1e versus 2e generatie langwerkende insuline analogen
Uitgangsvraag
Wat is de waarde van 2e generatie langwerkende insuline analogen?
Aanbeveling
Start in principe bij diabetes type 1 de novo met een eerste generatie langwerkend insuline analogon. Indien iemand met diabetes type 1 al goed ingesteld is op een tweede generatie langwerkend insuline analogon, is er geen reden om deze therapie te wijzigen in therapie met een eerste generatie langwerkend insuline analogon.
Overweeg bij mensen met type 1 diabetes bij wie de behandeling met eerste generatie langwerkende insuline analogen onbevredigend verloopt (ondanks gebruikelijke andere aanpassingen) om op proef over te stappen naar een tweede generatie langwerkende insuline analoog. Formuleer een behandeldoel en evalueer na een periode van 6 maanden het effect van de nieuwe behandeling op het behandeldoel.
Voorbeelden van situaties waarin dit kan worden overwogen zijn:
- Ernstige en onverwachte (nachtelijke) hypoglykemieën
- Wisselende diensten of regelmatig passeren van tijdzones
- Chronische toediening door zorgverleners of mantelzorgers
- Grote moeite met dagelijks spuiten van insuline
- Situaties waarin hoge insulinedoseringen gewenst zijn
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek verricht naar de gunstige en ongunstige effecten van behandeling met tweede generatie langwerkende insulineanalogen (glargine U300 of degludec) vergeleken met eerste generatie langwerkende insulinepreparaten bij volwassenen en kinderen met type 1 diabetes mellitus. Analyse van de literatuur leidde tot 2 meta-analyses (met systematische review bij Hemmingsen 2021, en een post hoc meta-analyse bij Danne 2020) waarin in totaal 9 klinische trials werden geïncludeerd.
Als cruciale uitkomstmaten voor de besluitvorming zijn HbA1c, ernstige hypoglykemie, diabetische ketoacidose (DKA), en kwaliteit van leven onderzocht. Daarnaast werd een aantal niet-cruciale maar belangrijke uitkomstmaten onderzocht: hypoglykemie, nachtelijke hypoglykemie, serious adverse events (SAEs), non-SAEs, locale reactie op de injectieplek en totale sterfte.
Uit deze literatuuranalyse komt naar voren dat behandeling van personen met type 1 diabetes mellitus met degludec of glargine U300, in vergelijking met de eerstegeneratie langwerkende insulineanalogen glargine U100 of detemir, resulteert in:
- geen verbetering of verslechtering in HbA1c (GRADE beoordeling REDELIJK);
- geen verschil in risico op risico op ernstige hypoglykemieën (GRADE beoordeling REDELIJK);
- geen verschil in risico op DKA (GRADE beoordeling LAAG)
- geen effect op kwaliteit van leven (GRADE beoordeling REDELIJK)
- geen verschil in risico op hypoglykemieën (GRADE beoordeling REDELIJK);
- geen verschil in risico op nachtelijke hypoglykemieën (GRADE beoordeling REDELIJK);
- geen verschil in risico op ernstige of niet-ernstige bijwerkingen (GRADE beoordeling REDELIJK)
- geen verschil in lokale reactie op de injectieplaats (GRADE beoordeling LAAG)
Door een gebrek aan onderzoeksgegevens is er geen uitspraak mogelijk over het effect van een behandeling met glargine U300 of degludec vergeleken met eerste generatie langwerkende insulines op de tijd binnen, boven en onder de streefwaarden van bloedglucose, en op de kwaliteit van leven. Evenmin valt een uitspraak te doen over het effect op totale sterfte.
De belangrijkste conclusie van de literatuursearch is dat zowel voor de vooraf bepaalde cruciale uitkomstmaten als voor de vooraf bepaalde belangrijke uitkomstenmaten geen klinisch relevante verschillen werden gevonden afhankelijk van soort van insulinebehandeling. Deze uitkomsten gelden zowel voor volwassenen als voor kinderen met type 1 diabetes. Ofschoon voor HbA1C een statistisch significant verschil werd gevonden (ten voordele van behandeling met 1ste generatie langwerkende insuline analogen) was het gevonden effect (0.07%) niet klinisch relevant. Voor de vooraf vastgestelde cruciale uitkomstmaten ‘tijd binnen de glucose-streefwaarden, tijd boven de glucose-streefwaarden, tijd onder de glucose-streefwaarden,’ was geen informatie beschikbaar in de systematische review van Hemmingsen (2021) en die van Danne (2020). De bewijskracht voor de cruciale uitkomstmaten was matig tot laag. De inconsistentie in de uitkomst ernstige hypoglykemie dient besproken te worden. In de glargine U300 trials (de zogenoemde EDITION trials, gerapporteerd in de systematische review van Danne [2020]) werd een lager risico gevonden op het krijgen van ernstige hypoglykemie bij gebruik van de 2de generatie langwerkende insuline analoog glargine U300 terwijl voor de andere trials (gerapporteerd in de systematische review van Hemmingsen [2021]) geen verschil in risico werd gevonden op het krijgen van ernstige hypoglykemie. Daarnaast blijkt uit de publicatie van Danne (2020) dat de positieve effecten van glargine U300 op ernstige hypoglykemie voornamelijk in de eerste 8 weken van behandeling aanwezig zijn en daarna uit lijken te doven. Gezien de EDITION trials gemiddeld genomen een kortere follow-up duur hadden dan de andere geïncludeerde trials zou mogelijk ook dit verschil in effectiviteit kunnen verklaren. In de originele trial resultaten van EDITION 4, EDITION JP1 en EDITION Junior werden overigens geen verschillen in hypoglykemie gevonden (maar wel voor nachtelijke hypoglykemie in EDITION JP1).
Aanvullende argumenten
Op grond van de uitkomsten van onze analyse van de studies biedt het gebruik van tweede generatie langwerkende insuline-analogen degludec en glargine U300 dus geen voordelen boven de eerste generatie langwerkende insulineanalogen bij mensen met type 1 diabetes. Daarnaast blijkt uit de systematische review van Hemmingsen (2021) dat er ook geen verschil is in effectiviteit en veiligheid tussen de 2e generatie insulineanalogen onderling. Farmacokinetisch en –dynamisch onderzoek laat zien dat tweede generatie insuline-analogen leiden tot een meer stabiele en langer durende beschikbaarheid van insuline, waardoor de kans op schommelingen in insulinespiegels afneemt (Haahr, 2014). Hierdoor zou de kans op het vóórkomen van (ernstige) hypoglykemieën afnemen, zonder dat daarbij de gemiddelde glucosewaarden, en daarmee het HbA1c stijgen. Hiermee zou het mogelijk moeten zijn om de glucosewaarden te optimaliseren (Porcelatti, 2019; Owens, 2019).
Hypoglykemie is een belangrijk probleem bij de behandeling van type 1 diabetes. Hypoglykemieën kunnen acuut ontregelend zijn en de kwaliteit van leven verminderen. Voorts zijn hypoglykemieën geassocieerd met cardiovasculaire complicaties, cognitieve achteruitgang en mortaliteit, en zijn de voornaamste barriere voor goede glucoseregulatie. Hierdoor hebben mensen met type 1 diabetes gemiddeld te hoge glucose- en HbA1c waarden, en daardoor een verhoogde kans op lange termijn complicaties zoals retinopathie, nefropathie en neuropathie. Hypoglykemieën kunnen echter onverwacht optreden, ernstig verlopen, en zowel de hypoglykemieën als de angst daarvoor spelen een belangrijke rol in het leven van mensen met type 1 diabetes. Iedere interventie die de kans op hypoglykemieën vermindert moet dan ook worden gezien als een belangrijke factor in de verbetering van kwaliteit van leven.
Insuline degludec en insuline glargine U300 worden in de dagelijkse praktijk bij de behandeling van type 1 diabetes vaak gebruikt. Hierbij is het argument van de verbeterde farmacokinetiek t.o.v. de eerste generatie langwerkende insulines een veel gebruikt argument. Bij patiënten met een al langer bestaande type 1 diabetes en een verminderde kwaliteit van leven door optreden van hypoglykemieën lijkt het voor behandelaar en patiënt een voor de hand liggende keuze om over te stappen op de tweede generatie insuline-analogen. Bij mensen met type 1 diabetes wordt soms primair voor de tweede generatie langwerkende insuline-analogen degludec en glargine U300 gekozen.
Op grond van de beschikbare data lijken er geen argumenten te bestaan die het switchen van eerste- naar tweede generatie langwerkende insuline-analogen ondersteunen om de hypoglykemiefrequentie te verminderen, het HbA1c te verlagen en/of de kwaliteit van leven te verbeteren. Echter, de beschikbare data zijn beperkt en het verschil in ernst en frequentie van hypoglykemie verschilt sterk per individuele patiënt. Bovendien worden mensen met een verhoogd risico op ernstige hypoglykemieën (zoals bij ‘hypoglycemia unawareness’ of bij ernstige hypoglykemieën in het recente verleden) maar ook ouderen en patiënten met ernstige cardiovasculaire aandoeningen vaak niet in de studies geïncludeerd. Daarom kan er nog steeds een reden zijn om in specifieke gevallen, met name bij mensen met problematische hypoglykemieën, een proefbehandeling met tweede generatie langwerkende insuline-analogen te proberen en kritisch te beoordelen of hiermee de ernst en frequentie van hypoglykemieën afneemt en de kwaliteit van leven verbetert. Iedere behandelaar van patiënten met type 1 diabetes kent individuele gevallen waarbij de switch van eerste- naar tweede generatie langwerkende insuline-analogen leidde tot klinisch relevante verbetering. De beschikbare data ondersteunen echter niet een primaire en brede inzet van tweede generatie langwerkende insuline-analogen. Indien iemand met diabetes type 1 al goed ingesteld is op een tweede generatie langwerkend insuline analogon, is er geen reden om deze therapie te wijzigen in therapie met een eerste generatie langwerkend insuline analogon. Reden daarvoor is dat een dergelijke switch bijna altijd gepaard gaat met een verslechtering van de glucoseregulatie, hetgeen ook kosten met zich meebrengt en waardoor mensen weer terug zullen worden gezet naar de oorspronkelijke behandeling. Het geringe prijsverschil tussen de eerste en tweede generatie insuline analogen rechtvaardigt deze switch volgens de commissie evenmin.
Alvorens er wordt overgegaan op een proefbehandeling met tweede generatie langwerkende insuline-analogen dient men zich ervan te vergewissen dat geen verdere winst valt te behalen van betere afstemming van medicatie, voeding en beweging om stabielere glucoseregulatie met minder hypo- en hyperglykemieën te bewerkstelligen.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Slechts een kleine minderheid van alle mensen met type 1 diabetes slaagt er in om de HbA1c streefwaarde lager dan 53 mmol/mol en/of time in range boven de 70% te behalen. De belangrijkste beperking hierbij is het optreden van vaak onverwachte, storende, en soms levensbedreigende hypoglykemieën. Iedere strategie die kan leiden tot afname van hypoglykemieën zonder stijging van het HbA1c en/of een afname in time in range van de glucosewaarden zal voor de meeste patiënten als belangrijke winst worden beschouwd bij het voorkómen van complicaties en het verbeteren van kwaliteit van leven. Hierbij moet rekening worden gehouden met de extra belasting die een nieuwe interventie (denk bv. aan een insulinepomp) met zich meebrengt. Vervanging van een eerste- door een tweede-generatie langwerkend insuline-analoog is een eenvoudige interventie met weinig tot geen extra belasting. Om deze reden zal er voor individuele patiënten uit oogpunt van belasting en gebruiksgemak geen reden zijn om een voorkeur te hebben voor eerste- of tweede generatie insuline analogen. Indien er ook geen voordeel mee kan worden gehaald, en het bijwerkingenprofiel vergelijkbaar is, is het onwaarschijnlijk dat mensen met type 1 diabetes een specifieke voorkeur zullen hebben. In specifieke situaties kan een tweede generatie insuline-analoog voordelen hebben. Het gaat dan bijvoorbeeld om patiënten die moeite hebben met het toedienen van de langwerkende insuline op een vast tijdstip (bijvoorbeeld door wisselende diensten of vanwege vaak passeren van tijdzones) of wanneer bijvoorbeeld de thuiszorg niet kan garanderen dat de langwerkende insuline altijd op hetzelfde tijdstip wordt toegediend of andere redenen waardoor patiënten moeilijk of nauwelijks in staat zijn om dagelijks insuline te spuiten.
Kosten (middelenbeslag)
De prijs van insuline glargine U300 vergeleken met insuline glargine U100 ligt ongeveer 50% hoger (Farmacotherapeutisch Kompas 2021, wegwerpspuit). Bij een gebruik van 24 E glargine/dag bedragen de kosten op jaarbasis 252,58 vs 379,89 euro. De kosten van insuline degludec bedragen volgens dezelfde berekening 334,92 euro/jaar, van dezelfde producent kost de eerste generatie langwerkende insuline analoog detemir 303,97 euro/jaar, een verschil van 10%. Vaak worden door ziektekostenverzekeraars afspraken met fabrikanten gemaakt waardoor deze gemelde verschillen in werkelijkheid anders kunnen uitvallen.
In het algemeen kan worden gesteld dat de uitkomsten van onze literatuur-analyse de hogere prijs van de tweedegeneratie langwerkende insuline-analogen niet rechtvaardigen. Echter, zoals eerder aangegeven, kan er in individuele patiënten gezondheidswinst worden bereikt. In deze gevallen is het verschil in kosten gerechtvaardigd. Het huidige preferentiebeleid van ziektekostenverzekeraars bemoeilijkt soms de volledige vergoeding van tweedegeneratie langwerkende insuline-analogen; in deze individuele gevallen dient hiervoor een oplossing te worden gevonden.
Aanvaardbaarheid, haalbaarheid en implementatie
Behoudens het preferentiebeleid van en de vergoeding door ziektekostenverzekeraars zijn er geen belemmeringen t.a.v. aanvaardbaarheid, haalbaarheid en implementatie
Onderbouwing
Achtergrond
- Wat is de huidige situatie?
- Beschrijf kort het knelpunt dat ten grondslag ligt aan de uitgangsvraag (bijv. praktijkvariatie, kosten, nieuwe therapie, acceptatie door patiënten)
Type 1 diabetes wordt gekenmerkt door (vrijwel) volledige afwezigheid van endogene insulineproductie door de betacellen in de eilandjes van Langerhans in de pancreas. Mensen met type 1 diabetes zijn daarom afhankelijk van insulinebehandeling, middels subcutane injecties meerdere malen per dag of middels een continue subcutane pomp. Ondanks intensieve insulinetherapie in combinatie met frequente glucosecontrole is optimale glucoseregulatie slechts voor een minderheid van de type 1 diabetespopulatie bereikbaar en zijn zowel hyper- als hypoglykemieën een dagelijkse realiteit.
Patiënten met type 1 diabetes die met multipele subcutane injecties worden behandeld gebruiken meerdere malen per dag een kortwerkende insuline-analoog, en daarnaast een langwerkende insuline-analoog die meestal 1x daags wordt geïnjecteerd. In de afgelopen jaren zijn er nieuwe langwerkende insuline-analogen beschikbaar gekomen. Deze uitgangsvraag gaat over de bijdrage van deze nieuwe insuline-analogen, die een ander werkingsprofiel hebben dan de al langer bestaande langwerkende insuline-analogen, aan de diabetesregulatie. Hierbij gaat het zowel om verbetering van de glucoseregulatie uitgedrukt in HbA1c, time in range (TIR) en hypoglykemieën, kwaliteit van leven, alsook om eventuele nadelige effecten. Aangezien de nieuwere langwerkende insuline-analogen in het algemeen duurder zijn dan de oudere, dient ook het kosten-aspect te worden beoordeeld
Conclusies / Summary of Findings
HbA1c levels (crucial outcome)
Severe hypoglycemia (crucial outcome)
|
Moderate GRADE |
Use of 2nd generation long-acting insulin analogues probably results in little to no difference in the incidence of severe hypoglycemia as compared with 1st generation long-acting insulin analogues in people with type 1 diabetes
Hemmingsen (2021), Danne (2020) |
Ketoacidosis (crucial outcome)
|
Low GRADE |
Use of 2nd generation long-acting insulin analogues may result in little to no difference in the incidence of diabetic ketoacidosis as compared with 1st generation long-acting insulin analogues in people with type 1 diabetes
Hemmingsen (2021) |
Health related quality of life (crucial outcome)
|
Moderate GRADE |
Use of 2nd generation long-acting insulin analogues probably results in little to no difference in health related quality of life as compared with 1st generation long-acting insulin analogues in people with type 1 diabetes
Hemmingsen (2021) |
Hypoglycemia (important outcome)
|
Moderate GRADE |
Use of 2nd generation long-acting insulin analogues probably results in little to no difference in the incidence of hypoglycemia as compared with 1st generation long-acting insulin analogues in people with type 1 diabetes
Hemmingsen (2021), Holmes (2015), Matsuhisa (2016), Danne (2020) |
Nocturnal hypoglycemia (important outcome)
|
Moderate GRADE |
Use of 2nd generation long-acting insulin analogues probably results in little to no difference in the incidence of nocturnal hypoglycemia as compared with 1st generation long-acting insulin analogues in people with type 1 diabetes
Hemmingsen (2021), Holmes (2015), Matsuhisa (2016), Danne (2020) |
Serious adverse events (important outcome)
|
Moderate GRADE |
Use of 2nd generation long-acting insulin analogues probably results in little to no difference in the onset of serious adverse events as compared with 1st generation long-acting insulin analogues in people with type 1 diabetes
Hemmingsen (2021), Holmes (2015), Matsuhisa (2016), Danne (2020) |
Non-serious adverse events (important outcome)
|
Moderate GRADE |
Use of 2nd generation long-acting insulin analogues probably results in little to no difference in the onset of non-serious adverse events as compared with 1st generation long-acting insulin analogues in people with type 1 diabetes
Hemmingsen (2021), Holmes (2015), Matsuhisa (2016), Danne (2020) |
Injection site reactions (important outcome)
|
Low GRADE |
Use of 2nd generation long-acting insulin analogues probably results in little to no difference in the onset of injection site reactions as compared with 1st generation long-acting insulin analogues in people with type 1 diabetes
Davies (2014), Heller (2012), Holmes (2015), Mathieu C (2013), Matsuhisa (2016) |
All-cause mortality (important outcome)
|
- GRADE |
Due to the lack of data, it is not possible to draw a conclusion about the effect of 2nd generation long-acting insulin analogues as compared with 1st generation long-acting insulin on all-cause mortality in people with type 1 diabetes
Hemmingsen (2021) |
Samenvatting literatuur
Design
Hemmingsen (2021) performed a systematic review with meta-analysis of 26 randomized controlled trials with a duration of 24 weeks or more comparing one long-acting insulin to NPH insulin or another long-acting insulin in people with type 1 diabetes mellitus. The literature search was performed until 24 August 2020.
Participants
Non-pregnant people (adults and children) with type 1 diabetes were included.
Exposure definitions
In Hemmingsen (2021), the intervention was defined as long-term treatment with (ultra-)long-acting insulin analogues and compared with NPH insulin (neutral protamine Hagedorn) or another (ultra-)long-acting insulin analogue. In order to make the exposure definition of Hemmingsen (2021) compatible to the PICO, the exposure definitions were adapted as follows:
The intervention treatment was defined as 2nd generation long-acting insulin analogues: Insulin degludec or Insulin glargine U300.
The comparator treatment was defined as 1st generation long acting insulin analogues: Insulin glargine U100, Insulin detemir.
Comparisons included
2nd generation 1st generation
Insulin degludec with Insulin detemir
Insulin degludec with Insulin glargine U100
One study (Urakami, 2017) did not provide the concentration of glargine (neither in the SR of Hemmingsen (2021) nor in the original publication, nor in clinicaltrials.gov). In this trial the concentration of glargine was hand searched through other publications and it was found that glargine U100 was given (Danne 2020).
In Hemmingsen (2021), no studies on glargine U300 were reported. We have sent a mail to the authors to ask why these trials were not included. Their answer was that the trials on glargine U300 were compared with glargine U100 trials and that they did not include trials comparing same type of insulin in different concentration. Since another meta-analysis did compare glargine U300 with glargine U100 in type 1 diabetes (Danne 2020) and fits the PICO, this meta-analysis was added. Its design is described below.
Design
Danne (2020) performed a post hoc meta-analysis from three randomized, multicenter, 6-month similarly designed phase 3 trials in which insulin glargine U300 was compared to glargine U100 in type 1 diabetes.
Participants
People (adults and children) with type 1 diabetes were included.
Exposure definitions
The intervention treatment was defined as 2nd generation long-acting insulin analogue: Insulin glargine U300.
The comparator treatment was 1st generation long acting insulin analogue: Insulin glargine U100
The total follow-up in all 3 studies was 6 months.
Outcomes
The systematic review of Hemmingsen (2021) reported the following outcomes: HbA1c levels, severe hypoglycemia, hypoglycemia, nocturnal hypoglycemia, diabetic ketoacidosis (DKA), health-related quality of life, serious adverse effects, adverse events, socioeconomic effects, all-cause mortality
The post hoc meta-analysis of Danne (2020) reported the following outcomes per trial: HbA1c levels, severe hypoglycemia. The original trials that were meta-analyzed by Danne (Home 2015, Mathuhisa 2016 and Danne 2020) also reported on the outcomes, hypoglycemia, nocturnal hypoglycemia, serious adverse effects and all cause-mortality. These outcomes from the original trials were added as well.
Conflicts of interest
In the study from Hemmingsen (2021), the first author was funded by The Leona M. and Harry B. Helmsley Charitable Trust as part of the Addressing the Challenge and Constraints of Insulin Sources and Supply (ACCISS) Study. The study from Danne (2020) was sponsored by the manufacturer of glargine (Sanofi) and writing assistance was provided by Sanofi.
Study characteristics
In the pooled study from Hemmingsen (2021) and Danne (2020), a total of 3538 participants were randomized: 2001 participants were randomized to second generation long-acting insulin analogues and 1537 were randomized to 1st generation long-acting insulin analogues. Three of the studies included children only and randomized 831 children, i.e. 23% of all participants. The remaining six studies included adults only. The proportion of participants finishing the studies varied from 81% to 100%. One study had a cross-over design (Urakami 2017). The remaining studies were parallel-group RCTs. All studies had an open-label design. All studies except one were multicenter studies (Urakami 2017). All studies included both genders. The mean age of the participants varied from 10 to 54 years. The average duration of type 1 diabetes varied from 3.9 to 23.2 years. All studies had predefined HbA1c as the primary outcome.
Results
In the systematic review of Hemmingsen (2021) and post hoc meta-anlysis of Danne (2020) an interaction analysis showed that results were similar in adults and children, after which the results were pooled. This strategy is also followed in the current systematic review.
HbA1c levels (critical outcome)
The mean difference in HbA1c levels at time of baseline and end of follow-up were expressed as percentages and were reported in 8 studies. The included studies showed a mean difference of 0.07% (95% CI 0.01 to 0.19), indicating a higher, but clinically non-important reduction of HbA1c in the control group compared to the intervention group. Effects were similar for adults and children. Lost to follow-up for this outcome was >14% in some of these studies (i.e., BEGIN Basal-Bolus type 1, BEGIN Flex T1, EDITION 4, EDITION JP)
Figure 1. Forest plot of comparison 2nd generation vs 1st generation long acting insulins, outcome HbA1c*
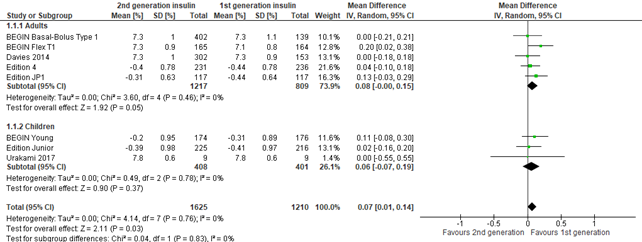
* The minus signs in the forest plot (see ‘mean’ HbA1C level for the studies Edition 4, Edition JP1, BEGIN Young and Edition Junior) denote mean differences within each treatment arm
Severe hypoglycemia (critical outcome)
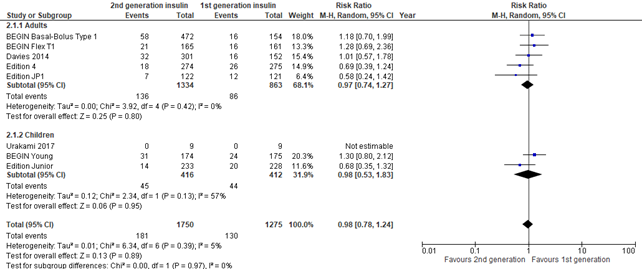
In the systematic reviews of Hemmingsen (2021) and post hoc meta-analysis of Danne (2020), severe hypoglycemia was defined as: requiring assistance from another person (was planned to be further categorized into 'assistance from other persons', assistance from medical staff, intravenous glucose administration, subcutaneous glucagon administration, hospitalization, intensive-care unit stay, coma). Overall, no differences in severe hypoglycemia were found between intervention and control group (risk ratio 0.98; 95% CI, 0.78-1.24) . Results were similar for adults and children. There was inconsistency between the two meta-analyses, where the three glargine U300 trials combined (reported by Danne, 2020) showed a lower risk for severe hypoglycemia for second generation long-acting insulin analogues (pooled risk ratio 0.67; 95% CI, 0.67-0.99), while the other trials (reported by Hemmingsen, 2021) showed no difference for severe hypoglycemia (pooled risk ratio 1.19; 95% CI, 0.91-1.56).
Figure 2. Forest plot of comparison 2nd generation vs 1st generation long acting insulins, outcome severe hypoglycemia
Diabetic ketoacidosis (critical outcome)
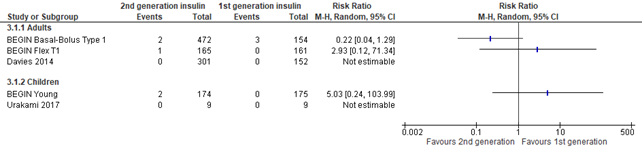
In the systematic review of Hemmingsen (2021), diabetic ketoacidosis was defined as potentially life-threatening condition with high levels of ketones in the body which when building up in the blood make the blood more acidic. This outcome was reported in 5 studies. Only few events were noted (n=8) making the results imprecise as shown by the large confidence intervals. Due to small numbers and imprecision the results were not pooled in the Forest plot.
Figure 3. Forest plot of comparison 2nd generation vs 1st generation long acting insulins, outcome diabetic ketoacidosis
Health-related quality of life (critical outcome)
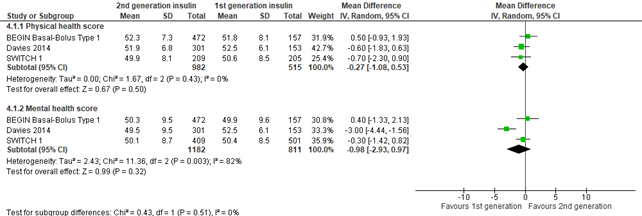
In the systematic review of Hemmingsen (2021), health-related quality of life was defined as mental and physical health-related quality of life and evaluated by a validated instrument: Short-Form-36 (higher values mean better health related quality of life)). The 3 included studies showed a similar physical health score in those randomized to second generation versus first generation long-acting insulin analogues (mean difference -0.27; 95% CI, -1.08 to 0.53). The 3 studies also showed a similar mental health score in those randomized to second generation versus first generation long-acting insulin analogues (mean difference -0.98; 95% CI, -2.93 to 0.97). The results on mental health score were inconsistent, i.e., Davies (2014) found a statistically significant effect where mental health was better in first generation long-acting insulin analogues, but the effect was small (-3.00; 95% CI, -4.44 to -1.56) and did not reach the threshold of clinical significance (0.5*SD). Results from SWITCH 1 concerned unpublished data.
Figure 4. Forest plot of comparison 2nd generation vs 1st generation long acting insulins, outcome health related quality of life
Hypoglycemia (important outcome)
In the systematic review of Hemmingsen (2021) hypoglycemia was defined as: hypoglycemic episodes not requiring assistance from another person. In the 3 trials that were reported by Danne (2020), hypoglycemia was reported as symptomatic hypoglycemic episode plus a glucose measurement of < 3.9 mmol/L (< 70 mg/dL ). All 8 included studies showed a consistent similar risk of hypoglycemia in those randomized to second generation versus first generation long-acting insulin analogues (pooled risk ratio 1.01; 95% CI, 1.00-1.03).
Figure 5. Forest plot of comparison 2nd generation vs 1st generation long acting insulins, outcome hypoglycemia
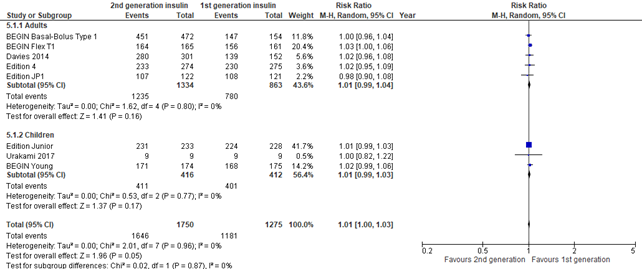
Nocturnal hypoglycemia (important outcome)
In the systematic review of Hemmingsen (2021) and the 3 trials that were reported by Danne (2020), nocturnal hypoglycemia was defined as hypoglycemia during night-time and defined as reported in studies. All 8 included studies showed no increased risk of nocturnal hypoglycemia in those randomized to second generation versus first generation long-acting insulin analogues (risk ratio 1.01; 95% CI, 0.95-1.17). There was some inconsistency as the results of Edition JP1 showed a protective effect of nocturnal hypoglycemia in those randomized to second generation long-acting insulin analogues (risk ratio 0.77; 95% CI, 0.62-0.95).
Figure 6. Forest plot of comparison 2nd generation vs 1st generation long acting insulins, outcome nocturnal hypoglycemia
Socioeconomic effects (important outcome)
In the systematic review of Hemmingsen (2021), socioeconomic effects were defined as direct costs defined as a consequence of admission or readmission rates; average length of stay; visits to general practitioner; accident or emergency visits; medication consumption; indirect costs defined as resources lost due to illness by the participant or their family member.
No studies reported the costs of the intervention during the study period.
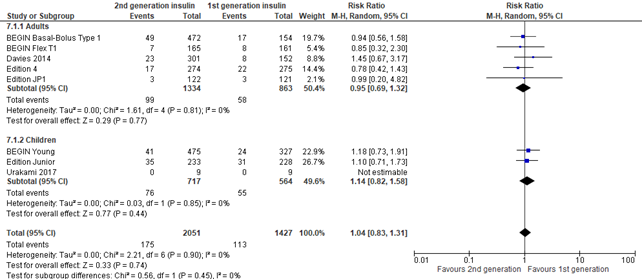
Serious adverse events (important outcome)
In the systematic review of Hemmingsen (2021) and the 3 trials that were reported by Danne (2020), serious adverse events (SAE) were defined according to the International Conference on Harmonization (ICH) guidelines as: any event that leads to death, that is life-threatening, required in-patient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability, and any important medical event which may have had jeopardized the patient or required intervention to prevent it (ICH 1997) or as reported in studies. Overall no difference was seen in SAEs between intervention and control group (risk ratio 1.04; 95% CI, 0.83-1.31), but there was imprecision as shown by the large pooled confidence interval.
Figure 7. Forest plot of comparison 2nd generation vs 1st generation long acting insulins, outcome serious adverse events
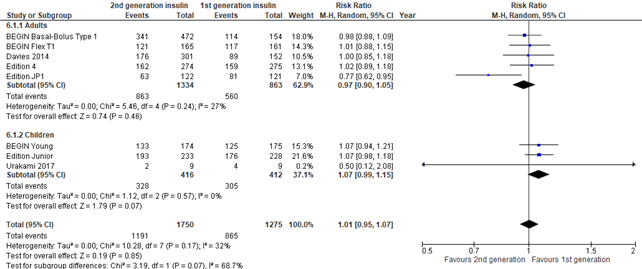
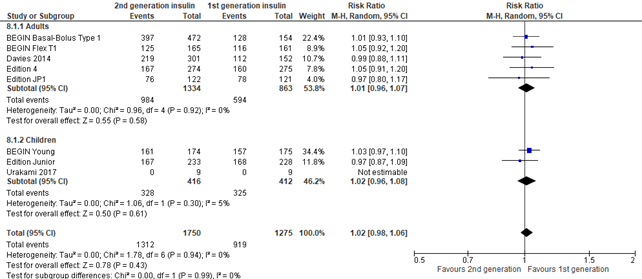
Non-serious adverse events (important outcome)
In the systematic review of Hemmingsen (2021), non-serious adverse events were defined as all adverse events, not classified as SAEs. In the 3 trials that were reported by Danne (2020), non-serious adverse events were defined as all adverse events that were experienced in > 5.0% of participants. Overall no difference was seen in non-serious adverse events between intervention and control group (risk ratio 1.02; 95% CI, 0.98-1.06).
Figure 8. Forest plot of comparison 2nd generation vs 1st generation long acting insulins, outcome non-serious adverse events
Injection site reactions (important outcome)
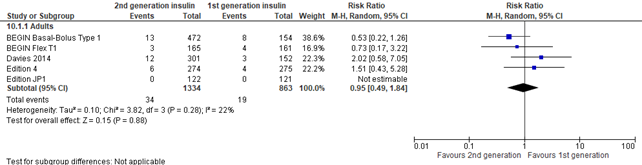
The outcome ‘injection site reaction’ was not reported in the systematic reviews of Hemmingsen (2021) and Danne (2020). Therefore, we searched in the original 8 studies that were reported by Hemmingsen (2021) and Danne (2020) to see if they reported injection site reactions. This outcome was reported by all 5 studies in adults (BEGIN Basal-Bolus type 1, Begin Flex T1, Davies (2014), Edition 4, Edition JP1). The outcome ‘injection site reaction’ was not reported in the 3 studies in children (BEGIN Young, Edition Junior, Urakami (2017)). Overall no difference was seen in injection site reactions between intervention and control group (risk ratio 0,95; 95% CI, 0.49-1.84), but the result was imprecise as shown by the pooled large 95% confidence interval.
Figure 9. Forest plot of comparison 2nd generation vs 1st generation long acting insulins, outcome injection site reactions
All-cause mortality (important outcome)
All-cause mortality was defined as death from any cause. Only few events were noted (n=6) and the follow-up of the studies to report on this outcome was relatively short (6 months to 2 years). Due to small numbers and imprecision the results were not pooled in the Forest plot.
Figure 10. Forest plot of comparison 2nd generation vs 1st generation long acting insulins, outcome all-cause mortality
Level of evidence of the literature
The level of evidence (GRADE method) is determined per comparison and outcome measure and is based on results from systematic review of randomized trials and therefore starts at level “high”. Subsequently, the level of evidence was downgraded if there were relevant shortcomings in one of the several GRADE domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias.
The level of evidence regarding the outcome measure HbA1c levels was downgraded by 1 level because of risk of bias (1 level, incomplete outcome data).
The level of evidence regarding the outcome measure severe hypoglycemia was downgraded by 1 level because of inconsistency).
The level of evidence regarding the outcome measure diabetic ketoacidosis was downgraded by 2 levels because of imprecision (2 levels, < 10 events in all 5 included studies, in total only 8 events)
The level of evidence regarding the outcome measure health related quality of life was downgraded by 1 level because of inconsistency.
The level of evidence regarding the outcome measure hypoglycemia was downgraded by 1 level because of risk of bias (1 level outcome event somewhat subjective).
The level of evidence regarding the outcome measure nocturnal hypoglycemia was downgraded by 1 level because of risk of bias (1 level outcome event somewhat subjective). Although one trial result was inconsistent to the pooled effect, this was not taken into account in further down grading the evidence, as it was only one trial, and results were adjusted for random effects.
The level of evidence regarding the outcome measure serious adverse event was downgraded by 1 level because of imprecision (1 level, large pooled confidence interval could not rule out a harmful effect).
The level of evidence regarding the outcome measure non-serious adverse event was downgraded by 1 level because of bias (1 level outcome event somewhat subjective).
The level of evidence regarding the outcome measure injection site reaction was downgraded by 2 levels because of imprecision (1 level, large pooled confidence interval could not rule out a protective [or harmful] effect) and indirectness (1 level, outcome not studied in children).
The level of evidence regarding the outcome measure all-cause mortality was ungraded due to lack of data.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the (un)favorable effects of 2nd generation long-acting insulin analogues compared to 1st generation long-acting insulin analogues in patients with Diabetes mellitus type 1?
PICO
P (Patients) : Children and adults with (incident) type 1 diabetes mellitus
I (Intervention) : 2nd generation long-acting insulin analogues: Insulin degludec, Insulin glargine U300
C (Comparison) : 1st generation long acting insulin analogues: Insulin, Insulin detemir, Insulin glargine U100
O (Outcomes): : HbA1c levels, severe hypoglycemia, hypoglycemia, nocturnal hypoglycemia, diabetic ketoacidosis (DKA), time within the target range of blood glucose, time above the target range of blood glucose, time below the target range of blood glucose, health-related quality of life, patient satisfaction (with treatment), serious adverse effects, adverse events/injection site reactions, socioeconomic effects, all-cause mortality.
Relevant outcome measures
The guideline development group a-priori considered HbA1c, severe hypoglycemia, DKA, time within normal range of blood glucose, time above normal range, time below normal range and health related quality of life as critical outcomes for decision making.
Important outcome measures included hypoglycemia, nocturnal hypoglycemia, patient satisfaction (with treatment), serious adverse effects, adverse events/injection site reactions, socioeconomic effects and all-cause mortality
The working group did not define the outcome measures listed above but used the definitions used in the studies.
The working group did not define a minimal clinically (patient) important difference for all outcomes. Therefore, the default thresholds proposed by the international GRADE working group were used: a 25% difference in relative risk (RR< 0.8 or RR>1.25) for dichotomous outcomes, and 0.5 standard deviations (SD) for continuous outcomes.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 14 July 2021. This search strategy can be found in the Methods section. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 607 hits, including 247 systematic reviews and 360 randomized controlled trials (RCTs)
A total of 31 articles were initially selected based on title and abstract screening. After reading the full text, 29 studies were excluded (see the table with reason for exclusion under the tab Methods), and 2 studies were included.
Results
Two meta-analyses were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Danne T, Tamborlane WV, Malievsky OA, Franco DR, Kawamura T, Demissie M, Niemoeller E, Goyeau H, Wardecki M, Battelino T. Efficacy and Safety of Insulin Glargine 300 Units/mL (Gla-300) Versus Insulin Glargine 100 Units/mL (Gla-100) in Children and Adolescents (6-17 years) With Type 1 Diabetes: Results of the EDITION JUNIOR Randomized Controlled Trial. Diabetes Care. 2020 Jul;43(7):1512-1519. doi: 10.2337/dc19-1926. Epub 2020 May 19. PMID: 32430458; PMCID: PMC7305011.
- Danne T, Matsuhisa M, Sussebach C, Goyeau H, Lauand F, Niemoeller E, Bolli GB. Lower risk of severe hypoglycaemia with insulin glargine 300 U/mL versus glargine 100 U/mL in participants with type 1 diabetes: A meta-analysis of 6-month phase 3 clinical trials. Diabetes Obes Metab. 2020 Oct;22(10):1880-1885. doi: 10.1111/dom.14109. Epub 2020 Jul 21. PMID: 32515543; PMCID: PMC7540568.
- Davies MJ, Gross JL, Ono Y, Sasaki T, Bantwal G, Gall MA, Niemeyer M, Seino H; BEGIN BB T1 Study Group. Efficacy and safety of insulin degludec given as part of basal-bolus treatment with mealtime insulin aspart in type 1 diabetes: a 26-week randomized, open-label, treat-to-target non-inferiority trial. Diabetes Obes Metab. 2014 Oct;16(10):922-30. doi: 10.1111/dom.12298. Epub 2014 May 8. PMID: 24702700; PMCID: PMC4237553.
- Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L, Renard E, Russell-Jones D, Philotheou A, Francisco AM, Pei H, Bode B; BEGIN Basal-Bolus Type 1 Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012 Apr 21;379(9825):1489-97. doi: 10.1016/S0140-6736(12)60204-9. PMID: 22521071.
- Haahr, H., & Heise, T. (2014). A review of the pharmacological properties of insulin degludec and their clinical relevance. Clinical pharmacokinetics, 53(9), 787–800. https://doi.org/10.1007/s40262-014-0165-y
- Hemmingsen B, Metzendorf MI, Richter B. (Ultra-)long-acting insulin analogues for people with type 1 diabetes mellitus. Cochrane Database Syst Rev. 2021 Mar 4;3(3):CD013498. doi: 10.1002/14651858.CD013498.pub2. PMID: 33662147; PMCID: PMC8094220.
- Home PD, Bergenstal RM, Bolli GB, Ziemen M, Rojeski M, Espinasse M, Riddle MC. New Insulin Glargine 300 Units/mL Versus Glargine 100 Units/mL in People With Type 1 Diabetes: A Randomized, Phase 3a, Open-Label Clinical Trial (EDITION 4). Diabetes Care. 2015 Dec;38(12):2217-25. doi: 10.2337/dc15-0249. Epub 2015 Jun 17. PMID: 26084341.
- Mathieu C, Hollander P, Miranda-Palma B, Cooper J, Franek E, Russell-Jones D, Larsen J, Tamer SC, Bain SC; NN1250-3770 (BEGIN: Flex T1) Trial Investigators. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013 Mar;98(3):1154-62. doi: 10.1210/jc.2012-3249. Epub 2013 Feb 7. PMID: 23393185; PMCID: PMC3612802.
- Matsuhisa M, Koyama M, Cheng X, Takahashi Y, Riddle MC, Bolli GB, Hirose T; EDITION JP 1 study group. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 1). Diabetes Obes Metab. 2016 Apr;18(4):375-83. doi: 10.1111/dom.12619. Epub 2016 Feb 1. PMID: 26662964; PMCID: PMC5066635.
- Owens DR, S Bailey T, Fanelli CG, Yale JF, Bolli GB. Clinical relevance of pharmacokinetic and pharmacodynamic profiles of insulin degludec (100, 200 U/mL) and insulin glargine (100, 300 U/mL) - a review of evidence and clinical interpretation. Diabetes Metab. 2019 Sep;45(4):330-340. doi: 10.1016/j.diabet.2018.11.004. Epub 2018 Nov 26. PMID: 30496834.
- Porcellati F, Lucidi P, Candeloro P, et al. Pharmacokinetics, pharmacodynamics, and modulation of hepatic glucose production with insulin Glargine U300 and Glargine U100 at steady state with individualized clinical doses in type 1 diabetes. Diabetes Care. 2019;42(1):85-92.
- Thalange N, Deeb L, Iotova V, Kawamura T, Klingensmith G, Philotheou A, Silverstein J, Tumini S, Ocampo Francisco AM, Kinduryte O, Danne T. Insulin degludec in combination with bolus insulin aspart is safe and effective in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2015 May;16(3):164-76. doi: 10.1111/pedi.12263. Epub 2015 Feb 12. PMID: 25683037; PMCID: PMC4413367.
- Urakami T, Mine Y, Aoki M, Okuno M, Suzuki J. A randomized crossover study of the efficacy and safety of switching from insulin glargine to insulin degludec in children with type 1 diabetes. Endocr J. 2017 Feb 27;64(2):133-140. doi: 10.1507/endocrj.EJ16-0294. Epub 2016 Oct 14. PMID: 27746408.
Evidence tabellen
Tabel 1. Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question: Are there (un)favorable effects of 2nd generation long-acting insulin analogues compared to 1st generation long-acting insulin analogues in Diabetes mellitus type 1?
Tabel 2. Table of quality assessment for systematic reviews of RCTs
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/not applicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Hemmingsen (2014) |
Yes |
Unclear. Comparisons of glargine U300 vs glargine U100 were not included in the search strategy |
Yes |
Yes |
not applicable |
Yes |
Yes |
Yes. Publication bias could not be assessed because there were fewer than 10 included studies |
Yes. Sponsored The Leona M. and Harry B. Helmsley Charitable Trust as part of the Addressing the Challenge and Constraints of Insulin Sources and Supply (ACCISS) Study. |
|
Danne (2020) |
Yes |
Unclear. Only trials from the EDITION program were added |
No. Only trials from the EDITION program were added |
No. Only study characteristics of the pooled trials were provided in the appendix |
not applicable |
Yes |
Yes |
Yes. Publication bias could not be assessed because there were fewer than 10 included studies |
Yes. Sponsored by Sanofi |
- Research question (PICO) and inclusion criteria should be appropriate and predefined
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs)
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table etc.)
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (e.g. Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (e.g., funnel plot, other available tests) and/or statistical tests (e.g., Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Tabel 3. Table of excluded studies
|
Author |
Reason for exclusion |
|
Alves (2017) |
Congress abstract only, too few information to know if it fits PICO |
|
Atkin (2013) |
more recent SR |
|
Bolli (2016) |
Abstract only, too few information to know if it fits PICO |
|
Chubb (2013) |
more recent SR |
|
Culic (2016) |
more recent SR |
|
Dawoud (2015) |
more recent SR |
|
Dawoud (2018) |
more recent SR |
|
Diez-Fernandez (2019) |
more recentSR |
|
Dzygalo (2014) |
more recent SR |
|
Dzygalo (2014) |
more recent SR |
|
Einhorn (2015) |
Abstract only, too few information to know if it fits PICO |
|
Ericsson (2013) |
more recent SR |
|
Freemantle (2013) |
more recent SR |
|
Heller (2016) |
more recent SR |
|
Holmes (2019) |
more recent SR |
|
Liu (2018) |
more recent SR |
|
Mathews (2016) |
more recent SR |
|
Meneghini (2016) |
more recent SR |
|
Monami (2013) |
more recent SR |
|
Philis-Tsimikas (2016) |
more recent SR |
|
Ratner (2013) |
more recent SR |
|
Rodbard (2013) |
more recent SR |
|
Russell-Jones (2015) |
more recent SR |
|
Siegmund (2017) |
more recent SR |
|
Sorli (2013) |
more recent SR |
|
Vora (2014) |
more recent SR |
|
Wang (2012) |
more recent SR |
|
Yfantopoulos (2017) |
more recent SR |
|
Zhang (2018) |
Abstract only, too few information to know if it fits PICO |
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld :
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
