Antipsychotica en zwangerschaps- en baringscomplicaties
Uitgangsvraag
Welke antipsychotica hebben de voorkeur voor gebruik in de zwangerschap met betrekking tot het risico op zwangerschaps- en baringscomplicaties?
Aanbeveling
Overweeg starten met haloperidol als eerste keus bij indicatie voor antipsychoticum in de fertiele levensfase. Binnen de groep van atypische antipsychotica is er geen specifieke voorkeur. Wees terughoudend bij het voorschrijven van clozapine.
Overweeg bij antipsychoticagebruik in de zwangerschap een orale glucosetolerantietest.
Overwegingen
De kwaliteit van het bewijs
De kwaliteit van het voorhanden zijnde bewijs is zeer laag. Het bewijs is voornamelijk gebaseerd op cohortstudies, waarbij retrospectief verzameld of retrospectief geanalyseerd werd. Er zijn geen RCT’s beschikbaar. De analyses zijn grotendeels op het niveau van groepen medicatie gedaan en het aantal cases van individuele middelen is beperkt. Ook is de informatie over confounders, ernst van maternale ziekte, polyfarmacie en kenmerken van de controlegroepen zeer beperkt. Het is onduidelijk in hoeverre beëindigde zwangerschappen in de verschillende studies geïncludeerd zijn. Er is geen informatie over de ernst van de psychiatrische ziekte, het beloop in de zwangerschap of het beloop van het medicatiegebruik tijdens de zwangerschap. Dit is bij deze uitgangsvraag van extra meerwaarde aangezien de ernst van de psychiatrische ziekte ook informatie geeft over het ziekte-inzicht, de behandeltrouw en de mate van instrueerbaarheid, met name durante partu.
Op basis van de gerapporteerde studies kan geen eenduidige conclusie getrokken worden ten aanzien van een eventuele verhoogde kans op obstetrische complicaties. Er zijn enige aanwijzingen dat er mogelijk een verhoogd risico op diabetes gravidarum of vroeggeboorte is.
Overige relevante bronnen
Ook buitenlandse richtlijnen en beschouwende artikelen refereren aan het gebrek aan voldoende conclusieve studies. De NICE-richtlijn van 2014 adviseert ‘When choosing an antipsychotic, take into account that there are limited data on the safety of these drugs in pregnancy and the postnatal period.’ (National Institute for Health and Care Excellence 2014).
De richtlijn van de American College of Obstetricians and Gynecologists dateert van 2008 en maakte geen gebruik van een systematische literatuurstudie. Deze richtlijn adviseert ten aanzien van typische antipsychotica ‘Typical antipsychotics have a larger reproductive safety profile; no significant teratogenic effect has been documented with chlorpromazine (Thorazine), haloperidol (Haldol), or perphenazine (Trilafon). Doses of typical antipsychotics should be minimized during the peri-partum period to limit the necessity of using additional medications to manage extrapyramidal side effects’ (American College of Obstetricians and Gynecologists, 2008). Ten aanzien van atypische antipsychotica: ‘Therefore, the routine use of these drugs during pregnancy and lactation is not recommended’ (American College of Obstetricians and Gynecologists, 2008).
The richtlijn van de British Association of Pharmacologists (BAP) dateert van 2017 (McAllister-Williams, 2017) en verwijst naar literatuur die niet bleek te voldoen aan de kwaliteitscriteria die gehanteerd werden voor deze richtlijn. Ten aanzien van het monitoren van eventuele zwangerschapscomplicaties noemen zij het volgende: “Monitor for excessive weight gain and ensure this is managed in line with appropriate guidance (bijvoorbeeld (NICE, 2010c)). Also monitor for gestational diabetes, particularly for women on a second generation antipsychotic (SGA), (request an oral glucose tolerance test) and ensure this is managed in line with appropriate guidance (bijvoorbeeld (NICE, 2015b)).”
Het teratologie informatie centrum (TIS) van Lareb benoemt voor de typische antipsychotica: ‘Bij de toepassing van klassieke antipsychotica tijdens de zwangerschap gaat de voorkeur uit naar haloperidol. Het is onbekend of de overige klassieke antipsychotica gebruikt kunnen worden tijdens de zwangerschap.’ Voor de atypische antipsychotica benoemt het TIS dat ‘quetiapine, olanzapine en aripiprazol gebruikt kunnen worden tijdens de zwangerschap. Het is onbekend of de overige middelen veilig gebruikt kunnen worden tijdens de zwangerschap’. Voor asenapine, clozapine, lurasidon, paliperidon, risperidon en sertindol wordt vermeld dat het risico onbekend is.
Literatuur wijst uit dat de placentaire ontwikkeling en daarmee de kans op foetale en maternale complicaties samenhangt met de preconceptionele cardiovasculaire en metabole status. Derhalve is het van belang dat bij het voorschrijven van medicatie aan vrouwen in de fertiele levensfase medicatie met een ongunstige invloed op het cardiovasculaire en metabole status, alleen op strikte indicatie voorgeschreven wordt.
Wat betreft de beschreven zwangerschapscomplicaties in deze uitgangsvraag, verdient het risico op diabetes in combinatie met antipsychotica speciale aandacht. Immers, antipsychotica worden veelal voorgeschreven aan patiënten met een psychotische stoornis. In deze groep zien we een hoger risico op metabole ontregeling (Osborn, 2008). Verder is het bekend dat behandeling met antipsychotica geassocieerd is met metabole ontregeling, waaronder diabetes (Regenold, 2002; Meltzer, 2013). Deze associatie is, naast de algemene populatie, specifiek beschreven bij adolescenten (Patel, 2009), maar er zijn geen data over de zwangere populatie. Binnen de groep van antipsychotica hebben clozapine en olanzapine het grootste risico op metabole ontregeling. Quetiapine en risperidon laten deze ontregeling in mindere mate zien en aripiprazol en haloperidol nog minder (Patel, 2009; De Hert, 2008, Nederlandse Vereniging voor Psychiatrie, 2012). Bij antipsychotica gebruik wordt, ongeacht de onderliggende stoornis, geadviseerd om eventuele somatische complicaties, waaronder metabole ontregeling, in een vroeg stadium op te sporen. De Nederlandse richtlijn raadt aan om een metabole screening voor start van antipsychotica te verrichten, in het eerste jaar van gebruik in ieder geval na 3 en 6 maanden en vervolgens jaarlijks danwel extra op indicatie (Cahn, 2008; Nederlandse Vereniging voor Psychiatrie, 2012). In lijn hiermee adviseert de NICE-richtlijn van 2014 screening op diabetes bij zwangere vrouwen die antipsychotica gebruiken; “Monitor for gestational diabetes in pregnant women taking antipsychotic medication in line with the NICE guideline on diabetes in pregnancy and offer an oral glucose tolerance test” (National Institute for Health and Care Excellence, 2014). De richtlijn ‘Diabetes mellitus en zwangerschap’ van de Nederlandse Vereniging van Obstetrie en Gynaecologie vermeldt dat behandeling van een eventuele diabetes gravidarum in het algemeen het aantal ernstige perinatale complicaties verlaagt van 4% naar 1% (met een number needed to treat van 34). Daarnaast wordt melding gemaakt dat er een relatie beschreven is tussen milde hyperglykemie (dus nog geen gevonden diabetes) en de kans op perinatale en maternale complicaties. Er werd een lineaire relatie gevonden tussen hogere nuchtere, 1-uurs, en 2-uursglucosewaarde (van de 75 grams orale glucosetolerantietest (OGTT)) en een grotere kans op perinatale complicaties (onder andere macrosomie, geboortetrauma, neonatale hypoglykemie). OGTT is een betrouwbare test en goede voorspeller.
Een zeer recente systematische review die een duidelijke overlap liet zien met de studies die wij hebben geïncludeerd, kwam tot dezelfde conclusies. Hoewel de evidentie ten aanzien van een verhoogd risico op diabetes gravidarum bij antipsychotica gebruik in de zwangerschap beperkt is, vooral voor de specifieke middelen, is er voldoende indicatie op een diabetische ontregeling wel te screenen (Wang, 2020).
Farmacologische overwegingen
Bij de keuze van een antipsychoticum worden verschillende factoren, zoals de effectiviteit (bij terugvalpreventie), methode van toediening, incidentie dan wel ervaring van bijwerkingen en veiligheid tijdens de zwangerschap individueel afgewogen. Hiernaast dient de behandelend psychiater rekening te houden met verschillen tussen antipsychotica in het risico op ontregeling van metabole waarden. Dit weegt het zwaarst bij clozapine en olanzapine en in mindere mate bij quetiapine en risperidon (Patel, 2009; De Hert, 2008; Nederlandse Vereniging voor Psychiatrie, 2012).
Waarden en voorkeuren van patiënten
Er is vrijwel geen literatuur ten aanzien van de waarden en voorkeuren van patiënten, die een rol spelen bij de besluitvorming rondom het gebruik van antipsychotica ten tijde van de zwangerschap. Vanzelfsprekend speelt het bijwerkingenprofiel een rol bij de voorkeuren van patiënten, evenals buiten de zwangerschap. Onderzoek bij zwangeren die anxiolytica en antidepressiva gebruiken en zwanger worden, laat zien dat de mogelijke invloed op het (ongeboren) kind een belangrijke overweging is om medicatie te staken (Kothari, 2019). Het afwegen van de risico’s en de meest veilige beslissing nemen wordt ook genoemd als een belangrijk thema (Nygaard, 2015). In welke verhouding de eventuele zwangerschapscomplicaties liggen ten opzichte van mogelijke aangeboren afwijkingen bij het maken van een medicatie keuze is niet duidelijk.
Een nationale studie in de VS laat zien dat 50% van de vrouwen die atypische antipsychotica gebruikt in de drie maanden voorafgaand aan de zwangerschap, deze staakt tijdens de zwangerschap. Het is niet waarschijnlijk dat al deze vrouwen de antipsychotica gebruikten in verband met een psychotische stoornis. Aangezien het een registerstudie betrof, is de reden van staken niet bekend (Park, 2017). Vanuit de richtlijn antipsychotica gebruik blijkt dat subjectief welbevinden een belangrijke maat is voor het continueren van het antipsychoticum (richtlijnen database schizofrenie FMS). Vanzelfsprekend speelt ook het bijwerkingen profiel hierin mee. De ‘betere naam' die de atypische antipsychotica kregen vergeleken met de typische antipsychotica is een factor geweest bij de acceptatie van gebruik (Courtet, 2001). Daarnaast speelt in vrouwen met depressie het stigma van de psychiatrische stoornis een rol bij besluitvorming rondom het gebruik van psychotrope medicatie (Hippman,2018). Dit zal bij patiënten met een psychose gevoeligheid niet anders liggen, hoewel niet specifiek onderzocht. Onderzoek naar activiteit op sociale media in relatie met wetenschappelijke publicaties rondom antidepressivagebruik tijdens de zwangerschap liet een verdubbeling zien van deze activiteit bij studies, waarbij er een associatie was met studies die een risico beschreven en er alleen relatieve risico’s (en niet de absolute risico’s) genoemd worden in de samenvatting. Dit suggereert dat een mogelijk risico van medicatiegebruik tijdens de zwangerschap een belangrijk thema is (Vigod, 2018). Een recente studie vanuit het Radboud UMC liet zien dat veel zwangeren in hun zoektocht naar informatie over zwangerschap en medicatiegebruik veel onjuiste informatie tegenkomen, hetgeen hun beslisvorming in negatieve zin kan beïnvloeden (van Gelder, 2019).
Samenvattend lijkt de veiligheid van medicatie gedurende de zwangerschap een belangrijke overweging voor zwangeren in de besluitvorming rondom het gebruik van psychotrope medicatie tijdens de zwangerschap. Hiernaast speelt bijwerkingen profiel, subjectief welbevinden en stigma een rol. Ten slotte is er een toename van activiteit op sociale media na publicaties van studies ten aanzien van risico, en staakt 50% van de vrouwen die antipsychotica gebruikt in de drie maanden voorafgaand aan de zwangerschap, deze bij zwangerschap.
Informatie over het gebruik van geneesmiddelen rondom de zwangerschap wordt verzameld door middel van patiëntenvragenlijsten via https://www.pregnant.nl/ en https://www.moedersvanmorgen.nl/.
Kosten
De kosten van de verschillende antipsychotica liggen dusdanig dicht bij elkaar dat de kosten per middel geen rol van betekenis spelen bij de medicatie keuze. Het staken van de antipsychotica tijdens de zwangerschap of het kraambed kan in sommige situaties leiden tot ernstige ontsporing, met mogelijk klinische opname en zorgmijding met alle kosten van dien. Daarnaast kan daaruit voortvloeiende ernstige stress en verminderde capaciteit tot adequate interactie met het kind de ontwikkeling van het kind in de cruciale periode van de eerste 1000 dagen ongunstig beïnvloeden met verhoogde kans op ontwikkelings- gedrags- en emotionele problemen later in het leven, hetgeen zal leiden tot middelenbeslag. Ook kan de noodzaak tot het tijdelijk of langdurig overnemen van de zorg voor het kind door derden leiden tot een aanzienlijk middelenbeslag.
Aanvaardbaarheid, haalbaarheid en implementatie
De werkgroep vindt antipsychotica gebruik tijdens zwangerschap met betrekking tot het risico op zwangerschapscomplicaties een aanvaardbare interventie mits er een juiste indicatie is en er een goede afweging is gemaakt ten aanzien van eventuele alternatieve behandelstrategieën.
Door implementatie van netwerkzorg voor zwangere vrouwen met antipsychoticagebruik, zoals bijvoorbeeld een multidisciplinaire POP-poli/ team, zullen alle betrokkenen (huisarts, verloskundig zorgverlener, GGZ zorgverlener (psychiater, psycholoog, verpleegkundig specialist, sociaal-psychiatrisch verpleegkundige (SPV), physician assistant (PA)), kraamverzorgster, maatschappelijke werker, medewerkers van jeugdgezondheidszorg (CB/CJG) en kinderarts) tijdig geïnformeerd zijn over de afwegingen voor de individuele zwangere om een antipsychoticum wel of niet te continueren en eventuele extra controles die in de zwangerschap of rondom de bevalling dienen plaats te vinden. Ook maatschappelijk bestaat er een inzicht in en groeiend draagvlak voor het belang van geïntegreerde zorg voor moeder en kind gedurende de eerste 1000 dagen van de ontwikkeling, zoals ook tot uitdrukking komt in het landelijke programma ‘kansrijke start’ (Ministerie van Volksgezondheid, Welzijn en Sport, 2018).
Rationale/ balans tussen de argumenten voor en tegen de interventie
Op basis van de momenteel voor handen zijnde gegevens ten aanzien van het risico op zwangerschaps- en bevallingscomplicaties, zoals risico op zwangerschapsdiabetes, zwangerschaps-geïnduceerde hypertensie, pre-eclampsie, spontane abortus, placentaire afwijkingen, ectopische zwangerschap, intra-uteriene vruchtdood, vroeggeboorte (< 37 weken), sectio caesarea, assisted vaginal delivery (forceps, vacuüm extractie), postpartum fluxus (total blood loss, TBL> 500 ml) geldt dat het relatieve onbekende risico afgewogen moet worden tegen de noodzaak van het gebruik tijdens de zwangerschap. Daarbij moet in acht worden genomen dat er gekeken moet worden naar een maximale inzet van niet-medicamenteuze interventies en de voorkeuren van de (toekomstige) zwangere. Te allen tijde dient gestreefd te worden naar een zo laag mogelijke dosering met een voldoende veilig maar ook doelmatig/ effectief antipsychoticum.
Als gevolg van het ontbreken van eenduidige evidence uit de literatuur, kan de werkgroep op de meeste gebieden geen harde aanbeveling doen over het gebruik van antipsychotica tijdens de zwangerschap, wat betreft de zwangerschapscomplicaties. Zwangerschapsdiabetes vormt hier naar onze mening een uitzondering op, zoals beschreven onder “overige relevante bronnen”. Screening op diabetes met een orale glucose tolerantie test is hierbij een aanbeveling.
Vanuit de voor deze uitgangsvraag gebruikte literatuurstudie is er niet een specifiek antipsychoticum met een sterke voorkeur. De voorkeuren vanuit het TIS, i.e. haloperidol, olanzapine, quetiapine of aripiprazol hebben waarschijnlijk te maken met de hoeveelheid onderzoek. Als we alleen kijken naar het metabole profiel kunnen haloperidol en aripiprazol als meer voordelig worden aangerekend in vergelijking met bijvoorbeeld clozapine, olanzapine, quetiapine en risperidon.
Clozapine vormt ook een speciale groep binnen de antipsychotica. Ten eerste omdat deze behandeling pas een optie wordt als er op de andere antipsychotica voldoende therapie resistentie is aangewezen (Essali, 2009). Om deze reden is gebruik binnen de zwangere populatie eerder uitzondering dan regel. Daarnaast heeft clozapine een afwijkend bijwerkingenprofiel ten opzichte van de andere antipsychotica, vanwege potentieel ernstige bijwerkingen, zoals agranulocytose (tot 1% en reversibel na staken), sedatie, orthostatische hypotensie (vooral in het begin van de behandeling), speekselvloed, obstipatie en gewichtstoename (Tiihonen, 2009). Ook zal tijdens de zwangerschap aandacht moeten zijn voor de veranderde farmacokinetiek van onder andere CYP2D6, CYP1A2 en CYP3A4. Rookgedrag, en voornamelijk het plots staken tijdens de zwangerschap, heeft effect op de plasmaspiegel van clozapine (spiegel zal stijgen als het roken stopt; Kroon, 2007). De ervaring met clozapine tijdens de zwangerschap en lactatie is zeer beperkt. Er is onvoldoende bekend om definitieve uitspraken te doen (Metha, 2017).
Onderbouwing
Achtergrond
Antipsychotica zijn over het algemeen effectief in de behandeling en preventie van psychotische stoornissen. Stabiliteit is van belang voor zowel de zwangere als het (ongeboren) kind. Antipsychotica bereiken, middels trans-placentaire passage, ook het (ongeboren) kind. Vanuit de praktijk is de ervaring dat de impact op het kind een belangrijke overweging is voor zwangeren in het starten of continueren van antipsychotica. Voor een goede afweging en shared decision-making is adequate informatie over de impact op de ontwikkeling van het kind van groot belang. Antipsychotica worden in de wetenschappelijke literatuur meestal ingedeeld in typische en atypische antipsychotica.
Conclusies / Summary of Findings
|
Very low GRADE |
The evidence is very uncertain about the effect of antipsychotics on the risk of gestational diabetes in pregnant women with psychiatric disorders, compared to women with a history of psychiatric disorders who discontinued antipsychotics during pregnancy, and to healthy pregnant women.
Bronnen: (Kucukgoncu, 2019) |
|
Very low GRADE |
The evidence is very uncertain about the effect of antipsychotics on the risk of pre-eclampsia in pregnant women with psychiatric disorders, compared to women who discontinued antipsychotics during pregnancy, and to healthy pregnant women.
Bronnen: (Reis, 2008; Petersen, 2016; Bellet, 2015) |
|
Very low GRADE |
The evidence is very uncertain about the effect of antipsychotics on the risk of spontaneous abortion in pregnant women with psychiatric disorders, compared to healthy pregnant women.
Bronnen: (Habermann, 2013; Bellet, 2015; McKenna, 2005; Sorensen, 2015) |
|
Very low GRADE |
The evidence is very uncertain about the effect of antipsychotics on the risk of placental abnormalities (placenta previa, abruptio placentae) in pregnant women with psychiatric disorders, compared to healthy pregnant women.
Bronnen: (Reis, 2008) |
|
Very low GRADE |
The evidence is very uncertain about the effect of antipsychotics on delivery by caesarean section in pregnant women with psychiatric disorders, compared to women who discontinued antipsychotics during pregnancy.
Bronnen: (Petersen, 2016; Reis, 2008) |
|
Very low GRADE |
The evidence is very uncertain about the effect of antipsychotics on the risk of stillbirth in pregnant women with psychiatric disorders, compared to healthy pregnant women and women who discontinued antipsychotics during pregnancy.
Bronnen: (Reis, 2008; Habermann, 2013; McKenna, 2005; Sorensen, 2015) |
|
Very low GRADE |
The evidence is very uncertain about the effect of antipsychotics on the risk of preterm delivery (< 37 weeks of gestation) in pregnant women with psychiatric disorders, compared to healthy pregnant women and women who discontinued antipsychotics during pregnancy.
Bronnen: (Reis, 2008; Lin, 2010; Habermann, 2013; Bellet, 2015; Sadowski, 2013; Vigod, 2015) |
|
- GRADE |
No conclusions could be drawn about the effect of antipsychotic exposure during pregnancy on the risk of ectopic pregnancy, pregnancy-induced hypertension, assisted vaginal delivery (forceps and vacuum extraction) and postpartum hemorrhage (> 500 ml TBL), because of the absence of relevant comparative studies. |
Samenvatting literatuur
Description of studies
The studies in the meta-analysis of gestational diabetes by Kucukgoncu (2019) were retrospective analyses of prospectively collected data from hospitals, registries of medical and prescription data, or teratology information services. The total number of subjects in the meta-analysis was 6213 in the antipsychotic-exposed group, 6836 in the antipsychotic-ceased control group, and 1.677.087 in the healthy unexposed control group (Kucukgoncu, 2019). Eight studies in the meta-analysis compared antipsychotic-exposed group with healthy unexposed controls (McKenna, 2005; Reis, 2008; Boden, 2012; Sadowski, 2013; Bellet, 2015; Vigod, 2015; Petersen, 2016; Panchaud, 2017). Three studies had an antipsychotic-ceased control group (Petersen, 2016; Park, 2018; Galbally, 2018), but only two studies (Petersen, 2016; Park, 2018) reported adjusted relative risks for the comparison with the antipsychotic-ceased group.
The window of antipsychotic exposure in the included studies was defined as either at least one exposure during first trimester (Bellet, 2015; Galbally, 2018; McKenna, 2005; Panchaud, 2017; Reis, 2008) or at least one exposure during first or second trimester (Park, 2018; Petersen, 2016; Vigod, 2015), or exposure any time during pregnancy (Boden, 2012; Sadowski, 2013).
Four studies investigated atypical (second-generation) antipsychotics as a group (Park, 2018; McKenna, 2005; Panchaud, 2017; Sadowksi, 2013). Individual atypical antipsychotics were not investigated in most studies, except aripiprazole in two studies (Bellet, 2015 and Galbally, 2018), and clozapine and olanzapine in one study (Boden, 2012). Four other studies investigated both atypical and typical (first generation) antipsychotics as a group (Reis, 2008, Boden, 2012; Vigod, 2015; Petersen, 2016). A stratification by type of antipsychotics (typical versus atypical) had not been performed (Kucukgoncu, 2019).
Only four out of eight studies reported adjusted effect estimates for the comparison of the antipsychotic exposed group and the unexposed healthy controls (Reis, 2008; Boden, 2012; Vigod, 2015; Petersen, 2016). The clinical definition of gestational diabetes was not provided in all of the studies. The outcome was defined based on medical charts, delivery records, patient reports verified by general practitioners/health providers or using ICD-10 codes for gestational diabetes contained within the databases.
Six studies describing other relevant outcome measures were included in the meta-analysis by Kucukgoncu (2019) (McKenna, 2005; Reis, 2008; Sadowski, 2013; Bellet, 2015; Vigod, 2015; Petersen, 2016), except for the studies by Habermann (2013), Lin (2010) and Sorensen (2015). Habermann (2013) was a retrospective analysis of 845 pregnant women in a Teratology information service database that were exposed to typical and atypical antipsychotics, and were compared to women who had called Teratology Information System (TIS) about other known non-teratogenic medications. Habermann (2013) investigated the cumulative incidence of spontaneous abortions and reported frequencies of stillbirth and preterm birth.
Lin (2010) was a retrospective analysis of a population-based prospective cohort in Taiwan National Health Insurance Research Database. This study in 242 pregnant women exposed to atypical antipsychotics during pregnancy reported on the risk of preterm birth (Lin, 2010).
Sorensen (2015) was a retrospective cohort study on the risk of spontaneous abortion and stillbirth conducted in nationwide registries of medical and prescription records in Denmark. It included 1881 women exposed to antipsychotics during pregnancy. The definition of stillbirth in Sorensen (2015) was based on gestational age of 22 weeks or more. The definitions of stillbirth used in other studies were different. In Reis (2008) the definition was > 28 gestational weeks, in Habermann (2013) and in McKenna (2005) no definitions were provided (the variables were coded as live birth or stillbirth).
Results
Results are presented by outcome measure, starting with critical outcomes.
Risk of gestational diabetes mellitus (GDM) (critical outcome)
Adjusted RR for GDM was nominally statistically significantly increased in the antipsychotic exposure group compared to healthy controls (RR 1.30, 95% CI 1.02 to 1.66, Figure 1; Kucukgoncu 2019). However, when the antipsychotic-exposed group was compared to controls with psychiatric illness who discontinued antipsychotics during pregnancy, this effect was not present any more (RR 0.78, 95% CI 0.28 to 2.16), Figure 2, Kucukgoncu 2019, Supplement).
Figure 1 Adjusted relative risk of GDM for comparison of antipsychotic users with healthy unexposed group (random effects model; Kucukgoncu, 2019)
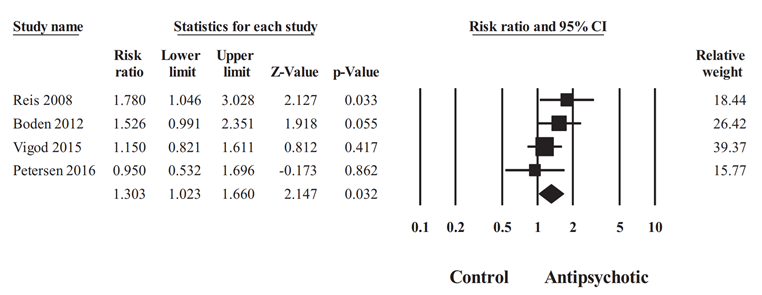
Heterogeneity: Tau2= 0.009; I2 = 14.37%; Q = 3.504, df = 3, P=0.032. Overall effect (fixed model):
1.29 (95% CI 1.04 to 1.61), Z = 2.321, P =0.02
Figure 2 Adjusted relative risk of GDM for comparison of antipsychotic users (continuers) with a group of women with psychiatric disorders that discontinued antipsychotics during pregnancy (random effects model; Kucukgoncu, 2019)
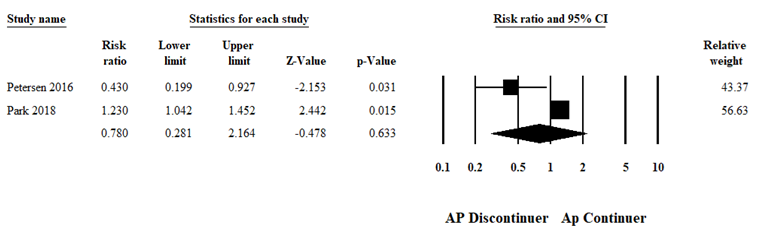
Heterogeneity: Tau2 =0.47; I2=85.43%; Q=6.86, d.f=1, p=0.009. Overall effect (fixed model): RR 1.17 (95% CI 0.99 to 1.38), Z=1.932, p=0.053
An estimation of RR separately for typical and atypical antipsychotics could not be performed, because stratification by type of antipsychotic was not provided in the studies in the meta-analysis by Kucukgoncu (2019). One of the studies in the meta-analysis (Petersen, 2016) did report on both types of antipsychotics, but had a low number of events per group, and RR was estimated for the whole group.
Risk of (pre-)eclampsia (critical outcome)
Petersen (2016) compared women taking antipsychotics with women that discontinued antipsychotics before pregnancy and healthy women (without prescriptions for antipsychotics within 2 years before pregnancy and throughout pregnancy) (Petersen, 2016).
The absolute risk of pre-eclampsia in the antipsychotic-exposed group was 4,3% (Petersen, 2016). When stratified by the type of antipsychotics (typical and atypical), the absolute risk of pre-eclampsia was 5.1% in the typical antipsychotics group, and 4.3% in the atypical antipsychotics group (Petersen, 2016). There was no statistically significant difference in absolute risk of pre-eclampsia between exposed and unexposed patients.
The adjusted relative risk compared to discontinuers was not increased, RR 0.69 (95% CI 0.37 to 1.29, p=0.25; Petersen, 2016). Compared to healthy women who were not on antipsychotic treatment before pregnancy, it was also not statistically significantly higher, RR 1.24 (95% CI 0.79 to 1.96, p=0.34; Petersen, 2016).
Reis (2008) performed analysis in a Swedish population-based register of births and found 27 cases of pre-eclampsia among 570 women taking antipsychotics during pregnancy, compared to other women in the database: OR 1.09 (95% CI 0.74 to 1.62), adjusted for BMI (Reis, 2008).
Bellet (2015) found no increase in rates of pre-eclampsia in women taking aripiprazole (65% in the first trimester only) compared to unexposed women, crude OR 0.66 (95% CI 0.07 to 6.47; n=1 in exposed and n=3 in unexposed).
Figure 3 Adjusted relative risk of pre-eclamsia for comparison of antipsychotic users with women not taking antipsychotics (Petersen, 2016; Reis, 2008)
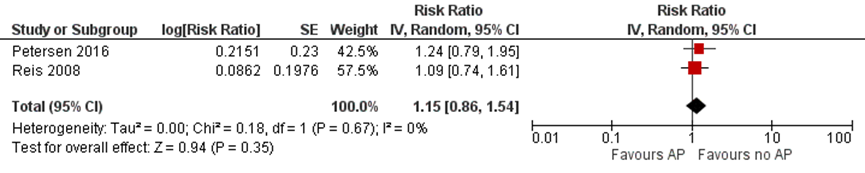
*Bellet (2015) was not included because only unadjusted effect estimate was reported and the event numbers were extremely small
In summary, there were three studies investigating the risk of pre-eclampsia in women taking antipsychotics during pregnancy (Petersen, 2016; Reis, 2008; Bellet, 2015). None of the studies reported an increased risk, compared with healthy unexposed controls and patients who discontinued antipsychotics before pregnancy. Also the pooled adjusted estimate for comparison with healthy women was not statistically significant (Figure 3).
Risk of spontaneous abortion (critical outcome)
In this literature analysis the term ‘spontaneous abortion’ was used for reporting the risk of miscarriage, because this term was used in the included studies.
In the study by Habermann (2013), the cumulative incidence of spontaneous abortions differed slightly, but not statistically significantly, among women taking atypical, typical and no antipsychotics: 24% (95% CI 14 to 39), 16% (95% CI 10 to 26) and 20% (95% CI 15 to 26), respectively (Habermann, 2013).
In the study by Bellet (2015) there were no differences in the rates of spontaneous abortion between the group exposed to aripiprazole and the unexposed group, OR 1.66 (95%CI 0.63 to 4.38) (Bellet, 2015).
McKenna (2005) found no differences in the rates of spontaneous abortions between women exposed to atypical antipsychotics and unexposed women (crude OR 1.81 (95% CI 0.88 to 3.74); McKenna, 2005).
However, in a large database study, Sorensen (2015) showed that women exposed to antipsychotics during pregnancy had a 34% higher risk of spontaneous abortion (adjusted RR 1.34 (95% CI 1.22 to 1.46)), compared to unexposed women, but a similar risk compared to women exposed prior to (but not during) pregnancy (adjusted RR 1.04 (95% CI 0.93 to 1.17). The adjustment was performed on maternal age, history of misuse, cohabitation, income and level of education (Sorensen, 2015).
In summary, four studies reported on spontaneous abortion in women taking antipsychotics during pregnancy (Habermann, 2013; Bellet, 2015; McKenna, 2005; Sorensen, 2015). Three studies found no differences in the rates of spontaneous abortions between exposed and unexposed women, and one study found an increased risk compared to healthy unexposed women, but not to women who previously took antipsychotics (Sorensen, 2015). The authors of the study reporting the increased risk indicated that residual confounding may be responsible for the observed effect (Sorensen, 2015).
Risk of placental abnormalities (placenta previa, abruptio placentae) (critical outcome)
Reis (2008) found 1 case of placenta previa and 5 cases of abruptio placentae in 570 women taking antipsychotics during pregnancy (RR 0.48 (95%CI 0.01 to 2.05) and RR 1.12 (95% CI 0.36 to 2.62), respectively, compared to other women in the database without antipsychotic exposure (Reis, 2008).
Risk of delivery by caesarean section (critical outcome)
The absolute risk of delivery by caesarean section in the antipsychotic-exposed group was 25%, compared with 21.6% and 18.4% in the discontinued and healthy groups, respectively (Petersen, 2016). The risk difference was statistically significant, showing a higher risk of delivery by caesarean section in antipsychotic users compared to healthy controls: 6.6 (95% CI 2.5 to 10.8) (Petersen, 2016). After stratifying on typical and atypical antipsychotics, the absolute risk of delivery by caesarean section was higher in women exposed to typical antipsychotics compared to healthy controls (26.8% versus 18.4%, risk difference 8.4 (95% CI 1.4 to 15.3), but it was not statistically significantly increased compared to discontinuers of antipsychotics (20.9%, risk difference 5.8 (95% CI -2.2 to 13.8); Petersen, 2016). The absolute risk of delivery by caesarean section in users of atypical antipsychotics was 24.6%. It was statistically significantly higher compared to healthy controls (18.4%, risk difference 6.3 (95% CI 1.2 to 11.3), but not increased compared to the discontinuers (22.2%, risk difference 2.5 (95% CI -4.4 to 9.3); Petersen, 2016). However, the relative risk of delivery by caesarean section adjusted for age, obesity, concomitant medications, excessive alcohol, smoking and illicit drug use, was not statistically significantly increased, RR 1.05 (95% CI 0.82 to 1.34; p=0.67), compared with discontinuers, and RR 1.09 (95% CI 0.92 to 1.30; p=0.28) compared with healthy controls (Petersen, 2016).
In the study by Reis (2008), the risk of delivering by caesarean section, adjusted for BMI, in women exposed to antipsychotics during pregnancy, compared to healthy controls, was also not statistically significantly increased (OR 1.43 (95% CI 1.17 to 1.74) (Reis, 2008).
Figure 4 Adjusted relative risk of caesarean section for comparison of antipsychotic users with women not taking antipsychotics (Petersen, 2016; Reis, 2008)
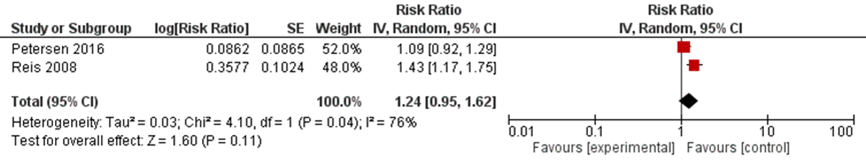
In summary, two studies reported on the risk of delivery by caesarean section (Petersen, 2016; Reis, 2008). Adjusted effect estimates suggest no increase in the risk of delivery by caesarean section. The pooled adjusted estimate also shows no statistically significant difference between antipsychotic drug-exposed and unexposed women (Figure 4).
However, the absolute risk of delivery by caesarian section in users of typical and atypical antipsychotics was increased compared to healthy unexposed controls (Petersen, 2016).
Risk of stillbirth (critical outcome)
Reis (2008) reported 5 cases of stillbirth among 576 children of women who received antipsychotics in the first trimester of pregnancy. There was no increase in the risk of stillbirth in the antipsychotics-exposed group, compared to the unexposed, OR 1.48 (95% CI 0.48 to 3.47), after adjustment for the year of birth, maternal age, parity, smoking and previous miscarriages (Reis, 2008).
In the study by Habermann (2013) that utilized data from a TIS, no cases of stillbirth occurred in 561 women taking atypical antipsychotics during pregnancy, compared to 2 cases 284 women taking typical antipsychotics and 88 cases in 1122 women not taking antipsychotics (Habermann, 2013).
McKenna (2005) found no differences in the percentages of stillbirths between the exposed and unexposed groups (n=4 (2.6%) versus n=4 (2.6%); crude OR 1.00 (95% CI 0.25 to 4.07).
However, in a large database study, a twofold higher risk of stillbirth (> 22 weeks gestation) was found in women exposed to antipsychotic medications compared with unexposed women (crude RR 2.27 (95% CI 1.45 to 3.55) and compared with women exposed only prior to pregnancy (crude RR 2.06 (95% CI 1.01 to 4.19) (Sorensen, 2015).
In summary, the risk of stillbirth was described in four studies (Reis, 2008; Habermann, 2013; McKenna, 2005; Sorensen, 2015). Three studies found no increase in risk, however the definitions of stillbirth were not available in most studies. Sorensen (2015) provided crude relative risks which indicated an increase in risk compared to healthy controls and discontinuers of antipsychotics, however the authors of the study indicated a strong possibility of inflated results due to confounding.
Risk of preterm delivery (< 37 weeks gestation) (critical outcome)
Including only singleton pregnancies in the analysis (n=563), Reis (2008) found an increased risk for preterm birth (< 37 weeks gestation) in women taking any antipsychotics in the first trimester of pregnancy, compared to women not taking antipsychotics (adjusted OR 1.73 (95% CI 1.31 to 2.29), Reis, 2008).
In a group of women with schizophrenia treated with typical antipsychotics during pregnancy, Lin (2010) found an increased risk of preterm birth (OR adj. 2.46 (95% CI 1.50 to 4.11)), compared to women with schizophrenia not taking antipsychotics during pregnancy (Lin, 2010). The risk was not increased in women with schizophrenia treated with atypical antipsychotics during pregnancy compared to untreated women with schizophrenia (OR adj. 1.61 (95% CI 0.63 to 4.12), Lin, 2010).
Habermann (2013) reported crude OR’s for preterm deliveries in women taking atypical, typical and no antipsychotics. There was no difference between the risk of preterm delivery between women taking atypical antipsychotics and no antipsychotics (crude OR 1.06 (95% CI 0.72 to 1.56). However, women taking typical antipsychotics during pregnancy seemed to have a higher risk of preterm delivery compared with women not taking antipsychotics (crude OR 1.96 (95% CI 1.29 to 2.98); Habermann, 2013). Compared to users of typical antipsychotics, users of atypical antipsychotics had a lower (unadjusted) risk of preterm delivery (crude OR 0.54 (95% CI 0.33 to 0.87); Habermann, 2013).
Bellet (2015) found an increased rate of preterm delivery in women taking aripiprazole (atypical antipsychotic) compared to unexposed women, crude OR 2.57 (95% CI 1.06 to 6.27).
Compared to a healthy control group, women exposed to atypical antipsychotics in the study by Sadowski (2013) did not have statistically significantly higher rates of preterm deliveries (12 (10.6%)/ 5 (4.3%), p=0.071; crude OR 2.54 (95% CI 0.87 to 7.42); Sadowski, 2013).
Vigod (2015) showed that women exposed to any type of antipsychotics (n=1209) had an increased risk of preterm delivery (crude RR 1.51 (95% CI 1.29 to 1.78)), compared to unmatched healthy controls (n=40 314). However, compared to matched unexposed controls (n=1021) with psychiatric disorders, the risk of preterm delivery in the antipsychotic-exposed group (n=1021) was not increased (adjusted RR 0.99 (95% CI 0.78 to 1.26). Furthermore, when the analysis was restricted to 923 (out of 1021) cases exposed only to atypical antipsychotics, compared to matched unexposed controls with psychiatric disorders, there was also no increased risk of preterm delivery (Figure 5; Vigod, 2015).
Figure 5 Adjusted relative risks for preterm birth (< 37 weeks) by individual atypical antipsychotic in the matched cohort (restricted to users of atypical antipsychotics, n=923; Vigod, 2015). The risk of preterm delivery was not significantly different between antipsychotic users and matched non-users (Vigod, 2015)
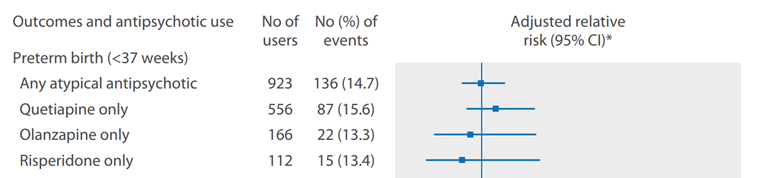
*Adjusted for a prescribed selective serotonin reuptake inhibitor (SSRI), non-SSRI, mood stabiliser, and/or benzodiazepine medication during the index pregnancy (Vigod, 2015)
The six studies investigating preterm delivery showed varying results. Four studies using a healthy control group reported an increased risk of preterm delivery in antipsychotic-exposed women (Reis, 2008; Vigod, 2015; Habermann, 2013; Bellet, 2015). One of the four studies reported an increased risk only for typical antipsychotics (Habermann, 2013), another two for any antipsychotics (as a group) (Vigod, 2015; Reis, 2008) and one study reported an increased risk for aripiprazole (Bellet, 2015). The risk of preterm delivery in users of typical antipsychotics was increased in comparison to untreated women with psychiatric disorders in one study (Lin, 2010). Four studies found no increase in risk of preterm delivery with atypical antipsychotics: two of these studies (Habermann, 2013; Sadowski, 2013) in comparison to healthy controls, an two studies (Lin, 2010; Vigod, 2015) compared to matched controls with psychiatric disorders.
Ectopic pregnancy (important outcome)
No studies assessed the association of antipsychotic use with the risk of ectopic pregnancy in women with psychiatric disorders, compared to women not taking antipsychotics.
Pregnancy-induced hypertension (important outcome)
No studies assessed the association of antipsychotic use with the risk of pregnancy-induced hypertension in women with psychiatric disorders, compared to women not taking antipsychotics.
Postpartum hemorrhage (TBL>500 ml) (important outcome)
No studies assessed the association of antipsychotic use with the risk of postpartum hemorrhage in women with psychiatric disorders, compared to women not taking antipsychotics.
Forceps delivery (important outcome)
No studies assessed the association of antipsychotic use with the risk of forceps delivery in women with psychiatric disorders, compared to women not taking antipsychotics.
Vacuum-assisted vaginal delivery (important outcome)
No studies assessed the association of antipsychotic use with the risk of vacuum-assisted vaginal delivery in women with psychiatric disorders, compared to women not taking antipsychotics.
Level of evidence of the literature
We started with a low level of evidence for observational studies. The quality of evidence regarding the outcome measure ‘risk of gestational diabetes’ was downgraded to very low, because of bias due to serious indirectness (comparison to healthy control group, multiple antipsychotics from different classes combined into one group, differences in exposure), inconsistency (varying results) and study limitations (risk of bias due to confounding in the included studies).
The quality of evidence regarding the outcome measure ‘risk of pre-eclampsia’ was downgraded to very low, because of study limitations (risk of bias), and bias due to indirectness (comparison to healthy controls).
The quality of evidence regarding the outcome measure ‘risk of spontaneous abortion’ was downgraded to very low, because of serious study limitations (high risk of bias), bias due to indirectness (comparison to healthy controls) and inconsistency (conflicting results).
The quality of evidence regarding the outcome measure ‘risk of placental abnormalities’ was downgraded to very low, because of serious imprecision (only one study), bias due to indirectness (comparison to healthy controls) and study limitations (risk of bias).
The quality of evidence regarding the outcome measure ‘risk of delivery by caesarean section’ was downgraded to very low, because of study limitations (risk of bias) and bias due to indirectness (comparison to healthy controls).
The quality of evidence regarding the outcome measure ‘risk of stillbirth’ was downgraded to very low, because of serious study limitations (high risk of bias), conflicting results (inconsistency) and bias due to indirectness (comparison to healthy controls).
The quality of evidence regarding the outcome measure ‘risk of preterm delivery (< 37 weeks)’ was downgraded to very low, because of study limitations (risk of bias), indirectness (comparison to healthy controls) and inconsistency (conflicting results).
The assessment of the level of the quality of evidence for the outcomes ‘risk of ectopic pregnancy’, ‘risk of pregnancy-induced hypertension’, ‘risk of forceps delivery’, ‘risk of vacuum-assisted delivery)’ and ‘risk of postpartum hemorrhage (>500 ml total blood loss (TBL))’ was not performed due to the absence of relevant literature.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following questions:
1. What is the risk of pregnancy and labor complications in women taking antipsychotics during pregnancy, compared to healthy pregnant women?
P: patients pregnant women;
I: intervention antipsychotic use during pregnancy;
C: control no antipsychotic use during pregnancy;
O: outcome risk of gestational diabetes, pregnancy-induced hypertension, pre-eclampsia, spontaneous abortion, placental abnormalities (placenta previa, abruptio placentae), ectopic pregnancy, stillbirth, risk of preterm delivery (< 37 weeks), risk of delivery by caesarean section, risk of forceps delivery, risk of vacuum-assisted delivery, risk of postpartum hemorrhage (total blood loss (TBL)> 500 ml).
2. What is the effect of antipsychotic use during pregnancy on the risk of pregnancy and labor complications in women with psychiatric disorders, compared to pregnant women with psychiatric disorders not taking antipsychotics or taking a different antipsychotic?
P: patients pregnant women with psychiatric disorders;
I: intervention antipsychotic use during pregnancy;
C: control no antipsychotic use during pregnancy (discontinuation) or use of a different antipsychotic drug (comparison between different antipsychotics);
O: outcome risk of gestational diabetes, pregnancy-induced hypertension, pre-eclampsia, spontaneous abortion, placental abnormalities (placenta previa, abruptio placentae), ectopic pregnancy, stillbirth, risk of preterm delivery (< 37 weeks), risk of delivery by caesarean section, risk of forceps delivery, risk of vacuum-assisted delivery, risk of postpartum hemorrhage (total blood loss (TBL)> 500 ml).
Relevant outcome measures
The guideline development group considered the risk of gestational diabetes, pre-eclampsia, spontaneous abortion, placental abnormalities (placenta previa, abruptio placentae), stillbirth, preterm delivery (< 37 weeks) and delivery by caesarean section as critical outcome measures, and the risk of ectopic pregnancy, pregnancy-induced hypertension, postpartum hemorrhage (TBL> 500 ml), forceps delivery and vacuum-assisted vaginal delivery as important outcome measures for decision making.
The minimal (clinically) important difference was defined according to the default recommendations of the international GRADE working group, as follows: for dichotomous outcomes as a relative risk reduction or an increase of 25% or more, and for continuous outcomes as a difference of half (0.5) a standard deviation.
A priori, the working group did not define the outcome measures listed above but used the definitions used in the studies.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from the 1st of January 1960 until the 29th of July 2019. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 427 hits. Studies were selected based on the following criteria: systematic reviews (with meta-analyses) and comparative observational (case-control and cohort) studies, investigating the risk of pregnancy and labor complications in women with psychiatric disorders taking antipsychotics during pregnancy, compared with healthy pregnant women, and pregnant women with psychiatric disorders not taking antipsychotics or taking a different antipsychotic. Twenty-six studies were initially selected based on title and abstract screening. After reading the full text, 12 studies were excluded (see the table with reasons for exclusion under the tab Methods) and 14 studies were included.
Results
Fourteen studies were included in the analysis of literature. One of them was a meta-analysis of studies reporting on the risk of gestational diabetes in women exposed to antipsychotics during pregnancy (Kucukgoncu, 2019). The meta-analysis included ten studies identified by the systematic search for this PICO and forms the basis of the literature analysis for the outcome ‘gestational diabetes’.
Three studies investigated the risk of pre-eclampsia (Petersen, 2016; Reis, 2008; Bellet, 2015), four studies reported on spontaneous abortion (Habermann, 2013; Bellet, 2015; McKenna, 2005; Sorensen, 2015), one study reported on placental abnormalities (Reis, 2008), two studies investigated the risk of delivery by caesarean section (Petersen, 2016; Reis, 2008), four studies assessed the risk of stillbirth (Reis, 2008; Habermann, 2013; McKenna, 2005; Sorensen, 2015), and six studies investigated the risk of premature birth (< 37 weeks of gestation) after antipsychotic treatment during pregnancy (Reis, 2008; Lin, 2010; Habermann, 2013; Bellet, 2015; Sadowski, 2013; Vigod, 2015).
Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- American College of Obstetricians and Gynecologists (ACOG) (2008). Use of psychiatric medications during pregnancy and lactation Clinical Practice Guidelines. Retrieved from https://www.guidelinecentral.com/summaries/use-of-psychiatric-medications-during-pregnancy-and-lactation/#section-society.
- Bellet, F., Beyens, M. N., Bernard, N., Beghin, D., Elefant, E., & Vial, T. (2015). Exposure to aripiprazole during embryogenesis: a prospective multicenter cohort study. Pharmacoepidemiology and drug safety, 24(4), 368-380.
- Bodén, R., Lundgren, M., Brandt, L., Reutfors, J., & Kieler, H. (2012). Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Archives of general psychiatry, 69(7), 715-721.
- Cahn, W., Ramlal, D., Bruggeman, R., De Haan, L., Scheepers, F. E., Van Soest, M. M.,... & Slooff, C. J. (2008). Preventie en behandeling van somatische complicaties bij antipsychoticagebruik. Tijdschr Psychiatr, 50(9), 579-91.
- Courtet, P. (2001). Viewpoint of schizophrenic patients: a European survey. L'Encephale, 27(1), 28-38.
- De Hert, M., Schreurs, V., Sweers, K., Van Eyck, D., Hanssens, L., Šinko, S.,... & van Winkel, R. (2008). Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: a retrospective chart review. Schizophrenia research, 101(1-3), 295-303.
- Essali, A., Haasan, N. A. H., Li, C., & Rathbone, J. (2009). Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database of Systematic Reviews, (1).
- Galbally, M., Frayne, J., Watson, S. J., & Snellen, M. (2018). Aripiprazole and pregnancy: a retrospective, multicentre study. Journal of affective disorders, 238, 593-596.
- Habermann, F., Fritzsche, J., Fuhlbrück, F., Wacker, E., Allignol, A., Weber-Schoendorfer, C.,... & Schaefer, C. (2013). Atypical antipsychotic drugs and pregnancy outcome: a prospective, cohort study. Journal of clinical psychopharmacology, 33(4), 453-462.
- Hippman, C., & Balneaves, L. G. (2018). Women's decision making about antidepressant use during pregnancy: A narrative review. Depression and anxiety, 35(12), 1158-1167.
- Kothari, A., de Laat, J., Dulhunty, J. M., & Bruxner, G. (2019). Perceptions of pregnant women regarding antidepressant and anxiolytic medication use during pregnancy. Australasian Psychiatry, 27(2), 117-120.
- Kroon, L. A. (2007). Drug interactions with smoking. American Journal of Health-System Pharmacy, 64(18), 1917-1921.
- Kucukgoncu, S., Guloksuz, S., Celik, K., Bahtiyar, M. O., Luykx, J. J., Rutten, B. P., & Tek, C. (2019). Antipsychotic Exposure in Pregnancy and the Risk of Gestational Diabetes: A Systematic Review and Meta-analysis. Schizophrenia Bulletin.
- McAllister-Williams, R. H., Baldwin, D. S., Cantwell, R., Easter, A., Gilvarry, E., Glover, V.,... & Khalifeh, H. (2017). British Association for Psychopharmacology consensus guidance on the use of psychotropic medication preconception, in pregnancy and postpartum 2017. Journal of Psychopharmacology, 31(5), 519-552.
- McKenna, K., Koren, G., Tetelbaum, M., Wilton, L., Shakir, S., Diav-Citrin, O.,... & Einarson, A. (2005). Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. The Journal of clinical psychiatry, 66(4), 444-9.
- Mehta, T. M., & Van Lieshout, R. J. (2017). A review of the safety of clozapine during pregnancy and lactation. Archives of women's mental health, 20(1), 1-9.
- Meltzer, H. Y. (2013). Update on typical and atypical antipsychotic drugs. Annual review of medicine, 64, 393-406.
- Ministerie van Volksgezondheid, Welzijn en Sport (2018). Richtlijn ‘Bijwerkingen antipsychotica bij schizofrenie’. Retrieved from https://kansrijkestart.nl/.
- National Institute for Health and Care Excellence. (2014) Antenatal and postnatal mental health: clinical management and service guidance (NICE Clinical guideline CG192). Retrieved from https://www.nice.org.uk/guidance/cg192.
- National Institute for Health and Care Excellence. (2015) Diabetes in pregnancy: management from preconception to the postnatal period (NICE guideline NG3). Retrieved from https://www.nice.org.uk/guidance/ng3.
- Nederlandse Vereniging voor Psychiatrie. (2012) Richtlijn Schizofrenie. Retrieved from https://richtlijnendatabase.nl/richtlijn/schizofrenie/schizofrenie_-_startpagina.html#tab-content-general.
- Nygaard, L., Rossen, C. B., & Buus, N. (2015). Balancing risk: a grounded theory study of pregnant women's decisions to (dis) continue antidepressant therapy. Issues in mental health nursing, 36(7), 485-492.
- Osborn, D. P. J. (2008). Wright C a, Levy G, King MB, Deo R, Nazareth I. Relative risk of diabetes, dyslipidaemia, hypertension and the metabolic syndrome in people with severe mental illnesses: systematic review and metaanalysis. BMC Psychiatry, 8, 84.
- Panchaud, A., Hernandez-Diaz, S., Freeman, M. P., Viguera, A. C., MacDonald, S. C., Sosinsky, A. Z., & Cohen, L. S. (2017). Use of atypical antipsychotics in pregnancy and maternal gestational diabetes. Journal of psychiatric research, 95, 84-90.
- Park, Y., Hernandez-Diaz, S., Bateman, B. T., Cohen, J. M., Desai, R. J., Patorno, E.,... & Huybrechts, K. F. (2018). Continuation of atypical antipsychotic medication during early pregnancy and the risk of gestational diabetes. American Journal of Psychiatry, 175(6), 564-574.
- Park, Y., Huybrechts, K. F., Cohen, J. M., Bateman, B. T., Desai, R. J., Patorno, E.,... & Hernandez-Diaz, S. (2017). Antipsychotic medication use among publicly insured pregnant women in the United States. Psychiatric services, 68(11), 1112-1119.
- Patel, J. K., Buckley, P. F., Woolson, S., Hamer, R. M., McEvoy, J. P., Perkins, D. O.,... & Cafe Investigators. (2009). Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophrenia research, 111(1-3), 9-16.
- Petersen, I., Sammon, C. J., McCrea, R. L., Osborn, D. P., Evans, S. J., Cowen, P. J., & Nazareth, I. (2016). Risks associated with antipsychotic treatment in pregnancy: comparative cohort studies based on electronic health records. Schizophrenia research, 176(2-3), 349-356.
- Regenold, W. T., Thapar, R. K., Marano, C., Gavirneni, S., & Kondapavuluru, P. V. (2002). Increased prevalence of type 2-diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. Journal of affective disorders, 70(1), 19-26.
- Reis, M., & Källén, B. (2008). Maternal use of antipsychotics in early pregnancy and delivery outcome. Journal of clinical psychopharmacology, 28(3), 279-288.
- Sadowski, A., Todorow, M., Brojeni, P. Y., Koren, G., & Nulman, I. (2013). Pregnancy outcomes following maternal exposure to second-generation antipsychotics given with other psychotropic drugs: a cohort study. BMJ open, 3(7), e003062.
- Sørensen, M. J., Kjaersgaard, M. I. S., Pedersen, H. S., Vestergaard, M., Christensen, J., Olsen, J.,... & Bech, B. H. (2015). Risk of fetal death after treatment with antipsychotic medications during pregnancy. PloS one, 10(7).
- Tiihonen, J., Lönnqvist, J., Wahlbeck, K., Klaukka, T., Niskanen, L., Tanskanen, A., & Haukka, J. (2009). 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). The Lancet, 374(9690), 620-627.
- van Gelder, M. M., Rog, A., Bredie, S. J., Kievit, W., Nordeng, H., & van de Belt, T. H. (2019). Social media monitoring on the perceived safety of medication use during pregnancy: A case study from the Netherlands. British journal of clinical pharmacology, 85(11), 2580-2590.
- Vigod, S. N., Bagheri, E., Zarrinkalam, F., Brown, H. K., Mamdani, M., & Ray, J. G. (2018). Online social network response to studies on antidepressant use in pregnancy. Journal of psychosomatic research, 106, 70-72.
- Vigod, S. N., Gomes, T., Wilton, A. S., Taylor, V. H., & Ray, J. G. (2015). Antipsychotic drug use in pregnancy: high dimensional, propensity matched, population based cohort study. bmj, 350, h2298.
- Wang, Z., Wong, I. C., Man, K. K., Alfageh, B. H., Mongkhon, P., & Brauer, R. (2019). The Use of Antipsychotic Agents During Pregnancy and the Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Available at SSRN 3408070.
Evidence tabellen
Evidence tables for systematic reviews of RCTs and observational studies (intervention studies)
Research question: What is the risk of pregnancy and labor complications in women with psychiatric disorders taking antipsychotics during pregnancy, compared to women with psychiatric disorders not taking antipsychotics and/or to healthy women?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison /control (C) |
Follow-up
|
Outcome measures and effect size |
Comments |
|
Kucukgoncu, 2019
individual study characteristics extracted from original studies included in the SR; effect measures extracted from Kucukgoncu, 2019
|
SR and meta-analysis of cohort studies
Literature search up to August, 2018
A: McKenna 2005 B: Reis 2008 C: Boden 2012 D: Sadowski 2013 E: Bellet 2015 F: Vigod 2015 G: Petersen 2016 H: Panchaud 2017 I: Park 2018 J: Galbally 2018
Study design: prospective cohort/retrospective analyses in different registries of prospectively collected data (A, B, C, D, E, F, G, H, I); retrospective cohort (J)
Setting and country: A: Teratogen information centers, including Motherisk Program at the Hospital for Sick Children in Toronto and an independent medical charity ‘Drug safety research unit’ in Southampton); Canada, Israel, England B: Swedish Medical Birth Register, Swedish Register of Congenital Malformations, the Hospital Discharge Register, Sweden C: Swedish Prescribed Drug Register, Medical Birth Register, National Patient Register, Sweden D: Motherisk Program at the Hospital for Sick Children, Toronto, Canada E: pharmacovigilance and teratogenic risk reporting databases, France F: multiple linked population health databases housed at the Institute for Clinical Evaluative Sciences (ICES) in Toronto, Ontario, Canada G: The Health Improvement Network (THIN) and the Clinical Practice Research Datalink (CPRD), UK H: Massachusetts General Hospital (MGH) National Pregnancy Registry for Atypical Antipsychotics; US I: Medicaid Analytic eXtract, a nationwide insurance claims database, US J: Mercy Hospital for Women in Victoria, King Edward Memorial Hospital in Western Australia, Australia
Source of funding and conflicts of interest: A, B, C, D, E, F, G, H, J: non-commercial; none reported I: grants from National institute of mental health and individual research grants from pharmaceutical companies; the sponsors had no roll in the design and conduct of the study |
Inclusion criteria SR: 1. randomized, controlled trials, case–control, or cohort studies that included an antipsychotic (AP)-exposed and non-exposed group 2. reported AP exposure during pregnancy and gestational diabetes outcomes 3. human research
Exclusion criteria SR: animal or in vitro studies, narrative reviews, case reports, book chapters, conference presentations; absence of a control group; exposure to AP unclear, overlap in patient data
8 of 10 studies included in the main analysis
Important patient characteristics at baseline:
N (exposed/unexposed) A: N=151/N=151 B: N= 570/N=958 729 C: N=169 (olanzapine+clozapine)/ N=338 (other AP)/ N=357 696 D: N=133/N=133 E: N=86/N=172 F: N=1209/N=40 314 (unmatched cohort) n=1021/n=1021 (matched cohort) G: (exposed/ discontinued/ unexposed) N=290/ N=492/ N=210 966 H: N=303/N=149 (for GDM N=33/N=16) I: N=2872/N=7507 -aripiprazole 419/1505 -ziprasidone 167/506 -quetiapine 1543/ 2990 -risperidone 359/1465 -olanzapine 384/1041 J: N=12/N=14
Mean age ± SD (years) (exposed/unexposed) A: NA B: NA C: NA D: 31.5±5.15/32.0±4.49 E: 31.8 ± 5.8/31.4 ± 5.4 F: 28.8±6.1/26.7±6.3 (unmatched cohort) 28.8±6.2/28.8±6.2 (matched cohort) G: NA (only categories) H: median age (IQR) 32.8 (29.4-36.1)/33.9 (31.3-36.4) I: unexposed: aripiprazole 24.8±7.2/ 24.4±6.7 ziprasidone 25.0±6.4/ 25.0±5.4 quetiapine 26.8±6.4/ 26.5±6.3 risperidone 25.3±7.4/ 25.5±7.5 olanzapine 28.5±6.9/ 27.1±6.4 J: 28.4±7.1/ 28.5±4.74
Co-medications (exposed group, %)#: A: 17% anti-epileptics; 12.4% valproate; 6% lithium; 57% anti-depressants; 34% benzodiazepines B: NA (for users of AP excluding dixyrazine or prochlorperoxine) C: NA; 87.9% used only 1 AP throughout pregnancy D: SSRI 34.6, benzodiazepine 15.8, anticonvulsants 14.3, SNRI 12.0, atypical antidepressants 9, non-benzo hypnotic 6, typical antipsychotic 3.8, serotonin antagonist and reuptake inhibitor 3, tetracyclic antidepressant 3, tricyclic antidepressant 2.3, norepinephrine reuptake inhibitor 1.5, synthetic cannabinoid 1.5, psychostimulant 0.8, GABA analogue 0.8, other dopamine antagonist 0.8 E: 72.1% of exposed patients had co-medications (benzodiazepines, antidepressants, other antipsychotics and anticonvulsants) F: 1.unmatched cohort SSRI 30.1; non-SSRI 26.6; mood stabilisers 13.8; benzodiazepines 25.3 2. matched cohort: SSRI 29.7; non-SSRI 25.9; mood stabilisers 10.3; benzodiazepines 21.7 G: anticonvulsant mood stabilisers 9.3; lithium 3.8); antidepressants 58.3; anxiolytics 10.7; hypnotics 14.1 H: antidepressants 54; Anticonvulsants 42 I: antidepressants, benzodiazepines, mood stabilizers, opioids J: NA
Indications, (%)#: A: depression (29), schizophrenia (24), bipolar disorder (18), schizoaffective (2), psychotic episode (7), psychotic depression (5), obsessive-compulsive disorder (2), PTSD (1), schizophreniform disorder (1) B: NA C: (olanzapine + clozapine)/other AP) schizophrenia: 24.9/18.9; other nonaffective psychosis: 20.1/16.3; bipolar disorder: 11.8/10.9 D: bipolar disorder (36.8), depression (27.1), anxiety and depression (9.8) sleep disorders (9.8), schizophrenia (3), schizoaffective disorders (1.5) E: schizophrenia (30.6), psychotic disorders not otherwise specified (19.4), bipolar disorders (6.7), depression (15.3) F: 1. unmatched cohort: psychotic disorder (35.5); bipolar disorder or major depression (77.5); personality disorder (32.5) 2. matched cohort: psychotic disorder (31.2); bipolar disorder or major depression (74.2); personality disorder (28.9) G: depression (27.2); epilepsy (5.9); SMI (62.1) H: bipolar disorder (62), depression (16), psychosis (7), anxiety (5), other (10), missing data (1) I: schizophrenia/ psychosis, bipolar disorder, depression, anxiety disorder, ADHD J: psychotic disorders (75), bipolar disorder (16.7), non-psychotic SMI (8.3) |
Exposure:
A: atypical AP during the 1st trimester of pregnancy (olanzapine n=60, risperidone n=49, quetiapine n=36, clozapine n=6) B: AP during the 1st trimester of pregnancy C: AP at any time during pregnancy 1. olanzapine (n=159) and/or clozapine (n=11) 2. all other AP, excluding olanzapine and clozapine (n, %): -quetiapine 90 (17.8) -risperidone 72 (14.2) -flupentixol 58 (11.4) -haloperidol 52 (10.3) -aripiprazole 38 (7.5) -perphenazine 35 (6.9) -zuclopenthixol 30 (5.9) -ziprasidone 18 (3.6) -chlorprothixene 9 (1.8) -fluphenazine 2 (0.4) -pimozide 1 (0.2) D: atypical AP for a minimum of 4 weeks of pregnancy (called TIS about safety of atypical AP) E: aripiprazole (5 to 30 mg/day) during pregnancy F: ≥2 consecutive prescriptions for AP between the conception date (estimated using the gestational age at birth) and the delivery date; at least one of the prescriptions was filled in the 1st or 2nd trimester G: prescriptions for AP during the 1st trimester of pregnancy H: atypical AP during pregnancy I: women without pre-existing diabetes, with a live-born infant, with ≥ 2 dispensings of aripiprazole, ziprasidone, quetiapine, risperidone, or olanzapine during the first 140 days of pregnancy, they used these drugs before pregnancy and did not switch to a different AP in the first 140 days J: aripirazole during pregnancy
|
Non-exposure:
A: no AP during pregnancy; taking drugs known to be non-teratogenic, also excluding other psychotropic drugs; B: all other women in the database (not using AP in the 1st trimester of pregnancy) C: no AP during pregnancy D: no AP during pregnancy, (called TIS about safety of exposure to non-teratogenic agents) E: no aripiprazole or exposed to agents known to be non-teratogenic F: not exposed to AP during Pregnancy G: 1. no AP in 2 years before pregnancy and during the whole pregnancy (through the delivery date); 2. AP in the 2 years before start of pregnancy, but no AP prescriptions issued after 4 weeks prior to pregnancy start H: no atypical AP during whole pregnancy I: discontinued aripiprazole, ziprasidone, quetiapine, risperidone, or olanzapine before the start of pregnancy and had no dispensing during the first 140 days of pregnancy J: discontinuation of aripiprazole during pregnancy
|
End-point of follow-up:
A: 3-4 months after delivery B: NA (used data from 1994-2005) C: NA (used data from 2005-2009) D: NA (used data from 2009-2012) E: within 2 months of the expected delivery date F: 180 days after delivery for exposure, 42 days for the outcome data G: NA H: assessment of outcome at 7 months gestation, end of follow-up 8-12 weeks postpartum I: NA (used data from 2000-2010) J: NA
For how many participants were no complete outcome data available? (exposed/unexposed) A: NA B: NA C: NA D: NA E: 15/11 F: NA G: NA H: total: 63 dropped out or lost to follow-up, 24 had an abortion I: NA J: NA
|
Outcome measure: gestational diabetes mellitus (GDM)
Effect measures:
Crude relative risk of GDM (comparison with healthy controls) A: RR 1.19 (95% CI 0.40-3.55) B: RR 2.58 (95% CI 1.54-4.33) C: RR 2.59 (95% CI 1.72-3.91) D: RR2.20 (95% CI 0.79-6.16) E: RR 1.14 (95% CI 0.34-3.79) F: RR 1.24 (95% CI 1.02-1.52) G: RR 1.61 (95% CI 0.89-2.89) H: RR 1.01 (95% CI 0.58-1.78)
Pooled effect -random effects model: RR 1.63 (95% CI 1.20-2.23) -fixed effects model: RR 1.48 (95% CI 1.27-1.72) Heterogeneity (I2): 59.64%
Adjusted relative risk of GDM (comparison with healthy controls) B: RR 1.78 (95% CI 1.05-3.03) C: RR 1.53 (95% CI 0.99-2.35) F: RR 1.15 (95% CI 0.82-1.61) G: RR 0.95 (95% CI 0.53-1.70)
Pooled effect -random effects model: RR 1.30 (95% CI 1.02-1.66) -fixed effects model: RR 1.29 (95% CI 1.04-1.61) Heterogeneity (I2): 14.37%;
*** Crude relative risk of GDM (comparison with AP discontinuers) G: RR 0.98 (95% CI 0.46-2.08) I: aripiprazole RR 1.06 (95% CI 0.65-1.72) ziprasidone RR 1.12 (95% CI 0.48-2.61) quetiapine RR 1.75 (95% CI 1.36-2.25) risperidone RR 1.56 (95% CI 0.98-2.49) olanzapine RR 2.55 (95% CI 1.73-3.75) J: RR 1.17 (95% CI 0.08-16.72)
Pooled effect (for comparison with discontinuers) -random effects model: RR 1.55 (95% CI 1.19-2.03) -fixed effects model: RR 1.66 (95% CI 1.40-1.96) Heterogeneity (I2): 45.75%
*** Adjusted relative risk of GDM (comparison with AP discontinuers) G: RR 0.43 (95% CI 0.20-0.93) I: RR 1.23 (95% CI 1.04-1.45)
Pooled effect (for comparison with discontinuers) -random effects model: RR 0.78 (95% CI 0.28-2.16) -fixed effects model: RR 1.17 (95% CI 0.99-1.38) Heterogeneity (I2): 85.43%
|
author’s conclusion: -“an increased risk of GDM with antipsychotic exposure in pregnant women, who may benefit from close pregnancy monitoring, early testing for GDM, targeting modifiable risk factors, and lifestyle modifications” -“if antipsychotic medication is needed during pregnancy, using medications with lower weight gain liabilities, while considering patients’ clinical stability, may decrease the risk of GDM development”
*clinical definition criteria for GDM were not reported in most studies *outcome GDM deduced from medical records, charts, ICD codes
|
|
Petersen, 2016 |
Type of study: retrospective analysis of prospective primary care data (prescription and electronic health records database)
Setting and country: The Health Improvement Network (THIN) and the Clinical Practice Research Datalink (CPRD), UK
Funding and conflicts of interest: non-commercial; funded by National Institute for Health Research Health Technology Assessment program (Grant 11/35/06); none reported
|
Inclusion criteria: all pregnant women and their children Identified in the databases between 1 January 1995 to 31 December 2012
Exclusion criteria: not described
N total at baseline: -all antipsychotics (exposed/discontinued/unexposed) N=416/ N=670/ N=318 434 (used for maternal outcomes) -typical AP N=157/ N=406 /N=318 434 -atypical AP N=280/N=302/N=318 434
Mean age ± SD (yrs): only age categories
Mean BMI ± SD, kg/m2: (exposed/discontinued/unexposed) 28±6.7/ 27±6.8/ 26±6.4
Obesity, N (%): (exposed/discontinued/unexposed) 53 (18.3)/ 62 (12.6)/ 15,363 (7.3)
Alcohol, N (%): (exposed/discontinued/unexposed) 23 (7.9)/ 28 (5.7)/ 1,124 (0.5)
Smoking N (%): (exposed/discontinued/unexposed) 139 (47.9)/ 183 (37.2)/ 42,502 (20.1)
Co-medications (only psychotropic) In the 1st trimester, N(%): (exposed/discontinued/unexposed) -anticonvulsant mood stabilisers 27 (9.3)/ 14 (2.8)/ 887 (0.4) -lithium 11 (3.8)/ 2 (0.4)/ 7 (0) -antidepressants 169 (58.3)/ 124 (25.2)/ 4,351 (2.1) -anxiolytics 31 (10.7)/ 24 (4.9)/ 523 (0.2) -hypnotics 41 (14.1)/ 28 (5.7)/ 423 (0.2)
Pre-existing psychiatric conditions, n (%): (exposed/discontinued/unexposed) depression 79 (27.2)/ 152 (30.9)/ 14,626 (6.9) epilepsy 17 (5.9)/22 (4.5)/ 3,254 (1.5 SMI 180 (62.1)/ 144 (29.3)/ 882 (0.4) |
Exposure:
records of prescriptions for antipsychotics during the 1st trimester of pregnancy (cohort A)
(prescribed AP 2 years before the start of pregnancy and between day 31 and 105 of pregnancy)
(all AP, except prochlorperazine)
|
Non-exposure:
1. discontinued AP: women with records of antipsychotic treatment in the 2 years before start of pregnancy, but no prescriptions issued after 4 weeks prior to pregnancy start (Cohort B)
2. no AP use 2 years before pregnancy and during the whole pregnancy (through the delivery date) (Cohort C)
|
Duration or endpoint of follow-up: NA, used data from 1995 to 2012
For how many participants were no complete outcome data available? N (%): NA
Reasons for incomplete outcome data described? NA |
Outcomes defined using CPRD read codes and ICD codes for respective disorders.
Outcome measures and effect estimates:
Pre-eclampsia -cohort A versus cohort B (discontinued) RR 1.03 (95% CI 0.57-1.87), p=0.91 RR adj. 0.69 (95% CI 0.37- 1.29), p=0.25
-cohort A versus cohort C (unexposed) RR 1.47 (95% CI 0.92-2.33), p=0.10 RR adj. 1.24 (95% CI 0.79-1.96), p=0.34
Caesarean section -cohort A versus cohort B (discontinued) RR 1.15 (95% CI 0.89-1.48), p=0.26 RR adj. 1.05 (95% CI 0.82-1.34), p= 0.67
-cohort A versus cohort C (unexposed) RR 1.36 (95% CI 1.12-1.64), p=0.001 RR adj. 1.09 (95% CI 0.92-1.30), P=0.23
Gestational diabetes (see meta-analysis above) |
author’s conclusion: -“women receiving antipsychotic treatment in pregnancy are of higher risk of a range of adverse pregnancy outcomes” -“rather than being specific associations/effects with antipsychotics, these increased risks may be associated with other health and lifestyle factors which are more common in this group of women (greater levels of obesity, smoking, alcohol problems, concomitant medication and illicit drug use”
analyses adjusted for age, obesity, alcohol problems, smoking, illicit drug use, and antidepressant prescribing and anticonvulsant mood stabilizers |
|
Reis, 2008 |
Type of study: prospective cohort (retrospective analysis of prospective data from a drug prescription database)
Setting and country: Swedish Medical Birth Register, Swedish Register of Congenital Malformations, the Hospital Discharge Register, Sweden
Funding and conflicts of interest: none reported
|
Inclusion criteria: all women giving birth in Sweden
Exclusion criteria: not reported
N total at baseline: (exposed/unexposed) N=2908 /N=958,729
N exposed (after excluding lithium, dixyrazine and prochlorperoxine): n (women)=570 n (children)=576
Mean age ± SD: NA
BMI≥26 (exposed/unexposed): n=225/n=215,796 (OR 2.06 (95%CI 1.72-2.47) versus normal BMI range)
Smoking (exposed/unexposed) <10 cigarettes/day n=77/n=72,882 ≥10 cigarettes/day N=142/n=33,623
Alcohol: not reported
Co-medications (only psychotropic): (n, exposed/unexposed, for all exposed, i.e. including dixyrazine or prochlorperoxine) anticonvulsants 23/2467 sedatives, hypnotics 141/3953 antidepressants 172/10,276 TCA 33/1578 SSRI 101/7835 other 31/971
Indications: not reported |
Exposure:
women who used AP during the 1st trimester of pregnancy
|
Non-exposure:
all other women in the database (1994-2005) |
Duration or endpoint of follow-up: NA (used data from 1994 to 2005)
For how many participants were no complete outcome data available? N (%): NA
Reasons for incomplete outcome data described? NA |
Outcome measures and effect estimates:
(95% CI
Pre-eclampsia OR 1.09 (95% CI 0.74–1.62)
Caesarean section OR 1.43 (95% CI 1.17–1.74)
Placental abnormalities -Placenta previa (n=1 in exposed group; n=6 in the unexposed group) RR adj. 0.48 (95% CI 0.01–2.05)
-Abruptio placentae (n=5 in exposed group; n=7 in the unexposed group) RR adj. 1.12 (95% CI 0.36–2.62)
Preterm birth (<37 weeks) OR 1.73 (95% CI 1.31–2.29)
Stillbirth OR 1.48 (95% CI 0.48–3.47)
Gestational diabetes (see meta-analysis above)
|
author’s conclusion: -no specific conclusions regarding complications of pregnancy and labor
analysis for risk of delivery by caesarean section and placental abnormalities adjusted for BMI
analysis for risk of preterm birth and stillbirth adjusted for the year of birth, maternal age, parity, smoking, and previous miscarriages. |
|
Lin, 2010 |
Type of study: Retrospective analysis of a population-based prospective cohort
Setting and country: Taiwan National Health Insurance Research Database (1996-2003) and birth certificate registry (2001-2003); School of Health Care Administration, Taipei Medical University, Taiwan
Funding and conflicts of interest: non-commercial; none
|
Inclusion criteria: women who had singleton live births from 1 January 2001 to 31 December 2003, with at least 3 consecutive records of diagnostic codes for schizophrenia within 3 years preceding pregnancy, AP prescription > 30 days during pregnancy
Exclusion criteria: (exposed) taking both typical and atypical AP during pregnancy, injectable AP, antiepileptics, lithium, atypical or atypical AP for less than 30 days during pregnancy (unexposed): records of mental illness or chronic diseases systemic lupus erythematosus, rheumatoid arthritis, gout, sarcoidosis, ankylosing spondylitis
N total at baseline (maternal): (exposed/unexposed diseased/ unexposed) N=242/N=454/N=3480
Mean maternal age: N/A (categories only)
Paternal age (%), p=0.004: (exposed/unexposed) <30: 32.8/ 28 30–34: 37.8/ 36.6 ≥34: 29.4/ 35.3 Indications: schizophrenia (any ICD-9-CM 295 code other than 295.7-schizoaffective disorder)
Smoking: N/A Alcohol: N/A Substance abuse: N/A Co-medications: N/A |
Exposure: 1. typical AP (n=194) 2. atypical AP (n=48)
Exposed were randomly matched by age categories (<20, 20–24, 25–29, 30–34, ≥35 yrs), year of delivery, hypertension, diabetes in a 1:5 ratio
|
Non-exposure: 1. diseased + no AP during pregnancy (n=454) 2. healthy + no AP (n=3480) |
Duration or endpoint of follow-up: NA (data for 2001 and 2003 were used)
For how many participants were no complete outcome data available? N (%): NA
Reasons for incomplete outcome data described? NA |
Outcome measures and effect estimates: preterm birth (<37 weeks gestation)
1. typical AP versus diseased unexposed OR adj 2.46 (95% CI 1.50–4.11)
2. atypical AP versus diseased unexposed OR adj 1.61 (95% CI 0.63–4.12)
|
author’s conclusion: -typical AP during pregnancy were associated with an increased risk of preterm birth (after adjusting for confounders)
adjusted for infant gender, parity, maternal age, highest maternal and paternal educational levels, hypertension, gestational diabetes, parental age difference, mother marital status, family monthly income
parental ages were defined as each parent's age at the time of birth |
|
Habermann, 2013 |
Type of study: Retrospective analysis of a prospective cohort
Setting and country: Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy, using data from Teratology Information Service (TIS), Germany
Funding and conflicts of interest: non-commercial; none reported
|
Inclusion criteria: all pregnant women consulted at Teratology information service from January 1997 to March 2009
Exclusion criteria: presence of prenatal pathologic findings; outcome of the pregnancy was known at the time of inclusion
(exposed/unexposed) N=845 (study cohort n=561 + cohort 1 n=284))/ N=1122 (cohort 2)
Median age: (exposed/unexposed) 32/32 yrs
Median BMI: (exposed/unexposed) 24.2/23.7 kg/m2
Smoking (study cohort/cohort 1/cohort2 (unexp.)) (n=529/n=264/n=1111) ≤5 cigarettes/day 27 (5.1%)/ 15 (5.7%)/ 42 (3.8%) >5 cigarettes/day (n=562) 176 (33.3%)/ 98 (37.1%)/ 58 (5.2%)
Alcohol (study cohort/cohort 1/cohort2 (unexp.)) (n=526/n=263/n=1112) ≤1 drink/day 23 (4.4%)/ 11 (4.2%)/ 24 (2.2%) >1 drink/day 14 (2.7%)/ 13 (4.9%)/ 3 (0.3%)
Co-medications (only psychotropic): (exposed) total other psychoactive drugs, including antidepressants, anticonvulsants, benzodiazepines, anxiolytics/sedatives, opioids (58%)
Indications: psychotic disorders, schizophrenia, depression, bipolar affective disorders, anxiety disorders |
Exposure:
study cohort: exposed to SGA (concomitant FGA allowed): olanzapine (n = 187), quetiapine (n = 185), clozapine (n = 73), risperidone (n = 64), aripiprazole (n = 60), ziprasidone (n = 37), amisulpride (n = 16), zotepine (n = 2)
cohort 1: (also a comparison group) exposed only to FGA (the most frequent were: haloperidol (n = 64), promethazine (n = 86), flupentixol (n = 44))
|
Non-exposure:
cohort 2: no antipsychotic use; excluded all women exposed to known teratogenic, fetotoxic or insufficiently studied drugs, allowed drugs known to be nonteratogenic; used a random sample from this cohort in 2:1 ratio
|
Duration or endpoint of follow-up: 8 weeks after the estimated date of birth (via hospital discharge summaries)
For how many participants were no complete outcome data available? N (%): (exposed/unexposed) 18.3%/17.4% lost to follow-up
Reasons for incomplete outcome data described? NA |
Outcome measures and effect estimates:
Spontaneous abortion Atypical AP: 46/561 Typical AP: 2/284 No AP: 3/1122 Cumulative incidences: SGA 24% (95% CI 14%-39%) FGA 16% (95% CI 10%-26%) No AP 20% (95% CI 15%-26%)
Stillbirth Atypical AP: 0/561 Typical AP: 2/284 No AP: 3/1122
Preterm birth (<37 weeks gestation) Atypical AP: 41/561 Typical AP: 36/284 No AP: 88/1122 *SGA versus FGA: Crude OR 0.54 (95% CI 0.33-0.87) *FGA versus no AP: Crude OR 1.96 (95% CI 1.29-2.98) SGA versus no AP: Crude OR 1.06 (95% CI 0.72-1.56) |
author’s conclusion: -no stat. sig. differences in cumulative incidences of spontaneous abortions;
|
|
Sorensen, 2015 |
Type of study: Historical (retrospective) cohort study
Setting and country: nationwide Danish registries (1997-2008): Danish National Hospital Register, Danish Medical Birth Register, Danish Prescription register; Denmark
Funding and conflicts of interest: The Regional Center for Child and Adolescent Psychiatry, Aarhus University Hospital, Risskov, Denmark; the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. |
Inclusion criteria: all pregnancies in the database
Exclusion criteria: molar and ectopic pregnancies, failed induced abortion
(exposed/unexposed) N=3164/ N=1 002 155 (exposed during pregnancy) N=1881 (exposed only prior to pregnancy) N=2745
Mean age ± SD: NA (only categories)
Maternal history of severe mental disorder (exposed/unexposed): 794 (25.1%)/1942 (0.2%)
Maternal psychiatric history (exposed/unexposed): 2369 (74.9%)/ 61 084 (6.1%)
Co-medication (psychotropic): Antiepileptic drugs 371 (11.7%)/ 4397 (0.4%) Antidepressant drugs 1539 (48.6%)/ 20522 (2.0%)
|
Exposure:
prescription of antipsychotic medications redeemed by the pregnant women during the exposure window (from 30 days before the estimated conception date to one day prior to spontaneous abortion/ stillbirth/ live birth), and recorded in the Danish National Prescription Register
|
Non-exposure:
1. no prescriptions for antipsychotics (and about 94% without history of psychiatric disorders) 2. discontinued before pregnancy (used antipsychotic medications during the preceding year but not 30 days prior to the estimated conception date and during the pregnancy) |
Duration or endpoint of follow-up: NA (used data from 1997 to 2008)
For how many participants were no complete outcome data available? N (%): NA
Reasons for incomplete outcome data described? NA |
Outcome measures and effect estimates:
spontaneous abortion, defined by ICD-10 codes O02.0-O03.9
Spontaneous abortion (n) (exposed/unexposed) 407/114,314 1. RR adj 1.34 (95% CI 1.22-1.46) (compared with unexposed) 2. RR adj. 1.04 (95% CI 0.93-1.17) (compared with discontinuers before pregnancy)
Stillbirth** Stillbirth cases, n (exposed/unexposed) 19/3515
1. crude RR 2.27 (95% CI 1.45-3.55) (compared with unexposed) 2. crude RR = 2.06 (95% CI 1.01-4.19) (compared with discontinuers before pregnancy)
|
author’s conclusion: -“The increased risk of spontaneous abortion found in women treated with antipsychotic medications during pregnancy is most likely due to confounding factors. The risk of stillbirth was twofold higher in pregnancies exposed to antipsychotic medication during pregnancy.”
**Definition of stillbirth was fetal death after 28 weeks of gestation, but because it was changed in 2004 to 22 weeks, spontaneous abortions with gestational age of 22 weeks or more, were recoded as stillbirths in this study.
*for spontaneous abortions adjustment was performed on maternal age, history of misuse (yes/no), cohabitation (yes/no), income (dichotomized at the median) and level of education (<10, 10–12, >12 years)
*adjusted analyses for stillbirth included one covariate at a time owing to the low number of exposed stillbirths; adjustment did not change the estimates |
|
Bellet, 2015 |
Type of study: Cohort (retrospective analysis of prospectively collected data)
Setting and country: pharmacovigilance and teratogenic risk reporting databases, France
Funding and conflicts of interest: non-commercial, none reported
|
Inclusion criteria: pregnant women who had contacted pharmacovigilance centres about the safety of their medication in pregnancy
Exclusion criteria: Duplicate database entries, not pregnant at the time of the first contact (preventive and retrospective cases), known developmental anomaly in fetus before the first contact (retrospective cases), ongoing pregnancy at 31 December 2011, follow-up data not available; patients were excluded from the exposed group if they were taking known teratogen(s) (e.g. carbamazepine, carbimazole, cyclophosphamide, lithium, methotrexate, misoprostol, mycophenolate, phenobarbital, retinoids, thalidomide, valproic acid and vitamin K antagonists)
N total at baseline: (exposed/unexposed) N=86/N=172 *exposed only in the 1st trimester: n=56 *exposed in trimester 2 and 3: n= 22 *exposed only in trimester 2: n=8
Mean age ± SD: (exposed/unexposed) N=77/N=172 31.8 ± 5.8/31.4 ± 5.4
Smoking (exposed/unexposed n=30/n=93) <5 cigarettes/day 10% / 5.4% ≥5 cigarettes/day 36.7% / 5.4% Alcohol (exposed/unexposed n=29/n=90) <2 drinks/day 17.2% / 8.9% ≥2 drinks/day 3.4% / 0%
Co-medications: 72.1% of exposed patients had co-medications (benzodiazepines, antidepressants, other antipsychotics and anticonvulsants)
Indications: schizophrenia (n=22; 30.6%), psychotic disorders not otherwise specified (n= 14; 19.4%), bipolar disorders (n= 12; 16.7%), depression (n = 11; 15.3%) |
Exposure
use of aripiprazole (5 to 30 mg/day) during pregnancy
|
Non-exposure
no use of aripiprazole or exposed to agents known to be non-teratogenic
2 random unexposed patients were matched for age (± 2 years) and gestational age at the first call (± 2weeks) with each exposed patient
|
Duration or endpoint of follow-up: ascertainment of outcomes within 2 months of the expected date of delivery
For how many participants were no complete outcome data available? (exposed/unexposed) N: 15/11
Reasons for incomplete outcome data described? Yes (exposed: 8 miscarriages (9.3%), 8 voluntary abortions (9.3%) and 1 therapeutic abortion) (unexposed: 10 miscarriages (5.8%) and 3 voluntary abortions (1.7%))
|
Outcome measures and effect estimates:
Spontaneous abortion (miscarriage) Crude OR 1.66 (95%CI 0.63–4.38)
Preterm delivery (<37 weeks) Crude OR 2.57 (95%CI 1.06–6.27)
Pre-eclampsia/eclampsia Crude OR 0.66 (95%CI 0.07–6.47)
Gestational diabetes (see meta-analysis above) |
-author’s conclusion: Interpret the results with caution, because analyses were not adjusted (also not for smoking) |
|
McKenna, 2005 |
Type of study: prospective cohort (retrospective analysis of a prospective TIS database)
Setting and country: multicentre; Canada, Israel, England (teratogen information centers, including Motherisk Program at the Hospital for Sick Children in Toronto and an independent medical charity ‘Drug safety research unit’ in Southampton)
Funding and conflicts of interest: non-commercial; none reported |
Inclusion criteria: all women who contacted the services regarding the use of antipsychotics and other drugs in the current pregnancy (assuming until 2004)
Exclusion criteria: women who reported a psychiatric illness or the use of antipsychotics were excluded from the comparison group
N total at baseline: (exposed/unexposed) N=151 /N=151
Mean age ± SD: not reported
median BMI: 27.8/ 22.92 kg/m2 sig. different between exposed and unexposed
smoking: (exposed/unexposed) n=38/ n=13
Alcohol (exposed /unexposed) heavy/binge n=9/ n=1 casual n=9/ n=11
Co-medications (only psychotropic): (exposed) 17% anti-epileptics; 12.4% valproate; 6% lithium; 57% anti-depressants; 34% benzodiazepines
Indications (%): depression (29), schizophrenia (24), bipolar disorder (18), schizoaffective (2), psychotic episode (7), psychotic depression (5), obsessive-compulsive disorder (2), PTSD (1), schizophreniform disorder (1) |
Exposure: Antipsychotics (SGA) during the 1st trimester of pregnancy (olanzapine n=60, risperidone n=49, quetiapine n=36, clozapine n=6)
|
Non-exposure: no antipsychotics during pregnancy; taking drugs known to be non-teratogenic, also excluding other psychotropic drugs;
matched with exposed on maternal age ± 2 yrs and gestational age ± 2 weeks |
Duration or endpoint of follow-up: 3-4 months after delivery
For how many participants were no complete outcome data available? N (%): NA
Reasons for incomplete outcome data described? NA |
Outcome measures and effect estimates:
Spontaneous abortion, n (%) (exposed/unexposed) 22 (14.5)/ 13 (8.6), p=0.15
Stillbirth, n (%) (exposed/unexposed) 4 (2.6) / 4 (2.6), p=1.00
Gestational diabetes (see meta-analysis above) |
author’s conclusion: -the rate of spontaneous abortion was higher in the exposed group, but not statistically significantly -pregnancies in which a woman requires antipsychotic medication should be considered high risk and closely monitored |
|
Sadowski, 2013 |
Type of study: retrospective analysis of a prospective cohort (prescription database data)
Setting and country: Motherisk Program at the Hospital for Sick Children, Toronto, Canada
Funding and conflicts of interest: non-commercial; none reported
|
Inclusion criteria: all women who contacted the Motherisk services between 2005 and 2009 regarding the use of antipsychotics and other drugs in the current pregnancy
Exclusion criteria: exposure to teratogenic medications unrelated to the psychiatric disorder treatment; substance abuse (eg, alcohol, marijuana, cocaine, heroin, etc); fertility-assisted pregnancies, twin or triplet pregnancies, pregnancies with known outcomes at the initial time of contact (eg, contacted Motherisk following the birth, reported abnormal pregnancy screening tests and/or ultrasounds); presence of psychiatric disorders in women of the comparison cohort
N total at baseline: (exposed/unexposed) N=133/ N=133
Mean age at conception ± SD (months): (exposed/unexposed) 378.3±61.8/ 383.6±53.9
BMI: not reported Pre-pregnancy weight (kg): (exposed/unexposed) 74.3±20.9/ 64.6±13.0, p<0.001
Smoking N (%) (exposed/unexposed) 24 (18)/ 0 (0), p<0.001
Alcohol, N (%) (exposed /unexposed) 8 (6)/12 (9.1)
Co-medications (only psychotropic), N(%): (exposed)c 72.2% total; 54.1% combination with antidepressants; SSRI n=46 (34.6), benzodiazepine n=21 (15.8), anticonvulsants n=19 (14.3), SNRI n=16 (12.0), atypical antidepressant n=12 (9), non-benzo hypnotic n=8 (6), typical antipsychotic n=5 (3.8), serotonin antagonist and reuptake inhibitor n=4 (3), tetracyclic antidepressant n=4 (3), tricyclic antidepressant n=3 (2.3), norepinephrine reuptake inhibitor n=2 (1.5), synthetic cannabinoid n=2 (1.5), psychostimulant n=1 (0.8), GABA analogue n=1 (0.8), other dopamine antagonist n=1 (0.8)
Indications (%): bipolar disorder (36.8), depression (27.1), anxiety and depression (9.8) sleep disorders (9.8), schizophrenia (3), schizoaffective disorders (1.5) |
Exposure:
quetiapine (69.9%), olanzapine (16.5%), risperidone (10.5%), aripiprazole (1.5%), paliperidone (0.8%), quetiapine plus olanzapine (0.8%)
women who initially called the service to inquire about the safety of an SGA and who confirmed the use of this medication for a minimum of 4 weeks of pregnancy
|
Non-exposure:
no AP use
women who contacted Motherisk between 2005 and 2009 and reported exposure to non-teratogenic agents
matched for age at conception (±3 years) and pregnancy duration at the initial time of contact (±2 weeks) |
Duration of end-point follow-up: between 2009 and 2012
For how many participants were no complete outcome data available? of 370 women who contacted Motherisk in the study period, 107 women could not be reached at their last reported contact details, 20 refused participation and 110 did not meet the inclusion criteria
Reasons for incomplete outcome data described? yes |
Outcome measures and effect estimates:
Preterm delivery (<37 weeks), n(%) (exposed/unexposed) 12 (10.6)/ 5 (4.3), p=0.071
Gestational diabetes (see meta-analysis above) |
author’s conclusion: The increased proportion of preterm deliveries observed in the exposed group may be influenced by maternal pre-pregnancy weight and co-morbidities that were more frequent in the exposed group |
|
Vigod, 2015 |
Type of study: population based cohort study (retrospective analysis of prescription and medical records databases)
Setting and country: multiple linked population health databases housed at the Institute for Clinical Evaluative Sciences (ICES) in Toronto, Ontario, Canada
Funding and conflicts of interest: grant from the Canadian Institutes of Health Research (CIHR), and by an annual grant from the Ontario Ministry of Health and Long-Term Care; author’s funding had no impact on the results and interpretation of the study |
women who delivered a singleton infant in Ontario between 2003 and 2012, who were eligible for provincially funded drug coverage, and who had filled a provincially funded drug prescription within 180 days before pregnancy and one during pregnancy or within 180 days of delivery
Exclusion criteria: absence of provincially funded drug coverage
N total at baseline: (exposed/unexposed) n=1,209/n=40,314 (unmatched cohort) n=1,021/n=1,021 (matched cohort)
Mean age ± SD: (exposed/unexposed) 28.8±6.1/ 26.7±6.3 yrs (unmatched cohort) 28.8±6.2/ 28.8±6.2 yrs (matched cohort) BMI: not reported
Alcohol and smoking (substance abuse), n(%) (exposed/unexposed) 569 (47.1)/ 5523 (13.7) (unmatched cohort) 458 (44.9)/ 415 (40.7) (matched cohort)
Co-medications, n(%): (exposed/unexposed)
1.unmatched cohort -SSRI 364 (30.1)/ 1981 (4.9) -Non-SSRI 322 (26.6)/ 989 (2.5) -Mood stabilisers 167 (13.8)/ 331 (0.8) -Benzodiazepines 306 (25.3)/ 630 (1.6)
2. matched cohort: -SSRI 303 (29.7)/ 204 (20) -Non-SSRI 264 (25.9)/ 149 (14.6) -Mood stabilisers 105 (10.3)/ 62 (6.1) -Benzodiazepines 222 (21.7)/ 135 (13.2)
Indications, n(%): (exposed/unexposed)
1. unmatched cohort: -Psychotic disorder 429 (35.5)/ 675 (1.69) -Bipolar disorder or major depression 937 (77.5)/ 8708 (21.6) -Personality disorder 393 (32.5)/ 1816 (4.5)
2. matched cohort: -Psychotic disorder 319 (31.2)/ 160 (15.7) -Bipolar disorder or major depression 758 (74.2)/ 673 (65.9) -Personality disorder 295 (28.9)/ 231 (22.6) |
Exposure:
≥2 consecutive prescriptions for AP between the conception date (estimated using the gestational age at birth) and the delivery date; at least one of the prescriptions was filled in the 1st or 2nd trimester
|
Non-exposure:
not exposed to any AP during pregnancy
Patients in the matched control group had a history of either psychotic disorder, bipolar disorder or major depression, alcohol or substance abuse, or a personality disorder, however it is unclear whether all of the patients in the control group had some sort of psychiatric disorders.
*matched 1:1 to AP patients using propensity scores (based on the HDPS score within 0.2 SD) and on maternal age at delivery within 3 years
|
Duration of end-point follow-up: 180 days after delivery for exposure, 42 days for the outcome data
For how many participants were no complete outcome data available? N (%): NA
Reasons for incomplete outcome data described? NA |
Outcome measures and effect estimates:
Preterm delivery (<37 weeks) matched cohort: crude RR 1.01 (95% CI 0.81-1.27) adj. RR 0.99 (95% CI 0.78-1.26
unmatched cohort (unadjusted): crude RR 1.51 (95% CI 1.29-1.78)
Gestational diabetes (see meta-analysis above) |
author’s conclusion: -preterm birth rate of 14% is almost twice that of the rate in general population -pregnant women taking antipsychotics are at higher risk of pregnancy complications and require monitoring
Adjusted for a prescribed selective serotonin reuptake inhibitor (SSRI), non-SSRI, mood stabilizers, or benzodiazepine during the index pregnancy. |
Risk of bias tables
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea, 2007; BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009; PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/not applicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Kucukgoncu, 2019 |
yes |
yes |
yes |
yes |
no, main analysis is for unadjusted effect estimates, in adjusted analysis only 4 out of 10 included studies |
yes, using NOS |
unclear* |
yes, using Egger’s regression test |
no |
NOS, Newcastle-Ottawa Scale. *In adjusted analysis, statistical heterogeneity was not significant; data were pooled for all types of antipsychotics and psychiatric disorders.
- Research question (PICO) and inclusion criteria should be appropriate and predefined.
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched.
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons.
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported.
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs).
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table et cetera.).
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (for example Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (for example funnel plot, other available tests) and/or statistical tests (for example Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias table for intervention studies (observational: non-randomized clinical trials, cohort and case-control studies)
Research question: What is the risk of pregnancy and labor complications in women with psychiatric disorders taking antipsychotics during pregnancy, compared to women with psychiatric disorders not taking antipsychotics and/or to healthy women?
|
Study reference
(first author, year of publication) |
Bias due to a non-representative or ill-defined sample of patients?1
(unlikely/likely/unclear) |
Bias due to insufficiently long, or incomplete follow-up, or differences in follow-up between treatment groups?2
(unlikely/likely/unclear)
|
Bias due to ill-defined or inadequately measured outcome ?3
(unlikely/likely/unclear) |
Bias due to inadequate adjustment for all important prognostic factors?4
(unlikely/likely/unclear) |
|
Bellet, 2015 |
Likely |
unlikely |
likely |
likely |
|
Habermann, 2013 |
likely |
likely |
unlikely |
likely |
|
Reis, 2008 |
likely |
likely |
unlikely |
likely |
|
McKenna, 2005 |
likely |
likely |
unlikely |
likely |
|
Petersen, 2016 |
likely |
unlikely |
unlikely |
likely |
|
Sadowski, 2013 |
likely |
unlikely |
unlikely |
likely |
|
Vigod, 2015 |
likely |
unlikely |
unlikely |
likely |
|
Sorensen 2015 |
unlikely |
unclear |
likely |
likely |
|
Lin 2010 |
unlikely |
unclear |
likely |
likely |
|
Panchaud 2017 |
likely |
unlikely |
unlikely |
likely |
|
Galbally 2018 |
likely |
unlikely |
unlikely |
likely |
|
Park 2018 |
likely |
likely |
likely |
likely |
- Failure to develop and apply appropriate eligibility criteria: a) case-control study: under- or over-matching in case-control studies; b) cohort study: selection of exposed and unexposed from different populations.
- 2 Bias is likely if: the percentage of patients lost to follow-up is large; or differs between treatment groups; or the reasons for loss to follow-up differ between treatment groups; or length of follow-up differs between treatment groups or is too short. The risk of bias is unclear if: the number of patients lost to follow-up; or the reasons why, are not reported.
- Flawed measurement, or differences in measurement of outcome in treatment and control group; bias may also result from a lack of blinding of those assessing outcomes (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Failure to adequately measure all known prognostic factors and/or failure to adequately adjust for these factors in multivariate statistical analysis.
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Terrana 2015 |
SR, meta-analysis; individual studies do not fit the pico (for the outcome preterm births patients were excluded) |
|
Coughlin 2015 |
SR, meta-analysis: includes studies for which no full text is available (risk of bias cannot be assessed) |
|
Schaffer 2019 |
wrong study design, compares outcomes across different patterns of AP use before/during/after pregnancy |
|
Galbally 2019 |
Retrospective with many subgroups; does not fit the pico (comparison: patients with psychosis versus patients without psychose; comparison 2: antipsychotics versus different medication) |
|
Kulkarni 2014 |
Non-comparative; reports descriptive statistics for medication use from a database, comparison with the general population |
|
Tosato 2017 |
SR, no meta-analysis |
|
Frayne 2018 |
Dow snot fir the pico (outcome is placental weight to birth weight ratio), retrospective data |
|
Sakai 2017 |
Non-comparative; analysis of reports from an adverse reactions database |
|
Heller 2017 |
Does not fit the pico (exposure unclear) |
|
Lopez-Yarto 2012 |
Does not fit the pico (only SSRI) |
|
Newham 2008 |
Does not fit the pico, excluded patients with the outcome of interest (preterm births) |
|
Newport 2007 |
Non-comparative |
|
Diav-Citrin 2005 |
descriptive statistics, small numbers |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 14-10-2021
Beoordeeld op geldigheid : 23-07-2021
Bij het opstellen van de modules heeft de werkgroep een inschatting gemaakt over de maximale termijn waarop herbeoordeling moet plaatsvinden. De geldigheid van de richtlijnmodules komt eerder te vervallen indien nieuwe ontwikkelingen aanleiding zijn een herzieningstraject te starten.
|
Module1 |
Regiehouder (s)i |
Jaar van autorisatie |
Eerstvolgende beoordeling actualiteit richtlijn2 |
Frequentie van beoordeling op actualiteit3 |
Wie houdt er toezicht op actualiteit4 |
Relevante factoren voor wijzigingen in aanbeveling5 |
|
Antipsychotica en zwangerschaps- en baringscomplicaties |
NVOG |
2021 |
2026 |
Eens in vijf jaar |
NVOG |
Nieuw onderzoek, beschikbaarheid nieuwe middelen |
1 Naam van de module
i Regiehouder van de module (deze kan verschillen per module en kan ook verdeeld zijn over meerdere regiehouders)
2 Maximaal na vijf jaar
3 (Half)Jaarlijks, eens in twee jaar, eens in vijf jaar
4 Regievoerende vereniging, gedeelde regievoerende verenigingen, of (multidisciplinaire) werkgroep die in stand blijft
5 Lopend onderzoek, wijzigingen in vergoeding/organisatie, beschikbaarheid nieuwe middelen
Algemene gegevens
De ontwikkeling van deze richtlijn werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodules.
De richtlijn is ontwikkeld in samenwerking met:
- Patiëntenfederatie Nederland
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijn is in 2018 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen die betrokken zijn bij de zorg voor zwangere patiënten die ‘niet-SSRI’ antidepressiva en/of antipsychotica gebruiken. De werkgroepleden zijn door hun beroepsverenigingen gemandateerd voor deelname. De werkgroep is verantwoordelijk voor de integrale tekst van deze richtlijn.
Werkgroep
- Dr. A. Coumans, gynaecoloog-perinatoloog, Maastricht UMC+, Maastricht, NVOG
- Dr. H.H. Bijma, gynaecoloog, Erasmus Medisch Centrum, Rotterdam, NVOG
- Drs. R.C. Dullemond, gynaecoloog, Jeroen Bosch Ziekenhuis, Den Bosch, NVOG
- Drs. S. Meijer, gynaecoloog, Gelre Ziekenhuis, Apeldoorn, NVOG
- Dr. M.G. van Pampus, gynaecoloog, OLVG, Amsterdam, NVOG
- Drs. M.E.N. van den Heuvel, neonatoloog, OLVG Amsterdam, NVK
- Drs. E.G.J. Rijntjes-Jacobs, neonatoloog, Leids Universitair Medisch Centrum, Leiden, NVK
- Dr. K.M. Burgerhout, psychiater, Amphia Ziekenhuis, Breda, NVvP
- Dr. E.M. Knijff, psychiater, Erasmus Medisch Centrum, Rotterdam, NVvP
Meelezers
- Dr. A.J. Risselada, klinisch farmacoloog, Wilhelmina Ziekenhuis Assen
Met ondersteuning van
- Dr. E. van Dorp-Baranova, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. M. Moret-Hartman, senior-adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- N. Verheijen, senior-adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- M. Wessels, literatuurspecialist, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Dr. A. Coumans |
gynaecoloog-perinatoloog Maastricht UMC+ |
geen |
geen |
geen |
|
Dr. H. H. Bijma |
Gynaecoloog, Erasmus MC, afdeling verloskunde en gynaecologie, subafdeling verloskunde en prenatale geneeskunde |
lid werkgroep wetenschap LKPZ, onbetaald |
geen |
geen |
|
Drs. S. Meijer |
gynaecoloog Gelre Ziekenhuis, Apeldoorn |
geen |
geen |
geen |
|
Drs. R. C. Dullemond |
gynaecoloog- perinatoloog, Jeroen Bosch Ziekenhuis |
LKPZ bestuur - voorzitter, onbetaald mind 2 care - raad van toezicht, onbetaald dagelijks bestuur @verlosdenbosch (integrale geboortezorg organisatie) als gynaecoloog uit het JBZ, onbetaald danwel deels in werktijd |
geen |
geen |
|
Drs. E. G. J. Rijntjes-Jacobs |
kinderarts-neonatoloog, afdeling neonatologie, Leids Universitair Medisch Centrum |
LKPZ bestuur – secretaris, onbetaald |
geen |
geen |
|
Dr. M. G. van Pampus |
Gynaecoloog, OLVG Amsterdam |
onbetaalde nevenfuncties |
geen |
geen |
|
Dr. E. M. Knijff |
Psychiater, Erasmus MC polikliniek psychiatrie & zwangerschap medisch coördinator polikliniek Erasmus MC |
geen |
geen |
geen |
|
Drs. M. E. N. van den Heuvel |
kinderarts-neonatoloog, OLVG Amsterdam |
geen |
geen |
geen |
|
Dr. K. Burgerhout |
Psychiater, Reinier van Arkel, POP-poli |
geen |
geen |
geen |
Inbreng patiëntenperspectief
Er is een aantal acties uitgevoerd om het patiëntperspectief mee te nemen bij het ontwikkelen van deze richtlijn. Allereerst is contact gezocht met het MIND Platform voor afvaardiging van een patiëntvertegenwoordiger in de werkgroep. Zij hebben ons in contact gebracht met de Stichting Me Mam. Het bleek niet mogelijk een patiëntvertegenwoordiger voor de werkgroep te vinden. Daarna is een focusgroepbijeenkomst voor patiënten georganiseerd, maar deze is geannuleerd vanwege onvoldoende aanmeldingen. Tot slot is een schriftelijke enquête voor patiënten in samenwerking met de Patiëntenfederatie Nederland opgesteld en uitgezet. Dit heeft helaas nauwelijks reactie opgeleverd, de enquête is door twee patiënten ingevuld. Voor de ontwikkeling van het product voor patiënten (informatie op de website www.Thuisarts.nl) is een ervaringsdeskundige van de patiëntenvereniging ‘Plusminus-leven met bipolariteit’ afgevaardigd. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland en het MIND Platform.
Methode ontwikkeling
Evidence based
Implementatie
In de verschillende fasen van de richtlijnontwikkeling is rekening gehouden met de implementatie van de richtlijnmodules en de praktische uitvoerbaarheid van de aanbevelingen. Daarbij is uitdrukkelijk gelet op factoren die de invoering van de richtlijn in de praktijk kunnen bevorderen of belemmeren. Het implementatieplan is te vinden in de bijlagen.
Werkwijze
AGREE
Deze richtlijn is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor vrouwen die antipsychotica of niet-SSRI antidepressiva tijdens zwangerschap en lactatie gebruiken. Tevens zijn er knelpunten aangedragen door IGJ, NHG, V&VN, Zorginstituut Nederland, Lareb, KNOV, NVvP, NVOG en NVK via een Invitational conference. Een verslag hiervan is opgenomen in de bijlagen. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijn is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module 'Organisatie van zorg'.
Commentaar- en autorisatiefase
De conceptrichtlijn werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijn aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijn werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. https://richtlijnendatabase.nl/over_deze_site/richtlijnontwikkeling.html.
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008 May 24;336(7654). doi: 10.1136/bmj.a139.
Schünemann, A Holger J (corrected to Schünemann, Holger J). PubMed PMID: 18483053; PubMed Central PMCID: PMC2386626. BMJ. 2008 May 24;336(7654). doi: 10.1136/bmj.a139. Schünemann, A Holger J [corrected to Schünemann, Holger J]
Wessels M, Hielkema L, van der Weijden T. How to identify existing literature on patients' knowledge, views, and values: the development of a validated search filter. J Med Libr Assoc. 2016 Oct;104(4):320-324. PubMed PMID: 27822157; PubMed Central PMCID: PMC5079497.
Zoekverantwoording
|
Database |
Search terms |
Total |
|
Medline (OVID)
1960-juli 2019
Engels |
1 exp antipsychotic agents/ or exp Butyrophenones/ or (antipsychotic* or anti-psychotic* or neuroleptic* or "major tranquilli*" or Bromperidol* or chlorpromazine* or chlorprothixene* or fluphenazine* or flupenthixol* or haloperidol* or penfluridol* or pimozide* or pipamperon* or aripiprazole* or clozapine* or olanzapine* or paliperidon* or quetiapine* or risperidone* or sulpiride*).ti,ab,kf. (aripiprazole/ or chlorpromazine/ or chlorprothixene/ or clozapine/ or flupenthixol/ or fluphenazine/ or haloperidol/ or olanzapine/ or paliperidone palmitate/ or penfluridol/ or pimozide/ or quetiapine fumarate/ or risperidone/ or sulpiride/ zijn onderliggende termen en worden automatische meegenomen met explode (exp)) (151189) 2 pregnancy/ or gravidity/ or exp labor, obstetric/ or exp parturition/ or exp pregnancy in adolescence/ or exp pregnancy, high-risk/ or exp pregnancy maintenance/ or exp pregnancy outcome/ or exp pregnancy, unplanned/ or exp pregnancy, unwanted/ or pregnan*.ti. or Maternal Exposure/ or pregnan*.ti,ab,kf. or ("exposure* in utero" or ((intrauterine or intra-uterine or fetal or foetal or maternal) adj2 exposure*)).ti,ab,kf. (971297) 3 1 and 2 (3536) 4 exp *pregnancy complications/ or exp abortion, spontaneous/ or diabetes, gestational/ or fetal macrosomia/ or fetal death/ or fetal resorption/ or stillbirth/ or hypertension, pregnancy-induced/ or eclampsia/ or hellp syndrome/ or pre-eclampsia/ or fetal membranes, premature rupture/ or chorioamnionitis/ or obstetric labor, premature/ or premature birth/ or placenta previa/ or postpartum hemorrhage/ or oligohydramnios/ or perinatal death/ or placenta, retained/ or polyhydramnios/ or exp pregnancy, ectopic/ or exp infant, low birth weight/ or exp infant, small for gestational age/ or exp infant, very low birth weight/ or Fetal Growth Retardation/ or delivery, obstetric/ or cesarean section/ or extraction, obstetrical/ or vacuum extraction, obstetrical/ or exp Blood Transfusion/ (499305) 5 (stillbirth or miscarriage or (spontaneous adj3 abortion) or (pregnancy adj3 loss*) or "gestational diabetes" or ((f?etal or intra-uterine) adj3 death*) or pre-eclampsia or "pregnancy induced hypertension" or HELLP or ((preterm or premature) adj3 (delivery or birth or labo?r)) or "preterm rupture of membranes" or chorioamnionitis or "placenta previa" or "postpartum h?emorrhage" or oligohydramnios or (perinatal adj3 (death or mortality)) or "retained placenta" or polyhydramnios or ((ectopic or extrauterine) adj3 pregnanc*) or ("f?etal growth" adj3 (restriction or retardation)) or "small for gestational age" or "low birth weight" or FGR or SGA or "caeserean section" or C-section or ((instrumental or obstetric*) adj3 "vaginal deliver*") or (blood adj3 transfusion*) or "maternal intensive care").ti,ab,kf. (216225) 6 4 or 5 (578685) 7 3 and 6 (1391) 8 limit 7 to (english language and yr="1960 -Current") (983) 9 (meta-analysis/ or meta-analysis as topic/ or (metaanaly* or metanaly* or meta-analy* or meta synthes* or metasynthes* or meta ethnograph* or metaethnograph* or meta summar* or metasummar* or meta-aggregation or metareview or meta-review or overview of reviews).ti,ab,kf. or ((systematic* or scoping or umbrella or meta-narrative or metanarrative or evidence based) and (review* or overview*)).ti,kf. or ((evidence or narrative or metanarrative or qualitative) adj3 synthesis).ti,kf. or systematic review.pt. or (prisma or preferred reporting items or quadas*).ti,ab,kf. or ((systematic* or literature) adj2 review*).ti,ab,kf. or cochrane.ab. or cochrane.jw. or embase.ab. or medline.ab. or ((selection criteria or data extraction).ab. and "review"/)) not (Comment/ or Letter/ or (animals/ not humans/)) (431600) 10 8 and 9 (63) > 62 uniek 11 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (1880967) 12 8 and 11 (132) 13 Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw,kw. or (cohort adj (study or studies)).tw,kw. or Cohort analy$.tw,kw. or (Follow up adj (study or studies)).tw,kw. or (observational adj (study or studies)).tw,kw. or Longitudinal.tw,kw. or Retrospective.tw,kw. or Prospective.tw,kw. or Cross sectional.tw,kw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ (Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies) (2942479) 14 8 and 13 (209) 15 10 or 12 or 14 (316) 21 12 or 14 (286) 22 21 not 10 (253) |
427 |
|
Embase (Elsevier) |
(((('neuroleptic agent'/exp/mj OR 'butyrophenone derivative'/exp/mj OR antipsychotic*:ti,ab OR 'anti psychotic*':ti,ab OR neurolep* OR 'major tranquili*' OR bromperidol*:ti,ab OR chlorpromazine*:ti,ab OR chlorprothixene*:ti,ab OR fluphenazine*:ti,ab OR flupenthixol*:ti,ab OR haloperidol*:ti,ab OR penfluridol*:ti,ab OR pimozide*:ti,ab OR pipamperon*:ti,ab OR aripiprazole*:ti,ab OR clozapine*:ti,ab OR olanzapine*:ti,ab OR paliperidon*:ti,ab OR quetiapine*:ti,ab OR risperidone*:ti,ab OR sulpiride*:ti,ab) AND ('pregnancy'/exp/mj OR 'pregnancy complication'/exp/mj OR pregnan*:ti,ab OR 'exposure* in utero':ti,ab OR (((intrauterine OR 'intra uterine' OR fetal OR foetal OR maternal) NEAR/3 exposure*):ti,ab) OR 'prenatal drug exposure'/exp)) AND (('pregnancy complication'/exp OR 'spontaneous abortion'/exp OR 'pregnancy diabetes mellitus'/mj OR 'fetus death'/exp OR 'eclampsia and preeclampsia'/exp OR 'maternal hypertension'/exp OR 'premature fetus membrane rupture'/exp OR 'chorioamnionitis'/exp OR 'premature labor'/exp OR 'immature and premature labor'/exp OR 'placenta previa'/exp OR 'postpartum hemorrhage'/exp OR 'oligohydramnios'/exp OR 'perinatal death'/exp OR 'retained placenta'/exp OR 'hydramnios'/exp OR 'ectopic pregnancy'/exp OR 'low birth weight'/exp OR 'intrauterine growth retardation'/exp OR 'instrumental delivery'/exp OR 'blood transfusion'/exp) OR (stillbirth:ti,ab OR miscarriage:ti,ab OR ((spontaneous NEAR/3 abortion):ti,ab) OR ((pregnancy NEAR/3 loss*):ti,ab) OR 'gestational diabetes':ti,ab OR (((f?etal OR 'intra uterine') NEAR/3 death*):ti,ab) OR 'pre eclampsia':ti,ab OR 'pregnancy induced hypertension':ti,ab OR hellp:ti,ab OR (((preterm OR premature) NEAR/3 (delivery OR birth OR labo?r)):ti,ab) OR 'preterm rupture of membranes':ti,ab OR chorioamnionitis:ti,ab OR 'placenta previa':ti,ab OR 'postpartum h?emorrhage':ti,ab OR oligohydramnios:ti,ab OR ((perinatal NEAR/3 (death OR mortality)):ti,ab) OR 'retained placenta':ti,ab OR polyhydramnios:ti,ab OR (((ectopic OR extrauterine) NEAR/3 pregnanc*):ti,ab) OR (('f?etal growth' NEAR/3 (restriction OR retardation)):ti,ab) OR 'small for gestational age':ti,ab OR 'low birth weight':ti,ab OR fgr:ti,ab OR sga:ti,ab OR 'caeserean section':ti,ab OR 'c section':ti,ab OR (((instrumental OR obstetric*) NEAR/3 'vaginal deliver*'):ti,ab) OR ((blood NEAR/3 transfusion*):ti,ab) OR 'maternal intensive care':ti,ab))) NOT 'conference abstract':it AND (english)/lim AND ((embase)/lim OR (pubmed-not-medline)/lim) AND (<1966-2019)/py) AND (('meta analysis'/de OR cochrane:ab OR embase:ab OR psycinfo:ab OR cinahl:ab OR medline:ab OR ((systematic NEAR/1 (review OR overview)):ab,ti) OR ((meta NEAR/1 analy*):ab,ti) OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp)) (42) > 22 uniek AND (('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it)) OR ('major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti)))) (130) > 90 uniek |
|
