Verlengde tocolyse
Uitgangsvraag
Wat is de waarde van het langer dan 48 uur behandelen met tocolytica van een zwangeren met een dreigende vroeggeboorte?
Aanbeveling
Geef geen verlengde tocolyse bij vrouwen met een dreigende vroeggeboorte die na 48 uur tocolyse nog niet bevallen zijn.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
In de literatuuranalyse werd onderzocht wat de waarde is van het langer dan 48 uur behandelen met tocolytica van een zwangere met een dreigende vroeggeboorte onder de 34 weken. Drie RCT’s onderzochten het effect van een verlengde tocolyse na 48 uur in zwangeren met een dreigende vroeggeboorte tussen de 26 en 34 weken (Aggarwal, 2018; Roos, 2013; Van Vliet, 2016). Daarnaast onderzocht één RCT de waarde van een verlengde tocolyse na 48 uur bij zwangeren met een dreigende vroeggeboorte tussen de 24 en 34 voltooide weken (Parry, 2014). De bewijskracht voor de cruciale uitkomstmaten ‘neonatale mortaliteit’ en ‘overleving zonder neurologische ontwikkelingsstoornissen’ was laag tot zeer laag vanwege tegenstrijdige resultaten en grote spreiding in de richting van het effect. Er is geen sprake van een klinisch relevant effect. Andere studies kunnen leiden tot nieuwe inzichten. Er kunnen op basis van alleen de literatuur geen sterke aanbevelingen geformuleerd worden over de waarde van het langer dan 48 uur behandelen met tocolytica bij een zwangere met een dreigende vroeggeboorte onder de 34 weken.
Het toedienen van tocolyse in de vorm van nifedipine leidt vaak tot bijwerkingen (oa hypotensie, malaise, oedeem, hoofdpijn, obstipatie). En daarom is het van belang om deze medicatie alleen te geven als er een duidelijk positief effect is.
Voor de uitkomst intra-ventriculaire bloeding werd een, weliswaar niet statistisch significant, effect gevonden, van 2/227 in de verlengde tocolyse groep versus 6/236 in de geen verlengde tocolyse groep. Gezien de zeer lage aantallen kunnen hier geen conclusies uit getrokken worden, maar het is niet uitgesloten dat in grotere groepen er een positief effect zou zijn van verlengde tocolyse op de uitkomst IVH. Echter er wordt geen verschil gezien in vroeggeboorte, de uitkomst waar IVH sterk mee is geassocieerd. Aldus is een verschil tussen wel en niet verlengde tocolyse niet aannemelijk.
Uit de huidige studies is geen voordeel te zien in specifieke subgroepen. Voor de groep vrouwen met een extreme dreigende vroeggeboorte van 24 tot 26 weken AD zou het potentiële effect het grootst zijn, echter werden er geen studies gevonden voor specifiek die subgroep, en aldus kan geen uitspraak worden gedaan over een effect bij die groep.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Hoewel er bij de patient een nadrukkelijk voorkeur zou kunnen bestaan om iets te doen om het risico op vroeggeboorte kleiner te maken, is er geen bewijs gevonden dat verlengde tocolyse een voordeel heeft ten opzichte van placebo. Dus ook bij verzoek vanuit de patient lijkt voorschrijven van nifedipine niet aangewezen.
Aangezien het blijvende verhoogde risico op vroeggeboorte bij vrouwen na een episode met dreigende vroeggeboorte en een korte cervix en/of een verhoogd fibronectine, en de angst die mogelijk ontstaan is na de episode van dreigende vroeggeboorte is het van belang om vrouwen psychisch te blijven ondersteunen na ontslag en duidelijke instructies te geven wanneer zich weer te melden in geval van klachten (Hermans, 2015). Tevens is het goed om te bespreken wat patiente wel en niet kan doen qua fysieke belasting na de episode van dreigende vroeggeboorte.
Kosten (middelenbeslag)
Niet van toepassing gezien behandeling niet wordt aanbevolen.
Aanvaardbaarheid, haalbaarheid en implementatie
Aangezien er geen behandeling wordt aangeraden worden er geen problemen voorzien in de implementatie. Mogelijk zijn er klinieken die deze behandeling reeds aanboden. Aangezien er ook geen enkele trend is waargenomen richting een positief effect van verlengde tocolyse, zou het geen probleem moeten opleveren om deze behandeling niet meer aan te bieden.
Helaas is er geen bewezen effectieve behandeling bij vrouwen die na initiële tocolyse nog niet zijn bevallen, maar nog wel een verhoogd risico hebben op vroeggeboorte. Ook het gebruik van een Arabin pessarium is helaas bewezen niet effectief, zoals aangetoond in de Apostel VI studie (Hermans, 2018). Zoals in de module progesteron is beschreven is het nut van het gebruik van progesteron bij vrouwen na een episode van dreigende vroeggeboorte niet duidelijk. Ten aanzien van restricties van fysieke activiteit, is er voldoende bewijs van het gebrek aan effect. Het beperken van fysieke activiteit moet dan ook niet worden aangemoedigd in deze groep zwangeren. Te meer omdat het beperken van fysieke activiteit duidelijke nadelen heeft, waaronder verminderde spierkracht, verminderde botmineralisatie, verminderde cardiovasculaire conditie, meer obstipatie, risico op trombose en veranderingen in het endocrine en immuun systeem. Tevens heefte het beperken van fysieke activiteit psychologische impact. Hiermee is er bij vrouwen die met ontslag gaan na een episode van dreigende vroeggeboorte geen aanvullend advies, anders dan wat voor elke zwangere als gezond wordt beschouwd. Blijft nog het overwegen van psychische ondersteuning en instructies ten aanzien van blijvend verhoogd risico op vroeggeboorte en wanneer contact op te nemen bij opnieuw tekenen van dreigende vroeggeboorte (Saccone, 2023; Gascoigne 2022).
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Gezien het ontbreken van enig effect van verlengde tocolyse is er geen reden om deze behandeling aan te raden.
Er is dus ook geen reden om tocolyse langer dan 48 uur te geven als het stop tijdstip midden in de nacht zou vallen, of op een ander minder gunstig moment, gezien het ontbreken van enig effect daarvan.
Onderbouwing
Achtergrond
Currently in most hospitals tocolysis is given for the maximal duration of 48 hours. This is the time it takes for the antenatal corticosteroids to have its optimal function. Because women with a threatened preterm birth remain high risk for preterm delivery, giving a prolonged treatment might be beneficial in preventing of postponing preterm birth.
Conclusies / Summary of Findings
|
Very low GRADE |
The evidence is very uncertain about the effect of prolonged tocolysis after 48 hours on neonatal mortality when compared with tocolysis stop after 48 hours in pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours.
Source: Aggarwal, 2018; Parry, 2014; Roos, 2013 |
|
Low GRADE |
The evidence suggests that prolonged tocolysis after 48 hours results in little to no difference in neonatal sepsis when compared with tocolysis stop after 48 hours in pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours.
Source: Aggarwal, 2018; Roos, 2013 |
|
Low GRADE |
The evidence suggests that prolonged tocolysis after 48 hours results in little to no difference in composite outcome of neonatal morbidity and mortality when compared with tocolysis stop after 48 hours in pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours.
Source: Aggarwal, 2018; Roos, 2013 |
|
Very low GRADE |
The evidence is very uncertain about the effect of prolonged tocolysis after 48 hours on respiratory distress syndrome when compared with tocolysis stop after 48 hours in pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours.
Source: Aggarwal, 2018; Roos, 2013 |
|
Very low GRADE |
The evidence is very uncertain about the effect of prolonged tocolysis after 48 hours on intraventricular hemorrhage when compared with tocolysis stop after 48 hours in pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours.
Source: Aggarwal, 2018; Roos, 2013 |
|
Very low GRADE |
The evidence is very uncertain about the effect of prolonged tocolysis after 48 hours on periventricular leukomalacia when compared with tocolysis stop after 48 hours in pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours.
Source: Roos, 2013 |
Preterm birth
|
Very low GRADE |
The evidence is very uncertain about the effect of prolonged tocolysis after 48 hours on preterm birth before 32 weeks when compared with tocolysis stop after 48 hours in pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours.
Source: Parry, 2014; Roos, 2013 |
|
Moderate GRADE |
Prolonged tocolysis after 48 hours probably results in little to no difference in in preterm birth before 34 weeks when compared with tocolysis stop after 48 hours in pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours.
Source: Aggarwal, 2018; Parry, 2014; Roos, 2013 |
|
Low GRADE |
The evidence suggests that prolonged tocolysis after 48 hours results in little to no difference in preterm birth before 37 weeks when compared with tocolysis stop after 48 hours in pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours.
Source: Aggarwal, 2018; Parry, 2014; Roos, 2013 |
|
Low GRADE |
The evidence suggests that prolonged tocolysis after 48 hours results in little to no difference in survival without neurodevelopmental impairment when compared with tocolysis stop after 48 hours in pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours.
Source: Van Vliet, 2016 |
Samenvatting literatuur
Description of studies
Aggarwal (2018) performed a randomized open-label study to assess the effect of maintenance tocolysis with nifedipine after acute tocolysis was used to arrest an episode of preterm labor. Women with a singleton pregnancy between 26+0 and 33+6 weeks of gestation were included. Exclusion criteria were women with antepartum hemorrhage, fetal malformation or demise, severe fetal growth restriction, advanced preterm labor (cervical dilatation >4 cm), ruptured membranes, contraindication to nifedipine or any indication necessitating delivery. In total, 25 women received maintenance tocolysis with nifedipine (20 mg 8th hourly orally for 12 more days) and 25 women did not receive maintenance tocolysis. Groups were comparable at baseline. Outcomes of interest were neonatal mortality, neonatal sepsis, respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), a composite of neonatal morbidity (development of RDS, IVH, NEC and sepsis/meningitis in the neonate) and preterm birth before 34 and 37 weeks.
Parry (2014) performed a randomized controlled trial to examine whether maintenance tocolysis with nifedipine in women in spontaneous preterm labor would prolong pregnancy and decrease neonatal unit admission. Women with threatened preterm labor and a singleton pregnancy between 24+0 and 33+6 completed weeks who were positive for fetal fibronectin and had intact membranes were included. The women had completed treatment with corticosteroids and then received rescue tocolysis with nifedipine for 48 hours. Exclusion criteria were ruptured membranes, suspicion of chorioamnionitis, antepartum hemorrhage or suspected concealed abruption, major fetal anomaly or a contraindication to nifedipine treatment. In total, 29 women received nifedipine and 31 women received placebo until 36 completed weeks’ gestation. Slow-release nifedipine was administered in a dose of 20 mg eight hourly up to a maximum of 160 mg in 24 hours. The maintenance dosage was similar to that at the time of randomization (dosage that had been used over the previous 48 hours for acute tocolysis). Groups were similar at baseline. Outcomes of interest were perinatal death and preterm birth before 32, 34 and 37 weeks.
Roos (2013) performed a randomized controlled trial (APOSTEL II trial) to examine whether maintenance tocolysis with nifedipine will decrease adverse perinatal outcomes because of preterm birth. Women with singleton and multiple pregnancies with and without ruptured membranes and threatened preterm labor with a gestational age between 26+0 and 32+2 weeks who had not delivered after a complete 48-hour course of tocolytics and corticosteroids were included. Besides, women who were transferred to a tertiary center during the first 48 hours were also included. Exclusion criteria for women were signs of intrauterine infection, hypertension (140/90 mm Hg), preeclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count), placenta previa, and contraindications for nifedipine. In addition, a fetus with signs of fetal distress, known lethal congenital anomalies, and intrauterine death were excluded. In total, 201 women received oral nifedipine (80 mg/day) and 205 women received placebo for 12 days. Groups were comparable at baseline. Outcomes of interest were neonatal mortality, neonatal sepsis, respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), a composite of adverse perinatal outcomes (perinatal death, chronic lung disease, neonatal sepsis, IVH grade 2, PVL grade 1, or necrotizing enterocolitis), and preterm birth before 32, 34 and 37 weeks.
Van Vliet (2016) performed a follow-up study of the APOSTEL II trial (Roos 2013) to examine wheter maintenance tocolysis with nifedipine was associated with long-term neurodevelopmental outcomes at 2 years of. In total, 276 women were eligible for follow-up but follow-up was completed by 145 women of which 78 received nifedipine and 66 received placebo. Groups were comparable at baseline. Data of 169 infants were included in this study with 90 infants whose mother received nifedipine and 79 infants whose mother received placebo. Infant development was assessed on five subscales with the Ages and Stages Questionnaire (ASQ).
Results
Short-term outcomes
1. Neonatal mortality
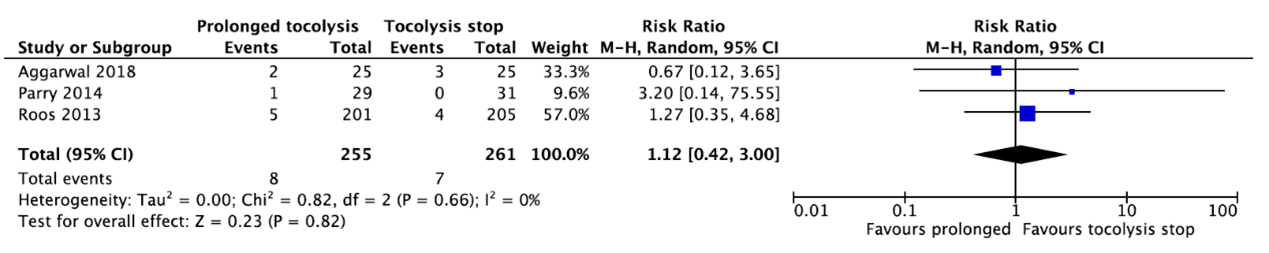
Three studies reported neonatal mortality (Aggarwal, 2018; Parry, 2014; Roos, 2013) (Figure 1). In total, 8 of the 255 infants (3.1%) whose mother received prolonged tocolysis with nifedipine died as compared to 7 of the 261 infants (2.7%) whose mother did not receive prolonged tocolysis (RR=1.12, 95%CI 0.42 to 3.00). This difference is clinically relevant favoring tocolysis stop.
Figure 1. Neonatal mortality.
2. Neonatal sepsis
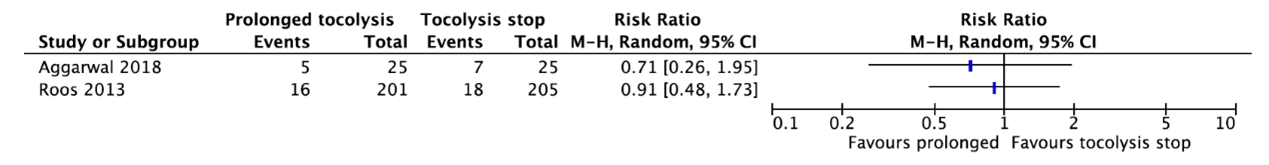
Two studies reported neonatal sepsis (Aggarwal, 2018; Roos, 2013) (Figure 2.)
Aggarwal (2018) reported sepsis/meningitis. Five of the 25 neonates (20%) whose mother received maintenance tocolysis with nifedipine had sepsis/meningitis as compared to 7 of the 25 neonates (28%) whose mother did not receive maintenance tocolysis (RR=0.71, 95%CI 0.26 to 1.95).
Roos (2013) reported that 16 of the 201 babies (8.0%) whose mother received prolonged tocolysis with nifedipine had neonatal sepsis as compared to 18 of the 205 babies (8.8%) whose mother received placebo (RR=0.91, 95%CI 0.48 to 1.73).
These data were not pooled as the agreement is to start pooling when including at least three studies. Figure 2 shows that there is probably no clinically relevant difference in neonatal sepsis between prolonged tocolysis and tocolysis stop.
Figure 2. Neonatal sepsis.
3. Composite outcome of neonatal morbidity and mortality
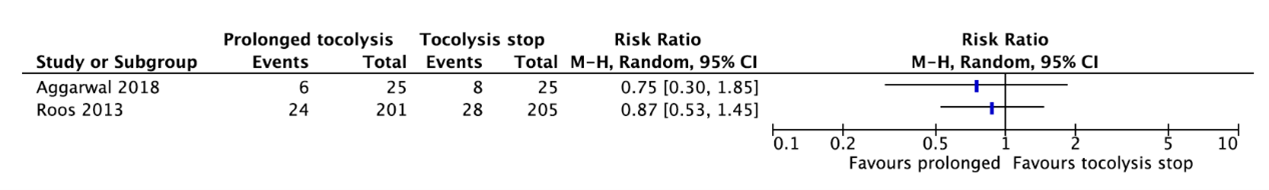
Two studies reported a composite outcome of neonatal morbidity and mortality (Aggarwal, 2018; Roos, 2013) (Figure 3.)
Aggarwal (2018) reported a composite of neonatal morbidity with the development of respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and sepsis/meningitis in the neonate. Six of the 25 neonates (24%) whose mother received prolonged tocolysis with nifedipine were positive for this composite outcome as compared to 8 of the 25 neonates (33.3%) whose mother did not receive maintenance tocolysis (RR=0.75, 95%CI 0.30 to 1.85).
Roos (2013) reported a composite of adverse perinatal outcomes due to premature birth, defined as perinatal mortality and serious morbidity including chronic lung
disease, neonatal sepsis (proven with a positive blood culture), severe intraventricular hemorrhage greater than grade 2, periventricular leukomalacia
greater than grade 1, and necrotizing enterocolitis. Twenty-four of the 201 babies (11.9%) whose mother received prolonged tocolysis with nifedipine were positive for this composite outcome as compared to 28 of the 205 babies (13.7%) whose mother received placebo (RR=0.87, 95%CI 0.53 to 1.45).
These data were not pooled as the agreement is to start pooling when including at least three studies. Figure 3 shows that there is probably no clinically relevant difference in the composite outcome of neonatal morbidity and mortality between prolonged tocolysis and tocolysis stop.
Figure 3. Composite outcome of neonatal morbidity and mortality.
4. Respiratory distress syndrome (RDS)
Two studies reported respiratory distress syndrome (Aggarwal, 2018; Roos, 2013) (Figure 4.)
Aggarwal (2018) reported RDS. Three of the 25 neonates (12%) whose mother received maintenance tocolysis with nifedipine had RDS as compared to 2 of the 25 neonates (8%) whose mother did not receive maintenance tocolysis (RR=1.50, 95%CI 0.27 to 8.22).
Roos (2013) reported chronic lung disease. Five of the 201 babies (2.5%) whose mother received prolonged tocolysis with nifedipine had chronic lung disease as compared to 6 of the 205 babies (2.9%) whose mother received placebo (RR=0.85, 95%CI 0.26 to 2.74).
These data were not pooled as the agreement is to start pooling when including at least three studies. Figure 4 shows that there is probably no clinically relevant difference in respiratory distress syndrome between prolonged tocolysis and tocolysis stop.
Figure 4. Respiratory distress syndrome.
5. Intraventricular hemorrhage (IVH)
Two studies reported intraventricular hemorrhage (Aggarwal, 2018; Roos, 2013) (Figure 5.)
Aggarwal (2018) reported that 1 of the 25 neonates (4%) whose mother did not receive maintenance tocolysis had IVH, while no cases of IVH occurred in neonates whose mother received maintenance tocolysis with nifedipine (RR=0.33, 95%CI 0.01 to 7.81).
Roos (2013) reported that 2 of the 201 babies (1.0%) whose mother received prolonged tocolysis with nifedipine had IVH > grade 2 as compared to 5 of the 205 babies (2.4%) whose mother received placebo (RR=0.41, 95%CI 0.08 to 2.08).
These data were not pooled as the agreement is to start pooling when including at least three studies. Figure 5 shows that there may be a clinically relevant difference in intraventricular hemorrhage between prolonged tocolysis and tocolysis stop.
Figure 5. Intraventricular hemorrhage.
6. Periventricular leukomalacia (PVL)
Roos (2013) reported that none of the babies whose mother received either prolonged tocolysis with nifedipine or no prolonged tocolysis had PVL > grade 1.
7. Preterm birth
7.1. Preterm birth before 32 weeks
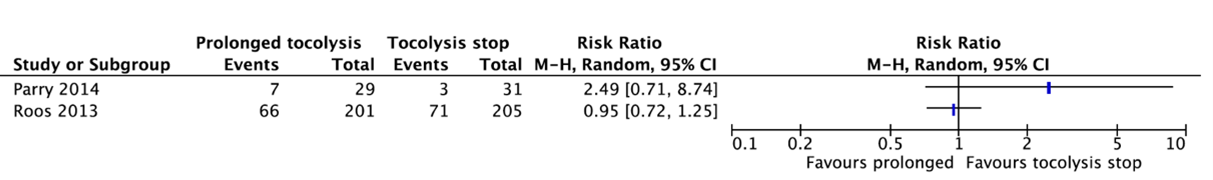
Two studies reported a preterm birth before 32 weeks (Parry, 2014; Roos, 2013) (Figure 6).
Parry (2014) reported that 7 of the 29 babies (24.1%) whose mother received prolonged tocolysis with nifedipine had a preterm birth before 32 weeks as compared to 3 of the 31 babies (9.7%) whose mother received placebo (RR=2.49, 95%CI 0.71 to 8.74).
Roos (2013) reported that 66 of the 201 babies (32.8%) whose mother received prolonged tocolysis with nifedipine had a preterm birth before 32 weeks as compared to 71 of the 205 babies (34.6%) whose mother received placebo (RR=0.72, 95%CI 0.72 to 1.25).
These data were not pooled as the agreement is to start pooling when including at least three studies. Figure 6 shows that there is probably no clinically relevant difference in preterm birth before 32 weeks between prolonged tocolysis and tocolysis stop.
Figure 6. Preterm birth before 32 weeks.
7.2. Preterm birth before 34 weeks
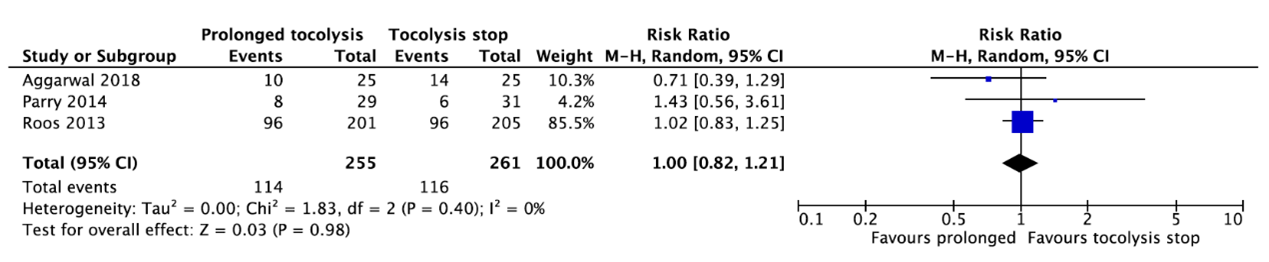
Three studies reported a preterm birth before 34 weeks (Aggarwal, 2018; Parry, 2014; Roos, 2013) (Figure 7). In total, 114 of the 255 infants (44.7%) whose mother received prolonged tocolysis with nifedipine had a preterm birth before 34 weeks as compared to 116 of the 261 infants (44.4%) whose mother did not receive prolonged tocolysis (RR=1.00, 95%CI 0.82 to 1.21). This difference is not clinically relevant.
Figure 7. Preterm birth before 34 weeks.
7.3. Preterm birth before 37 weeks
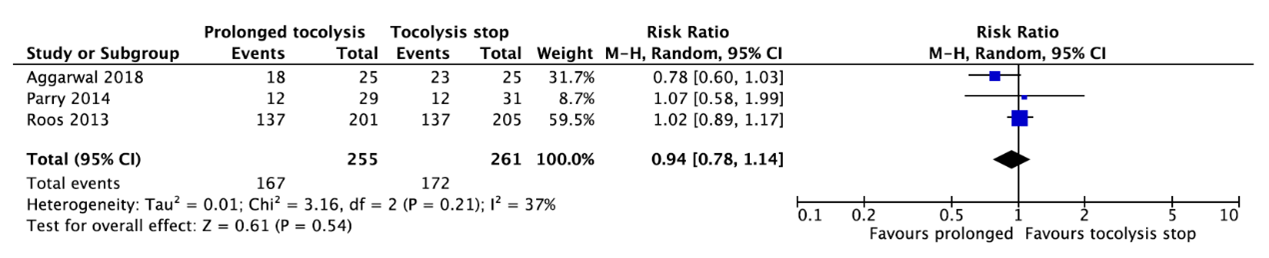
Three studies reported a preterm birth before 37 weeks (Aggarwal, 2018; Parry, 2014; Roos, 2013) (Figure 8). In total, 167 of the 255 infants (65.5%) whose mother received prolonged tocolysis with nifedipine died as compared to 172 of the 261 infants (65.9%) whose mother did not receive prolonged tocolysis (RR=0.94, 95%CI 0.78 to 1.14). This difference is not clinically relevant.
Figure 8. Preterm birth before 37 weeks.
Long-term outcomes
1. Survival without neurodevelopmental impairment
Van Vliet (2016) reported infant development with the Ages and Stages Questionnaire (ASQ) consisting of five subscales: communication scale, gross motor scale, fine motor scale, problem solving scale, and personal social scale. For all these subscales, the number of infants with a poor outcome (defined as a score lower than 1 standard deviation below the mean of the norm group) were reported. Besides, the developmental delay was reported, defined as performance < 1 SD below the mean score at one or more subscales. Developmental delay was positive for 53 of the 90 infants (58.9%) whose mother received maintenance tocolysis with nifedipine as compared to 51 of the 69 infants (64.6%) whose mother did not receive maintenance tocolysis (RR=0.91, 95%CI 0.72 to 1.16). This difference is not clinically relevant.
Level of evidence of the literature
According to GRADE, the level of evidence of randomized controlled trials start at high.
The level of evidence regarding the outcome measure neonatal mortality was downgraded by three levels to very low because of conflicting results (-1, inconsistency) and the 95% confidence interval crossed both lines of no (clinically relevant) effect (-2, imprecision).
The level of evidence regarding the outcome measure neonatal sepsis was downgraded by two levels to low because of the broad confidence intervals crossing both lines of no (clinically relevant) effect (-2, imprecision).
The level of evidence regarding the outcome measure composite outcome of neonatal morbidity and mortality was downgraded by two levels to low because of the broad confidence intervals crossing both lines of no (clinically relevant) effect (-2, imprecision).
The level of evidence regarding the outcome measure respiratory distress syndrome was downgraded by three levels to very low because of conflicting results (-1, inconsistency) and the broad confidence intervals crossing both lines of no (clinically relevant) effect (-2, imprecision).
The level of evidence regarding the outcome measure intraventricular hemorrhage was downgraded by three levels to very low because of the broad confidence intervals crossing both lines of no (clinically relevant) effect and the optimal information size was not achieved (-3, imprecision).
The level of evidence regarding the outcome measure periventricular leukomalacia was downgraded by three levels to very low because no events occurred and the optimal information size was not achieved (-3, imprecision).
Preterm birth
The level of evidence regarding the outcome measure preterm birth before 32 weeks was downgraded by three levels to very low because of conflicting results (-1, inconsistency) and the broad confidence intervals crossing the lines of no (clinically relevant) effect (-2, imprecision).
The level of evidence regarding the outcome measure preterm birth before 34 weeks was downgraded by one level to moderate because of conflicting results (-1, inconsistency).
The level of evidence regarding the outcome measure preterm birth before 37 weeks was downgraded by two levels to low because of conflicting results (-1, inconsistency) and the 95% confidence interval crossed the line of no (clinically relevant) effect (-1, imprecision).
The level of evidence regarding the outcome measure survival without neurodevelopmental impairment was downgraded by two levels to low because the 95% confidence interval crossed the line of no (clinically relevant) effect and the optimal information size was not achieved (-2, imprecision).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the (un)favorable effects of treating women with threatened preterm birth under 34 weeks with tocolytics for longer than 48 hours compared to no extended tocolysis (up to 48 hours) on the morbidity and mortality of the child?
| P: | pregnant women with threatened premature birth under 34 weeks, with indication for corticosteroids and tocolysis, who have not given birth after 48 hours |
| I: | prolonged tocolysis after 48 hours (atosiban, adalat/nifedipine) |
| C: | tocolysis stop after 48 hours |
| O: |
= short term: neonatal mortality, neonatal sepsis, composite outcome of neonatal morbidity and mortality (respiratory distress syndrome, bronchopulmonary dysplasia, retinopathy of prematurity, periventricular leukomalacia, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis, neonatal death), RDS (respiratory distress syndrome), IVH (intraventricular hemorrhage), PVL (periventricular leukomalacia), preterm birth = long term: survival without neurodevelopmental impairment |
Relevant outcome measures
The guideline development group considered neonatal mortality and survival without neurodevelopmental impairment as critical outcome measures for decision making; and preterm birth, neonatal sepsis, composite outcome of neonatal morbidity and mortality, respiratory distress syndrome, intraventricular hemorrhage, periventricular leukomalacia, and gestational age at delivery as important outcome measures for decision making.
The working group defined preterm birth as a gestational age before 32 weeks, before 34 weeks or before 37 weeks. For the other outcome measures, the working group did not define the outcome a priori, but used the definitions used in the studies.
The working group defined a 1% difference in neonatal mortality (RR < 0.99 or > 1.01) as minimal clinically (patient) important difference. For survival without neurodevelopmental impairment, a 10% difference (RR < 0.9 or > 1.1) was chosen as minimal clinically (patient) important difference. For the other outcomes, a 25% difference for dichotomous outcomes (RR < 0.8 or > 1.25) and 0.5 SD for continuous outcomes was taken as minimal clinically (patient) important difference.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 2010 until the 14th of July, 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 192 hits. Studies were selected based on the following criteria:
- Systematic review (searched in at least two databases, and detailed search strategy, risk of bias assessment and results of individual studies available), randomized controlled trial, or observational studies comparing prolonged tocolysis after 48 hours with stop of tocolysis after 48 hours;
- The study population had to meet the criteria as defined in the PICO; and
- Full-text English language publication.
Fourteen studies were initially selected based on title and abstract screening. After reading the full text, ten studies were excluded (see the table with reasons for exclusion under the tab Methods), and four studies were included.
Results
Four studies were included in the analysis of the literature (Aggarwal, 2018; Parry, 2014; Roos, 2013; Van Vliet, 2016). All studies used nifedipine as tocolytic drug. Important study characteristics and results are summarized in the evidence table. The assessment of the risk of bias is summarized in the risk of bias table.
Referenties
- Aggarwal A, Bagga R, Girish B, Kalra J, Kumar P. Effect of maintenance tocolysis with nifedipine in established preterm labour on pregnancy prolongation and neonatal outcome. J Obstet Gynaecol. 2018 Feb;38(2):177-184. doi: 10.1080/01443615.2017.1331340. Epub 2017 Aug 8. PMID: 28784001.
- Gascoigne EL, Webster CM, Honart AW, Wang P, Smith-Ryan A, Manuck TA. Physical activity and pregnancy outcomes: an expert review. Am J Obstet Gynecol MFM. 2023 Jan;5(1):100758. doi: 10.1016/j.ajogmf.2022.100758. Epub 2022 Sep 26. PMID: 36174931; PMCID: PMC9772147.
- Hermans FJR, Bruijn MMC, Vis JY, Wilms FF, Oudijk MA, Porath MM, Scheepers HCJ, Bloemenkamp KWM, Bax CJ, Cornette JMJ, Nij Bijvanck BWA, Franssen MTM, Vandenbussche FPHA, Kok M, Grobman WA, Van Der Post JAM, Bossuyt PMM, Opmeer BC, Mol BWJ, Schuit E, Van Baaren GJ. Risk stratification with cervical length and fetal fibronectin in women with threatened preterm labor before 34 weeks and not delivering within 7 days. Acta Obstet Gynecol Scand. 2015 Jul;94(7):715-721. doi: 10.1111/aogs.12643. Epub 2015 Apr 29. PMID: 25845495.
- Hermans FJR, Schuit E, Bekker MN, Woiski M, de Boer MA, Sueters M, Scheepers HCJ, Franssen MTM, Pajkrt E, Mol BWJ, Kok M. Cervical Pessary After Arrested Preterm Labor: A Randomized Controlled Trial. Obstet Gynecol. 2018 Sep;132(3):741-749. doi: 10.1097/AOG.0000000000002798. PMID: 30095769.
- Parry E, Roos C, Stone P, Hayward L, Mol BW, McCowan L. The NIFTY study: a multicentre randomised double-blind placebo-controlled trial of nifedipine maintenance tocolysis in fetal fibronectin-positive women in threatened preterm labour. Aust N Z J Obstet Gynaecol. 2014 Jun;54(3):231-6. doi: 10.1111/ajo.12179. Epub 2014 Feb 8. PMID: 24506318.
- Roos C, Spaanderman ME, Schuit E, Bloemenkamp KW, Bolte AC, Cornette J, Duvekot JJ, van Eyck J, Franssen MT, de Groot CJ, Kok JH, Kwee A, Merién A, Nij Bijvank B, Opmeer BC, Oudijk MA, van Pampus MG, Papatsonis DN, Porath MM, Scheepers HC, Scherjon SA, Sollie KM, Vijgen SM, Willekes C, Mol BW, van der Post JA, Lotgering FK; APOSTEL-II Study Group. Effect of maintenance tocolysis with nifedipine in threatened preterm labor on perinatal outcomes: a randomized controlled trial. JAMA. 2013 Jan 2;309(1):41-7. doi: 10.1001/jama.2012.153817. PMID: 23280223.
- Saccone G, Della Corte L, Cuomo L, Reppuccia S, Murolo C, Napoli FD, Locci M, Bifulco G. Activity restriction for women with arrested preterm labor: a randomized controlled trial. Am J Obstet Gynecol MFM. 2023 Aug;5(8):100954. doi: 10.1016/j.ajogmf.2023.100954. Epub 2023 Apr 18. Erratum in: Am J Obstet Gynecol MFM. 2023 Dec;5(12):101199. PMID: 37080296.
- van Vliet E, Seinen L, Roos C, Schuit E, Scheepers H, Bloemenkamp K, Duvekot JJ, van Eyck J, Kok JH, Lotgering FK, van Baar A, van Wassenaer-Leemhuis AG, Franssen MT, Porath MM, van der Post J, Franx A, Mol B, Oudijk MA. Maintenance tocolysis with nifedipine in threatened preterm labour: 2-year follow up of the offspring in the APOSTEL II trial. BJOG. 2016 Jun;123(7):1107-14. doi: 10.1111/1471-0528.13586. Epub 2015 Aug 27. PMID: 26330379.
Evidence tabellen
Evidence table for intervention studies
Research question: What are the (un)favorable effects of treating women with threatened preterm birth under 34 weeks with tocolytics for longer than 48 hours compared to no extended tocolysis (up to 48 hours) on the morbidity and mortality of the child?
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Aggarwal, 2018 |
Type of study: Randomised open-label study
Setting and country: Tertiary care hospital in north India
Funding and conflicts of interest: Source of funding not reported. No potential conflict of interest was reported by the authors. |
Inclusion criteria: Women with a singleton pregnancy between 26+0 and 33+6 weeks of gestation and arrested preterm labor.
Exclusion criteria: - Women with antepartum hemorrhage - Fetal malformation or demise - Severe fetal growth restriction - Advanced preterm labor (cervical dilatation >4 cm) - Ruptured membranes - Contraindication to nifedipine - Any indication necessitating delivery
N total at baseline: Intervention: 25 Control: 25
Important prognostic factors2: Age ± SD: I: 25.40 ± 4.29 C: 24.16 ± 2.93
Gestation at admission in weeks I: 30+4/7 ± 2 C: 30+6/7 ± 2+2/7
Groups comparable at baseline
|
Maintenance tocolysis with tablet nifedipine (retard) 20mg 8th hourly orally for 12 more days. Women who did not achieve 34 weeks of gestation after 12 days continued to take nifedipine till 34 weeks of gestation.
Women with a repeat episode of preterm labor (uterine contractions four per 20 min or eight per 60 min) were re-treated with acute tocolysis if cervical dilatation was <4 cm. Oral maintenance therapy was restarted after successful repeat acute tocolysis.
|
None (did not receive maintenance tocolysis)
Treated with acute tocolysis in case of recurrent preterm labor. |
Length of follow-up: Till delivery
Loss-to-follow-up: No loss-to-follow-up
Incomplete outcome data: No missing data
|
Perinatal mortality I: 2/25 (8%) C: 3/25 (12%)
RDS I: 3/25 (12%) C: 2/25 (8%)
IVH I: 0 C: 1/25 (4%)
Sepsis/meningitis I: 5/25 (20%) C: 7/25 (28%)
Composite outcome I: 6/25 (24%) C: 8/25 (33.3%)
Preterm birth before 34 weeks I: 10/25 (40%) C: 14/25 (56%)
Preterm birth before 37 weeks I: 18/25 (72%) C: 23/25 (92%) |
Author’s conclusion Maintenance tocolysis did not result in statistically significant prolongation of pregnancy or reduction in neonatal hospital stay.
Remarks - Small sample size - Open label study where a placebo was not used in the control group
|
|
Parry, 2014 |
Type of study: Multicentre double-blind placebo controlled trial
Setting and country: Two tertiary referral centres in Auckland, New Zealand.
Funding and conflicts of interest: Funding was provided by Auckland Medical Research Foundation for the midwifery salaries and Douglas Pharmaceuticals who provided the placebo gratis. Conflicts of interest were not reported. |
Inclusion criteria: - Women with threatened preterm labor - Singleton pregnancy - Between 24+0 and 34+6 completed weeks - Positive fetal fibronectin - Intact membranes - Completed treatment with corticosteroids and received rescue tocolysis with nifedipine for 48 hours.
Exclusion criteria: - Ruptured membranes - Suspicion of chorioamnionitis - Antepartum hemorrhage or suspected concealed abruption - Major fetal anomaly
N total at baseline: Intervention: 29 Control: 31
Important prognostic factors2: Age ± SD: I: 29.0 ± 5.8 C: 29.7 ± 6.4
Gestational age at study entry I: 29.8 ± 3.6 C: 29.5 ± 2.6
Groups comparable at baseline
|
Slow-release nifedipine was administered in a dose of 20 mg eight hourly up to a maximum of 160 mg in 24 h. The maintenance dosage was similar to that which women were prescribed at the time of randomisation, the dosage that had been used over the previous 48 h for acute tocolysis until 36 completed weeks.
|
Placebo until 36 completed weeks |
Length of follow-up: Until delivery
Loss-to-follow-up: Intervention: 1 (3.4%) Control: 3 (9.7%)
Incomplete outcome data: No missing data
|
Neonatal death I: 1/29 (3.4%) C: 0
Preterm birth before 32 weeks I: 7/29 (24.1%) C: 3/31 (9.7%)
Preterm birth before 34 weeks I: 8/29 (27.6%) C: 6/31 (19.4%)
Preterm birth before 37 weeks I: 12/29 (41.4%) C: 12/31 (38.7%)
|
Author’s conclusion In women with threatened preterm labour who are fetal fibronectin positive, maintenance tocolysis with nifedipine does not seem to prolong pregnancy, nor reduce length of NICU admission.
Remarks Low number of participants, due to recruitment and funding difficulties
|
|
Roos, 2013 |
Type of study: Double-blind, placebo-controlled trial
Setting and country: 11 perinatal units including all tertiary centres in the Netherlands from June 2008 and February 2010.
Funding and conflicts of interest: This trial was funded by ZonMw, the Netherlands Organization for Health Research and Development Healthcare Efficiency Program grant 80-82310-98-08210. No conflicts of interest.
|
Inclusion criteria: - Singleton and multiple pregnancies - With or without ruptured membranes - Threatened preterm labor - Gestational age between 26+0 and 32+2 weeks - Women who had not delivered after a complete 48-hour course of tocolytics and corticosteroids
Exclusion criteria: Maternal: - Signs of intrauterine infection - Hypertension (140/90 mm Hg) - Preeclampsia - Placenta previa - Contraindications for nifedipine
Fetal: - Signs of fetal distress - Known lethal congenital anomalies - Intrauterine death
N total at baseline: Intervention: 201 Control: 205
Important prognostic factors2: Age ± SD: I: 30.2 ± 5.1 C: 30.2 ± 5.1
Gestational age at study entry I: 29.2 ± 1.7 C: 29.2 ± 1.7
Groups comparable at baseline
|
20 mg of nifedipine slow-release tablets every 6 hours, resulting in a total daily dose of 80 mg.
For all women, initiation of the study drugs was 48 to 72 hours after the start of initial tocolysis. The protocol allowed the treating physicians to decrease the dosing interval to every 4 hours based on symptoms. The treatment was phased out from day 10 until day 12 (total daily dose 60 mg; total daily dose 20 mg) and discontinued on day 13. Once the study treatment was complete, a repeat course of tocolysis and corticosteroids for 48 hours was allowed in cases of recurrent threatened preterm labour.
|
20 mg of placebo tablets every 6 hours, resulting in a total daily dose of 80 mg.
|
Length of follow-up: Until 6 months after birth
Loss-to-follow-up: No loss-to-follow-up
Incomplete outcome data: No missing data
|
Adverse perinatal outcome (perinatal death, chronic lung disease, neonatal sepsis, IVH > grade 2, PVL > grade 1, necrotizing enterocolitis) I: 24/201 (11.9%) C: 28/205 (13.7%)
Neonatal mortality: I: 5/201 (2.5%) C: 4/205 (2.0%)
Neonatal sepsis: I: 16/201 (8.0%) C:18/205 (8.8%)
Chronic lung disease I: 5/201 (2.5%) C: 6/205 (2.9%)
IVH (> grade 2) I: 2/201 (1.0%) C: 5/205 (2.4%)
PVL (> grade 1) I: 0 C: 0
Preterm birth before 32 weeks I: 66/201 (32.8%) C: 71/205 (34.6%)
Preterm birth before 34 weeks I: 96/201 (47.8%) C: 96/205 (46.8%)
Preterm birth before 37 weeks I: 137/201 (68.2%) C: 137/205 (66.8%)
|
Author’s conclusion In patients with threatened preterm labor, nifedipine maintained tocolysis did not result in a statistically significant reduction in adverse perinatal outcomes when compared with placebo. Although the lower than anticipated rate of adverse perinatal outcomes in the control group indicates that a benefit of nifedipine cannot completely be excluded, its use for maintenance tocolysis does not appear beneficial at this time.
Remarks - Lower than planned power for primary and secondary end points in the study design due to a lower than anticipated control event rate - Inclusion of both singletons and multiples in the study. A differential effect is possible because multiples have an increased risk of delivering preterm. The authors did not observe any difference between the subgroups, but the numbers per subgroup were too small to exclude a possible difference. |
|
Van Vliet, 2016 |
Type of study: Follow-up of double-blind, placebo-controlled trial (APOSTEL II trail by Roos 2013)
Setting and country: 10 perinatal centres and one large teaching hospital in the Netherlands.
Funding and conflicts of interest: APOSTEL II trial was funded by ZonMw, the Netherlands Organization for Health Research and Development Healthcare Efficiency Program grant 80-82310-98-08210. No additional funding was obtained for the follow-up study. No conflicts of interest.
|
Inclusion criteria: See Roos 2013
Exclusion criteria: See Roos 2013 N total at baseline: Intervention: 90 Control: 79
Important prognostic factors2: Birthweight (gram) ± SD: I: 2304.2 ± 98.0 C: 2152.7 ± 100.3
NICU admittance I: 37 (41.1%) C: 36 (45.6%)
Ventilation support I: 7 (8.9%) C: 16 (17.8%)
Groups comparable at baseline |
Mother received 20 mg nifedipine slow-release tablets every 6 hours |
Mother received placebo |
Length of follow-up: 2 years
Loss-to-follow-up: 131 (47.5%) Did not respond to request to fill out the ASQ
Incomplete outcome data: No missing data about infants |
Neurodevelopmental outcome (ASQ) Communication scale I: 14/90 (15.6%) C: 20/79 (25.3%)
Gross motor scale I: 31/90 (34.4%) C: 30/79 (38.0%)
Fine motor scale I: 20/90 (22.2%) C: 6/79 (7.6%)
Problem solving scale I: 19/90 (21.1%) C: 23/79 (29.1%)
Personal social scale I: 25/90 (27.8%) C: 51/79 (32.9%)
Developmental delay (performance <1 SD below the mean score at 1 or more subscales) I: 53/90 (58.9%) C: 51/79 (64.6%) |
Author’s conclusion This follow-up study revealed no clear benefit of nifedipine maintenance tocolysis at 2 years of age. As short-term adverse perinatal outcome was not reduced in the original APOSTEL II trial, we conclude that maintenance tocolysis does not appear to be beneficial at this time.
Remarks: - Questionnaire data - Selection bias (not greatly affected results) - Some of the parents participating in this follow-up study did not fill out the questionnaire within the correct time frame (not greatly affected results)
|
Risk of bias table for intervention studies
Research question: What are the (un)favorable effects of treating women with threatened preterm birth under 34 weeks with tocolytics for longer than 48 hours compared to no extended tocolysis (up to 48 hours) on the morbidity and mortality of the child?
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Aggarwal, 2018 |
Definitely yes;
Reason: Computer generated randomisation table was used.
|
No information |
Probably no;
Reason: Open-label trial. |
Definitely yes;
Reason: No missing data. |
Probably yes;
Reason: All relevant outcomes were reported. |
Probably yes;
Reason: No other problems noted. |
Low (all outcomes) |
|
Parry, 2014 |
Definitely yes;
Reason: Computer-generated numbers were used with a block size of 10.
|
Definitely yes;
Reason: Randomization performed at pharmacy and blinded to allocation.
|
Definitely yes;
Reason: Participants, clinicians and dispensing pharmacists were blinded to the treatment group allocation.
|
Probably yes;
Reason: No missing data. Loss to follow-up was infrequent in both groups. Used intention-to-treat analysis. |
Probably yes;
Reason: All relevant outcomes were reported. |
Probably no;
Reason: Trial was stopped before the full sample size was recruited. |
Low (all outcomes) |
|
Roos, 2013 |
Definitely yes;
Reason: Group assignment was based on a computer-generated random sequence in blocks of 4 participants.
|
Definitely yes;
Reason: Treatment assignment was blinded. |
Definitely yes;
Reason: Treatment assignment and block size were blinded to investigators, participants, clinicians, and research nurses. |
Definitely yes;
Reason: No missing data. |
Probably yes;
Reason: All relevant outcomes were reported. |
Probably yes;
Reason: No other problems noted. |
LOW (all outcomes) |
|
Van Vliet, 2016 (follow-up study of Roos 2013) |
Definitely yes;
Reason: Group assignment was based on a computer-generated random sequence in blocks of 4 participants.
|
Definitely yes;
Reason: Treatment assignment was blinded. |
Definitely yes;
Reason: Treatment assignment and block size were blinded to investigators, participants, clinicians, and research nurses. |
Definitely yes;
Reason: No missing data. |
Probably yes;
Reason: Intention for follow-up described in protocol. |
Probably yes;
Reason: No other problems noted. |
LOW (all outcomes) |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Ahmed D, Ahmed F. Vaginal progesterone for maintenance of tocolysis in a sample of iraqi women. European Journal of Molecular and Clinical Medicine. 2020; 7(10):172-184. |
Wrong intervention: vaginal progesterone |
|
Alavi A, Rajaee M, Amirian M, Mahboobi H, Jahanshahi KA, Faghihi A. Effect of Maintenance Therapy with Isoxsuprine in the Prevention of Preterm Labor: Randomized controlled trial. Electron Physician. 2015 Aug 10;7(4):1144-9. doi: 10.14661/2015.1144-1149. PMID: 26396726; PMCID: PMC4578532. |
Wrong intervention: isoxsuprine with acute tocolysis with magnesium sulfate for 12 hours
|
|
Arikan I, Barut A, Harma M, Harma IM. Effect of progesterone as a tocolytic and in maintenance therapy during preterm labor. Gynecol Obstet Invest. 2011;72(4):269-73. doi: 10.1159/000328719. Epub 2011 Nov 12. PMID: 22086108. |
Wrong intervention: vaginal micronized natural progesterone |
|
Ashraf B. Efficacy and safety of oral nifedipine with or without vaginal progesterone in the management of threatened preterm labor. Int J Reprod Biomed. 2019 Sep 22;17(9):629-636. doi: 10.18502/ijrm.v17i9.5098. PMID: 31646257; PMCID: PMC6804328. |
Wrong comparison: nifedipine versus nifedipine and vaginal progesterone |
|
Barnett SD, Asif H, Buxton ILO. Novel identification and modulation of the mechanosensitive Piezo1 channel in human myometrium. J Physiol. 2023 May;601(9):1675-1690. doi: 10.1113/JP283299. Epub 2022 Aug 8. PMID: 35941750; PMCID: PMC9905381. |
Other study aim: Piezo1 channel in human myometrium |
|
Breuking SH, De Ruigh AA, Hermans FJR, Schuit E, Combs CA, de Tejada BM, Oudijk MA, Mol BW, Pajkrt E. Progestogen maintenance therapy for prolongation of pregnancy after an episode of preterm labour: A systematic review and meta-analysis. BJOG. 2023 Oct;130(11):1306-1316. doi: 10.1111/1471-0528.17499. Epub 2023 Apr 19. PMID: 37077041. |
Wrong intervention: progestogen maintenance therapy |
|
Chawanpaiboon S, Laopaiboon M, Lumbiganon P, Sangkomkamhang US, Dowswell T. Terbutaline pump maintenance therapy after threatened preterm labour for reducing adverse neonatal outcomes. Cochrane Database Syst Rev. 2014 Mar 23;2014(3):CD010800. doi: 10.1002/14651858.CD010800.pub2. PMID: 24659357; PMCID: PMC11193541. |
Wrong study design: protocol |
|
Choudhary M, Suneja A, Vaid NB, Guleria K, Faridi MM. Maintenance tocolysis with oral micronized progesterone for prevention of preterm birth after arrested preterm labor. Int J Gynaecol Obstet. 2014 Jul;126(1):60-3. doi: 10.1016/j.ijgo.2014.01.019. Epub 2014 Apr 3. PMID: 24807871. |
Wrong intervention: oral micronized progesterone |
|
Conde-Agudelo A, Romero R, Kusanovic JP. Nifedipine in the management of preterm labor: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011 Feb;204(2):134.e1-20. doi: 10.1016/j.ajog.2010.11.038. PMID: 21284967; PMCID: PMC3437772. |
Included only studies <2010 |
|
Dehaene I, Bergman L, Turtiainen P, Ridout A, Mol BW, Lorthe E; from the International Spontaneous Preterm birth Young Investigators group (I-SPY). Maintaining and repeating tocolysis: A reflection on evidence. Semin Perinatol. 2017 Dec;41(8):468-476. doi: 10.1053/j.semperi.2017.08.005. Epub 2017 Sep 22. PMID: 28943054. |
Wrong study design: narrative review |
|
Ding MX, Luo X, Zhang XM, Bai B, Sun JX, Qi HB. Progesterone and nifedipine for maintenance tocolysis after arrested preterm labor: A systematic review and meta-analysis of randomized controlled trial. Taiwan J Obstet Gynecol. 2016 Jun;55(3):399-404. doi: 10.1016/j.tjog.2015.07.005. PMID: 27343323. |
Suitable studies from systematic review were analyzed separately |
|
Dodd JM, Crowther CA, Middleton P. Oral betamimetics for maintenance therapy after threatened preterm labour. Cochrane Database Syst Rev. 2012 Dec 12;12:CD003927. doi: 10.1002/14651858.CD003927.pub3. PMID: 23235600. |
Included only studies <2010 |
|
Doret M, Kayem G. La tocolyse en cas de menace d’accouchement prématuré à membranes intactes [Tocolysis for preterm labor without premature preterm rupture of membranes]. J Gynecol Obstet Biol Reprod (Paris). 2016 Dec;45(10):1374-1398. French. doi: 10.1016/j.jgyn.2016.09.018. Epub 2016 Oct 28. PMID: 28029463. |
Article in French |
|
Dutta EH, Behnia F, Harirah H, Costantine M, Saade G. Perinatal Outcomes after Short versus Prolonged Indomethacin for Tocolysis in Women with Preterm Labor. Am J Perinatol. 2016 Jul;33(9):844-8. doi: 10.1055/s-0036-1579647. Epub 2016 Mar 9. PMID: 26960702. |
Study design: retrospective study |
|
Eke AC, Chalaan T, Shukr G, Eleje GU, Okafor CI. A systematic review and meta-analysis of progestogen use for maintenance tocolysis after preterm labor in women with intact membranes. Int J Gynaecol Obstet. 2016 Jan;132(1):11-6. doi: 10.1016/j.ijgo.2015.06.058. Epub 2015 Oct 18. PMID: 26489489; PMCID: PMC9941008. |
Wrong intervention: progestational agents for maintenance tocolysis |
|
Facchinetti F, Vergani P, Di Tommaso M, Marozio L, Acaia B, Vicini R, Pignatti L, Locatelli A, Spitaleri M, Benedetto C, Zaina B, DʼAmico R. Progestogens for Maintenance Tocolysis in Women With a Short Cervix: A Randomized Controlled Trial. Obstet Gynecol. 2017 Jul;130(1):64-70. doi: 10.1097/AOG.0000000000002065. PMID: 28594783. |
Wrong intervention: progestogens |
|
Ferrari F, Minozzi S, Basile L, Chiossi G, Facchinetti F. Progestogens for maintenance tocolysis in symptomatic women. A systematic review and meta-analysis. PLoS One. 2023 Feb 22;18(2):e0277563. doi: 10.1371/journal.pone.0277563. PMID: 36812243; PMCID: PMC9946203. |
Wrong intervention: 17-alpha-hydroxyprogesterone caproate (17-HP), vaginal progesterone (Vaginal P) and oral progesterone (Oral P). |
|
Frey HA, Stout MJ, Abdelwahab M, Tuuli MG, Woolfolk C, Shamshirsaz AA, Macones GA, Cahill AG. Vaginal progesterone for preterm birth prevention in women with arrested preterm labor. J Matern Fetal Neonatal Med. 2022 Dec;35(25):8160-8168. doi: 10.1080/14767058.2021.1963705. Epub 2021 Aug 18. PMID: 34407736. |
Wrong intervention: vaginal progesterone |
|
Gaudet LM, Singh K, Weeks L, Skidmore B, Tsertsvadze A, Ansari MT. Effectiveness of terbutaline pump for the prevention of preterm birth. A systematic review and meta-analysis. PLoS One. 2012;7(2):e31679. doi: 10.1371/journal.pone.0031679. Epub 2012 Feb 21. PMID: 22363704; PMCID: PMC3283660. |
Only two RCTs, before 2010 |
|
Grzesiak M, Ahmed RB, Wilczynski J. 48-hours administration of nifedipine in spontaneous preterm labor - Doppler blood flow assessment of placental and fetal circulation. Neuro Endocrinol Lett. 2013;34(7):687-92. PMID: 24463995. |
Other study aim: placental and fetal circulation during nifedipine tocolysis within the first 48 hours of therapy |
|
Han S, Crowther CA, Moore V. Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database Syst Rev. 2010 Jul 7;(7):CD000940. doi: 10.1002/14651858.CD000940.pub2. Update in: Cochrane Database Syst Rev. 2013 May 31;(5):CD000940. doi: 10.1002/14651858.CD000940.pub3. PMID: 20614423. |
Updated version Han 2013 |
|
Han S, Crowther CA, Moore V. Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database Syst Rev. 2013 May 31;2013(5):CD000940. doi: 10.1002/14651858.CD000940.pub3. PMID: 23728634; PMCID: PMC7063385. |
Included only studies <2010 |
|
Hermans FJ, Schuit E, Opmeer BC, Oudijk MA, Bekker M, Woiski M, Bax CJ, Sueters M, Scheepers HC, Franssen MT, Pajkrt E, Mol BW, Kok M. Effectiveness of a cervical pessary for women who did not deliver 48 h after threatened preterm labor (Assessment of perinatal outcome after specific treatment in early labor: Apostel VI trial). BMC Pregnancy Childbirth. 2016 Jul 12;16(1):154. doi: 10.1186/s12884-016-0935-7. PMID: 27405353; PMCID: PMC4942883. |
Wrong intervention: pessary |
|
Hyett J, Asadi N, Zare Khafri M, Vafaei H, Kasraeian M, Salehi A, Saadati N, Bazrafshan K. The use of vaginal progesterone as a maintenance therapy in women with arrested preterm labor: a double-blind placebo-randomized controlled trial. J Matern Fetal Neonatal Med. 2022 Mar;35(6):1134-1140. doi: 10.1080/14767058.2020.1743662. Epub 2020 Mar 26. PMID: 32216490. |
Wrong intervention: vaginal progesterone |
|
Hyuga S, Parry RC, Danielsson J, Vink J, Fu XW, Wu A, Dan W, Yim PD, Gallos G. Anoctamin 1 antagonism potentiates conventional tocolytic-mediated relaxation of pregnant human uterine smooth muscle. J Physiol Sci. 2021 Feb 22;71(1):7. doi: 10.1186/s12576-021-00792-3. PMID: 33618673; PMCID: PMC9352361. |
Other study aim: anoctamin 1 antagonism |
|
Jaju PB. Effectiveness and Safety of Isoxsuprine Hydrochloride as Tocolytic Agent in Arresting Active/Threatened Preterm Labor and Its Role in Maintenance Tocolysis: A Prospective, Open-Label Study. Am J Perinatol. 2021 Feb;38(3):291-295. doi: 10.1055/s-0039-1696720. Epub 2019 Sep 24. PMID: 31550735. |
No comparison: effectiveness isoxsuprine hydrochloride |
|
Kamat S, Veena P, Rani R. Comparison of nifedipine and progesterone for maintenance tocolysis after arrested preterm labour. J Obstet Gynaecol. 2014 May;34(4):322-5. doi: 10.3109/01443615.2013.874407. Epub 2014 Jan 31. PMID: 24483757. |
Wrong comparison: nifedipine versus progesterone |
|
Kashanian M, KaramiAbd T, Sheikhansari N, AminiMoghaddam S, Jangjoo S. Efficacy of daily rectal micronized progesterone for prevention of preterm delivery: a randomized clinical trial. J Matern Fetal Neonatal Med. 2022 Jan;35(1):122-128. doi: 10.1080/14767058.2020.1712709. Epub 2020 Jan 14. PMID: 31937160. |
Wrong intervention: rectal progesterone |
|
Kim SH, Pohl O, Chollet A, Gotteland JP, Fairhurst AD, Bennett PR, Terzidou V. Differential Effects of Oxytocin Receptor Antagonists, Atosiban and Nolasiban, on Oxytocin Receptor-Mediated Signaling in Human Amnion and Myometrium. Mol Pharmacol. 2017 Apr;91(4):403-415. doi: 10.1124/mol.116.106013. Epub 2017 Feb 10. PMID: 28188254; PMCID: PMC5363712. |
Other study aim: oxytocin receptor-mediated signaling |
|
Lamont RF, Jørgensen JS. Safety and Efficacy of Tocolytics for the Treatment of Spontaneous Preterm Labour. Curr Pharm Des. 2019;25(5):577-592. doi: 10.2174/1381612825666190329124214. PMID: 30931850. |
Wrong study design: narrative review |
|
Li Q, Li C, Jin H. Efficacy of allylestrenol combined with ritodrine on threatened premature labor and its influence on inflammatory factors in peripheral blood. Exp Ther Med. 2020 Feb;19(2):907-912. doi: 10.3892/etm.2019.8273. Epub 2019 Dec 3. PMID: 32010251; PMCID: PMC6966111. |
Wrong comparison: allylestrenol combined with ritodrine versus allylestrenol combined with magnesium sulfate |
|
Lucovnik M, Trojner Bregar A, Bombac L, Gersak K, Garfield RE. Effects of vaginal progesterone for maintenance tocolysis on uterine electrical activity. J Obstet Gynaecol Res. 2018 Mar;44(3):408-416. doi: 10.1111/jog.13545. Epub 2018 Jan 3. PMID: 29297950. |
Wrong intervention: vaginal progesterone |
|
Maisonneuve E, Carbonne B. Tocolyse d'entretien par les inhibiteurs calciques [Maintenance tocolysis with calcium channel blockers]. J Gynecol Obstet Biol Reprod (Paris). 2015 Apr;44(4):357-62. French. doi: 10.1016/j.jgyn.2014.12.009. Epub 2015 Feb 26. PMID: 25728781. |
Article in French |
|
Moraitis AA, Cordeaux Y, Charnock-Jones DS, Smith GC. The Effect of an Oxytocin Receptor Antagonist (Retosiban, GSK221149A) on the Response of Human Myometrial Explants to Prolonged Mechanical Stretch. Endocrinology. 2015 Oct;156(10):3511-6. doi: 10.1210/en.2015-1378. Epub 2015 Jul 24. PMID: 26207346. |
Other study aim: oxytocin receptor antagonist, retosiban (GSK221149A), inhibited the procontractile effect of stretch on human myometrium |
|
Murillo C, Migliorelli F, Nieto C, Martínez H, Rueda C, Bermejo R, Corrales A, Palacio M. A multi-centre, open-label, prospective, observational study to assess the safety of a nifedipine oral solution in the treatment of preterm labor. Clínica e Investigación en Ginecología y Obstetricia. 2023 Oct 1;50(4):100883. |
No comparison: effectiveness nifedipine |
|
Naik Gaunekar N, Raman P, Bain E, Crowther CA. Maintenance therapy with calcium channel blockers for preventing preterm birth after threatened preterm labour. Cochrane Database Syst Rev. 2013 Oct 31;(10):CD004071. doi: 10.1002/14651858.CD004071.pub3. PMID: 24173691. |
More recent systematic review with same studies available |
|
Navathe R, Berghella V. Progesterone as a tocolytic agent for preterm labor: a systematic review. Curr Opin Obstet Gynecol. 2016 Dec;28(6):464-469. doi: 10.1097/GCO.0000000000000327. PMID: 27764015. |
Wrong intervention: progesterone |
|
Nijman TA, van Vliet EO, Naaktgeboren CA, Oude Rengerink K, de Lange TS, Bax CJ, Bloemenkamp KW, van Eyck J, Kok M, Scheepers HC, Woiski M, Franx A, Mol BW, Oudijk MA. Nifedipine versus placebo in the treatment of preterm prelabor rupture of membranes: a randomized controlled trial: Assessment of perinatal outcome by use of tocolysis in early labor-APOSTEL IV trial. Eur J Obstet Gynecol Reprod Biol. 2016 Oct;205:79-84. doi: 10.1016/j.ejogrb.2016.08.024. Epub 2016 Aug 9. PMID: 27567363. |
Wrong intervention: no maintenance tocolysis |
|
Okuda A, Inayama Y, Mizuno K, Takeuchi M, Kawakami K, Mandai M, Higuchi T. Long-term vs short-term tocolysis with ritodrine hydrochloride: Propensity score-matched analysis. Eur J Obstet Gynecol Reprod Biol. 2023 Mar;282:77-82. doi: 10.1016/j.ejogrb.2023.01.011. Epub 2023 Jan 13. PMID: 36682208. |
Study design: retrospective study |
|
Palacio M, Cobo T, Antolín E, Ramirez M, Cabrera F, Mozo de Rosales F, Bartha JL, Juan M, Martí A, Oros D, Rodríguez À, Scazzocchio E, Olivares JM, Varea S, Ríos J, Gratacós E; PROMISE Collaborative Group. Vaginal progesterone as maintenance treatment after an episode of preterm labour (PROMISE) study: a multicentre, double-blind, randomised, placebo-controlled trial. BJOG. 2016 Nov;123(12):1990-1999. doi: 10.1111/1471-0528.13956. Epub 2016 Mar 30. PMID: 27028759. |
Wrong intervention: vaginal progesterone |
|
Palacio M, Ronzoni S, Sánchez-Ramos L, Murphy KE. Progestogens as Maintenance Treatment in Arrested Preterm Labor: A Systematic Review and Meta-analysis. Obstet Gynecol. 2016 Nov;128(5):989-1000. doi: 10.1097/AOG.0000000000001676. PMID: 27741193. |
Wrong intervention: progestogens as a maintenance treatment |
|
Papatsonis DN, Flenady V, Liley HG. Maintenance therapy with oxytocin antagonists for inhibiting preterm birth after threatened preterm labour. Cochrane Database Syst Rev. 2013 Oct 13;(10):CD005938. doi: 10.1002/14651858.CD005938.pub3. PMID: 24122673. |
Included only 1 study before 2010 |
|
Plummer CP. Evaluation of maternal and neonatal outcomes after maintenance tocolysis: a retrospective study. J Obstet Gynaecol Res. 2012 Jan;38(1):198-202. doi: 10.1111/j.1447-0756.2011.01675.x. Epub 2011 Oct 14. PMID: 21995784. |
Study design: retrospective study |
|
Ragunath MP, Sasmal D. Comparative evaluation of hematology and biochemistry before and after administration of nifedipine and isoxsuprine in the treatment of preterm labor. International Journal of Pharmaceutical and Clinical Research. 2015; 7(3): 212-215 |
Wrong comparison: nifedipine versus isoxsuprine |
|
Rath W. Treatment of preterm birth. Tocolysis, progesterone, RDS-prophylaxis. Padiatrische Praxis. 2017; 87(2):253-264. |
Article in German |
|
Rath W, Kuon RJ. Progesterone - Effective for Tocolysis and Maintenance Treatment After Arrested Preterm Labour?: Critical Analysis of the Evidence. Geburtshilfe Frauenheilkd. 2019 Aug;79(8):834-843. doi: 10.1055/a-0829-3992. Epub 2019 May 13. PMID: 31423018; PMCID: PMC6690738. |
Wrong study design: narrative review |
|
Roos C, Borowiack E, Kowalska M, Zapalska A, Mol B, Mignini L, Meads C, Walczak J, Khan K; EBM CONNECT collaboration. What do we know about tocolytic effectiveness and how do we use this information in guidelines? A comparison of evidence grading. BJOG. 2013 Dec;120(13):1588-96; discussion 1597-8. doi: 10.1111/1471-0528.12388. Epub 2013 Sep 10. PMID: 24020895. |
Wrong comparison: comparing tocolytics with either placebo or betamimetics. |
|
Roos C, Vis JY, Scheepers HC, Bloemenkamp KW, Duvekot HJ, van Eyck J, de Groot C, Kok JH, Opmeer BC, Oudijk MA, Papatsonis DN, Porath MM, Sollie K, Spaanderman ME, Lotgering FK, van der Post JA, Mol BW. Fetal fibronectin status and cervical length in women with threatened preterm labor and the effectiveness of maintenance tocolysis. J Matern Fetal Neonatal Med. 2016;29(10):1556-61. doi: 10.3109/14767058.2015.1053863. Epub 2015 Jun 24. PMID: 26103778. |
Wrong comparison: high- and low-risk women |
|
Saccone G, Suhag A, Berghella V. 17-alpha-hydroxyprogesterone caproate for maintenance tocolysis: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015 Jul;213(1):16-22. doi: 10.1016/j.ajog.2015.01.054. Epub 2015 Feb 4. PMID: 25659469. |
Wrong intervention: maintenance tocolysis with 17-alpha-hydroxyprogesterone caproate (17P) |
|
Schulz D, Schlieckau F, Fill Malfertheiner S, Reuschel E, Seelbach-Göbel B, Ernst W. Effect of betamethasone, indomethacin and fenoterol on neonatal and maternal mononuclear cells stimulated with Escherichia coli. Cytokine. 2019 Apr;116:97-105. doi: 10.1016/j.cyto.2018.12.017. Epub 2019 Jan 28. PMID: 30703694. |
Other study aim: effects on the immune system of mothers and neonates |
|
Sebastian E, Bykersma C, Eggleston A, Eddy KE, Chim ST, Zahroh RI, Scott N, Chou D, Oladapo OT, Vogel JP. Cost-effectiveness of antenatal corticosteroids and tocolytic agents in the management of preterm birth: A systematic review. EClinicalMedicine. 2022 Jun 3;49:101496. doi: 10.1016/j.eclinm.2022.101496. PMID: 35747187; PMCID: PMC9167884. |
Other study aim: costeffectiveness ACS and/or tocolytics |
|
Sentilhes L, Sénat MV, Ancel PY, Azria E, Benoist G, Blanc J, Brabant G, Bretelle F, Brun S, Doret M, Ducroux-Schouwey C, Evrard A, Kayem G, Maisonneuve E, Marcellin L, Marret S, Mottet N, Paysant S, Riethmuller D, Rozenberg P, Schmitz T, Torchin H, Langer B. Recommandations pour la pratique clinique : prévention de la prématurité spontanée et de ses conséquences (hors rupture des membranes) — Texte des recommandations (texte court) [Prevention of spontaneous preterm birth (excluding preterm premature rupture of membranes): Guidelines for clinical practice - Text of the Guidelines (short text)]. J Gynecol Obstet Biol Reprod (Paris). 2016 Dec;45(10):1446-1456. French. doi: 10.1016/j.jgyn.2016.09.011. Epub 2016 Nov 9. PMID: 27836377. |
Article in French |
|
Sharami SH, Zahiri Z, Shakiba M, Milani F. Maintenance therapy by vaginal progesterone after threatened idiopathic preterm labor: a randomized placebo-controlled double-blind trial. International Journal of Fertility and Sterility. 2010 Jul 1;4(2):45-50. |
Wrong intervention: vaginal progesterone |
|
SOMA M, KONDA A, FUJIEDA S, SASAKI Y, WATARI M, KEIRA M, YOSHIDA H, TODA T, HAYAKAWA T, KISHIMOTO S, FUKUSHIMA S. Difference in ritodrine pharmacokinetics between singleton and twin pregnancies. Rinsho yakuri/Japanese Journal of Clinical Pharmacology and Therapeutics. 2013 Sep 30;44(5):389-94. |
Other study aim: pharmacokinetics of ritodrine in singleton and twin pregnancies |
|
Stelzl P, Kehl S, Rath W. Maintenance tocolysis: a reappraisal of clinical evidence. Arch Gynecol Obstet. 2019 Nov;300(5):1189-1199. doi: 10.1007/s00404-019-05313-7. Epub 2019 Oct 1. PMID: 31576452. |
Wrong study design: narrative review |
|
Su LL, Samuel M, Chong YS. Progestational agents for treating threatened or established preterm labour. Cochrane Database Syst Rev. 2014 Jan 31;2014(1):CD006770. doi: 10.1002/14651858.CD006770.pub3. PMID: 24482121; PMCID: PMC11031808. |
Wrong intervention: progestational agents |
|
Suhag A, Saccone G, Berghella V. Vaginal progesterone for maintenance tocolysis: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015 Oct;213(4):479-87. doi: 10.1016/j.ajog.2015.03.031. Epub 2015 Mar 19. PMID: 25797233. |
Wrong intervention: maintenance tocolysis with vaginal progesterone |
|
Usta IM, Khalil A, Nassar AH. Oxytocin antagonists for the management of preterm birth: a review. Am J Perinatol. 2011 Jun;28(6):449-60. doi: 10.1055/s-0030-1270111. Epub 2010 Dec 17. PMID: 21170825. |
Wrong study design: narrative review |
|
van Vliet E, Dijkema GH, Schuit E, Heida KY, Roos C, van der Post J, Parry EC, McCowan L, Lyell DJ, El-Sayed YY, Carr DB, Clark AL, Mahdy ZA, Uma M, Sayin NC, Varol GF, Mol BW, Oudijk MA. Nifedipine maintenance tocolysis and perinatal outcome: an individual participant data meta-analysis. BJOG. 2016 Oct;123(11):1753-60. doi: 10.1111/1471-0528.14249. Epub 2016 Aug 23. PMID: 27550838. |
No data of individual studies presented |
|
van Vliet EO, Schuit E, Heida KY, Opmeer BC, Kok M, Gyselaers W, Porath MM, Woiski M, Bax CJ, Bloemenkamp KW, Scheepers HC, Jaquemyn Y, van Beek E, Duvekot HJ, Franssen MT, Bijvank BN, Kok JH, Franx A, Mol BW, Oudijk MA. Nifedipine versus atosiban in the treatment of threatened preterm labour (Assessment of Perinatal Outcome after Specific Tocolysis in Early Labour: APOSTEL III-Trial). BMC Pregnancy Childbirth. 2014 Mar 3;14:93. doi: 10.1186/1471-2393-14-93. PMID: 24589124; PMCID: PMC3944539. |
Wrong comparison: nifedipine versus atosiban |
|
Vladic Stjernholm Y, Vladic T, Marchini G. Progesterone Gel and Placebo Prolonged Pregnancy More Effectively Than Intravenous Tocolysis Alone in Women with Preterm Labor. Gels. 2022 Apr 26;8(5):272. doi: 10.3390/gels8050272. PMID: 35621570; PMCID: PMC9141710. |
Wrong intervention: progesterone |
|
Wagner P, Sonek J, Abele H, Sarah L, Hoopmann M, Brucker S, Wu Q, Kagan KO. Effectiveness of the contemporary treatment of preterm labor: a comparison with a historical cohort. Arch Gynecol Obstet. 2017 Jul;296(1):27-34. doi: 10.1007/s00404-017-4389-6. Epub 2017 May 8. PMID: 28484835. |
Study design: observational study |
|
Wood S, Rabi Y, Tang S, Brant R, Ross S. Progesterone in women with arrested premature labor, a report of a randomised clinical trial and updated meta-analysis. BMC Pregnancy Childbirth. 2017 Aug 2;17(1):258. doi: 10.1186/s12884-017-1400-y. PMID: 28768474; PMCID: PMC5541428. |
Wrong intervention: progesterone |
|
Yadav G, Gupta S, Singh P, Kansara M, Kathuria P, Gothwal M, Sharma C. The role of vaginal progesterone in established pre-term labor: A randomized controlled trial. J Family Med Prim Care. 2022 Nov;11(11):7042-7047. doi: 10.4103/jfmpc.jfmpc_884_22. Epub 2022 Dec 16. PMID: 36993040; PMCID: PMC10041234. |
Wrong intervention: progesterone |
|
Yokoyama K, Takahashi N, Yada Y, Koike Y, Kawamata R, Uehara R, Kono Y, Honma Y, Momoi MY. Prolonged maternal magnesium administration and bone metabolism in neonates. Early Hum Dev. 2010 Mar;86(3):187-91. doi: 10.1016/j.earlhumdev.2010.02.007. Epub 2010 Mar 12. PMID: 20226604. |
Other study aim: prolonged maternal administration of MgSO(4) on fetal bone metabolism according to serum biochemistry in infants |
|
Yoneda S, Yoneda N, Fukuta K, Shima T, Nakashima A, Shiozaki A, Yoshino O, Kigawa M, Yoshida T, Saito S. In which preterm labor-patients is intravenous maintenance tocolysis effective? J Obstet Gynaecol Res. 2018 Mar;44(3):397-407. doi: 10.1111/jog.13547. Epub 2017 Dec 14. PMID: 29239057. |
No comparison: effectiveness intravenous ritodrine hydrochloride and/or magnesium sulfate |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 28-08-2025
Beoordeeld op geldigheid : 03-06-2025
De Koninklijke Nederlandse Organisatie van Verloskundigen (KNOV) heeft een formele verklaring van geen bezwaar gegeven.
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor zwangeren waarbij sprake is van een dreigende vroeggeboorte.
Werkgroep
- Dr. C.J. (Caroline) Bax, gynaecoloog-perinatoloog, NVOG (voorzitter)
- Dr. J.B. (Jan) Derks, gynaecoloog-perinatoloog, NVOG
- Dr. A. (Ayten) Elvan-Taşpınar, gynaecoloog-perinatoloog, NVOG
- Dr. H.M. (Marieke) Knol, gynaecoloog-perinatoloog, NVOG
- Dr. M.A. (Marjon) de Boer, gynaecoloog-perinatoloog, NVOG
- Dr. D.N.M. (Dimitri) Papatsonis, gynaecoloog, NVOG
- Dr. D.E. (Lia) Wijnberger, gynaecoloog, NVOG
- Dr. P.H. (Dijk), kinderarts-neonatoloog, NVK
- Drs. L. (Leanne) Erkelens-de Vetten, kinderarts-neonataloog, NVK
- Drs. C. (Christel) Rolf, klinisch verloskundige, KNOV (tot maart 2023)
- Drs. C. (Cedric) van Uytrecht, klinisch verloskundige, KNOV (tot 15 augustus 2023)
- Drs. D. (Daphne) de Jong, eerstelijns verloskundige, KNOV (vanaf september 2023)
- Drs. M.A.M. (Machteld) van der Noll, verloskundige, KNOV
- Dr. I.F. (Igna) Kwint-Reijnders, patiëntenvertegenwoordiging Care4Neo
Klankbordgroep
- Drs. H.I. (Herma) Davelaar – van Zanten, V&VN Voortplanting, Obstetrie & Gynaecologie (tot mei 2024)
- Dhr. M. (Maikel) Hustinx, bestuurslid afdeling Vrouw & Kind V&VN (vanaf mei 2024)
Met ondersteuning van
- Drs. D.A.M. (Danique) Middelhuis, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. T. (Tessa) Geltink, adviseur, Kennisinstituut van de Federatie Medsich Specialisten (tot april 2023)
- Dr. M.L. (Marja) Molag, adviseur, Kennisinstituut van de Federatie Medsich Specialisten (vanaf april 2023)
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroep |
||||
|
Achternaam werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Bax (voorzitter) |
Gynaecoloog-perinatoloog AmsterdamUMC |
Allen onbetaald: Adviesraad MADAM project Lid Raad kwaliteit FMS Organisatie en docent basiscursus prenatale counseling Amsterdam UMC Audit voorzitter in regio Amsterdam Lid Dagelijks bestuur koepel kwaliteit |
ZonMW subsidie voor onderzoek naar NIPT |
Geen restricties |
|
Knol |
Perinataloog Isala Kliniek Zwolle |
Lid werkgroep Otterlo NVOG Lid wetenschapscommissie NVOG Lokale hoofdonderzoeker consortiumstudie apostel 8 |
Geen |
Geen restricties |
|
Elvan-Taspinar |
Perinatoloog UMCG |
Instructeur MOET onbetaald |
Geen |
Geen restricties |
|
Van Uytrecht |
Physician Assistant- Obstetrie |
Training acute verloskunde te Medsim. Verloskundige/ Physician Assistant te Maxima Medisch Centrum te Veldhoven |
Geen |
Geen restricties |
|
Rolf |
Physician Assistant Obstetrie; functie van afdelingsarts op de high care verloskunde( OHC), Máxima MC. Betaalde functie |
Klinisch verloskundige, Máxima MC, betaalde functie |
Geen |
Geen restricties |
|
Papatsonis |
Gynaecoloog Amphia Ziekenhuis Breda |
Geen |
Geen |
Geen restricties |
|
Kwint-Reijnders |
Patientvertegenwoordiger namens Care4Neo, experienced expert |
Gynaecoloog i.o. VAGO afgevaardigde in het pijlerbestuur NVOG werkgroep foetomaternale geneeskunde |
In mijn werkzaamheden als gynaecoloog in opleiding werk ik zelf met dreigende vroeggeboorte casuïstiek en met collega's die uitvoering geven aan deze richtlijn. Daarnaast heb ik zitting als VAGO-afgevaardigde in het pijlerbestuur van de NVOG werkgroep foetomaternale geneeskunde, waarin ook onderwerpen geadresseerd worden die gerelateerd zijn aan dreigende vroeggeboorte. |
Geen restricties |
|
Derks |
Gynaecoloog, afdeling verloskunde, WKZ, UMCU.
|
Betrokken bij de richtlijn preventie vroeggeboorte, onderdeel van de Otterlo, deze commissie schrijft de verloskunde richtlijnen voor de NVOG |
Ik ben binnen mijn kliniek betrokken bij de behandeling van patienten met vroeggeboorte (in de anamnese). Gezien mijn expertise op dit gebied zie ik veel patienten met vroeggeboorte |
Geen retricties |
|
De Vetten |
Kinderarts-neonatoloog, Martini ziekenhuis Groningen |
Geen |
Geen |
Geen restricties |
|
Wijnberger |
Gynaecoloog en perinatoloog Rijnstate Ziekenhuis Arnhem |
Lid werkgroep Otterlo (richtlijnontwikkeling) onbetaald Opleider, onbetaald |
Geen |
Geen restricties |
|
Van der Noll |
Klinisch verloskundige - Master Physician Assistant (inactief) Docent Verloskunde Ba-VKV Rotterdam (actief) |
Geen |
Geen |
Geen restricties |
|
De Boer |
Gynaecoloog |
Geen |
Geen |
Geen restricties |
|
Dijk |
Kinderarts-neonatoloog UMC Groningen |
Lidmaatschap Neonatologie Netwerk Nederland Lid LNR werkgroep Perined/NVK Lid werkgroep Nedederlands Kinderformularium NKFK Lid consortium PedMed-Nl Lid werkgroep revisie RL Hyperbilirubinemie Adviesraad N3 Adviesraad Zwangerschap en Geboorte Consortium Noord Nederland Lid werkgroep Kinderformularium Lid Pedmed Lid sectie Neonatologie Lid werkgroep SPIN |
Geen |
Geen restricties |
|
De Jong |
Eerstelijns verloskundige De Geboortezaak Nieuwegein Klinisch epidemioloog |
Klinisch epidemioloog Lid werkgroep HPP in de 1e lijn Lid werkgroep Handreiking indicaties vitaliteitsecho |
Geen |
Geen restricties |
|
Klankbordgroep |
||||
|
Achternaam klankbordgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Davelaar-Van Zanten |
Adviseur kwaliteit en veiligheid (betaalde functie/reguliere baan) Spaarne Gasthuis |
Geen |
Geen |
Geen restricties |
|
Maikel Hustinx |
Verpleegkundig Specialist, Albert Schweitzer Ziekenhuis, 36u p.w. |
Algemeen bestuurslid V&VN afdeling Vrouw en Kind, vrijwillig. Vice-voorzitter Vereniging Verpleegkundig Specialisten Albert Schweitzer Ziekenhuis, vrijwillig Lid landelijke tafel College Perinatale Zorg, Utrecht, vrijwillig |
Geen |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door uitnodigen van Patiëntenfederatie Nederland en Care4Neo voor de schriftelijke knelpuntenanalyse en afvaardiging namens Care4Neo in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen zie per module ook “Waarden en voorkeuren van patiënten”). De conceptrichtlijn is tevens voor commentaar voorgelegd aan Patiëntenfederatie Nederland en Care4Neo en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Module |
Uitkomst raming |
Toelichting |
|
Verlengde tocolyse
|
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat [het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet OF het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft]. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 3.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor zwangeren waarbij sprake is van dreigende vroeggeboorte. Tevens zijn er knelpunten aangedragen door Inspectie Gezondheidszorg en Jeugd, Nederlandse Vereniging voor Obstetrie en Gynaecologie, de Koninklijke Nederlandse Organisatie van Verloskundigen en Care4Neo via een schriftelijke knelpuntenanalyse. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy
|
Richtlijn: Dreigende vroeggeboorte |
|
|
Uitgangsvraag: Wat is de waarde van het langer dan 48 uur behandelen met tocolytica van een zwangere vrouw met een dreigende vroeggeboorte? |
|
|
Database(s): Medline (OVID), Embase |
Datum: 14-07-2023 |
|
Periode: > 2010 |
Talen: geen beperking |
|
Literatuurspecialist: Laura Boerboom |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting en opmerkingen:
→ Voor deze vraag is gezocht op de elementen dreigende vroeggeboorte (in het blauw), tocolytica (in het groen) en verlengde behandeling (in het rood).
à Er waren 4 sleutelartikelen en deze zitten allemaal in de resultaten.
à Resultaten staan in Rayyan.
|
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase (via embase.com) en Medline (via OVID) is op 14-07-2023 met relevante zoektermen gezocht naar systematische reviews, RCT’s en observationele studies over wat de waarde is van het langer dan 48 uur behandelen met tocolytica van een zwangere vrouw met een dreigende vroeggeboorte. De literatuurzoekactie leverde 192 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR’s |
40 |
36 |
46 |
|
RCT’s |
88 |
46 |
101 |
|
Observationele designs |
38 |
23 |
45 |
Zoekstrategie
|
Database |
Zoektermen |
|||||||||||||||||||||||||||||||||||||||
|
Embase
|
|
|||||||||||||||||||||||||||||||||||||||
|
Medline (OVID)
|
1 exp Obstetric Labor, Premature/ or ((labo*r or deliver* or parturition* or birth*) adj3 (premature or preterm or 'pre term' or early or prior)).ti,ab,kf. (81485) 2 exp Tocolysis/ or tocoly*.ti,ab,kf. or 'uterine relaxant*'.ti,ab,kf. or 'uterus spasmolytic agent*'.ti,ab,kf. or exp Calcium Channel Blockers/ or exp Nifedipine/ or exp Nicardipine/ or nifedipine.ti,ab,kf. or adalat.ti,ab,kf. or nicardipine.ti,ab,kf. or 'calcium channel blocker*'.ti,ab,kf. or 'calcium channel blocking agent*'.ti,ab,kf. or 'calcium channel antagonist*'.ti,ab,kf. or (atosiban or cligosiban or nolasiban or barusiban or 'oxytocin antagonis*').ti,ab,kf. (108315) 3 (maintenance or prolonged or continuation).ti,ab,kf. (700388) 4 (1 and 2 and 3) not (comment/ or editorial/ or letter/) (349) 5 limit 4 to yr="2010-Current" (148) 6 meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. (679945) 7 exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. (2609254) 8 Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) (5464844) 9 5 and 6 (36) SR’s 10 (5 and 7) not 9 (46) RCT’s 11 (5 and 8) not 9 not 10 (23) Observationele studies 12 9 or 10 or 11 (105) |