Dosering cisplatin lokaal gevorderde tumoren
Uitgangsvraag
Welke dosering van cisplatin heeft de voorkeur in combinatie met radiotherapie bij de definitieve behandeling van lokaal gevorderde hoofd-halstumoren?
Aanbeveling
Geef patiënten tot en met 70 jaar met een locoregionaal vergevorderd plaveiselcelcarcinoom van het hoofdhalsgebied (Stadium III-IV) die een indicatie hebben voor chemoradiatie bij voorkeur concomitante chemotherapie met cisplatin (100 mg/m2 op dag 1, 22 en 43) in combinatie met conventioneel gefractioneerde radiotherapie (voorbeeld 70 Gy in zeven weken).
Op basis van bijvoorbeeld ingeschat toxiciteitsrisico kan een wekelijks (40 mg/m2) schema een te verdedigen alternatieve optie zijn.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Onze systematische zoekactie in Medline en Embase resulteerde in acht RCT’s (Mashhour, 2020; Sahoo, 2017; Noronha, 2018; Rawat, 2016; Kiyota, 2022; Nanda, 2019; Nair, 2017; Tsan, 2012). In vier van deze RCT’s werden (ook) patiënten geïncludeerd die adjuvant behandeld werden (Mashhour, 2020; Noronha, 2018; Kiyota, 2022; Tsan, 2012).
Voor beide cruciale uitkomsten (overleving en terugkeer van de kanker) werden resultaten gerapporteerd. Overleving werd gerapporteerd in vijf studies, één studie rapporteerde een slechtere overleving in de groep die wekelijks 40 mg/m2 cisplatin kreeg, vergeleken met driewekelijks cisplatin. Het ging hierbij echter om zeer kleine aantallen patiënten die waren overleden en een ongelijke grootte van de studiearmen (10/25 versus 9/31). Eén studie rapporteerde een betere overleving in de groep die wekelijks 40 mg/m2 cisplatin kreeg in de adjuvante setting, vergeleken met driewekelijks cisplatin.
Terugkeer van de kanker werd op twee verschillende manieren gerapporteerd: het wel of niet bereiken van een complete respons op de behandeling of locoregionale controle. Informatie over het bereiken van een complete respons was beschikbaar in vijf studies. Eén studie liet zien dat de frequentie van complete respons hoger was in de groep die wekelijks cisplatin kreeg (Nanda, 2020, 81% versus 75%; 40 mg/m2, 100% definitieve behandeling).
Twee studies lieten geen verschil zien (Mashhour, 2020, 30 mg/m2, 50% adjuvant behandeld en 50% definitief; Rawat, 2016, 35 mg/m2, 100% definitieve behandeling). Twee studies lieten zien dat de frequentie van complete respons lager was in de groep die wekelijks cisplatin kreeg (Sahoo, 2017, 72% versus 86%; 30 mg/m2, 100% definitieve behandeling; Nair, 2017, 75% versus 90%, 40 mg/m2, 100% definitieve behandeling). Locoregionale controle werd gerapporteerd in zes studies. In drie studies was de frequentie van locoregionale controle lager in de groep die wekelijks cisplatin kreeg, waarbij het in twee studies ging om een zeer klein aantal patiënten waarbij de kanker terugkeerde (4/29 versus 2/31 en 13/30 versus 8/30). In één van deze studies werd een dosering van 40 mg/m2 gebruikt in de definitieve setting en in de overige twee studies werd in de definitieve of adjuvante setting een dosering van 30 mg/m2 gebruikt. In één studie was de frequentie van locoregionale controle hoger in de groep die wekelijks 40 mg/m2 cisplatin kreeg in de adjuvante setting, vergeleken met driewekelijks cisplatin.
Ook voor alle belangrijke uitkomsten (ziektevrije overleving, kwaliteit van leven en bijwerkingen) werden resultaten gerapporteerd. Ziektevrije overleving werd in vier studies gerapporteerd. Eén studie (40 mg/m2) liet een slechtere 2-jaars overleving zien in de wekelijkse behandelgroep (53% versus 65%), twee studies (30 mg/m2 en 40 mg/m2 ) lieten geen verschil zien en in één studie (40 mg/m2) was het niet mogelijk om te bepalen of het verschil klinisch relevant was, de mediane ziektevrije overleving was echter vrijwel gelijk tussen de groepen (26,4 maanden versus 27,4 maanden).
Bijwerkingen werden in alle studies gerapporteerd. De frequentie acute bijwerkingen van graad 3 of hoger lag in één studie (30 mg/m2) lager in de wekelijkse behandelgroep (72% versus 85%; p=0.006), terwijl in een andere studie (40 mg/m2) geen verschil werd gevonden in de frequentie van bijwerkingen van graad 3 of hoger (81% versus 80%; p=0.87), maar wel in de frequentie van graad 4 bijwerkingen (8% versus 19%; p=0.017). Een andere studie analyseerde de frequentie van niet-hematologische bijwerkingen van graad 3 of hoger,
waarbij een lagere frequentie werd gerapporteerd in de wekelijkse behandelgroep (57% versus 77%). In sommige studies werd voor afzonderlijke niet-hematologische bijwerkingen van graad 3 of hoger een lagere frequentie in de wekelijkse groep gerapporteerd, bijvoorbeeld een verschil in de frequentie van dysfagie (63% versus 26%), maar dit werd niet consistent in alle studies teruggezien. Voor hematologische bijwerkingen werd in de meerderheid van de studies geen verschil in bijwerkingen van graad 3 of hoger tussen de groepen gerapporteerd.
Kwaliteit van leven werd in één studie gerapporteerd, waarbij zowel de scores voor de Trial Outcome Index (een combinatie van drie subschalen) als de scores voor vijf subschalen werden gerapporteerd. Scores op de Trial Outcome Index lagen op alle vier de meetmomenten wat lager (wat een lagere kwaliteit van leven inhoudt) in de wekelijkse behandelgroep. Alleen op het laatste meetmoment, drie maanden na het afronden van de behandeling, ging het om een klinisch relevant verschil tussen de groepen (9.7 punten verschil op een schaal van 0 tot 96). Voor de subschalen werd een wisselend beeld gezien.
De bewijskracht voor alle uitkomstmaten was zeer laag. Er werd afgewaardeerd wegens een risico op bias omdat de randomisatie en allocatie niet beschreven waren, omdat er niet geblindeerd was (voor de uitkomst kwaliteit van leven) en omdat één van de studies voortijdig stopgezet was wegens tegenvallende inclusie. Daarnaast werd in twee gevallen afgewaardeerd voor inconsistentie wegens verschillen in gerapporteerde effecten tussen de studies. Voor alle uitkomsten werd afgewaardeerd wegens indirectheid, omdat vier studies (ook) patiënten includeerden die in de adjuvante setting behandeling werden met chemoradiatie en omdat zeven van de acht studies waren uitgevoerd in Azië. Daarnaast ging het in zes van de acht studies om kleine patiëntaantallen (range 30 tot 71) wat leidde tot brede betrouwbaarheidsintervallen waardoor afgewaardeerd werd wegens imprecisie.
Uit de geïncludeerde studies bleek dat patiënten die wekelijks cisplatin kregen over het algemeen een lagere cumulatieve dosis ontvingen vergeleken met patiënten die driewekelijks cisplatin kregen. In twee Nederlandse retrospectieve studies werd voor patiënten waarbij dosisbeperkende toxiciteit optrad een minder goede overleving gerapporteerd (Bril, 2022; Wendrich, 2017).
In de literatuursamenvatting zijn alleen RCT’s geïncludeerd. De RCT’s zijn over het algemeen relatief klein en hebben hun beperkingen. Er zijn diverse niet-gerandomiseerde studies verschenen die zich met name op een vergelijking van toxiciteit hebben gericht. Hieruit komen aanwijzingen naar voren dat het wekelijks toedienen van cisplatin (40 mg/m2) tot minder (renale) toxiciteit zou kunnen leiden (Bauml, 2019; Driessen, 2016; Espeli, 2012; Ho, 2008). Daarnaast werd er één RCT geëxcludeerd omdat deze was uitgevoerd onder patiënten met een nasofarynxcarcinoom (Lee, 2016). Deze kleine RCT uit Korea suggereerde dat een wekelijkse dosis van 40 mg/m2 niet inferieur zou zijn aan driewekelijkse toediening van cisplatin.
Waarden en voorkeuren van patiënten (en eventueel hun verzorgers)
Het doel van het toedienen van cisplatin in een wekelijks schema in plaats van een driewekelijks schema is het verminderen van toxiciteit, zonder duidelijke afname van de effectiviteit. Er is geen onderzoek gedaan naar de waarden en voorkeuren van patiënten wat betreft deze twee doseringsschema’s. Uit een meta-analyse van 107 RCT’s naar de effecten van chemotherapie bij hoofd-halstumoren (Lacas, 2021) bleek dat met het toenemen van de leeftijd het effect van het toevoegen van cisplatin aan radiotherapie afneemt, waarbij er in de leeftijdsgroep boven de 70 jaar geen positief effect is op overleving en dit positieve effect in de leeftijdsgroep 60-69 ook aanzienlijk lager is dan in de groep jonger dan 50 jaar. Het is goed om te realiseren dat in de studies ook selectie heeft plaatsgevonden, bijvoorbeeld op basis van leeftijd en de aan- of afwezigheid van ernstige comorbiditeit. Met de patiënt moet duidelijk gecommuniceerd worden wat met de huidige behandelopties bereikt kan worden, en tegen welke prijs. Op basis hiervan en de eigen doelen van de patiënt kan een gewogen beslissing worden genomen.
Kosten (middelenbeslag)
De werkgroep heeft geen informatie gevonden over de kosteneffectiviteit van het wekelijkse doseringsschema ten opzichte van het driewekelijkse schema. De werkgroep heeft dit aspect daarom niet meegewogen bij het formuleren van de aanbeveling. De werkgroep verwacht dat de aanbeveling geen relevante impact heeft op de zorgkosten.
Aanvaardbaarheid, haalbaarheid en implementatie
Patiënten bezoeken het ziekenhuis dagelijks voor de radiotherapie, daarom is de belasting van wekelijkse ten opzichte van driewekelijkse toediening wat minder groot. .
In Nederland is de hoofd-halsoncologie gecentreerd in 8 werkgroepen, waardoor een hoge mate van expertise is gewaarborgd. Voor sommige patiënten betekent dit langere reistijden wat een belasting kan zijn. Er zijn geen aanwijzingen dat dit de therapie trouw ten nadele beïnvloedt. In het algemeen is in de centra sprake van voldoende capaciteit, hoewel er binnen financiële kaders spanningen op kunnen treden. Gezien de zeer goede onderlinge samenwerking binnen een groot team, waarvan de samenstelling en benodigde expertise is vastgesteld in de SONCOS-normen, is de kwaliteit gewaarborgd. De centra worden regelmatig gevisiteerd. Alle patiënten worden besproken binnen het multidisciplinaire overleg (MDO), waarbij ook de behandelaar aanwezig is. Hierna vindt overleg plaats met de patiënt, waarbij op basis van de adviezen uit het MDO met aandacht voor de eigen voorkeur van de patiënt een beleid wordt vastgesteld.
De werkgroep is van mening dat de aanbeveling aanvaardbaar is voor zowel zorgverleners als patiënten. De werkgroep verwacht dat het uitvoeren van de aanbeveling haalbaar en implementeerbaar is. De aanbeveling sluit aan bij de huidige werkwijze in de praktijk.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Concomitante chemotherapie met cisplatin (100 mg/m2 dag 1,22 en 43) in combinatie met conventioneel gefractioneerde radiotherapie (voorbeeld 70 Gy in zeven weken) wordt beschouwd als de standaard in de definitieve behandeling van het plaveiselcelcarcinoom van het hoofd-halsgebied. Op basis van de beschikbare literatuur is het onzeker of het wekelijkse schema tenminste net zo effectief is als het driewekelijkse schema met minder bijwerkingen.
Retrospectieve studies geven aanwijzingen dat de wekelijkse toediening gepaard zou kunnen gaan met minder (met name nefro-)toxiciteit. Een studie met dezelfde behandeling, maar voor een andere indicatie (nasofarynxcarcinoom) suggereerde dat wekelijkse toediening in een dosis van 40 mg/m2 niet inferieur is aan driewekelijkse toediening. De weging van argumenten voor en tegen de ene dan wel de andere behandeling dient besproken te worden met patiënten.
Onderbouwing
Achtergrond
Bij de definitieve behandeling van lokaal gevorderde hoofdhals-tumoren leidt chemoradiatie met cisplatin tot betere uitkomsten dan radiotherapie alleen. Van oudsher wordt een driewekelijks schema gebruikt met een dosering van 100 mg/m2 cisplatin op dag 1, 22 en 43 van de radiotherapie. Echter, dit gaat gepaard met aanzienlijke toxiciteit (in het bijzonder renale toxiciteit). In Nederland krijgt tegenwoordig ongeveer de helft van de patiënten cisplatin toegediend in een wekelijks schema, waarbij vaak een dosis van 40 mg/m2 wordt gegeven. Er zijn ook studies gedaan met een lagere wekelijkse dosis van bijvoorbeeld 30 of 35 mg/m2. De vraag is echter of een wekelijks doseringsschema even effectief is als het driewekelijkse schema en minder toxiciteit geeft.
Conclusies / Summary of Findings
Overall survival (critical outcome)
|
Very low GRADE |
The evidence is very uncertain about the effect of weekly cisplatin concurrent with definitive radiotherapy on overall survival when compared with three-weekly cisplatin concurrent with definitive radiotherapy in patients with locally advanced head and neck squamous cell carcinoma.
Sources: (Noronha, 2018; Kiyota, 2022; Nanda, 2019; Nair, 2017; Tsan, 2012) |
Recurrence (complete tumour response) (critical outcome)
|
Very low GRADE |
The evidence is very uncertain about the effect of weekly cisplatin concurrent with definitive radiotherapy on recurrence (complete tumour response) when compared with three-weekly cisplatin concurrent with definitive radiotherapy in patients with locally advanced head and neck squamous cell carcinoma.
Sources: (Mashhour, 2020; Sahoo, 2017; Rawat, 2016; Nanda, 2019; Nair, 2017; Tsan, 2012) |
Recurrence (locoregional control) (critical outcome)
|
Very low GRADE |
The evidence is very uncertain about the effect of weekly cisplatin concurrent with definitive radiotherapy on recurrence (locoregional control) when compared with three-weekly cisplatin concurrent with definitive radiotherapy in patients with locally advanced head and neck squamous cell carcinoma.
Sources: (Mashhour, 2020; Kiyota, 2022; Noronha, 2018; Nanda, 2019; Nair, 2017; Tsan, 2012) |
Disease-free survival (important outcome)
|
Very low GRADE |
The evidence is very uncertain about the effect of weekly cisplatin concurrent with definitive radiotherapy on disease-free survival when compared with three-weekly cisplatin concurrent with definitive radiotherapy in patients with locally advanced head and neck squamous cell carcinoma.
Sources: (Noronha, 2018; Kiyota, 2022; Nanda, 2019; Nair, 2017) |
Adverse events (important outcome)
|
Very low GRADE |
The evidence is very uncertain about the effect of weekly cisplatin concurrent with definitive radiotherapy on adverse events when compared with three-weekly cisplatin concurrent with definitive radiotherapy in patients with locally advanced head and neck squamous cell carcinoma.
Sources: (Mashhour, 2020; Sahoo, 2017; Noronha, 2018; Rawat, 2016; Kiyota, 2022; Nanda, 2019; Nair, 2017; Tsan, 2012) |
Quality of life (important outcome)
|
Very low GRADE |
The evidence is very uncertain about the effect of weekly cisplatin concurrent with definitive radiotherapy on quality of life when compared with three-weekly cisplatin concurrent with definitive radiotherapy in patients with locally advanced head and neck squamous cell carcinoma.
Sources: (Tsan, 2012) |
Samenvatting literatuur
Description of studies
Eight randomized controlled trials were included. Different doses of cisplatin (30, 35, or 40 mg/m2) were used in the weekly treatment arms. Studies are grouped according to the dose provided. This clinical question is focused on patients treated with definitive chemoradiation. Four studies (also) included patients who received cisplatin in adjuvant setting (Mashhour, 2020; Noronha, 2018; Kiyota, 2022; Tsan, 2012). Most studies included patients with a carcinoma of the oral cavity, oropharynx, hypopharynx or larynx, however the study of Nanda (2019) only included patients with an oropharyngeal carcinoma and Tsan (2012) only included patients with an oral cavity carcinoma. One study was performed in Egypt, the other seven studies were conducted in Asia.
30 mg/m2
Mashhour (2020) conducted a randomized controlled trial in Egypt. Patients with a locally advanced head and neck squamous cell carcinoma, aged between 18 to 70 years, with an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2 were eligible. Patients were treated with adjuvant (52%) or definitive (48%) intent. Patients were randomly assigned (1:1) to receive either cisplatin at a planned dose of 30 mg/m2 weekly (n=30) or at a planned dose of 100 mg/m2 every three weeks (on days 1, 22, and 43) (n=30). Both groups received cisplatin concurrently with intensity modulated radiation therapy. Radiotherapy was given in a total dose of 70Gy in 33 fractions, delivered five days a week. Treatment compliance in terms of completing all planned cycles was higher in the weekly treatment group, where 70% of patients received at least six cycles of weekly chemotherapy with minor dose reductions because of toxicity. In the group receiving cisplatin every three weeks, 60% of patients completed three cycles of treatment and 40% received only two cycles. However, the median cumulative cisplatin dose was lower in the weekly treatment group (170 mg/m2 versus 200 mg/m2). In the weekly treatment group, 46% of patients received at least 200 mg/m2, while in the three-weekly treatment group 75% received at least 200 mg/2. Outcome measures included tumour response, locoregional control, and treatment toxicities. Toxicity was assessed using the Common Terminology Criteria for Adverse Events (CTCAE version 4.03).
Sahoo (2017) conducted a randomized controlled trial at a regional cancer centre in India. Patients with advanced stage squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx and larynx, aged between 18 and 70 years, with an ECOG performance status ≤ 2 were eligible. All patients received treatment with definitive intent. Patients were randomly assigned (1:1) to receive either cisplatin at a planned dose of 30 mg/m2 weekly (n=15) or at a planned dose of 100 mg/m2 every three weeks (on days 1, 22, and 43) (n=15). External beam radiotherapy was delivered to a dose of 66 Gy in a conventional fractionation schedule. Treatment compliance in terms of completing all planned cycles was 67% in the weekly treatment arm (six cycles) and 47% in the three-weekly treatment arm (three cycles). Completion of 66 Gy radiotherapy was 87% in the weekly treatment group and 80% in the three-weekly treatment group. Outcome measures included tumour response, locoregional control, and acute and late toxicity. Toxicities were assessed using the Radiation Therapy Oncology Group Acute Radiation Morbidity Criteria.
Noronha (2018) conducted a randomized controlled trial at an academic oncology hospital in India. Patients with locally advanced squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx or larynx or metastatic cervical lymphadenopathy of unknown primary, aged between 18 and 70 years, with an ECOG performance status ≤ 2 were eligible. Patients were treated with adjuvant (93%) or definitive (7%) intent. Patients were randomly assigned (1:1) to receive either cisplatin at a planned dose of 30 mg/m2 weekly (n=150) or at a planned dose of 100 mg/m2 every three weeks (on days 1, 22, and 43) (n=150). External beam radiotherapy in a conventional fractionation schedule was delivered to a dose of 70 Gy in 35 fractions for patients receiving definitive treatment, and a dose of 60 Gy for patients receiving adjuvant treatment. Treatment compliance in terms of completing the planned chemoradiation was 89% in the weekly treatment arm and 94% in the three-weekly treatment arm. The chemotherapy dose was reduced in 9% of patients in the weekly treatment arm and 8% of patients in the three-weekly treatment arm, while dosing was delayed for 25% in the weekly arm and 28% in the three-weekly arm. The median cumulative cisplatin dose was 210 mg/m2 in the weekly arm and 300 mg/m2 in the three-weekly arm. Outcome measures included overall survival, tumour response, locoregional control, progression-free survival and acute and chronic toxicity. Toxicities were assessed using the Common Terminology Criteria for Adverse Events (version 4.03).
35 mg/m2
Rawat (2016) conducted a randomized controlled trial at a single centre in India. Patients with locally advanced (stage III – IV B) squamous cell carcinoma of the head and neck, aged between 18 and 65 years were eligible. All patients received treatment with definitive intent. Patients were randomly assigned (1:1) to receive either cisplatin at a planned dose of 35 mg/m2 weekly (n=30) or at a planned dose of 100 mg/m2 every three weeks (on days 1, 22, and 43) (n=30). Radiotherapy was given in a total dose of 70 Gy in 35 fractions. Treatment compliance in terms of completing all planned chemotherapy cycles was 90% in the weekly arm and 79% in the three-weekly arm. Mean cisplatin dose received was lower in the weekly arm as compared with the three-weekly arm (292 mg/m2 versus 438 mg/m2).
The mean dose of radiotherapy received was comparable between the arms (69.86 Gy versus 69.22 Gy). Radiotherapy had to be interrupted for 17% of patients in the weekly arm and 34% of patients in the three-weekly arm. Outcome measures included tumour response and toxicity. Tumour response was evaluated according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1. Toxicity was assessed using the CTCAE version 4.03.
40 mg/m2
Kiyota (2022) conducted a randomized controlled non-inferiority trial in 28 centres in Japan. Patients with postoperative high-risk locally advanced squamous cell carcinoma of the head and neck, aged between 20 and 75 years, with an ECOG performance score of 0 or 1 were eligible. All patients received treatment with adjuvant intent. Patients were randomly assigned (1:1) to receive either cisplatin at a planned dose of 40 mg/m2 weekly (n=129) or at a planned dose of 100 mg/m2 every three weeks (n=132). Radiotherapy was given in a total dose of 66 Gy in 33 fractions. The median number of chemotherapy cycles received was 6 (IQR 5 to 7) in the weekly arm and 3 (IQR 3 to 3) in the three-weekly arm. The median cumulative cisplatin dose was lower in the weekly treatment group (239 mg/m2 [IQR 199 to 277] versus 280 mg/m2 [IQR 250 to 299]). The median total radiotherapy dose was 66 Gy in both groups (IQR 66 to 66). Outcome measures included overall survival, relapse-free survival, local relapse-free survival, and adverse events. Toxicity was assessed using the CTACE version 4.0.
Nanda (2019) conducted a randomized controlled trial at a single centre in India. Patients with locally advanced oropharyngeal carcinoma, aged between 20 and 70 years, with a Karnofsky performance score > 70 were eligible. All patients received treatment with definitive intent. Patients were randomly assigned (1:1) to receive either cisplatin at a planned dose of 40 mg/m2 weekly (n=39) or at a planned dose of 100 mg/m2 every three weeks (n=31). Radiotherapy was given in a total dose of 70 Gy in 35 fractions. The median number of chemotherapy cycles received was five in the weekly arm and two in the three-weekly arm. The median cumulative cisplatin dose was lower in the weekly treatment group (272 mg/m2 versus 303 mg/m2). Fewer patients in the weekly treatment group as compared with the three-weekly group received at least 200 mg/m2 cisplatin (89% versus 97%). In the weekly arm, 54% of patients discontinued chemotherapy beyond four cycles, mostly because of toxicity. All patients received the planned radiation dose of 70 Gy. Outcome measures included overall survival, tumour response, locoregional control, disease-free survival, and toxicities. Tumour response was evaluated according to the WHO criteria. Toxicity was assessed using the Radiation Therapy Oncology Group criteria for radiotherapy-induced acute toxicities, and Common Toxicity Criteria for chemotherapy-induced toxicity.
Nair (2017) conducted a randomized controlled trial at a regional cancer centre in India. Patients with locally advanced squamous cell carcinoma of the oropharynx, hypopharynx or larynx, aged between 18 and 70 years, with an ECOG performance status 0 or 1 were eligible. All patients received treatment with definitive intent. Patients were randomly assigned (1:1) to receive either cisplatin at a planned dose of 40 mg/m2 weekly (n=25) or at a planned dose of 100 mg/m2 every three weeks (n=31). Radiotherapy was given in a total dose of 66 Gy in 33 fractions. Treatment compliance in terms of completing all planned chemotherapy cycles was 63% in the weekly treatment group (six cycles) and 35% in the three-weekly treatment group (three cycles). The mean cumulative cisplatin dose was slightly lower in the weekly treatment group (339 mg/m2 versus 357 mg/m2). All patients completed radiation apart from one patient who died during treatment. Outcome measures included overall survival, locoregional control, tumour response, disease-free survival, and toxicities. Tumour response was evaluated using RECIST criteria. Toxicity was assessed using the Radiation Therapy Oncology Group criteria for radiotherapy-induced toxicities, and Common Terminology Criteria version 4 for chemotherapy-induced toxicity.
Tsan (2012) conducted a randomized controlled trial at a single centre in Taiwan. Patients with high-risk oral cavity squamous cell carcinoma, aged between 18-70 years, with an ECOG performance status 0 to 2 were eligible. All patients received treatment with adjuvant intent. The trial aimed to recruit 371 patients but the trial was stopped after recruiting only 55 patients (of which 50 were randomized) over 30 months. Patients were randomly assigned (1:1) to receive either cisplatin at a planned dose of 40 mg/m2 weekly (n=24) or at a planned dose of 100 mg/m2 every three weeks (n=26). Radiotherapy was given in a total dose of 66 Gy in 33 fractions. The mean cumulative doses of cisplatin and radiotherapy were comparable between the groups. However, fewer patients in the weekly treatment group received at least 200 mg/m2 cisplatin (63% versus 89%). Outcome measures included (preliminary) overall survival, (preliminary) locoregional recurrence-free survival, quality of life (Chinese version of the Functional Assessment of Cancer Therapy - Head and Neck (FACT-H&N) questionnaire) and adverse events. Toxicity was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Results
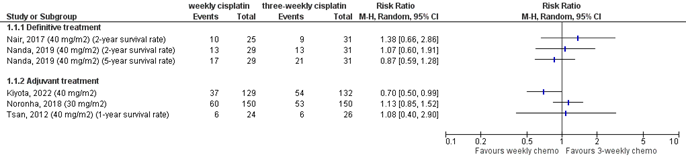
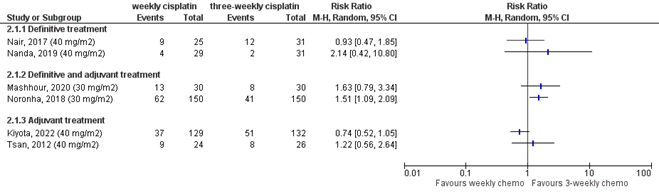
No meta-analysis was performed because of clinical and methodological heterogeneity, but the results for overall survival and locoregional recurrence are shown in Figures 13.9.1 and 13.9.2 to provide more insight in the effects found.
30 mg/m2
Overall survival
Noronha (2018) observed 60 deaths in the weekly treatment arm (40%) and 53 deaths in the three-weekly treatment arm (35%). Median overall survival was 39.5 months in the weekly treatment arm, while median overall survival was not reached in the three-weekly treatment arm (HR 1.14 (95%CI 0.79 to 1.65; p=0.48).
Recurrence (tumour response)
Mashhour reported tumour response, a complete response was seen in 77% of patients receiving weekly cisplatin and 76% of patients receiving three-weekly cisplatin. A partial response was seen in 13.2% of patients receiving weekly cisplatin and 12.6% of patients receiving three-weekly cisplatin. Two months after treatment, stable disease was observed in 4.6% of patients in the weekly treatment group and 4.1% of patients in the three-weekly treatment group.
Sahoo (2017) reported that after a median follow-up of seven months, complete response was achieved by 73% of patients in the weekly arm and 86% of patients in the three-weekly arm (not statistically significant).
Recurrence (locoregional control)
Mashhour (2020) reported that after a median follow-up of 24 months, locoregional control rates were 57.6% in the weekly treatment group and 72.8% in the three-weekly treatment group (HR 1.78; p=0.015).
Noronha (2018) reported that the 2-year locoregional control rate was 58.5% in the study arm receiving weekly cisplatin and 73.1% in the group receiving three-weekly cisplatin (HR 1.76 (95%CI 1.11 to 2.79; p=0.014).
Disease-free survival
In the trial by Noronha (2018), the estimated median progression-free survival was 17.7 months (95%CI 0.42 to 35.05) in the weekly treatment arm and 28.6 months (95%CI 15.9 to 41.3) in the three-weekly treatment arm (HR 1.24 (95%CI 0.89 to 1.73); p=0.21).
Quality of life
Quality of life was not reported in any of the RCTs using a dose of 30 mg/m2.
Adverse events
Mashhour (2020) reported non-haematological and haematological adverse events. For non-haematological adverse events, acute toxicities grade ≥ 3 were observed less frequently in the weekly treatment group (56.6%) compared with the three-weekly treatment group (76.6%) (p=0.007). No statistically significant differences were found for individual grade ≥ 3 toxicities, including mucositis (53% in the weekly cisplatin group versus 47% in the three-weekly cisplatin group), dysphagia (47% versus 67%) , nausea/vomiting (13% versus 20%), xerostomia (17% versus 20%), dermatitis (13% versus 10%), and laryngeal oedema (17% versus 17%).
For haematological adverse events, grade ≥ 3 leukopenia (20% versus 37%; p<0.05) and neutropenia (10% versus 20%; p<0.05) occurred less frequently in the weekly cisplatin group compared with the three-weekly cisplatin group. No differences were found between the frequency of grade ≥ 3 anemia (17% versus 30%) and thrombocytopenia (3% versus 10%) in the weekly and three-weekly treatment groups.
Sahoo (2017) reported that grade 3 mucositis and vomiting were less frequent in the weekly cisplatin arm compared with the three-weekly cisplatin arm (53% versus 40%; p=0.729) and (20% versus 7%; p=0.360). In contrast, grade 3 dermatitis was more frequent in the weekly arm compared with the three-weekly arm (27% versus 7%; p=0.360). The frequencies of grade 3 dysphagia, anemia, and leukopenia were (almost) similar between the arms (0% versus 7%, 7% versus 7%, 13% versus 7%). The frequency of late toxicities (xerostomia and skin fibrosis) was comparable between the study arms.
Noronha (2018) reported acute (within 90 days from the start of treatment) and chronic (more than 90 days from the start of treatment) toxicities. For acute toxicities, any acute toxicity grade ≥ 3 was observed in 72% of patients receiving weekly cisplatin and 85% of patients receiving three-weekly cisplatin (p=0.006). Toxicities that occurred less frequently in the weekly treatment group included vomiting (1% versus 7%; p=0.019), infection (21% versus 34%; p=0.015), deafness (5% versus 13%; p=0.013), hyponatremia (23% versus 52%; p<0.001), leukopenia (3% versus16%; p<0.001), neutropenia (1% versus 13%; p<0.001), febrile neutropenia (1% versus 6%; p=0.019), and lymphocytopenia (72% versus 89%; p=0.001). No differences between the weekly and three-weekly treatment group were observed in the frequency of mucositis, dysphagia, odynophagia, xerostomia, dysgeusia, dermatitis, diarrhoea, fatigue, weight loss, hoarseness, hypertension, hypokalemia, transaminase elevation, anemia and thrombocytopenia. There were no patients experiencing ≥ 3 neuropathy or renal dysfunction.
For chronic toxicities, any chronic toxicity grade ≥ 3 was observed in 10% of patients receiving weekly cisplatin and 14% of patients receiving three-weekly cisplatin (p=0.55). The only toxicity that occurred less frequency in the weekly treatment group was deafness (4% versus 16%; p=0.004). No differences between the weekly and three-weekly treatment group were observed in the frequency of mucositis, dysphagia, odynophagia, infection, xerostomia, subcutaneous, trismus, and hypertriglyceridemia. There were no patients experiencing ≥ 3 dysgeusia, skin toxicity, hypothyroidism, or thromboembolic events.
35 mg/m2
Overall survival
Rawat (2016) did not report on overall survival.
Recurrence (tumour response)
Rawat (2016) reported that three months after treatment completion, complete response was 67% in the group receiving weekly cisplatin and 62% in the group receiving three-weekly cisplatin. Partial responses were received in 33% of patients receiving weekly treatment and 38% of patients receiving three-weekly treatment. No statistically significant differences were found between the arms (p=0.20).
Recurrence (locoregional control)
Rawat (2016) did not report on locoregional control.
Disease-free survival
Rawat (2016) did not report on disease-free survival.
Quality of life
Rawat (2016) did not report on quality of life.
Adverse events
For non-haematological toxicities, Rawat (2016) reported that the frequency of grade 3 mucositis (70% versus 76%; p=0.20) was similar between the groups, while the frequency of grade 3 vomiting was lower in the group receiving weekly treatment (20% versus 35%; p=0.03). For haematological toxicities, no differences were found for grade 3 anemia (33% versus 31%; p=0.22) and thrombocytopenia (7% versus10%; p=0.32), while grade 3 neutropenia was less frequent in the group receiving weekly treatment (27% versus 55%; p=0.02). Rawat (2016) also reported on a number of other toxicities. For acute renal toxicity, only mild toxicity was observed, while for significant weight loss, hyponatremia and hypomagnesemia it was not clear whether the frequencies involved grade 3 toxicity.
40 mg/m2
Overall survival
Kiyota (2022) reported estimated 2-year and 3-year survival rates of 77.7% and 71.6% in the weekly treatment arm and 74.5% and 59.1% in the three-weekly treatment arm. The hazard ratio was 0.69 (99.1%CI 0.37 to 1.27; one-sided p-value for non-inferiority=0.0027). Since the upper limit of the confidence interval was below the prespecified threshold of 1.32, the authors concluded that weekly treatment is non-inferior with regard to survival.
Nanda (2020) reported that median overall survival was 35.4 months in the weekly treatment group and 32.9 months in the three-weekly treatment group (p=0.303). The two-year and five-year survival rates were 55% and 42% in the weekly treatment group and 58% and 32% in the three-weekly treatment group (not statistically significant, no p-value provided).
Nair (2017) reported two-year survival rates of 61% in the weekly treatment arm and 71% in the three-weekly treatment arm (p=0.610).
Tsan (2012) reported preliminary overall survival after a median follow-up of 12 months. In each group, six patients had died. One-year overall survival rates were 72% in the weekly treatment group and 79% in the three-weekly treatment group (p=0.978).
Recurrence (tumour response)
Nanda (2020) reported that complete response was seen in 81% of patients receiving weekly treatment and 75% of patients receiving three-weekly treatment. Partial responses were seen in 14% of patients receiving weekly treatment and 13% of patients receiving three-weekly treatment. Eight weeks after completion of treatment, stable disease was 5% in the weekly treatment group and 4% in the three-weekly treatment group.
Nair (2017) reported that complete responses were observed in 75% of patients in the weekly treatment arm and 90% of patients in the three-weekly arm. Partial response rates were 12% and 6%. Twelve weeks after completion of treatment, two patients in each arm (8% versus 6%) had residual disease.
Recurrence (locoregional control)
Kiyota (2022) reported recurrences in 29% of patients receiving weekly treatment and 39% of patients received three-weekly treatment.
Nanda (2020) observed locoregional relapses in 14% of patients receiving weekly treatment and 6% of patients receiving three-weekly treatment. Three months after completion of treatment, stable or progressive disease was observed in 29% of patients in the weekly treatment group and 42% of patients in the three-weekly treatment group.
Nair (2017) reported that two patients in the weekly treatment group developed local recurrence and one patient developed lung metastasis (13%), while four patients in the three-weekly treatment group developed local recurrence (13%). In the weekly treatment group, three patients developed a second primary tumour ( in the esophagus or tongue) (13%), while in the three-weekly group two patients developed a second primary tumour in the esophagus (6%). Two-year locoregional control rates were 63% in the weekly cisplatin arm and 61% in the three-weekly cisplatin arm.
Tsan (2012) reported preliminary locoregional recurrence-free survival after a median follow-up of 12 months. In the weekly arm, 9 patients had experienced a recurrence while in the three-weekly arm, 8 patients had experienced a recurrence. One-year locoregional recurrence-free survival rates were 60% in the weekly treatment group and 71% in the three-weekly treatment group (p=0.806).
Disease-free survival
Kiyota (2022) reported hazard ratios of 0.71 (95%CI 0.48 to 1.06) for relapse-free survival and 0.73 (95%CI 0.47 to 1.13) for local relapse-free survival.
Nanda (2020) reported that median progression-free survival was 26.4 months in the weekly treatment group and 27.4 months in the three-weekly treatment group (p=0.953).
Nair (2017) reported that two-year disease-free survival rates were 53% in the weekly arm and 65% in the three-weekly arm (p=0.674).
Adverse events
Kiyota (2022) reported no difference in the proportion of patients experiencing at least one grade ≥ 3 event (81.1% versus 79.8%; p=0.87), while fewer patients in the weekly group experienced a grade 4 event (8.2% versus 18.6%; p=0.017). For haematological adverse events, there were no differences in the frequency of grade ≥ 3 events (64.8% versus 61.2%; p=0.06) and grade 4 events (7.4% versus 14.7%; p=0.07). Specific grade ≥ 3 adverse events that were reported to be lower in the weekly treatment group included neutropenia (35% versus 49%) and infection (7% versus 12%).
Nanda (2020) observed no statistically significant differences in the frequency of grade ≥ 3 radiation toxicities and haematological toxicities between the two groups. Radiation toxicities included mucositis (32% versus 29%; p=1.00), dysphagia (46% versus 32%; p=0.27), dermatitis (14% versus 19%; p=0.73), larynx (11% versus 10%; p=1.00), and nausea/vomiting (7% versus 0%; p=0.22). Haematological toxicities included anemia (0% versus 3%; p=1.00), leukopenia (25% versus 13%; p=0.32), neutropenia (18% versus 7%; p=0.24), and thrombocytopenia (0% versus 3%; p=1.00).
Nair (2017) reported a lower frequency of grade ≥ 3 dysphagia (63% versus 26%; p<0.05) in the weekly cisplatin group. No other statistically significant differences were reported in the frequency of grade ≥ 3 non-haematological and haematological toxicities between the two groups. Non-haematological toxicities included mucositis (54% versus 52%; p>0.05), and dermatitis (13% versus3%; p>0.05). Haematological toxicities included anemia (4% versus 0%; p>0.05), neutropenia (8% versus 3%; p>0.05), and thrombocytopenia (no grade 3 adverse events observed). No grade ≥ 3 renal toxicity was observed.
Tsan (2012) reported that overall, more grade ≥ 3 toxicities were observed in the weekly group (92%) compared with the three-weekly group (81%) (p=0.02). For non-haematological toxicities, mucositis was reported more frequency in the weekly treatment group (75%) compared with the three-weekly group (39%) (p=0.012). The frequencies of the following toxicities were comparable between the groups: pharyngitis (54% versus 54%; p=1.0), stomatitis (54% versus 54%; p=1.0), laryngeal edema (4% versus 12%; p=0.611), dermatitis (8% versus 8%; p=1.0), and nausea/vomiting (21% versus 12%; p=0.456).
For haematological toxicities, no differences were observed between the groups for anemia (4% versus 4%; p=1.0), leukopenia (13% versus 0%; p=0.103), neutropenia (4% versus 0%; p=0.480), and thrombocytopenia (0% versus 0%).
Quality of life
Tsan (2012) reported results for five subscales of the FACT H&N questionnaire and the Trial Outcome Index (TOI) which is a combined scale for the subscales physical well-being, functional well-being and the head and neck subscale. Higher scores represent better QoL. It was not reported how many patients completed the questionnaires at each time point.
For the physical well-being scale (range 0 to 28), lower scores were seen in the weekly treatment group at week 2 (difference of 4.5 points between the groups), week 4 (5.7 points), at the end of radiotherapy (7.8 points) and follow-up after three months (4.3 points). For the social well-being scale (range 0 to 28), higher scores were seen in the weekly treatment group at week 4 (difference of 2.9 points between the groups), at the end of radiotherapy (5.7 points), and follow-up after three months (5 points). Emotional well-being scores were comparable between the groups at all time points. Functional well-being scores (range 0 to 28) were only different between the groups at three months follow-up, with lower scores seen in the weekly treatment group (3.3 points difference between the groups). Scores on the head and neck subscale were comparable between the groups at all time points. TOI scores (range 0 to 96) were only different between the groups at three months follow-up, with lower scores seen in the weekly treatment group (difference 9.7 points).
Level of evidence of the literature
All studies were RCTs, therefore the level of evidence started at ‘high’ for all outcome measures.
The level of evidence was downgraded for all outcomes because most (7/8) studies were conducted in Asia (India, Taiwan, and Japan), while it has been described that head and neck cancers in Asian countries have a different etiology and molecular biology. Publication bias was not assessed because of the low number of studies found.
The level of evidence regarding the outcome measure overall survival was downgraded by four levels because of study limitations (-1; risk of bias because of incomplete reporting of study methodology and one trial was stopped prematurely); applicability (-1; bias due to indirectness because three studies were conducted among patients who were treated (mainly) with adjuvant intent and all studies were conducted in Asia); and number of included patients (-2; imprecision because of wide confidence intervals including the possibility of a negative effect, no effect, and a positive effect). Publication bias was not assessed.
The level of evidence regarding the outcome measure recurrence (tumour response) was downgraded by five levels because of study limitations (-1; risk of bias because of incomplete reporting of study methodology); conflicting results (-1; inconsistency because two studies showed worse tumour response rates, two studies showed no effect and one study showed better tumour response rates); applicability (-1; bias due to indirectness because in one study 52% of patients were treated with adjuvant intent and most studies were conducted in Asia); and number of included patients (-2; imprecision because of the low sample sizes). Publication bias was not assessed.
The level of evidence regarding the outcome measure recurrence (locoregional control) was downgraded by five levels because of study limitations (-1; risk of bias because of incomplete reporting of study methodology and one trial was stopped prematurely); conflicting results (-1; inconsistency because three studies showed worse locoregional control, two studies showed no effect, and one study showed better locoregional control); applicability (-1; bias due to indirectness because four studies were conducted among patients who were treated (mainly) with adjuvant intent and most studies were conducted in Asia); and number of included patients (-2; imprecision because of wide confidence intervals including the possibility of a negative effect, no effect, and a positive effect). Publication bias was not assessed.
The level of evidence regarding the outcome measure disease-free survival was downgraded by three levels because of conflicting results (-1; inconsistency because one study showed worse DFS and two showed no difference); applicability (-1; bias due to indirectness because two studies were conducted among patients who were treated (mainly) with adjuvant intent and all studies were conducted in Asia); and number of included patients (-1; imprecision because of confidence intervals including the possibility of a negative effect and no effect (and a positive effect)). Publication bias was not assessed.
The level of evidence regarding the outcome measure adverse events was downgraded by three levels because of study limitations (-1; risk of bias because of incomplete reporting of study methodology and one study was stopped prematurely); applicability (-1; bias due to indirectness because four studies were conducted among patients who were treated (mainly) with adjuvant intent and most studies were conducted in Asia); and number of included patients (-1; imprecision because of the low number of patients included in individual studies). Publication bias was not assessed.
The level of evidence regarding the outcome measure quality of life was downgraded by five levels because of study limitations (-2 risk of bias because of incomplete reporting of study methodology, lack of blinding, and study stopped prematurely); applicability (-1; bias due to indirectness because the study was conducted among patients who were treated with adjuvant intent and was conducted in Asia); number of included patients (-2; imprecision because of the low sample size in a single study). Publication bias was not assessed.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the benefits and risks of weekly versus three-weekly cisplatin concurrent with definitive radiotherapy for patients with locally advanced head and neck squamous cell carcinoma?
P: Patients with locally advanced head and neck squamous cell carcinoma.
I: Weekly cisplatin concurrent with definitive radiotherapy.
C: Three-weekly cisplatin concurrent with definitive radiotherapy.
O: Overall survival, recurrence (tumour response and locoregional control), disease-free survival, quality of life, adverse events.
Relevant outcome measures
The guideline development group considered overall survival and recurrence as critical outcome measures for decision making; and disease-free survival, adverse events, and quality of life as important outcome measures for decision making.
A priori, the working group did not define the outcome measures listed above but used the definitions used in the studies.
The working group defined a minimal clinically important difference as follows:
- Overall survival: absolute difference > 5%, or absolute difference > 3% and hazard ratio (HR) < 0.7.
- Tumour response: absolute difference > 5% in complete response rates
- Local recurrence: 0.8 or 1.25 as borders for risk or odds ratios.
- Locoregional control: absolute difference > 5%, or absolute difference > 3% and HR < 0.7.
- Disease-free survival: absolute difference > 5%, or absolute difference > 3% and HR < 0.7.
- Progression-free survival: absolute difference > 5%, or absolute difference > 3% and HR < 0.7.
- Quality of life: absolute difference ≥ 10 points on the EORTC QLQ-C30 or a difference of a similar magnitude on other disease-specific quality of life questionnaires.
- Adverse events: statistically significant difference in grade ≥ 3 adverse event rate.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 12 November 2020. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 534 hits (60 SRs and 474 RCTs). Studies were selected based on the following criteria: (1) patients with a locally advanced squamous cell carcinoma in the head and neck region; (2) comparison between radiotherapy combined with weekly or three-weekly cisplatin; (3) systematic review or randomized controlled trial; (4) full-text English language publication. Studies including only patients with nasopharyngeal cancer were excluded.
24 studies were initially selected based on title and abstract screening. After reading the full text, 17 studies were excluded (see the table with reasons for exclusion under the tab Methods) and seven studies were included. The working group identified an additional RCT that was published after the search date. This RCT was also included in the summary of literature. We cannot exclude the possibility that other relevant reviews or RCTs were published after the search date.
Results
Eight original studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Kiyota N, Tahara M, Mizusawa J, Kodaira T, Fujii H, Yamazaki T, Mitani H, Iwae S, Fujimoto Y, Onozawa Y, Hanai N, Ogawa T, Hara H, Monden N, Shimura E, Minami S, Fujii T, Tanaka K, Homma A, Yoshimoto S, Oridate N, Omori K, Ueda T, Okami K, Ota I, Shiga K, Sugasawa M, Asakage T, Saito Y, Murono S, Nishimura Y, Nakamura K, Hayashi R; Head and Neck Cancer Study Group of the Japan Clinical Oncology Group (JCOG-HNCSG). Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J Clin Oncol. 2022 Mar 1:JCO2101293. doi: 10.1200/JCO.21.01293. Epub ahead of print. PMID: 35230884.
- Lacas B, Carmel A, Landais C, Wong SJ, Licitra L, Tobias JS, Burtness B, Ghi MG, Cohen EEW, Grau C, Wolf G, Hitt R, Corvò R, Budach V, Kumar S, Laskar SG, Mazeron JJ, Zhong LP, Dobrowsky W, Ghadjar P, Fallai C, Zakotnik B, Sharma A, Bensadoun RJ, Ruo Redda MG, Racadot S, Fountzilas G, Brizel D, Rovea P, Argiris A, Nagy ZT, Lee JW, Fortpied C, Harris J, Bourhis J, Aupérin A, Blanchard P, Pignon JP; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother Oncol. 2021 Mar;156:281-293. doi: 10.1016/j.radonc.2021.01.013. Epub 2021 Jan 27. PMID: 33515668; PMCID: PMC8386522.
- Mashhour K, Hashem W. Cisplatin Weekly Versus Every 3 Weeks Concurrently with Radiotherapy in the Treatment of Locally Advanced Head and Neck Squamous Cell Carcinomas: What Is the Best Dosing and Schedule? Asian Pac J Cancer Prev. 2020 Mar 1;21(3):799-807. doi: 10.31557/APJCP.2020.21.3.799. PMID: 32212810; PMCID: PMC7437345.
- Nair LM, Kumar RR, Thomachan KC, Rafi M, George PS, Krishna KMJ, Ramadas K. Phase IIb trial comparing two concurrent cisplatin schedules in locally advanced head and neck cancer. South Asian J Cancer. 2017 Apr-Jun;6(2):64-68. doi: 10.4103/2278-330X.208840. PMID: 28702409; PMCID: PMC5506812.
- Nanda R, Katke A, Suneetha N, Thejaswini B, Pasha T, Jagannath KP, Giri GV, Babu KG. A prospective randomized study comparing concurrent chemoradiation with weekly and 3 weekly cisplatin in locally advanced oropharyngeal carcinoma. South Asian J Cancer. 2019 Jul-Sep;8(3):178-182. doi: 10.4103/sajc.sajc_270_18. PMID: 31489293; PMCID: PMC6699241.
- Noronha V, Joshi A, Patil VM, Agarwal J, Ghosh-Laskar S, Budrukkar A, Murthy V, Gupta T, D'Cruz AK, Banavali S, Pai PS, Chaturvedi P, Chaukar D, Pande N, Chandrasekharan A, Talreja V, Vallathol DH, Mathrudev V, Manjrekar A, Maske K, Bhelekar AS, Nawale K, Kannan S, Gota V, Bhattacharjee A, Kane S, Juvekar SL, Prabhash K. Once-a-Week Versus Once-Every-3-Weeks Cisplatin Chemoradiation for Locally Advanced Head and Neck Cancer: A Phase III Randomized Noninferiority Trial. J Clin Oncol. 2018 Apr 10;36(11):1064-1072. doi: 10.1200/JCO.2017.74.9457. Epub 2017 Dec 8. PMID: 29220295.
- Rawat S, Srivastava H, Ahlawat P, Pal M, Gupta G, Chauhan D, Tandon S, Khurana R. Weekly versus Three-Weekly Cisplatin-based Concurrent Chemoradiotherapy as definitive treatment in Head and Neck Cancer- Where do we stand? Gulf J Oncolog. 2016 May;1(21):6-11. PMID: 27250881.
- Sahoo TK, Samanta DR, Senapati SN, Parida K. A Comparative Study on Weekly Versus Three Weekly Cisplatinum Based Chemoradiation in Locally Advanced Head and Neck Cancers. J Clin Diagn Res. 2017 Jan;11(1):XC07-XC11. doi: 10.7860/JCDR/2017/24765.9293. Epub 2017 Jan 1. PMID: 28274031; PMCID: PMC5324476.
- Tsan DL, Lin CY, Kang CJ, Huang SF, Fan KH, Liao CT, Chen IH, Lee LY, Wang HM, Chang JT. The comparison between weekly and three-weekly cisplatin delivered concurrently with radiotherapy for patients with postoperative high-risk squamous cell carcinoma of the oral cavity. Radiat Oncol. 2012 Dec 18;7:215. doi: 10.1186/1748-717X-7-215. PMID: 23245290; PMCID: PMC3564896.
- Espeli V, Zucca E, Ghielmini M, Giannini O, Salatino A, Martucci F, Richetti A. Weekly and 3-weekly cisplatin concurrent with intensity-modulated radiotherapy in locally advanced head and neck squamous cell cancer. Oral Oncol. 2012 Mar;48(3):266-71. doi: 10.1016/j.oraloncology.2011.10.005. Epub 2011 Nov 11. PMID: 22079100.
- Driessen CM, Janssens GO, van der Graaf WT, Takes RP, Merkx TA, Melchers WJ, Kaanders HA, van Herpen CM. Toxicity and efficacy of accelerated radiotherapy with concurrent weekly cisplatin for locally advanced head and neck carcinoma. Head Neck. 2016 Apr;38 Suppl 1:E559-65. doi: 10.1002/hed.24039. Epub 2015 Jul 6. PMID: 25810154.
- Ho KF, Swindell R, Brammer CV. Dose intensity comparison between weekly and 3-weekly Cisplatin delivered concurrently with radical radiotherapy for head and neck cancer: a retrospective comparison from New Cross Hospital, Wolverhampton, UK. Acta Oncol. 2008;47(8):1513-8. doi: 10.1080/02841860701846160. PMID: 18607863.
- Bril SI, Al-Mamgani A, Chargi N, Remeijer P, Devriese LA, de Boer JP, de Bree R. The association of pretreatment low skeletal muscle mass with chemotherapy dose-limiting toxicity in patients with head and neck cancer undergoing primary chemoradiotherapy with high-dose cisplatin. Head Neck. 2022 Jan;44(1):189-200. doi: 10.1002/hed.26919. Epub 2021 Oct 29. PMID: 34713519.
- Wendrich AW, Swartz JE, Bril SI, Wegner I, de Graeff A, Smid EJ, de Bree R, Pothen AJ. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017 Aug;71:26-33. doi: 10.1016/j.oraloncology.2017.05.012. Epub 2017 Jun 5. PMID: 28688687.
Evidence tabellen
Evidence table
Research question: What are the benefits and risks of weekly versus three-weekly cisplatin concurrent with definitive radiotherapy for patients with locally advanced head and neck squamous cell carcinoma?
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3 |
Follow-up |
Outcome measures and effect size 4 |
Comments |
|||||||
|
30 mg/m2 |
||||||||||||||
|
Mashhour, 2020 |
Type of study: Randomized controlled trial
Setting and country: Egypt
Funding and conflicts of interest: The authors reported there was no funding for the trial. No conflicts of interest were reported. |
Patients with locally advanced head and neck squamous cell carcinomas
Intent of treatment Adjuvant I: 53% / C: 50% Definitive I: 47% / C: 50%
Inclusion criteria: - patients with locally advanced head and neck cancers (stages 3 and 4) - histo-pathologically confirmed squamous or undifferentiated carcinoma - age range from 18 to 70 years - Eastern Co-operative Oncology Group (ECOG) performance status (PS) from 0-2 - creatinine clearance> 60 ml/min
Exclusion criteria: - metastatic disease - neo-adjuvant chemotherapy - history of another malignant disease
N total at baseline: 60 (I: 30 / C: 30)
Important characteristics:
Age in both groups: median 61 years (range 56-65)
Male gender I: 73% / C: 80%
Primary tumour site Oral cavity I: 17% / C: 20% Oropharynx I: 20% / C: 17% Nasopharynx I: 13% / C: 23% Larynx I: 33% / 27% Hypopharynx I: 17% / 13%
Stage at diagnosis Stage III I: 33% / C: 30% Stage IVa I: 37% / C: 37% Stage IVb I: 30% / C: 33%
Groups were comparable at baseline |
30 mg/m2 cisplatin once a week (for 6-7 weeks) given concurrently with intensity modulated radiation therapy (IMRT)
Radiotherapy prescribed dose was 70Gy in 33 fractions, delivered 5 days a week.
Supportive treatment: Pre and post- treatment hydration, corticosteroids, antiemetics, intravenous Mannitol 20% and supportive treatment was administrated for each patient. Potassium chloride and magnesium sulfate infused over 60 minutes each was necessary with each infusion. |
100 mg/m2 cisplatin every three weeks (on days 1, 22 , and 43) given concurrently with intensity modulated radiation therapy (IMRT)
Radiotherapy prescribed dose was 70Gy in 33 fractions, delivered 5 days a week.
Supportive treatment: Pre and post- treatment hydration, corticosteroids, antiemetics, intravenous Mannitol 20% and supportive treatment was administrated for each patient. Potassium chloride and magnesium sulfate infused over 60 minutes each was necessary with each infusion. Cisplatin at a dose of 100mg/m2 was given intravenously in 1 litre 0.9% sodium chloride over 2 hours every 3 weeks at days1, 22 and 43. More vigorous hydration and anti-emetics were adjusted to the every 3 weeks protocol in view of being a higher nephrotoxic and ematogenic risk protocol. |
Length of follow-up: Median 24 months (range 15-37 months)
Loss-to-follow-up: none |
Overall survival Not reported
Recurrence
Complete response I: 77% / C: 76% Partial response I: 13.2% / 12.6% Stable disease 2 months after treatment I: 4.6% / 4.1%
2 year locoregional control rate I: 57.6% / C: 72.8% HR: 1.78, p=0.015
Disease-free survival Not reported
Quality of life Not reported
Adverse events (% grade ≥3)
Non-haematological
Mucositis I: 53% / C: 47% p>0.05
Dysphagia I: 47% / C: 67% p>0.05
Nausea/vomiting I: 13% / C: 20% p>0.05
Xerostomia I: 17% / C: 20% p>0.05
Dermatitis I: 13% / C: 10% p>0.05
Laryngeal oedema I: 17% / C: 17% p>0.05
Acute toxicity grade 3 or higher I: 57% / C: 77% p=0.007
Haematological
Anemia I: 17% / C: 30% p>0.05
Leukopenia I: 20% / C: 37% P<0.05
Neutropenia I: 10% / C: 20% P<0.05
Thrombocytopenia I: 3% / C: 10% p>0.05 |
Human papilloma virus (P16) testing was not done due to unavailability.
Evaluation of toxicity was based on the fourth version of Common Toxicity Criteria for Adverse Events (CTCAE v. 4.03)
Compliance to treatment 75% of the patients who received cisplatin high dose every 3 weeks had a higher cumulative cisplatin dose (at least 200 mg/m2) versus 46% of patients who received weekly low dose cisplatin with a statistically significant p-value of 0.003 (Figure 2).
|
|||||||
|
Sahoo, 2017 |
Type of study: randomized controlled trial
Setting and country: Regional Cancer Centre, India
Funding and conflicts of interest: The authors reported no competing interests |
Patients with locally advanced head and neck squamous cell carcinomas
Intent of treatment: 100% definitive in both groups
Inclusion criteria: - histo-pathologically proven advanced stage (T3-4, N0-3, M0) squamous cell carcinoma of oral cavity, oropharynx, hypopharynx and larynx - age more than 18 years and less than 70 years - normal haematological and biochemical parameters, - Karnofsky Performance score of 70 or above - no history of prior chemotherapy or radiotherapy
Exclusion criteria: - evidence of distant metastases by clinical or radiological examination - recurrent disease - prior radiotherapy or chemotherapy to head and neck, widely disseminated diseases - synchronous double primary malignancies - pregnant women - simultaneous participation in another clinical study
N total at baseline: 30 (I: 15 / C: 15)
Important characteristics:
Age: median 57.6 years (only reported for total of 30 patients)
Male gender: 90% (only reported for total of 30 patients)
Primary tumour site (only reported for total of 30 patients): Oropharynx 53% Hypopharynx 30% Larynx 13% Oral cavity 3%
Stage (only reported for total of 30 patients) Stage III 57% Stage IV 43%
Histology grading (only reported for total of 30 patients) Well differentiated 50% Moderately differentiated 43% Poorly differentiated 7%
It was not possible to assess whether the groups were comparable at baseline, because demographics were only reported for the total group and not per study arm |
30 mg/m2 cisplatin once a week (for 6 weeks) along with radiation
Radiotherapy was delivered to a total dose of 66 Gy in conventional fractionation schedule
|
100 mg/m2 cisplatin every three weeks (on days 1, 22 , and 43) along with radiation
Radiotherapy was delivered to a total dose of 66 Gy in conventional fractionation schedule
|
Length of follow-up: Median 7 months
Loss-to-follow-up: Intervention: N=0 (0%)
Control: N=1 (7%) Reasons: one patient did not come for follow up.
|
Overall survival Not reported
Recurrence
Complete response after a median follow up of seven months: I: 73% / C: 86% p>0.05
Disease-free survival Not reported
Quality of life Not reported
Adverse events (% grade ≥3)
Mucositis I: 40% / C: 60% p=0.729
Dermatitis I: 27% / C: 7% p=0.360
Dysphagia I: 0% / C: 7% p>0.05
Vomiting I: 7% / C: 20% p=0.360
Anaemia I: 7% / C: 7% p>0.05
Leukopenia I: 13% / C: 7% p>0.05
There was almost no difference in late toxicities in both the arms (xerostomia and skin fibrosis). No further details were provided. |
Compliance: A 66.67% (10/15) patients completed six cycles of CT in weekly cisplatin arm, whereas, only 46.67% (7/15) patients were able to complete three cycles of three weekly cisplatin in arm B. A total of 13 patients (86.67%) completed 66 Gy RT in weekly arm, whereas, 12 patients (80%) in three weekly arm completed 66 Gy RT.
Toxicities were assessed using the Radiation Therapy Oncology Group Acute Radiation Morbidity Criteria. |
|||||||
|
Noronha, 2018 |
Type of study: phase III randomized noninferiority trial
Setting and country: academic oncology hospital in India
Funding and conflicts of interest: The Tata Memorial Center Research Administration Council funded the study. No conflicts of interest were reported by the authors. |
Patients with locally advanced head and neck squamous cell cancer (mainly oral cavity)
Intent of treatment Adjuvant, for high-risk features I: 93% / C: 93% Definitive I: 7% / C: 7%
Inclusion criteria: HNSCC of the oral cavity, oropharynx, hypopharynx, or larynx, or metastatic cervical lymphadenopathy of unknown primary - disease had to be locally advanced (ie, stage III or IV), with no distant metastases - planned for curative CRT, either adjuvant for one or more high-risk features (extracapsular extension, close (ie,≤ 5mm), or positive margins, more than two lymph nodes positive, T4 primary) or for definitive CRT for unresectable disease or organ preservation - ≤70 years of age - Eastern Cooperative Oncology Group performance status ≤2 - calculated creatinine clearance ≥ 50mL/min - no moderate or severe sensorineural Deafness - adequate organ function - no induction chemotherapy
N total at baseline: 300 (I: 150 / C: 150)
Important characteristics:
Median age (range) I: 41.5 (26-65) C: 47 (25-67)
Male gender I: 90% / C: 88%
Primary tumour site Oral cavity I: 91% / C: 84% Oropharynx I: 1% / C: 2% Larynx I: 2% / 5% Hypopharynx I: 1% / 2% Cervical lymphadenopathy of unknown primary I: 5% / C: 7%
T-stage Tx or T0 I: 5% / C: 7% T1 I: 9% / C: 8% T2 I: 19% / C: 17% T3 I: 7% / C: 7% T4 I: 60% / C: 61%
N-stage N0 I: 3% / C: 7% N1 I: 27% / C: 21% N2 I: 69% / C: 70% N3 I: 1% / C: 1%
TNM stage Stage III I: 9% / C: 9% Stage IV I: 91% / 91%
Histologic differentiation Well differentiated I: 4% / C: 7% Moderately differentiated I: 53% / C: 59% Poorly differentiated I: 40% / C: 31% Unknown I: 3% / C: 3%
Groups were comparable at baseline |
30 mg/m2 cisplatin once a week (for 6 or 7 weeks) along with radiation
Patients received conventional external-beam radiotherapy. The total dose planned was 70 Gy in 35 fractions over 7 weeks for definitive CRT; 60 Gy was planned for high-risk areas (tumour bed and involved nodal regions) for adjuvant treatment.
Patients received routine hydration, antiemetics, corticosteroids, and other supportive medications.
|
100 mg/m2 cisplatin every three weeks (on days 1, 22, and 43) along with radiation
Patients received conventional external-beam radiotherapy. The total dose planned was 70 Gy in 35 fractions over 7 weeks for definitive CRT; 60 Gy was planned for high-risk areas (tumour bed and involved nodal regions) for adjuvant treatment.
Patients received routine hydration, antiemetics, corticosteroids, and other supportive medications. |
Length of follow-up: Median 22 months
Loss-to-follow-up: none
Incomplete outcome data: Intervention: N=2 (1%) Reasons: two patients were not included in the analysis of toxicity because they did not start treatment with cisplatin
Control: N =1 (%) Reasons: one patient was not included in the analysis of toxicity because the patient did not start treatment with cisplatin
|
Overall survival
Median overall survival I: 39.5 months C: not reached HR 1.14 (95%CI 0.79 to 1.65) P=0.48
Recurrence
Locoregional relapse I: 38% / C: 24%
2-year locoregional control rate I: 59% / C: 73% p=0.014; HR 1.76 (95%CI 1.11 to 2.79)
Disease-free survival
Disease progression I: 49% / C: 39%
Median progression-free survival I: 17.7 months (95%CI 0.42 to 35.1) C: 28.6 months (95%CI 15.9 to 41.3) HR 1.24 (95%CI, 0.89 to 1.73); P = 0.21.
Quality of life Not reported
Adverse events
Acute (first 90 days from the start of treatment)
Any acute toxicity grade 3 or higher I: 72% / C: 85% p=0.006
Statistically significant differences were found for: Vomiting (I: 1% / C: 7%) Infection (I: 21% / C: 34%) Deafness (I: 5% / C: 13%) Hyponatremia (I: 23% / C: 52%) Leukopenia (I: 3% / C: 16%) Neutropenia (I: 1% / C: 12%) Febrile neutropenia (I: 1% / C: 6%) Lymphocytopenia (I: 72% / C: 89%)
No statistically significant differences were found for: Mucositis (I: 17% C: 18 %) Dysphagia (I: 42% / C: 39%) Odynophagia (I: 41% / C: 52 %) Xerostomia (I: 0% / C: 1%) Dysgeusia (I: 0% / C: 1%) Dermatitis (I: 7% / C: 8%) Diarrhoea (I: 1% / C: 5%) Fatigue (I: 1% / C: 1%) Weight loss (I: 1% / C: 0%) Hoarseness (I: 9% / C: 9%) Hypertension (I: 4% / C: 7%) Hypokalemia (I: 1% / C: 5%) Transaminase elevation (I: 1% / C: 5%) Anemia (I: 2% / C: 5%) Thrombocytopenia (I: 3% / C: 2%)
No grade ≥3 toxicity was observed for neuropathy and renal dysfunction.
Chronic (> 90 days from the start of treatment)
Any chronic toxicity grade 3 or higher I: 10% / C: 14% (p=0.55)
A statistically significant difference was found for: Deafness (I: 4% / C: 16%)
No statistically significant differences were found for: Mucositis (I: 0% / C: 1%) Dysphagia (I: 4% / C: 4%) Odynophagia (I: 2 % / C: 4%) Infection (I: 5% / C: 5%) Xerostomia (I: 1% / C: 1%) Subcutaneous (I: 0% / C: 2%) Trismus (I: 2% / C: 0%) Hypertriglyceridemia (I: 2% / C: 1%)
No grade ≥3 toxicity was observed for dysgeusia, skin, hypothyroidism, and thromboembolic events.
|
Compliance There were no significant differences between the two arms in terms of treatment completion and compliance to therapy (P = .1). Overall compliance was 91%.
The median cumulative cisplatin dose was 300 mg/m2 (IQR, 200-300 mg/m2) in the weekly treatment group and 210 mg/m2 (IQR, 180-210 mg/m2) in the three-weekly treatment group.
The study was powered for the primary outcome locoregional control, therefore the study was not powered to show statistically significant differences in progression-free survival and overall survival.
Toxicities were assessed using the Common Terminology Criteria for Adverse Events (version 4.03). |
|||||||
|
35 mg/m2 |
||||||||||||||
|
Rawat, 2016 |
Type of study: randomized controlled trial
Setting and country: single centre study, India
Funding and conflicts of interest: no information provided by authors |
Patients with locally advanced squamous cell carcinoma of the head and neck
Intent of treatment: 100% definitive in both groups
Inclusion criteria: - histologically proven squamous cell carcinoma - locally advanced (stage III - IV B) disease - oral cavity, oropharynx, hypopharynx and larynx primary tumours - age 18 – 65 years - normal haemogram, renal and liver function tests
Exclusion criteria: - previously treated head and neck malignancy - evidence of distant metastasis - patients with comorbid conditions, such as uncontrolled hypertension, uncontrolled diabetes, or active cardiac disease
N total at baseline: 60 (I: 30 / C: 30)
Important characteristics:
Age (mean ± SD) I: 50.7 ± 8.1 C: 51.4 (10.1)
Male gender I: 90% / C: 100%
Primary tumour site Oral cavity I: 20% / C: 30% Oropharynx I: 60% / C: 47% Larynx I: 17% / 17% Hypopharynx I: 3% / 7%
T-stage T1/Ts I: 40% / C: 20% T3/T4 I: 60% / 80% p=0.04
N-stage N0/N1 I: 30% / C: 60% N2/N3 I: 70% / C: 40% P=0.01
Stage Stage III I: 23% / C: 40% Stage IV (non-metastatic) I: 77% / C: 60%
Differentiation Well I: 17% / C: 17% Moderate I: 57% / C: 70% Poorly I: 27% / C: 13%
The weekly arm had fewer men and more advanced stage patients (N2/N3) as compared to the three-weekly arm. |
35 mg/m2 cisplatin once a week (for 6 weeks) along with radiation Radiotherapy was delivered to a total dose of 70 Gy in 35 fractions.
Weekly cisplatin was provided on an outpatient basis in day care ward with similar hydration and other measures, as compared to the control group.
Supportive treatment like hospitalization, administration of colony stimulating factor and insertion of Ryle’s tube feeding was done as and when indicated. |
100 mg/m2 cisplatin every three weeks (on days 1, 22 , and 43) along with radiation
In the 3-weekly arm, the patient was admitted one day before and cisplatin was given intravenously over 2 days in equal divided dose in 500 ml of normal saline with adequate hydration, anti-emetic prophylaxis, and mannitol infusion while
Supportive treatment like hospitalization, administration of colony stimulating factor and insertion of Ryle’s tube feeding was done as and when indicated. |
Length of follow-up: Median 8 months (range 4-13)
Loss-to-follow-up: Intervention: N=0 (0%)
Control: N =1 (3%) Reasons: one patient died during treatment because of a medical cause unrelated to the treatment (dengue)
|
Overall survival Not reported
Recurrence
Complete response three months after treatment: I: 67% / C: 62% Partial response three months after treatment: I: 33% / C: 38% p=0.20
Disease-free survival Not reported
Quality of life Not reported
Adverse events (% grade ≥3 3)
Mucositis I: 70% / C: 76% p=0.20
Vomiting I: 20% / 35% P=0.03
Anemia I: 33% / C: 31% P=0.22
Neutropenia I: 27% / C: 55% p=0.01
Thrombocytopenia I: 7% / C: 10% P=0.32
Hypomagnesemia (20% versus 60%; p=0.001) occurred less frequently in the study arm receiving weekly cisplatin. However, it was not clear whether this involved grade ≥3 adverse events.
The frequency of significant weight loss (23% versus 41%; p=0.08) and hyponatremia (33% versus 40%; p=0.18) was comparable between the groups. It was not clear whether this involved grade ≥3 adverse events. |
Compliance Mean RT dose was similar between the weekly (69.86 Gy ± 0.73) and three-weekly (69.22 Gy ± 4.38) study arms. However, a higher mean cisplatin dose was received by patients receiving three-weekly treatment (438.27 mg ± 65.03) as compared to patients receiving weekly treatment (291.66 mg ± 23.05) during RT. Five patients in arm A and 10 patients in arm B had RT interruption due to various reasons like mucositis, haematological toxicity and non-compliance. Although not statistically significant, 10% and 20.7% of patients receiving weekly or three-weekly treatment did not complete the planned course of CT (p = 0.15).
Need for supportive treatment Supportive treatment in the form of feeding procedure (such as percutaneous endoscopic gastrostomy or Ryle’s tube) (p = 0.05), use of colony-stimulating factors (p =0.05) and hospitalization for supportive care during CRT were more for 3-weekly arm patients as compared to weekly arm (p = 0.05).
Tumour response was evaluated according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1.
Toxicity was assessed using the CTCAE v. 4.03.
|
|||||||
|
40 mg/m2 |
||||||||||||||
|
Kiyota, 2022 |
Type of study: Randomized controlled non-inferiority trial
Setting and country: 28 institutions in Japan
Funding and conflicts of interest: Supported by the National Cancer Center Research and Development Funds (29-A-3, 25-B-2, and 2020-J-3), a Grant-in-Aid for Clinical Cancer Research (H23-009 and H26-052) from the Ministry of Health, Labor and Welfare of Japan, and by AMED (Japan Agency for Medical Research and Development).
Several authors, including the first author, disclosed receiving honorary an research funding from the pharmaceutical industry (including Novartis). |
Patients with postoperative locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN) with high risk for recurrence
Intent of treatment: 100% adjuvant in both groups
Inclusion criteria: - the presence of histologically proven squamous cell carcinoma in the resected specimen - primary lesion located in the oral cavity, oropharynx, hypopharynx, or larynx - pathologic stages III, IVA, or IVB (UICC seventh edition) - high-risk factors for recurrence (microscopically positive margin and/or extranodular extension) - within 56 days of surgery - without distant metastasis - age 20-75 years - an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1 - adequate organ function - no clinically significant abnormal findings on electrocardiography
A microscopically positive margin was defined as an invasive cancer at or close to the resection margin (, 5 mm) on microscopic evaluation, with no evidence of residual gross tumor.
Exclusion criteria: - active multiple primary cancers (synchronous primary double cancers and metachronous double cancers with 5 years or less of disease-free survival; carcinoma in situ and lesions consistent with intramucosal carcinoma judged to be curable with topical treatments, and lesions diagnosed as esophageal, gastric, or colon carcinoma in situ or intramucosal carcinoma based on endoscopic findings before registration are not regarded as active primary double cancers) - infection requiring systemic treatment - fever exceeding 38°C at registration - women who are or may be pregnant, or who are nursing - psychosis or psychiatric symptoms/signs that are judged to make participation in the study difficult - long-term use of systemic steroidal treatment (oral/intravenous) - uncontrolled diabetes mellitus - complication with unstable angina (new onset within the last 3 weeks or aggravation of angina pectoris), or history of myocardial infarction within the last 6 months - uncontrolled hypertension - pleural effusion, pericardial effusion, or ascites that requires drainage - HBs antigen-positive - judged to have difficulty in abstaining from smoking or alcohol during the protocol treatment
N total at baseline: I: 129 / C: 132
Important characteristics:
Age, median (IQR) I: 61 (53 to 66) C: 62 (55 to 68)
Male gender I: 85% / C: 85%
Primary tumour site Oral cavity I: 46% / C: 46% Larynx I: 9% / C: 9% Oropharynx I: 16% / C: 11% Hypopharynx I: 29% / C: 34%
T-stage T1 I: 5% / C: 10% T2 I: 31% / C: 20% T3 I: 18% / C: 19% T4 I: 46% / C: 51%
N-stage No I: 5% / C: 7% N1 I: 12% / C: 7% N2 I: 81% / C: 81% N3 I: 1% / C: 4% Nx I: 1% / C: 1%
Stage Stage III I: 9% / C: 7% Stage IVA I: 88% / C: 88% Stage IVB I: 2% / C: 4% Unknown I: 1% / C: 1%
|
40 mg/m2 cisplatin once a week (for 7 weeks) along with radiation
Radiotherapy was delivered to a total dose of 66 Gy in 33 fractions over 6.5 weeks.
|
100 mg/m2 cisplatin every three weeks (on days 1, 22 , and 43) along with radiation
Radiotherapy was delivered to a total dose of 66 Gy in 33 fractions over 6.5 weeks.
|
Length of follow-up: median follow-up of 2.2 (interquartile range 1.19-3.56) years
Loss-to-follow-up:
All patients were included in the survival analysis.
Intervention: N=7 (5%) Control: N =3 (2%) Reasons: these patients were not included in the analysis of adverse events because they did not start treatment with cisplatin
|
Overall survival HR 0.69 (99.1% CI, 0.37 to 1.27 One-sided P for noninferiority = .0027 , .0043).
Recurrence I: 29% / C: 39%
Disease-free survival
Relapse-free survival HR 0.71 (95% CI, 0.48 to 1.06) (in favour of weekly treatment)
Local relapse-free survival HR 0.73 (95% CI, 0.47 to 1.13
Quality of life Not reported
Adverse events (% grade ≥3 3)
Haematological
Anemia I: 13% / C: 14% Leukocytopenia I: 62% / 55% Neutropenia I: 35% / 49% Thrombocytopenia I: 3% / 2%
Non-haematological
Mucositis I: 28% / C: 23% Radiation dermatitis I: 12% / C: 15% Nausea I: 5% / C: 13% Dysphagia I: 12% / C: 19% Infection I: 7% / C: 12% Hearing disturbance I: 2% / C: 4%
Proportion of patients with at least one grade ≥3 adverse event I: 81.1% / C 79.8%
Proportion of patients with at least one grade ≥3 haematological adverse event I: 64.8% / C 61.2%
|
Compliance
Total RT dose in both groups: median 66 (IQR 66 to 66)
Cycles of cisplatin: I: median 6 (IQR 5 to 7) C: median 3 (IQR 3 to 3)
Cumulative dose of cisplatin I: 239 mg/m2 (IQR 199 to 277) C: 280 mg/m2 (IQR 250 to 299)
Toxicity was assessed using CTACE version 4.0.
|
|||||||
|
Nanda, 2019 |
Type of study: randomized controlled trial
Setting and country: regional cancer centre in India
Funding and conflicts of interest: nil financial support and sponsorship, the authors reported no conflicts of interest |
Patients with locally advanced oropharyngeal squamous cell carcinoma
Intent of treatment: 100% definitive in both groups
Inclusion criteria: - Histologically proven newly diagnosed oropharyngeal SCC Stage III–IV - age 20–70 years - Karnofsky Performance Scale >70 - well‑controlled medical Comorbidities - normal baseline haematological, hepatic, and renal functions
Exclusion criteria: - active tuberculosis - retroviral infection - cardiac abnormalities with ejection fraction <60 - received neoadjuvant chemotherapy or had undergone surgery
N total at baseline: 60 (I: 29 / C: 31)
Important characteristics:
Median age I: 54.5 ± 7.9 C: 55.0 ± 6.5
Male gender I: 93% / C: 97%
Primary tumour site (in oropharynx): Base of tongue I: 48% / C: 54% Tonsil I: 35% / C:23% Soft palate I: 10% / C: 16% Posterior pharyngeal wall I: 7% / C: 7%
T status T2 I: 7% / C: 3% T3 I: 62% / C: 55% T4 I: 31% / C: 42% p=0.67
N status N0 I: 17% / C: 23% N1 I: 31% / C: 32% N2 I: 45% / C: 36% N3 I: 7% / C: 10% p=0.35
Stage III I: 35% / C: 36% IV I: 66% / C: 65% p=0.91
Groups were comparable at baseline
|
40 mg/m2 cisplatin once a week (for a minimum of four cycles and maximum of six cycles) along with radiation
Radiotherapy was delivered to a total dose of 70 Gy in 35 fractions, delivered 5 days a week.
All patients received prechemotherapy hydration 24 h prior. Premedication with antiemetics, ondansetron 8 mg and dexamethasone 16 mg were given before chemotherapy, and 20‑mmol potassium chloride and a vial of magnesium sulfate (1000 mg) diluted with saline were infused over 1 h each.
In the weekly arm, cisplatin of 40 mg/m2 was given along with saline over 3 h.
Postchemotherapy hydration was given and antiemetics continued for the following 2 days.
|
100 mg/m2 cisplatin every three weeks (for a maximum of two cycles) along with radiation
Radiotherapy was delivered to a total dose of 70 Gy in 35 fractions, delivered 5 days a week.
All patients received prechemotherapy hydration 24 h prior. Premedication with antiemetics, ondansetron 8 mg and dexamethasone 16 mg were given before chemotherapy, and 20‑mmol potassium chloride and a vial of magnesium sulfate (1000 mg) diluted with saline were infused over 1 h each.
In the three-weekly arm, cisplatin dose of 100 mg/m2 was given in divided doses over 2–3 days.
Postchemotherapy hydration was given and antiemetics continued for the following 2 days. |
Length of follow-up: Median 28 months (range 2.8-66.9 months)
Incomplete outcome data: Intervention: N=1 (3%) Reasons not described, but toxicities were reported for 28/29 patients in the weekly treatment group
Control: N=0 (0%)
|
Overall survival
Median overall survival I: 35.4 months C: 32.9 months p=0.303
2-year survival rate I: 55% / C: 58% No statistically significant difference, p-value not provided
5-year survival rate I: 42% / 32% No statistically significant difference, p-value not provided
Recurrence
Complete response three months after treatment: I: 81% / C: 75% Partial response three months after treatment: I: 14% / C: 13% Stable disease 8 weeks after completion of treatment: I: 5% / C: 4% Stable or progressive disease three months after completion of treatment: I: 29% / C: 42%
Locoregional relapses I: 14% / C: 6%
Disease-free survival
Median disease‑free survival at two years I: 26.4 months / C: 27.4 months P=0.953
There were two nondisease‑related deaths in the weekly treatment arm and five in the three-weekly treatment arm.
Quality of life Not reported
Adverse events (% grade ≥3)
Non- haematological
Mucositis I: 32% / C: 29% p=1.00
Dysphagia I: 46% / C: 32% p=0.27
Dermatitis I: 14% / C: 19% p=0.73
Larynx I: 11%% / C: 10% p=1.00
Nausea/vomiting I: 7% / C: 0% p=0.22
Haematological
Anemia I: 0% / C: 3% p=1.00
Leukopenia I: 25% / C: 13% p=0.32
Neutropenia I: 18% / C: 7% p=0.24
Thrombocytopenia I: 0% / C: 3% p=1.00
Two patients of Arm B died due to neutropenic sepsis and one in Arm A due to aspiration pneumonia. |
Compliance The median total cumulative dose of cisplatin was 271.8 mgs (range: 120–320 mgs) in the weekly arm and 303 mgs (range: 180–350 mgs) in the three-weekly arm (p=0.02). Twenty‑five (89%) in Arm A and thirty (96.8%) in Arm B received cisplatin >200 mg/m².
Both the groups of patients received radiation dose of 70 Gy in 35 fractions over 7 weeks. There was no difference in the overall treatment time (OTT) in both the arms (weekly arm: 53.4 days (±5.2) and three-weekly arm: 53.8 days (±7.7)).
Tumour response was evaluated according to the WHO criteria.
Toxicity was assessed using the Radiation Therapy Oncology Group criteria for radiotherapy-induced acute toxicities, and Common Toxicity Criteria for chemotherapy-induced toxicity. |
|||||||
|
Nair, 2017 |
Type of study: randomized controlled phase IIb trial
Setting and country: regional cancer centre, India
Funding and conflicts of interest: nil financial support and sponsorship, the authors reported no conflicts of interest |
Patients with locally advanced head and neck squamous cell carcinoma
Intent of treatment: 100% definitive in both groups
Inclusion criteria: - Previously untreated patients with stage III and IV squamous cell carcinoma of oropharynx, hypopharynx, and larynx - age in the range of 18–70 years - Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1 - baseline hemoglobin ≥10 g/dl, white blood cells count ≥4000 cells per cubic millimeter - platelet count ≥100,000 cells per cubic millimeter - creatinine clearance of ≥60 ml/min
Exclusion criteria: - bone or cartilage involvement - distant metastasis
N total at baseline: 56 (I: 25 / C: 31)
Important characteristics:
Age > 60 I: 42% / C: 45%
Male gender I: 96% / C: 90%
Primary tumour site Oropharynx I: 42% / C: 55% Larynx I: 42% / C: 23% Hypopharynx I: 17% / 23%
T status T2 I: 21% / C: 35% T3 I: 58% / C: 55% T4 I: 21% / C: 10% p=0.30
N status N0 I: 29% / C: 23% N1 I: 29% / C: 39% N2 I: 42% / C: 39% p=0.73
Stage III I: 63% / C: 58% IV I: 38% / C: 42% p=0.78
Groups were comparable at baseline
|
40 mg/m2 cisplatin once a week (for 6 weeks) along with radiation
Radiotherapy was delivered to a total dose of 66 Gy in 33 fractions over 6.5 weeks
For 40 mg/m2 cisplatin, 500 ml NS and 200 ml 10% mannitol were given as prehydration followed by cisplatin in 1000 ml NS. Posthydration consisted of 500 ml oral fluids.
Antiemetic prophylaxis consisted of 8 mg ondansetron, 12 mg dexamethasone as intravenous bolus, and NK1 antagonist 125 mg orally on the day of chemotherapy. Dexamethasone 8 mg per day and NK1 antagonist 80 mg were continued on the second and third days after each cycle. |
100 mg/m2 cisplatin every three weeks along with radiation
Radiotherapy was delivered to a total dose of 66 Gy in 33 fractions over 6.5 weeks
For 3 weekly cisplatin, 1000 ml normal saline (NS) with 20 mmol potassium chloride (KCl) and 10 mmol magnesium sulfate (MgSO4) followed by 200 ml 10% mannitol were given as prehydration. This was followed by cisplatin 100 mg/m2 in 1000 ml NS. Posthydration consisted of 1000 ml NS with 20 mmol KCl and 10 mmol MgSO4.
Antiemetic prophylaxis consisted of 8 mg ondansetron, 12 mg dexamethasone as intravenous bolus, and NK1 antagonist 125 mg orally on the day of chemotherapy. Dexamethasone 8 mg per day and NK1 antagonist 80 mg were continued on the second and third days after each cycle.
|
Length of follow-up: Median 26 months
Incomplete outcome data: Intervention: N=1 (4%) Reasons : One patient in the weekly arm did not complete the treatment and was excluded from the analysis.
Control: N=0 (0%)
|
Overall survival
2 year overall survival rate I: 61% / C: 71% P=0.610
Recurrence
Complete response six months after treatment: I: 75% / C: 90% Partial response six months after treatment: I: 12% / C: 6% Residual disease at 12 weeks I: 8% / C: 6%
Local recurrence or metastasized disease I: 13% / C: 13%
Second primary tumour I: 8% / C: 3%
Locoregional control at 2 years I: 63% / C: 61%
Disease-free survival
2 year disease-free survival rate I: 53% / C: 65% P=0.674
Quality of life Not reported
Adverse events (% grade ≥3)
Non- haematological
Mucositis I: 54% / C: 52% P>0.05
Dysphagia I: 63% / C: 26% P<0.05
Dermatitis I: 13% / C: 3% P>0.05
Haematological
Anemia I: 4% / C: 0 % P>0.05
Neutropenia I: 8% / C: 3% P>0.05
|
Compliance The mean dose of cisplatin in 3 weekly arm was 356.6 mg and 339.1 mg in the weekly arm
Tumour response was evaluated using RECIST criteria.
Toxicity was assessed using the Radiation Therapy Oncology Group criteria for radiotherapy-induced toxicities, and Common Terminology Criteria version 4 for chemotherapy-induced toxicity. |
|||||||
|
Tsan, 2012 |
Type of study: phase III randomized trial
Setting and country: Taiwan
Funding and conflicts of interest: Funded by grants from Chang Gung Memorial Hospital.
The authors reported no conflicts of interest |
Patients with high-risk oral cavity squamous cell carcinoma
Intent of treatment: 100% adjuvant in both groups
Inclusion criteria: - histologically confirmed primary oral cavity squamous cell carcinoma, pathologic documentation for extracapsular spreading of the involved lymph node, a positive surgical margin, or lymph node staging ≥ N2 - between 18 and 70 years old - Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2 - adequate bone marrow, liver, and renal function
Exclusion criteria: - suspected distant metastatic lesions - prior chemotherapy or RT - serious concomitant illness, such as active cardiac disease, severe uncontrolled hypertension, uncontrolled infection, or a history of other head and neck malignancies
N total at baseline: 50 (I: 24/ C: 26)
Important characteristics:
Mean age (range) I: 49.0 (32-65) C: 49.2 (33-63)
Male gender I: 96% / C: 96%
Primary tumour site (in oral cavity) Buccal I: 38% / C: 39% Tongue I: 46% / C: 39% Gum I: 13% / C: 19% Others I: 4% / C: 4%
T status T1/T2 I: 46% / C: 54% T3/T4 I: 54% / C: 46% p=0.778
N status N0 I: 8% / C: 0% N1 I: 33% / C: 19% N2 I: 58% / C: 81% p=0.125
Stage II I: 4% / C: 0% III I: 17% / C: 12% IV I: 79% / C: 89% p=0.549
Differentiation Well I: 21% / C: 27% Moderate I: 71% / C: 58% Poor I: 8% / C: 15%
Groups were comparable at baseline
|
40 mg/m2 cisplatin once a week along with radiation
Radiotherapy was delivered to a total dose of 66 Gy in 33 fractions
|
100 mg/m2 cisplatin every three weeks along with radiation
Radiotherapy was delivered to a total dose of 66 Gy in 33 fractions
Forced hydration with normal saline 500 ml infusion over 2 hours before and after cisplatin infusion
|
Length of follow-up: Median 12.0 months (range 2.7 to 32.8)
Loss-to-follow-up: none
Incomplete outcome data: none
|
Overall survival
1-yr overall survival rates I: 72% / C: 79% P=0.978
Recurrence
1-year locoregional recurrence-free survival rates: I: 60% / C: 71% P=0.806
Disease-free survival Not reported
Quality of life
FACT-H&N physical well-being subscale (range 0-28)
Week 2 (mean change from baseline) I: -8.3 / C: -3.8 Difference: 4.5 (p=0.03)
Week 4 (mean change from baseline) I: -9.9 / C: -4.2 Difference: 5.7 (p=0.02)
End of radiotherapy (mean change from baseline) I: -10.3 / C: -2.5 Difference: 7.8 (p=0.007)
Follow-up at 3 months (mean change from baseline) I: -3.8 / C: 0.5 Difference: 4.3 (p=0.04)
FACT-H&N social well-being subscale (range 0-28)
Week 2 (mean change from baseline) I: -0.4 / C: -2.4 Difference: 2 (p=0.47)
Week 4 (mean change from baseline) I: -0.2 / C: -3.1 Difference: 2.9 (p=0.29)
End of radiotherapy (mean change from baseline) I: -1.6 / C: -7.3 Difference: 5.7 (p=0.02)
Follow-up at 3 months (mean change from baseline) I: -0.7 / C: -5.7 Difference: 5 (p=0.10)
FACT-H&N emotional well-being subscale (range 0-24)
Week 2 (mean change from baseline) I: -0.9 / C: -1.2 Difference: 0.3 (p=0.79)
Week 4 (mean change from baseline) I: -0.8 / C: -1.4 Difference: 0.6 (p=0.46)
End of radiotherapy (mean change from baseline) I: -1.1 / C: -1.0 Difference: 0.1 (p=0.98)
Follow-up at 3 months (mean change from baseline) I: 0.1 / C: 0.5 Difference: 0.4 (p=0.70)
FACT-H&N functional well-being subscale (range 0-28)
Week 2 (mean change from baseline) I: -1.9 / C: -2.0 Difference: 0.1 (p=0.74)
Week 4 (mean change from baseline) I: -1.7 / C: -3.2 Difference: 1.5 (p=0.65)
End of radiotherapy (mean change from baseline) I: -2.8 / C: -3.2 Difference: 0.4 (p=0.90)
Follow-up at 3 months (mean change from baseline) I: -1.4 / C: 1.9 Difference: 3.3 (p=0.15)
FACT-H&N Head and neck cancer subscale (range 0-40)
Week 2 (mean change from baseline) I: -3.7 / C: -3.0 Difference: 0.7 (p=0.48)
Week 4 (mean change from baseline) I: -5.6 / C: -4.9 Difference: 0.7 (p=0.46)
End of radiotherapy (mean change from baseline) I: -5.2 / C: -3.4 Difference: 1.8 (p=0.47)
Follow-up at 3 months (mean change from baseline) I: -2.4 / C: -0.3 Difference: 2.1 (p=0.24)
FACT-H&N Trial Outcome Index (range 0-96)
Week 2 (mean change from baseline) I: -14.6 / C: -8.8 Difference: 5.8 (p=0.14)
Week 4 (mean change from baseline) I: -17.2 / C: -12.3 Difference: 4.9 (p=0.31)
End of radiotherapy (mean change from baseline) I: -18.4 / C: -9.1 Difference: 9.3 (p=0.14)
Follow-up at 3 months (mean change from baseline) I: -7.6 / C: 2.1 Difference: 9.7 (p=0.06)
Adverse events (% grade ≥3)
Overall toxicity I: 92% / C 81% P=0.020
Non-haematological
Mucositis I: 75% / C: 39% p=0.012
Pharyngitis I: 54% / C: 54% p=1.0
Stomatitis I: 54% / C: 54% p=1.0
Laryngeal oedema I: 4% / C: 12% p=0.611
Dermatitis I: 8% / C: 8% p=1.0
Nausea/vomiting I: 21% / C: 12% p=0.456
Haematological
Anemia I: 4% / C: 4% p=1.0
Leukopenia I: 13% / C: 0% p=0.103
Neutropenia I: 4% / C: 0% p=0.480
Thrombocytopenia I: 0% / C: 0% P not computed |
The trial aimed to recruit 371 patients but due to slow recruitment, the trial was ended after only 55 patients were recruited over 30 months.
Compliance Both groups of patients received the same mean doses of RT and cisplatin, and they also did not differ regarding the inadequate RT (RT dose < 60 Gy) or interrupted RT (RT duration > 8 weeks) rate. However, in terms of adequate cisplatin dose, which was defined as cisplatin ≥ 200 mg/m2, more patients in the three-weekly arm received an adequate cisplatin dose than did patients in the weekly treatment arm. In the three-weekly arm, 88.5% of patients received an adequate dose of cisplatin, whereas only 62.5% of those in the weekly treatment arm received an adequate dose (p = 0.047).
Toxicity was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. |
|||||||
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures.
- Provide data per treatment group on the most important prognostic factors ((potential) confounders).
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls.
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders.
Figure 13.9.1 Forest plot for overall survival
Figure 13.9.2 Forest plot for locoregional control (recurrence)
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
Research question: What are the benefits and risks of weekly versus three-weekly cisplatin concurrent with definitive radiotherapy for patients with locally advanced head and neck squamous cell carcinoma?
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
||||||
|
30 mg/m2 |
|||||||||||||
|
Mashhour, 2020 |
Probably yes;
Reason: With the aid of a randomization table, patients were randomly assigned 1:1 into two arms ( Arm A: low dose cisplatin and Arm B: high dose cisplatin). |
No information; |
Probably no;
Reason: No details were provided about blinding, but it is difficult to blind patients to the frequency of cisplatin infusions. Also, patients in the three-weekly group received additional supportive treatment. |
Probably yes;
Reason: all patients were included in the analysis of adverse events. It was not explicitly described how many patients were included in the analysis of loco-regional control and tumour response. |
Probably yes;
Reason: Outcomes listed in the methods section were all reported. However, this selection of outcomes could not be compared against a trial registration or study protocol. |
Probably yes;
Reasons: no other problems were noted. |
Some concerns (recurrence, adverse events) |
||||||
|
Sahoo, 2017 |
No information;
|
No information;
|
Probably no;
Reason: No details were provided about blinding, but it is difficult to blind patients to the frequency of cisplatin infusions.
|
Probably yes;
Reason: One patient in the control group was lost to follow up (7%) |
Probably yes;
Reason: Outcomes listed in the methods section were all reported. However, this selection of outcomes could not be compared against a trial registration or study protocol. |
Probably yes;
Reasons: no other problems were noted. |
Some concerns (locoregional control, adverse events)
|
||||||
|
Noronha, 2018 |
Definitely yes;
Reason: Immediately before the start of CRT, patients were stratified by T stage (T0, T1, or T2 v T3 or T4), N stage (N0 or N1 v N2 or N3), and intent of therapy (adjuvant v definitive) and were randomly assigned (computer-generated block random assignment by independent statistician) 1:1 to cisplatin 30 mg/m2 once a week or cisplatin100 mg/m2 once every 3 weeks (on days 1, 22, and 43) with curative radiotherapy. |
No information;
|
Probably no;
Reason: No details were provided about blinding, but it is difficult to blind patients to the frequency of cisplatin infusions.
|
Probably yes;
Reason: No patients were lost to follow up, a few patients were not included in the analysis of toxicity because they did not start treatment (I: 1% / C: 1%) |
Probably no;
Reason: The outcome Quality of life was mentioned as a secondary outcome in the study protocol and the Methods section but no results were reported. |
Probably yes;
Reasons: no other problems were noted. |
Some concerns (overall survival, locoregional control, progression-free survival, adverse events) |
||||||
|
35 mg/m2 |
|||||||||||||
|
Rawat, 2016 |
No information;
Reason: After pre-treatment evaluation and staging, patients were randomized into two arms by sequential randomization according to their first visit in the RT department. No specific information about how the allocation sequence was generated. |
No information;
|
Probably no;
Reason: No details were provided about blinding, but it is difficult to blind patients to the frequency of cisplatin infusions.
|
Probably yes;
Reason: One patient in the three-weekly treatment group was lost to follow up (3%) |
Probably yes;
Reason: Outcomes listed in the methods section were all reported. However, this selection of outcomes could not be compared against a trial registration or study protocol. |
Probably yes;
Reasons: no other problems were noted. |
Some concerns (locoregional control, adverse events)
|
||||||
|
40 mg/m2 |
|||||||||||||
|
Kiyota, 2022 |
Probably yes;
Reason: Patients were randomly assigned in a 1:1 ratio to chemoradiotherapy with 3-weekly cisplatin or weekly cisplatin by the minimization method using a random component, with adjustment to balance high-risk factors for recurrence and institution |
Definitely yes;
Reason: Investigators or subinvestigators must confirm that a patient fulfills all of the eligibility criteria and does not meet any of the exclusion criteria, and then register the patient via the JCOG Web Entry System. Details of the randomized assignment method will not be disclosed to the investigators at participating institutions.
|
Definitely no;
Reason: open-label study. It is difficult to blind patients to the frequency of cisplatin infusions. |
Definitely yes;
Reason: all patients were included in the survival analysis, only a few patients did not receive cisplatin (I: 5% / C: 2%) and were excluded from the toxicity analysis |
Definitely yes;
Reason: Outcomes listed in the methods section were all reported and were in accordance with the study protocol.
|
Probably yes;
Reasons: no other problems were noted.
|
LOW (OS, recurrence, disease-free survival, adverse events) |
||||||
|
Nanda, 2019 |
Probably yes;
Reason: Patients were prospectively randomized to two arms using randomization table. |
No information;
|
Probably no;
Reason: No details were provided about blinding, but it is difficult to blind patients to the frequency of cisplatin infusions.
|
Probably yes;
Reason: One patient in the weekly treatment group was lost to follow up (3%)
|
Probably yes;
Reason: Outcomes listed in the methods section were all reported. However, this selection of outcomes could not be compared against a trial registration or study protocol.
|
Probably yes;
Reasons: no other problems were noted.
|
Some concerns (overall survival, tumour response, locoregional control, disease-free survival, adverse events) |
||||||
|
Nair, 2017 |
Probably yes;
Reason: Patients were randomized into either Arm A or Arm B using a computer‑generated randomization chart. |
No information;
|
Probably no;
Reason: No details were provided about blinding, but it is difficult to blind patients to the frequency of cisplatin infusions.
|
Probably yes;
Reason: One patient in the weekly treatment group was lost to follow up (4%)
|
Probably no;
Reason: Outcomes listed in the methods section were all reported. However, the trial registration did not include overall survival, and did include chronic toxicity and quality of life. |
Probably yes;
Reasons: no other problems were noted.
|
Some concerns (overall survival, tumour response, locoregional control, disease-free survival, adverse events) |
||||||
|
Tsan, 2012 |
No information;
|
No information;
|
Probably no;
Reason: No details were provided about blinding, but it is difficult to blind patients to the frequency of cisplatin infusions.
|
Probably yes;
Reason: no patients were lost to follow-up, outcome data were presented for all randomized patients for the outcomes OS, locoregional recurrence-free survival and toxicities. It was not reported how many patients were included in the analysis of quality of life. |
Probably yes;
Reason: Outcomes listed in the methods section were all reported. However, this selection of outcomes could not be compared against a trial registration or study protocol.
|
Definitely no;
Reason: The sample size calculation showed that 371 patients had to be recruited but due to slow recruitment, the trial was stopped after 55 patients were recruited (50 were randomized) |
HIGH (overall survival, locoregional recurrence, quality of life, adverse events) |
||||||
- Randomization: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomization process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomization (performed at a site remote from trial location). Inadequate procedures are all procedures based on inadequate randomization procedures or open allocation schedules.
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments, but this should not affect the risk of bias judgement. Blinding of those assessing and collecting outcomes prevents that the knowledge of patient assignment influences the process of outcome assessment or data collection (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is usually not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary. Finally, data analysts should be blinded to patient assignment to prevents that knowledge of patient assignment influences data analysis.
- Lost to follow-up: If the percentage of patients lost to follow-up or the percentage of missing outcome data is large, or differs between treatment groups, or the reasons for loss to follow-up or missing outcome data differ between treatment groups, bias is likely unless the proportion of missing outcomes compared with observed event risk is not enough to have an important impact on the intervention effect estimate or appropriate imputation methods have been used.
- Selective outcome reporting: Results of all predefined outcome measures should be reported; if the protocol is available (in publication or trial registry), then outcomes in the protocol and published report can be compared; if not, outcomes listed in the methods section of an article can be compared with those whose results are reported.
- Other biases: Problems may include: a potential source of bias related to the specific study design used (for example lead-time bias or survivor bias); trial stopped early due to some data-dependent process (including formal stopping rules); relevant baseline imbalance between intervention groups; claims of fraudulent behavior; deviations from intention-to-treat (ITT) analysis; (the role of the) funding body (see also downgrading due to industry funding https://kennisinstituut.viadesk.com/do/document?id=1607796-646f63756d656e74). Note: The principles of an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
- Overall judgement of risk of bias per study and per outcome measure, including predicted direction of bias (for example favors experimental, or favors comparator). Note: the decision to downgrade the certainty of the evidence for a particular outcome measure is taken based on the body of evidence, i.e. considering potential bias and its impact on the certainty of the evidence in all included studies reporting on the outcome.
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Mohamed (2019) |
Review providing only indirect comparisons, problems with confounding |
|
Strojan (2016) |
Review providing only indirect comparisons, problems with confounding |
|
Guan (2016) |
Review including mainly retrospective studies, no correction for confounding. |
|
Szturz (2017) |
Review providing only indirect comparisons, problems with confounding |
|
Jacinto (2017) |
Review providing only indirect comparisons, problems with confounding. One RCT in postoperative setting. |
|
Negi (2016) |
Review, no systematic search |
|
Szturz (2019) |
Review, no systematic search |
|
Jeremic (2019) |
Review, no systematic search |
|
Jeremic (2020) |
Review, no systematic search |
|
Shivakumar (2015) |
Review protocol |
|
Lee (2016) |
RCT in which only patients with nasopharyngeal cancer were included |
|
Bauml (2019) |
Not an RCT |
|
Uygun (2009) |
Not an RCT, treatment was based on age and/or performance status |
|
Morse (2019) |
Retrospective study |
|
MacKiewicz (2017) |
Retrospective study |
|
Chang (2017) |
Retrospective study |
|
Beryhy (2017) |
Abstract only |
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 20-09-2023
De geldigheid van de richtlijnmodule komt te vervallen indien nieuwe ontwikkelingen aanleiding zijn een herzieningstraject te starten.
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2019 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met hoofd-halstumoren.
Werkgroep
- Prof. Dr. R. de Bree, KNO-arts/hoofd-halschirurg, UMC Utrecht, Utrecht, NVKNO (voorzitter)
- Dr. M.B. Karakullukcu, KNO-arts/hoofd-halschirurg, NKI, Amsterdam, NVKNO
- Dr. H.P. Verschuur, KNO-arts/hoofd-halschirurg, Haaglanden MC, Den Haag, NVKNO
- Dr. M. Walenkamp, AIOS-KNO, LUMC, Leiden, NVKNO
- Dr. A. Sewnaik, KNO-arts/hoofd-halschirurg, Erasmus MC, Rotterdam, NVKNO
- Drs. L.H.E. Karssemakers, MKA-chirurg-oncoloog/hoofd-hals chirurg, NKI, Amsterdam, NVMKA
- Prof. dr. M.J.H. Witjes, MKA-chirurg-oncoloog, UMC Groningen, Groningen, NVMKA
- Drs. L.A.A. Vaassen, MKA-chirurg-oncoloog, Maastricht UMC+, Maastricht, NVMKA
- Drs. W.L.J. Weijs, MKA-chirurg-oncoloog, Radboud UMC, Nijmegen, NVKMA
- Drs. E.M. Zwijnenburg, Radiotherapeut-oncoloog, Radboud UMC, Nijmegen, NVRO
- Dr. A. Al-Mamgani, Radiotherapeut-oncoloog, NKI, Amsterdam, NVRO
- Prof. Dr. C.H.J. Terhaard, Radiotherapeut-oncoloog, UMC Utrecht, Utrecht, NVRO
- Drs. J.G.M. Van den Hoek, Radiotherapeut-oncoloog, UMC Groningen, Groningen, NVRO
- Dr. E. Van Meerten, Internist-oncoloog, Erasmus MC Kanker Instituut, Rotterdam, NIV
- Dr. M. Slingerland, Internist-oncoloog, LUMC, Leiden, NIV
- Drs. M.A. Huijing, Plastisch Chirurg, UMC Groningen, Groningen, NVPC
- Prof. Dr. S.M. Willems, Klinisch patholoog, UMC Groningen, Groningen, NVVP
- Prof. Dr. E. Bloemena, Klinisch patholoog, Amsterdam UMC, locatie Vumc, Amsterdam, NVVP
- R.A. Burdorf, Voorzitter dagelijks bestuur patiëntenvereniging, Patiëntenvereniging HOOFD-HALS, PvHH
- P.S. Verdouw, Hoofd infocentrum patiëntenvereniging, Patiëntenvereniging HOOFD-HALS, PvHH
- A.A.M. Goossens, Verpleegkundig specialist oncologie, Haaglanden MC, Den Haag, V&VN
- Dr. P. de Graaf, Radioloog, Amsterdam UMC, Amsterdam, NVvR
- Dr. W.V. Vogel, Nucleair geneeskundige/radiotherapeut-oncoloog, NKI, Amsterdam, NVNG
- Drs. G.J.C. Zwezerijnen, Nucleair geneeskundige, Amsterdam UMC, Amsterdam, NVNG
Klankbordgroep
- Dr. C.M. Speksnijder, Fysiotherapeut/Bewegingswetenschapper/Epidemioloog, UMC Utrecht, Utrecht, KNGF
- Ir. A. Kok, Diëtist, UMC Utrecht, Utrecht, NVD
- Dr. M.M. Hakkesteegt, Logopedist, Erasmus MC, Rotterdam, NVvLF
- Drs. D.J.M. Buurman, Tandarts-MFP, Maastricht UMC+, Maastricht, KNMT
- W. Van der Groot-Roggen, Mondhygiënist, UMC Groningen, Groningen, NVvM
- Drs. D.J.S. Dona, Bedrijfsarts/Klinisch arbeidsgeneeskundige oncologie, Radboud UMC, Nijmegen, NVKA
- Dr. M. Sloots, Ergotherapeut, UMC Utrecht, Utrecht (tot november 2021), EN
- A.C.P. Kauerz-de Rooij, Ergotherapeut, UMC Utrecht, Utrecht (vanaf januari 2022), EN
- J. Poelstra, Medisch maatschappelijk werkster, op persoonlijke titel
- Dr. K.S. Versteeg, Internist, Amsterdam UMC, Amsterdam, NIV ouderengeneeskunde
Met dank aan
- Drs. Maarten Donswijk, Nucleair geneeskundige, AVL
- Dr. José Hardillo, KNO-arts/hoofd-halschirurg, Erasmus MC, Rotterdam
- Drs. Dominique Monserez, KNO-arts/hoofd-halschirurg, Erasmus MC, Rotterdam
Met ondersteuning van
- Dr. J. Boschman, Senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. C. Gaasterland, Adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. A. Van der Hout, Adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. L. Oostendorp, Adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. M. Oerbekke, Adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. A. Hoeven, Junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. N. Elbert, Adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Bree, de |
KNO-arts/hoofd-halschirurg, UMC Utrecht |
* Lid Algemeen Bestuur Patiëntenvereniging Hoofd-Hals (onbetaald) * Voorzitter Research Stuurgroep NWHHT * Lid Richtlijnen commissie NWHHT * Lid dagelijks bestuur NWHHT * Lid Clinical Audit Board van de Dutch Head and Neck Audit (DHNA) * Lid wetenschappelijk adviescommissie DORP * Voorzitter Adviescommissie onderzoek hoofd-halskanker (IKNL/PALGA/DHNA/NWHHT) |
Geen |
Geen |
|
Slingerland |
Internist-oncoloog, LUMC |
* 2018-present: Treasurer of the "Dutch Association of Medical Oncology"(NVMO - vacancy fees) * 2018-present: Member of the "Dutch Working Group for Head-Neck Tumors" (NWHHT-Systemic therapy) * 2016-present: Member of the 'Dutch Working Group for Head-Neck Tumors" (NWHHT - study group steering group (coordinating)) * 2016-present: Member of the "Dutch Working Group for Head-Neck Tumors" (NWHHT - Elderly Platform) * 2012-present: Member "Working Group for Head-Neck Tumors" (WHHT) "University Cancer Centre"(UCK) Leiden - Den Haag * 2019: Member CAB DHNA |
Deelname Nationaal expert forum hoofd-halskanker MSD dd 2-5-2018
* Deelname Checkmate studie, sponsor Bristol-Myers Squibb (BMS): An open label, randomized phase 3 clinical trial of nivolumab versus therapy of investigator's choice in recurrent or metastatic platinum-refractory squamous cell carcinoma of the head and neck (SCCHN) * Deelname Commence studie, sponsor Radboud University, in collaboration with Merck Serono International SA (among several Dutch medical centers): A phase lB-II study of the combination of cetuximab and methotrexate in recurrent of metastatic squamous cell carcinoma of the head and neck. A study of the Dutch Head and Neck Society, MOHN01/COMMENCE study. * Deelname HESPECTA studie: Phase I study: to determine the biological activity of two HPV16E6 specific peptides coupled to Amplivant®, a Toll-like receptor ligand in non-metastatic patients treated for HPV16-positive head and neck cancer. * Deelname PINCH studie (nog niet open): PD-L1 ImagiNg to predict durvalumab treatment response in HNSCC (PINCH) trial; patiënten met biopt bewezen locally recurrent of gemetastaseerd HNSCC * Deelname ISA 101b-HN-01-17 studie (nog niet open): A randomized, Double-blind, Placebo-Controlled, Phase 2 Study of Cemiplimab versus the combination of Cemiplimab with ISA101b in the Treatment of Subjects. |
In de werkgroep participeren 2 internist-oncologen, zodat één van beide de voortrekker is van modules over systemische therapie. Actie: werkgroeplid is uitgesloten van besluitvorming bij modules die betrekking hebben op de onderwerpen van de gemelde onderzoeken: nivolumab, cetuximab + methotrexaat, Amplivant, durvalumab, cemiplimab. |
|
Meerten, van |
Internist-oncoloog, Erasmus MC Kanker Instituut |
Geen |
Op dit moment Principal Investigator voor NL van gerandomiseerde fase III trial naar toegevoegde waarde van pembrolizumab aan chemoradiotherapie bij patiënten met gevorderd hoofdhalskanker. Sponsor: GlaxoSmithKline Research & Development Ltd. Studie is nog lopend, resultaten zullen pas bekend zijn na verschijning van de richtlijn.
In toekomst mogelijk participatie aan door industrie gesponsorde studies op gebied van behandeling van hoofdhalskanker |
In de werkgroep participeren 2 internist-oncologen, zodat één van beide de voortrekker is van modules over systemische therapie. Actie: werkgroeplid is uitgesloten van besluitvorming bij modules die betrekking hebben op het onderwerp van het gemelde onderzoeken: de toegevoegde waarde van pembrolizumab bij patiënten met gevorderd hoofdhalskanker. |
|
Huijing |
Plastisch chirurg, UMC Groningen |
Geen |
Geen |
Geen |
|
Sewnaik |
KNO-arts/hoofd Hals chirurg, Erasmus MC |
Sectorhoofd Hoofd-Hals chirurgie |
Geen |
Geen |
|
Vaassen |
MKA-chirurg-oncoloog, Maastricht UMC+ / CBT Zuid-Limburg |
*Lid Bestuur NVMKA *Waarnemend hoofd MKA-chirurgie MUMC |
Geen |
Geen |
|
Witjes |
MKA-chirurg-oncoloog, UMC Groningen |
Geen |
PI van KWF grant: RUG 2015 -8084: Image guided surgery for margin assessment of head & neck Cancer using cetuximab-IRDye800 cONjugate (ICON)
geen financieel belang |
Geen. Financiering door KWF werd niet als een belang ingeschat. |
|
Bloemena |
Klinisch patholoog, Amsterdam UMC (locatie Vumc) / Radboud UMC / Academisch Centrum voor Tandheelkunde Amsterdam (ACTA) |
* Lid bestuur Nederlandse Vereniging voor Pathologie (NVVP) – vacatiegeld (tot 1-12-20) * Voorzitter Commissie Bij- en Nascholing (NVVP) * Voorzitter (tot 1-12-20) Wetenschappelijke Raad PALGA - onbezoldigd |
Geen |
Geen |
|
Willems |
Klinisch patholoog, UMC Groningen |
Vice-vz PALGA, AB NWHHT, CAB DHNA, mede-vz en oprichter expertisegroep HH pathologie NL, Hoofdhalspathologie UMC Groningen |
PDL1 trainer NL voor MSD Onderzoeksfinanciering van Pfizer, Roche, MSD, BMS, Lilly, Novartis, Bayer, Amge, AstraZeneca |
Geen |
|
Karakullukcu |
KNO-arts/hoofd-hals chirurg, NKI/AVL |
Geen |
Geen |
Geen |
|
Verschuur |
KNO-arts/Hoofd-hals chirurg, Haaglanden MC |
* Opleider KNO-artsen |
Geen |
Geen |
|
Walenkamp |
AIOS KNO, LUMC |
Geen |
Geen |
Geen |
|
Al-Mamgani |
Radiotherapeut-oncoloog, NKI/AVL |
Geen |
Geen |
Geen |
|
Terhaard |
Radiotherapeut-oncoloog, UMC Utrecht |
Niet van toepassing |
Geen |
Geen |
|
Hoek, van den |
Radiotherapeut-oncoloog UMCG |
Niet van toepassing |
Geen |
Geen |
|
Zwijnenburg |
Radiotherapeut, Hoofd-hals Radboud UMC |
Geen |
Geen |
Geen |
|
Burdorf |
Patiëntvertegenwoordiger |
Geen |
Geen |
Geen |
|
Verdouw |
Hoofd Infocentrum patiëntenvereniging HOOFD HALS |
Geen |
Werkzaam bij de patiëntenvereniging. De achterban heeft baat bij een herziening van de richtlijn |
Geen |
|
Karssemakers |
Hoofd-hals chirurg NKI/AVL
MKA-chirurg-oncoloog Amsterdam UMC (locatie AMC) / vakgroep kaakchirurgie Amsterdam West |
Niet van toepassing |
Geen |
Geen |
|
Goossens |
Verpleegkundig specialist, Haaglanden Medisch Centrum (HMC) |
* Bestuurslid (penningmeester) PWHHT (onbetaald) * Lid Commissie voorlichting PVHH (onbetaald) |
Geen |
Geen |
|
Zwezerijnen |
Nucleair geneeskundige, Amsterdam UMC (locatie Vumc)
PhD kandidaat, Amsterdam UMC (locatie Vumc) |
Lid als nucleair geneeskundige in HOVON imaging werkgroep (bespreken van richtlijnen en opzetten/uitvoeren van wetenschappelijke studies met betrekking tot beeldvorming in de hematologie); onbetaald |
Geen |
Geen |
|
Vogel |
Nucleair geneeskundige/radiotherapeut-oncoloog, AVL |
Geen |
In de afgelopen jaren incidenteel advies of onderwijs, betaald door Bayer, maar niet gerelateerd aan hoofd-hals
KWF-grant speekselklier toxiteit na behandeling. Geen belang bij de richtlijn |
Geen |
|
Graaf, de |
Radioloog, Amsterdam UMC (locatie Vumc) |
Bestuurslid sectie Hoofd-Hals radiologie (onbetaald) |
Geen |
Geen |
|
Weijs |
MKA-chirurg-oncoloog, Radboudumc |
MKA-chirurg, Weijsheidstand B.V. Werkzaam als algemeen praktiserend MKA-chirurg, betaald (0,1 fte) |
Geen |
Geen |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door het uitnodigen van de patiëntenvereniging HOOFD-HALS (PVHH) voor de Invitational conference en met afgevaardigden van de PVHH in de werkgroep. Het verslag hiervan (zie aanverwante producten) is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de patiëntenvereniging HOOFD-HALS en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met hoofd-halstumoren. De werkgroep beoordeelde de aanbeveling(en) uit de eerdere richtlijnmodule (NVKNO, 2014) op noodzaak tot revisie. Tevens zijn er knelpunten aangedragen door de patiëntenvereniging en genodigde partijen tijdens de Invitational conference (zie aanverwante producten voor het verslag van de Invitational conference). Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Waar relevant is er specifieke aandacht voor de (oudere) kwetsbare patiëntengroep in de overwegingen en wordt er ingegaan op de begeleiding en behandeling van deze patiënten.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE-gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodules is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen.
Herziening 2023
De werkgroep besloot na het bestuderen van alle aanbevelingen van de richtlijn Hoofd-halstumoren om in de periode 2019-2023 te werken aan de volgende updates:
- De indeling van de richtlijn is aangepast en per tumortype zijn alle relevante modules te vinden. Sommige modules (zoals Systemische therapie bij radiotherapie lokaal gevorderde tumoren) zijn daarom bij zowel Orofarynxcarcinoom, Hypofarynxcarcinoom, als Larynxcarcinoom in de richtlijn te vinden.
- In de meest recente UICC/AJCC classificatie is lipcarcinoom niet langer ondergebracht bij mondholte (TNM7) maar bij huid (TNM8). Dit brengt een verandering in stadiëring (volgens TNM8) met zich mee, maar niet in behandeling (volgens TNM7).
- Nieuwe modules zijn ontwikkeld over het bepalen van botinvasie, bepalen HPV-status, indicaties voor onderzoek naar afstandsmetastasen en het diagnostisch onderzoek naar afstandsmetastasen, de behandeling van HPV-positieve orofarynxtumoren, dosering cisplatin en systemische therapie bij radiotherapie voor lokaal gevorderde tumoren, en Tis/T1 supgraglottisch larynxcarcinoom.
- Een groot aantal modules zijn herzien. Literatuuronderbouwingen, overwegingen en aanbevelingen zijn geupdate.
- Een aantal modules zijn herbevestigd en waar nodig tekstueel verbeterd, waaronder de modules Diagnostiek hypofarynxcarcinoom en Premaligne afwijkingen larynx.
- Een aantal modules zijn vervallen: Indicaties FDG PET-CT-scan, Behandeling per lokalisatie en T-classificatie, Reconstructieve chirurgie mondholtecarcinoom, Invasieve chirurgie bij orofarynxcarcinoom, Reconstructieve chirurgie orofarynxcarcinoom, T1-T4N+ hypofarynxcarcinoom, Stemkwaliteit als uitkomstmaat na behandeling, T2- en kleine T3 larynxcarcinomen, Niet gemetastaseerde speekselklier tumoren. Deze modules bleken moeilijk te vatten in richtlijn, of zijn samengevoegd in een (nieuwe) module.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html.
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008 May 24;336(7654). doi: 10.1136/bmj.a139.
Schünemann, A Holger J (corrected to Schünemann, Holger J). PubMed PMID: 18483053; PubMed Central PMCID: PMC2386626.
Wessels M, Hielkema L, van der Weijden T. How to identify existing literature on patients' knowledge, views, and values: the development of a validated search filter. J Med Libr Assoc. 2016 Oct;104(4):320-324. PubMed PMID: 27822157; PubMed Central PMCID: PMC5079497.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.