Therapeutic drug monitoring
Uitgangsvraag
Wat is de beste monitoringsstrategie om de effectiviteit van anti-TNF-α medicatie te waarborgen bij kinderen met inflammatoire darmziekten (IBD)?
Clinical question
What is the optimal monitoring strategy to secure effectivity of anti-TNF-α medication in children with inflammatory bowel disease (IBD)?
Aanbeveling
Pas proactieve therapeutic drug monitoring (TDM) in ieder geval toe bij kinderen met IBD en ernstige ontstekingsactiviteit:
- Pan-enterische Crohn
- Diepe ulceraties in het colon
- Acute ernstige colitis
- Perianale fisteling
en/of (een risico op) hoge medicatieklaring:
- Lichaamsgewicht <30 kg
- Laag serumalbumine
Pas reactieve TDM altijd toe bij kinderen met IBD in geval van primaire non-response of secundaire loss-of-response op anti-TNF-α therapie.
Recommendations
Apply proactive therapeutic drug monitoring (TDM) in any case for children with IBD and a high inflammatory burden:
- Pan-enteric Crohn's disease
- Deep ulcerations in the colon
- Acute severe colitis
- Perianal fistulizing disease
and/or (a risk of) high medication clearance:
- Body weight <30 kg
- Low serum albumin
Apply reactive TDM in all cases for children with IBD in the event of primary non-response or secondary loss of response to anti-TNF-α therapy.
Overwegingen
Advantages and disadvantages of the intervention and quality of the evidence
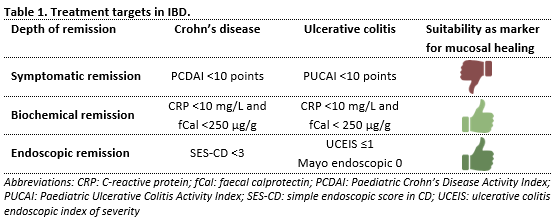
Therapeutic drug monitoring (TDM) is a valuable tool to enhance the efficacy of anti-TNF-α medication in patients with inflammatory bowel disease (IBD). It involves measuring serum trough concentrations and interpreting these concentrations for adjusting further drug dosages. The aim is to ensure that drug concentrations are within the therapeutic window. The critical threshold trough concentration above which the drug exerts its effect varies based on treatment target and disease phenotype. Typically, higher drug concentrations are associated with deeper forms of remission (table 1). For IBD phenotypes with a greater inflammatory burden, including fistulizing Crohn’s disease and acute severe colitis, even higher drug concentrations might be necessary.
Proactive TDM, i.e. measuring drug concentrations at predetermined intervals, provides added value especially in situations where early high drug concentrations are necessary to induce remission (with patients being at risk for underexposure).
Infliximab
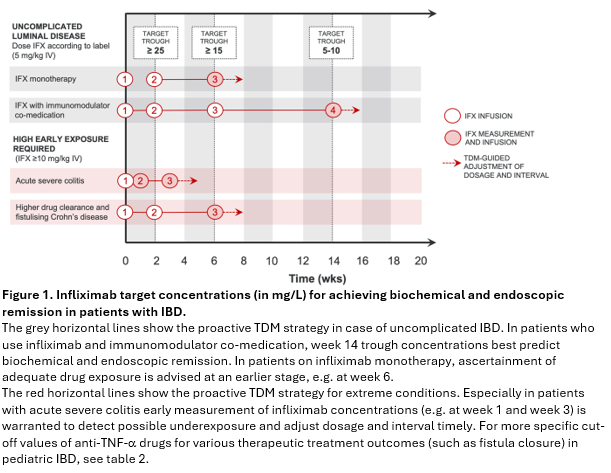
Figure 1 is a graphical representation of how proactive TDM can be employed during the induction with infliximab. While lower dosing (5 mg/kg) can be used in uncomplicated luminal Crohn’s disease and ulcerative colitis, higher induction doses (³10 mg/kg) are required in children and adolescents with panenteric Crohn’s disease, deep colonic ulcers, acute severe colitis, fistulizing Crohn’s disease, low weight (<30 kg) or serum albumin concentrations below 30 g/L. Infliximab concentrations measured in the induction phase will be helpful to guide any further dose adjustments.
Please note that the target trough concentrations mentioned in figure 1 relate to the treatment of uncomplicated luminal disease and require prospective validation.
Reactive TDM is the standard of care for optimizing anti-TNF-α therapy in the following conditions:
(1) In children and adolescents with mild to moderate luminal disease who are treated with a standard dose of infliximab (5 mg/kg) on week 0, 2 and 6, and who do not achieve biochemical remission on week 10. In such a scenario, also called primary non-response, measure the infliximab trough concentration before the fourth infusion (week 14). If the trough level is found to be less than 5 mg/L (in the absence of significant anti-infliximab-antibody titer), ensure that the next dose is administered earlier than after the standard interval, and double the dose. A 25% reduction of the dosing interval is generally as effective as a 50% increase of the dosage.
(2) In patients who initially achieved biochemical remission on anti-TNF-α therapy, but subsequently experience a flare during the maintenance phase. In such a scenario, also called secondary loss-of-response, measure the infliximab or adalimumab concentration prior to the next administration. The therapeutic options in case of subtherapeutic concentrations are the same as under (1).
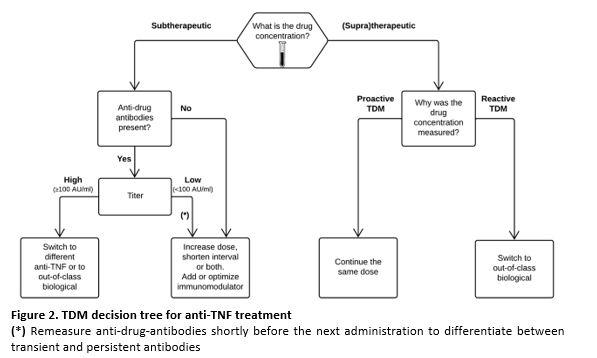
Figure 2 shows a treatment algorithm for tailoring anti-TNF-α therapy based on drug concentrations and can be used in both proactive and reactive TDM.
Infliximab Subcutaneous
The current recommendations are limited to TDM for intravenously administered infliximab. At the time of the literature search, no exposure-response data had been published for subcutaneously administered infliximab. However, a recent French study described an exposure-response relationship for this form of administration (Roblin, 2024).In this French study, all patients had previously been treated with intravenous infliximab and were in clinical remission at the time of switching to the subcutaneous route. Infliximab levels were measured after at least 5 subcutaneous injections of 120 mg infliximab, administered at 2-week intervals. The researchers identified a cutoff value of 20 mg/L, above which patients had a significantly higher chance of maintaining deep remission. Deep remission was defined as the absence of symptoms, CRP < 5 mg/L, and fecal calprotectin < 250 µg/g. These results suggest that the target range during the maintenance phase for subcutaneously administered infliximab is substantially higher than for intravenous administration. Further research is needed to establish recommendations for the target concentration and the optimal timing for proactive or reactive TDM in patients receiving subcutaneous infliximab.
Adalimumab
The lack of data on TDM in children with IBD being treated with adalimumab is evident in this module. The PAILOT trial by Assa (2019) demonstrated that the implementation of proactive TDM in children with luminal Crohn's disease led to a significantly longer-lasting biochemical remission. In this study, the target trough concentration for adalimumab was ≥5 mg/L. The ECCO-ESPGHAN guideline (van Rheenen, 2021) for the treatment of children with Crohn's disease sets a minimum adalimumab concentration of 7.5 mg/L as the therapeutic window's lower limit in the maintenance phase, starting at week 4. In the case of a too low adalimumab trough level, there are two treatment options: doubling the dose from 40 to 80 mg or shortening the dosing interval from 2 weeks to 1 week. In the case of adalimumab, both options are equally effective for higher drug exposure. Consider adalimumab ineffective if there is no treatment response despite a trough level ≥12,5 mg/L (Samuels, 2023) and co-medication with an immunomodulator.
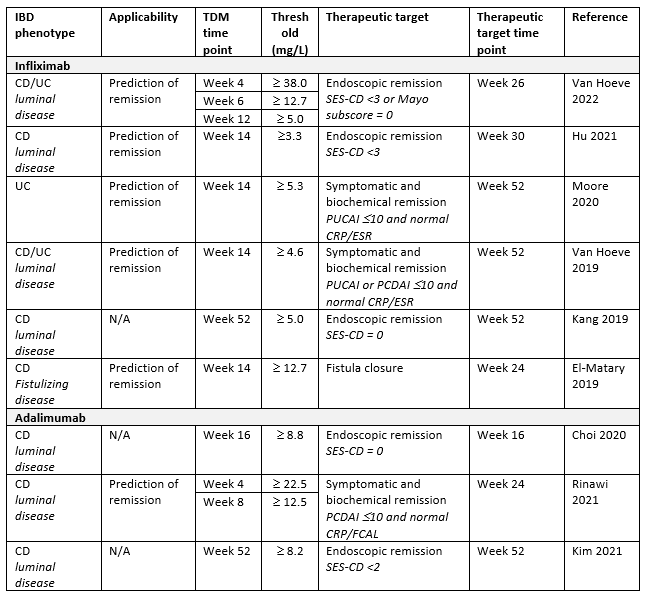
Table 2. Anti-TNF-α drug concentration thresholds in relation to the therapeutic targets in pediatric IBD
Patient values and preferences
Opinions regarding the use of TDM in pediatric patients may vary. Some patients and parents view TDM as a valuable tool that can improve treatment outcomes and create greater confidence in the drug they are using. Others find it inconvenient that TDM leads to changes in dosing regimens which can disrupt their daily lives and routines. Open and transparent communication between patient, parents and healthcare provider is crucial to address any concerns and ensure that patients feel informed and supported in their treatment journey.
To better understand the patient viewpoint, Crohn & Colitis NL evaluated patient preferences (bijlage Resultaten vragenlijst Crohn Colitis) including those concerning TDM. A total of 86 questionnaires were completed, of which 31 (36%) were filled out by the child with IBD alone, in 14 cases (16%), the child answered the questions together with one of the parents, and 41 times (48%) the questionnaire was completed solely by the parent. The questions about TDM were answered by 77 respondents. Two scenarios were presented sequentially. In the first scenario, the patient feels completely well, but the fecal calprotectin level is too high and the medication level in the blood is too low. The caregiver advises a dose escalation. 45 of 77 respondents (58%) agreed with this advice, 17 (22%) were neutral, while 15 (19%) did not want a dose increase. In the second scenario, the caregiver and patient together decide that a dose escalation is necessary. In this scenario, 37 of the 76 respondents (49%) preferred a dose escalation with the same dosing interval, while 16 (21%) preferred the same dose with shorter intervals. The remaining 23 respondents (30%) had no preference. It is crucial that healthcare providers and patients engage in discussions to make decisions that balance medical necessity with personal preferences.
Costs
Recently, two systematic reviews of cost-effectiveness of TDM in patients with IBD have been published (Yao, 2021a; Marquez-Megias, 2022). TDM-guided strategies were consistently found to be cost-saving or cost-effective compared to standard treatment without TDM (Yao, 2021). Among the studies, only one focused on a pediatric population, using approximate utility values from adult patients (Yao, 2021b). In the first year, higher costs were estimated for the proactive TDM group compared to the reactive TDM group, because of a more frequent TMD testing schedule (every 8 weeks). However, in years 2 and 3, the proactive TDM group showed a higher sustained remission rate on adalimumab therapy, resulting in cost avoidance related to hospitalization, change of therapy, and surgery. By year 3, the cumulative cost difference between proactive and reactive TDM strategies became cost-saving. The applicability of these findings to a clinical setting depends on TDM frequency, drug cost, time frame of follow-up, and a country’s healthcare context.
Furthermore, there is a paucity of information on the cost-effectiveness of TDM in patients with ulcerative colitis. Differences in the response to infliximab could significantly affect the cost-effectiveness of TDM for anti-TNF in patients with ulcerative colitis.
Medical resources
We want to emphasize that adopting a proactive TDM strategy without considering suitable markers of mucosal healing may lead to early exhaustion of the medical arsenal. This can make healthcare more expensive without necessarily improving its quality. The application of objective markers for mucosal healing, with strict targets for biochemical and endoscopic remission (see table 1), is key.
Acceptability, feasibility and implementation
Although reactive TDM has become the standard of care for optimizing anti-TNF therapy in IBD, recent data demonstrate the benefits and important role of proactive TDM in patient management. Apart from that, proactive TDM is not an entirely new concept. Clinicians and pharmacists have been doing it for decades with other medications, usually antibiotics, antiepileptics and immunosuppressives.
Onderbouwing
Achtergrond
Anti-tumor necrosis factor (TNF)-α drugs (including infliximab and adalimumab) have demonstrated effectiveness in treating children with IBD. A significant proportion of patients do not respond to the treatment initially (referred to as primary non-responders), and approximately 50% of children eventually lose response over time (known as secondary loss of response, LOR) (van Rheenen, 2020). Subtherapeutic drug concentrations, with or without the development of antidrug antibodies, play a considerable role in these outcomes.
Therapeutic drug monitoring (TDM) has emerged as a valuable tool to enhance the efficacy of anti-TNF-α in patients with IBD (Papamichael, 2019). It involves measuring serum drug concentrations and interpreting these concentrations for adjusting further drug dosages. The aim is to ensure that drug trough concentrations are within the therapeutic window. There is an ongoing debate when and how to perform TDM in clinical practice. Both reactive and proactive TDM strategies involve measuring drug concentrations followed by an interpretation and a dosing advice, but they differ in their context and purpose. Reactive TDM is used to investigate the cause of primary non-response or secondary LOR, whereas proactive TDM is a preventive approach aimed at reaching sufficiently high drug concentrations to achieve therapeutic success.
In this module we sought to evaluate which of the two monitoring strategies, reactive or proactive TDM, is the best with respect to disease control. Secondly, we aimed to identify clinically relevant anti-TNF-α concentrations and establish consensus on the timing of TDM in children with IBD.
Conclusies / Summary of Findings
1a. Achievement of remission
|
Very low GRADE |
The evidence is very uncertain about the effect of proactive compared to reactive Therapeutic Drug Monitoring in achieving remission in children with Inflammatory Bowel Disease treated with infliximab.
Source: Lawrence (2022) |
1b. Sustained remission
|
Low GRADE |
The evidence suggests that proactive Therapeutic Drug Monitoring (TDM) improves sustained remission in children with Inflammatory Bowel Disease treated with adalimumab, compared to reactive TDM.
Source: Assa (2019) |
2. Fistula healing
|
Very low GRADE |
The evidence is very uncertain about the effect of proactive Therapeutic Drug Montioring (TDM) compared to reactive TDM on fistula healing in children with Crohn’s Disease treated with infliximab.
Source: Singer (2022) |
Samenvatting literatuur
Description of studies
The selected studies have been categorized based on the timing of the drug concentration measurement, namely:
- Induction phase: up to week 6 (infliximab) and up to week 2 (adalimumab)
- Post-induction phase: between week 6 and 14 (infliximab) and between week 2 and 6 (adalimumab)
- Maintenance phase: from 14 weeks onward (infliximab) and 6 weeks onward (adalimumab)
Original PICO
Three studies met the initial stringent PICO criteria.
Assa (2019) performed an RCT to investigate the effect of proactive TDM compared with reactive TDM. A total of 78 pediatric patients (6-17 years) with luminal Crohn’s Disease who had responded well on standard adalimumab dosing at weeks 0 and 2 were randomised at week 4, prior to the third injection. In the proactive TDM group (n=38), physicians were informed of all adalimumab trough concentrations (TC), and treatment was intensified based on adalimumab TC of <5mg/L (regardless of disease activity; PCDAI, CRP and calprotectin). In the reactive TDM group (n=40), physicians were informed of adalimumab TC only in cases of clinical or biologic loss of response, with subsequent intensification of adalimumab treatment only when TC <5mg/L. After 72 weeks, biological remission (as sustained CRP £5 mg/L or as sustained fecal calprotectin £150 mg/kg) and combined remission (clinical remission as PCDAI <10 and above biological remission) were assessed.
Lawrence (2022) performed a prospective cohort study, comparing optimized infliximab induction therapy (n = 78) to standard induction therapy (n = 62) in children ≤18 years with IBD. Optimized induction therapy consisted of dose optimization (to a maximum of 10 mg/kg) and interval shortening between the third and fourth dose (to a minimum of 4 weeks). The decision to optimize was based on lack of clinical response (decrease in PCDAI/PUCAI <15 points or total score >30/>35) and lack of CRP decrease at week 2 and 6, or on low pre-third dose trough levels (≤15 mg/L). Standard induction therapy consisted of 5 mg/kg infliximab at 0, 2, and 6 weeks, with thereafter 8 weekly maintenance dosing. Dose changes were at the physician’s discretion. After 52 weeks, biomarker remission (CRP <5 mg/L) and combined remission (clinical remission as PCDAI/PUCAI <10 and CRP <5) were assessed.
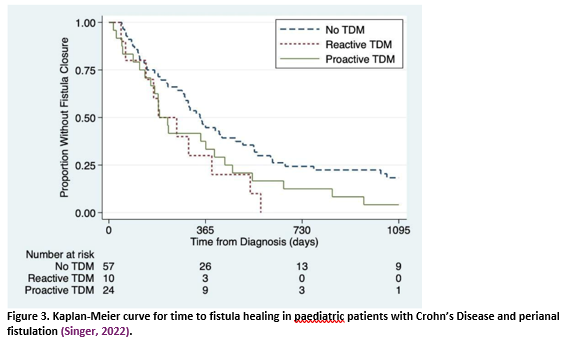
Singer (2022) conducted a retrospective cohort study in paediatric patients with newly diagnosed perianal fistulizing Crohn’s disease (n = 91). A minority had TDM within the first 6 months of anti-TNF-α therapy: 24 had proactive TDM and 10 had reactive TDM (no further definitions provided). The primary outcome of interest was time-to-fistula-healing, defined as the period between CD diagnosis and resolution of the fistulous tract on cross-sectional imaging, or in case no pelvic cross-sectional imaging was performed, as resolution of symptoms (including drainage) and fistula closure on visual inspection.
Pragmatic approach
From the pragmatic approach, nine studies were included. Their characteristics can be found in Table 3.
All studies evaluating infliximab therapy used standard dosing in the induction phase: 5mg/kg intravenously at week 0, 2, and 6. Maintenance therapy with the same dose was started on week 14, with 8-weekly intervals thereafter. Dose and/or interval adjustments during the maintenance phase were based on trough level concentrations or clinical parameters, at the discretion of the treating physician.
For adalimumab, induction therapy was given subcutaneously at week 0 (160 mg), 2 (80 mg), and 4 (40 mg); doses were halved for patients weighing <40kg. Maintenance dose was 40mg (or 20 mg for patients <40 kg) on alternate weeks. Interval shortenings during maintenance therapy to 1 week could be decided upon by the treating physician in case of loss of response.
Deviations from these dosing schemes are reported in Table 3.
Results
The results will be discussed in the order of the following clinical scenarios:
(1) anti-TNF-α treatment to induce remission,
(2) anti-TNF-α treatment to maintain remission, and
(3) perianal fistulizing Crohn's disease.
We did not identify studies that focused on anti-TNF-α treatment for conditions with higher drug clearance.
1. Remission
1a. Achievement of remission – infliximab
One study (Lawrence, 2022) evaluated the proportion of patients in remission at 52 weeks in the proactive TDM group compared to the reactive TDM group. Combined (symptomatic and biochemical) remission at week 52 was significantly higher in the optimized infliximab group (65/78; 83%) compared to the standard treated cohort (32/62; 52%), resulting in a relative risk (RR) of 2.07 (95% CI 1.50 to 2.84) (Table 3). The results were not (multivariate) adjusted for confounders, and baseline characteristics were not similar at baseline. Therefore, the results should be interpreted with extreme caution.
Table 4 Biomarker remission and corticosteroid-free combined remission after 52 week, for dose optimization during infliximab induction compared to standard induction therapy
|
|
Optimized induction therapy cohort (n = 78) |
Standard induction therapy cohort (n = 62) |
RR (95% CI) |
|
Biomarker remission (CRP <5) |
69 (88%) |
44 (71%) |
1.25 (1.04 to 1.49) |
|
Combined remission (PCDAI/PUCAI <10 and CRP <5) |
65 (83%) |
25 (40%) |
2.07 (1.50 to 2.84) |
1b. Sustained remission – adalimumab
One study (Assa, 2019) evaluated the proportion of patients with Crohn’s disease in remission at week 72 in the proactive TDM group compared to reactive TDM. Combined (symptomatic and biochemical) remission from week 48 to week 72 was significantly higher in the proactive group (16/38; 42%) than in the reactive TDM group (5/40; 12%), resulting in an RR of 3.36 (95% CI 1.37 to 8.29).
The risk ratios of remission for the different outcome measures at different time points can be found in Table 5.
Table 5 Biomarker remission and corticosteroid-free combined remission at different time points, for adalimumab proactive TDM compared to reactive TDM (NB: total numbers differ per outcome, based on availability of measurements)
|
|
At week 48 |
At week 72 |
Sustained from week 48 to 72 |
|||
|
|
Proactive |
Reactive |
Proactive |
Reactive |
Proactive |
Reactive |
|
Biological remission (CRP £5 mg/L) |
34 (94%) |
35 (97%) |
31 (94%) |
29 (88%) |
28 (74%) |
23 (57%) |
|
RR (95% CI) |
0.97 (0.88 to 1.07) |
1.07 (0.92 to 1.25) |
1.28 (0.92 to 1.78) |
|||
|
Biological remission (fecal calprotectin £150 mg/kg) |
18 (62%) |
11 (37%) |
24 (80%) |
8 (30%) |
18 (47%) |
9 (22%) |
|
RR (95% CI) |
1.69 (0.98 to 1.93) |
2.70 (1.47 to 4.96) |
2.1 (1.08 to 1.10) |
|||
|
Combined remission (PCDAI <10, CRP <5 mg/L and fecal calprotectin £150 mg/kg) |
18 (62%) |
8 (27%) |
24 (80%) |
6 (22%) |
16 (42%) |
5 (12%) |
|
RR (95% CI) |
2.33 (1.21 to 4.49) |
3.60 (1.74 to 7.46) |
3.36 1.37 to 8.29) |
|||
2. Fistula healing
One study (Singer, 2022) retrospectively compared proactive and reactive TDM (no definitions provided) in paediatric patients treated with infliximab for perianal fistulising Crohn’s disease. For time-to-fistula-healing, only in the supplementary material of the study, a Kaplan Meier curve (figure 3) is provided.
Level of evidence of the literature
1. Remission
The level of evidence regarding the outcome measure remission was downgraded:
- For achievement of remission: from starting point low as it was based on an observational study, by 4 levels to very low, because of study limitations (-2, risk of bias, for reasons see risk of bias table) and because of a single study with a low number of patients, and with large confidence intervals crossing the border of clinical relevance (-2, imprecision)
- For sustained remission: from starting point high by 2 levels to low, because of study limitations (-1, risk of bias, for reasons see risk of bias table) and because of a single study with a low number of patients, and with large confidence intervals crossing the border of clinical relevance (-1, imprecision)
No downgrading took place for inconsistency, indirectness, or publication bias.
2. Fistula healing
The level of evidence regarding the outcome measure fistula healing was downgraded from starting point low as it was based on an observational study, by 4 levels to very low, because of study limitations (-2, risk of bias, for reasons see risk of bias table) and because of a single study with a low number of patients, and with large confidence intervals crossing the border of clinical relevance (-2, imprecision).
No GRADE assessment was performed for the studies from the pragmatic selection, as none met all the elements of the PICO.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the positive and negative effects of proactive TDM compared to usual care (with or without reactive TDM) in children with IBD using anti-TNF-α medication?
| P: | Children with IBD using anti-TNF-α medication |
| I: | Proactive TDM |
| C: | Usual care (with or without reactive TDM) |
| O: | Achievement of remission (in induction phase), staying in remission (time to flare, in maintenance phase), fistula healing |
Relevant outcome measures
The guideline development group considered biochemical and/or endoscopic remission and fistula closure as critical outcome measures for decision making.
The working group used table 1 to assess remission. Biochemical and endoscopic remission are well-validated outcome measures, whereas symptomatic remission was considered an insufficient proxy for mucosal healing, except for the Paediatric Ulcerative Colitis Activity Index (PUCAI).
The working group defined a 25% difference for dichotomous outcome measures informing on relative risk (RR ≤ 0.8 or RR ≥ 1.25) as minimal clinically (patient) important differences.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 2000 until December 20th, 2022. The detailed search strategy is available upon request. The systematic literature search resulted in 475 hits. Studies were selected based on the following criteria:
- Design: Systematic review and/or meta-analysis, or randomized controlled trial (RCT);
- Patients: children with IBD using anti-TNF-α medication;
- Intervention: proactive TDM;
- Comparison: usual care (with or without reactive TDM);
- Outcomes: biochemical and/or endoscopic remission and fistula closure;
- Representing one of the following four clinical scenarios: (1) anti-TNF-α treatment to induce remission, (2) anti-TNF-α treatment to maintain remission, (3) conditions known to be associated with higher drug clearance, or (4) perianal fistulizing Crohn’s disease.
A total of 41 studies was initially selected based on title and abstract screening. After reading the full text, 38 studies were excluded (see the table with reasons for exclusion under the heading Evidence Tables), and three studies were included.
Due to the limited number of studies selected based on our initial strict criteria, an additional pragmatic selection of observational studies was performed, based on the following criteria:
- Design: studies assessing multivariable associations between trough levels of infliximab (IFX) and adalimumab (ADA) and biochemical and/or endoscopic remission (exposure-response)
- Patients: children with IBD using anti-TNF-α medication;
- Reporting a cut-off value for infliximab or adalimumab
- Language: English.
The previously assessed 41 studies were reassessed according to the pragmatic selection criteria. After reading the full text, nine studies were included (see the table with reasons for exclusion under the heading Evidence tables). An interpretation of the relevant study results is provided in the section “considerations”.
Results
Three studies were included in the initial analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Assa A, Matar M, Turner D, Broide E, Weiss B, Ledder O, Guz-Mark A, Rinawi F, Cohen S, Topf-Olivestone C, Shaoul R, Yerushalmi B, Shamir R. Proactive Monitoring of Adalimumab Trough Concentration Associated With Increased Clinical Remission in Children With Crohn's Disease Compared With Reactive Monitoring. Gastroenterology. 2019 Oct;157(4):985-996.e2. doi: 10.1053/j.gastro.2019.06.003. Epub 2019 Jun 10. PMID: 31194979.
- Cheifetz AS, Abreu MT, Afif W, Cross RK, Dubinsky MC, Loftus EV Jr, Osterman MT, Saroufim A, Siegel CA, Yarur AJ, Melmed GY, Papamichael K. A Comprehensive Literature Review and Expert Consensus Statement on Therapeutic Drug Monitoring of Biologics in Inflammatory Bowel Disease. Am J Gastroenterol. 2021 Oct 1;116(10):2014-2025. doi: 10.14309/ajg.0000000000001396. PMID: 34388143; PMCID: PMC9674375.
- Choi SY, Choi YO, Choe YH, Kang B. Potential Utility of Therapeutic Drug Monitoring of Adalimumab in Predicting Short-Term Mucosal Healing and Histologic Remission in Pediatric Crohn's Disease Patients. J Korean Med Sci. 2020 Apr 27;35(16):e114. doi: 10.3346/jkms.2020.35.e114. PMID: 32329259; PMCID: PMC7183843.
- El-Matary W, Walters TD, Huynh HQ, deBruyn J, Mack DR, Jacobson K, Sherlock ME, Church P, Wine E, Carroll MW, Benchimol EI, Lawrence S, Griffiths AM. Higher Postinduction Infliximab Serum Trough Levels Are Associated With Healing of Fistulizing Perianal Crohn's Disease in Children. Inflamm Bowel Dis. 2019 Jan 1;25(1):150-155. doi: 10.1093/ibd/izy217. PMID: 29912413; PMCID: PMC6290776.
- Hu W, Feng Y, Ye Z, Tang Z, Qian L, Wang Y, Huang Y. The Association Between Genetic Variants, Pharmacokinetics, and Infliximab Efficacy in Pediatric Patients With Crohn's Disease in China. Front Pediatr. 2021 Dec 13;9:744599. doi: 10.3389/fped.2021.744599. PMID: 34966700; PMCID: PMC8711600.
- Kang B, Choi SY, Choi YO, Lee SY, Baek SY, Sohn I, Choe BH, Lee HJ, Choe YH. Infliximab Trough Levels Are Associated With Mucosal Healing During Maintenance Treatment With Infliximab in Paediatric Crohn's Disease. J Crohns Colitis. 2019 Feb 1;13(2):189-197. doi: 10.1093/ecco-jcc/jjy155. PMID: 30452616.
- Kim MJ, Kim E, Kang B, Choe YH. Therapeutic Drug Monitoring of Adalimumab During Long-term Follow-up in Paediatric Patients With Crohn Disease. J Pediatr Gastroenterol Nutr. 2021 Jun 1;72(6):870-876. doi: 10.1097/MPG.0000000000003070. PMID: 33908743.
- Lawrence S, Faytrouni F, Harris RE, Irvine M, Carrion E, Scott G, Clarke B, Garrick V, Curtis L, Gervais L, Tayler R, Riou M, Hansen R, Jacobson K, Russell RK. Optimized Infliximab Induction Predicts Better Long-Term Clinical and Biomarker Outcomes Compared to Standard Induction Dosing. J Pediatr Gastroenterol Nutr. 2022 Nov 1;75(5):601-607. doi: 10.1097/MPG.0000000000003587. Epub 2022 Aug 22. PMID: 36048178.
- Lucidarme C, Petitcollin A, Brochard C, Siproudhis L, Dewitte M, Landemaine A, Bellissant E, Bouguen G. Predictors of relapse following infliximab de-escalation in patients with inflammatory bowel disease: the value of a strategy based on therapeutic drug monitoring. Aliment Pharmacol Ther. 2019 Jan;49(2):147-154. doi: 10.1111/apt.15046. PMID: 30589970.
- Marquez-Megias S, Nalda-Molina R, Sanz-Valero J, Más-Serrano P, Diaz-Gonzalez M, Candela-Boix MR, Ramon-Lopez A. Cost-Effectiveness of Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease: A Systematic Review. Pharmaceutics. 2022 May 7;14(5):1009. doi: 10.3390/pharmaceutics14051009. PMID: 35631594; PMCID: PMC9145467.
- Moore H, Dolce P, Devas N, Baldassano R, Martinelli M. Post-induction infliximab trough levels and disease activity in the clinical evolution of pediatric ulcerative colitis. United European Gastroenterol J. 2020 May;8(4):425-435. doi: 10.1177/2050640620912877. Epub 2020 Mar 12. PMID: 32213038; PMCID: PMC7226697.
- Papamichael K, Cheifetz AS, Melmed GY, Irving PM, Vande Casteele N, Kozuch PL, Raffals LE, Baidoo L, Bressler B, Devlin SM, Jones J, Kaplan GG, Sparrow MP, Velayos FS, Ullman T, Siegel CA. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2019 Aug;17(9):1655-1668.e3. doi: 10.1016/j.cgh.2019.03.037. Epub 2019 Mar 27. PMID: 30928454; PMCID: PMC6661210.
- Rinawi F, Ricciuto A, Church PC, Frost K, Crowley E, Walters TD, Griffiths AM. Association of Early Postinduction Adalimumab Exposure With Subsequent Clinical and Biomarker Remission in Children with Crohn's Disease. Inflamm Bowel Dis. 2021 Jun 15;27(7):1079-1087. doi: 10.1093/ibd/izaa247. PMID: 32978946.
- Roblin X, Nancey S, Papamichael K, Duru G, Flamand M, Kwiatek S, Cheifetz A, Fabien N, Barrau M, Paul S. Higher Serum Infliximab Concentrations Following Subcutaneous Dosing are Associated with Deep Remission in Patients with Inflammatory Bowel Disease. J Crohns Colitis. 2024 May 31;18(5):679-685. doi: 10.1093/ecco-jcc/jjad188. PMID: 37934041.
- Samuels A, Whaley KG, Minar P. Precision Dosing of Anti-TNF Therapy in Pediatric Inflammatory Bowel Disease. Curr Gastroenterol Rep. 2023 Sep 11. doi: 10.1007/s11894-023-00895-4. Epub ahead of print. PMID: 37695555.
- Sethi S, Dias S, Kumar A, Blackwell J, Brookes MJ, Segal JP. Meta-analysis: The efficacy of therapeutic drug monitoring of anti-TNF-therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2023 Jun;57(12):1362-1374. doi: 10.1111/apt.17313. Epub 2022 Dec 9. PMID: 36495020.
- Singer AAM, Rompca A, Gadepalli SK, Adler J. Predictors of Perianal Fistula Healing in Children With Newly Diagnosed Crohn Disease. J Pediatr Gastroenterol Nutr. 2022 Dec 1;75(6):709-716. doi: 10.1097/MPG.0000000000003595. Epub 2022 Aug 24. PMID: 36399175.
- van Hoeve K, Dreesen E, Hoffman I, Van Assche G, Ferrante M, Gils A, Vermeire S. Adequate Infliximab Exposure During Induction Predicts Remission in Paediatric Patients With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2019 Jun;68(6):847-853. doi: 10.1097/MPG.0000000000002265. PMID: 30633108.
- van Hoeve K, Seyed Tabib NS, Dreesen E, Tops S, Hoffman I, Gils A, Ferrante M, Vermeire S. Infliximab Concentrations during Induction Are Predictive for Endoscopic Remission in Pediatric Patients with Inflammatory Bowel Disease under Combination Therapy. J Pediatr. 2022 Jan;240:150-157.e4. doi: 10.1016/j.jpeds.2021.08.079. Epub 2021 Sep 3. PMID: 34481805.
- van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, Gasparetto M, Gerasimidis K, Griffiths A, Henderson P, Koletzko S, Kolho KL, Levine A, van Limbergen J, Martin de Carpi FJ, Navas-López VM, Oliva S, de Ridder L, Russell RK, Shouval D, Spinelli A, Turner D, Wilson D, Wine E, Ruemmele FM. The Medical Management of Paediatric Crohn's Disease: an ECCO-ESPGHAN Guideline Update. J Crohns Colitis. 2021 Feb;15(2):171-194. doi: 10.1093/ecco-jcc/jjaa161. PMID: 33026087.
- van Rheenen H, van Rheenen PF. Long-Term Efficacy of Anti-Tumor Necrosis Factor Agents in Pediatric Luminal Crohn's Disease: A Systematic Review of Real-World Evidence Studies. Pediatr Gastroenterol Hepatol Nutr. 2020 Mar;23(2):121-131. doi: 10.5223/pghn.2020.23.2.121. Epub 2020 Mar 4. PMID: 32206624; PMCID: PMC7073369.
- Yao J, Jiang X, You JHS. A Systematic Review on Cost-effectiveness Analyses of Therapeutic Drug Monitoring for Patients with Inflammatory Bowel Disease: From Immunosuppressive to Anti-TNF Therapy. Inflamm Bowel Dis. 2021a Jan 19;27(2):275-282. doi: 10.1093/ibd/izaa073. PMID: 32311018.
- Yao J, Jiang X, You JHS. Proactive therapeutic drug monitoring of adalimumab for pediatric Crohn's disease patients: A cost-effectiveness analysis. J Gastroenterol Hepatol. 2021b Sep;36(9):2397-2407. doi: 10.1111/jgh.15373. Epub 2021 Jan 23. PMID: 33326123.
Evidence tabellen
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Assa, 2019 |
Type of study: Nonblinded RCT
Setting and country: Multicenter, Israel
Funding and conflicts of interest: Authors report individual fees and grants from pharmaceutical companies. The study was supported by an unrestricted educational grant by AbbVie Pharmaceutical. |
Inclusion criteria: Biologic-naïve children (6–17 years) with luminal CD who had responded at week 4 to standard adalimumab induction by a reduction of PCDAI of ³15 points from adalimumab initiation, or PCDAI <10 points. Elevated CRP or fecal calprotectin were permitted at inclusion.
Exclusion criteria: 1) prior exposure to anti-TNF-a agents, 2) current or previous bowel perforation, 3) small bowel obstruction in last 3 months, 4) fixed noninflammatory stricture, 5) complicated or draining perianal fistula, 6) previous malignancy, 7) sepsis or active bacterial infection/ infection with hepatitis B/C/ tuberculosis/ clostridium difficile, 8) previous surgical bowel resection, 9) diagnosis of IBD-unclassified, 10) treatment with 5-ASA, exclusive enteral nutrition, or systemic antibiotics
N total at baseline: 78 Intervention: 38| Control: 40
Important prognostic factors2: age ± SD | % male: I: 14.0 ± 2.6 | 68% C: 14.6 ± 2.6 | 72%
Groups comparable at baseline? Yes |
Assessment during each visit (at week 4, week 8 and every 8 weeks thereafter) for clinical and biologic disease activity
|
Length of follow-up: 72 weeks
Loss-to-follow-up: Intervention: 5 (13.2%) Reasons: loss of response and low adalimumab levels despite intensification (n=3), adverse events (n=2)
Control: 8 (20%) Reasons: loss of response despite adalimumab TC >5mg/ml (n = 4), loss of response and low adalimumab levels despite intensification (n=2), penetrating disease (n=2)
Incomplete outcome data: See above.
|
Outcome measures and effect size (95%CI):
Biological remission -1 Definition: sustained CRP £0.5 mg/dL
RR: 0.97 (95% CI 0.88 to 1.07)
RR: 1.07 (95% CI 0.92 to 1.25)
I: 28 (74%)| C: 23 (57%) RR: 1.28 (95% CI 0.92 to 1.78)
Biological remission -2 Definition: sustained fecal calprotectin £150 mg/g
RR: 1.69 (95% CI 0.98 to 1.93)
RR: 2.70 (95% CI 1.47 to 4.96)
I: 18 (47%)| C: 9 (22%) RR: 2.1 (95% CI 1.08 to 1.10)
Sustained corticosteroid-free combined remission Definition: clinical remission (PCDAI <10) and CRP £0.5 and fecal calprotectin £150
RR: 2.33 (95% CI 1.21 to 4.49)
RR: 3.60 (95% CI 1.74 to 7.46)
I: 16 (42%) | C: 5 (12%) RR: 3.36 ( 95% CI 1.37 to 8.29) |
Author’s conclusions: The study, the first of its kind performed in the pediatric population, shows that proactive trough measurements are superior to reactive measurements, resulting in higher corticosteroid-free sustained remission and biologic remission rates.
|
|
|
Proactive TDM: treating physicians were informed of adalimumab TC for all patients within 2 weeks of sampling. Adalimumab treatment was intensified based on plasmatic TC <5mg/ml (regardless of disease activity; PCDAI, CRP and calprotectin) |
Reactive TMD: physicians were informed of adalimumab TC only in cases of clinical or biologic loss of response, with subsequent intensification of adalimumab treatment only when TC <5mg/ml |
||||||
|
Lawrence, 2022 |
Type of study: Prospective cohort study
Setting and country: Multicentre, Canada and United Kingdom
Funding and conflicts of interest: Several authors received non-commercial research grants, but also fees from pharmaceutical companies. |
Inclusion criteria: children ≤18 years of age, with IBD who were started on infliximab therapy in two pediatric IBD centers between August 1, 2016, and August 1, 2018
Exclusion criteria: None reported.
N total at baseline: 140 Intervention: 78| Control: 62
Important prognostic factors2: age (median, IQR) | % male: I: 12 (9-14) | 54% C: 12 (10-14) | 55%
Groups comparable at baseline? Difference in PUCAI at infliximab start between groups, but not in wPUCAI. Difference in CRP, fecal calprotectin and immunomodulatory medication at infliximab start. |
Infliximab therapy: standard induction of 5mg/kg. Induction dose optimization to 10 mg/kg or fourth dose shortening to (minimally) 4 weeks took place: - Based on lack of clinical response (decrease in PCDAI/PUCAI <15 points or total score >30/>35) and biomarker response (downward trending CRP based on physician discretion) at week 2 and 6, or - Low pre-third dose trough levels (≤15 μg/g)
|
Infliximab standard induction of 5mg/kg at 0, 2, and 6 weeks. Thereafter maintenance 8 weekly dosing. Infliximab trough levels and antibodies were measured immediately prior to the fourth dose. Dose changes post-fourth infusion were at the physician’s discretion secondary to clinical and biomarker non-remission or low infliximab trough levels with or without anti-drug antibody formation |
Length of follow-up: 52 weeks
Loss-to-follow-up: Not reported, only patients with 52-week follow-up included.
Incomplete outcome data: Not reported.
|
Outcome measures and effect size (95%CI):
Biomarker remission Definition: CRP <5 mg/L at 52 weeks I: 69 (88%) | C: 44 (71%) RR: 1.25 (95% CI 1.04 to 1.49)
Corticosteroid-free combined remission Definition: clinical remission (PCDAI/PUCAI <10 and CRP <5) at 52 weeks I: 65 (83%) | C: 25 (40%) RR: 2.07 (95% CI 1.50 to 2.84)
Post-induction trough-level threshold that predicted week 52 corticosteroid-free clinical remission: >4.25 mg/L (AUC: 0.622; sensitivity 0.45, specificity 0.80)
|
Author’s conclusions: A standard infliximab induction regimen, associated with lower median post-induction trough levels, results in less favorable long-term clinical outcomes for pediatric IBD patients. Timely induction dose escalation based on clinical indices combined with biomarkers and proactive TDM, results in significantly higher post-induction trough levels and better long-term clinical outcomes.
Risk of bias: unclear if selection of only patients with 1 year follow-up. Stratified treatment per center (different countries): possible that conditions other than form of TDM influenced treatment. |
|
Singer, 2022 |
Type of study: Retrospective cohort study
Setting and country: Single centre, United States of America
Funding and conflicts of interest: Last author received non-commercial funding for other research. Rest of authors report no conflict of interest. Funding information not disclosed. |
Inclusion criteria: Paediatric patients with newly diagnosed CD from Jan 2009 to Dec 2018 seen in the centre, with perianal fistula based on physical examination, endoscopy and cross-sectional imaging.
Exclusion criteria: (1) first identification of fistula later in disease course after diagnosis, (2) ambiguity regarding timing of fistula diagnosis, (3) incomplete documentation
N total at baseline: 34 Intervention: 24 | Control: 10
Important prognostic factors (for whole cohort, n = 91): age (median, IQR) | % male: 13.9 (11.9 to 15.8) | 70%
Groups comparable at baseline? Unclear, no information provided for proactive versus reactive TDM. |
Proactive TDM within first 6 months of anti-TNF-a therapy. |
Reactive TDM within first 6 months of anti-TNF-a therapy.
|
Length of follow-up: Unclear, reported “until time of analysis”
Loss-to-follow-up: For whole cohort: 3 (censored)
Incomplete outcome data: Only patients with sufficient documentation included.
|
Outcome measures and effect size (95%CI):
Time to fistula healing Definition: resolution of the fistulous tract on cross-sectional imaging (or resolution of symptoms and visual inspection) à no numbers reported; authors report: There was no significant difference in proactive versus reactive TDM, though both were associated with improved healing as compared to no TDM (HR 1.29)
|
Author’s conclusions: Early treatment with anti-TNFα medications with subsequent TDM may improve fistula outcomes.
Risk of bias: only those included with sufficient records available, but unclear whether incomplete data is MCAR (probably not). |
|
Decisions regarding treatment including medication choice, dosing and intervals, use of cross-sectional imaging, and surgical procedures were at the discretion of the treating physician
|
|||||||
Abbreviations: CD: Crohn’s disease, CI: confidence interval, CRP: C-reactive protein, PCDAI: Paediatric Crohn’s Disease Activity Index, PUCAI: Paediatric Ulcerative Colitis Activity Index, RCT: randomized controlled trial, RR: relative risk, SD: standard deviation, TC: trough concentrations, TDM: therapeutic drug monitoring
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
|
Was the allocation adequately concealed?
|
Blinding:
|
Was loss to follow-up (missing outcome data) infrequent?
|
Are reports of the study free of selective outcome reporting?
|
Was the study apparently free of other problems that could put it at a risk of bias?
|
Overall risk of bias If applicable/necessary, per outcome measure
|
|
|
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
LOW Some concerns HIGH
|
|
Assa, 2019 |
No information
Reason: randomization and coding were performed by a dedicated clinical trial assistant for all centres in blocks of 4 (1:1 ratio). No information on how sequence was generated. |
Probably yes
Reason: randomization performed by dedicated clinical trial assistant |
Definitely no
Reason: no blinding took place in the study |
Probably no
Reason: Loss to follow-up was frequent both groups. No imputation methods (multiple imputation) were used. Numbers/reasons for incomplete data not mentioned. |
Probably no
Reason: protocol mentions loss of response as primary outcome measure (in study clinical remission). Relevant outcomes are mentioned. |
Probably yes
Reason: No other problems noted |
Some concerns
|
Risk of bias table for interventions studies (cohort studies based on risk of bias tool by the CLARITY Group at McMaster University)
|
Author, year |
Selection of participants
Was selection of exposed and non-exposed cohorts drawn from the same population?
|
Exposure
Can we be confident in the assessment of exposure?
|
Outcome of interest
Can we be confident that the outcome of interest was not present at start of study?
|
Confounding-assessment
Can we be confident in the assessment of confounding factors?
|
Confounding-analysis
Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables?
|
Assessment of outcome
Can we be confident in the assessment of outcome?
|
Follow up
Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed?
|
Co-interventions
Were co-interventions similar between groups?
|
Overall Risk of bias
|
|
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Definitely yes Probably yes Probably no Definitely no |
Low, Some concerns High |
|
|
Lawrence, 2019 |
Definitely no
Reason: patients not presenting at same point of care |
Definitely yes
Reason: information about dose and interval of infliximab extracted from medical records. |
Probably no
Reason: Indication for infliximab not provided, median CRP at start for standard group 4.5 (= biological remission already present) |
Probably yes
Reason: Extraction from medical records. |
Probably no
Reason: Difference in CRP and fecal calprotectin at infliximab start. No multivariate analysis for biological and combined remission (only clinical remission). Unclear if same indication for infliximab therapy was used. |
For bological remission: Definitely yes
For combined remission: probably yes
Reason: Laboratory values from medical records. Unclear if wPCDAI/PUCAI score was independent or reproducible. |
Probably no
Reason: Follow up duration was adequate, but information on missing data was not provided |
Definitely no
Reason: immunomodulatory medication at start of study was unbalanced. |
High - I and C treated in different centres - Groups not balanced at baseline - No multivariate adjusments for outcomes of interest (biological and combined remission) - No information on missing data - unbalanced co-interventions |
|
Singer, 2022 |
Definitely yes
Reason: all patients seen in same medical cenre in same time frame
|
Probably yes
Reason: Use of medical records, but no definition how proactive TDM was assessed
|
Probably yes
Reason: Presence of fistula based on documentation from physical examination, endoscopy and imaging, but unclear how far before diagnosis these where assessed (and hence whether healing was present at inclusion) |
Probably yes
Reason: Review of charts.
|
Definitely no
Reason: proactive versus reactive TDM not of primary interest. No data provided on matching of confounders between these groups or adjustment in analysis
|
Probably no
Reason: preference for medical imaging, but when not available, visual inspection by physician sufficed (but fistula tract can run deeper)
|
Probably no
Reason: Unclear when time of analysis was. No imputation methods used (censoring of data).
|
Unclear
Reason: no information provided for co-interventions in the groups proactive versus reactive TDM
|
High - No adjustment for confounding for comparison of interest - Unclear timepoint of analysis - Unclear similar co-interventions |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Nguyen, 2022. DOI: 10.1053/j.gastro.2022.06.052 |
Wrong outcome |
|
Pigneur, 2022. DOI: 10.1080/17474124.2022.2149489 |
Wrong study design |
|
Ricciuto, 2018. DOI: 10.1093/ecco-jcc/jjy109 |
Wrong population |
|
Barauna, 2021. DOI: 10.1590/S0004-2803.202100000-18 |
Wrong comparison, wrong population |
|
Sharma, 2015. DOI: 10.1097/MIB.0000000000000327 |
Wrong outcome |
|
Adedokun, 2013. DOI: 10.1097/01.MIB.0000435438.84365.f7 |
Wrong intervention: no proactive TDM |
|
Burgess, 2019. DOI: 10.1136/archdischild-2018-315100 |
Pragmatic: no prediction modelling |
|
Clarkston, 2019. doi: 10.1097/MPG.0000000000002304 |
Pragmatic: no prediction modelling |
|
Drobne, 2018. DOI: 10.1080/00365521.2018.1486882 |
Wrong population |
|
Feng, 2019. DOI: 10.1093/ibd/izz061 |
Wrong population |
|
Guidi, 2018. DOI: 10.1093/ecco-jcc/jjy076 |
Wrong population |
|
Liefferinckx, 2017. DOI: 10.1097/MIB.0000000000001120 |
Wrong population |
|
Tajiri, 2019. DOI: 10.1186/s12887-019-1739-5 |
Pragmatic: no prediction modelling |
|
Yanai, 2015. DOI: 10.1016/j.cgh.2014.07.029 |
Wrong population |
|
Dubinsky, 2022. DOI: 10.1093/ibd/izab285 |
Wrong outcome |
|
Fernandes, 2020. DOI: 10.1093/ibd/izz131 |
Wrong population |
|
Van Hoeve, 2018. DOI: 10.1093/ecco-jcc/jjy111 |
Duplicate patient population |
|
Whaley, 2022. DOI: 10.1016/j.cgh.2022.08.016 |
Pragmatic: no prediction modelling |
|
Carlsen, 2018. DOI: 10.1177/1756284818759930 |
Wrong population |
|
Deora, 2017. DOI: 10.1111/apa.14008 |
Wrong outcome |
|
Hämäläinen, 2013. DOI: 10.3109/00365521.2012.741619 |
Insufficient reporting of outcome of interest |
|
Merras-Salmio, 2017. DOI: 10.1097/MPG.0000000000001258 |
Pragmatic: no prediction modelling |
|
Minar, 2016. DOI: 10.1097/MPG.0000000000001029 |
Wrong outcome |
|
Stein, 2016. DOI: 10.1097/MIB.0000000000000769 |
Wrong outcome |
|
Ohem, 2017. DOI: 10.1159/000477962 |
Pragmatic: unclear methods |
|
Singh, 2014. DOI: 10.1097/MIB.0000000000000137 |
Pragmatic: no calculation of cut-off values |
|
Rolandsdotter, 2017. DOI: 10.3390/ijms18030575 |
Pragmatic: no calculation of cut-off values |
|
Rinawi, 2022. DOI: 10.1097/MPG.0000000000003366 |
Pragmatic: no calculation of cut-off values |
|
Rodriguez Azor, 2022. DOI: 10.1016/j.anpede.2023.01.007 |
Pragmatic: unclear methods |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 10-02-2025
Beoordeeld op geldigheid : 07-02-2025
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodules werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). Patiëntenparticipatie (in de vorm van een achterbanuitvraag) bij deze richtlijn werd medegefinancierd uit de Kwaliteitsgelden Patiënten Consumenten (SKPC) binnen het programma KIDZ.
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor kinderen met inflammatoire darmziekten.
Werkgroep
- prof. dr. J.C. (Hankje) Escher, kinderarts-MDL, Erasmus MC, Rotterdam, namens de NVK
- dr. J.E. (Johan) van Limbergen, kinderarts-MDL, Amsterdam UMC, namens de NVK
- dr. L. (Lissy) de Ridder, kinderarts-MDL, Erasmus MC, Rotterdam, namens de NVK
- dr. L.J.J. (Luc) Derijks, ziekenhuisapotheker - klinisch farmacoloog, Máxima MC en Maastricht UMC, namens de NVZA
- drs. M.P. (Menne) Scherpenzeel, patiëntvertegenwoordiger, namens Crohn & Colitis Nederland
- dr. P.F. (Patrick) van Rheenen (voorzitter), kinderarts-MDL, UMC Groningen, namens de NVK
- S. (Suzanne) van Zundert, diëtist kindergeneeskunde, Amsterdam UMC, namens de NVD
- dr. T.G.J. (Tim) de Meij, kinderarts-MDL, Amsterdam UMC, namens de NVK
Klankbordgroep
De klankbordgroepleden hebben gedurende de ontwikkeling van de richtlijn meegelezen met de conceptteksten en deze becommentarieerd.
- drs. C. (Carmen) Willemsen-Vermeer, diëtist, Radboud UMC, Nijmegen, namens de NVD
- dr. D.R. (Dennis) Wong, ziekenhuisapotheker - klinisch farmacoloog, Zuyderland Medisch Centrum, locatie Sittard-Geleen, namens de NVZA
- drs. F.D.M. (Fiona) van Schaik, arts-MDL, UMC Utrecht, namens de NVMDL
- drs. I.A. (Imke) Bertrams-Maartens, kinderarts-MDL, Máxima MC, namens de NVK
- drs. M. (Marieke) Zijlstra, kinderarts-MDL, Maasstad ziekenhuis, Rotterdam, namens de NVK
- drs. M.A.C. (Martha) van Gaalen, verpleegkundig specialist kinder-MDL, Erasmus MC, Rotterdam, namens de V&VN
Met ondersteuning van
- dr. M.M.J. (Machteld) van Rooijen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- drs. L.C. (Laura) van Wijngaarden, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
J.C. (Hankje) Escher |
Kinderarts-MDL, Erasmus MC Sophia |
E-dokter bij Cyberpoli van Stichting Artsen voor Kinderen (onbetaald) Scientific Advisory Committee Develop Registry - Jansen (betaling via ziekenhuis, ter ondersteuning van research) Scientific Advisory Committee van Cape Registry - Abbvie (betaling via ziekenhuis, ter ondersteuning van research) Programma commissie Jeugdartsen congres - Nutricia (betaling via ziekenhuis, ter ondersteuning van research) |
Abbvie, project Trasnitie coordinator Erasmus MC, projectleider MSD, project Biomarkers voor anti-TNF respons bij kinder-IBD, projectleider Stichting Theia, project HAPPY-IBD screen, angst en depressie bij kinder-IBD |
Restricties t.a.v. besluitvorming rondom Biomarkers voor anti-TNF respons. |
|
J.E. (Johan) van Limbergen |
Kinderarts-MDL, Amsterdam UMC |
Nestle Health Science: seminarie 4x/jaar |
Janssen, Nestle Health Science, Novalac Klinisch, translationeel en fundamenteel onderzoek naar voedingstherapie bij kinderen en volwassenen: genetica, microbioom, metaboloom, biomarkers. |
Restricties t.a.v. besluitvorming rondom voedingsinterventies. |
|
L. (Lissy) de Ridder |
Kinderarts-MDL, Erasmus MC |
Bestuurslid NVK (onbezoldigd) Scientific secretary ESPGHAN (onbezoldigd) Voorzitter P-ECCO (onbezoldigd) |
PIBD congres met symposium over integratie wetenschappelijk onderzoek binnen de kinder IBD patiëntenzorg (Janssen); webinars over biosimilarts (speaker's fee Pfizer).
Projectleider (PI) van investigator-initiated TISKids trial (ZonMW, Pfizer levert medicatie en restricted grant voor follow-up studie; heeft geen inbreng op protocol, data-analyse). Verdere medewerking (local investigator) aan klinische trials (Takeda, Abbvie, Ei Lilly). |
Restricties t.a.v. besluitvorming rondom TNF-alpha medicatie/ TDM |
|
L. (Luc) Derijks |
Ziekenhuisapotheker - klinisch farmacoloog, Máxima MC en Maastricht UMC |
Onderwijs (webinar, e-learning, college): webinar in opdracht van Takeda (betaald), e-learning in opdracht van Ferring (betaald), hoorcolleges UU (onbetaald). |
Geen. |
Geen. |
|
M.P. (Menne) Scherpenzeel |
Directeur, Crohn & Colitis Nederland |
Partner adviesbureau Blauwe Noordzee Diverse onbezoldigde bestuursfuncties |
Wij worden betrokken bij extern gefinancierd onderzoek van derden voor het patiëntenperspectief. Het werk van Crohn & Colitis NL wordt mede mogelijk gemaakt door pharma. |
Input patiëntenorganisatie voor alle te ontwikkelen modules middels uitvraag via achterban. |
|
P.F. (Patrick) van Rheenen |
Kinderarts-MDL, UMC Groningen |
Kinderarts lid van Medisch-Ethische Toetsingscomissie van UMCG (vacatiegelden) |
PI van een investigator-initiated onderzoeksproject mede gefinacierd door Europese Crohn en Colitis Organisatie (ECCO). Materiaal voor calprotectine sneltests zijn gedoneerd door BÜHLMANN Laboratories (beide geen invloed op opzet, uitvoering, analyse en rapportage van onderzoek). |
Geen. |
|
S. (Suzanne) van Zundert |
Diëtist kindergeneeskunde, Amsterdam UMC |
Bestuursfunctie Nederlandse KinderDiëtisten (NKD, onder de NVD) |
PECDED, Nestle Health Science: Patiëntervaring van ouders/kinderen bij voedingstherapie CDED. Participatie in wetenschappelijke onderzoeken die binnen de kinder-MDL afdeling van het Amsterdam UMC worden gedaan. |
Geen. |
|
T.G.J. (Tim) de Meij |
Kinderarts-MDL, Amsterdam UMC |
Lid advisory board Nutricia (vergoeding voor onderzoek) |
Principal investigator (PI) van industry-initiated study fase 3 naar tofacitinib (Pfizer). co-PI van investigator-initiated RCT naar probiotica bij antibiotica-geassocieerde diarree gedeeltelijk gesponsord door Winclove (restricted grant, toegekend bij minimum aantal inclusies). Unrestricted grant van Nutricia voor microbioomanalyse op fecessample van kinderen bij wie moeder antibiotica heeft gehad tijdens sectio caesaria. |
Restricties t.a.v. besluitvorming rondom voedingsinterventies. |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door een afgevaardigde van de patiëntenvereniging Crohn&Colitis NL in de werkgroep en een enquête onder alle leden van Crohn&Colitis NL. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen (zie kop ‘Waarden en voorkeuren van patiënten’). De conceptrichtlijn is tevens ter commentaar voorgelegd aan Crohn&Colitis NL en Patiëntenfederatie Nederland, en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Binnen het SKMS project “Inventarisatie en optimalisatie modulair onderhoud richtlijn kindergeneeskunde” is breed geïnventariseerd welke kindergeneeskundige modules toe waren aan herziening, en er is een onderhoudsplan opgeleverd. De vijf modules van deze richtlijn, herzien in 2022-2024, kwamen uit dit project naar voren. De modules zijn kritisch beoordeeld en de uitgangsvraag en zoekvraag werden aangepast of aangescherpt.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit.
http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Algemene informatie
|
Richtlijn: NVK-Inflammatoire darmziekten bij kinderen en adolescenten |
|
|
Uitgangsvraag: UV4 Wat is de beste monitoringsstrategie om de effectiviteit en veiligheid van anti-TNF alpha medicatie te waarborgen bij kinderen met IBD? |
|
|
Database(s): Ovid/Medline, Embase.com |
Datum: 20-12-2022 |
|
Periode: 2000 - heden |
Talen: geen limitering |
|
Literatuurspecialist: Alies van der Wal |
|
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: → Voor deze vraag is gezocht op de elementen IBD, anti-TNF alpha, drug monitoring en kinderen. → De sleutelartikelen worden gevonden met de zoekopdracht. |
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase.com en Medline (Ovid) is op 20 december 2022 met relevante zoektermen gezocht naar systematische reviews, RCTs en observationele studies over monitoringsstrategie bij anti-TNF alpha medicatie bij kinderen met IBD. De literatuurzoekactie leverde 457 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
MEDLINE (Ovid) |
Ontdubbeld |
|
SRs |
75 |
24 |
76 |
|
RCT |
51 |
18 |
54 |
|
Observationele studies |
328 |
120 |
345 |
|
Totaal |
454 |
162 |
475 |
Zoekstrategie
Embase.com
|
No. |
Query |
Results |
|
#15 |
#12 OR #13 OR #14 |
454 |
|
#14 |
#6 AND #11 NOT (#12 OR #13) = observationeel |
328 |
|
#13 |
#6 AND #8 NOT #12 = RCT |
51 |
|
#12 |
#6 AND #7 = SR |
75 |
|
#11 |
#9 OR #10 |
15485573 |
|
#10 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
13701577 |
|
#9 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
7405824 |
|
#8 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1994989 |
|
#7 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
885419 |
|
#6 |
#5 AND [1-1-2000]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
663 |
|
#5 |
#1 AND #2 AND #3 AND #4 |
1358 |
|
#4 |
'adolescent'/exp OR 'baby'/exp OR 'boy'/exp OR 'child'/exp OR 'minors'/exp/mj OR 'pediatric patient'/exp OR 'pediatrics'/exp OR 'schoolchild'/exp OR infan*:ti,ab OR newborn*:ti,ab OR 'new born*':ti,ab OR perinat*:ti,ab OR neonat*:ti,ab OR baby*:ti,ab OR babies:ti,ab OR toddler*:ti,ab OR minors*:ti,ab OR boy:ti,ab OR boys:ti,ab OR boyfriend:ti,ab OR boyhood:ti,ab OR girl*:ti,ab OR kid:ti,ab OR kids:ti,ab OR child*:ti,ab OR children*:ti,ab OR schoolchild*:ti,ab OR adolescen*:ti,ab OR juvenil*:ti,ab OR youth*:ti,ab OR teen*:ti,ab OR pubescen*:ti,ab OR pediatric*:ti,ab OR paediatric*:ti,ab OR peadiatric*:ti,ab OR school*:ti,ab OR prematur*:ti,ab OR preterm*:ti,ab |
5578559 |
|
#3 |
'drug monitoring'/exp OR (((drug* OR medication*) NEAR/3 monitor*):ti,ab,kw) OR 'drug level*':ti,ab,kw OR 'trough-level*':ti,ab,kw OR 'trough concentration*':ti,ab,kw OR ctrough:ti,ab,kw OR 'anti-drug-antibody level*':ti,ab,kw OR adab:ti,ab,kw OR 'exposure-response*':ti,ab,kw OR 'pharmacokinetics'/exp OR 'body drug relation*':ti,ab,kw OR 'drug body relation*':ti,ab,kw OR 'drug kinetic*':ti,ab,kw OR 'pharmaceutical kinetic*':ti,ab,kw OR 'pharmaco-kinetic*':ti,ab,kw OR 'pharmacokinetic*':ti,ab,kw OR 'pharmacological kinetic*':ti,ab,kw OR pharmacodynamic*:ti,ab,kw |
1006895 |
|
#2 |
('tumor necrosis factor inhibitor'/exp OR 'tnf alpha inhibitor*':ti,ab,kw OR 'tnf α inhibitor*':ti,ab,kw OR 'tnf-alpha blocker*':ti,ab,kw OR 'tnf α blocker*':ti,ab,kw OR 'tnf inhibitor*':ti,ab,kw OR 'anti tnf':ti,ab,kw OR 'tnf antagonist':ti,ab,kw OR 'tnf blocker*':ti,ab,kw OR (((tumour OR tumor) NEAR/3 necro* NEAR/3 factor* NEAR/3 (inhibitor* OR agent* OR blocker* OR antagonist*)):ti,ab,kw) OR ((anti NEAR/3 (tumour OR tumor) NEAR/3 necro* NEAR/3 factor*):ti,ab,kw) OR 'adalimumab'/exp OR 'abrilada':ti,ab,kw OR 'adalimumab':ti,ab,kw OR 'adaly':ti,ab,kw OR 'amgevita':ti,ab,kw OR 'amjevita':ti,ab,kw OR 'amsparity':ti,ab,kw OR 'cinnora':ti,ab,kw OR cyltezo:ti,ab,kw OR 'exemptia':ti,ab,kw OR 'fyzoclad':ti,ab,kw OR 'hadlima':ti,ab,kw OR 'halimatoz':ti,ab,kw OR 'hefiya':ti,ab,kw OR 'hukyndra':ti,ab,kw OR 'hulio':ti,ab,kw OR 'humira':ti,ab,kw OR 'hyrimoz':ti,ab,kw OR 'idacio':ti,ab,kw OR 'imraldi':ti,ab,kw OR 'kromeya':ti,ab,kw OR 'libmyris':ti,ab,kw OR 'mabura':ti,ab,kw OR 'qletli':ti,ab,kw OR 'raheara':ti,ab,kw OR 'solymbic':ti,ab,kw OR 'sulinno':ti,ab,kw OR 'trudexa':ti,ab,kw OR 'yuflyma':ti,ab,kw OR 'yusimry':ti,ab,kw OR 'infliximab'/exp OR 'avakine':ti,ab,kw OR 'avsola':ti,ab,kw OR 'flixabi':ti,ab,kw OR 'inflectra':ti,ab,kw OR 'infliximab':ti,ab,kw OR 'ixifi':ti,ab,kw OR 'remicade':ti,ab,kw OR 'remsima':ti,ab,kw OR 'renflexis':ti,ab,kw OR 'revellex':ti,ab,kw OR 'zessly':ti,ab,kw OR 'biological therapy'/exp OR 'biologic therap*':ti,ab,kw OR biotherap*:ti,ab,kw OR 'cell and tissue-based therap*':ti,ab,kw OR 'tissue therapy':ti,ab,kw OR 'immunomodulating agent'/exp OR 'immunomodulating agent*':ti,ab,kw OR 'immunomodulant agent*':ti,ab,kw OR 'immunomodulating drug*':ti,ab,kw OR 'immunomodulator*':ti,ab,kw OR 'immunotherapeutic agent*':ti,ab,kw OR 'biological product'/exp OR ((biologic* NEAR/2 (agent* OR therap* OR treatment* OR medic* OR drug* OR product* OR pharmaceutic*)):ti,ab,kw) OR 'biologicals':ti,ab,kw OR biologics:ti,ab,kw) |
4388897 |
|
#1 |
'inflammatory bowel disease'/exp OR 'ulcerative colitis'/exp OR 'crohn disease'/exp OR 'colon crohn disease'/exp OR (('inflammatory bowel' NEAR/3 dis*):ti,ab,kw) OR ibd:ti,ab,kw OR crohn*:ti,ab,kw OR 'cleron disease*':ti,ab,kw OR 'colitis ulcer*':ti,ab,kw OR 'ulcerative col*':ti,ab,kw OR 'idiopathic proctocol*':ti,ab,kw OR 'colitis gravis':ti,ab,kw OR 'ulcerative proctocol*':ti,ab,kw OR 'ulcerative procto colit*':ti,ab,kw OR 'ulcerous colit*':ti,ab,kw OR (((granulomatous OR terminal OR regional* OR mucosal) NEAR/3 (ileit* OR enterit* OR enterocolit* OR colit*)):ti,ab,kw) OR ileocolit*:ti,ab,kw |
236,315 |
Medline (Ovid)
|
# |
Searches |
Results |
|
14 |
11 or 12 or 13 |
162 |
|
13 |
(7 and 10) not (11 or 12) = observationeel |
120 |
|
12 |
(7 and 9) not 11 = RCT |
18 |
|
11 |
7 and 8 = SR |
24 |
|
10 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) or Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort*.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ |
7081522 |
|
9 |
exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1570887 |
|
8 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
636786 |
|
7 |
6 not (comment/ or editorial/ or letter/) not ((exp animals/ or exp models, animal/) not humans/) |
217 |
|
6 |
limit 5 to yr="2000 -Current" |
217 |
|
5 |
1 and 2 and 3 and 4 |
217 |
|
4 |
(child* or schoolchild* or infan* or adolescen* or pediatri* or paediatr* or neonat* or boy or boys or boyhood or girl or girls or girlhood or youth or youths or baby or babies or toddler* or childhood or teen or teens or teenager* or newborn* or postneonat* or postnat* or puberty or preschool* or suckling* or picu or nicu or juvenile?).tw. |
2832276 |
|
3 |
Drug Monitoring/ or ((drug* or medication*) adj3 monitor*).ti,ab,kf. or 'drug level*'.ti,ab,kf. or 'trough-level*'.ti,ab,kf. or 'trough concentration*'.ti,ab,kf. or Ctrough.ti,ab,kf. or 'anti-drug-antibody level*'.ti,ab,kf. or ADAB.ti,ab,kf. or 'exposure-response*'.ti,ab,kf. or exp Pharmacokinetics/ or 'body drug relation'.ti,ab,kf. or 'drug body relation'.ti,ab,kf. or 'drug kinetic*'.ti,ab,kf. or 'pharmaceutical kinetic*'.ti,ab,kf. or 'pharmaco-kinetic*'.ti,ab,kf. or 'pharmacokinetic*'.ti,ab,kf. or 'pharmacological kinetic*'.ti,ab,kf. or pharmacodynamic*.ti,ab,kf. |
526042 |
|
2 |
exp Tumor Necrosis Factor Inhibitors/ or 'tnf alpha inhibitor*'.ti,ab,kf. or 'TNF-alpha blocker*'.ti,ab,kf. or 'tnf inhibitor*'.ti,ab,kf. or 'anti tnf'.ti,ab,kf. or 'tnf antagonist'.ti,ab,kf. or 'tnf blocker*'.ti,ab,kf. or ((tumour or tumor) adj3 necro* adj3 factor* adj3 (inhibitor* or agent* or blocker* or antagonist*)).ti,ab,kf. or (anti adj3 (tumour or tumor) adj3 necro* adj3 factor*).ti,ab,kf. or Adalimumab/ or 'abrilada'.ti,ab,kf. or 'adalimumab'.ti,ab,kf. or 'adaly'.ti,ab,kf. or 'amgevita'.ti,ab,kf. or 'amjevita'.ti,ab,kf. or 'amsparity'.ti,ab,kf. or 'cinnora'.ti,ab,kf. or cyltezo.ti,ab,kf. or 'exemptia'.ti,ab,kf. or 'fyzoclad'.ti,ab,kf. or 'hadlima'.ti,ab,kf. or 'halimatoz'.ti,ab,kf. or 'hefiya'.ti,ab,kf. or 'hukyndra'.ti,ab,kf. or 'hulio'.ti,ab,kf. or 'humira'.ti,ab,kf. or 'hyrimoz'.ti,ab,kf. or 'idacio'.ti,ab,kf. or 'imraldi'.ti,ab,kf. or 'kromeya'.ti,ab,kf. or 'libmyris'.ti,ab,kf. or 'mabura'.ti,ab,kf. or 'qletli'.ti,ab,kf. or 'raheara'.ti,ab,kf. or 'solymbic'.ti,ab,kf. or 'sulinno'.ti,ab,kf. or 'trudexa'.ti,ab,kf. or 'yuflyma'.ti,ab,kf. or 'yusimry'.ti,ab,kf. or Infliximab/ or 'avakine'.ti,ab,kf. or 'avsola'.ti,ab,kf. or 'flixabi'.ti,ab,kf. or 'inflectra'.ti,ab,kf. or 'infliximab'.ti,ab,kf. or 'ixifi'.ti,ab,kf. or 'remicade'.ti,ab,kf. or 'remsima'.ti,ab,kf. or 'renflexis'.ti,ab,kf. or 'revellex'.ti,ab,kf. or 'zessly'.ti,ab,kf. or Biological Therapy/ or 'biological therap*'.ti,ab,kf. or 'biologic therap*'.ti,ab,kf. or biotherap*.ti,ab,kf. or 'cell-and-tissue-based-therap*'.ti,ab,kf. or 'tissue therapy'.ti,ab,kf. or Immunomodulating Agents/ or 'immunomodulating agent*'.ti,ab,kf. or 'immunomodulant agent*'.ti,ab,kf. or 'immunomodulating drug*'.ti,ab,kf. or 'immunomodulator*'.ti,ab,kf. or 'immunotherapeutic agent*'.ti,ab,kf. or (biologic* adj2 (agent* or therap* or treatment* or medic* or drug* or product* or pharmaceutic*)).ti,ab,kf. or 'biologicals'.ti,ab,kf. or biologics.ti,ab,kf. |
166261 |
|
1 |
exp Inflammatory Bowel Diseases/ or Colitis, Ulcerative/ or ('inflammatory bowel' adj3 dis*).ti,ab,kf. or ibd.ti,ab,kf. or crohn*.ti,ab,kf. or 'cleron disease*'.ti,ab,kf. or 'colitis ulcer*'.ti,ab,kf. or 'ulcerative col*'.ti,ab,kf. or 'idiopathic proctocol*'.ti,ab,kf. or 'colitis gravis'.ti,ab,kf. or 'ulcerative proctocol*'.ti,ab,kf. or 'ulcerative procto colit*'.ti,ab,kf. or 'ulcerous colit*'.ti,ab,kf. or ((granulomatous or terminal or regional* or mucosal) adj3 (ileit* or enteriti* or enterocolit* or colit*)).ti,ab,kf. or ileocolit*.ti,ab,kf. |
135215 |