Verneveling bij bronchiolitis
Uitgangsvraag
Wat is de rol van vernevelingen (met normotoon zout, hypertoon zout, salbutamol en/of ipratropiumbromide, adrenaline) bij de behandeling van bronchiolitis in de eerste uren na opvang bij kinderen die zich melden in het ziekenhuis (op de spoedeisende hulp (SEH))?
Aanbeveling
Aanbeveling 1
Geef niet standaard verneveling met normotoon zout bij kinderen die zich met bronchiolitis presenteren op de SEH.
Aanbeveling 2
Geef niet standaard verneveling met hypertoon zout bij kinderen met bronchiolitis in de eerste uren na presentatie op de SEH.
Aanbeveling 3
Geef niet standaard (proef)verneveling met salbutamol/ ipratropiumbromide bij kinderen die zich met bronchiolitis presenteren op de SEH.
Aanbeveling 4
Geef niet standaard adrenalineverneveling bij kinderen ≥1 maand oud, die zich met bronchiolitis op de SEH presenteren.
Overweeg verneveling met adrenaline indien op korte termijn verlichting van klachten wenselijk is (eenmalige dosis van 3 tot 5 mg; 1mg/1ml oplossing).
Houd rekening met de mogelijkheid dat het effect van adrenaline kortdurend van aard kan zijn.
Overwegingen
Effecten van de interventies en de kwaliteit van het bewijs
Voor het effect van verneveling bij kinderen met bronchiolitis is in de literatuur evidence based data van lage kwaliteit beschikbaar. Er zijn geen studies die het effect van normotoon zout verneveling op relevante uitkomstmaten bij bronchiolitis beschrijven. De gevonden literatuur wijst erop dat voor kinderen met bronchiolitis, verneveling met salbutamol/ipratropiumbromide geen of weinig effect heeft op uitkomstmaten. De literatuur laat voor verneveling met hypertoon zout – alleen of in een mix – wel een indicatie voor een positief effect op opnameduur, kans op opname en klinische symptomen zien, maar de bewijskracht is laag tot zeer laag. De data ondersteunen wel met enige zekerheid (matige bewijskracht) dat verneveling met adrenaline de kans op een ziekenhuisopname, m.n. op de korte termijn (binnen 24 uur) verkleint en klinische symptomen binnen twee dagen kan verminderen. Mogelijke effecten van de vernevelingen op de risico’s van intensive care opname en bijwerkingen zijn niet systematisch in de literatuur onderzocht.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Voor kinderen en hun ouders/verzorgers die zich met bronchiolitis in het ziekenhuis presenteren is het voornaamste doel de klachten van dyspnoe te verminderen, op een wijze, die zo min mogelijk invasief is, en het voorkomen van een opname. Verneveling is een niet invasieve therapie, al kan het soms door kinderen als vervelend ervaren worden. Het plaatsen van het mondmasker is voor de kinderen het vervelendst, het ligt niet in de lijn der verwachting dat ouders/ kinderen een voorkeur hebben voor welk type medicatie vervolgens wordt verneveld, mits de effectiviteit gelijk is.
Kosten (middelenbeslag)
Kosten van toepassing van verneveling met medicatie, waaronder ook natriumchloride zijn relatief laag. Een verneveling met natriumchloride, hypertoon zout, salbutamol en ipratropium bromide kost minder dan 1 euro per verneveling. Een verneveling met adrenaline kost circa 6 euro per verneveling. Zowel materialen voor verneveling als ook de medicatie zijn reeds in het ziekenhuis voorradig.
Aanvaardbaarheid, haalbaarheid en implementatie
De genoemde vernevelingen (normotoon en hypertoon zout, salbutamol, ipratropiumbromide, adrenaline) zijn beschikbaar in elk Nederlands ziekenhuis en de kosten zijn relatief laag. Verneveling is in principe haalbaar bij elke patiënt en is niet invasief. Het is wel een behandeling op de eerste hulp die een tijdsinvestering vraagt van de verpleegkundigen. Daarnaast kan verneveling voor kinderen ook stress/discomfort geven, wat als negatief effect een toename van ademarbeid kan veroorzaken.
Rationale van de aanbeveling 1: weging van argumenten voor en tegen de interventies
Er zijn onvoldoende data om een uitspraak te doen over effectiviteit van verneveling met normotoon zout bij kinderen die zich presenteren met bronchiolitis. In de praktijk lijken kinderen soms een positief effect te ondervinden van de toediening van bevochtigde lucht/ zuurstof die vaak gepaard gaat met de verneveling.
Rationale van de aanbeveling 2: weging van argumenten voor en tegen de interventie
Hypertoon zout lijkt in sommige studies een positief effect te hebben op de verblijfstijd in het ziekenhuis en bij langduriger gebruik (vanaf twee dagen) in ernst van de klachten; Het bewijs hiervoor is echter laag tot zeer laag. Gezien deze combinatie wordt niet geadviseerd om kinderen die zich met bronchiolitis op de SEH presenteren met hypertoon zout te behandelen.
Rationale van de aanbeveling 3: weging van de argumenten voor en tegen de interventie
Verneveling met salbutamol/ ipratropiumbromide lijkt geen positief effect te hebben in de behandeling van bronchiolitis in de eerste uren na presentatie. In de huidige literatuur is onvoldoende bewijs om een proefverneveling met salbutamol/ ipratropiumbromide, zoals geadviseerd in de vorige versie van de richtlijn, te onderbouwen. Echter, de aanwezige data zijn van lage kwaliteit, en klinische beoordeling dient de doorslag hiervoor te geven.
Rationale van de aanbeveling 4: weging van de argumenten voor en tegen de interventie
- Uit meerdere studies lijkt verneveling met adrenaline bij patiënten die zich met bronchiolitis op de SEH presenteren een risicoreductie voor opname <24 uur na presentatie te geven, als ook verkorting van de verblijfstijd in het ziekenhuis. Kanttekeningen bij deze bevinding zijn dat 1) de evidence matig is, 2) het gunstige effect veel zwakker is wanneer vergeleken wordt met salbutamol dan met normotoon zout en 3) het gunstige effect niet geldt voor risico op opname na 7 dagen. Daarom is de werkgroep van mening dat adrenalineverneveling niet standaard voorgeschreven moet worden, maar dat op individuele basis besloten kan worden om dit op de SEH toe te passen met aandacht voor het mogelijk slechts kortdurende effect.
- Gezien het bewijs voor een positief effect van adrenaline op clinical severity scores en opnames binnen 24 uur kan overwogen worden om dit in te zetten indien op korte termijn klinische verlichting van de klachten wenselijk is.
- Op basis van toegepaste doseringen in de literatuur kan geen duidelijke dosis- effect relatie worden geconstateerd. Gekeken naar toegepaste doseringen bij andere indicaties, zoals bijvoorbeeld laryngitis subglottica, zou de volgende dosering kunnen worden overwogen: eenmalige dosis van 5mg (1mg/1ml oplossing) bij kinderen ≥1 maand oud. Risico op (ernstige) bijwerkingen wordt bij deze dosering minimaal geacht.
- In de huidige literatuurstudie is de combinatie van adrenaline verneveling en orale corticosteroïden niet meegenomen; hoewel dit een veelbelovende interventie leek is er onvoldoende bewijs om dit aan te bevelen (kennislacune).
Onderbouwing
Achtergrond
Bronchiolitis wordt gekenmerkt door aanwezigheid van inflammatie, zwelling en slijmproductie. Bij de acute behandeling van kinderen met bronchiolitis zou theoretisch een positief effect verwacht kunnen worden van verneveling met middelen die:
- een anti-inflammatoir effect hebben, waaronder corticosteroïden;
- luchtwegverwijdend werken, waaronder salbutamol / ipratropiumbromide en adrenaline;
- de slijmklaring kunnen verbeteren en daardoor atelectase kunnen voorkomen/ verminderen, waaronder fysiologisch zout, hypertoon zout en desoxyribonuclease (Dnase).
Met betrekking tot vernevelingen in bij behandeling van kinderen met bronchiolitis op de SEH vermeldt de NVK richtlijn bronchiolitis van 2012 het volgende:
- Salbutamol/ ipratropiumbromide verneveling: advies tot proefverneveling, stop indien niet effectief.
- Corticosteroïden po, iv of inhalatie als monotherapie zijn niet effectief gebleken bij RSV bronchiolitis.
- DNase is niet effectief gebleken bij RSV bronchiolitis.
- Hypertoon zout en combinatiebehandeling van adrenaline verneveling en dexamethason oraal zijn mogelijk veelbelovende toekomstige interventies.
In de praktijk wordt bij kinderen met bronchiolitis in de kliniek naast de proefverneveling met salbutamol/ipratropiumbromide ook verneveling met diverse andere middelen toegepast, waaronder normotoon zout (NaCl 0,9%), hypertoon zout (bijv. NaCl 3%, NaCl 6%, NaCl 7%) en adrenaline verneveling. Doel van deze zoekopdracht is om te achterhalen of er actueel voldoende onderbouwing is om deze vormen van verneveling op te nemen in de richtlijn behandeling van kinderen met bronchiolitis op de SEH. Naar de combinatiebehandeling van adrenaline en dexamethason is sinds het uitkomen van de oude richtlijn in 2012 geen nieuw onderzoek verschenen; deze vorm van verneveling is daarom niet meegenomen in de zoekopdracht (wel adrenaline verneveling als monotherapie). Daarnaast willen wij de actuele ‘evidence’ voor proefverneveling met salbutamol/ ipratropiumbromide onderzoeken.
Conclusies / Summary of Findings
Conclusions: overview
For an overview of conclusions with regard to possible effect and the level of evidence per patient population, outcome and type of nebulization, see Table 1.
Table 1. Conclusions on possible effect and level of evidence per patient population, outcome, and type of nebulization
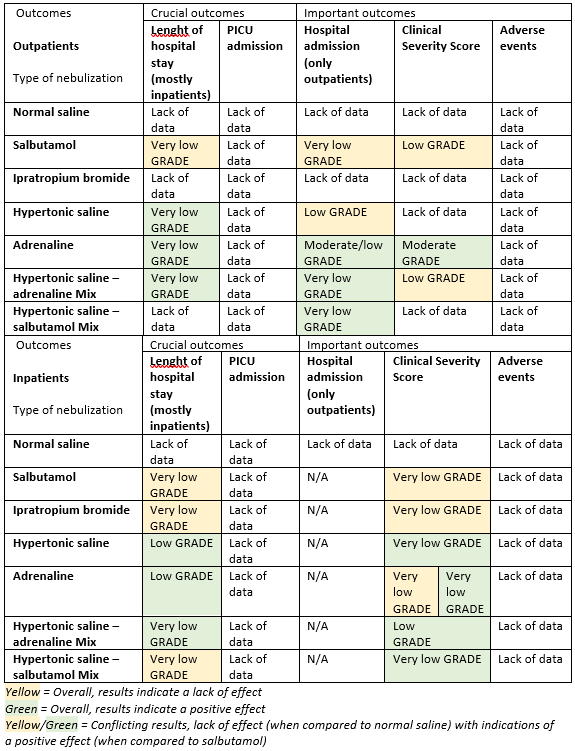
Conclusions: written out
|
GRADE |
Outcome |
Conclusions normal saline |
|
No GRADE |
All outcomes (all patients) |
For children with bronchiolitis (in- and outpatients), no data were found with regard to the effect of nebulization with normal saline on the outcomes of interest; therefore, no conclusions could be drawn with regard to the effects. |
|
GRADE |
Outcome |
Conclusions salbutamol |
|
Low |
CSS (all patients) |
For children with bronchiolitis, nebulization with salbutamol as compared to normal saline may have little to no effect on clinical severity score (CSS; outpatients and inpatients).
For children with bronchiolitis, nebulization with salbutamol as compared to normal saline may have little to no effect on length of hospital stay (LOS; outpatients and inpatients) and hospital admissions (HA; outpatients only), but the evidence is very uncertain.
For children with bronchiolitis (outpatients and inpatients), no (systematic) data were found with regard to the effect of nebulization with salbutamol on PICU admission (PICU) and adverse events (AE); therefore no conclusions could be drawn with regard to the effects.
Sources: Gadomski, 2014; Uysalol, 2017. |
|
Very low |
LOS (all patients), HA |
|
|
No GRADE |
PICU, AE (all patients) |
|
GRADE |
Outcome |
Conclusions ipratropium bromide |
|
Very low |
LOS, CSS (inpatients) |
For children with bronchiolitis (inpatients), nebulization with ipratropium bromide as compared to normal saline may have little to no effect on length of hospital stay (LOS) and clinical severity score (CSS), but the evidence is very uncertain.
For children with bronchiolitis (outpatients), no data were found with regard to the effect of nebulization with ipratropium bromide; therefore, no conclusions could be drawn with regard to the effect on LOS, hospital admissions (HA), and CSS.
For children with bronchiolitis (outpatients and inpatients), no (systematic) data were found with regard to the effect of nebulization with ipratropium bromide on PICU admission (PICU) and adverse events (AE); therefore, no conclusions could be drawn with regard to the effects.
Sources: Gadomski, 2014. |
|
No GRADE |
LOS, HA, CSS (outpatients)
PICU, AE (all patients) |
|
GRADE |
Outcome |
Conclusions hypertonic saline |
|
Low |
LOS (inpatients), HA (outpatients) |
For children with bronchiolitis, nebulization with hypertonic saline as compared to normal saline (not to standard care) may reduce length of hospital stay (LOS; outpatients and inpatients), but the evidence is very uncertain for outpatients.
For children with bronchiolitis, nebulization with hypertonic saline as compared to normal saline may have little to no effect on hospital admissions (HA; outpatients only).
For children with bronchiolitis, nebulization with hypertonic saline as compared to normal saline may reduce clinical severity score (CSS; inpatients), but the evidence is very uncertain.
For children with bronchiolitis, no data were found and therefore no conclusion could be drawn with regard to the effect of nebulization with hypertonic saline on CSS for outpatients.
For children with bronchiolitis (outpatients and inpatients), no (systematic) data were found with regard to the effect of nebulization with hypertonic saline on PICU admission (PICU) and adverse events (AE); therefore, no conclusions could be drawn with regard to the effects.
Sources: Zhang, 2017; Hsieh, 2020; Faten, 2014; Uysalol, 2017; Bashir, 2018, Jaquet-Pilloud, 2020. |
|
Very low |
LOS (outpatients), CSS (inpatients) |
|
|
No GRADE |
CSS (outpatients)
PICU, AE (all patients) |
|
GRADE |
Outcome |
Conclusions adrenaline |
|
Moderate |
HA (outpatients; within 24 hours), CSS (outpatients) |
For children with bronchiolitis, nebulization with adrenaline as compared to normal saline (and salbutamol) may reduce hospital admissions (HA; outpatients only), even likely within 24 hours.
For children with bronchiolitis, nebulization with adrenaline as compared to normal saline (and salbutamol and/or ipratropium bromide) may reduce clinical severity score (CSS; outpatients and inpatients), but the evidence is inconsistent and very uncertain for inpatients.
For children with bronchiolitis, nebulization with adrenaline as compared to normal saline (and salbutamol) may reduce length of hospital stay (LOS; outpatients and inpatients), but the evidence is very uncertain for outpatients.
For children with bronchiolitis (outpatients and inpatients), no (systematic) data were found with regard to the effect of nebulization with adrenaline on PICU admission (PICU) and adverse events (AE); therefore, no conclusions could be drawn with regard to the effects.
Sources: 1e Hartling, 2011; Modaressi, 2012; Skjerven, 2013; Uysalol, 2017. |
|
Low |
LOS (inpatients), HA (outpatients; within one week) |
|
|
Very Low |
LOS (outpatients), CSS (inpatients) |
|
|
No GRADE |
PICU, AE (all patients) |
|
GRADE |
Outcome |
Conclusions hypertonic saline - adrenaline mix |
|
Low |
CSS (all patients) |
For children with bronchiolitis, nebulization with hypertonic saline - adrenaline mix as compared to normal saline, hypertonic saline, adrenaline or normal saline-adrenaline mix may reduce clinical severity score (CSS) in inpatients, but may have little to no effect on CSS in outpatients (only comparisons to normal saline-adrenaline mix).
For children with bronchiolitis, nebulization with hypertonic saline - adrenaline mix as compared to normal saline, hypertonic saline, adrenaline or normal saline-adrenaline mix may reduce length of hospital stay (LOS; outpatients and inpatients), hospital admissions (HA; outpatients only; only comparisons to normal saline-adrenaline mix), but the evidence is very uncertain.
For children with bronchiolitis (outpatients and inpatients), no (systematic) data were found with regard to the effect of nebulization with hypertonic saline – adrenaline mix on PICU admission (PICU) and adverse events (AE); therefore, no conclusions could be drawn with regard to the effects.
Sources: 1e Zhang, 2017; Faten, 2014; Flores-Gonzalez, 2015; Uysalol, 2017. |
|
Very low |
LOS (all patients), HA |
|
|
No GRADE |
PICU, AE (all patients) |
|
GRADE |
Outcome |
Conclusions hypertonic saline – salbutamol mix |
|
Very low |
LOS, CSS (inpatients), HA |
For children with bronchiolitis (inpatients), nebulization with hypertonic saline – salbutamol mix as compared to normal saline – salbutamol mix may have little to no effect on length of hospital stay (LOS; inpatients) and may reduce hospital admissions (HA; outpatients) and clinical severity score (CSS; inpatients), but the evidence is very uncertain. No data were found and therefore no conclusion could be drawn with regard to the effect on LOS and CSS for outpatients.
For children with bronchiolitis (outpatients and inpatients), no (systematic) data were found with regard to the effect of nebulization with hypertonic saline – salbutamol mix on PICU admission (PICU) and adverse events (AE); therefore no conclusions could be drawn with regard to the effects.
Sources: Zhang, 2017; Hsieh, 2020. |
|
No GRADE |
LOS, CSS (outpatients)
PICU, AE (all patients) |
Samenvatting literatuur
Eleven articles: four SRs (Hartling, 2011; Gadomski, 2014; Zhang, 2017; Hsieh, 2020) and seven RCTs (Bashir, 2018; Faten, 2014; Flores-Gonzalez, 2015; Jaquet-Pilloud, 2020; Modaressi, 2012; Skjerven, 2013; Uysalol, 2017) were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables (one for SRs, one for RCTs). The assessment of the risk of bias is summarized in the risk of bias tables.
Description of studies: Systematic Reviews
Hartling (2011) is a Cochrane systematic review examining the efficacy and safety of adrenaline nebulization in children under two years of age with bronchiolitis. They included 19 RCTs (2256 patients). Adrenaline nebulization was compared to a placebo 0.9% saline nebulization in 9 studies, 6 studies in an outpatient setting (Anil, 2010; Barlas, 1998; Khashabi, 2005; Okutan, 1998; Plint, 2009; Ralston, 2005) and 3 studies in an inpatient setting (Abul-Ainine, 2002; Patel, 2002; Wainwright, 2003). In 15 studies, they (also) compared adrenaline to salbutamol, 9 were outpatient studies (Anil, 2010; Barlas, 1998; Beck, 2007; Khashabi, 2005; Kuyucu, 2004; Menon, 1995; Mull, 2004; Okutan, 1998; Ralston, 2005), 6 were inpatient studies (Abu-Shukair, 2001; Bertrand, 2001; Bilan, 2007; John, 2006; Patel, 2002; Sanchez, 1993). Relevant outcomes measured were “admissions at enrolment, within 24 hours” and “admissions within 7 days” (outpatients), “length of hospital stay” (inpatients), “clinical score at 60 and at 120 minutes, at 12-24 hours, and at 3-10 days” (outpatients and inpatients). Adverse effects were described narratively.
Gadomski (2014) is a Cochrane systematic review examining the efficacy of bronchodilators, mainly, and relevant to our research question, salbutamol and ipratropium bromide nebulization, compared to normal saline placebo in infants aged 0 to 12 months old with bronchiolitis. They included 30 RCTs with 35 datasets representing 1992 infants. Six studies examined salbutamol in outpatients (Can, 1998; Gadomski, 1994 – nebulization; Klassen, 1991; Ralston, 2005; Schuh, 1990; Schweich, 1992), eight studies in inpatients (Chevallier, 1995; Dobson, 1998; Gurkan, 2004; Ho, 1991; Karadag, 2005 (SAL alone); Lines, 1990; Patel, 2002; Scarlett, 2012). Five studies examined salbutamol and/or ipratropium bromide in inpatients (Chowdhury, 1995; Goh, 1997; Karadag, 2005 (IPR alone); Lines, 1992 (IPR alone); Wang, 1992). Two studies examined salbutamol plus hypertonic saline or salbutamol plus normal saline in outpatients (Anil, 2010; Ipek, 2011). They included 9 studies examining medications or a population not relevant to our research question (Alario, 1992; Gadomski, 1994 – oral; Gupta, 2008 – oral; Levin, 2008 – population on mechanical ventilation; Mallol, 1987; Patel, 2003; Tal, 1983; Tinsa, 2009; Totapally, 2002). Relevant outcomes measured were “hospital admission after treatment”(outpatients), “duration of hospitalization” (inpatients), and “clinical severity score after treatment” (outpatients and inpatients). Adverse effects were described narratively.
Zhang (2017) is a Cochrane systematic review examining the efficacy of nebulized hypertonic (≥ 3%) saline (HS) as compared to nebulized 0.9% saline (NS) in children under 24 months of age with bronchiolitis. Studies included arms comparing hypertonic and normal saline solutions alone or in conjunction with bronchodilators. They included 28 RCTs involving 4195 patients. Four studies examined HS compared with NS, without added bronchodilators, in outpatient/ED populations (Angoulvant, 2017; Florin, 2014; Li, 2014; Wu, 2014), three in inpatient populations (Kuzik, 2007; Luo, 2011; Ojha, 2014). Four studies examined HS vs. NS + adrenaline in outpatient/ED populations (Al-Ansari, 2010; Grewal, 2009; Jacobs, 2014; Khanal, 2015), five in inpatient populations (Mandelberg, 2003; Miraglia Del Giudice, 2012; Pandit, 2013; Tal, 2006; Tinsa, 2014). HS vs. NS + salbutamol was examined in outpatients by one study (Ipek, 2011) and in inpatients by eight studies (Flores, 2016; Köse, 2016; Luo, 2010; Mahesh Kumar, 2013; NCT01238848; Ratajczyk-Pekrul, 2016; Sharma, 2013; Teunissen, 2014). HS + salbutamol and/or adrenaline was examined by one study (Anil, 2010). Everard, 2014 compared HS with standard care and Sarrell, 2002 compared HS vs. NS + terbutaline (not relevant to our research question). Relevant outcomes measured were “rate of hospitalization” (outpatients), “length of hospital stay” (days; inpatients), and “clinical severity score (post-treatment) at day 1, 2, and 3.” Adverse effects were described narratively.
Hsieh (2020) is a systematic review examining the efficacy of nebulized HS (≥ 3%) as compared to NS (0.9%) in children with acute bronchiolitis. This study was included to add to Zhang, 2017. We included two studies from Hsieh, 2020 to our meta-analysis: Islam, 2018 examined HS vs. NS in 90 children aged 1-24 months, and Morikawa, 2018 examined HS + salbutamol vs. NS + salbutamol in 128 children aged < 12 months, both studies were performed in an inpatient setting. Relevant outcomes were “length of hospital stay” and “clinical severity score after treatment.”
Description of studies: Randomized Controlled Trials
Modaressi (2012) examined nebulized adrenaline vs. salbutamol in 40 children aged 1 month to 2 years with acute bronchiolitis, in an inpatient setting. Relevant outcomes measured were “length of stay (days)” and a “clinical severity score (RDAI).”
Skjerven (2013) examined nebulized adrenaline dissolved in 0.9% saline vs. 0.9% NS alone in 404 children under a year of age with acute bronchiolitis in an inpatient setting. Relevant outcomes measured were “length of stay (hours)”, “change in clinical severity score, 30 minutes after treatment”, and “need for ventilatory support.”
Faten (2014) examined nebulized 5% HS, 5% HS with adrenaline vs. NS in 94 children aged 1 month to 2 years with acute bronchiolitis in an inpatient setting. Relevant outcomes measured were “clinical severity score (Wang) 30, 60, and 120 minutes after treatment” and” time to discharge (length of stay in days).”
Flores-Gonzalez (2015) examined nebulized adrenaline in a 3% HS solution vs. 3% HS alone. They analyzed an inpatient population consisting of 185 children aged < 24 months with acute bronchiolitis. Relevant outcomes measured were “length of stay” and a “clinical severity score (WDF) measured at day 3 and 5.”
Uysalol (2017) examined nebulized 3% HS alone, adrenaline alone, and 3% HS with adrenaline vs. 0.9% NS. They analyzed an emergency department population consisting of 378 children aged 2-24 months with bronchiolitis. Relevant outcomes measured were “length of stay (hours)” and “adverse events.”
Bashir (2018) examined nebulized 3% HS vs. 0.9% NS in 189 children aged 2-18 months with bronchiolitis in an inpatient setting. Relevant outcomes measured were “length of stay (days)” and a “reduction in clinical severity score (Wang).”
Jaquet-Pilloud (2020) examined 3% HS + standard care vs. standard care alone in an inpatient population of 120 children aged 6 weeks to 24 months with acute bronchiolitis. Relevant outcomes measured were “length of stay” and “number of PICU transfers.”
Clinical Severity Score (CSS): overview of included scores
The studies included in the analysis of the literature (including those within the SRs) varied in the CSS used. At least six different scores were used:
Respiratory Distress Assessment Instrument (RDAI)
The RDAI score consists of wheezing (expiration, inspiration, location) and retractions (supraclavicular, intercostal, subcostal) 0-4 scores per row score, total range 0-17, with a higher score indicating increased severity. Eleven included studies used the RDAI score (Abul-Ainine, 2002; Anil, 2010; Khashabi, 2005; Klassen, 1991; Modaressi, 2012; Okutan, 1998; Patel, 2002; Plint, 2009; Ralston 2005; Scarlett, 2012; Schweich, 1992).
Wang 1992 score
The Wang 1992 score grades respiratory rate, wheezing, retraction, and general condition on a scale from 0 to 3, with a higher score indicating increased severity. Four studies used the Wang 1992 score (Bashir, 2018; Faten, 2014; Karadag, 2005; Luo, 2011).
Clinical Bronchiolitis Severity Score (CBSS)
The CBSS consists of row scores for respiratory rate (<30, 30-45, 46-60, >60), wheezing (none, terminal expiration or only with stethoscope, entire expiration or audible on expiration without stethoscope, inspiration and expiration without stethoscope), retraction (none, intercostal, tracheosternal), general condition (normal or irritability, lethargy, poor feeding), score 0-3 per row, with a higher score indicating increased severity. Two studies used the CBSS (Ipek, 2011; Islam, 2018).
Wood-Downes Scale modified by Ferres (WDF)
The WDF consists of row scores for wheezing (none, end expiration, entire expiratory phase, inspiration and expiration; 0-3), retractions (none, subcostal or lower intercostal, 1 + supraclavicular + nasal flaring, 2 + suprasternal + lower intercostal; 0-3), respiratory rate (breaths/min < 30, 31-45, 46-60, > 60; 0-3), heart rate (< 120 or > 120; 0-1), inspiratory breath sounds, cyanosis (present or not; 0-1), range 0-14, with a higher score indicating increased severity. One study used the WDF (Flores-Gonzalez, 2015).
Two studies used study-specific clinical scores:
- a 34-point scale for each degree of grunting, nasal flaring, supraclavicular retractions, intercostal retraction, chest indrawing, air entry, air hunger, wheezing, and general appearance (Gadomski 1994);
- a 4-point scale for each of general appearance, accessory muscle use, and wheezing (Gurkan, 2004).
Four studies did not specify the CSS used (Barlas, 1998; Can, 1998; Skjerven, 2013; Wainwright, 2003 used an unspecified respiratory effort score).
To be able to combine results of the different CSS scales in the meta-analyses, standardized mean differences were used.
Inpatient and outpatient populations
In all included articles, results were divided in results for outpatients (e.g. emergency department) and inpatients. Although, it might seem that only the outpatient populations would meet our inclusion criteria (P of our PICO), the distinction between inpatient and outpatient populations is not clear-cut: In the included articles, patients who were referred to as ‘inpatients’ were often patients who were randomized and received nebulization directly after reporting to a hospital, and patients who were referred to as ‘ outpatients’ were often directly admitted to an observational ward, even for days on end. Therefore, in our analysis, we decided to report on both inpatient and outpatient populations, maintaining the division as reported by the included articles, although, with these heterogenic populations, the true effect of nebulization in the first hours after presentation at the hospital / at the ED on outcomes such as hospital admission is difficult to derive from the current analysis.
Results
All outcomes
Normal saline vs. placebo or no nebulization
With regard to the effect of normal saline on the outcomes of interest, no data were found. Included studies used normal saline as the placebo intervention.
Crucial outcome - Length of Stay (LOS)
Salbutamol or ipratropium bromide vs. normal saline
Results from the Gadomski (2014) meta-analysis of two studies (Karadag, 2005; Patel, 2002), including 168 inpatients, indicated that salbutamol (n=75) and ipratropium bromide (n=22) as compared to normal saline (n=60 and n=11 resp.) do not significantly reduce length of stay in inpatients (salbutamol mean difference -0.19 days, 95% CI -0.78 to 0.39; ipratropium bromide mean difference 0.43 days, 95% CI -0.56 to 1.42; see figure 1.1). The same meta-analysis, but now including two other inpatient studies (Chowdhury, 1995; Wang, 1992) comparing salbutamol, ipratropium or a mix thereof to normal saline also showed no clinically relevant between-group difference in length of stay (total n=146; mean difference 0.06, 95% CI -0.40 to 0.52). Also in Uysalol (2017; emergency department setting - outpatients), the salbutamol (n=72) and normal saline (n=79) groups did not differ in length of stay (median 16 hours, IQR 20 hours in both groups). No data were found regarding the effect of ipratropium bromide on LOS for outpatients.
Figure 1.1. Length of stay (days), comparisons to normal saline placebo
Salbutamol vs. Normal saline placebo (inpatients)
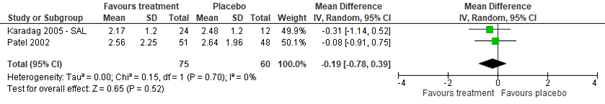
Ipratropium bromide vs. Normal saline placebo (inpatients)
Hypertonic saline or adrenaline vs. normal saline
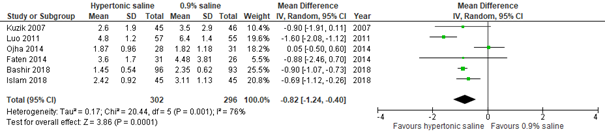
Meta-analysis of six studies (Zhang, 2017: Kuzik, 2007; Luo, 2011; Ojha, 2014. Additional RCTs: Faten, 2014; Bashir, 2018; Islam, 2018), including 598 inpatients, showed that compared to normal saline nebulization (n=296), nebulization with hypertonic saline (n=302) may reduce length of stay for inpatients with a clinically relevant ~ 20 hours (95% CI ranges from 10 to 30 hour reduction; see figure 1.2).
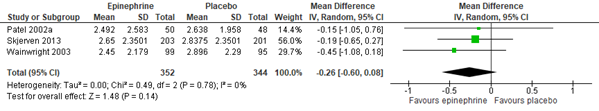
Meta-analysis of three studies (Hartling, 2011: Patel, 2002; Wainwright, 2003. Additional RCT: Skjerven, 2013), including 696 inpatients, showed that nebulization with adrenaline (n=352) might also reduce length of stay for inpatients with a clinically relevant ~ 6 hours as compared to normal saline nebulization (n= 344), but the width of the confidence interval also included the possibility that the efficacy of adrenaline is not better than that of normal saline (95% CI ranges from a 14 hour reduction to a 2 hour increase; see figure 1.2).
Results from Uysalol (2017) supported a positive effect of both hypertonic saline (n=77, median LOS 8 hours, IQR 12 hours) and adrenaline (n=75, median LOS 4 hours, IQR 12 hours) on length of stay as compared to normal saline (n=79, median LOS 16 hours, IQR 20 hours) in an outpatient (emergency department) population.
Figure 1.2. Length of stay (days), comparisons to normal saline placebo
Hypertonic Saline vs. Normal saline placebo (inpatients)
Adrenaline vs. Normal saline placebo (inpatients)
Hypertonic saline or adrenaline vs. standard care or salbutamol
Results from a study in 120 inpatients (Jacquet-Pilloud, 2019) do not support the efficacy of hypertonic saline nebulization in reducing length of stay as compared to standard care (mean difference -2.8 hours; 95% CI -11 to 16 hours; p=0.33).
Additional support for an efficacy of nebulization with adrenaline comes from studies performed in inpatient settings comparing adrenaline to salbutamol nebulization. Meta-analysis of 4 studies (Hartling, 2011: Bertrand, 2001; Bilan, 2007; John, 2006; Patel, 2002), including 261 patients (adrenaline group n=131, salbutamol group n=130), indicated that adrenaline might be more effective than salbutamol in reducing length of stay (mean difference -0.28 days, CI -0.46 to -0.09, p=0.003), so did the results of Modaressi (2012) in 40 inpatients (adrenaline mean 3 days, SD 0.9; salbutamol mean 3.7 days, SD 1.1; p=0.03). However, one study (Menon, 1995) in 42 outpatients did not find a difference in length of stay between adrenaline and salbutamol (mean difference 0.46 days, CI -0.27 to 1.20, p=0.22).
Hypertonic saline and adrenaline mix
There is some support in the literature that nebulization with a hypertonic saline-adrenaline mix could be superior in reducing length of stay for inpatients as compared to nebulization with a normal saline-adrenaline mix (Zhang, 2017: Mandelberg, 2003; Tal, 2006; Miraglia Del Giudice, 2012; Pandit, 2013; Tinsa, 2014: total n=356; mean difference -0.65 days; 95% CI -1.01 days to -0.30 days), or compared to hypertonic saline alone (Flores-Gonzalez, 2015: mix n=94, mean 3.94 days, SD 1.37 vs. hypertonic saline n=91 mean 4.82 days, SD 2.3, p=0.011). Uysalol (2017) also reported a superior efficacy of a hypertonic saline-adrenaline mix in comparison with hypertonic saline, adrenaline, or normal saline in reducing length of stay in an emergency department setting (mix n=75, median 4 hours, IQR 8; hypertonic saline n=77, median 8 hours, IQR 12; adrenaline n=75, median 4 hours, IQR 12; p<0.001 for mix compared with normal saline). However, Faten (2014) did not find a difference in length of stay between inpatient groups receiving a hypertonic saline-adrenaline mix (n=37) or a normal saline nebulization (n=26; mean difference 0.98 days, 95% CI -0.49 to 2.45).
Hypertonic saline and salbutamol mix
Zang (2017) and Hsieh (2020) included studies comparing a mix of hypertonic saline and salbutamol with a mix of normal saline and salbutamol. No studies were found reporting on any other comparisons (such as a comparison between the hypertonic saline - salbutamol mix and normal saline/placebo alone). For outcome LOS, there appeared to be no clinically relevant difference in effectivity between the hypertonic saline - salbutamol mix and the normal saline - salbutamol mix (LOS – inpatients only, eight studies taken from Zhang 2017 and one from Hsieh 2020, total n=1086, mean difference -0.12, 95% CI -0.54 to 0.30).
Crucial outcome: PICU admission
Few studies reported on the occurrence of PICU admissions. Gadomski (2014) reported on one RCT (Patel, 2002) in which one infant receiving albuterol was transferred to the intensive care unit for 48 hours, however without requiring mechanical ventilation. Jaquet-Piloud (2020) reported no PICU transfers in the hypertonic saline group and 5% in the standard care group (RR 0.138 (95%CI 0.007 to 2.620)). Skjerven (2013) reported no differences in need for ventilatory support between inpatients receiving adrenaline nebulization and those receiving normal saline placebo (RR 0.99 (95%CI 0.5 to 1.97)). Flores-Gonzalez (2015) did not include infants in their analyses that were admitted to intensive care; they did mention that both in the intervention (i.e. hypertonic saline plus adrenaline) and the control group (i.e. hypertonic saline plus water placebo), six out of the 104 infants assigned per group were admitted to intensive care.
Important outcome: Hospital admission (for outpatients only)
Salbutamol or ipratropium bromide vs. normal saline
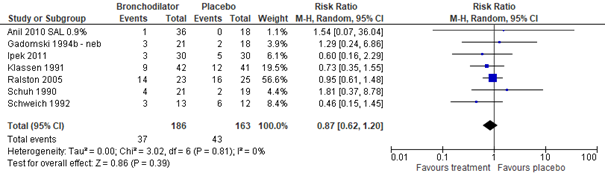
Meta-analysis of seven studies (Gadomski, 2014: Anil, 2010; Gadomski, 1994; Ipek, 2011; Klassen, 1991; Ralston, 2005; Schuh, 1990; Schweich, 1992), including a total of 349 outpatients, showed that the risk of hospital admission was not significantly lower in outpatients treated with salbutamol than in those treated with normal saline nebulization (RR 0.87, 95% CI 0.62 to 1.20; see figure 2.1). No data were found on the effects of ipratropium bromide on hospital admission.
Figure 2.1. Hospital admissions, comparisons to normal saline placebo
Salbutamol vs. Normal saline placebo (outpatients)
Hypertonic saline or adrenaline vs. normal saline
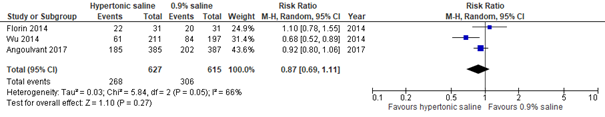
Meta-analysis of three studies in emergency department populations (Zhang, 2017: Florin, 2014; Wu, 2014; Angoulvant, 2017), including a total of 1242 patients, showed that the risk of hospital admission was not significantly lower for patients treated with hypertonic saline as compared to those treated with normal saline nebulization (RR 0.87, 95% CI 0.69 to 1.11; see figure 2.2).
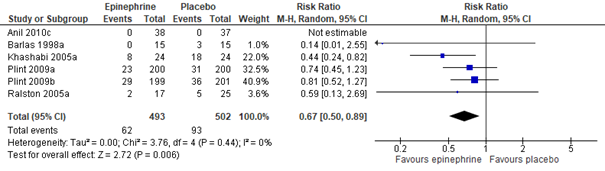
Meta-analysis of five studies (Hartling, 2011: Anil, 2010; Barlas, 1998; Khasabi, 2005; Plint, 2009; Ralston, 2005), including a total of 995 patients, showed that, as compared to treatment with normal saline nebulization, nebulization with adrenaline may result in a clinically important 33% risk reduction of being hospitalized within 24 hours (RR 0.67, 95% CI 0.50 to 0.89; see figure 2.2). Effectivity of adrenaline nebulization on reducing hospitalization risk in the long run (within 7 days) was not proven by this same meta-analysis (total n=875, RR 0.81, 95% CI 0.63 to 1.03; see figure 2.2).
Figure 2.2. Hospital admissions, comparisons to normal saline placebo
Hypertonic Saline vs. Normal saline placebo (outpatients: setting Emergency Department)
Adrenaline vs. Normal saline placebo (outpatients) - hospital admissions within 24 hours
Adrenaline vs. Normal saline placebo (outpatients) - hospital admissions within 7 days
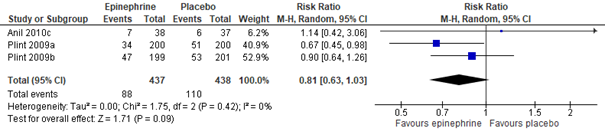
Adrenaline vs. salbutamol
As additional proof of some efficacy of adrenaline nebulization, when compared to salbutamol nebulization, adrenaline might be more effective in reducing the risk of hospitalization within 24 hours, but the confidence interval also included the possibility that both medications were equally (in)effective (Hartling, 2011: Anil, 2010; Barlas, 1998; Khashabi, 2005; Menon, 1995; Mull, 2004; Ralston, 2005; total n=375, RR 0.67, 95% CI 0.41 to 1.09). Treatment with adrenaline, as compared to treatment with salbutamol, did not seem more effective in reducing the risk of hospitalization within 7 days (Hartling, 2011: Anil, 2010; Mull, 2004; total n=212, RR 1.05, 95% CI 0.71 to 1.54).
Hypertonic saline and adrenaline mix
With respect to the risk of hospitalization, no data were found on the effectivity of a hypertonic saline – adrenaline mix as compared to normal saline, hypertonic saline, or adrenaline alone. Results from a meta-analysis showed that outpatients treated with a hypertonic saline-adrenaline mix had a (marginally) clinically relevant lower risk of being hospitalized than outpatients treated with a normal saline-adrenaline mix, but the confidence interval included the possibility that these treatments did not differ in effect (Zhang, 2017: Grewal, 2009; Jacobs, 2014; total n=147, RR 0.78, 95% CI 0.55 to 1.12).
Hypertonic saline and salbutamol mix
Zang (2017) included studies comparing a mix of hypertonic saline and salbutamol with a mix of normal saline and salbutamol. No studies were found reporting on any other comparisons (such as a comparison between the hypertonic saline - salbutamol mix and normal saline/placebo alone). For outcome hospital admissions, a clinically relevant between-group difference was found favoring the hypertonic saline - salbutamol mix over the normal saline - salbutamol mix, but the confidence interval also included the possibility that the normal saline mix was actually better (hospital admissions – outpatients only, one study from Zhang 2017, total n=120, mean difference 0.63, 95% CI 0.22 to 1.80).
Important outcome: Clinical Severity Score (CSS; after treatment)
Salbutamol or ipratropium bromide vs. normal saline
Meta-analysis of outpatient studies (Gadomski, 2014: Anil, 2010; Can, 1998; Gadomski, 1994; Ipek, 2011; Klassen, 1991; Ralston, 2005; Schweich, 1992), including 477 outpatients, and inpatient studies (Gadomski, 2014: Gurkan, 2004; Karadag, 2005; Patel, 2002; Scarlett, 2012), including 185 inpatients, showed no clinically important between-group difference in CSS after treatment with salbutamol or normal saline nebulization (outpatient std. mean difference -0.46, 95% CI -0.94 to 0.02; inpatient std. mean. -0.29, 95% CI -0.84 to 0.26; see figure 3.1).
Regarding the effect of ipratropium bromide on CSS for inpatients, as compared to normal saline placebo, no clinically relevant effect was found (Gadomski, 2014: Karadag, 2005; total n=33, std. mean difference -0.23, 95% CI -0.96 to 0.49, see figure 3.1). Also studies comparing inpatient groups treated with salbutamol, ipratropium bromide, or a mix thereof to normal saline (Gadomski, 2014: Goh, 1997; Wang, 1992) did not find a significant difference in effect on CSS (total n=144, std. mean difference -0.06, 95% CI -0.41 to 0.29). No data were found regarding the effect of ipratropium bromide on CSS for outpatients.
Figure 3.1. Clinical Severity Score (after treatment), comparisons to normal saline placebo
Salbutamol vs. Normal saline placebo (outpatients)
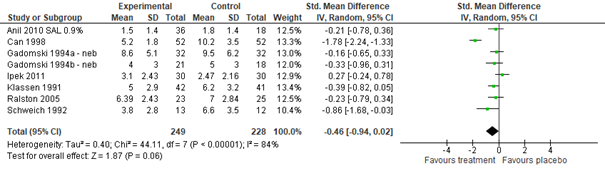
Salbutamol vs. Normal saline placebo (inpatients)
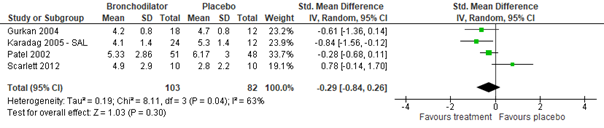
Ipratropium bromide vs. Normal saline placebo (inpatients)
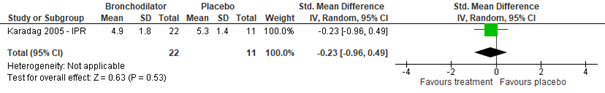
Hypertonic saline or adrenaline vs. normal saline
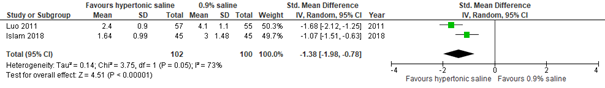
Regarding the effect of hypertonic saline on CSS, no data were found for outpatient populations. With regard to an inpatient population, results from two studies did not show a clinically important difference in effect between hypertonic and normal saline nebulization within 24 hours (Zhang, 2017: Luo, 2011. Additional RCT: Faten, 2014; total n=169, std. mean difference -0.43, 95% CI -1.56 to 0.69; see figure 3.2.1). However, after 24 hours, a clinically important difference was found favoring hypertonic saline nebulization (Zhang, 2017: Luo, 2011; total n=112, std. mean difference at day 2 -1.82, 95% CI -2.26 to -1.37; see figure 3.2.1). Results indicated that this difference favoring hypertonic saline nebulization may be maintained after 48 hours (Zhang, 2017: Luo, 2011; additional RCT from Hsieh, 2020: Islam, 2018; total n=202, std. mean difference -1.38, 95% CI -1.98 to -0.78; see figure 3.2). Results from Bashir (2018) confirmed a possible positive effect within 48 hours of hypertonic saline on CSS (total n=189 inpatients, mean reduction HS group 2.26 on Wang CSS, SD 0.68; mean reduction NS group 1.23, SD 0.49; p<0.001).
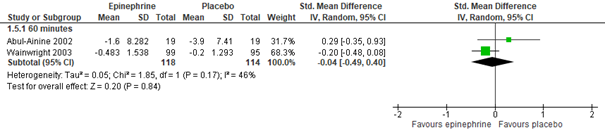
For outpatients, adrenaline nebulization may be more effective in reducing CSS than normal saline nebulization, as was shown in a meta-analysis of five studies (Hartling, 2011: Anil, 2010; Barlas, 1998; Khasabi, 2005; Okutan, 1998; Plint, 2009), both at 60 (n=975, std. mean difference -0.40, 95% CI -0.58 to -0.23) and 120 minutes (n=105, std. mean difference -0.73, 95% CI -1.13 to -0.33) after nebulization (see figure 3.2.2). However, for inpatients, an effect of nebulization with adrenaline on CSS was not proven in comparison with normal saline, at least not within 60 minutes after treatment (Hartling, 2011: Abul-Ainine, 2002; Wainwright, 2003; total n= 232, std. mean difference -0.04, 95% CI -0.49 to 0.40, see figure 3.2.2; results from a separate RCT, Skjerven, 2013; total n=404, mean inhaled racemic adrenaline group -1.26, range -1.44 to -1.08, and mean inhaled normal saline group -1.08, range -1.23 to -0.92, p=ns). For inpatients, no data were found on the effectivity of adrenaline on CSS after more than 60 minutes, at least not in comparison with normal saline.
Figure 3.2.1. Clinical Severity Score (after treatment), comparisons to normal saline placebo
Hypertonic Saline vs. Normal saline placebo (inpatients) - day 1
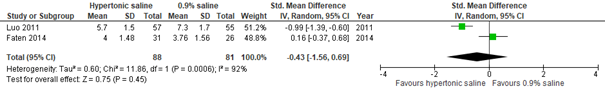
Hypertonic Saline vs. Normal saline placebo (inpatients) - day 2

Hypertonic Saline vs. Normal saline placebo (inpatients) - day 3
Adrenaline vs. salbutamol (mixed with ipratropium bromide)
In contrast to the positive effect on CSS found in outpatient populations for adrenaline and the lack of effect found for salbutamol when compared to normal saline nebulization, comparisons between adrenaline and salbutamol in outpatients showed that adrenaline and salbutamol were about equally (in)effective in reducing CSS at 60 minutes (Hartling, 2011: Anil, 2010; Barlas, 1998; Beck, 2007; Khashabi, 2005; Menon, 1995; Mull, 2004; Okutan, 1998; total n=397, std. mean difference -0.12, 95% CI -0.32 to 0.08), at 120 minutes (Hartling, 2011: Anil, 2010; Barlas, 1998; Kuyucu, 2004; Menon, 1995; Mull, 2004; total n=356, std. mean difference -0.10, 95% CI -0.31 to 0.11), and within 12-14 hours (Hartling, 2011: Kuyucu, 2004; total n=69, std. mean difference -0.21, 95% CI -0.86 to 0.44). Although at all time points the overall effect leaned towards favoring adrenaline, only after 3 days (therefore not clinically relevant), adrenaline was shown to be more effective than salbutamol in improving CSS in outpatients (Hartling, 2011: Kuyucu, 2004; total n=69, std. mean difference -0.50, 95% CI -0.98 to -0.02).
In contrast with the lack of effect on CSS at 60 minutes after treatment, found in inpatients for adrenaline when compared to normal saline nebulization, comparisons with salbutamol showed that adrenaline may have a clinically important effect on CSS at 60 minutes (Hartling, 2011: Abu-Shukair, 2001; Bertrand, 2001; John, 2006; Sanchez, 1993; total n=248, std. mean difference -0.79, 95% CI -1.45 to -0.13) and at 120 minutes after treatment (Hartling, 2011: Abu-Shukair, 2001; total n=140, std. mean difference -0.52, 95% CI -0.86 to -0.18). At 6-12 hours, one study (Hartling, 2011: Kadir, 2009) found adrenaline to resort more effect at 6-12 hours after treatment than salbutamol mixed with ipratropium bromide (total inpatients n=60, std. mean difference -0.60, 95% CI -1.12 to -0.09). RCT results from Modaressi (2012) in 40 inpatients support a possible efficacy of adrenaline superior to salbutamol (mean RDAI scores at 10-180 minutes and after 1-5 days were lower in adrenaline group compared to salbutamol group, overall p=0.02).
Figure 3.2.2. Clinical Severity Score (after treatment), comparisons to normal saline placebo
Adrenaline vs. Normal saline placebo (outpatients) – 60 and 120 minutes
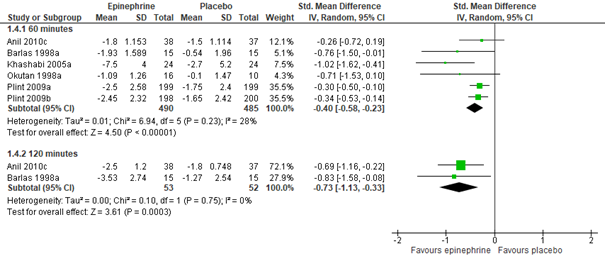
Adrenaline vs. Normal saline placebo (inpatients) – 60 minutes
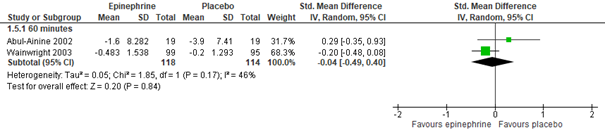
Hypertonic saline and adrenaline mix
For outpatients, no data were found on the effectivity of a hypertonic saline-adrenaline mix as compared to normal saline or hypertonic saline. As compared to a normal saline-adrenaline mix, no clinically relevant effects on CSS were found at day 1 (Zhang, 2017: Al-Ansari, 2010; total n=171, std. mean difference -0.07, 95% CI -0.39 to 0.25) nor at day 2 (Zhang, 2017: Al-Ansari, 2010; total n=171, std. mean difference -0.23, 95% CI -0.56 to 0.09).
In inpatients, Faten (2014) found a hypertonic saline-adrenaline mix to be no more effective than a normal saline nebulization in reducing Wang’s CSS at 30 to 120 minutes after treatment (inpatients n=94, p≥0.56). A hypertonic saline-adrenaline mix did appear more effective than a normal saline-adrenaline in a meta-analysis, both at day 1 (Zhang, 2017: Mandelberg, 2003; Tal, 2006; Miraglia del Giudice, 2012; total n=199, std. mean difference -0.44, 95% CI -0.78 to -0.10) and day 2 (Zhang, 2017: Mandelberg, 2003; Tal, 2006; Miraglia del Giudice, 2012; total n=195, std. mean difference -0.73, 95% CI -1.10 to -0.37). Also, Flores-Gonzalez (2015) found a hypertonic saline-adrenaline mix to be more effective than hypertonic saline alone in reducing the WDF severity score at day 3 (total inpatients n=185, mix mean 3.93, 95% CI 3.68 to 4.17, hypertonic saline mean 4.31, 95% CI 4.01 to 4.59, p=0.029; comparisons before day 3 not available, results at day 3 are too late to be considered clinically relevant).
Hypertonic saline and salbutamol mix
Zang (2017) included studies comparing a mix of hypertonic saline and salbutamol with a mix of normal saline and salbutamol. No studies were found reporting on any other comparisons (such as a comparison between the hypertonic saline - salbutamol mix and normal saline/placebo alone). For outcome CSS, the hypertonic saline - salbutamol mix was found to be marginally more effective on day two than the normal saline - salbutamol mix, but the confidence interval also included the possibility that both mixes were of equal effect (day 1 CSS – inpatients only, 3 studies from Zhang 2017, total n=265, std. mean difference -0.48, 95% CI -1.03 to 0.08; day 2 CSS – inpatients only, 2 studies from Zhang 2017, total n=160, std. mean difference -0.80, 95% CI -1.63 to 0.02).
Important outcome: Adverse events
Adverse events were not systematically addressed or not (completely) reported by the individual RCTs. Therefore, the systematic reviews only provided a narrative account of adverse events.
Gadomski (2014) and Uysalol (2017) reported on adverse effects of salbutamol and ipratropium bromide.
Gadomski (2014) reported that adverse events were exclusively found in study groups receiving bronchodilators and included tachycardia with or without prolonged cough, decreased oxygen saturation, flushing, hyperactivity, mild hypertension, and tremor.
Uysalol (2017) reported on tachycardia, pallor, tremor, nausea, and vomiting and found 7/72 (9.7%) adverse events in the salbutamol group and, in comparison, 2/79 (2.5%) in the normal saline control group.
Zhang (2017) and Hsieh (2020) reported on possible adverse effects of hypertonic saline.
Zhang (2017) reported that in 13 RCTs (n=1363) no adverse events were observed, and 11 RCTs (n=2360) observed one or more adverse events.
Both Zhang (2017) and Hsieh (2020) stated that when adverse events were observed, they were mild and resolved spontaneously; adverse events included cough, bronchospasm, vomiting, diarrhea, desaturation, agitation, rhinorrhea, tachycardia, hoarse voice, and vigorous crying. Both systematic reviews report on one study (n=285) with one serious adverse event of bradycardia and desaturation in the hypertonic saline group, which resolved the following day. The RCTs of Faten (2014; total n=94; hypertonic saline vs. normal saline), Bashir (2018; total n=189; hypertonic saline vs. normal saline), and Jaquet-Pilloud (2020; total n=120; HS vs. standard care) reported no adverse event in either treatment group (i.e. no reports of tachycardia, flushing, pallor, tremor, bronchospasm, hypertension, excessive coughing, apnea, cyanosis, sweating). Uysalol (2017) also reported no (0/77) adverse events in the hypertonic saline group.
Hartling (2011) reported on adverse events of adrenaline, occurrences of vomiting (1.5-2%), pallor (8-11%), tremor (1-2%), and hypertension (0.5%) were observed in one (n=unknown) out of four RCTs (n=982) comparing adrenaline to normal saline placebo, stating that there were no between-group differences in the occurrence of adverse events. Also in Hartling (2011), five RCTs (n=368) comparing adrenaline to salbutamol reported on adverse events. One RCT (n=66) observed one case of pallor and one case of vomiting in the adrenaline group and five cases of vomiting in the albuterol group. One RCT (n=40) only observed two cases of tachycardia in the albuterol group. In all five RCTs, no cases of tremor or increased blood pressure were observed. The RCT of Skjerven (2013; n=404) reported no serious adverse events in the adrenaline group and three children discontinuing treatment because of moderate tachycardia (including one child in the normal saline group).
Uysalol (2017) reported 7/75 (9.3%) adverse events in the adrenaline group, and 5/75 (6.7%) in the adrenaline + hypertonic saline group. Flores-Gonzalez (2015; total n=185; adrenaline + hypertonic saline vs. hypertonic saline) reported no adverse events (i.e., tachycardia, sweating, pallor, trembling, or hypertension).
Supplementary evidence table: Adrenaline dose regimens
A supplementary evidence table was added to obtain an overview of adrenaline dose regimens across studies to assess a possible effect of dose. An effect could not be assessed because of heterogeneity observed with regard to type of adrenaline (unspecified adrenaline, L-adrenaline, or racemic adrenaline), dose, number and timing (see Supplementary Evidence Table 1. Adrenaline: study specifications with regard to dose).
Level of evidence of the literature
All outcomes
For all reported outcomes, the level of evidence started as high, because all included studies were (SRs of) RCTs. For all reported outcomes, the level of evidence regarding the possible effect of nebulization with normal saline was not assessed due to lack of data.
Crucial outcome - Length of Stay (LOS)
The level of evidence regarding the possible effect of nebulization with salbutamol on the outcome measure LOS for outpatients and inpatients was downgraded by the maximum of three levels because of study limitations (risk of bias) and serious imprecision with small study samples and confidence intervals including the possibilities that salbutamol had a better, equal or worse effect compared to normal saline placebo. The level of evidence for outpatients and inpatients was assessed as “very low.”
The level of evidence regarding the possible effect of nebulization with ipratropium bromide on the outcome measure LOS for inpatients was downgraded by the maximum of three levels because of study limitations (risk of bias) and serious imprecision with small study samples and confidence intervals including the possibilities that ipratropium bromide had a better, equal or worse effect compared to normal saline placebo. The level of evidence for inpatients was assessed as “very low.” For outpatients, the level of evidence was not assessed due to lack of data.
The level of evidence regarding the possible effect of nebulization with hypertonic saline on the outcome measure LOS for inpatients was downgraded by two levels because of study limitations (risk of bias) and inconsistent results between studies. The level of evidence for inpatients was assessed as “low.” For outpatients, with data from only one small study with a high risk of bias, the level of evidence was assessed as “very low.”
The level of evidence regarding the possible effect of nebulization with adrenaline on the outcome measure LOS for inpatients was downgraded by two levels because of study limitations (risk of bias) and imprecision with confidence intervals including the possibilities that adrenaline had a better or equal effect compared to normal saline (or salbutamol). The level of evidence for inpatients was assessed as “low.” For outpatients, with data from two small studies, one with serious imprecision and only comparing adrenaline to salbutamol, the other with a high risk of bias, the level of evidence was assessed as “very low.”
The level of evidence regarding the possible effect of nebulization with a mix of hypertonic saline and adrenaline on the outcome measure LOS for outpatients and inpatients was downgraded by the maximum of three levels because of study limitations (risk of bias), heterogeneity and inconsistent results between studies, and imprecision. For inpatients, only one small study with a risk of bias and serious imprecision compared the mix to normal saline placebo, other studies (in a meta-analysis) with a risk of bias compared it to an adrenaline-normal saline mix, and one small study with a risk of bias to hypertonic saline. For outpatients only one small study with a high risk of bias compared the mix to normal saline placebo. The level of evidence for outpatients and inpatients was assessed as “very low.”
The level of evidence regarding the possible effect of nebulization with a mix of hypertonic saline and salbutamol on the outcome measure LOS for inpatients was downgraded by the maximum of three levels because of study limitations (risk of bias), inconsistent results between studies and indirectness (only comparisons between mixes, not to normal saline placebo). The level of evidence for inpatients was assessed as “very low.” For outpatients, the level of evidence was not assessed due to lack of data.
Crucial outcome: PICU admission
The level of evidence regarding the possible effect of all the nebulization medications of interest on outcome measure PICU admission was not assessed due to lack of (systematically reported) data.
Important outcome: Hospital admission (for outpatients only)
The level of evidence regarding the possible effect of nebulization with salbutamol on the outcome measure hospital admission for outpatients was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency) and number of included patients (imprecision). The level of evidence for outpatients was assessed as “very low.”
The level of evidence regarding the possible effect of nebulization with ipratropium bromide on the outcome measure hospital admission for outpatients was not assessed due to lack of data.
The level of evidence regarding the possible effect of nebulization with hypertonic saline on the outcome measure hospital admission for outpatients was downgraded by two levels because of study limitations (risk of bias) and conflicting results (inconsistency). The level of evidence for outpatients was assessed as “low.”
The level of evidence regarding the possible effect of nebulization with adrenaline on the outcome measure hospital admission within 24 hours was downgraded by one level because of study limitations (risk of bias). The level of evidence regarding the effect of adrenaline within 24 hours for outpatients was assessed as “moderate.” Because of imprecision in addition to risk of bias, the level of evidence regarding the effect adrenaline on hospital admission within 7 days for outpatients was assessed as “low.”
The level of evidence regarding the possible effect of nebulization with a mix of hypertonic-saline and adrenaline on the outcome measure hospital admission for outpatients was downgraded by three levels because of study limitations (risk of bias) and serious imprecision due to small sample size and wide overall confidence interval. The level of evidence was assessed as “very low.”
The level of evidence regarding the possible effect of nebulization with a mix of hypertonic saline and salbutamol on the outcome measure hospital admission for outpatients was downgraded by the maximum of three levels because of study limitations (risk of bias), serious imprecision and indirectness (only comparisons between mixes, not to normal saline placebo). The level of evidence was assessed as “very low.”
Important outcome: Clinical Severity Score (CSS)
The level of evidence regarding the possible effect of nebulization with salbutamol on the outcome measure CSS for outpatients was downgraded by two levels because of study limitations (risk of bias) and conflicting results (inconsistency) and for inpatients by one more level because of number of included patients (imprecision). The level of evidence for outpatients was assessed as “low”, for inpatients as “very low.”
The level of evidence regarding the possible effect of nebulization with ipratropium bromide on the outcome measure CSS for inpatients was downgraded by the maximum of three levels because of study limitations (risk of bias) and serious imprecision due to one small study sample and wide confidence interval. For inpatients, the level of evidence was assessed as “very low.” For outpatients, the level of evidence was not assessed due to lack of data.
The level of evidence regarding the possible effect of nebulization with hypertonic saline on the outcome measure CSS for inpatients was downgraded by the maximum of three levels because of study limitations (risk of bias), heterogeneity of studies and conflicting results (inconsistency) and number of included patients (imprecision). The level of evidence for inpatients was assessed as “very low.” For outpatients, the level of evidence was not assessed due to lack of data.
The level of evidence regarding the possible effect of nebulization with adrenaline on the outcome measure CSS for outpatients was downgraded by one level because of study limitations (risk of bias). The level of evidence for outpatients was assessed as “moderate.” For inpatients the level of evidence was further downgraded for serious inconsistency of results to “very low.”
The level of evidence regarding the possible effect of nebulization with a mix of hypertonic-saline and adrenaline on the outcome measure CSS for outpatients was downgraded by two levels because of study limitations (risk of bias) and number of included patients (imprecision) and for inpatients also by two levels for study limitations (risk of bias) and inconsistent results. The level of evidence for outpatients and inpatients was assessed as “low.”
The level of evidence regarding the possible effect of nebulization with a mix of hypertonic saline and salbutamol on the outcome measure CSS for inpatients was downgraded by the maximum of three levels because of study limitations (risk of bias), imprecision and indirectness (only comparisons between mixes, not to normal saline placebo). The level of evidence for inpatients was assessed as “very low.” For outpatients, the level of evidence was not assessed due to lack of data.
Important outcome: Adverse events
The level of evidence regarding the possible effect of all the nebulization medications of interest on outcome measure adverse events was not assessed due to lack of (systematically reported) data.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: For children with bronchiolitis in the first hours after presentation at the hospital, does nebulization with adrenaline, normal saline, hypertonic saline, and/or salbutamol/ ipratropium bromide improve clinical outcome, reduce hospitalization and length of stay?
P: Children with bronchiolitis (0-3 years) in the first hours after presentation at the hospital / at the Emergency Department (ED)
I: Nebulization with normal saline (NaCl 0.9%), salbutamol, ipratropium bromide, hypertonic saline (NaCl ≥ 3%), adrenaline, or a combination thereof
C: Nebulization with placebo or with one of the other intervention substances (i.e. normal saline, salbutamol, ipratropium bromide, hypertonic saline, adrenaline, or a combination thereof)
O: (a reduction of) length of hospital stay (LOS), Pediatric Intensive Care Unit (PICU) admissions, hospital admissions, clinical symptoms / clinical severity score (CSS), adverse events
Relevant outcome measures
The guideline development group considered LOS and PICU admissions as crucial outcome measures for decision making, hospital admissions, CSS and adverse events as important outcome measures for decision making.
The working group did not predefine values for minimal clinically (patient) important differences per outcome measure. For the dichotomous variables PICU admissions, hospital admissions and adverse events, a Risk Ratio (RR) above 1.25 or below 0.8 was considered to indicate a clinically important difference between groups. For continuous variable LOS, a reduction of ≥ 6 hours was considered as possibly clinically relevant. For continuous variable CSS for which results from different scales were combined, standardized mean differences are reported and half the standard deviation was used as an estimate of a clinically important difference between groups. Furthermore, the guideline development group decided that differences in CSS should have occurred within 48 hours to be clinically relevant.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms for normal saline, hypertonic saline, salbutamol and/or ipratropium bromide nebulization until January 26st, 2021. A second search, now including adrenaline, was done on February 2nd, 2021. The detailed search strategy is depicted under the tab Methods.
The initial systematic literature search resulted in 389 unique hits. The second search added 144 unique hits. Studies were selected based on the following criteria:
- They should be a systematic review (SR), randomized controlled trial (RCT), or comparative observational study (i.e. prospective or retrospective cohort study);
- published between the year 2000 and January 26st, 2021, and for the additional search directed at finding studies on adrenaline nebulization, until February 2nd, 2021;
- be in line with our PICO.
Initially, 81 papers were selected based on title and abstract screening (75 in the first search and an additional 6 papers in the second, adrenaline search). After reading the full text, four systematic reviews (SRs), including three Cochrane SRs with available data files, and seven randomized controlled trials (RCTs), adding to (information from) the studies included in the SRs, were selected. Twenty-eight papers were read full text and excluded (see the table with reasons for exclusion under the tab Methods). Forty-two RCTs found by our search were covered by (one of the) selected SRs and were not read full text and not included separately in our analyses. In the end, eleven papers were included.
Referenties
- Bashir, T., Reddy, K. V., Ahmed, K., & Shafi, S. (2018). Comparative Study of 3% Hypertonic Saline Nebulization Versus 0.9% Normal Saline Nebulization for Treating Acute Bronchiolitis. Journal of Clinical & Diagnostic Research, 12(6).
- Faten, T., Sana, A., Imen, B. H., Samia, H., Ines, B., Bechir, Z., & Khadija, B. (2014). A randomized, controlled trial of nebulized 5% hypertonic saline and mixed 5% hypertonic saline with epinephrine in bronchiolitis. La Tunisie medicale, 92(11).
- Flores-González, J. C., Matamala-Morillo, M. A., Rodríguez-Campoy, P., Pérez-Guerrero, J. J., Serrano-Moyano, B., Comino-Vazquez, P., ... & Bronchiolitis of Cadiz Study group (BronCaS). (2015). Epinephrine improves the efficacy of nebulized hypertonic saline in moderate bronchiolitis: a randomized clinical trial. PloS one, 10(11), e0142847.
- Gadomski, A. M., & Scribani, M. B. (2014). Bronchodilators for bronchiolitis. Cochrane database of systematic reviews, (6).
- Hartling, L., Bialy, L. M., Vandermeer, B., Tjosvold, L., Johnson, D. W., Plint, A. C., ... & Fernandes, R. M. (2011). Epinephrine for bronchiolitis. Cochrane Database of Systematic Reviews, (6).
- Hsieh, C. W., Chen, C., Su, H. C., & Chen, K. H. (2020). Exploring the efficacy of using hypertonic saline for nebulizing treatment in children with bronchiolitis: a meta-analysis of randomized controlled trials. BMC pediatrics, 20(1), 1-15.
- Jaquet-Pilloud, R., Verga, M. E., Russo, M., Gehri, M., & Pauchard, J. Y. (2020). Nebulized hypertonic saline in moderate-to-severe bronchiolitis: a randomized clinical trial. Archives of disease in childhood, 105(3), 236-240.
- Modaressi, M. R., Asadian, A., Faghihinia, J., Arashpour, M., & Mousavinasab, F. (2012). Comparison of epinephrine to salbutamol in acute bronchiolitis. Iranian journal of pediatrics, 22(2), 241.
- Skjerven, H. O., Hunderi, J. O. G., Brügmann-Pieper, S. K., Brun, A. C., Engen, H., Eskedal, L., ... & Lødrup Carlsen, K. C. (2013). Racemic adrenaline and inhalation strategies in acute bronchiolitis. New England Journal of Medicine, 368(24), 2286-2293.
- Uysalol, M., Ha?lak, F., Özünal, Z. G., Vehid, H., & Uzel, N. (2017). Rational drug use for acute bronchiolitis in emergency care. The Turkish journal of pediatrics, 59(2), 155-161.
- Zhang, L., Mendoza?Sassi, R. A., Wainwright, C., & Klassen, T. P. (2017). Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane database of systematic reviews, (12).
Evidence tabellen
Evidence table for systematic review of RCTs
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Hartling, 2011
PS: individual study characteristics and results are extracted from the SR (unless stated otherwise) |
Cochrane SR and meta-analysis of RCTs
Literature search up to September 2010
Inpatients: A: Abu-Shukair, 2001 B: Abul-Ainine, 2002 C: Bertrand, 2001 D: Bilan, 2007 E: John, 2006 F: Kadir, 2009 G: Patel, 2002 H: Sanchez, 1993 I: Wainwright, 2003
Outpatients: J: Anil, 2010 K: Barlas, 1998 L: Beck, 2007 M: Khashabi, 2005 N: Kuyucu, 2004 O: Menon, 1995 P: Mull, 2004 Q: Okutan, 1998 R: Plint, 2009 S: Ralston, 2005
High income countries: Australia: I Canada: G, H, O, R Chile: C England: B Israel: L Turkey: J, K, N, Q United States: P, S Low income countries: Jordan: A Iran: D, M India: E Bangladesh: F
Source of funding and conflicts of interest: ACP, HP, DWJ and TPK are authors and/or co-authors on trials included in this review. No other declarations of interest are noted. Funding: Internal sources • Alberta Research Centre for Health Evidence (ARCHE), Canada. External sources • Canadian Institutes of Health Research, Canada. • Programme for Advanced Medical Education (Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde and Fundação para a Ciência e Tecnologia), Portugal. Ricardo M Fernandes (Fellowship) |
Inclusion criteria SR:
Exclusion criteria SR: Studies in the intensive care setting or with intubated or ventilated participants (or both).
19 studies included, with a total of 2256 participants
Important patient characteristics at baseline: See the Cochrane review for all study details.
Groups comparable at baseline? Yes, on relevant variables |
Describe intervention:
In all 19 studies epinephrine was administered via nebulization.
Racemic epinephrine (5 studies): G, H, K, P, S
L-epinephrine 12 studies): B, C, E, F, I, J, L, M, N, O, Q, R
Type of epinephrine unclear (2 studies): A, D
Dexamethasone added to epinephrine (2 studies): N, R (subgroup in these studies) NB: we were not interested in this intervention.
Most trials reported administering epinephrine in multiple doses (n = 12) with all others being delivered as a single dose (n = seven). |
Describe control:
Placebo (0.9% saline; 9 studies): B, G, I, J, K, M, Q, R, S
Salbutamol/albuterol (15 studies): A, C, D, E, G, H, J, K, L, M, N, O, P, Q, S
Steroid (prednisolone (n=15) or budesonide (n=15); dexamethasone (n=200)); 2 studies): K, R
NB: We were not interested in this comparison.
Salbutamol and ipratropium bromide (1 study): F |
End-point of follow-up:
Not specifically mentioned per study. In general, children were monitored until hospital discharge and some examined admission and re-admission.
Incomplete outcome data Incomplete outcome reporting was adequately addressed in 11 of 13 for the review primary outcomes; 13 of 18 for clinical severity score.
|
They combined results using random-effects models regardless of heterogeneity, due to expected differences in interventions, outcomes and measurement instruments (for example, clinical scores measuring different clinical features or weighting these differently).
Comparison 1. Epinephrine versus placebo Nine studies compared epinephrine and placebo; these comparisons involved a total of 1354 patients (677 epinephrine; 677 placebo).
Adverse events, narrative Inpatients: One study (n=38) reported on adverse events and found no occurrences of vomiting, pallor, tremor or arrhythmias. Outpatients: Three studies reported on adverse events (n = 944). One study observed pallor (11% epinephrine, 8% placebo), vomiting (2% epinephrine, 1.5% placebo), tremors (2% epinephrine, 1% placebo) and hypertension (0.5% epinephrine, 0% placebo). However, the occurrence was not significantly different between groups. The other study found no occurrences of tachycardia, withdrawal due to worsening clinical status, or discontinuation of study medications due to adverse events.
Admissions at enrolment or < 24 hours (outpatients only) 6 studies, n=995, RR 0.67 (95% CI 0.50 to 0.89) Total events: 62 (Epinephrine), 93 (Placebo) I2=0% Test for overall effect: Z=2.72(P=0.01)
Admissions overall up to 7 days (outpatients only) 3 studies, n=875, RR 0.81 (95% CI 0.63 to 1.03) Total events: 88 (Epinephrine), 110 (Placebo) I2=0% Test for overall effect: Z=1.71(P=0.09)
Length of stay, days (inpatients only, Mean Difference (95% CI)) 2 studies, n=292, -0.35 (-0.87 to 0.17) Total: 149 (Epinephrine), 143 (Placebo) I2=0% Test for overall effect: Z=1.32(P=0.19)
Clinical score - all (outpatients) At 60 minutes, Std. Mean Difference (95% CI) 6 studies, n=975, -0.40 (-0.58 to -0.23) Total: 490 (Epinephrine), 485 (Placebo) I2=27.91% Test for overall effect: Z=4.5(P<0.0001) At 120 minutes, Std. Mean Difference (95% CI) 2 studies, n=105, -0.73 (-1.13 to -0.33) Total: 53 (Epinephrine), 52 (Placebo) I2=0% Test for overall effect: Z=3.61(P=0)
Clinical score – all (inpatients) At 60 minutes, Std. Mean Difference (95% CI) 2 studies, n=232, -0.04 (-0.49 to 0.40) Total: 118 (Epinephrine), 114 (Placebo) I2=45.84% Test for overall effect: Z=0.2(P=0.84)
Comparison 2. Epinephrine versus salbutamol/albuterol Fifteen studies compared epinephrine versus salbutamol/ albuterol; these comparisons involved 957 randomized participants (480 epinephrine; 477 salbutamol).
Adverse events Inpatients: Two studies reported on adverse events. One study found no cases of pallor, vomiting or tremors (n = 46). The second study (n =30) reported no cases of tremor, increased blood pressure after nebulization or tachycardia among the epinephrine group; the authors did not report on adverse events for the salbutamol group. Outpatients: Three studies reported on adverse events. One study (n = 66) reported on pallor (one epinephrine, zero albuterol), vomiting (one epinephrine, five albuterol), and tremors (zero epinephrine, zero albuterol). The second study (n = 40) reported cases of tachycardia (zero epinephrine, two albuterol) but reported no cases of withdrawal due to worsening clinical status or discontinuation of study medications due to adverse events. The third study (n=186) reported no cases of tremor or increased heart rate in the epinephrine or albuterol groups.
Admissions at enrolment or < 24 hours (outpatients only) 9 studies, n=444, 0.67 (0.41 to 1.09) Total: 222 (Epinephrine), 222 (Salbutamol/Albuterol) I2=30.88% Test for overall effect: Z=1.6(P=0.11)
Admissions overall up to 7 days (outpatients only) 3 studies, n=212, 1.05 (0.71 to 1.54) Total: 109 (Epinephrine), 103 (Salbutamol/Albuterol) I2=0% Test for overall effect: Z=0.23(P=0.82)
Length of stay, days (inpatients only, Mean Difference (95% CI)) 4 studies, n=261, -0.28 (-0.46 to -0.09) Total: 131 (Epinephrine), 130 (Salbutamol/Albuterol) I2=0% Test for overall effect: Z=2.95(P=0)
Length of stay, days (outpatients only, Mean Difference (95% CI)) 1 study, n=42, 0.46 (-0.27 to 1.20) Total: 21 (Epinephrine), 21 (Salbutamol/Albuterol) I2=not applicable Test for overall effect: Z=1.23(P=0.22)
Clinical score - all (outpatients) At 60 minutes, Std. Mean Difference (95% CI) 8 studies, n=397, -0.12 (-0.32 to 0.08) Total: 199 (Epinephrine), 175 (Salbutamol/Albuterol) I2=0% Test for overall effect: Z=1.2(P=0.23) At 120 minutes, Std. Mean Difference (95% CI) 7 studies, n=356, -0.10 (-0.31 to 0.11) Total: 181 (Epinephrine), 130 (Salbutamol/Albuterol) I2=0% Test for overall effect: Z=0.93(P=0.35) At 12 to 24 hours, Std. Mean Difference (95% CI) 2 studies, n=69, -0.21 (-0.86 to 0.44) Total: 34 (Epinephrine), 35 (Salbutamol/Albuterol) I2=41.27% Test for overall effect: Z=0.64(P=0.52) At 3 to 10 days, Std. Mean Difference (95% CI) 2 studies, n=69, -0.50 (-0.98 to -0.02) Total: 34 (Epinephrine), 35 (Salbutamol/Albuterol) I2=0% Test for overall effect: Z=2.04(P=0.04)
Clinical score – all (inpatients) At 60 minutes, Std. Mean Difference (95% CI) 4 studies, n=248, -0.79 (-1.45 to -0.13) Total: 127 (Epinephrine), 121 (Salbutamol/Albuterol) I2=79.23% Test for overall effect: Z=2.36(P=0.02) At 120 minutes, Std. Mean Difference (95% CI) 1 study, n=140, -0.52 (-0.86 to -0.18) Total: 72 (Epinephrine), 68 (Salbutamol/Albuterol) I2=not applicable Test for overall effect: Z=3.02(P=0)
Comparison 3. Epinephrine versus salbutamol and ipratropium bromide One study compared epinephrine versus salbutamol and ipratropium bromide among 60 inpatients.
Clinical score (inpatients) At 6 to 12 hours, Std. Mean Difference (95% CI) 1 study, n=60, -0.60 (-1.12 to -0.09) Total: 30 (Epinephrine), 30 (Salbutamol+IB) I2=not applicable Test for overall effect: Z=2.28(P=0.02) |
Of the 19 included studies
Brief description of author’s conclusion: This systematic review provides evidence that epinephrine is more effective than placebo for bronchiolitis in outpatients. Recent research suggests combined epinephrine and steroids may be effective for outpatients. There is no evidence to support the use of epinephrine for inpatients.
Sensitivity analyses (excluding low quality studies; relevant subgroup-analyses)
Epinephrine vs placebo
When analyses for admission at Day 1 were restricted to trials with low risk of bias, results were no longer statistically significant. There was no change in the results for LOS and clinical scores when only low risk of bias studies were included.
Epinephrine vs salbutamol/albuterol
|
|
Gadomski, 2014
PS: individual study characteristics and results are extracted from the SR (unless stated otherwise) |
Cochrane SR and meta-analysis of RCTs
Literature search up to January 2014
Country: See the Cochrane review; several countries worldwide.
Setting: Outpatients / Emergency Department (ED):
Inpatients:
Source of funding and conflicts of interest: Potential biases in the review process: Gadomski is a trialist and a member of the American Academy of Pediatrics Subcommittee on the Diagnosis and Management of Bronchiolitis. Internal sources of funding: • National Prescribing Service Pty Ltd, Australia. External sources • No sources of support supplied
|
Inclusion criteria SR: - Randomized, placebo-controlled trials of bronchodilators for bronchiolitis. - Infants and young children up to 24 months with bronchiolitis. Bronchodilator therapy, including albuterol, salbutamol, terbutaline, ipratropium bromide and adrenergic agents.
Exclusion criteria SR: Studies of inhaled steroids were not included. Routes of administration were: nebulized, oral and subcutaneous. Although included in the original review, authors excluded studies of epinephrine in bronchiolitis from the update.
30 studies included, with a total of 1922 participants
Important patient characteristics at baseline: See the Cochrane review for all study details.
Fourteen studies included infants aged less than or equal to 12 months (Chevallier 1995; Chowdhury 1995; Gupta 2008; Henry 1983; Ho 1991; Karadag 2008; Levin 2008; Mallol 1987; Patel 2002; Patel 2003; Scarlett 2012; Tal 1983; Tinsa 2009; Totapally 2002).
Groups comparable at baseline? Yes, on relevant variables |
Describe intervention:
Salbutamol/albuterol: Outpatients: Can 1998, Gadomski 1994 – nebulization, Klassen 1991, Ralston 2005, Schuh 1990, Schweich 1992 Inpatients: Chevallier 1995, Dobson 1998, Gurkan 2004, Ho 1991, Karadag 2005 (SAL alone), Lines 1990, Patel 2002, Scarlett 2012
Salbutamol and/or ipratropium bromide Inpatients: Chowdhury 1995, Goh 1997, Karadag 2005 (IPR alone), Lines 1992 (IPR only), Wang 1992
Salbutamol plus hypertonic saline or salbutamol plus normal saline Outpatients: Anil 2010, Ipek 2011
Other medications (not relevant to us): Alario 1992, Gadomski 1994 – oral, Gupta 2008 – oral, Levin 2008 – population on mechanical ventilation, Mallol 1987, Patel 2003, Tal 1983, Tinsa 2009, Totapally 2002 |
Describe control:
Normal saline
|
End-point of follow-up:
Not specifically mentioned per study. In general, children were monitored until hospital discharge and some examined admission and re-admission.
Incomplete outcome data In the outpatient studies, there tended to be more missing data for follow-up measurements beyond 60 minutes because many patients were discharged from these settings before 90 or 120- minute assessments could be done. Bronchodilators have short term effects, therefore some outpatient trialists did not include measurement of outcomes longer than 60 minutes post-treatment. Therefore, the outpatient results are biased towards those data measured at a shorter interval from treatment administration, so Sustained outcomes may have been missed.
Details regarding study attrition were often not well described in the included studies. Drop out rates range from 0% to 13% (Gupta 2008; Patel 2003; Scarlett 2012). Few studies included study flow diagrams that could be used to assess differential drop out from the study groups (Anil 2010 SAL 0.9%; Gupta 2008; Patel 2003; Ralston 2005). Few studies employed intention-to-treat (ITT) analysis when study participant attrition occurred (Patel 2002; Patel 2003). Possible attrition bias might be a factor in three studies that excluded participants from analysis because they were 'therapeutic failures' (Tal 1983), or that withdrew participants for other reasons (Dobson 1998; Goh 1997; Scarlett 2012). |
Comparison: Salbutamol/albuterol (and/or ipratropium bromide) vs. normal saline NB: - For the inpatients analyses, the total /overall effect meta-analytic results in Gadomski include studies that are not relevant to our research question (i.e. studies with wrong drugs / methods or study design). - Three trials employed cross-over designs (Alario 1992; Ho 1991; Totapally 2002). - Ipratropium bromide studies are only inpatients studies. - Two outpatient studies are included with hypertonic saline with salbutamol in experimental groups. - Not all included studies reported on outcomes relevant to us.
Hospital admission after treatment (outpatients), OR, fixed, (95% CI) 7 studies Total: 222 salbutamol/albuterol and 182 normal saline Total events: 37 (Bronchodilator), 43 (Placebo) 0.77 (0.44 to 1.33) I2=0% Test for overall effect: Z=0.94(P=0.35)
Duration of hospitalization (inpatients), days, Std. Mean Difference, fixed, (95% CI) 5 studies Total: 220 (Bronchodilator), 129 (Placebo) 0.06 (-0.27 to 0.39) I2=0% Test for overall effect: Z=0.38 (p=0.7)
Clinical Severity Score after treatment (outpatients), Std. Mean Difference, random, (95% CI) 7 studies Total: 285 salbutamol/albuterol and 247 normal saline -0.36 (-0.83 to0.11) I2=85.08% Test for overall effect: Z=1.49(P=0.13)
Clinical Severity Score after treatment (inpatients), Std. Mean Difference, random, (95% CI) 9 studies Total: 249 (Bronchodilator), 167 (Placebo) -0.14 (-0.41 to 0.12) I2=35.96% Test for overall effect: Z=1.06 (p=0.29)
Adverse effects (only narrative results) Where adverse effects were reported, the authors note that these were exclusively found in the study groups receiving bronchodilators and they included: tachycardia (P value less than 0.05) (Klassen 1991; Lines 1990), decreased oxygen saturation (P value less than 0.05) (Ho 1991; Schweich 1992), flushing (four participants) (Gadomski 1994b - neb), hyperactivity (three participants) (Gadomski 1994b - neb), tachycardia and prolonged cough (two participants) (Henry 1983) and tremor (one participant) (Wang 1992). Patel 2002 reported tachycardia, mild hypertension and slight tremor. One infant receiving albuterol was transferred to the intensive care unit for 48 hours but did not require mechanical ventilation.
Significant tachycardia (sustained heart rate over 200 beats per minute for more than 30 minutes) was reported in two infants receiving albuterol nebulization (Ralston 2005). Scarlett 2012 reported a paradoxical response to albuterol in an infant whose phase angle increased after receiving albuterol (the expected response was a decrease).
No side effects were reported by Karadag 2005 (IPR), Can 1998, Anil 2010. |
Risk of bias of included and relevant studies: Low: Anil 2010, Gadomski 1994, Karadag 2005, Klassen 1991, Levin 2008, Patel 2002, Ralston 2005, Scarlett 2012, Schuh 1990, Schweich 1992, Wang 1992 High: Can 1998, Goh 1997, Henry 1983 Unclear: Chowdhury 1995, Gurkan 2004, Ho 1991, Ipek 2011, Lines 1990
Problems in Gadomski 2014: CSS/hospital admission after treatment: not specified how long after treatment. Numbers per treatment arm are not specified. Not specified which treatment arms were included (epinephrine arms were not included). Treatment arms are not separately compared to one another or to placebo; results of all different treatment arms are combined and compared to placebo. For some reason , for Karadag 2005 the results are split per treatment arm vs placebo. Wang 1992; I checked the original study => number of patients (2 or 3 treatment arms taken together; the placebo group alone) do not match in any way with numbers in Gadomki 2014. Studies with treatment arms with oral medications are also included in their meta-analyses.
Sensitivity analyses (only low risk studies CSS (RDAI)(outpatients)), Std. Mean Difference, random, (95% CI)
3 studies Total: 137 salbutamol vs. 103 normal saline -0.11(-0.48 to 0.25) I2=46.79% Test for overall effect: Z=0.62(P=0.54)
CSS (inpatients), Std. Mean Difference, random, (95% CI) 5 studies Total: 125 salbutamol vs. 103 normal saline 0.01(-0.35 to 0.37) I2=36.79% Test for overall effect: Z=0.05 (P=0.96)
Brief description of author’s conclusion: Given their high cost, adverse effects and lack of effect on oxygen saturation and other outcomes included in this meta-analysis, bronchodilators are not effective in the routine management of first-time wheezers who present with the clinical findings of bronchiolitis, in either inpatient or outpatient settings. |
|
Zhang, 2017
PS: individual study characteristics and results are extracted from the SR (unless stated otherwise) |
Cochrane SR and meta-analysis of RCTs
Literature search up to August 2017
Studies and Setting:
Countries:
Sharma 2013
Source of funding and conflicts of interest: Funding: • Faculty of Medicine, Universidade Federal do Rio Grande, Brazil. • National Council for Scientific and Technological Development (CNPq), Brazil. Fellowship of research productivity (PQ) Authors declare no conflicts of interest except for Wainwright: She received travel grants from the North American Cystic Fibrosis Foundation and the European Cystic Fibrosis Society, travel and accommodation expenses from Novartis, Vertex and the University of Miami; has served as a consultant for/an advisory board member for Vertex; has previously and is currently receiving grants or grants pending from the National Health and Medical Research Council (Australia), and has previously and is currently receiving funds from Vertex, previously from Ablynx, and Novo Nordisk Phamaceuticals for site costs associated with clinical trial participation; her institution has previously received payment for her providing lectures from Novartis and Vertex Pharmaceuticals. None of the declared benefits were received in relation to this review or review topic or scope. |
Inclusion criteria SR: - randomized controlled trials and quasi-randomized controlled trials (in which there is alternate allocation to treatment and control groups) - Children up to 24 months of age diagnosed with acute bronchiolitis. Acute bronchiolitis was defined as the first episode of acute wheezing associated with clinical evidence of a viral infection (cough, coryza, or fever). Confirmation of viral aetiology was not necessary for study inclusion. - studies of inpatients, emergency department patients, or outpatients - comparison of nebulized hypertonic (K 3%) saline solution with nebulized normal (0.9%) saline
Exclusion criteria SR: - participants who had recurrent wheezing or who were intubated and ventilated, and studies that assessed pulmonary function alone
Included: 28 trials involving 4195 infants with acute bronchiolitis, of whom 2222 infants received hypertonic saline
Important patient characteristics at baseline: See the Cochrane review for all study details.
All 28 included studies were randomized, parallel-group, controlled trials. All but two trials were double-blinded (NCT01238848; Everard 2014). Four studies were multicentre: a hospital in the United Arab Emirates and two hospitals in Canada in Kuzik 2007; 10 centres in the UK in Everard 2014; two centres in the USA in Wu 2014; and 24 centres in France in Angoulvant 2017.
Infants hospitalized with severe bronchiolitis (requiring mechanical ventilation or intensive care, or oxygen saturation <85% on room air) were excluded from all but two trials (Teunissen 2014; Wu 2014).
Groups comparable at baseline? The baseline characteristics of participants were similar between treatment groups in all 28 trials. |
Describe intervention:
(1) Hypertonic Saline (HS)
(2) HS + epinephrine / adrenaline
(3) HS + salbutamol / albuterol
(4) HS + salbutamol / albuterol and/or epi-nephrine/adrenaline
Everard 2014 (inpatient) – open, wrong comparison Intervention group: 3% + standard care Control group: standard care
Sarrell 2002 (outpatient) – wrong drug Intervention group: nebulized 3% + terbutaline Control group: nebulized 0.9% + terbutaline |
Describe control:
(1) Normal Saline (NS) (2) NS + epinephrine / adrenaline (3) NS + salbutamol / albuterol (4) NS + salbutamol/albuterol and/or epinephrine/adrenaline
|
End-point of follow-up:
Not specifically mentioned per study. In general, children were monitored until hospital discharge and some examined admission and re-admission.
Incomplete outcome data
The number of withdrawals after randomization was small in all but two trials; the withdrawal rate was 18% in NCT01238848 and Ojha 2014.
Seven trials reported using intention-to-treat analysis (Everard 2014; Florin 2014; Grewal 2009; Kuzik 2007; Mandelberg 2003; Sarrell 2002; Wu 2014).
|
- For outpatient or emergency department participants, rate of hospitalization was measured in Angoulvant 2017, Anil 2010, Florin 2014, Grewal 2009, Ipek 2011, Jacobs 2014, Sarrell 2002. - All 17 inpatient trials except Tinsa 2014 used length of hospital stay as the primary outcome measure. - All outpatient or emergency department trials measured clinical severity score. - The same clinical severity score was used by 11 inpatient trials as a secondary outcome measure (Flores 2016; Köse 2016; Luo 2010; Luo 2011; Mahesh Kumar 2013; Mandelberg 2003; Miraglia Del Giudice 2012; Ratajczyk-Pekrul 2016; Sharma 2013; Tal 2006; Tinsa 2014). This clinical score was initially described by Wang 1992. Other clinical scoring systems were used by two inpatient trials (Kuzik 2007; Ojha 2014). - NB: in MA of Zhang 2017 Sarrell 2002 and Everard 2014 are included in out- and inpatients analyses (i.e. wrong drug and wrong control).
Rate of hospitalization (outpatients / ED), Risk Ratio (M-H, Random, 95% CI) 8 studies, n=1723 0.86 (0.76 to 0.98) I2=7.01% Test for overall effect: Z=2.28(P=0.02)
Length of Hospital Stay (days) (inpatients), Mean Difference (Random, 95% CI) 17 studies, n=1867 -0.41 (-0.75 to -0.07) I2=79.07% Test for overall effect: Z=2.36(P=0.02)
Clinical severity score (post-treatment) at day 1 / day 2 / day 3, Mean Difference (Random, 95% CI)
CSS at day 1: Outpatients, 1 study, n=65, 33 HS and 32 NS -1.28 (-1.92 to -0.64) Test for overall effect: Z=3.9 (P<0.0001)
ED, 1 study, n= 171, 115 HS and 56 NS -0.09(-0.51 to 0.33) Test for overall effect: Z=0.42 (P=0.68)
Inpatients, 7 studies, n=576, 309 HS and 267 NS -0.82 (-1.25 to -0.38) I2=67.33% Test for overall effect: Z=3.64 (P=0)
All patients, 9 studies, n=812 -0.77 (-1.18 to -0.36) I2=74.44% Test for overall effect: Z=3.67 (P=0)
CSS at day 2: Outpatients, 1 study, n=65, 33 HS and 32 NS -2(-2.93 to -1.07) Test for overall effect: Z=4.21(P<0.0001)
ED, 1 study, n= 171, 115 HS and 56 NS -0.27(-0.63 to 0.09) Test for overall effect: Z=1.47(P=0.14)
Inpatients, 6 studies, n=467, 236 HS and 231 NS -1.39(-1.95 to -0.84) I2=75.53% Test for overall effect: Z=4.92(P<0.0001)
All patients, 8 studies, n=703 -1.28(-1.91 to -0.65) I2=87.72% Test for overall effect: Z=3.99(P<0.0001)
CSS at day 3: Outpatients, 1 study, n=65 -2.64(-3.85 to -1.43) Test for overall effect: Z=4.28(P<0.0001)
Inpatients, 6 studies, n=434 -1.35(-1.72 to -0.98) Test for overall effect: Z=7.13(P<0.0001) I2=56.89%
All patients, 7 studies, n=499 -1.43(-1.82 to -1.04) I2=60.53% Test for overall effect: Z=7.17(P<0.0001)
Adverse events (narrative report only): Twenty-four trials presented safety data: 13 trials (1363 infants, 703 treated with hypertonic saline) did not report any adverse events, and 11 trials (2360 infants, 1265 treated with hypertonic saline) reported at least one adverse event, most of which were mild and resolved spontaneously.
|
GRADE (according to Zhang 2017):
=> downgraded for high clinical heterogeneity between studies.
=> downgraded for inconsistent results between studies (high heterogeneity) and risk of bias.
=> downgraded for inconsistent results between studies (high heterogeneity) and risk of bias.
Problems with Zhang 2017: - Takes results of different treatment arms together, for example Anil 2010, they take the epinephrine + HS together with the salbutamol + HS, just as the epi +NS with the sal +NS. Also Ipek 2011 salbutamol + HS and HS alone groups taken together, and salbutamol + NS and NS alone taken together. - All outpatient or emergency department trials measured clinical severity score at 30 or 120 minutes, but post-treatment results are not reported. Variation in scoring methods and assessment time points made conducting meta-analyses inappropriate according to Zhang 2017. Only for Sarrell 2002 (outpatient) and Al-Ansari 2010 (ED) they are reported. - The study of Li 2014 is missing in the analyses (only adverse events are reported).
Brief description of author’s conclusion: Nebulized hypertonic saline may modestly reduce length of stay among infants hospitalized with acute bronchiolitis and improve clinical severity score. Treatment with nebulized hypertonic saline may also reduce the risk of hospitalization among outpatients and emergency department patients. However, authors assessed the quality of the evidence as low to moderate. |
|
Hsieh, 2020
PS: individual study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs
Literature search up to July 2019
Included studies:
Study design: RCT
Setting and Country: 20 (62.5%) were conducted in the Asian region, and six (18.8%) were conducted in the Americas or European countries. Regarding the research setting, 22 (68.8%) studies were conducted in hospital wards with study targets being hospitalized children, and 10 studies (31.3%) were conducted in emergency wards of outpatient departments.
Source of funding and conflicts of interest: Funding for this research was provided mainly by Taipei Medical University (no. TMU106-AE1-B12, 109TMU-WFH-06) and Taiwan Ministry of Science and Technology (MOST 107–2320-B038–018-MY2). The authors declare no conflicts of interests relevant to this article. |
Inclusion criteria SR: (1) population: children aged < 18 years with bronchiolitis; (2) intervention: hypertonic saline (3%); (3) control intervention: normal saline (0.9%); (4) results: severity of respiratory distress, length of hospital stay (LOS), rate of hospitalization, rate of readmission, time of sleeping, frequency of waking up in the night, drug side effects, etc.; (5) study design: RCTs.
Exclusion criteria SR: Patients with other comorbidities such as congenital respiratory tract disease, cardiac insufficiency and immunodeficiency.
32 RCTs included involving 4186 subjects with acute bronchiolitis, of which 2100 were treated with hypertonic saline (3%) abd 2086 were treated with normal saline.
Important patient characteristics at baseline: The mean age of the two population groups were 6.3 months vs. 6.5 months, the sex ratio were 58.3% males vs. 41.7% females, and there were no significant differences regarding the age or sex between these two groups (p > 0.05).
Studies included which add to Zhang 2017:
Islam 2018 Country: Bangladesh Setting: Inpatients Patients: n = 90, age 1 ~ 24 months Average Age (% male) 5.4 mon (56.6%)
Morikawa 2018 Country: Japan Setting: inpatients Patients, n=128 Age < 12 months, Average age (% Male) 4.3 months (39.2%)
Groups comparable at baseline? Yes on sex ratio and age, but the severity of respiratory syncytial virus (RSV) infection was inconsistent, and this might have affected the effects of the interventions. |
Describe intervention:
Hypertonic Saline 3%
Regarding required treatments according to different clinical symptoms, 22 studies (68.8%) combined treatment with epinephrine, bronchodilators, or steroids.
The nebulization treatment time lasted for 20 ~ 30 min, but the saline dosage used for nebulizing ranged from 2 to 5 ml.
Studies included which add to Zhang 2017:
Islam 2018 Country: Bangladesh 4mL 3% HS
Morikawa 2018 Intervention: 2mL HS (n = 63) + 0.5% 0.1 mL salbutamol
|
Describe control:
Normal saline 0.9%
Regarding required treatments according to different clinical symptoms, 22 studies (68.8%) combined treatment with epinephrine, bronchodilators, or steroids.
Islam 2018 4 mL NS (n = 45)
Morikawa 2018 2mL NS (n = 65) + 0.5% 0.1 mL salbutamol
|
End-point of follow-up:
Not specifically mentioned per study. In general, children were monitored until hospital discharge and some examined admission and re-admission.
For how many participants were no complete outcome data available? For bias due to missing outcome data, 20 studies (62.5%) conformed to the intention-to-treat principle, and although there were certain data losses during the study process, those did not affect the balance of the subjects’ basic characteristics, and these were determined to be with low risk of bias; five studies (15.6%) had no information on whether loss of data affected the results, and these were assessed to be with some concern of bias.
|
Summary SR results per outcome, mean difference or Risk Ratio, Random (95% CI)
CSS: N=2010 (11 RCTs) Risk with 0.9% Normal Saline: The mean CSS was −3.57 to 8.8 point Risk with 3% Hypertonic Saline: MD 0.93 point lower (1.23 lower to 0.62 lower)
RDAI: N=1369 (5 RCTs) Risk with 0.9% Normal Saline: The mean RDAI was −4.7 to 5.32 point Risk with 3% Hypertonic Saline: MD 0.6 point lower (0.95 lower to 0.26 lower)
LOS: N=2055 (20 RCTs) Risk with 0.9% Normal Saline: The mean LOS was 1.4 to 7.49 days Risk with 3% Hypertonic Saline: MD 0.54 days lower (0.86 lower to 0.23 lower)
Rate of hospitalization: N=1710 (8 RCTs) Risk with 0.9% Normal Saline: 402 per 1000 Risk with 3% Hypertonic Saline: 342 per 1000 (298 to 394) RR 0.85 (0.74 to 0.98)
Adverse events: narrative results only: Twelve studies reported mild adverse events, including cough (6 studies), bronchospasm (2 studies), vomiting and diarrhea (2 studies), desaturation (1 study), agitation (2 studies), rhinorrhea (1 study), tachycardia (1 study), hoarse voices (1 study), vigorous crying (1 study), vomiting and diarrhea (2 studies). One study reported adverse event (bradycardia and desaturation) in hypertonic saline group. However, these were mild and resolved naturally and all subjects completed the trial process.
Results of studies adding to Zhang 2017:
CSS > 3 days, mean (SD), mean difference, Random (95% CI) Islam 2018, HS, n= 45, 1.64 (0.99); NS, n=45, 3 (1.48); -1.36 (-1.88, -0.84)
LOS, days, mean (SD), mean difference, Random (95% CI) Islam 2018, HS, n=45, 2.42 (0.92); NS, n=45, 3.11 (1.13); -0.69 (-1.12,-0.26) Morikawa 2018, HS, n=63, 4.81 (2.14); NS, n=65, 4.61 (2.18); 0.20 (-0.55, 0.95) |
GRADE CSS: LOW a,b RDAI: MODERATE a LOS: LOW a,b Rate of hospitalization: MODERATE a
a. The overall of Risk of Bias was some concern b. I2 > 75% (statistically significant)
Islam 2018: Some concerns for bias in measurement of the outcome. Other RoB low.
Morikawa 2018: Bias due to deviations from intended interventions, other RoB low.
Limitations of SR: (1) inconsistent disease severity in the included subjects; (2) differences between studies with respect to dosage of hypertonic saline used for the intervention and the combined use of drugs such as bronchodilators; (3) evaluators of the severity of respiratory distress were either medical personnel or research personnel who were not blinded.
Article conclusion: Using hypertonic saline for nebulizing treatment in children with bronchiolitis can significantly improve the severity of respiratory distress, shorten the LOS, and increase the children’s night-time sleep quality. It is recommended that a large-scale randomized clinical trial with a standardized design be conducted in the future to investigate the effects of hypertonic saline in children with bronchiolitis. |
arch question: For children with bronchiolitis in the first hours after presentation at the hospital, does nebulization with epinephrine, normal saline,
Evidence table for intervention studies: RCTs
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Modaressi, 2012 |
Type of study: RCT
Setting and country: - Inpatients - Iran
Funding and conflicts of interest: Conflict of Interest: The authors declare that they have no competing interests. No funding reported. |
Inclusion criteria: The target population included 1 month to 2 year old infants admitted to Amin and Al-Zahra hospitals and diagnosed as acute bronchiolitis by the ICU or ward physicians.
Exclusion criteria: Children with history of two or more respiratory distresses, wheezing, family history of asthma, those who suffered from chronic pulmonary heart disease, suspected heart disease, bronchomalacia, previous use of bronchodilator and glucocorticoids, those treated with monoamino oxydase inhibitors (MAOI), tachycardia >180/min, and respiratory rate >100/min,(4,10-16) were not included in the study.
N total at baseline: - 40 were randomized
Intervention: N = unknown
Control: N = unknown
Important prognostic factors2: age days, Mean (± SD): I: 364±210.4 days C: 409.6±207.6 days
Sex % M: Overall: 20 (50%) I: unknown C: unknown
RDAI severity score, Mean (± SD): I: 12.8 ± 2.4 C: 14.3 ± 1.8
Groups comparable at baseline? yes |
The first group was given one dose of 0.1 ml/kg l-epinephrine in a concentration of 1.10000.
Each volume was 3 cc, which was nebulized using oxygen flow 8 liters per minute. Three doses of each medication at intervals of 20 minutes were prescribed; 10 minutes after the third dose, the patient was rated again by Respiratory Distress Assessment Instrument (RDAI)
During this medication, no other medications like antibiotics and steroids were prescribed for them. |
The other group received salbutamol 0.15 mg/kg at a minimum volume of 1 mg mixed with normal saline.
Each volume was 3 cc, which was nebulized using oxygen flow 8 liters per minute. Three doses of each medication at intervals of 20 minutes were prescribed; 10 minutes after the third dose, the patient was rated again by Respiratory Distress Assessment Instrument (RDAI)
During this medication, no other medications like antibiotics and steroids were prescribed for them. |
Length of follow-up No follow-up or incomplete data reported.
Patients were monitored until discharged from hospital (max 5 days).
|
LOS days, Mean ± SD I - Epinephrine: 3±0.9 C - Salbutamol: 3.7±1.1 p=0.03
RDAI, Mean ± SD
After 10 minutes I - Epinephrine: 10.6 ± 2.1 C - Salbutamol: 12.6 ± 1.4
After 180 minutes I - Epinephrine: 8.2 ± 2.2 C - Salbutamol: 10 ± 1.5
After 1 day I - Epinephrine: 4.5 ± 1.5 C - Salbutamol: 7.3 ± 2
After 2 days I - Epinephrine: 3.1 ± 2.2 C - Salbutamol: 4.3 ± 2.6
After 3 days I - Epinephrine: 1.8 ± 2.4 C - Salbutamol: 3 ± 2
After 4 days I - Epinephrine: 0 C - Salbutamol: 1.3 ± 1.3
After 5 days All zero
p=0.02
|
This was a triple-blind study, i.e. the patient, physician and statistical analyst were unaware of the treatment.
N per group and most baseline characteristics are not mentioned.
Article conclusion: Regarding the effect of epinephrine on reduction of hospitalization duration and the RDAI index in patients with acute bronchiolitis, it seems that using epinephrine instead of salbutamol could be more effective in the management of the disease. |
|
Skjerven, 2013 |
Type of study: RCT
Setting and country: - Inpatients - Norway
Funding and conflicts of interest: Supported by Medicines for Children, a publicly funded body administered by Haukeland University Hospital. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. |
Inclusion criteria: The inclusion criteria were clinical signs of bronchiolitis as defined by Court, Med J 1973;49:771-6, an age of less than 12 months, and an overall clinical score of at least 4 on a scale of 0 to 10. The clinical score was the sum of points allotted, from 0 (indicating normal findings) to 2 (indicating severe illness), for each of the following: general condition, skin color, findings on auscultation, respiratory rate, and retractions.
Exclusion criteria: The exclusion criteria were the presence of any serious cardiac, immunologic, neurologic, or oncologic disease or any serious pulmonary disease other than bronchiolitis; more than one previous episode of obstructive airway disease; symptoms of disease of the lower airway (e.g., coughing) for more than 4 weeks; and receipt of any glucocorticoid therapy in the preceding 4 weeks.
N total at baseline: 404 Infants underwent randomization
Intervention: - adrenaline on demand: 102 - adrenaline fixed schedule: 101
Control: - NS on demand: 98 - NS fixed schedule: 103
Important prognostic factors2: age days, Mean (SD): Intervention: - adrenaline on demand: 134.9±91.6 - adrenaline fixed schedule: 116.9±87.8
Control: - NS on demand: 117.8±68.1 - NS fixed schedule: 136.0±97.0
Sex % M: Intervention: - adrenaline on demand: 63 (61.8) - adrenaline fixed schedule: 60 (59.4)
Control: - NS on demand: 54 (55.1) - NS fixed schedule: 63 (61.2)
Groups comparable at baseline? yes |
10 ml of racemic adrenaline dissolved in 0.9% saline to form a solution of 20 mg per milliliter.
on demand or on a fixed schedule
The dose administered was based on the infant’s weight: 0.10 ml for infants weighing less than 5 kg, 0.15 ml for those weighing 5 to 6.9 kg, 0.20 ml for those weighing 7 to 9.9 kg, and 0.25 ml for those weighing 10 kg or more. The medications were diluted in 2 ml of saline before nebulization and were administered with a Respironics Facemask.
No other inhaled medications, with the exception of 0.9% inhaled saline could be administered during the period when the infant was participating in the trial. Supportive therapy and any other treatments were provided in accordance with routine care.
|
0.9% saline alone.
The medications were diluted in 2 ml of saline before nebulization and were administered with a Respironics Facemask.
on demand or on a fixed schedule
No other inhaled medications, with the exception of 0.9% inhaled saline could be administered during the period when the infant was participating in the trial. Supportive therapy and any other treatments were provided in accordance with routine care.
|
Follow-up: No Follow-up, only monitored children until discharge.
Incomplete data: Intervention: - adrenaline on demand 17 Discontinued study 11 Had treatment failure 4 Were withdrawn by parent 2 Were inappropriately Withdrawn
- adrenaline fixed schedule 19 Discontinued study 12 Had treatment failure 2 Had side effects 4 Were withdrawn by parent 1 Was inappropriately Withdrawn
167 Completed inhaled RA
Control: - NS on demand 20 Discontinued study 15 Had treatment failure 1 Had side effects 3 Were withdrawn by parent 1 Was inappropriately Withdrawn
- NS fixed schedule 27 Discontinued study 21 Had treatment failure 5 Were withdrawn by parent 1 Was inappropriately Withdrawn
154 Completed inhaled saline
The study medication was discontinued in 83 children (20.5%) for the reasons listed above.
321 Completed study
|
LOS in hours The mean (±SD) length of stay for all infants was 80±67 hours
I: Inhaled Racemic Adrenaline (N=203), Mean (range) 63.6 (46.2 to 81.0)
C: Inhaled Saline (N=201), Mean (range) 68.1 (49.8 to 86.4)
Mean difference (95% CI): 4.5 (-6.5 to 15.5) P=0.42
change in the clinical score 30 minutes after the first inhalation
I: Inhaled Racemic Adrenaline (N=203), Mean (range) -1.26 (-1.44 to -1.08)
C: Inhaled Saline (N=201), Mean (range) -1.08 (-1.23 to -0.92)
Mean difference (95% CI): Not reported.
Ventilatory support I : Inhaled Racemic Adrenaline (N=203), n/N (%) 15/203 (7.4)
C: Inhaled Saline (N=201), n/N (%) 15/201 (7.5)
Rate Ratio (95% CI): 0.99 (0.50 to 1.97)
|
The primary outcome, length of hospital stay, was defined as the time from the first study inhalation until discharge from the hospital. No serious adverse events were reported. Three children (including one who was receiving inhaled saline) discontinued treatment because of moderate tachycardia, which may have been due to the study medication.
Article conclusion: There was no significant difference in length of hospital stay between children treated with inhaled racemic adrenaline and those treated with inhaled saline (P = 0.43). There were also no significant between-group differences in the use of nasogastric-tube feeding, supplemental oxygen, or ventilatory support; clinical scores before and after the first inhalation of the study medication; or the number of children in whom the study medication was discontinued. |
|
Faten, 2014 |
Type of study: RCT
Setting and country: - Inpatients - Tunis
Funding and conflicts of interest: Not reported |
Inclusion criteria: Eligible infants included all previously well infants aged between one month old and 12 months old with a clinical diagnosis of first acute viral bronchiolitis and who are hospitalized during the study period.
Exclusion criteria: Children were excluded from the study if they had a gestational age at birth <34 weeks, or underlying chronic cardiac or pulmonary disease (eg, broncho-pulmonary dysplasia, cystic fibrosis), recurrent wheezing, severe respiratory distress, as evidence by apnea, heart rate> 200 beats per minute, respiratory rate >80 breath/minute, profound lethargy, duration of illness exceeding 15 days.
N total at baseline: 97 infants were randomized to the study protocol 94 patients achieved the study.
Intervention: - Group 1: 31 patients received 5% hypertonic saline - Group 2: 37 a mixed 5% hypertonic saline and standard epinephrine
Control: - 26 received normal saline (placebo)
Important prognostic factors2: age ± SD: I: Group 1: 3,76±2,8 Group 2: 3,28±2,53 C: 3,06±2,47
Sex % M: I: Group 1: 71% Group 2: 59% C: 54%
Baseline Wang Severity score: I: Group 1: 5,35±1,4 Group 2: 5,76±1,84 C: 4,28±1,53
Groups comparable at baseline? No |
Group 1: nebulized 5% hypertonic saline (4ml)
Group 2: mixed 5% hypertonic saline with standard epinephrine ( 2ml standard epinephrine + 2 ml 5% hypersaline) |
Control: normal saline placebo (4ml of normal saline) |
Length of follow-up: Infants were only monitored until hospital discharge; no follow-up.
Loss-to-follow-up / incomplete data: Three patients were excluded from statistical analyses: Two patients were withdrawn by the pediatric inpatient team because of worsening clinical status during the first 24 hours. These patients had been randomized to receive placebo. Another patient was withdrawn at parents’ request because the parents refused the hospitalization.
NB: Only those who completed the study were included in the analyses. |
Wang clinical severity score, 30, 60, and 120 minutes after start treatment, mean (SD)
T30 Intervention: Group 1: 4,74±1,3 Group 2: 4,54±1,53 Control: 4,42±1,8 p=0,74
T60 Intervention: Group 1: 4,42±1,4 Group 2: 4,3±1,45 Control: 4 ± 1,55 p=0,56
T120 Intervention: Group 1: 4±1,48 Group 2: 3,68±1,25 Control: 3,76±1,56 p=0,63
The mean time for discharge (SD) days
Intervention: Group 1: 3,6± 1,7 Group 2: 3,5±1,973 Control: 4,48±3,81 p=0,32 |
No patients in either treatment group experienced clinically significant adverse side effects (tachycardia, flushing, tremor or bronchospasm)
Criteria of discharge from the hospital included: no need for supplemental oxygen, Wang severity score less than 3 and adequate fluid intake.
NB: Patients in control group were less often male and had a lower severity score at baseline than intervention groups.
Article conclusion: Nebulized 5% hypertonic saline or mixed 5% hypertonic saline with epinephrine are safe but do not appear effective in treating moderately ill infants with the first acute bronchiolitis. |
|
Flores-Gonzalez, 2015 |
Type of study: RCT
Setting and country: - Inpatients - Spain
Funding and conflicts of interest: Funding: This work was supported by grants from the Spanish Ministry of Health, Social Politics and Equality for the promotion of independent clinical research of 2010 (EC10-180). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist. |
Inclusion criteria: Eligible patients included infants aged under 24 months admitted to Hospital with a clinical diagnosis of acute bronchiolitis classified as moderate in severity. The diagnosis was based on a first episode of respiratory distress with wheezing and/or crackles, preceded by an infection of the upper airways.
Exclusion criteria: Infants were excluded if they had any of the following risk factors: premature birth as defined by the World Health Organization (< 37 weeks), in infants with an adjusted age of less than 6 weeks at the time of enrollment, chronic respiratory disease, hemodynamically significant heart disease, immunodeficiency, and neuromuscular disease. Infants with previous episodes of wheezing or a physician’s diagnosis of asthma were also excluded. Finally, we also excluded patients receiving other non-study treatments during hospitalization.
N total at baseline: - 208 were randomized - 185 in analyses
Intervention: 104 randomized, 94 in analyses
Control: 104 randomized, 91 in analyses
Important prognostic factors2: age Mean months (SD): I: 2.10±2.37 C: 2.12±2.08
Sex % M: I: 46 (48.9) C: 46 (50.5)
Wood-Downes Scale modified by Ferres (WDF) severity score, N (%) I: 5.36±0.98 C: 5.24±1.17
Groups comparable at baseline? yes |
Patients received nebulized epinephrine (3 ml of a 1:1000 solution), in 3% hypertonic saline (7 mL).
The nebulized solution was administered by means of a mask using an ultrasonic hospital nebulizer (Shinmed model Sw918) with a frequency of 1.7 MHz and a mist particle size of 1 to 5 μm.
The solutions were administered initially every 4 hours.
Infants received the same standard support (elevation of the head of bed, supplemental oxygen when oxygen saturation dropped below 94%, acetaminophen if fever, and a nasal lavage with sterile saline before and after the administration of the nebulized solution). |
Patients received nebulized 3% hypertonic saline (7 mL) plus 3 mL placebo (sterile water).
The nebulized solution was administered by means of a mask using an ultrasonic hospital nebulizer (Shinmed model Sw918) with a frequency of 1.7 MHz and a mist particle size of 1 to 5 μm.
The solutions were administered initially every 4 hours.
Infants received the same standard support (elevation of the head of bed, supplemental oxygen when oxygen saturation dropped below 94%, acetaminophen if fever, and a nasal lavage with sterile saline before and after the administration of the nebulized solution). |
Follow-up: Patients were monitored only until hospital discharge.
Lost in follow-up: I: 10/104 lost - 6 were admitted to intensive care - 2 were withdrawn by the pediatrician - 1 was withdrawn by parent - 1 failed to fulfil inclusion criteria
C: 13/104 lost - 6 were admitted to intensive care - 2 were withdrawn by pediatrician - 3 were withdrawn by parent - 2 failed to fulfil inclusion criteria
NB: Only those who completed the study were included in the analyses. I: 94 C: 91
|
LOS days, mean (SD) I: 3.94±1.37 C: 4.82±2.3 P = 0.011
Required more than 4 days of hospitalization, n (%) I: 13 (13.8) C: 28 (30.8) P = 0.006 RR: 0.45, 95% CI: 0.25 to 0.81
Comparison of survival curves showed significant differences in the LOS from day 4 onwards (P = 0.001)
WDF severity score at day 3, mean (95% CI) I: 3.93 (3.68 to 4.17) C: 4.31 (4.01 to 4.59) p = 0.029
WDF severity score at day 5 I: 3.37 (3.02 to 4.72) C: 4.03 (3.67 to 4.40) p = 0.036
|
The primary efficacy outcome was length of hospital stay (LOS), defined as the number of days from admission to the time at which the patient fulfilled the study discharge criteria: aWDF score of 3 or less, an oxygen saturation of 97% or more without supplemental oxygen, adequate oral tolerance, and no further need for nebulized therapy.
Authors report no adverse events (i.e. tachycardia, sweating, pallor, trembling, or hypertension), during hospitalization. NB: However, patients with such severe adverse events that PICU admission was necessary (6 in each group), were excluded from analyses.
Group n confusing: figure group data are swapped.
Article conclusion: Nebulized epinephrine in 3% saline significantly shortens the length of hospital stay of infants with acute moderate bronchiolitis in our setting, where it normally exceeds 4 days, and reduces the risk of a prolonged stay, without any increase in the occurrence of adverse events, when compared with placebo in 3% saline. |
|
Uysalol, 2017 |
Type of study: RCT
Setting and country: - Emergency Department (ED) - Turkey
Funding and conflicts of interest: Not reported |
Inclusion criteria: Children with acute bronchiolitis, aged between 2-24 months with a score as moderate (4-8) in the bronchiolitis clinical score (BCS) system were included.
Exclusion criteria: Exclusion criteria were being younger than 2 months old, prematurity (less than 36th gestational week), low birth weight (less than 2,500 g), history of admission in neonatal intensive care unit due to respiratory distress, history of intubation in the intensive care unit, congenital heart/lung/neurologic or immunologic disease, history of atopic disease or recurrent wheezing, clinical or radiologic findings of bacterial infections, atelectasis or consolidations on X-ray and refusal to consent by parents.
N total at baseline: - 386 patients were randomized. - 378 patients were able to complete the trial and only these were included in statistical analyses.
Intervention: Group 1 (HS): 77 Group 2 (ADR): 75 Group 3 (ADR+HS): 75 Group 4 (Salbutamol): 72
Control: Group control (NS): 79
Important prognostic factors2: age months, Median (IQR): I: 7 (4-10) months (all groups the same) C: 7 (4-10) months
Sex % M: Intervention: Group 1 (HS): 55.8% Group 2 (ADR): 54.7% Group 3 (ADR+HS): 54.7% Group 4 (Salbutamol): 54.2%
Control: Group control (NS): 54.4%
Groups comparable at baseline? Yes |
Drugs were administered by means of standard hospital nebulizers through a firmly applied face mask with an oxygen flow of 6 liters per minute within 6-8 minutes.
Administration at at 0, 30, and 60 minutes, and every 4 hours thereafter if needed to a maximum of 24 h.
Group 1: 3% hypertonic saline (HS); Group HS was given 4 ml HS
Group 2: nebulized adrenaline (ADR); group ADR received 4 ml NS with ADR 0.1 mg/kg
Group 3: nebulized adrenaline mixed with 3% hypertonic saline (ADR+HS); group ADR+HS received 4 ml HS with 0.1 mg/kg/dose ADR
Group 4: nebulized salbutamol; group Salbutamol had nebulized salbutamol 0.15 mg/kg with 4 ml NS
|
Control Group: normal saline (0.9% NaCl) (NS); group NS was administered 5 ml NS
Administration at at 0, 30, and 60 minutes, and every 4 hours thereafter if needed to a maximum of 24 h.
|
Length of follow-up: Readmission to the hospital within first 15 days was recorded.
Loss-to-follow-up / incomplete data: During the study, infants whose BCS had deteriorated worse than 9 were excluded from the study (2 in HS group, 1 in ADR group, 2 in salbutamol group and 3 in NS group). At the end, 378 patients were able to complete the trial
NB: Only those who completed the study were included in the analyses. |
Comparison of Discharge Rates at 4 Hours of Treatment Options (Reference Group: Normal saline) Odds Ratio (OR), 95%CI Group 1 (HS): 1.595 (0.841 to 3.024) p=0.153 Group 2 (ADR): 2.194 (1.150 to 4.186) p=0.017 Group 3 (ADR+HS): 3.898 (1.993 to 7.625) p<0.001 Group 4 (Salbut): 1.034 (0.534 to 2.004) p=0.920
Discharge rate at 4 hrs, n (%) Intervention: Group 1 (HS): 37/77 (48.1%) Group 2 (ADR): 42/75 (56%) Group 3 (ADR+HS): 52/75 (69.3%) Group 4 (Salbut): 27/72 (37.5%) Control: Group control (NS): 29/79 (36.7%) p=0.001
Discharge rate at 24 hrs, n (%) Intervention: Group 1 (HS): 69 (77%) Group 2 (ADR): 66 (88%) Group 3 (ADR+HS): 71 (94.7%) Group 4 (Salbut): 63 (87.5%) Control: Group control (NS): 66 (83.5%) p=0.294
Length of Stay (LOS) (hours), median (IQR) Intervention: Group 1 (HS): 8 (12) Group 2 (ADR): 4 (12) Group 3 (ADR+HS): 4 (8) Group 4 (Salbut): 16 (20) Control: Group control (NS): 16 (20) p=0.039
Adverse events (tachycardia, pallor, tremor, nausea, vomiting), n (%) Intervention: Group 1 (HS): 0 (0%) Group 2 (ADR): 7 (9.3%) Group 3 (ADR+HS): 5 (6.7%) Group 4 (Salbut): 7 (9.7%) Control: Group control (NS): 2 (2.5%) p=0.079
|
Primary outcomes: LOS and Discharge rate. The study was not powered to detect differences in secondary outcome measures.
Only those who completed the study were included in the analyses.
Criteria for discharge: Infants were evaluated using BCS at 4-hour intervals and a score less than 3 were considered for discharge decision.
Article conclusion: Nebulized adrenaline mixed with 3% hypertonic saline, as compared with other options, were associated with a significantly higher discharge rate at 4th hours (p<0.001) and shorter length of hospital stay (p=0.039). However, there was no significant difference between options with regard to adverse events, discharge rates at 24th hours, and readmission rates within the first fifteen days.
|
|
Bashir, 2018 |
Type of study: RCT
Setting and country: - Inpatients, tertiary care hospital - India
Funding and conflicts of interest: None |
Inclusion criteria: Previously healthy infants and children of two months to 18 months of age, getting admitted with first episode of respiratory tract infection with wheeze, starting as a viral upper respiratory infection (coryza, cough, or fever), and a clinical score between 4 and 8 were included in the study.
Exclusion criteria: Includes a history of any of the following: previous episode of wheezing, chronic cardiopul monary disease or immunodeficiency; critical illness at presentation requiring admission to intensive care; the use of nebulized HS within the previous 12 hours; or premature birth (gestational age 34 weeks).
N total at baseline: 189 Intervention: 96 Control: 93
Important prognostic factors2: age median months (IQR): I: 4.0 (2.63-8.0) C: 4.0 (2.0-7.0)
Sex % M: I: 64.6% C: 69.9%
Groups comparable at baseline? yes |
Treatment with 4 mL of nebulized study solution containing 3% HS.
Every two hourly for three doses, followed by every four hourly for six doses, followed by every six hourly until discharge.
|
Treatment with 4 mL of nebulized study solution containing 0.9% NS.
Every two hourly for three doses, followed by every four hourly for six doses, followed by every six hourly until discharge. |
Length of follow-up: Until hospital discharge.
Loss-to-follow-up / incomplete data: Five infants (one from the HS group and four from the NS group) were withdrawn before study completion but were included in the final intention to treat analysis and were counted as treatment failures. |
Discharge within 1 day, n (%), RR (95%CI) I: 55 (57.3%) C: 4 (4.3%) 13.32 (5.03 to 35.28), p<.0001
Discharge within 2 days, n (%), RR (95%CI) I: 94 (97.92%) C: 59 (63.44%) 1.54 (1.32 to 1.81), p<.0001
Discharge within 3 days, n (%), RR (95%CI) I: 96 (100%) C: 90 (96.77%) 1.03 (0.996 to 1.072), p=0.083
100% Discharge within 4 days for all.
LOS mean days (SD) I: 1.45 (0.54) C: 2.35 (0.62) p<.001
Reduction in Wang clinical severity score within 48 hours, mean (SD) I: 2.26 (0.68) C: 1.23 (0.49) p<0.001 favouring 3% HS group |
Children showing worsening of clinical scores and general condition during the course of the stay were excluded from the study and treated as the condition necessitates. However, these patients were included in the final analysis and were counted as treatment failures.
Criteria for discharge: patients were discharged once they were off oxygen support, maintaining saturations without any respiratory distress and accepting feeds well.
There were no adverse events noted in either of the groups in present study.
Article conclusion: This study demonstrates that 3% HS nebulization is safe and effective treatment for infants up to the age of 18 months hospitalized with acute bronchiolitis and decreases hospital stay by about one day. |
|
Jaquet-Pilloud, 2020 |
Type of study: RCT
Setting and country: - Inpatients - Switzerland
Funding and conflicts of interest:
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. Competing interests: None declared. |
Inclusion criteria: Eligible patients included children aged from 6 weeks up to 24 months coming to the emergency department (ED) with a first episode of acute bronchiolitis, defined as symptoms of upper respiratory tract infection in addition to tachypnoea, wheezing and widespread crackles at auscultation. Further inclusion criteria were a Wang Score of 5–12 (moderate to severe) on arrival.
Exclusion criteria: Exclusion criteria were children with mild bronchiolitis (Wang Score <5), previous episodes of wheezing, cardiac or chronic respiratory disease, immunocompromised children, gestational age <34 weeks and children with critical illness requiring immediate admission to intensive care unit (ICU). Children who received RSV immunoglobulin therapy, corticotherapy in any form in the preceding 2 weeks or bronchodilators within 24 hours prior to presentation, were also excluded.
N total at baseline: - 122 were randomized - 120 in analyses
Intervention: 61 randomized, 60 in analyses
Control: 61 randomized, 60 in analyses
Important prognostic factors2: age Mean months (95% CI): I: 7.7 (6.4; 9.1) C: 7.5 (6.2; 8.9)
Sex % M: I: 39 (64%) C: 37 (63%)
Wang severity score, N (%) I: 15 (24%) C: 14 (23%)
Wang severity score, N (%) I: 46 (76%) C: 45 (77%)
Groups comparable at baseline? yes |
HS 3% Group received 4 mL of NaCl 3% (MucoClear 3%) every 6 hours until discharge in addition to standard care.
Standard therapy includes suctioning nasal secretions, water-electrolyte balance maintenance and oxygen supplementation when needed.
If any child showed signs of respiratory failure including either persistent major respiratory distress, signs of exhaustion with a partial pressure of carbon dioxide above >50 mm Hg on the capillary blood gas, a nebulization of 4 mg of epinephrine was given. Nebulized epinephrine could be administered up to three times within the hour. If respiratory failure continued despite a total of three nebulizations of epinephrine / in the absence of response, the patient was admitted to ICU.
|
Standard Care group with no inhalation.
Standard therapy includes suctioning nasal secretions, water-electrolyte balance maintenance and oxygen supplementation when needed.
If any child showed signs of respiratory failure including either persistent major respiratory distress, signs of exhaustion with a partial pressure of carbon dioxide above >50 mm Hg on the capillary blood gas, a nebulization of 4 mg of epinephrine was given. Nebulized epinephrine could be administered up to three times within the hour. If respiratory failure continued despite a total of three nebulizations of epinephrine / in the absence of response, the patient was admitted to ICU.
|
Length of follow-up: Readmission rate in the next 7 days following discharge from hospital was studied.
Loss-to-follow-up: None
Incomplete data: HS was discontinued in 10 patients at parents’ request (sleep preservation (n=5), agitation with the inhalation facemask (n=5).
Two patients were excluded after randomization, one for misdiagnosis (pneumonia) and the other for decompensation of an unknown neurological disease and excluded from the intention to treat analyses. |
Hospital LOS (hours), Mean (95% CI) I: 47 (39 to 56) C: 50.4 (39 to 61) Difference -2.8 (-11 to 16) p=0.33
Duration oxygen therapy (hours), mean (95% CI) I: 29.5 (22 to 36) C: 31.1 (22 to 39) Difference -1.5 (-9.6 to 12) p=0.6
Transfers to PICU, N (%) I: 0 (0%) C: 3 (5%) RR: 0.138 (95%CI 0.007 to 2.620) p=0.187
Racemic epinephrine nebulization rescue therapy, N (%) I: 5 (8.2) C (9 (15%) RR: 0.537 (95%CI 0.191 to 1.510) p=0.239
|
No serious adverse events were observed (bronchospasm, excessive couching, infection, apnea and cyanosis) during the study.
Authors report: Sixty-one patients were allocated to the intervention group (HS) and 61 were allocated to the control group (standard care alone). One hundred and twenty patients completed the whole study. However, all Tables in article state that the HS group analyses were with n=61 and the standard care group with n=59 => included N is unclear.
Article conclusion: There were no differences in oxygen therapy duration, transfer to ICU, readmission rate or adverse events. Study does not support the use of HS nebulization in children with moderate to severe bronchiolitis. |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors ((potential) confounders)
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
Supplementary Evidence Table 1. Adrenaline: study specifications with regard to dose
|
Adrenaline Studies |
Type of adrenaline (L- or racemic) |
Number and timing of doses |
Single dose in mg (children of 3 kg – 12 kg range) |
Compared with (normal saline, salbutamol) |
Length of Stay |
Hospital admission
|
CSS |
|
From SR Hartling, 2011: |
|||||||
|
Abul-Ainine, 2002 |
L-adrenaline |
1 dose |
3 mg |
normal saline |
|
|
X |
|
Abu-Shukair, 2001 |
adrenaline |
2 doses at 0 and 30 min |
3 mg |
salbutamol |
|
|
X |
|
Anil, 2010 |
adrenaline |
2 doses at 0 and 30 min |
1,5 mg |
normal saline, salbutamol |
|
X |
X |
|
Barlas, 1998 |
racemic adrenaline |
Doses every 2 h, the first 4 h |
unclear |
normal saline, salbutamol |
|
X |
X |
|
Beck, 2007 |
adrenaline |
1 dose |
1 mg |
salbutamol |
|
|
X |
|
Bertrand, 2001 |
adrenaline |
Doses every 2-4 h |
0.5 mg |
salbutamol |
X |
|
X |
|
Bilan, 2007 |
adrenaline |
Doses every 4 h |
0.6 mg – 2.4 mg |
salbutamol |
X |
|
|
|
John, 2006 |
adrenaline |
Doses at 0, 30, 60 min, and then every 4 h |
unclear |
salbutamol |
X |
|
X |
|
Kadir, 2009 |
L-adrenaline |
2 doses at 0 and 6 h |
0.03 mg - 0.12 mg |
salbutamol |
|
|
X |
|
Khasabi, 2005 |
adrenaline |
3 doses, every 20 min |
0.3 mg - 1.2 mg |
normal saline, salbutamol |
|
X |
X |
|
Kuyucu, 2004 |
L-adrenaline |
3 doses |
3 mg |
salbutamol |
|
|
X |
|
Menon, 1995 |
L-adrenaline |
1 dose |
3 mg |
salbutamol |
X |
X |
X |
|
Mull, 2004 |
racemic adrenaline |
3 doses at 0, 30 and 60 min |
2.7 mg - 10.8 mg |
salbutamol |
|
X |
X |
|
Okutan, 1998 |
adrenaline |
Number and timing of doses unclear |
0.6 mg - 2.4 mg |
normal saline, salbutamol |
|
|
X |
|
Patel, 2002 |
racemic adrenaline |
Doses every 1-6 h |
2.025 mg - 8.1 mg |
normal saline, salbutamol |
X |
|
|
|
Plint, 2009 |
adrenaline |
2 doses, 30 min apart |
3 mg |
normal saline |
|
X |
X |
|
Ralston, 2005 |
racemic adrenaline |
2 doses at 0 and 30 min, optional 3rd dose at 60 min |
5 mg |
normal saline, salbutamol |
|
X |
|
|
Sanchez, 1993 |
racemic adrenaline |
1 dose |
11.25 mg |
salbutamol |
|
|
X |
|
Wainwright, 2003 |
adrenaline |
3 doses every 4 h |
40 mg |
normal saline |
X |
|
X |
|
Extra RCT’s: |
|||||||
|
Modaressi, 2012 |
adrenaline |
3 doses every 20 min, and every 10 min thereafter |
0.03 mg - 0.12 mg |
salbutamol |
|
|
X |
|
Skjerven, 2013 |
racemic adrenaline |
Doses on demand or every 2 hours |
1 mg – 2.5 mg |
normal saline |
X |
|
X |
|
Uysalol, 2017 |
adrenaline |
Doses at 0, 30, 60 min and every 4 h thereafter |
0.3 mg – 1.2 mg |
normal saline, salbutamol |
X |
|
|
X = study is in analysis for this outcome
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea ; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/not applicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Hartling, 2011 |
Yes |
Yes |
Yes |
Yes |
Not applicable, only RCTs. |
Yes |
Unclear; Authors report variation between the trials in delivery of interventions, type of adrenaline, administrations, dosage, patient characteristics such as presence of co-morbidities, age, stage of illness, etc. They report insufficient data presented at the trial level to assess the impact of these variations or allow for subgroup comparisons. |
Yes, authors were unable to test for publication bias due to the small number of studies within each category of comparison, outcome and clinical setting. |
Yes |
|
Gadomski, 2014 |
Yes |
Yes |
Yes |
No, not all relevant characteristics are described, such as number per treatment arm. Not always baseline patient characteristics described for all studies. |
Not applicable, only RCTs. |
Yes |
No. Treatment arms are not separately compared to one another or to placebo; results of all different treatment arms, including oral medication, are combined and compared to placebo. Probably a wide variety of assessment time points in their analyses were combinefor outcome “CSS after treatment” |
No. |
Yes |
|
Zhang, 2017 |
Yes |
Yes |
Yes |
Yes |
Not applicable, only RCTs. |
Yes |
No. Takes results of different treatment arms together. Same problem as Gadomski, 2014. |
Yes |
Yes |
|
Hsieh, 2020 |
Yes |
Yes |
Yes |
Yes |
Not applicable, only RCTs. |
Yes |
Yes |
Yes |
Yes |
- Research question (PICO) and inclusion criteria should be appropriate and predefined
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs)
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table etc.)
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (e.g. Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (e.g., funnel plot, other available tests) and/or statistical tests (e.g., Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias table for intervention studies (randomized controlled trials)
|
Study reference
(first author, publication year) |
Describe method of randomization |
Bias due to inadequate concealment of allocation?
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?
(unlikely/likely/ unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation? (unlikely/likely/ unclear) |
Bias due to selective outcome reporting on basis of the results?
(unlikely/likely/ unclear) |
Bias due to loss to follow-up?
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?
(unlikely/likely/unclear) |
|
Modaressi, 2012 |
old infants admitted to Amin and Al-Zahra hospitals and diagnosed as acute bronchiolitis by the ICU or ward physicians.
children were placed in one of the two groups by the random allocation software.
|
Unclear |
Unlikely |
Unclear |
Unclear |
Unlikely |
Unclear |
Unclear |
|
Skjerven, 2013 |
to the hospital as long as attending personnel (a physician and a nurse) were available.
schedule).
|
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Likely |
Unlikely |
|
Faten, 2014 |
prepared each morning solutions which were similar in appearance and smell, stored in identical syringes, labeled only by a code number.
|
Unclear |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Likely |
Likely |
|
Flores-Gonzalez, 2015 |
withdrew consent or received any other non-study medications.
|
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Likely |
|
Uysalol, 2017 |
|
Unlikely |
Unclear |
Unclear |
Unclear |
Unlikely |
Unlikely |
Likely |
|
Bashir, 2018 |
|
Unclear |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
|
Jaquet-Pilloud, 2020 |
as expected, but it is unclear in which group they were. |
Unlikely |
Likely |
Likely |
Likely |
Unlikely |
Unclear |
Unclear |
- Randomization: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomization process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomization (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomization procedures or open allocation schedules..
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the risk of bias is unclear
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the risk of bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Guo, 2018 |
Meta-analysis with all relevant studies already covered by the 3 Cochrane reviews. |
|
House, 2020 |
No relevant outcome measures. |
|
Simsek-Kiper, 2011 |
RCT that added to Hartling 2011 adrenaline, or so I thought, but did not present relevant outcome data. |
|
Flores-Gonzalez, 2016 |
Adds to Hartling 2011 adrenaline, but seems to be the same study as Flores-Gonzalez 2015, but with older/incomplete data of the same trial - data until 2014 |
|
Morikawa, 2018 |
Adds to Zhang 2017, but already in Hsieh 2020 => data taken from there |
|
Saseen, 2004 |
Is a summary of Hartling 2003 |
|
Bourke, 2011 |
SR excluded because everything in Hartling 2011, Gadomski 2014, or Zhang 2017 |
|
Chen, 2014 |
SR of which all relevant studies included in Zhang 2017 |
|
Castro-Rodriguez, 2015 |
Doesn't add any relevant information; just gives a summary of results of different SRs we already have |
|
Everard, 2015 |
SR of which all relevant studies included in Zhang 2017 |
|
Maguire, 2015 |
SR of which all relevant studies included in Zhang 2017 |
|
Zhang, 2015 |
SR of which all studies included in Zhang 2017 |
|
Brooks, 2016 |
SR of which all studies included in Zhang 2017 |
|
Heikkilä, 2018 |
SR of which all relevant studies included in Zhang 2017 |
|
Zhang, 2018 |
SR of which all studies included in Zhang 2017 |
|
Wang, 2019 |
SR of which all studies included in Zhang 2017 |
|
Hartling, 2003 |
See Hartling 2011 for new Cochrane |
|
Absar, 2008 |
Short communications, not all data present. Prospective Cohort. |
|
Baron, 2016 |
Review is not systematic; not all necessary data |
|
Unknown, 2010 |
Is a summary of Grewal 2009 |
|
Yasin, 2020 |
No RCT: quasi-randomized unblinded trial; allocation not blinded; prospective |
|
Hartling, 2011 |
SR excluded because everything in Hartling 2011, Gadomski 2014, or Zhang 2017 |
|
Khanal, 2015 |
RCT included in Zhang 2017 |
|
King, 2004 |
SR of which all studies included in Hartling 2011 |
|
Ralston, 2014 |
No relevant data provided |
|
Seiden, 2009 |
Narrative review |
|
Wright, 2011 |
Narrative review |
|
Panitch, 2003 |
Narrative review |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 02-08-2023
Beoordeeld op geldigheid : 28-06-2023
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2020 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor kinderen met bronchiolitis in de tweede lijn.
Werkgroep
- Dr. C.C. (Chris) de Kruiff, kinderarts, werkzaam in het Amsterdam UMC, afgevaardigd namens NVK (voorzitter)
- Dr. T. (Tessa) Sieswerda, kinderarts, werkzaam in het Amsterdam UMC, afgevaardigd namens NVK (vice voorzitter)
- Drs. M. (Miranda) Wiggelinkhuizen, kinderarts en fellow kinderintensivist, werkzaam in het RadboudUMC Amalia kinderziekenhuis te Nijmgen, afgevaardigd namens NVK SICK
- Dr. E.M. (Marije) van den Beukel-Bakker, kinderlongarts, werkzaam in het ErasmusUMC Sophia kinderziekenhuis te Rotterdam, afgevaardigd namens NVK
- A. (Annelies) van der Kolk, verpleegkundig specialist kinderallergologie, werkzaam in het Deventer ziekenhuis, afgevaardigd namens V&VN Verpleegkundig specialisten
Klankbordgroep
- J. (Janine) Pingen, junior projectmanager en beleidsmedewerker, Stichting Kind en Ziekenhuis, afgevaardigd namens Stichting Kind en Ziekenhuis (tot 01-12-2020)
- R. (Rowy) Uitzinger, junior projectmanager en beleidsmedewerker, Stichting Kind en Ziekenhuis, afgevaardigd namens Stichting Kind en Ziekenhuis (van 01-12-2020 tot 01-06-2022)
- E.C. (Esen) Doganer, junior projectmanager en beleidsmedewerker, Stichting Kind en Ziekenhuis, afgevaardigd namens Stichting Kind en Ziekenhuis (vanaf 01-06-2022)
- M. (Maria) van ’t Erve, verpleegkundig specialist kindergeneeskunde, werkzaam bij het Isala ziekenhuis te Zwolle, afgevaardigd namens V&VN
- Dr. M.J.C. (Marjolein) Schot, huisarts, werkzaam bij Groepspraktijk d’n Iemhof te Oss, afgevaardigd namens NHG
- Drs. P.M. (Petra-Marije) Greidanus, algemeen kinderarts, werkzaam in ziekenhuis Amstelland te Amstelveen, afgevaardigd namens NVK
Met ondersteuning van
- Dr. J. (Janneke) Hoogervorst – Schilp, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. M. (Mattias) Göthlin, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. T. (Tim) Christen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
de Kruiff |
Functie: Kinderarts algemene pediatrie, Hoofd vakgroep algemene kindergeneeskunde, Amsterdam UMC |
Redactielid Praktische Pediatrie, waarvoor honorarium ontvangen wordt; Voorzitter NVK congres (onbetaald). |
Geen |
Geen actie |
|
Sieswerda |
Kinderarts, algemene pediatrie, AUMC Per 01-07-2020 fellow sociale pediatrie, AUMC |
Lid NVK commissie richtlijnen, onbetaald Vice-voorzitter revisie NVK richtlijn dehydratie en bronchiolitis, waarvoor vacatiegelden worden ontvangen |
Geen |
Geen actie |
|
Van den Beukel-Bakker |
Kinderlongarts in het ErasmusMC - Sophia kinderziekenhuis |
CSO visitaties voor kinderlongarts, onkostenvergoeding |
Geen |
Geen actie |
|
van der Kolk |
Verpleegkundig specialist kinderallergologie, Deventer ziekenhuis |
Lid van de stichting verpleegkundig specialisten kinderlongziekten en aanverwante aandoeningen (onbetaald). |
Studie met sponsoring van Teva Pharma |
Geen actie |
|
Wiggelinkhuizen |
Kinderarts, kinderintensivist in opleiding Radboudumc – Amalia kinderziekenhuis |
Commissielid commissie PatientVeiligheid NVK (onbetaalde functie) |
Geen |
Geen actie |
|
Klankbordgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Pingen |
Junior projectmanager en beleidsmedewerker Stichting Kind en Ziekenhuis |
Geen |
Geen |
Geen actie |
|
Uitzinger (tot 10 mei 2022) |
Junior projectmanager en beleidsmedewerker Stichting Kind en Ziekenhuis |
Geen |
Geen |
Geen actie |
|
Cingir-Doganer (vanaf 10 mei 2022) |
Junior projectmanager en beleidsmedewerker Stichting Kind en Ziekenhuis |
Geen |
Geen |
Geen actie |
|
Schot |
Praktijkhoudend huisarts |
Geen |
Geen |
Geen actie |
|
van ’t Erve |
Verpleegkundig specialist kindergeneeskunde Isala ziekenhuis Zwolle |
Geen |
Geen |
Geen actie |
|
Greidanus |
Kinderarts Ziekenhuis Amsterland |
Geen |
Geen |
Geen actie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntperspectief door afgevaardigde patiëntenvereniging Stichting Kind & Ziekenhuis in de klankbordgroep. Het verslag hiervan (zie aanverwante producten) is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan Stichting Kind en Ziekenhuis en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Implementatie
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er (waarschijnlijk geen) substantiële financiële gevolgen zijn, zie onderstaande tabel.
|
Module |
Uitkomst raming |
Toelichting |
|
Module Vernevelingen bij bronchiolitis |
geen financiële gevolgen |
Aantal patiënten |
|
Module Indicatie zuurstoftoediening |
geen financiële gevolgen |
Aantal patiënten |
|
Module Apneurisico |
geen financiële gevolgen |
Aantal patiënten |
|
Module Cohortverpleging bij bronchiolitis |
geen financiële gevolgen |
Aantal patiënten |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor kinderen met bronchiolitis. De werkgroep beoordeelde de aanbeveling(en) uit de eerdere richtlijnmodule (Nederlandse Vereniging voor Kindergeneeskunde, 2011) op noodzaak tot revisie. Tevens zijn er via een enquête knelpunten aangedragen door de stakeholders in onderstaande tabel. Een verslag hiervan is opgenomen onder aanverwante producten.
|
Nederlandse Vereniging voor Kindergeneeskunde (NVK) |
|
Nederlands Huisartsen Genootschap (NHG) |
|
Verpleegkundigen & Verzorgenden Nederland (V&VN) |
|
Stichting Kind en Ziekenhuis, |
|
Inspectie Gezondheidszorg en Jeugd (IGJ) |
|
Nederlandse Vereniging van Ziekenhuizen (NVZ) |
|
Zorginstituut Nederland (ZiNL) |
|
Zelfstandige Klinieken Nederland (ZKN) |
|
Zorgverzekeraars Nederland (ZN) |
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. Doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. Doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. Doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. Doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. Doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. Doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy
Embase
|
No. |
Query |
Results |
|
#18 |
#15 OR #16 OR #17 |
365 |
|
#17 |
#11 AND #14 NOT (#15 OR #16) = observationeel |
76 |
|
#16 |
#11 AND #13 NOT #15 = RCT |
216 |
|
#15 |
#11 AND #12 = SR |
73 |
|
#14 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6318857 |
|
#13 |
('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomized controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it |
2513361 |
|
#12 |
('meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) |
538676 |
|
#11 |
#5 AND #6 AND (#7 OR #8 OR #9 OR #10) AND ((english)/lim OR (dutch)/lim) AND (2000-2021)/py NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) |
503 |
|
#10 |
'ipratropium bromide plus salbutamol'/exp/mj OR 'ipratropium bromide'/exp/mj OR ipratropium:ti,ab,kw OR ipraxa:ti,ab,kw |
4814 |
|
#9 |
'salbutamol'/exp/mj OR salbutamol:ti,ab,kw OR albuterol:ti,ab,kw |
20641 |
|
#8 |
'sodium chloride'/exp/mj OR 'sodium chloride':ti,ab,kw OR saline:ti,ab,kw OR nacl:ti,ab,kw OR sodiumchloride:ti,ab,kw OR 'hypertonic salt':ti,ab,kw |
346639 |
|
#7 |
'nebulization'/exp OR 'nebulizer'/exp/mj OR nebuliz*:ti,ab,kw OR nebuliz*:ti,ab,kw |
21011 |
|
#6 |
'bronchiolitis'/exp OR bronchiolitis:ti,ab,kw OR 'human respiratory syncytial virus'/exp OR 'respiratory syncytial virus infection'/exp OR 'respiratory syncytial virus*':ti,ab,kw OR rsv:ti,ab,kw OR 'rs-virus*':ti,ab,kw |
50230 |
|
#5 |
infan*:ti,ab OR newborn*:ti,ab OR 'new born*':ti,ab OR perinat*:ti,ab OR neonat*:ti,ab OR picu:ti,ab,kw OR nicu:ti,ab,kw OR 'baby'/exp OR baby*:ti,ab OR babies:ti,ab OR toddler*:ti,ab OR 'minors'/exp/mj OR minors*:ti,ab OR 'boy'/exp OR boy*:ti,ab OR girl*:ti,ab OR kid:ti,ab OR kids:ti,ab OR 'child'/exp OR child*:ti,ab OR children*:ti,ab OR pediatric*:ti,ab OR paediatric*:ti,ab OR peadiatric*:ti,ab OR prematur*:ti,ab OR preterm*:ti,ab OR 'pediatrics'/exp OR 'pediatric patient'/exp |
4124588 |
Ovid/Medline
|
# |
Searches |
Results |
|
15 |
12 or 13 or 14 |
271 |
|
14 |
(8 and 11) not (12 or 13) = observationeel |
61 |
|
13 |
(8 and 10) not 12 = RCT |
146 |
|
12 |
8 and 9 = SR |
64 |
|
11 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort*.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ |
3783627 |
|
10 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2264049 |
|
9 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
502650 |
|
8 |
limit 7 to (english language and yr="2000 -Current") |
415 |
|
7 |
1 and 2 and (3 or 4 or 5 or 6) |
584 |
|
6 |
exp Albuterol, Ipratropium Drug Combination/ or exp Ipratropium/ or ipratropium.ti,ab,kf. |
2599 |
|
5 |
exp Albuterol/ or salbutamol.ti,ab,kf. or albuterol.ti,ab,kf. |
13520 |
|
4 |
exp Saline Solution/ or exp "Saline Solution, Hypertonic"/ or 'sodium chloride'.ti,ab,kf. or saline.ti,ab,kf. or NaCl.ti,ab,kf. or sodiumchloride.ti,ab,kf. or 'hypertonic salt'.ti,ab,kf. |
258117 |
|
3 |
exp "Nebulizers and Vaporizers"/ or nebuliz*.ti,ab,kf. or nebuliz*.ti,ab,kf. |
18816 |
|
2 |
exp Bronchiolitis/ or bronchiolitis.ti,ab,kf. or exp "Respiratory Syncytial Virus, Human"/ or exp "Respiratory Syncytial Virus Infections"/ or 'respiratory syncytial virus*'.ti,ab,kf. or RSV.ti,ab,kf. or 'RS-virus*'.ti,ab,kf. |
30282 |
|
1 |
neonates/ or premature infants/ or preterm*.ti,ab. or prematur*.ti,ab. or perinat*.ti,ab. or neonat*.ti,ab. or newborn*.ti,ab. or "new-born".ti,ab. or infant, newborn/ or picu.ti,ab. or nicu.ti,ab. or infant/ or infan*.ti,ab. or toddler*.ti,ab. or baby*.ti,ab. or babies.ti,ab. or pediatrics/ or paediat*.ti,ab. or pediat*.ti,ab. or child/ or child*.ti,ab. or kid.ti,ab. or kids.ti,ab. or boy*.ti,ab. or girl*.ti,ab,kf. or child, preschool/ or preschool*.ti,ab. |
3424613 |
