Corticosteroïden
Uitgangsvraag
Wat is de plaats van corticosteroïden bij de behandeling van COVID-19 patiënten?
Aanbeveling
Behandel patiënten met COVID-19 bij wie zuurstoftoediening geïndiceerd is en met name bij patiënten waarbij de zuurstoftherapie geëscaleerd moet worden naar invasieve respiratoire ondersteuning met dexamethason 6 mg per dag gedurende maximaal 10 dagen.
Bij patiënten zonder extra zuurstofbehoefte wordt behandeling met corticosteroïden niet aangeraden.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek verricht naar de verschillen in klinische uitkomsten tussen behandeling met en zonder corticosteroïden. Corticosteroïden die moesten worden geïnhaleerd werden in dit literatuuronderzoek niet opgenomen. Tot en met 2 september 2021 werden er 11 gerandomiseerde gecontroleerde studies (RCTs) gevonden in patiënten die waren opgenomen in het ziekenhuis (n=2989 in de interventiegroep en n=5018 in de controlegroep). De grootste studie die werd meegenomen was de RECOVERY trial (Horby, 2021), met meer dan 6000 geïncludeerde patiënten. Er werden geen studies gevonden die aan de selectiecriteria voldeden met ambulante patiënten.
De geïncludeerde studies onderzochten de volgende corticosteroïden: dexamethason (Tomazini, 2020; Jamaati, 2021; Horby, 2021), hydrocortison (Angus, 2020; Dequin, 2020; Munch, 2021), of methylprednisolon (Jeronimo, 2020; Edalatifard, 2020; Corral-Gudino, 2021; Solanich, 2021; Tang, 2021). De cruciale uitkomstmaten voor de besluitvorming waren mortaliteit en de noodzaak voor invasieve respiratoire ondersteuning. Daar waar mogelijk werd data van verschillende typen corticosteroïden gecombineerd om tot overkoepelende literatuurconclusies te komen over de effecten van corticosteroïden. Wanneer er enkel data aanwezig was over één type corticosteroïd werd het middel in de literatuurconclusies specifiek benoemd. Ook werden de effecten van corticosteroïden op de mortaliteit, in een subgroep analyse, opgesplitst naar ernst van ziekte: patiënten met milde, matige en ernstige COVID-19 symptomen op basis van respiratoire ondersteuning op moment van inclusie.
Er werden alleen randomiseerde trials geïncludeerd in de analyse, waardoor de kwaliteit van bewijs initieel hoog was. Omdat dat er een aantal open-label trials waren (waaronder de RECOVERY trial), met een mogelijk risico op vertekening van de studieresultaten (risk of bias) bij subjectieve uitkomstmaten, werd de kwaliteit van dit bewijs waar nodig naar beneden bijgesteld. Daarnaast waren er meerdere studies met een relatief kleine populatie en mede hierdoor een grote spreiding van het betrouwbaarheidsinterval rondom de puntschatter van de uitkomstmaat (imprecision), waardoor de kwaliteit van dit bewijs ook naar beneden werd bijgesteld.
Systemische corticosteroïden bij patiënten die waren opgenomen in het ziekenhuis
Met een redelijke zekerheid kan worden geconcludeerd dat er een reductie in de mortaliteit optreedt bij het gebruik van corticosteroïden (risicoverschil: -4,33%, 95% CI: -8,58% tot -0,07%; relatief risico: 0,89, 95% CI: 0,79 tot 1,02). Dit verschil werd door de werkgroep gedefinieerd als klinisch relevant. De data betroffen grotendeels patiënten met redelijke tot ernstige COVID-19; alleen de RECOVERY trial (Horby, 2021) includeerde ook patiënten met milde COVID-19.
De RECOVERY trial van Horby (2021) splitste de data van de mortaliteit ook op naar ernst van ziekte. De subgroep met ernstige ziekte kon worden gepoold in een meta-analyse samen met data van andere studies die enkel patiënten met ernstige ziekte includeerden (Tomazini, 2020; Angus, 2020). Bij het gebruik van corticosteroïden in de groep patiënten die als ernstig ziek kon worden aangemerkt, werd met een hoge zekerheid geconcludeerd dat er een klinisch relevante reductie in de mortaliteit optreedt (risicoverschil: -9,18%, 95% CI: -14,13% tot -4,23%; relatief risico: 0,82, 95%CI: 0,68 tot 0,98). Dit effect was minder groot, maar nog steeds statistisch significant, bij de patiënten met een matig-ernstige COVID (risicoverschil: -2,9%). Omdat dit verschil minder was dan de vooraf gedefinieerde grens van klinische relevantie (3% punten verschil), wordt in de conclusie beschreven dat dexamethason nauwelijks verschil maakt in de mortaliteit na 28 dagen. Echter, vanwege de grootte en de kwaliteit van de studie van Horby (2021), en de relatief beperkte bijwerkingen van dexamethason, wordt de risicoreductie van 2,9% nog steeds als relevant en betrouwbaar beschouwd. In de groep patiënten met milde COVID-19 (waarbij geen zuurstof suppletie nodig was) was er mogelijk een kleine, maar klinisch relevante, toename in de mortaliteit bij het gebruik van corticosteroïden (in dit geval dexamethason).
Het is nog niet duidelijk waarom er geen mortaliteitswinst is in de ‘milde groep’ zonder zuurstofbehoefte of bij personen die minder dan 7 dagen ziek waren. Het is daarbij niet helder wat daarbij het meest bepalend was: de ernst van infectie (dus de daling van de zuurstofsaturatie bij een aantal patiënten in die totale groep met een duur van symptomen van 7 dagen of minder) of alleen de duur van de symptomen. Deze bevindingen geven richting aan gebruik van dexamethason: vooral in de latere fase bij matig of ernstig zieke patiënten met extra zuurstofbehoefte waar (hyper)inflammatie op de voorgrond staat. Die zuurstofbehoefte is leidend vanwege de subgroep analyse in de studie van Horby (2021), maar mogelijk is gebruik van dexamethason ook bij slechts een korte duur (< 7 dagen) van symptomen niet effectief. Preventief gebruik in een vroege fase van infectie moet worden afgeraden, behalve als dat vanwege een andere indicatie (bijv. exacerbatie COPD) moet worden voorgeschreven. Een retrospectieve studie pleit voor gebruik van corticosteroïden alleen bij ernstige infecties, hier gedefinieerd door CRP >200 mg/L (vs. CRP <100 mg/L) (Keller, 2020). De RCTs in deze richtlijn gebruiken deze criteria niet en in het advies wordt een dergelijk afkappunt dan ook niet geadviseerd.
Uitkomsten betreffende invasieve respiratoire ondersteuning werden heterogeen gerapporteerd in de studies. Zo werd bijvoorbeeld de noodzaak voor invasieve ventilatie gerapporteerd, maar ook het aantal dagen aan mechanische ventilatie, het aantal dagen zonder respiratoire ondersteuning, de noodzaak voor high flow zuurstof of ventilatoire ondersteuning en de noodzaak voor respiratoire ondersteuning. Hierdoor zijn voor deze specifieke uitkomsten veelal weinig data beschikbaar. Met een lage tot zeer lage zekerheid werd geconcludeerd dat er bij verschillende typen corticosteroïden een klein tot geen effect zou kunnen optreden voor de verschillende uitkomsten die voor invasieve respiratoire ondersteuning werden gerapporteerd of dat het bewijs onzeker was over het effect. De studie van Horby (2021) liet wel een puntschatter zien in het voordeel van corticosteroïden.
Belangrijke uitkomstmaten waren de duur van hospitalisatie en de tijd tot klinische verbetering. Met een lage zekerheid werd geconcludeerd dat er een klein tot geen effect zou kunnen optreden op de duur van hospitalisatie. Het bewijs over het effect van corticosteroïden (in dit geval alleen methylprednisolon) op de tijd tot symptoomresolutie was erg onzeker, waarbij data over andere middelen ontbrak.
Soort corticosteroïden bij patiënten die waren opgenomen in het ziekenhuis
Omdat de geïncludeerde studies verschillende middelen onderzochten werden ze ook geclusterd geanalyseerd op basis van het middel dat verstrekt werd in de studie: dexamethason (Tomazini, 2020; Jamaati, 2021; Horby, 2021), hydrocortison (Angus, 2020; Dequin, 2020; Munch, 2021), of methylprednisolon (Jeronimo, 2020; Edalatifard, 2020; Corral-Gudino, 2021; Solanich, 2021; Tang, 2021). Zie de bijlage voor de literatuurbeoordelingen en -conclusies per middel. Een head-to-head vergelijking van de verschillende corticosteroïden is op dit moment niet beschikbaar.
Voor alle verschillende corticosteroïden was de gepoolde puntschatter van de risk ratio in het voordeel van het betreffende middel. Op basis van deze risk ratio’s is er geen voordeel uit te spreken voor een bepaald type corticosteroïden. Wel is er veruit het meeste bewijs beschikbaar voor dexamethason, omdat de grootste geïncludeerde studie (Horby, 2021) hier onderzoek naar deed. In het advies wordt er om deze reden een voorkeur voor dexamethason uitgesproken.
Duur van de behandeling met corticosteroïden
In de geïncludeerde studies worden niet alleen verschillende middelen onderzocht, ook de duur en de dosis van de behandeling is verschillend. De behandelduur varieert van enkele dagen tot 28 dagen. Het meeste bewijs komt uit de studie van Horby (2021), waar patiënten gedurende 10 dagen met 6 mg dexamethason werden behandeld of tot en met de dag dat ze uit het ziekenhuis werden ontslagen. In het advies zullen wij deze behandelduur aanhouden.
Overige overwegingen
Dosering
Klinische dose-finding studies voor corticosteroïden zijn niet gedaan. Er is op dit moment onvoldoende data over het gebruik van hogere doses corticosteroïden dan de dosis gebruikt in de RECOVERY-studie. Een recente RCT uit Iran met 86 patiënten laat zien dat 2 mg/kg/dag methylprednisolon geassocieerd is met een sneller klinisch herstel, kortere opname duur en kleinere kans op progressie naar invasieve beademing, vergeleken met 6 mg dexamethason per dag (Ranibar, 2021). Echter, deze studie is relatief klein en van matige kwaliteit en vergelijkt bovendien twee verschillende doseringen van twee verschillende middelen. Een grote RCT in Europa en India met 982 deelnemers met tenminste 10 liter zuurstofbehoefte (COVID STEROID 2 Trial group, 2021), randomiseerde naar 12 mg of 6 mg dexamethason. Er werd geen statistisch significant verschil gezien qua dagen vrij van orgaanondersteuning (22,0 dagen bij 12 mg versus 20,5 dagen bij 6 mg dexamethason) of mortaliteit na 28 dagen (27,1% bij 12 mg versus 32,3% bij 6 mg dexamethason). Wel rapporteren de auteurs dat de uitkomsten allebei neigen naar een voordeel van een hogere dosering dexamethason zonder dat er een verschil was in de hoeveelheid bijwerkingen. Een Italiaanse RCT randomiseerde 301 patiënten naar methylprednisolon boven op de standaard behandeling met dexamethason 6 mg (Salvarani, 2022). In deze studie werd geen voordeel gezien van aanvullende behandeling met methylprednisolon.
Al met al is er op dit moment geen positief bewijs voor behandeling met een hogere dosering corticosteroïden dan 6 mg dexamethason. Daarnaast is het nog onduidelijk of een hogere dosering corticosteroïden de toevoeging van een IL-6 remmer (zoals tocilizumab) onnodig maakt bij ernstig zieke patiënten, zoals dat op dit moment gebruikelijk is. Ook is het onduidelijk of een hogere dosering corticosteroïden in combinatie met een IL-6 remmer tot meer complicaties zou leiden in deze populatie. In de COVID STEROID 2 studie werd IL-6 therapie toegepast in een minderheid van de patiënten (11% van de 12 mg dexamethason groep en 10% van de 6 mg dexamethason groep). Eerdere onderzoeken bij andere virale luchtweginfecties lieten zien dat de mortaliteit toenam bij het gebruik van hogere doseringen corticosteroïden. Vandaar dat er op dit moment wordt afgeraden om hogere doseringen corticosteroïden toe te passen buiten studieverband.
Bijwerkingen
Omdat er zeer heterogene definities werden gebruikt bij het rapporteren van bijwerkingen en er een hoog risico op bias was, werden deze gegevens niet systematisch weergegeven in de module. Een Cochrane review zet de bijwerkingen van diverse studies op een rij (Wagner, 2021). Er wordt geen meta-analyse verricht, maar een analyse van de descriptieve statistiek van de studies resulteert wel in de conclusie dat er geen grote verschillen in (ernstige) bijwerkingen werden gezien in de groep met en zonder corticosteroïden. Ook als er specifiek naar in het ziekenhuis opgelopen infecties werd gekeken, werden er geen grote verschillen gezien.
Ondanks dat de verschillen in bijwerkingen en ongewenste effecten van corticosteroïden niet in deze studies naar voren komen, is het wel bekend dat corticosteroïden geassocieerd zijn met onder andere een ontregeling van diabetes mellitus, het optreden van neuropsychiatrische symptomen en predisponeren voor infecties. Bij hoge doseringen corticosteroiden werd er ook een associate met ernstige schimmelinfecties beschreven zoals aspergillose en mucormycose (Hoenigl, 2022).
Speciale patiëntengroepen
Het is logisch om het advies over corticosteroïden voor volwassenen naar zeer ernstig zieke kinderen te extrapoleren. Helaas kan dit niet goed worden onderbouwd met gerandomiseerd onderzoek. Bij kinderen is er nu nog weinig bewijs dat COVID-19 met meer complicaties gepaard gaat, de ziekte lijkt bij kinderen juist met minder complicaties gepaard te gaan. Dat zou pleiten voor terughoudendheid voor het voorschrijven van corticosteroïden bij minder zieke pediatrische patiënten (niet op IC opgenomen).
In de bovengenoemde trials werden ook oudere patiënten geïncludeerd. In een subgroep analyse van de RECOVERY trial, bij ruim 900 patiënten die ouder waren dan 70 jaar, werd een mogelijk minder sterk voordeel van dexamethason zien op de 28-dagen mortaliteit (Horby, 2021). Bij de kwetsbare oudere patiënt kan dexamethason een hoger risico geven op een delier, waardoor een afweging van de baten en te verwachte bijwerkingen extra belangrijk is.
Inhalatiecorticosteroïden
Therapie met inhalatiecorticosteroïden (ICS) is tot nu toe vooral overwogen bij ambulante patiënten. Het gebruik van ICS is relatief eenvoudig en kent, zeker als de behandeling kortdurend is, weinig bijwerkingen. Er zijn tot nu toe vier gerandomiseerde studies gepubliceerd die rapporteren over het effect van ICS bij patiënten met COVID-19.
In een open-label fase 2-gerandomiseerd onderzoek (de STOIC-trial) werd het effect van de inhalatie van budesonide (2dd 800 ug) onderzocht bij patiënten die waren gediagnosticeerd met covid-19 en nog geen opname-indicatie hadden (Ramakrishnan, 2021). Deze studie werd voortijdig gestopt na inclusie en 1:1-randomisatie van 146 patiënten. Het primaire eindpunt was de behoefte aan ‘urgente medische zorg’. Dit kwam frequenter voor in de groep met standaardzorg dan in de groep die budesonide gebruikte (11 versus 2 maal, dit was statistisch significant). Patiënten die ICS gebruikte waren gemiddeld 1 dag eerder klachtenvrij. De hoeveelheid virus die op verschillende momenten was gemeten middels nasofarynxuitstrijk verschilde niet tussen beide groepen. Een andere open-label fase 2-gerandomiseerde studie onderzocht het effect van ciclesonide inhalatie (2dd 320 ug) gedurende 14 dagen (Song, 2021). Patiënten waren ambulant en hadden een recente diagnose COVID-19 (binnen 7 dagen na start symptomen of binnen 3 dagen na diagnose). Er werden 61 patiënten geïncludeerd. In de groep met ICS werd een snellere daling van de viral load gezien en een kleinere kans op klinisch falen. Echter, het aantal patiënten in de studie was klein en deze positieve effecten werden niet in alle secundaire uitkomstmaten terug gezien.
In de grootste trial, de open-label PRINCIPLE trial (Yu, 2021), werden ambulante patiënten geïncludeerd met verhoogd risico op een ernstig beloop. Inclusiecriteria waren o.a. een leeftijd ≥ 50 jaar met minimaal 1 comorbiditeit, of een leeftijd ≥ 65 jaar, met of zonder comorbiditeit. Eindpunten waren de door de patiënt zelf gerapporteerde tijd tot herstel, en noodzaak tot ziekenhuisopname in de eerste 28 dagen. Na randomisatie en exclusie vanwege het ontbreken van een positieve test voor SARS-CoV-2, werden er 787 personen in de budesonide behandelgroep en 1069 in de controlegroep geanalyseerd. Patiënten die budesonide turbuhaler gebruikten rapporteerden gemiddeld 3 dagen eerder hersteld te zijn. In de standaardzorg groep werden iets meer patiënten binnen 28 dagen na randomisatie in het ziekenhuis opgenomen (8,8% vs. 6,8%; OR 0,75 in het voordeel van budesonide; CI 95% 0,55-1,03). Superioriteit van budesonide voor deze uitkomstmaat werd statistisch niet aangetoond.
De RCT van Clemency (2022) randomiseerde 400 ambulante patiënten naar 2 maal per dag ciclesonide 320 ug of placebo gedurende 30 dagen. Inclusie in de studie was onafhankelijk van de duur van de klachten of onderliggend lijden. Gemiddeld waren de patiënten 43 jaar, 55% was vrouw. Het primaire eindpunt van de studie was de tijd tot alle symptomen van COVID-19 verdwenen waren. In beide groepen was dit 19 dagen. Er werd ook geen verschil gezien in het percentage mensen dat op dag 30 nog symptomen had: 70,6% was klachtenvrij in de ciclesonide groep versus 63,5% in de placebo groep (OR 1,28; 95% CI 0,84-1,97). Wel werden er minder bezoeken aan de spoedeisende hulp of opnames gezien in de groep met ciclesonide: 1% versus 5,4% (OR 0,18; 95% CI 0,04-0,85). Niemand overleed tijdens de studie.
De vier gerapporteerde RCT’s beschrijven allen in meer of mindere mate een positief effect van ICS. Twee studies waren relatief klein; de PRINCIPLE studie had methodologische beperkingen, onder meer omdat de behandeling niet geblindeerd was en de tijd tot herstel door patiënten ‘self-reported’ was. De RCT van Clemency (2022) heeft door de blindering beduidend minder beperkingen. In zowel de studie van Clemency (2022) als de PRINCIPLE trial (Yu, 2021) was het aantal patiënten dat zou zijn behandeld zijn om 1 ziekenhuisopname te voorkomen echter hoog, 50 (number needed to treat). De STOIC trial bevatte te weinig inclusies om de werkzaamheid betrouwbaar vast te stellen. Data over het voorkomen van harde eindpunten als IC opname, noodzaak tot mechanische ventilatie en overlijden, ontbreken. Er zijn geen gegevens uit deze of andere studies die wijzen op een schadelijk effect van ICS, ook een Cochrane review die 3 RCT’s includeerde, vond geen verhoogd aantal ‘adverse events’ bij het gebruik van ICS (Griesel, 2022).
Op basis van bovengenoemde data heeft de NHG een behandeladvies opgesteld voor ambulante patiënten (zie NHG standaard COVID-19, corona.nhg.org. Bij opgenomen patiënten is er geen plaats voor behandeling met inhalatiecorticosteroïden.
In maart 2022 adviseerde de Amerikaanse NIH (National Institutes of Health) niet voor of tegen het gebruikt van ICS, de Amerikaanse IDSA (Infectious Diseases Society of America) adviseerde tegen het gebruik van ICS. De IDSA nam in haar advies ook de studie van Ezer (2021) mee, die geen positief effect van ciclesonide liet zien. Deze studie was echter vroegtijdig gestopt en was mogelijk ‘underpowered’.
Virusvarianten
Sinds de opkomst van de omikron variant van SARS-CoV-2 in Nederland eind 2021, is de kans op een ernstig beloop van COVID-19 op populatieniveau zeer sterk gedaald. Het is van belang om op te merken dat de besproken gerandomiseerde studies werden verricht voor de opkomst van de omikron variant. Het is onduidelijk wat de invloed is van deze variant op het effect van anti-inflammatoire therapie, al wordt aangenomen dat patiënten die door de omikron variant een ernstige COVID-19 infectie ontwikkelen nog steeds baat hebben bij anti-inflammatoire therapie. De ‘number needed to treat’ zou wel anders (vermoedelijk hoger) kunnen zijn.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Er werd een klinisch relevant voordeel gevonden bij opgenomen patiënten met een ernstige COVID-19 infectie die behandeld werden met dexamethason, vergeleken met de groep die dit niet kreeg. In patiënten met een matig ernstige COVID-19 infectie werd er ook voordeel vastgesteld, al was dit minder groot dan bij patiënten met een ernstige ziekte.
Het is voor patiënten belangrijk om te weten wat de voor en nadelen van dexamethason zijn, zoals bijvoorbeeld de ontregeling van de glucose waarden bij patiënten met pre-existente diabetes mellitus (zie ook het kopje ‘bijwerkingen’). Deze nadelen zullen in de groep patiënten met een matig ernstige COVID-19 infectie zwaarder wegen dan bij patiënten met een ernstige COVID-19, waar deze mogelijke bijwerkingen veelal opwegen tegen de sterke voordelen.
Kosten (middelenbeslag)
Een behandeling met dexamethason gedurende 10 dagen kost ongeveer 50 euro indien dit intraveneus gegeven wordt en 10 euro indien dit oraal gegeven wordt. Dit is relatief weinig vergeleken met de kosten die gemaakt worden bij de opname van patiënten met COVID-19.
Aanvaardbaarheid, haalbaarheid en implementatie
Dexamethason lijkt effectief om een ernstig beloop van de ziekte te voorkomen en de kosten zijn beperkt. De bijwerkingen, zoals bij de overwegingen beschreven, kunnen echter de aanvaardbaarheid beperken.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Bij patiënten met COVID-19 bij wie zuurstoftoediening geïndiceerd is vanwege saturatiedaling, en met name bij patiënten waarbij de zuurstoftherapie geëscaleerd moet worden naar invasieve respiratoire ondersteuning, is behandeling met dexamethason 6 mg per dag (of een equivalente dosis hydrocortison/prednison) gedurende 10 dagen of tot ontslag uit het ziekenhuis, aangewezen. Bij patiënten zonder extra zuurstofbehoefte wordt behandeling met corticosteroïden niet aangeraden. Voor de behandeling van kinderen kunnen de doseringen beschreven in het Kinderformularium worden gebruikt.
Onderbouwing
Achtergrond
Een dysregulatie van de immuunrespons lijkt bij COVID-19 een belangrijke rol in de pathofysiologie te spelen (Veerdonk, 2021). Er worden verschillende middelen ingezet en onderzocht met anti-inflammatoire werking. Corticosteroïden zijn goed beschikbaar en werden bij infecties met SARS-CoV-1 en MERS-CoV virussen frequent voorgeschreven in de hoop dat daarmee immuun-gemedieerde schade voorkomen kon worden. Omdat corticosteroïden ook kunnen zorgen voor een toename of langere duur van virale replicatie was er veel twijfel over gebruik ervan als behandeling (Lee, 2004; Arabi, 2018). In algemene zin wijzen de verschillende meta-analyses naar de toepassing van corticosteroïden bij ARDS in de richting van verbeterde uitkomsten met corticosteroïden (Meduri, 2016; Peter, 2008; Yang, 2017). Bij influenza geassocieerde ARDS is er echter een aanwijzing voor verhoogde mortaliteit (Tsai, 2020).
Een eerste retrospectieve analyse van opgenomen SARS-CoV-2 patiënten in Wuhan, China, toonde dat de sterfte van patiënten die een ARDS ontwikkelden lager was in de groep die behandeld werd met methylprednisolon dan in de groep zonder (Wu, 2020). De gebruikte dosis werd niet vermeld. Klinische dose-finding studies zijn niet gedaan. Inmiddels is in diverse gerandomiseerde studies (RCTs) de effectiviteit van corticosteroïden onderzocht om de plaats van corticosteroïden bij de behandeling van COVID-19 patiënten te bepalen.
Conclusies / Summary of Findings
Systemic corticosteroids in hospitalized COVID-19 patients
Mortality (crucial)
|
Moderate GRADE |
Treatment with corticosteroids (all combined) probably reduces mortality (overall) when compared with treatment without corticosteroids in hospitalized COVID-19 patients.
Source: Angus, 2020; Corral-Gudino, 2021; Dequin,2020; Edalatifard, 2020; Horby, 2021; Jamaati, 2021; Jeronimo, 2020; Much, 2021; Solanich, 2021; Tang, 2021; Tomazini, 2020 |
|
High GRADE |
Treatment with corticosteroids (all combined) reduces mortality when compared with treatment without corticosteroids in hospitalized patients with severe COVID-19.
Source: Angus, 2020; Horby, 2021; Tomazini, 2020 |
|
Moderate GRADE |
Treatment with dexamethasone probably results in limited difference of mortality when compared with treatment without dexamethasone in hospitalized patients with moderate COVID-19.
Source: Horby, 2021 |
|
Low GRADE |
Treatment with dexamethasone may increase mortality when compared with treatment without dexamethasone in hospitalized patients with mild COVID-19.
Source: Horby, 2021 |
Extensive respiratory support (crucial)
|
Low GRADE |
Treatment with corticosteroids may result in little to no difference in the need for extensive respiratory support when compared with treatment without corticosteroids in hospitalized COVID-19 patients.
Source: Angus, 2020; Corral-Gudino, 2021; Dequin, 2020; Edalatifard, 2021; Horby, 2021; Jamaati, 2021; Jeronimo, 2020; Solanich 2021; Tang, 2021; Tomazini, 2020 |
Duration of hospitalization
|
Low GRADE |
Treatment with corticosteroids may result in little to no difference in the length of stay when compared with treatment without corticosteroids in hospitalized COVID-19 patients.
Source: Horby, 2021; Angus, 2020; Jamaati, 2021; Jeronimo, 2020; Tang, 2021 |
Time to clinical improvement
|
Very low GRADE |
The evidence is very uncertain about the effects of methylprednisolone on the time to clinical improvement when compared with treatment without methylprednisolone in hospitalized COVID-19 patients.
Source: Solanich, 2021; Tang, 2021 |
Corticosteroids in non-hospitalized COVID-19 patients
|
No GRADE |
No studies were found investigating the effect of corticosteroids on mortality, respiratory support, duration of hospitalization, or time to clinical improvement compared to standard care in non-hospitalized COVID-19 patients.
Sources: - |
Samenvatting literatuur
Systemic corticosteroids in hospitalized COVID-19 patients
DEXAMETHASONE
Tomazini (2020) (CoDEX trial) described a multicenter, open-label randomized clinical trial in Brazil comparing intravenous dexamethasone and standard care with standard care only in patients admitted to an ICU with suspected or confirmed COVID-19. The trial was terminated early. Patients were included when intubated and mechanically ventilated within 48 hours of meeting the criteria for moderate or severe ARDS. Patients were excluded when they e.g. used corticosteroids in the past 15 days (when non-hospitalized) or were immunosuppressed. A total of 299 patients were randomized to receive standard care and dexamethasone or to receive standard care only. The dexamethasone-group received 20 mg dexamethasone once daily intravenously for the first five days, followed by 10 mg intravenously for the following 5 days or until ICU discharge. In the standard care only-group 35.1% of the patients had deviations from the assigned control intervention and received corticosteroids as well. Follow-up in both the intervention and control group was 28 days. The following outcomes relevant to this guideline module were reported: mortality, respiratory support, and serious adverse events. Following the predefined criteria by the working group for mild, moderate, and severe disease status at randomization, the sample in Tomazini (2020) contained patients with severe disease at baseline. The primary outcome was mechanical ventilatory-free days during the first 28 days. The corticosteroid group had a mean ventilatory-free days of 6.6 (95% CI: 5.0 to 8.2), compared to 4.0 days (95% CI: 2.9 to 5.4) in the group not receiving corticosteroids (difference: 2.26 days, 95% CI: 0.2 to 4.38).
Jamaati (2021) reported the preliminary results of a randomized controlled trial in a hospital in Iran comparing intravenous dexamethasone and standard care to standard care only. The trial was terminated after no significant clinical response was observed in 50 patients. Patients were included when e.g. they had a PaO2/FiO2 ratio between 100-300mmHg. Exclusion criteria were e.g. chronic kidney or liver disease, or hyperglycemia. The 50 included patients were randomized to dexamethasone with standard care (n=25) or to standard care only (n=25). Standard care consisted of oxygen support, fluid support, and lopinavir/ritonavir (200/50 mg, two tablets twice daily) according to Iranian guidelines. Dexamethasone at a dose of 20mg per day was administered intravenously up to day 5, whereafter 10mg per day was administered intravenously up to day 10. At baseline, the standard care only-group had more patients with pulmonary disease (36%) compared to the dexamethasone-group (4%). Follow-up in both groups was 28 days. The following outcomes relevant to this guideline module were reported: mortality, need for non-invasive ventilation, and duration of hospitalization. The disease status in Jamaati (2020) was mild to moderate disease, patients were included when the PaO2/FiO2 ratio was between 100 and 300 mmHg. The primary outcomes were both the need for extensive mechanical ventilation and the death rate. In the corticosteroids group 52.0% required invasive mechanical ventilation compared to 44.0% in the control group (RR=1.18, 95% CI: 0.66 to 2.11; RD=8%. 95% CI: -19.6% to 35.6%). Sixteen deaths (16/25, 64.0%) were observed in the corticosteroids group, compared to 15 deaths (15/25, 60.0%) in the group not receiving corticosteroids (RR=1.07, 95% CI: 0.69 to 1.65; RD=4%, 95% CI: -22.9% to 30.9%).
Horby (202) (RECOVERY trial) described an open-label randomized clinical trial in the United Kingdom assessing the effect of dexamethasone and standard care compared to standard care only. Patients were included when hospitalized, and had no medical history that would put the patient substantially at risk when participating in the study. Exclusion criteria were not specified. A total of 6.425 patients were included and randomized to dexamethasone and standard care or to standard care only. Standard care was provided as the usual standard of care in the participating hospital but was not further defined in the trial report. Patients in the dexamethasone-group received 6mg oral or intravenous dexamethasone daily for up to 10 days or until discharge, whichever came first. Here, 95% of the patients received at least one dose of a glucocorticoid. The standard care group received the usual care, however 8% of these patients also received a glucocorticoid. Follow-up in both groups was 28 days. The following outcomes relevant to this guideline module were reported: mortality, respiratory support, and serious adverse events. Following the predefined criteria by the working group for mild, moderate, and severe disease status at randomization, the sample in Horby (2020) contained patients with mild to severe disease at baseline. The primary outcome was the 28-day all-cause mortality. Here, 482 deaths (482/2104, 22.9%) were observed in the dexamethasone group, compared to 1.110 deaths (1110/4321, 25.7%) in the usual care group (RR=0.89, 95% CI: 0.81 to 0.98; RD=-2.8%, 95% CI: -5% to -0.6%).
HYDROCORTISONE
Angus (2020) (REMAP-CAP) examined the effects of hydrocortisone compared to usual care as part of a multicenter open-label adaptive platform randomized controlled trial. Centers were located in Australia, Canada, France, Ireland, the Netherlands, New Zealand, the United Kingdom, and the United States of America. The domain examining corticosteroids was terminated early due to a loss of equipoise, while no study data were reviewed prior to the decision to stop enrollment of patients. Patients were included when admitted to the intensive care unit for respiratory or cardiovascular organ support. Patients were excluded when e.g. death was deemed imminent and inevitable, or soon discharge was expected, or when more than 36 hours elapsed since admission to the intensive care unit, or when the treating clinician believed that participating would not be in the best interest of the patient. Patients were randomly allocated to one of the three study-arms: fixed dose hydrocortisone, shock-dependent hydrocortisone, and a standard of care without hydrocortisone. Standard care was provided as per each center’s standard. Systemic corticosteroids were permitted in all groups when new indications developed for which corticosteroids would be an established treatment. In the group who were randomized to standard care without hydrocortisone, 15% (n=15) received a systemic corticosteroid (of which n=6 received hydrocortisone). Patients in the fixed dose-group received 50 mg hydrocortisone intravenously every 6 hours for 7 days (n=2 received 100 mg fixed dose). Patients in the shock-dependent-group received 50 mg hydrocortisone intravenously every 6 hours while in shock and up to 28 days. Shock was defined as the requirement for treatment for shock due to COVID-19 by intravenous vasopressor infusion. Hydrocortisone was discontinued when the shock was considered to be resolved or when vasopressors were discontinued for 24 hours. Follow-up of the primary outcome was 21 days. The following outcomes relevant to this guideline module were reported: mortality, respiratory support, duration of hospitalization and serious adverse events. Following the predefined criteria by the working group for mild, moderate, and severe disease status at randomization, the sample in Angus (2020) contained patients with severe disease at baseline (with the exception of one patient in the shock-dependent-group who did not receive any acute respiratory support or received supplemental oxygen only). The primary outcome was alive and free of respiratory or cardiovascular organ support- up to 21 days. Median organ support-free days were reported for the fixed-dose group (0 days, IQR: -1 to 15), the shock-dependent group (0 days, IQR: -1 to 13), and the no hydrocortisone group (0 days, IQR: -1 to 11).
Dequin (2020) (CAPECOVID trial) described a multicenter randomized controlled trial examining the effects of hydrocortisone compared to a placebo on intensive care units in France. The trial was terminated early pending the results of the RECOVERY trial and changes in treatment recommendations. Patients were included when admitted to a participating intensive care unit for acute respiratory distress syndrome, and when the experimental treatment was administered within 24 hours of the onset of one of the severity criteria (or within 48 hours when patients were referred from another hospital). Patients were excluded when in septic shock or when there were do-not-intubate orders. Included patients were randomized to hydrocortisone (n=76) or to placebo (n=73). The hydrocortisone dose was 200 mg/day until day 7, 100 mg/day for the next 4 days, and 50 mg/day for the last 3 days of a 14-day treatment regime. When the patient’s respiratory status had sufficiently improved by day 4 a short treatment regime of 8 days was initiated instead (200 mg/day until day 4, 100 mg/day for the next 2 days, and 50 mg/day for the last 2 days). Patients in the placebo-group received saline. Adjunctive therapy was allowed in both groups at the discretion of the treating primary physicians. In the hydrocortisone group, n=44 (57.9%) received one or more adjunctive therapies, compared to n=47 (64.4%) in the placebo-group. Adjunctive therapies provided consisted of hydroxycholoquine (whether or not in combination with azithromycin), ritonavir-lopinavir, eculizumab, remdesivir, and/or tocilizumab. The follow-up was 21 days for the post-hoc outcomes. The following outcomes relevant to this guideline module were reported: mortality, respiratory support, and serious adverse events. Following the predefined criteria by the working group for mild, moderate, and severe disease status at randomization, the sample in Dequin (2020) contained patients with moderate (n=9 had a non-rebreathing mask) to severe disease at baseline. The primary outcome was treatment failure on day 21, a composite of death or the persistent dependency on mechanical ventilation or high-flow oxygen therapy. Treatment failure was observed in 32 patients (32/76, 42.1%) receiving hydrocortisone, compared to 37 patients (37/73, 50.7%) in the placebo group (difference: -8.6%, 95% CI: -24.9% to 7.7%, p=0.29).
Munch (2021) (COVID STEROID trial) described a multicenter randomized controlled trial examining the effects of hydrocortisone compared to a placebo in Denmark. The trial was terminated early due to an unexpected inability to enroll patients. Patients were included when they had severe hypoxia (i.e. use of mechanical or non-mechanical ventilation, or continuous use of CPAP for hypoxia, or oxygen supplementation of at least 10 L/minute independent of delivery system). Patients were excluded when e.g. they used systemic corticosteroids, had invasive mechanical ventilation more than 48 hours before screening. Participating patients were randomized to hydrocortisone (n=16) or placebo (n=14), both with standard care. The hydrocortisone-group received 200 mg per day using continuous infusion over the course of 24 hours or per bolus injection of 50mg each 6 hours. Treatment continued up to 7 days or until hospital discharge. The placebo-group received 0.9% saline continuously infused over the course of 24 hours or as bolus injections every 6 hours. Additional antiviral treatment provided were remdesivir (n=4) or convalescent plasma (n=2). Antibacterial agents were provided in 12 participants (86%). In the hydrocortisone group, n=8 had major protocol deviations, compared to n=3 in the placebo group. The follow-up was 90 days. The following outcomes relevant to this guideline module were reported: mortality and serious adverse events. Following the predefined criteria by the working group for mild, moderate, and severe disease status at randomization, the sample in Munch (2020) contained patients with moderate to severe disease at baseline. The primary outcome was days alive without the use of life support up to day 28. No difference was observed between groups (adjusted mean difference: -1.1 days, 95% CI: -9.5 to 7.3).
METHYLPREDNISOLONE
Jeronimo (2020) (Metcovid trial) reported a randomized controlled trial examining the effects of sodium succinate methylprednisolone compared to a placebo in Brazil. Patients were included when they had a clinical suspicion of COVID-19 (fever and any respiratory symptom), were 18 years or older, had an SpO2 ≤ 94% with room air, and required supplemental oxygen or invasive mechanical ventilation. Patients were excluded when they had e.g. chronic use of corticosteroid or immunosuppressive agents, had decompensated cirrhosis, or had chronic renal failure. Participating patients were randomized to sodium succinate methylprednisolone (n=209) or placebo (n=207). The methylprednisolone group received 0.5 mg/kg intravenously twice daily over the course of five days. Patients in the placebo group received a saline solution intravenously twice daily for five days. All participating patients meeting criteria for acute respiratory distress syndrome received preemptive ceftriaxone (1g twice daily, 7 days) plus azithromycin (500 mg/day, 5 days) or clarithromycin (500 mg twice daily, 7 days) intravenously. The follow-up was 28 days. The following outcomes relevant to this guideline module were reported: mortality, respiratory support, duration of hospitalization, and viral clearance. Following the predefined criteria by the working group for mild, moderate, and severe disease status at randomization, the cohort contained patients with moderate to severe disease or had an unclear respiratory status (33.8% received invasive mechanical ventilation, 47.8% received non-invasive oxygen therapy, 18.4% unreported). The primary outcome was the 28-day mortality. There were 72 observed deaths (72/194, 37.1%) in the methylprednisolone group compared to 76 (76/199, 38.2%) observed deaths in the placebo group (RR=0.97, 95% CI 0.75 to 1.25; RD=-1.1%, 95% CI -10.7% to 8.5%).
Edalatifard (2020) reported a randomized controlled trial examining the effects of methylprednisolone compared to no methylprednisolone in Iran. Patients were included when 18 years or older, had confirmed COVID-19 (positive RT-PCR and abnormal CT-scan findings) with an SpO2 <90% at rest, had C-reactive protein > 10 mg/L and interleukin-6 >6 pg/ml before connecting to the ventilator and intubation, and when agreed to give informed consent. Patients were excluded when they had e.g. an SpO2 < 70%, had a positive pro-calcitonin and troponin test, had acute respiratory distress syndrome, uncontrolled diabetes mellitus, gastrointestinal problems or bleeding history, heart failure, or active malignancies, or received any immunosuppressive agents. Participating patients were randomized to receive standard care with methylprednisolone pulse (n=34) or standard care without methylprednisolone or other glucocorticoids (n=34). The standard care consisted of hydroxychloroquine sulfate, lopinavir, and naproxen. Patients allocated to methylprednisolone pulse received 250 mg per day from an intravenous injection for three days. Six persons in the group receiving standard care without methylprednisolone had received corticosteroids and were excluded. The follow-up was 3 days. The following outcomes relevant to this guideline module were reported: mortality, respiratory support, and serious adverse events. Following the predefined criteria by the working group for mild, moderate, and severe disease status at randomization, the sample in Edalatifard (2020) contained patients with moderate to severe disease at baseline. The primary outcomes were the time to clinical improvement, and a composite of the time to hospital discharge or death (whichever came first). Median time to improvement was 11.84 days in the methylprednisolone group compared to 16.44 days in the standard care group (p=0.011). Median time to discharge or death was 11.62 days versus 17.61 days for the methylprednisolone group and the standard care group, respectively.
Corral-Gudino (2021) (GLUCOCOVID trial) described a multicenter open-label randomized controlled trial assessing the effect of methylprednisolone with standard care compared to standard care only in Spain. The trial was terminated before the intended sample size was achieved. Patients were included when they e.g. had symptoms for at least 7 days, had moderate to severe disease with abnormal gas exchange (PaO2/FiO2 or PaFi < 300, SaO2/FiO2 or SaFi <400, or at least two criteria of the BRESCIA-COVID Respiratory Severity Scale), and had evidence of a systemic inflammatory response (any criterium: CRP >150 mg/L, D-dimer >800 ng/ml, ferritin >100 mg/dl, IL-6 >20 pg/ml). Exclusions were made when patients were mechanically ventilated, hospitalized in the intensive care unit, were treated with corticosteroids or immunosuppressive agents at the time of enrollment, had chronic kidney disease on dialysis, or were pregnant. Sixty-four participants were randomized to receive methylprednisolone with standard care (n=35) or to receive standard care only (n=29). Standard care was provided according to the local hospital protocols based on the recommendations of the Spanish Ministry of Health and the World Health Organization. The authors stated that the local standard of care protocols among the participating hospitals were similar. The trial also set up a preference arm which allocated the treatment based on preferences rather than randomization. Patients receiving methylprednisolone were administered 40 mg intravenously twice per day for the first three days, whereafter the dose reduced to 20 mg twice per day for the next three days. Additional therapies provided in both groups were azithromycin, hydroxychloroquine, lopinavir/ritonavir, and low molecular weight heparin. The follow-up was 28 days. The following outcomes relevant to this guideline module were reported: mortality and respiratory support. Following the predefined criteria by the working group for mild, moderate, and severe disease status at randomization, the sample in Corral-Gudino (2021) contained patients with unclear disease severity at baseline. The respiratory status at inclusion or randomization was not reported, although patients receiving mechanical ventilation or when admitted to the intensive care unit were excluded. The primary outcome was a composite consisting of in-hospital all-cause mortality, escalation to ICU admission, and progression of respiratory insufficiency which would require non-invasive ventilatory support. Although events occurred less frequently in the methylprednisolone group, no statistical differences between groups were found (RR=0.68, 95% CI: 0.37 to 1.26).
Solanich (2021) reported a single-center open-label randomized controlled trial assessing the effects of methylprednisolone and tacrolimus with standard care compared to standard care only in Spain. The trial was terminated early. Patients were included when they had respiratory failure (PaO2/FiO2 <300, or SpO2/FiO2 < 220), and had high inflammatory parameters (CRP >100 mg/L, or D-dimer >1000 µg/L, or ferritin >1000µg/L). Patients were excluded when they had e.g. a glomerular infiltration of 30 ml/min/1.73m2 or less, had leukopenia of 4000 cells/µl or less, had other conditions that cause immunosuppression, had a concomitant potentially serious infection, had contraindications for corticosteroid or tacrolimus use. The intervention-arm (n=27) received 120 mg/day methylprednisolone pulses on three consecutive days. Longer duration or a higher dose was allowed when considered appropriate by the treating physician. Besides methylprednisolone, tacrolimus was administered twice daily at 0.05 mg/kg as a starting dose. Tacrolimus dose was thereafter adjusted to achieve 8-10 ng/ml levels in the patient’s blood. Standard care could consist of supplemental oxygen and respiratory support, fluid therapy, anti-pyretic treatment, postural interventions, low molecular weight heparin, antiviral drugs (e.g. lopinavir/ritonavir, hydroxychloroquine), and/or immunosuppressive drugs (e.g. corticosteroids, tocilizumab, anakinra) at the discretion of the treating physician. The standard care-only group (n=28) could not receive cyclosporine or tacrolimus. All patients in the standard care-only group received corticosteroids during hospitalization (median duration of corticosteroid therapy: 18.5 days, IQR: 3.00-53.2 days). The follow-up was 58 days. The following outcomes relevant to this guideline module were reported: mortality, respiratory support, duration of hospitalization, time to clinical improvement. Following the predefined criteria by the working group for mild, moderate, and severe disease status at randomization, the cohort contained patients with moderate to severe disease severity at baseline. The primary outcome was days to reach clinical stability up to 56 days. No statistical differences between groups were found (HR=0.73, 95%CI: 0.39 to 1.37), where the methylprednisolone group had a median of 10.0 days (IQR: 7.0 to 13.0) compared to 11.0 days (IQR: 8.0 to 18.0).
Tang (2021) described a multicenter single-blind randomized controlled trial assessing the effects of methylprednisolone and standard care compared to a placebo and standard care in China. The trial was terminated before the intended sample size was reached. Patients were included when they had a laboratory confirmed SARS-CoV-2 infection, had pneumonia as confirmed by CT, were 18 years or older, were admitted to the general ward for less than 72 hours, and when they were able to sign the informed consent. Patients were excluded when there was e.g. severe immunosuppression, when corticosteroids were needed for other disease, had refractory hypertension or hypokalemia, epilepsy, delirium, glaucoma, active gastrointestinal bleeding (within the last 3 months), or secondary bacterial or fungal infections. Patients allocated to receive methylprednisolone and standard care (n=43) or placebo and standard care (n=43). Standard care was provided according to version 6 of the Chinese Diagnosis and Treatment Plan for COVID-19. The methylprednisolone-group received 1 mg/kg methylprednisolone per day for 7 days. Patients in the placebo-group received 100 ml 0.9% saline intravenously per day for 7 days. The majority (>70%) of patients received additional therapies: such as antiviral and/or antibacterial drugs. The follow-up was 14 days. The following outcomes relevant to this guideline module were reported: mortality, respiratory support, duration of hospitalization, time to clinical improvement. Following the predefined criteria by the working group for mild, moderate, and severe disease status at randomization, the cohort contained patients with moderate to severe or unclear disease severity at baseline as 70.9% received oxygen therapy via nasal cannula and 47.7% had hypoxic respiratory failure. It was not found whether there were patients without any oxygen supplementation. The primary outcome was the occurrence of clinical deterioration within 14 days. Both groups had a clinical deterioration rate of 4.8% (OR=1.00, 95% CI: 0.13 to 7.44).
Table 1. Overview of RCTs comparing corticosteroids with standard care in hospitalized COVID-19 patients.
|
Author (year, trial name) |
Disease severity* |
Sample size |
Dosage/regime |
|
Dexamethasone |
|||
|
Tomazini (2020, CoDEX trial) |
Severe |
N = 299 I: 151 C: 148
|
I: 20 mg intravenously once daily for 5 days, followed by 10 mg intravenously once daily for additional 5 days or until ICU discharge, whichever occurred first, plus standard care.
C: Standard care only |
|
Jamaati (2021) |
Mild to moderate |
N = 50 I: 25 C: 25 |
I: Intravenous dexamethasone at a dose of 20 mg/day from day 1–5 and then at 10 mg/day from day 6–10, plus standard care
C: Standard care only |
|
Horby (2021, RECOVERY trial) |
Mild to severe (sub-group analyses for mild, moderate, and severe disease) |
N = 6425 I: 2104 C: 4321 |
I: 6 mg given once daily for up to 10 days, plus standard care
C: Standard care only |
|
Hydrocortisone |
|||
|
Angus (2020, REMAP-CAP) |
Severe |
N = 614 I: 137 II: 146 Control: 101
|
I: fixed-dose hydrocortisone: Patients received a fixed dose of intravenous hydrocortisone, 50 mg or 100 mg, every 6 hours for 7 days.
II: shock-dependent hydrocortisone: intravenous hydrocortisone, 50 mg, every 6 hours while in shock for up to 28 days.
C: Standard care only |
|
Dequin (2020, CAPECOVID trial) |
Moderate to severe |
N = 149 I: 76 C: 73
|
I: Continuous intravenous infusion of hydrocortisone 200mg/day. Treatment was continued at 200mg/d until day 7 and then decreased to 100 mg/d for 4 days and 50 mg/d for 3 days, for a total of 14 days. If the patient’s respiratory and general status had sufficiently improved by day 4, a short treatment regimen was used (200mg/d for 4 days, followed by 100mg/d for 2 days and then 50 mg/d for the next 2 days, for a total of 8 days).
C: Continuous intravenous infusion of saline, plus standard care |
|
Munch (2021, COVID STEROID trial) |
Moderate to severe |
N = 30 I: 16 C: 14
|
I: Intravenous hydrocortisone (200 mg/day) for 7 days or until hospital discharge in addition to standard care; continuous infusion over 24 hrs or as bolus injections every 6 hrs (50 mg per bolus).
C: Intravenous saline solution, plus standard care |
|
Methylprednisolone |
|||
|
Jeronimo (2020, Metcovid trial) |
Moderate to severe |
N = 397 I: 195 C 202
|
I: Intravenous sodium succinate MP (0.5 mg/kg), twice daily for 5 days
C: Intravenous saline solution, plus standard care |
|
Edalatifard (2020) |
Moderate to severe |
N = 68 I: 34 C: 34
|
I: Intravenous methylprednisolone (MP) Injection (250 mg/day for 3 days), plus standard care
C: Standard care and no methylprednisolone or other glucocorticoids |
|
Corral-Gudino (2021, GLUCOCOVID trial) |
Unclear |
N = 64 I: 35 C: 29
|
I: Intravenous methylprednisolone (MP) 40 mg, twice a day, for 3 days and then 20 mg, twice a day, for 3 more days.
C: Standard care |
|
Solanich (2021) |
Moderate to severe |
N = 55 I: 27 C: 28 |
I: Methylprednisolone pulses (120 mg/day) and tacrolimus, plus standard of care
C: Standard care |
|
Tang (2021) |
Moderate to severe, partially unclear |
N = 86 I: 43 C: 43
|
I: 1 mg/kg per day of methylprednisolone administered intravenously for 7 days, plus standard care
C: intravenous saline solution, plus standard care |
*Disease severity categories:
- mild disease (no supplemental oxygen);
- moderate disease (supplemental oxygen: low flow oxygen, non-rebreathing mask);
- severe disease (supplemental oxygen: high flow oxygen [high flow nasal cannula (HFNC)/Optiflow], continuous positive airway pressure [CPAP], non-invasive ventilation [NIV], mechanical ventilation, extracorporeal membrane oxygenation [ECMO or ECLS]).
N: Total sample size; I: Intervention; C: Control
Results – Systemic corticosteroids in hospitalized COVID-19 patients
Mortality (crucial)
All of the included RCTs investigated the effect of corticosteroids on mortality. Figure 1 shows the overall pooled estimate and sub-group analysis by overall disease severity. Overall, there were 763 observed deaths in the corticosteroids group (763/2983, 25.6%) and 1371 observed deaths in the group not receiving corticosteroids (1371/5009, 27.4%). The pooled relative risk was 0.89 (95% CI 0.79 to 1.02), with the summary point estimate favoring the use of corticosteroids. Relative risks for mortality in all individual studies are shown in Figure 1 as well. The pooled risk difference was -4.33% (95% CI -8.58% to -0.07%), with the summary point estimate favoring the use of corticosteroids. When using a risk difference of 3% as a minimally clinically important difference, the pooled point estimate shows a clinically relevant difference.
When studies administering dexamethasone were pooled, 583 deaths were observed (583/2280, 25.6%) in the dexamethasone group compared to 1216 (1216/4494, 27.1%) in the control group. The relative risk was 0.90 (95%CI: 0.83 to 0.98) and the risk difference was -2.83% (95%CI: -5.00% to -0.66%), with the point estimates favoring the use of dexamethasone. The pooled risk difference point estimate was not larger than the -3% minimally clinically important difference, indicating that the point estimate is not clinically relevant. For hydrocortisone, 95 deaths were observed (95/370, 25.7%) compared to 55 (55/188, 29.3%) in the control group. This resulted in a relative risk of 0.84 (95%CI: 0.47 to 1.49) and a risk difference of -3.49 (95%CI: -17.40% to 10.42%). The pooled point estimates favored the use of methylprednisolone. In studies administering methylprednisolone a total of 85 deaths were observed (85/333, 25.5%) in the methylprednisolone group, compared to 100 (100/327, 30.6%) in the control group. This resulted in a pooled relative risk of 0.68 (95%CI: 0.35 to 1.32) and a risk difference of -6.86% (95%CI: -16.88% to 3.17%), where both point estimates favored the use of methylprednisolone. When using a risk difference of 3% as a minimally clinically important difference, the pooled point estimates for hydrocortisone and methylprednisolone show a clinically relevant difference albeit there are wide confidence intervals around these estimates.
Figure 1: Mortality (28-30days) in hospitalized patients.
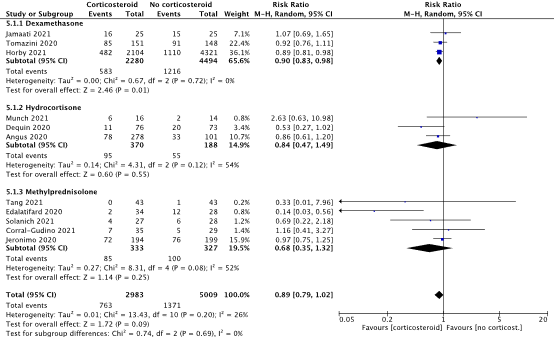
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval
Tomazini (2020) observed 85 death (85/151, 56.3% in the dexamethasone group, compared to 91 death (91/148, 61.5%) in the usual care group. This resulted in a risk difference of -5.2% (95% CI -1.6% to 5.9%). The point estimate favors dexamethasone.
Jamaati (2021) observed 16 deaths (16/25, 64.0%) in the dexamethasone group, while 15 deaths (15/25, 60.0%) were observed in the usual care group. The corresponding risk difference was 4% (95% CI: -22.9% to 30.9%).
Jeronimo (2020) included suspected cases and reported the 7-day mortality (16.5% vs. 23.6%) and 14-day mortality (27.3% vs. 31.7%) in the methylprednisolone vs. placebo groups. On day 28 the mortality increased to 72 observed deaths (72/194, 37.1%) in the methylprednisolone group compared to 76 (76/199, 38.2%) observed deaths in the placebo group. This resulted in a risk difference of -1.1% (95% CI -10.7% to 8.5%), with the point estimates favoring methylprednisolone.
Horby (2021) observed 482 deaths (482/2104, 22.9%) in the dexamethasone group, compared to 1110 deaths (1110/4321, 25.7%) in the usual care group. The corresponding risk difference was -2.8% (95% CI: -5% to -0.6%). Horby (2021) also presented sub-group analyses based on respiratory support status at randomization (see Figure 2). In this figure the risk ratio is depicted, in line with the other studies. The original article displays the rate ratio.
Figure 2: Mortality (day 28) by respiration support status at randomization in hospitalized COVID-19 patients (Horby, 2021) in hospitalized patients.

CI: confidence interval
Angus (2020) observed 41 in-hospital deaths in the fixed-dose hydrocortisone-group (41/137, 30%; RD = -2.8% [95% CI -14.7% to 9.2%]), while in the shock-dependent hydrocortisone-group 37 (37/141, 26%; RD = -6.4% [95% CI -18.1% to 5.3%]) in-hospital deaths were observed compared to 33 deaths (33/101, 32.7%) in the no-hydrocortisone group. Together, 78 deaths occurred (78/278, 28%) in both hydrocortisone groups. In the control group 33 (33/101, 33%) in-hospital deaths occurred (RD = -4.6% [95% CI -15.2% to 6.0%]; favoring hydrocortisone).
Dequin (2020) included both suspected and confirmed cases. Over the course of 21 days, 11 deaths (11/76, 14.5%) were observed in the hydrocortisone-group, compared to 20 deaths (20/73, 27.4%) in the placebo-group. This resulted in a risk difference of -12.9% (95% CI -25.9% to 0.0%), with the point estimate favoring hydrocortisone.
Munch (2021) solely included confirmed cases and reported the all-cause mortality on both day 28 and day 90. Over the course of 28 days, 6 deaths (6/16, 37.5%) were observed in the hydrocortisone-group, compared to 2 deaths (2/14, 14.3%) in the placebo-group. The risk difference was 23.2% (95% CI -6.8% to 53.2%), favoring placebo. On day 90, the observed deaths increased to 7 (7/16, 44%) and 3 (3/14, 21%), respectively.
Jeronimo (2020) included suspected cases and reported the 7-day mortality (16.5% vs. 23.6%) and 14-day mortality (27.3% vs. 31.7%) in the methylprednisolone vs. placebo groups. On day 28 the mortality increased to 72 observed deaths (72/194, 37.1%) in the methylprednisolone group compared to 76 (76/199, 38.2%) observed deaths in the placebo group. This resulted in a risk difference of -1.1% (95% CI -10.7% to 8.5%), with the point estimates favoring methylprednisolone.
Edalatifard (2020) recruited confirmed cases only and reported the mortality for circa up to 28 days in both groups (approximated from a figure). Two deaths (2/34, 5.9%) were observed in the methylprednisolone group, compared to twelve deaths (12/28, 42.9%) in the standard care-only group. This resulted in a risk difference of -37% (95% CI -56.9% to -17%), with the point estimates favoring methylprednisolone.
Corral-Gudino (2021) recruited confirmed cases only. There were 7 deaths (7/35, 20%) observed in the methylprednisolone group on day 28, compared to 5 (5/29, 17.2%) in the standard care-only group. This resulted in a risk difference of 2.8% (95% CI -16.3% to 21.9%), with the point estimates favoring no methylprednisolone.
Solanich (2021) recruited confirmed cases only. Both COVID-related mortality and all-cause mortality were reported. For COVID-related mortality was reported for day 28 (11.1% vs. 14.3%) and for day 56 (14.8% vs. 14.3%) in the methylprednisolone group vs. standard care-only group, respectively. For the 28-day all-cause mortality, 4 deaths were observed (4/27, 14.8%) in the methylprednisolone group, compared to 6 deaths (6/28, 21.4%) in the standard care-only group. This resulted in a risk difference of -6.6% (95% CI -26.9% to 13.7%), with the point estimates favoring methylprednisolone. At day 56 there were 5 observed deaths (18.5%, methylprednisolone) compared to 6 deaths (21.4%, standard care-only) for mortality.
Tang (2021) recruited confirmed cases only and reported the in-hospital mortality up to 14 days. No deaths were observed in the methylprednisolone group, compared to one death (1/43, 2.3%) in the placebo and standard care group. This resulted in a risk difference of -2.3% (95% CI -16.9% to 3.2%), with the point estimates favoring methylprednisolone.
Systemic corticosteroid use in patients with severe disease
The RECOVERY trial (Horby, 2021), included a subgroup analysis reporting the mortality in patient receiving invasive mechanical ventilation at baseline (IMV, n=1007), see Figure 2. The working group considered this sub-group as having severe disease. The risk difference calculated from the reported events was -12.1% (95% CI -18.3% to -5.9%). This sub-group was furthermore used in the meta-analysis to pool the effect of corticosteroids on mortality in the severe disease group along with the data from Angus (2020) and Tomazini (2020). Figure 3 shows the pooled relative risk in the severe disease group regardless of the type of corticosteroid provided (Angus, 2020; Horby, 2021; Tomazini, 2020). The corresponding pooled risk difference was -9.18% (95% CI: -14.13% to -4.23%), favoring the use of corticosteroids. The pooled point estimate shows a clinically relevant difference.
Figure 3: Mortality in hospitalized patients with severe disease.
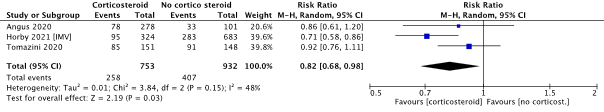
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval
Systemic corticosteroid use in patients with moderate disease
The only RCT including only moderate disease or specifically reporting the moderate disease subgroup seemed to be the RECOVERY trial (Horby, 2021). Here, the mortality was reported in a sub-group of patients receiving oxygen therapy only at randomization while receiving dexamethasone (n=1279) or usual care only (n=2604) during the study period. See Figure 2 for the relative risk in this sub-group. The risk difference was -2.9% (95% CI: -5.8% to -0.0%), indicating with the point estimate that there is no clinically relevant difference.
Systemic corticosteroid use in patients with mild disease
Horby (2021) also presented the results of the sub-group not receiving any oxygen support at randomization in the RECOVERY trial. The working group considered this subgroup as having mild disease. In this sub-group (n=1535) the risk difference calculated from the reported events was 3.7% (95% CI -0.2% to 7.7%), with the point estimate favoring standard care. The point estimate shows a clinically relevant difference. The relative risks of this subgroup receiving no oxygen at randomization is reported in Figure 2. Horby (2021) was the only study that included patients with mild disease.
Level of evidence of the literature
The certainty of evidence started as high since the body of evidence consisted solely of RCTs. The level of evidence regarding the outcome measure mortality (overall) was downgraded by 1 level due to imprecision (reason: the confidence interval of the pooled risk difference crossed the predefined border of clinical relevance [RD 3% points difference]). We did not downgrade for study limitations (not downgraded for risk of bias: lack of blinding probably does not affect a hard outcome such as mortality). Publication bias was not assessed. The level of evidence for the outcome mortality in hospitalized patients is moderate.
The certainty of evidence started as high since the body of evidence consisted solely of RCTs. The level of evidence regarding the outcome measure mortality (severe disease only) was not downgraded. We did not downgrade for study limitations (not downgraded for risk of bias: lack of blinding probably does not affect a hard outcome such as mortality) and imprecision (reason: using the risks in both groups [34.3% vs 43.7%] and the observed proportion in both groups [q1=0.447, q2=0.553], the calculated needed sample size is n=897 [using: alpha=0.05, beta=0.2]). Publication bias was not assessed. The level of evidence for the outcome mortality (severe disease) in hospitalized patients is high.
The certainty of evidence started as high since the body of evidence consisted solely of RCTs. The level of evidence regarding the outcome measure mortality (moderate disease only) was downgraded 1 level due to imprecision (reason: confidence interval of the risk difference crosses the clinical border of 3%). We did not downgrade for study limitations (not downgraded for risk of bias: lack of blinding probably does not affect a hard outcome such as mortality) and publication bias was not assessed. The level of evidence for the outcome mortality (moderate disease) in hospitalized patients is moderate.
The certainty of evidence started as high since the body of evidence consisted solely of RCTs. The level of evidence regarding the outcome measure mortality (mild disease only) was downgraded by 2 levels due to imprecision (reason: using the risks in both groups [17.8% vs 14.0%] and the 1:2 allocation scheme [q1=0.33, q2=0.67], the calculated needed sample size in the intervention group is n=1137 and for the control group n= 2308 [using: alpha=0.05, beta=0.2]). We did not downgrade for study limitations (not downgraded for risk of bias: lack of blinding probably does not affect a hard outcome such as mortality). Publication bias was not assessed. The level of evidence for the outcome mortality (mild disease) in hospitalized patients is low.
Extensive respiratory support
Five RCTs reported the need for extensive respiratory support (Corral-Gudino, 2021; Dequin, 2020; Horby, 2021; Jamaati, 2020; Jeronimo, 2020). Figure 4 shows an overview of the relative risks.
Figure 4: Need for extensive respiratory support in hospitalized patients.
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IV: Invasive (mechanical) ventilation; NIV: Non-invasive ventilation
Tomazini (2020), including confirmed or suspected cases, reported the number of days on mechanical ventilation. The dexamethasone group had a mean duration of 12.5 days (95% CI 11.2 to 13.8) compared to 13.9 days (95% CI 12.7 to 15.1). The adjusted mean difference was -1.54 days (95%CI -3.24 to 0.12, adjusted for age and baseline PaO2/FiO2 ratio).
The relative risk from the data reported in Jamaati (2021) was 1.18 (95% CI 0.66 to 2.11) and the risk difference was 8% (95% CI -19.6% to 35.6%) with the point estimate favoring standard care, which is considered a clinically relevant difference. Jamaati (2021) also reported the need for non-invasive mechanical ventilation and observed that 23 patients (23/25, 92%) in the dexamethasone-group and 24 patients (24/25, 96%) in the standard care only-group needed non-invasive ventilation (RR = 0.96, 95% CI 0.83 to 1.10). The risk difference was -4% (95% CI -17.1% to 9.1%), where the point estimate does not indicate a clinically relevant difference.
Horby (2021) included both suspected and confirmed cases resulting in a relative risk of 0.75 (95% CI 0.61 to 0.93) and a risk difference of -2% (95% CI -3.4% to -0.6), with the point estimates favoring dexamethasone while not being considered a clinically relevant difference.
Angus (2020) reported the respiratory support-free days in the sample of both suspected and confirmed cases. The mean adjusted odds ratio in the fixed dose hydrocortisone group compared to the no-hydrocortisone group was 1.45, with the point estimate favoring hydrocortisone. For the shock dependent hydrocortisone group the mean adjusted odds ratio was 1.31 compared to the no-hydrocortisone group, with the point estimate favoring hydrocortisone.
Dequin (2020) included both suspected and confirmed cases. Respiratory support status on day 21 was reported. In the hydrocortisone group 17 patients (17/76, 22.3%) had mechanical ventilation on day 21, compared to 17 patients (17/73, 23.3%) in the placebo group (RR = 0.96 [95% CI 0.53 to 1.73]; RD = 0.9% [95% CI -14.4% to 12.6%]; favoring hydrocortisone, no clinically relevant difference). High-flow oxygen therapy on day 21 was provided for 3 patients (3/76, 4%) in the hydrocortisone group, while none of the patients received this type of support in the placebo group (RD = 4% [95% CI -1.1% to 9.0%]; favoring placebo, no clinically relevant difference).
Jeronimo (2020) only recruited suspected cases and reported the need for invasive mechanical ventilation until day 7. In the methylprednisolone group, 18 patients (18/93, 19.4%) needed invasive mechanical ventilation, compared to 16 patients (16/95, 16.8%) in the placebo group. The calculated relative risk from this data was 1.15 (95% CI 0.62 to 2.11) and the risk difference was 2.6% (95% CI -7.5% to 14.7%), with the point estimates favoring the placebo group while not indicating a clinically relevant difference.
Edalatifard (2020) recruited confirmed cases only and reported the need for oxygen therapy on day 3. Oxygen therapy was needed in 28 patients (28/34, 82.4%) in the methylprednisolone group versus 26 patients (26/28, 92.8%) in the standard care-only group. The need for oxygen therapy was a composite of the need for nasal cannula, mask oxygen, reservoir mask, non-invasive ventilation, and invasive ventilation. The calculated relative risk from this data was 0.89 (95% CI 0.74 to 1.07) and the risk difference was -10.5% (95% CI -26.5% to 5.5%), with the point estimates favoring the methylprednisolone group and indicating a clinically relevant difference.
Corral-Gudino (2021) reported the progression of respiratory insufficiency which required non-invasive ventilation. Requirement of non-invasive ventilation due to progression was observed in 10 patients (10/35, 28.6%) in the methylprednisolone group compared to 7 patients (7/29, 24.1%) in the standard care-only group. The calculated relative risk from this data was 1.18 (95% CI 0.52 to 2.72) and the risk difference was 4.4% (95% CI -17.2% to 26.0%), with the point estimate favoring the standard-care only group while not indicating a clinical relevant difference.
Solanich (2021) included confirmed cases only and reported the duration of oxygen support, the need for high-flow oxygen or ventilatory support, and the duration of high-flow or ventilatory support. The methylprednisolone group had a median of 11.0 days (IQR: 8.0-19.5) of oxygen support compared to 13 days (IQR: 7.75-23.0) in the standard care-only group. High-flow or ventilatory support was provided to 14 patients in the methylprednisolone group (14/27, 51.9%) versus 18 patients (18/28, 64.3%) in the standard care-only group. The calculated relative risk from this data was 0.81 (95% CI 0.51 to 1.27) and the risk difference was -12.4% (95% CI -38.3% to 13.5%), with the point estimates favoring the methylprednisolone group. The risk difference point estimate indicates a clinically relevant difference. Median duration of high-flow or ventilatory support was 8 days (IQR: 5.0-27.2) in the methylprednisolone group versus a median of 6.5 days (IQR: 4.25-14.20) in the standard care only-group.
Tang (2021) recruited confirmed cases only and reported the need for respiratory support therapies. Patients received high-flow oxygen (4.6% vs. 2.3%), non-invasive positive pressure ventilation (0% vs. 2.3%), invasive mechanical ventilation (4.6% vs. 2.3%), and/or extracorporeal membrane oxygenation (4.6% vs. 0%) in the methylprednisolone group versus the placebo group, respectively.
Level of evidence of the literature
The certainty of evidence started as high since the body of evidence consisted solely of RCTs. The level of evidence regarding the outcome measure extensive respiratory support was downgraded by 2 levels because of study limitations (1 level for risk of bias, several (potential) flaws in the included studies: lack of blinding of several roles (e.g. open label, or health care providers not blinded), ITT analyses were not followed); number of included patients (1 level for imprecision: relatively small number of patients in the study); publication bias was not assessed. The level of evidence for the outcome respiratory support in hospitalized patients is low.
Duration of hospitalization (important)
Horby (2021) found that patients receiving dexamethasone had a shorter length of stay (median of 12 days) compared to patients receiving the usual care only (median of 13 days), however this is not considered to be a clinically relevant difference.
Jamaati (2021) included confirmed SARS-CoV2 cases and found a median duration of hospitalization of 11 days (IQR: 6 to 16) in the dexamethasone-group (n=25) compared to 6 days (IQR: 4 to 9) in the standard care only-group (n=25). In the subgroup that survived, the median duration of hospitalization for the dexamethasone-group (n=9) was 11 days (IQR: 9 to 21) compared to 8.5 days (IQR: 5 to 13) in the standard care only-group (n=10). This was not considered to be a clinically relevant difference.
Angus (2020) reported the hazard ratio of duration of hospitalization in the sample of both suspected and confirmed cases. The median adjusted hazard ratio in the fixed dose hydrocortisone group compared to the control group was 0.97 (95% credible interval: 0.72 to 1.32), with the point estimate favoring hydrocortisone. For the shock dependent hydrocortisone group the median adjusted hazard ratio was 0.93 (95% credible interval: 0.69 to 1.26) compared to the hydrocortisone group, with the point estimate favoring no-hydrocortisone.
Jeronimo (2020) included suspected cases only. The median days of hospitalization was 10 days (IQR 7-13) in the methylprednisolone group. In the placebo group, the median duration of hospitalization was 9 days (IQR: 7-11). No significant (p=0.296) and clinically relevant differences between groups were found.
Solanich (2021) included confirmed cases only and observed a median duration of hospitalization of 13 days (IQR: 8.5-21) in the methylprednisolone group, compared to a median of 14 days (IQR: 9-22.5) in the standard care group. No significant (p=0.933, Wilcoxon test) and clinically relevant differences between groups were found.
Tang (2021) included confirmed cases only. The median duration of hospitalization was 17 days (IQR: 13-22) and 13 days (IQR: 10-20) in the methylprednisolone and placebo groups, respectively. No significant differences between groups were found (p=0.314, HR = 1.3 [95% CI 0.84-2.00]), however the difference in median duration (i.e. 4 days) is considered to be clinically relevant.
Level of evidence of the literature
The certainty of evidence started as high since the body of evidence consisted solely of RCTs. The level of evidence regarding the outcome measure duration of hospitalization was downgraded by 2 levels because of study limitations (2 levels for risk of bias: potential risk of bias since four trials had no or unclear blinding of health care providers). Publication bias was not assessed. The level of evidence for the outcome respiratory support (need for respiratory support therapies) in hospitalized patients is low.
Time to clinical improvement (important)
Solanich (2021) included confirmed cases only and reported the number of patients reaching clinical stability. Here, clinical stability was defined as meeting all 4 criteria for 48 hours: body temperature of 37.5°C or less; PaO2/FiO2 ratio over400 and/or SpO2/FiO2 ratio over 300; and a respiratory rate of 24 rpm or less. In the methylprednisolone group 21 patients (21/27, 77.8%) achieved clinical stability at day 56 compared to 22 patients (22/28, 78.6%) in the standard care-only group (RR = 1.04, 95% CI 0.38 to 2.82; RD = 0.8%, 95% CI -21.0% to 22.6%; point estimates favour methylprednisolone). Median time to reach clinical stability in the methylprednisolone group was 10 days (IQR: 7-13) versus 11 days (IQR: 8-18.8) in the standard care-only group (HR = 0.73, 95% CI 0.39-1.37), which indicates no clinically relevant difference.
Tang (2021) included confirmed cases and reported the number of patients reaching clinical cure. Clinical cure was defined as meeting all of the following criteria: the clinical signs and symptoms of COVID-19 are improved or alleviated (i.e. body temperature for 3 consecutive days, respiratory symptoms improved significantly, CT images showed absorption and/or consolidation of bilateral ground-glass opacification), and no additional treatment was necessary. At day 14 after randomization there were 22 patients (22/43, 51.2%) in the methylprednisolone group achieving clinical cure compared to 25 patients (25/43, 58.1%) in the placebo group (RR = 1.17, 95% CI 0.73-1.86; RD = 7.0%, 95% CI -14.0% to 28.0%; point estimates favor placebo). The median time to achieve clinical cure was 14 days (IQR: 10-19) versus 12 days (IQR: 9-17) in the methylprednisolone and placebo groups, respectively (HR = 1.04, 95% CI 0.67-1.62), which indicates no clinically relevant difference.
Level of evidence of the literature
The certainty of evidence started as high since the body of evidence consisted solely of RCTs. The level of evidence regarding the outcome measure time to clinical improvement was downgraded by 3 levels because of study limitations (1 level for risk of bias: open-label study, lack of blinding of care providers) and number of included patients (2 levels for imprecision: very low number of included patients); Publication bias was not assessed. The level of evidence for the outcome time to clinical improvement in hospitalized patients is very low.
Corticosteroids in non-hospitalized COVID-19 patients
No studies were found investigating the effect of corticosteroids in non-hospitalized COVID-19 patients.
Results
No studies were found investigating the effect of corticosteroids on mortality, respiratory support, duration of hospitalization, or time to clinical improvement in non-hospitalized COVID-19 patients.
Level of evidence of the literature
GRADE assessment could not be performed. No studies were found investigating the effect of corticosteroids on mortality, respiratory support, duration of hospitalization, or time to clinical improvement in non-hospitalized COVID-19 patients.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the effectivity of treatment with corticosteroids compared to treatment without corticosteroids in patients with COVID-19?
PICO 1
P: hospitalized with COVID-19 (subgroups mild, moderate, severe)
I: systemic corticosteroid use + standard care
C: standard care only or placebo treatment + standard care
O: 28-30 day mortality (if not available, any other reports of mortality), extensive respiratory support, duration of hospitalization, time to clinical improvement
PICO 2
P: non-hospitalized patients with COVID-19
I: systemic corticosteroid use + standard care
C: standard care only or placebo treatment + standard care
O: 28-30 day mortality (if not available, any other reports of mortality), respiratory support, hospitalization, time to clinical improvement
Relevant outcome measures
PICO 1: For hospitalized COVID-19 patients, mortality and need for extensive respiratory support were considered as crucial outcome measures for decision making; and duration of hospitalization, and time to clinical improvement as important outcome measures for decision making.
PICO 2: For non-hospitalized COVID-19 patients, mortality was considered as a critical outcome measure for decision making. Hospitalization, respiratory support, and time to clinical improvement were considered as important outcome measures for decision making.
Extensive respiratory support was defined as high flow nasal cannula (HFNC)/Optiflow, continuous positive airway pressure (CPAP), non-invasive ventilation (NIV), mechanical ventilation or extracorporeal membrane oxygenation (ECMO or ECLS).
The working group defined 3% points absolute difference as a minimal clinically important difference for mortality (resulting in a NNT of 33), 3 days difference for duration of hospitalization and time to clinical improvement, and a 5% points absolute difference need for respiratory support and ICU admission (resulting in a NNT of 20).
The results of studies in non-hospitalized and hospitalized patients are summarized separately. Studies of hospitalized patients were, when possible, categorized based on respiratory support that was needed at baseline (preferably based on patient inclusion/exclusion criteria; otherwise on baseline characteristics). The following categories were used:
- mild disease (no supplemental oxygen);
- moderate disease (supplemental oxygen: low flow oxygen, non-rebreathing mask);
- severe disease (supplemental oxygen: high flow oxygen [high flow nasal cannula (HFNC)/Optiflow], continuous positive airway pressure [CPAP], non-invasive ventilation [NIV], mechanical ventilation, extracorporeal membrane oxygenation [ECMO or ECLS]).
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms up to and including 2 September 2021. The detailed search strategy is outlined under the tab Methods. The systematic literature search resulted in 75.172 hits. Studies were selected based on the following criteria: peer-reviewed randomized controlled trials published in an indexed journal, comparing the addition of any non-inhaled corticosteroid to standard care with standard care (whether or not with the addition of a placebo) in patients with COVID-19. Studies investigating inhaled corticosteroids were excluded. Eventually, eleven studies were included.
Statistical methods
Statistical analyses were conducted using Review Manager (RevMan) software 5.4. For dichotomous outcomes, Mantel Haenszel random‐effects risk ratios (RRs) and risk differences (RDs) were calculated. For continuous outcomes, a random‐effects mean difference (MD) weighted by the inverse variance was calculated. The random-effects model estimates the mean of a distribution of effects.
Results
In total, eleven RCTs were included in the analysis of the literature concerning hospitalized patients. These studies investigated dexamethasone (n=3), hydrocortisone (n=3), or methylprednisolone (n=5). Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, van Bentum-Puijk W, Berry L, Bhimani Z, Bonten M, Bradbury C, Brunkhorst F, Buxton M, Buzgau A, Cheng AC, de Jong M, Detry M, Estcourt L, Fitzgerald M, Goossens H, Green C, Haniffa R, Higgins AM, Horvat C, Hullegie SJ, Kruger P, Lamontagne F, Lawler PR, Linstrum K, Litton E, Lorenzi E, Marshall J, McAuley D, McGlothin A, McGuinness S, McVerry B, Montgomery S, Mouncey P, Murthy S, Nichol A, Parke R, Parker J, Rowan K, Sanil A, Santos M, Saunders C, Seymour C, Turner A, van de Veerdonk F, Venkatesh B, Zarychanski R, Berry S, Lewis RJ, McArthur C, Webb SA, Gordon AC; Writing Committee for the REMAP-CAP Investigators, Al-Beidh F, Angus D, Annane D, Arabi Y, van Bentum-Puijk W, Berry S, Beane A, Bhimani Z, Bonten M, Bradbury C, Brunkhorst F, Buxton M, Cheng A, De Jong M, Derde L, Estcourt L, Goossens H, Gordon A, Green C, Haniffa R, Lamontagne F, Lawler P, Litton E, Marshall J, McArthur C, McAuley D, McGuinness S, McVerry B, Montgomery S, Mouncey P, Murthy S, Nichol A, Parke R, Rowan K, Seymour C, Turner A, van de Veerdonk F, Webb S, Zarychanski R, Campbell L, Forbes A, Gattas D, Heritier S, Higgins L, Kruger P, Peake S, Presneill J, Seppelt I, Trapani T, Young P, Bagshaw S, Daneman N, Ferguson N, Misak C, Santos M, Hullegie S, Pletz M, Rohde G, Rowan K, Alexander B, Basile K, Girard T, Horvat C, Huang D, Linstrum K, Vates J, Beasley R, Fowler R, McGloughlin S, Morpeth S, Paterson D, Venkatesh B, Uyeki T, Baillie K, Duffy E, Fowler R, Hills T, Orr K, Patanwala A, Tong S, Netea M, Bihari S, Carrier M, Fergusson D, Goligher E, Haidar G, Hunt B, Kumar A, Laffan M, Lawless P, Lother S, McCallum P, Middeldopr S, McQuilten Z, Neal M, Pasi J, Schutgens R, Stanworth S, Turgeon A, Weissman A, Adhikari N, Anstey M, Brant E, de Man A, Lamonagne F, Masse MH, Udy A, Arnold D, Begin P, Charlewood R, Chasse M, Coyne M, Cooper J, Daly J, Gosbell I, Harvala-Simmonds H, Hills T, MacLennan S, Menon D, McDyer J, Pridee N, Roberts D, Shankar-Hari M, Thomas H, Tinmouth A, Triulzi D, Walsh T, Wood E, Calfee C, O’Kane C, Shyamsundar M, Sinha P, Thompson T, Young I, Bihari S, Hodgson C, Laffey J, McAuley D, Orford N, Neto A, Detry M, Fitzgerald M, Lewis R, McGlothlin A, Sanil A, Saunders C, Berry L, Lorenzi E, Miller E, Singh V, Zammit C, van Bentum Puijk W, Bouwman W, Mangindaan Y, Parker L, Peters S, Rietveld I, Raymakers K, Ganpat R, Brillinger N, Markgraf R, Ainscough K, Brickell K, Anjum A, Lane JB, Richards-Belle A, Saull M, Wiley D, Bion J, Connor J, Gates S, Manax V, van der Poll T, Reynolds J, van Beurden M, Effelaar E, Schotsman J, Boyd C, Harland C, Shearer A, Wren J, Clermont G, Garrard W, Kalchthaler K, King A, Ricketts D, Malakoutis S, Marroquin O, Music E, Quinn K, Cate H, Pearson K, Collins J, Hanson J, Williams P, Jackson S, Asghar A, Dyas S, Sutu M, Murphy S, Williamson D, Mguni N, Potter A, Porter D, Goodwin J, Rook C, Harrison S, Williams H, Campbell H, Lomme K, Williamson J, Sheffield J, van’t Hoff W, McCracken P, Young M, Board J, Mart E, Knott C, Smith J, Boschert C, Affleck J, Ramanan M, D’Souza R, Pateman K, Shakih A, Cheung W, Kol M, Wong H, Shah A, Wagh A, Simpson J, Duke G, Chan P, Cartner B, Hunter S, Laver R, Shrestha T, Regli A, Pellicano A, McCullough J, Tallott M, Kumar N, Panwar R, Brinkerhoff G, Koppen C, Cazzola F, Brain M, Mineall S, Fischer R, Biradar V, Soar N, White H, Estensen K, Morrison L, Smith J, Cooper M, Health M, Shehabi Y, Al-Bassam W, Hulley A, Whitehead C, Lowrey J, Gresha R, Walsham J, Meyer J, Harward M, Venz E, Williams P, Kurenda C, Smith K, Smith M, Garcia R, Barge D, Byrne D, Byrne K, Driscoll A, Fortune L, Janin P, Yarad E, Hammond N, Bass F, Ashelford A, Waterson S, Wedd S, McNamara R, Buhr H, Coles J, Schweikert S, Wibrow B, Rauniyar R, Myers E, Fysh E, Dawda A, Mevavala B, Litton E, Ferrier J, Nair P, Buscher H, Reynolds C, Santamaria J, Barbazza L, Homes J, Smith R, Murray L, Brailsford J, Forbes L, Maguire T, Mariappa V, Smith J, Simpson S, Maiden M, Bone A, Horton M, Salerno T, Sterba M, Geng W, Depuydt P, De Waele J, De Bus L, Fierens J, Bracke S, Reeve B, Dechert W, Chassé M, Carrier FM, Boumahni D, Benettaib F, Ghamraoui A, Bellemare D, Cloutier È, Francoeur C, Lamontagne F, D’Aragon F, Carbonneau E, Leblond J, Vazquez-Grande G, Marten N, Wilson M, Albert M, Serri K, Cavayas A, Duplaix M, Williams V, Rochwerg B, Karachi T, Oczkowski S, Centofanti J, Millen T, Duan E, Tsang J, Patterson L, English S, Watpool I, Porteous R, Miezitis S, McIntyre L, Brochard L, Burns K, Sandhu G, Khalid I, Binnie A, Powell E, McMillan A, Luk T, Aref N, Andric Z, Cviljevic S, Đimoti R, Zapalac M, Mirković G, Baršić B, Kutleša M, Kotarski V, Vujaklija Brajković A, Babel J, Sever H, Dragija L, Kušan I, Vaara S, Pettilä L, Heinonen J, Kuitunen A, Karlsson S, Vahtera A, Kiiski H, Ristimäki S, Azaiz A, Charron C, Godement M, Geri G, Vieillard-Baron A, Pourcine F, Monchi M, Luis D, Mercier R, Sagnier A, Verrier N, Caplin C, Siami S, Aparicio C, Vautier S, Jeblaoui A, Fartoukh M, Courtin L, Labbe V, Leparco C, Muller G, Nay MA, Kamel T, Benzekri D, Jacquier S, Mercier E, Chartier D, Salmon C, Dequin P, Schneider F, Morel G, L’Hotellier S, Badie J, Berdaguer FD, Malfroy S, Mezher C, Bourgoin C, Megarbane B, Voicu S, Deye N, Malissin I, Sutterlin L, Guitton C, Darreau C, Landais M, Chudeau N, Robert A, Moine P, Heming N, Maxime V, Bossard I, Nicholier TB, Colin G, Zinzoni V, Maquigneau N, Finn A, Kreß G, Hoff U, Friedrich Hinrichs C, Nee J, Pletz M, Hagel S, Ankert J, Kolanos S, Bloos F, Petros S, Pasieka B, Kunz K, Appelt P, Schütze B, Kluge S, Nierhaus A, Jarczak D, Roedl K, Weismann D, Frey A, Klinikum Neukölln V, Reill L, Distler M, Maselli A, Bélteczki J, Magyar I, Fazekas Á, Kovács S, Szőke V, Szigligeti G, Leszkoven J, Collins D, Breen P, Frohlich S, Whelan R, McNicholas B, Scully M, Casey S, Kernan M, Doran P, O’Dywer M, Smyth M, Hayes L, Hoiting O, Peters M, Rengers E, Evers M, Prinssen A, Bosch Ziekenhuis J, Simons K, Rozendaal W, Polderman F, de Jager P, Moviat M, Paling A, Salet A, Rademaker E, Peters AL, de Jonge E, Wigbers J, Guilder E, Butler M, Cowdrey KA, Newby L, Chen Y, Simmonds C, McConnochie R, Ritzema Carter J, Henderson S, Van Der Heyden K, Mehrtens J, Williams T, Kazemi A, Song R, Lai V, Girijadevi D, Everitt R, Russell R, Hacking D, Buehner U, Williams E, Browne T, Grimwade K, Goodson J, Keet O, Callender O, Martynoga R, Trask K, Butler A, Schischka L, Young C, Lesona E, Olatunji S, Robertson Y, José N, Amaro dos Santos Catorze T, de Lima Pereira TNA, Neves Pessoa LM, Castro Ferreira RM, Pereira Sousa Bastos JM, Aysel Florescu S, Stanciu D, Zaharia MF, Kosa AG, Codreanu D, Marabi Y, Al Qasim E, Moneer Hagazy M, Al Swaidan L, Arishi H, Muñoz-Bermúdez R, Marin-Corral J, Salazar Degracia A, Parrilla Gómez F, Mateo López MI, Rodriguez Fernandez J, Cárcel Fernández S, Carmona Flores R, León López R, de la Fuente Martos C, Allan A, Polgarova P, Farahi N, McWilliam S, Hawcutt D, Rad L, O’Malley L, Whitbread J, Kelsall O, Wild L, Thrush J, Wood H, Austin K, Donnelly A, Kelly M, O’Kane S, McClintock D, Warnock M, Johnston P, Gallagher LJ, Mc Goldrick C, Mc Master M, Strzelecka A, Jha R, Kalogirou M, Ellis C, Krishnamurthy V, Deelchand V, Silversides J, McGuigan P, Ward K, O’Neill A, Finn S, Phillips B, Mullan D, Oritz-Ruiz de Gordoa L, Thomas M, Sweet K, Grimmer L, Johnson R, Pinnell J, Robinson M, Gledhill L, Wood T, Morgan M, Cole J, Hill H, Davies M, Antcliffe D, Templeton M, Rojo R, Coghlan P, Smee J, Mackay E, Cort J, Whileman A, Spencer T, Spittle N, Kasipandian V, Patel A, Allibone S, Genetu RM, Ramali M, Ghosh A, Bamford P, London E, Cawley K, Faulkner M, Jeffrey H, Smith T, Brewer C, Gregory J, Limb J, Cowton A, O’Brien J, Nikitas N, Wells C, Lankester L, Pulletz M, Williams P, Birch J, Wiseman S, Horton S, Alegria A, Turki S, Elsefi T, Crisp N, Allen L, McCullagh I, Robinson P, Hays C, Babio-Galan M, Stevenson H, Khare D, Pinder M, Selvamoni S, Gopinath A, Pugh R, Menzies D, Mackay C, Allan E, Davies G, Puxty K, McCue C, Cathcart S, Hickey N, Ireland J, Yusuff H, Isgro G, Brightling C, Bourne M, Craner M, Watters M, Prout R, Davies L, Pegler S, Kyeremeh L, Arbane G, Wilson K, Gomm L, Francia F, Brett S, Sousa Arias S, Elin Hall R, Budd J, Small C, Birch J, Collins E, Henning J, Bonner S, Hugill K, Cirstea E, Wilkinson D, Karlikowski M, Sutherland H, Wilhelmsen E, Woods J, North J, Sundaran D, Hollos L, Coburn S, Walsh J, Turns M, Hopkins P, Smith J, Noble H, Depante MT, Clarey E, Laha S, Verlander M, Williams A, Huckle A, Hall A, Cooke J, Gardiner-Hill C, Maloney C, Qureshi H, Flint N, Nicholson S, Southin S, Nicholson A, Borgatta B, Turner-Bone I, Reddy A, Wilding L, Chamara Warnapura L, Agno Sathianathan R, Golden D, Hart C, Jones J, Bannard-Smith J, Henry J, Birchall K, Pomeroy F, Quayle R, Makowski A, Misztal B, Ahmed I, KyereDiabour T, Naiker K, Stewart R, Mwaura E, Mew L, Wren L, Willams F, Innes R, Doble P, Hutter J, Shovelton C, Plumb B, Szakmany T, Hamlyn V, Hawkins N, Lewis S, Dell A, Gopal S, Ganguly S, Smallwood A, Harris N, Metherell S, Lazaro JM, Newman T, Fletcher S, Nortje J, Fottrell-Gould D, Randell G, Zaman M, Elmahi E, Jones A, Hall K, Mills G, Ryalls K, Bowler H, Sall J, Bourne R, Borrill Z, Duncan T, Lamb T, Shaw J, Fox C, Moreno Cuesta J, Xavier K, Purohit D, Elhassan M, Bakthavatsalam D, Rowland M, Hutton P, Bashyal A, Davidson N, Hird C, Chhablani M, Phalod G, Kirkby A, Archer S, Netherton K, Reschreiter H, Camsooksai J, Patch S, Jenkins S, Pogson D, Rose S, Daly Z, Brimfield L, Claridge H, Parekh D, Bergin C, Bates M, Dasgin J, McGhee C, Sim M, Hay SK, Henderson S, Phull MK, Zaidi A, Pogreban T, Rosaroso LP, Harvey D, Lowe B, Meredith M, Ryan L, Hormis A, Walker R, Collier D, Kimpton S, Oakley S, Rooney K, Rodden N, Hughes E, Thomson N, McGlynn D, Walden A, Jacques N, Coles H, Tilney E, Vowell E, Schuster-Bruce M, Pitts S, Miln R, Purandare L, Vamplew L, Spivey M, Bean S, Burt K, Moore L, Day C, Gibson C, Gordon E, Zitter L, Keenan S, Baker E, Cherian S, Cutler S, Roynon-Reed A, Harrington K, Raithatha A, Bauchmuller K, Ahmad N, Grecu I, Trodd D, Martin J, Wrey Brown C, Arias AM, Craven T, Hope D, Singleton J, Clark S, Rae N, Welters I, Hamilton DO, Williams K, Waugh V, Shaw D, Puthucheary Z, Martin T, Santos F, Uddin R, Somerville A, Tatham KC, Jhanji S, Black E, Dela Rosa A, Howle R, Tully R, Drummond A, Dearden J, Philbin J, Munt S, Vuylsteke A, Chan C, Victor S, Matsa R, Gellamucho M, Creagh-Brown B, Tooley J, Montague L, De Beaux F, Bullman L, Kersiake I, Demetriou C, Mitchard S, Ramos L, White K, Donnison P, Johns M, Casey R, Mattocks L, Salisbury S, Dark P, Claxton A, McLachlan D, Slevin K, Lee S, Hulme J, Joseph S, Kinney F, Senya HJ, Oborska A, Kayani A, Hadebe B, Orath Prabakaran R, Nichols L, Thomas M, Worner R, Faulkner B, Gendall E, Hayes K, Hamilton-Davies C, Chan C, Mfuko C, Abbass H, Mandadapu V, Leaver S, Forton D, Patel K, Paramasivam E, Powell M, Gould R, Wilby E, Howcroft C, Banach D, Fernández de Pinedo Artaraz Z, Cabreros L, White I, Croft M, Holland N, Pereira R, Zaki A, Johnson D, Jackson M, Garrard H, Juhaz V, Roy A, Rostron A, Woods L, Cornell S, Pillai S, Harford R, Rees T, Ivatt H, Sundara Raman A, Davey M, Lee K, Barber R, Chablani M, Brohi F, Jagannathan V, Clark M, Purvis S, Wetherill B, Dushianthan A, Cusack R, de Courcy-Golder K, Smith S, Jackson S, Attwood B, Parsons P, Page V, Zhao XB, Oza D, Rhodes J, Anderson T, Morris S, Xia Le Tai C, Thomas A, Keen A, Digby S, Cowley N, Wild L, Southern D, Reddy H, Campbell A, Watkins C, Smuts S, Touma O, Barnes N, Alexander P, Felton T, Ferguson S, Sellers K, Bradley-Potts J, Yates D, Birkinshaw I, Kell K, Marshall N, Carr-Knott L, Summers C. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. 2020 Oct 6;324(13):1317-1329. doi: 10.1001/jama.2020.17022. PMID: 32876697; PMCID: PMC7489418.
- Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al-Dawood A, Merson L, Hayden FG, Fowler RA; Saudi Critical Care Trial Group. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018 Mar 15;197(6):757-767. doi: 10.1164/rccm.201706-1172OC. PMID: 29161116.
- Clemency BM, Varughese R, Gonzalez-Rojas Y, Morse CG, Phipatanakul W, Koster DJ, Blaiss MS. Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA Intern Med. 2022 Jan 1;182(1):42-49. doi: 10.1001/jamainternmed.2021.6759. PMID: 34807241; PMCID: PMC8609464.
- Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, Gómez-Barquero J, Abadía-Otero J, García-Ibarbia C, Mora V, Cerezo-Hernández A, Hernández JL, López-Muñíz G, Hernández-Blanco F, Cifrián JM, Olmos JM, Carrascosa M, Nieto L, Fariñas MC, Riancho JA; GLUCOCOVID investigators. Methylprednisolone in adults hospitalized with COVID-19 pneumonia : An open-label randomized trial (GLUCOCOVID). Wien Klin Wochenschr. 2021 Apr;133(7-8):303-311. doi: 10.1007/s00508-020-01805-8. Epub 2021 Feb 3. PMID: 33534047; PMCID: PMC7854876.
- COVID STEROID 2 Trial Group, Munch MW, Myatra SN, Vijayaraghavan BKT, Saseedharan S, Benfield T, Wahlin RR, Rasmussen BS, Andreasen AS, Poulsen LM, Cioccari L, Khan MS, Kapadia F, Divatia JV, Brøchner AC, Bestle MH, Helleberg M, Michelsen J, Padmanaban A, Bose N, Møller A, Borawake K, Kristiansen KT, Shukla U, Chew MS, Dixit S, Ulrik CS, Amin PR, Chawla R, Wamberg CA, Shah MS, Darfelt IS, Jørgensen VL, Smitt M, Granholm A, Kjær MN, Møller MH, Meyhoff TS, Vesterlund GK, Hammond NE, Micallef S, Bassi A, John O, Jha A, Cronhjort M, Jakob SM, Gluud C, Lange T, Kadam V, Marcussen KV, Hollenberg J, Hedman A, Nielsen H, Schjørring OL, Jensen MQ, Leistner JW, Jonassen TB, Kristensen CM, Clapp EC, Hjortsø CJS, Jensen TS, Halstad LS, Bak ERB, Zaabalawi R, Metcalf-Clausen M, Abdi S, Hatley EV, Aksnes TS, Gleipner-Andersen E, Alarcón AF, Yamin G, Heymowski A, Berggren A, La Cour K, Weihe S, Pind AH, Engstrøm J, Jha V, Venkatesh B, Perner A. Effect of 12 mg vs 6 mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA. 2021 Nov 9;326(18):1807-1817. doi: 10.1001/jama.2021.18295. Erratum in: JAMA. 2021 Dec 14;326(22):2333. PMID: 34673895; PMCID: PMC8532039.
- Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, François B, Aubron C, Ricard JD, Ehrmann S, Jouan Y, Guillon A, Leclerc M, Coffre C, Bourgoin H, Lengellé C, Caille-Fénérol C, Tavernier E, Zohar S, Giraudeau B, Annane D, Le Gouge A; CAPE COVID Trial Group and the CRICS-TriGGERSep Network. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA. 2020 Oct 6;324(13):1298-1306. doi: 10.1001/jama.2020.16761. PMID: 32876689; PMCID: PMC7489432.
- Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, Najafizadeh SR, Farhadi E, Jalili N, Esfahani M, Rahimi B, Kazemzadeh H, Mahmoodi Aliabadi M, Ghazanfari T, Sattarian M, Ebrahimi Louyeh H, Raeeskarami SR, Jamalimoghadamsiahkali S, Khajavirad N, Mahmoudi M, Rostamian A. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020 Dec 24;56(6):2002808. doi: 10.1183/13993003.02808-2020. PMID: 32943404; PMCID: PMC7758541.
- Ezer N, Belga S, Daneman N, Chan A, Smith BM, Daniels SA, Moran K, Besson C, Smyth LY, Bartlett SJ, Benedetti A, Martin JG, Lee TC, McDonald EG. Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ. 2021 Nov 2;375:e068060. doi: 10.1136/bmj-2021-068060. PMID: 34728476; PMCID: PMC8561042.
- Griesel M, Wagner C, Mikolajewska A, Stegemann M, Fichtner F, Metzendorf MI, Nair AA, Daniel J, Fischer AL, Skoetz N. Inhaled corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2022 Mar 9;3(3):CD015125. doi: 10.1002/14651858.CD015125. PMID: 35262185; PMCID: PMC8905579.
- Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux JP, Nasir N, Bonifaz A, Araiza J, Klimko N, Serris A, Lagrou K, Meis JF, Cornely OA, Perfect JR, White PL, Chakrabarti A; ECMM and ISHAM collaborators. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022 Jan 25. doi: 10.1016/S2666-5247(21)00237-8. Epub ahead of print. PMID: 35098179; PMCID: PMC8789240.
- Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, Moniri A, Abtahian Z, Haseli S, Mortaz E, Dastan A, Mohamadnia A, Vahedi A, Monjazebi F, Yassari F, Fadaeizadeh L, Saffaei A, Dastan F. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: A preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021 Apr 15;897:173947. doi: 10.1016/j.ejphar.2021.173947. Epub 2021 Feb 16. PMID: 33607104; PMCID: PMC7885705.
- Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Safe IP, Borba MGS, Netto RLA, Maciel ABS, Neto JRS, Oliveira LB, Figueiredo EFG, Oliveira Dinelly KM, de Almeida Rodrigues MG, Brito M, Mourão MPG, Pivoto João GA, Hajjar LA, Bassat Q, Romero GAS, Naveca FG, Vasconcelos HL, de Araújo Tavares M, Brito-Sousa JD, Costa FTM, Nogueira ML, Baía-da-Silva DC, Xavier MS, Monteiro WM, Lacerda MVG; Metcovid Team. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With Coronavirus Disease 2019 (COVID-19; Metcovid): A Randomized, Double-blind, Phase IIb, Placebo-controlled Trial. Clin Infect Dis. 2021 May 4;72(9):e373-e381. doi: 10.1093/cid/ciaa1177. PMID: 32785710; PMCID: PMC7454320.
- Keller MJ, Kitsis EA, Arora S, Chen JT, Agarwal S, Ross MJ, Tomer Y, Southern W. Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19. J Hosp Med. 2020 Aug;15(8):489-493. doi: 10.12788/jhm.3497. PMID: 32804611; PMCID: PMC7518134.
- Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJ, Lo YM. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004 Dec;31(4):304-9. doi: 10.1016/j.jcv.2004.07.006. PMID: 15494274; PMCID: PMC7108318.
- Meduri GU, Bridges L, Shih MC, Marik PE, Siemieniuk RAC, Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016 May;42(5):829-840. doi: 10.1007/s00134-015-4095-4. Epub 2015 Oct 27. PMID: 26508525.
- Munch MW, Meyhoff TS, Helleberg M, Kjaer MN, Granholm A, Hjortsø CJS, Jensen TS, Møller MH, Hjortrup PB, Wetterslev M, Vesterlund GK, Russell L, Jørgensen VL, Kristiansen KT, Benfield T, Ulrik CS, Andreasen AS, Bestle MH, Poulsen LM, Hildebrandt T, Knudsen LS, Møller A, Sølling CG, Brøchner AC, Rasmussen BS, Nielsen H, Christensen S, Strøm T, Cronhjort M, Wahlin RR, Jakob SM, Cioccari L, Venkatesh B, Hammond N, Jha V, Myatra SN, Jensen MQ, Leistner JW, Mikkelsen VS, Svenningsen JS, Laursen SB, Hatley EV, Kristensen CM, Al-Alak A, Clapp E, Jonassen TB, Bjerregaard CL, Østerby NCH, Jespersen MM, Abou-Kassem D, Lassen ML, Zaabalawi R, Daoud MM, Abdi S, Meier N, la Cour K, Derby CB, Damlund BR, Laigaard J, Andersen LL, Mikkelsen J, Jensen JLS, Rasmussen AH, Arnerlöv E, Lykke M, Holst-Hansen MZB, Tøstesen BW, Schwab J, Madsen EK, Gluud C, Lange T, Perner A. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: The COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiol Scand. 2021 Nov;65(10):1421-1430. doi: 10.1111/aas.13941. Epub 2021 Sep 20. PMID: 34138478; PMCID: PMC8441888.
- Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008 May 3;336(7651):1006-9. doi: 10.1136/bmj.39537.939039.BE. Epub 2008 Apr 23. PMID: 18434379; PMCID: PMC2364864.
- Ramakrishnan S, Nicolau DV Jr, Langford B, Mahdi M, Jeffers H, Mwasuku C, Krassowska K, Fox R, Binnian I, Glover V, Bright S, Butler C, Cane JL, Halner A, Matthews PC, Donnelly LE, Simpson JL, Baker JR, Fadai NT, Peterson S, Bengtsson T, Barnes PJ, Russell REK, Bafadhel M. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021 Jul;9(7):763-772. doi: 10.1016/S2213-2600(21)00160-0. Epub 2021 Apr 9. Erratum in: Lancet Respir Med. 2021 Jun;9(6):e55. PMID: 33844996; PMCID: PMC8040526.
- Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, Erfani A, Khodamoradi Z, Gholampoor Saadi MH. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021 Apr 10;21(1):337. doi: 10.1186/s12879-021-06045-3. Erratum in: BMC Infect Dis. 2021 May 11;21(1):436. PMID: 33838657; PMCID: PMC8035859.
- RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021 Feb 25;384(8):693-704. doi: 10.1056/NEJMoa2021436. Epub 2020 Jul 17. PMID: 32678530; PMCID: PMC7383595.
- Salvarani C, Massari M, Costantini M, Franco Merlo D, Lucia Mariani G, Viale P, Nava S, Guaraldi G, Dolci G, Boni L, Savoldi L, Bruzzi P, Turrà C, Catanoso M, Maria Marata A, Barbieri C, Valcavi A, Franzoni F, Cavuto S, Mazzi G, Corsini R, Trapani F, Bartoloni A, Barisione E, Barbieri C, Jole Burastero G, Pan A, Inojosa W, Scala R, Burattini C, Luppi F, Codeluppi M, Eldin Tarek K, Cenderello G, Salio M, Foti G, Dongilli R, Bajocchi G, Alberto Negri E, Ciusa G, Fornaro G, Bassi I, Zammarchi L, Aloè T, Facciolongo N. Intravenous methylprednisolone pulses in hospitalised patients with severe COVID-19 pneumonia, A double-blind, randomised, placebo-controlled trial. Eur Respir J. 2022 Mar 31:2200025. doi: 10.1183/13993003.00025-2022. Epub ahead of print. PMID: 35361632; PMCID: PMC8971731.
- Solanich X, Antolí A, Rocamora-Blanch G, Padullés N, Fanlo-Maresma M, Iriarte A, Mitjavila F, Capdevila O, Riera-Mestre A, Bas J, Vicens-Zygmunt V, Niubó J, Calvo N, Bolivar S, Rigo-Bonnin R, Mensa-Vilaró A, Arregui L, Tebe C, Videla S, Hereu P, Corbella X. Methylprednisolone Pulses Plus Tacrolimus in Addition to Standard of Care vs. Standard of Care Alone in Patients With Severe COVID-19. A Randomized Controlled Trial. Front Med (Lausanne). 2021 Jun 14;8:691712. doi: 10.3389/fmed.2021.691712. PMID: 34195214; PMCID: PMC8236585.
- Song JY, Yoon JG, Seo YB, et al. Ciclesonide Inhaler Treatment for Mild-to-Moderate COVID-19: A Randomized, Open-Label, Phase 2 Trial. Journal of Clinical Medicine. 2021 Aug;10(16). DOI: 10.3390/jcm10163545. PMID: 34441840; PMCID: PMC8396813.
- Tang X, Feng YM, Ni JX, Zhang JY, Liu LM, Hu K, Wu XZ, Zhang JX, Chen JW, Zhang JC, Su J, Li YL, Zhao Y, Xie J, Ding Z, He XL, Wang W, Jin RH, Shi HZ, Sun B. Early Use of Corticosteroid May Prolong SARS-CoV-2 Shedding in Non-Intensive Care Unit Patients with COVID-19 Pneumonia: A Multicenter, Single-Blind, Randomized Control Trial. Respiration. 2021;100(2):116-126. doi: 10.1159/000512063. Epub 2021 Jan 22. PMID: 33486496; PMCID: PMC7900459.
- Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP; COALITION COVID-19 Brazil III Investigators. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020 Oct 6;324(13):1307-1316. doi: 10.1001/jama.2020.17021. PMID: 32876695; PMCID: PMC7489411.
- Tsai MJ, Yang KY, Chan MC, Kao KC, Wang HC, Perng WC, Wu CL, Liang SJ, Fang WF, Tsai JR, Chang WA, Chien YC, Chen WC, Hu HC, Lin CY, Chao WC, Sheu CC; for Taiwan Severe Influenza Research Consortium (TSIRC) Investigators. Impact of corticosteroid treatment on clinical outcomes of influenza-associated ARDS: a nationwide multicenter study. Ann Intensive Care. 2020 Feb 27;10(1):26. doi: 10.1186/s13613-020-0642-4. PMID: 32107651; PMCID: PMC7046839.
- Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, Metzendorf MI, Fischer AL, Kopp M, Stegemann M, Skoetz N, Fichtner F. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021 Aug 16;8(8):CD014963. doi: 10.1002/14651858.CD014963. PMID: 34396514; PMCID: PMC8406706.
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul 1;180(7):934-943. doi: 10.1001/jamainternmed.2020.0994. Erratum in: JAMA Intern Med. 2020 Jul 1;180(7):1031. PMID: 32167524; PMCID: PMC7070509
- Yang ZG, Lei XL, Li XL. Early application of low-dose glucocorticoid improves acute respiratory distress syndrome: A meta-analysis of randomized controlled trials. Exp Ther Med. 2017 Apr;13(4):1215-1224. doi: 10.3892/etm.2017.4154. Epub 2017 Feb 22. PMID: 28413460; PMCID: PMC5377286.
- Yu LM, Bafadhel M, Dorward J, Hayward G, Saville BR, Gbinigie O, Van Hecke O, Ogburn E, Evans PH, Thomas NPB, Patel MG, Richards D, Berry N, Detry MA, Saunders C, Fitzgerald M, Harris V, Shanyinde M, de Lusignan S, Andersson MI, Barnes PJ, Russell REK, Nicolau DV Jr, Ramakrishnan S, Hobbs FDR, Butler CC; PRINCIPLE Trial Collaborative Group. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021 Sep 4;398(10303):843-855. doi: 10.1016/S0140-6736(21)01744-X. Epub 2021 Aug 10. Erratum in: Lancet. 2021 Aug 18;: PMID: 34388395; PMCID: PMC8354567.
Evidence tabellen
|
First author, year |
Describe method of randomisation1 |
Bias due to inadequate concealment of allocation?2
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?4
(unlikely/likely/unclear) |
Bias due to loss to follow-up?5
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?6
(unlikely/likely/unclear) |
|
Dexamethasone |
||||||||
|
Tomazini et al. 2020
|
The randomization list was generated by the trial statistician, not involved in patient care or enrolment, using the R software. The list comprised random blocks of two and four, unknown to researchers and stratified at center level and was uploaded and implemented in the on-line web-based system used for randomization data collection. |
Unlikely
The randomization list was generated by the trial statistician, not involved in patient care or enrolment, using the R software.
The group treatment was disclosed to the investigator only after all information regarding patient enrolment was recorded in the online system. |
Likely
Physicians, patients, and individuals who assessed the outcomes were not blinded for the assigned treatment
|
Likely
Physicians, patients, and individuals who assessed the outcomes were not blinded for the assigned treatment
|
Likely
Physicians, patients, and individuals who assessed the outcomes were not blinded for the assigned treatment
|
Unlikely
All predefined outcome measures were reported |
Unlikely
All patients were included in the primary analysis. There was no loss to follow-up, and data on the primary outcome, mortality within 28 days, clinical status at day 15, ICU-free days at 28 days, and mechanical ventilation duration were available for all patients.
|
Unlikely
ITT analysis performed. |
|
Jamaati, 2021
|
Computerized
The selected patients were allocated to either the dexamethasone group or the control group by block randomization. Ten blocks were generated by the Online Randomizer website. Each block included five patients; of these, two patients were assigned to the dexamethasone group and three patients were assigned to the control group or vice versa. |
Unclear
It is unclear whether allocation of treatment was concealed to the patients and caretakers. |
Unlikely
The authors do not report whether the patients were blind to the randomization. However, it is not expected that patients influenced the reported outcome measures (need for invasive mechanical ventilation, death rate, length of hospital / ICU stay, and radiological changes in the CT scan). |
Unclear
The authors do not report whether any blinding occurred. This may have affected the more subjective outcome measures such as duration of hospital stay, and whether ventilation was started or maintained. |
Unlikely
The authors report that the radiologist who assessed the CT scans was blinded to the lab data and clinical findings. |
Unlikely
All outcome measures stated in the methods section were reported in the results. |
Unlikely
No patients seem to have been lost to follow up (except for those who died). |
Unlikely
No mention of ITT analysis, however the participants seem to be analyzed as allocated. |
|
Horby et al., 2021
|
Randomization was performed with the use of a Web-based system with concealment of the trial-group assignment |
Unlikely
Adequate concealment
“webbased system randomization with concealment of the trial-group assignment”. |
Likely
Patients not blinded, primary outcome not susceptible for bias (death) |
Likely
Study staff not blinded to allocated treatment |
Likely
Study staff not blinded to allocated treatment |
Unlikely
Relevant outcomes reported; protocol and analysis plan available |
Unlikely
Completed follow-up forms were available for 2095 of 2104 patients (99.6%) in the intervention group and 4306 of 4321 patients (99.7%) in the control group.
All information is based on a data cut-off of December 14, 2020. Information regarding the primary and secondary outcomes is complete for 99.9% of trial participants. |
Unlikely
Analyses performed according to intention-to-treat protocol |
|
Hydrocortisone |
||||||||
|
Angus et al. 2020 |
Randomization will be conducted through a password-protected, secure website using a central, computer-based randomization program. |
Unlikely
Randomization will be at the patient level and occur after data necessary to implement the inclusion and exclusion criteria have been entered into the secure randomization website. The RAR will occur centrally as part of the computerized randomization process. Sites will receive the allocation status and will not be informed of the randomization proportions. |
Unclear
Not reported in the study
|
Unlikely
The default position within the REMAP is that treatments determined by randomization will be provided on an open-label basis. However, the blinding of treatment status is not precluded within the REMAP. If required, details related to blinding of interventions will be specified in the DSAs. |
Unlikely
The primary outcome of all-cause mortality censored at 90 days is not subject to ascertainment bias. Wherever possible, trial management personnel, who are blinded to allocation status, will conduct any follow up after discharge. |
Unlikely
Wherever possible, trial management personnel, who are blinded to allocation status, will conduct any follow up after discharge. |
Unlikely
Patients missing the primary end point (n = 5) were ignored; there was no imputation of missing primary (or secondary) end point values. A patient who survived to hospital discharge was assumed to be free of organ support through 21 days (last status carried forward). |
Unlikely
Analysis of the primary outcome was then repeated in a second model using only data from those patients enrolled in the corticosteroid domain with no adjustment for assignment to interventions in other domains. Although using less information, this analysis is more typical for an RCT. Further secondary analyses explored the effects of excluding patients who were ruled out for COVID-19 (defined as documented negative test results for SARS-CoV-2 infection and no positive test results), of excluding adjustment for site and time epoch, and of combining the fixed-dose and shock-dependent hydrocortisone groups.
|
|
Dequin et al., 2020
|
Computerized
“Randomization was centralized and performed electronically. Allocation sequences were generated in a 1:1 ratio by a computer-generated random number using a blocking schema; the range of block sizes remains confidential until the completion of the parent trial. Randomization was stratified by center and by use of mechanical ventilation at the time of inclusion.” |
Likely
Randomization stratified by center |
Unlikely
Patients in placebo group in identical protocol |
Unlikely
“Both hydrocortisone and placebo were provided in industrially prepared packaging (Serb Specialty Pharmaceuticals).” |
Unclear
|
Unlikely
Trial registered; all relevant outcomes reported |
Unlikely
1 patient in the intervention group withdrew consent; this patient was considered to have experiences treatment failure on day 21 (primary outcome) |
Unlikely
Analyses performed according to intention-to-treat protocol |
|
Munch, 2021 |
Computerised
Participants were randomised (1:1) using a centralised and web-based randomisation system at the Copenhagen Trial Unit (CTU. The randomisation was performed according to a computer-generated allocation sequence, stratification variables, and varying block sizes. |
Unlikely
The allocation sequence was only known by the data manager at the CTU. |
Unlikely
Participants were blinded to treatment allocation. |
Unlikely
Clinical staff members were blinded to the allocation. |
Unlikely
Management Committee, investigators, trial site staff registering outcome data and the trial statistician were blinded to the allocation. |
Unlikely
All outcome measures described in the methods are reported in the results. |
Unlikely
No participants were lost to follow-up. |
Unlikely
All analyses were done in the intention-to-treat population, defined as all randomised participants for whom there were consent to use data. |
|
Methylprednisolone |
||||||||
|
Edalatifard et al., 2021
|
Method not described
“the patients randomly allocated in control (n=34) and intervention group (n=34), in a 1:1 ratio by block randomization method.” |
Unclear
not described |
Unlikely
Patients were blinded to treatment |
Likely
“ Physicians and clinicians team know about the medicine and intervention groups” |
Likely
“ Physicians and clinicians team know about the medicine and intervention groups” |
Unlikely
Relevant outcomes reported
Trial registered: Iranian Registry of Clinical Trials on 15 April 2020 (IRCT ID: IRCT20200404046947N1 |
Likely
34/34 (100%) of patients of the intervention and 28/34 (82.4%) of patients of the control group included in analysis |
Likely
6 patients (17.8%) discontinued treatment of control group and received intervention. They were excluded from the analysis.
Analyses performed according to intention-to-treat protocol |
|
Jeronimo et al., 2020
|
An independent statistician prepared an electronically generated randomization list with 14 blocks of 30 participants per block, |
Unlikely
The list was accessible only to nonblinded pharmacists in the study. Participants were randomized by the study pharmacist to their designated treatment regimen at the time of inclusion and were subsequently identified throughout the study only by their allocated study number. |
Unlikely
Patients were considered to be blinded for the interventional drug
|
Unclear
No blinding of care providers |
Unlikely:
Radiologists and other assessors were blinded |
Unlikely
Primary outcome was mortality
|
Unlikely
Low loss to follow up: I: 0.5% |
Unlikely
A modified and non-modified intention-to-treat analyses was conducted |
|
Corral-Gudino et al., 2021
|
Patients were randomized based on a spreadsheet that transformed every medical record number into a group allocation using a concealed mathematical formula
Partly randomized; i.e. some participants received treatment according to the clinician’s preference, the other participants were randomized.
If the clinical team decided that a strong preference for glucocorticoid therapy existed, the patient was allocated to the preference arm. Otherwise, the patient was randomized (1:1) and allocated to the MP or control arm accordingly.. |
Unclear
Note: allocation concealment not described. allocation. |
Unclear
Note: Blinding of participants was not described
|
Unclear
Note: Blinding of care providers was not described
|
Unclear
Note: Blinding of outcome assessors was not described
|
Unclear
Note: Protocol not available
|
Unclear
Note: Loss to follow-up was not reported
|
Likely
Note: In the intention-to-treat analysis, only patients who received at least one dose of treatment were included in the treatment arm. |
|
Tang, 2021 |
Computerized
Randomization was stratified by the statistician of the leading site, who produced computer- generated block randomization lists with a block size of 4 patients. |
Unlikely
Allocation of treatment seems to have been concealed properly. |
Unlikely
Patients were blind to treatment allocation.
|
Likely
Care providers were not blind to treatment allocation. Therefore, they could have biased the outcome measures that are prone to subjectivity, such as the duration of hospitalization and ICU admission.
Unlikely
It is unlikely that care providers biased objective outcome measures such as mortality, clinical deterioration or clinical cure, and virus shedding.
|
Unlikely
During the study, data collection and end point judgement were blinded, and the statisticians were also blinded during the statistical analysis. |
Unlikely
All outcome measures stated in the methods section were reported in the results. |
Unlikely
No patients were lost to follow up. |
Unlikely
Authors indicate that an ITT was not necessary and the included patients seem to be analyzed as allocated. |
|
Solanich, 2021
|
Computerized
Patients were randomized using the RedCap, a secure web application for building and managing electronic case report forms (eCRF). Patients were randomly (1:1) assigned to one of the study arms with no baseline stratification. |
Unclear
No information on concealment |
Likely
Open-label RCT |
Likely
Open-label RCT |
Unlikely
The IDIBELL Biostatistical Unit performed the analysis and analysts were blinded to the treatment received by patients (intervention vs. usual care) |
Unlikely
All outcomes were reported in main article of appendix
|
Unlikely
Similar loss to follow-up, no indications for bias
|
Unlikely
Except for outcome on viral load, but no comparisons were done.
|
Table of excluded studies
On request.
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 07-11-2022
Beoordeeld op geldigheid : 03-10-2022
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut). Deze ondersteuning werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De werkgroep werd gefinancierd uit een VWS subsidie.
De financiers hebben geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodules is in 2020 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de behandeling van patiënten met COVID-19.
In 2020 is een multidisciplinair expertiseteam behandeling ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van het expertiseteam behandeling) die betrokken zijn bij de zorg voor patiënten met COVID-19. Dit expertiseteam fungeerde als stuurgroep, welke opdracht heeft gegeven tot het ontwikkelen van de module, alsmede fungeerde als klankbordgroep.
Werkgroep
- Dr. Marjolein Hensgens, internist-infectioloog, Afdeling Infectieziekten, UMC Utrecht en LUMC Leiden (Stichting Werkgroep Antibiotica Beleid)
- Drs. Emilie Gieling, apotheker, Afdeling Klinische Farmacie, UMC Utrecht.
- Prof. Dr. Dylan de Lange, intensivist, Afdeling Intensive Care, UMC Utrecht.
- Dr. Wim Boersma, longarts, Afdeling Longziekten, Noordwest Ziekenhuisgroep, Alkmaar.
- Dr. Paul van der Linden, apotheker, Afdeling Klinische Farmacie, Tergooi MC, Hilversum (Stichting Werkgroep Antibiotica Beleid).
- Prof. Dr. Bhanu Sinha, arts-microbioloog, Afdeling Medische Microbiologie & Infectiepreventie, UMCG, Groningen (Stichting Werkgroep Antibiotica Beleid).
- Dr. Mark de Boer, internist-infectioloog, Afdelingen Infectieziekten en Klinische Epidemiologie, LUMC, Leiden (Stichting Werkgroep Antibiotica Beleid).
- Tot 1-11-2021 tevens deel van de werkgroep: Dr. Albert Vollaard, internist-infectioloog, LCI, RIVM
Stuurgroep (expertiseteam Behandeling COVID-19)
- Dr. L.M. van den Toorn (voorzitter), longarts, Erasmus Medisch Centrum (Erasmus MC), NVALT
- Dr. M.G.J. de Boer, internist-infectioloog, Leids Universitair Medisch Centrum (LUMC), SWAB/NIV)
- Drs. A.J. Meinders, internist-intensivist, St. Antonius Ziekenhuis, NVIC
- Prof. dr. D.W. de Lange, intensivist-toxicoloog, Universitair Medisch Centrum Utrecht (UMC Utrecht), NVIC
- Dr. C.H.S.B. van den Berg, infectioloog-intensivist Universitair Medisch Centrum Groningen (UMCG), NVIC
- Dr. S.U.C. Sankatsing, internist-infectioloog, Diakonessenhuis, NIV
- Dr. E.J.G. Peters, internist-infectioloog, Amsterdam University Medical Centers (Amsterdam UMC), NIV
- Drs. M.S. Boddaert, arts palliatieve geneeskunde, Leids Universitair Medisch Centrum (LUMC), IKNL
- Dr. P.L.A. Fraaij, kinderarts-infectioloog, Erasmus Medisch Centrum (Erasmus MC), Sophia Kinderziekenhuis, NVK
- Dr. E. van Leeuwen, gynaecoloog, Amsterdam University Medical Centers (Amsterdam UMC), NVOG
- Dr. J.J. van Kampen, arts-microbioloog, Erasmus Medisch Centrum (Erasmus MC), NVMM
- Dr. M. Bulatović-Ćalasan, internist allergoloog-immunoloog en klinisch farmacoloog, Universitair Medisch Centrum Utrecht (UMC Utrecht), Amsterdam University Medical Centers (Amsterdam UMC), NIV
- Drs. A.F.J. de Bruin, anesthesioloog-intensivist, St. Antonius Ziekenhuis, NVA
- Drs. A. Jacobs, klinisch geriater, Catharina Ziekenhuis, NVKG
- Drs. B. Hendriks, ziekenhuisapotheker, Leids Universitair Medisch Centrum (LUMC), NVZA
- Drs. M. Nijs, huisarts, NHG
- Dr. S.N. Hofstede, senior adviseur, Kennisinstituut van Medisch Specialisten
Meelezer
- Drs. K. (Klaartje) Spijkers, senior adviseur patiëntenbelang, Patiëntenfederatie Nederland, Utrecht
Met ondersteuning van:
- dr. S.N. Hofstede, senior adviseur, Kennisinstituut van Medisch Specialisten
- dr. L.M.P. Wesselman, adviseur, Kennisinstituut van Medisch Specialisten
- dr. D. Nieboer, adviseur, Kennisinstituut van Medisch Specialisten
- drs. A.L.J. (Andrea) Kortlever - van der Spek, adviseur, Kennisinstituut van Medisch Specialisten
-
M. Griekspoor MSc., junior adviseur, Kennisinstituut van Medisch Specialisten
- drs. I. van Dusseldorp, senior literatuurspecialist, Kennisinstituut van Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
De Lange |
1. Afdelingshoofd Nationaal Vergiftigingen Informatie Centrum (NVIC) van het UMC Utrecht (0,6 fte) |
Secretaris Stichting Nationale Intensive Care Evaluatie (Stichting NICE), onbezoldigd. |
Geen |
Geen actie nodig |
|
De Boer |
Internist-Infectioloog, klinisch epidemioloog, senior medisch specialist, Leids Universitair Medisch Centrum, afdeling Infectieziekten |
- Voorzitter Stichting Werkgroep Antibioticabeleid (onkostenvergoeding) |
Geen |
Geen actie nodig |
|
Sinha |
Arts-microbioloog/hoogleraar, Universitair Medisch Centrum Groningen (voltijd) (zie ook https;//www.rug.nl/staff/b.sinha/) |
- SWAB-bestuur: secretaris [onbetaald; vacatiegeld voor instelling] |
- Projectsubsidie EU (Cofund): deelprojecten, cofinanciering
Mogelijk boedbeeldfunctie SWAB |
Geen actie nodig |
|
Van der Linden |
Ziekenhuisapotheker |
Penningmeester SWAB, vacatiegeld |
Geen |
Geen actie nodig |
|
Vollaard |
Internist-infectioloog, Landelijke Coordinatie Infectieziektebestrijding, RIVM |
Arts voor ongedocumenteerde migranten, Dokters van de Wereld, Amsterdam (onbetaald) |
Geen |
Geen actie nodig |
|
Gieling |
Ziekenhuisapotheker - Klinisch Farmacoloog, UMC Utrecht |
Lid OMT Nederlandse Vereniging voor Ziekenhuisapothekers (onbetaald) |
Geen |
Geen actie nodig |
|
Boersma |
Longarts Noordwest Ziekhuisgroep |
Lid sectie infectieziekten NVALT, onbetaald |
Eenmalige digitale deelname aan adviesraad MSD Pneumovax over Pneumococcal disease, betaald
|
Geen actie nodig |
|
Hensgens |
Internist-infectioloog, UMC Utrecht (0.8 aanstelling, waarvan nu 0.4 gedetacheerd naar LUMC) Internist-infectioloog, LUMC (via detachering, zie boven) |
Geen |
Geen |
Geen actie nodig |
Stuurgroep
|
Achternaam stuurgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Van den Toorn (voorzitter) |
Voorzitter NVALT |
Geen |
Geen |
Geen actie nodig |
|
De Boer |
Internist-Infectioloog, senior medisch specialist, LUMC, afdeling infectieziekten |
- Voorzitter Stichting Werkgroep Antibioticabeleid (onkostenvergoeding) |
Geen |
Geen actie nodig |
|
Meinders |
Internist-intensivist, St.-Antonius ziekenhuis, Nieuwegein |
commissie werk |
Geen |
Geen actie nodig |
|
De Lange |
Afdelingshoofd Nationaal Vergiftigingen Informatie Centrum (NVIC) van het UMC Utrecht |
secretaris Stichting Nationale Intensive Care Evaluatie (Stichting NICE) (onbetaald) |
Geen |
Geen actie nodig |
|
Van den Berg |
Infectioloog-intensivist, UMCG |
Geen |
Geen |
Geen actie nodig |
|
Sankatsing |
Internist-infectioloog/internist-acute geneeskunde, Diakonessenhuis, Utrecht |
- Bestuurslid Nederlandse Vereniging van Internist-Infectiologen (NVII) (onbetaald). |
Geen |
Geen actie nodig |
|
Peters |
Internist - aandachtsgebieden infectieziekten en Acute Geneeskunde Amsterdam UMC, locatie Vumc |
Wetenschappelijk Secretaris International Working Group on the Diabetic Foot (onbetaald) |
Geen |
Geen actie nodig |
|
Boddaert |
Medisch adviseur bij Integraal Kankercentrum Nederland (IKNL) en Palliatieve Zorg Nederland (PZNL) Arts palliatieve geneeskunde in LUMC |
Geen |
Geen |
Geen actie nodig |
|
Fraaij |
Kinderarts infectioloog- immunoloog, Erasmus MC-Sophia, Rotterdam |
Bestuur Stichting Infecties bij Kinderen (onbetaald) |
deelname aan RECOVER, European Union's Horizon 2020 research |
Geen actie nodig |
|
Van Leeuwen |
Gyaecoloog Amsterdam Universitair Medisch Centra |
Geen |
Geen |
Geen actie nodig |
|
Van Kampen |
Arts-microbioloog, afdeling Viroscience, Erasmus MC |
- associate editor antimicrobial resistance & infection control (onbetaald) - lid antibioticacommissie Erasmus MC (onbetaald) |
1. Mede uitvinder patent: 1519780601-1408/3023503 2. R01AI147330 (NIAID/NH) (HN onderzoek (1+2 niet gerelateerd aan COVID-19)
|
Geen actie nodig |
|
Bulatovic |
Internist allergoloog-immunoloog en klinische farmacoloog, UMC Utrecht en Diakonessenhuis Utrecht |
Functie 1: arts |
Geen |
Geen actie nodig |
|
De Bruin |
Anesthesioloog - Intensivist St. Antonius ziekenhuis Nieuwegein en Utrecht |
Geen |
Geen |
Geen actie nodig |
|
Jacobs |
Klinisch geriater en klinisch farmacoloog |
Geen |
Geen |
Geen actie nodig |
|
Hendriks |
Ziekenhuisapotheker farmaceutische patiëntenzorg, afd. Kiinische Farmacie en Toxicoiogie, Leids Universitair Medisch Centrum |
Lid SWAB werkgroep surveillance antibioticagebruik, onbetaald Lid SWAB richtlijncommissie antibiotica allergie, onbetaald |
Geen |
Geen actie nodig |
|
Nijs |
Huisarts |
Geen |
Geen |
Geen actie nodig |
|
Hofstede |
Senior adviseur Kennisinstituut van Medisch Specialisten |
Geen |
Geen |
Geen actie nodig |
Meelezer
|
Achternaam |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Spijkers |
Senior adviseur patiëntenbelang |
Voorzitter Stichting Samen voor Duchenne |
Geen |
Geen actie nodig |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door een afgevaardigde patiëntenvereniging in de klankbordgroep. De verkregen input is meegenomen bij het opstellen van de module. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Werkwijze
Van leidraad naar richtlijnmodules
Bij aanvang van de pandemie in 2020 was het onduidelijk of bestaande of nieuwe medicijnen een relevante bijdrage konden leveren aan het herstel van patiënten geïnfecteerd met het SARS-CoV-2. Vandaar dat eind februari 2020 werd aangevangen met de eerste versie van de leidraad ‘Medicamenteuze behandeling voor patiënten met COVID-19 (infectie met SARS–CoV-2)’, welke begin maart 2020 online beschikbaar werd gesteld op de website van de SWAB (https://swab.nl/nl/covid-19). Sindsdien werd het adviesdocument op wekelijkse basis gereviseerd en indien nodig op basis van nieuwe publicaties van onderzoek aangepast. Het initiatief en de coördinatie hiertoe werden genomen door de SWAB Leidraadcommissie, ondersteund door het kennisinstituut van de Federatie Medisch Specialisten en een brede klankbordgroep waarbinnen de betrokken specialisten(verenigingen) zijn vertegenwoordigd. In september 2021 is gestart met het doorontwikkelen van de leidraad naar richtlijnmodules.
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de COVID-19 pandemie zijn knelpunten op verschillende manieren geïnventariseerd:
1. De expertiseteams benoemde de knelpunten in de zorg voor patiënten met COVID-19.
2. Er is een mailadres geopend (covid19@demedischspecialist.nl) waar verschillende partijen knelpunten konden aandragen, die vervolgens door de expertiseteams geprioriteerd werden.
3. Door de Federatie van Medisch Specialisten zijn webinars georganiseerd waarbij vragen konden worden ingestuurd. Deze vragen zijn na afloop van de webinars voorgelegd aan de expertiseteams en geprioriteerd.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Wanneer mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
Definitie |
|
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
Bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
On request.
