Inleiding bij zwangerschap na IVF of ICSI
Uitgangsvraag
Wat is de toegevoegde waarde van het aanbieden van inleiding van de bevalling bij 39 weken zwangerschapsduur aan vrouwen die zwanger zijn geworden na IVF/ICSI?
Aanbeveling
Geef zwangere vrouwen die zwanger zijn geworden na IVF/ICSI dezelfde informatie over inductie van de bevalling na 39 weken als gebruikelijk is voor vrouwen die op natuurlijke wijze zwanger zijn geworden. De werkgroep adviseert om kwantitatieve informatie over het verhoogde risico op doodgeboorte toe te voegen aan de counseling in deze subgroep van vrouwen.
Overwegingen
Considerations – evidence to decision
Pros and cons of the intervention
It is well known that induction of labour near term has a beneficial impact on the reduction of perinatal morbidity and mortality. However, the Number Needed to Treat (NNT) is around 400 (Middleton, 2018). However, in a low-risk obstetric population current evidence states that induction of labour at 39 weeks has no (dis-) advantages as compared to expectant management (Grobman, 2018; Nielsen, 2005; Miller, 2015; NVOG 2020). It is feasible to suppose that uncomplicated pregnancies may be considered as low-risk pregnancies. The latter may also be true for pregnancies after IVF/ICSI if uncomplicated.
A historical cohort study found a systematically increased risk of stillbirth in low‐risk term pregnancies following IVF/ICSI (Bay, 2019). The risk of stillbirth in singleton low-risk pregnancies in spontaneous pregnancies has a prevalence of 0.1% and in pregnancies after IVF/ICSI of 0.3 %, leading to ORs for IVF of 1.7 (95% CI 0.9 to 3.1) and for ICSI of 2.2 (95% CI 1.2 to 3.1). The following hazard ratios for stillbirth (adjusted for fetal sex, maternal age, parity, smoking during pregnancy, and year of birth) were found when comparing pregnancies after IVF/ICSI versus spontaneously conceived pregnancies (Bay, 2019):
>37 weeks HR 2.4 (1.6 to 3.6)
>38 weeks HR 2.3 (1.5 to 3.6)
>39 weeks HR 2.5 (1.5 to 4.1)
> 40 weeks HR 3.0 (1.7 to 5.2)
>41 weeks HR 2.4 (0.9 to 5.9)
>42 weeks HR 6.8 (1.3 to 37)
On the other hand, induction of labour may imply a longer duration of labour, more involvement of midwives, nursing staff and occupation of labour ward rooms. Planned induction of these pregnancies therefore challenges the health care system in logistics and organization.
The experiences of the pregnant woman and her birth partner must also be taken into account.
Values and preferences of patients (and their caregivers)
From the women’s perspective it can be reasoned that they want to give birth to their child in the best possible health. The potential advantages of induction of labour at 39 weeks in pregnant women after IVF/ICSI have to be weighed against women’s preferences.
Any improvement in perinatal outcomes should be balanced against a possibly more technical development and longer duration of labour, which necessitates shared decision making. At minimum, information about the possibility of induction of labour has to be addressed by the healthcare professional in a timely manner. As for all interventions that have to be instituted, it is of utmost importance to inform the women and their partners in order for informed consent to be given. The woman and her partner have to make the final decision for a particular intervention. Of course, this may include the advice from a healthcare professional. Retrospectively, this decision process should be documented in the clinical charts.
Costs
The potential of an overcrowding of birthing rooms, the need for more care staff and an increase in hospital costs must be taken into account.
Acceptability, feasibility and implementation
This aspect can vary greatly between different health care systems. There is currently no evidence of the acceptability and feasibility of systematic elective induction at term. Due to a longer stay in the birthing room or maternity unit, the accessibility to this intervention may not be equally feasible. A number of studies have been carried out and are currently underway to check the safety of induction, in part, at home or at least outside of a hospital setting, but currently it is unclear whether this practice has the same degree of safety as in-hospital care. There is a political trend, especially in the context of pregnancies and childbirth, to work on the shortest possible admission and low variable care, which is in contrast to the rising trend towards more inductions. Considering the primary perinatal outcomes, there is also no disadvantage in elective induction.
Differences between countries
It appeared that regional differences within and between the countries exist in their policy of induction of labour at term for women whose pregnancies conceived after IVF/ICSI.
This is a brief overview of education and preferred management according to the knowledge of the guideline authors in order to describe the differences between countries. Irrespective of which country, all interventions should be made after informed consent has been given.
In the United Kingdom, there are no formal recommendations to guide timing of birth in pregnancies conceived by IVF/ICSI. Nonetheless, the association between assisted conception and higher stillbirth rates means that many clinicians discuss IOL (or planned caesarean birth if appropriate) with women around 40 weeks of gestation.
In the Netherlands, planned IOL before 41 weeks of gestation is not routinely discussed or offered to pregnant women after IVF/ICSI.
In Belgium, conception through IVF/ICSI is not a reason for planned IOL at 39 weeks of gestation.
In Germany, there are no systematic recommendations to indicate IOL directly due to IVF/ICSI. However, due to other commonly associated risk factors leading to IVF/ICSI, these pregnancies are classified as high risk and are commonly scheduled for IOL after 39 weeks, if not before due to other commonly associated risk factors such as gestational diabetes, preeclampsia or advanced maternal age.
Recommendation
Rationale of the recommendation: weighing arguments in favour and against the intervention
To date, there is no evidence that induction at 39 weeks in pregnancies after IVF/ICSI has benefits or harms for both woman and baby. The strength of the recommendation is based on current evidence that induction of labour at 39 weeks in a low-risk obstetric population has no (dis-)advantages when compared to expectant management. Nevertheless, in pregnancies after IVF/ICSI, induction of labour at 39 weeks might be beneficial because of the increased stillbirth rate in those pregnancies compared to pregnancies after spontaneous conception (1/1000 versus 3/1000 pregnancies)(Bay, 2019).
It seems reasonable to discuss the possibility of elective IOL in low-risk women at or beyond 39 weeks of gestation. More specifically, in pregnancies after IVF/ICSI, induction of labour at 39 weeks of gestation should be considered given the elevated risk of stillbirth in this subgroup of women.
|
Advice about induction of labour at 39 weeks for women who conceived after IVF/ICS should be the same as the advice offered to women who conceived spontaneously. However, the guideline development panel suggests adding quantitative information about the elevated stillbirth risk to the counseling in this subgroup of women. |
Onderbouwing
Achtergrond
Introduction
In high resource settings, 1-5% of all children are born after IVF/ICS treatment of their mothers. Currently, in the decision making of inducing labour, no distinction is made between women who conceived spontaneously or after IVF/ICSI. However, health care providers may be prone to induce labour in pregnancies after IVF/ICSI at an earlier gestation than 41 weeks, often at request of the pregnant woman.
Is there a rationale to induce labour in women who conceived by IVF/ICSI? What are the (possible) adverse effects on maternal and neonatal outcomes when women who conceived by IVF/ICSI are induced and spontaneous labour is not awaited?
Zoeken en selecteren
Search and select
A systematic review of the literature was performed to answer the following question:
What are the consequences of induction of labour at 39 weeks of gestation in women who conceived after IVF/ICSI on maternal and fetal outcomes?
P: term pregnant women with singleton pregnancies who conceived after IVF or ICSI;
I: induction of labour at 39 weeks of gestation;
C: expectant management;
O: instrumental birth, cesarean birth, neonatal mortality and morbidity, postpartum hemorrhage, women’s experience, pain, perineal trauma, psychological trauma, mother-child bonding, infant feeding.
Relevant outcome measures
The guideline development group considered neonatal mortality and morbidity as a crucial outcome measures for decision making. Caesarean birth, postpartum hemorrhage, women’s experience, pain, perineal trauma, psychological trauma, mother-child bonding and infant feeding are other important outcome measures for decision making.
A priori, the guideline development group did not define the outcome measures listed above but used the definitions used in the studies.
The guideline development group defined a Relative Risk (RR) <0.8 or >1.25 as a minimal clinically important effect.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched using relevant search terms until October 2019. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 409 hits. Studies were selected based on the following criteria: randomised or non-randomised studies comparing induction of labour at 39 weeks of gestation with expectant management in women who conceived after IVF or ICSI. Twelve studies were initially selected based on title and abstract screening. After reading the full text, all 12 studies were excluded (see the table with reasons for exclusion under the tab Methods), and no studies were included.
Results
No studies were included in the analysis of the literature, since none of the studies answered the search question.
Referenties
- Bay, B., Boie, S., & Kesmodel, U. S. (2019). Risk of stillbirth in low‐risk singleton term pregnancies following fertility treatment: a national cohort study. BJOG: An International Journal of Obstetrics & Gynaecology, 126(2), 253-260.
- Dutch Society of Obstetrics and Gynaecology (NVOG). Electieve inductie van de baring bij a terme zwangeren/Elective induction of parturition in term pregnant women. 2020. Available from: https://richtlijnendatabase.nl/richtlijn/electieve_inductie_module/electieve_inductie_van_de_baring_bij_aterme_zwangeren.html
- Grobman WA, Rice MM, Reddy UM , et al. Labor induction versus expaectnt management in low-risk nulliparous women. N Engl J Med. 2018 Aug 9;379(6):513-523.
- Middleton, P., Shepherd, E., & Crowther, C. A. (2018). Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews, (5).
- Miller NR, Cypher RL, Foglia LM, Pates JA, Nielsen PE. Elective Induction of Labor Compared With Expectant Management of Nulliparous Women at 39 Weeks of Gestation: A Randomized Controlled Trial. Obstet Gynecol. 2015 Dec;126(6):1258- 64.
- Nielsen PE, Howard BC, Hill CC, Larson PL, Holland RH, Smith PN. Comparison of elective induction of labor with favorable Bishop scores versusexpectant management: a randomized clinical trial. Journal of Maternal-Fetal & Neonatal Medicine. 2005; 18(1):59–64.
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 30-12-2022
Beoordeeld op geldigheid : 30-12-2022
Validity period
The Board of the Dutch Society of Obstetrics and Gynaecology (NVOG) will assess whether these guidelines are still up-to-date in 2027 at the latest. If necessary, a new working group will be appointed to revise the guideline. The guideline’s validity may lapse earlier if new developments demand revision at an earlier date.
As the holder of this guideline, the NVOG is chiefly responsible for keeping the guideline up to date. Other scientific organizations participating in the guideline or users of the guideline share the responsibility to inform the chiefly responsible party about relevant developments within their fields.
Algemene gegevens
Er is meegelezen vanuit de Nederlandse Vereniging voor Kindergeneeskunde (NVK). De NVK heeft de richtlijn niet geautoriseerd, maar heeft geen bezwaar tegen publicatie.
De Koninklijke Nederlandse Organisatie van Verloskundigen (KNOV) is betrokken geweest bij de ontwikkeling van de richtlijn.
De Patiëntenfederatie Nederland heeft de richtlijn goedgekeurd.
De Vlaamse Vereniging voor Obstetrie en Gynaecologie (VVOG), Royal College of Obstetrics and Gynaecology (RCOG) en Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (DGGG) zijn betrokken geweest bij de ontwikkeling van de richtlijn.
Regiehouder: NVOG
Samenstelling werkgroep
Composition guideline development panel
An international panel for the development of the guidelines was formed in 2019. The panel consisted of representatives from all relevant medical disciplines that are involved in medical care for pregnant women.
All panel members have been officially delegated for participation in the guideline development panel by their (scientific) societies. The panel developed the guidelines in the period from May 2019 until March 2021.
The guideline development panel is responsible for the entire text of this guideline.
All panel members have been officially delegated for participation in the guideline development panel by their scientific societies. The guideline development panel is responsible for the entire text of this guideline.
Guideline development panel
- J.J. Duvekot, obstetrician, Consultant Obstetrics and Gynaecology, Erasmus Medical Centre, Rotterdam, the Netherlands (chair)
- I. Dehaene, obstetrician, Consultant Obstetrics and Gynaecology, Ghent University Hospital Belgium
- S. Galjaard, obstetrician, Consultant Obstetrics and Gynaecology, Erasmus Medical Centre, Rotterdam, the Netherlands
- A. Hamza, obstetrician, Consultant Obstetrics and Gynaecology, University Medical Center of Saarland, Homburg an der Saar, Germany
- S.V. Koenen, obstetrician, Consultant Obstetrics and Gynaecology, ETZ, locatie Elisabeth Ziekenhuis Tilburg, the Netherlands
- M. Kunze, obstetrician, Consultant Obstetrics and Gynaecology, Department of Gynecology& Obstetrics University of Freiburg, Germany
- M.A. Ledingham, obstetrician, Consultant Obstetrics and Gynaecology, the Queen Elizabeth Hospital Glasgow, UK
- B. Magowan, obstetrician, Consultant Obstetrics and Gynaecology, and Co-Chair UK RCOG Guidelines Committee, NHS Borders, Scotland, UK
- G. Page, obstetrician, Consultant Obstetrics and Gynaecology, Jan Yperman Hospital, Ypres, Belgium
- S.J. Stock, Reader and Consultant in Maternal and Fetal Medicine, University of Edinburgh Usher Institute and NHS Lothian, Edinburgh, Scotland, UK
- A.J. Thomson, obstetrician, Consultant Obstetrics and Gynaecology, Royal Alexandra Hospital (NHS Greater Glasgow and Clyde), UK
- G. Verhulst, obstetrician, Consultant Obstetrics and Gynaecology, ASZ Aalst/Geraardsbergen/Wetteren, Belgium
- D.C. Zondag, midwife/practice owner verloskundige praktijk De Toekomst-Geldermalsen, the Netherlands
Methodological support
- E. den Breejen, senior advisor, Knowledge Institute of the Dutch Association of Medical Specialists (until June 2019)
- J.H. van der Lee, senior advisor, Knowledge Institute of the Dutch Association of Medical Specialists (since May 2019)
- Y. Labeur, junior advisor, Knowledge Institute of the Dutch Association of Medical Specialists
Belangenverklaringen
Declarations of interests
The Code for the prevention of improper influence due to conflicts of interest was followed (https://storage.knaw.nl/2022-08/Code-for-the-prevention-of-improper-influence-due-to-conflicts-of-interest.pdf).
The working group members have provided written statements about (financially supported) relations with commercial companies, organisations or institutions related to the subject matter of the guideline during the past three years. Furthermore, inquiries have been made regarding personal financial interests, interests due to personal relationships, interests related to reputation management, interest related to externally financed research and interests related to knowledge valorisation. The chair of the guideline development panel is informed about changes in interests during the development process. The declarations of interests are reconfirmed during the commentary phase. The declarations of interests can be requested at the administrative office of the Knowledge Institute of the Dutch Association of Medical Specialists and are summarised below.
|
Last name |
Principal position |
Ancillary position(s) |
Declared interests |
Action |
|
Duvekot (chair) |
Consultant Obstetrics and Gynaecology, Erasmus MC, Rotterdam |
Director Medisch Advies en Expertise Bureau Duvekot, Ridderkerk |
none |
none |
|
Dehaene |
Consultant Obstetrics and Gynaecology, Ghent University Hospital |
none |
none |
none |
|
Galjaard |
Consultant Obstetrics and Gynaecology, Erasmus MC, Rotterdam |
Associated member of Diabetes in Pregnancy Group (DPSG) |
none |
none |
|
Hamza |
Consultant Obstetrics and Gynaecology, University Medical Center of Saarland, Homburg |
part of the advisory board of clinical innovations, which produces Kiwi-Vacuum Extractors® and Ebb Balloon Catheter®;
|
gave ultrasound courses sponsored by ultrasound producing companies: Samsung Germany and Matramed |
Recommendations do not involve either vacuum extractor or Ebb catheter (which is used for postpartum hemorrhage); therefore no actions |
|
Koenen |
Consultant Obstetrics and Gynaecology, ETZ, locatie Elisabeth Ziekenhuis Tilburg |
Chairman 'Koepel Kwaliteit' NVOG |
none |
none |
|
Kunze |
Divison Chief, Maternal-Fetal Medicine and Obstetrics, Departement of Gynecology & Obstetrics, University of Freiburg |
none |
none |
none |
|
Ledingham |
Consultant in Maternal and Fetal Medicine, Queen Elizabeth Hospital, Glasgow |
Co-chair RCOG Guidelines committee, Guideline developer for sign (scottisch intercollegiate guidelines group) |
none |
none |
|
Magowan |
Consultant Obstetrics and Gynaecology, and Co-Chair UK RCOG Guidelines Committee, NHS Borders, Scotland |
Co-chair RCOG Guidelines committee |
none |
none |
|
Page |
Consultant Obstetrics and Gynaecology, Jan Yperman Hospital, Ypres |
none |
none |
none |
|
Stock |
Reader and Consultant in Maternal and Fetal Medicine, University of Edinburgh and NHS Lothian, Edinburgh, Scotland, UK |
Consultant Obstetrician and Subspecialist Maternal and Fetal Medicine, member of the NIHR HTA General committee (grant funding board) and Chair of the RCOG Stillbirth Clinical Studies Group |
Research grants paid to the institution for research into pregnancy problems from National Institute of Healthcare Research (NIHR) Health Technology Assessment (HTA), NIHR Global Research Fund, Wellcome Trust, Medical Research Council, Tommy's Baby Charity, Cheif Scientist Office Scotland. Some of this work focuses on improving risk prediction of preterm labour and researching the benefits and harms of antenatal corticosteroids. Non-financial support from HOLOGIC, non-financial support from PARSAGEN, non-financial support from MEDIX BIOCHEMICA during the conduct of an NIHR HTA study in the form of provision of reduced cost assay kits to participating sites and blinded test assay analysers |
none |
|
Thomson |
Consultant Obstetrics and Gynaecology, Royal Alexandra Hospital (NHS Greater Glasgow and Clyde) |
Guideline developer for the RCOG |
none |
none |
|
Verhulst |
Head of Department of Gynaecology and Obstetrics, ASZ Aalst/Geraardsbergen/Wetteren |
none |
none |
none |
|
Zondag |
Midwife/practice owner verloskundige praktijk De Toekomst-Geldermalsen |
Policy adviser at the Dutch association of midwives (KNOV). Teacher at PA clinical midwives - Hogeschool Rotterdam |
none |
none |
Inbreng patiëntenperspectief
Representation of the patient perspective
Involvement of patient representatives from all four participating countries was challenging. Representatives of patient organisations from three countries (UK, Belgium, the Netherlands) commented on the draft guideline texts and discussed these during an online meeting. They represented the RCOG Women’s Network, the Flemish organisation for people with fertility problems ‘De verdwaalde ooievaar’, the Netherlands Patient Federation, and the Dutch association for people with fertility problems ‘Freya’. The comments were discussed and where relevant incorporated by the guideline development panel.
Methode ontwikkeling
Evidence based
Implementatie
Implementation
Guideline implementation and practical applicability of the recommendations was taken into consideration during various stages of guideline development. Factors that may promote or hinder implementation of the guideline in daily practice were given specific attention.
The guideline is distributed digitally among all relevant professional groups. The guideline can also be downloaded from the following websites: www.nvog.nl, www.vvog.be, www.rcog.org.uk, www.dggg.de, and the Dutch guideline website: www.richtlijnendatabase.nl.
Werkwijze
Method
AGREE
This guideline has been developed conforming to the requirements of the report of Guidelines for Medical Specialists 2.0 by the advisory committee of the Quality Counsel. This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (www.agreetrust.org)(Brouwers, 2010), a broadly accepted instrument in the international community and on the national quality standards for guidelines: “Guidelines for guidelines” (www.zorginstituutnederland.nl).
Identification of subject matter
During the initial phase of the guideline development the chairman, guideline development panel and the advisor inventoried the relevant subject matter for the guideline. Since this was a pilot project, the content of the questions and the support base in clinical practice was considered of less importance than the process of international collaboration and learning from each other. Key questions were selected in such a way that:
-
-
- they were relevant for obstetric practice in all collaborating countries;
- it was expected that the amount of literature identified for each question would be reasonable, i.e. some literature was expected, but not much;
- the recommendations were expected not to lead to extensive discussion among working group members because no major controversy was expected;
- there were no recent guidelines available for these particular topics in any of the four countries.
-
Clinical questions and outcomes
The guideline development panel then formulated definitive clinical questions and defined relevant outcome measures (both beneficial and harmful effects). The working group rated the outcome measures as critical, important and not important. Furthermore, where applicable, the working group defined relevant clinical differences.
Strategy for search and selection of literature
For the separate clinical questions, specific search terms were formulated and published scientific articles were searched for in (several) electronic databases. Furthermore, studies were scrutinized by cross-referencing for other included studies. The studies with potentially the highest quality of research were searched for first. The panel members selected literature in pairs (independently of each other) based on title and abstract. A second selection was performed based on full text. The databases, search terms and selection criteria are described in the modules containing the clinical questions.
Quality assessment of individual studies
Individual studies were systematically assessed, based on methodological quality criteria that were determined prior to the search, so that risk of bias could be estimated. This is described in the “risk of bias” tables.
Summary of literature
The relevant research findings of all selected articles are shown in evidence tables. The most important findings in literature are described in literature summaries. In case there were enough similarities between studies, the study data were pooled.
Grading quality of evidence and strength of recommendations
The strength of the conclusions of the scientific publications was determined using the GRADE-method. GRADE stands for Grading Recommendations Assessment, Development and Evaluation (see http://www.gradeworkinggroup.org/).
GRADE defines four gradations for the quality of scientific evidence: high, moderate, low or very low. These gradations provide information about the amount of certainty about the literature conclusions (http://www.guidelinedevelopment.org/handbook/).
The basic principles of the GRADE method are: formulating and prioritising clinical (patient) relevant outcome measures, a systematic review for each outcome measure, and appraisal of the evidence for each outcome measure based on the eight GRADE domains (domains for downgrading: risk of bias, inconsistency, indirectness, imprecision, and publication bias; domains for upgrading: dose-effect association, large effect, and residual plausible confounding).
GRADE distinguishes four levels for the quality of the scientific evidence: high, moderate, low and very low. These levels refer to the amount of certainty about the conclusion based on the literature, in particular the amount of certainty that the conclusion based on the literature adequately supports the recommendation (Schünemann, 2013; Hultcrantz, 2017).
|
Definition |
|
|
High |
|
|
Moderate |
|
|
Low |
|
|
Very low |
|
For the wording of the conclusions we used the statements suggested by the GRADE working group (Santesso, 2020), as shown below.
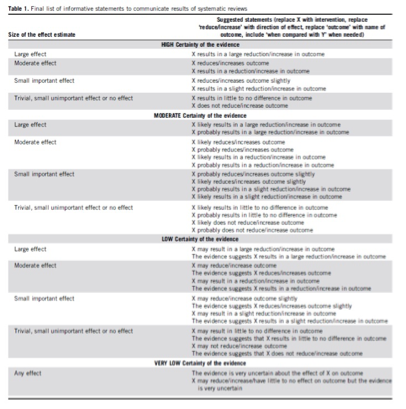
Source: Santesso (2020)
The limits of clinical decision making are very important in grading the evidence in guideline development according to the GRADE methodology (Hultcrantz, 2017). Exceedance of these limits would give rise to adaptation of the recommendation. All relevant outcome measures and considerations need to be taken into account to define the limits of clinical decision making. Therefore, the limits of clinical decision making are not one to one comparable to the minimal clinically relevant difference. In particular for interventions of low costs and without important drawbacks the limit of clinical decision making regarding the effectiveness of the intervention may be lower (i.e. closer to no effect) than the Minimal Clinically Important Difference (MCID) (Hultcrantz, 2017).
Considerations (evidence to decision)
Aspects such as expertise of working group members, patient preferences, costs, availability of facilities, and organisation of healthcare aspects are important to consider when formulating a recommendation. For each clinical question, these aspects are discussed in the paragraph Considerations, using a structured format based on the evidence-to-decision framework of the international GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). The evidence-to-decision framework is an integral part of the GRADE methodology.
Recommendations provide an answer to the primary question, and are based on the best scientific evidence available and the most important considerations. The level of scientific evidence and the importance given to considerations by the working group jointly determine the strength of the recommendation. In accordance with the GRADE method, a low level of evidence for conclusions in the systematic literature review does not rule out a strong recommendation, while a high level of evidence may be accompanied by weak recommendations. The strength of the recommendation is always determined by weighing all relevant arguments.
Knowledge gaps
During the development of this guideline, systematic searches were conducted for research contributing to answering the primary questions. For each primary question, the working group determined whether (additional) scientific research is desirable.
Commentary and authorisation phase
The concept guideline was subjected to commentaries by the scientific societies and patient organisations involved. The draft guideline was also submitted to the following organisations for comment: RCOG Guideline Committee and RCOG Patient Information Committee, German Neonatology and Peaediatric Intensive Care Association (Gesellschaft für Neonatologie und pädiatrische Intensivmedzin e.V.), German Midwives Society (Deutscher Hebammenverband), Flemish Midwives Society (VBOV), Belgian Federal Knowledge Centre for Health Care (KCE), Flemish College of Maternity and Neonatal Medicine (College Moeder Kind), Flemish patient organization for fertility problems (De Verdwaalde Ooievaar), Dutch Pediatric Society (NVK), Dutch College of General Practitioners (NHG), Healthcare Insurers Netherlands (ZN), The Dutch Healthcare Authority (NZA), the Health Care Inspectorate (IGJ), Netherlands Care Institute (ZIN), Dutch Organisation of Midwives (KNOV), Hospital organization (NVZ), Patient organisations Dutch Patient Federation and Freya. The comments were collected and discussed with the working group. The feedback was used to improve the guideline; afterwards the working group made the guideline definitive. The final version of the guideline was offered for authorization to the involved scientific societies and patient organisations and was authorized or approved, respectively.
Legal standing of guidelines
Guidelines are not legal prescriptions but contain evidence-based insights and recommendations that care providers should meet in order to provide high quality care. As these recommendations are primarily based on ‘general evidence for optimal care for the average patient’, care providers may deviate from the guideline based on their professional autonomy when they deem it necessary for individual cases. Deviating from the guideline may even be necessary in some situations. If care providers choose to deviate from the guideline, this should be done in consultation with the patient, where relevant. Deviation from the guideline should always be justified and documented.
Literature
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open 2017;7:e018593.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016;353:i2016.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Santesso, N., Glenton, C., Dahm, P., Garner, P., Akl, E. A., Alper, B., ... & GRADE Working Group. (2020). GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of clinical epidemiology, 119, 126-135.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10.
Schünemann, A Holger J [corrected to Schünemann, Holger J]. PubMed PMID: 18483053; PubMed Central PMCID: PMC2386626.
Wessels M, Hielkema L, van der Weijden T. How to identify existing literature on patients' knowledge, views, and values: the development of a validated search filter. J Med Libr Assoc. 2016 Oct;104(4):320-324.
Zoekverantwoording
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Allen, 2006 |
no comparison induction versus expectant management |
|
Henningsen, 2014 |
no information about induction |
|
Jackson, 2004 |
no comparison induction versus expectant management |
|
Jenabi, 2018 |
no comparison induction versus expectant management |
|
Källén, 2005 |
no comparison induction versus expectant management |
|
McGovern, 2004 |
no comparison induction versus expectant management |
|
Pandey, 2012 |
no comparison induction versus expectant management |
|
Sun, 2014 |
no comparison induction versus expectant management |
|
Szymankiewicz, 2004 |
no information about induction |
|
Tallo, 1995 |
no comparison induction versus expectant management |
|
Vannuccini, 2018 |
no comparison induction versus expectant management |
|
Yang, 2014 |
no information about induction |
Literature search strategy
|
Database |
Search elements |
Total |
|
Medline (OVID)
|
1 exp Reproductive Techniques, Assisted/ or ivf.ti,ab,kw. or icsi.ti,ab,kw. or ((extracorporeal or 'in vitro' or invitro) and fertili*).ti,ab,kw. or (embryo* and (transfer* or implantat*)).ti,ab,kw. or (intracytoplas* and sperm).ti,ab,kw. or (((assisted and reproducti*) or (infertility or fertility)) and (technique* or technolog* or therap* or treatment*)).ti,ab,kw. or art.ti,ab,kw. or 'artificial insemination'.ti,ab,kw. (229091) 2 exp Labor, Induced/ or ((labo*r or delivery or parturition) adj3 (induc* or accelerat* or premature or stimulat*)).ti,ab,kw. or priming.ti,ab,kw. or planned early deliver*.ti,ab,kw. or interventionist*.ti,ab,kw. (61761) 3 1 and 2 (1013) 4 limit 3 to english language (907) 5 (meta-analysis/ or meta-analysis as topic/ or (meta adj analy$).tw. or ((systematic* or literature) adj2 review$1).tw. or (systematic adj overview$1).tw. or exp "Review Literature as Topic"/ or cochrane.ab. or cochrane.jw. or embase.ab. or medline.ab. or (psychlit or psyclit).ab. or (cinahl or cinhal).ab. or cancerlit.ab. or ((selection criteria or data extraction).ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (417946) 6 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (1912744) 7 Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or (cohort adj (study or studies)).tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] (3292331) 8 4 and 5 (34) 9 (4 and 6) not 8 (103) 10 (4 and 7) not (8 or 9) (182) 11 8 or 9 or 10 (319)
= 319 (94 unique) |
409 |
|
Embase
|
'infertility therapy'/exp/mj OR ivf:ti,ab OR icsi:ti,ab OR ((extracorporeal:ti,ab OR 'in vitro':ti,ab OR invitro:ti,ab) AND fertili*:ti,ab) OR (embryo*:ti,ab AND (transfer*:ti,ab OR implantat*:ti,ab)) OR (intracytoplas*:ti,ab AND sperm:ti,ab) OR ((assisted:ti,ab AND reproducti*:ti,ab OR infertility:ti,ab OR fertility:ti,ab) AND (technique*:ti,ab OR technolog*:ti,ab OR therap*:ti,ab OR treatment*:ti,ab)) OR art:ti,ab OR 'artificial insemination':ti,ab AND 'labor induction'/exp/mj OR (((labo*r OR delivery OR parturition) NEAR/3 (induction OR induce* OR inducing OR accelerat* OR premature OR stimulat* OR immediate)):ti,ab) OR priming:ti,ab OR 'planned early deliver*':ti,ab OR interventionist*:ti,ab AND [english]/lim NOT 'conference abstract':it
Used filters: Sytematic reviews ('meta analysis'/de OR cochrane:ab OR embase:ab OR psycinfo:ab OR cinahl:ab OR medline:ab OR ((systematic NEAR/1 (review OR overview)):ab,ti) OR ((meta NEAR/1 analy*):ab,ti) OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp)
RCT’s ('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it
Other controlled studies 'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti)
= 320 (315 unique) |
