Laat afnavelen bij premature neonaat
Uitgangsvraag
Wat is de waarde van laat afnavelen bij een premature neonaat?
Aanbeveling
Wacht minimaal 60 seconden met het afklemmen van de navelstreng na de geboorte van een pasgeborene met een goede start, bij zwangerschapsduur tussen de 24 - 37 weken.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
In de literatuuranalyse werd onderzocht wat de waarde is van laat afnavelen (ten minste 60 seconden wachten met afnavelen of wachten tot volledig gestopt met pulseren) bij een premature neonaat (<37 weken zwangerschapsduur) met een goede start. Er werd één systematische review (Rabe, 2019) gevonden met acht RCT’s die voldeden aan de PICO (Dipak, 2017; Duley, 2017; Rana, 2017; Ranjit, 2015; Salae, 2016; Strauss, 2008; Tarnow-Mordi, 2017; Ultee, 2008). Daarnaast werden drie RCT’s gevonden die na de zoekdatum van Rabe 2019 zijn gepubliceerd (Armstrong-Buisseret, 2020; Robledo, 2021; Yunis, 2020). Armstrong-Buisseret (2020) en Robledo (2021) onderzochten de lange termijn effecten.
In deze module zijn de maternale uitkomsten niet meegenomen. Uit de literatuur blijkt wel dat er geen verschil lijkt te zijn tussen de hoeveelheid maternaal bloedverlies en het vroeg en laat afnavelen (McDonald, 2013). Uterotonica kunnen direct worden gegeven na de geboorte van het kind of na het afnavelen. Zie de NVOG richtlijn Haemorrhagia postpartum 2013-2015 (dit lijkt een kennis lacune).
Zwangerschapstermijn tussen de 24 - 37 weken
Elf studies rapporteerden het effect van laat afnavelen bij een premature neonaat geboren tussen 24 en 37 weken (Armstrong-Buisseret, 2020; Dipak, 2017; Duley, 2017; Rana, 2017; Ranjit, 2015; Robledo, 2021; Salea, 2016; Strauss, 2008; Tarnow-Mordi, 2017; Ultee, 2008; Yunis, 2020).
Met betrekking tot de cruciale uitkomstmaten blijkt dat laat afnavelen de perinatale sterfte vermindert en zijn er aanwijzingen dat het de neurologische uitkomsten bij 2 jaar verbetert. Door het beperkte aantal studies naar het effect van laat afnavelen op neurologische uitkomsten bij 2 jaar en door heterogeniteit tussen de studies kon de data niet gepoold worden. De bewijskracht voor deze uitkomstmaat is daarom laag.
Wat betreft belangrijke uitkomstmaten wordt in deze literatuuranalyse een klinisch relevant (doch statistisch niet significant) gunstig effect gevonden van laat afnavelen op het voorkomen van anemie bij de pasgeboren prematuur. Dit leidt volgens onze verdere analyse niet tot een vermindering van het aantal bloedtransfusies of een klinisch relevant verschil in ferritine gehalte op de termijn van 2-4 maanden postpartum.
Ten aanzien van potentieel nadelige effecten van laat afnavelen, te weten intra-ventriculaire bloedingen (door volume shifts direct na de geboorte) danwel hyperbilirubinemie (door verhoging van het hematocriet) vonden wij in deze literatuur analyse geen verschillen van laat afnavelen ten opzichte van vroeg afnavelen.
Zwangerschapstermijn tussen de 24 - 30 weken
Twee studies rapporteerden het effect van laat afnavelen bij de specifieke patiëntengroep prematuren geboren vóór 30 weken zwangerschapsduur (Tarnow-Mordi, 2017; Robledo, 2021).
Met betrekking tot de cruciale uitkomstmaten lijkt laat afnavelen te resulteren in een vermindering van perinatale sterfte vergeleken met vroeg afnavelen en zijn er aanwijzingen dat het de neurologische uitkomsten bij 2 jaar verbetert.
Wat betreft belangrijke uitkomstmaten werd in deze subgroep geen literatuur gevonden ten aanzien van het voorkomen van anemie bij prematuren < 30 weken die laat vs. vroeg werden afgenaveld. De literatuuranalyse toont geen verschil in het aantal gegeven bloedtransfusies. Het ferritine gehalte op de termijn van 2-4 maanden postpartum werd in deze subgroep niet beschreven.
Ten aanzien van potentieel nadelige effecten van laat afnavelen, te weten intra-ventriculaire bloedingen danwel hyperbilirubinemie, werd in deze subgroep <30 weken geen verschil gevonden van laat afnavelen ten opzichte van vroeg afnavelen.
Zwangerschapstermijn tussen de 30 - 37 weken
Drie studies rapporteerden het effect van laat afnavelen bij specifiek prematuren geboren na 30 weken zwangerschapsduur (Ranjit, 2015; Salae, 2016; Ultee, 2008).
Met betrekking tot cruciale uitkomstmaten lijkt laat afnavelen te resulteren in een vermindering van perinatale sterfte vergeleken met vroeg afnavelen. De bewijskracht voor de cruciale uitkomstmaten ‘perinatale sterfte’ was echter zeer laag vanwege methodologische beperkingen en spreiding in de richting van het effect. Voor de cruciale uitkomstmaat ‘neurologische uitkomsten op 2 jaar’ werd geen bewijs gevonden. Andere studies kunnen leiden tot nieuwe inzichten.
Wat betreft belangrijke uitkomstmaten wordt in deze literatuuranalyse geen statistisch significant gunstig effect gevonden van laat afnavelen op het voorkomen van anemie bij de pasgeboren prematuur. Echter neigen de gerapporteerde getallen van 12% vs. 0% anemie (Ranjit, 2015) en 6.8% vs. 0% (Salae, 2016) in het voordeel van laat afnavelen naar een klinisch relevant verschil. De data konden niet worden gepoold door het beperkte aantal artikelen en daarmee heterogeniteit tussen de studies.
De mogelijke vermindering van voorkomen van anemie in de groep prematuren >30 weken die behandeld werden met laat afnavelen, leidt volgens onze verdere analyse niet tot een vermindering van het aantal bloedtransfusies of een klinisch relevant verschil in ferritine gehalte op de termijn van 2-4 maanden postpartum.
Ten aanzien van potentieel nadelige effecten van laat afnavelen, te weten intra-ventriculaire bloedingen danwel hyperbilirubinemie, werd in deze subgroep >30 weken geen verschil gevonden van laat afnavelen ten opzichte van vroeg afnavelen.
De pasgeborene met een slechte start:
Alle beschreven literatuur gaat over laat afnavelen bij pasgeborenen met een goede start. Wanneer een kind met een slechte start geboren wordt, ontstaat het dilemma of het kind direct afgenaveld moet worden om adequate respiratoire ondersteuning te krijgen. Tot op heden is onvoldoende wetenschappelijk bewijs dat het positieve effect van laat afnavelen opweegt tegen later starten met respiratoire ondersteuning als het kind met een slechte start geboren wordt. Daarom wordt dit nog niet als zodanig geadviseerd in internationale richtlijnen met betrekking tot neonatale reanimatie.
In sommige centra wordt om deze reden een aangepaste opvangtafel gebruikt die naast moeder of over moeder heen geplaatst kan worden (o.a. Life Start Trolley of Concord Table). Deze manier van opvangen maakt het mogelijk om de neonatale reanimatie op te starten terwijl de navelstreng van de pasgeborene nog intact is. Het gebruik van een dergelijke opvangtafel wordt over het algemeen in onderzoeksverband gedaan in academische centra; het is nog geen standaard zorg. De recent gepubliceerde Nederlandse ABC3-trial naar rescusitatie met intacte navelstreng bij prematuren toont geen verschil in overleving zonder grote intra-cerebrale bloedingen of necrotiserende enterocolitis (Knol, 2024). Een mogelijk gunstig effect in mannelijke pasgeborenen moet verder onderzocht worden. De techniek was veilig om uit te voeren en ouders rapporteerde meer tevredenheid en minder angstklachten.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Wanneer wordt gewacht met afnavelen van een premature pasgeborene, betekent dat over het algemeen dat de pasgeborene op de borst/ in de buurt van de moeder kan blijven liggen. Naar verwachting zullen ouders het op prijs stellen om op deze manier het eerste contact met hun kindje te hebben. Wetenschappelijk onderzoek toont aan dat direct huidcontact na de geboorte positieve effecten heeft op het slagen van borstvoeding, mortaliteit, thermoregulatie van de pasgeborene en daarnaast op de binding tussen ouder en kind en het geestelijk welzijn van ouders (Altit 2024).
Gezien bovendien het gunstige effect van laat afnavelen op perinatale mortaliteit en mogelijk ook op neurologische uitkomst bij 2 jaar, is onze verwachting dat ouders het belang hiervan volledig ondersteunen.
Het is belangrijk om ouders vooraf aan de partus voor te lichten over de mogelijkheid en de procedure van laat afnavelen. Door dit vooraf met ouders te bespreken, is het duidelijk naar ouders toe en kan geen verkeerde verwachting, teleurstelling of zelfs trauma ontstaan wanneer de opvang anders verloopt dan gedacht.
Kosten (middelenbeslag)
Van het wachten met afnavelen tot tenminste 60 seconden na de geboorte, zijn geen of nihil extra kosten te verwachten. Gezien het potentieel gunstige effect op perinatale sterfte, zouden eventuele minimale kosten de verandering in werkwijze zeker waard zijn.
Aanvaardbaarheid, haalbaarheid en implementatie
Sinds 2021 wordt vanuit de Nederlandse Reanimatie Raad de aanbeveling gedaan om na de geboorte van onbedreigde premature of a-terme pasgeborene, minimaal 60 seconden te wachten met het afklemmen van de navelstreng. Het is een simpele handeling die weinig tijdverlies of kosten met zich meebrengt, maar wel potentieel gunstige gevolgen heeft voor de pasgeborene. Derhalve is het laat afnavelen in de meeste klinieken in Nederland al dagelijkse gang van zaken. Er zijn dan ook geen grote bezwaren of belemmerende factoren te verwachten om dit beleid landelijk te implementeren.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Gezien het gunstige effect op neonatale mortaliteit, de mogelijke verbetering van neurologische uitkomsten bij 2 jaar en het ontbreken van nadelige effecten (IVH en hyperbilirubinemie) is er voldoende argumentatie om minimaal 60 seconden te wachten met afnavelen na de geboorte van een pasgeborene met een goede start met een zwangerschapsduur ³ 24 en -<37 weken.
Onderbouwing
Achtergrond
Clamping the umbilical cord can take place at different times after birth: immediately, within 60 seconds, after 60-180 seconds or after the umbilical cord completely stopped pulsating. Ideally the lungs will be inflated before the umbilical cord is clamped. At that stage, oxygen supply to the newborn is not depending on the umbilical flow anymore.
A number of guidelines (WHO, European resuscitation guideline, Dutch resuscitation guideline) already recommend delayed cord clamping (after 60 seconds) in case of a premature birth and a vital child. It has not yet been described in an NVOG guideline. It is therefore still dependent on local agreements/customs whether everyone adheres to this recommendation.
Conclusies / Summary of Findings
PICO 1: premature birth between 24 and 37 weeks
|
Low GRADE |
The evidence suggests that delayed cord clamping reduces perinatal death when compared with early cord clamping in premature births between 24 and 37 weeks.
Source: Duley, 2017; Ranjit, 2015; Strauss, 2008; Tarnow-Mordi, 2017; Yunis, 2020 |
|
Low GRADE |
The evidence suggests that delayed cord clamping reduces adverse neurological outcome at 2 years when compared with early cord clamping in premature births between 24 and 37 weeks.
Source: Armstrong-Buisseret, 2020; Robledo, 2021 |
|
Very low GRADE |
The evidence is very uncertain about the effect of delayed cord clamping on intraventricular hemorrhage when compared with early cord clamping in premature births between 24 and 37 weeks.
Source: Duley, 2017; Rana, 2017; Ranjit, 2015; Tarnow-Mordi, 2017; Yunis, 2020 |
|
Low GRADE |
The evidence suggests that delayed cord clamping results in little to no difference on need for erythrocyte transfusion when compared with early cord clamping in premature births between 24 and 37 weeks.
Source: Dipak, 2017; Duley, 2017; Rana, 2017; Ranjit, 2015; Salae, 2016; Strauss, 2008; Tarnow-Mordi, 2017; Yunis, 2020 |
|
Very low GRADE |
The evidence is very uncertain about the effect of delayed cord clamping on anemia when compared with early cord clamping in premature births between 24 and 37 weeks.
Source: Duley, 2017; Ranjit, 2015; Salae, 2016 |
|
Low GRADE |
The evidence suggests that delayed cord clamping results in little to no difference on bilirubin level when compared with early cord clamping in premature births between 24 and 37 weeks.
Source: Ranjit, 2015; Tarnow-Mordi, 2017; Yunis, 2020 |
|
Very low GRADE |
The evidence is very uncertain about the effect of delayed cord clamping on ferritin level when compared with early cord clamping in premature births between 24 and 37 weeks.
Source: Ranjit, 2015; Ultee, 2008 |
PICO 2: premature birth between 24 and 30 weeks
|
Low GRADE |
The evidence suggests that delayed cord clamping results in a reduction in perinatal death when compared with early cord clamping in premature births between 24 and 30 weeks.
Source: Tarnow-Mordi, 2017 |
|
Low GRADE |
The evidence suggests that delayed cord clamping results in a reduction in adverse neurological outcome at 2 years when compared with early cord clamping in premature births between 24 and 30 weeks.
Source: Robledo, 2021 |
|
Very low GRADE |
The evidence is very uncertain about the effect of delayed cord clamping on intraventricular hemorrhage when compared with early cord clamping in premature births between 24 and 30 weeks.
Source: Tarnow-Mordi, 2017 |
|
Low GRADE |
The evidence suggests that delayed cord clamping results in little to no difference in the need for erythrocyte transfusion when compared with early cord clamping in premature births between 24 and 30 weeks.
Source: Tarnow-Mordi, 2017 |
|
NO GRADE |
No evidence was found regarding the effect of delayed cord clamping on anemia when compared with early cord clamping in premature births between 24 and 30 weeks. |
|
Low GRADE |
The evidence suggests that delayed cord clamping results in little to no difference in bilirubin level when compared with early cord clamping in premature births between 24 and 30 weeks.
Source: Tarnow-Mordi, 2017 |
|
NO GRADE |
No evidence was found regarding the effect of delayed cord clamping on ferritin level when compared with early cord clamping in premature births between 24 and 30 weeks. |
PICO 3: premature birth between 30 and 37 weeks
|
Very low GRADE |
The evidence is very uncertain about the effect of delayed cord clamping on perinatal death when compared with early cord clamping in premature births between 30 and 37 weeks.
Source: Ranjit, 2015 |
|
No GRADE |
No evidence was found regarding the effect of delayed cord clamping on neurological outcome at 2 years when compared with early cord clamping in premature births between 30 and 37 weeks. |
|
Very low GRADE |
The evidence is very uncertain about the effect of delayed cord clamping on intraventricular hemorrhage when compared with early cord clamping in premature births between 30 and 37 weeks. |
|
Very low GRADE |
The evidence is very uncertain about the effect of delayed cord clamping on need for erythrocyte transfusion when compared with early cord clamping in premature births between 30 and 37 weeks.
Source: Ranjit, 2015; Salae, 2016 |
|
Very low GRADE |
The evidence is very uncertain about the effect of delayed cord clamping on anemia when compared with early cord clamping in premature births between 30 and 37 weeks.
Source: Ranjit, 2015; Salae, 2016 |
|
Very low GRADE |
The evidence is very uncertain about the effect of delayed cord clamping on bilirubin level when compared with early cord clamping in premature births between 30 and 37 weeks.
Source: Ranjit, 2015 |
|
Very low GRADE |
The evidence is very uncertain about the effect of delayed cord clamping on ferritin level when compared with early cord clamping in premature births between 30 and 37 weeks.
Source: Ranjit, 2015; Ultee, 2008 |
Samenvatting literatuur
Description of studies
The Cochrane review of Rabe (2019) assessed the effects of delayed cord clamping, early cord clamping and umbilical cord milking on infants born at less than 37 weeks' gestation and their mothers. In November 2018, the Cochrane Pregnancy and Childbirth Group Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) were searched for (cluster) randomized controlled trials and reference lists of retrieved studies were checked. Quasi-randomized trials were excluded. For the purpose of this guideline, only studies comparing delayed cord clamping (defined as after 60 seconds or completely stopped pulsating) with early cord clamping were included. This resulted in eight studies matching with the PICO (Dipak, 2017; Duley, 2017; Rana, 2017; Ranjit, 2015; Salae, 2016; Strauss, 2008; Tarnow-Mordi, 2017; Ultee, 2008). These studies are described in more detail below and in table 1.
Armstrong-Buisseret (2020) performed a parallel group randomized trial to assess outcomes at 2 years corrected age for children of women recruited to a trial comparing alternative policies for timing of cord clamping and immediate neonatal care at very preterm birth (follow-up Duley). Women expected to have a live birth before 32+0 weeks’ gestation were included. In total, 261 women were randomized to either delayed cord clamping (cord clamping after ³ 2 minutes; neonatal care with cord intact) or immediate cord clamping (cord clamping £ 20 seconds; neonatal care after clamping). One hundred and thirty-seven children had their cord clamped for ³ 2 minutes and 139 children had their cord clamped for less than 20 seconds. Groups were comparable at baseline. The outcome of interest was adverse neurodevelopmental outcome at 2 years corrected age.
Dipak (2017) performed a randomized controlled trial to determine short term clinical effects of delayed cord clamping. Women with a gestational age between 27 and 32 weeks with preterm onset of labor were included. Exclusion criteria were women with multiple gestation, Rh-ve status, placenta previa or abruption-placenta, and those having a fetus with major congenital anomalies, hydrops, fetal growth restriction with abnormal Doppler waveforms, or evidence of fetal distress. In total, 26 infants received delayed cord clamping (at 60 seconds) and 27 infants had immediate cord clamping (at 10 seconds). Groups were comparable at baseline. Outcomes of interest were bilirubin level, transfusion, and intraventricular hemorrhage.
Duley (2017) performed a parallel group randomized trial to compare alternative policies for umbilical cord clamping and immediate neonatal care for very preterm births. Women who expected a live birth before 32 weeks of gestation regardless of mode of delivery or fetal presentation were included. Exclusion criteria were monochorionic twins, triplets, or higher-order multiple pregnancy and known major congenital malformation. In total, 130 women had their cord clamped after at least 2 minutes and their infants, if needed, received immediate neonatal stabilization and resuscitation with cord intact. These women were compared with 124 women who had their cord clamped within 20 seconds and their infants, if needed, received immediate neonatal stabilization and resuscitation after clamping. Groups were comparable at baseline. Outcomes of interest were perinatal death, intraventricular hemorrhage grade 3 or 4 and blood transfusion.
Rana (2017) performed a randomized controlled trial to determine the safety of delayed cord clamping in infants born at less than 34 weeks of gestation. Women with a gestational age of less than 34 weeks and who were in the late first stage of labor were included. Exclusion criteria were congenital malformations, serious maternal illnesses (such as severe preeclampsia or eclampsia, uncompensated heart disease, or any abnormal bleeding before cord clamping), pregnant with twins or triplets, and infants who required immediate resuscitation at birth. Fifty infants underwent delayed cord clamping (DCC: after 120 seconds) and fifty infants had early cord clamping (ECC: within 30 seconds of birth). Groups were comparable at baseline except for birth weight and small for gestational age (SGA). Infants who underwent DCC had a higher birth weight and had no SGA. Outcomes of interest were bilirubin levels and need for blood transfusion.
Ranjit (2015) performed a randomized controlled trial to assess the benefits and safety of delayed cord clamping. Neonates born between 30+0 and 36+6 weeks were included. Exclusion criteria were mothers with Rhesus negative blood group and monoamniotic/
monochorionic twins. Besides, infants who were randomized to delayed cord clamping, but needed resuscitation at birth, were excluded from the analysis. In total, 44 infants received delayed cord clamping (>2 minutes) and 50 infants had their cord clamped immediately after delivery. Groups were comparable at baseline except for maternal hemoglobin. Women who had delayed cord clamping had lower hemoglobin levels. Outcomes of interest were death anemia, blood transfusion, bilirubin level and ferritin level at 6 weeks.
Robledo (2021) performed a multicenter randomized clinical trial to determine whether delayed umbilical cord clamping decrease mortality or major disability at 2 years (follow-up Tarnow-Mordi). Infants from women who expected to deliver before 30 weeks of gestation were included. Exclusion criteria were fetal hemolytic disease, hydrops fetalis, twin-twin transfusion, genetic syndromes, and potentially lethal malformations. In total, 767 infants had delayed cord clamping (³60 seconds) and 764 infants received immediate cord clamping (within 10 seconds). Groups were comparable at baseline. The outcome of interest was major disability at 2 years of age.
Salae (2016) performed a randomized controlled trial to assess the hematocrit and microbilirubin levels after delayed and immediate cord clamping in late preterm neonates born by vaginal delivery. Women between 18 and 45 years with a gestational age between 34 and 36+6 weeks who were admitted for preterm delivery were included. Exclusion criteria were thalassemia syndrome, preeclampsia, gestational diabetes mellitus, renal impairment, placental abnormalities, fetus with major congenital anomalies, multiple
gestations, instrumental delivery and-or abnormal fetal tracing (severe fetal bradycardia, fetal distress and non-reassuring fetal heart rate). In total, 42 infants received delayed cord clamping (within 2 minutes after delivery) and 44 infants had immediate cord clamping (not defined). Groups were comparable at baseline. The outcome of interest was bilirubin level.
Strauss (2008) performed a randomized controlled trial to compare delayed with immediate cord clamping with respect to hematologic and clinical effects. Infants born before 37 weeks of gestation were included. In total, 45 infants had delayed cord clamping (at 60 seconds) and 60 infants received immediate cord clamping (within 2 to 5 seconds, but not to exceed 15 seconds after delivery). It was unclear if groups were comparable at baseline. Outcomes of interest were death, intraventricular hemorrhage, and blood transfusion of the infant.
Tarnow-Mordi (2017) performed a randomized controlled trial to determine the effects of delayed versus immediate cord clamping on neonatal outcomes. Women who were expected to deliver before 30 weeks of gestation were included. Exclusion criteria were fetal hemolytic disease, hydrops fetalis, twin–twin transfusion, genetic syndromes, and potentially lethal malformations. In total, 784 infants received delayed clamping (≥60 seconds after delivery) and 782 infants received immediate clamping of the umbilical cord (≤10 seconds after delivery). Groups were comparable at baseline. Outcomes of interest were death, intraventricular hemorrhage, bilirubin level, and transfusion.
Ultee (2008) performed a randomized controlled trial to assess the effects of delayed or early cord clamping. Infants born between 34+0 and 36+6 weeks of gestation who were delivered vaginally were included. Exclusion criteria were overt diabetes or gestational diabetes and pregnancy-induced hypertension (>20 mm Hg rise of diastole during pregnancy in combination with albuminuria). In total, 21 infants had delayed cord clamping (after 180 seconds) and 20 infants received immediate cord clamping (within 30 seconds). Groups were comparable at baseline. The outcome of interest was ferritin level at 10 weeks.
Yunis (2020) performed a pilot randomized controlled trial to assess the effect of delayed cord clamping in infants born to mothers with placental insufficiency. Infants born at less than 34 weeks of gestation whose mother had placental insufficiency were included. Exclusion criteria were infants with congenital anomalies or suspected chromosomal anomalies, and infants who needed major resuscitation steps at birth in whom delay of resuscitation measures was not possible. In total, 38 infants had delayed cord clamping (at 60 seconds) and 30 infants received immediate cord clamping (10 seconds after delivery). Groups were comparable at baseline. Outcomes of interest were death, bilirubin level, intraventricular hemorrhage, and blood transfusion.
Table 1. Description of included studies.
|
Study |
Gestation (inclusion criteria) |
Intervention |
Comparator |
Outcomes |
||
|
|
Characteristics |
Intervention |
Characteristics |
Control |
|
|
|
Armstrong-Buisseret, 2020 (follow-up Duley, 2017) |
<32 weeks |
Arm 1 (n= 132) Gestational age (median) at birth: 29 weeks (27.1 to 30.7 weeks) |
Delayed cord clamping: ³ 2 minutes |
Arm 2 (n= 129) Gestational age (median) at birth: 29.1 weeks (27.6 to 30.4 weeks) |
Immediate cord clamping: within 10 seconds |
Major disability at 2 years |
|
Dipak, 2017 |
27 to 316/7 |
Arm 1 (n= 26) Gestational age (mean±SD): 29.9 ± 1.4 weeks |
Delayed cord clamping: at 60 seconds |
Arm 2 (n= 27) Gestational age (mean±SD): 30.1 ± 1.2 weeks |
Immediate cord clamping: at 10 seconds |
Bilirubin level, blood transfusion |
|
Duley, 2017 |
<32 weeks |
Arm 1 (n= 130) Gestational age at randomisation: <26 weeks: 22 (17%) 26 to 27+6 weeks: 25 (19%) 28 to 29+6 weeks: 38 (29%) 30 to 31+6 weeks: 44 (34%) ³32 weeks: 1 (1%) |
Delayed cord clamping: ³ 2 minutes |
Arm 2 (n= 124) Gestational age at randomisation: <26 weeks: 14 (11%) 26 to 27+6 weeks: 21 (17%) 28 to 29+6 weeks: 42 (34%) 30 to 31+6 weeks: 46 (37%) ³32 weeks: 1 (1%) |
Immediate cord clamping: within 20 seconds |
Death, IVH grade 3 or 4, blood transfusion |
|
Rana, 2017 |
<34 weeks |
Arm 1 (n= 50) Gestational age (mean±SD) at baseline: 32.3 ± 1.1 weeks |
Delayed cord clamping: after 120 seconds |
Arm 2 (n= 50) Gestational age (mean±SD) at baseline: 32.4 ± 1.0 weeks |
Immediate cord clamping: within 30 seconds |
Blood transfusion, bilirubin level |
|
Ranjit, 2015 |
300/7 to 366/7 weeks |
Arm 1 (n= 50) Gestational age (mean±SD): 34.0 ± 1.6 weeks |
Delayed cord clamping: >2 minutes |
Arm 2 (n= 50) Gestational age (mean±SD): 34.1 ± 2.0 weeks |
Immediately after birth |
Death, anemia, bilirubin- and ferritin level, blood transfusion |
|
Robledo, 2021 (follow-up Tarnow-Mordi, 2017) |
<30 weeks |
Arm 1 (n= 767) Gestational age (mean±SD) at randomisation: 28 ± 2 weeks |
Delayed cord clamping: ³60 seconds |
Arm 2 (n= 764) Gestational age (mean±SD) at randomisation: 28 ± 2 weeks |
Immediate cord clamping: within 10 seconds |
Major disability at 2 years |
|
Salae, 2016 |
34 to 36+6 weeks |
Arm 1 (n= 42) Gestational age (mean±SD): 35.7 ± 1.0 weeks |
Delayed cord clamping: at 120 seconds |
Arm 2 (n= 44) Gestational age (mean±SD): 36.0 ± 0.8 weeks |
Immediate cord clamping (not defined) |
Bilirubin level |
|
Strauss, 2008 |
≤36 weeks |
Arm 1 (n= 45) Gestational age (mean±SD): NR Between 30 and 36 weeks |
Delayed cord clamping: 60 seconds |
Arm 2 (n= 60) Gestational age (mean±SD): NR Between 30 and 36 weeks |
Immediate cord clamping: within 2-5 sec, but not to exceed 15 seconds after delivery |
Death, blood transfusion, bilirubin level |
|
Tarnow-Mordi, 2017 |
<30 weeks |
Arm 1 (n= 784) Gestational age (mean±SD) at randomisation: 28 ± 2 weeks |
Delayed cord clamping: ³60 seconds |
Arm 2 (n= 782) Gestational age (mean±SD) at randomisation: 28 ± 2 weeks |
Immediate cord clamping: ≤10 seconds
|
Death, IVH, blood transfusion, bilirubin level |
|
Ultee, 2008 |
34+0 weeks to 36+6 weeks |
Arm 1 (n= 21) Gestational age (mean±SD): 36.05 ± 0.65 weeks |
Delayed cord clamping: after 180 seconds |
Arm 2 (n= 20) Gestational age (mean±SD): 36.08 ± 0.74 weeks |
Immediate cord clamping: within 30 secs (mean of 13.4 seconds (SD 5.6)) |
Ferritin level |
|
Yunis, 2020 |
<34 weeks |
Arm 1 (n= 30) Gestational age (mean±SD): 29.7 ± 1.7 weeks |
Delayed cord clamping: at 60 seconds |
Arm 2 (n= 30) Gestational age (mean±SD): 30.4 ± 1.2 weeks |
Immediate cord clamping: within 10 seconds after delivery |
Death, bilirubin level, IVH, transfusion |
Abbreviations: IVH=intraventricular haemorrhage; NR=not reported
PICO 1: premature birth between 24 and 37 weeks
Results
1. Perinatal death
Five studies reported perinatal death (Duley, 2017; Ranjit, 2015; Strauss, 2008; Tarnow-Mordi, 2017; Yunis, 2020) (Figure 1.1). In total, 61 of the 1038 infants (5.9%) who received delayed cord clamping died as compared to 93 of the 1057 infants (8.8%) who had early cord clamping (RR=0.66, 95%CI 0.44 to 1.00). This difference is clinically relevant favoring delayed cord clamping.
Figure 1.1. Perinatal death for gestational age between 24 and 37 weeks.
2. Neurological outcome at 2 years
Two studies reported neurological outcomes at 2 years (Armstrong-Buisseret, 2020; Robledo, 2021) (Figure 1.2). These data were not pooled as the agreement is to start pooling when including at least three studies and because of heterogeneity between the studies.
Armstrong-Buisseret (2020) reported adverse neurodevelopmental outcome at 2 years corrected age. This was defined as having met the criteria for a moderate/severe impairment in any one of five functions: motor, cognitive, speech/language, hearing or vision. Adverse neurodevelopmental outcomes occurred in 16 of the 107 infants (15%) who had delayed cord clamping as compared to 19 of the 87 infants (21.8%) who had immediate cord clamping (RR=0.68, 95%CI 0.38 to 1.25). This difference is clinically relevant favoring delayed cord clamping.
Robledo (2021) reported major disability at 2 years defined as one or more of the following: cerebral palsy, severe visual loss, deafness, major problems with language or speech, or cognitive delay. Major disability occurred in 144 of the 627 infants (23%) who had delayed cord clamping as compared to 159 of the 603 infants (26%) who had immediate cord clamping (RR=0.87, 95%CI 0.72 to 1.06). This difference is clinically relevant favoring delayed cord clamping.
Figure 1.2. Neurological outcomes at 2 years for gestational age between 24 and 37 weeks.
3. Intraventricular hemorrhage
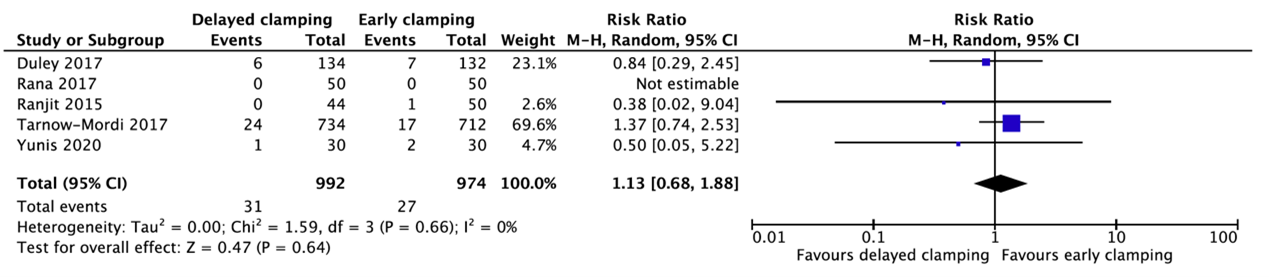
Five studies reported intraventricular hemorrhage (Duley, 2017; Rana, 2017; Ranjit, 2015; Tarnow-Mordi, 2017; Yunis, 2020) (Figure 1.3). In total, 31 of the 992 infants (3.1%) who received delayed cord clamping had an intraventricular hemorrhage as compared to 27 of the 974 infants (2.8%) who had early cord clamping (RR=1.13, 95%CI 0.68 to 1.88).
Figure 1.3. Intraventricular hemorrhage for gestational age between 24 and 37 weeks.
4. Need for erythrocyte transfusion
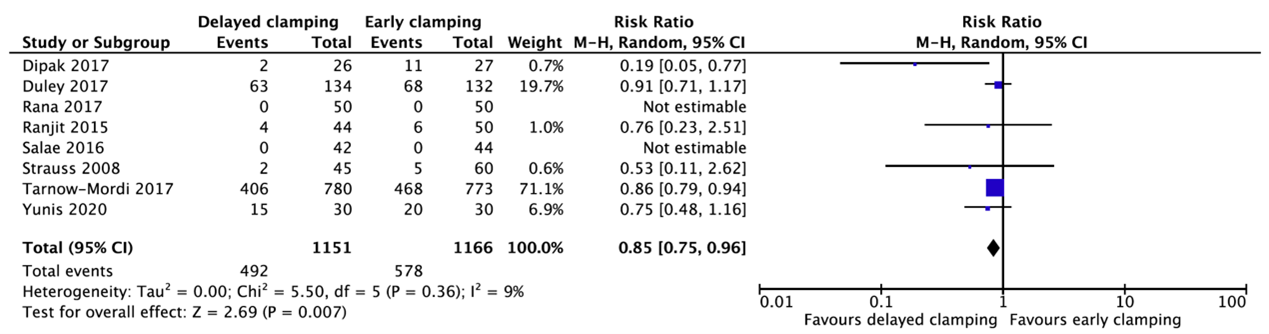
Eight studies reported about the need for erythrocyte transfusion (Dipak, 2017; Duley, 2017; Rana, 2017; Ranjit, 2015; Salae, 2016; Strauss, 2008; Tarnow-Mordi, 2017; Yunis, 2020) (Figure 1.4). In total, 492 of the 1151 infants (42.7%) who received delayed cord clamping needed a transfusion as compared to 578 of the 1166 infants (49.6%) who had early cord clamping (RR=0.85, 95%CI 0.75 to 0.96). This difference is not clinically relevant favoring delayed cord clamping.
Figure 1.4. Need for erythrocyte transfusion for gestational age between 24 and 37 weeks.
5. Anemia
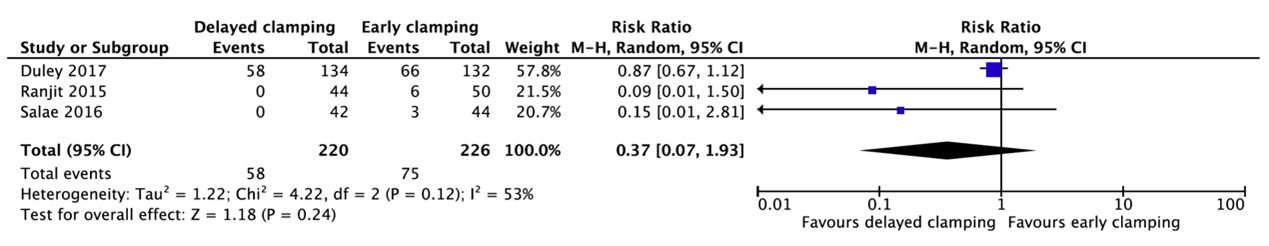
Three studies reported anemia (Duley, 2017; Ranjit, 2015; Salae, 2016) (Figure 1.5). In total, 58 of the 220 infants (26.4%) who had delayed cord clamping had anemia as compared to 75 of the 226 infants (33.2%) (RR=0.37, 95%CI 0.07 to 1.93).
Figure 1.5. Anemia for gestational age between 24 and 37 weeks.
6. Bilirubin level
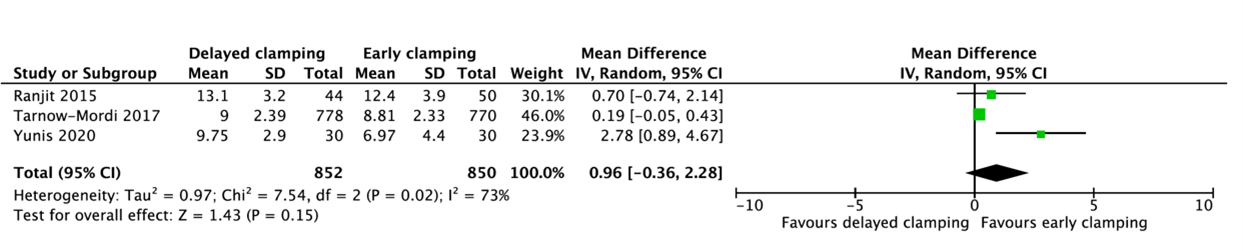
Three studies reported the peak serum bilirubin (Ranjit, 2015; Tarnow-Mordi, 2017; Yunis, 2020) (figure 1.6). A pooled mean difference of 0.96 mg/dL (95%CI -0.36 to 2.28) in peak bilirubin was found between delayed cord clamping and early cord clamping. This difference is not clinically relevant.
Figure 1.6. Peak serum bilirubin for gestational age between 24 and 37 weeks.
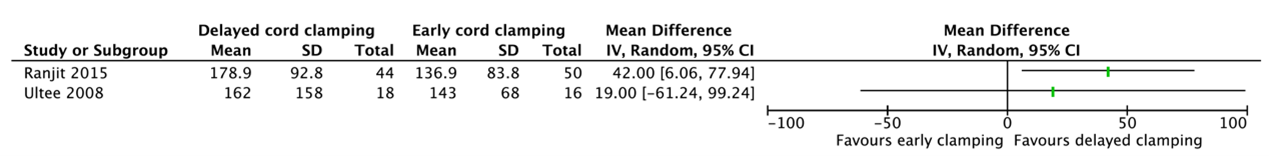
7. Ferritin level
Two studies reported a ferritin level (Ranjit, 2015; Ultee, 2008) (Figure 1.7). These data were not pooled as the agreement is to start pooling when including at least three studies and because of heterogeneity between the studies
Ranjit (2015) reported the ferritin level in infants at 6 weeks of age. Infants who had delayed cord clamping (n=44) had a mean ferritin level of 178.9 ng/mL (SD=92.8) as compared to 136.9 ng/mL (SD=83.8) for infants who had early cord clamping (n=50) (MD=42.00, 95%CI 6.06 to 77.94).
Ultee (2008) reported the ferritin level in infants at 10 weeks. Infants who had delayed cord clamping (n=18) had a mean ferritin level of 162 mg/L (SD=158) as compared to 143 mg/L (SD=68) for infants who had early cord clamping (n=16) (MD=19.00, 95%CI -61.24 to 99.24).
Figure 1.7. Ferritin level for gestational age between 24 and 37 weeks.
Level of evidence of the literature
According to GRADE, the level of evidence of randomized controlled trials start high.
The level of evidence regarding the outcome measure perinatal death was downgraded by two levels to low because of study limitations (-1, risk of bias) and the 95% confidence interval crossed the line of no (clinically relevant) effect (-1, imprecision).
The level of evidence regarding the outcome measure neurological outcome at 2 years was downgraded by two levels to low because the optimal information size was not achieved (-2, imprecision).
The level of evidence regarding the outcome measure intraventricular hemorrhage was downgraded by three levels to very low because of study limitations (-1, risk of bias) and the 95% confidence interval crossed the lines of no (clinically relevant) effect (-2, imprecision).
The level of evidence regarding the outcome measure need for erythrocyte transfusion was downgraded by two levels to low because of study limitations regarding the study population and incomplete outcome data (-1, risk of bias), and the 95% confidence interval crossed the line of no (clinically relevant) effect (-1, imprecision).
The level of evidence regarding the outcome measure anemia was downgraded by three levels to very low because of study limitations (-1, risk of bias), and the 95% confidence interval crossed both lines of no (clinically relevant) effect (-2, imprecision).
The level of evidence regarding the outcome measure bilirubin level was downgraded by three levels to low because of study limitations (-1, risk of bias) and heterogeneity between the studies (-1, inconsistency).
The level of evidence regarding the outcome measure ferritin level was downgraded by three levels to very low because of study limitations (-1, risk of bias), differences in the definition of the outcome (-1, indirectness) and the optimal information size was not achieved (-1, imprecision).
PICO 2: premature birth between 24 and 30 weeks
Results
1. Perinatal death
Tarnow-Mordi (2017) reported that 50 of 784 infants (6.4%) who had delayed cord clamping died as compared to 70 of the 782 infants (9.0%) who had early cord clamping (RR=0.71, 95%CI 0.50 to 1.01). This difference is clinically relevant favoring delayed cord clamping.
2. Neurological outcome at 2 years
Robledo (2021) reported major disability at 2 years defined as one or more of the following: cerebral palsy, severe visual loss, deafness, major problems with language or speech, or cognitive delay. Major disability occurred in 144 of the 627 infants (23%) who had delayed cord clamping as compared to 159 of the 603 infants (26%) who had immediate cord clamping (RR=0.87, 95%CI 0.72 to 1.06). This difference is clinically relevant favoring delayed cord clamping.
3. Intraventricular hemorrhage
Tarnow-Mordi (2017) reported that 24 of 734 infants (3.3%) who had delayed cord clamping had an intraventricular hemorrhage of grade 3 or 4 as compared to 17 of the 712 infants (2.4%) who had early cord clamping (RR=1.37, 95%CI 0.74 to 2.53).
4. Need for erythrocyte transfusion
Tarnow-Mordi (2017) reported that 406 of 780 infants (52.1%) who had delayed cord clamping received a red cell transfusion (of whole blood or packed cells) as compared to 468 of the 773 infants (60.5%) who had early cord clamping (RR=0.86, 95%CI 0.79 to 0.94). This difference is not clinically relevant.
5. Anemia
Not reported.
6. Bilirubin level
Tarnow-Mordi (2017) reported the peak bilirubin in the first 7 days. Infants who had delayed cord clamping had a mean bilirubin level of 153.9 µmol/L (SD=40.9) as compared to 150.6 µmol/L (SD=39.9) (MD=3.30, 95%CI -0.73 to 7.33). This difference is not clinically relevant.
7. Ferritin level
Not reported.
Level of evidence of the literature
According to GRADE, the level of evidence of randomized controlled trials start high.
The level of evidence regarding the outcome measure perinatal death was downgraded by two levels to low because the 95% confidence interval crossed the line of no (clinically relevant) effect and the optimal information size was not achieved (-2, imprecision).
The level of evidence regarding the outcome measure neurological outcome at 2 years was downgraded by two levels to low because the 95% confidence interval crossed the line of no (clinically relevant) effect and the optimal information size was not achieved (-2, imprecision).
The level of evidence regarding the outcome measure intraventricular hemorrhage was downgraded by two levels to very low because the 95% confidence interval crossed both lines of no (clinically relevant) effect and the optimal information size was not achieved (-3, imprecision).
The level of evidence regarding the outcome measure need for erythrocyte transfusion was downgraded by two levels to low because the 95% confidence interval crossed the line of no (clinically relevant) effect and the optimal information size was not achieved (-2, imprecision).
The level of evidence regarding the outcome measure anemia could not be assessed with GRADE as this outcome measure was not studied in the included studies.
The level of evidence regarding the outcome measure bilirubin level was downgraded by two levels to low because the optimal information size was not achieved (-2, imprecision).
The level of evidence regarding the outcome measure ferritin level could not be assessed with GRADE as this outcome measure was not studied in the included studies.
PICO 3: premature birth between 30 and 37 weeks
Results
1. Perinatal death
Ranjit (2015) reported that 5 of the 50 infants (10%) who had early cord clamping died, while no deaths occurred in infants who had delayed cord clamping (RR=0.10, 95%CI 0.01 to 1.81).
2. Neurological outcome at 2 years
Not reported.
3. Intraventricular hemorrhage
Ranjit (2015) reported that 1 of the 50 infants (2%) who had early cord clamping had an intraventricular hemorrhage, while no intraventricular hemorrhage occurred in infants who had delayed cord clamping (RR=0.38, 95%CI 0.02 to 9.04).
4. Need for erythrocyte transfusion
Ranjit (2015) reported that 4 of the 44 infants (9.1%) who had delayed cord clamping received packed cell transfusion as compared to 6 of the 50 infants (12%) who had early cord clamping (RR=0.76, 95%CI 0.23 to 2.51).
Salae (2016) reported that no blood transfusions were needed in infants who had either delayed or early cord clamping.
5. Anemia
Two studies reported anemia (Ranjit, 2015; Salae, 2016) (Figure 3.1). These data were not pooled as the agreement is to start pooling when including at least three studies.
Ranjit (2015) reported that 6 of the 50 infants (12%) who had early cord clamping had anemia on day 1, while this did not occur in infants who had delayed cord clamping (RR=0.09, 95%CI 0.01 to 1.50).
Salae (2016) reported that 3 of the 44 infants (6.8%) who had early cord clamping were anemic (Hct <40%), while this did not occur in infants who had delayed cord clamping (RR=0.15, 95%CI 0.01 to 2.18).
Figure 3.1. Anemia for gestational age between 30 and 37 weeks.
6. Bilirubin level
Ranjit (2015) reported the mean peak bilirubin. Infants who had delayed cord clamping had a mean peak bilirubin of 13.1 mg/dL (SD=3.2) as compared to 12.4 mg/dL (SD=3.9) (MD=0.70, 95%CI -0.74 to 2.14).
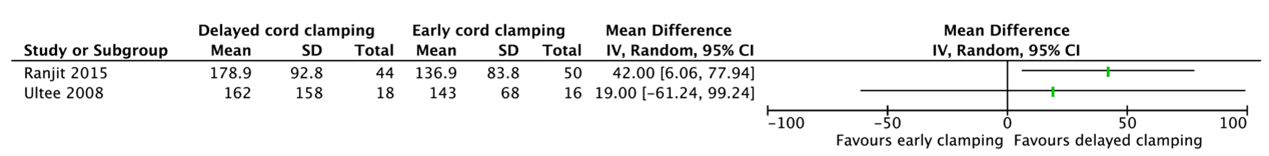
7. Ferritin level
Two studies reported a ferritin level (Ranjit, 2015; Ultee, 2008) (Figure 3.2). These data were not pooled as the agreement is to start pooling when including at least three studies and because of heterogeneity between the studies.
Ranjit (2015) reported the ferritin level in infants at 6 weeks of age. Infants who had delayed cord clamping (n=44) had a mean ferritin level of 178.9 ng/mL (SD=92.8) as compared to 136.9 ng/mL (SD=83.8) for infants who had early cord clamping (n=50) (MD=42.00, 95%CI 6.06 to 77.94).
Ultee (2008) reported the ferritin level in infants at 10 weeks. Infants who had delayed cord clamping (n=18) had a mean ferritin level of 162 mg/L (SD=158) as compared to 143 mg/L (SD=68) for infants who had early cord clamping (n=16) (MD=19.00, 95%CI -61.24 to 99.24).
Figure 3.2. Ferritin level for gestational age between 30 and 37 weeks.
Level of evidence of the literature
According to GRADE, the level of evidence of randomized controlled trials start high.
The level of evidence regarding the outcome measure perinatal death was downgraded by three levels to very low because of study limitations (-1, risk of bias) and the 95% confidence interval crossed both lines of no (clinically relevant) effect (-2, imprecision).
The level of evidence regarding the outcome measure neurological outcome at 2 years could not be assessed with GRADE as this outcome measure was not studied in the included studies.
The level of evidence regarding the outcome measure intraventricular hemorrhage was downgraded by three levels to very low because of study limitations (-1, risk of bias) and the 95% confidence interval crossed both lines of no (clinically relevant) effect (-2, imprecision).
The level of evidence regarding the outcome measure need for erythrocyte transfusion was downgraded by three levels to very low because of study limitations (-1, risk of bias), differences in the direction of the effect (-1, inconsistency) and the optimal information size was not achieved (-1, imprecision).
The level of evidence regarding the outcome measure anemia was downgraded by three levels to very low because of study limitations (-1, risk of bias) and the optimal information size was not achieved (-2, imprecision).
The level of evidence regarding the outcome measure bilirubin level was downgraded by three levels to very low because of study limitations (-1, risk of bias) and the optimal information size was not achieved (-2, imprecision).
The level of evidence regarding the outcome measure ferritin level was downgraded by three levels to very low because of study limitations (-1, risk of bias) and the optimal information size was not achieved (-2, imprecision).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the (un)favorable effects of delayed cord clamping in premature neonates compared to early cord clamping on the morbidity and mortality of the child?
| P: |
P1 = premature birth between 24 and 37 weeks P2 = 24 to 30 weeks P3 = 30 to 37 weeks (subgroup: 30 to 34 weeks and 34 to 37 weeks) |
| I: |
delayed cord clamping (after at least 60 seconds or after the umbilical cord completely stopped pulsating; no cord milking) |
| C: | early cord clamping |
| O: | perinatal death, intraventricular hemorrhage, need for erythrocyte transfusion, anemia, bilirubin level, ferritin level and neurological outcome at 2 years |
Relevant outcome measures
The guideline development group considered perinatal death and neurological outcome at 2 years as a critical outcome measure for decision making; and intraventricular hemorrhage (IVH), need for erythrocyte transfusion, anemia, bilirubin level and ferritin level as an important outcome measure for decision making.
The working group defined the outcome measures as follows:
- Intraventricular hemorrhage: from grade 3
- Ferritin level: in the newborn between 2 and 4 months
For the other outcomes, the working group did not define the outcome measures a priori but used the definitions used in the studies.
The working group defined a 1% difference for perinatal death (RR < 0.99 or > 1.01) and 10% for neurological outcomes at 2 years (RR < 0.90 to >1.10) as a minimal clinically (patient) important difference. For the other outcomes, a 25% difference for dichotomous outcomes (RR < 0.8 or > 1.25) and 0.5 SD for continuous outcomes was taken as minimal clinically (patient) important difference.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 2010 until the 20th of July, 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 819 hits. Studies were selected based on the following criteria:
- Systematic review (searched in at least two databases, and detailed search strategy, risk of bias assessment and results of individual studies available), randomized controlled trial, or observational studies comparing delayed cord clamping with early cord clamping;
- The study population had to meet the criteria as defined in the PICO; and
- Full-text English language publication.
Eighty-one studies were initially selected based on title and abstract screening. After reading the full text, 77 studies were excluded (see the table with reasons for exclusion under the tab Methods), and four studies were included (one systematic review of Rabe 2019 and three other randomized controlled trials performed by Armstrong-Buisseret 2020, Robledo 2021 and Yunis 2020). Rabe 2019 defined a broader PICO than the PICO defined for this module (it also included studies about umbilical cord milking and less than 60 seconds for delayed cord clamping). Therefore, eight randomized controlled trials included in the review were selected for the literature analysis (Dipak, 2017; Duley, 2017; Rana, 2017; Ranjit, 2015; Salae, 2016; Strauss, 2008; Tarnow-Mordi, 2017; Ultee, 2008).
After our search date, two systematic reviews performed by Seidler 2023 were published. However, since the same studies were included in these systematic reviews of Seidler as in Rabe 2019, this had no consequences for the literature analysis.
Studies on intact cord rescusitation (as the ABC3-trial by Knol 2024) where not included for analysis, yet described in the discussion, as this new technique is mostly used in an academic setting and is not yet general practice.
Results
The eight randomized controlled trials included in the systematic review of Rabe 2019 and three randomized controlled trials performed by Armstrong-Buisseret 2020, Robledo 2021 and Yunis 2020 were included in the analysis of the literature. Important study characteristics and results are summarized in table 1 and the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables. A subgroup analysis was performed based on gestational age: <30 weeks (24 to 30 weeks) and >30 weeks (to 37 weeks) of gestation. It was not possible to perform a subgroup analysis for 30 to 34 weeks and 34 to 37 weeks because of lack of data.
Referenties
- Altit G, Hamilton D, O’Brien K. Skin-to-skin care (SSC) for term and preterm infants. Paediatr Child 2024 Jul 22; 29(4): 238-254.
- Paediatr Child 2024 Jul 22; 29(4): 238-254.
- Armstrong-Buisseret L, Powers K, Dorling J, Bradshaw L, Johnson S, Mitchell E, Duley L. Randomised trial of cord clamping at very preterm birth: outcomes at 2 years. Arch Dis Child Fetal Neonatal Ed. 2020 May;105(3):292-298. doi: 10.1136/archdischild-2019-316912. Epub 2019 Aug 1. PMID: 31371434; PMCID: PMC7363783.
- Duley L, Dorling J, Pushpa-Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, Bradshaw L, Mitchell EJ, Fawke JA; Cord Pilot Trial Collaborative Group. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Arch Dis Child Fetal Neonatal Ed. 2018 Jan;103(1):F6-F14. doi: 10.1136/archdischild-2016-312567. Epub 2017 Sep 18. PMID: 28923985; PMCID: PMC5750367.
- Knol R, Brouwer E, van den Akker T, DeKoninck PLJ, Onland W, Vermeulen MJ, de Boode WP, van Kaam AH, Lopriore E, Reiss IKM, Hutten GJ, Prins SA, Mulder EEM, d'Haens EJ, Hulzebos CV, Bouma HA, van Sambeeck SJ, Niemarkt HJ, van der Putten ME, Lebon T, Zonnenberg IA, Nuytemans DH, Willemsen SP, Polglase GR, Steggerda SJ, Hooper SB, Te Pas AB. Physiological versus time based cord clamping in very preterm infants (ABC3): a parallel-group, multicentre, randomised, controlled superiority trial. Lancet Reg Health Eur. 2024 Dec 4;48:101146. doi: 10.1016/j.lanepe.2024.101146. PMID: 39717227; PMCID: PMC11664066.
- Madar J, Roehr CC, Ainsworth S, Ersdal H, Morley C, Rüdiger M, Skåre C, Szczapa T, Te Pas A, Trevisanuto D, Urlesberger B, Wilkinson D, Wyllie JP. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation. 2021 Apr;161:291-326. doi: 10.1016/j.resuscitation.2021.02.014. Epub 2021 Mar 24. PMID: 33773829.
- McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013 Jul 11;2013(7):CD004074. doi: 10.1002/14651858.CD004074.pub3. PMID: 23843134; PMCID: PMC6544813.
- Nederlandse Reanimatie Raad. Richtlijnen Reanimatie in Nederland. Reanimatie en ondersteuning van de transitie van het kind direct na de geboorte. Available from: www.reanimatieraad.nl
- Rana A, Agarwal K, Ramji S, Gandhi G, Sahu L. Safety of delayed umbilical cord clamping in preterm neonates of less than 34 weeks of gestation: a randomized controlled trial. Obstet Gynecol Sci. 2018 Nov;61(6):655-661. doi: 10.5468/ogs.2018.61.6.655. Epub 2018 Oct 29. PMID: 30474011; PMCID: PMC6236088.
- Ranjit T, Nesargi S, Rao PN, Sahoo JP, Ashok C, Chandrakala BS, Bhat S. Effect of early versus delayed cord clamping on hematological status of preterm infants at 6 wk of age. Indian J Pediatr. 2015 Jan;82(1):29-34. doi: 10.1007/s12098-013-1329-8. Epub 2014 Feb 6. PMID: 24496587.
- Rabe H, Gyte GM, Díaz-Rossello JL, Duley L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2019 Sep 17;9(9):CD003248. doi: 10.1002/14651858.CD003248.pub4. PMID: 31529790; PMCID: PMC6748404.
- Rana A, Agarwal K, Ramji S, Gandhi G, Sahu L. Safety of delayed umbilical cord clamping in preterm neonates of less than 34 weeks of gestation: a randomized controlled trial. Obstet Gynecol Sci. 2018 Nov;61(6):655-661. doi: 10.5468/ogs.2018.61.6.655. Epub 2018 Oct 29. PMID: 30474011; PMCID: PMC6236088.
- Robledo KP, Tarnow-Mordi WO, Rieger I, Suresh P, Martin A, Yeung C, Ghadge A, Liley HG, Osborn D, Morris J, Hague W, Kluckow M, Lui K, Soll R, Cruz M, Keech A, Kirby A, Simes J; APTS Childhood Follow-up Study collaborators. Effects of delayed versus immediate umbilical cord clamping in reducing death or major disability at 2 years corrected age among very preterm infants (APTS): a multicentre, randomised clinical trial. Lancet Child Adolesc Health. 2022 Mar;6(3):150-157. doi: 10.1016/S2352-4642(21)00373-4. Epub 2021 Dec 8. Erratum in: Lancet Child Adolesc Health. 2022 Jan 21;: PMID: 34895510.
- Strauss RG, Mock DM, Johnson KJ, Cress GA, Burmeister LF, Zimmerman MB, Bell EF, Rijhsinghani A. A randomized clinical trial comparing immediate versus delayed clamping of the umbilical cord in preterm infants: short-term clinical and laboratory endpoints. Transfusion. 2008 Apr;48(4):658-65. doi: 10.1111/j.1537-2995.2007.01589.x. Epub 2008 Jan 10. PMID: 18194383; PMCID: PMC2883857.
- Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, Evans N, Finlayson S, Fogarty M, Gebski V, Ghadge A, Hague W, Isaacs D, Jeffery M, Keech A, Kluckow M, Popat H, Sebastian L, Aagaard K, Belfort M, Pammi M, Abdel-Latif M, Reynolds G, Ariff S, Sheikh L, Chen Y, Colditz P, Liley H, Pritchard M, de Luca D, de Waal K, Forder P, Duley L, El-Naggar W, Gill A, Newnham J, Simmer K, Groom K, Weston P, Gullam J, Patel H, Koh G, Lui K, Marlow N, Morris S, Sehgal A, Wallace E, Soll R, Young L, Sweet D, Walker S, Watkins A, Wright I, Osborn D, Simes J; Australian Placental Transfusion Study Collaborative Group. Delayed versus Immediate Cord Clamping in Preterm Infants. N Engl J Med. 2017 Dec 21;377(25):2445-2455. doi: 10.1056/NEJMoa1711281. Epub 2017 Oct 29. PMID: 29081267.
- Ultee CA, van der Deure J, Swart J, Lasham C, van Baar AL. Delayed cord clamping in preterm infants delivered at 34 36 weeks' gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008 Jan;93(1):F20-3. doi: 10.1136/adc.2006.100354. Epub 2007 Feb 16. PMID: 17307809.
- WHO. Guideline: Delayed umbilical cord clamping for improved maternal and infant health and nutrition outcomes. Geneva: World Health Organization; 2014
Evidence tabellen
Evidence table for intervention studies
Research question: What are the (un)favorable effects of delayed cord clamping in premature neonates compared to early cord clamping on the morbidity and mortality of the child?
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Armstrong-Buisseret, 2020 |
Type of study: Parallel group randomised trial
Setting and country: Eight tertiary maternity units, UK.
Funding and conflicts of interest: This trial is independent research funded by the NIHR under its Programme Grants for Applied Research funding scheme (RPPG0609-10107). One author reports grants from NIHR during the conduct of the trial; another author reports memberships to CTUs funded by NIHR. All other authors have nothing to disclose.
|
Inclusion criteria: Women expected to have a live birth before 32+0 weeks’ gestation (very preterm)
Exclusion criteria: Not reported
N total at baseline: Intervention: 132 women / 137 babies Control: 129 women / 139 babies
Important prognostic factors2: Women’s age mean ± SD: I: 30.5 ± 6.3 years C: 29.4 ± 6.7 years
Gestation at birth (median) I: 29 weeks (27.1 to 30.7 weeks) C: 29.1 weeks (27.6 to 30.4 weeks)
Groups comparable at baseline
|
Delayed cord clamping: cord clamping after ³ 2 minutes; neonatal care with cord intact
|
Immediate cord clamping: cord clamping £ 20 seconds; neonatal care after clamping
|
Length of follow-up: 2 years
Loss-to-follow-up: Intervention: 16 (11.9%) Reasons: oral consent only, withdrew or lost to follow-up
Control: 27 (20%) Reasons: lost to follow-up or withdrew
Incomplete outcome data: Intervention: 4 (3.0%) Reasons: insufficient data
Control: 5 (3.7%) Reasons: insufficient data
|
Adverse neurodevelopmental outcome at 2 years: I: 16/107 (15.0%) C: 19/87 (21.8%%)
|
Author’s conclusion “Deferred clamping and immediate neonatal care with cord intact may reduce the risk of death or adverse neurodevelopmental outcome at 2 years of age for children born very premature”.
Limitation - Low long-term follow-up rates - Screening test with poor diagnostic accuracy and routine clinical assessments have poor sensitivity for evaluating cognitive outcomes |
|
Dipak, 2017 |
Type of study: Randomized controlled trial
Setting and country: A tertiary care hospital in Mumbai, India
Funding and conflicts of interest: No funding. No conflicts of interest were stated.
|
Inclusion criteria: Women with a gestational age between 27 and 32 weeks with preterm onset of labor
Exclusion criteria - Multiple gestation - Placenta previa - Abruption-placenta - Major congenital anomalies - Hydrops - Fetal growth restriction with abnormal Doppler waveforms - Evidence of fetal distress
N total at baseline: Intervention: 26 Control: 27
Important prognostic factors2: Age I: 26.6 (SD=3.9) C: 26.6 (SD=4.2)
Gestational age I: 30.1 (SD=1.2) C: 29.9 (SD=1.4)
Groups were comparable at baseline.
|
Delayed cord clamping: at 60 seconds
Infant was held in a pre-warmed towel approximately 10-15 inches below the introitus at vaginal delivery/below the level of placental incision in caesarean delivery |
Immediate cord clamping: at 10 seconds
Infant was held supine at level of introitus/placental incision |
Length of follow-up Up to 72 hours after birth
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported |
Red cells transfusion I: 2/26 (7.7%) C: 11/27 (40.7%)
Mean total serum bilirubin at 72 hours I: 9.4 ± 3.1 mg/dL C: 5.6 ± 1.7 mg/dL
Peak serum bilirubin (weighted mean difference ± SD) 2.1 ± 0.9 mg/dL
|
Author’s conclusion “In preterm neonates delayed cord clamping along with lowering the infant below perineum or incision site and administration of ergometrine to mother has significant benefits in terms of increase in hematocrit, higher temperature on admission, and higher blood pressure and urinary output during perinatal transition”.
Limitations: Small sample size |
|
Duley, 2017 |
Type of study: Parallel group randomised trial
Setting and country: Eight tertiary maternity units, UK
Funding and conflicts of interest: This trial is independent research funded by the National Institute for HealthResearch (NIHR) under its Programme Grants for Applied Research funding scheme (RPPG-0609-10107). No conflicts of interest.
|
Inclusion criteria: Women who expected a live birth before 32 weeks of gestation regardless of mode of delivery or fetal presentation
Exclusion criteria - Monochorionic twins, triplets, or higher-order multiple pregnancy -Known major congenital malformation
N total at baseline: Intervention: 130 Control: 124
Important prognostic factors2: Age I: 30.3 (SD=6.1) C:29.2 (SD=6.6)
Gestation <26 weeks I: 22 (17%) C: 14 (11%)
Gestation 26 to 27+6 weeks I: 25 (19%) C: 21 (17%)
Gestation 28 to 29+6 weeks I: 38 (29%) C: 42 (34%)
Gestation 30 to 31+6 weeks I: 44 (34%) C: 46 (37%)
Groups comparable at baseline
|
Delayed cord clamping (³2 minutes)
Received immediate neonatal stabilization and resuscitation with cord intact |
Immediate cord clamping (within 20 seconds)
Received immediate neonatal stabilization and resuscitation after clamping. |
Length of follow-up: Until discharge
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Death I: 7/135 (5.2%) C: 15/135 (11.1%)
IVH grade 3-4 I: 6/134 (4.5%) C: 7/132 (5.3%)
Blood transfusion in infants I: 63/134 (47.0%) C: 68/132 (51.5%)
Blood transfusion for anemia: I: 58/134 (43.3%) C: 66/132 (50%)
|
Author’s conclusion “This is promising evidence that clamping after at least 2 min and immediate neonatal care with cord intact at very preterm birth may improve outcome”.
Remarks:
|
|
Rana, 2017 |
Type of study: Randomized controlled trial
Setting and country: Department of Obstetrics and Gynecology of a tertiary-level teaching hospital in India
Funding and conflicts of interest: Source of funding was not reported. No potential conflict of interest relevant to this article was reported.
|
Inclusion criteria: Pregnant women whose pregnancies had reached less than 34 weeks’ gestation and were in the late first stage of labor
Exclusion criteria: - Any known congenital malformations - Serious maternal illnesses: severe preeclampsia or eclampsia, uncompensated heart disease, any abnormal bleeding before cord clamping - Twins or triplets - Babies requiring immediate resuscitation at birth
N total at baseline: Intervention: 50 Control: 50
Important prognostic factors2: Age I: 32.3± 1.1 weeks C: 32.4± 1.0 weeks
Birth weight I: 1,818±282 C: 1,679±373
Small for gestational age (SGA) I: 0 C: 4 (8%)
Groups comparable at baseline, except for birth weight and SGA
|
Delayed cord clamping: after 120 seconds
|
Early cord clamping: within 30 seconds of birth |
Length of follow-up:
Loss-to-follow-up: No
Incomplete outcome data: No
|
Bilirubin levels (mg/dL) at 72 hours of birth) I: 6.6 ± 1.2 mg/dL C: 8.7±1.6 mg/dL
Intraventricular hemorrhage (not defined) I: 0/50 C: 0 /50
Need for blood transfusion I: 0/50 C: 0/50
|
Author’s conclusion: “DCC benefits preterm neonates with no significant adverse effects”.
Remarks: - Small number of study subjects - Some pregnant women were excluded after randomization which might result in bias
|
|
Ranjit, 2015 |
Type of study: Randomized controlled trial
Setting and country: Tertiary care hospital in South India
Funding and conflicts of interest: Source of funding not reported. No conflicts of interest.
|
Inclusion criteria: Neonates born between 30+0 and 36+6 weeks
Exclusion criteria: - Mothers with Rhesus negative blood group - Monoamniotic/ monochorionic twins - Infants who were randomized to delayed cord clamping, but needed resuscitation at birth
N total at baseline: Intervention: 44 Control: 50
Important prognostic factors2: Age I: 34.0 ± 1.6 weeks C: 34.1 ± 2.0 weeks
Groups comparable at baseline, except for maternal hemoglobin (lower in delayed cord clamping group)
|
Delayed cord clamping; >2 minutes |
Immediate cord clamping: immediate after birth |
Length of follow-up:
Loss-to-follow-up: At follow-up: Intervention: 3 (6.8%) Reasons: lost to follow-up
Control: 9 (18%) Reasons: lost to follow-up or death
Incomplete outcome data: No
|
Death I: 0/44 C: 5/50 (10%)
Intraventricular hemorrhage (not defined) I: 0/44 C: 1/50 (1%)
Anemia on day 1 I: 0/44 C: 6/50 (12%)
Mean peak bilirubin levels (mg/dL) I: 13.1 ± 3.2 mg/dL C: 12.4±3.9 mg/dL
Ferritin (ng/mL) I: 178.9±92.8 ng/mL C: 136.9±83.8 ng/mL
Blood transfusion I: 4/44 (9.1%) C: 6/50 (12%) |
Author’s conclusion “Delaying the cord clamping by 2 min, significantly improves the hematocrit value at birth and this beneficial effect continues till at least 2nd month of life”
Limitations - Small sample size - No long term developmental outcomes |
|
Robledo, 2021 (follow-up Tarnow-mordi) |
Type of study: International, open-label, parallel, pragmatic, randomised, controlled, superiority trial.
Setting and country: 25 centres in seven countries.
Funding and conflicts of interest: Funded by Australian National Health and Medical Research Council. The authors declare no competing interests.
|
Inclusion criteria: Fetuses were eligible if obstetricians or maternal–fetal medicine specialists considered that they might be delivered before 30 weeks of gestation
Exclusion criteria: - Fetal haemolytic disease - Hydrops fetalis - Genetic syndromes - Potentially lethal malformations
N total at baseline: Intervention: 767 Control: 764
Important prognostic factors2: Gestational age I: 28 ± 2 weeks C: 28 ± 2 weeks
Groups comparable at baseline |
Delayed cord clamping (³60 seconds) |
Immediate cord clamping (within 10 seconds) |
Length of follow-up: 2 years
Loss-to-follow-up: Not reported
Incomplete outcome data: Intervention: 58 (7.6%) Reasons: missing at least one component of primary outcome
Control: 54 (7.1%) Reasons: missing at least one component of primary outcome |
Major disability at 2 years I: 144/627 (23%) C: 159/603 (26%)
|
Author’s conclusion “Clamping the umbilical cord at least 60 s after birth reduced the risk of death or major disability at 2 years by 17%, reflecting a 30% reduction in relative mortality with no difference in major disability”.
Remarks - Staff and parents were not blinded to the intervention and assessments of outcome, but researchers who assessed disability were unaware of randomised group and death is an outcome at low risk of observer bias - Did not record heart rate or time to first breath or to regular breathing - Clamping occurred before 60 s in 26% of infants assigned to delayed clamping, largely reflecting clinical concerns for the infant. |
|
Salae, 2016 |
Type of study: Randomized controlled trial
Setting and country: Department of Obstetrics and Gynecology, Thammasat University Hospital in Pathumthani, Thailand
Funding and conflicts of interest: Funding for this study was supported by the Faculty of Medicine, Thammasat University, Pathumthani, Thailand. No conflicts of interest.
|
Inclusion criteria: Women between 18 and 45 years with a gestational age between 34 and 36+6 weeks who were admitted for preterm delivery
Exclusion criteria - Thalassemia syndrome - Preeclampsia - Gestational diabetes mellitus - Placental abnormalities - Fetus with major congenital anomalies - Multiple gestations - Instrumental delivery and-or abnormal fetal tracing (severe fetal bradycardia, fetal distress and non-reassuring fetal heart rate).
N total at baseline: Intervention: 42 Control: 44
Important prognostic factors2: Age I: 26.2 ± 6.2 years C: 28.7 ± 5.0 years
Gestational age I: 35.7 ± 1.0 years C: 36.0 ± 0.8 years
Groups were comparable at baseline
|
Delayed cord clamping: within 2 minutes
“Following birth, the babies were placed at the same level of maternal body trunk. The babies in the DCC group were wrapped with a sterile towel. During the process, care was taken not to allow the umbilical cord to be overstretched”.
“Neonates from either group were transferred to the newborn unit and underwent routine standard management by a pediatrician who attended the newborn unit”. |
Immediate cord clamping (not defined) |
Length of follow-up: Loss-to-follow-up: Intervention: 8 (16%) Reasons: Apgar score at 1 min <7 or non-reassuring fetal heart rate
Control: 6 (12%) Reasons: Apgar score at 1 min <7 or non-reassuring fetal heart rate
Incomplete outcome data: No
|
Microbilirubin at 48 hours I: 9.4±1.3 mg/dL C: 8.6 ±2.1 mg/dL
Blood transfusion I: 0/42 C: 0/44
Anemia (Hct <40%) I: 0 C: 3 |
Author’s conclusion “The DCC procedure could raise the Hct level in the late preterm newborns without serious adverse effects”.
Limitations - No long-term follow-up - High drop-out rate
|
|
Strauss, 2008 |
Type of study: Prospective randomized clinical trial
Setting and country: US
Funding and conflicts of interest: Source of funding not reported. No conflicts of interest.
|
Inclusion criteria: Infants born before 37 weeks
Exclusion criteria: Not reported
N total at baseline: Intervention: 45 Control: 60
Important prognostic factors2: Not reported
Unclear if groups were comparable at baseline
|
Delayed cord clamping (60 seconds) |
Immediate cord clamping (within 2 to 5 seconds, but not to exceed 15 seconds after delivery) |
Length of follow-up: Not reported
Loss-to-follow-up: Not reported
Incomplete outcome data: Some missing data: for blood transfusion and bilirubin levels is data missing for one patient in the immediate cord clamping group. For outcome haematocrit, missing data is almost similar for both groups. |
Perinatal death I: 0/45 C: 0 /60
Red blood cell transfusion in infant I: 2/45 (4.4%) C: 5/60 (8.3%)
Serum bilirubin levels (at start of phototherapy) I: 11 mg/dL C: 11 mg/dL
|
Author’s conclusion Although a 1-minute delay in cord clamping significantly increased RBC volume/ mass and Hct, clinical benefits were modest. Clinically significant adverse effects were not detected. Consider a 1-minute delay in cord clamping to increase RBC volume/mass and RBC iron, for neonates 30 to 36 weeks' gestation, who do not need immediate resuscitation.
Remarks: No patient characteristics were presented |
|
Tarnow-Mordi, 2017 |
Type of study: Randomized pilot trial
Setting and country: 25 centres in 7 countries
Funding and conflicts of interest: Funded by supported by the National Health and Medical Research Council (NHMRC) and by the NHMRC Clinical Trials Centre, University of Sydney. No conflicts of interest.
|
Inclusion criteria: Women who were expected to deliver before 30 weeks of gestation
Exclusion criteria: - Fetal hemolytic disease - Hydrops fetalis - Twin–twin transfusion - Genetic syndromes - Potentially lethal malformations
N total at baseline: Intervention: 784 Control: 782
Important prognostic factors2: Gestational age I: 28 ± 2 weeks C: 28 ± 2 weeks
Groups comparable at baseline
|
Delayed clamping (≥60 seconds after delivery) |
Immediate clamping of the umbilical cord (≤10 seconds after delivery) |
Length of follow-up: Not reported
Loss-to-follow-up: Not reported
Incomplete outcome data: I: 36 had missing data C: 33 had missing data |
Perinatal death I: 50/784 (6.4%) C: 70/782 (9.0%)
IVH grade 3 or 4 I: 24/734 (3.3%) C: 17/712 (2.4%)
Blood transfusion in infant (whole blood or packed cells) I: 406/780 (52.1%) C: 468/773 (60.5%)
Peak bilirubin in first 7 days I: 153.9 µmol/L (SD=40.9) à 9 mg/dL (SD=2.39) C: 150.6 µmol/L (SD=39.9) à 8.81 mg/dL (SD=2.33) |
Author’s conclusion “Among preterm infants, delayed cord clamping did not result in a lower incidence of the combined outcome of death or major morbidity at 36 weeks of gestation than immediate cord clamping”.
Remarks Unblinded trial |
|
Ultee, 2008 |
Type of study: Randomized controlled trial
Setting and country: Netherlands
Funding and conflicts of interest: Source of funding not reported; report no competing interests. |
Inclusion criteria: - Infants born at 34 weeks and 0 days to 36 weeks and 6 days gestational age - Delivered vaginally - Only Caucasian parents
Exclusion criteria: - Overt diabetes or gestational diabetes (>20 mm Hg rise of diastole during pregnancy in combination with albuminuria)
N total at baseline: Intervention: 21 Control: 20
Important prognostic factors2: Gestational age (weeks) I: 36.05 ± 0.65 weeks C: 36.08 ± 0.74 weeks
Groups were comparable at baseline
|
Delayed cord clamping after 180 seconds
|
Immediate cord clamping within 30 secs (mean of 13.4 seconds (SD 5.6))
|
Length of follow-up: 10 weeks
Loss-to-follow-up: I: 2 C: 1
Incomplete outcome data: I: 2 C: 1 |
Ferritin levels I: 162 ± 158 mg/L C: 143 ± 68 mg/L
|
Author’s conclusion Immediate clamping of the umbilical cord should be discouraged.
Remarks: Small number of study subjects |
|
Yunis, 2020 |
Type of study: Pilot, prospective, non-blinded, randomized controlled trial
Setting and country: Neonatal Intensive Care Unit (NICU) of Mansoura University Children’s Hospital, Mansoura, Egypt
Funding and conflicts of interest: Source of funding not reported. The authors declare that they have no conflict of interest.
|
Inclusion criteria: Preterm infants, less than 34 weeks’ gestation, delivered to mothers with antenatal diagnosis of placental insufficiency
Exclusion criteria: - Infants with a congenital anomaly or suspected chromosomal anomaly - Infants who required major resuscitation steps at birth in whom delay of resuscitation measures was not possible
N total at baseline: Intervention: 30 Control: 30
Important prognostic factors2: Gestational age (weeks) I: 29.7 ± 1.7 weeks C: 30.4 ± 1.2 weeks
Groups were comparable at baseline
|
Delayed cord clamping: 60 seconds
Keeping the infant 2–3 in. below the level of the maternal introitus or placenta, with caution not to put traction on the cord, for 60 s with an intact umbilical cord followed by ligation of the cord at 2–3 cm from the umbilical stump without cord milking |
Immediate cord clamping: 10 seconds after delivery
Infant’s umbilical cord was immediately clamped within 10 s after delivery of the whole body of the infant at 2–3 cm from the umbilical stump without cord milking |
Length of follow-up: 2 months
Loss-to-follow-up: Intervention: 8 (21%) Required immediate resuscitation
Control: 0
Incomplete outcome data: Not reported
|
Neonatal mortality (before hospital discharge) I: 4/30 (13.3%) C: 3/30 (10%)
Packed red blood cell transfusion I: 15/30 (50%) C: 20/30 (66.7%)
Peak serum bilirubin I: 9.75 ± 2.90 mg/dL C: 6.97 ± 4.40 mg/dL
IVH grades 3 or 4 I: 1/30 (3%) C: 2/30 (7%)
|
Author’s conclusion In conclusion, DCC compared with ICC increased stem cell transfusion and decreased early- and late-onset anemia in preterm infants with placental insufficiency.
Limitations - Small sample size - No intention-to-treat |
Risk of bias table for intervention studies
Research question: What are the (un)favorable effects of delayed cord clamping in premature neonates compared to early cord clamping on the morbidity and mortality of the child?
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Armstrong-Buisseret, 2020 (follow-up of Cord Pilot Trial) |
Definitely yes;
Reason: Computer was used for sequence generation and stratified by centre with balanced blocks of randomly varying size, created by NCTU
|
Definitely yes;
Reason: Sealed envelopes were used. Once the label was completed, the patient was considered randomized, even if the envelope was not opened. |
Probably no;
Reason: Attending clinicians could not be blinded. It was unclear whether the mother knew or not. Outcome assessors were blinded. |
Probably yes;
Reason: No loss to follow-up or missing data. |
No information
Reason: The trial protocol was for a feasibility study and clinical outcomes are unclear. |
Probably yes;
Reason: No other problems noted. |
Some concerns |
|
Dipak, 2017 |
Definitely yes;
Reason: A random number sequence with variable block size of 3 or 6 using a ‘Random Allocation Software’ program was used.
|
Definitely yes;
Reason: Serially numbered, opaque, sealed and identical envelopes were used. The random allocation sequence was generated by a statistician who was not a part of the study.
|
No information |
Probably yes;
Reason: No loss to follow-up or missing data. |
Probably yes;
Reason: All relevant outcomes were reported. |
Probably yes;
Reason: No other problems noted. |
Some concerns |
|
Duley, 2017 |
Definitely yes;
Reason: Sequence generation was by computer, stratified by centre with balanced blocks of randomly varying size, created by NCTU.
|
Definitely yes;
Reason: Sealed envelopes. On the envelope was a label to record the date, time, woman’s initials, her date of birth and gestation. Once this label was completed, she was considered randomized, even if the envelope was not opened.
|
Probably no;
Reason: Assessor and clinicians were blinded, but patients could probably not be blinded. |
Probably yes;
Reason: No loss to follow-up or missing data. |
No information
Reason: The trial protocol was for a feasibility study and clinical outcomes are unclear. |
Probably yes;
Reason: No other problems noted. |
Some concerns |
|
Rana, 2017 |
Definitely yes;
Reason: Uninvolved statistician generated 2 randomization sequences in variable 4 by 6 block sizes.
|
Definitely yes;
Reason: The random sequence was kept in serially numbered, sealed, and opaque envelopes.
|
Probably no;
Reason: Outcome assessors were blinded but unknown if staff was blinded and patients knew. |
Probably yes;
Reason: No loss to follow-up or missing data. |
Probably yes;
Reason: All relevant outcomes were reported. |
Probably no;
Reason: Some of the babies had to be excluded from the study after the pregnant women were enrolled and randomized in the late first stage of labor which might result in some bias.
|
Some concerns |
|
Ranjit, 2015 |
Definitely yes;
Reason: Computer generated random numbers were used.
|
Definitely yes;
Reason: Allocation concealment was achieved by sequentially numbered opaque sealed envelopes containing the codes for intervention.
|
Probably no;
Reason: No blinding possible. |
Probably no;
Reason: More lost to follow-up in delayed cord clamping group. Unclear whether infants who were excluded from analysis had died. |
Probably yes;
Reason: All relevant outcomes were reported. |
Probably no;
Reason: Six infants in delayed cord clamping group were excluded because they needed resuscitation. |
Some concerns |
|
Robledo, 2021 (follow-up Tarnow-Mordi) |
Probably yes
Reason: Infants were randomly assigned (1:1). Randomization was performed centrally by a clinician calling an automated telephone service that used computer-based minimisation methods, stratified for gestation, centre, and multiple birth status.
|
Definitely yes
Reason: Allocation concealment was ensured by central randomization. |
Probably no
Reason: Parents and care providers in the delivery room were not blinded to the intervention. Outcome assessors were unaware of the randomised group. |
Probably no
Reason: Missing data was frequent but similar in both groups. Missing data group were similar to group with outcome data available. |
Probably yes
Reason: Protocol was available and all relevant outcome measures were reported. |
Probably yes
Reason: No other problems noted. |
LOW |
|
Salae, 2016 |
Probably yes;
Reason: Simple randomization was performed and generated by a statistical computer program.
|
Probably yes:
Reason: Sealed envelopes were used. |
No information |
Probably no;
Reason: Lost to follow-up was frequent but similar for both groups. |
Probably yes;
Reason: All relevant outcomes were reported, but maternal and neonatal complications were not defined in methods. |
Probably yes;
Reason: No other problems noted. |
Some concerns |
|
Strauss, 2008 |
Definitely yes;
Reason: Group assignment was generated with a table of random numbers. |
Definitely yes;
Reason: Written instructions were kept in sealed envelopes and were opened immediately before delivery. |
Probably no;
Reason: Laboratory staff were unaware of group assignment. Unknown if other staff was blinded. |
Probably yes;
Reason: Some missing data but for outcomes of interest this was similar in both groups. |
Probably yes;
Reason: No protocol was available, but all relevant outcomes mentioned in the methods were described under results.
|
Probably no;
Reason: No patient characteristics were provided, so unclear if groups were comparable. Groups were also not balanced in size. |
Some concerns |
|
Tarnow-Mordi, 2017 |
Definitely yes;
Reason: Randomization was performed centrally by a computer.
|
Definitely yes;
Reason: Use of an interactive voice-response system with minimization and with stratification according to gestational age (<27 weeks vs. ≥27 weeks), center, and multiple birth status (singleton birth vs. multiple birth).
|
Definitely no;
Reason: Unblinded trial |
Probably yes
Reason: Missing data was frequent but similar in both groups. |
Definitely yes;
Reason: Protocol was available and all relevant outcome measures were reported.
|
Probably yes;
Reason: No other problems noted. |
Some concerns |
|
Ultee, 2008 |
Probably yes;
Reason: Subjects were randomly assigned to one of the two experimental groups by pulling the category out of a blinded box with loose papers.
|
Probably no;
Reason: Same person was responsible for randomisation, delivering clinical care and collected data. |
Probably no;
Reason: Some clinical staff were blinded, but also same person delivered care and collected data. |
Probably yes;
Reason: Missing data was small and similar for both groups. |
Probably yes;
Reason: No protocol was available, but all relevant outcomes mentioned in the methods were described under results.
|
Probably no;
Reason: Exclusion of patients after randomisation might lead to bias. |
Some concerns |
|
Yunis, 2020 |
Probably yes;
Reason: Infants were randomly assigned using internet-based random table technique.
|
Definitely yes;
Reason: Opaque, sealed envelopes were used. |
Definitely no;
Reason: Non-blinded trial. |
Probably yes;
Reason: Lost to follow-up was frequent (21%) and only for infants first allocated to delayed cord clamping. |
Probably yes;
Reason: All relevant outcome measures were reported. |
Probably yes;
Reason: No other problems noted. |
Some concerns |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Agarwal S, Jaiswal V, Singh D, Jaiswal P, Garg A, Upadhyay A. Randomised control trial showed that delayed cord clamping and milking resulted in no significant differences in iron stores and physical growth parameters at one year of age. Acta Paediatr. 2016 Nov;105(11):e526-e530. doi: 10.1111/apa.13559. PMID: 27564579. |
Wrong population: term-born infants |
|
Al-Tawil MM, Abdel-Aal MR, Kaddah MA. A randomized controlled trial on delayed cord clamping and iron status at 3–5 months in term neonates held at the level of maternal pelvis. Journal of Neonatal-Perinatal Medicine. 2012 Jan 1;5(4):319-26. |
Wrong population: born at term |
|
Alzaree F, Elbohoty A, Abdellatif M. Early Versus Delayed Umbilical Cord Clamping on Physiologic Anemia of the Term Newborn Infant. Open Access Maced J Med Sci. 2018 Aug 15;6(8):1399-1404. doi: 10.3889/oamjms.2018.286. PMID: 30159064; PMCID: PMC6108792. |
Wrong population: term neonates |
|
Amendolia B, Kilic N, Afridi F, Qari O, Bhat V, Nakhla D, Sadre S, Eckardt R, Nakhla T, Bhandari V, Aghai ZH. Delayed Cord Clamping for 45 Seconds in Very Low Birth Weight Infants: Impact on Hemoglobin at Birth and Close to Discharge. Am J Perinatol. 2024 May;41(S 01):e126-e132. doi: 10.1055/a-1845-1816. Epub 2022 May 6. PMID: 35523407. |
Wrong intervention: 45 seconds |
|
Andersson O, Hellström-Westas L, Andersson D, Clausen J, Domellöf M. Effects of delayed compared with early umbilical cord clamping on maternal postpartum hemorrhage and cord blood gas sampling: a randomized trial. Acta Obstet Gynecol Scand. 2013 May;92(5):567-74. doi: 10.1111/j.1600-0412.2012.01530.x. Epub 2012 Oct 17. PMID: 22913332. |
Wrong population: term deliveries |
|
Andersson O, Hellström-Westas L, Andersson D, Domellöf M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. BMJ. 2011 Nov 15;343:d7157. doi: 10.1136/bmj.d7157. PMID: 22089242; PMCID: PMC3217058. |
Wrong population: full term infants |
|
Andersson O, Lindquist B, Lindgren M, Stjernqvist K, Domellöf M, Hellström-Westas L. Effect of Delayed Cord Clamping on Neurodevelopment at 4 Years of Age: A Randomized Clinical Trial. JAMA Pediatr. 2015 Jul;169(7):631-8. doi: 10.1001/jamapediatrics.2015.0358. PMID: 26010418. |
Wrong population: full term infants |
|
Backes CH, Rivera BK, Haque U, Bridge JA, Smith CV, Hutchon DJR, Mercer JS. Placental transfusion strategies in very preterm neonates: a systematic review and meta-analysis. Obstet Gynecol. 2014 Jul;124(1):47-56. doi: 10.1097/AOG.0000000000000324. PMID: 24901269. |
Better systematic review available. No suitable studies |
|
Bates SE, Isaac TCW, Marion RL, Norman V, Gumley JS, Sullivan CD. Delayed cord clamping with stabilisation at all preterm births - feasibility and efficacy of a low cost technique. Eur J Obstet Gynecol Reprod Biol. 2019 May;236:109-115. doi: 10.1016/j.ejogrb.2019.03.012. Epub 2019 Mar 15. PMID: 30903883. |
Other study aim: evaluate feasibility and efficacy of a simple, low cost technique to deliver respiratory support and thermal care during DCC at all preterm deliveries |
|
Bharati R, Rani S. Assessment of Cord Clamping in Preterm Infants Less Than 37 Weeks and its Impact on the Outcomes. International Journal of Pharmaceutical and Clinical Research. 2021;13(3): 184-191. |
Wrong intervention: 45 seconds |
|
Bitler CK, Rivera BK, Godavarthi S, Stehle CG, Smith CV, Halling C, Backes CH. Evaluating the evidence behind umbilical cord clamping practices in at-risk neonatal populations. Semin Perinatol. 2023 Jun;47(4):151745. doi: 10.1016/j.semperi.2023.151745. Epub 2023 Mar 17. PMID: 37012137. |
Wrong study design: narrative review |
|
Brown BE, Shah PS, Afifi JK, Sherlock RL, Adie MA, Monterrosa LA, Crane JM, Ye XY, El-Naggar WI; Canadian Neonatal Network; Canadian Preterm Birth Network Investigators. Delayed cord clamping in small for gestational age preterm infants. Am J Obstet Gynecol. 2022 Feb;226(2):247.e1-247.e10. doi: 10.1016/j.ajog.2021.08.003. Epub 2021 Aug 9. PMID: 34384773. |
Wrong intervention: ≥30 seconds |
|
Carvalho OMC, Augusto MCC, Medeiros MQ, Lima HMP, Viana Junior AB, Araujo Júnior E, Carvalho FHC. Late umbilical cord clamping does not increase rates of jaundice and the need for phototherapy in pregnancies at normal risk. J Matern Fetal Neonatal Med. 2019 Nov;32(22):3824-3829. doi: 10.1080/14767058.2018.1473367. Epub 2018 May 17. PMID: 29732948. |
Study design: retrospective study |
|
Cavallin F, Galeazzo B, Loretelli V, Madella S, Pizzolato M, Visentin S, Trevisanuto D. Delayed Cord Clamping versus Early Cord Clamping in Elective Cesarean Section: A Randomized Controlled Trial. Neonatology. 2019;116(3):252-259. doi: 10.1159/000500325. Epub 2019 Jul 2. PMID: 31266035. |
Wrong population: ≥39 weeks gestation |
|
Chapman J, Marfurt S, Reid J. Effectiveness of Delayed Cord Clamping in Reducing Postdelivery Complications in Preterm Infants: A Systematic Review. J Perinat Neonatal Nurs. 2016 Oct/Dec;30(4):372-378. doi: 10.1097/JPN.0000000000000215. PMID: 27776037. |
Better systematic review with meta-analysis available. No description of studies or risk of bias assessment |
|
Chaudhary P, Priyadarshi M, Singh P, Chaurasia S, Chaturvedi J, Basu S. Effects of delayed cord clamping at different time intervals in late preterm and term neonates: a randomized controlled trial. Eur J Pediatr. 2023 Aug;182(8):3701-3711. doi: 10.1007/s00431-023-05053-6. Epub 2023 Jun 6. PMID: 37278737; PMCID: PMC10243262. |
No comparison with immediate cord clamping |
|
Chiruvolu A, Daoud Y, Inzer RW. Effect of delayed cord clamping on very preterm twins. Early Hum Dev. 2018 Sep;124:22-25. doi: 10.1016/j.earlhumdev.2018.08.002. Epub 2018 Aug 10. PMID: 30099274. |
Study design: retrospective study |
|
Chiruvolu A, Tolia VN, Qin H, Stone GL, Rich D, Conant RJ, Inzer RW. Effect of delayed cord clamping on very preterm infants. Am J Obstet Gynecol. 2015 Nov;213(5):676.e1-7. doi: 10.1016/j.ajog.2015.07.016. Epub 2015 Jul 18. PMID: 26196456. |
Wrong intervention: 45 seconds |
|
Chopra A, Thakur A, Garg P, Kler N, Gujral K. Early versus delayed cord clamping in small for gestational age infants and iron stores at 3 months of age - a randomized controlled trial. BMC Pediatr. 2018 Jul 18;18(1):234. doi: 10.1186/s12887-018-1214-8. PMID: 30021580; PMCID: PMC6052555. |
Wrong population: SGA infants born at ≥35 weeks; Wrong outcomes: serum ferritin |
|
Chu KS, Shah PS, Whittle WL, Windrim R, Murphy KE. The "DUC" trial: a pilot randomized controlled trial of immediate versus delayed cord clamping in preterm infants born between 24 and 32 weeks gestation. J Matern Fetal Neonatal Med. 2021 Dec;34(24):4049-4052. doi: 10.1080/14767058.2019.1702959. Epub 2019 Dec 25. PMID: 31875737. |
Wrong intervention: 30-45 seconds |
|
De Bernardo G, Giordano M, De Santis R, Castelli P, Sordino D, Trevisanuto D, Buonocore G, Perrone S. A randomized controlled study of immediate versus delayed umbilical cord clamping in infants born by elective caesarean section. Ital J Pediatr. 2020 May 24;46(1):71. doi: 10.1186/s13052-020-00835-2. PMID: 32448358; PMCID: PMC7247269. |
Wrong population: in term infants born by elective caesarean section |
|
Dipak NK, Nanavat RN, Kabra NK, Srinivasan A, Ananthan A. Effect of Delayed Cord Clamping on Hematocrit, and Thermal and Hemodynamic Stability in Preterm Neonates: A Randomized Controlled Trial. Indian Pediatr. 2017 Feb 15;54(2):112-115. doi: 10.1007/s13312-017-1011-8. PMID: 28285280. |
No outcomes relevant to the guideline |
|
Duley L, Dorling J, Pushpa-Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, Bradshaw L, Mitchell EJ, Fawke JA; Cord Pilot Trial Collaborative Group. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Arch Dis Child Fetal Neonatal Ed. 2018 Jan;103(1):F6-F14. doi: 10.1136/archdischild-2016-312567. Epub 2017 Sep 18. PMID: 28923985; PMCID: PMC5750367. |
Included in systematic review of Rabe 2019 |
|
Elimian A, Goodman J, Escobedo M, Nightingale L, Knudtson E, Williams M. Immediate compared with delayed cord clamping in the preterm neonate: a randomized controlled trial. Obstet Gynecol. 2014 Dec;124(6):1075-1079. doi: 10.1097/AOG.0000000000000556. PMID: 25415157. |
Wrong intervention: 30 seconds |
|
El-Naggar W, Afifi J, Dorling J, Bodani J, Cieslak Z, Canning R, Ye XY, Crane J, Lee SK, Shah PS; Canadian Neonatal Network and the Canadian Preterm Birth Network Investigators. A Comparison of Strategies for Managing the Umbilical Cord at Birth in Preterm Infants. J Pediatr. 2020 Oct;225:58-64.e4. doi: 10.1016/j.jpeds.2020.05.018. Epub 2020 May 20. PMID: 32442446. |
Study design: retrospective study |
|
Fawzy AE, Moustafa AA, El-Kassar YS, Swelem MS, El-Agwany AS, Diab DA. Early versus delayed cord clamping of term births in Shatby Maternity University Hospital. Progresos de Obstetricia y Ginecología. 2015 Nov 1;58(9):389-92. |
Wrong population: term infants |
|
Fenton C, McNinch NL, Bieda A, Dowling D, Damato E. Clinical Outcomes in Preterm Infants Following Institution of a Delayed Umbilical Cord Clamping Practice Change. Adv Neonatal Care. 2018 Jun;18(3):223-231. doi: 10.1097/ANC.0000000000000492. PMID: 29794839. |
Wrong intervention: 45-60 seconds |
|
Fogarty M, Osborn DA, Askie L, Seidler AL, Hunter K, Lui K, Simes J, Tarnow-Mordi W. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018 Jan;218(1):1-18. doi: 10.1016/j.ajog.2017.10.231. Epub 2017 Oct 30. PMID: 29097178. |
More recent systematic review available with the same studies |
|
Fu X, Dang D, Li S, Xu Z, Wu H. Effect of Delayed Versus Early Cord Clamping on Improving Anemia in Term Infants Aged Two Months or Older - A Meta-analysis. Indian Pediatr. 2020 Sep 15;57(9):815-819. PMID: 32999110. |
Wrong population: term infants |
|
Garabedian C, Rakza T, Drumez E, Poleszczuk M, Ghesquiere L, Wibaut B, Depoortere MH, Vaast P, Storme L, Houfflin-Debarge V. Benefits of Delayed Cord Clamping in Red Blood Cell Alloimmunization. Pediatrics. 2016 Mar;137(3):e20153236. doi: 10.1542/peds.2015-3236. Epub 2016 Feb 18. PMID: 26908660. |
Wrong intervention: 30 seconds |
|
García C, Prieto MT, Escudero F, Bosh-Giménez V, Quesada L, Lewanczyk M, Pertegal M, Delgado JL, Blanco-Carnero JE, De Paco Matallana C. The impact of early versus delayed cord clamping on hematological and cardiovascular changes in preterm newborns between 24 and 34 weeks' gestation: a randomized clinical trial. Arch Gynecol Obstet. 2024 Jun;309(6):2483-2490. doi: 10.1007/s00404-023-07119-0. Epub 2023 Jul 12. PMID: 37436461. |
Wrong intervention: 45-60 seconds |
|
Garofalo M, Abenhaim HA. Early versus delayed cord clamping in term and preterm births: a review. J Obstet Gynaecol Can. 2012 Jun;34(6):525-531. doi: 10.1016/S1701-2163(16)35268-9. PMID: 22673168. |
Wrong study design: narrative review |
|
Gomersall J, Berber S, Middleton P, McDonald SJ, Niermeyer S, El-Naggar W, Davis PG, Schmölzer GM, Ovelman C, Soll RF. Umbilical cord management at term and late preterm birth: a meta-analysis. Pediatrics. 2021 Mar 1;147(3). |
≥34 weeks’ gestational age; ≥30 seconds delayed cord clamping |
|
Grabovac M, Beltempo M, Lodha A, O'Quinn C, Grigoriu A, Barrington K, Yang J, McDonald SD. Impact of Deferred Cord Clamping on Mortality and Severe Neurologic Injury in Twins Born at <30 Weeks of Gestation. J Pediatr. 2021 Nov;238:118-123.e3. doi: 10.1016/j.jpeds.2021.07.058. Epub 2021 Jul 30. PMID: 34332971. |
Wrong intervention: ≥30 seconds |
|
Güner S, Saydam BK. The Impact of Umbilical Cord Clamping Time on the Infant Anemia: A Randomized Controlled Trial. Iran J Public Health. 2021 May;50(5):990-998. doi: 10.18502/ijph.v50i5.6116. PMID: 34183957; PMCID: PMC8223556. |
Wrong population: ≥ 37 weeks |
|
Handley SC, Kumbhat N, Eggleston B, Foglia EE, Davis AS, Van Meurs K, Lakshminrusimha S, Walsh M, Watterberg KL, Wyckoff MH, Das A, DeMauro SB. Exposure to umbilical cord management approaches and death or neurodevelopmental impairment at 22-26 months' corrected age after extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2023 May;108(3):224-231. doi: 10.1136/archdischild-2022-324565. Epub 2022 Oct 17. PMID: 36253076; PMCID: PMC10108713. |
Study design: retrospective study |
|
Hemmati F, Sharma D, Namavar Jahromi B, Salarian L, Farahbakhsh N. Delayed cord clamping for prevention of intraventricular hemorrhage in preterm neonates: a randomized control trial. J Matern Fetal Neonatal Med. 2022 Oct;35(19):3633-3639. doi: 10.1080/14767058.2020.1836148. Epub 2020 Oct 22. PMID: 33092420. |
Wrong intervention: 30-45 seconds |
|
Jasani B, Torgalkar R, Ye XY, Syed S, Shah PS. Association of Umbilical Cord Management Strategies With Outcomes of Preterm Infants: A Systematic Review and Network Meta-analysis. JAMA Pediatr. 2021 Apr 1;175(4):e210102. doi: 10.1001/jamapediatrics.2021.0102. Epub 2021 Apr 5. PMID: 33683307; PMCID: PMC7941254. |
Better review available. No data for individual studies provided |
|
Jenusaitis L, Keplinger KB, Dean K, Madan I, Shepherd JP. Impact of a delayed cord clamping protocol on maternal and neonatal outcomes in patients undergoing term cesarean section. J Matern Fetal Neonatal Med. 2022 Dec;35(23):4607-4611. doi: 10.1080/14767058.2020.1857357. Epub 2020 Dec 7. PMID: 33287591. |
Wrong population: term cesarean section |
|
Jomjak P, Inploy N, Prommas S, Smanchat B, Pongrojpaw D, Bhamarapravatana K, Suwannaruk K. Effect of Delayed Cord Clamping Reduced Anemic Outcome in Preterm Neonate. Journal of the Medical Association of Thailand. 2021 May 1;104(5). |
Article unavailable |
|
Kaempf JW, Tomlinson MW, Kaempf AJ, Wu Y, Wang L, Tipping N, Grunkemeier G. Delayed umbilical cord clamping in premature neonates. Obstet Gynecol. 2012 Aug;120(2 Pt 1):325-30. doi: 10.1097/AOG.0b013e31825f269f. PMID: 22825092. |
Wrong intervention: 45 seconds |
|
Kazemi MV, Akbarianrad Z, Zahedpasha Y, Mehraein R, Mojaveri MH. Effects of delayed cord clamping on intraventricular hemorrhage in preterm infants. Iranian Journal of Pediatrics. 2017 Oct 31;27(5) |
Wrong intervention: 30-45 seconds |
|
Li J, Yang S, Yang F, Wu J, Xiong F. Immediate vs delayed cord clamping in preterm infants: A systematic review and meta-analysis. Int J Clin Pract. 2021 Nov;75(11):e14709. doi: 10.1111/ijcp.14709. Epub 2021 Sep 1. PMID: 34370357. |
Better review with more studies available |
|
Liyanage SK, Ninan K, McDonald SD. Guidelines on Deferred Cord Clamping and Cord Milking: A Systematic Review. Pediatrics. 2020 Nov;146(5):e20201429. doi: 10.1542/peds.2020-1429. PMID: 33087551. |
Wrong study design: systematically review clinical practice guidelines and other statements |
|
Lodha A, Shah PS, Soraisham AS, Rabi Y, Abou Mehrem A, Singhal N; Canadian Neonatal Network Investigators. Association of Deferred vs Immediate Cord Clamping With Severe Neurological Injury and Survival in Extremely Low-Gestational-Age Neonates. JAMA Netw Open. 2019 Mar 1;2(3):e191286. doi: 10.1001/jamanetworkopen.2019.1286. PMID: 30924898; PMCID: PMC6450317. |
Study design: retrospective study |
|
Mahli K, Kakar FA, Jaffar M. Early versus late clamping of the umbilical cord in full-term neonates. PJMHS. 2015 Jul 1;9(3):1083-5. |
Wrong population: full-term neonates |
|
Mathew JL. Timing of umbilical cord clamping in term and preterm deliveries and infant and maternal outcomes: a systematic review of randomized controlled trials. Indian Pediatr. 2011 Feb;48(2):123-9. doi: 10.1007/s13312-011-0031-z. PMID: 21378422. |
Better systematic review available. No suitable studies |
|
Malik AU, Shahnawaz, KHURRAM, Riaz ADEEL. Comparison between the efficacy of early and delayed umbilical cord clamping in preterm infants. PJMHS 2013, 7(4), 992-995. |
No outcomes relevant to the guideline |
|
Mangla MK, Thukral A, Sankar MJ, Agarwal R, Deorari AK, Paul VK. Effect of umbilical cord milking vs delayed cord clamping on venous hematocrit at 48 hours in late preterm and term neonates: a randomized controlled trial. Indian pediatrics. 2020 Dec;57:1119-23 |
Wrong control: umbilical cord milking |
|
McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Evid Based Child Health. 2014 Jun;9(2):303-97. doi: 10.1002/ebch.1971. PMID: 25404605. |
Wrong population: term infants |
|
Mercer JS, Vohr BR, Erickson-Owens DA, Padbury JF, Oh W. Seven-month developmental outcomes of very low birth weight infants enrolled in a randomized controlled trial of delayed versus immediate cord clamping. J Perinatol. 2010 Jan;30(1):11-6. doi: 10.1038/jp.2009.170. Epub 2009 Oct 22. PMID: 19847185; PMCID: PMC2799542. |
Wrong intervention: 30-45 seconds |
|
Mohammad K, Tailakh S, Fram K, Creedy D. Effects of early umbilical cord clamping versus delayed clamping on maternal and neonatal outcomes: a Jordanian study. J Matern Fetal Neonatal Med. 2021 Jan;34(2):231-237. doi: 10.1080/14767058.2019.1602603. Epub 2019 Apr 15. PMID: 30931665. |
Wrong population: term births |
|
Nesheli HM, Esmailzadeh S, Haghshenas M, Bijani A, Moghaddams TG. Effect of late vs early clamping of the umbilical cord (on haemoglobin level) in full-term neonates. J Pak Med Assoc. 2014 Nov;64(11):1303-5. PMID: 25831651. |
Wrong population: full term infants |
|
Nouraie S, AMIRALIl Akbari S, Vameghi R, Akbarzade Baghban A. The Effect of the Timing of Umbilical Cord Clamping on Hemoglobin Levels, Neonatal Outcomes and Developmental Status in Infants at 4 Months Old. Iran J Child Neurol. 2019 Winter;13(1):45-55. PMID: 30598672; PMCID: PMC6296705. |
Wrong population: no preterm birth |
|
Nudelman MJ, Goel K, Jegatheesan P, Song D, Huang A, Govindaswami B. Haematocrit in <35 weeks preterm infants who received at least 60 seconds of delayed cord clamping: a retrospective observational study. BMJ Paediatr Open. 2019 Sep 24;3(1):e000531. doi: 10.1136/bmjpo-2019-000531. PMID: 31646196; PMCID: PMC6782040. |
No comparison: ≥60 s of delayed cord clamping |
|
Ofojebe CJ, Eleje GU, Ikechebelu JI, Okpala BC, Ofojebe BA, Ugwu EO, Igbodike EP, Onwuegbuna AA, Ikwuka DC, Anikwe CC, Ejikeme TB. A randomized controlled clinical trial on peripartum effects of delayed versus immediate umbilical cord clamping on term newborns. Eur J Obstet Gynecol Reprod Biol. 2021 Jul;262:99-104. doi: 10.1016/j.ejogrb.2021.04.038. Epub 2021 May 7. PMID: 34004481. |
Wrong population: term newborn |
|
Oh W, Fanaroff AA, Carlo WA, Donovan EF, McDonald SA, Poole WK; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Effects of delayed cord clamping in very-low-birth-weight infants. J Perinatol. 2011 Apr;31 Suppl 1(Suppl 1):S68-71. doi: 10.1038/jp.2010.186. PMID: 21448208; PMCID: PMC3327157. |
Wrong intervention: 30-45 seconds |
|
Panda S, Mishra NR, Shukla P, Nayak BK. Early versus delayed cord clamping among preterms: a cohort study. Perinatology. 2020;21(2):50-6. |
Study design: prospective study |
|
Patel M, Gopalakrishnan M, Sundararajan S. Impact of Delayed Cord Clamping on Red Blood Cell Transfusion and Related Outcomes in Very Low Birth Weight Infants. Am J Perinatol. 2024 May;41(S 01):e2444-e2453. doi: 10.1055/a-2115-4360. Epub 2023 Jun 22. PMID: 37348546. |
Wrong intervention: 30-60 seconds |
|
Patel S, Patil N. Effect of Early Versus Delayed Cord Clamping on Hematocrit and Serum Bilirubin Levels. Perinatology. 2021; 22(1):15-19 |
Wrong population: term low-birth-weight babies |
|
Perretta LJ, Spaight M, Yap V, Perlman J. Randomized Study of Delayed Cord Clamping of 30 to 60 Seconds in the Larger Infant Born Preterm. J Pediatr. 2020 Sep;224:153-157. doi: 10.1016/j.jpeds.2020.04.058. Epub 2020 Jul 7. PMID: 32651013. |
Wrong comparison: 30 versus 60 seconds |
|
Popat H, Robledo KP, Kirby A, Sebastian L, Evans N, Gill A, Kluckow M, Sinhal S, de Waal K, Tarnow-Mordi W, Osborn D. Associations of measures of systemic blood flow used in a randomized trial of delayed cord clamping in preterm infants. Pediatr Res. 2019 Jul;86(1):71-76. doi: 10.1038/s41390-019-0348-1. Epub 2019 Feb 21. Erratum in: Pediatr Res. 2019 Apr 15;: PMID: 30791040. |
Other study aim: association of low superior vena cava flow and low right ventricular output |
|
Purisch SE, Ananth CV, Arditi B, Mauney L, Ajemian B, Heiderich A, Leone T, Gyamfi-Bannerman C. Effect of Delayed vs Immediate Umbilical Cord Clamping on Maternal Blood Loss in Term Cesarean Delivery: A Randomized Clinical Trial. JAMA. 2019 Nov 19;322(19):1869-1876. doi: 10.1001/jama.2019.15995. PMID: 31742629; PMCID: PMC6865311. |
Wrong population: term cesarean section |
|
Qian Y, Ying X, Wang P, Lu Z, Hua Y. Early versus delayed umbilical cord clamping on maternal and neonatal outcomes. Arch Gynecol Obstet. 2019 Sep;300(3):531-543. doi: 10.1007/s00404-019-05215-8. Epub 2019 Jun 15. PMID: 31203386; PMCID: PMC6694086. |
Wrong study design: narrative review |
|
Quinn MK, Katheria A, Bennett M, Lu T, Lee H. Delayed Cord Clamping Uptake and Outcomes for Infants Born Very Preterm in California. Am J Perinatol. 2024 May;41(S 01):e981-e987. doi: 10.1055/a-1975-4607. Epub 2022 Nov 9. PMID: 36351446. |
Study design: cohort study |
|
Rana A, Agarwal K, Ramji S, Gandhi G, Sahu L. Safety of delayed umbilical cord clamping in preterm neonates of less than 34 weeks of gestation: a randomized controlled trial. Obstet Gynecol Sci. 2018 Nov;61(6):655-661. doi: 10.5468/ogs.2018.61.6.655. Epub 2018 Oct 29. PMID: 30474011; PMCID: PMC6236088. |
Wrong intervention: 120 seconds |
|
Ranjit T, Nesargi S, Rao PN, Sahoo JP, Ashok C, Chandrakala BS, Bhat S. Effect of early versus delayed cord clamping on hematological status of preterm infants at 6 wk of age. Indian J Pediatr. 2015 Jan;82(1):29-34. doi: 10.1007/s12098-013-1329-8. Epub 2014 Feb 6. PMID: 24496587. |
Included in systematic review of Rabe 2019 |
|
Rhoades JS, Bierut T, Conner SN, Tuuli MG, Vesoulis ZA, Macones GA, Cahill AG. Delayed Umbilical Cord Clamping at <32 Weeks' Gestation: Implementation and Outcomes. Am J Perinatol. 2017 Sep;34(11):1048-1053. doi: 10.1055/s-0037-1603591. Epub 2017 May 25. PMID: 28561189; PMCID: PMC5578907. |
Study design: retrospective study |
|
Rhoades JS, Wesevich VG, Tuuli MG, Macones GA, Cahill AG. Implementation and Outcomes of Universal Delayed Umbilical Cord Clamping at Term. Am J Perinatol. 2019 Feb;36(3):233-242. doi: 10.1055/s-0038-1669908. Epub 2018 Sep 12. PMID: 30208504. |
Wrong population: at term |
|
Rincón D, Foguet A, Rojas M, Segarra E, Sacristán E, Teixidor R, Ortega A. Tiempo de pinzamiento del cordón umbilical y complicaciones neonatales, un estudio prospectivo [Time of cord clamping and neonatal complications, a prospective study]. An Pediatr (Barc). 2014 Sep;81(3):142-8. Spanish. doi: 10.1016/j.anpedi.2013.10.051. Epub 2013 Dec 4. PMID: 24315426. |
Article in Spanish |
|
Ruangkit C, Bumrungphuet S, Panburana P, Khositseth A, Nuntnarumit P. A Randomized Controlled Trial of Immediate versus Delayed Umbilical Cord Clamping in Multiple-Birth Infants Born Preterm. Neonatology. 2019;115(2):156-163. doi: 10.1159/000494132. Epub 2018 Nov 27. PMID: 30481760. |
Wrong intervention: 30-60 seconds |
|
Salcido C, Shahidi SA, Poeltler DM, Gollin Y, Johnston LA, Katheria AC. Maternal bleeding complications and neonatal outcomes following early versus delayed umbilical cord clamping in cesarean deliveries for very low birthweight infants. J Perinatol. 2023 Jan;43(1):39-43. doi: 10.1038/s41372-022-01558-4. Epub 2022 Nov 10. PMID: 36357575. |
Study design: retrospective study |
|
Shinohara E, Kataoka Y, Yaju Y. Effects of timing of umbilical cord clamping on preventing early infancy anemia in low-risk Japanese term infants with planned breastfeeding: a randomized controlled trial. Matern Health Neonatol Perinatol. 2021 Jan 19;7(1):5. doi: 10.1186/s40748-021-00125-7. PMID: 33468261; PMCID: PMC7814648. |
Wrong population: term infants |
|
Singh N, Brammer D. Delayed cord clamping in infants born less than 35 weeks: A retrospective study. J Neonatal Perinatal Med. 2021;14(3):391-395. doi: 10.3233/NPM-200497. PMID: 33325400. |
Wrong intervention: 30-45 seconds |
|
Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, Evans N, Finlayson S, Fogarty M, Gebski V, Ghadge A, Hague W, Isaacs D, Jeffery M, Keech A, Kluckow M, Popat H, Sebastian L, Aagaard K, Belfort M, Pammi M, Abdel-Latif M, Reynolds G, Ariff S, Sheikh L, Chen Y, Colditz P, Liley H, Pritchard M, de Luca D, de Waal K, Forder P, Duley L, El-Naggar W, Gill A, Newnham J, Simmer K, Groom K, Weston P, Gullam J, Patel H, Koh G, Lui K, Marlow N, Morris S, Sehgal A, Wallace E, Soll R, Young L, Sweet D, Walker S, Watkins A, Wright I, Osborn D, Simes J; Australian Placental Transfusion Study Collaborative Group. Delayed versus Immediate Cord Clamping in Preterm Infants. N Engl J Med. 2017 Dec 21;377(25):2445-2455. doi: 10.1056/NEJMoa1711281. Epub 2017 Oct 29. PMID: 29081267. |
Included in systematic review of Rabe 2019 |
|
Wang L, Ou J, Wu Y, Xiao G, Gong H, Chen W, Zhou L, Zhong X. Delayed versus immediate cord clamping in dichorionic twins <32 weeks: a retrospective study. J Matern Fetal Neonatal Med. 2023 Dec;36(1):2203300. doi: 10.1080/14767058.2023.2203300. PMID: 37120713. |
Study design: retrospective study |
|
Yu L, Sun Y, Shang Y, Yin M. Effect of timing of umbilical cord clamping on maternal and neonatal outcomes: a protocol for systematic review and network meta-analysis. Medicine. 2019 Apr 1;98(16):e15283 |
Wrong study design: protocol |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 28-08-2025
Beoordeeld op geldigheid : 03-06-2025
De Koninklijke Nederlandse Organisatie van Verloskundigen (KNOV) heeft een formele verklaring van geen bezwaar gegeven.
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor zwangeren waarbij sprake is van een dreigende vroeggeboorte.
Werkgroep
- Dr. C.J. (Caroline) Bax, gynaecoloog-perinatoloog, NVOG (voorzitter)
- Dr. J.B. (Jan) Derks, gynaecoloog-perinatoloog, NVOG
- Dr. A. (Ayten) Elvan-Taşpınar, gynaecoloog-perinatoloog, NVOG
- Dr. H.M. (Marieke) Knol, gynaecoloog-perinatoloog, NVOG
- Dr. M.A. (Marjon) de Boer, gynaecoloog-perinatoloog, NVOG
- Dr. D.N.M. (Dimitri) Papatsonis, gynaecoloog, NVOG
- Dr. D.E. (Lia) Wijnberger, gynaecoloog, NVOG
- Dr. P.H. (Dijk), kinderarts-neonatoloog, NVK
- Drs. L. (Leanne) Erkelens-de Vetten, kinderarts-neonataloog, NVK
- Drs. C. (Christel) Rolf, klinisch verloskundige, KNOV (tot maart 2023)
- Drs. C. (Cedric) van Uytrecht, klinisch verloskundige, KNOV (tot 15 augustus 2023)
- Drs. D. (Daphne) de Jong, eerstelijns verloskundige, KNOV (vanaf september 2023)
- Drs. M.A.M. (Machteld) van der Noll, verloskundige, KNOV
- Dr. I.F. (Igna) Kwint-Reijnders, patiëntenvertegenwoordiging Care4Neo
Klankbordgroep
- Drs. H.I. (Herma) Davelaar – van Zanten, V&VN Voortplanting, Obstetrie & Gynaecologie (tot mei 2024)
- Dhr. M. (Maikel) Hustinx, bestuurslid afdeling Vrouw & Kind V&VN (vanaf mei 2024)
Met ondersteuning van
- Drs. D.A.M. (Danique) Middelhuis, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. T. (Tessa) Geltink, adviseur, Kennisinstituut van de Federatie Medsich Specialisten (tot april 2023)
- Dr. M.L. (Marja) Molag, adviseur, Kennisinstituut van de Federatie Medsich Specialisten (vanaf april 2023)
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroep |
||||
|
Achternaam werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Bax (voorzitter) |
Gynaecoloog-perinatoloog AmsterdamUMC |
Allen onbetaald: Adviesraad MADAM project Lid Raad kwaliteit FMS Organisatie en docent basiscursus prenatale counseling Amsterdam UMC Audit voorzitter in regio Amsterdam Lid Dagelijks bestuur koepel kwaliteit |
ZonMW subsidie voor onderzoek naar NIPT |
Geen restricties |
|
Knol |
Perinataloog Isala Kliniek Zwolle |
Lid werkgroep Otterlo NVOG Lid wetenschapscommissie NVOG Lokale hoofdonderzoeker consortiumstudie apostel 8 |
Geen |
Geen restricties |
|
Elvan-Taspinar |
Perinatoloog UMCG |
Instructeur MOET onbetaald |
Geen |
Geen restricties |
|
Van Uytrecht |
Physician Assistant- Obstetrie |
Training acute verloskunde te Medsim. Verloskundige/ Physician Assistant te Maxima Medisch Centrum te Veldhoven |
Geen |
Geen restricties |
|
Rolf |
Physician Assistant Obstetrie; functie van afdelingsarts op de high care verloskunde( OHC), Máxima MC. Betaalde functie |
Klinisch verloskundige, Máxima MC, betaalde functie |
Geen |
Geen restricties |
|
Papatsonis |
Gynaecoloog Amphia Ziekenhuis Breda |
Geen |
Geen |
Geen restricties |
|
Kwint-Reijnders |
Patientvertegenwoordiger namens Care4Neo, experienced expert |
Gynaecoloog i.o. VAGO afgevaardigde in het pijlerbestuur NVOG werkgroep foetomaternale geneeskunde |
In mijn werkzaamheden als gynaecoloog in opleiding werk ik zelf met dreigende vroeggeboorte casuïstiek en met collega's die uitvoering geven aan deze richtlijn. Daarnaast heb ik zitting als VAGO-afgevaardigde in het pijlerbestuur van de NVOG werkgroep foetomaternale geneeskunde, waarin ook onderwerpen geadresseerd worden die gerelateerd zijn aan dreigende vroeggeboorte. |
Geen restricties |
|
Derks |
Gynaecoloog, afdeling verloskunde, WKZ, UMCU.
|
Betrokken bij de richtlijn preventie vroeggeboorte, onderdeel van de Otterlo, deze commissie schrijft de verloskunde richtlijnen voor de NVOG |
Ik ben binnen mijn kliniek betrokken bij de behandeling van patienten met vroeggeboorte (in de anamnese). Gezien mijn expertise op dit gebied zie ik veel patienten met vroeggeboorte |
Geen retricties |
|
De Vetten |
Kinderarts-neonatoloog, Martini ziekenhuis Groningen |
Geen |
Geen |
Geen restricties |
|
Wijnberger |
Gynaecoloog en perinatoloog Rijnstate Ziekenhuis Arnhem |
Lid werkgroep Otterlo (richtlijnontwikkeling) onbetaald Opleider, onbetaald |
Geen |
Geen restricties |
|
Van der Noll |
Klinisch verloskundige - Master Physician Assistant (inactief) Docent Verloskunde Ba-VKV Rotterdam (actief) |
Geen |
Geen |
Geen restricties |
|
De Boer |
Gynaecoloog |
Geen |
Geen |
Geen restricties |
|
Dijk |
Kinderarts-neonatoloog UMC Groningen |
Lidmaatschap Neonatologie Netwerk Nederland Lid LNR werkgroep Perined/NVK Lid werkgroep Nedederlands Kinderformularium NKFK Lid consortium PedMed-Nl Lid werkgroep revisie RL Hyperbilirubinemie Adviesraad N3 Adviesraad Zwangerschap en Geboorte Consortium Noord Nederland Lid werkgroep Kinderformularium Lid Pedmed Lid sectie Neonatologie Lid werkgroep SPIN |
Geen |
Geen restricties |
|
De Jong |
Eerstelijns verloskundige De Geboortezaak Nieuwegein Klinisch epidemioloog |
Klinisch epidemioloog Lid werkgroep HPP in de 1e lijn Lid werkgroep Handreiking indicaties vitaliteitsecho |
Geen |
Geen restricties |
|
Klankbordgroep |
||||
|
Achternaam klankbordgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Davelaar-Van Zanten |
Adviseur kwaliteit en veiligheid (betaalde functie/reguliere baan) Spaarne Gasthuis |
Geen |
Geen |
Geen restricties |
|
Maikel Hustinx |
Verpleegkundig Specialist, Albert Schweitzer Ziekenhuis, 36u p.w. |
Algemeen bestuurslid V&VN afdeling Vrouw en Kind, vrijwillig. Vice-voorzitter Vereniging Verpleegkundig Specialisten Albert Schweitzer Ziekenhuis, vrijwillig Lid landelijke tafel College Perinatale Zorg, Utrecht, vrijwillig |
Geen |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door uitnodigen van Patiëntenfederatie Nederland en Care4Neo voor de schriftelijke knelpuntenanalyse en afvaardiging namens Care4Neo in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen zie per module ook “Waarden en voorkeuren van patiënten”). De conceptrichtlijn is tevens voor commentaar voorgelegd aan Patiëntenfederatie Nederland en Care4Neo en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Module |
Uitkomst raming |
Toelichting |
|
Laat afnavelen bij premature neonaat
|
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat [het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet OF het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft]. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 3.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor zwangeren waarbij sprake is van dreigende vroeggeboorte. Tevens zijn er knelpunten aangedragen door Inspectie Gezondheidszorg en Jeugd, Nederlandse Vereniging voor Obstetrie en Gynaecologie, de Koninklijke Nederlandse Organisatie van Verloskundigen en Care4Neo via een schriftelijke knelpuntenanalyse. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy
|
Richtlijn: Dreigende vroeggeboorte |
|
|
Uitgangsvraag: Wat is de waarde van laat afnavelen (volledig gestopt met pulseren of ten minste 60 seconden wachten) bij een premature neonaat? |
|
|
Database(s): Medline (OVID), Embase |
Datum: 20-07-2023 |
|
Periode: > 2010 |
Talen: geen beperking |
|
Literatuurspecialist: Laura Boerboom |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting en opmerkingen:
→ Voor deze vraag is gezocht op het element laat afnavelen (in het blauw).
à Er waren 3 sleutelartikelen en deze zitten allemaal in de resultaten.
à Resultaten staan in Rayyan. |
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase (via embase.com) en Medline (via OVID) is op 20-07-2023 met relevante zoektermen gezocht naar systematische reviews, RCT’s en observationele studies over wat de waarde is van laat afnavelen (volledig gestopt met pulseren of ten minste 60 seconden wachten) bij een premature neonaat. De literatuurzoekactie leverde 819 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR’s |
155 |
121 |
154 |
|
RCT’s |
398 |
329 |
448 |
|
Observationele designs |
206 |
145 |
217 |
Zoekstrategie
|
Database |
Zoektermen |
|||||||||||||||||||||||||||||||||
|
Embase
|
|
|||||||||||||||||||||||||||||||||
|
Medline (OVID)
|
1 exp Umbilical Cord Clamping/ or ('cord' adj3 clamping).ti,ab,kf. (1395) |