mj OR 'ultrasound scanner'/exp/mj) AND ('central venous catheter'/exp/mj OR 'central venous catheterization'/exp/mj OR 'peripherally inserted central venous catheter'/exp/mj OR 'peripheral venous catheter'/exp/mj OR 'peripheral vein'/exp/mj OR 'cannulation'/mj OR 'vascular access'/exp/mj OR 'vascular puncture (procedure)'/exp/mj) |
1507 |
|
#4 |
(#1 AND #2 OR #3) NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT (('adolescent'/exp OR 'child'/exp OR adolescent*:ti,ab,kw OR child*:ti,ab,kw OR schoolchild*:ti,ab,kw OR infant*:ti,ab,kw OR girl*:ti,ab,kw OR boy*:ti,ab,kw OR teen:ti,ab,kw OR teens:ti,ab,kw OR teenager*:ti,ab,kw OR youth*:ti,ab,kw OR pediatr*:ti,ab,kw OR paediatr*:ti,ab,kw OR puber*:ti,ab,kw) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp OR adult*:ti,ab,kw OR man:ti,ab,kw OR men:ti,ab,kw OR woman:ti,ab,kw OR women:ti,ab,kw)) |
2184 |
|
#5 |
#4 AND [2012-2024]/py |
1627 |
|
#6 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
999373 |
|
#7 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
3963906 |
|
#8 |
#5 AND #6 - SR |
103 |
|
#9 |
#5 AND #7 NOT #8 - RCT |
495 |
|
#10 |
#8 OR #9 |
598 |
|
#11 |
23249991:ui OR 30032874:ui OR 25656255:ui - sleutelartikelen |
3 |
|
#12 |
#10 AND #11 – sleutelartikelen worden gevonden |
3 |
Ovid/Medline
|
# |
Searches |
Results |
|
1 |
exp Catheterization, Central Venous/ or exp Central Venous Catheters/ or ((central* or peripheral*) adj3 (venous or vein or intravenous or vascular) adj3 (catheter* or access or line* or device* or cannulation)).ti,ab,kf. or cvc.ti,ab,kf. or cvcs.ti,ab,kf. or pivc.ti,ab,kf. or pivcs.ti,ab,kf. or (central adj3 (cath* or line*)).ti,ab,kf. or exp Catheterization, Peripheral/ or (peripheral* adj3 (insert* or catheter* or line or infusion or access or cannulation)).ti,ab,kf. or picc*.ti,ab,kf. or 'port a cath'.ti,ab,kf. or portacath.ti,ab,kf. or (('venous access' or 'central venous' or cv or implant* or catheter*) adj2 port*).ti,ab,kf. or (implant* adj2 venous adj2 (device* or port*)).ti,ab,kf. or tivad*.ti,ab,kf. or tivap*.ti,ab,kf. or hickman*.ti,ab,kf. or ((tunnel* or cuffed) adj3 (catheter* or 'central catheter*' or line* or cvad*)).ti,ab,kf. |
56560 |
|
2 |
(((ultrasound or ultrasonograph* or ultrasonic or echo* or sonograph*) adj3 (guidance or guided)) or 'point of care ultrasound*' or pocus).ti,ab,kf. |
55540 |
|
3 |
Ultrasonography/ and (exp Catheterization, Central Venous/ or exp Central Venous Catheters/ or exp Catheterization, Peripheral/) |
1249 |
|
4 |
((1 and 2) or 3) not (comment/ or editorial/ or letter/) not ((exp animals/ or exp models, animal/) not humans/) not ((Adolescent/ or Child/ or Infant/ or adolescen*.ti,ab,kf. or child*.ti,ab,kf. or schoolchild*.ti,ab,kf. or infant*.ti,ab,kf. or girl*.ti,ab,kf. or boy*.ti,ab,kf. or teen.ti,ab,kf. or teens.ti,ab,kf. or teenager*.ti,ab,kf. or youth*.ti,ab,kf. or pediatr*.ti,ab,kf. or paediatr*.ti,ab,kf. or puber*.ti,ab,kf.) not (Adult/ or adult*.ti,ab,kf. or man.ti,ab,kf. or men.ti,ab,kf. or woman.ti,ab,kf. or women.ti,ab,kf.)) |
2476 |
|
5 |
limit 4 to yr="2012 -Current" |
1651 |
|
6 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
725135 |
|
7 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2688744 |
|
8 |
5 and 6 - SR |
88 |
|
9 |
(5 and 7) not 8 - RCT |
340 |
|
10 |
8 or 9 |
428 |
|
11 |
("23249991" or "30032874" or "25656255").ui. - sleutelartikelen |
3 |
|
12 |
10 and 11 – sleutelartikelen worden gevonden |
3 |