Tromboseprofylaxe op de Intensive Care bij COVID-19
Uitgangsvraag
Wat is de plaats van tromboseprofylaxe bij COVID-19 patiënten op de intensive care?
Aanbeveling
Geef een standaard profylactische dosis antistolling aan patiënten met COVID-19 op de intensive care.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek verricht naar de verschillen in klinische uitkomsten tussen 1) behandeling met therapeutische dosis antistolling versus standaard of intermediaire dosis tromboseprofylaxe, en 2) behandeling met intermediaire dosis tromboseprofylaxe versus standaard dosis tromboseprofylaxe, bij patiënten met COVID-19 op de intensive care (IC). Tot op heden zijn 3 RCTs gevonden die de vergelijking in de eerste PICO – therapeutische dosis versus profylactische of intermediaire dosis - hebben onderzocht. Er is één studie gevonden, waarvan de resultaten op twee meetmomenten in twee artikelen beschreven werden, die de vergelijking in de tweede PICO – intermediaire dosis versus profylactische dosis - heeft onderzocht. Tabel 1 geeft een overzicht van voorbeelddoseringen voor drie soorten low-molecular-weight heparines (LMWHs).
Tabel 1. Voorbeelddoseringen voor de meest gebruikte LMWHs in Nederland
|
|
Doseringen |
||
|
Nadroparine |
Dalteparine |
Enoxaparine |
|
|
Standaard profylactische dosering |
2850 IU eenmaal daags |
2500 IU eenmaal daags |
20 mg eenmaal daags
|
|
Intermediair profylactische dosering |
5700 IU eenmaal daags (of 2 dd 2850 IU) |
5000 IU eenmaal daags |
40 mg eenmaal daags
|
|
Therapeutische dosering |
11.400 IU per dag voor gewicht tussen 50 en 70 kg; 15.200 IU per dag voor gewicht boven de 70 kg; en 17.100 IU per dag voor gewicht boven de 90 kg |
200 IU per kg per dag met een maximum van 18.000 IU per dag |
1,5 mg per kg per dag of 1,0 mg tweemaal daags |
LMWH, low-molecular-weight heparin; IU, International Unit.
Cruciale uitkomstmaten
Voor de cruciale uitkomstmaten mortaliteit, veneuze trombo-embolie, en het aantal dagen vrij van mechanische ventilatie is het op basis van de gevonden resultaten onzeker of het gebruik van een therapeutische dosis antistolling zou kunnen resulteren in een verschil in deze uitkomstmaten, vergeleken met een profylactische dosis antistolling. Hetzelfde geldt voor de vergelijking tussen een intermediaire dosis en profylactische dosis antistolling. De bewijskrachten voor beide vergelijkingen waren voor deze drie uitkomstmaten zeer laag.
Voor trombo-embolische complicaties samengenomen (arteriële en veneuze trombo-embolie) werd gevonden dat behandeling met een therapeutische dosis antistolling mogelijk kan resulteren in een reductie in trombo-embolische complicaties vergeleken met een profylactische dosis antistolling. De bewijskracht hiervoor was laag. Het is onduidelijk of een intermediaire dosis antistolling zou kunnen leiden tot een verschil in trombo-embolische complicaties vergeleken met een profylactische dosis antistolling. De bewijskracht was zeer laag.
Behandeling met therapeutische dosis antistolling zou kunnen resulteren in geen tot een klein verschil in ernstige bloedingen vergeleken met een profylactische of intermediaire dosis antistolling. De bewijskracht hiervoor was laag. Het is onduidelijk of behandeling met een intermediaire dosis antistolling zou ook kunnen resulteren in een verschil in ernstige bloedingen vergeleken met een profylactische dosis antistolling. De bewijskracht hiervoor was zeer laag.
Belangrijke uitkomstmaten
Voor de belangrijke uitkomstmaat duur van ziekenhuisopname is het onduidelijk of behandeling met een therapeutische dosis antistolling zou kunnen resulteren in een verschil in de duur van ziekenhuisopname vergeleken met een profylactische dosis antistolling. De bewijskracht hiervoor was zeer laag. Er is geen bewijs gevonden voor het effect van een intermediaire dosis antistolling vergeleken met en profylactische dosis antistolling op de duur van ziekenhuisopname. Voor de duur van IC opname is het ook onduidelijk of behandeling met een therapeutische dosis antistolling zou kunnen resulteren in een verschil in de duur van IC opname vergeleken met een profylactische dosis antistolling. Hetzelfde geldt voor de vergelijking tussen een intermediaire dosis antistolling vergeleken met en profylactische dosis antistolling. De bewijskracht voor beide vergelijkingen was zeer laag.
Er is geen bewijs gevonden voor het effect van een therapeutische dosis antistolling vergeleken met een profylactische dosis antistolling op orgaan support anders dan mechanische ventilatie. Het is onduidelijk of behandeling met een intermediaire dosis antistolling vergeleken met een profylactische dosis antistolling zou kunnen resulteren in een verschil in orgaan support anders dan mechanische ventilatie, meer specifiek nier vervangende therapie. De bewijskracht hiervoor was zeer laag.
Er is geen bewijs gevonden voor het effect op het aantal dagen vrij van orgaan ondersteuning anders dan mechanische ventilatie voor de vergelijking tussen 1) een therapeutische dosis antistolling vergeleken met een profylactische dosis antistolling, en 2) een intermediaire dosis antistolling vergeleken met een profylactische dosis antistolling. Voor de uitkomstmaten heparine geïnduceerde trombocytopenie en cumulatieve transfusie zijn geen resultaten beschreven in de geselecteerde studies.
Interpretatie
De interpretatie van de studies is om meerdere redenen complex. De INSPIRATION studie komt qua populatie niet overeen met de Nederlandse populatie op de Intensive Care Unit (ICU), gezien het relatief lage percentage invasieve beademing (dat samengenomen met non-invasieve beademing rond de 50% lag) en de lage APACHE II score van de geïncludeerde patiënten (mediaan 8, IQR 5-11). In de multiplatform studie van Goligher (2021) is dit percentage ook laag, maar daar kregen patiënten high flow nasal oxygen of non invasive ventilation en slechts 1,5% van de patiënten zuurstof via masker of low flow nasal oxygen. Ook was de APACHE II score beduidend hoger (mediaan 14, IQR 8-21), hetgeen meer in lijn is met de Nederlandse situatie op de ICU. De studie van Lemos (2020) uit Brazilië met tweemaal 10 patiënten is heel klein en daarom niet representatief. Met betrekking tot bepaalde subgroepen, zoals leeftijd of D-dimeer niveau bij presentatie, kon geen onderscheid worden gevonden in de analyse van de studies. Daarom konden geen aanbevelingen worden gedaan voor deze subgroepen. Alle cruciale uitkomsten waren secundaire uitkomsten in de studies. De studies hadden samengestelde primaire eindpunten die onderling onvergelijkbaar bleken. Zo werden bijvoorbeeld non-invasieve en invasieve beademing samen genomen, of sterfte en trombotische complicaties. Door deze verschillende samengestelde uitkomstmaten kon deze data niet gepoold worden. Een ander belangrijk punt van overweging is het feit dat de behandeling van patiënten met COVID-19 in 2021 in Nederland veranderd is ten opzichte van 2020, het jaar waarin de studies zijn verricht. Zo is er nu standaardbehandeling met IL-6 remmers en monoklonale antistoffen. In verschillende van de gevonden studies kreeg een substantieel deel van de patiënten bijvoorbeeld geen behandeling met steroïden en werd slechts een kleine minderheid behandeld met IL-6 remmers. Alle studies hadden een zogenaamd ‘open label design’, waardoor bias kan zijn opgetreden bij zachtere uitkomstmaten als veneuze trombo-embolie en bloeding. De artsen wisten welke behandeling een patiënt kreeg, hetgeen de klinische verdenking en diagnostische strategie heeft kunnen beïnvloeden. Eindpunten werden slechts deels centraal of lokaal geadjudiceerd, waardoor de validiteit van de diagnose longembolie (overgrote meerderheid van de trombotische events) niet in alle studies is na te gaan. De inclusiecriteria tussen de studies waren ook erg wisselend, met soms -maar niet altijd - selectie van patiënten met hoge tot zeer hoge D-dimeerwaarden. Patiënten met een van tevoren ingeschat hoog bloedingsrisico werden uitgesloten. De incidentie van bloedingscomplicaties zou daarom bij toepassing van therapeutische antistolling in de dagelijkse praktijk hoger kunnen uitvallen. Naast beademing was er voor andere orgaanondersteuning (zoals nierfunctievervangende therapie en extracorporele membraanoxygenatie (ECMO)) alleen literatuur beschikbaar met een zeer lage bewijskracht. Wat betreft veiligheid was er alleen rapportage van bloedingen en niet van cumulatieve transfusie, en kan er niet uitgesloten worden dat er een onderrapportage is geweest van milde bloedingscomplicaties. Ook is er geen data gerapporteerd over het voorkomen van van heparine geïnduceerde trombocytopenie (HIT). Dit alles maakt dat er nog kennislacunes zijn. Het zal moeten blijken of resultaten van nieuwe studies de conclusies van de samenvatting van de literatuur zullen veranderen.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Het doel van toedienen van antistolling is het voorkómen van veneuze en, in mindere mate, arteriële trombose. Er is geen verschil in toediening of impact van de profylactische of intermediaire versus de therapeutische dosis LMWH; beide worden op dezelfde manier subcutaan geïnjecteerd. Er zijn geen subgroepen met andere uitkomsten gevonden.
Patiënten die een behandeling met medicijnen voor antistolling krijgen vinden complete en eenduidige informatievoorziening belangrijk, o.a. over de indicatie en de veiligheid en risico’s van de behandeling (onderwerpen die van belang zijn in de communicatie met patiënten zijn terug te vinden in de Landelijke Transmurale Afspraak antistollingszorg (https://lta-antistollingszorg.nl/communicatie-met-patienten).
Kosten (middelenbeslag)
Er is geen doorslaggevend verschil in de kosten voor de profylactisch of therapeutisch gedoseerde LMWH of ongefractioneerde heparine.
Aanvaardbaarheid, haalbaarheid en implementatie
Door de opzet en uitkomsten van de onderzochte studies is geen definitief bewijs gevonden voor de cruciale uitkomsten. Het was volgens de werkgroep onzeker of het gebruik van therapeutische of intermediaire dosis antistolling tot een klinisch relevant verschil in cruciale uitkomsten zou leiden.
Rationale van de aanbeveling
De individuele studies rapporteerden vaak gecombineerde uitkomstmaten, waarbij (niet-invasieve) beademing, IC opnameduur, trombotische complicaties, ECMO, nierfunctievervangende therapie en sterfte in verschillende combinaties waren samengenomen. Bij de analyses van de individuele eindpunten bleek het voordeel van therapeutische antistolling onzeker te zijn of onder de van tevoren vastgestelde grens van klinische relevantie te liggen ten opzichte van een profylactische of intermediaire dosis antistolling. Ook bleek het voordeel van een intermediaire dosis antistolling onzeker te zijn of onder de van tevoren vastgestelde grens van klinische relevantie te liggen ten opzichte van een profylactische dosis antistolling. Eerder waren voor de Nederlandse situatie geen grenzen van klinische relevantie vastgesteld voor het instellen van tromboseprofylaxe. De werkgroep heeft zich bij het vaststellen van die grenzen geconformeerd aan de grenzen voor sterfte en IC opname, zoals vastgesteld door de werkgroep voor medicamenteuze behandeling van COVID-19. De grens voor een klinisch relevant verschil in trombotische complicaties werd indirect afgeleid uit de ACCP richtlijn tromboseprofylaxe uit 2012. Uit de onderzochte studies bleek ook dat therapeutische antistolling niet klinisch relevant meer schade berokkende dan een profylactische of intermediaire dosis: het optreden van bloedingen is mogelijk niet klinisch relevant verschillend tussen de groepen. De werkgroep kwam tot de conclusie dat er op grond van de onderzochte studies onvoldoende grond is om een therapeutische of een intermediair profylactische dosis antistolling te adviseren boven een standaard profylactische dosering antistolling voor patiënten met COVID-19 op de intensive care.
Onderbouwing
Achtergrond
Ondanks tromboseprofylaxe en een verbeterde behandeling van COVID-19 komen trombotische complicaties nog frequent voor, met een geschatte incidentie van 23-28% in ICU patiënten en 7-9% in afdelingspatiënten (Jiménez, 2021; Tan, 2021; Nopp, 2021). Het is niet bekend wat de beste dosis van tromboseprofylaxe is (laag, intermediair of therapeutisch). Mede hierdoor verschillen ziekenhuisprotocollen.
Conclusies / Summary of Findings
1. Mortality
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with therapeutic dose of anticoagulation on mortality when compared to standard (or intermediate) dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Sources: Goligher, 2021; Lemos, 2020. |
Intermediate dose versus standard dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with intermediate dose of prophylactic anticoagulation on mortality when compared to standard dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Source: Sadeghipour, 2021. |
2. Length of hospital or ICU stay
Length of hospital stay
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with therapeutic dose of anticoagulation on length of hospital stay when compared to standard (or intermediate) dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Sources: Lemos, 2020. |
Intermediate dose versus standard dose of prophylactic anticoagulation
|
- GRADE |
No studies were found that could answer the question what the effect is of intermediate dose of prophylactic anticoagulation when compared to standard dose of prophylactic anticoagulation on length of hospital stay in adult COVID-19 patients admitted to the ICU.
|
Length of ICU stay
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with therapeutic dose of anticoagulation on length of ICU stay when compared to standard (or intermediate) dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Sources: Lemos, 2020. |
Intermediate dose versus standard dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with intermediate dose of prophylactic anticoagulation on length of ICU stay when compared to standard dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Source: Sadeghipour, 2021. |
3. Organ support
Ventilator-free days
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with therapeutic dose of anticoagulation on ventilator-free days when compared to standard (or intermediate) dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Sources: Lemos, 2020. |
Intermediate dose versus standard dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with intermediate dose of prophylactic anticoagulation on ventilator-free days when compared to standard dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Source: Sadeghipour, 2021. |
Other organ support free days
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
- GRADE |
No studies were found that could answer the question what the effect is of therapeutic dose of anticoagulation when compared to standard (or intermediate) dose of prophylactic anticoagulation on other organ support free days in adult COVID-19 patients admitted to the ICU.
|
Intermediate dose versus standard dose of prophylactic anticoagulation
|
- GRADE |
No studies were found that could answer the question what the effect is of intermediate dose of prophylactic anticoagulation when compared to standard dose of prophylactic anticoagulation on other organ support free days in adult COVID-19 patients admitted to the ICU.
|
Organ support other than mechanical ventilation
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
- GRADE |
No studies were found that could answer the question what the effect is of therapeutic dose of anticoagulation when compared to standard prophylactic anticoagulation on organ support other than mechanical ventilation in adult COVID-19 patients admitted to the ICU.
|
Intermediate dose versus standard dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with intermediate dose of prophylactic anticoagulation on organ support other than mechanical ventilation when compared to standard dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Source: Sadeghipour, 2021. |
4. Venous thromboembolism
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with therapeutic dose of anticoagulation on venous thromboembolism when compared to standard (or intermediate) dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Source: Goligher, 2021. |
Intermediate dose versus standard dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with intermediate dose of prophylactic anticoagulation on venous thromboembolism when compared to standard dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Source: Sadeghipour, 2021. |
Thromboembolic complications (VTE/ATE)
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
Low GRADE |
Treatment with therapeutic dose of anticoagulation may result in a decrease in thromboembolic complications (VTE/ATE) when compared to standard (or intermediate) dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Sources: Goligher, 2021. |
Intermediate dose versus standard dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with intermediate dose of prophylactic anticoagulation on thromboembolic complications (VTE/ATE) when compared to standard dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Source: Sadeghipour, 2021. |
5. Major bleeding
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
Low GRADE |
Treatment with therapeutic dose of anticoagulation may result in little to no difference in major bleedings when compared to standard (or intermediate) dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Sources: Goligher, 2021; Lemos, 2020; Spyropoulos, 2021. |
Intermediate dose versus standard dose of prophylactic anticoagulation
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with intermediate dose of prophylactic anticoagulation on major bleedings when compared to standard dose of prophylactic anticoagulation in adult COVID-19 patients admitted to the ICU.
Source: Sadeghipour, 2021. |
6. Heparin induced thrombocytopenia (HIT)
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
- GRADE |
No studies were found that could answer the question what the effect is of therapeutic dose of anticoagulation when compared to standard (or intermediate) dose of prophylactic anticoagulation on HIT in adult COVID-19 patients admitted to the ICU.
|
Intermediate dose versus standard dose of prophylactic anticoagulation
|
- GRADE |
No studies were found that could answer the question what the effect is of intermediate dose of prophylactic anticoagulation when compared to standard dose of prophylactic anticoagulation on HIT in adult COVID-19 patients admitted to the ICU.
|
7.Cumulative transfusion
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
|
- GRADE |
No studies were found that could answer the question what the effect is of therapeutic dose of anticoagulation when compared to standard prophylactic (or intermediate) dose of anticoagulation on cumulative transfusion in adult COVID-19 patients admitted to the ICU.
|
Intermediate dose versus standard dose of prophylactic anticoagulation
|
- GRADE |
No studies were found that could answer the question what the effect is of intermediate dose of prophylactic anticoagulation when compared to standard dose of prophylactic anticoagulation on cumulative transfusion in adult COVID-19 patients admitted to the ICU.
|
Samenvatting literatuur
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Spyropoulos (2021) describes a multicenter open label randomized clinical trial evaluating the effects of therapeutic-dose low-molecular-weight heparin vs institutional standard or intermediate prophylactic dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19. Patients were enrolled from March 8, 2020, through May 14, 2021, at 12 centers in the US. A total of 11649 patients were assessed for eligibility. Eligible patients consisted of hospitalized nonpregnant adults 18 years or older with COVID-19 diagnosed by nasal swab or serologic testing. Moreover, there was a requirement for supplemental oxygen per investigator judgment and a plasma D-dimer level greater than 4 times the upper limit of normal based on local laboratory criteria or a sepsis-induced coagulopathy score of 4 or greater. 257 patients were randomized into the therapeutic heparin dose group (n= 130) or standard/intermediate prophylactic heparin dose group (n = 127). 45 out of 129 patients in the therapeutic dose group were admitted to the ICU, versus 38 out of 124 in the standard prophylactic dose group. Treatment began after randomization and was stopped at hospital discharge or upon occurrence of a primary efficacy outcome, key secondary outcome, or principal safety outcome requiring study drug discontinuation. All patients without a primary or key secondary outcome event underwent lower extremity Doppler compression ultrasonography at hospital day 10 + 4 or at discharge if sooner. The length of follow-up was 30 +2 days after randomization. For the primary efficacy outcome (venous thromboembolism, arterial thromboembolism or death) and major bleeding, patients were stratified based on intensive care unit (ICU) or non-ICU status. For all other outcomes, no stratification was performed. Patients in the therapeutic dose group had a mean age of 65.8 years (SD 13.9) versus 67.7 years (SD 14.1) in the standard prophylactic dose group and the small majority was male (52.7% in the intervention versus 54.8% in the control group). The study groups were comparable with respect to baseline characteristics.
Goligher (2021) describes an open-label, adaptive, multiplatform RCT (mpRCT). In this mpRCT, three platforms (REMAPCAP, ATTACC and ACTIV-4a) were integrated to evaluate therapeutic dose anticoagulation versus usual-care pharmacologic thromboprophylaxis in critically ill patients with COVID-19. Patients were recruited from different countries, e.g. the United Kingdom, United States, Canada, Brazil. Patients were included in the mpRCT if they had a laboratory confirmed COVID-19 infection, they had severe COVID-19 (which was defined as COVID-19 that led to receipt of ICU-level respiratory or cardiovascular organ support in an ICU). A total of 1207 patients underwent randomization at 393 sites in 10 countries, until the pre-specified criterion for futility had been met. Patients were randomized to receive therapeutic dose anticoagulation with heparin (n=591) or usual-case thromboprophylaxis (n=616). After exclusion, 534 patients in the therapeutic dose group and 564 patients in the usual-care group were included in the primary analysis. The mean age of patients in the therapeutic dose group was 60.4 years (SD 13.1) versus 61.7 years (SD 12.5) in the usual-care group. The majority in both groups was male: 387 patients (72.2%) in the therapeutic dose group, versus 385 patients (67.9%) in the usual-care group. The study groups were comparable with respect to baseline characteristics.
Lemos (2020) describes a randomized, controlled, open-label, single-center, phase II study conducted in Brazil, evaluating therapeutic dose enoxaparin versus standard anticoagulant thromboprophylaxis in COVID-19 patients requiring mechanical ventilation. Adult patients (>18 years of age) were included in this study if they suffered from respiratory failure requiring mechanical ventilation and laboratory confirmed SARS-CoV-2 infection. Twenty patients were randomized to receive therapeutic enoxaparin (n=10) or prophylactic anticoagulation (UFH and LMWH, n=10). All patients who were randomized were included in the primary analysis. The mean age of patients in the therapeutic dose group was 55 years (SD 10) versus 58 years (SD 16) in the prophylactic dose group. The majority was male: nine out of ten patients in the therapeutic dose group, versus seven out of ten patients in the prophylactic dose group. The study groups were comparable with respect to baseline characteristics.
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) describes a multicenter open-label randomized clinical trial (INSPIRATION trial) comparing intermediate versus standard dose of prophylactic anticoagulation in adult patients with COVID-19 admitted to the ICU. Patients were recruited between July 29, 2020, and November 19, 2020, conducted in Iran at 10 academic centers in Tehran and Tabriz. Patients were eligible if they were admitted to the ICU with polymerase chain reaction testing–confirmed COVID-19 within 7 days of the index hospitalization. Patients were excluded when their life expectancy was less than 24 hours. Also, patients with an established indication for therapeutic-dose anticoagulation, weight less than 40 kg, pregnancy, history of heparin-induced thrombocytopenia, platelet count less than 50 ×103 /μL, or overt bleeding were excluded. A total of 600 patients were randomized into the intermediate prophylactic dose group (n=299) or the standard dose prophylactic anticoagulation group (n=299). The primary anticoagulant agent in both groups was enoxaparin. In case a patient had severe kidney insufficiency, an unfractionated heparin was used. Treatments were continued until 30 days of follow-up, irrespective of hospital discharge status. Ultimately, 562 patients were included in the prespecified primary analysis. The median age of patients in the intermediate prophylactic dose group (n=276) was 62 years (IQR 51 to 70.7), versus 61 years (IQR 47 to 71) in the standard prophylactic dose group (n=286). The majority in both groups was male: 162 patients (58.7%) in the intermediate prophylactic dose group, versus 163 patients (57.0%) in the standard prophylactic dose group. Except for history of cigarette smoking, which was more frequent in the intermediate prophylactic dose group, the study groups were comparable with respect to baseline characteristics.
Bikdeli (2021) reports the final 90-day follow-up results of the INSPIRATION trial by Sadeghipour (2021). Therefore, the study design and included patients were the same as described above. The number of patients included in the primary analysis were not altered (n=276 in the intermediate prophylactic dose group versus n=286 in the standard prophylactic dose group). These articles are therefore taken together for the literature analysis.
Table 2. Overview of included RCTs that compared therapeutic dose anticoagulation with standard (or intermediate) dose anticoagulation and intermediate dose anticoagulation with standard dose anticoagulation in COVID-19 patients admitted to the ICU
|
Author, year and trial name |
Intervention (I) and control (C) |
Sample size for analysis |
Doses |
Duration |
|
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation |
||||
|
Spyropoulos, 2021
HEP-COVID Randomized Clinical Trial |
I: therapeutic-dose low-molecular-weight heparin (enoxaparin)
C: institutional standard prophylactic or intermediate-dose heparins for thromboprophylaxis
|
I: N= 45 ICU admitted patients C: N= 38 ICU admitted patients Total = 83
|
I: 1 mg/kg subcutaneously twice daily if CrCl was 30 mL/min/1.73 m2 or greater or 0.5 mg/kg twice daily if CrCl was 15-29 mL/min/ 1.73 m2
C: could include unfractionated heparin (UFH), up to 22 500 IU subcutaneously (divided twice or thrice daily); enoxaparin, 30 mg or 40 mg subcutaneously once or twice daily (weight based enoxaparin 0.5 mg/kg subcutaneously twice daily was permitted but strongly discouraged); or dalteparin, 2500 IU or 5000 IU subcutaneously daily |
Study drug was administered for the duration of hospitalization, including patient transfers to ICU settings. |
|
Goligher, 2021
The REMAP-CAP, ACTIV-4a, and ATTACC Investigators |
I: therapeutic-dose anticoagulation with heparin
C: pharmacologic thromboprophylaxis in accordance with local usual care |
I: N= 534 C: N= 564 Total = 1098
|
I: Therapeutic-dose anticoagulation was administered according to local site protocols for the treatment of acute venous thromboembolism
C: Usual-care thromboprophylaxis was administered at a dose and duration determined by the treating clinician according to local practice, which included either standard low-dose thromboprophylaxis or enhanced intermediate-dose thromboprophylaxis.
The anticoagulation and thromboprophylaxis regimens that were specified by each platform are detailed in the Supplementary Appendix (page 49-51 and Table S1 at page 65). |
I: Up to 14 days or until recovery (defined as either hospital discharge or discontinuation of supplemental oxygen for at least 24 hours).
C: Up to 14 days or hospital discharge, whichever comes first. After this period, decisions regarding thromboprophylaxis are at discretion of treating clinician
The trial was stopped when the prespecified criterion for futility was met for therapeutic-dose anticoagulation. |
|
Lemos, 2020
HESACOVID trial |
I: therapeutic enoxaparin
C: standard anticoagulant thromboprophylaxis |
I: N= 10 C: N= 10 Total = 20 |
I: subcutaneous (SC) enoxaparin with the dose according to age and adjusted daily by the creatinine clearance (CrCl) estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. See article for exact doses per age group.
C: subcutaneous unfractionated heparin (UFH) at a dose of 5000 IU TID (if weight < 120 kg) and 7500 IU TID (if weight > 120 kg) or enoxaparin at a dose of 40 mg OD (if weight < 120 kg) and 40 mg BID (if weight > 120 Kg) according to the doctor's judgment. |
I: The median of the therapeutic enoxaparin treatment duration was 14 days, ranging from 9 to 14 days
C: ? |
|
Intermediate dose versus standard dose of prophylactic anticoagulation |
||||
|
INSPIRATION trial
Sadeghipour, 2021 (initial study)
Bikdeli, 2021 (extension of initial study/follow-up measurements at day 90) |
I: intermediate-dose enoxaparin
C: standard prophylactic anticoagulation enoxaparin |
I: N= 276 C: N= 286 Total = 562
|
I: 1 mg/kg daily, with modification according to body weight and creatinine clearance.
C: 40 mg daily, with modification according to body weight and creatinine clearance. |
Irrespectively of hospital discharge status, the assigned treatments were planned to be continued until the 30-day follow-up (Sadeghipour, 2021) or 90-day follow-up (Bikdeli, 2021).
|
Table 3. Overview of composite outcomes and results per study
|
Study |
Primary composite outcome |
Results |
|
INSPIRATION trial
|
Composite of adjudicated acute venous thromboembolism, arterial thrombosis, treatment with extracorporeal membrane oxygenation, or all-cause mortality. |
|
|
Goligher, 2021 Multiplatform trial |
Median number of days free of cardiovascular or respiratory organ support. This was evaluated on an ordinal scale that combined in-hospital death and the number of organ support free days up to day 21 among patients who survived to hospital discharge. If a patient died, they were assigned a value of -1.
|
In the therapeutic dose group, the median number of organ support-free days was 1 day (IQR -1 to 16), versus 4 days (IQR -1 to 16) in the standard prophylactic dose group. |
|
Spyropoulos, 2021 HEP-COVID trial |
Composite outcome: VTE, ATE, or death Measured in the ICU-stratum |
Therapeutic dose group: 23 out of 45 (51.1%) patients Standard prophylactic dose group: 21 out of 38 (55.3%) patients |
Results
1. Mortality
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Goligher (2021) reported death in hospital. A total of 199 out of 534 (37.3%) patients died in the therapeutic dose group, versus 200 out of 564 (35.5%) in the standard prophylactic dose group. The risk difference (RD) was 1.8% in favor of the standard prophylactic dose group (95%CI -3.9% to 7.5%). The corresponding NNT was 56. This was not considered to be a clinically relevant difference.
Lemos (2020) reported in-hospital mortality. A total of 2 out of 10 (20.0%) patients died in the therapeutic dose group, versus 5 out of 10 (50.0%) in the standard prophylactic dose group. The RD was 30.0% in favor of the therapeutic dose group (95%CI -69.7% to 9.7%). The corresponding NNT was 3. This was considered to be a clinically relevant difference.
Level of evidence
The level of evidence regarding the outcome measure mortality was downgraded from high to very low because in Goligher (2021) some patients are missing from the analyses and it is not clear why, and many patients were excluded after randomization in both groups (risk of bias, -1), and the confidence interval around the RD crossing the upper and lower thresholds for clinical relevance (imprecision, -2). Lemos (2020) was given few weight in the determination of the level of evidence, because of the very small study population.
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) reported all-cause mortality at day 30. In the intermediate prophylactic dose group, 119 out of 276 (43.1%) patients died, versus 117 out of 286 (40.9%) patients in the standard prophylactic dose group. The RD was 2.2% in favor of the standard prophylactic dose group (95%CI -6.0% to 10.4%). The corresponding NNT was 45. This was not considered to be a clinically relevant difference.
At day 90, Bikdeli (2021) reported that 127 out of 276 (46.0%) patients died, versus 123 out of 286 (43.0%) patients in the standard prophylactic dose group.
Level of evidence
The level of evidence regarding the outcome measure mortality was downgraded from high to very low because of the study population differing from what was defined in the PICO (indirectness, -1), the confidence interval around the RD crossing the upper and lower thresholds for clinical relevance (imprecision, -2). The level of evidence is based on the results reported in Sadeghipour (2021).
2. Length of hospital or ICU stay
Length of hospital stay
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Lemos (2020) reported the median length of hospital stay. The median length of hospital stay was 31 days (IQR 22 to 35) in the therapeutic dose group, versus 30 days (IQR 23 to 38) in the standard prophylactic dose group. The difference in median days was 1 day. This was not considered to be a clinically relevant difference.
Goligher (2021) did not report length of hospital stay.
Level of evidence
The level of evidence regarding the outcome measure length of hospital stay was downgraded from high to very low because of the small sample size and wide interquartile range (imprecision, -3).
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) and Bikdeli (2021) did not report length of hospital stay.
Length of ICU stay
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Lemos (2020) also reported the median number of ICU-free days. This was 12 days (IQR 2 to 12) in the therapeutic dose group, versus 0 days (IQR 0 to 10) in the standard prophylactic dose group.
Goligher (2021) did not report length of ICU stay.
Level of evidence
The level of evidence regarding the outcome measure length of ICU stay was downgraded from high to very low because of the outcome measure not being the same as defined in the PICO (indirectness, -1), and the small sample size and wide interquartile range (imprecision, -2).
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) reported the median length of ICU stay. The median length of ICU stay for the intermediate prophylactic dose group was 5 days (IQR 2 to 10), versus 6 days (IQR 3 to 11) in the standard prophylactic dose group. The difference in median days was 1 day. This was not considered to be a clinically relevant difference.
Sadeghipour (2021) also reported the number of patients discharged from the ICU. The number of patients discharged from the ICU in the intermediate prophylactic dose group was 169 out of 276 (61.2%), versus 174 out of 286 (60.8%) in the standard prophylactic dose group.
Bikdeli (2021) did not report length of ICU stay at day 90.
Level of evidence
The level of evidence regarding the outcome measure length of ICU stay was downgraded from high to very low because of the study population differing from what was defined in the PICO (indirectness, -1), and the wide interquartile range in both groups (imprecision, -2).
3. Organ support free days
Ventilator-free days
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Lemos (2020) reported the median number of ventilator-free days. In the therapeutic dose group, the median number of ventilator-free days was 15 days (IQR 6 to 16), versus 0 days (IQR 0 to 11) in the standard prophylactic dose group.
Goligher (2021) did not report ventilator-free days.
Level of evidence
The level of evidence regarding the outcome measure ventilator-free days was downgraded from high to very low because of the small sample size and the wide interquartile range in both groups (imprecision, -3).
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) reported the median number of ventilator-free days. In the intermediate prophylactic dose group, the median number of ventilator-free days was 30 days (IQR 3 to 30), versus 30 days (IQR 1 to 30) in the standard prophylactic dose group. The difference in median days was zero days. This was not considered to be a clinically relevant difference.
Bikdeli (2021) did not report ventilator-free days at day 90.
Level of evidence
The level of evidence regarding the outcome measure ventilator-free days was downgraded from high to very low because of the study population differing from what was defined in the PICO (indirectness, -1), and the wide interquartile range in both groups (imprecision, -2).
Other organ support free days
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Goligher (2021) and Lemos (2020) did not report on other organ support free days.
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) and Bikdeli (2021) did not report on other organ support free days.
Organ support other than mechanical ventilation
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Goligher (2021) and Lemos (2020) did not report on other organ support than mechanical ventilation.
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) and Bikdeli (2021) reported extracorporeal membrane oxygenation (ECMO) as part of the primary composite outcome (see Table 2), but not on its own. In addition, Sadeghipour (2021) reported new in-hospital kidney replacement therapy. In the intermediate prophylactic dose group, 10 out of 276 (3.6%) patients needed kidney replacement therapy, versus 7 out of 286 (2.4%) patients in the standard prophylactic dose group. The difference was 1.2% in favor of the standard prophylactic dose group (95%CI -1.7% to 4.0%). This was not considered to be a clinically relevant difference. Bikdeli (2021) reported the same numbers at day 90.
Level of evidence
The level of evidence regarding the outcome measure organ support other than mechanical ventilation was downgraded from high to very low because of the study population differing from what was defined in the PICO (indirectness, -1), and the low number of events (imprecision, -2).
4. Venous thromboembolism
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Goligher (2021) and Lemos (2020) did not report the number of patients with VTE.
However, Goligher (2021) did report the number of events for deep venous thrombosis and pulmonary embolism separately. In the therapeutic dose group 6 events for deep venous thrombosis were reported, versus 6 events in the standard prophylactic dose group. For pulmonary embolism, 13 events were reported in the therapeutic dose group, versus 42 events in the standard prophylactic dose group.
Level of evidence
The level of evidence regarding the outcome measure venous thromboembolism was downgraded from high to very low because in Goligher (2021) some patients are missing from the analyses and it is not clear why, and many patients were excluded after randomization in both groups (risk of bias, -1), indirectness in the outcome measure (indirectness, -1), the low number of events (imprecision, -1).
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) reported adjudicated venous thromboembolism at day 30. In the intermediate prophylactic dose group 9 out of 276 patients (3.3%) developed adjudicated venous thromboembolism, versus 10 out of 286 (3.5%) in the standard prophylactic dose group. The RD was 0.24% in favor of the intermediate prophylactic dose group (95%CI -3.2% to 2.8%). The NNT was 417. This was not considered to be a clinically relevant difference.
Bikdeli (2021) reported the same outcome in the same patients at day 90. No differences occurred in the incidence between day 30 and 90.
Level of evidence
The level of evidence regarding the outcome measure venous thromboembolism was downgraded from high to very low because of the open-label study design (risk of bias, -1), the study population differing from what was defined in the PICO (indirectness, -1), and the confidence interval around the RD crossing the lower threshold for clinical relevance (imprecision, -1).
Thromboembolic complications (VTE/ATE)
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Goligher (2021) reported the number of patients with any thrombotic event (defined as pulmonary embolism, myocardial infarction, ischemic cerebrovascular event, systemic arterial thromboembolism): 38 out of 530 patients (7.2%) experienced a thrombotic event in the therapeutic dose group, versus 62 out of 559 patients (11.1%) in the standard prophylactic dose group. The RD was 3.9% in favor of the therapeutic dose group (95%CI -7.3% to 0.5%). The corresponding NNT was 26. This was considered to be a clinically relevant difference.
Lemos (2020) reported the number of patients with thrombotic events: 2 out of 10 (2.0%) patients experienced a thrombotic event in the therapeutic dose group, versus 2 out of 10 (2.0%) patients in the standard prophylactic dose group. The RD was 0.0% (95%CI -35.1% to 35.1%). This was not considered to be a clinically relevant difference.
Level of evidence
The level of evidence regarding the outcome measure thromboembolic complications was downgraded from high to low because in Goligher (2021) some patients are missing from the analyses and it is not clear why, and many patients were excluded after randomization in both groups (risk of bias, -1), and the confidence interval around the RD crossing the lower threshold for clinical relevance (imprecision, -1). Lemos (2020) was given no weight in the determination of the level of evidence, because of the very small study population.
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) and Bikdeli (2021) did not report the number of patients with thromboembolic events (VTE/ATE).
However, Sadeghipour (2021) reported the number of patients with acute peripheral arterial thrombosis, ischemic stroke, and type I acute myocardial infarction, all three objectively clinically diagnosed. None of the 276 patients in the intermediate prophylactic dose group and none of the 286 patients in the standard prophylactic dose group were diagnosed with acute peripheral arterial thrombosis and/or type I acute myocardial infarction. In the intermediate prophylactic dose group, 1 out of 276 (0.4%) patients developed ischemic stroke, versus 1 out of 286 (0.3%) patients in the standard prophylactic dose group.
Level of evidence
The level of evidence regarding the outcome measure thromboembolic complications was downgraded from high to very low because of the open-label study design (risk of bias, -1), indirectness in the outcome measure and study population (indirectness, -1), and the low number of events (imprecision, -1).
5. Major bleeding
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Figure 1: Major bleeding in COVID-19 patients admitted to the ICU
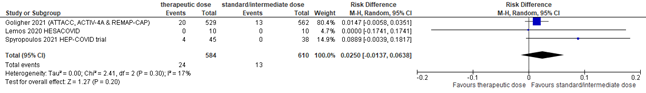
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval
A total of 24 out of 584 (4.1%) patients developed a major bleeding in the therapeutic dose group, versus 13 out of 610 (2.1%) in the standard/intermediate prophylactic dose group. The pooled risk difference (RD) was 2.5% in favor of the standard/intermediate prophylactic dose group (95%CI -1.4% to 6.4%; figure 1). The corresponding NNH was 40. This was not considered to be a clinically relevant difference.
Level of evidence
The level of evidence regarding the outcome measure major bleeding was downgraded from high to low because in Goligher (2021) some patients are missing from the analyses and it is not clear why, and many patients were excluded after randomization in both groups (risk of bias, -1), and the confidence interval around the RD crossing the upper threshold for clinical relevance (imprecision, -1).
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) reported major bleeding. In the intermediate prophylactic dose group 7 out of 276 (2.5%) patients developed a major bleeding, versus 4 out of 286 (1.4%) patients in the standard prophylactic dose group. The RD was 1.14% in favor of the standard prophylactic dose group (95%CI -1.16% to 3.44%). The corresponding NNH was 88. This was not considered to be a clinically relevant difference. Bikdeli (2021) reports the same numbers at day 90.
Level of evidence
The level of evidence regarding the outcome measure major bleeding was downgraded from high to very low because of the open-label study designs (risk of bias, -1), the study population differing from what was defined in the PICO (indirectness, -1), and the confidence interval around the RD crossing the upper threshold for clinical relevance (imprecision, -1).
6. Heparin induced thrombocytopenia (HIT)
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Goligher (2021) and Lemos (2020) did not report HIT.
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) and Bikdeli (2021) did not report HIT.
7. Cumulative transfusion
Therapeutic dose versus standard (or intermediate) dose of prophylactic anticoagulation
Goligher (2021) and Lemos (2020) did not report cumulative transfusion.
Intermediate dose versus standard dose of prophylactic anticoagulation
Sadeghipour (2021) and Bikdeli (2021) did not report cumulative transfusion.
However, Sadeghipour (2021) reported a sub-category of major bleeding: ‘BARC Type 3a- hemoglobin drop of 3 to 5 g/dL or any transfusion’. In the intermediate prophylactic dose group, 3 out of 276 (1.1%) patients reported this outcome, versus 4 out of 286 (1.4%) patients in the standard prophylactic dose group. Bikdeli (2021) reported the same numbers at day 90.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the efficacy and safety of anticoagulation therapy in COVID-19 patients at the ICU?
PICO 1
P: all adult COVID-19 patients admitted to the ICU who are not already on chronic therapeutic anticoagulants
I: therapeutic dose (low molecular weight heparin, unfractionated heparin, direct oral anticoagulants, vitamin K-antagonists, aspirin)
C: standard or intermediate prophylactic dose (low molecular weight heparin, unfractionated heparin, direct oral anticoagulants, vitamin K-antagonists, aspirin) or no use of standard or intermediate prophylactic dose (low molecular weight heparin, unfractionated heparin, direct oral anticoagulants, vitamin K-antagonists, aspirin)
O: mortality, major bleeding, venous thromboembolism, thromboembolic complications, length of hospital or ICU stay, organ support (ventilator-free days, other organ support free days, and organ support other than mechanical ventilation), heparin induced thrombocytopenia, and cumulative transfusion
PICO 2
P: all adult COVID-19 patients admitted to the ICU who are not already on chronic therapeutic anticoagulants
I: intermediate prophylactic dose (low molecular weight heparin, unfractionated heparin, direct oral anticoagulants, vitamin K-antagonists, aspirin)
C: standard prophylactic dose (low molecular weight heparin, unfractionated heparin, direct oral anticoagulants, vitamin K-antagonists, aspirin) or no use of standard or intermediate prophylactic dose (low molecular weight heparin, unfractionated heparin, direct oral anticoagulants, vitamin K-antagonists, aspirin)
O: mortality, major bleeding, venous thromboembolism, thromboembolic complications, length of hospital or ICU stay, organ support (ventilator-free days, other organ support free days, and organ support other than mechanical ventilation), heparin induced thrombocytopenia, and cumulative transfusion
We searched for standard dose of prophylaxis and intermediate dose of prophylaxis; the latter is typically a doubling of the standard dose of prophylaxis. We also searched for a therapeutic dose of prophylaxis (intensive dose of prophylaxis; referred to as therapeutic dose). For aspirin only one dose (80-100 mg per day) was searched.
Relevant outcome measures
The guideline development group considered mortality, ventilator-free days, venous thromboembolism, thromboembolic complications (venous and arterial thrombotic complications combined) and major bleeding as critical outcome measures for decision making; and length of hospital or ICU stay, other organ support free days, organ support other than mechanical ventilation, heparin induced thrombocytopenia, and cumulative transfusion as important outcome measures for decision making.
The working group defined a risk difference of 3% as a minimal clinically (patient) important difference for mortality, venous thromboembolism, thromboembolic complications (venous and arterial thrombotic complications combined) and major bleeding; 3 days for length of hospital(/ICU) stay, ventilator-free days, and other organ support free days; a risk difference of 5% for organ support other than mechanical ventilation (yes/no), an absolute risk difference of 0.5 % for heparin induced thrombocytopenia; and an absolute risk difference of 10% for cumulative transfusion.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until October 18th 2021. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 686 hits. Studies were selected based on the following criteria:
- randomized controlled trial (RCT)
- peer reviewed and published in indexed journal or pre-published
- comparing treatment with
- a therapeutic dose of anticoagulant (low molecular weight heparin, unfractionated heparin, direct oral anticoagulants, vitamin K-antagonists, aspirin) with a prophylactic dose or no dose of anticoagulant (low molecular weight heparin, unfractionated heparin, direct oral anticoagulants, vitamin K-antagonists, aspirin)
- an intermediate prophylactic dose of anticoagulant (low molecular weight heparin, unfractionated heparin, direct oral anticoagulants, vitamin K-antagonists, aspirin) with a standard prophylactic dose or no dose of anticoagulant (low molecular weight heparin, unfractionated heparin, direct oral anticoagulants, vitamin K-antagonists, aspirin)
- in critically ill patients with COVID-19 admitted to the ICU.
Fourteen studies were initially selected based on title and abstract screening. After reading the full text, nine studies were excluded (see the table with reasons for exclusion under the tab Methods), and five studies were included.
Results
Five studies were included in the analysis of the literature. Three studies investigated a therapeutic dose anticoagulant versus standard (or intermediate) prophylactic dose anticoagulant in COVID-19 patients admitted to the ICU (PICO 1). One study (for which different follow-up times – day 30 and day 90 – are described in two articles) investigated an intermediate prophylactic dose anticoagulant versus standard prophylactic dose anticoagulant in COVID-19 patients admitted to the ICU (PICO 2). No subgroups were made based on the type of anticoagulant used, as all studies used a heparin. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Bikdeli B, Talasaz AH, Rashidi F, Bakhshandeh H, Rafiee F, Rezaeifar P, Baghizadeh E, Matin S, Jamalkhani S, Tahamtan O, Sharif-Kashani B, Beigmohammadi MT, Farrokhpour M, Sezavar SH, Payandemehr P, Dabbagh A, Moghadam KG, Khalili H, Yadollahzadeh M, Riahi T, Abedini A, Lookzadeh S, Rahmani H, Zoghi E, Mohammadi K, Sadeghipour P, Abri H, Tabrizi S, Mousavian SM, Shahmirzaei S, Amin A, Mohebbi B, Parhizgar SE, Aliannejad R, Eslami V, Kashefizadeh A, Dobesh PP, Kakavand H, Hosseini SH, Shafaghi S, Ghazi SF, Najafi A, Jimenez D, Gupta A, Madhavan MV, Sethi SS, Parikh SA, Monreal M, Hadavand N, Hajighasemi A, Maleki M, Sadeghian S, Piazza G, Kirtane AJ, Van Tassell BW, Stone GW, Lip GYH, Krumholz HM, Goldhaber SZ, Sadeghipour P. Intermediate-Dose versus Standard-Dose Prophylactic Anticoagulation in Patients with COVID-19 Admitted to the Intensive Care Unit: 90-Day Results from the INSPIRATION Randomized Trial. Thromb Haemost. 2021 Apr 17. doi: 10.1055/a-1485-2372. Epub ahead of print. PMID: 33865239.
- REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators, Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, Carrier M, Reynolds HR, Kumar A, Turgeon AF, Kornblith LZ, Kahn SR, Marshall JC, Kim KS, Houston BL, Derde LPG, Cushman M, Tritschler T, Angus DC, Godoy LC, McQuilten Z, Kirwan BA, Farkouh ME, Brooks MM, Lewis RJ, Berry LR, Lorenzi E, Gordon AC, Ahuja T, Al-Beidh F, Annane D, Arabi YM, Aryal D, Baumann Kreuziger L, Beane A, Bhimani Z, Bihari S, Billett HH, Bond L, Bonten M, Brunkhorst F, Buxton M, Buzgau A, Castellucci LA, Chekuri S, Chen JT, Cheng AC, Chkhikvadze T, Coiffard B, Contreras A, Costantini TW, de Brouwer S, Detry MA, Duggal A, Džavík V, Effron MB, Eng HF, Escobedo J, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald M, Fowler RA, Froess JD, Fu Z, Galanaud JP, Galen BT, Gandotra S, Girard TD, Goodman AL, Goossens H, Green C, Greenstein YY, Gross PL, Haniffa R, Hegde SM, Hendrickson CM, Higgins AM, Hindenburg AA, Hope AA, Horowitz JM, Horvat CM, Huang DT, Hudock K, Hunt BJ, Husain M, Hyzy RC, Jacobson JR, Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski A, King AJ, Knudson MM, Kornblith AE, Kutcher ME, Laffan MA, Lamontagne F, Le Gal G, Leeper CM, Leifer ES, Lim G, Gallego Lima F, Linstrum K, Litton E, Lopez-Sendon J, Lother SA, Marten N, Saud Marinez A, Martinez M, Mateos Garcia E, Mavromichalis S, McAuley DF, McDonald EG, McGlothlin A, McGuinness SP, Middeldorp S, Montgomery SK, Mouncey PR, Murthy S, Nair GB, Nair R, Nichol AD, Nicolau JC, Nunez-Garcia B, Park JJ, Park PK, Parke RL, Parker JC, Parnia S, Paul JD, Pompilio M, Quigley JG, Rosenson RS, Rost NS, Rowan K, Santos FO, Santos M, Santos MO, Satterwhite L, Saunders CT, Schreiber J, Schutgens REG, Seymour CW, Siegal DM, Silva DG Jr, Singhal AB, Slutsky AS, Solvason D, Stanworth SJ, Turner AM, van Bentum-Puijk W, van de Veerdonk FL, van Diepen S, Vazquez-Grande G, Wahid L, Wareham V, Widmer RJ, Wilson JG, Yuriditsky E, Zhong Y, Berry SM, McArthur CJ, Neal MD, Hochman JS, Webb SA, Zarychanski R. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med. 2021 Aug 26;385(9):777-789. doi: 10.1056/NEJMoa2103417. Epub 2021 Aug 4. PMID: 34351722; PMCID: PMC8362592
- Jiménez D, García-Sanchez A, Rali P, Muriel A, Bikdeli B, Ruiz-Artacho P, Le Mao R, Rodríguez C, Hunt BJ, Monreal M. Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest. 2021 Mar;159(3):1182-1196. doi: 10.1016/j.chest.2020.11.005. Epub 2020 Nov 17. PMID: 33217420; PMCID: PMC7670889.
- Lemos ACB, do Espírito Santo DA, Salvetti MC, Gilio RN, Agra LB, Pazin-Filho A, Miranda CH. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb Res. 2020 Dec;196:359-366. doi: 10.1016/j.thromres.2020.09.026. Epub 2020 Sep 21. PMID: 32977137; PMCID: PMC7503069.
- Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res Pract Thromb Haemost. 2020 Sep 25;4(7):1178–91. doi: 10.1002/rth2.12439. Epub ahead of print. PMID: 33043231; PMCID: PMC7537137.
- INSPIRATION Investigators, Sadeghipour P, Talasaz AH, Rashidi F, Sharif-Kashani B, Beigmohammadi MT, Farrokhpour M, Sezavar SH, Payandemehr P, Dabbagh A, Moghadam KG, Jamalkhani S, Khalili H, Yadollahzadeh M, Riahi T, Rezaeifar P, Tahamtan O, Matin S, Abedini A, Lookzadeh S, Rahmani H, Zoghi E, Mohammadi K, Sadeghipour P, Abri H, Tabrizi S, Mousavian SM, Shahmirzaei S, Bakhshandeh H, Amin A, Rafiee F, Baghizadeh E, Mohebbi B, Parhizgar SE, Aliannejad R, Eslami V, Kashefizadeh A, Kakavand H, Hosseini SH, Shafaghi S, Ghazi SF, Najafi A, Jimenez D, Gupta A, Madhavan MV, Sethi SS, Parikh SA, Monreal M, Hadavand N, Hajighasemi A, Maleki M, Sadeghian S, Piazza G, Kirtane AJ, Van Tassell BW, Dobesh PP, Stone GW, Lip GYH, Krumholz HM, Goldhaber SZ, Bikdeli B. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA. 2021 Apr 27;325(16):1620-1630. doi: 10.1001/jama.2021.4152. PMID: 33734299; PMCID: PMC7974835.
- Spyropoulos AC, Goldin M, Giannis D, Diab W, Wang J, Khanijo S, Mignatti A, Gianos E, Cohen M, Sharifova G, Lund JM, Tafur A, Lewis PA, Cohoon KP, Rahman H, Sison CP, Lesser ML, Ochani K, Agrawal N, Hsia J, Anderson VE, Bonaca M, Halperin JL, Weitz JI; HEP-COVID Investigators. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern Med. 2021 Dec 1;181(12):1612-1620. doi: 10.1001/jamainternmed.2021.6203. PMID: 34617959; PMCID: PMC8498934.
- Tan BK, Mainbourg S, Friggeri A, Bertoletti L, Douplat M, Dargaud Y, Grange C, Lobbes H, Provencher S, Lega JC. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax. 2021 Oct;76(10):970-979. doi: 10.1136/thoraxjnl-2020-215383. Epub 2021 Feb 23. PMID: 33622981; PMCID: PMC7907632.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size
|
Comments |
|
Spyropoulos, 2021
HEP-COVID Randomized Clinical Trial
AND
corresponding Trial protocol design paper (Goldin, 2021) |
Type of study: Randomized controlled trial
Setting and country: Multicenter study in the US (12 centers)
Funding and conflicts of interest: Support from Feinstein Institutes for Medical Research, the Broxmeyer Fellowship in Clinical Thrombosis, and grant R24AG064191 from the National Institute on Aging. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. |
Inclusion criteria:
Exclusion criteria:
Patients were stratified into two subgroups: non-intensive care unit patients and intensive care unit patients. ICU status was defined by mechanical ventilation, noninvasive positive pressure ventilation or high-flow nasal cannula, vasopressors, or vital sign monitoring more often than every 4 hours. ICU: 83 patients (32.8%) Non-ICU: 170 patients (67.2%)
N total at baseline: N = 257 participants randomized (130 to the intervention group and 127 to the control group) at baseline.
Important prognostic factors2:
Age, mean (SD): I: 65.8 (13.9) C: 67.7 (14.1)
Sex, No./total No. (%) male I: 68/129 (52.7%) C: 68/124 (54.8%)
BMI, mean (SD) I: 31.2 (9.3) C: 29.8 (13.6)
Race and ethnicity, No. (%) (I/C) Asian 11 (8.5) / 14 (11.3) Black 33 (25.6) / 37 (29.8) White 56 (43.4) / 46 (37.1) Multiracial/unknown 29 (22.5) / 27 (21.8)
ICU, No./total No. (%) I: 45/129 (34.9) C: 38/124 (30.6)
Comorbidities, No./total No. (%) (I/C) Hypertension 81/129 (62.8) / 70/123 (56.9) Heart failure 0 / 2/124 (1.6) Diabetes mellitus 51/128 (39.8) / 43/124 (34.7) Dyslipidemia 48/129 (37.2) / 39/124 (31.5) Coronary artery disease 7/129 (5.4) / 11/124 (8.9) Valvular heart disease 1/129 (0.8) / 3/124 (2.4) History of ischemic stroke 5/129 (3.9) / 3/124 (2.4) History of carotid occlusive disease 0 / 0 Peripheral artery disease 4/129 (3.1) / 1/124 (0.8) Chronic kidney disease 5/129 (3.9) / 4/124 (3.2) Chronic lung disease 9/129 (7.0) / 8/124 (6.5) Chronic liver disease/cirrhosis 2/129 (1.6) / 1/124 (0.8) Pulmonary hypertension 1/127 (0.8) / 2/124 (1.6)
VTE risk factors, , No./total No. (%) (I/C) Personal history of VTE 6/129 (4.7) / 2/124 (1.6) History of cancer 16/129 (12.4) / 10/124 (8.1) Active cancer 1/129 (0.8) / 4/124 (3.2) Autoimmune disease 1/128 (0.8) / 2/124 (1.6) Hormonal therapy/oral contraceptives 1/129 (0.8) / 1/124 (0.8) Known thrombophilia 0 / 0 Recent stroke with paresis 1/129 (0.8) / 1/124 (0.8)
Clinical scores, mean (SD) (I/C) IMPROVEDD VTE risk score 4.33 (1.48) / 4.22 (1.36) Sepsis-induced coagulopathy score 2.35 (0.73) / 2.31 (0.85)
Laboratory parameters, mean (SD) (I/C) White blood cell count, /μL 9600 (5800) / 9800 (8200) Platelets, ×103/μL 287.7 (119.8) / 269.7 (108.2) Serum creatinine, mg/dL 0.94 (0.45) / 1.00 (0.50) Prothrombin time, s 13.5 (1.6) /13.6 (2.6) D-dimer, ng/mL 3837 (6166) / 3183 (5409)
Medications prior to randomization, No./total No. (%)(I/C) Low-molecular-weight heparin 106/128 (82.8) / 97/124 (78.2) Unfractionated heparin 18/127 (14.2) / 23/121 (19.0) Remdesivir 93/129 (72.1) / 85/124 (68.6) Glucocorticoids 111/127 (87.4) / 93/123 (75.6) Antiplatelets 40/129 (31.0) / 24/124 (19.4)
Oxygen therapy, No./total No. (%) (I/C) Nasal cannula 80/129 (62.0) / 83/124 (66.9) Nonrebreather mask 12/129 (9.3) / 11/124 (8.9) Ventilation mask 4/129 (3.1) / 2/124 (1.6) High-flow or noninvasive positive-pressure ventilation 20/129 (15.5) / 19/124 (15.3) Invasive mechanical ventilation 8/129 (6.2) / 5/124 (4.0)
Length of hospital stay, mean (SD), d I: 12.2 (9.3)
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
Patients in the therapeutic dose group received enoxaparin at a dose of 1 mg/kg subcutaneously twice daily if CrCl was 30 L/min/1.73m2 or greater or 0.5 mg/kg twice daily if CrCl was 15-29 mL/min/1.73m2. Study drug was administered for the duration of hospitalization, including patient transfers to ICU settings.
Study protocol (Goldin, 2021): Individual dose modification is not permitted unless the CrCl falls below 15 mL/min in the treatment arm (arm 0). In that case, conversion to dose-adjusted intravenous (IV) UFH is acceptable. The investigator is encouraged to convert back to treatment-dose enoxaparin as per protocol once the CrCl returns to values higher than or equal to 15 mL/min.
|
Describe control (treatment/procedure/test):
Patients in the standard-dose group received prophylactic or intermediate-dose heparin regimens per local institutional standard and could include UFH, up to 22500 IU subcutaneously (divided twice or thrice daily); enoxaparin, 30 mg or 40 mg subcutaneously once or twice daily (weight based enoxaparin 0.5mg/kg subcutaneously twice daily was permitted but strongly discouraged); or dalteparin, 2500 IU or 5000 IU subcutaneously daily. If CrCl fell below15 mL/min/ 1.73 m2, enoxaparin was converted to treatment-dose intravenous UFH until kidney function improved to CrCl greater than 15 mL/min/1.73 m2, when blinded-dose subcutaneous enoxaparin was resumed. Study drug was administered for the duration of hospitalization, including patient transfers to ICU settings. In the standarddose group, 76 patients (61.3%) received prophylactic doses of heparin (enoxaparin, ≤40mg daily), while 48 patients (38.7%) received intermediate doses of heparin (enoxaparin, 30 mg twice daily, 3 patients [2.4%]; enoxaparin, 40mg twice daily, 43 patients [34.7%]; enoxaparin, 0.5mg/kg twice daily, 2 patients [1.6%]).
Goldin, 2021: Dose modification is allowed in the prophylactic/intermediate group (arm 1) if the CrCl falls below15 mL/min so that UFH up to 22,500 U daily (i.e., UFH 5,000 U SQ BID or TID or 7,500 IU SQ BID or TID) can be used. The investigator is encouraged to convert back to prophylactic-/intermediate dose LMWH/UFH as per protocol once the CrCl returns to values higher than or equal to 15 mL/min. |
Length of follow-up: Until 30 + 2 days after randomization.
Loss-to-follow-up: 4 patients did not receive study drug (2 withdrew consent and 2 reached end points prior to the first dose). That resulted in 253 patients in the modified intention-to-treat population for analysis: Intervention: 129 Control: 124
The primary analysis was based on the modified intention to-treat population, followed by the per-protocol population.
|
Clinical Outcomes During the 30-Day, stratified for ICU and non-ICU:
Mortality Not reported
Length of hospital stay Not reported
ICU-admission Not reported
Organ support free days Not reported
Venous thromboembolism Not reported
Major bleeding I: 4/45 (8.9) C: 0 RR (95% CI): 7.62 (0.42-137.03)
Non-ICU patients: I: 2/84 (2.4) C: 2/86 (2.3) RR (95% CI): 1.02 (0.15-7.10)
|
Author’s conclusions: Therapeutic dose LMWH reduced the composite of thromboembolism and death compared with standard heparin thromboprophylaxis without increased major bleeding among hospitalized patients with COVID-19 with very elevated D-dimer levels. The treatment effect was not seen in ICU patients. |
|
Sadeghipour, 2021
INSPIRATION trial – initial study |
Type of study: Open-label RCT [multicenter]
Setting: 10 academic centers in Tehran and Tabriz; recruitment: July 29, 2020, and Nov 19, 2020. Final follow-up date for 30-day primary outcome: Dec 19, 2020
Country: Iran
Source of funding: “The study was funded by the Rajaie Cardio-vascular Medical and Research Center. Some study authors, including the lead author, are affiliated with the Rajaie Cardio-vascular Medical and Research Center. Enoxaparin was provided through Alborz Darou, Pooyesh Darou, and Caspian Pharmaceuticals companies, and atorvastatin and matching placebo was provided by Sobhan Darou. None of these companies were study sponsors.”
|
COVID-19 patients admitted to ICU, within 7 days of hospitalization
Inclusion criteria:
Exclusion criteria:
N total at baseline: N = 600 randomized, after eligibility check and consent; n=566 Intervention: 280 (276 included in primary analysis) Control: 286 (286 included in primary analysis)
Important characteristics: Age, median (IQR: I: 62 (51-70.7) y C: 61 (47-71) y Sex, n/N (%) male: I: 162/276 (58.7%) C: 163/286 (57.0%)
Duration of symptoms prior to hospitalization, d, median (IQR) I: 7 (4-8) C: 7 (5-10)
Duration of hospitalization before randomization, d, median (IQR) I: 4 (2-6) C: 4 (3-6)
Baseline indicators of illness severity, No. (%) Patients with systolic blood pressure <100mmHg at the time of randomization I: 25/276 (9.0%) C: 33/286 (11.5%)
Vasopressor agent support within 72 h of enrolment I: 63/276 (22.8%) C: 64/286 (22.3%)
Fraction of inspired oxygen >50% at the time of randomization I: 112/276 (40.5%) C: 122/286 (42.6%)
Acute Physiology and Chronic Health Evaluation II score at the time of randomization I: median 8 (IQR 5-11) C: median 8 (IQR 5-11)
Acute respiratory support, No. (%) Nasal cannula I: 10/276 (3.6%) C: 14/286 (4.9%)
Face mask I: 33/276 (12.0%) C: 27/286 (9.4%)
Reservoir mask I: 76/276 (27.5%) C: 96/286 (33.6%)
High-flow nasal cannula I: 9/276 (3.3%) C: 6/286 (2.1%)
Non-invasive positive pressure ventilation I: 93/276 (33.7%) C: 85/286 (29.7%)
Invasive positive pressure ventilation (endotracheal intubation) I: 55/276 (19.9%) C: 58 (20.3%)
Groups comparable at baseline? Yes, expect for history of cigarette smoking, which was more frequent in the intermediate dose group. |
Intermediate-dose Prophylactic Anticoagulation
Enoxaparin, 1mg/kg daily)
|
Standard-dose Prophylactic Anticoagulation
Enoxaparin, 40mg daily |
Length of follow up: 30 days
Not included in follow-up: I: 4/280 (1.4%) Reasons: ‘did not receive at least 1 dose of the assigned treatment’ C: 0/286 (0%)
|
Clinical outcomes at 30 days follow-up
Mortality All-cause mortality, 30 days I: 119/276 (43.1%) C: 117/286 (40.9%) Diff 2.2 (−5.9 to 10.3) OR 1.09 (0.78 to 1.53) P=0.50
Length of ICU stay ICU length of stay, median (IQR) I: 5 (2-10) C: 6 (3-11)
Patients discharged from the ICU, n/total (%) I: 169 / 276 (61.2%) C: 174 / 286 (60.8%)
Organ support free days Ventilator-free days, median (IQR) I: 30 (3 to 30) C: 30 (1 to 30)
New in-hospital kidney replacement therapy, n (%) I: 10 out of 276 (3.6%) patients C: 7 out of 286 (2.4%) patients
Venous thromboembolism Adjudicated venous thromboembolism, n/total (%) I: 9 / 276 (3.3%) C: 10 / 286 (3.5%)
Objectively clinically diagnosed acute peripheral arterial thrombosis I: 0 / 276 C: 0 / 286
Objectively clinically diagnosed stroke, n/total (%) I: 1/276 (0.4%) C: 1/286 (0.3%)
Objectively clinically diagnosed type I acute myocardial infarction, n/total I: 0/276 C: 0/286
Major bleeding*, n/total (%) I: 7/276 (2.5%) C: 4/286 (1.4%) |
Definitions: Definitions of outcome Events are described in the study protocol
Remarks: * Major bleeding consisted of Bleeding Academic Research Consortium (BARC) type 3 and 5, which defines type 3a as overt bleeding plus hemoglobin drop of 3 to 5 g/dL or any transfusion with overt bleeding; type 3b as overt bleeding plus hemoglobin drop 5 g/dL, cardiac tamponade, or bleeding requiring surgical intervention for control; type 3c as intracranial hemorrhage; and type 5 as fatal bleeding
Authors conclusion: Among patients admitted to the ICU with COVID-19, intermediate-dose prophylactic anticoagulation, compared with standard-dose prophylactic anticoagulation, did not result in a significant difference in the primary outcome of a composite of adjudicated venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days. These results do not support the routine empirical use of intermediate-dose prophylactic anticoagulation in unselected patients admitted to the ICU with COVID-19.
|
|
Bikdeli, 2021
INSPIRATION trial – extension 90 days follow-up of Sadeghipour (2021)(follow-up 30 days) |
See Sadeghipour (2021) |
See Sadeghipour (2021) |
See Sadeghipour (2021) |
See Sadeghipour (2021) |
Length of follow up: 90 days
90-day outcome data were available for all 562 participants.
Not included in follow-up See Sadeghipour (2021)
|
Clinical outcomes at 90 days follow-up
Mortality All-cause mortality, no./total no. of patients (%) I: 127 276 (46.0%) C: 123/286 (43.0%) HR (95%CI): 1.24 (0.97–1.60)
Length of ICU stay Not reported
Organ support free days Not reported
Venous thromboembolism Adjudicated venous thromboembolism, no./total no. of patients (%) I: 9/276 (3.3%) C: 10/286 (3.5%) HR (95%CI): 0.93 (0.48–1.76)
Objectively clinically diagnosed acute peripheral arterial thrombosis, no./total no. of patients (%) I: 0/276 C: 0/286
Objectively clinically diagnosed stroke, no./total no. of patients (%) I: 1/276 (0.4) C: 1/286 (0.3) HR (95%CI): 1.03 (0.06–16.56)
Objectively clinically diagnosed type I acute myocardial infarction, no./total no. of patients (%) I: 0/276 C: 0/286
Major bleeding n/total (%) I: 7/276 (2.5%) C: 4/286 (1.4%) |
Author’s conclusions: By the end of 90-day clinical follow-up, intermediate dose prophylactic anticoagulation compared with standard-dose prophylactic anticoagulation did not result in a reduction in the 90-day composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or all-cause mortality. Collectively, these findings do not support the routine use of intermediate-dose prophylactic anticoagulation in ICU patients with COVID-19. |
|
Goligher, 2021
The REMAP-CAP, ACTIV-4a, and ATTACC Investigators |
Type of study: open-label, international, adaptive, multiplatform RCT (mpRCR)
Setting and country: The lead investigators of three international adaptive platform trials harmonized their protocols to study the effect of therapeutic anticoagulation in patients hospitalized for Covid-19 into one integrated mpRCT. The participating platforms included the REMAP-CAP; ACTIV-4a andATTACC. Patients were included in the UK, USA, Canada, Brazil, Ireland, Netherlands, Australia, Nepal, Saudi Arabia, and Mexico.
Funding and conflicts of interest: The trial was supported by multiple international funding organizations who had no role in the design, analysis or reporting of the trial result.
REMAP-CAP was supported by the European Union through FP7-HEALTH-2013-INNOVATION: the PREPARE consortium (grant 602525) and the Horizon 2020 research and innovation program: the RECOVER consortium (grant 101003589) and by grants from the Australian National Health and Medical Research Council (APP1101719 and APP1116530), the Health Research Council of New Zealand (16/631), the Canadian Institutes of Health Research (Strategy for Patient-Oriented Research Innovative Clinical Trials Program Grant 158584 and COVID-19 Rapid Research Operating Grant 447335), the U.K. National Institute for Health Research (NIHR) and the NIHR Imperial Biomedical Research Centre, the Health Research Board of Ireland (CTN 2014-012), the UPMC Learning While Doing Program, the Translational Breast Cancer Research Consortium, the French Ministry of Health (PHRC-20-0147), the Minderoo Foundation, Amgen, Eisai, the Global Coalition for Adaptive Research, and the Wellcome Trust Innovations Project (215522).
The ATTACC platform was supported by grants from the Canadian Institutes of Health Research, LifeArc, Thistledown Foundation, Research Manitoba, CancerCare Manitoba Foundation, Victoria General Hospital Foundation, Ontario Ministry of Health, and the Peter Munk Cardiac Centre.
The ACTIV-4a platform was supported by the National Heart, Lung, and Blood Institute of the NIH and administered through OTA-20-011 and was supported in part by NIH agreement 1OT2HL156812-01.
|
Inclusion criteria: Patients hospitalized for severe COVID-19* with confirmed infection.
Exclusion criteria: Patients were ineligible if they had been admitted to the ICU with Covid-19 for 48 hours or longer (in REMAP-CAP) or to a hospital for 72 hours or longer (in ACTIV-4a and ATTACC) before randomization.
REMAP-CAP:
ACTIV-4a:
ATTACC:
N total at baseline: N = 1207 randomized; primary analysis involved 1103 patients Intervention: 591 (534 with data on primary outcome available) Control: 616 (564 with data on primary outcome available)
Important characteristics: Age, mean (SD) I: 60.4 y (13.1) C: 61.7 y (12.5)
Sex, n/N (%) male I: 387/536 (72.2%) C: 385/567 (67.9%)
Country, n (%) (i/C) UK: 389 (72.6%)/395 (69.7%) USA: 79 (14.7%)/97 (17.1%) Canada: 40 (7.5%)/54 (9.5%) Brazil: 12 (2.2%)/6 (1.1%) Other: 16 (3.0%/15 (2.6%)
BMI, median (IQR) I: 30.4 (26.9-36.1) C: 30.2 (26.4-34.9)
Baseline organ support, n/N (%) Low-flow nasal cannula or face mask or no supplemental oxygen I: 8/536 (1.5%) C: 7/567 (1.2%) High-flow nasal cannula I: 170/536 (31.7%) C: 188/567 (33.2%) Non-invasive ventilation I: 215/536 (40.1%) C: 200/567 (35.3%) Invasive mechanical ventilation I: 143/536 (26.7%) C: 172/567 (30.3%) Vasopressors or inotropes I: 94/536 (17.5%) C: 109/567 (19.2)
Pre-existing conditions, n/ N (%) Diabetes mellitus (type 1 or 2) I: 171/536 (31.9%) C: 191/567 (33.7%) Severe cardiovascular disease I: 44/524 (8.4%) C: 45/558 (8.1%) Chronic kidney disease I: 58/509 (11.4%) C: 43/521 (8.3%) Chronic respiratory disease I: 129/517 (25.0%) C: 129/537 (24.0%) Chronic liver disease I: 6/516 (1.2%) C: 3/548 (0.5%)
Baseline treatments, n/N (%) Antiplatelet agent I 37/485 (7.6%) C: 38/494 (7.7%) Remdesivir I: 174/532 (32.7%) C: 172/564 (30.5%) Glucocorticoids I: 426/522 (81.6%) C: 458/555 (82.5%) Tocilizumab I: 11/532 (2.1%) C: 9/564 (1.6%)
D-dimer level ≥ 2 times ULN at site, n/N (%) I: 100/210 (47.6%) C: 107/223 (48.0%)
Laboratory values, median (IQR) D-dimer (ng/ml) I: 823 (433–1740) (n = 189) C: 890 (386–1844) (n = 196)
International normalized ratio I: 1.1 (1-1.2) (n = 327) C: 1.1 (1-1.2) (n = 324)
Neutrophil count per mm3 I: 7900 (5500-10,600) (n = 446) C: 7800 (5600-10,700) (n = 478)
Lymphocyte count per mm3 I: 700 (500-1000) (n = 447) C: 700 (500-900) (n = 482)
Platelet count per mm3 I: 247,000 (190,200-3167,500) (n = 530) C: 244.000 (182,000–312,000) (n = 561)
Groups comparable at baseline? Yes |
Therapeutic-dose anticoagulation with unfractionated or low-molecular-weight heparin, administered according to local site protocols for the treatment of acute venous thromboembolism for up to 14 days or until recovery (defined as either hospital discharge or discontinuation of supplemental oxygen for at least 24 hours). |
Usual-care pharmacologic thromboprophylaxis, administered at a dose and duration determined by the treating clinician according to local practice, which included either standard low-dose thromboprophylaxis or enhanced intermediate-dose thromboprophylaxis. |
Length of follow-up: The primary outcome, organ support–free days, was evaluated on an ordinal scale indicating the number of days free of cardiovascular or respiratory organ support up to day 21 among patients who survived to hospital discharge; patients who died in the hospital by day 90 were assigned a value of –1.
Loss-to-follow-up: 1207 patients initially included and randomized
Intervention: N = 591 Exclusion: 57 (9.6%) Reasons: 10 withdrew consent 2 did not have outcome data available 45 did not have confirmed COVID-19
Control: N = 616 Exclusie: 52 (8.4%) Reasons 13 withdrew consent 3 did not have outcome data available 36 did not have confirmed COVID-19 |
Clinical outcomes Mortality Death in hospital, n/total (%) I: 199/534 (37.3) C: 200/564 (35.5)
Length of ICU stay Not reported
Organ support Organ support–free days up to day 21, median (IQR) I: 1 day (–1 to 16) C: 4 days (–1 to 16)
Venous thromboembolism Number of patients with VTE not specifically reported.
Deep venous thrombosis events I: 6 C: 6
Systemic Arterial Thromboembolism events I: 8 C: 3
Pulmonary embolism events I: 13 C: 42
Ischemic cerebrovascular events I: 8 C: 9
Myocardial infarction events I: 7 C: 10
Number of patients with any thrombotic event (defined as pulmonary embolism, myocardial infarction, ischemic cerebrovascular event, systemic arterial thromboembolism). I: 38/530 (7.2%) C: 62/559 (11.1%)
Major bleeding I: 20/529 (3.8%) C: 13/562 (2.3%)
|
Definitions: * Severe COVID-19 was defined as COVID-19 that led to receipt of ICU-level respiratory or cardiovascular organ support (oxygen through a highflow nasal cannula, noninvasive or invasive mechanical ventilation, extracorporeal life support, vasopressors, or inotropes) in an ICU.
Definitions of major thrombotic and any thrombotic events are described in the study protocol.
Remarks: -
Authors conclusion: In critically ill patients with Covid-19, an initial strategy of therapeutic-dose anticoagulation with heparin did not result in a greater probability of survival to hospital discharge or a greater number of days free of cardiovascular or respiratory organ support than did usual-care pharmacologic thromboprophylaxis. |
|
Lemos, 2020
|
Type of study: randomized, open-label, phase II study
Setting and country: Probably Brazil, but not mentioned in the article
Funding and conflicts of interest: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors declare no conflicts of interest. |
Inclusion criteria: Patients with age over 18 years-old, SARS-CoV-2 infection confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR), presence of acute respiratory distress syndrome (ARDS) according to the Berlin definition, severe clinical presentation with respiratory failure requiring mechanical ventilation, D-dimer levels greater than 1000 μg/L; prothrombin time/international normalized ratio (INR) < 1.5; activated partial thromboplastin time (aPTT)/ratio < 1.5, and platelet count greater than 100,000/mm3.
Exclusion criteria: Patients with age greater than 85 years-old, creatinine clearance (CrCl) < 10 mL/min, severe circulatory shock with a dose of norepinephrine higher than 1.0 μg/kg/min, chronic renal failure in renal replacement therapy, Child B and C chronic liver disease, advanced diseases, such as active cancer, heart failure with functional class III and IV (New York Heart Failure Association), chronic obstructive pulmonary disease using home oxygen, advanced dementia, significant disability from stroke or severe head injury, cardiorespiratory arrest, pregnant women, recent major surgery or severe trauma in the last 3 weeks, recent stroke in the last 3 months, active bleeding, blood dyscrasia such as hemophilia, Von Willebrand factor deficiency, participation in another clinical investigation, indication for therapeutic anticoagulation due to pulmonary embolism, and acute coronary syndrome.
N total at baseline: Intervention: 10 Control: 10
Important prognostic factors2: Age, mean (SD): I: 55 (10) C: 58 (16)
Sex, male, n (%): I: 9 (90%) C: 7 (70%)
Clinical features Fever, n (%) I: 9 (90%) C: 8 (80%)
Cough, n (%) I: 9 (90%) C: 8 (80%)
Dyspnea, n (%) I: 9 (90%) C: 9 (90%)
Myalgia, n (%) I: 5 (50%) C: 4 (40%)
Time from illness onset to hospital admission (days), mean (SD) I: 7 (2) C: 6 (3)
Medical history
Diabetes mellitus, n (%) I: 4 (40%) C: 3 (30%)
Hypertension, n (%) I: 4 (40%) C: 3 (30%)
Cardiovascular disease, n (%) I: 1 (10%) C: 1 (10%)
Immunocompromise, n (%) I: 1 (10%) C: 0 (0%)
BMI (Kg/m2), mean (SD) I: 33 (8) C: 34 (8)
Physical examination
Systolic blood pressure (mmHg), mean (SD) I: 125 (20) C: 120 (25)
Diastolic blood pressure (mmHg), mean (SD) I: 78 (12) C: 72 (14)
Heart rate (beats per minute), mean (SD) I: 80 (20) C: 77 (14)
Mechanical ventilation, n (%) I: 10 (100%) C: 10 (100%)
Previous condition before enrollment
ICU stay before enrollment (days), median (IQR) I: 0 (0–2) C: 1 (0–2)
Prophylactic anticoagulation, n (%) I: 4 (40%) C: 7 (70%)
Therapeutic anticoagulation, n (%) I: 0 (0%) C: 0 (0%)
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
therapeutic dose subcutaneous (SC) enoxaparin with the dose according to age and adjusted daily by the creatinine clearance (CrCl) estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. See article for elaboration on the doses per age.
|
Describe control (treatment/procedure/test):
prophylactic anticoagulation (UFH and LMWH): subcutaneous unfractionated heparin (UFH) at a dose of 5000 IU TID (if weight < 120 kg) and 7500 IU TID (if weight > 120 kg) or enoxaparin at a dose of 40 mg OD (if weight < 120 kg) and 40 mg BID (if weight > 120 Kg) according to the doctor's judgment. |
Length of follow-up: 28 days
Loss-to-follow-up: No loss to follow-up, 10 patients in each treatment arm.
|
Mortality All cause 28-day mortality, n (%) I: 1/10 (10%) C: 3/10 (30%)
In-hospital mortality, n (%) I: 2/10 (20%) C: 5/10 (50%)
Length of ICU stay Length of hospital stay; days, median (IQR) I: 31 (22–35) C: 30 (23–38)
ICU-free days, median (IQR) I: 12 (2−12) C: 0 (0−10)
Organ support Ventilator-free days, median (IQR) I: 15 (6–16) C: 0 (0−11)
Venous thromboembolism VTE not reported
Thrombotic events, n (%) I: 2 (20%) C: 2 (20%)
Major bleeding, m (%) I: 0 (0%) C: 0 (0%) |
Author’s conclusions: Therapeutic enoxaparin improved gas exchange over time and increased the ratio of successful liberation from mechanical ventilation. |
Risk of bias table for intervention studies (randomized controlled trials)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Spyropoulos, 2021
HEP COVID trial |
Definitely yes
Reason: Randomization well-described and well-performed
Study protocol The Feinstein Institutes Biostatistics Unit (Northwell Health) developed and implemented the randomization procedure using the Biostatistics Randomization Management System (BRMS). BRMS is a secure, HIPAA-compliant, web-based application that allows investigators to randomize subjects into randomized clinical trials (RCTs) using their personal computer. BRMS allows for multicenter, stratified, and single/double-blinded RCTs, using permuted blocks. Eligible patients will be stratified according to whether their level of care corresponds to intensive care unit (ICU) care or not. Subjects will be randomly assigned to the treatment arm (arm 0: treatment dose of LMWH) or the prophylactic-/ intermediate-dose arm (arm 1: prophylactic/intermediate dose of LMWH or UFH) in a 1:1 ratio.
Spyropoulos, 2021: Randomization was performed using a secure web application and was stratified based on noncritical care (non– intensive care unit [ICU]) or critical care (ICU) status at the time of randomization. Participants were randomly assigned 1:1 to therapeutic-dose enoxaparin or institutional standard prophylactic or intermediate-dose heparins. |
Definitely yes
Reason:
Study protocol BRMS is a secure, HIPAA-compliant, web-based application that allows investigators to randomize subjects into randomized clinical trials (RCTs) using their personal computer.
Spyropoulos, 2021: The study pharmacists as well as data abstractors and designated randomization personnel (i.e., research coordinators and/or research nurses performing the randomization process) will be unblinded. |
Were patients blinded? Definitely yes
Were healthcare providers blinded? Definitely no
Were data collectors blinded? Definitely yes
Were outcome assessors blinded? Unknown
Were data analysts blinded? Unknown
Reason: Study protocol (Goldin, 2021): Due to the pragmatic nature of this study and pseudoblinded trial design, at the time of randomization the study subject and corresponding site PIs will be blinded (unaware of specific treatment arm to which the patient is assigned). The study pharmacists as well as data abstractors and designated randomization personnel (i.e., research coordinators and/or research nurses performing the randomization process) will be unblinded.
Spyropoulos, 2021: Patients and investigators were blinded to treatment assignment as much as possible.
Data were collected and adjudicated locally by blinded investigators via imaging, laboratory, and health record data.
Discussion part: Although both investigators and patients were blinded to study drug regimen, other unblinded personnel may have introduced bias affecting outcome ascertainment. |
Probably yes
Reason: There was no loss to follow-up. However, the primary analysis was based on the modified intention to-treat population, followed by the per-protocol population. We assumed that the modified ITT did not have an influence on the risk of bias, as the number of patients that was different between the ITT and modified ITT population was very small (1 in the intervention group and 3 in the control group). |
Definitely yes
Reason: Study protocol available, all reported outcomes in study protocol reported in trial. |
Probably yes
Reason: Funding/Support: This work was supported by the Feinstein Institutes for Medical Research, the Broxmeyer Fellowship in Clinical Thrombosis, and grant R24AG064191 from the National Institute on Aging. Role of the funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Modified intention-to-treat analysis performed. However, only 1 and 3 patients in the intervention and control group, respectively, were not analysed. |
LOW (mortality, length of hospital or ICU stay, organ support and organ support free days, ventilator-free days)
HIGH (venous thromboembolism, thromboembolic complications, major bleeding, heparin induced thrombocytopenia, and cumulative transfusion)
Reasons: - patients and data collections blinded, but all other personnel including the healthcare providers were not blinded and could therefore have influenced the ‘soft’ outcome measures
|
|
Sadeghipour, 2021
INSPIRATION trial – initial study |
Definitely yes
Reason: ‘Randomization was done using an electronic web-based system with permuted blocks of 4 and allocation sequence concealment. Eligible patients were allocated in 1:1 ratio to receive intermediate-dose or standard-dose prophylactic anticoagulation.’ |
Definitely yes
Reason: Protocol: ‘1:1 multicenter open-label 2x2 factorial design randomized controlled trial with allocation sequence concealment and blinded endpoint adjudication.’ |
Hard clinical outcomes and laboratory values: Probably yes
Other clinical outcomes: Probably no
Reason: ‘anticoagulation assignment was open-label. Using a double-dummy design during the COVID-19 pandemic was not considered feasible. However, the allocation sequence was concealed and outcomes were blindly adjudicated and analyzed.’ |
Probably yes
Reason: 4% of patients in the intervention group was not included in the primary analysis and 0% of patients in the control group. Reasons for not including patients are described. Unlikely to induce a bias in the results.
|
Probably yes
Reason: Elaborate protocol; publication in line with protocol; distinction made between prespecified and post hoc subgroup analyses.
|
Probably yes
Reason: Some study authors are affiliated with the study funder (Rajaie Cardiovascular and Medical Research Center). Neither the funder, nor the companies who donated the study drugs (Alborz Darou, Pooyesh Darou and Caspian Pharmaceuticals) had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Analyses not performed according to intention-to-treat protocol, however small number of patients excluded; I: N=280 met criteria and did not withdraw consent, of which 276 (98.6%) included in primary analysis C: 286 met criteria and did not withdraw consent, of which 286 (100%) included in primary analysis
|
LOW (mortality, length of hospital or ICU stay, organ support and organ support free days, ventilator-free days)
HIGH (venous thromboembolism, thromboembolic complications, major bleeding, heparin induced thrombocytopenia, and cumulative transfusion)
Reasons: - open-label trial, no use of blinding could have influenced the ‘soft’ outcome measures
|
|
Bikdeli, 2021
INSPIRATION trial – extension 90 day follow-up Sadeghipour (2021)(follow-up 30 days) |
See Sadeghipour, 2021
|
See Sadeghipour, 2021
|
See Sadeghipour, 2021
|
See Sadeghipour, 2021 |
See Sadeghipour, 2021
|
See Sadeghipour, 2021
|
LOW (mortality, length of hospital or ICU stay, organ support and organ support free days, ventilator-free days)
HIGH (venous thromboembolism, thromboembolic complications, major bleeding, heparin induced thrombocytopenia, and cumulative transfusion)
Reasons: - open-label trial, no use of blinding could have influenced the ‘soft’ outcome measures
|
|
Goligher, 2021
The REMAP-CAP, ACTIV-4a, and ATTACC Investigators |
Definitely yes
ReasonL Randomization was performed with the use of separate central Web-based systems for each platform. |
Definitely yes
Reason: Protocol: ‘Allocation concealment will be maintained by using centralized randomization that is remote from study sites.’ |
Hard clinical outcomes and laboratory values: Probably yes
Other clinical outcomes: Probably no
Reason: Open-label trial |
Probably yes
Reason: Loss to follow-up was comparable in both groups and reasons are described.
Missing data were queried with the site. Data imputed where appropriate. |
Probably yes
Reason: Elaborate protocol; publication in line with protocol
|
Definitely no
Reason: The trial was supported by multiple international funding organizations that had no role in the design, analysis, or reporting of the trial results, with the exception of the ACTIV-4a protocol, which received input on design from professional staff members at the National Institutes of Health and from peer reviewers.
High numbers of patients excluded in both groups after randomization. No ITT analysis performed. Different numbers of total patients in Table 2.
Studie is stopped due to meeting the stopping criteria for futility. However, this would probably not have caused risk of bias, as the study was not stopped in a ‘random high’ effect. |
HIGH (all outcome measures)
Reasons: - open-label trial, no use of blinding could have influenced the ‘soft’ outcome measures - No ITT performed and many patients excluded after randomization in both groups |
|
Lemos, 2021 |
Defnitely yes
Reason: We used blocked randomization, and the participants were randomized in a 1:1 ratio within two blocks of ten patients each. The patients were assigned to each treatment by drawing the sequential numbering of opaque envelopes containing the treatment allocation |
Probably yes
Reason: We used blocked randomization, and the participants were randomized in a 1:1 ratio within two blocks of ten patients each. The patients were assigned to each treatment by drawing the sequential numbering of opaque envelopes containing the treatment allocation |
Definitely no
Reason: Open-label trial |
Definitely yes
Reason: No loss to follow-up |
Unknown
No protocol |
Definitely yes
Reason: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors declare no conflicts of interest.
|
LOW (mortality, length of hospital or ICU stay, organ support and organ support free days, ventilator-free days)
HIGH (venous thromboembolism, thromboembolic complications, major bleeding, heparin induced thrombocytopenia, and cumulative transfusion)
Reason: open-label trial, no use of blinding could have influenced the ‘soft’ outcome measures |
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Connors, 2021 |
Outpatients, does not fit PICO |
|
Perepu, 2021 |
Exclusion, no difference made between ICU and non-ICU patients |
|
Diep, 2021 |
No publication available |
|
Ananworanich, 2021 |
Outpatients, does not fit PICO |
|
Cuker, 2021 |
Is not an RCT but a guideline based on observational studies |
|
Sholzberg, 2021 |
Included in non-ICU submodule, but not in the ICU submodule |
|
Lawler, 2021 |
Included in non-ICU submodule, but not in the ICU submodule |
|
Lopes, 2021 |
Included in non-ICU submodule, but not in the ICU submodule |
|
Marcos, 2021 |
Included in non-ICU submodule, but not in the ICU submodule |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 14-04-2022
Beoordeeld op geldigheid : 14-04-2022
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS).
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Deze richtlijnmodule is ontwikkeld in samenwerking met:
- Nederlandse Vereniging voor Intensive Care
- Nederlandse Vereniging van Artsen voor Longziekten en Tuberculose
- Nederlandse Vereniging voor Cardiologie
- Nederlandse Vereniging voor Ziekenhuisapothekers
- Nederlandse Vereniging van Spoedeisende Hulp Artsen
- Nederlandse Vereniging voor Klinische Chemie en Laboratoriumgeneeskunde
- Harteraad
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodules is in 2020 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen die betrokken zijn bij de behandeling van patiënten met COVID-19.
Werkgroep
- Prof. dr. M.V. Huisman (voorzitter), internist-vasculair geneeskundige, Leids Universitair Medisch Centrum (LUMC), NIV
- Dr. F.A. Klok, internist-vasculair geneeskundige, Leids Universitair Medisch Centrum (LUMC), NIV
- Prof. dr. H.C.J. Eikenboom, internist-vasculair geneeskundige/hematoloog, Leids Universitair Medisch Centrum (LUMC), NIV
- Prof. dr. S. Middeldorp, internist-vasculair geneeskundige, Radboud Universitair Medisch Centrum (Radboudumc), NIV
- Dr. M.J.H.A. Kruip, internist-hematoloog, Erasmus Medisch Centrum (Erasmus MC), NIV
- Prof. dr. K. Meijer, internist-hematoloog, Universitair Medisch Centrum Groningen (UMCG), NIV
- Dr. M. Coppens, internist-vasculair geneeskundige, Amsterdam University Medical Centers (Amsterdam UMC), NIV
- Prof. dr. P.W. Kamphuisen, internist-vasculair geneeskundige, Tergooi Medisch Centrum (Tergooi MC), Amsterdam University Medical Centers (Amsterdam UMC), NIV
- Dr. M.C.A. Müller, internist-intensivist, Amsterdam University Medical Centers (Amsterdam UMC), NVIC
- Dr. J.P.J. Wester, internist-intensivist, OLVG, NVIC
- Dr. R. Vink, internist-intensivist, Tergooi Medisch Centrum (Tergooi MC), NVIC
- Dr. L.M. van den Toorn, longarts, Erasmus Medisch Centrum (Erasmus MC), NVALT
- Dr. R.G. Tieleman, cardioloog, Martini Ziekenhuis, NVVC
- Dr. J. Diepstraten, ziekenhuisapotheker, Amphia ziekenhuis, NVZA
- Dr. N. van Rein, ziekenhuisapotheker, Leids Universitair Medisch Centrum (LUMC), NVZA
- Drs. N. Buenen, SEH-arts, Máxima Medisch Centrum (MMC), NVSHA
- Prof. dr. Ir. Y.C.M. Henskens, klinisch chemicus, Maastricht Universitair Medisch Centrum (Maastricht UMC), NVKC
Meelezer:
- Dr. I. Schalkers, Beleidsadviseur Patientenparticipatie, Harteraad
Onafhankelijke reviewers:
- Dr. E. van Dijk, neuroloog, Radboud Universitair Medisch Centrum, (Radboudumc), NVN
- Drs. P.R. van der Valk, internist-vasculair geneeskundige, Universitair Medisch Centrum Utrecht (UMC Utrecht), NIV
Met ondersteuning van:
- F.M. Janssen, MSc, junior adviseur, Kennisinstituut van Medisch Specialisten
- dr. S.N. Hofstede, senior adviseur, Kennisinstituut van Medisch Specialisten
- drs. I. van Dusseldorp, senior literatuurspecialist, Kennisinstituut van Medisch Specialisten
In 2020 is een multidisciplinair expertiseteam behandeling COVID-19 ingesteld, bestaande uit vertegenwoordigers van alle relevante die betrokken zijn bij de zorg voor patiënten met COVID-19. Dit expertiseteam fungeerde als stuurgroep, welke opdracht heeft gegeven tot het ontwikkelen van de module, alsmede fungeerde als klankbordgroep.
Stuurgroep (expertiseteam Behandeling COVID-19)
- Dr. L.M. van den Toorn (voorzitter), longarts, Erasmus Medisch Centrum (Erasmus MC), NVALT
- Dr. M.G.J. de Boer, internist-infectioloog, Leids Universitair Medisch Centrum (LUMC), SWAB/NIV)
- Drs. A.J. Meinders, internist-intensivist, St. Antonius Ziekenhuis, NVIC
- Prof. dr. D.W. de Lange, intensivist-toxicoloog, Universitair Medisch Centrum Utrecht (UMC Utrecht), NVIC
- Dr. C.H.S.B. van den Berg, infectioloog-intensivist Universitair Medisch Centrum Groningen (UMCG), NVIC
- Dr. S.U.C. Sankatsing, internist-infectioloog, Diakonessenhuis, NIV
- Dr. E.J.G. Peters, internist-infectioloog, Amsterdam University Medical Centers (Amsterdam UMC), NIV
- Drs. M.S. Boddaert, arts palliatieve geneeskunde, Leids Universitair Medisch Centrum (LUMC), IKNL
- Dr. P.L.A. Fraaij, kinderarts-infectioloog, Erasmus Medisch Centrum (Erasmus MC), Sophia Kinderziekenhuis, NVK
- Dr. E. van Leeuwen, gynaecoloog, Amsterdam University Medical Centers (Amsterdam UMC), NVOG
- Dr. J.J. van Kampen, arts-microbioloog, Erasmus Medisch Centrum (Erasmus MC), NVMM
- Dr. M. Bulatović-Ćalasan, internist allergoloog-immunoloog en klinisch farmacoloog, Universitair Medisch Centrum Utrecht (UMC Utrecht), Amsterdam University Medical Centers (Amsterdam UMC), NIV
- Drs. A.F.J. de Bruin, anesthesioloog-intensivist, St. Antonius Ziekenhuis, NVA
- Drs. A. Jacobs, klinisch geriater, Catharina Ziekenhuis, NVKG
- Prof. dr. P.H.M. van der Kuy, ziekenhuisapotheker, Erasmus Medisch Centrum (Erasmus MC), NVZA
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
Werkgroep
|
Achternaam werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Huisman (voorzitter) |
internist vasculaire geneeskunde Leids Universitair Medisch Centrum hoogleraar Interne Geneeskunde |
voorzitter commissie herziening Antitrombotisch Beleid - onbetaald voorzitter Dutch Thrombosis Network – onbetaald member European Society of Cardiology Guideline on Pulmonary Embolism – onbetaald member American College of chest Physicians VTE update – onbetaald |
- adviseur farmaceutische bedrijven die (nieuwe) antistollingsmiddelen maken - betaald; gelden gaan naar afdeling Interne Geneeskunde LUMC
- ZoNMw grant Dutch-AF - registry met onderzoek op het gebied van antistolling bij patienten met atriumfibrilleren; betaald, gelden gaan naar afdeling Interne Geneeskunde LUMC - ZonMw - Hartstichting grant Coronis - onderzoek naar herseninfarct bij COVID -19
- Research grants van farmaceutische bedrijven die (nieuwe) antistollingsmiddelen maken - betaald; gelden gaan naar afdeling Interne Geneeskunde LUMC |
Zie overkoepelende actie |
|
Klok |
Internist, LUMC, Leiden |
Algemeen commissiewerk voor ISTH, ESC, ERS. Richtlijncommissie voor ESC. Richtlijncommissie voor ASH die zich buigt over tromboseprofylaxe bij COVID-19 |
Unrestricted research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, Actelion, the Dutch thrombosis association, The Netherlands Organisation for Health Research and Development and the Dutch Heart foundation |
Zie overkoepelende actie |
|
Eikenboom |
Hoogleraar medisch specialist (fte 1,0) |
Sectie editor van tijdschrift HemaSphere (honorarium naar instituut)
|
- LSBR: Modelling Von Willebrand disease with patient-specific
- Sprekersgeld: Roche, Cellgene |
Zie overkoepelende actie |
|
Middeldorp |
- Internist-vasculaire geneeskunde |
Onbetaald: |
- Betaald adviseurschap NB - alle honoraria (personal fees) gaan naar mijn ziekenhuis, geen persoonlijk financieel belang of eigen stichting:
- Financiele bijdragen aan investigator-initiated onderzoek:
- Academisch belang:
Lid richtlijn COVID coagulopathie ISTH (gestart december 2021). |
Zie overkoepelende actie |
|
Kruip |
Internist-hematoloog Erasmus MC |
Medisch leider trombosedienst Star-shl, gedetacheerd vanuit het Erasmus MC (betaald) voorzitter Federatie Nederlandse Trombosediensten (FNT, onbetaald) |
- "Caging the dragon: translation approach to unravel and prevent COVID-19 associated thrombosis" gefinancieerd door ZonMW en Trombose Stichting Nederland
- Financiële bijdrage aan investigator-initiated onderzoek, gelden ten gunste van afdeling hematologie Erasmus MC: Sobi
- Sprekersvergoeding, gelden ten gunste van afdeling hematologie Erasmus MC: Bayer, Roche, Sobi, BMS
|
Zie overkoepelende actie |
|
Meijer |
Internist-hematoloog, UMCG |
Voorzitter Nederlandse Vereniging voor Hematologie, onbetaald |
- NWO: Ethische aspecten van gentherapie, binnen Symphony project - projectleider voor Symphony
- Rollen als voorzitter van de NVvH en gedeeld voorzitter Trombose Expertise Centrum Noord Nederland kunnen gezien worden als boegbeeldfunctie, ik heb daarmee belang DAT er een richtlijn komt, maar niet bij hoe de inhoud is.
- In de afgelopen drie jaar |
Zie overkoepelende actie |
|
Coppens |
Internist-vasculaire geneeskunde en hemofilie. Hoofd Antistollingscommissie. Amsterdam |
Voorzitter werkgroep trombose en hemostase, Nederlandse Vereniging van Internisten |
- Daiichi Sankyo. Lid Steering Committee van de ETNA-VTE Europe studie. Een - Bayer. Fase 1-2 onderzoek naar gentherapie voor hemofilie A.
- Het hoge risico op veneuze trombose bij COVID-19 en de zeldzame, maar ernstige |
Zie overkoepelende actie |
|
Kamphuisen |
Internist, Tergooi MC, 0,8 fte |
Geen |
Mijn wetenschappelijk onderzoek wordt gesubsidieerd door Tergooi MC, Trombose stichting Nederland, Nederlandse Hartstichting. Verder investigator grant van Daiichi Sankyo en Roche diagnostics |
Zie overkoepelende actie |
|
Müller |
internist-intensivist, Amsterdam UMC, lokatie AMC |
Geen |
Geen |
Geen |
|
Wester |
Internist-intensivist |
Geen |
Geen |
Geen |
|
Vink |
Internist-intensivist |
Geen |
Geen |
Geen |
|
Van den Toorn |
Voorzitter NVALT |
Geen |
Voorzitter NVALT, ik heb geen baat bij welke uitkomst dan ook |
Geen |
|
Tieleman |
Cardioloog, electrofysioloog Martiniziekenhuis Groningen 0,8 FTE en UMCG 0,2 FTE |
Voorzitter pijler Implementatie DCVA 0,1 FTE |
- Ik heb sponsoring, onderzoeksgeld en honoraria voor nascholing ontvangen van Boehringer Ingelheim, Bayer en BMS/Pfizer, Daichi Sankyo
- Boehringer Ingelheim: Covid en cardiovasculair lijden (CAPACITY studie) - geen projectleider |
Zie overkoepelende actie |
|
Diepstraten |
Ziekenhuisapotheker en Medisch manager Amphia Ziekenhuis |
SIG Hematologie NVZA onbetaald |
Stichting Phoenix |
Zie overkoepelende actie |
|
Van Rein |
Ziekenhuisapotheker, Klinische Farmacie en Toxicologie, LUMC (32 uur per week) |
Geen |
Trombose Stichting Nederland: Balans bloedingen trombose atrium fibrilleren patienten tijdens behandeling met antistolling optimaliseren - projectleider |
Geen |
|
Buenen |
SEH-arts KNMG - Máxima MC - vaste dienst |
Geen |
Geen |
Geen |
|
Henskens |
0,9 fte Klinisch chemicus, waarnemend hoofd Centraal Diagnostisch Laboratorium Maastricht UMC+ |
Voorzitter concilium NVKC sinds 2020, vacatiegelden |
Geen |
Geen |
Overkoepelende actie
Om potentiële belangenverstrengelingen te voorkomen is de module tegengelezen door twee onafhankelijke reviewers. Dit betreft één onafhankelijke reviewer van de regiehoudende vereniging (Nederlandse Internisten Vereniging) en één onafhankelijke reviewer van een wetenschappelijke vereniging die niet in de werkgroep zitting heeft genomen (Nederlandse Vereniging voor Neurologie). Zij controleren of de aanbeveling duidelijk volgt uit de overwegingen en niet mogelijk gekleurd wordt door potentiële belangen. Daarnaast is de module tegengelezen door de stuurgroep.
Stuurgroep
|
Achternaam stuurgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Van den Toorn (voorzitter) |
Voorzitter NVALT |
Geen |
Geen |
Geen actie nodig |
|
De Boer |
Internist-Infectioloog, senior medisch specialist, LUMC, afdeling infectieziekten |
- Voorzitter Stichting Werkgroep Antibioticabeleid (onkostenvergoeding) |
Geen |
Geen actie nodig |
|
Meinders |
Internist-intensivist, St.-Antonius ziekenhuis, Nieuwegein |
commissie werk |
Geen |
Geen actie nodig |
|
De Lange |
Afdelingshoofd Nationaal Vergiftigingen Informatie Centrum (NVIC) van het UMC Utrecht |
secretaris Stichting Nationale Intensive Care Evaluatie (Stichting NICE) (onbetaald) |
Geen |
Geen actie nodig |
|
Van den Berg |
Infectioloog-intensivist, UMCG |
Geen |
Geen |
Geen actie nodig |
|
Sankatsing |
Internist-infectioloog/internist-acute geneeskunde, Diakonessenhuis, Utrecht |
- Bestuurslid Nederlandse Vereniging van Internist-Infectiologen (NVII) (onbetaald). |
Geen |
Geen actie nodig |
|
Peters |
Internist - aandachtsgebieden infectieziekten en Acute Geneeskunde Amsterdam UMC, locatie Vumc. |
Wetenschappelijk Secretaris International Working Group on the Diabetic Foot (onbetaald) |
Geen |
Geen actie nodig |
|
Boddaert |
Medisch adviseur bij Integraal Kankercentrum Nederland (IKNL) en Palliatieve Zorg Nederland (PZNL) Arts palliatieve geneeskunde in LUMC |
Geen |
Geen |
Geen actie nodig |
|
Fraaij |
Kinderarts infectioloog- immunoloog, Erasmus MC-Sophia, Rotterdam |
Bestuur Stichting Infecties bij Kinderen (onbetaald) |
deelname aan RECOVER, European Union's Horizon 2020 research |
Geen actie nodig |
|
Van Leeuwen |
Gyaecoloog Amsterdam Universitair Medisch Centra |
Geen |
Geen |
Geen actie nodig |
|
Van Kampen |
Arts-microbioloog, afdeling Viroscience, Erasmus MC |
- associate editor antimicrobial resistance & infection control (onbetaald) - lid antibioticacommissie Erasmus MC (onbetaald) |
1. Mede uitvinder patent: 1519780601-1408/3023503 2. R01AI147330 (NIAID/NH) (HN onderzoek (1+2 niet gerelateerd aan COVID-19)
|
Geen actie nodig |
|
Bulatovic |
Internist allergoloog-immunoloog en klinische farmacoloog, UMC Utrecht en Diakonessenhuis Utrecht |
Functie 1: arts |
Geen |
Geen actie nodig |
|
De Bruin |
Anesthesioloog - Intensivist St. Antonius ziekenhuis Nieuwegein en Utrecht |
Geen |
Geen |
Geen actie nodig |
|
Jacobs |
Klinisch geriater en klinisch farmacoloog |
Geen |
Geen |
Geen actie nodig |
|
Van der Kuy |
Afdelingshoofd Ziekenhuisapotheek Erasmus MC |
Ziekenhuisapotheker/onder-zoeker Zuyderland MC (betaald) |
betrokkenheid bij 2 ZonMw gefinancieerde studies (CHECkUP, AMUSE) |
Geen actie nodig |
Meelezer
|
Achternaam meelezer |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Schalkers |
Beleidsadviseur bij Harteraad |
Geen |
Geen |
Geen actie nodig |
Onafhankelijke reviewers
|
Achternaam onafhankelijke reviewer |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Van der Valk |
Als internist werkzaam in het UMC Utrecht, bij van Creveldkliniek - centrum voor benigne hematologie. Expert centrum voor o.a. stollingsstoornissen. Het behelst patientenzorg en onderzoek (PI voor studies naar cardiovasculaire ziekten bij Hemofilie, gentherapie bij hemofilie) |
Lid van de richtlijn commissie NIV. Onbetaald. |
Geen |
Geen |
|
Van Dijk |
Neuroloog, Radboudumc |
Voorzitter bestuur Nederlandse Vereniging voor Neurologie (0,1fte, betaald aan Radboudumc) |
Geen |
Geen |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door het meelezen van de patiëntenvereniging Harteraad. De verkregen input is meegenomen bij het opstellen van de module. De conceptrichtlijn is tevens voor commentaar voorgelegd aan Harteraad en de Patiëntenfederatie Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
Module |
Uitkomst raming |
Toelichting |
|
Module Tromboseprofylaxe op de afdeling |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbevelingen breed toepasbaar zijn (>40.000 patiënten), volgt uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen financiële gevolgen verwacht. |
Methode ontwikkeling
Evidence based
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de COVID-19 pandemie zijn knelpunten op verschillende manieren geïnventariseerd:
- De expertiseteams benoemde de knelpunten in de zorg voor patiënten met COVID-19.
- Er is een mailadres geopend (covid19@demedischspecialist.nl) waar verschillende partijen knelpunten konden aandragen, die vervolgens door de expertiseteams geprioriteerd werden.
- Door de Federatie van Medisch Specialisten zijn webinars georganiseerd waarbij vragen konden worden ingestuurd. Deze vragen zijn na afloop van de webinars voorgelegd aan de expertiseteams en geprioriteerd.
Op basis van de uitkomsten van de bovenstaande knelpuntenanalyses zijn door de expertiseteams concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
Bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd. Betrokken partijen (NVIC en NVVC) hebben geen bezwaar geuit tegen de inhoud van deze module.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. https://richtlijnendatabase.nl/over_deze_site/richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
|
# |
Searches |
Results |
|
9 |
8 not 7 |
210 |
|
8 |
4 and 6 |
255 |
|
7 |
4 and 5 |
128 |
|
6 |
(exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) |
2098400 |
|
5 |
(meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) |
486511 |
|
4 |
2 and 3 |
1897 |
|
3 |
exp Anticoagulants/ or exp Heparin, Low-Molecular-Weight/ or (anti coagulant* or anticoagulant* or anticoagulat* or anit coagulat* or antivitamin k or vitamin k antagonist* or choay or depolymerized heparin or low molecular heparin or low molecular weight heparin or traxyparine or unfractionated heparin).ti,ab,kf. |
273695 |
|
2 |
limit 1 to dt="20191201-20220101" |
118848 |
|
1 |
((exp Coronavirus/ or Coronavirus Infections/ or pneumonia virus*.ti,ab,kf. or cov.ti,ab,kf.) and ((outbreak or wuhan).ti,ab,kf. or novel.af. or '19'.ti,ab,kf. or '2019'.ti,ab,kf. or epidem*.af. or epidemy.af. or epidemic*.af. or pandem*.af. or new.ti,ab,kf.)) or (coronavirus* or 'corona virus*' or ncov or "2019ncov" or "covid" or "covid19" or "covid 19*" or "sarscov2*" or "sarscov-2*" or "sars cov 2*" or "sars cov2*" or 'sars2' or "ncov 2019" or "sars coronavirus 2*" or "sars corona virus 2*" or "severe acute respiratory syndrome cov 2*" or "severe acute respiratory syndrome cov2*" or "severe acute respiratory syndrome cov*").ti,ab,kf. |
131474 |
OVID/Medline 17-7-2020
1 ((exp Coronavirus/ or Coronavirus Infections/ or pneumonia virus*.ti,ab,kf. or cov.ti,ab,kf.) and ((outbreak or wuhan).ti,ab,kf. or novel.af. or '19'.ti,ab,kf. or '2019'.ti,ab,kf. or epidem*.af. or epidemy.af. or epidemic*.af. or pandem*.af. or new.ti,ab,kf.)) or (coronavirus* or 'corona virus*' or ncov or '2019ncov' or 'covid19' or "covid 19" or "sars cov 2" or 'sars2' or "ncov 2019" or "sars coronavirus 2" or "sars corona virus 2" or "severe acute respiratory syndrome cov 2" or "severe acute respiratory syndrome cov2" or "severe acute respiratory syndrome cov*").ti,ab,kf. (46908)
2 limit 1 to dt="20191201-20220101" (34250)
5 letter/ (1089279)
7 (anti coagulant* or anticoagulant* or anticoagulat* or anit coagulat* or antivitamin k or vitamin k antagonist* or choay or depolymerized heparin or low molecular heparin or low molecular weight heparin or traxyparine or unfractionated heparin).ti. (37017)
8 2 and 7 (24)
9 from 8 keep 1-24 (24)
Ovid/Medline 16-7-2020
1 ((exp Coronavirus/ or Coronavirus Infections/ or pneumonia virus*.ti,ab,kf. or cov.ti,ab,kf.) and ((outbreak or wuhan).ti,ab,kf. or novel.af. or '19'.ti,ab,kf. or '2019'.ti,ab,kf. or epidem*.af. or epidemy.af. or epidemic*.af. or pandem*.af. or new.ti,ab,kf.)) or (coronavirus* or 'corona virus*' or ncov or '2019ncov' or 'covid19' or "covid 19" or "sars cov 2" or 'sars2' or "ncov 2019" or "sars coronavirus 2" or "sars corona virus 2" or "severe acute respiratory syndrome cov 2" or "severe acute respiratory syndrome cov2" or "severe acute respiratory syndrome cov*").ti,ab,kf. (46908)
2 limit 1 to dt="20191201-20220101" (34250)
3 exp Anticoagulants/ or exp Heparin, Low-Molecular-Weight/ or (anti coagulant* or anticoagulant* or anticoagulat* or anit coagulat* or antivitamin k or vitamin k antagonist* or choay or depolymerized heparin or low molecular heparin or low molecular weight heparin or traxyparine or unfractionated heparin).ti,ab,kf. (265577)
4 1 and 2 and 3 (338)
5 4 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) (259)
6 (meta-analysis/ or meta-analysis as topic/ or (meta adj analy$).tw. or (systematic*or literature adj2 review$1).tw. or (systematic adj overview$1).tw. or exp "Review Literature as Topic"/ or cochrane.ab. or cochrane.jw. or embase.ab. or medline.ab. or (psychlit or psyclit).ab. or (cinahl or cinhal).ab. or cancerlit.ab. or ((selection criteria or data extraction).ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (295132)
7 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (2003494)
8 Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or (cohort adj (study or studies)).tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] (3474976)
9 5 and 6 (5)
10 5 and 7 (30)
11 5 and 8 (61)
12 9 or 10 or 11 (83)
13 5 not 12 (176)
Ovid/Medline 9-7-2020
1 ((exp Coronavirus/ or Coronavirus Infections/ or pneumonia virus*.ti,ab,kf. or cov.ti,ab,kf.) and ((outbreak or wuhan).ti,ab,kf. or novel.af. or '19'.ti,ab,kf. or '2019'.ti,ab,kf. or epidem*.af. or epidemy.af. or epidemic*.af. or pandem*.af. or new.ti,ab,kf.)) or (coronavirus* or 'corona virus*' or ncov or '2019ncov' or 'covid19' or "covid 19" or "sars cov 2" or 'sars2' or "ncov 2019" or "sars coronavirus 2" or "sars corona virus 2" or "severe acute respiratory syndrome cov 2" or "severe acute respiratory syndrome cov2" or "severe acute respiratory syndrome cov*").ti,ab,kf. (45175)
2 limit 1 to dt="20191201-20220101" (32507)
3 exp Anticoagulants/ or exp Heparin, Low-Molecular-Weight/ or (anti coagulant* or anticoagulant* or anticoagulat* or anit coagulat* or antivitamin k or vitamin k antagonist* or choay or depolymerized heparin or low molecular heparin or low molecular weight heparin or traxyparine or unfractionated heparin).ti,ab,kf. (249345)
4 2 and 3 (254)
5 (meta-analysis/ or meta-analysis as topic/ or (meta adj analy$).tw. or (systematic*or literature adj2 review$1).tw. or (systematic adj overview$1).tw. or exp "Review Literature as Topic"/ or cochrane.ab. or cochrane.jw. or embase.ab. or medline.ab. or (psychlit or psyclit).ab. or (cinahl or cinhal).ab. or cancerlit.ab. or ((selection criteria or data extraction).ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (294638)
6 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (2001874)
7 Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or (cohort adj (study or studies)).tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] (3471012)
8 4 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) (185)
9 5 and 8 (4)
10 6 and 8 (21)
11 7 and 8 (48)
12 10 not 9 (21)
13 11 not 10 not 9 (36)
14 9 or 10 or 11 (61)
15 8 not 14 (124)
Embase 25-3-2021
|
No. |
Query |
Results |
|
#10 |
#8 NOT #7 |
348 |
|
#9 |
#7 OR #8 |
550 |
|
#8 |
#4 AND #6 |
396 |
|
#7 |
#3 AND #6 |
204 |
|
#6 |
#5 AND [1-7-2020]/sd NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
4071 |
|
#5 |
#1 AND #2 |
4333 |
|
#4 |
('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it |
2500470 |
|
#3 |
('meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) |
533576 |
|
#2 |
'anticoagulant agent'/exp OR 'anticoagulation'/exp OR 'anticoagulant therapy'/exp OR 'anti coagulant*':ti,ab,kw OR 'anticoagulant*':ti,ab,kw OR 'anticoagulative*':ti,ab,kw OR 'antithrombotic*':ti,ab,kw |
755890 |
|
#1 |
('coronavirus disease 2019'/exp OR 'covid-19 testing'/exp OR 'sars-cov-2 convalescent plasma'/exp OR 'coronavirus disease 2019 breathalyzer'/exp OR covid19:ti,ab,kw OR 'covid 19':ti,ab,kw OR 'sars-cov-2 vaccine'/exp OR 'sars-cov-2 antibody'/exp OR 'severe acute respiratory syndrome coronavirus 2'/exp OR 'sars coronavirus test kit'/exp OR 'sars cov 2':ti,ab,kw OR sars2:ti,ab,kw OR 'ncov 2019':ti,ab,kw OR 'sars coronavirus 2':ti,ab,kw OR 'sars corona virus 2':ti,ab,kw OR 'severe acute respiratory syndrome cov 2':ti,ab,kw OR 'severe acute respiratory syndrome cov2':ti,ab,kw OR 'coronavirinae'/exp OR 'coronavirus infection'/de OR coronavirus*:ti,ab,kw OR 'corona virus*':ti,ab,kw OR 'pneumonia virus*':ti,ab,kw OR cov:ti,ab,kw OR ncov:ti,ab,kw OR wuhan:ti,ab,kw) AND [2019-2030]/py |
123949 |
Embase 17-7-2020
|
No. |
Query |
Results |
|
#15 |
#4 AND #13 AND #14 |
8 |
|
#14 |
'anti coagulant*':ti OR 'anticoagulant*':ti OR 'antithrombotic*':ti OR anticoagulat*:ti |
52776 |
|
#13 |
'letter'/it |
1108495 |
|
#4 |
('coronavirus disease 2019'/exp OR (('coronavirinae'/exp OR 'coronavirus infection'/de OR coronavirus*:ti,ab,kw OR 'corona virus*':ti,ab,kw OR 'pneumonia virus*':ti,ab,kw OR cov:ti,ab,kw OR ncov:ti,ab,kw) AND (outbreak:ti,ab,kw OR wuhan:ti,ab,kw)) OR covid19:ti,ab,kw OR 'covid 19':ti,ab,kw OR ((coronavirus*:ti,ab,kw OR 'corona virus*':ti,ab,kw) AND 2019:ti,ab,kw) OR 'sars cov 2':ti,ab,kw OR sars2:ti,ab,kw OR 'coronavirus*':ti,ab,kw OR 'corona virus*':ti,ab,kw OR 'ncov 2019':ti,ab,kw OR ncov:ti,ab,kw OR 'sars coronavirus 2':ti,ab,kw OR 'sars corona virus 2':ti,ab,kw OR 'severe acute respiratory syndrome cov 2':ti,ab,kw OR 'severe acute respiratory syndrome cov2':ti,ab,kw) AND [2019-2020]/py |
25663 |
Embase 16-7-2020
|
No. |
Query |
Results |
|
#12 |
#7 NOT #11 |
276 |
|
#11 |
#8 OR #9 OR #10 |
89 |
|
#10 |
#3 AND #7 |
67 |
|
#9 |
#2 AND #7 |
36 |
|
#8 |
#1 AND #7 |
12 |
|
#7 |
#6 NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
365 |
|
#6 |
#4 AND #5 |
346 |
|
#5 |
'anticoagulant agent'/exp OR 'anti coagulant*':ti,ab,kw OR 'anticoagulant*':ti,ab,kw OR 'antithrombotic*':ti,ab,kw OR anticoagulat*:ti,ab,kw OR 'anticoagulation'/exp |
729222 |
|
#4 |
('coronavirus disease 2019'/exp OR (('coronavirinae'/exp OR 'coronavirus infection'/de OR coronavirus*:ti,ab,kw OR 'corona virus*':ti,ab,kw OR 'pneumonia virus*':ti,ab,kw OR cov:ti,ab,kw OR ncov:ti,ab,kw) AND (outbreak:ti,ab,kw OR wuhan:ti,ab,kw)) OR covid19:ti,ab,kw OR 'covid 19':ti,ab,kw OR ((coronavirus*:ti,ab,kw OR 'corona virus*':ti,ab,kw) AND 2019:ti,ab,kw) OR 'sars cov 2':ti,ab,kw OR sars2:ti,ab,kw OR 'coronavirus*':ti,ab,kw OR 'corona virus*':ti,ab,kw OR 'ncov 2019':ti,ab,kw OR ncov:ti,ab,kw OR 'sars coronavirus 2':ti,ab,kw OR 'sars corona virus 2':ti,ab,kw OR 'severe acute respiratory syndrome cov 2':ti,ab,kw OR 'severe acute respiratory syndrome cov2':ti,ab,kw) AND [2019-2020]/py |
25663 |
|
#3 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
5306977 |
|
#2 |
('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it |
2340458 |
|
#1 |
('meta analysis'/de OR cochrane:ab OR embase:ab OR psycinfo:ab OR cinahl:ab OR medline:ab OR ((systematic NEAR/1 (review OR overview)):ab,ti) OR ((meta NEAR/1 analy*):ab,ti) OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
473856 |
Embase 9-7-2020
|
No. |
Query |
Results |
|
#14 |
#7 NOT #13 |
210 |
|
#13 |
#8 OR #9 OR #10 |
68 |
|
#12 |
#10 NOT #9 NOT #8 |
30 |
|
#11 |
#9 NOT #8 |
28 |
|
#10 |
#3 AND #7 |
50 |
|
#9 |
#2 AND #7 |
29 |
|
#8 |
#1 AND #7 |
10 |
|
#7 |
#6 NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
278 |
|
#6 |
#4 AND #5 |
346 |
|
#5 |
'anticoagulant agent'/exp OR 'low molecular weight heparin'/exp OR 'anti coagulant*':ti,ab,kw OR 'anticoagulant*':ti,ab,kw OR 'anticoagulative*':ti,ab,kw OR 'antithrombotic*':ti,ab,kw OR 'antivitamin k':ti,ab,kw OR 'vitamin k antagonist*':ti,ab,kw OR 'choay':ti,ab,kw OR 'depolymerized heparin':ti,ab,kw OR 'low molecular heparin':ti,ab,kw OR 'low molecular weight heparin':ti,ab,kw OR 'traxyparine':ti,ab,kw OR 'unfractionated heparin':ti,ab,kw |
703517 |
|
#4 |
('coronavirus disease 2019'/exp OR (('coronavirinae'/exp OR 'coronavirus infection'/de OR coronavirus*:ti,ab,kw OR 'corona virus*':ti,ab,kw OR 'pneumonia virus*':ti,ab,kw OR cov:ti,ab,kw OR ncov:ti,ab,kw) AND (outbreak:ti,ab,kw OR wuhan:ti,ab,kw)) OR covid19:ti,ab,kw OR 'covid 19':ti,ab,kw OR ((coronavirus*:ti,ab,kw OR 'corona virus*':ti,ab,kw) AND 2019:ti,ab,kw) OR 'sars cov 2':ti,ab,kw OR sars2:ti,ab,kw OR 'coronavirus*':ti,ab,kw OR 'corona virus*':ti,ab,kw OR 'ncov 2019':ti,ab,kw OR ncov:ti,ab,kw OR 'sars coronavirus 2':ti,ab,kw OR 'sars corona virus 2':ti,ab,kw OR 'severe acute respiratory syndrome cov 2':ti,ab,kw OR 'severe acute respiratory syndrome cov2':ti,ab,kw) AND [2019-2020]/py |
25663 |
|
#3 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
5306977 |
|
#2 |
('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it |
2340458 |
|
#1 |
('meta analysis'/de OR cochrane:ab OR embase:ab OR psycinfo:ab OR cinahl:ab OR medline:ab OR ((systematic NEAR/1 (review OR overview)):ab,ti) OR ((meta NEAR/1 analy*):ab,ti) OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
473856 |
