Bepaling HPV-status
Uitgangsvraag
Hoe moet de HPV-status bepaald worden?
De uitgangsvraag omvat de volgende deelvragen:
- Hoe moet de HPV-status op histologisch materiaal bepaald worden bij patiënten met een gediagnosticeerd orofarynx carcinoom?
- Hoe moet de HPV-status bepaald worden bij patiënten met een lymfekliermetastase in de hals van een onbekende primaire tumor?
Aanbeveling
Aanbeveling-1
Onafhankelijkheid
Voer een HPV-test onafhankelijk van kennis over het anamnestische rookgedrag van de patiënt uit.
Aanbeveling-2
Bij (klinische verdenking op) nieuwe orofarynxcarcinomen
- Voer een hoog risico (HR)-HPV test uit op alle nieuw gediagnosticeerde plaveiselcelcarcinomen van de orofarynx, onafhankelijk van het histologische subtype.
- Voer de HR-HPV test uit op de primaire tumor of op een metastase indien deze metastase klinisch afkomstig is van het orofarynxcarcinoom.
- Voer p16 immunohistochemie uit op histologisch materiaal van een orofarynxcarcinoom als HR-HPV test. Overweeg een additionele specifieke test als bevestiging.
- Voer HR-HPV testen uit op cytologisch materiaal van een lymfklierpunctaat indien er geen histologisch materiaal aanwezig is en histologisch materiaal niet te verkrijgen is bij patiënten met een orofarynxcarcinoom dat nog niet eerder getest is of bij patiënten met een klinische verdenking op een orofarynxcarcinoom.
Aanbeveling-3
Bij metastasen van onbekende primaire tumor
- Voer routinematig een HR-HPV test uit op materiaal van patiënten met een plaveiselcelcarcinoommetastase van onbekende primaire tumor bij metastasen in Level II of III van de hals.
- Voer p16 immunohistochemie uit op histologisch materiaal uit een level II of III lymfekliermetastase met onbekende primaire tumor.
- Voer p16 immunohistochemie uit op histologisch materiaal van lymfekliermetastase buiten level II of III met onbekende primaire tumor in geval van niet-keratiniserende morfologie. Overweeg een additionele specifieke test als bevestiging.
- Voer HR-HPV testen uit op cytologisch materiaal van een lymfklierpunctaat indien er geen histologisch materiaal aanwezig is en dit materiaal niet te verkrijgen is bij patiënten met een onbekende primaire tumor.
NB: er wordt geen aanbeveling gegeven over de te gebruiken test op cytologisch materiaal.
Aanbeveling-4
Niet routinematig onderzoek
- Voer niet routinematig HR-HPV testen uit voor niet-plaveiselcelcarcinomen.
- Voer niet routinematig HR-HPV testen uit op andere primaire hoofd-hals carcinomen dan orofarynx.
- Overweeg geen HR-HPV testen uit te voeren bij patiënten met een residu of recidiverende tumor waarvan de HPV status initieel al was vastgesteld. Overweeg bij twijfel of het een recidiverende eerste tumor is om wél een HR-HPV test uit te voeren.
- Voer niet routinematig laag risico HPV testen uit op plaveiselcelcarcinomen van het hoofd-halsgebied.
Aanbeveling-5
Rapportage
- Rapporteer p16-positiviteit in het histologisch materiaal bij ten minste matige tot sterke aankleuring van 70% van de cellen als surrogaat voor HR-HPV.
- Rapporteer p16 immunohistochemie-positieve of HR-HPV-positieve primaire orofarynxcarcinomen als p16-positief of HPV-positief.
- Gradeer de HPV/p16-positieve orofarynxcarcinomen niet.
Overwegingen
Prigge (2017) vond een hoge sensitiviteit (0,93; 95%BHI: 0,87 to 0,97; I2: 23,4%) en een hoge specificiteit (0,96; 95%BHI: 0,89 to 1,00; I2: 68,4%) voor het gecombineerde gebruik van p16INK4a met een HPV DNA PCR op histologisch materiaal van mensen met een plaveiselcarcinoom van de orofarynx. Er werden geen positief en negatief voorspellende waarden gerapporteerd. De zekerheid in deze diagnostische accuratesse werd echter als zeer laag beoordeeld, gezien het onduidelijk was of de geïncludeerde studies onwenselijke exclusies vermeden, er enige zorgen waren over de toepasbaarheid met betrekking tot te patiënten in de steekproeven en vanwege de kleine informatie grootte door het lage deelnemersaantal (imprecisie).
Er werden veel verschillende methoden en procedures gevonden voor testen op cytologisch materiaal van patiënten met een plaveiselcelcarcinoom metastase in een hals lymfklier. Zo werd er, bijvoorbeeld, getest met verschillende indextesten, verschillende referentietesten, verschillende afkapwaarden voor positieve uitslagen, verschillend materiaal voor referentietesten en/of verschillende fixatiemiddelen. Door deze heterogeniteit werd geacht dat de data uit deze studies niet samen te voegen waren tot gepoolde schatters voor sensitiviteit en specificiteit. Hierdoor varieerden de geobserveerde sensitiviteit (range: 0,00 tot 1,00), de specificiteit (range: 0,16 tot 1,00), de positief voorspellende waarde (range: 0,00 tot 1,00) en de negatief voorspellende waarde (range: 0,00 tot 1,00), afhankelijk van de tests en procedures. De zekerheid in het gevonden bewijs was zeer laag door risico’s op vertekening van uitkomsten, door enige zorgen over de toepasbaarheid en door de zeer kleine informatie grootte in elke afzonderlijke vergelijking (imprecisie).
The College of American Pathologists (CAP) ontwikkelde een richtlijn over HPV diagnostiek bij hoofd-hals carcinomen (Lewis, 2018). De CAP-richtlijn werd multidisciplinair ontwikkeld, met medische expertise, expertise op het gebied van hoofd, hals en moleculaire pathologie, en chirurgische, medische en radiatie oncologie (Lewis, 2018). Ook werd er een methodoloog aan de multidisciplinaire werkgroep toegevoegd en werd er een adviesgroep opgericht. De adviesgroep bestond uit patiëntvertegenwoordigers, pathologen, een medisch oncoloog en moleculair epidemioloog, een radiotherapeut-oncoloog en een methodoloog. Eventuele financiële belangen van de werkgroep werden in kaart gebracht. Twee (van de elf) deelnemers hadden potentiële belangen, maar specifieke acties hierop werden niet gerapporteerd. De ontwikkelmethodologie van de richtlijn werd in een supplement gerapporteerd (Lewis, 2018). Uitgangsvragen werden opgesteld en een systematisch zoekopdracht en literatuurselectie werden uitgevoerd. De in- en exclusiecriteria zijn vermeld, maar kunnen wellicht niet voor elke uitgangsvraag volledig reproduceerbaar zijn. Data werd vervolgens uit de geselecteerde studies geëxtraheerd en de studies werden op kwaliteit beoordeeld. Systematische reviews werden met de AMSTAR-tool beoordeeld en observationele studies met de Newcastle-Ottawa quality assessment scale. (Her)analyses van RCT’s werden niet met een specifiek kwaliteitsinstrument beoordeeld. De richtlijnwerkgroep moest vier specifieke overwegingen maken op tot aanbevelingen te komen. De overwegingen betroffen significante bevindingen, de algehele sterkte van het bewijs, de sterkte van de te maken aanbeveling, en de balans tussen schade en voordelen. Er werd geen formeel framework gebruikt om deze beslissingen expliciet en/of transparant te maken. De CAP voorzag de werkgroep van geld voor de projectadministratie en er werden geen gelden uit de industrie gebruik. Werkgroepleden van de CAP-richtlijn werden niet gecompenseerd voor hun betrokkenheid en investeerden kosteloos hun tijd.
De CAP-richtlijn rapporteert een algoritme voor de work-up van patiënt monster (Lewis, 2018). Het algoritme start met een monster door biopsie of resectie van een gediagnosticeerd plaveiselcarcinoom en vertakt afhankelijk van de tumorlocatie (i.e. multi-site met een betrokken oropharynx, cervicale lymfklier, non-orofaryngeale primaire tumor, en orofaryngeale tumor). In het algoritme is de eerste test uit de work-up p16 immunohistochemie wanneer een HPV test geïndiceerd is. Hierin wordt ≥ 70% kleuring van de nuclei en cytoplasma als een positief resultaat gezien. Alleen wanneer het carcinoom keratinizerend is, de metastase zich niet in level II of level III van de hals bevindt, en/of wanneer de betrokken lymfklier niet bepaald kan worden stellen de CAP-richtlijn auteurs dat een HR HPV-specifieke test noodzakelijk is om een positieve p16 immunohistochemische test te bevestigen (Lewis, 2018). De diagnostische accuratesse van het voorgestelde algoritme werd niet onderzocht. Figuur 1 in de CAP-richtlijn laat het gehele voorgestelde algoritme zien van de diagnostische work-up (Lewis, 2018). De richtlijn werd als een open access artikel gepubliceerd (zie de URLs in de bijlagen van deze module).
De CAP-richtlijn vermeldde, als aanbeveling, dat op materiaal van patiënten met een cervicale plaveiselcelcarcinoom metastase van een onbekend primair carcinoom routinematig HR HPV testen zou moeten worden gedaan (Lewis, 2018). Deze vermelding als aanbeveling betekent dat er enkele limitaties aan de kwaliteit van het bewijs, balans tussen schade en voordelen, waarden of kosten zitten. Verder werd er een expert consensus uitspraak gedaan over HR HPV tests op materiaal afgenomen via een fijne naald aspiraat bij patiënten met een plaveiselcarcinoom metastase van een onbekende primaire tumor. De consensus onder de experts in de CAP-richtlijnwerkgroep was dat HR HPV tests bij alle patiënten met plaveiselcelcarcinoom metastasen van een onbekende primaire tumor zouden moeten worden uitgevoerd (Lewis, 2018). Een expert consensus uitspraak in de CAP-richtlijn betekent dat de werkgroep van de CAP het noodzakelijk achtte om over het onderwerp een uitspraak te doen, maar dat er ernstige limitaties zijn aan de kwaliteit van het bewijs, balans tussen schade en voordelen, waarden of kosten. De CAP-richtlijn vermeldde verder dat er geen specifieke aanbevelingen gegeven konden worden over de test methodologie en dat testen op weefsel (wanneer beschikbaar) uitgevoerd zouden moeten worden indien de HR HPV test op het cytologische materiaal negatief was (Lewis, 2018). Verder merkt de CAP-richtlijnwerkgroep op dat pathologen de afkapwaarden (voor positieve/negatieve uitslagen) zouden moeten valideren wanneer men p16 immunohistochemie op cytologisch materiaal gebruikt (Lewis, 2018).
Uit het literatuuronderzoek zijn geen eenduidige aanbevelingen te destilleren met betrekking tot de “beste” test of combinatie van testen om HR-HPV aan te tonen. Ook het literatuuronderzoek dat verricht is voor het schrijven van de CAP richtlijn heeft dit niet kunnen aantonen. Derhalve sluit de werkgroep zich aan bij de aanbevelingen uit de CAP richtlijn die wel beschrijven in welke situaties er wel en niet getest moet worden voor HPV door de patholoog, maar niet expliciet voorschrijven welke test daarvoor gebruikt moet worden (Lewis, 2018). De makkelijke beschikbaar, relatieve eenvoud en brede beschikbaarheid van p16 immunohistochemie geldt daarbij wel als minimum basis wat elk laboratorium moet kunnen uitvoeren.
Het doel van de PA diagnose is om met het beschikbare materiaal de beste diagnose voor de patiënt te stellen. Voor patiënten is het van belang dat de tumor juist wordt geclassificeerd voor de best mogelijke behandeling. Hierbij is het bepalen van de HPV-status van belang, omdat deze tumor een aparte classificatie heeft gekregen in de TNM 8e editie. Eventueel kan de hoog-risico HPV nog nader getypeerd worden.
P16 immunohistochemie is een relatief simpele en snel uit te voeren test die elk PA laboratorium in Nederland standaard in zijn pakket heeft. Voor de overige (moleculaire) technieken zijn er verschillen welke test ter beschikking is. Alle PA laboratoria in Nederland zijn ISO15189 geaccrediteerd en voeren dus geregeld kwaliteitscontroles en interlaboratorium vergelijkingen uit voor al hun beschikbare testen, zodat onafhankelijk van het platform de kwaliteit van de uitslag geborgd is.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Aanbeveling-1
De aanbevelingen zijn met enige aanpassingen voor de Nederlandse situatie overgenomen uit de richtlijn van de College of American Pathologists (Lewis, 2018). Kennis van het rookgedrag van de patiënt mag het uitvoeren van de HPV-test niet beïnvloeden.
Aanbeveling-2
De aanbevelingen zijn met enige aanpassingen voor de Nederlandse situatie overgenomen uit de richtlijn van de College of American Pathologists (Lewis, 2018). Het is belangrijk om, daar waar mogelijk, op histologisch materiaal te testen. Voor het gebruik van p16 immunohistochemie als eerste test op histologisch materiaal werd gekozen omdat deze test makkelijk beschikbaar en relatieve eenvoudig uit te voeren is. Indien er geen histologisch materiaal beschikbaar is of beschikbaar komt, is het testen op cytologisch materiaal van een lymfklierpunctaat een alternatief. Het is onduidelijk vanuit de literatuur welke test op cytologisch materiaal gebruikt zou moeten worden.
Aanbeveling-3
De aanbevelingen zijn met enige aanpassingen voor de Nederlandse situatie overgenomen uit de richtlijn van de College of American Pathologists (Lewis, 2018). Indien er histologisch materiaal beschikbaar is uit een Level II of III lymfekliermetastase werd er voor p16 als eerste test gekozen omdat deze test makkelijk beschikbaar en relatieve eenvoudig uit te voeren is. Omdat er in deze situatie niet altijd histologisch materiaal beschikbaar is, worden testen op cytologisch materiaal uit een lymfklierpunctaat als alternatief gezien. Het is onduidelijk vanuit de literatuur met welke test er op cytologisch materiaal gebruikt zou moeten worden.
Aanbeveling-4
De aanbevelingen zijn met enige aanpassingen voor de Nederlandse situatie overgenomen uit de richtlijn van de College of American Pathologists (Lewis, 2018). Routinematige HR-HPV testen voor niet-plaveiselcelcarcinomen, andere primaire hoofd-halscarcinomen, en residu van of recidiverende eerste tumoren (waarvan de HPV status al initieel bepaald werd) worden niet aangeraden. Voor plaveiselcelcarcinomen van het hoofd-halsgebied werd routinematige laag risico HPV (LR-HPV) testen niet aanbevolen.
Aanbeveling-5
De aanbevelingen zijn met enige aanpassingen voor de Nederlandse situatie overgenomen uit de richtlijn van de College of American Pathologists (Lewis, 2018).
Onderbouwing
Achtergrond
De Humaan Papillomavirus (HPV) status van orofarynx carcinomen kan bepaald worden met verschillende testen. Op dit moment is het onduidelijk welke op histopathologie gebaseerde teststrategie de beste diagnostische accuratesse voor het bepalen van de HPV-status van gediagnosticeerde orofarynx carcinomen heeft. In sommige omstandigheden is histopathologisch materiaal niet beschikbaar, bijvoorbeeld wanneer de primaire tumor onbekend is. De HPV-status zou dan wellicht op basis van cytologische tests kunnen worden vastgesteld op cytologisch materiaal dat met een lymfklierpunctaat is verkregen bij patiënten met een onbekende primaire tumor en een gediagnosticeerde halsmetastase. Echter is het op dit moment nog onduidelijk wat de diagnostische accuratesse van HPV-testen op cytologisch materiaal van een positieve lymfeklier uit de hals is.
Conclusies / Summary of Findings
|
Very low GRADE |
There is a very low confidence in the sensitivity (0.93, 95%CI: 0.87 to 0.97) and specificity (0.96, 95%CI: 0.89 to 1.00) of p16INK4a combined with an HPV DNA PCR on histological material as a test strategy.
Sources: (Prigge, 2017) |
|
- GRADE |
Positive and negative predictive values were not reported for p16INK4a combined with an HPV DNA PCR on histological material as a test strategy.
Sources: (Prigge, 2017) |
|
Very low GRADE |
There is a very low confidence in the sensitivity (range: 0.00 to 1.00) and specificity (range: 0.16 to 1.00) of the tests performed on cytologic material.
Sources: (Baldassari, 2015; Begum, 2007; Bishop, 2012; Buonocore, 2019; Chute, 2014; Hou, 2016; Jalaly, 2015; Jannapureddy, 2010; Sivars, 2017; Smith, 2014; Takes, 2016; Xu, 2016) |
|
Very low GRADE |
There is a very low confidence in the positive predictive value (range: 0.00 to 1.00) and negative predictive value (range: 0.16 to 1.00) of the tests performed on cytologic material.
Sources: (Baldassari, 2015; Begum, 2007; Bishop, 2012; Buonocore, 2019; Chute, 2014; Hou, 2016; Jalaly, 2015; Jannapureddy, 2010; Sivars, 2017; Smith, 2014; Takes, 2016; Xu, 2016) |
Samenvatting literatuur
Description of studies
Diagnostic algorithms on histological material to determine the HPV-status in patients with a confirmed oropharyngeal carcinoma (PICO 1)
Prigge (2017) conducted a systematic review where the diagnostic accuracy of a strategy was assessed where p16INK4a immunohistochemistry and an HPV DNA PCR was combined for HPV-testing in oropharyngeal squamous cell carcinomas. MEDLINE was searched through PubMed on the 8th of January 2016. Studies were included when persons in the sample were diagnosed with oropharyngeal squamous cell carcinoma, p16INK4a was the index test, a reference test was used that detected E6 and/or E7 mRNA and when the study design was prospective or retrospective. Studies were excluded when the authors of the original studies did not respond to inquiries about the presented data, when the sample size was smaller than 10, and when the study did not report primary data. Eleven of the included studies reported the use of p16INK4a and HPV DNA PCR as a combined test for diagnostic test accuracy evaluation against a reference standard that detected E6 and/or E7 mRNA. The eleven studies comprised of a sample of 509 persons (as reported in the study characteristics table in the systematic review). A case was defined as positive when both the p16INK4a and the HPV DNA PCR returned positive results. When one of both or both tests returned a negative result, the case was defined as negative. Studies were assessed with the QUADAS-2 tool for risk of bias and applicability. Most of the 11 studies scored ‘unclear’ in the patient selection domain, where it was mostly unclear whether studies avoided inappropriate exclusions. The applicability regarding the patient selection was generally judged as having moderate concerns. The cut-off for defining positive/negative cases by using p16INK4a varied among the included studies and was tested on whole tissue section FFPE material (with one exception, where tissue microarray was used on FFPE material). Five studies used the G175-405 antibody clone for p16INK4a, while the other six studies used E6H4. Variation in procedures was also observed in the HPV DNA PCR methods. Here, GP5+/6+ (reverse line blot genotyping or bead-based genotyping), HPV 16 primers, HPV 16 E6/E7 primers, MY09/MY11/HMB01 (dot blot hybridization genotyping), BSGP5+/6+ (bead-based genotyping) were the described methods. Reference tests also had variation in their procedures. Transcript types used were E6, E6*I, E7, or combinations thereof. Reference tests also showed variation in the detected HPV types. HPV 16 was sought for detection in all studies, however some studies added HPV 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68b, 70, 73, and/or 82 for detection as well. Four of the eleven studies used whole tissue section FFPE material for the reference test, while the remaining seven studies used fresh frozen material.
Tests on cytological material in patients with positive neck nodes and CUP (PICO 2)
Baldassari (2015) used a cobas HPV assay on fine needle aspirates and compared it to a combined test of p16 and HPV ISH on paraffin-embedded formalin-fixed surgical tissue of the primary and/or metastatic tumor. Specimens were collected prospectively, but it remained unclear whether this was consecutively. Inclusion and exclusion criteria were not described. Air-dried and alcohol fixed smears were prepared and stained. A slide was prepared after centrifugation and the cobas HPV assay was performed according to the manufacturer’s protocol. Fourteen HPV types were targeted for amplification (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68). Both p16 IHC and HPV ISH were performed on deparaffinized 5-micrometer sections. For p16, sections were incubated with a mouse monoclonal antibody (E6H4). Sections for HPV ISH were incubated with the INFORM HPV III Family 16 probe, detecting HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66. The cut-off points for a positive/negative case in the cobas HPV assay, p16, and HPV ISH were unclear. Thirty-seven participants were recruited and forty-two fine needle aspirates were taken. Participants had a mean age of 60.4 (SD: 11.6) years. Seventeen participants had a history of head and neck squamous cell carcinomas. Fine needle sample sites were the neck mass (n=36), the mediastinal lymph node (n=5), or a left parapharyngeal mass (n=1).
Begum (2007) searched a database and selected cases when the processing of the initial fine needle aspirate included the preparation of a cellblock by spinning the cell block in a cellular pellet. The index test was p16 on cell blocks, where 5-micron sections were deparaffinized. Sections were then incubated with a mouse monoclonal antibody. Observing any staining in the squamous cells was considered to be positive for HPV. Material from fine needle aspirates were also tested with HPV16 ISH (considered as a reference test). HPV16 ISH was performed on cell blocks for signal amplification and on resections of the primary tumor. Signals visualized as dots in nuclei of the squamous cells were considered positive for HPV. Nineteen participants with oropharyngeal tumors and ten participants with an unknown primary were selected. No other patient characteristics were described.
Bishop (2012) consecutively recruited participants to assess the accuracy of the Hybrid Capture 2 assay (HC2), although inclusion and exclusion criteria were not reported. Metastatic tumors were aspirated using a 12 gauge needle and multiple passes. HC2 detected HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68. Specimen DNA was denatured into single stranded DNA. RNA/DNA hybrids were then immobilized onto a microplate surface. Light is emitted and measured in relative light units. A case with ≥ 3 RLU/CO was considered positive for HPV, 0.85-3 RLU/CO was considered equivocal, and < 0.85 RLU/CO was considered negative. HPV ISH was used as the reference test. Hybridization was performed using the HPV III Family 16 probe (HPV16, 18, 33, 35, 45, 51, 52, 56, 66) on 5-micron section from the tissue microarray was assessed. HPV ISH was considered positive when signals localized to tumor cell nuclei. Participant recruitment resulted in 24 participants (27 cytologic preparations), of which 12 had a lymph node sample site. From these 12 participants, the tumor site was the skin (n=2), larynx (n=2, floor of mouth (n=1), tongue (n=1), base of tongue (n=1), tonsil (n=4), and unknown (n-1).
Buonocore (2019) recruited 25 participants consecutively (n=24 were positive for HPV by HPV ISH, n=1 was non-contributory). Participants were included when they had previous or unknown oropharyngeal head and neck squamous cell carcinoma (or this was determined at the time of the procedure) and an unknown p16 status. Exclusion criteria were not reported. Fine needle aspirates were performed with a 25 gauge needle. Diff-Quik-stained and ethanol fixed smears were prepared. Passes were allowed to clot before fixed in formalin. From this material both a CytoLyt-fixed and a formalin-fixed cell block were made. Both the CytoLyt-fixed and formalin-fixed cell blocks were tested with p16 (indextest) against HPV ISH (reference test). Mouse monoclonal antibodies (E6H4) were used for p16. A cut-off of ÷70% staining in nuclei and cytoplasm was used to define a HPV-positive case. It was unclear on which material the HPV ISH was performed and it was unclear how an HPV-positive case was defined. HPV ISH targeted HPV 16, 18, 26, 31, 33, 35, 39, 41, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and E6/E7 mRNA. Participants (22 male; 3 female) had a mean age of 60 (range: 47 to 76) years and a variety in smoking history (never smoked: n=14, smoking history: n=11 (range: 0.5-40 pack years)). Similarly, a variety of alcohol use was observed: never (n=1), abstinent (n=1), occasional (n=2), social (n=15), daily (n=3), heavy (n=3).
Chute (2014) recruited 95 participants (resulting in 96 fine needle aspirates) prospectively. Participants were eligible when a fine needle aspirate from a head and neck-site was interpreted as being a squamous cell carcinoma, atypical, or suspicious for squamous cell carcinoma. Exclusion criteria were not reported. A cell block was made in an automated system according to its manufacturer’s directions. However, methylene blue was replaced by eosin. HC2 and CISH were performed on cytological material. HC2 was performed according to the manufacturer’s instructions, targeting HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68. A RLU/CO ≥ 1 was defined as a HPV-positive case. For, CISH, the HPV III Family 16 probe was used (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58). A discrete blue dot-like staining in the tumor nuclei was defines as an HPV-positive case. CISH combined with p16 was performed on a surgical biopsy. An HPV-positive case for p16 was defined as > 75% strong and diffuse cytoplasmic and nuclear staining. For the CISH and p16 combined test, an HPV-positive case was defined as having a positive test result from both CISH and p16. Formalin-fixed paraffin-embedded tissue of the excised primary tumor or neck metastasis were used for testing in the CISH and p16 combined test. Participants (72 male; 21 female) had a median age of 60 (range: 17-93) years. The primary tumor location was oropharyngeal (n=32), non-oropharyngeal (n=32), non-head and neck (n=18), or unknown (n=13).
Hou (2016) searched a database to select cases with metastatic head and neck squamous cell carcinomas in cervical lymph nodes, diagnosed by a fine needle aspirate. HPV ISH and p16 had to be performed on fine needle aspirate material to be selected from the database. No exclusion criteria were reported. Both p16 (index test) and HPV ISH (reference test) were performed on cytologic material. Fine needle aspirates were centrifuged and the specimen was clot dried. The specimen was then placed in a CellSafe mesh capsule and fixated in formalin (10% neutral-buffered). For p16, monoclonal antibodies (E6H4) were used. An HPV-positive case by p16 was defined as ≥ 70% diffuse or strong nuclear or cytoplasmic staining. HPV ISH was performed according to the manufacturer’s protocol on 4-micrometer sections of the cell block. HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 68, and Y1443 were targeted. Presence of staining in the nuclei defined an HPV-positive case for HPV ISH. Participants (80 male; 7 female) had a mean age of 59 (range: 38 to 86) years. The primary site of the tumor was the base of the tongue (n=32), tonsil (n=19), other oropharyngeal sites (not specified, n=4), non-oropharyngeal (not specified, n=25), or unknown (n=7).
Jalaly (2015) searched a database to select cases that had a metastatic cervical lymph node (proven by fine needle aspirates) with a corresponding surgical specimen (either biopsy or resection). P16 and HPV ISH were performed on the cell blocks created from the fine needle aspirate. Fine needle aspirate material was centrifuged for 2 minutes. The specimen clot was allowed to dry on tissue paper. Thereafter, it was placed in a CellSafe capsule and fixed in formalin (10% neutral-buffered). For p16, a monoclonal antibody (E6H4) was used and an HPV-positive case for p16 was defined as >70% nuclear and cytoplasmic staining of the tumor cells. HPV ISH detected E6/E7 mRNA and was performed according to the manufacturer’s instruction and 4-millimeter formalin-fixed paraffin-embedded cell block sections were prepared. Red punctate dots in the nucleus and/or cytoplasm signals higher than the signal on a DapB-negative control slide was defined as a HPV-positive case. Forty-eight participants were recruited (44 male; 4 female). The fine needle aspirate sample site was the neck (n=41), subcarinal (n=2), mediastinal (n=1), submandibular (n=2), chest wall (n=1), or supraclavicular (n=1). The specimen was either resected (n=32) or a biopsy was made (n=16). The tumor site of the primary tumor was at the base of the tongue (n=14), tonsil (n=15), other oropharyngeal (not specified, n=8), oral cavity (n=6), larynx (n=1), maxilla (n=1), or unknown (n=3).
Janapureddy (2010) searched a database to select participants that had a cell block cytologic diagnosis of metastatic squamous cell carcinoma in a cervical lymph node. Participants with inadequate cell block material were excluded. Cytologic material was tested with p16INK4a and ProExC as index tests and compared to HPV ISH on cell block sections. Material from fine needle aspirates were fixed in formalin (10% neutral-buffered). After centrifugation the supernatant was discarded and the resulting content was assessed. Cell block tissue was created (5-micrometer) from the formalin-fixed paraffin-embedded tissue. Incubation was performed with E6H4 monoclonal p16INK4a at room temperature. An HPV-positive case for p16 was defined as the presence of nuclear and cytoplasmic staining. ProExC also had in incubation period at room temperature. Presence of nuclear staining defined an HPV-positive case for ProExC. HPV ISH detected HPV 16, 18, 31, 33, and 51. Cell block tissue was deparaffinized and rehydrated. Slides were air dried, sections were denatured and hybridized. HPV-positive cases by HPV ISH were defined as the presence of punctate or dot-like nuclear staining. Participants (36 male; 4 female) had a mean age of 58.2 (reange 25 to 87) years. The primary tumor site was oropharyngeal (not specified, n=11), nasopharyngeal (n=2), other (not specified, n=5), or was not determined (n=9).
Sivars (2017) prospectively obtained fine needle aspirate material. Participants were recruited when they were suspected of head and neck carcinoma or had a neck mass suspicious for metastasis, and when there was not enough material left for HPV testing after cytological diagnosis. The HPV-status was tested on cytological material from fine needle aspirates and/or formalin-fixed paraffin-embedded material (either resection or biopsy). For cytologic testing, DNA was extracted from fine needle aspirates. The DNA multiplex assay was performed by using GP5+/GP6+ primers and additionally E6 (HPV 16, 33) was amplified. DNA detection was performed on a bead-based multiplex using mean fluorescent intensity. An HPV-positive case for the multiplex assay was defined as a mean fluorescent intensity above the background * 1.5 + 15. Furthermore, a Real-Time PCR was used in the clinic to detect seven common high-risk HPV genotypes. Is was unclear how a positive/negative case was defined vor the Real-Time PCR in the clinic. Sixty-six patients (35 male; 31 female) participated. Fine needle aspirate sample sites were the primary tumor (n=2) or neck masses (n=64). The mean age was 61 year for persons with an oropharyngeal tumor (n=20), 71.5 years for persons with other malignancies (n=17), and 53 years for persons with benign conditions (n=29).
Smith (2014) recruited participants prospectively when a cervical lymph node was swollen to one centimeter or larger (exclusion criteria were not reported). A modified HC2 HPV assay detected HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 in cytologic material. Fine needle biopsies of cervical metastases were performed with a 25 gauge needle. The aspirate was placed on a slide and was air-dried and stained. A final pass was made with a fresh needle in to the lymph node, which was stored until used for HC2. DNA was denatured and incubated for 45 minutes. Samples were applied to hybrid-specific antibodiy coated microplate wells. Signal amplification was performed with Detection Reagent II and light emission was used to detect HPV DNA. An HPV-positive case for HC2 was defined as ≥ 2.5 RLO/CO, an equivocal case as 0.85 to 2.5 RLU/CO, and a negative case as < 0.85 RLU/CO. HPV ISH was performed on tissue specimen from resected participants. Five-micron formalin-fixed paraffin-embedded tissue sections from either tumors or biopsies were used for HPV ISH. The HPV III Family 16 probe set was used to detect HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66). Punctate signals in the nuclei defined a positive HPV case for HPV ISH. The mean age and sex distribution were not reported for the participants, however 25 persons were recruited. The tumor location was on the palatine tonsil (n=6), base of the tongue (n=8), hypopharynx (n=1), skin of the auricle (n=1), or unknown (n=2).
Takes (2016) searched a database for cases where formalin-fixed paraffin-embedded histological material was available and was tested positive or negative for both HPV and p16. Cases were excluded when there was a secondary tumor in the head and neck region, when there was not enough cytological material, or when there was previous exposure to radiotherapy. A HPV PCR was performed on fine needle aspirates material scraped from archival slides. The DNA was purified, diluted and stored until tested by HPV PCR. A broad-spectrum DNA amplification was performed in the HPV PCR. Probes were used in a micro titer hybridization assay. Cases positive in the micro titer hybridization assay were tested with line-specific probes (LiPA25) for detection of HPV 1, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, and 74. LiPA strips were visually inspected and interpreted. Interpretation was performed following the standardized reference guide. It was unclear how an HPV-positive case was defined. Both the same HPV PCR and p16 were performed. The HPV PCR was performed on DNA isolated from formalin-fixed paraffin-embedded tissue sections of 4-micrometer. The p16 procedures were not reported. It was unclear, for both the HPV PCR and p16, how a HPV-positive case was defined. Participants (33 male; 14 female) had a mean age of 58 (range: 28.9 to 77.2) years. Their N-stage was N0 (n=6), N1 (n=7), or N2 (n=38). The primary tumor site was on the tonsils (n=19), the base of the tongue (n=12), other oropharyngeal (not specified, n=10), or unknown (n=6). Formalin-fixed paraffin-embedded material originated from the tonsils (n=21), base of the tongue (n=9), neck dissection (n=7), other oropharyngeal (not specified, n=10).
Xu (2016) searched a database and selected cases with cervical metastatic squamous cell carcinomas diagnosed with fine needle aspirates. For the selection, cases had to have p16 performed on both the cytological material and corresponding surgical material. Cases were excluded when tumors originated from outside the head and neck region. Cytologic material was prepared from fine needle aspirates for cell blocks and ThinPrep in CytoLyt solution. The p16 index test was performed on cell bock, smear or ThinPrep with pre-defined thresholds of 1%, 5%, 10%, 15%, and 70% nuclear and cytoplasmic staining to define an HPV-positive case. HPV CISH was performed on cytologic material using high risk HPV probes for detecting HPV 16, 18, 31, 33, and 51. An HPV-positive case for CISH was defined as discrete dot-like stippled nuclear labelling. How HPV-negative and equivocal cases were defined was not reported.
Results
Diagnostic algorithms on histological material to determine the HPV-status in patients with a confirmed oropharyngeal carcinoma (PICO 1)
Sensitivity
Prigge (2017) calculated a summary estimate for the sensitivity of p16INK4a combined with HPV DNA PCR from 11 studies (n=509). There was variation in underlying procedures (for example cut-offs for p16 positivity, materials, reference standards). Prigge (2017) found a pooled sensitivity 0.93 (95%CI: 0.87 to 0.97). Statistical heterogeneity (I2) was 23.39%.
Specificity
Prigge (2017) pooled the specificity of a p16INK4a and HPV DNA PCR combined test from 11 studies (n=509). Underlying procedures showed variation in procedures (for example cut-offs for p16 positivity, materials, reference standards). A summary estimate was calculated and Prigge (2017) reported a specificity of 0.96 (0.89 to 1.00). Statistical heterogeneity (I2) was 68.4%.
Positive predictive value
Positive predictive values were not reported.
Negative predictive value
Negative predictive values were not reported.
Tests on cytological material in patients with positive neck nodes and CUP (PICO 2)
Sensitivity
A variety of index tests on cytologic material were found. Tests were evaluated using several different methods and procedures: p16 (on CUP only, on oropharyngeal carcinoma only, on CytoLyt or formalin fixed cytologic material, at several thresholds, reference test on cytological or histological material), HC2 (various reference tests, reference test on cytological or histological material), PCR or RT-PCR (various reference tests, reference test on cytological or histological material), ProExC, HPV CISH, and cobas 4800. Data could not be pooled due to heterogeneity in the study procedures. Sensitivity ranged from 0.00 to 1.00. An overview of the sensitivities (including 95% confidence intervals) are found in Figure 1. Sensitivity and/or 95% confidence intervals were calculated when not reported in the original study. Cases were excluded in most analyses (described in the evidence table, for example due to invalid or equivocal test results, no reference test performed, or inadequate specimens).
Specificity
A variety of index tests on cytologic material were found. A variety of index tests on cytological material were found, identical to as described under the sensitivity results. Data could not be pooled due to heterogeneity in the study procedures and tests. Specificity and/or 95% confidence intervals were calculated when not reported in the original study. Specificity ranged from 0.16 to 1.00. An overview of the specificities (including 95% confidence intervals) are found in Figure 1 Cases were excluded in most analyses (described in the evidence table, for example due to invalid or equivocal test results, no reference test performed, or inadequate specimens).
Positive and negative predictive value
Most studies did not report the positive and/or negative predictive values. When not reported, positive and negative predictive values were calculated from the extracted data. Positive and negative predictive values ranged from 0.00 to 1.00. Cases were excluded in most analyses (described in the evidence table, for example due to invalid or equivocal test results, no reference test performed, or inadequate specimens).
Figure 1 An overview of the sensitivity and specificity of the tests reported in the included references by index test, reference test and testing material. Category titles show the used index test (type of material) versus reference test (type of material)
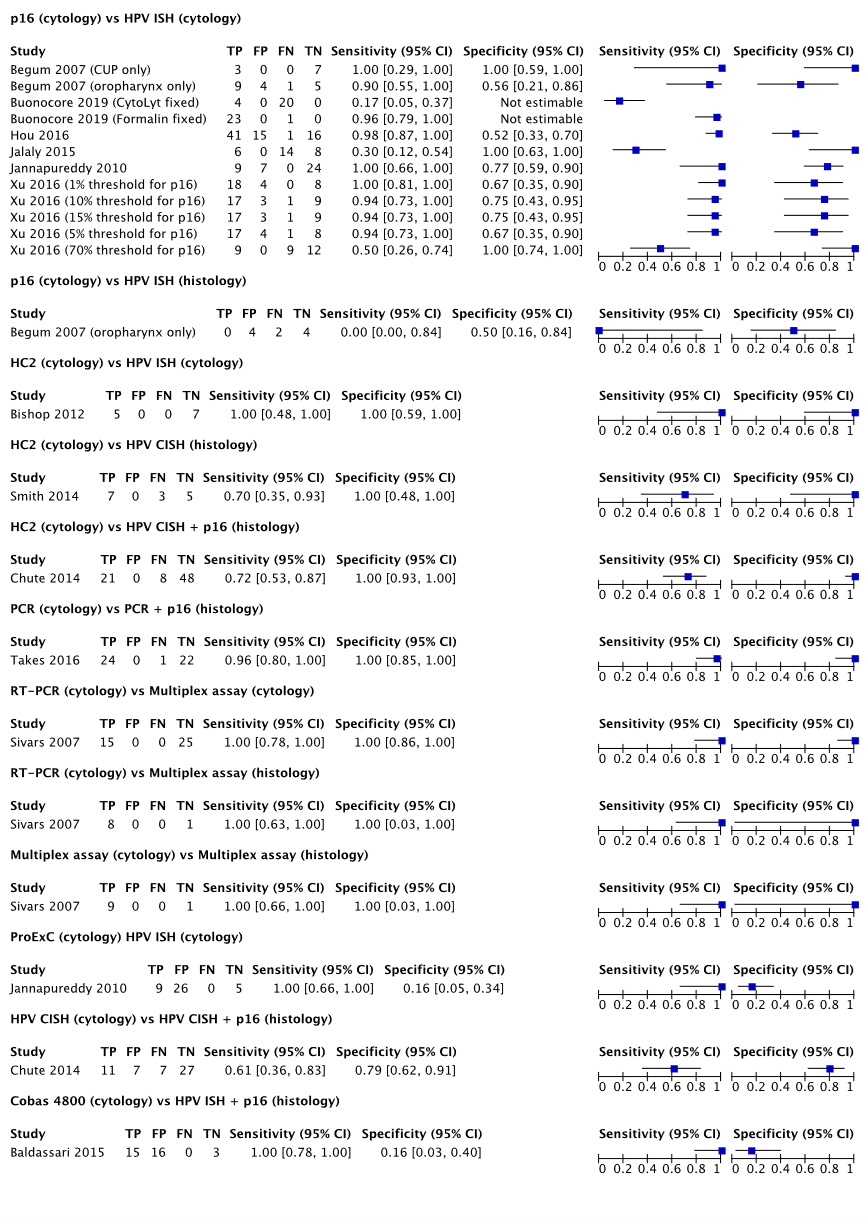
Table 1 Overview of the positive and negative predictive values of the tests on cytologic material by index test, reference test and testing material
|
Author, year (condition) |
TP |
FP |
FN |
TN |
PPV |
NPV |
|
P16 (cytology) versusHPV ISH (cytology) |
||||||
|
Begum 2007 (CUP only) |
3 |
0 |
0 |
7 |
1.00* |
1.00* |
|
Begum 2007 (oropharynx only) |
9 |
4 |
1 |
5 |
0.69 |
0.833 |
|
Buonocore 2019 (CytoLyt fixed) |
4 |
0 |
20 |
0 |
1.00* |
0.00* |
|
Buonocore 2019 (Formalin fixed) |
23 |
0 |
1 |
0 |
1.00* |
0.00* |
|
Hou 2016 |
41 |
15 |
1 |
16 |
0.73 |
0.94 |
|
Jalaly 2015 |
6 |
0 |
14 |
8 |
1.00* |
0.36 |
|
Jannapureddy 2010 |
9 |
7 |
0 |
24 |
0.56 |
1.00* |
|
Xu 2016 (1% threshold for p16) |
18 |
4 |
0 |
8 |
0.82 |
1.00* |
|
Xu 2016 (10% threshold for p16) |
17 |
3 |
1 |
9 |
0.85 |
0.90 |
|
Xu 2016 (15% threshold for p16) |
17 |
3 |
1 |
9 |
0.85 |
0.90 |
|
Xu 2016 (5% threshold for p16) |
17 |
4 |
1 |
8 |
0.81 |
0.89 |
|
Xu 2016 (70% threshold for p16) |
9 |
0 |
9 |
12 |
1.00* |
0.57 |
|
P16 (cytology) versus HPV ISH (histology) |
||||||
|
Begum 2007 (oropharynx only) |
0 |
4 |
2 |
4 |
0.00* |
0.67 |
|
HC2 (cytology) versus HPV ISH (cytology) |
||||||
|
Bishop 2012 |
5 |
0 |
0 |
7 |
1.00* |
1.00* |
|
HC2 (cytology) versus HPV CISH (histology) |
||||||
|
Smith 2014 |
7 |
0 |
3 |
5 |
1.00* |
0.63 |
|
HC2 (cytology) versus HPV CISH = p16 (histology) |
||||||
|
Chute 2014 |
21 |
0 |
8 |
48 |
1.00* |
0.86 |
|
PCR (cytology) versus PCR + p16 (histology) |
||||||
|
Takes 2016 |
24 |
0 |
1 |
22 |
1.00* |
0.96 |
|
RT-PCR (cytology) versus Multiplex assay (cytology) |
||||||
|
Sivars 2007 |
15 |
0 |
0 |
25 |
1.00* |
1.00* |
|
RT-PCR (cytology) versus Multiplex assay (histology) |
||||||
|
Sivars 2007 |
8 |
0 |
0 |
1 |
1.00* |
1.00* |
|
Multiplex assay (cytology) versus Multiplex assay (histology) |
||||||
|
Sivars 2007 |
9 |
0 |
0 |
1 |
1.00* |
1.00* |
|
ProExC (cytology) versus HPV ISH (cytology) |
||||||
|
Jannapureddy 2010 |
9 |
26 |
0 |
5 |
0.26 |
1.00* |
|
Cobas 4800 (cytology) versus HPV ISH + p16 (histology) |
||||||
|
Baldassari 2015 |
15 |
16 |
0 |
3 |
0.48 |
1.00* |
|
HPV ISH (cytology) versus HPV ISH (histology) |
||||||
|
Begum 2007 (oropharynx only) |
1 |
0 |
1 |
8 |
1.00* |
0.89 |
|
*Calculation contained a cell value of zero |
||||||
Level of evidence of the literature
Diagnostic algorithms on histological material to determine the HPV-status in patients with a confirmed oropharyngeal carcinoma (PICO 1)
The level of evidence regarding the outcome measure sensitivity (for p16INK4a + HPV DNA PCR) was downgraded by 3 levels because of study limitations (1 level for risk of bias: most of the relevant studies were appraised by the authors as unclear regarding inappropriate exclusions); applicability (1 level for bias due to indirectness: most of the relevant studies were appraised by the authors as having moderate applicability concerns regarding patient selection); number of included patients (1 level for imprecision: n=509 according to the general characteristics table); publication bias was not assessed.
The level of evidence regarding the outcome measure specificity (for p16INK4a + HPV DNA PCR) was downgraded by 3 levels because of study limitations (1 level for risk of bias: most of the relevant studies were appraised by the authors as unclear regarding inappropriate exclusions); applicability (1 level for bias due to indirectness: most of the relevant studies were appraised by the authors as having moderate applicability concerns regarding patient selection); number of included patients (1 level for imprecision: n=509 according to the general characteristics table); publication bias was not assessed.
The positive and negative predictive value was not reported and therefore GRADE was not performed.
Tests on cytological material in patients with positive neck nodes and CUP (PICO 2)
The level of evidence regarding the outcome measure sensitivity was downgraded by 4 levels because of study limitations (1 levels for risk of bias: 27.1% judgements in the QUADAS-2 were high risk of bias (predominantly in patient selection and flow/timing), 41.7% judgements were unclear risk (predominantly in index test and reference standard)); not downgraded for conflicting results (inconsistency: observed heterogeneity might possibly be explained by differences in procedures and thresholds); applicability (1 level for bias due to indirectness: mostly unclear whether the included patients matched the review question); number of included patients (2 levels for imprecision: very low number of participants per comparison); publication bias was not assessed.
The level of evidence regarding the outcome measure specificity was downgraded by 4 levels because of study limitations (1 levels for risk of bias: 27.1% judgements in the QUADAS-2 were high risk of bias (predominantly in patient selection and flow/timing), 41.7% judgements were unclear risk (predominantly in index test and reference standard)); not downgraded for conflicting results (inconsistency: observed heterogeneity might possibly be explained by differences in procedures and thresholds); applicability (1 level for bias due to indirectness: mostly unclear whether the included patients matched the review question); number of included patients (2 levels for imprecision: very low number of participants per comparison); publication bias was not assessed.
The level of evidence regarding the outcome measure positive predictive value was downgraded by 4 levels because of study limitations (1 levels for risk of bias: 27.1% judgements in the QUADAS-2 were high risk of bias (predominantly in patient selection and flow/timing), 41.7% judgements were unclear risk (predominantly in index test and reference standard)); not downgraded for conflicting results (inconsistency: observed heterogeneity might possibly be explained by differences in procedures and thresholds); applicability (1 level for bias due to indirectness: mostly unclear whether the included patients matched the review question); number of included patients (2 levels for imprecision: very low number of participants per comparison); publication bias was not assessed.
The level of evidence regarding the outcome measure negative predictive value was downgraded by 4 levels because of study limitations (1 levels for risk of bias: 27.1% judgements in the QUADAS-2 were high risk of bias (predominantly in patient selection and flow/timing), 41.7% judgements were unclear risk (predominantly in index test and reference standard)); not downgraded for conflicting results (inconsistency: observed heterogeneity might possibly be explained by differences in procedures and thresholds); applicability (1 level for bias due to indirectness: mostly unclear whether the included patients matched the review question); number of included patients (2 levels for imprecision: very low number of participants per comparison); publication bias was not assessed.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following questions:
PICO 1
What is the diagnostic accuracy of diagnostic test algorithms to determine the HPV-status on histological material in patients with an oropharyngeal carcinoma?
P: patients with an oropharyngeal carcinoma;
I: diagnostic strategies/algorithms to determine the HPV-status based on histopathologic tests;
C: diagnostic strategies/algorithms compared;
R: a test to detect HPV-DNA and/or E6/E7 mRNA;
O: sensitivity, specificity, positive predictive value, negative predictive value.
PICO 2
What is the diagnostic accuracy of tests on cytologic material to determine the HPV-status in patients with a carcinoma of unknown primary?
P: patients with a carcinoma of unknown primary and a positive neck node;
I: diagnostic tests to determine the HPV-status based on cytologic material;
C: comparison of tests on cytologic material;
R: a test to detect HPV-DNA and/or E6/E7 mRNA;
O: sensitivity, specificity, positive predictive value, negative predictive value.
Relevant outcome measures
The guideline development group considered sensitivity and negative predictive value as a critical outcome measures for decision making; and specificity and positive predictive value as an important outcome measures for decision making.
A priori, the working group did not define the outcome measures listed above but used the definitions used in the studies.
Search and select (Methods)
PICO 1
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 2016 until the 10th of April 2020 for PICO 1 (histology). The time limiter was chosen because the guideline “Human Papillomavirus Testing in Head and Neck Cancers” from the College of American Pathologists (CAP) had their latest searched performed in 2016 (Lewis, 2018). The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 251 hits. Studies were selected based on the following criteria: patients had an oropharyngeal carcinoma, diagnostic strategies or algorithms were used to determine the HPV-status with histopathological tests, the reference test was a test that detected HPV DNA and/or mRNA, and at least one of the outcomes of interest was reported or it could be calculated manually from the presented data. Conference abstracts and non-systematic reviews were excluded. A total of 6 studies were initially selected based on title and abstract screening. After reading the full text, 5 studies were excluded (see the table with reasons for exclusion under the tab Evidence tables), and 1 systematic review (which included 24 primary studies) was included.
PICO 2
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until the 27th of February 2020 for PICO 2 (cytology). The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 448 hits. Studies were selected based on the following criteria: patients had a (suspected) neck metastasis, patients had (suspected) primary head and neck squamous cell carcinoma at the time material was taken for cytologic tests, the reference test was a test that detected HPV DNA and/or mRNA, and at least one of the outcomes of interest were reported or it could be calculated manually from the presented data. Conference abstracts and non-systematic reviews were excluded. A total of 74 studies were initially selected based on title and abstract screening. After reading the full text, 62 studies were excluded (see the table with reasons for exclusion under the tab Evidence tables) and 12 studies were included.
Results
One systematic review (including 24 primary studies) was included in the analysis of the literature for the histopathology-based diagnostic algorithms. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables (under the tab Evidence tables).
Thirteen studies were included in the analysis of the literature for the cytology-based tests. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables. Data regarding the classification of cases by the index test compared to the reference test were extracted from each of the included studies. Diagnostic accuracy parameters and/or 95% confidence intervals were calculated based on the extracted data when not reported in the original study.
Referenties
- Baldassarri R, Aronberg R, Levi AW, Yarbrough WG, Kowalski D, Chhieng D. Detection and genotype of high-risk human papillomavirus in fine-needle aspirates of patients with metastatic squamous cell carcinoma is helpful in determining tumor origin. Am J Clin Pathol. 2015 May;143(5):694-700. doi: 10.1309/AJCPCZA4PSZCFHQ4. PMID: 25873503.
- Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007 Feb 15;13(4):1186-91. doi: 10.1158/1078-0432.CCR-06-1690. PMID: 17317828.
- Bishop JA, Maleki Z, Valsamakis A, Ogawa T, Chang X, Pai SI, Westra WH. Application of the hybrid capture 2 assay to squamous cell carcinomas of the head and neck: a convenient liquid-phase approach for the reliable determination of human papillomavirus status. Cancer Cytopathol. 2012 Feb 25;120(1):18-25. doi: 10.1002/cncy.20175. Epub 2011 Jul 12. PMID: 21751428; PMCID: PMC3197962.
- Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. PMID: 20603348; PMCID: PMC3001530.
- Buonocore DJ, Fowle E, Lin O, Xu B, Katabi N, Cohen JM. Cytologic evaluation of p16 staining in head and neck squamous cell carcinoma in CytoLyt versus formalin-fixed material. Cancer Cytopathol. 2019 Dec;127(12):750-756. doi: 10.1002/cncy.22191. Epub 2019 Oct 10. PMID: 31600033; PMCID: PMC6906234.
- Chute DJ, Aramouni GT, Brainard JA, Hoschar AP, Kroeger A, Yen-Lieberman B. Hybrid Capture 2 human papilloma virus testing for head and neck cytology specimens. J Am Soc Cytopathol. 2014;3(4):173-182. doi:10.1016/j.jasc.2014.02.004.
- Hou Y, Chaudhary S, Shen R, Li Z. Fine-needle aspiration of cervical lymph nodes yields adequate materials for accurate HPV testing in metastatic head and neck squamous cell carcinomas. Diagn Cytopathol. 2016 Oct;44(10):792-8. doi: 10.1002/dc.23548. Epub 2016 Jul 28. PMID: 27465660.
- Jalaly JB, Lewis JS Jr, Collins BT, Wu X, Ma XJ, Luo Y, Bernadt CT. Correlation of p16 immunohistochemistry in FNA biopsies with corresponding tissue specimens in HPV-related squamous cell carcinomas of the oropharynx. Cancer Cytopathol. 2015 Dec;123(12):723-31. doi: 10.1002/cncy.21600. Epub 2015 Aug 4. PMID: 26242494.
- Jannapureddy S, Cohen C, Lau S, Beitler JJ, Siddiqui MT. Assessing for primary oropharyngeal or nasopharyngeal squamous cell carcinoma from fine needle aspiration of cervical lymph node metastases. Diagn Cytopathol. 2010 Nov;38(11):795-800. doi: 10.1002/dc.21293. PMID: 20014308.
- Lewis JS Jr, Beadle B, Bishop JA, Chernock RD, Colasacco C, Lacchetti C, Moncur JT, Rocco JW, Schwartz MR, Seethala RR, Thomas NE, Westra WH, Faquin WC. Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Arch Pathol Lab Med. 2018 May;142(5):559-597. doi: 10.5858/arpa.2017-0286-CP. Epub 2017 Dec 18. PMID: 29251996.
- Prigge ES, Arbyn M, von Knebel Doeberitz M, Reuschenbach M. Diagnostic accuracy of p16INK4a immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int J Cancer. 2017 Mar 1;140(5):1186-1198. doi: 10.1002/ijc.30516. Epub 2016 Dec 2. PMID: 27859245.
- Sivars L, Landin D, Haeggblom L, Tertipis N, Grün N, Bersani C, Marklund L, Ghaderi M, Näsman A, Ramqvist T, Nordfors C, Munck-Wikland E, Tani E, Dalianis T. Human papillomavirus DNA detection in fine-needle aspirates as indicator of human papillomavirus-positive oropharyngeal squamous cell carcinoma: A prospective study. Head Neck. 2017 Mar;39(3):419-426. doi: 10.1002/hed.24641. Epub 2016 Nov 29. PMID: 27898186.
- Smith DF, Maleki Z, Coughlan D, Gooi Z, Akpeng B, Ogawa T, Bishop JA, Frick KD, Agrawal N, Gourin CG, Ha PK, Koch WM, Richmon JD, Westra WH, Pai SI. Human papillomavirus status of head and neck cancer as determined in cytologic specimens using the hybrid-capture 2 assay. Oral Oncol. 2014 Jun;50(6):600-4. doi: 10.1016/j.oraloncology.2014.02.011. Epub 2014 Mar 12. PMID: 24630260; PMCID: PMC4318229.
- Takes RP, Kaanders JH, van Herpen CM, Merkx MA, Slootweg PJ, Melchers WJ. Human papillomavirus detection in fine needle aspiration cytology of lymph node metastasis of head and neck squamous cell cancer. J Clin Virol. 2016 Dec;85:22-26. doi: 10.1016/j.jcv.2016.10.008. Epub 2016 Oct 27. PMID: 27816020.
- Xu B, Ghossein R, Lane J, Lin O, Katabi N. The utility of p16 immunostaining in fine needle aspiration in p16-positive head and neck squamous cell carcinoma. Hum Pathol. 2016 Aug;54:193-200. doi: 10.1016/j.humpath.2016.04.002. Epub 2016 Apr 19. PMID: 27105759.
Evidence tabellen
Evidence table for algorithms (PICO 1)
|
Study reference |
Study characteristics |
Patient characteristics
|
Index test (test of interest) |
Reference test
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Prigge 2017
(individual study characteristics deduced from Prigge 2017)
|
SR and meta-analysis
Literature searches up to the 8th of January 2016.
Included articles for p16INK4a + HPV DNA PCR A: Smeets 2007 B: Schache 2011 C: Schlecht 2011 D: Rotnaglova 2011 E: Hoffmann 2012 F: Rietbergen 2013 G: Bussu 2013 H: Bussu 2014 I: Lukesova 2014 J: Masterson 2015 K: Vojtechova 2016
Study design: prospective and retrospective designs were included (unclear whether case-control designs could be included. Specific designs not reported in the SR)
Setting and Country: A: Netherlands B: UK C: USA D: Czech Republic E: Germany F: Netherlands G: Italy H: Italy I: Czech Republic J: UK K: Czech Republic
Source of funding and conflicts of interest: One author is a co-inventor of various patents regarding the diagnostic use op p16INK4a antibodies / was co-founder, shareholder and member of a company that developed and marketed p16INK4a related reagents (later aquired by Roche) / holds a patent regarding therapeutic use of p16INK4a / received honoraria as a scientific advisor and received research funds frum Oryx GmbH & Co. The salary of another author was partially funded by the research funds received from Oryx GmbH & Co. Both authors are inventors of a patent related to the therapeutic use of p16INK4a.
|
Inclusion criteria SR: men and women diagnosed with oropharyngeal squamous cell carcinoma, p16INK4a IHC as index test, A reference test that would detect E6 and/or E7 HPV mRNA, sensitivity and specificity as outcomes, prospective or retrospective studydesign.
Exclusion criteria SR: No author response when inquiry was made about data, samplesize <10, no primary data.
11 studies included for p16INK4a + HPV DNA PCR (24 studies in total)
Important patient characteristics: Patient characteristics are not reported by the systematic review authors.
Sample size, n: A: 15 B: 82 C: 19 D: 45 E: 20 F: 86 G: 21 H: 22 I: 52 J: 24 K: 123
|
Describe index and comparator tests* and cut-off point(s):
All studies described here used p16INK4a + HPV DNA PCR as an index test.
Cut-off points (p16INK4a + HPV DNA PCR): Positive: p16INK4a and HPV DNA PCR both positive Negative: Either one or both of the tests (i.e., p16INK4a and/or HPV DNA PCR) was negative.
Cut-off points for p16INK4a: A: Staining intensity above background of negative control B: ≥70% strong and diffuse nuclear and cytoplasmic staining C: Mean intensity of ≥2 and ≥75% staining in either the nuclei or cytoplasm D: >50% nuclear and/or cytoplasmic staining E: strong nuclear and cytoplasmic staining in focal or diffuse distribution F: >70% moderate to strong diffuse nuclear and cytoplasmic staining G: ≥70% strong and diffuse nuclear and cytoplasmic staining H: ≥70% strong and diffuse nuclear and cytoplasmic staining I: >50% nuclear and/or cytoplasmic staining J: >70% staining K: >50% nuclear and/or cytoplasmic staining
Material for p16INK4a: A: FFPE (whole tissue section) B: FFPE (tissue microarray) C: FFPE (whole tissue section) D: FFPE (whole tissue section E: FFPE (whole tissue section) F: FFPE (whole tissue section) G: FFPE (whole tissue section) H: FFPE (whole tissue section) I: FFPE (whole tissue section) J: FFPE (whole tissue section) K: FFPE (whole tissue section)
Antibody clone p16INK4a: A: E6H4 B: E6H4 C: G175-405 D: G175-405 E: E6H4 F: E6H4 G: E6H4 H: E6H4 I: G175-405 J: G175-405 K: G175-405 .....
HPV-DNA PCR method: A: GP5+/6+ reverse line blot genotyping B: HPV16 primers C: MY09/MY11/HMB01 dot blot hybridization genotyping D: GP5+/6+ reverse line blot genotyping E: BSGP5+/6+ bead-based genotyping F: GP5+/6+ and EIA readout with bead-based genotyping G: Hybrid capture 2 H: Hybrid capture 2 I: PF5+/6+ reverse line blot genotyping J: HPV16 E6/E7 primers K: GP5+/6+ reverse line blot genotyping |
Describe reference test and cut-off point(s):
The exact reference test per study is unclear from the systematic review. Nonetheless, it was a test for E6 and/or E7 mRNA.
Detection HPV-DNA (transcript types / HPV types): A: E6*I / HPV 16 B: E6 / HPV 16 C: E6, E7, E6*I / HPV 16 D: E6*I / HPV 16 E: E6*I / HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68b, 70, 73, 82 F: E6*I, E6 / HPV 16, 33 G: E6, E7 / HPV 16, 18, 31, 33, 45 H: E6, E7 / HPV 16, 18, 31, 33, 35 I: E6*I / HPV 16 J: E6, E7 / HPV 16 K: E6*I / HPV 16
Material for reference test: A: FFPE (whole tissue section) B: Fresh frozen C: Fresh frozen D: FFPE (whole tissue section E: Fresh frozen F: Fresh frozen G: Fresh frozen H: Fresh frozen I: FFPE (whole tissue section) J: Fresh frozen K: FFPE (whole tissue section)
Prevalence (%) (based on refence test at specified cut-off point) Not reported in the SR
For how many participants were no complete outcome data available? Not reported in the SR
Reasons for incomplete outcome data described. Not reported in the SR
|
Endpoint of follow-up: N/A |
Outcome measures and effect size (include 95%CI and p-value if available):
Sensitivity P16INK4a + HPV DNA PCS versus a reference test detecting E6 and/or E7 mRNA: sensitivity (95%CI) A: 1.00 (0.57-1.00) B: 0.96 (0.82-0.99) C: 0.91 (0.76-0.97) D: 0.89 (0.57-0.98) E: 0.82 (0.52-0.95) F: 0.96 (0.80-0.99) G: 0.70 (0.40-0.89) H: 0.82 (0.52-0.95) I: 0.81 (0.63-0.92) J: 1.00 (0.70-1.00) K: 0.96 (0.88-0.99) Pooled characteristic (bivariate analysis) per index test and cut-off point: Index test (various cut-offs) 0.93 (95% CI 0.87 to 0.97) Heterogeneity (reasons): I2 = 23.39% (p=0.22)
Specificity P16INK4a + HPV DNA PCS versus a reference test detecting E6 and/or E7 mRNA: Specificity (95%CI) A: 1.00 (0.72-1.00) B: 0.94 (0.74-0.99) C: 0.94 (0.83-0.98) D: 1.00 (0.72-1.00) E: 1.00 (0.70-1.00) F: 0.98 (0.91-1.00) G: 1.00 (0.74-1.00) H: 1.00 (0.74-1.00) I: 1.00 (0.87-1.00) J: 0.53 (0.30-0.75) K: 0.81 (0.68-0.89) Pooled characteristic (bivariate analysis) per index test and cut-off point: Index test (various cut-offs) 0.96 (95% CI 0.89 to 1.00) Heterogeneity (reasons): I2= 68.4% (p=0.00) |
Study quality (ROB): QUADAS-2
Place of the index test in the clinical pathway: Unclear
Choice of cut-off point: Various cut-off points were observed for p16INK4a. Various HPV genes were targeted.
Facultative:
Brief description of author’s conclusion
Personal remarks on study quality, conclusions, and other issues (potentially) relevant to the research question
Sensitivity analyses (excluding small studies; excluding low quality studies; excluding case-control type of studies; relevant subgroup-analyses); mention only analyses which are of potential importance to the research question.
Heterogeneity: clinical and statistical heterogeneity; clinical: enough similarities in patient characteristics, diagnostic tests (strategy) to allow pooling? For pooled data: assessment of statistical heterogeneity and, more importantly, assessment of the reasons for heterogeneity (if present)? Note: sensitivity and specificity depend on the situation in which the test is being used and the thresholds that have been set, and sensitivity and specificity are correlated; therefore, the use of heterogeneity statistics (p-values; I2) is problematic, and rather than testing whether heterogeneity is present, the reasons for heterogeneity should be examined.
A: B: C: D: E: F: G: H: I: J: K: .....
|
*comparator test equals the C of the PICO; two or more index/ comparator tests may be compared; note that a comparator test is not the same as a reference test (golden standard)
Table of quality assessment for systematic reviews of diagnostic studies
Based on AMSTAR checklist (Shea, 2007; BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009; PLoS Med 6: e1000097; doi:10.1371/journal. pmed1000097)
Research question:
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Assessment of scientific quality of included studies?5
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?6
Yes/no/unclear |
Potential risk of publication bias taken into account?7
Yes/no/unclear |
Potential conflicts of interest reported?8
Yes/no/unclear |
|
Prigge |
Yes, PICO was also provided in the supplement |
Yes, search string was described. MEDLINE was searched. |
No, no references were made to the (full-text) excluded articles. |
No, sample characteristics were not described.
For testprocedures, most relevant characteristics are described (see supplement as well). However, cut-off point in the reference test and methods of the reference test should have been described as well. |
Yes, QUADAS-2 tool was used. |
Unclear, differences in cut-offs from the index/comparator tests were analysed in sub-analyses. Unclear what the impact of the variability in cut-offs is compared to eachother. |
No, publication bias was not discussed. |
No, the authors disclose their conflicts of interest. CoI of included studies were not reported. |
- Research question (PICO) and inclusion criteria should be appropriate (in relation to the research question to be answered in the clinical guideline) and predefined.
- Search period and strategy should be described; at least Medline searched.
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons.
- Characteristics of individual studies relevant to the research question (PICO) should be reported.
- Quality of individual studies should be assessed using a quality scoring tool or checklist (preferably QUADAS-2; COSMIN checklist for measuring instruments) and taken into account in the evidence synthesis.
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, diagnostic tests (strategy) to allow pooling? For pooled data: at least 5 studies available for pooling; assessment of statistical heterogeneity and, more importantly (see Note), assessment of the reasons for heterogeneity (if present)? Note: sensitivity and specificity depend on the situation in which the test is being used and the thresholds that have been set, and sensitivity and specificity are correlated; therefore, the use of heterogeneity statistics (p-values; I2) is problematic, and rather than testing whether heterogeneity is present, heterogeneity should be assessed by eye-balling (degree of overlap of confidence intervals in Forest plot), and the reasons for heterogeneity should be examined.
- There is no clear evidence for publication bias in diagnostic studies, and an ongoing discussion on which statistical method should be used. Tests to identify publication bias are likely to give false-positive results, among available tests, Deeks’ test is most valid. Irrespective of the use of statistical methods, you may score “Yes” if the authors discuss the potential risk of publication bias.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Evidence table for cytologic testing (PICO2)
|
Study reference |
Study characteristics |
Patient characteristics
|
Index test (test of interest) |
Reference test
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Baldassari 2015 |
Type of study: Prospective
Setting and country: University medical center, USA
Funding and conflicts of interest: funding and COI are not reported in the manuscript |
Inclusion criteria: Unclear
Exclusion criteria: Unclear
N=37 with 42 FNAs
Prevalence: Unclear, FNAs were tested, not patients. (20/41 FNAs were positive, 1 invalid result by the reference)
Mean age ± SD: 60.4 (11.8)
Sex: 31M / 6F
Other important characteristics:
History of HNSCC, n: 17
FNA location: Neck mass: 36 Mediastinal lymph nodes: 5 Left parapharyngeal mass: 1 |
Describe index test: cobas 4800 in cytologic material. FNA was performed with a 25-gauge needle (no imaging guidance). Direct air-dried and alcohol fixed smears were prepared and stained (Diff-Quik stain and Papanicolaou stain). One or two drops of the cell suspension was added to prepare a ThinPrep slide after centrifugation. The assay was performed according to the manufacturer’s protocol.
Cut-off point(s): Unclear, interpretation was carried out using proprietary software (provided with cobas z 480 analyzer). HPV 16, 18, pooled (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) DNA detection by amplification. Cycle thresholds <40.5 for HPV 16, <40 HPV 18 and pooled genotypes. |
Describe reference test: P16 IHC and HPV ISH on histologic samples (FFPE material).
P16: 5-micrometer sections were deparaffinized. Sections were incubated with a prediluted monoclinal antibody (mouse, E6H4) for 32 minutes.
HPV ISH: sections were incubated with INFORM HPV III family 16 probe for 4 minutes. Probes detected HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66.
Cut-off point(s): P16: unclear HPV ISH: unclear
|
Time between the index test en reference test: Unclear
For how many participants were no complete outcome data available?
Reasons for incomplete outcome data described? |
Outcome measures and effect size (include 95%CI and p-value if available):
Cobas 4800 versus p16+ISH, n=34: TP: 15 FP: 16 FN: 0 TN: 3 Excluded: 3 atypical cytological results, 4 cytological suspicious results, 1 invalid reference result Sensitivity: 1.00 (0.78-1.00) Specificity: 0.16 (0.03-0.40) *sens/spec calculated from presented data |
|
|
Begum 2007 |
Type of study: Database
Setting and country: Hospital, USA
Funding and conflicts of interest: funding and COI are not reported in the manuscript |
Inclusion criteria: when initial processing of the FNA included preparation of a cell block, if the preparation of the cell block was spun in a cellular pellet
Exclusion criteria: Unclear (hpv was detected in 13/77 FNAs)
N= 19
Prevalence: Unclear, not all n=19 had reference testing.
Mean age ± SD:
Sex: % M / % F
Other important characteristics:
|
Describe index test: P16: 5-micron sections were deparaffinized. Sections were incubated with mouse monoclonal antibodies.
Cut-off point(s): any staining in the squamous cells
|
Describe reference test: HPV 16 ISH for cytology: FFPE cell blocks. Signal amplification for visualization of HPV16 infected cells.
HPV16 for histology: 5-micron sections were deparaffinized. Slides were hybridized with HPV16 probes (and in specific cases with 6, 11, 18, 31, 33, 35, 45, 51, 52).
Cut-off point(s): hybridization signals visualized as dots in squamous cell nuclei.
|
Time between the index test en reference test: Unclear
For how many participants were no complete outcome data available? Reasons for incomplete outcome data described. NR |
Outcome measures and effect size (include 95%CI and p-value if available):
Oropharynx only (n=19), FNA material, p16 versus HPV16 ISH: TP: 9 FP: 4 FN: 1 TN: 5 Sensitivity: 0.90 (0.55-1.00) Specificity: 0.56 (0.21-0.86) *sens/spec calculated from presented data.
Oropharynx only (n=10), p16 (FNA material) versus HPV16 ISH (resection material): TP: 0 FP: 4 FN: 2 TN: 4 9 cases did not undergo reference testing and were excluded. Sensitivity: 0.00 (0.00-0.84) Specificity: 0.50 (0.16-0.84) *sens/spec calculated from presented data.
CUP only (n=10), FNA material, p16 versus HPV16 ISH: TP: 3 FP: 0 FN: 0 TN: 7 Sensitivity: 1.00 (0.29-1.00) Specificity: 1.00 (0.59-1.00) *sens/spec calculated from presented data.
|
Because a database was searched and FNA smeas had to be available as well as the surgical specimen, a selection of patients might occur. It is possible that not all patients suspected of HPV positivity presenting in the hospital are included in the sample.
|
|
Bishop 2012 |
Type of study: Consecutive
Setting and country: Hospital, USA
Funding and conflicts of interest: no financial disclosures. Funded by NIDCR. |
Inclusion criteria: not reported.
Exclusion criteria: Not reported
N= 24 (27 cytologic preparations), n=12 with a lymph node sample site
Prevalence: 5/12 were HPV positive by HPV ISH
Mean age ± SD: Not reported
Sex: not reported
Tumor site (n=12): Unknown: 1 Skin: 2 Larynx: 2 Floor of mouth: 1 Tongue: 1 Tongue base: 1 Tonsil: 4 |
A 12-gauge needle was used for aspirates. Multiple passes were made.
Hybrid Capture 2: Detects HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68. Specimen DNA was denatured into single stranded DNA and hybridized with specific probes. RNA/DNA hybrids are immobilized onto microplate surface. Light is emitted and relative light units are measured. The intensity of the light denotes the presence or absence of HPV DNA.
Cut-off point(s): HC2: ≥3 RLU/CO was positive, 0.85-3 was equivocal, <0.85 negative.
|
Describe reference test: HPV ISH. Hybridization was performed with the HPV III Family 16 probe set and captures HPV 16, 18, 33, 35, 45, 51, 52, 56, 66. 5-micron sections from tissue microarrays and FFPE tumor blocks were used.
Cut-off point(s): Hybridization signals localized to tumor cell nuclei defined an HPV positive tumor.
|
Time between the index test en reference test: Not reported
For how many participants were no complete outcome data available? Reasons for incomplete outcome data described. NA |
Outcome measures and effect size (include 95%CI and p-value if available):
HC2 versus HPV ISH, lymph node sample site only (n=12): TP: 5 FP: 0 FN: 0 TN: 7 Sensitivity: 1.00 (0.48-1.00) Specificity: 1.00 (0.59-1.00) *sens/spec calculated from presented data.
|
P16 was performed on resected HNSCCs.
Cytologic material from excised HNSCC of known HPV status |
|
Buonocore 2019 |
Type of study: Consecutive
Setting and country: Hospital, USA
Funding and conflicts of interest: authors had no COI to disclose, funding was supported by cancer center support grant for the national institutes of health/national cancer center institute |
Inclusion criteria: previous or unknown oropharyngeal HNSCC (or determined to have HNSCC at the time of procedure), unknown p16 status.
Exclusion criteria: Not reported.
N= 25
Prevalence: 24/24 positives for mRNA ISH (1 case was nonconstributory)
Mean age (range): 60 (47-76)
Sex: 22M / 3F
Smoking history: Never: 14 History 11 (pack-years range: 0.5-40, one person smoked 30 cigars per year)
Alcohol use: Never: 1 Abstinent: 1 (9 year abstinent) Occasional: 2 Social: 15 Daily: 3 Heavy: 3 |
FNA typically under ultrasound guidance with a 25-gauge needle. Initial passes were performed for Diff-Quik-staned and ethanol fixed smears. Multiple passes were made and allowed to clot before a transfer into formalin fixation. From this material a CytoLyt and a formalin-fixed cellblock were made.
Describe index test: P16 CytoLyt cellblock: Multiple passes were made and allowed to clot before a transfer into formalin fixation. From this material a CytoLyt and a formalin-fixed cellblock were made. E6H4 mouse antihuman monoclonal antibodies were used.
P16 formalin-fixed cellblock: Multiple passes were made and allowed to clot before a transfer into formalin fixation. From this material a CytoLyt and a formalin-fixed cellblock were made. E6H4 mouse antihuman monoclonal antibodies were used.
Cut-off point(s): ≥70% of the tumor cells showing nucleas and cytoplasmic staining.
|
Describe reference test: HPV ISH targeting HPV 16, 18, 26, 31, 33, 35, 39, 41, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82 and E6/E7 mRNA. Unclear on which material ISH was performed.
Cut-off point(s): Not described how a positive case was defined.
|
Time between the index test en reference test: Unclear
For how many participants were no complete outcome data available? 1/25
Reasons for incomplete outcome data described. Insufficient tumor remaining on deeper levels. |
Outcome measures and effect size (include 95%CI and p-value if available):
P16 versus HPV ISH, Formalin-fixed cell blocks (n=24): TP: 23 FP: 0 FN: 1 TN: 0 Sensitivity: 0.96 (0.79-1.00) Specificity: Not Estimable. (0 TN) One case had a non-contributory reference test and was excluded from analysis. *sens/spec calculated from presented data.
P16 versus HPV ISH, CytoLyt cell blocks (n=24): TP: 4 FP: 0 FN: 20 TN: 0 Sensitivity: 0.17 (0.05-0.37) Specificity: Not Estimable. (0 TN) One case had a non-contributory reference test and was excluded from analysis. *sens/spec calculated from presented data |
|
|
Chute 2014 |
Type of study: Prospective
Setting and country: Hospital, USA
Funding and conflicts of interest: authors had no COI to disclose, funding was supported by the American society of cytopathology foundation through an ASC cytology research seed grant. |
Inclusion criteria: HN-site FNA sample interpreted as SCC / atypical/ suspicious for SCC.
Exclusion criteria: Not reported
N=95 (96 FNAs)
Prevalence: 29/95, 30.5%
Median age (range): 60 (17-93)
Sex: 72M / 21F
Primary tumor location, n: Orophatynx: 32 Non-oropharyngeal: 32 Non-head/neck: 18 Unknown: 13 |
FNA
Describe index test: CISH for HR HPV: The Inform HPV III Family 16 probe was used, which recognizes HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58.
HC2 was performed according to the manufacturer’s instructions. HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 are detected. Microplate surfaces are coated with antibodies. Light is emitted and measured. Cut-off point(s): CISH: discrete dotlike blue staining in tumor nuclei was defines as a positive case.
HC2: ≥1 RLU/CO was defined as a positive case.
|
FFPE block of primary tumor or excised neck metastasis were used when available.
Describe reference test: P16 IHC was performed using the i-View DAB streptavidin-biotin-based detection kit.
CISH: The Inform HPV III Family 16 probe was used, which recognizes HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58.
Cut-off point(s): Positive results from both p16 and CISH defined a positive case. CISH: discrete dotlike blue staining in tumor nuclei was defines as a positive case. P16: >75% strong and diffuse tumor cytoplasmic and nuclear staining |
Time between the index test en reference test: Not reported
For how many participants were no complete outcome data available? 19 missings for HC2 cyto versus surgical 44 missings for CISH cyto versus surgical
Reasons for incomplete outcome data described? |
Outcome measures and effect size (include 95%CI and p-value if available):
HC2 cytology versus surgical specimen (p16+CISH) (n=77) TP: 21 FP: 0 FN: 8 TN: 48 Sensitivity: 0.72 (0.53-0.87) Specificity: 1.00 (0.93-1.00) 15 cases were not tested. 4 cases had equivocal outcomes on the reference test (1 tested positive, 3 tested negative on HC2) *sens/spec calculated from presented data.
CISH cytology versus surgical specimen (p16+CISH) (n=52) TP: 11 FP: 7 FN: 7 TN: 27 Sensitivity: 0.61 (0.36-0.83) Specificity: 0.79 (0.62-0.91) 7 cases were not tested (from the table), however 44 cases were not tested compared to the overall included sample. *sens/spec calculated from presented data.
HC2 cytology versus surgical specimen (p16+CISH) on the same sample (n=52) as tested with CISH cyto: TP: 17 FP: 0 FN: 1 TN: 34 Sensitivity: 0.94 (0.73-1.00) Specificity: 1.00 (0.90-1.00) 44 cases were not tested compared to the overall included sample. *sens/spec calculated from presented data |
Cytology and surgical specimen analyses were interpreted blinded to each other’s results. |
|
Hou 2016 |
Type of study: Database
Setting and country: Hospital, USA
Funding and conflicts of interest: authors had no COI to disclose. Funding was not reported in the manuscript |
Inclusion criteria: Metastatic HNSCC in cervical lymph nodes diagnosed by FNA, p16 and HPV ISH was performed on FNA material.
Exclusion criteria: Not reported.
N=87
Prevalence: 42/87
Mean age (range): 59 (38-86)
Sex: 80M / 7F
Other important characteristics:
Primary site, n: Base of tongue: 32 Tonsil: 19 Other oropharyngeal: 4 Non oropharyngeal: 25 Unknown: 7 |
FNA specimen were centrifuged. The specimen was clot dried and places in a CellSafe mesh calsule. The capsule was fixated in formalin (10% neutral buffered).
Describe index test: P16 on cell block sections. Monoclonal antibodies were used (E6H4).
Cut-off point(s): ≥ 70% diffuse or strong staining in nuclei and cytoplasm
|
Test on FNA material.
Describe reference test: HPV ISH was performed according to the manufacturer’s protocol on 4-micrometer microtome sections (FNA cell block). HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, code Y1443).
Cut-off point(s): Presence of staining in nuclei
|
Time between the index test en reference test: Not reported For how many participants were no complete outcome data available? 14 (14/87) were excluded form analyses because specimens were deemed inadequate.
Reasons for incomplete outcome data described? |
Outcome measures and effect size (include 95%CI and p-value if available):
P16 versus HPV ISH on FNA material (n=73) TP: 41 FP: 15 FN: 1 TN: 16 Sensitivity: 0.98 (0.87-0.1.00) Specificity: 0.52 (0.33-0.70 PPV: 0.73 NPV: 0.94 14 cases were not tested compared to the overall included sample (inadequate specimen).
|
Because a database was searched, both p16 and ISH had to be performed on FNA material. A selection of patients might occur. It is possible that not all patients suspected of HPV positivity presenting in the hospital are included in the sample.
|
|
Jalaly 2015 |
Type of study: Database
Setting and country: Hospital, USA
Funding and conflicts of interest: two authors are employees of Advanced Cell Diagnostics and own stock in the company. One author reported to receive research support from Advanced Cell Diagnostics outside the submitted work. The work was funded by the Washington University Department of Pathology and Immunology. ISH was performed and funded by Advanced Cell Diagnostics. |
Inclusion criteria: FNA diagnosis for metastatic SCC to cervical lymph nodes, clinically proven SCC with corresponding surgical specimen (either biopsy or resection)
Exclusion criteria: Cell blocks have no tumor cells.
N= 48
Prevalence: 20/28 cases were positive by ISH (n=20 did not undergo ISH)
Mean age ± SD: 60.8 (11.7)
Sex: 44M / 4F
FNA site, n: Neck: 41 Subcarinal: 2 Mediastinal: 1 Submandibular: 2 Chest wall: 1 Supraclavicular: 1
Primary site: Base of tongue: 14 Tonsil: 15 Other oropharyngeal: 8 Oral cavity: 6 Larynx: 1 Maxilla: 1 Unknown: 3
Type of specimen: Biopsy: 16 Resection 32 |
FNA specimens were centrifuged for 2 minutes (x800g). The sediment was evaluated, and CytoRich Red was added with repeated centrifuging when the sediment was bloody. Isotonic saline was added when the sediment was sparse, or CytoRich Red was used. When the specimen was sparse, toluidine blue was added as well. O plasma was added. The specimen clot was dried on tissue paper in placed in a CellSafe calsule. The capsule was inserted into a cassette and fixed in formalin (10% neutral-buffered)
Describe index test: P16 was performed in theFNA cell blocks using a monoclonal antibody (clone E6H4) following a standard protocol. Scoring of cell blocks were performed without the knowledge of the p16 status from tissue.
Cut-off point(s): P16: more than 70% nuclear and cytoplasmic staining of tumor cells
|
ISH on recuts of FNA cell blocks from specimen that had ≤50% p16 staining that had available tumor cells and on specimens >50% p16 staining that had abundant cellularity on the recut.
Describe reference test: ISH detected E6/E7 mRNA (RNAscope HPV kit). ISH was performed according to the manufacturer’s instruction. 4-mm FFPE cellblock sections were prepared. Cases were excluded when ubiquitin C signal was less or equal to Dap B signal. Staining was scored independently by 2 pathologists.
Cut-off point(s): Red, punctate dots present in the nucleus and/or the cytoplasm. Positive cases had to have granular cytoplasmin and/or nuclear red staining that was higher than the signal on the DapB-negative control slide.
|
Time between the index test en reference test: Unclear.
For how many participants were no complete outcome data available?
Reasons for incomplete outcome data described. ISH was not performed on 11 cases; Specimens we acellular for ISH in 6 cases; Specimens failed the quality control in 3 cases (ubiquitin C signal was less or equal to Dap B signal on ISH); Therefore, 20 cases were excluded from analysis.
|
Outcome measures and effect size (include 95%CI and p-value if available):
P16 (FNA) versus ISH (FNA), n=28: TP: 6 FP: 0 FN: 14 TN: 8 Sensitivity: 0.30 (0.12-0.54) Specificity: 1.00 (0.63-1.00) ISH was not performed on 11 cases; Specimens we acellular for ISH in 6 cases; Specimens failed the quality control in 3 cases; Therefore, 20 cases were excluded from analysis. *sens/spec calculated from presented data.
|
Because a database was searched it is possible that not all patients suspected of HPV positivity presenting in the hospital are included in the sample. Reference testing (ISH) was performed on 28/48 of the included participants.
P16 on cell blocks versus p16 on tissue was not extracted, since the reference test had to be a test that detected DNA/mRNA. |
|
Jannapureddy 2010 |
Type of study: Database
Setting and country: Hospital, USA
Funding and conflicts of interest: funding and CoI were not reported in the mansucript.
|
Inclusion criteria: Cell block cytologic diagnosis of metastatic SCC in cervical lymph nodes, between sept 2004-sept 2008
Exclusion criteria: inadequate cell block material
N=45 (n=40 selected, because 5 had inadequate cell block material)
Prevalence: 9/40 (22.5%)
Mean age: 58.2 (range: 25-87)
Sex: 36M / 4F
Primary tumor site, n: Oropharyngeal: 11 Nasopharyngeal: 2 Other: 5 Not determined: 9
|
FNA were fixed in 10% neutral-buffered formalin and centrifuged (7 min) Supernatant was discharged and the resulting content (collidon bag and contents) were submitted for routine histology.
Cell block tissue (5 micrometer) of FFPE tissue were tested.
Describe index test: P16: monoclonal p16(INK4a) (E6H4) incubation was performed for 30 minutes at room temperature.
ProExC: incubation was performed for 30 minutes at room temperature.
Cut-off point(s): P16: nuclear and cytoplasmic staining ProExC: nuclear staining.
|
Cell block tissue (5 micrometer) of FFPE tissue were tested.
Describe reference test: HPV ISH detected HPV 16, 18, 31, 33, 51. Cell block tissue was deparaffinized and rehydrated. Slides were air-dried after rinsing. For 5 minutes, sections were denatured (95 deg. Celsius). Sections were hybridized overnight at 37 deg. Celsius. Sections were incubated.
Cut-off point(s): Positive cases were defined as punctuate or dot-like nuclear staining.
|
Time between the index test en reference test: Unclear For how many participants were no complete outcome data available?
Reasons for incomplete outcome data described? |
Outcome measures and effect size (include 95%CI and p-value if available):
P16 (FNA) versus ISH (FNA), n=40: TP: 9 FP: 7 FN: 0 TN: 24 Sensitivity: 1.00 (0.66-0.1.00) Specificity: 0.77 (0.59-0.90) 5 cases were excluded from patient selection because they had inadequate cell blocks, therefore the analyses were performed on n=40 *sens/spec calculated from presented data.
ProExC (FNA) versus ISH (FNA), n=40: TP: 9 FP: 26 FN: 0 TN: 5 Sensitivity: 1.00 (0.66-0.1.00) Specificity: 0.16 (0.05-0.34) 5 cases were excluded from selection because they had inadequate cell blocks, therefore the analyses were performed on n=40 *sens/spec calculated from presented data |
Because a database was searched it is possible that not all patients suspected of HPV positivity presenting in the hospital are included in the sample. Participants needed to have had a cytologic diagnosis of metastatic SCC in cervical lymph nodes.
|
|
Sivars 2017 |
Type of study: Prospective
Setting and country: Hospital, Sweden
Funding and conflicts of interest: COI were not declared in the manuscript. Contract grant sponsor was the Swedish Cancer Society / The Stockholm Cancer Society, Ther cancer and Allergy foundation, Karolinska Institutet, The Stockholm City council
|
Inclusion criteria: Suspected of HNSCC or with neck masses suspicious for metastasis.
Exclusion criteria: Not enough material left for testing after cytological diagnosis.
N=66
Prevalence: 17/66 (23 cases not tested)
Mean age ± SD: 61.0 (oropharyngeal, n=20) 71.5 (other malignancies, n=17) 53.0 (benign conditions, n=29)
Sex: 35M / 31F
FNAC sites, n: Primary tumor: 2 Neck masses: 64
|
DNA was extracted from FNA cytology samples. QIAmp DNA micro kit was used to extract DNA from thawed aspirates.
Describe index test: FNAC material; HPV DNA multiplex assay (lab): 27 HPV types were tested using GP5+/GP6+ primers. Additionally, E6 (HPV 16, 33) were amplified. DNA detection was performed on bead-based multiplex was used. Mean fluorescent intensity was used.
HPV RT-PCR (clinic): FNAC material; a real-time TaqMan PCR was performed to detect the 7 most common HR-HPV genotypes.
Cut-off point(s): HPV DNA multiplex assay: mean fluorescent intensity above background*1.5+15 was considered positive for HPV. HPV PCR: unclear
|
Some cases had FFPE biopsies from a primary tumor.
Describe reference test: HPV DNA multiplex assay (histo): 27 HPV types were tested using GP5+/GP6+ primers. Additionally, E6 (HPV 16, 33) were amplified. DNA detection was performed on bead-based multiplex was used. Mean fluorescent intensity was used.
HPV RT-PCR (histo): tested on FFPE material. a real-time TaqMan PCR was performed to detect the 7 most common HR-HPV genotypes.
Cut-off point(s): mean fluorescent intensity above background*1.5+15 was considered positive for HPV.
|
Time between the index test en reference test:
For how many participants were no complete outcome data available? Reasons for incomplete outcome data described? |
Outcome measures and effect size (include 95%CI and p-value if available): HPV RT-PCR at clinic (cyto) versus HPV multiplex assay (cyto), n=40: TP: 15 FP: 0 FN: 0 TN: 25 Sensitivity: 1.00 (0.78-0.1.00) Specificity: 1.00 (0.86-1.00) 26 cases were not tested with the HPV RT-PCR *sens/spec calculated from presented data.
HPV RT-PCR at clinic (cyto) versus HPV multiplex assay (histo FFPE), n=9: TP: 8 FP:0 FN: 0 TN: 1 Sensitivity: 1.00 (0.63-0.1.00) Specificity: 1.00 (0.03-1.00) 56 cases were not tested on histopathology with HPV multiplex, 1 case was not tested with HPV RT-PCR. Therefore 57 cases were excluded from the analysis. *sens/spec calculated from presented data.
HPV multiplex assay (cyto) versus HPV multiplex assay (histo FFPE), n=10: TP: 9 FP: 0 FN: 0 TN: 1 Sensitivity: 1.00 (0.66-1.00) Specificity: 1.00 (0.03-1.00) 56 cases were not tested on histopathology with HPV multiplex; therefore 56 cases were excluded from the analysis. *sens/spec calculated from presented data |
Patients included in the sample with malignant conditions othat than oropharyngeal SCC or with benign conditions did not undergo HPV multiplex assay and HPV PCR on histopathologic FFPE tissue.
Results from HPV mRNA detection were not extracted, since this was only tested on HPV positive specimen. |
|
Smith 2014 |
Type of study: Prospective
Setting and country: Hospital, USA
Funding and conflicts of interest: Authors declared there were no COI. Funded by an NIH/NCI Grant
|
Inclusion criteria: Cervical lymph nodes ≥1cm,
Exclusion criteria: Not reported
N=25
Prevalence: 9 cases did not receive HPV ISH, 11/16 were HPV-positive
Mean age ± SD: NR.
Sex: NR
Cause of cervical lymph mass: Tumor/matastasis: 18 Lymphadenopathy: 2 Non-inflammatory process: 5
Tumor location, n: Palatine tonsils: 6 Base of tongue: 8 Unknown: 2 Hypopharynx: 1 Skin of the auricle: 1
|
FNA biopsies were performed of metastatic cervical lymph nodes (in the clinic or operating room). 25-gauge needle was used passing into the lymph node 3-5 times. The aspirate was places on a slide (charged with Vista Vision HistoBond). The slide was air-dried and stained with a Diff-Quik stain. A final pass was made with a fresh needle into the lymph node. This aspirate was placed into 1ml of transport medium and stored at -20 deg. Celsius until HC2 was performed.
Describe index test: A modified-HC2 HPV assay: The test detects HPV 16, 18, 31, 33, 35, 39, 45, 51, 52,56, 58, 59, 68. DNA was denatured to single standed DNA and incubated in 65 deg. Celsius water for 45 minutes. Samples were applied to microplate wells coaded with hybrid specific antibodies. Signal amplification was performed with Detection Reagent II. Light emission was used to detect HPV DNA
Cut-off point(s): A positive case was defined as RLU/CO ≥2.5. A negative case was defined as RLU/CO <0.85. Between 0.85-2.5 was considered equivocal. |
Reference test was performed on tissue specimen from patients who had a resection. 5-micron FFPE tissue sections from tumors/biopsies were conditioned.
Describe reference test: ISH hybridization was performed with HPV III Family16 probe set (HPV 16, 18, 31, 33, 35, 39, 45, 51,52, 56, 58, 66)/ Genotypes were detected with the ISH iVIEW Blue Plus Detection Kit.
Cut-off point(s): Punctate signals in the nuclei defined a positive case.
|
Time between the index test en reference test: Unclear
For how many participants were no complete outcome data available? N (%)
Reasons for incomplete outcome data described? |
Outcome measures and effect size (include 95%CI and p-value if available):
HC2 (cyto) versus HPV ISH (histo FFPE), n=15: TP: 7 FP: 0 FN: 3 TN: 5 Sensitivity: 0.70 (0.35-0.93) Specificity: 1.00 (0.48-1.00) 9 cases did not undergo the ISH reference testing; 1 case had an equivocal result for HC2 and was excluded from analysis. Therefore 10 cases were excluded from analysis. *sens/spec calculated from presented data.
|
Data for p16 was not extracted, since p16 was performed on tissue specimens from the resection. The reference test had to be a test that detects HPV DNA/mRNA.
|
|
Takes 2016 |
Type of study: Database
Setting and country: Hospital, the Netherlands
Funding and conflicts of interest: Authors declared there were no COI. No funding received.
|
Inclusion criteria: FFPE histological material from the primary tumor or cetastatic lymph node available, FFPE material was both hpv AND p16 negative or both hpv AND p16 positive.
Exclusion criteria: Secondary tumor in the head/neck region, insufficient cytological material, previous exposure of the neck to radiotherapy.
N=47
Prevalence: 25/47
Mean age: 58 (range: 28.9-77.2)
Sex: 33M / 14F
T-stage, n: T0: 6 T1: 7 T2: 16 T3: 11 T4: 7
N-stage, n: N0: 2 N1: 7 N2: 38 N3: 0
Primary tumor site, n: Tonsils: 19 Base of tongue: 12 Unknown: 6 Other oropharyngeal: 10
Origin of FFPE material: Tonsils: 21 Base of tongue: 9 Neck dissection: 7 Other oropharyngeal: 10
Histological tumor differentiation, n: Poor: 7 Moderate: 33 Well: 3 Unknown: 4 |
Scraped FNAC from archival slided were used and DNA was purified. DNA was diluted in elution buffer and stored at -20 deg. Celsius until processing by PCR.
Describe index test: HPV PCR testing combined (cyto): p16 procedures were not described. For the HPV PCR, a broad-spectrum DNA amplification was performed using a short-PCR-fragment assay. 9 conservative probes were used in a micro titer hybridization assay (DNA enzyme immunoassay, DEIA). Cases positive for HPV by DEIA were analysed with line-specific probes assay (LiPA25) for HPV 6, 11, 16, 18, 31, 33, 34,35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, 74. LiPA strips were visually inspected and interpreted following the standardized reference guide.
Cut-off point(s): Unclear
|
DNA was isolated from FFPE tissue sections (4 micrometer) and used for PCR analysis.
Describe reference test: P16 + HPV PCR testing combined (histo): p16 procedures were not described. For the HPV PCR, a broad-spectrum DNA amplification was performed using a short-PCR-fragment assay. 9 conservative probes were ised in a micro titer hybridization assay (DNA enzyme immunoassay, DEIA). Cases positive for HPV by DEIA were analysed with line-specific probes assay (LiPA25) for HPV 6, 11, 16, 18, 31, 33, 34,35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68.73, 70, 74. LiPA strips were visually inspected and interpreted dollowing the standardized reference guide.
Cut-off point(s): Unclear (both p16 and HPV PCR)
|
Time between the index test en reference test: Not reported
For how many participants were no complete outcome data available? Reasons for incomplete outcome data described. NA |
Outcome measures and effect size (include 95%CI and p-value if available):
HPV PCR (cyto) versus HPV PCR +p16 (histo FFPE), n=47: TP: 24 FP: 0 FN: 1 TN: 22 Sensitivity: 0.96 (0.80-1.00) Specificity: 1.00 (0.85-1.00) *sens/spec calculated from presented data.
|
Because a database was searched it is possible that not all patients suspected of HPV positivity presenting in the hospital are included in the sample. Specimen were selected only when FFPE material were both hpv and p16 positive or negative.
|
|
Xu 2016 |
Type of study: Database
Setting and country: Hospital, USA
Funding and conflicts of interest: Authors declared that there were no CoI. Partly supported by Cancer center support grant of the national institutes of health / national cancer institute. |
Inclusion criteria: Metastatic SCC of a cervical lymph node based of FNA between 2007 and 2015, p16 performed on both cytological material and corresponding surgical material.
Exclusion criteria: Tumors originating from outside head and neck region.
N = 60 (63 FNAs from neck lymph nodes, 66 surgical specimens (47 primary tumor, 19 metastases))
Prevalence: 18/30 in cytology p16 versus CISH
Mean age ± SD: Not reported
Sex: Not reported
Tumor location, n: Non-oropharyngeal: 6 Oropharyngeal: 48 Unknwon primary: 6
|
FNA specimens were collected for cell blocks in ThinPrep and CytoLyt solution. Direct smears were fixed using ethanol (95%)
Describe index test: P16 (cyto) was performed on cell block, smear, or ThinPrep.
Cut-off point(s): Several thresholds, P16 nuclear and cytoplasmic staining in 1%/ 5%/ 10%/ 15%/ 70%.
|
CISH was performed on cytologic material.
Describe reference test: CISH (cyto) was performed using high risk HPV probes (HPV 16, 18, 31, 33, 51).
Cut-off point(s): CISH was categorized as negative, positive or equivocal. Negative and equivocal cases were not defined. A positive case was defined as discrete dot-like stippled nuclear labelling.
|
Time between the index test en reference test:
For how many participants were no complete outcome data available?
Reasons for incomplete outcome data described. 8 FNAs were excluded because the percentage p16 was not documented and/or cases were interpreted as equivocal in CISH. 53 surgical specimen were excluded, since the presented data concerned testing on cytology only. (p16 on FNA was not related to CISH on surgical specimens). |
Outcome measures and effect size (include 95%CI and p-value if available):
P16 (cyto) versus HPV ISH (cyto), n=30, at 1% cut-off: TP: 18 FP: 4 FN: 0 TN: 8 Sensitivity: 1.00 (0.81-1.00) Specificity: 0.67 (0.35-0.90) PPV: 0.82 NPV: 1.00 8 cases did not have their p16 status documented and/or were interpreted as equivocal by CISH. *sens/spec calculated from presented data.
P16 (cyto) versus HPV ISH (cyto), n=30, at 5% cut-off: TP: 17 FP: 4 FN: 1 TN: 8 Sensitivity: 0.94 (0.73-1.00) Specificity: 0.67 (0.35-0.90) PPV: 0.81 NPV: 0.89 8 cases did not have their p16 status documented and/or were interpreted as equivocal by CISH. *sens/spec calculated from presented data.
P16 (cyto) versus HPV ISH (cyto), n=30, at 10% cut-off: TP: 17 FP: 3 FN: 1 TN: 9 Sensitivity: 0.94 (0.73-1.00) Specificity: 0.75 (0.43-0.95) PPV: 0.85 NPV: 0.90 8 cases did not have their p16 status documented and/or were interpreted as equivocal by CISH. *sens/spec calculated from presented data.
P16 (cyto) versus HPV ISH (cyto), n=30, at 15% cut-off: TP: 17 FP: 3 FN: 1 TN: 9 Sensitivity: 0.94 (0.73-1.00) Specificity: 0.75 (0.43-0.95) PPV: 0.85 NPV: 0.90 8 cases did not have their p16 status documented and/or were interpreted as equivocal by CISH. *sens/spec calculated from presented data.
P16 (cyto) versus HPV ISH (cyto), n=30, at 70% cut-off: TP: 9 FP: 0 FN: 9 TN: 12 Sensitivity: 0.50 (0.26-0.74) Specificity: 1.00 (0.74-1.00) PPV: 0.60 NPV: 0.58 8 cases did not have their p16 status documented and/or were interpreted as equivocal by CISH. *sens/spec calculated from presented data |
Because a database was searched it is possible that not all patients suspected of HPV positivity presenting in the hospital are included in the sample. Only cases with p16 performed on both the cytological and corresponding surgical materials were included.
CISH was performed in 38 cytological and 53 surgical samples.
Data for p16 on the surgical specimen were not extracted, since the reference test should be a test that detects HPV DNA/mRNA.
P16 on cytological material was performed on 3 different types of preparations (cell block, direct smear, ThinPrep). Sub-analyses are not reported for each preparation (and the data is not reported to perform such subanalyses). |
[1] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer,1999).
[2] De referentiestandaard is de test waarmee definitief wordt aangetoond of iemand al dan niet ziek is. Idealiter is de referentiestandaard de Gouden standaard (100% sensitief en 100% specifiek). Let op! dit is niet de “comparison test/index 2”.
4 Beschrijf de statistische parameters voor de vergelijking van de indextest(en) met de referentietest, en voor de vergelijking tussen de indextesten onderling (als er twee of meer indextesten worden vergeleken).
Risk of bias assessment diagnostic accuracy studies (QUADAS II, 2011)
|
Study reference |
Patient selection
|
Index test |
Reference standard |
Flow and timing |
Comments with respect to applicability |
|
Baldassari 2015 |
Was a consecutive or random sample of patients enrolled? Unclear, reason: data was collected prospectively but it was not reported this was done consecutively or randomly.
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Unclear, reason: in and exclusion criteria were not stated.
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? No, reason atypical and suspicious cytological results were excluded from analysis |
Are there concerns that the included patients do not match the review question? Unclear
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? Unclear, no threshold for the reference standard was provided.
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: UNCLEAR |
CONCLUSION Could the patient flow have introduced bias?
RISK: HIGH |
||
|
Begum 2007 |
Was a consecutive or random sample of patients enrolled? No, reason: a database was searched.
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Unclear, exclusion criteria were not reported.
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? No, reason: 9 cases did not receive the reference standard in some analyses.
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? No |
Are there concerns that the included patients do not match the review question? Unclear
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: HIGH |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: UNCLEAR |
CONCLUSION Could the patient flow have introduced bias?
RISK: HIGH |
||
|
Bishop 2012 |
Was a consecutive or random sample of patients enrolled? Yes, consecutive.
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Unclear, in and exclusion criteria were not described.
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? Unclear
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: UNCLEAR |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
||
|
Buonocore 2019 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Unclear, no exclusion criteria reported.
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? No, 1 non-contributory case was excluded. However, we judged that the exclusion of 1 case did not have a significant impact on the findings. |
Are there concerns that the included patients do not match the review question? Unclear
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? Unclear, no cut-off for reference was reported |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: UNCLEAR |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
||
|
Chute 2014 |
Was a consecutive or random sample of patients enrolled? Unclear, prospective data collection. It is not clear whether it was consecutive (or random).
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Unclear, no exclusion criteria are reported.
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? No, cases were excluded when there were equivocal results, no surgical hpv status could be determined, or when there were acellular cell blocks. |
Are there concerns that the included patients do not match the review question? Unclear
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: HIGH |
||
|
Hou 2016 |
Was a consecutive or random sample of patients enrolled? No, a database was searched.
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Unclear, exclusion criteria were not reported.
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? No, 14 cases were excluded because the specimen was deemed inadequate. It remains unclear what the influence of these exclusions are on the reported outcomes. |
Are there concerns that the included patients do not match the review question? Unclear
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: HIGH |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: UNCLEAR |
CONCLUSION Could the patient flow have introduced bias?
RISK: UNCLEAR |
||
|
Jalaly 2015 |
Was a consecutive or random sample of patients enrolled? No, a database was searched.
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? No, excluded patients could probably have a new pass to gather tumor cells by FNA when a prospective/consecutive design was used.
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes, but only blinded for p16 in tissue samples. Otherwise, it was unclear.
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? No
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? No, cases that received no reference test were excluded from analysis. Furthermore, acellular specimen and quality control-failed specimen were excluded as well |
Are there concerns that the included patients do not match the review question? Unclear
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: HIGH |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: UNCLEAR |
CONCLUSION Could the patient flow have introduced bias?
RISK: HIGH |
||
|
Jannapureddy 2010 |
Was a consecutive or random sample of patients enrolled? No, a database was searched.
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Unclear, no exclusion criteria were reported. However, 5 cases were not selected because they had inadequate cell block material. |
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? Unclear
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: HIGH |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: UNCLEAR |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
||
|
Sivars 2017 |
Was a consecutive or random sample of patients enrolled? Unclear, not reported as such, however it may have been consecutive.
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes, probably.
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? No, not for HPV PCR on cytologic material.
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? No (56 cases were not tested with the HPV multiplex PCR on histologic material)
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? No, 57 cases did not undergo the reference standard (HPV multiplex assay) |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: HIGH |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: HIGH |
||
|
Smith 2014 |
Was a consecutive or random sample of patients enrolled? Unclear
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Unclear, exclusion criteria were not reported.
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? No
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? No, 9 cases did not receive a reference test and 1 case had equivocal results and was excluded in analysis |
Are there concerns that the included patients do not match the review question? Unclear
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: UNCLEAR |
CONCLUSION Could the patient flow have introduced bias?
RISK: HIGH |
||
|
Takes 2016 |
Was a consecutive or random sample of patients enrolled? No, a database was searched.
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? No, cases were selected when both p16 and HPV DNA test were positive or negative. Therefore, cases with (for example) equivocal results or only 1 test positive/negative were excluded. This may not be representative for clinical practice. |
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes
|
Are there concerns that the included patients do not match the review question? Yes
Are there concerns that the index test, its conduct, or interpretation differ from the review question? Unclear
Are there concerns that the target condition as defined by the reference standard does not match the review question? Unclear
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: HIGH |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: UNCLEAR |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
||
|
Xu 2016 |
Was a consecutive or random sample of patients enrolled? No, a database was searched.
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Unclear, cases were selected when p16 was both performed on cytologic and resection material. Selected cases may or may not represent cases in clinical practice, depending how common it was that both specimen was tested with p16.
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear, p16 status from cytology was blinded for p16 and dna status from tumors. However, the comparison of interest was both performed on cytologic material and it was unclear whether this was blinded.
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Unclear: 8 cases were excluded because the p16 status was not documented
Did patients receive the same reference standard? Unclear: 8 cases were excluded because the p16 status was not documented
Were all patients included in the analysis? No: 8 cases were excluded because the p16 status was not documented |
Are there concerns that the included patients do not match the review question? Unclear
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: HIGH |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: UNCLEAR |
Judgments on risk of bias are dependent on the research question: some items are more likely to introduce bias than others, and may be given more weight in the final conclusion on the overall risk of bias per domain:
Patient selection:
- Consecutive or random sample has a low risk to introduce bias.
- A case control design is very likely to overestimate accuracy and thus introduce bias.
- Inappropriate exclusion is likely to introduce bias.
Index test:
- This item is similar to “blinding” in intervention studies. The potential for bias is related to the subjectivity of index test interpretation and the order of testing.
- Selecting the test threshold to optimise sensitivity and/or specificity may lead to overoptimistic estimates of test performance and introduce bias.
Reference standard:
- When the reference standard is not 100% sensitive and 100% specific, disagreements between the index test and reference standard may be incorrect, which increases the risk of bias.
- This item is similar to “blinding” in intervention studies. The potential for bias is related to the subjectivity of index test interpretation and the order of testing.
Flow and timing:
- If there is a delay or if treatment is started between index test and reference standard, misclassification may occur due to recovery or deterioration of the condition, which increases the risk of bias.
- If the results of the index test influence the decision on whether to perform the reference standard or which reference standard is used, estimated diagnostic accuracy may be biased.
- All patients who were recruited into the study should be included in the analysis, if not, the risk of bias is increased.
Judgement on applicability:
Patient selection: there may be concerns regarding applicability if patients included in the study differ from those targeted by the review question, in terms of severity of the target condition, demographic features, presence of differential diagnosis or co-morbidity, setting of the study and previous testing protocols.
Index test: if index tests methods differ from those specified in the review question there may be concerns regarding applicability.
Reference standard: the reference standard may be free of bias but the target condition that it defines may differ from the target condition specified in the review question.
Quality assessment with AGREE-II (Brouwers, 2010) for the CAP Guideline (Lewis, 2018)
|
Domain |
Item |
Score (1 strongly disagree - 7 strongly agree) |
Comments |
|
SCOPE AND PURPOSE |
The overall objective(s) of the guideline is (are) specifically described. |
7 |
Explicitly stated |
|
|
The health question(s) covered by the guideline is (are) specifically described. |
7 |
Six questions are explicitly stated |
|
|
The population (patients, public, etc.) to whom the guideline is meant to apply is specifically described. |
6 |
Deducible, patients with oropharyngeal tumors |
|
STAKEHOLDER INVOLVEMENT |
The guideline development group includes individuals from all relevant professional groups |
7 |
Members with molecular pathology, surgical, medical, radiation oncology experience were seated in the working group. A methodologist was also added. |
|
|
The views and preferences of the target population (patients, public, etc.) have been sought. |
7 |
An advisory panel was created. Two patient representatives took seat. |
|
|
The target users of the guideline are clearly defined. |
3 |
Deducible, however it does not seem to be explicitly stated. |
|
RIGOUR OF DEVELOPMENT |
Systematic methods were used to search for evidence. |
7 |
Search and selection are described in the methods supplement |
|
|
The criteria for selecting the evidence are clearly described. |
3 |
Selection criteria are described, but may not be reproducible for each search/key question. |
|
|
The strengths and limitations of the body of evidence are clearly described. |
3 |
Risk of bias of included literature is described in supplemental tables 2-6, however it may not cover all relevant domains per study design. |
|
|
The methods for formulating the recommendations are clearly described. |
5 |
It is described how the panel would develop recommendations, however no formal framework seems to be used (e.g. GRADE evidence to decision). The panel needed to identify the relevant evidence and make 4 key judgements. |
|
|
The health benefits, side effects, and risks have been considered in formulating the recommendations. |
7 |
Harms and benefits were one of the key judgements the panel had to make. (supplement) |
|
|
There is an explicit link between the recommendations and the supporting evidence. |
6 |
Not assessed for the full guideline. However, systematically searching and selecting evidence and making key judgements about this should ensure the link between recommendations |
|
|
The guideline has been externally reviewed by experts prior to its publication. |
7 |
The guideline was peer-reviewed |
|
|
A procedure for updating the guideline is provided. |
6 |
The guideline is updated every 4 years, or earlier when new evidence could alter the guideline recommendations. A specific procedure was not stated. |
|
CLARITY OF PRESENTATION |
The recommendations are specific and unambiguous. The different options for management of the condition or health issue are clearly presented |
7 |
Seem specific. Includes a figure describing a complex algorithm. |
|
|
Key recommendations are easily identifiable. |
7 |
Summarized in a box and clearly stated in the text. |
|
APPLICABILITY |
The guideline describes facilitators and barriers to its application. |
4 |
Describes implementation of the statements/recommendations in practice, rather than the use/uptake of the guideline. (supplement) |
|
|
The guideline provides advice and/or tools on how the recommendations can be put into practice. |
4 |
Describes implementation of the statements/recommendations in practice, rather than the use/uptake of the guideline. (supplement) |
|
|
The potential resource implications of applying the recommendations have been considered. |
5 |
This has been discussed (supplement), but could have been more extensively reported. |
|
|
The guideline presents monitoring and/or auditing criteria. |
1 |
There does not seem to be any monitoring/audit criteria. |
|
EDITORIAL INDEPENDENCE |
The views of the funding body have not influenced the content of the guideline. |
4 |
Unclear. It was stated that the CAP provided funding for the project and that no industry funds were used. However, it is not explicitly stated that the CAP did not have any influence on the contents of the guideline. |
|
|
Competing interests of guideline development group members have been recorded and addressed. |
6 |
Assessed and reported. Two participants were assessed to have potential conflicts of interest. It was not stated which actions followed the COI assessment. |
|
OVERALL GUIDELINE ASSESSMENT |
Rate the overall quality of this guideline. |
5 |
Overall, it seems to be a methodologically transparent guideline. PICOs and in/exclusion criteria per question should probably have been reported for reproducibility/updating purposes. |
|
|
I would recommend this guideline for use. (yes / yes, with modifications / no) |
Yes, with modifications |
For example, clear statements of PICOs per question, clear statements of in/exclusion criteria per key question, extended RoB assessments for RCTs, more extensive evidence tables, GRADEing of evidence, use of an explicit/formal evidence to decision framework (e.g. GRADE evidence-to-decision) |
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Allison 2015 |
conference abstract |
|
Alison 2016 |
FNA (25 gauge) and core biopsies (20 gauge) were not analyzed separately. |
|
Aramouni 2010 |
Probably a conference abstract from the American Society of Cytopathology 58th Annual Scientific Meeting Platform and Poster Presentations, Supplement to Cancer Cytopathology |
|
Bernadt 2017 |
narratieve review |
|
Bishop 2014 |
Editorial |
|
Buckley 2018 |
Not a systematic review |
|
Buonocore 2017 |
Conference abstract |
|
Channir 2016 |
Conference abstract |
|
Channir 2016 |
Reference test does not seem to be a DNA/mRNA test |
|
Chernesky 2018 |
Oral and oropharyngeal tumors |
|
Dictor 2011 |
sensitivity / specificity can not be calculated |
|
Dona 2014 |
research question is phrased differently |
|
El-Salem 2019 |
|
|
Faquin 2014 |
Editorial |
|
Faquin 2016 |
Conference abstract |
|
Datima 2013 |
Conference abstract |
|
Fatima 2012 |
Conference abstract |
|
Fatima 2013 |
Conference abstract |
|
Gargano 2019 |
Conference abstract |
|
Gipson 2018 |
No diagnostic accuracy of algorithms |
|
Griffith 2019 |
Editorial |
|
Grimes 2013 |
Does not compare p16 immunocytochemistry to a reference standard |
|
Guo 2012 |
conference abstract |
|
Guo 2014 |
Unclear whether this assay is used in the Netherlands |
|
Haeggblom 2017 |
No diagnostic accuracy of algorithms |
|
Hakima 2015 |
Reference test does not seem to be a DNA/mRNA test |
|
Han 2016 |
Unclear whether this assay is used in the Netherlands |
|
Holmes 2015 |
No sub-analyses for NFA only (FNA cyto + FNA biopsy were analysed together) |
|
Holmes 2014 |
Narrative review |
|
Hou 2016 |
Conference abstract |
|
Jalaly 2020 |
narative review |
|
Jannapureddy 2010 |
sens/spec gegeven, ook na te rekenen. Let op: spec voor p16 klopt niet. Sample uit database met cervical SCC metastasis. |
|
Jannapureddy 2009 |
Conference abstract |
|
Jarboe 2012 |
Narrative review |
|
Jensen 2018 |
No diagnostic accuracy of algorithms |
|
Kerr 2014 |
FNA were taken from resections or biopsies. Only 9/33 were taken from lymph nodes. |
|
Khazai 2017 |
Conference abstract |
|
Krame 2013 |
Narrative review |
|
Kwon 2018 |
Conference abstract |
|
Lastra 2013 |
research question is phrased differently |
|
Lewis 2018 |
tests on biopsies or resected tissue |
|
Lewis 2018 |
Guideline, no original accuracy research of the algorithm |
|
Linxweiler 2015 |
Mucosal swabs for cytological analyses in already diagnosed HNSCC patients (and healthy controls) |
|
Madrigal 2018 |
Narrative review |
|
Mehrotra 2012 |
Narrative review |
|
Miller 2017 |
No seprarate analyses for cell-blocks (cell-block + core biopsy analyzed together) |
|
Miller 2016 |
Conference abstract |
|
Pai 2009 |
DTA parameters unobtainable from presented data |
|
Patel 2019 |
Conference abstract |
|
Pusztaszeri 2015 |
Narrative review |
|
Qureishi 2017 |
Narrative review |
|
Rohrback 2017 |
Case-report of 2 cases |
|
Rollo 2018 |
Unclear whether this assay is used in the Netherlands |
|
Roy-Chowdhuri 2015 |
Narrative review |
|
Segura 2018 |
Conference abstract (via web of science) |
|
Shelton 2017 |
Tested on biopsies or resected tissue |
|
Shirsat 2018 |
Conference abstract |
|
Shirsat 2017 |
Conference abstract |
|
Thanky 2017 |
Niet te vinden via pubmed, google scholar en web of science. Referentie verwijst naar pagina E97 in vol 39 van Head & Neck, deze (E97 in vol 39) is niet te vinden op de website van het journal: pagina E97 is onderdeel van een case-report van andere auteurs. |
|
Troussier 2018 |
(Article in French) |
|
Virk 2015 |
Conference abstract |
|
Wilson 2019 |
Conference abstract |
|
Wong 2019 |
Only HPV positive patients were selected. |
|
Xu 2016 |
conference abstract |
|
Yang 2018 |
Conference abstract |
|
Yang 2019 |
Reference test does not seem to be a DNA/mRNA test |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 08-01-2024
Beoordeeld op geldigheid :
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2019 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen die betrokken zijn bij de zorg voor patiënten met hoofd-halstumoren.
Werkgroep
- Prof. Dr. R. de Bree, KNO-arts/hoofd-halschirurg, UMC Utrecht, Utrecht, NVKNO (voorzitter)
- Dr. M.B. Karakullukcu, KNO-arts/hoofd-halschirurg, NKI, Amsterdam, NVKNO
- Dr. H.P. Verschuur, KNO-arts/hoofd-halschirurg, Haaglanden MC, Den Haag, NVKNO
- Dr. M. Walenkamp, AIOS-KNO, LUMC, Leiden, NVKNO
- Dr. A. Sewnaik, KNO-arts/hoofd-halschirurg, Erasmus MC, Rotterdam, NVKNO
- Drs. L.H.E. Karssemakers, MKA-chirurg-oncoloog/hoofd-hals chirurg, NKI, Amsterdam, NVMKA
- Dr. M.J.H. Witjes, MKA-chirurg-oncoloog, UMC Groningen, Groningen, NVMKA
- Drs. L.A.A. Vaassen, MKA-chirurg-oncoloog, Maastricht UMC+, Maastricht, NVMKA
- Drs. W.L.J. Weijs, MKA-chirurg-oncoloog, Radboud UMC, Nijmegen, NVKMA
- Drs. E.M. Zwijnenburg, Radiotherapeut-oncoloog, Radboud UMC, Nijmegen, NVRO
- Dr. A. Al-Mamgani, Radiotherapeut-oncoloog, NKI, Amsterdam, NVRO
- Prof. Dr. C.H.J. Terhaard, Radiotherapeut-oncoloog, UMC Utrecht, Utrecht, NVRO
- Drs. J.G.M. Van den Hoek, Radiotherapeut-oncoloog, UMC Groningen, Groningen, NVRO
- Dr. E. Van Meerten, Internist-oncoloog, Erasmus MC Kanker Instituut, Rotterdam, NIV
- Dr. M. Slingerland, Internist-oncoloog, LUMC, Leiden, NIV
- Drs. M.A. Huijing, Plastisch Chirurg, UMC Groningen, Groningen, NVPC
- Prof. Dr. S.M. Willems, Klinisch patholoog, UMC Groningen, Groningen, NVVP
- Prof. Dr. E. Bloemena, Klinisch patholoog, Amsterdam UMC, locatie Vumc, Amsterdam, NVVP
- R.A. Burdorf, Voorzitter dagelijks bestuur patiëntenvereniging, Patiëntenvereniging HOOFD-HALS, PvHH
- P.S. Verdouw, Hoofd infocentrum patiëntenvereniging, Patiëntenvereniging HOOFD-HALS, PvHH
- A.A.M. Goossens, Verpleegkundig specialist oncologie, Haaglanden MC, Den Haag, V&VN
- Dr. P. de Graaf, Radioloog, Amsterdam UMC, Amsterdam, NVvR
- Dr. W.V. Vogel, Nucleair geneeskundige/radiotherapeut-oncoloog, NKI, Amsterdam, NVNG
- Drs. G.J.C. Zwezerijnen, Nucleair geneeskundige, Amsterdam UMC, Amsterdam, NVNG
Klankbordgroep
- Dr. C.M. Speksnijder, Fysiotherapeut, UMC Utrecht, Utrecht, KNGF
- Ir. A. Kok, Diëtist, UMC Utrecht, Utrecht, NVD
- Dr. M.M. Hakkesteegt, Logopedist, Erasmus MC, Rotterdam, NVLF
- Drs. D.J.M. Buurman, Tandarts-MFP, Maastricht UMC+, Maastricht, KNMT
- W. Van der Groot-Roggen, Mondhygiënist, UMC Groningen, Groningen, NVvM
- Drs. D.J.S. Dona, Bedrijfsarts/Klinisch arbeidsgeneeskundige oncologie, Radboud UMC, Nijmegen, NVKA
- Dr. M. Sloots, Ergotherapeut, UMC Utrecht, Utrecht
- J. Poelstra, Medisch maatschappelijk werkster, op persoonlijke titel
Met ondersteuning van
- Dr. J. Boschman, Senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. C. Gaasterland, Adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. A. Van der Hout, Adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. L. Oostendorp, Adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. M. Oerbekke, Adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. A. Hoeven, Junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Bree, de |
KNO-arts/hoofd-halschirurg, UMC Utrecht |
* Lid Algemeen Bestuur Patiëntenvereniging Hoofd-Hals (onbetaald) * Voorzitter Research Stuurgroep NWHHT * Lid Richtlijnen commissie NWHHT * Lid dagelijks bestuur NWHHT * Lid Clinical Audit Board van de Dutch Head and Neck Audit (DHNA) * Lid wetenschappelijk adviescommissie DORP * Voorzitter Adviescommissie onderzoek hoofd-halskanker (IKNL/PALGA/DHNA/NWHHT) |
Geen |
Geen |
|
Slingerland |
Internist-oncoloog, LUMC |
* 2018-present: Treasurer of the "Dutch Association of Medical Oncology"(NVMO - vacancy fees) * 2018-present: Member of the "Dutch Working Group for Head-Neck Tumors" (NWHHT-Systemic therapy) * 2016-present: Member of the 'Dutch Working Group for Head-Neck Tumors" (NWHHT - study group steering group (coordinating)) * 2016-present: Member of the "Dutch Working Group for Head-Neck Tumors" (NWHHT - Elderly Platform) * 2012-present: Member "Working Group for Head-Neck Tumors" (WHHT) "University Cancer Centre"(UCK) Leiden - Den Haag * 2019: Member CAB DHNA |
Deelname Nationaal expert forum hoofd-halskanker MSD dd 2-5-2018
* Deelname Checkmate studie, sponsor Bristol-Myers Squibb (BMS): An open label, randomized phase 3 clinical trial of nivolumab versus therapy of investigator's choice in recurrent or metastatic platinum-refractory squamous cell carcinoma of the head and neck (SCCHN) * Deelname Commence studie, sponsor Radboud University, in collaboration with Merck Serono International SA (among several Dutch medical centers): A phase lB-II study of the combination of cetuximab and methotrexate in recurrent of metastatic squamous cell carcinoma of the head and neck. A study of the Dutch Head and Neck Society, MOHN01/COMMENCE study. * Deelname HESPECTA studie: Phase I study: to determine the biological activity of two HPV16E6 specific peptides coupled to Amplivant®, a Toll-like receptor ligand in non-metastatic patients treated for HPV16-positive head and neck cancer. * Deelname PINCH studie (nog niet open): PD-L1 ImagiNg to predict durvalumab treatment response in HNSCC (PINCH) trial; patiënten met biopt bewezen locally recurrent of gemetastaseerd HNSCC * Deelname ISA 101b-HN-01-17 studie (nog niet open): A randomized, Double-blind, Placebo-Controlled, Phase 2 Study of Cemiplimab versus the combination of Cemiplimab with ISA101b in the Treatment of Subjects. |
In de werkgroep participeren 2 internist-oncologen, zodat één van beide de voortrekker is van modules over systemische therapie. Actie: werkgroeplid is uitgesloten van besluitvorming bij modules die betrekking hebben op de onderwerpen van de gemelde onderzoeken: nivolumab, cetuximab + methotrexaat, Amplivant, durvalumab, cemiplimab. |
|
Meerten, van |
Internist-oncoloog, Erasmus MC Kanker Instituut |
Geen |
Op dit moment Principal Investigator voor NL van gerandomiseerde fase III trial naar toegevoegde waarde van pembrolizumab aan chemoradiotherapie bij patiënten met gevorderd hoofdhalskanker. Sponsor: GlaxoSmithKline Research & Development Ltd. Studie is nog lopend, resultaten zullen pas bekend zijn na verschijning van de richtlijn.
In toekomst mogelijk participatie aan door industrie gesponsorde studies op gebied van behandeling van hoofdhalskanker |
In de werkgroep participeren 2 internist-oncologen, zodat één van beide de voortrekker is van modules over systemische therapie. Actie: werkgroeplid is uitgesloten van besluitvorming bij modules die betrekking hebben op het onderwerp van het gemelde onderzoeken: de toegevoegde waarde van pembrolizumab bij patiënten met gevorderd hoofdhalskanker. |
|
Huijing |
Plastisch chirurg, UMC Groningen |
Geen |
Geen |
Geen |
|
Sewnaik |
KNO-arts/hoofd Hals chirurg, Erasmus MC |
Sectorhoofd Hoofd-Hals chirurgie |
Geen |
Geen |
|
Vaassen |
MKA-chirurg-oncoloog, Maastricht UMC+ / CBT Zuid-Limburg |
*Lid Bestuur NVMKA *Waarnemend hoofd MKA-chirurgie MUMC |
Geen |
Geen |
|
Witjes |
MKA-chirurg-oncoloog, UMC Groningen |
Geen |
PI van KWF grant: RUG 2015 -8084: Image guided surgery for margin assessment of head & neck Cancer using cetuximab-IRDye800 cONjugate (ICON)
geen financieel belang |
Geen. Financiering door KWF werd niet als een belang ingeschat. |
|
Bloemena |
Klinisch patholoog, Amsterdam UMC (locatie Vumc) / Radboud UMC / Academisch Centrum voor Tandheelkunde Amsterdam (ACTA) |
* Lid bestuur Nederlandse Vereniging voor Pathologie (NVVP) – vacatiegeld (tot 1-12-20) * Voorzitter Commissie Bij- en Nascholing (NVVP) * Voorzitter (tot 1-12-20) Wetenschappelijke Raad PALGA - onbezoldigd |
Geen |
Geen |
|
Willems |
Klinisch patholoog, UMC Groningen |
Vice-vz PALGA, AB NWHHT, CAB DHNA, mede-vz en oprichter expertisegroep HH pathologie NL, Hoofdhalspathologie UMC Groningen |
PDL1 trainer NL voor MSD Onderzoeksfinanciering van Pfizer, Roche, MSD, BMS, Lilly, Novartis, Bayer, Amge, AstraZeneca |
Geen |
|
Karakullukcu |
KNO-arts/hoofd-hals chirurg, NKI/AVL |
Geen |
Geen |
Geen |
|
Verschuur |
KNO-arts/Hoofd-hals chirurg, Haaglanden MC |
* Opleider KNO-artsen |
Geen |
Geen |
|
Walenkamp |
AIOS KNO, LUMC |
Geen |
Geen |
Geen |
|
Al-Mamgani |
Radiotherapeut-oncoloog, NKI/AVL |
Geen |
Geen |
Geen |
|
Terhaard |
Radiotherapeut-oncoloog, UMC Utrecht |
Niet van toepassing |
Geen |
Geen |
|
Hoek, van den |
Radiotherapeut-oncoloog UMCG |
Niet van toepassing |
Geen |
Geen |
|
Zwijnenburg |
Radiotherapeut, Hoofd-hals Radboud UMC |
Geen |
Geen |
Geen |
|
Burdorf |
Patiëntvertegenwoordiger |
Geen |
Geen |
Geen |
|
Verdouw |
Hoofd Infocentrum patiëntenvereniging HOOFD HALS |
Geen |
Werkzaam bij de patiëntenvereniging. De achterban heeft baat bij een herziening van de richtlijn |
Geen |
|
Karssemakers |
Hoofd-hals chirurg NKI/AVL
MKA-chirurg-oncoloog Amsterdam UMC (locatie AMC) / vakgroep kaakchirurgie Amsterdam West |
Niet van toepassing |
Geen |
Geen |
|
Goossens |
Verpleegkundig specialist, Haaglanden Medisch Centrum (HMC) |
* Bestuurslid (penningmeester) PWHHT (onbetaald) * Lid Commissie voorlichting PVHH (onbetaald) |
Geen |
Geen |
|
Zwezerijnen |
Nucleair geneeskundige, Amsterdam UMC (locatie Vumc)
PhD kandidaat, Amsterdam UMC (locatie Vumc) |
Lid als nucleair geneeskundige in HOVON imaging werkgroep (bespreken van richtlijnen en opzetten/uitvoeren van wetenschappelijke studies met betrekking tot beeldvorming in de hematologie); onbetaald |
Geen |
Geen |
|
Vogel |
Nucleair geneeskundige/radiotherapeut-oncoloog, AVL |
Geen |
In de afgelopen jaren incidenteel advies of onderwijs, betaald door Bayer, maar niet gerelateerd aan hoofd-hals
KWF-grant speekselklier toxiteit na behandeling. Geen belang bij de richtlijn |
Geen |
|
Graaf, de |
Radioloog, Amsterdam UMC (locatie Vumc) |
Bestuurslid sectie Hoofd-Hals radiologie (onbetaald) |
Geen |
Geen |
|
Weijs |
MKA-chirurg-oncoloog, Radboudumc |
MKA-chirurg, Weijsheidstand B.V. Werkzaam als algemeen praktiserend MKA-chirurg, betaald (0,1 fte) |
Geen |
Geen |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door het uitnodigen van de patiëntenvereniging HOOFD-HALS (PVHH) voor de Invitational conference en met afgevaardigden van de PVHH in de werkgroep. Het verslag hiervan (zie bijlagen) is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de patiëntenvereniging HOOFD-HALS en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Methode ontwikkeling
Evidence based
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerden de werkgroep de knelpunten in de zorg voor patiënten met hoofd-halstumoren. De werkgroep beoordeelde de aanbeveling(en) uit de eerdere richtlijnmodule (NVKNO, 2014) op noodzaak tot revisie. Tevens zijn er knelpunten aangedragen door de patiëntenvereniging en genodigde partijen tijdens de invitational conference (zie de bijlagen voor het verslag van de invitational conference). Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE-gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. https://richtlijnendatabase.nl/over_deze_site/richtlijnontwikkeling.html.
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008 May 24;336(7654). doi: 10.1136/bmj.a139.
Schünemann, A Holger J (corrected to Schünemann, Holger J). PubMed PMID: 18483053; PubMed Central PMCID: PMC2386626.
Wessels M, Hielkema L, van der Weijden T. How to identify existing literature on patients' knowledge, views, and values: the development of a validated search filter. J Med Libr Assoc. 2016 Oct;104(4):320-324. PubMed PMID: 27822157; PubMed Central PMCID: PMC5079497.
Zoekverantwoording
Histopathology-based diagnostic algorithms
|
Uitgangsvraag: Welke teststrategie is het meest effectief om de HPV status te bepalen bij patiënten met een orofarynxcarcinoom? |
|
|
Database(s): PubMed, Embase |
Datum: 10-3-2020 |
|
Periode: niet van toepassing |
Talen: niet van toepassing |
|
Toelichting: Specifiek gezocht op orofarynx neoplasma. Met uitzondering van het artikel van Smeets worden alle sleutelartikelen gevonden. In het artikel van Smeets wordt gesproken over hoofd hals tumoren en niet specifiek orofarynx tumoren. Er is gezocht naar systematic reviews. Daarnaast is een diagnostisch filter gebruikt.
Met vriendelijke groet, Ingeborg van Dusseldorp |
|
|
|
Inclusief dubbele referenties |
Ontdubbeld |
|
SR |
47 |
36 |
|
Diagnostisch |
573 |
455 |
|
|
|
|
|
Totaal |
|
491 |
PubMed
|
Search |
Query |
Items found |
|
#62 |
Search #54 AND #61 |
275 |
|
#61 |
Search "Sensitivity and Specificity"(MeSH) OR specificit*(tw) OR screening(tw) OR accura*(tw) OR reference value*(tw) OR false positive(tw) OR false negative(tw) OR predictive value*(tw) OR roc(tw) OR likelyhood*(tw) OR likelihood*(tw) |
2683505 |
|
#57 |
Search #54 AND #55 |
23 |
|
#55 |
Search ("Meta-Analysis as Topic"(Mesh) OR “Meta-Analysis”(Publication Type) OR metaanaly*(tiab) OR metanaly*(tiab) OR meta-analy*(tiab) OR meta synthes*(tiab) OR metasynthes*(tiab) OR meta ethnograph*(tiab) OR metaethnograph*(tiab) OR meta summar*(tiab) OR metasummar*(tiab) OR meta-aggregation(tiab) OR metareview(tiab) OR meta-review(tiab) OR overview of reviews(tiab) OR ((systematic*(ti) OR scoping(ti) OR umbrella(ti) OR meta-narrative(ti) OR metanarrative(ti) OR evidence based(ti)) AND (review*(ti) OR overview*(ti))) OR ((evidence(ti) OR narrative(ti) OR metanarrative(ti) OR qualitative(ti)) AND synthesis(ti)) OR systematic review(pt) OR prisma(tiab) OR preferred reporting items(tiab) OR quadas*(tiab) OR systematic review*(tiab) OR systematic literature(tiab) OR structured literature search(tiab) OR systematic overview*(tiab) OR scoping review*(tiab) OR umbrella review*(tiab) OR mapping review*(tiab) OR systematic mapping(tiab) OR evidence synthes*(tiab) OR narrative synthesis(tiab) OR metanarrative synthesis(tiab) OR research synthesis(tiab) OR qualitative synthesis(tiab) OR realist synthesis(tiab) OR realist review(tiab) OR realist evaluation(tiab) OR systematic qualitative review(tiab) OR mixed studies review(tiab) OR mixed methods synthesis(tiab) OR mixed research synthesis(tiab) OR quantitative literature review(tiab) OR systematic evidence review(tiab) OR evidence-based review(tiab) OR comprehensive literature search(tiab) OR integrated review*(tiab) OR integrated literature review(tiab) OR integrative review*(tiab) OR integrative literature review*(tiab) OR structured literature review*(tiab) OR systematic search and review(tiab) OR meta-narrative review*(tiab) OR metanarrative review(tiab) OR systematic narrative review(tiab) OR systemic review(tiab) OR systematized review(tiab) OR systematic research synthesis(tiab) OR bibliographic*(tiab) OR hand-search*(tiab) OR handsearch*(tiab) OR manual search*(tiab) OR searched manually(tiab) OR manually searched(tiab) OR journal database*(tiab) OR review authors independently(tiab) OR reviewers independently(tiab) OR independent reviewers(tiab) OR independent review authors(tiab) OR electronic database search*(tiab) OR (study selection(tiab) AND data extraction(tiab)) OR (selection criteria(tiab) AND data collection(tiab)) OR (selection criteria(tiab) AND data analysis(tiab)) OR (evidence acquisition(tiab) AND evidence synthesis(tiab)) OR (pubmed(tiab) AND embase(tiab)) OR (medline(tiab) AND embase(tiab)) OR (pubmed(tiab) AND cochrane(tiab)) OR (medline(tiab) AND cochrane(tiab)) OR (embase(tiab) AND cochrane(tiab)) OR (pubmed(tiab) AND psycinfo(tiab)) OR (medline(tiab) AND psycinfo(tiab)) OR (embase(tiab) AND psycinfo(tiab)) OR (cochrane(tiab) AND psycinfo(tiab)) OR (pubmed(tiab) AND web of science(tiab)) OR (medline(tiab) AND web of science(tiab)) OR (embase(tiab) AND web of science(tiab)) OR (psycinfo(tiab) AND web of science(tiab)) OR (cochrane(tiab) AND web of science(tiab)) OR ((literature(ti) OR qualitative(ti) OR quantitative(ti) OR integrated(ti) OR integrative(tiab) OR rapid(ti) OR short(ti) OR critical*(ti) OR mixed stud*(ti) OR mixed method*(ti) OR focused(ti) OR focussed(ti) OR structured(ti) OR comparative(ti) OR comparitive(ti) OR evidence(ti) OR comprehensive(ti) OR realist(ti)) AND (review*(ti) OR overview*(ti)) AND (literature search(tiab) OR structured search(tiab) OR electronic search(tiab) OR search strategy(tiab) OR gray literature(tiab) OR grey literature(tiab) OR Review criteria(tiab) OR eligibility criteria(tiab) OR inclusion criteria(tiab) OR exclusion criteria(tiab) OR predetermined criteria(tiab) OR included studies(tiab) OR identified studies(tiab) OR (systematic search(tiab) AND literature(tiab)) OR strength of evidence(tiab) OR citation*(tiab) OR references(tiab) OR database search*(tiab) OR electronic database*(tiab) OR data base search*(tiab) OR electronic data-base*(tiab) OR search criteria(tiab) OR study selection(tiab) OR data extraction(tiab) OR methodological quality(tiab) OR methodological characteristics(tiab) OR methodologic quality(tiab) OR methodologic characteristics(tiab))) OR ((literature review(tiab) OR literature search*(tiab)) AND (structured search(tiab) OR electronic search(tiab) OR Search strategy(tiab) OR gray literature(tiab) OR grey literature(tiab) OR review criteria(tiab) OR eligibility criteria(tiab) OR inclusion criteria(tiab) OR exclusion criteria(tiab) OR predetermined criteria(tiab) OR included studies(tiab) OR identified studies(tiab) OR (systematic search(tiab) AND literature(tiab)) OR strength of evidence(tiab) OR citation*(tiab) OR references(tiab) OR database search*(tiab) OR electronic database*(tiab) OR data base search*(tiab) OR electronic data-base*(tiab) OR search criteria(tiab) OR study selection(tiab) OR data extraction(tiab) OR methodological quality(tiab) OR methodological characteristics(tiab) OR methodologic quality(tiab) OR methodologic characteristics(tiab)))) NOT ("Comment" (Publication Type) OR "Letter" (Publication Type)) NOT (“Animals”(Mesh) NOT “Humans”(Mesh)) |
335882 |
|
#54 |
Search #44 AND #45 AND #53 |
1030 |
|
#53 |
Search "Algorithms"(Mesh) OR "Diagnostic Techniques and Procedures"(Mesh) OR algorhythm(tiab) OR algorism(tiab) OR algorithm(tiab) OR algorithms(tiab) OR diagnostic test/exp OR test(tiab) OR tests(tiab) |
8648716 |
|
#44 |
Search "Oropharyngeal Neoplasms"(Mesh) OR oropharynx tumor*(tiab) OR oropharynx tumour*(tiab) OR (squamous cell(tiab) AND oropharyn*(tiab)) OR oropharynx cancer(tiab) OR oropharyngeal cancer(tiab) OR oropharynx carcinoma*(tiab) OR oropharyngeal carcinoma*(tiab) OR oropharynx neoplasm*(tiab) OR oropharyngeal neoplasm*(tiab) |
11717 |
|
#45 |
Search "Papillomaviridae"(Mesh) OR human papilloma virus(tiab) OR wart virus(tiab) OR condyloma virus(tiab) OR hpv(tiab) OR human papillomavirus(tiab) OR verruca virus(tiab) OR viral verruca(tiab) OR virus verruca(tiab) OR virus wart(tiab) |
55608 |
Embase
|
No. |
Query |
Results |
|
#20 |
#19 NOT #18 |
298 |
|
#19 |
#4 AND #5 |
307 |
|
#18 |
#4 AND #17 |
24 |
|
#17 |
('meta analysis'/de OR cochrane:ab OR embase:ab OR psycinfo:ab OR cinahl:ab OR medline:ab OR ((systematic NEAR/1 (review OR overview)):ab,ti) OR ((meta NEAR/1 analy*):ab,ti) OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
481200 |
|
#16 |
#14 NOT #15 |
1 |
|
#15 |
#6 AND #14 |
8 |
|
#14 |
#7 OR #8 OR #9 OR #10 OR #11 OR #12 |
9 |
|
#13 |
defining AND better AND algorithm AND for AND the AND accurate AND identification AND of AND hpv AND status AND among AND oropharyngeal AND 'squamous cell' AND carcinoma AND 2015 |
0 |
|
#12 |
diagnosis AND of AND hpv AND driven AND head AND neck AND cancer AND comparing AND p16 AND based AND algorithms AND with AND the AND rnascope AND 'hpv test' AND mirghani |
2 |
|
#11 |
double AND positivity AND is AND the AND biomarker AND with AND strongest AND diagnostic AND accuracy AND prognostic AND value AND for AND human AND papillomavirus AND related AND oropharyngeal AND cancer AND patients AND mena |
1 |
|
#10 |
developing AND a AND new AND diagnostic AND algorithm AND for AND human AND papilloma AND virus AND associated AND oropharyngeal AND carcinoma AND cohen AND 2017 |
1 |
|
#9 |
validation AND of AND a AND novel AND diagnostic AND standard AND in AND 'hpv positive' AND oropharyngeal AND squamous AND cell AND carcinoma AND schache AND 2013 AND british AND journal AND cancer |
1 |
|
#8 |
detection AND human AND papillomavirus AND in AND clinical AND samples AND evolving AND methods AND strategies AND for AND the AND accurate AND determination AND hpv AND status AND of AND head AND neck AND carcinomas AND westra AND 2014 |
3 |
|
#7 |
a AND novel AND algorithm AND for AND reliable AND detection AND of AND human AND papillomavirus AND in AND paraffin AND embedded AND head AND neck AND cancer AND specimen AND smeets |
1 |
|
#6 |
#4 AND #5 |
412 |
|
#5 |
'sensitivity and specificity'/exp OR 'screening'/exp OR 'reference value'/exp OR 'diagnostic error'/exp OR 'predictive value'/exp OR 'receiver operating characteristic'/exp OR specificit*:ab,ti OR screening:ab,ti OR accura*:ab,ti OR 'reference value*':ab,ti OR 'false positive':ab,ti OR 'false negative':ab,ti OR 'predictive value*':ab,ti OR roc:ab,ti OR likelyhood*:ab,ti OR likelihood*:ab,ti |
3030582 |
|
#4 |
#1 AND #2 AND #3 |
1378 |
|
#3 |
'algorithm'/exp OR 'algorhythm*':ti,ab,kw OR 'algorism*':ti,ab,kw OR 'algorithm*':ti,ab,kw OR 'diagnostic test'/exp OR 'test battery'/exp OR test:ti,ab,kw OR tests:ti,ab,kw OR 'assay combination':ti,ab,kw |
3687381 |
|
#2 |
'wart virus'/exp OR 'human papilloma virus':ti,ab OR 'wart virus':ti,ab OR 'condyloma virus':ti,ab OR 'hpv':ti,ab OR 'human papillomavirus':ti,ab OR 'verruca virus':ti,ab OR 'viral verruca':ti,ab OR 'virus verruca':ti,ab OR 'virus wart':ti,ab OR 'verruca, viral':ti,ab OR 'papillomaviridae'/exp |
77439 |
|
#1 |
'oropharynx cancer'/exp OR ((oropharyn* NEAR/3 (tumor* OR 'squamous cell' OR tumour* OR cancer* OR carcinoma* OR neoplasm*)):ti,ab,kw) |
14838 |
Cytology-based tests for positive neck nodes and CUP
Algemene informatie
|
Richtlijn: Hoofd- halstumoren |
|
|
Uitgangsvraag: Hoe dient de HPV-status bepaald te worden? |
|
|
Database(s): PubMed, Embase |
Datum: 27-2-2020 |
|
Periode: niet van toepassing |
Talen: niet van toepasing |
|
Literatuurspecialist: Ingeborg van Dusseldorp |
|
|
Toelichting en opmerkingen: Voor deze vraag is op een specifieke manier gezocht conform de opgave in het zoekformulier. Dat betekent concreet dat er een combinatie is gemaakt van: HHT tumoren EN cytologie, fine needle aspiration EN p16 staining, immunohistochemy et cetera zie zoekformulier EN HPV Vervolgens zijn de systematische reviews geselecteerd en is een combinatie gemaakt met een sensitief diagnostisch filter. Bij het specifieke diagnostische filter werd het sleutelartikel van Rollo niet gevonden, vandaar de keuze voor het sensitieve filter, waarmee alle sleutelartikelen werden gevonden. |
|
Zoekopbrengst
|
|
Embase |
PubMed |
Ontdubbeld |
|
SRs |
6 |
8 |
13 |
|
RCTs |
|
|
|
|
Observationele studies |
|
|
|
|
Diagnostische studies |
285 |
201 |
435 |
|
Totaal |
|
|
448 |
PubMed
|
Search |
Query |
Results |
|
#19 |
Search: #12 AND #17 |
201 |
|
#15 |
Search: #10 AND #13 |
8 |
|
#17 |
Search: "Immunohistochemistry"(Mesh) OR “22c3 pharmdx”(tiab) OR “28-8 pharmdx”(tiab) OR “er-pr pharmdx”(tiab) OR “envision duoflex”(tiab) OR “envision flex”(tiab) OR “pd-l1 22c3 pharmdx”(tiab) OR “pd-l1 ihc 22c3 pharmdx”(tiab) OR “pd-l1 ihc 28-8 pharmdx”(tiab) OR “ventana pd-l1”(tiab) OR “confirm id kit”(tiab) OR “immunohistochemical test kit”(tiab) OR pharmdx(tiab) OR antigen staining(tiab) OR immunohistochemistr*(tiab) OR immunostaining(tiab) OR "in situ hybridization"(MeSH Terms) OR “hybridization in situ”(tiab) OR “in situ hybridisation”(tiab) OR “hybridisation in situ”(tiab) OR "Polymerase Chain Reaction"(Mesh) OR “polymerase chain reaction*”(tiab) OR p16(tiab) OR "P16 protein, human" (Supplementary Concept) |
1,274,482 |
|
#13 |
Search: ("Meta-Analysis as Topic"(Mesh) OR “Meta-Analysis”(Publication Type) OR metaanaly*(tiab) OR metanaly*(tiab) OR meta-analy*(tiab) OR meta synthes*(tiab) OR metasynthes*(tiab) OR meta ethnograph*(tiab) OR metaethnograph*(tiab) OR meta summar*(tiab) OR metasummar*(tiab) OR meta-aggregation(tiab) OR metareview(tiab) OR meta-review(tiab) OR overview of reviews(tiab) OR ((systematic*(ti) OR scoping(ti) OR umbrella(ti) OR meta-narrative(ti) OR metanarrative(ti) OR evidence based(ti)) AND (review*(ti) OR overview*(ti))) OR ((evidence(ti) OR narrative(ti) OR metanarrative(ti) OR qualitative(ti)) AND synthesis(ti)) OR systematic review(pt) OR prisma(tiab) OR preferred reporting items(tiab) OR quadas*(tiab) OR systematic review*(tiab) OR systematic literature(tiab) OR structured literature search(tiab) OR systematic overview*(tiab) OR scoping review*(tiab) OR umbrella review*(tiab) OR mapping review*(tiab) OR systematic mapping(tiab) OR evidence synthes*(tiab) OR narrative synthesis(tiab) OR metanarrative synthesis(tiab) OR research synthesis(tiab) OR qualitative synthesis(tiab) OR realist synthesis(tiab) OR realist review(tiab) OR realist evaluation(tiab) OR systematic qualitative review(tiab) OR mixed studies review(tiab) OR mixed methods synthesis(tiab) OR mixed research synthesis(tiab) OR quantitative literature review(tiab) OR systematic evidence review(tiab) OR evidence-based review(tiab) OR comprehensive literature search(tiab) OR integrated review*(tiab) OR integrated literature review(tiab) OR integrative review*(tiab) OR integrative literature review*(tiab) OR structured literature review*(tiab) OR systematic search and review(tiab) OR meta-narrative review*(tiab) OR metanarrative review(tiab) OR systematic narrative review(tiab) OR systemic review(tiab) OR systematized review(tiab) OR systematic research synthesis(tiab) OR bibliographic*(tiab) OR hand-search*(tiab) OR handsearch*(tiab) OR manual search*(tiab) OR searched manually(tiab) OR manually searched(tiab) OR journal database*(tiab) OR review authors independently(tiab) OR reviewers independently(tiab) OR independent reviewers(tiab) OR independent review authors(tiab) OR electronic database search*(tiab) OR (study selection(tiab) AND data extraction(tiab)) OR (selection criteria(tiab) AND data collection(tiab)) OR (selection criteria(tiab) AND data analysis(tiab)) OR (evidence acquisition(tiab) AND evidence synthesis(tiab)) OR (pubmed(tiab) AND embase(tiab)) OR (medline(tiab) AND embase(tiab)) OR (pubmed(tiab) AND cochrane(tiab)) OR (medline(tiab) AND cochrane(tiab)) OR (embase(tiab) AND cochrane(tiab)) OR (pubmed(tiab) AND psycinfo(tiab)) OR (medline(tiab) AND psycinfo(tiab)) OR (embase(tiab) AND psycinfo(tiab)) OR (cochrane(tiab) AND psycinfo(tiab)) OR (pubmed(tiab) AND web of science(tiab)) OR (medline(tiab) AND web of science(tiab)) OR (embase(tiab) AND web of science(tiab)) OR (psycinfo(tiab) AND web of science(tiab)) OR (cochrane(tiab) AND web of science(tiab)) OR ((literature(ti) OR qualitative(ti) OR quantitative(ti) OR integrated(ti) OR integrative(tiab) OR rapid(ti) OR short(ti) OR critical*(ti) OR mixed stud*(ti) OR mixed method*(ti) OR focused(ti) OR focussed(ti) OR structured(ti) OR comparative(ti) OR comparitive(ti) OR evidence(ti) OR comprehensive(ti) OR realist(ti)) AND (review*(ti) OR overview*(ti)) AND (literature search(tiab) OR structured search(tiab) OR electronic search(tiab) OR search strategy(tiab) OR gray literature(tiab) OR grey literature(tiab) OR Review criteria(tiab) OR eligibility criteria(tiab) OR inclusion criteria(tiab) OR exclusion criteria(tiab) OR predetermined criteria(tiab) OR included studies(tiab) OR identified studies(tiab) OR (systematic search(tiab) AND literature(tiab)) OR strength of evidence(tiab) OR citation*(tiab) OR references(tiab) OR database search*(tiab) OR electronic database*(tiab) OR data base search*(tiab) OR electronic data-base*(tiab) OR search criteria(tiab) OR study selection(tiab) OR data extraction(tiab) OR methodological quality(tiab) OR methodological characteristics(tiab) OR methodologic quality(tiab) OR methodologic characteristics(tiab))) OR ((literature review(tiab) OR literature search*(tiab)) AND (structured search(tiab) OR electronic search(tiab) OR Search strategy(tiab) OR gray literature(tiab) OR grey literature(tiab) OR review criteria(tiab) OR eligibility criteria(tiab) OR inclusion criteria(tiab) OR exclusion criteria(tiab) OR predetermined criteria(tiab) OR included studies(tiab) OR identified studies(tiab) OR (systematic search(tiab) AND literature(tiab)) OR strength of evidence(tiab) OR citation*(tiab) OR references(tiab) OR database search*(tiab) OR electronic database*(tiab) OR data base search*(tiab) OR electronic data-base*(tiab) OR search criteria(tiab) OR study selection(tiab) OR data extraction(tiab) OR methodological quality(tiab) OR methodological characteristics(tiab) OR methodologic quality(tiab) OR methodologic characteristics(tiab)))) NOT ("Comment" (Publication Type) OR "Letter" (Publication Type)) NOT (“Animals”(Mesh) NOT “Humans”(Mesh)) |
334,389 |
|
#12 |
Search: #10 AND #11 |
239 |
|
#11 |
Search: "Sensitivity and Specificity"(MeSH) OR "Diagnostic Errors"(MeSH) OR sensitive(tw) OR sensitivity(tw) OR specificity(tw) OR accurate(tw) OR accuracy(tw) OR "golden standard" OR "gold standard" OR (reference(tw) AND (test(tw) OR standard(tw))) OR "index test" OR validity(tw) OR validation(tw) OR validate*(tw) OR valid(ti) OR validation studies(pt) OR verif*(ti) OR evaluation studies(pt) OR evaluat*(ti) OR (false(tw) AND (positive(tw) OR negative(tw))) OR pretest(tw) OR pre-test(tw) OR posttest(tw) OR post-test(tw) OR predictive value OR predict*(ti) OR roc(tw) OR likelyhood(tw) OR likelihood(tw) OR value*(ti) OR reference values(mesh) OR cutoff(tw) OR cut-off(tw) OR quality control(mesh) OR "reproducibility of results"(mesh) OR repeatability(tw) OR reproducibility(tw) OR efficacy(tw) OR reliability(tw) OR comparative study(pt) OR odds(tw) OR error*(tw) OR suitability(tw) OR utility(tw) |
7,058,350 |
|
#10 |
Search: #6 AND #7 AND #9 |
535 |
|
#9 |
Search: "cytology" (Subheading) OR "Cytological Techniques"(Mesh) OR "Cell Biology"(Mesh) OR “automated cytological technique*”(tiab) OR cell biology(tiab) OR cytolog*(tiab) OR cytotechnolog*(tiab) OR cytotest*(tiab) OR "Biopsy, Fine-Needle"(Mesh) OR fine core needle biop*(tiab) OR fine needle aspiration*(tiab) OR fine needle biop*(tiab) OR needle aspiration*(tiab) OR fnab(tiab) OR fnac(tiab) |
2,591,742 |
|
#7 |
Search: Head and Neck Neoplasms(Mesh:NoExp) OR Squamous Cell Carcinoma of Head and Neck(Mesh) OR neck mass*(tiab) OR neck tumor*(tiab) OR neck tumour*(tiab) OR neck cancer*(tiab) |
67,320 |
|
#6 |
Search: "Papillomaviridae"(Mesh) OR human papilloma virus(tiab) OR wart virus(tiab) OR condyloma virus(tiab) OR hpv(tiab) OR human papillomavirus(tiab) OR verruca virus(tiab) OR viral verruca(tiab) OR virus verruca(tiab) OR virus wart(tiab) |
55,513 |
Embase
Embase Session Results (27 Feb 2020)
|
No. |
Query |
Results |
|
#16 |
#12 AND #14 |
285 |
|
#15 |
#1 AND #14 |
6 |
|
#14 |
#7 AND #8 AND #10 AND #13 |
302 |
|
#13 |
'immunohistochemical test kit'/exp OR '22c3 pharmdx':ti,ab,kw OR '28-8 pharmdx':ti,ab,kw OR 'er-pr pharmdx':ti,ab,kw OR 'envision duoflex':ti,ab,kw OR 'envision flex':ti,ab,kw OR 'pd-l1 22c3 pharmdx':ti,ab,kw OR 'pd-l1 ihc 22c3 pharmdx':ti,ab,kw OR 'pd-l1 ihc 28-8 pharmdx':ti,ab,kw OR 'ventana pd-l1':ti,ab,kw OR 'confirm id kit':ti,ab,kw OR 'immunohistochemical test kit':ti,ab,kw OR 'immunohistochemistry test kit':ti,ab,kw OR 'pharmdx':ti,ab,kw OR 'immunohistochemistry'/exp OR 'antigen staining':ti,ab,kw OR 'immunohistochemistr*':ti,ab,kw OR 'immunostaining':ti,ab,kw OR 'in situ hybridization'/exp OR 'hybridization in situ':ti,ab,kw OR 'in situ hybridisation':ti,ab,kw OR 'hybridisation in situ':ti,ab,kw OR 'polymerase chain reaction'/exp OR 'polymerase chain reaction':ti,ab,kw OR p16:ti,ab,kw OR 'protein p16'/exp |
1557687 |
|
#12 |
sensitiv* OR detect* OR accura* OR specific* OR reliab* OR positive OR negative OR diagnos* |
14295362 |
|
#11 |
#9 AND #10 |
500 |
|
#10 |
'cytology'/exp OR 'automated cytological technique*':ti,ab,kw OR 'cell biology':ti,ab,kw OR 'cytolog*':ti,ab,kw OR 'cytotechnolog*':ti,ab,kw OR 'cytotest*':ti,ab,kw OR 'fine needle aspiration biopsy'/exp OR 'fine core needle biop*':ti,ab,kw OR 'fine needle aspiration*':ti,ab,kw OR 'fine needle biop*':ti,ab,kw OR 'needle aspiration*':ti,ab,kw OR fnab:ti,ab,kw OR fnac:ti,ab,kw |
980450 |
|
#9 |
#7 AND #8 |
6886 |
|
#8 |
'neck tumor'/exp OR 'neck mass*':ti,ab,kw OR 'neck tumor*':ti,ab,kw OR 'neck tumour*':ti,ab,kw OR 'neck cancer*':ti,ab,kw OR 'head and neck carcinoma'/exp OR 'neck carcinoma*':ti,ab,kw |
114000 |
|
#7 |
'wart virus'/exp OR 'human papilloma virus':ti,ab OR 'wart virus':ti,ab OR 'condyloma virus':ti,ab OR 'hpv':ti,ab OR 'human papillomavirus':ti,ab OR 'verruca virus':ti,ab OR 'viral verruca':ti,ab OR 'virus verruca':ti,ab OR 'virus wart':ti,ab OR 'verruca, viral':ti,ab OR 'papillomaviridae'/exp |
77320 |
|
#6 |
#2 OR #3 OR #4 OR #5 |
4 |
|
#5 |
27816020 |
1 |
|
#4 |
29873841 |
1 |
|
#3 |
31246356 |
1 |
|
#2 |
role AND cytology AND in AND the AND diagnosis AND management AND of AND 'hpv associated' AND head AND neck AND carcinoma |
1 |
|
#1 |
('meta analysis'/de OR cochrane:ab OR embase:ab OR psycinfo:ab OR cinahl:ab OR medline:ab OR ((systematic NEAR/1 (review OR overview)):ab,ti) OR ((meta NEAR/1 analy*):ab,ti) OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
479733 |
