Behandeling morbus Conn
Uitgangsvraag
Wat is de plaats van chirurgische behandeling versus medicamenteuze behandeling bij morbus Conn (primair hyperaldosteronisme)?
Aanbeveling
Behandel bij voorkeur patiënten met unilateraal primair hyperaldosteronisme (m. Conn) met adrenalectomie. Factoren die bij de individuele patiënt meegenomen moeten worden met betrekking tot de behandelbeslissing (samen beslissen) zijn:
- Biologische leeftijd (in combinatie met comorbiditeit)
- Comorbiditeit
- Operatierisico
- Respons op hypertensie behandeling voor operatie
- Bijwerkingen van medicatie
- Follow-up duur
- Wens van de patiënt
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Voor drie van de vier cruciale uitkomstmaten (bloeddrukcontrole, cardiovasculaire events en kwaliteit van leven) werden resultaten gerapporteerd in de geïncludeerde studies. Voor de uitkomst cardiovasculaire morbiditeit en mortaliteit, zijn geen resultaten gerapporteerd. Zeventien studies rapporteerden systolische bloeddruk en negen studies rapporteerden diastolische bloeddruk welke verschillende effecten lieten zien. De meeste studies lieten een effect zien in het voordeel van de chirurgische behandeling lieten zien. Slechts twee studies toonden lieten een klinisch relevant effect zien van 10mmHg of meer in het voordeel van de chirurgische behandeling.
Zeven studies rapporteerde cardiovasculaire events en lieten verschillende effecten zien. Vier studies vier studies rapporteerden een positief effect in het voordeel van de chirurgische behandeling. Daarnaast rapporteerden twee studies geen effect en één studie rapporteerde een negatief effect.
Drie studies rapporteerden kwaliteit van leven met behulp van de SF-36 (Buffolo, 2020; Velema, 2018; Tan, 2021). Voor de subschalen fysiek functioneren, pijn, algemene gezondheid, vitaliteit en sociaal en emotioneel functioneren was er een voordeel te zien voor de chirurgische behandeling. Voor de subschaal mentale gezondheid was er geen verschil te zien tussen beide behandelingen. Twee studies (Velema, 2018; Tan, 2021) hebben daarnaast ook EQ-5D gebruikt om kwaliteit van leven te meten waarbij er één studie wel een verschil vond en één studie geen verschil vond tussen de chirurgische behandeling en medicamenteuze behandeling. Dit kan verklaard worden tussen het verschil in studie populaties en de manier van rapporteren van de uitkomsten.
De overall bewijskracht voor alle uitkomstmaten was zeer laag omdat er in sommige gevallen mogelijk sprake was van selectie bias. Daarnaast zijn er enkele studies die de medicamenteuze behandeling niet goed beschreven hebben waardoor het niet duidelijk is wat patiënten precies voor medicatie ontvangen hebben. In veel studies zijn mogelijke covariabelen niet beschreven en/of is er niet gecorrigeerd voor mogelijke confounding. Daarnaast was er sprake van inconsistente resultaten tussen de studies vanwege verschillende studiepopulaties. Sommige studies hebben alleen patiënten met unilateraal primair hyperaldosteronisme geïncludeerd, terwijl de meeste studies zowel patiënten met unilateraal als bilateraal primair hyperaldosteronisme hebben geïncludeerd. Daarnaast verschilde de interventie ook per studie. In sommige studies werd de chirurgische behandeling alleen toegepast bij patiënten met unilateraal primair hyperaldosteronisme en de medicamenteuze behandeling bij bilateraal of unilateraal primair hyperaldosteronisme.
De hoofdvraag in deze module is of adrenalectomie te verkiezen is boven medicamenteuze therapie bij de behandeling van unilateraal primair hyperaldosteronisme (PHA) met betrekking tot effecten op (a) bloeddruk, (b) kaliumconcentratie, (c) cardiovasculaire morbiditeit en mortaliteit, (d) aantal antihypertensiva en (d) kwaliteit van leven.
Bij analyse van de beschikbare literatuur lijkt adrenalectomie een betere uitkomst te hebben op deze vier eindpunten dan medicamenteuze therapie. Dit wordt ook zo beschreven in een recente meta-analyse (Chen, 2022). Er zijn echter diverse factoren die de bewijskracht minder overtuigend maken:
- Heterogeniteit in de verschillende studies met betrekking tot patiënten selectie, uitvoering van de medicamenteuze therapie, follow-up duur etc.
- Verschillen in baseline karakteristieken tussen chirurgische en medicamenteus behandelde patiënten. Bijvoorbeeld in sommige studies zijn patiënten ouder, hebben diabetes en/of een hogere BMI in de medicamenteus behandelde groep
- Het voordeel van adrenalectomie is waarschijnlijk minder bij ouderen (Chen, 2022), mogelijk omdat zij al langer bloot hebben gestaan aan hypertensie en teven ook al cardiovasculaire comorbiditeit hebben. Dat jongere patiënten meer profijt hebben van adrenalectomie wordt ook gesuggereerd in de studie van Williams (2017).
- Veelal retrospectieve studies en het ontbreken van prospectieve, gerandomiseerde trials
Daarnaast zijn er twee conceptuele overwegingen die in ogenschouw genomen moeten worden. Allereerst wordt in alle studies niet de biochemische effectiviteit van mineralocorticoid receptor (MR) blokkade geëvalueerd. Bij effectieve MR blokkade dient de renine concentratie te stijgen tot in het normale gebied. Eén studie laat inderdaad zien dat bij patiënten met PHA die behandeld worden met MR blokkade er een verhoogde cardiovasculaire morbiditeit bij een onderdrukt renine vergeleken met patiënten met een normaal renine (Hundemer, 2018). Het is goed voor te stellen dat in de verrichtte studies bij een deel van de patiënten onvoldoende MR blokkade gegeven is waardoor er voortschrijdende negatieve effecten aanhielden als gevolg van van aldosteron overschot. Toekomstige studies zullen dus renine-geleide MR blokkade moeten evalueren.
De tweede conceptuele overweging sluit hier op aan. Adrenalectomie leidt vrijwel altijd tot volledige normalisatie van aldosteron concentraties. Bij medicamenteuze MR blokkade blijven echter chronisch verhoogde aldosteron concentraties bestaan. Ook al zou de MR blokkade leiden tot normale renine waarden (zie bovenstaande) dan zouden chronisch verhoogde aldosteron waarden theoretisch toch schadelijke effecten kunnen hebben. Zo is bijvoorbeeld niet bekend of MR blokkade even effectief is in ieder weefsel, differentiële effecten lijken meer voor de hand te liggen. En om het nog verder te nuanceren, het is ook niet bekend wat voor een bepaald individu een normaal renine is en zou een patiënt die met MR blokkade een laag-normaal renine bereikt nog steeds onder behandeld kunnen zijn.
Ten opzichte van medicamenteuze therapie geeft adrenalectomie een snelle definitieve remissie en maakt het in feite bovenstaande discussie overbodig.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Bij de beslissing tot chirurgische of medicamenteuze behandeling van primair hyper-aldosteronisme dient de voorkeur van de patiënt in ogenschouw genomen te worden (‘samen beslissen’). Voor- en nadelen van beide behandelmodaliteiten moeten vooraf met de patiënt besproken worden waarbij deze een persoonlijke afweging kan maken. Factoren die aan bod moeten komen zijn o.a. effectiviteit van de behandeling, operatie risico, bijwerkingen van medicatie en follow-up duur.
Kosten (middelenbeslag)
Er zijn geen kosten-baten analyse gegevens voorhanden van een chirurgische versus medicamenteuze behandeling van primair hyperaldosteronisme. Echter wanneer een patiënt langduriger of zelfs levenslang onder controle blijft in tweede of derde lijn ten behoeve van de medicamenteuze behandeling, is de verwachting dat de kosten hoger zullen zijn dan een eenmalige operatie. Dit betreft kosten voor geneesmiddelen, medisch-specialistische controle en laboratoriumonderzoek.
Aanvaardbaarheid, haalbaarheid en implementatie
In centra waar bijnieroperaties uitgevoerd worden conform de geldende afspraken en met de aanwezigheid van een multidisciplinair team met bijnierexpertise, zijn geen beperkingen te verwachten met betrekking tot aanvaardbaarheid, haalbaarheid en implementatie.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Op grond van de huidige literatuur, ofschoon het bewijs beperkt is, gaat de voorkeur van behandeling van unilateraal PHA uit naar een adrenalectomie boven medicamenteuze behandeling, zeker bij (biologisch) jonge patiënten. Dit zou idealiter nog moeten worden bevestigd in prospectieve studies met lange termijn follow-up waarbij patiënten gerandomiseerd worden tussen adrenalectomie en MR blokkade. Daarnaast zullen toekomstige studies de effectiviteit moeten evalueren van renine-geleide MR blokkade en deze vergelijken met de effectiviteit van adrenalectomie.
Factoren die bij de individuele patiënt in ogenschouw genomen moeten worden met betrekking tot de behandelbeslissing zijn leeftijd, comorbiditeit en operatierisico, respons op hypertensie behandeling voor operatie (aantal antihypertensiva en effectiviteit) en de wens van de patiënt.
Onderbouwing
Achtergrond
In de huidige situatie wordt een patiënt met M. Conn (primair hyperaldosteronisme) geopereerd als er een unilaterale aldosteron-overproductie is aangetoond met veneuze bijniervene sampling (AVS). Alle andere patiënten (met bilaterale afwijkingen of normale bijnieren op CT en/of AVS zonder lateralisatie of ongeschikte patiënten voor chirurgie) worden medicamenteus behandeld met een mineralocorticoïd receptor antagonist, eventueel aangevuld met andere antihypertensiva.
Met betrekking tot de behandeling van primair hyperaldosteronisme zijn er verschillende uitkomstmaten van belang: bloeddrukregulatie, kaliumbalans, lange termijn (cardiovasculaire) morbiditeit en mortaliteit en kwaliteit van leven. De vraag is of er verschillen zijn in deze uitkomstmaten tussen geopereerde patiënten en medicamenteus behandelde patiënten.
Conclusies / Summary of Findings
Blood pressure control
|
Very low GRADE |
The evidence is very uncertain about the effect of adrenalectomy on blood pressure control compared with MRA treatment in patients with primary aldosteronism.
Sources: Satoh, 2019; Araujo Castro, 2022; Buffolo, 2020; Chen, 2021; Haze, 2021; Katabami, 2019; Meng, 2019; Murck, 2021; Puar, 2020; Wada, 2017 |
Cardiovascular morbidity and mortality
|
No GRADE |
No evidence was found regarding the effect of adrenalectomy on cardiovascular morbidity and mortality compared with MRA treatment in patients with primary aldosteronism.
Source: - |
Cardiovascular events
|
Very low GRADE |
The evidence is very uncertain about the effect of adrenalectomy on cardiovascular events compared with MRA treatment in patients with primary aldosteronism.
Sources: Satoh, 2019; Aruajo Castro, 2022; Nakamaru, 2021; Puar, 2020 |
Quality of life
|
Very low GRADE |
The evidence is very uncertain about the effect of adrenalectomy on quality of life compared with MRA treatment in patients with primary aldosteronism.
Sources: Buffolo, 2020; Velema, 2018; Tan, 2021 |
Number of antihypertensive drugs
|
Very low GRADE |
The evidence is very uncertain about the effect of adrenalectomy on number of antihypertensive drugs compared with MRA treatment in patients with primary aldosteronism.
Sources: Satoh, 2019; Araujo Castro, 2022; Buffolo, 2020; Katabami, 2019; Meng, 2019; Puar, 2020; Wada, 2017; Zavatta, 2019 |
Normokalemia
|
Very low GRADE |
The evidence is very uncertain about the effect of adrenalectomy on normokalemia compared with MRA treatment in patients with primary aldosteronism.
Sources: Satoh, 2019; Katabami, 2019; Meng, 2019 |
Samenvatting literatuur
Description of studies
Satoh (2019) performed a systematic review of the literature. Randomized Controlled Trials (RCT), prospective cohort studies and retrospective cohort studies which compared the operative treatment with the medical treatment in patients with primary aldosteronism, were included in this review. Other inclusion criteria were that a study had to provide values (means with standard deviation) of at least one of the following variables: Left ventricular mass (LVM), serum potassium, systolic blood pressure (SBP), glomerular filtration ratio (GFR), the number of oral antihypertensive agents or incidence of cardiovascular events. No exclusion criteria were reported. The search was performed to articles published between 1985 and 2017. Satoh (2019) included sixteen studies in the review. All studies were cohort studies. Four studies with 2073 patients were included in the meta-analysis of cardiovascular events. Eight studies with 903 patients were included in the systolic blood pressure meta-analysis. Five studies with a total of 499 patients were included in the hypokalemia analysis and three studies with 265 patients were included in the meta-analysis of the number of antihypertensive agents.
Velema (2018) performed a post hoc comparative effectiveness study within the Subtyping Primary Aldosteronism: A Randomized Trial Comparing Adrenal Vein Sampling and Computed Tomography Scan (SPARTACUS) trial. Inclusion data were reported in the SPARTACUS trial (Dekkers, 2016). Regarding this post hoc study patients who underwent adrenalectomy were compared with patients who underwent mineralocortoid receptor antagonist (MRA) treatment. Both the adrenalectomy group and the MRA treatment group, consisted of 92 patients. Mean age in the adrenalectomy group was 51.8 years and 71.7% was male. Mean age in the MRA treatment group was 54.4 years and 84.8% was male. Median Body Mass Index (BMI) was 27.5 kilogram per square meter in the adrenalectomy group and 29.4 kilogram per square meter in the MRA treatment group. Mean serum potassium level in the adrenalectomy group was 3.5 mEq per liter and in the MRA treatment group 3.6 mEq per liter. Velema (2018) reported quality of life using the EQ-5D, which comprises five questions, and the 36-item Short Form Health Survey (SF-36) which consisted of eight subscales which are reported separately. Also, the physical component summary (PCS) and mental component summary (MCS) of the SF-36 are reported. A higher score indicates a better health condition.
Araujo Castro (2022) performed a retrospective cohort study using patient reports from the Spanish Primary Aldosteronism Registry of the Spanish Endocrinology and Nutrition Society (SPAIN-ALDO) with a follow-up between 2018 and 2020. Patients who underwent adrenalectomy or were under medical treatment with Mineralocorticoid Receptor Antagonist (MRA) and who had clinical, hormonal, and biochemical information during follow-up, were included. Patients with confirmed co-secretion of cortisol were excluded. The adrenal surgery group consisted of 100 patients with unilateral PA, a mean age of 52.7 years and a mean Body Mass Index (BMI) of 29.1 kilogram per square meter. The medication group consisted of 168 patients with bilateral PA, a mean age of 54.7 years and mean BMI of 30.0 kilogram per square meter. The adrenal surgery group consisted of 54 women (54.6%) and 63 patients (76.8%) experienced grade two or higher hypertension. The medication group consisted of 70 women (41.7%) and 108 patients (70.6%) experienced grade two or higher hypertension. Araujo Castro (2022) reported systolic blood pressure, diastolic blood pressure, cardiovascular events and number of antihypertensive drugs.
Buffolo (2020) performed a prospective cohort study including patients from the QUALITO study in Italy. The total cohort consisted of 70 patients with primary aldosteronism and 70 matched patients with essential hypertension, only data regarding patients with primary aldosteronism (PA), are taken into account. Mean age of the PA patients was 52 years and 35.7% was female. Mean BMI was 25.9 and 67 patients (95.7%) had type 2 diabetes. There were 37 patients included with unilateral PA who underwent laparoscopic adrenalectomy and 30 patients with unilateral or bilateral PA who received mineralocorticoid receptor antagonist (MRA) treatment. The MRA treatment consisted of spironolactone (n=14) or potassium canrenoate (n=16).
Buffolo (2020) reported systolic blood pressure, diastolic blood pressure, number of antihypertensive drugs and quality of life. Quality of life was measured using the 36-item Short Form Health Survey (SF-36) which consisted of eight subscales which are reported separately. A higher score indicates a better health condition.
Chen (2021) performed a prospective cohort study including patients with hypertension and primary aldosteronism in the inpatient ward from November 2018 to July 2020. Patients with other forms of secondary hypertension, ischemic heart disease, valvular heart disease, cardiomyopathy, pacemaker implantation, atrial fibrillation, or suboptimal echocardiographic windows, were excluded. They included 39 patients who underwent unilateral adrenalectomy. The mean age was 49.4 years and mean BMI was 25.7 kilogram per square meter. There were 28 patients with bilateral PA who underwent treatment with mineralocorticoid receptor antagonist (MRA) with a mean age of 48.8 years and mean BMI of 26.8 kilogram per square meter. Median plasma renin activity in the surgery group was 0.27 nanogram per millilitre per hour and 0.84 nanogram per millilitre per hour in the MRA treatment group. Chen (2021) reported systolic blood pressure and diastolic blood pressure.
Haze (2021) performed a retrospective cohort study using data from the Japan Rare/Interactable Adrenal Disease Study (JRAS). Patients aged between 20 and 90 years, enrolled in JRAS between 2006 and 2019, diagnosed with PA based on guidelines of the Japan Endocrine Society and Japanese Society of Hypertension, records with assessment of of plasma aldosterone concentration, plasma renin activity and blood pressure before treatment, treatment with unilateral adrenalectomy or MRA for unilateral or bilateral PA between month zero and six and observation data for more than six months from baseline, were included. The adrenalectomy group consisted of 740 patients with a mean age of 51.5 years, 49.7 percent was female and mean BMI was 24.2 kilograms per square meter. The MRA treatment group consisted of 1247 patients with a mean age of 54.2 years, 53.4 percent was female and mean BMI was 25.1 kilograms per square meter. Mean duration of hypertension in the adrenalectomy group was 10.1 years and in the MRA treatment group 8.0 years. Haze (2021) reported on systolic blood pressure and diastolic blood pressure.
Katabami (2019) performed a retrospective cohort study using data from the Japan Primary Aldosteronism Study (JPAS). Patients enrolled in the JPAS between January 2006 and October 2016 with primary aldosteronism with confirmed unilateral subtype. Patients were excluded in case of a bilateral subtype, unsuccessful adrenal vein sampling (AVS), AVS without adrenocorticotropic hormone stimulation, missing follow-up data, incomplete data on blood pressure and/or antihypertensive drugs or if patients in the medically treated group missed out on receiving MRAs. The unilateral adrenalectomy group consisted of 63 patients with median age of 54.0 years, 46% female, median duration of hypertension of 9.0 years and 17.3% diabetic. The mineralocortoid receptor antagonist (MRA) treatment group consisted of 276 patients with median age of 60.0 years, 32% female, median duration of hypertension of 12.5 years and 20.6% diabetic. Because groups were not comparable at baseline, propensity score matching was used to reduce bias associated with different prevalence of some baseline characteristics within the treatment groups. Therefore, in the analysis 55 patients in the adrenalectomy group and 55 patients in the MRA treatment group were included. Katabami (2019) reported systolic blood pressure, diastolic blood pressure, serum potassium normalization rate and daily defined dose of antihypertensive drugs. There was no clear definition of normalization of serum potassium rate.
Meng (2019) performed a retrospective cohort study, with data from the Fuwai Hospital in China. Patients who were hospitalized between January 2016 and December 2017, who had successful AVS proven unilateral PA, were included in this study. Surgical treatment consisted of total or partial laparoscopic adrenalectomy and medical treatment consisted of mineralocortoid receptor antagonist (MRA) treatment by spironolactone. Mean age in the adrenalectomy group was 44.6 years, 57.1% was female and mean body mass index (BMI) was 24.2 kilogram per square meter. Mean age in the MRA treatment group was 50.5 years, 33.3% was female and mean BMI was 27.0 kilogram per square meter. Mean duration of hypertension in the adrenalectomy group was 8.3 years and in the MRA treatment group 13.6 years. In the adrenalectomy group no patients had diabetes mellitus, in the MRA treatment group five patients (16.7%) had diabetes mellitus. Meng (2019) reported systolic blood pressure, diastolic blood pressure, number of antihypertensive drugs and number of patients with hypokalemia. There was no clear definition of hypokalemia.
Murck (2021) performed a retrospective cohort study using data from the German Conn registry. Patients with newly diagnosed primary aldosteronism were included. Unilateral adrenalectomy was performed in case of a unilateral tumor in 75 patients. Mineralocorticoid receptor antagonist (MRA) treatment, mainly with spironolactone was used for bilateral hyperplasia of the adrenal gland in 90 patients. The study stratified data according to gender. Therefore, baseline characteristics were not reported for patients in the adrenalectomy and the MRA treatment group. Regarding the scope of this summary, only systolic blood pressure and diastolic blood pressure were reported.
Nakamaru (2021) performed a retrospective cohort study using data from the Japan Primary Aldosteronism Study (JPAS). Patients aged between 20 and 90 years with PA who underwent adrenal vein sampling (AVS) were included. Patients with no follow-up data regarding blood pressure or estimated glomerular filtration rate, were excluded. The adrenalectomy treatment group consisted of 622 patients and the mineralocorticoid receptor antagonist (MRA) treatment group consisted of 233 patients. Nakamaru (2021) stratified data according to age (< 65 years versus ³ 65 years) therefore not all baseline and outcome data were available regarding the scope of this summary. Nakamaru (2021) reported number of cardiovascular events.
Puar (2020) performed a retrospective cohort study using data from two referral centers in Singapore between 2000 and 2019. Patients with confirmed unilateral PA by AVS and patients with likely unilateral PA by clinical prediction score, were included. Patients without adequate follow-up for at least six months post-treatment and patients with baseline estimated glomerular filtration rate (eGFR) under 45 milliliter per minute per 1.73 square meter, were excluded. The unilateral adrenalectomy group consisted of 86 patients with mean age of 51.0 years and mean eGFR of 90.7 ml/min/1.73 m2. The mineralocorticoid receptor antagonist (MRA) treatment group consisted of 68 patients with a mean age of 55.0 years and mean eGFR of 83.8 ml/min/1.73 m2. Puar (2020) reported systolic blood pressure, diastolic blood pressure, number of antihypertensive drugs and a composite outcome for cardiovascular events, consisting of acute myocardial infarction, coronary revascularization or coronary artery bypass graft, admission for congestive cardiac failure, incidence of atrial fibrillation or stroke.
Tan (2021) performed a prospective cohort study using data from the Changi General Hospital. Patients of age eighteen years or older, with confirmed diagnosis of PA and completion of baseline questionnaires, were included. Patients with adrenal carcinoma, severe or terminal co-morbidity that interfered with possible treatment or Health-Related Quality of Life (HRQoL) or glucocorticoid-remediable aldosteronism, were excluded. The unilateral adrenalectomy group consisted of 21 patients with a mean age of 48.1 years, mean body mass index of 26.9 kilogram per square meter and 38.1 percent was female. The medical treatment group consisted of 13 patients with a mean age of 56.4 years, mean body mass index of 28.6 kilogram per square meter and 15.4 percent was female.
Tan (2021) reported quality of life using the EQ-5D and the SF-36. The 36-item Short Form Health Survey (SF-36) consists of eight subscales, which were reported separately. A higher score indicates a better health condition.
Wada (2017) performed a retrospective cohort study using data from the West Japan Adrenal Vein Sampling study (WAVES-J). Patients with PA who underwent AVS from January 2006 to December 2013 and who had at least one of the recordings of data after treatment, were included. Patients who did not take Mineralocorticoid Receptor Antagonist after diagnosis of PA, were excluded. The surgery comprised unilateral adrenalectomy. The medical treatment comprised Mineralocorticoid Receptor Antagonist (MRA) treatment. The adrenalectomy group consisted of 142 patients with a mean age of 53 years. Of these patients 50% was female, median aldosterone renin ratio was 1085 and mean serum potassium level was 3.3 m Eg l-1 .
Wada (2017) reported systolic blood pressure, diastolic blood pressure, number of antihypertensive drugs and hyperkalemia.
Zavatta (2019) performed a prospective cohort study with patients from the Endocrinology unit of the S. Orsola-Malpighi University Hospital of Bologna, Italy. Patients diagnosed with PA according to current guidelines were included in the study undergoing a unilateral adrenalectomy or treatment with aldosterone antagonist (canrenone). The control group in this study consisted of consecutive hypertensive patients. Regarding the scope of this summary, only data and outcomes regarding patients with PA, were reported. The adrenalectomy group consisted of 12 patients, the aldosterone antagonist treatment group consisted of 33 patients. Because in this study patients with PA were compared to patients with hypertension, no baseline data of the adrenalectomy versus medication group, were available. Zavatta (2019) reported the number of anti-hypertensive drugs.
Results
Blood pressure control
Systolic blood pressure
Systolic blood pressure was reported in eight studies in the review of Satoh (2019) and nine additional studies (Araujo Castro, 2022; Buffolo, 2020; Chen, 2021; Haze, 2021; Katabami, 2019; Meng, 2019; Murck, 2021; Puar, 2020; Wada, 2017). Two studies reported in-between treatment differences in systolic blood pressure (Araujo Castro, 2022; Chen, 2021). The results of the studies that presented the mean systolic blood pressure, are presented in Figure 1. Because of the heterogeneity of the studies due to difference in study population, intervention and duration of follow-up, the pooled results are not displayed.
Katabami (2019) reported median between treatment difference in the adrenalectomy group of -9.0 mmHg (95%CI -22.0 – -3.0) and in the MRA treatment group -5.0 mmHg (95%CI -25.0 – 6.0).
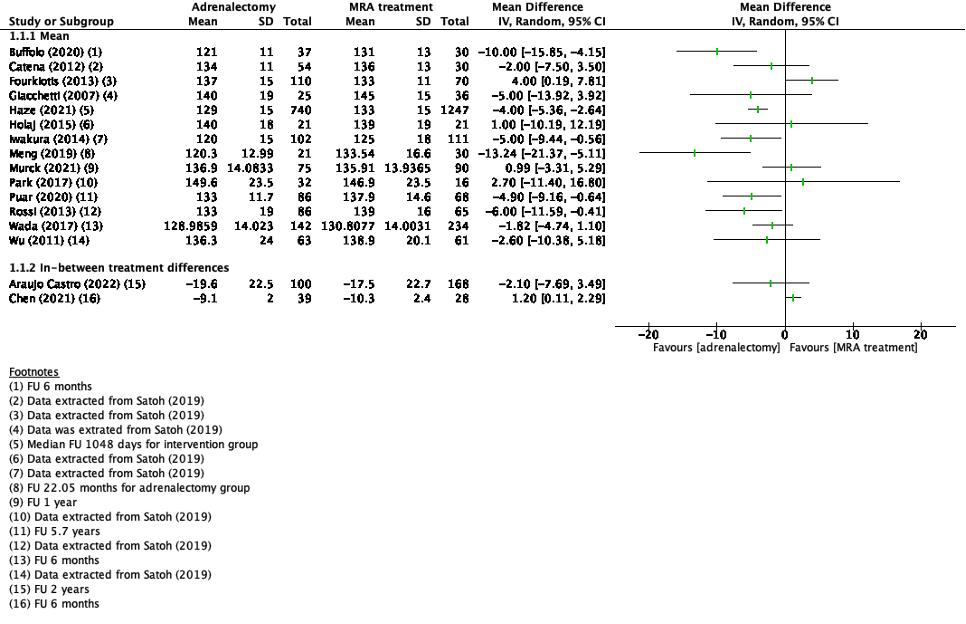
Figure 1. Outcome systolic blood pressure with adrenalectomy versus MRA
Z: p-value of pooled effect; df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval
Diastolic blood pressure
Diastolic blood pressure was reported in nine studies (Araujo Castro, 2022; Buffolo, 2020; Chen, 2021; Haze, 2021; Katabami, 2019; Meng, 2019; Murck, 2021; Puar, 2020; Wada, 2017). Two studies reported in-between treatment differences in diastolic blood pressure (Araujo Castro, 2022; Chen, 2021). The results of the studies that presented the mean systolic blood pressure, are presented in Figure 2. Because of the heterogeneity of the studies due to difference in study population, intervention and duration of follow-up, the pooled results are not displayed.
Katabami (2019) reported median between treatment difference in the adrenalectomy group of -4.5 mmHg (95%CI -12.0 – 7.0) in the adrenalectomy group and -7.0 mmHg (95%CI -13.0 – 4.0) in the MRA treatment group.
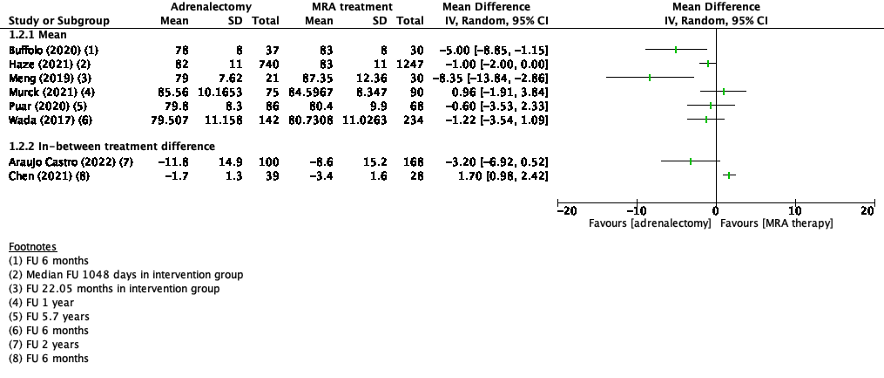
Figure 2. Outcome diastolic blood pressure with adrenalectomy versus MRA
Z: p-value of pooled effect; df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval
Cardiovascular morbidity and mortality
None of the included studies reported cardiovascular morbidity and mortality.
Cardiovascular events
Four studies in the review of Satoh (2019) and three additional studies reported cardiovascular events (Aruajo Castro, 2022; Nakamaru, 2021; Puar, 2020). The review of Satoh (2019) and Nakamaru (2021) reported number of cardiovascular events with a relative risk. Because of the heterogeneity of the studies due to difference in study population, intervention and duration of follow-up, the pooled results are not displayed (figure 3).
Araujo Castro (2022) reported new cardiovascular events using a hazard ratio. In the adrenalectomy group two events (3.9%) were reported and in the MRA treatment group seven events (6.4%) were reported (HR 0.5 [95%CI 0.1-2.2]).
Puar (2020) reported composite cardiovascular events using a hazard ratio (HR 0.93 [95%CI 0.32-2.67]).
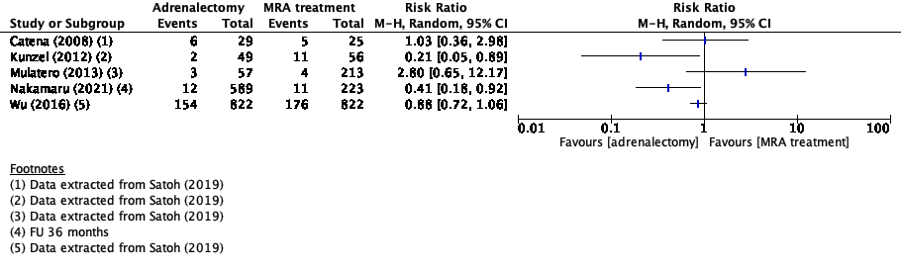
Figure 3. Outcome cardiovascular events with adrenalectomy versus MRA
Z: p-value of pooled effect; df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval
Quality of life
Three studies reported quality of life (Buffolo, 2020; Velema, 2018; Tan, 2021).
Velema (2018) reported SF-36 subscale scores as mean difference with a Dutch reference population for the adrenalectomy and the MRA treatment group. The physical component summary (PCS) of the SF-36 in the adrenalectomy was 2.4 (95%CI 0.3-4.5) and in the MRA treatment group -1.5 (95%CI -4.0-0.9). The mental component summary (MCS) in the adrenalectomy group was -0.2 (95%CI -2.4-2.1) and in the MRA treatment group -4.0 (95%CI -6.3 – -1.8).
The studies of Buffolo (2020) and Tan (2021) reported mean scores in the treatment groups for the different subscales (Figure 4). Because of the heterogeneity of the studies due to difference in study population, intervention and duration of follow-up, the pooled results are not displayed, and the results of Velema (2018) were not added to this figure.

Figure 4. Outcome quality of life (SF-36 subscales) with adrenalectomy versus MRA
Z: p-value of pooled effect; df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval
Two studies also reported the EQ-5D scores (Velema, 2018; Tan, 2021). Velema (2018) reported adjusted odds ratios for reporting problems on the EQ-5D during follow-up for adrenalectomy treatment versus MRA treatment.
Table 3. Odds Ratios EQ-5D, Velema (2018)
|
Dimension of EQ-5D |
Adrenalectomy/MRA, Adjusted Odds Ratio (95%CI) |
|
Mobility |
0.52 (0.23-1.20) |
|
Self-care |
0.14 (0.01-2.50) |
|
Usual activities |
0.35 (0.17-0.75) |
|
Pain/discomfort |
0.52 (0.30-0.91) |
|
Anxiety/depression |
0.79 (0.39-1.60) |
Tan (2021) reported that the median EQ-5D index score in the adrenalectomy group was 1.00 (IQR 1.00-1.00) and in the MRA treatment group 1.00 (IQR 0.838-1.00).
Number of antihypertensive drugs
Number of antihypertensive drugs was reported in three studies in the review of Satoh (2019) and seven additional studies (Araujo Castro, 2022; Buffolo, 2020; Katabami, 2019; Meng, 2019; Puar, 2020; Wada, 2017; Zavatta, 2019).
The review of Satoh (2019), Buffolo (2020), Meng (2019) and Wada (2017) reported mean number of antihypertensive drugs. Araujo Castro (2022) reported in-between treatment difference in number of antihypertensive drugs. Because of the heterogeneity of the studies due to difference in study population, intervention, reporting of the outcome and duration of follow-up, the pooled results are not displayed (Figure 5).
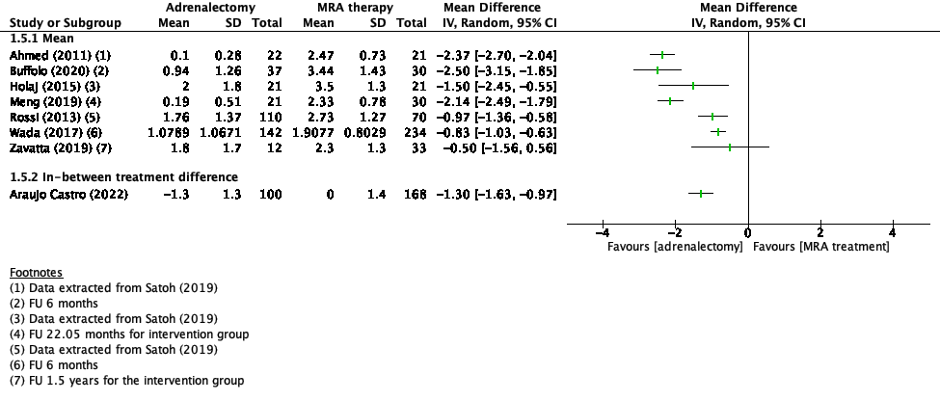
Figure 5. Outcome number of hypertensive drugs with adrenalectomy versus MRA
Z: p-value of pooled effect; df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval
Katabami (2019) reported median daily defined dose of antihypertensive drugs of -1.0 (IQR -1.9-0.0) in the adrenalectomy group and 0.5 (IQR -0.1-2.0) in the MRA treatment group.
Puar (2020) reported median change in number of antihypertensive drugs of -1.0 (IQR -2.0-0.0) in the adrenalectomy group and 0.0 (IQR -1.0-1.0) in the MRA treatment group.
Normokalemia
One study reported normalization of serum potassium levels (Katabami, 2019). One study described average serum potassium levels at follow-up in the different treatment groups (Satoh, 2019) and one study reported percentage of patients with hypokalemia at follow-up in both groups (Meng, 2019).
Katabami (2019) reported normalization of serum potassium levels in 55 patients (100%) in the adrenalectomy group and in 50 patients (90.9%) in the MRA treatment group.
The review of Satoh (2019) reported mean end-of-study serum potassium levels in the adrenalectomy group and MRA treatment group. Because of the heterogeneity of the studies due to difference in study population, intervention, reporting of the outcome and duration of follow-up, the pooled results are not displayed.
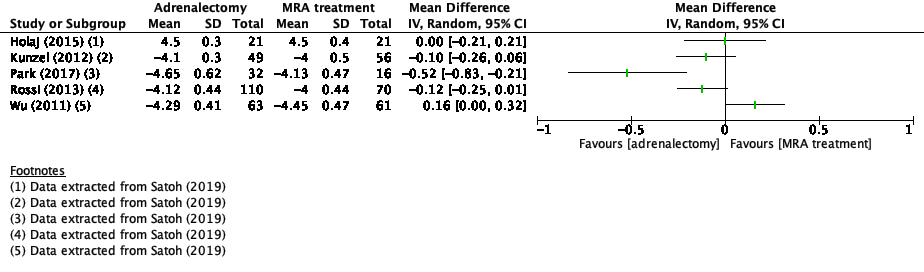
Figure 6. Outcome normokalemia with adrenalectomy versus MRA
Z: p-value of pooled effect; df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval
Meng (2019) reported the percentage of patients with hypokalemia at follow-up. In the adrenalectomy group no patients (0%) experience hypokalemia, in the MRA treatment group 4 patients (13.3%) experienced hypokalemia.
Level of evidence of the literature
The level of evidence of observational cohort studies is considered low according to the GRADE methodology. Therefore, the level of evidence of these cohort studies starts at low GRADE.
Blood pressure control
The level of evidence regarding the outcome measure blood pressure control was downgraded by three levels because of study limitations (-1; risk of bias regarding confounding reporting, confounding analysis and selection bias), conflicting results (-1; inconsistency because of clinical and methodological heterogeneity) and number of included patients (-1; imprecision because of low sample size). The level of evidence was therefore graded as very low.
Cardiovascular morbidity and mortality
None of the included studies reported cardiovascular morbidity and mortality.
Cardiovascular events
The level of evidence regarding the outcome measure cardiovascular events was downgraded by three levels because of study limitations (-1; risk of bias regarding confounding report, confounding analysis and adequate follow-up); conflicting results (-1; inconsistency because of clinical and methodological heterogeneity) and number of included patients (-1; imprecision because of low sample size and small number of events per arm). The level of evidence was therefore graded as very low.
Quality of life
The level of evidence regarding the outcome measure quality of life was downgraded by two levels because of study limitations (-1; risk of bias regarding selection bias, assessment of exposure, confounding assessment and analysis) and number of included patients (-1; imprecision because of low sample size and the confidence interval are including the possibility of a positive effect or no effect).
The level of evidence was therefore graded as very low.
Number of anti-hypertensive drugs
The level of evidence regarding the outcome measure number of antihypertensive drugs was downgraded by two levels because of study limitations (-1; risk of bias regarding selection bias, confounding assessment, confounding analysis and difference in follow-up) and number of included patients (-1; imprecision because of low sample size).
The level of evidence was therefore graded as very low.
Normokalemia
The level of evidence regarding the outcome measure normokalemia was downgraded by three levels because of study limitations (-1; risk of bias regarding confounding assessment and analysis), conflicting results (-1; inconsistency because of clinical and methodological heterogeneity) and number of included patients (-1; imprecision because of low sample size). The level of evidence was therefore graded as very low.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What are the advantages and disadvantages of surgery versus medication for the treatment of patients with M. Conn (primary aldosteronism) on blood pressure control, number of antihypertensive drugs, normokalemia, (long-term) cardiovascular morbidity and mortality and quality of life.
|
P (Patients) |
Patients with primary aldosteronism (M. Conn) |
|
I (Intervention) |
Adrenal surgery |
|
C (Control) |
Medication |
|
O (Outcomes) |
Blood pressure control, number of antihypertensive drugs, normokalemia, (long-term) cardiovascular morbidity and mortality, cardiovascular events and quality of life |
Relevant outcome measures
The guideline development group considered blood pressure control, cardiovascular morbidity and mortality, cardiovascular events and quality of life as a critical outcome measure for decision making and number of anti-hypertensive drugs and normokalemia as an important outcome measure for decision making.
The guideline development group defined the outcome measures as follows:
- Blood pressure control: Systolic and diastolic blood pressure.
- Number of antihypertensive drugs: daily defined dose of drugs to control hypertension.
- Normokalemia: Normalization of potassium levels in the blood.
A priori, the working group did not define the outcome measures cardiovascular morbidity and mortality, cardiovascular events and quality of life, but used the definitions used in the studies.
The working group defined the following differences as a minimal clinically (patient) important difference:
Dichotomous outcomes (absolute difference)
- Normokalemia: ³5%
- Cardiovascular morbidity ³1%
- Cardiovascular mortality: ³1%
- Cardiovascular events: ³5%
Continuous outcomes (mean difference):
- Blood pressure control: 10mmHg
- Number of antihypertensive drugs: daily defined dose of >1
- Quality of life: EQ5D MCID: 0.18 and SF-36: 5.00 (Coretti, 2014; Ogura, 2020)
Search and select (Methods)
Initially the databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 15 February 2022. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 114 hits. Studies were selected based on the following criteria:
- The study population had to meet the criteria as defined in the PICO;
- The intervention and comparison had to be as defined in the PICO;
- One or more reported outcomes had to be as defined in the PICO;
- Research type: Systematic review, randomized-controlled trial, prospective or retrospective observational cohort studies;
- Articles written in English or Dutch
Seven studies were initially selected based on title and abstract screening. After reading the full text, five studies were excluded (see the table with reasons for exclusion under the tab Methods). The review of Satoh (2019) and an RCT (Velema, 2018) were included. An update of the search was performed to search for observational studies after the search date of Satoh (2019) (24 August 2017). The updated systematic literature search resulted in another 542 hits. Twenty-seven studies were selected based on title and abstract screening and fifteen studies were excluded (see the table with reasons for exclusion under the tab Methods), twelve additional studies were included.
Results
One systematic review, one RCT and twelve additional cohort studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Ahmed AH, Gordon RD, Sukor N, Pimenta E, Stowasser M. Quality of life in patients with bilateral primary aldosteronism before and during treatment with spironolactone and/or amiloride, including a comparison with our previously published results in those with unilateral disease treated surgically. J Clin Endocrinol Metab. 2011 Sep;96(9):2904-11. doi: 10.1210/jc.2011-0138. Epub 2011 Jul 21. PMID: 21778218.
- Araujo-Castro M, Paja Fano M, González Boillos M, Pla Peris B, Pascual-Corrales E, García Cano AM, Parra Ramírez P, Rojas-Marcos PM, Ruiz-Sanchez JG, Vicente Delgado A, Gómez Hoyos E, Ferreira R, García Sanz I, Díaz Guardiola P, García González JJ, Perdomo CM, Morales M, Hanzu FA. Evolution of the cardiometabolic profile of primary hyperaldosteronism patients treated with adrenalectomy and with mineralocorticoid receptor antagonists: results from the SPAIN-ALDO Registry. Endocrine. 2022 Jun;76(3):687-696. doi: 10.1007/s12020-022-03029-4. Epub 2022 Mar 11. PMID: 35275344.
- Buffolo F, Cavaglià G, Burrello J, Amongero M, Tetti M, Pecori A, Sconfienza E, Veglio F, Mulatero P, Monticone S. Quality of life in primary aldosteronism: A prospective observational study. Eur J Clin Invest. 2021 Mar;51(3):e13419. doi: 10.1111/eci.13419. Epub 2020 Oct 14. PMID: 32997795.
- Catena C, Colussi G, Lapenna R, Nadalini E, Chiuch A, Gianfagna P, Sechi LA. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007 Nov;50(5):911-8. doi: 10.1161/HYPERTENSIONAHA.107.095448. Epub 2007 Sep 24. PMID: 17893375.
- Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008 Jan 14;168(1):80-5. doi: 10.1001/archinternmed.2007.33. PMID: 18195199.
- Catena C, Colussi GL, Marzano L, Sechi LA. Predictive factors of left ventricular mass changes after treatment of primary aldosteronism. Horm Metab Res. 2012 Mar;44(3):188-93. doi: 10.1055/s-0032-1301902. Epub 2012 Feb 20. PMID: 22351477.
- Chang YH, Chung SD, Wu CH, Chueh JS, Chen L, Lin PC, Lin YH, Huang KH, Wu VC, Chu TS; TAIPAI Study Group. Surgery decreases the long-term incident stroke risk in patients with primary aldosteronism. Surgery. 2020 Feb;167(2):367-377. doi: 10.1016/j.surg.2019.08.017. Epub 2019 Oct 29. PMID: 31676114.
- Chen YY, Lin YH, Huang WC, Chueh E, Chen L, Yang SY, Lin PC, Lin LY, Lin YH, Wu VC, Chu TS, Wu KD. Adrenalectomy Improves the Long-Term Risk of End-Stage Renal Disease and Mortality of Primary Aldosteronism. J Endocr Soc. 2019 Mar 25;3(6):1110-1126. doi: 10.1210/js.2019-00019. PMID: 31086833; PMCID: PMC6507624.
- Chen YL, Xu TY, Xu JZ, Zhu LM, Li Y, Wang JG. A Prospective Comparative Study on Cardiac Alterations After Surgery and Drug Treatment of Primary Aldosteronism. Front Endocrinol (Lausanne). 2021 Nov 11;12:770711. doi: 10.3389/fendo.2021.770711. PMID: 34867814; PMCID: PMC8632631.
- Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res. 2014 Apr;14(2):221-33. doi: 10.1586/14737167.2014.894462. PMID: 24625040.
- Dekkers T, Prejbisz A, Kool LJS, Groenewoud HJMM, Velema M, Spiering W, Kołodziejczyk-Kruk S, Arntz M, Kądziela J, Langenhuijsen JF, Kerstens MN, van den Meiracker AH, van den Born BJ, Sweep FCGJ, Hermus ARMM, Januszewicz A, Ligthart-Naber AF, Makai P, van der Wilt GJ, Lenders JWM, Deinum J; SPARTACUS Investigators. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016 Sep;4(9):739-746. doi: 10.1016/S2213-8587(16)30100-0. Epub 2016 Jun 17. PMID: 27325147.
- Fourkiotis V, Vonend O, Diederich S, Fischer E, Lang K, Endres S, Beuschlein F, Willenberg HS, Rump LC, Allolio B, Reincke M, Quinkler M; Mephisto Study Group. Effectiveness of eplerenone or spironolactone treatment in preserving renal function in primary aldosteronism. Eur J Endocrinol. 2012 Dec 10;168(1):75-81. doi: 10.1530/EJE-12-0631. PMID: 23033260.
- Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, Boscaro M. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007 Jan;25(1):177-86. doi: 10.1097/HJH.0b013e3280108e6f. PMID: 17143190.
- Haze T, Hirawa N, Yano Y, Tamura K, Kurihara I, Kobayashi H, Tsuiki M, Ichijo T, Wada N, Katabami T, Yamamoto K, Oki K, Inagaki N, Okamura S, Kai T, Izawa S, Yamada M, Chiba Y, Tanabe A, Naruse M. Association of aldosterone and blood pressure with the risk for cardiovascular events after treatments in primary aldosteronism. Atherosclerosis. 2021 May;324:84-90. doi: 10.1016/j.atherosclerosis.2021.03.033. Epub 2021 Mar 29. PMID: 33831673.
- Holaj R, Rosa J, Zelinka T, Štrauch B, Petrák O, Indra T, Šomlóová Z, Michalský D, Novák K, Wichterle D, Widimský J Jr. Long-term effect of specific treatment of primary aldosteronism on carotid intima-media thickness. J Hypertens. 2015 Apr;33(4):874-82; discussion 882. doi: 10.1097/HJH.0000000000000464. PMID: 25490707; PMCID: PMC4354456.
- Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal Outcomes in Medically and Surgically Treated Primary Aldosteronism. Hypertension. 2018 Sep;72(3):658-666. doi: 10.1161/HYPERTENSIONAHA.118.11568. PMID: 29987110; PMCID: PMC6202119.
- Indra T, Holaj R, Štrauch B, Rosa J, Petrák O, Šomlóová Z, Widimský J Jr. Long-term effects of adrenalectomy or spironolactone on blood pressure control and regression of left ventricle hypertrophy in patients with primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2015 Dec;16(4):1109-17. doi: 10.1177/1470320314549220. Epub 2014 Sep 30. PMID: 25271250.
- Iwakura Y, Morimoto R, Kudo M, Ono Y, Takase K, Seiji K, Arai Y, Nakamura Y, Sasano H, Ito S, Satoh F. Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: renal outcome of 213 cases. J Clin Endocrinol Metab. 2014 May;99(5):1593-8. doi: 10.1210/jc.2013-2180. Epub 2013 Nov 27. PMID: 24285678.
- Katabami T, Fukuda H, Tsukiyama H, Tanaka Y, Takeda Y, Kurihara I, Ito H, Tsuiki M, Ichijo T, Wada N, Shibayama Y, Yoshimoto T, Ogawa Y, Kawashima J, Sone M, Inagaki N, Takahashi K, Fujita M, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Fujii Y, Yamamoto K, Ogo A, Yanase T, Suzuki T, Naruse M; JPAS/JRAS Study Group. Clinical and biochemical outcomes after adrenalectomy and medical treatment in patients with unilateral primary aldosteronism. J Hypertens. 2019 Jul;37(7):1513-1520. doi: 10.1097/HJH.0000000000002070. PMID: 31145370.
- Künzel HE, Apostolopoulou K, Pallauf A, Gerum S, Merkle K, Schulz S, Fischer E, Brand V, Bidlingmaier M, Endres S, Beuschlein F, Reincke M. Quality of life in patients with primary aldosteronism: gender differences in untreated and long-term treated patients and associations with treatment and aldosterone. J Psychiatr Res. 2012 Dec;46(12):1650-4. doi: 10.1016/j.jpsychires.2012.08.025. Epub 2012 Sep 25. PMID: 23017810.
- Meng X, Ma WJ, Jiang XJ, Lu PP, Zhang Y, Fan P, Cai J, Zhang HM, Song L, Wu HY, Zhou XL, Lou Y. Long-term blood pressure outcomes of patients with adrenal venous sampling-proven unilateral primary aldosteronism. J Hum Hypertens. 2020 Jun;34(6):440-447. doi: 10.1038/s41371-019-0241-8. Epub 2019 Sep 5. PMID: 31488861.
- Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013 Dec;98(12):4826-33. doi: 10.1210/jc.2013-2805. Epub 2013 Sep 20. PMID: 24057288.
- Murck H, Adolf C, Schneider A, Schlageter L, Heinrich D, Ritzel K, Sturm L, Quinkler M, Beuschlein F, Reincke M, Künzel H. Differential effects of reduced mineralocorticoid receptor activation by unilateral adrenalectomy vs mineralocorticoid antagonist treatment in patients with primary aldosteronism - Implications for depression and anxiety. J Psychiatr Res. 2021 May;137:376-382. doi: 10.1016/j.jpsychires.2021.02.064. Epub 2021 Mar 13. PMID: 33761426.
- Nakamaru R, Yamamoto K, Akasaka H, Rakugi H, Kurihara I, Yoneda T, Ichijo T, Katabami T, Tsuiki M, Wada N, Yamada T, Kobayashi H, Tamura K, Ogawa Y, Kawashima J, Inagaki N, Fujita M, Watanabe M, Kamemura K, Okamura S, Tanabe A, Naruse M; JPAS/JRAS Study Group. Age-stratified comparison of clinical outcomes between medical and surgical treatments in patients with unilateral primary aldosteronism. Sci Rep. 2021 Mar 25;11(1):6925. doi: 10.1038/s41598-021-86290-3. PMID: 33767283; PMCID: PMC7994572.
- Ogura K, Yakoub MA, Christ AB, Fujiwara T, Nikolic Z, Boland PJ, Healey JH. What Are the Minimum Clinically Important Differences in SF-36 Scores in Patients with Orthopaedic Oncologic Conditions? Clin Orthop Relat Res. 2020 Sep;478(9):2148-2158. doi: 10.1097/CORR.0000000000001341. PMID: 32568896; PMCID: PMC7431256.
- Park KS, Kim JH, Yang YS, Hong AR, Lee DH, Moon MK, Choi SH, Shin CS, Kim SW, Kim SY. Outcomes analysis of surgical and medical treatments for patients with primary aldosteronism. Endocr J. 2017 Jun 29;64(6):623-632. doi: 10.1507/endocrj.EJ16-0530. Epub 2017 Apr 29. PMID: 28458337.
- Puar TH, Loh LM, Loh WJ, Lim DST, Zhang M, Tan PT, Lee L, Swee DS, Khoo J, Tay D, Tan SY, Zhu L, Gani L, King TF, Kek PC, Foo RS. Outcomes in unilateral primary aldosteronism after surgical or medical therapy. Clin Endocrinol (Oxf). 2021 Feb;94(2):158-167. doi: 10.1111/cen.14351. Epub 2020 Oct 26. PMID: 33058182.
- Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013 Jul;62(1):62-9. doi: 10.1161/HYPERTENSIONAHA.113.01316. Epub 2013 May 6. Erratum in: Hypertension. 2014 Dec;64(6):e7. PMID: 23648698.
- Satoh M, Maruhashi T, Yoshida Y, Shibata H. Systematic review of the clinical outcomes of mineralocorticoid receptor antagonist treatment versus adrenalectomy in patients with primary aldosteronism. Hypertens Res. 2019 Jun;42(6):817-824. doi: 10.1038/s41440-019-0244-4. Epub 2019 Apr 5. PMID: 30948836.
- Sechi LA, Di Fabio A, Bazzocchi M, Uzzau A, Catena C. Intrarenal hemodynamics in primary aldosteronism before and after treatment. J Clin Endocrinol Metab. 2009 Apr;94(4):1191-7. doi: 10.1210/jc.2008-2245. Epub 2009 Jan 13. PMID: 19141581; PMCID: PMC2682479.
- Tan YK, Kwan YH, Teo DCL, Velema M, Deinum J, Tan PT, Zhang M, Khoo JJC, Loh WJ, Gani L, King TFJ, Tan EJH, Soh SB, Au VSC, Tay TL, Dacay LMQ, Ng KS, Wong KM, Wong ASY, Ng FC, Aw TC, Chan YHB, Tong KL, Lee SSG, Chai SC, Puar THK. Improvement in quality of life and psychological symptoms after treatment for primary aldosteronism: Asian Cohort Study. Endocr Connect. 2021 Jul 26;10(8):834-844. doi: 10.1530/EC-21-0125. PMID: 34223820; PMCID: PMC8346187.
- Wada N, Shibayama Y, Umakoshi H, Ichijo T, Fujii Y, Kamemura K, Kai T, Sakamoto R, Ogo A, Matsuda Y, Fukuoka T, Tsuiki M, Suzuki T, Naruse M. Hyperkalemia in both surgically and medically treated patients with primary aldosteronism. J Hum Hypertens. 2017 Oct;31(10):627-632. doi: 10.1038/jhh.2017.38. Epub 2017 May 25. PMID: 28540931.
- Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF Jr, Gomez-Sanchez CE, Funder JW, Reincke M; Primary Aldosteronism Surgery Outcome (PASO) investigators. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017 Sep;5(9):689-699. doi: 10.1016/S2213-8587(17)30135-3. Epub 2017 May 30. PMID: 28576687; PMCID: PMC5572673.
- Wu VC, Kuo CC, Wang SM, Liu KL, Huang KH, Lin YH, Chu TS, Chang HW, Lin CY, Tsai CT, Lin LY, Chueh SC, Kao TW, Chen YM, Chiang WC, Tsai TJ, Ho YL, Lin SL, Wang WJ, Wu KD; TAIPAI Study Group. Primary aldosteronism: changes in cystatin C-based kidney filtration, proteinuria, and renal duplex indices with treatment. J Hypertens. 2011 Sep;29(9):1778-86. doi: 10.1097/HJH.0b013e3283495cbb. PMID: 21738054.
- Wu VC, Wang SM, Chang CH, Hu YH, Lin LY, Lin YH, Chueh SC, Chen L, Wu KD. Long term outcome of Aldosteronism after target treatments. Sci Rep. 2016 Sep 2;6:32103. doi: 10.1038/srep32103. Erratum in: Sci Rep. 2017 Mar 24;7:45249. PMID: 27586402; PMCID: PMC5009379.
- Zavatta G, Di Dalmazi G, Pizzi C, Bracchetti G, Mosconi C, Balacchi C, Pagotto U, Vicennati V. Larger ascending aorta in primary aldosteronism: a 3-year prospective evaluation of adrenalectomy vs. medical treatment. Endocrine. 2019 Mar;63(3):470-475. doi: 10.1007/s12020-018-1801-3. Epub 2018 Nov 14. PMID: 30430353.
Evidence tabellen
Evidence table for systematic review of RCTs and observational studies
Research question: What are the effects of surgery versus medication in patients with primary hyperaldosteronism?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Satoh, 2019
|
SR and meta-analysis of RCTs, prospective cohort studies and retrospective cohort studies
Literature search up to august 2017
A: Catena, 2008 B: Wu, 2016 C: Kunzel, 2012 D: Giacchetti, 2007 E: Catena, 2007 F: Rossi, 2013 G: Indra, 2015 H: Wu, 2011 I: Catena, 2012 J: Fourkiotis, 2013 K: Iwakura, 2014 L: Holaj, 2015 M: Park, 2017 N: Sechi, 2009 O: Ahmed, 2011 P: Mulatero, 2013
Study design: A: Prospective clinical trial B: Retrospective study C: Cross-sectional study D: Prospective clinical trial E: Prospective clinical trial F: Prospective trial G: Prospective clinical trial H: Prospective study I: Prospective study J: Prospective cohort study K: Prospective study L: Prospective study M: Retrospective study N: Prospective study O: Cohort study P: Retrospective cohort study
Setting and Country: A: Italy B: Taiwan C: Germany D: Italy E: Italy F: Italy G: Czech republic H: Taiwan I: Italy J: Germany K: Japan L: Czech republic M: South Korea N: Italy O: Australia P: Italy
Source of funding and conflicts of interest: Not reported for individual studies. The authors of the SR declare that they have no conflict of interest.
|
Inclusion criteria SR: - Comparing operative treatment with medical treatment - Study had to provide values (mean + SD) of at least one of the following variables: Left Ventricular (LV) Mass, serum potassium, systolic blood pressure (SBP), glomerular filtration ratio (GFR), number of oral antihypertensive agents or incidence of cardiovascular events - Articles published after 1985 - English articles - Studies in humans
Exclusion criteria SR: - Not specifically reported
16 studies included
Important patient characteristics at baseline:
N, mean agea A: 377 patients, 53 years B: 822 patients, 47 years C: 105 patients, 61 years D: 61 patients, 51 years E: 54 patients, 53 years F: 180 patients, 51 years G: 31 patients, 50 years H: 100 patients, 42 years I: 54 patients, 53 years J: 29 patients, 49 years K: 212 patients, 54 years L: 42 patients, 51 years M: 48 patients, 60 years N: 54 patients, 53 years O: 41 patients, age not reported P: 270 patients, 44 years
Sexa: A: 68% Male B: 44% Male C: 64% Male D: 60% Male E: 70% Male F: 57% male G: 64% male H: 42% male I: 70% male J: 59% male K: 42% male L: 57% male M: Not reported for subgroup N: 70% male O: Not reported for subgroup P: 60% male
No information on comparability of groups at baseline |
Describe intervention:
A: Adrenalectomy B: Adrenalectomy C: Adrenalectomy D: Adrenalectomy in patients with aldosterone-producing adenoma (APA) E: Unilateral adrenalectomy F: Adrenalectomy G: Adrenalectomy in patients with APA H: Adrenalectomy I: Adrenalectomy J: Unilateral adrenalectomy K: Patients with unilateral disease underwent laparoscopic adrenalectomy L: Patients with unilateral APA underwent adrenalectomy M: Adrenalectomy N: Adrenalectomy O: Adrenalectomy P: Patients with aldosterone producing adenoma underwent adrenalectomy within 3 months from the Adrenal Vein Sampling (AVS) diagnosis.
|
Describe control:
A: Treatment with spironolactone (100mg/day) B: Mineralcorticoid Receptor Antagonist (MRA) treatment C: MRA treatment D: Pharmalogical treatment of idiopathic hyperaldosteronism E: Spironolactone (50-300mg/day; average dose 121 mg/day) F: Mineralcorticoid receptor (MR) antagonist G: Spironolactone (50mg/day) H: Spironolactone (50mg/day) I: Spironolactone (50-300mg/day; average dose 121 mg/day) J: Spironolactone, eplerone or other antihypertensives K: MR antagonists L: Spironolactone M: MRA treatment N: Spironolactone (starting 100mg/day; average dose 121mg/day) O: Medical treatment with spironolactone (47%) or amiloride (43%) or both (10%) P: For patients with bilateral adrenal hyperplasia medical, MRA therapy was initiated immediately after subtype diagnosis. Dose was targeted to obtain normal potassium and BP levels in absence of side effects.
|
Duration of follow-upa:
A: 7.4 years (mean) B: 5.75 years (mean) C: 4.3 years (mean) D: 34.4 months (mean) E: 6.4 years (mean) F: 36.0 months (median)a G: 12 months H: 12 months I: 6.4 years (mean) J: 12 months K: 12 months L: 6 years M: Surgical: 3.8 years; Medical: 4.6 years N: 12 months O: 6 months P: 12 years (median)
For how many participants were no complete outcome data available? (intervention/control) Not reported in SR
|
Outcome measure-1: Systolic blood pressure Effect measure: MD (95% CI) D: -5.00 (-13.92-3.92) F: -6.00 (-11.59- -0.41) H: -2.60 (-10.38-5.18) I: -2.00 (-7.50-3.50) J: 4.00 (0.19-7.81) K: -5.00 (-9.44- -0.56) L: 1.00 (-10.19-12.19) M: 2.70 (-11.40-16.80)
Pooled effect (random effects model): MD: -1.88 (95% CI -5.16 to 1.39) favoring adrenalectomy Heterogeneity (I2): 50%
Outcome measure-2: Cardiovascular events Effect measure: RR (95% CI): A: 1.03 (0.36-2.98) B: 0.88 (0.72-1.06) C: 0.21 (0.05-0.89) P: 2.80 (0.65-12.17)
Pooled effect (random effects model): RR: 0.87 (95% CI 0.44 to 1.72) favoring adrenalectomy Heterogeneity (I2): 52%
Outcome measure-3: Number of antihypertensive agents Effect measure: MD (95% CI) F: -0.97 (-1.36- -0.58) O: -2.37 (-2.70- -2.04) L: -1.50 (-2.45- -0.55)
Pooled effect (random effects model): MD: -1.62 (95% CI -2.67 to -0.58) favoring adrenalectomy Heterogeneity (I2): 93%
Outcome measure-4: Hypokalemia Effect measure: MD (95% CI) C: 0.10 (-0.06-0.26) F: 0.12 (-0.01-0.25) H: -0.16 (-0.32-0.00) L: 0.00 (-0.21-0.21) M: 0.52 (0.21-0.83)
Pooled effect (random effects model): MD: 0.09 (95% CI -0.08 to 0.25) favoring medication Heterogeneity (I2): 77%
|
Facultative:
Brief description of author’s conclusion: Results indicate that surgery is associated with a reduced need for additional antihypertensive drugs than MR antagonist treatment in patients with PA.
Personal remarks on study quality, conclusions, and other issues (potentially) relevant to the research question: Due to heterogeneity within studies which compared adrenalectomy in APA patients and MR antagonist treatment in IHA patients, the comparison is not correct and therefore outcomes are not suitable for all PA patients.
Sensitivity analyses (excluding small studies; excluding studies with short follow-up; excluding low quality studies; relevant subgroup-analyses); mention only analyses which are of potential importance to the research question
Heterogeneity: Clinical heterogeneity due to subgroups within PA: APA and IHA: This review combined articles on APA treated with adrenalectomy and IHA treated with MR antagonist. |
|
Velema, 2018 |
Type of study: Post hoc comparative effectiveness study within the Subtyping Primary Aldosteronism: A Randomized Trail Comparing Adrenal Vein Sampling and Computed Tomography Scan (SPARTACUS) trial
Funding and conflicts of interest: The original SPARTACUS trial was supported by a grant from ZonMW Doelmatigheids Onderzoek 2010-2012 E&K (171002102) and by a grant from the Institute of Cardiology, Warsaw, Poland. The authors did not report conflict of interest
|
Inclusion criteria: - Legally capacitated - Age 18 years or older - Diagnosed with hypertension that is difficult to treat, or accompanied by hypokalemia, either spontaneous or induced by use of diuretics - Positive result on sodium loading test, i.e. insufficient suppression of aldosterone - Cooperating patients and willing to give written informed consent
Exclusion criteria: - Unsuitability for or objection to undergo AVS, CT or adrenal surgery - Pregnant - Glucocorticoid remediable aldosteronism or adrenal carcinoma - Severe or terminal co-morbidity with seriously interferes with possible treatment or HRQOL. - Requirement of certain medication that interacts with the prescribed treatments
N total at baseline: Intervention: 92 Control: 92
Important prognostic factors2: Mean age in years (SD): I: 51.8 (10.1) C: 54.4 (8.8)
Male sex: I: N=66 (71.7%) C: N=78 (84.8%)
Median Body Mass Index in kg/m2 (IQR): I: 27.5 (25.2-30.5) C: 29.4 (26.7-32.6)
Mean Serum potassium in mEq/L (SD): I: 3.5 (0.5) C: 3.6 (0.4)
Groups are not comparable at baseline
|
Describe intervention: Adrenalectomy
|
Describe control: Mineralocorticoid Receptor Antagonist (MRA) based treatment
|
Length of follow-up: 1 year
Incomplete outcome data: No incomplete outcome data
Loss-to-follow-up: No loss-to-follow-up
|
Outcome measures and effect size (include 95%CI and p-value if available):
Quality of life, SF-36 scores mean difference with reference population: Physical functioning, mean (95%CI): I: 2.5 (0.7-4.3) C: -0.4 (-2.8-2.0)
Role physical, mean (95%C(): C: -0.2 (-2.6-2.2)
Bodily pain, mean (95%CI):
General health, mean (95%CI): C: -4.4 (-6.6 – -2.2)
Vitality, mean (95%CI): I: -0.8 (-3.0-1.3)
Social functioning, mean (95%CI): I: 0.2 (-2.0-2.4) C: -3.0 (-5.6 – -0.5)
Role emotional, mean (95%CI): I: 0.7 (-1.6-2.9) C: -0.7 (-3.0-1.7)
Mental health, mean (95%CI): I: 0.8 (-1.4-3.1) C: -3.7 (-5.8 – -1.5)
Physical component summary (PCS), mean (95%CI): I: 2.4 (0.3-4.5) C: -1.5 (-4.0-0.9)
Mental component summary (MCS), mean (95%CI): I: -0.2 (-2.4 – 2.1) C: -4.0 (-6.3 – -1.8)
EQ-5D OR for reporting problems on EQ-5D dimension during follow-up according to generalized estimating equation analysis (Adrenalectomy versus MRA treatment), adjusted OR (95%CI): Mobility: 0.52 (0.23-1.20) Self-care: 0.14 (0.01-2.50) Usual activities: 0.35 (0.17-0.75) Pain/discomfort: 0.52 (0.30-0.91) Anxiety/depression: 0.79 (0.39-1.60)
|
Authors conclusion: In conclusion, QoL in PA is better 1 year after ADX than 1 year after initiation of MRAs. However, both treatment modalities improve QoL, which is relevantly impaired before treatment compared with the general population. Our findings underscore the need to identify patients with PA and support the practice to select patients who are amenable for ADX. Adrenalectomy treatment for unilateral adrenal enlargement or unilateral aldosterone hypersecretion with contralateral aldosterone suppression was demonstrated. All other patients received MRA based treatment.
All patients were further treated with conventional antihypertensive drugs according to a treatment algorithm targeting a blood pressure of <135/85 mmHg using semiautomatic device.
Study population are patients with hypertension, without adrenal carcinoma or remediable aldosteronism |
|
Araujo Castro, 2022 |
Type of study: Retrospective cohort study
Setting and country: SPAIN-ALDO registry, Spain
Funding and conflicts of interest: The research was funded by Sociedad Española de Endocrinología y Nutrición (SEEN). The authors declare no conflict of interest |
Inclusion criteria: - Patients with PA from the Spanish Primary Aldosteronism Registry of the Spanish Endocrinology and Nutrition Society (SPAIN-ALDO) who had follow-up between 2018 and 2020 - Patients who underwent adrenalectomy or were under medical treatment with MRA - Patients who had clinical, hormonal and biochemical information during follow-up
Exclusion criteria: - Patients with confirmed co-secretion of cortisol
N total at baseline: Intervention: 100 Control: 168
Important prognostic factors2: Mean age in years (SD): I: 52.7 (9.4) C: 54.7 (12.5)
Female sex: I: N=54 (54.6%) C: N=70 (41.7%)
Mean Body Mass Index in kg/m2 (SD): I: 29.1 (6.1) C: 30.0 (6.1)
Hypertension grade ³2: I: N=63 (76.8%) C: N=108 (70.6%)
Type 2 diabetes: I: N=15 (15%) C: N=33 (19.6%)
Groups are not comparable at baseline
|
Describe intervention: Adrenalectomy
|
Describe control: Mineralocorticoid Receptor Antagonist (MRA) in monotherapy or in combination with other hypertensive drugs
|
Length of follow-up: 2 years
Loss-to-follow-up: Loss-to-follow-up or incomplete outcome data were not included in the analysis of the study
|
Outcome measures and effect size (include 95%CI and p-value if available):
Systolic blood pressure, mean between treatment difference in mmHg (SD): I: -19.6 (22.5) C: -17.5 (22.7) MD -2.10 (95%CI -7.7-3.4)
Diastolic blood pressure, mean between treatment difference in mmHg (SD): I: -11.8 (14.9) C: -8.6 (15.2)
New cardiovascular events I: N=2 (3.9%) C: N=7 (6.4%) HR 0.5 (95%CI 0.1-2.2)
Number of antihypertensive drugs, mean between treatment difference (SD): I: -1.3 (1.3) C: 0.0 (1.4)
|
Authors conclusion: In patients with PA, MRA and surgery offer a similar car- diovascular, metabolic, and renal protection, in a short-term follow-up, but surgery improves biochemical control and reduces pill burden more commonly than MRA, leading to hypertension cure or improvement in up to 83% of the patients. Short follow-up period.
No correction for confounding for all outcomes. For cardiovascular events cox regression was performed but not reported which covariates were included in the analysis.
Adrenalectomy treatment for unilateral and bilateral PA |
|
Buffolo, 2020 |
Type of study: Prospective cohort study
Setting and country: QUALITY study, Italy
Funding and conflicts of interest: |
Inclusion criteria: - Patients in the QUALIty of Life study in Torino, Italy between 2017 and 2019 - Patients with primary aldosteronism (PA) confirmed by diagnosis according to the Endocrine Society guideline and ESH consensus
Exclusion criteria: Not reported
N total at baseline: Intervention: 37 Control: 30
Important prognostic factorsb: Mean age in years (SD): 52 (9)
Sex, female: N=25 (35.7%)
Mean Body Mass Index in kg/m2: 25.9 (4.1)
Type 2 diabetes: N=67 (95.7%)
Presence of comorbidity by CCI: N= 9 (12.8%)
No information on comparability of Groups at baseline
|
Describe intervention: Patients with unilateral PA underwent laparoscopic adrenalectomy
|
Describe control: Treatment with Mineralocorticoid Receptor Antagonist (MRA): Spironolactone (N=14) and potassium canrenoate (N=16)
|
Length of follow-up: 6 months
Incomplete outcome data: Intervention: N=0 (0%) Control: N=3 (10%) Reasons: No MRA medical treatment
Loss-to-follow-up: Intervention: N=1(2.7%) Reasons: Not reported
Control: N=1 (3%) Reasons: Not reported
Incomplete outcome data: No incomplete outcome data reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Systolic blood pressure, mean in mmHg (SD): I: 121 (11) C: 131 (13) MD -10.00 (95%CI -15.85- -4.15)
Diastolic blood pressure, mean in mmHg (SD): I: 78 (8) C: 83 (8) MD -5.00 (95%CI -8.85- -1.15)
Quality of life, SF-36 scores: Physical functioning, mean (SD): I: 94.4 (9.6) C: 92.2 (9.2)
Role limitations due to physical health problems, mean (SD): I: 91.4 (25.1) C: 91.4 (22.4)
Role limitations due to emotional problems, mean (SD): I: 91.7 (26.8) C: 81.6 (37.4)
Vitality, mean (SD): I: 69.4 (21.1) C: 65.7 (17.9)
General mental health, mean (SD): I: 77.9 (16.6) C: 79.1 (13.6)
Social functioning, mean (SD): I: 87.3 (14.3) C: 78.6 (18.9)
Bodily Pain, mean (SD): I: 87.5 (17.9) C: 84.0 (20.0)
General health perceptions, mean (SD): I: 70.3 (22.1) C: 63.1 (16.4)
Number of antihypertensive drugs, mean daily defined dose: I: 0.94 (1.26) C: 3.44 (1.43)
|
Study included patients with primary aldosteronism and matched controls with essential hypertension. Regarding this study and analysis, we only reported outcome data regarding adrenalectomy versus MRA therapy.
Patients who underwent laparoscopic adrenalectomy were only patients diagnosed with unilateral PA, MRA treatment was for unilateral (N=6) and bilateral PA (N=24). |
|
Chen, 2021 |
Type of study: Prospective cohort study
Setting and country: Shanghai, China Funding and conflicts of interest: The present study was financially supported by the Shanghai Municipal Commission of Health. Researchers were also financially supported by grants from the National Natural Science Foundation of China, Ministry of Science and Technology, Ministry of Health, Beijing, China, and the Shanghai Commissions of Science and Technology, Education and Health.
|
Inclusion criteria: - Patients with hypertension and primary aldosteronism who are surgically or medically treated in inpatient ward - In ward between November 2018 to July 2020
Exclusion criteria: - Patients with other forms of secondary hypertension, ischemic heart disease, valvular heart disease, cardiomyopathy, pacemaker implantation, atrial fibrillation or suboptimal echocardiographic windows
N total at baseline: Intervention: 39 Control: 28
Important prognostic factors2: Mean age in years (SD): I: 49.4 (10.2) C: 48.8 (11.5)
Male sex: I: N=26 (66.7%) C: N=22 (71.4%)
Mean Body Mass Index in kg/m2 (SD): I: 25.7 (3.3) C: 26.8 (2.4)
Median plasma Renin activity in ng/ml/h (IQR): I: 0.27 (0.14-0.47) C: 0.84 (0.31-1.2)
Mean serum potassium concentration in mmol/L (SD): I: 3.3 (0.4) C: 3.5 (0.4)
Groups were not comparable at baseline
|
Describe intervention: Unilateral adrenalectomy
|
Describe control: Treatment with mineralocortoid receptor antagonist
|
Length of follow-up: 6 months
Loss-to-follow-up: No loss-to-follow-up reported
Incomplete outcome data: No incomplete outcome data reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Systolic blood pressure, mean between treatment difference in mmHg (SD): I: -9.1 (2.0) C: -10.3 (2.4) MD: 1.2 (95%CI 0.11-2.29)
Diastolic blood pressure, mean between treatment difference in mmHg (SD): I: -1.7 (1.3) C: -3.4 (1.6) MD: 1.7 (95%CI 0.98-2.42)
|
Authors conclusion: Our study demonstrated that the surgical treatment with adrenalectomy had an effect of early regression of cardiac structure and definite improvement of cardiac function, although both surgery and drug treatment significantly reduced blood pressure and normalized serum potassium concentration. Intervention group consisted of 33 patients with aldosterone-producing adenoma and 6 with idiopathic hyperaldosteronism. Medication group consisted of 13 patients with bilateral primary aldosteronism and 15 with clinical requirement |
|
Haze, 2021 |
Type of study: Retrospective cohort study
Setting and country: Japan Rare/Intractable Adrenal Diseases Study (JRAS)
Funding and conflicts of interest: This study was conducted as a part of the JRAS by a Research Grant from the Japan Agency for Medical Research and Development The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
|
Inclusion criteria: - Patients aged 20-90 years - Enrolled in JRAS between 2006 and 2019 - PA diagnosed based on the guidelines of the Japan Endocrine Society and Japanese Society of Hypertension - Patients records who included assessments of plasma aldosterone concentration, plasma renin activity and blood pressure before treatment - Patients who underwent adrenalectomy or was initiated on MRA treatment between month 0 and 6 - Patients who had been observed for more than 6 months from baseline
Exclusion criteria: Not reported
N total at baseline: Intervention: 740 Control: 1247
Important prognostic factors2: Mean age in years (SD): I: 51.5 (11.7) C: 54.2 (10.5)
Female sex: I: N=368 (49.7%) C: N=666 (53.4%)
Mean Body Mass Index in kg/m2 (SD): I: 24.2 (4.1) C: 25.1 (4.1)
Mean duration of hypertension in years (SD): I: 10.1 (9.1) C: 8.0 (8.7)
History of diabetes: I: N=121 (16.4%) C: N=182 (14.6%)
Groups are not comparable at baseline
|
Describe intervention: Adrenalectomy for patients with a lateralized form of primary aldosteronism
|
Describe control: Treatment with mineralocortoid receptor antagonist (MRA)
|
Length of follow-up: Median follow-up period in days (IQR): I: 1048 (475-1901) C: 1126 (412-1855)
Loss-to-follow-up: No loss-to-follow-up reported
Incomplete outcome data: Systolic blood pressure N=247 (12.4%)
Diastolic blood pressure N=262 (13.2%)
No reason(s) reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Mean systolic blood pressure in mmHg (SD): I: 129 (15) C: 133 (15) MD: -4.00 (95%CI -5.36- -2.64)
Mean diastolic blood pressure in mmHg (SD): I: 82 (11) C: 83 (11) MD: -1.00 (95%CI -2.00 – 0.00)
|
Primary analysis was association between aldosterone to renin ratio (ARR) and pulse pressure with composite cardiovascular disease events only unadjusted data regarding blood pressure was used for this summary. |
|
Katabami, 2019 |
Type of study: Retrospective cohort study
Setting and country: Japan Primary Aldosteronism Study (JPAS)
Funding and conflicts of interest: This research was supported by grants-in-aid from the Practical Research Project for Rare/Intractable Diseases, funded by the Japan Agency for Medical Research and Development, AMED, Japan . This study was also supported by a grant from Ministry of Health, Labor, and Welfare, Japan.
|
Inclusion criteria: - Patients enrolled in the JPAS between January 2006 and October 2016 with primary aldosteronism with confirmed unilateral subtype
Exclusion criteria: - Bilateral subtype - Unsuccessful adrenal vein sampling (AVS) - AVS without adrenocorticotropic hormone stimulation - Missing follow-up data - Incomplete data on blood pressure and/or antihypertensive drugs - Patients in medically treated group who missed out on receiving MRAs
N total at baseline: Intervention: 63 Control: 276
Important prognostic factors2: Median age in years (IQR): I: 54.0 (42.4-61.0) C: 60.0 (54.0-64.0)
Female sex: I: N=127 (46%) C: N=20 (32%)
Median duration of hypertension in years (IQR): I: 9.0 (4.0-17.0) C: 12.5 (4.0-20.0)
Median serum potassium level in mmol/liter (IQR): I: 3.4 (3.0-3.7) C: 3.5 (3.1-3.8)
Diabetes mellitus I: N=47 (17.3%) C: N=13 (20.6%)
Groups are not comparable at baseline
|
Describe intervention: Unilateral adrenalectomy
|
Describe control: Treatment with mineralocortoid receptor antagonist for unilateral PA
|
Length of follow-up: 6 months
Loss-to-follow-up: Patients with missing data were excluded
Incomplete outcome data: Patients with incomplete outcome data regarding blood pressure and/or antihypertensive drugs were excluded
|
Outcome measures and effect size (include 95%CI and p-value if available):
Systolic blood pressure, median between treatment difference in mmHg (IQR): I: -9.0 (-22.0 – -3.0) C: -5.0 (-25.0 – 6.0)
Diastolic blood pressure, median between treatment difference in mmHg (IQR): I: -4.5 (-12.0 – 7.0) C: -7.0 (-13.0 – 4.0)
Serum potassium normalization rate: I: N=50 (90.9%) C: N=55 (100%)
Daily defined dose of antihypertensive drugs, median between treatment difference (IQR): I: -1.0 (-1.9 – 0.0) C: 0.5 (-0.1 – 2.0) |
Authors conclusion: The current study provides further evidence that AdX is the first choice of treatment in the patients with unilateral primary aldosteronism in terms of clinical and biochemical outcome. The superior effects of AdX on hyper- tension and hypokalemia should contribute to a better long- term prognosis in unilateral primary aldosteronism. Only participants with unilateral subtype of PA were included in this study.
Only propensity matched score outcomes were used for the analysis regarding the summary (I: N=55, C: N=55). |
|
Meng, 2019 |
Type of study: Retrospective cohort study
Setting and country: Fuwai Hospital, China
Funding and conflicts of interest: This work was supported by the National Key Research and Development Plan of China, the CAMS Innovation Fund for Medical Science and the PUMC Youth Fund. Authors declare they have no conflict of interest
|
Inclusion criteria: - Patients who were hospitalized at Fuwai hospital from 1 January 2016 to 31 december 2017 - Proven unilateral primary aldosteronism - Undergone successful adrenal vein sampling in Fuwai hospital
Exclusion criteria: Not reported
N total at baseline: Intervention: 21 Control: 30
Important prognostic factors2: Mean age in years (SD): I: 44.63 (10.55) C: 50.53 (12.45)
Female sex: I: N=12 (57.1%) C: N=10 (33.3%)
Mean Body Mass Index in kg/m2 (SD): I: 24.21 (3.78) C: 26.99 (3.16)
Mean duration of hypertension in years (SD): I: 8.3 (7.46) C: 13.60 (12.61)
Mean serum potassium level in mmol/liter (SD): I: 3.24 (0.44) C: 3.42 (0.53)
Diabetes mellitus: I: N=5 (16.7%) C: N=0 (0%)
Groups are not comparable at baseline
|
Describe intervention: Total or partial laparoscopic adrenalectomy (decision by experienced urologist). Spironolactone with or without other antihypertensive agents was administered for preoperative blood pressure control.
|
Describe control: Mineralocortoid receptor antagonist (MRA) treatment started with 60 milligram spironolactone. The dose of spironolactone was reduced when serum potassium level progressively increased or reached >5.0 mmol per liter. Eplerenone, the acknowledged substitute for spironolactone is not registered in China.
|
Length of follow-up: Mean follow-up in months (SD): I: 22.05 (6.26) C: 20.57 (4.63)
Loss-to-follow-up: No loss-to-follow-up reported
Incomplete outcome data: No incomplete outcome data reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Mean systolic blood pressure in mmHg (SD): I: 120.3 (12.99) C: 133.54 (16.60) MD: -13.24 (95%CI -21.37 - -5.11)
Mean diastolic blood pressure in mmHg (SD): I: 79.00 (7.62) C: 87.35 (12.36) MD: -8.35 (95%CI -13.84 - -2.86)
Mean number of antihypertensive drugs (SD): I: 0.19 (0.51) C: 2.33 (0.78)
Hypokalemia: I: N=0 (0%) |
Authors conclusion: The cure rate of hypertension in patients with AVS-proven unilateral PA who underwent laparoscopic adrenalectomy was higher than that in patients who underwent medical treatment.
Only patients with unilateral PA were included in this study |
|
Murck, 2021 |
Type of study: Retrospective cohort study
Setting and country: German Conn Registry Funding and conflicts of interest: This work was supported by the Else Kro ̈ner-Fresenius Stiftung in support of the German Conns Registry-Else-Kröner Hyperaldosteronism Registry, the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme and by the Deutsche Forschungsgemeinschaft (DFG). The authors report no conflicts of interest in this work. HM is owner of Murck-Neuroscience LLC and holds a patent in the area of treatment refractory depression.
|
Inclusion criteria: - Patients with newly diagnosed PA
Exclusion criteria: Not reported
N total at baseline: Intervention: 75 Control: 90
Important prognostic factors2: Female Sex: N=82 (39%)
No information on comparability of Groups at baseline
|
Describe intervention: Adrenalectomy in case of a unilateral tumor
|
Describe control: Mineralocortoid receptor antagonist (MRA) treatment, mainly spironolactone (25-50 milligram per day) for bilateral hyperplasia of the adrenal gland
|
Length of follow-up: 1 year
Loss-to-follow-up: No loss-to-follow-up regarding blood pressure outcome
Incomplete outcome data: No reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Mean 24-hour systolic blood pressure in mmHg (SD): I: 136.9 (14.1) C: 135.91 (13.9) MD: 0.99 (95%CI -3.31 – 5.29)
Mean 24-hour diastolic blood pressure in mmHg (SD): I: 85.56 (10.2) C: 84.60 (8.3) MD: 0.96 (95%CI -1.91 – 3.84) |
Study invested four different groups: Male and female after surgical or medical treatment. Regarding this study and analysis, we only reported outcome data regarding adrenalectomy versus MRA therapy.
|
|
Nakamaru, 2021 |
Type of study: Retrospective cohort study
Setting and country: Japan Primary Aldosteronism Study (JPAS) Funding and conflicts of interest: This study was supported in part by grants-in-aid for the Japan Primary Aldosteronism Study and the Japan Rare Adrenal Diseases Study from the Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development and grants from the National Center for Global Health and Medicine, Japan. The authors declared no competing interests.
|
Inclusion criteria: - Patients aged between 20 and 90 years with PA - Underwent adrenal vein sampling (AVS)
Exclusion criteria: - No follow-up data regarding blood pressure or estimated glomerular filtration rate
N total at baseline: Intervention: 622 Control: 233
Important prognostic factors2: Female sex: I: N=300 (45%) C: N=98 (42%)
No information on comparability of Groups at baseline
|
Describe intervention: Adrenalectomy
|
Describe control: Mineralocortoid receptor antagonist (MRA) treatment
|
Length of follow-up: 36 months
Loss-to-follow-up: Not reported
Incomplete outcome data: Regarding outcome number of cardiovascular events: I: N=33 (5.3%) C: N=10 (4.3%)
Regarding outcome hyperkalemia: I: N=14 (2.3%) C: N=2 (0.8%)
No reason(s) reported |
Outcome measures and effect size (include 95%CI and p-value if available):
Number of cardiovascular events: I: N=12 (2%) C: N=11 (4.9%)
Hyperkalemia I: N=43 (7%) |
Study stratified data according to age (< 65 years versus ³ 65 years) therefore not all baseline data was available regarding the scope of this summary. |
|
Puar, 2020 |
Type of study: Retrospective cohort study
Setting and country: Two referral centres, Singapore
Funding and conflicts of interest: This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. TH Puar was supported by the Singapore National Medical Research Council Research Training Fellowship award. RS Foo was supported by Singapore National Medical Research Council and A*Star Biomedical Research Council.
|
Inclusion criteria: - Patients with PA fulfilling diagnostic criteria recommended by the Endocrine Society guidelines - Managed at referral centre between 2000 and 2019 - Confirmed unilateral PA by adrenal vein sampling - Likely unilateral PA by clinical prediction score (APR)
Exclusion criteria: - Patients without adequate follow-up for at least six months post-treatment - Patients with baseline estimated glomerular filtration rate (eGFR) < 45 ml/min/1.73m2
N total at baseline: Intervention: 86 Control: 68
Important prognostic factors2: Mean age in years (SD): I: 51.0 (10.1) C: 55.0 (9.1)
Female sex: I: N=37 (43.0%) C: N=22 (32.4%)
Mean Body Mass Index in kg/m2 (SD): I: 26.1 (4.5) C: 26.0 (3.8)
Median duration of hypertension in years (IQR): I: 7 (3-11) C: 8 (5-10)
Mean estimated glomerular filtration rate in ml/min per 1.73m2 (SD): I: 90.7 (19.4) C: 83.8 (18.2)
Diabetes mellitus: I: N=19 (22.1%) C: N=23 (33.8%)
Groups are not comparable at baseline
|
Describe intervention: Unilateral adrenalectomy
|
Describe control: Mineralocortoid receptor antagonist (MRA) treatment with spironolactone, eplerenone or amiloride
|
Length of follow-up: Mean duration of follow-up (SD): 5.7 years (4.5)
Loss-to-follow-up: Not reported
Incomplete outcome data: N=12 from MRA treatment group classified in surgery group
Reason(s): Opting for surgery in view of medication side effects or patient preference
|
Outcome measures and effect size (include 95%CI and p-value if available):
Mean systolic blood pressure in mmHg (SD): I: 134.4 (14.1) C: 140.0 (19.2)
Mean change in systolic blood pressure in mmHg (SD): I: -17.3 (20.3) C: 12.1 (22.5)
Mean diastolic blood pressure in mmHg (SD): C: 83.3 (11.4)
Mean change in diastolic blood pressure in mmHg (SD): C: -2.9 (12.2)
Composite cardiovascular events: HR 0.93 (95%CI 0.32-2.67)
Median number of anti-hypertensive drugs (IQR): I: 2.0 (1.0-3.0) C: 1.0 (0.0-2.0)
Median change in anti-hypertensive drugs (IQR): I: -1.0 (-2.0-0.0) C: 0.0 (-1.0-1.0)
|
Authors conclusion: We demonstrated that in patients with confirmed or likely unilateral PA, who are averse to surgery and are tolerant of medications, medical therapy may provide a viable treatment option. Medical therapy improves BP and biochemical control and may offer similar cardiovascular protection. This underscores the importance of diagnosing PA and instituting targeted therapy with MR antagonists. Surgery should be the first-line treatment for unilateral PA as it re- duces pill burden and offers the opportunity for cure of hypertension altogether. Patients with (likely) confirmed unilateral PA
|
|
Tan, 2021 |
Type of study: Prospective cohort study
Setting and country: Changi General Hospital
Funding and conflicts of interest: This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported
|
Inclusion criteria: - Patients of age 18 years or older - Confirmed diagnosis of PA in accordance with the 2016 Endocrine Society Guidelines - Completion of baseline questionnaires
Exclusion criteria: - Patients with adrenal carcinoma, severe or terminal co-morbidity that interfered with possible treatment or HRQoL or glucocorticoid-remediable aldosteronism
N total at baseline: Intervention: 21 Control: 13
Important prognostic factors2: Mean age in years (SD): I: 48.1 (11.4) C: 56.4 (9.6)
Female sex: I: N=8 (38.1%) C: N=2 (15.4%)
Mean Body Mass Index in kg/m2 (SD): I: 26.9 (5.3) C: 28.6 (5.6)
Median duration of hypertension in years (IQR): I: 8.0 (0-23.0) C: 20.0 (3.0-37.0)
Mean estimated glomerular filtration rate in ml/min per 1.73m2 (SD): I: 88.45 (20.3) C: 88.8 (16.9)
Diabetes mellitus: I: N=7 (33.3%) C: N=5 (38.5%)
Groups are not comparable at baseline
|
Describe intervention: Unilateral adrenalectomy via a minimally invasive transabdominal laparoscopic approach.
|
Describe control: Medical therapy
|
Length of follow-up: 1 year
Loss-to-follow-up: Not reported
Incomplete outcome data: SF-36 I: N=0 C: N=1 (9.1%)
EQ-5D I: N=1 (4.7%) C: N=1 (7.7%)
Reason(s): Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Quality of life, SF-36 scores: Physical functioning, mean (SD): I: 91.4 (12.5) C: 86.0 (18.7) MD 5.40 (95%CI -7.55 – 18.35)
Role physical, mean (SD): C: 77.5 (38.1) MD 16.90 (95%CI -7.87-41.67)
Bodily pain, mean (SD): MD 1.30 (-11.55-14.15)
General health, mean (SD): C: 66.5 (21.2) MD 9.30 (95%CI -4.94-23.54)
Vitality, mean (SD): I: 75.3 (10.8) MD 5.80 (95%CI -11.01-22.61)
Social functioning, mean (SD): I: 91.0 (14.1) C: 87.5 (16.7) MD 3.50 (95%CI -8.73-15.73)
Role emotional, mean (SD): I: 94.4 (17.2) C: 93.3 (21.1) MD 1.10 (95%CI -14.20-16.40)
Mental health, mean (SD): I: 84.4 (14.8) C: 83.2 (14.9) MD 1.20 (95%CI -10.29-12.69)
EQ-5D index score, median (IQR): I: 1.00 (1.00-1.00) C: 1.0 (0.838-1.0)
|
Authors conclusion: In conclusion, we found an improvement in HRQoL and depressive symptoms in patients with PA after surgical and medical treatment, with better outcomes observed after surgery. No specific description of medical therapy.
|
|
Wada, 2017 |
Type of study: Retrospective cohort study
Setting and country: West Japan Adrenal Vein Sampling study (WAVES-J), Japan
Funding and conflicts of interest: This study was supported in part by grants-in-aid for the study of primary aldosteronism in Japan (JPAS), including a Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development and a Grant for National Center for Global Health and Medicine.
|
Inclusion criteria: - Patients with PA who underwent adrenal vein sampling from January 2006 to December 2013. - Diagnosis of PA was established by at least one positive result of confirmatory testing. - Patients who had at least one of the recordings of the data after the treatment
Exclusion criteria: - Patients who did not take MRA’s after diagnosis of PA
N total at baseline: Intervention: 142 Control: 234
Important prognostic factors2: Mean age in years (SD): I: 53 (11) C: 55 (12)
Female sex: I: N=71 (50%) C: N=136 (58%)
Mean duration of hypertension in years (SD): I: 9 (8) C: 8 (8)
Median aldosterone renin ratio (IQR): C: 420 (275-695)
Mean serum potassium in m Eq l-1 (SD): C: 3.8 (0.4)
Groups are not comparable at baseline
|
Describe intervention:
|
Describe control: Mineralocortoid Receptor Antagonist (MRA) treatment
|
Length of follow-up: 6 months
Loss-to-follow-up: Intervention: N (%) Reasons (describe)
Control: N (%) Reasons (describe)
Incomplete outcome data: Intervention: N (%) Reasons (describe)
Control: N (%) Reasons (describe)
|
Outcome measures and effect size (include 95%CI and p-value if available):
Mean systolic blood pressure in mmHg (SD): C: 130.81 (14.0) MD -1.82 (95%CI -4.74-1.10)
Mean diastolic blood pressure in mmHg (SD): I: 79.51 (11.2) C: 80.73 (11.0) MD -1.22 (95%CI -3.54-1.09)
Mean number of anti-hypertensive drugs (SD): C: 1.9 (0.8) MD -0.83 (95%CI -1.03- - 0.63)
Hyperkalemia I: N=14 (9.8%) C: N=9 (3.8%) |
Authors conclusion: the potential occurrence of hyperkalemia should be considered after medical treatment as well as surgical treatment for PA, especially in patients with older age (460 years) and impaired renal function (eGFR < 70 ml min-1 per 1.73 m2). No specific description of medical therapy. Data is stratified according to presence or absence of hyperkalemia (serum potassium > 5 mEq l-1). |
|
Zavatta, 2019 |
Type of study: Prospective cohort study
Setting and country: Endocrinology unit of the S. Orsola-Malpighi University Hospital of Bologna, Italy
Funding and conflicts of interest: This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. The authors declare that they have no conflict of interest. |
Inclusion criteria: - Patients diagnosed with PA according to current guidelines
Exclusion criteria: - Patients diagnosed with pheochromocytoma - Patients with known aortic bicuspid valve or connective tissue disorders
N total at baseline: Intervention: 12 Control: 33
Important prognostic factors2:
No information on comparability of Groups at baseline |
Describe intervention: Unilateral adrenalectomy
|
Describe control: Treatment with aldosterone antagonist (canrenone) with a mean dosage of 61,5 milligram (SD 31.4)
|
Length of follow-up: I: 1.5 years C: 3.5 years
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Mean number of antihypertensive drugs (SD): I: 1.8 (1.7) C: 2.3 (1.3) |
Study compared PA with essential hypertension. Therefore, no baseline data of the intervention and control group within patients with PA, were available. |
|
a; Results are extracted from individual studies; b no prognostic factors available for subgroups, only for general PA patients |
|||||||
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Satoh, 2019 |
Yes |
Yes |
Yes |
No |
Unclear |
Yes |
Yes |
Yes |
Yes |
Risk of bias table for interventions studies (cohort studies based on risk of bias tool by the CLARITY Group at McMaster University)
|
Author, year |
Selection of participants
Was selection of exposed and non-exposed cohorts drawn from the same population?
|
Exposure
Can we be confident in the assessment of exposure?
|
Outcome of interest
Can we be confident that the outcome of interest was not present at start of study?
|
Confounding-assessment
Can we be confident in the assessment of confounding factors?
|
Confounding-analysis
Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables? |
Assessment of outcome
Can we be confident in the assessment of outcome?
|
Follow up
Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed?
|
Co-interventions
Were co-interventions similar between groups?
|
Overall Risk of bias
|
|
|
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Low, Some concerns, High |
|
Araujo Castro, 2022 |
Definitely yes
Reason: Participants were selected from a registry using clear inclusion and exclusion criteria |
Probably yes
Reason: Exposure is surgery or medication, data from records |
Probably yes
Reason: Outcome data was measured pre- and post-intervention, regarding |
Probably yes
Reason: Possible confounding factors were reported |
Definitely no
Reason: No adjustment for confounding factors in analysis |
Probably yes
Reason: Clear definition of outcome, data from records. Regarding cardiovascular event no exact definition |
Probably no
Reason: Follow-up was too short for the outcome cardiovascular events, for other outcomes it could be sufficient
|
No information
|
High (regarding confounding analysis, assessment of outcome and follow-up #cardiovascular events)
Some concerns (regarding confounding analysis #bloodpressure and number of antihypertensive drugs)
|
|
Buffolo, 2020 |
Definitely no
Reason: Participants for the surgical treatment were differently selected then participants for the medical treatment
|
Definitely yes
Reason: Exposure is surgery or medication
|
Probably yes
Reason: Before screening tests all interfering antihypertensive drugs were stopped and outcomes were measured pre- and post intervention
|
Definitely no
Reason: Possible confounding factors were reported
|
Definitely no
Reason: No adjustment for confounding factors in analysis
|
Probably yes
Reason: Regarding QoL standardized questionnaire was used
|
Probably yes
Reason: Follow-up was sufficient for outcomes
|
No information
|
High (regarding selection bias and confounding analysis)
|
|
Chen, 2021 |
Probably yes
Reason: Participants were prospectively selected with in- and exclusion criteria
|
Definitely yes
Reason: Exposure is surgery or medication
|
Probably yes
Reason: Outcome data were measured pre- and post intervention with clear definition and instruction
|
Probably yes
Reason: Possible confounding factors were reported
|
Definitely no
Reason: No adjustment for confounding factors in analysis
|
Probably yes
Reason: Clear definitions and protocols for measuring outcomes
|
Probably yes
Reason: Follow-up was six months, sufficient for outcome blood pressure
|
No information
|
Some concerns (regarding confounding analysis because groups were not comparable at baseline)
|
|
Haze, 2021 |
Probably yes
Reason: Participants were retrospectively selected from registry with clear in- and exclusion criteria
|
Definitely yes
Reason: Exposure is surgery or medication, data is from records |
Probably yes
Reason: Outcome data were measured pre- and post intervention
|
Definitely yes
Reason: Possible confounding factors were reported
|
Probably no
Reason: Regarding analysis for outcome blood pressure there was no adjustment for confounders
|
Probably yes
Reason: Clear definition and protocol for measuring outcome |
Probably yes
Reason: Follow-up was six months, sufficient for outcome blood pressure
|
No information
|
Some concerns (regarding confounding analysis because groups were not comparable at baseline)
|
|
Katabami, 2019 |
Definitely yes
Reason: Participants were retrospectively selected from registry with clear in- and exclusion criteria
|
Definitely yes
Reason: Exposure is surgery or medication, data is from records
|
Probably yes
Reason: Outcome data were measured pre- and post-intervention
|
Definitely yes / Probably yes / Probably no / Definitely no
Reason: Confouding factors were reported
|
Definitely yes
Reason: Propensity score matching was used to correct for confounding variables
|
Probably no
Reason: No clear definition for outcome #normalization of serum potassium levels
|
Definetly yes
Reason: Follow-up was six months, sufficient for outcome blood pressure, normokalemia and antihypertensive drugs and missing data were imputed
|
No information
|
Some concerns (regarding outcome measurement #normokalemia)
Low (#blood pressure and number of antihypertensive drugs)
|
|
Meng, 2019 |
Probably yes
Reason: Participants were retrospectively selected from registry with clear inclusion criteria
|
Definitely yes
Reason: Exposure is surgery or medication, data is from medical records
|
Probably yes
Reason: Outcome data were measured pre- and post-intervention
|
Probably yes
Reason: Confouding factors were reported
|
Definitely no
Reason: No adjustment for confounding factors in analysis
|
Probably no
Reason: No clear definition for outcome #hypokalemia, clear definition and protocol for measuring other outcomes
|
Probably yes
Reason: Follow-up was sufficient for outcomes, no missing data
|
No information
|
Some concerns (regarding confounding analysis and outcome assessment) |
|
Murck, 2021 |
Probably no
Reason: Subgroup of participants (newly diagnosed) were selected from registry with no clear in- and exclusion criteria
|
Probably yes
Reason: Exposure is surgery or medication, data is from medical records
|
Probably yes
Reason: Outcome data were measured pre- and post-intervention
|
Probably no
Reason: Not all confounding factors were reported
|
Definitely no
Reason: No adjustment for confounding factors in analysis
|
Probably yes
Reason: Clear protocol for measuring outcome data
|
Probably yes
Reason: Follow-up was sufficient for outcome
|
No information
|
High (Regarding selection bias, confounding assessment and analysis)
|
|
Nakamaru, 2021 |
Probably yes
Reason: Patients were selected from JPAS registry with in- and exclusion criteria
|
Probably yes
Reason: Exposure is surgery or medication, data is from medical records
|
Probably yes
Reason: Outcome data were measured pre- and post-intervention
|
Probably no
Reason: Not all confounding factors were reported because of stratification of data
|
Definitely no
Reason: No adjustment for confounding factors in analysis regarding outcomes cardiovascular events or hyperkalemia
|
Probably yes
Reason: Clear protocol for measuring outcome data
|
Probably yes
Reason: Follow-up was sufficient for outcomes
|
No information
|
Some concerns (regarding confounding assessment and analysis)
|
|
Puar, 2020 |
Probably no
Reason: Proportion of patients were selected according to primary aldosteronism prediction score
|
Probably yes
Reason: Exposure is sugery or medication, data is from medical records
|
Probably yes
Reason: Outcome data were measured pre- and post-intervention
|
Probably yes /Probably no
Reason: Confounding factors were reported for outcome #cardiovascular events and not for outcome #blood pressure and #number of antihypertensive drugs
|
Definitely yes / Definitely no
Reason: Adjustment for confounding factors in analysis regarding outcome #cardiovascular events not for outcome #blood pressure
|
Probably yes
Reason: Outcome data was obtained from medical records |
Probably yes
Reason: Follow-up was sufficient for outcomes
|
No information
|
Some concerns (regarding selection bias for outcome #cardiovascular events)
High (regarding selection bias and confounding assessment and analysis for outcome #blood pressure and #number of antihypertensive drugs)
|
|
Tan, 2021 |
Probably yes
Reason: Patients were prospectively selected with clear in- and exclusion criteria
|
Probably no
Reason: No clear definition of exposure of medical therapy (control)
|
Probably yes
Reason: Outcome data were measured pre- and post-intervention
|
Probably no
Reason: Not all confounding factors were reported
|
Definitely no
Reason: No adjustment for confounding factors in analysis
|
Probably yes
Reason: Outcome data was measured using standardized questionnaires
|
Probably yes
Reason: Follow-up was sufficient for outcomes
|
No information
|
Some concerns (regarding assessment of exposure and confounding assessment and analysis) |
|
Velema, 2019 |
Probably yes
Patients were prospectively selected with clear in- and exclusion criteria |
Probably yes
Reason: Clear definition of exposures |
Probably yes
Reason: Outcome data were measured pre- and post-intervention |
Probably yes
Reason: Confounding factors were reported |
Probably yes
Reason: Mixed model analysis was used to compare outcomes before and after the two treatments |
Probably yes
Reason: Outcome data was measured using standardized questionnaires |
Probably yes
Reason: Follow-up was sufficient for outcomes
|
Probably yes
Reason: Study reported all patients were further treated with conventional antihypertensive drugs
|
Low |
|
Wada, 2017 |
Probably yes
Reason: Patients were selected from WAVES-J study with in- and exclusion criteria
|
Probably yes
Reason: Exposure is sugery or medication, data is from medical records
|
Probably yes
Reason: Outcome data were measured pre- and post-intervention
|
Probably yes
Reason: Confouding factors were reported
|
Definitely no
Reason: No adjustment for confounding factors regarding outcomes of interest in analysis
|
Probably yes
Reason: Outcome data was measured using a clear definition
|
Probably yes
Reason: Follow-up was sufficient for outcomes
|
No information
|
Some concerns (regarding confounding analysis) |
|
Zavatta, 2019 |
Probably yes
Reason: Patients were selected prospectively with in- and exclusion criteria
|
Probably yes
Reason: Exposure is surgery or medication with clear definitions
|
Probably yes
Reason: Outcome data were measured pre- and post-intervention
|
Definitely no
Reason: No confounding factors were reported
|
Definitely no
Reason: No adjustment for confounding factors regarding outcomes of interest in analysis
|
Probably yes
Reason: Outcome data was measured using a clear definition
|
Definitely no
Reason: Follow-up for intervention and control group was different, no report of missing data
|
No information
|
High (regarding confounding assessment and analysis and follow-up)
|
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Chang YH, Chung SD, Wu CH, Chueh JS, Chen L, Lin PC, Lin YH, Huang KH, Wu VC, Chu TS; TAIPAI Study Group. Surgery decreases the long-term incident stroke risk in patients with primary aldosteronism. Surgery. 2020 Feb;167(2):367-377. doi: 10.1016/j.surg.2019.08.017. Epub 2019 Oct 29. PMID: 31676114.
|
Wrong intervention versus control and no outcome of interest reported |
|
Chen YY, Lin YH, Huang WC, Chueh E, Chen L, Yang SY, Lin PC, Lin LY, Lin YH, Wu VC, Chu TS, Wu KD. Adrenalectomy Improves the Long-Term Risk of End-Stage Renal Disease and Mortality of Primary Aldosteronism. J Endocr Soc. 2019 Mar 25;3(6):1110-1126. doi: 10.1210/js.2019-00019. PMID: 31086833; PMCID: PMC6507624.
|
No outcome of interest reported |
|
W. Grira, F. Chaker, M. Yazidi, C. Denguir, M. Tebib, M. Chihaoui, H. Slimane, P-158: Long-term evolution of blood pressure and kalemia in patients with primary aldosteronism: surgical treatment versus medical treatment, Annales de Cardiologie et d'Angéiologie, Volume 64, Supplement 1, 2015, Page S76, ISSN 0003-3928, |
Article not available in English |
|
Holaj R, Rosa J, Zelinka T, Štrauch B, Petrák O, Indra T, Šomlóová Z, Michalský D, Novák K, Wichterle D, Widimský J Jr. Long-term effect of specific treatment of primary aldosteronism on carotid intima-media thickness. J Hypertens. 2015 Apr;33(4):874-82; discussion 882. doi: 10.1097/HJH.0000000000000464. PMID: 25490707; PMCID: PMC4354456.
|
Already included in review of Satoh (2019) |
|
Huang WC, Chen YY, Lin YH, Chueh JS. Composite Cardiovascular Outcomes in Patients With Primary Aldosteronism Undergoing Medical Versus Surgical Treatment: A Meta-Analysis. Front Endocrinol (Lausanne). 2021 May 17;12:644260. doi: 10.3389/fendo.2021.644260. PMID: 34079522; PMCID: PMC8165438.
|
Only meta-analysis for composite outcome |
|
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal Outcomes in Medically and Surgically Treated Primary Aldosteronism. Hypertension. 2018 Sep;72(3):658-666. doi: 10.1161/HYPERTENSIONAHA.118.11568. PMID: 29987110; PMCID: PMC6202119.
|
No outcome of interest reported |
|
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of Atrial Fibrillation and Mineralocorticoid Receptor Activity in Patients With Medically and Surgically Treated Primary Aldosteronism. JAMA Cardiol. 2018 Aug 1;3(8):768-774. doi: 10.1001/jamacardio.2018.2003. PMID: 30027227; PMCID: PMC6143072.
|
Wrong intervention versus control |
|
Jing Y, Liao K, Li R, Yang S, Song Y, He W, Wang K, Yang J, Li Q, Hu J. Cardiovascular events and all-cause mortality in surgically or medically treated primary aldosteronism: A Meta-analysis. J Renin Angiotensin Aldosterone Syst. 2021 Jan-Dec;22(1):14703203211003781. doi: 10.1177/14703203211003781. PMID: 33752505; PMCID: PMC8880484.
|
Systematic review reported only one outcome of interest |
|
Kwak MK, Lee JY, Kim BJ, Lee SH, Koh JM. Effects of Primary Aldosteronism and Different Therapeutic Modalities on Glucose Metabolism. J Clin Med. 2019 Dec 12;8(12):2194. doi: 10.3390/jcm8122194. PMID: 31842354; PMCID: PMC6947343.
|
No outcome of interest reported |
|
Lin YF, Peng KY, Chang CH, Hu YH, Wu VC, Chung SD; Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group. Changes in Glucose Metabolism after Adrenalectomy or Treatment with a Mineralocorticoid Receptor Antagonist for Primary Aldosteronism. Endocrinol Metab (Seoul). 2020 Dec;35(4):838-846. doi: 10.3803/EnM.2020.797. Epub 2020 Dec 2. PMID: 33261310; PMCID: PMC7803597.
|
No outcome of interest reported |
|
Marzano L, Colussi G, Sechi LA, Catena C. Adrenalectomy is comparable with medical treatment for reduction of left ventricular mass in primary aldosteronism: meta-analysis of long-term studies. Am J Hypertens. 2015 Mar;28(3):312-8. doi: 10.1093/ajh/hpu154. Epub 2014 Oct 21. PMID: 25336498.
|
No outcome of interest in meta-analysis |
|
Muth A, Ragnarsson O, Johannsson G, Wängberg B. Systematic review of surgery and outcomes in patients with primary aldosteronism. Br J Surg. 2015 Mar;102(4):307-17. doi: 10.1002/bjs.9744. Epub 2015 Jan 20. PMID: 25605481.
|
No meta-analysis performed because of methodological heterogeneity |
|
Pan CT, Liao CW, Tsai CH, Chen ZW, Chen L, Hung CS, Liu YC, Lin PC, Chang CC, Chang YY, Wu VC, Lin YH; TAIPAI study group †. Influence of Different Treatment Strategies on New-Onset Atrial Fibrillation Among Patients With Primary Aldosteronism: A Nationwide Longitudinal Cohort-Based Study. J Am Heart Assoc. 2020 Mar 3;9(5):e013699. doi: 10.1161/JAHA.119.013699. Epub 2020 Feb 19. PMID: 32070205; PMCID: PMC7335564.
|
Wrong intervention versus control |
|
Park KS, Kim JH, Yang YS, Hong AR, Lee DH, Moon MK, Choi SH, Shin CS, Kim SW, Kim SY. Outcomes analysis of surgical and medical treatments for patients with primary aldosteronism. Endocr J. 2017 Jun 29;64(6):623-632. doi: 10.1507/endocrj.EJ16-0530. Epub 2017 Apr 29. PMID: 28458337.
|
Is already included in review of Satoh (2019) |
|
Rossi GP, Maiolino G, Flego A, Belfiore A, Bernini G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Muiesan ML, Mannelli M, Negro A, Palumbo G, Parenti G, Rossi E, Mantero F; PAPY Study Investigators. Adrenalectomy Lowers Incident Atrial Fibrillation in Primary Aldosteronism Patients at Long Term. Hypertension. 2018 Apr;71(4):585-591. doi: 10.1161/HYPERTENSIONAHA.117.10596. Epub 2018 Feb 26. PMID: 29483224.
|
Wrong intervention versus control |
|
Tsai CH, Chen YL, Pan CT, Lin YT, Lee PC, Chiu YW, Liao CW, Chen ZW, Chang CC, Chang YY, Hung CS, Lin YH. New-Onset Atrial Fibrillation in Patients With Primary Aldosteronism Receiving Different Treatment Strategies: Systematic Review and Pooled Analysis of Three Studies. Front Endocrinol (Lausanne). 2021 May 24;12:646933. doi: 10.3389/fendo.2021.646933. PMID: 34108934; PMCID: PMC8181760.
|
No outcome of interest reported |
|
Velema MS, Terlouw JM, de Nooijer AH, Nijkamp MD, Jacobs N, Deinum J. Psychological Symptoms and Well-Being After Treatment for Primary Aldosteronism. Horm Metab Res. 2018 Aug;50(8):620-626. doi: 10.1055/a-0628-6847. Epub 2018 Jun 12. PMID: 29895075.
|
No comparison between intervention and control for outcomes of interest |
|
Wu CH, Yang YW, Hung SC, Tsai YC, Hu YH, Lin YH, Chu TS, Wu KD, Wu VC. Effect of Treatment on Body Fluid in Patients with Unilateral Aldosterone Producing Adenoma: Adrenalectomy versus Spironolactone. Sci Rep. 2015 Oct 19;5:15297. doi: 10.1038/srep15297. PMID: 26477337; PMCID: PMC4609981.
|
No outcome of interest reported |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 19-03-2024
Beoordeeld op geldigheid : 07-05-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen en patiëntvertegenwoordigers (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met bijniertumoren.
Werkgroep
- Prof. dr. M.R. (Menno) Vriens, endocrien oncologisch chirurg, werkzaam in het UMC Utrecht te Utrecht, NVvH (voorzitter)
- Prof. dr. S. (Schelto) Kruijff, endocrien oncologisch chirurg, werkzaam in het UMCG te Groningen, NVvH (voorzitter)
- Prof. dr. R.A. (Richard) Feelders, internist-endocrinoloog, werkzaam in het Erasmus MC te Rotterdam, NIV
- Prof. dr. H.R. (Harm) Haak, internist, werkzaam in het Máxima MC te Eindhoven, NIV
- Drs. J.F. (Julia) Heusdens, anesthesioloog, werkzaam in het UMC Utrecht te Utrecht, NVA
- Prof. dr. R.R. (Ronald) de Krijger, patholoog, werkzaam in het UMC Utrecht/Prinses Máxima Centrum te Utrecht, NVVP
- Drs. J. (Jeroen) Vister, radioloog, werkzaam in het Universitair Medisch Centrum Groningen te Groningen, NVvR
- Dr. M.R. (Max) Dahele, radiotherapeut-oncoloog, werkzaam in het Amsterdam UMC te Amsterdam, NVRO
- Dr. J.F. (Hans) Langenhuijsen, uroloog, werkzaam in het Radboudumc te Nijmegen, NVU
- Dr. B.P.M. (Bernadette) van Nesselrooij, klinisch geneticus, werkzaam in het UMC Utrecht te Utrecht, VKGN
- J.G. (Johan) Beun, manager/coördinator BijnierNET, BijnierNET
- D.D. (Diana) Kwast-Hoekstra, MScN.RN. Verplegingswetenschapper en patientvertegenwoordiger, Bijniervereniging NVACP (tot 31-12-2022)
- Drs. N.T.M. (Nick) van der Meij, verpleegkundig specialist, werkzaam in het UMC Utrecht, te Utrecht, LWEV
Met ondersteuning van
- dr. A. (Anja) van der Hout, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- drs. S. (Sarah) van Duijn, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- drs. M. (Miriam) te Lintel Hekkert, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- drs. I. (Ingeborg) van Dusseldorp, medisch informatiespecialist, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Vriens (voorzitter) |
Chirurg UMC Utrecht |
Bestuurslid NVvH (tot mei 2021) |
Geen |
Geen restricties |
|
Kruijff (voorzitter) |
Endocrien chirurg UMCG Groningen |
Geen |
Geen |
Geen restricties |
|
Feelders |
- Professor -internist-endocrinoloog Erasmus MC - Adjunct Professor of Medicine New York University U.S.A. |
- Medisch adviseur NVCAP, onbetaald - Bestuurslid Dutch Adrenal Network, onbetaald - Consultant Recordati, betaald |
Geen |
Geen restricties |
|
Beun |
Coordinator van de Stichting BijnierNET, parttime |
Geen |
Geen |
Geen restricties |
|
Langenhuijsen |
Uroloog Radboudumc, Niijmegen |
Bestuurslid Radboudumc Expertisecentrum Bijnierziekten Voorzitter eUROGEN WS 3 Rare genito-urological cancers en Expertise Area coordinator Adrenal tumours |
ZonMw gefinancieerd onderzoek, DoelmatigheidsOnderzoek "Pentixafor PET/CT vs veneuze bijniervenesampling bij subtypering primair hyperaldosteronisme" i..s.m. PentixaPharm GmBH |
Geen restricties |
|
De Krijger |
- Patholoog, UMC Utrecht, 0,2 fte |
- Board member of Perined, Dutch organization supporting perinatal registries (vacatiegeld) - Council Member European Society of Pathology (onbetaald) - International Panel Member of Wilms tumor panel of SIOP Renal Tumor Study Group (onbetaald) - Chair International (European) pediatrie liver tumor panel (PHITT trial) (onbetaald) - Chairmen Dutch/Belgian working group on Pediatrie Pathology (onbetaald) - Associate editor Pediatrie and Developmental Pathology (onbetaald) - Member editorial board Endocrine Pathology (onbetaald) - Member editorial board Virchows Archiv (onbetaald) - Member editorial board Frontiers in Endocrinology (onbetaald) - Editor-in-Chief Cancers, section Pediatrie Oncology (honorarium) - Member editorial board WHO Endocrine and Neuroendocrine Tumors, 5th edition (onbetaald) |
Geen |
Geen restricties |
|
Heusdens |
Anesthesioloog UMC Utrecht
|
Geen |
Geen |
Geen restricties |
|
Haak |
- Internist- endocrinoloog Maxima MC tot 01-09-2023, daarna nul-aanstelling en pensioen - Hoogleraar acute interne geneeskunde MUMC/UM, tot 01-02-2024 |
- Lid algemeen bestuurd BijnierNET - Voorzitter Bijniernetwerk Nederland D.A.N. - Raad van Toezicht Kempenhaeghe, betaald |
Incidenteel grant van HRA
|
Geen restricties |
|
Dahele |
Radiotherapeut/VHD afdeling radiotherapie Amsterdam UMC (locatie VUmc)
|
Geen |
Onderzoek financiering van: Varian Medical Systems (niet gerelateerd aan bijniertumoren) |
Geen restricties |
|
Van Nesselrooij |
Klinisch Genetica, UMC Utrecht (0,8fte)
|
Secretaris van de VKGN (tot 01-01-2023)
|
Geen |
Geen restricties |
|
Kwast (tot 13-12-2022)
|
Bestuurslid Bijniervereniging NVACP te Nijkerk (onbetaald) (tot 13-12-2022) |
Redactielid Bijniervereniging NVACP (onbetaald) (tot 13-12-2022) |
Geen |
Geen restricties |
|
Vister
|
Radioloog, UMCG
|
Geen |
Geen |
Geen restricties |
|
van der Meij
|
Verpleegkundig specialist AGZ, UMC Utrecht, afdeling Endocriene oncologie |
Geen |
Geen |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door Patiëntenfederatie Nederland, BijnierNET, Bijniervereniging NVACP, Nederlandse Federatie van Kankerpatiënten organisaties (NFK), Nierstichting, Nierpatiëntenvereniging Nederland uit te nodigen voor de invitational conference en afgevaardigden van BijnierNET en Bijniervereniging NVACP in de werkgroep. Het verslag van de invitational conference (zie bijlage) is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de patiëntenorganisaties: Bijniervereniging NVACP, Patiëntenfederatie Nederland, BijnierNET, Nederlandse Federatie van Kankerpatiënten organisaties (NFK), Nierstichting, Nierpatiëntenvereniging Nederland, Nederlandse Hypofyse Stichting en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
|
Module |
Uitkomst raming |
Toelichting |
|
Module Diagnostiek morbus Conn |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Behandeling morbus Conn |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Behandeling Cushing |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Behandeling feochromocytoom |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Expertisecentrum ACC |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Biopsie bij ongedefinieerde retroperitoneale massa |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Kenmerken CT-scan incidentaloom |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Autonome cortisol (hyper)secretie (subklinische Cushing) |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Behandeling bijniermetastasen |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Minimaal invasieve chirurgie |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Genetisch testen en chirurgisch beleid |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Pathologieverslag |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Radiologieverslag |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Follow-up |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
|
Module Aandacht bijnierschorsinsufficiëntie |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling niet breed toepasbaar is (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met bijniertumoren. Tevens zijn er knelpunten aangedragen door de NVvH, NVU, NOV, NVRO, VKGN, Bijniervereniging NVACP, IKNL, NAPA (vakgroep interne geneeskunde), Belangenvereniging Von Hippel-Lindau via een invitational conference. Een verslag hiervan is opgenomen in de bijlage.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
Definitie |
|
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Algemene informatie
|
Richtlijn: NVVH bijniertumoren |
|
|
Uitgangsvraag: Wat zijn de voor- en nadelen van chirurgie ten opzichte van medicatie bij patiënten met M. Conn, op de bloeddrukcontrole (inclusief aantal antihypertensiva), normale kaliumwaarden in het bloed (normokalemia), (lange termijn) cardiovasculaire morbiditeit en mortaliteit en kwaliteit van leven
|
|
|
Database(s): Ovid/Medline, Embase |
Datum: 15-2-2022, 14-3-2022 |
|
Periode: 2000- |
Talen: nvt |
|
Literatuurspecialist: Ingeborg van Dusseldorp |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: 14-3-2022 Naar aanleiding van een gevonden SR van Satoh, is gekozen om de observationele studies vanaf 2017 toe te voegen. Daarnaast is een update uitgevoerd voor de SRs en RCTs en is 1 SR toegevoegd.
15-2-2022 Voor deze vraag is gezocht met de volgende elementen: M. Conn EN adrenalectomy 4 van de sleutelartikelen worden gevonden met het studiedesign SR, RCT. De overige sleutelreferenties worden gevonden met het observationele filter, met uitzondering van de richtlijn. |
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase en Ovid/Medline is op 15 februari 2022 met relevante zoektermen gezocht naar systematische reviews en RCTs over de chirurgische behandeling van M. Conn. De literatuurzoekactie leverde 114 unieke treffers op. Op 14 maart wordt naar aanleiding van de gevonden SR van Satoh, besloten om een update uit te voeren en de observationele studies toe te voegen. Het totaal aantal gevonden unieke treffers is 542. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs 15-2-2022 |
65 |
36 |
67 |
|
RCTs 15-2-2022 |
56 |
40 |
47 |
|
Observationele studies 14-3-2022 |
401 |
264 |
427 |
|
Update SR 14-3-2022 |
1 |
1 |
1 |
|
Totaal |
|
|
542 |
Zoekstrategie
Embase
14-3-2022
|
No. |
Query |
Results |
|
#29 |
#4 AND #25 NOT #7 NOT #8 |
401 |
|
#28 |
#4 AND #25 |
439 |
|
#27 |
#22 NOT #26 |
1 |
|
#26 |
#22 AND #25 |
4 |
|
#25 |
#23 OR #24 |
14595627 |
|
#24 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
12886408 |
|
#23 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6767914 |
|
#22 |
#19 NOT #21 |
5 |
|
#21 |
#19 AND #20 |
4 |
|
#20 |
#7 OR #8 |
109 |
|
#19 |
#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 |
9 |
|
#18 |
composite AND cardiovascular AND outcomes AND in AND patients AND with AND primary AND aldosteronism AND undergoing AND medical AND versus AND surgical AND treatment AND huang |
1 |
|
#17 |
mineralocorticoid AND antagonists AND treatment AND versus AND surgery AND in AND primary AND aldosteronism AND catena |
1 |
|
#16 |
(8. AND medical OR surgical) AND therapy AND for AND primary AND aldosteronism AND 'post treatment' AND 'follow up' AND as AND a AND surrogate AND measure AND of AND comparative AND outcomes AND kline |
1 |
|
#15 |
the AND management AND of AND primary AND aldosteronism AND funder AND 2016 AND case AND detection, AND diagnosis, AND treatment:ti NOT 2008 |
1 |
|
#14 |
management AND of AND endocrine AND disease AND guideline AND williams AND aldosteronism AND 2018 |
1 |
|
#13 |
systematic AND review AND the AND clinical AND outcomes AND of AND mineralocorticoid AND receptor AND antagonist AND treatment AND versus AND adrenalectomy AND in AND patients AND with AND primary AND aldosteronism AND satoh AND 2019 |
1 |
|
#12 |
puar AND 2021 AND aldosteronism AND outcomes:ti |
1 |
|
#11 |
composite AND cardiovascular AND outcomes AND in AND patients AND with AND primary AND aldosteronism AND undergoing AND medical AND versus AND surgical AND treatment |
1 |
|
#10 |
outcomes:ti AND after AND adrenalectomy AND for AND unilateral AND primary AND aldosteronism:ti AND lenders AND 2017 |
1 |
|
#9 |
. AND quality AND life AND in AND primary AND aldosteronism AND a AND comparative AND effectiveness AND study AND of AND adrenalectomy AND medical AND treatment. AND dekkers AND 2018 |
1 |
|
#8 |
#4 AND #6 |
29 |
|
#7 |
#4 AND #5 |
43 |
|
#6 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1874875 |
|
#5 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#4 |
#3 AND [1-1-2017]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
710 |
|
#3 |
#1 AND #2 |
2962 |
|
#2 |
'adrenalectomy'/exp OR 'endoscopic surgery'/exp OR 'laparoscopy'/exp OR 'minimally invasive surgery'/exp OR 'adrenal enucleation':ti,ab,kw OR 'adrenalectom*':ti,ab,kw OR 'hemiadrenalectom*':ti,ab,kw OR 'retroperitoneoscopy'/exp OR retroperioneoscop*:ti,ab,kw OR (((adrenal OR 'minimally invasive') NEAR/3 surger*):ti,ab,kw) OR laparoscop*:ti,ab,kw OR endoscopic*:ti,ab,kw |
645497 |
|
#1 |
'primary hyperaldosteronism'/exp OR 'aldosterone producing adenoma':ti,ab,kw OR 'aldosteronism primary':ti,ab,kw OR 'primary aldosteronism':ti,ab,kw OR 'primary hyperaldosteronism':ti,ab,kw OR ((conn NEAR/3 (morbus OR disease OR syndrome)):ti,ab,kw) |
9501 |
|
No. |
Query |
Results |
|
#22 |
#19 NOT #21 |
5 |
|
#21 |
#19 AND #20 |
4 |
|
#20 |
#7 OR #8 |
109 |
|
#19 |
#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 |
9 |
|
#18 |
composite AND cardiovascular AND outcomes AND in AND patients AND with AND primary AND aldosteronism AND undergoing AND medical AND versus AND surgical AND treatment AND huang |
1 |
|
#17 |
mineralocorticoid AND antagonists AND treatment AND versus AND surgery AND in AND primary AND aldosteronism AND catena |
1 |
|
#16 |
(8. AND medical OR surgical) AND therapy AND for AND primary AND aldosteronism AND 'post treatment' AND 'follow up' AND as AND a AND surrogate AND measure AND of AND comparative AND outcomes AND kline |
1 |
|
#15 |
the AND management AND of AND primary AND aldosteronism AND funder AND 2016 AND case AND detection, AND diagnosis, AND treatment:ti NOT 2008 |
1 |
|
#14 |
management AND of AND endocrine AND disease AND guideline AND williams AND aldosteronism AND 2018 |
1 |
|
#13 |
systematic AND review AND the AND clinical AND outcomes AND of AND mineralocorticoid AND receptor AND antagonist AND treatment AND versus AND adrenalectomy AND in AND patients AND with AND primary AND aldosteronism AND satoh AND 2019 |
1 |
|
#12 |
puar AND 2021 AND aldosteronism AND outcomes:ti |
1 |
|
#11 |
composite AND cardiovascular AND outcomes AND in AND patients AND with AND primary AND aldosteronism AND undergoing AND medical AND versus AND surgical AND treatment |
1 |
|
#10 |
outcomes:ti AND after AND adrenalectomy AND for AND unilateral AND primary AND aldosteronism:ti AND lenders AND 2017 |
1 |
|
#9 |
. AND quality AND life AND in AND primary AND aldosteronism AND a AND comparative AND effectiveness AND study AND of AND adrenalectomy AND medical AND treatment. AND dekkers AND 2018 |
1 |
|
#8 |
#4 AND #6 RCT |
56 |
|
#7 |
#4 AND #5 SR |
65 |
|
#6 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1874875 |
|
#5 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#4 |
#3 AND [1-1-2000]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
1684 |
|
#3 |
#1 AND #2 |
2962 |
|
#2 |
'adrenalectomy'/exp OR 'endoscopic surgery'/exp OR 'laparoscopy'/exp OR 'minimally invasive surgery'/exp OR 'adrenal enucleation':ti,ab,kw OR 'adrenalectom*':ti,ab,kw OR 'hemiadrenalectom*':ti,ab,kw OR 'retroperitoneoscopy'/exp OR retroperioneoscop*:ti,ab,kw OR (((adrenal OR 'minimally invasive') NEAR/3 surger*):ti,ab,kw) OR laparocop*:ti,ab,kw OR endoscopic*:ti,ab,kw |
574911 |
|
#1 |
'primary hyperaldosteronism'/exp OR 'aldosterone producing adenoma':ti,ab,kw OR 'aldosteronism primary':ti,ab,kw OR 'primary aldosteronism':ti,ab,kw OR 'primary hyperaldosteronism':ti,ab,kw OR ((conn NEAR/3 (morbus OR disease OR syndrome)):ti,ab,kw) |
9503 |
Ovid/Medline
14-3-2022
|
# |
Searches |
Results |
|
14 |
13 not 9 not 8 |
264 |
|
13 |
5 and (11 or 12) |
287 |
|
12 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5104379 |
|
11 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4091551 |
|
10 |
9 not 8 |
10 |
|
9 |
5 and 7 |
19 |
|
8 |
5 and 6 |
24 |
|
7 |
(exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf.) not (animals/ not humans/) |
1358363 |
|
6 |
(meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) |
552308 |
|
5 |
4 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
465 |
|
4 |
limit 3 to yr="2017 -Current" |
497 |
|
3 |
1 and 2 |
1845 |
|
2 |
Adrenalectomy/ or exp Laparoscopy/ or Endoscopy/ or Minimally Invasive Surgical Procedures/ or adrenal enucleation.ti,ab,kf. or adrenalectom*.ti,ab,kf. or hemiadrenalectom*.ti,ab,kf. or retroperioneoscop*.ti,ab,kf. or ((adrenal or minimally invasive) adj3 surger*).ti,ab,kf. or laparocop*.ti,ab,kf. or endoscopic*.ti,ab,kf. |
357645 |
|
1 |
exp Hyperaldosteronism/ or aldosterone producing adenoma.ti,ab,kf. or aldosteronism primary.ti,ab,kf. or primary aldosteronism.ti,ab,kf. or primary hyperaldosteronism.ti,ab,kf. or (conn adj3 (morbus or disease or syndrome)).ti,ab,kf. |
10735 |
15-2-2022
|
# |
Searches |
Results |
|
9 |
5 and 7 RCT |
40 |
|
8 |
5 and 6 SR |
36 |
|
7 |
(exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf.) not (animals/ not humans/) |
1352557 |
|
6 |
(meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) |
547875 |
|
5 |
4 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
1190 |
|
4 |
limit 3 to yr="2000 -Current" |
1265 |
|
3 |
1 and 2 |
1836 |
|
2 |
Adrenalectomy/ or exp Laparoscopy/ or Endoscopy/ or Minimally Invasive Surgical Procedures/ or adrenal enucleation.ti,ab,kf. or adrenalectom*.ti,ab,kf. or hemiadrenalectom*.ti,ab,kf. or retroperioneoscop*.ti,ab,kf. or ((adrenal or minimally invasive) adj3 surger*).ti,ab,kf. or laparocop*.ti,ab,kf. or endoscopic*.ti,ab,kf. |
356286 |
|
1 |
exp Hyperaldosteronism/ or aldosterone producing adenoma.ti,ab,kf. or aldosteronism primary.ti,ab,kf. or primary aldosteronism.ti,ab,kf. or primary hyperaldosteronism.ti,ab,kf. or (conn adj3 (morbus or disease or syndrome)).ti,ab,kf. |
10721 |
