Antidepressiva (niet-SSRI’s) en aangeboren afwijkingen
Uitgangsvraag
Welke niet-SSRI antidepressiva hebben de voorkeur voor gebruik in de zwangerschap met betrekking tot het risico op aangeboren afwijkingen?
Aanbeveling
Bespreek met de zwangere dat bij gebruik van een niet-SSRI antidepressivum, met uitzondering van MAO-remmers, er geen reden is om te switchen naar een ander middel op basis van de huidige gegevens met betrekking tot het risico op aangeboren afwijkingen. MAO-remmers worden in principe afgeraden vanwege hun bijwerkingenprofiel, interactie met andere medicatie, specifiek dieet en specifieke maatregelen bij anesthesie. Er is geen voorkeursmiddel voor de overige niet-SSRI antidepressiva vanuit oogpunt van aangeboren afwijkingen voorhanden op basis van de literatuur.
Gebruik de laagste effectieve dosis, houd er rekening mee dat de dosering soms aangepast moet worden tijdens de zwangerschap. Absolute risico’s ten aanzien aangeboren afwijkingen zijn laag. Er is geen evidence dat doseringen hierop van invloed zijn.
Overwegingen
De kwaliteit van het bewijs
Er zijn geen aanwijzingen dat antidepressiva een sterk verhoogd risico geven op ernstige aangeboren afwijkingen in het algemeen of specifieke afwijkingen in het bijzonder. Interpretatie van studies over dit onderwerp zijn lastig, omdat optimale correctie voor verstorende variabelen zoals middelengebruik veelal niet mogelijk is. Een licht verhoogd risico op specifieke afwijkingen is niet helemaal uit te sluiten (Berard, 2017). Als er al een causaal verband bestaat, dan zijn de absolute risico's gering, omdat de prevalentie van deze specifieke afwijkingen laag is. De uitkomsten van de verschillende publicaties zijn niet eenduidig. In het merendeel van de studies is geen duidelijk effect gezien. In een klein aantal studies is een hoger risico op hartafwijkingen of een specifieke groep afwijkingen gevonden. In grote studie naar hartafwijkingen werd dit risico niet teruggevonden (Huybrechts, 2014). Als een antidepressivum geassocieerd wordt met een verhoogd risico op congenitale afwijking, is het absolute risico in elk geval nog laag.
Een aantal middelen van de antidepressiva vereisen meer hoog specialistische zorg dan anderen, waarbij gebruik tijdens de zwangerschap geen voorkeur heeft. Dit geldt bijvoorbeeld voor het gebruik van MAO-remmers tijdens de zwangerschap. Dit wordt afgeraden vanwege eerder beschreven bijwerkingenprofiel, mogelijke interactie met andere medicatie, specifiek dieet en specifieke maatregelen bij anesthesie. Indien toch een MAO-remmer wordt gebruikt, is het zinvol de zwangere te begeleiden in gespecialiseerd centrum.
Overige geraadpleegde bronnen
De NICE richtlijn benoemt dat er studies zijn die een minimale toename laten zien van congenitale afwijkingen (met name cardiale afwijkingen). De NICE richtlijn benoemt hierbij dat er twijfel is of dit effect toe te schrijven is aan medicatiegebruik tijdens de zwangerschap vanwege beperkingen ten aanzien van controle groepen (niet altijd zwangeren met depressie als controle groep, geen informatieve of ernst van de ziekte vergelijkbaar is in controle groep).
Guidelines on treatment of perinatal depression with antidepressants: an international review door Nina Molenaar (2018)
In een recente review van internationale richtlijnen ten aanzien van antidepressiva gebruik tijdens de zwangerschap beschrijft Molenaar dat vier richtlijnen adviseren antidepressiva gedurende zwangerschap te continueren (Molenaar, 2018). Vijf andere richtlijnen geven geen specifiek advies ten aanzien van het continueren of staken van antidepressiva tijdens zwangerschap. Drie richtlijnen raden af om van antidepressivum te veranderen gedurende de zwangerschap. Algemeen is er consensus dat voor- en nadelen van antidepressiva gebruik tijdens de zwangerschap door de arts met de patiënt besproken dienen te worden.
Lareb
Naar het gebruik van TCA’s tijdens de zwangerschap is veel onderzoek gedaan. Er zijn meer dan 12.000 zwangerschappen beschreven met blootstelling tijdens het eerste trimester. In de studies zijn de TCA's vaak als gehele groep onderzocht en niet de afzonderlijke middelen. De meeste ervaring is met clomipramine, amitryptiline, imipramine en nortryptiline. Met de andere TCA’s is weinig ervaring (LAREB, 2020).
In onderzoek naar het gebruik van TCA’s wordt geen hoger risico gezien op aangeboren afwijkingen. Ook onderzoek naar specifieke orgaanafwijkingen wijst niet op een duidelijk verhoogd risico. Alleen een studie van het Zweedse gezondheidsregister meldt bij clomipramine een licht verhoogd risico op met name ventrikel- en atriumseptumdefecten. Dit wordt niet in andere onderzoeken gezien.
Er is redelijke hoeveelheid onderzoek naar het gebruik van mirtazapine in de zwangerschap. Uit de onderzoeken komt geen hoger risico op aangeboren afwijkingen naar voren. In een twintigtal cases wordt mirtazapine gebruikt voor de behandeling van hyperemesis, vaak in het 2e trimester.
Met het gebruik van bupropion tijdens de zwangerschap is redelijk veel ervaring opgedaan. De beschikbare gegevens wijzen niet op een eenduidig verhoogd risico op aangeboren afwijkingen, specifieke hartafwijkingen of andere nadelige effecten op de zwangerschap en het kind.
De beperkte ervaring met het gebruik van duloxetine of trazodon tijdens de zwangerschap wijst niet op een verhoogd risico op aangeboren afwijkingen. Maar er zijn nog te weinig zwangerschappen gevolgd om op basis hiervan al een conclusie te kunnen trekken over de veiligheid van deze middelen.
Gebruik bij voorkeur geen MAO-remmer tijdens de zwangerschap. Het is onbekend of fenelzine, moclobemide en tranylcypromine veilig gebruikt kunnen worden tijdens de zwangerschap (aangezien dat deze middelen een afwijkend bijwerkingenprofiel ten opzichte van de andere antidepressiva hebben).
De NVOG Leidraad indicatiestelling prenatale diagnostiek beschrijft de indicaties voor geavanceerd ultrageluidsonderzoek bij gebruik van (teratogene) medicatie of genotsmiddelen.
Farmacologische overwegingen
Bij vrouwen die een (niet-SSRI) antidepressivum gebruiken en zwanger willen worden is het advies om per patiënt een zorgvuldig overzicht te maken van de diagnose, ziekteperiode(s), gebruikte psychofarmaca, eventueel ervaren bijwerkingen en de huidige klachten en medicatie. Er is weinig bekend over de kans op terugval na staken medicatie in de zwangerschap en postpartum periode. Er is weinig bekend over de risico’s.
Indien er wordt besloten medicatie te gebruiken is het goed om een middel van voorkeur tijdens de zwangerschap en zo mogelijk lactatie proberen te gebruiken. Dit zijn medicijnen waarmee al langere tijd ervaring is tijdens de zwangerschap en kraamperiode en derhalve als veilig voor moeder en kind worden beschouwd. Er kan niet altijd voor een van de voorkeursmedicijnen gekozen worden, bijvoorbeeld als patiënte in het verleden, ondanks adequate behandeling, geen effect van de medicatie heeft gehad, dan wel forse bijwerkingen heeft ervaren. Tevens wordt ook altijd geadviseerd de laagst mogelijke, effectieve dosis gedurende zwangerschap en eventueel tijdens de lactatieperiode te gebruiken.
Zwangerschap is vaak een periode van toegenomen stress vanwege de nieuwe levensfase, lichamelijke veranderingen en zorgen om de toekomst. Daarom zouden wij adviseren medicatie te continueren na een goede afweging van de risico’s. Hierbij dient gekozen te worden voor medicatie die beschouwd wordt als het meest veilig voor de foetus.
Waarden en voorkeuren van patiënten
De manier en inhoud van de voorlichting aan patiënte heeft grote invloed op de keuze al dan niet door te gaan met medicatie. In de praktijk blijkt vaak dat behandelaren met minder ervaring met psychofarmaca en zwangerschap eerder geneigd zijn te stoppen met psychofarmaca. Vanuit de POP-poli/ team is na goede counseling de ervaring dat patiënten juist wél hiermee doorgaan. De balans tussen de toxicologische risico’s voor het kind en de kans op ziekte terugval, dan wel exacerbatie van de ziekte en de gevolgen hiervan voor het kind zijn belangrijke redenen in deze afweging om te stoppen of door te gaan.
Kosten (middelenbeslag)
Psychofarmaca gebruiken en poliklinische begeleiding door een psychiatrisch team, kinderarts en gynaecoloog zijn in verhouding goedkoper dan een spoedopname bij ernstige terugval van ziekte. Een ander belangrijk aspect is de emotionele schade voor de moeder en de eventuele gevolgen voor het ongeboren kind als zij een forse ziekteperiode doormaakt in haar zwangerschap. Daarnaast zal naar verwachting adequate behandeling van moeder leiden tot verminderd voorkomen van ontwikkelingsproblematiek bij het kind. De kosten van de verschillende antidepressiva liggen dusdanig dicht bij elkaar dat de kosten per middel geen rol van betekenis spelen bij de medicatie keuze.
Aanvaardbaarheid, haalbaarheid en implementatie
De expertise van de betrokken behandelaren in dit land is meegenomen in deze overweging. Aangezien er weinig evidence based literatuur beschikbaar is voor dit onderwerp hebben betrokken experts uit geheel Nederland samen besproken wat de aanbeveling is. Behalve de werkgroep hebben ook andere experts uit het land de mogelijkheid gekregen hun visie op deze aanbeveling te geven. De werkgroep verwacht hierna geen bezwaren tegen implementatie van deze richtlijn.
Rationale/ balans tussen de argumenten voor en tegen de interventie
Op basis van de momenteel voor handen zijnde gegevens ten aanzien van het risico op congenitale afwijkingen geldt dat het onbekende risico op congenitale afwijkingen afgewogen moet worden tegen de noodzaak van het gebruik tijdens de zwangerschap, daarbij in acht nemende een maximale inzet van niet-medicamenteuze interventies, de voorkeuren van de (toekomstige) zwangere, waarbij gestreefd wordt naar een zo laag mogelijke effectieve dosering en het gebruik van eenzelfde medicament tijdens de periconceptionele periode, de zwangerschap, de kraamtijd en 6 maanden postpartum.
Onderbouwing
Achtergrond
Tijdens de zwangerschap is bij 7 tot 20% van de vrouwen sprake van klinisch relevante angst- en/of stemmingsklachten (Biaggi, 2016). Antidepressiva passeren de placenta en hebben derhalve potentiële gevolgen voor de foetus en neonaat (Ewing, 2015). Het is onduidelijk wat de neonatale effecten en symptomen zijn van niet-SSRI-antidepressiva op de neonaat. Niet-SSRI antidepressiva bestaan uit diverse groepen zoals de tricyclische antidepressiva (amitriptyline, clomipramine, imipramine, nortriptyline), serotonineheropnameremmers (SNRI’s: venlafaxine, duloxetine),de MAO-remmers (moclebemide, fenelzine, tranylcypromine) en overige antidepressiva (bupropion, mirtazapine, trazodon, vortioxine). Vrouwen met een kinderwens of prille zwangerschap staken nu soms de niet-SSRI antidepressiva uit angst voor de mogelijke foetale effecten. Daartegenover staat dat het niet behandelen van een depressie of angststoornis tijdens de zwangerschap een ongunstige invloed kan hebben op het beloop van de zwangerschap en complicaties bij het kind kan geven. Stabiliteit is dus van belang voor zowel de zwangere als het (ongeboren) kind. Vanuit de praktijk is de ervaring dat de gevolgen voor het kind een belangrijke overweging is voor zwangeren in de beslissing over het wel of niet starten of het continueren van antidepressiva. Voor een goede afweging en shared decision-making is adequate informatie over de gevolgen voor het kind van groot belang.
Conclusies / Summary of Findings
|
Very low GRADE |
The evidence is very uncertain about the risk of major congenital malformations in children exposed to TCAs during (the first trimester of) pregnancy as compared to children of women with unmedicated depression.
Bronnen: (Ben, 2014; Berard, 2017; Simon, 2002) |
|
Very low GRADE |
The evidence is very uncertain about the risk of major congenital malformations in children exposed to SNRIs during the first trimester of pregnancy as compared to the children not exposed to antidepressants during the first trimester of pregnancy.
Bronnen: (Berard, 2017) |
|
Very low GRADE |
The evidence is very uncertain about the risk of major congenital malformations in children exposed to bupropion during pregnancy as compared to the children of women who did not use any teratogens.
Bronnen: (Chun-Fai-Chan, 2005) |
|
Very low GRADE |
The evidence is very uncertain about the risk of major congenital malformations in children exposed to mirtazapine during pregnancy as compared to the children of women not exposed to antidepressants.
Bronnen: (Djulus, 2006; Winterfield, 2015) |
|
Very low GRADE |
The evidence is very uncertain about the risk of major congenital malformations in children exposed to trazodone or nefazodone during pregnancy as compared to children of women not exposed to antidepressants.
Bronnen: (Einarson, 2003) |
|
Very low GRADE |
The evidence is very uncertain about the risk of major cardiac malformations in children exposed to SNRIs or TCAs during the first trimester of pregnancy as compared to children not exposed to antidepressants.
Bronnen: (Huybrechts, 2014; Louik, 2014) |
|
Very low GRADE |
The evidence is very uncertain about the risk of major cardiac malformations in children exposed to bupropion during pregnancy as compared to children not exposed to antidepressants.
Bronnen: (Alwan, 2010; Louik, 2014; Huybrechts, 2014) |
|
Very low GRADE |
The evidence is very uncertain about the risk for specific organ malformations in children exposed to TCAs during pregnancies. There might be an increased risk of malformations in the digestive system, in the eye, ear, face and neck and an increased risk for spina bifida after exposure to TCAs, but the results of the studies were inconsistent.
Bronnen: (Berard, 2017; Davis, 2007; Simon, 2002) |
Samenvatting literatuur
Important study characteristics and results of the twelve included studies are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Two studies reported on the risk of any congenital anomalies after exposure to TCAs (Davis, 2007 (TCAs), Vasilakis (2013) (TCAs)). Davis (2007) reported the results of a retrospective study using data from a population-based cohort. For this study, the data from five health maintenance organizations (HMOs) were used. Female members, > 15 years of age, admitted to a hospital between 1996 and 2000 for delivery of an infant and who were continuously enrolled with prescription drug coverage for 1 year prior to the admission were included. The outcome of women exposed to antidepressants, TCAs or SSRIs, during the first trimester of pregnancy were compared to women who were not prescribed antidepressants at any time during pregnancy.
Vasilakis (2013) reported on a matched cohort study using data from the General Practice Research Database in the UK. All pregnancies of more than 20 weeks’ gestation and included live births, stillbirths, and therapeutic abortions were included. The outcome of women exposed to TCAs and SSRIs in early pregnancy were compared to women with no exposure to any antidepressants during pregnancy, matched by age, year of pregnancy outcome, and general practice.
Seven studies reported on the risk of major congenital anomalies after exposure to TCAs, bupropion or other non-SSRI antidepressants (Ban, 2014 (TCAs); Berard, 2017 (TCAs; SNRIs); Chun-Fai-Chan, 2005 (bupropion); Djulus, 2006 (mirtazapine); Einarson, 2003 (trazodone or nefazodone); Simon, 2002 (TCAs), Winterfield, 2015 (mirtazapine)).
Ban (2014) reported the results of a population-based cohort study. Data were used from a nationally representative database of computerized primary care records in Canada. Herein information was prospectively recorded throughout pregnancy and in the year before pregnancy. All singleton live births for women aged 15 to 45 years between 1990 and 2009 were included in the analysis. Women with antenatal exposure to SSRIs and TCAs during the first trimester of pregnancy children into 5 exposure groups: no maternal depression, maternal depression but no antidepressants in the first trimester (unmedicated depression), first-trimester exposure to SSRIs alone, first-trimester exposure to TCAs alone, dual exposure to both SSRIs and TCAs in the first trimester.
Berard (2017) reported on a register-based cohort study using data from the Quebec Pregnancy Cohort (Canada), in which prospective data on all pregnancies in a certain period in a region was collected. All pregnancies with a diagnosis of depression or anxiety or exposed to antidepressants in the 12 months before pregnancy, and ending with a live-born singleton were included. Women with exposure to SSRI / SNRI /TCA /other antidepressants during the first trimester were included as cases. Controls were women with no exposure to antidepressants during the first trimester.
Chun-Fai-Chan (2005), Djulus (2006), and Einarson (2003) reported on the risk of specific antidepressant drugs using data from The Motherisk Program in Canada. Women who contacted The Motherisk Program in the first trimester of pregnancy, taking the drug of interest (Chun-Fai-Chan: bupropion, Djulus: mirtazapine, Einarson: trazodone/ nefazodone) and were pregnant or planning a pregnancy at the time of call were enrolled. Two comparison groups of women were included: 1) Women taking other antidepressants; 2) women who contacted Motherisk but were not exposed to any teratogens.
Simon (TCAs) performed a cohort study, for which a sample was drawn from a prepaid health plan serving approximately 400,000 members in Washington State. All live births between January 1986 and December 1998 were identified using Hospital discharge records. Pharmacy records were used to identify all antidepressant prescriptions filled or refilled during the 360 days before delivery. Mothers using TCAs (n=209) or SSRIs were included as cases. Mothers with no antidepressant prescriptions during this period were considered unexposed (n=209).
A multicenter, observational prospective cohort study addresses the risk associated with exposure to mirtazapine during pregnancy (Winterfield 2015). Pregnant women who themselves or whose physician contacted one of the eleven participating Teratology Information Services (TIS) between January 1995 and December 2011 seeking counsel about safety of exposure to therapeutic agents during pregnancy. Pregnancy outcomes after exposure to mirtazapine were compared with 2 matched control groups: exposure to a SSRI, or no exposure to medication known to be teratogenic.
Huybrechts (2014) assessed the risk of cardiac malformations after the use of TCAs, SNRI, buproprion or other antidepressants in an observational study. 64,389 women who used antidepressants during the first trimester of their pregnancy were included. women without exposure to antidepressants during the first trimester were used as control group. The authors also presented the results of an analysis restricted to women with depression.
Three studies reported on the risk of developing major heart defects after exposure to bupropion (Ban, 2014; Alwan, 2010; Louik, 2014). Louik (2014) reported results of an observational study using data from the Slone Epidemiology Center’s Birth Defects Study (USA). Infants with malformations were identified at study centers. The risk of malformations was compared between children from women with any exposure to bupropion (alone or in combination with other antidepressants) with children from women with exposure to any other antidepressant (SSRI, TCA, or other antidepressants).
Alwan (2010) presented the results of a retrospective case-control study. Data from the National Births Defects Prevention Study (USA) were used. Infants with major heart defects were included as cases and without major birth defects as controls. Exposure to bupropion was defined as any reported use between one month before and three months after conception.
Finally, three studies also reported on the risk of malformations in specific organs, Berard (2017), Davis (2007) and Simon (2002), after exposure to TCAs or SNRIs.
Risk of any congenital malformations (critical outcome)
Davis (2007) found no increased risk of any congenital malformations after exposure to TCAs (RR= 0.86, 95%CI 0.57 to 1.30). One or more malformations in 20 of the 167 children (12%) exposed to TCA’s during the first trimester of pregnancy and in 6811 of 49669 children not exposed to antidepressants at any time during pregnancy (14%).
Vasilakis (2013) reported that 42 infants with congenital malformations were found in 1608 infants exposed to TCA (2.6%). 205 children with congenital malformations were found in 6617 children of women with no exposure to any antidepressants during pregnancy (3.1%). They only reported the crude Relative Risk (95% CI), TCAs) no exposure: 0.9 (0.6 to 1.2) and remarked that “adjustment for confounders did not alter the crude results by 10% or more”.
Risk of major congenital malformations (critical outcome)
Seven studies reporting on the risk of major congenital anomalies but differed in the source of the population, the antidepressants and the control group. The main characteristics are summarized in Table 1. Ban (2014), Berard (2017), Simon (2002) reported on groups of antidepressants: TCAs and SNRIs. Chun-Fai-Chan (2005), Djulus (2006), Winterfield (2015), and Einarson (2003) reported on the risk of specific drugs.
Table 1 Studies reporting on the risk of major congenital anomalies
|
reference |
source population |
exposed |
non-exposed |
|
Ban 2014 |
Population-based cohort study; all singleton live births for women in a specific network |
TCAs during first trimester |
Women with unmedicated depression |
|
Berard 2017 |
register-based cohort study; prospective data collection on all pregnancies in a specific province |
TCAs/ SNRIs during first trimester |
No exposure to antidepressants during 1st trimester |
|
Simon 2002 |
sample drawn from a prepaid health plan; mothers with antidepressant prescriptions |
Exposure to TCAs during pregnancy |
Mothers with no antidepressant prescriptions |
|
Chun-Fai-Chan 2005 |
Women who contacted counselling services for pregnant and lactating women and their health professionals |
Women using bupropion for depression |
Women not using any teratogens |
|
Djulus 2006 |
Women who contacted counselling services for pregnant and lactating women and their health professionals or teratology information services |
Mirtazapine during pregnancy |
Pregnant women exposed to nonteratogens |
|
Winterfield 2015 |
Pregnant women who themselves or whose physician contacted Teratology Information Services |
Mirtazapine during pregnancy |
No exposure to medication known to be teratogenic or any antidepressant |
|
Einarson 2003 |
Women who contacted counselling services for pregnant and lactating women and their health professionals |
Trazodone/ nefazodone during the first trimester |
Women exposed to other Nonteratogenic drugs |
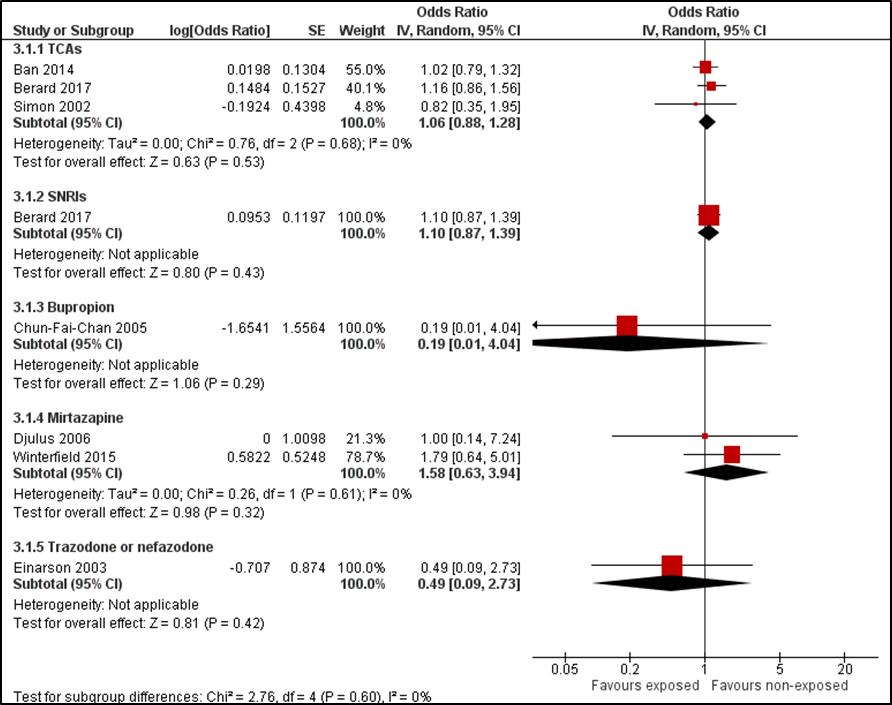
The risk of major congenital anomalies after exposure to TCAs, SNRIs, or specific antidepressant drugs is presented in Figure 1. The results show no increased risk of major congenital anomalies after exposure to TCAs (OR = 1.06 (95%CI 0.88 to 1.28)) or SNRIs (OR = 1.10 (95%CI 0.87 to 1.39)).
Figure 1 Forest plot. Risk of major congenital anomalies after exposure to antidepressants (non-SSRIs)
Risk of congenital cardiac malformations (important outcome)
Huybrecht (2014) reported adjusted odds ratios for any cardiac malformation, right outflow heart defects and septal heart defects after using TCAs, SNRI, bupropion, or other antidepressants in a subgroup of women with depression. The adjusted odds ratio (OR) for any cardiac malformation was 0.77 (0.52 to 1.14) for TCAs, 1.20 (0.91 to 1.57) for SNRIs, 1.21 (0.91 to 1.60) for other antidepressants, and 0.92 (0.69 to 1.22) for bupropion.
Table 2 Risk of major heart defects after exposure to TCAs
|
reference |
major heart defects |
Odds ratio (95% CI) |
|
Louik 2014 |
ventricular septal defect any left-sided cardiac defect coarctation of the aorta hypoplastic LHS |
0.8 (0.4, 1.8) 0.7 (0.2, 2.3) 1.3 (0.3, 5.5) 0.9 (0.1, 7.1) |
|
Huybrechts 2014 |
any cardiac malformation ventricular septal defect right ventricular outflow tract obstruction other cardiac malformations |
0.8 (0.5-1.1) 0.9 (0.5-1.5) 0.9 (0.4-2.4)
0.8 (0.5-1.40) |
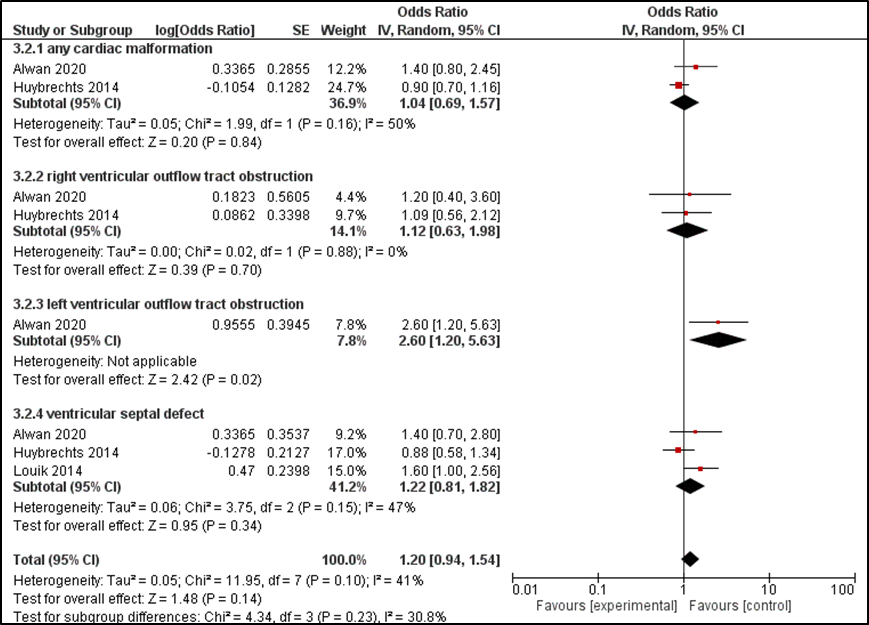
Alwan (2010) reported the overall risk and the risk for 4 categories of heart defects, including conotruncal heart defects, left outflow tract heart defects, right outflow heart defects and septal heart defects. Louik (2014) only reported the risk of specific types of cardiac malformations, including ventricular septal defect, left-sided defects, coarctation aorta and hypoplastic LHS. The ORs for each of these outcome measures are summarized in Table 2. Alwan (2010) found no overall increased risk in heart defects (OR=1.4 (0.8 to 2.5)) but did find an increased risk left outflow tract heart defects (OR=2.6 (1.2 to 5.7)). Louik (2014) found an increased risk of ventricular septal defects, but no increased risk of any left-sided cardiac defects, coarctation of the aorta or hypoplastic left heart syndrome (HLHS). Two studies (Alwan, 2010; Louik, 2014) reported an increased risk of a septal defect. Huybrechts (2014), however, did not find an increased risk for ventricular septal defects after exposure to bupropion. Combining these data, the overall risks of a ventricular septal defect after exposure to bupropion during pregnancy is not increased (OR= 1.22 (95%CI 0.81 to 1.82)).
Table 3 Risk of major heart defects after exposure to SNRIs
|
reference |
major heart defects |
Odds ratio (95% CI) |
|
Huybrechts 2014 |
any cardiac malformation ventricular septal defect right ventricular outflow tract obstruction other cardiac malformations |
1.2 (0.9-1.6) 1.2 (0.9-1.8)
1.3 (0.9-1.9) |
Figure 2 Risk of major heart defects after exposure to bupropion
Other specific organ malformations (important outcome)
Finally, four studies also reported on the risk of malformations in specific organs.
Berard (2017) reported an increased risk for some organ-specific malformations. The exposure to TCAs was related to an increased risk malformation in the digestive system (OR = 2.55 (95%CI 1.40 to 4.66)) and in the eye, ear, face and neck (OR=2.45 (95%CI 1.05 to 5.72)). The exposure to venlafaxine was related to an increased risk of malformations in the respiratory system OR=2.17 (95%CI 1.07 to 4.38). No increased risks were found for other organ-specific malformations.
Ban (2014) found no increased risk of specific major congenital anomalies (such as the heart, limb, genital system, urinary system) in children exposed to TCAs alone, as compared with children born to women with unmedicated depression. They did find an increase of nervous system anomalies in children with joint SSRI and TCA exposure in the first trimester (OR 4.6, 95% CI 1.10 to 19.06).
Davis (2007) found an increased risk for spina bifida after exposure to TCAs (RR = 12.43; 95%CI 1.70 to 90.66), but no increased risk for other specific malformations (heart, respiratory system, genital organs, urinary system).
Simon (2002) found no increased risk of specific malformations (genitourinary, cardiac, skeletal, vascular, craniofacial) after expose to TCAs during pregnancy.
Level of evidence of the literature
In some studies, the exposure to antidepressants during pregnancy were extracted from registration data in databases, which yields a risk of misclassification. In other studies, the population consisted of women who contacted a counselling services or teratology information services and women who were exposed to other nonteratogenic drugs were used as control group, which deviated from the population we are interested in (indirectness).
We started with a low level of evidence for observational studies. The level of evidence regarding the outcome measure ‘risk of major congenital malformations’ for TCAs was downgraded by two levels because of study limitations (risk of bias) and imprecision, resulting in a very low level of evidence.
The level of evidence regarding the outcome measures ‘risk of major congenital malformations’ for SNRI, bupropion, trazodone and nefazodone was downgraded by three levels because of study limitations (risk of bias; risk of misclassification) indirectness (control group of women without depression), and imprecision (one study per drug with wide confidence interval).
The level of evidence regarding the outcome measure ‘risk of major congenital malformations’ for mirtazapine was downgraded by three levels because of study limitations (risk of bias; risk of misclassification), indirectness (control group of women without depression) and imprecision.
The level of evidence regarding the outcome measure ‘risk of congenital cardiac malformations’ was downgraded by two levels because of study limitations (risk of bias; risk of misclassification) and imprecision.
The level of evidence regarding the outcome measure ‘other specific organ malformations’ was downgraded by three levels because of study limitations (risk of bias; risk of misclassification), inconsistency, and imprecision.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the effect of non-SSRI antidepressant use during pregnancy on the risk of congenital malformations in children of women with psychiatric disorders, compared to pregnant women not taking non-SSRI antidepressants?
P (patients): pregnant women with psychiatric disorders;
I (intervention): antidepressant use (non-SSRIs);
C (control): no antidepressant use;
O (outcome): congenital malformations.
Relevant outcome measures
The guideline development group considered major congenital malformations as a critical outcome measure for decision making; and the risks of congenital malformations in specific organs as important outcome measures for decision making.
The guideline working group did not define a minimally clinically important difference.
A priori, the working group did not define the outcome measures listed above but used the definitions used in the studies.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 4th of April 2019. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 550 hits. Studies were selected based on the following criteria: original research comparing the outcome in women who used antidepressants and women who did not use antidepressants, reporting the outcomes for relevant (subgroups of) antidepressants, including SNRIs, TCAs and other antidepressants (no SSRIs). 79 studies were initially selected based on title and abstract screening. The systematic reviews did not report their results usefully. After reading the full text, 67 studies were excluded (see the table with reasons for exclusion under the tab Methods) and 12 original studies were included.
Four of these studies reported on results using data from the same program (the Motherrisk Program in Canada; Chun-Fai-Chan, 2005; Djulus, 2006; Einarson, 2003; and Einarson, 2009. Neither of the two studies by Einarson reported from which period women had been included. Thus, there is a risk of overlapping populations between the results reported by Einarson in 2009 and the three other studies (Chun-Fai-Chan, 2005; Djulus, 2006 and Einarson, 2003). Therefore, the data from Einerson (2009) were excluded from this literature analysis.
Referenties
- Alwan, S., Reefhuis, J., Botto, L. D., Rasmussen, S. A., Correa, A., Friedman, J. M., & Study, N. B. D. P. (2010). Maternal use of bupropion and risk for congenital heart defects. American journal of obstetrics and gynecology, 203(1), 52-e1.
- Ban, L., Gibson, J. E., West, J., Fiaschi, L., Sokal, R., Smeeth, L.,... & Tata, L. J. (2014). Maternal depression, antidepressant prescriptions, and congenital anomaly risk in offspring: a population‐based cohort study. BJOG: An International Journal of Obstetrics & Gynaecology, 121(12), 1471-1481.
- Bérard, A., Zhao, J. P., & Sheehy, O. (2017). Antidepressant use during pregnancy and the risk of major congenital malformations in a cohort of depressed pregnant women: an updated analysis of the Quebec Pregnancy Cohort. Bmj Open, 7(1), e013372.
- Biaggi, A., Conroy, S., Pawlby, S., & Pariante, C. M. (2016). Identifying the women at risk of antenatal anxiety and depression: a systematic review. Journal of affective disorders, 191, 62-77.
- Chun-Fai-Chan, B., Koren, G., Fayez, I., Kalra, S., Voyer-Lavigne, S., Boshier, A.,... & Einarson, A. (2005). Pregnancy outcome of women exposed to bupropion during pregnancy: a prospective comparative study. American journal of obstetrics and gynecology, 192(3), 932-936.
- Davis, R. L., Rubanowice, D., McPhillips, H., Raebel, M. A., Andrade, S. E., Smith, D.,... & Platt, R. (2007). Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiology and drug safety, 16(10), 1086-1094.
- Djulus, J., Koren, G., Einarson, T. R., Wilton, L., Shakir, S., Diav-Citrin, O.,... & Einarson, A. (2006). Exposure to mirtazapine during pregnancy: a prospective, comparative study of birth outcomes. The Journal of clinical psychiatry, 67(8), 1280-1284.
- Einarson, A., Bonari, L., Voyer-Lavigne, S., Addis, A., Matsui, D., Johnson, Y., & Koren, G. (2003). A multicentre prospective controlled study to determine the safety of trazodone and nefazodone use during pregnancy. The Canadian Journal of Psychiatry, 48(2), 106-110.
- Einarson, A., Choi, J., Einarson, T. R., & Koren, G. (2009). Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. The Canadian Journal of Psychiatry, 54(4), 242-246.
- Ewing, G., Tatarchuk, Y., Appleby, D., Schwartz, N., & Kim, D. (2015). Placental transfer of antidepressant medications: implications for postnatal adaptation syndrome. Clinical pharmacokinetics, 54(4), 359-370.
- Huybrechts, K. F., Palmsten, K., Avorn, J., Cohen, L. S., Holmes, L. B., Franklin, J. M.,... & Hernández-Díaz, S. (2014). Antidepressant use in pregnancy and the risk of cardiac defects. New England Journal of Medicine, 370(25), 2397-2407.
- LAREB. (2020) Tricyclische antidepressiva tijdens de zwangerschap. Retrieved from https://www.lareb.nl/tis-knowledge-screen?id=423
- Leidraad indicatiestelling prenatale diagnostiek https://www.nvog.nl/wp-content/uploads/2019/02/definitief-NVOG-Leidraad-indicatiestelling-PND-versie-feb.-2019.pdf
- Louik, C., Kerr, S., & Mitchell, A. A. (2014). First‐trimester exposure to bupropion and risk of cardiac malformations. Pharmacoepidemiology and drug safety, 23(10), 1066-1075.
- Molenaar, N. M., Kamperman, A. M., Boyce, P., & Bergink, V. (2018). Guidelines on treatment of perinatal depression with antidepressants: An international review. Australian & New Zealand Journal of Psychiatry, 52(4), 320-327.
- Simon, G. E., Cunningham, M. L., & Davis, R. L. (2002). Outcomes of prenatal antidepressant exposure. American Journal of Psychiatry, 159(12), 2055-2061.
- Vasilakis‐Scaramozza, C., Aschengrau, A., Cabral, H., & Jick, S. S. (2013). Antidepressant use during early pregnancy and the risk of congenital anomalies. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 33(7), 693-700.
Evidence tabellen
Evidence tables for intervention studies (randomized controlled trials and non-randomized observational studies (cohort studies, case-control studies, case series))
Research question: What is the effect of non-SSRI antidepressant use during pregnancy on the risk of congenital malformations in children of women with psychiatric disorders, compared to pregnant women not taking non-SSRI antidepressants?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison/control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Alwan 2010
USA |
retrospective case-control
data from National Births Defects Prevention Study; population- based case control study
population based birth defects surveillance systems at 10 sites
|
cases: 12383: -6853 infants with major heart defects (live births, fetal death >20 weeks or electively terminated pregnancies with reliably ascertained defects); -5763 cases with noncardiac defects (6 categories)
|
bupropion exposure = any reported use between 1 month before and 3 months after conception
data on exposure collected by standardized telephone interviews with mothers; 6 weeks to 2 years after delivery. Asked if they took any of a list of medications
mothers having depression but did not report use of antidepressant during pregnancy were excluded
n=90 (0,5%) reported use bupropion in period 1 month before – 3 months after conception
n=64 mothers of cases; 26 mothers of controls |
controls: 5869 control infants born 1997-2004; no major birth defects |
Duration or endpoint of follow-up: N/A For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A
|
risk of major heart defects
all heart defects, n=34 in exposed; n=26 in controls OR=1.4 (95%CI 0.8 – 2.5) (adjusted analysis)
categories: conotruncal heart defects, n=4 in exposed OR=0.9(95%CI 0.3-2.6)
left outflow tract heart defects, n=10 in exposed OR=2.6 (95%CI 1.2-5.7)
Right outflow heart defects, n=4 in exposed OR=1.2 (95%CI 0.4-3.4)
Septal heart defects, n=15 in exposed OR=1.4 (95%CI 0.7-2.8) |
exposure: measured via telephone interviews
only heart defects / birth defects that had at least 3 cases exposed to bupropion were analysed
potential confounders: maternal age, race, education, obesity before pregnancy, smoking, alcohol use, folic acid use, annual family income, plurality, and parity.
GRADE: - risk of misclassification (non)exposed - uncertainty due to low number of cases
276 mothers excluded; no complete interview;
6 mothers excluded with depression but no use of antidepressants |
|
Ban 2014
UK
|
Population-based cohort study
The Health Improvement Network (THIN): medical records of mothers and the children were linked; prospectively recorded information throughout pregnancy and in the year before pregnancy. A nationally representative database of computerised primary care records from across the UK, validated for pharmacoepidemiology studies, contains diagnoses, events, symptoms, and drug prescriptions |
all singleton live births for women aged 15–45 years between 1990 and 2009
excluded children whose mothers had bipolar disorder, schizophrenia, other serious psychotic disorders, or prescriptions for antimanic and antipsychotic drugs before childbirth
excluded children with records of genetic malformations or malformations attributed to known teratogens (e.g. Read codes for malformations arising from maternal infections or fetal alcohol syndrome).
n=349.127 singleton live births
|
Antenatal exposure to SSRIs and TCAs during the first trimester of pregnancy: presence or absence of relevant drug prescriptions in women’s records from 4 weeks before to 12 weeks after the first day of the estimated last menstrual period.
grouped children into 5 exposure groups: - no maternal depression; - maternal depression but no antidepressants in the first trimester (unmedicated depression); - first-trimester exposure to SSRIs alone; - first-trimester exposure to TCAs alone; - dual exposure to both SSRIs and TCAs in the first trimester.
TCAs alone: n = 2.428
|
unmedicated depression: n = 13.432 no depression: n=325.294 |
Duration or endpoint of follow-up: N/A For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A
|
major congenital anomaly (MCA)
identified in the children’s medical records using Read codes, classified into 14 system-specific groups according to the European Surveillance of Congenital Malformations (EUROCAT) subgroups
overall prevalence of MCA = 2.7% (95% CI 2.6–2.8%).
absolute risks of MCAs - Children of women with unmedicated depression: 283/10.000 - children of women with no depression: 268/10.000 adjusted OR = 1.07, 95% CI 0.96–1.18
risk in children exposed to TCAs alone, compared with children of women with unmedicated depression - all MCAs OR=1.02 95%CI 0.79–1.32 - Heart OR=0.90 95%CI 0.54–1.50 - Limb OR=1.08 95%CI 0.60–1.93 - Genital system OR=0.62 95%CI 0.29–1.30
risk in children exposed to TCAs alone, compared with children born to mothers without depression - all MCAs OR=1.09 95%CI 0.87–1.38 - heart OR=1.03 95%CI 0.65–1.63 -limb OR=1.04 95%CI 0.61–1.77 -genital system OR=0.81 95%CI 0.41–1.63
ORs adjusted for maternal age at the end of pregnancy, year of childbirth, Townsend deprivation quintile, maternal smoking history, body mass index before pregnancy, and maternal diabetes, hypertension, asthma, and epilepsy in the year before conception or during pregnancy. |
|
|
Berard 2017
Canada |
register-based cohort study
the Quebec Pregnancy Cohort
prospective data collection on all pregnancies that occurred between January 1998 and December 2009 in the province of Quebec. |
All pregnancies with a diagnosis of depression or anxiety, or exposed to antidepressants in the 12 months before pregnancy, and ending with a live-born singleton
only considered those who had a diagnosis or were treated with antidepressants in the year before their pregnancy
n=18 487 women |
cases (n=3640): -exposure to SSRI / SNRI /TCA /other antidepressants -exposed to only one type of antidepressants - prescription fillings for any antidepressant dispensed to women in the study - relevant exposure time window = first trimester (0–14 weeks)
SSRIs n=2327 SNRIs n=738 TCAs n=382 other antidepressants n=193
excluded pregnancies exposed to known teratogens during the first trimester of pregnancy and pregnancies with newborn diagnoses of chromosomal abnormalities. |
controls: -no exposure to antidepressants during 1st trimester
not exposed n=14847
|
Duration or endpoint of follow-up: up to 11 years of follow-up For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A
|
Major congenital malformations diagnosed in the first year of life
risk major congenital malformations SSRI: n=279/2327 SNRI; n=91/738 TCA n=51/382 non-exposed n=1650/14847
risk major congenital malformations) non-exposed: SSRI OR=1.07 (95%CI 0.93 to 1.22) SNRI OR= 1.10 (95%CI 0.87 to 1.38) TCA OR= 1.16 (95%CI 0.86 to 1.56) other antidepressants OR =0.93 (0.59 to 1.47)
OR’s adjusted for maternal age, welfare status, diabetes, hypertension, asthma and other medication uses incl benzodiazepines and healthcare usage
Increased risks for organ-specific malformations (only increased risks): Eye, ear, face and neck - TCAs OR=2.45 (95%CI 1.05 to 5.72)
Respiratory system - Venlafaxine OR=2.17 (95%CI 1.07 to 4.38)
Digestive system - TCAs OR = 2.55 (95%CI 1.40 to 4.66) |
selection: very few excluded pregnancies because the total number of combined antidepressants (AD) uses or switches were small
potential confounders: maternal age, maternal marital status, welfare status, education level and place of residence, maternal chronic comorbidities during the 12 months prior to pregnancy including hypertension, diabetes, asthma; other medication uses including benzodiazepines.
missing information on potentially important confounders such as smoking, folic acid intake and alcohol intake performed probabilistic sensitivity analyses to quantify the likely effects of misclassifications of exposure and outcome.
up to 11 years of follow-up |
|
Chun-Fai-Chan 2005
Canada
|
observational study
The Motherisk Program
period not reported |
Women who contacted The Motherisk Program in Toronto, Canada, and The Pregnancy Riskline in Farmington, Conn, in the first trimester of pregnancy, taking bupropion, and were pregnant or planning a pregnancy at the time of call were enrolled
Women also recruited from The Drug Safety Research Unit in Southampton, UK, a prescription event monitoring database of new drugs on the market. When a woman became pregnant while taking a new drug, the physician is contacted and asked to prospectively monitor these women |
exposed to Bupropion
n=136 exposed during the first trimester; n=91 used bupropion for depression
matching criteria: age of the participant, alcohol consumption, and smoking. Gestational age at the time of call to Motherisk program was also matched. Unable to do this at the other 2 sites. |
Two comparison groups: 1) Women taking other antidepressants; n=89 2) women who contacted Motherisk, but were not exposed to any teratogens; n=89
|
Duration or endpoint of follow-up: Follow-up of pregnancy outcome between 4 months and 1 year after delivery For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A
|
risks for major malformations, n (%) (definition not reported)
bupropion: n=0/91 other antidepressant: n=1/89 (1.1%) no teratogens: n=2/89 (2.2%)
|
outcomes: collected by telephone interview using a questionnaire; the researcher subsequently sent a letter to the patient’s physician asking for verification of the information
Follow-up of pregnancy outcome between 4 months and 1 year after delivery.
|
|
Davis 2007
USA |
restrospecticve study
population-based cohort
data from five health maintenance organizations (HMOs)
|
female members > 15 years of age, admitted to a hospital 1996 - 2000 for delivery of an infant and were continuously enrolled with prescription drug coverage for 1 year prior to the admission. |
exposed: use of antidepressants, TCAs or SSRIs, dispensing during trimester one
SSRI n=1602 TCAs n=339 other antidepressants n=260
Maternal prescription medication dispensings prior to the infant’s birth were obtained from these health systems’ administrative pharmacy records |
non-exposed: infants born to mothers who were not prescribed antidepressants at any time during pregnancy, but who might have had other medications prescribed
non-exposed n=75833
|
Duration or endpoint of follow-up: limited study to those infants that had a requisite follow-up 365 days For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A
|
congenital malformations
Inpatient (including birth hospitalization), outpatient, and emergency department databases were evaluated for diagnoses of any congenital malformations of interest
overall, one or more malformations exposed TCA n= 20 / 167 non-exposed n= 6811 / 49669 RR = 0.86 (95%CI 0.57, 1.30)
many specific malformations reported including:
- spina bifida; exposed TCA n=1/167 non-exposed n=28/49669 RR = 12.43 (95%CI 1.70, 90.66)
-Bulbus cordis malformations and malformations of cardiac septal closure; RR=0.92 (0.23, 3.70) -Congenital malformations of respiratory system; RR=1.09 (0.15, 7.72) -Congenital malformations of genital organs; 0.77 (0.25, 2.38) -Congenital malformations of urinary system; 0.64 (0.09, 4.53) -congenital musculoskeletal deformities; RR=1.42 (0.65, 3.12)
risks were stratified by health system, maternal age, and birth season |
confidence intervals unadjusted for multiple testing
limited study to those infants that had a requisite follow-up 365 days |
|
Djulus 2006
Canada, Israel, Australia, USA, Italy |
observational study, prospective
The Motherisk Program and 4 other teratology information services in Israel, Australia, USA, Italy;
June 2002 to August 2005 |
Pregnant women who were exposed to mirtazapine during pregnancy and who contacted (directly or indirectly through their health care providers) 1 of the 5 participating centers were asked to participate in the study |
Mirtazapine during pregnancy; n=104 women
matched for maternal age at the time of conception, gestational age at the first contact, tobacco use, alcohol consumption, and chronic conditions |
2 comparison groups: 1) disease-matched pregnant women diagnosed with depression taking other antidepressants and 2) pregnant women exposed to nonteratogens |
Duration or endpoint of follow-up: contacted 2 to 6 months after their expected date of delivery to assess pregnancy outcome For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A |
risk for major malformations (=a structural abnormality that was either lethal, required treatment, or was of cosmetic importance and would interfere with quality of life), n (%)
mirtazapine; n=2 (1.9%), 95%CI (0.5%, 3.6%) other antidepressant: n=1 (1.0%), 95%CI (0.2%, 2.7%) Nonteratogen: n=2 (1.9%), 95%CI (0.5%, 3.6%)
|
fetal outcomes were collected by telephone interview; Each mother’s report was corroborated with the report of physician caring for the infant
analysis: excluded chromosomal defects and genetic disorders
contacted 2 to 6 months after their expected date of delivery to assess pregnancy outcome |
|
Einarson 2003 |
observational study
sample from Motherisk Program; counselling services for pregnant and lactating women and their health professionals
period not reported |
all women who had called each service requesting information about the safety of trazodone or nefazodone
147 pregnancies
|
use of trazodone or nefazodone
All women used the drugs during the first trimester, with 52 (35%) using the drug throughout their pregnancies.
|
2 groups of women to compare: - disease-matched women with depression taking other nonteratogenic antidepressants - women exposed to other nonteratogenic drugs (sumatriptan, dextromethorphan, diclectin, and clarithromycin) |
Duration or endpoint of follow-up: most women were followed up between 4 and 6 months after their expected confinement date For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A
|
incidence of major malformations = presence of any anomaly with an adverse effect on either the function or the social acceptability of the child
definition major malformation not reported
exposed to trazodone or nefazodone: n=121 (82.4%) live births, n= 2 (1.6%) major malformations (Hirschsprung disease, Neural tube defect)
comparison groups: - Antidepressants group: n=121 live births, n=3 (2.4%) major malformations - Nonteratogen group: n=131 live births, n=4 (3.0%) major malformations |
used a structured questionnaire to obtain a history of drug exposure and pregnancy outcome; At follow-up, we questioned women regarding the course of their pregnancy, the health of their child, and the specific details of their exposure to trazodone or nefazodone and any concomitant drugs or other exposures; Outcomes were confirmed by a letter asking the child’s primary care physician
no multivariate analysis; disease matched; compared for age, smoking, and alcohol use and matched for time of call (early or late in pregnancy)
Most women were followed up between 4 and 6 months after their expected confinement date |
|
Einarson 2009
Canada |
Prospective Cohort Study
Motherisk Program a teratogenic information service; provides evidence-based information on the safety and risks associated with exposures to drugs, chemicals, radiations, and infectious diseases during pregnancy and lactation to pregnant women, lactating mothers, and their health care providers.
period not reported |
women who contacted us for the antidepressant exposure
|
data from women who contacted Motherisk Program for the antidepressant exposure (n = 1243) including: bupropion (n=113) mirtazepine (n=68) trazodone (n=17) venlafaxine (n=154) Nefazodone (n = 49)
remaining antidepressants: Citalopram, Fluvoxamine, Fluoxetine, Paroxetine, Sertraline
only included women exposed to the antidepressant during the first trimester
|
data from women who contacted Motherisk Program; control: women who were not exposed to antidepressants and who had called Motherisk for information regarding nonteratogenic drugs, matched for maternal age, smoking, and alcohol use. |
Duration or endpoint of follow-up: N/A For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A |
major malformations: the antidepressant group n= 24 (2.5%) comparison group: n=25 (2.6%) risk ratio = 0.96 (95% CI 0.55 to 1.67).
major malformations in specific drugs: bupropion: 0/ 113 mirtazapine: 2/68 =2.9% (Trachomalacia Vesicoureteral reflux) trazodone: 0/ 17 venlafaxine: 2/154=1.3% (Hypospadias, Club foot) nefazodone:1/49 (Hirschsprung disease)
At follow-up interview, gestational findings, fetal outcomes, and neonatal health are documented on a structured form by telephone interview with each mother. The details are then, with her permission, corroborated with the report of the physician caring for the baby. |
geen gegevens over jaartallen inclusie; mogelijk overlap met Chun-Fai-Chan 2005 (bupropion), Djulus 2006 (mirtazapine), Einarson 2003 (trazodone or nefazodone)
matched for maternal age, smoking, and alcohol use.
no definitions of major malformations
able to analyze 928 women in each group (75%) no multivariate analysis / matched for maternal age, smoking, and alcohol use.
able to analyze 928 women in each group (75%) |
|
Huybrechts 2014
USA |
cohort study nested in the 2000–2007 nationwide Medicaid Analytic eXtract (AX) contains individual-level demographic and Medicaid information, physician services and hospitalizations, diagnoses and procedures, and outpatient medication prescriptions |
all completed pregnancies in women aged 12 to 55 years; linked these pregnancies to live-born infants
excluded pregnancies in which baby diagnosed with chromosomal abnormality and pregnancies in which mother treated with known teratogens during the 1st trimester |
exposure to antidepressants during the 1st trimester (n=64,389) - SSRIs (n=46,144) -TCAs (n=5,954) -SNRIs (n=6,904) -bupropion (n=8,856) -other antidepressants (n=7,055)
estimated date of last menstrual period using the delivery date combined with diagnostic codes indicative of pre-term delivery
determined maternal use of antidepressants through pharmacy dispensing records, using the dispensing date and number of days supply
Depression restricted cohort, non-exposed, n=180,564
|
non-exposed: women who took no antidepressant during 1st trimester (n=885,115)
|
Duration or endpoint of follow-up: N/A For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A |
risk of Congenital cardiac malformations, defined as the presence of in- or outpatient ICD-9 diagnostic codes in the maternal or infant records during the first 90 days post delivery
P=cohort to women with a depression diagnosis:
- any cardiac malformation, OR (95%CI) SSRIs, n=416, 1.06 (0.93–1.22) TCAs n=42, 0.77 (0.52–1.14) SNRIs n=75, 1.20 (0.91–1.57) other antidepressants n=74, 1.21 (0.91–1.60) bupropion n=76, 0.92 (0.69–1.22)
- right ventricular outflow tract obstruction (RVOTO) OR (95%CI) TCAs 0.8 (0.5-1.40) SNRIs 1.1 (0.6-2.1) other antidepressants 0.61 (0.24-1.52) bupropion 1.1 (0.6-2.1)
- ventricular septal defect (VSD), OR (95%CI) TCAs 0.9 (0.5-1.5) SNRIs 1.2 (0.9-1.8) other antidepressants 0.99 (0.64-1.53) bupropion 0.9 (0.6-1.3)
- other cardiac malformation OR (95%CI) TCAs 0.8 (0.5-1.40) SNRIs 1.3 (0.9-1.9) other antidepressants 1.65 (1.15-2.37) bupropion 1.2 (0.8-1.7)
Adjustment for the propensity score, to control for proxies of depression severity and other potential confounders |
Logistic regression analysis was used to estimate odds ratios for cardiac malformations and their corresponding 95% confidence intervals (CI). Because the odds ratio is an excellent approximation of the risk ratio in the case of rare outcomes, the results are referred to as relative risks |
|
Louik 2014
USA |
observational study
Using data from the Slone Epidemiology Center’s Birth Defects Study (BDS, also known as the Pregnancy Health Interview Study); a multi-center case-control surveillance program for birth defects that began in 1976
data from February, 1993 - December 2011 |
Infants with any of a wide range of malformations are identified at study centers (areas surrounding Boston, Philadelphia, Toronto, San Diego, a portion of New York State and the entire state of Massachusetts)
The BDS also systematically enrolls mothers of non-malformed infants; initially, exclusively at study hospitals, where non-malformed infants are selected in proportion to the number of malformed infants identified and are matched to cases on age within 2months. In 1998, enhanced inclusion of non-malformed infants by enrolling a population-based random sample of newborns in Massachusetts. |
exposure to bupropion during 1st trimester
(1) Any exposure to bupropion 1a. Exposure to bupropion bupropion alone 1b. Exposure to bupropion and other antidepressants
(2) Exposure to any other antidepressant 2a. Exposure to an SSRI 2b. Exposure to a TCA 2c. Exposure to an antidepressant other than bupropion, SSRI, or TCA
data collected with interviews, conducted by trained nurses who are unaware of the study hypotheses; collects detailed data on all medications used anytime from 2months prior to conception through the pregnancy.
|
Control: not exposed to antidepressants (women with no exposure to any antidepressant at any time from 56 days prior to LMP to the end of pregnancy).
|
Duration or endpoint of follow-up: mothers of identified infants are contacted within 5 months of delivery and invited to participate in a telephone interview within 6 months following delivery
For how many participants were no complete outcome data available? N (%): N/A response rates among women we were able to contact were 70% for mothers of cases and 68% for mothers of controls Reasons for incomplete outcome data described? N/A |
risk of cardiac malformations (certain cardiac defects: VSD, left outflow tract heart defects as a group, coarctation of the aorta, and HLHS)
risk of VSD, adjusted OR for first-trimester exposure (95%CI) - any bupropion: 1.6 (1.0, 2.8) - bupropion alone: 2.5 (1.3, 5.0) - TCAs: 0.8 (0.4, 1.8) - any other antidepressant: 1.0 (0.5, 1.6)
risk of Left-sided defects, adjusted OR for first-trimester exposure (95%CI) - any bupropion: 0.4 (0.1, 1.6) - bupropion alone: 0.5 (0.1, 3.4) - TCAs: 0.7 (0.2, 2.3) - any other antidepressant:2.0 (1.1, 3.8)
risk of Coarctation of aorta, adjusted OR for first-trimester exposure (95%CI) - any bupropion: - - bupropion alone: - - TCAs: 1.3 (0.3, 5.5) - any other antidepressant:2.9 (1.2, 6.8)
risk of Hypoplastic LHS, adjusted OR for first-trimester exposure (95%CI) - any bupropion: 0.8 (0.0, 4.6) - bupropion alone: 2.0 (0.3, 15.3)** - TCAs: 0.9 (0.1, 7.1) - any other antidepressant:0.6 (0.1, 4.5)
*)VSD ventricular septal defect **) based on only one exposed case |
Research staff identify malformed subjects by reviewing hospital admission and discharge lists.
Interviewed women were asked to sign a medical record release form allowing study staff to obtain the infant’s hospital record to confirm the diagnosis
Women with exposure to antidepressants only outside of the first trimester and women who reported a drug not classified as an antidepressant for the indication “depression” were excluded from all analyses
potential confounders: during interview data collected: demographic, reproductive, and medical factors; health behaviors such as cigarette smoking, alcohol, and caffeine consumption; occupational exposures; and dietary intake.
Mothers of identified infants are contacted within 5months of delivery and invited to participate in a telephone interview within 6months following delivery
response rates among women we were able to contact were 70% for mothers of cases and 68% for mothers of controls |
|
Simon 2002
USA |
cohort study, sample drawn from Group Health Cooperative, a prepaid health plan serving approximately 400,000 members in Washington State. |
all live births between January 1, 1986, and December 31, 1998
identified using Hospital discharge records
sample limited to -those enrolled at primary care facilities owned by Group Health Cooperative. - mothers continuously enrolled in Group Health Cooperative for 360 days before delivery
|
Exposure to TCAs or SSRIs
Pharmacy records were used to identify all antidepressant prescriptions filled or refilled during the 360 days before delivery
Those with any antidepressant prescriptions during the 270 days before delivery were considered exposed. patients with antidepressant prescriptions filled in the period between 270 and 360 days before delivery were classified as indeterminate and excluded
population: TCAs n=209 unexposed infants, n=209 SSRI: n=185 |
Mothers with no antidepressant prescriptions during this period were considered unexposed.
unexposed infants, n=209
|
Duration or endpoint of follow-up: up to 2 years For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A |
Congenital Malformations
Major malformations TCAs n=10 (4.8%) Unexposed n= 12 (5.7%) RR*= 0.82, 95%CI 0.35 to 1.95
Minor malformations TCAs n=14 (6.7%) Unexposed n=18 (8.6%) RR=0.76, 95%CI 0.37 to 1.58
Specific malformations Genitourinary, RR= 0.66, 95%CI 0.23 to 1.88 Cardiac, RR= 0.50, 95%CI 0.05 to 5.53 Skeletal, RR= 0.80, 95%CI 0.21 to 3.00 Vascular, RR= 1.34, 95%CI 0.30 to 6.06 Craniofacial, RR= 1.26, 95%CI 0.33 to 4.75
*) authors reported RRs, but calculating the crude OR results in the same numbers (OR=0.82, 95%CI 0.35 to 1.95) |
Chart reviewers and investigators remained blind to exposure status throughout chart reviews and primary data analyses
no correction for potential confounding
Group Health Cooperative’s computerized information systems record outpatient prescriptions, outpatient visits, and hospital discharges. Except for Medicare members, all Group Health Cooperative plans include prescription drug coverage.
authors conclusion: no association between tricyclic antidepressant exposure and congenital malformations
up to 2 years;
no data on completeness of follow up |
|
Vasilakis 2013
United Kingdom |
Matched cohort study
data from the General Practice Research Database; These records describe medical diagnoses and prescribed drugs
a family identification number, date of birth in the baby’s record, and the date of delivery in the mother’s record were used to link mothers with their offspring |
singleton pregnancies among women aged 15–45 years that occurred from January 1991 through April 2002
pregnancies of more than 20 weeks’ gestation and included live births, stillbirths, and therapeutic abortions. All subjects were required to have a full year of medical data prior to the delivery date for entry into the study.
|
exposed to TCAs and SSRIs in early pregnancy n=3276 women; TCAs n=1608
by age, year of pregnancy outcome, and general practice.
Women exposed to antidepressants were required to have had a diagnosis of depression prior to the delivery and at least one prescription for a TCA or SSRI during the first trimester of pregnancy
Exposure to antidepressants defined as receipt of a prescription from 180 to 335 days prior to the delivery date for live births and from 70 to 225 days prior to the delivery or termination date for stillbirths and therapeutic abortions. |
women with no exposure to any antidepressants during pregnancy n=6617 women
|
Duration or endpoint of follow-up: N/A For how many participants were no complete outcome data available? N (%): 21% of subjects did not have a complete year of follow-up Reasons for incomplete outcome data described? N/A
|
prevalence of congenital malformations, within a year after birth
reviewed the records of all babies born to mothers in the study population by using a computer search for all congenital anomaly codes (ICD-9) codes (740.0–759.9, excluding chromosomal malformations). all potential cases of a congenital anomaly were reviewed by hand, without knowledge of the mother’s exposure
Chromosomal malformations were also excluded, as were malformations associated with prematurity
No. of Infants with Congenital Malformations No exposure: 205/6617 any antidepressant: 89/3267 TCAs: 42/1608
Crude Relative Risk (95% CI), TCAs) no exposure: 0.9 (0.6–1.2). “Adjustment for confounders did not alter the crude results by 10% or more”
the crude RRs for a central nervous system anomaly were 4.2 (95% CI 0.6–29.5) for TCAs |
matched by age, year of pregnancy outcome, and general practice
TCAs: more smokers; higher BMI
matching: for whole group antidepressant users; here only data TCA users used
21% of subjects did not have a complete year of follow-up |
|
Winterfield 2015
Switzerland; Israel; United Kingdom; Finland; the Netherlands; Italy; Turkey |
multicenter, observational prospective cohort study |
pregnant women who themselves or whose physician contacted 1 of the 11 participating Teratology Information Services (TIS) during the period ranging from January 1995 to December 2011 seeking counsel about safety of exposure to therapeutic agents during pregnancy |
exposure to mirtazapine 357 women exposed to mirtazapine during pregnancy; 91% of the patients in the mirtazapine group were exposed during the first trimester
Maternal characteristics (age, tobacco use, alcohol consumption, and medical and obstetric history) as well as details of medication exposure (indication, timing in pregnancy, duration, dose, and concomitant medication) were also collected at initial TIS contact.
|
2 matched control groups: (1) exposure to any SSRI, control subjects with a psychiatric condition) (2) no exposure to medication known to be teratogenic or any antidepressant (general control subjects). 357 women
|
Duration or endpoint of follow-up: N/A For how many participants were no complete outcome data available? N (%): N/A Reasons for incomplete outcome data described? N/A |
rate of major birth defects after first-trimester exposure, defined as severe structural impairments or anomalies requiring surgical correction and were diagnosed prenatally by targeted ultrasound or amniocentesis or at birth by physical examination of the infant and appropriate imaging methods
Major birth defects,n (%) Mirtazapine; 13/292 (4.5) SSRI; 13/307 (4.2) Control Subjects; 6/309 (1.9)
Mirtazapine) General Control Subjects OR (95% CI) 2.35 (0.88–6.28)
Major birth defects, 1st-trimester exposure, ‡n (%) Mirtazapine; 10/292 (3.4) SSRI; 13/307 (4.2)) Control Subjects; 6/309 (1.9)
Mirtazapine) General Control Subjects OR (95% CI) 1.79 (0.64–4.99) |
Control subjects were selected randomly, and cases and control subjects were matched by TIS center, year of TIS contact (±2 years), maternal age (±2 years), and gestational age at time of call (±4 weeks). Data collections at first contact and follow-up were performed in the same way for the 3 groups.
follow-up was achieved through a structured telephone interview and/or mailed questionnaire to the patient and/or her health care professional
The rate of loss to follow-up differed between centers and ranged from 0% to 76% (median, 35%) in the mirtazapine and 5%to 77% (median, 37.5%) in the control groups. |
Risk of bias table for intervention studies (observational: non-randomized clinical trials, cohort and case-control studies)
Research question: What is the effect of non-SSRI antidepressant use during pregnancy on the risk of congenital malformations in children of women with psychiatric disorders, compared to pregnant women not taking non-SSRI antidepressants?
|
Study reference
|
Bias due to a non-representative or ill-defined sample of patients?
(unlikely/likely/unclear) |
Bias due to insufficiently long, or incomplete follow-up, or differences in follow-up between treatment groups?
(unlikely/likely/unclear) |
Bias due to ill-defined or inadequately measured outcome?
(unlikely/likely/unclear) |
Bias due to inadequate adjustment for all important prognostic factors?
(unlikely/likely/unclear) |
|
Alwan 2010 |
likely |
likely |
unlikely |
likely |
|
Ban 2014 |
likely |
unlikely |
unlikely |
likely |
|
Berard 2017 |
likely |
unlikely |
unlikely |
likely |
|
Chun-Fai-Chan 2005 |
likely |
likely |
unlikely |
likely |
|
Davis 2007 |
likely |
likely |
unlikely |
likely |
|
Djulus 2006 |
likely |
likely |
unlikely |
likely |
|
Einarson 2003 |
likely |
likely |
unlikely |
likely |
|
Louik 2014 |
likely |
likely |
unlikely |
unlikely |
|
Simon 2002 |
likely |
likely |
unlikely |
likely |
|
Vasilakis 2013 |
unlikely |
likely |
unlikely |
likely |
|
Winterfield 2015 |
likely |
likely |
unlikely |
likely |
- Failure to develop and apply appropriate eligibility criteria: a) case-control study: under- or over-matching in case-control studies; b) cohort study: selection of exposed and unexposed from different populations.
- Bias is likely if: the percentage of patients lost to follow-up is large; or differs between treatment groups; or the reasons for loss to follow-up differ between treatment groups; or length of follow-up differs between treatment groups or is too short. The risk of bias is unclear if: the number of patients lost to follow-up; or the reasons why, are not reported.
- Flawed measurement, or differences in measurement of outcome in treatment and control group; bias may also result from a lack of blinding of those assessing outcomes (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Failure to adequately measure all known prognostic factors and/or failure to adequately adjust for these factors in multivariate statistical analysis.
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Anonymous 2012 |
double |
|
Anonymous 2012 |
no original study |
|
Achar 2012 |
not relevant for PICO |
|
Adam 2011 |
not relevant for PICO |
|
Altshuler 1996 |
review, limited information methods |
|
Andrade 2008 |
not relevant for PICO |
|
Austin 1998 |
narrative review |
|
Baylor 2009 |
narrative review |
|
Boshier 2003 |
not relevant for PICO |
|
Bracken 1981 |
not relevant for PICO |
|
Broy 2010 |
not relevant for PICO |
|
Carvalho 2016 |
narrative review |
|
de Jonge 2013 |
not relevant for PICO |
|
Eberhard-Gran 2005 |
narrative review |
|
Einarson 2012 |
letter |
|
Einarson 2005 |
review, limited information methods |
|
Einarson 2012 |
not relevant for PICO |
|
Forrester 2004 |
not relevant for PICO |
|
Gentile, 2010 |
narrative review |
|
Gentile 2005 |
narrative review |
|
Goldaber 1997 |
narrative review |
|
Goldstein 2000 |
not relevant for PICO |
|
Grigoriadis 2013 |
narrative review |
|
Hill 1988 |
not relevant for PICO |
|
Jain 2005 |
narrative review |
|
Jimenez-Solem 2014 |
not relevant for PICO |
|
Kalra. 2005 |
narrative review |
|
Khalifeh 2015 |
not relevant for PICO |
|
Kirsch 2019 |
not relevant for PICO |
|
Kuller 1996 |
narrative review |
|
Lewis 2010 |
not relevant for PICO |
|
Lorenzo 2011 |
narrative review |
|
Manakova 2011 |
not relevant for PICO |
|
Maschi 2008 |
not relevant for PICO |
|
Mauck 2004 |
narrative review |
|
McDonagh 2014 |
not relevant for PICO |
|
Olshan 1989 |
not relevant for PICO |
|
Patkar 2004 |
narrative review |
|
Pearson 2007 |
not relevant for PICO |
|
Pinto 2003 |
letter |
|
Rajkumar 2008 |
letter |
|
Ram 2015 |
narrative review |
|
Ramos 2008 |
not relevant for PICO |
|
Raudzus 2009 |
not relevant for PICO |
|
Reiff-Eldridge 2000 |
not relevant for PICO |
|
Rzewuska 2009 |
narrative review |
|
Santone 2009 |
not relevant for PICO |
|
Simoncelli 2010 |
review, limited information methods and included studies |
|
Smit 2015 |
not relevant for PICO |
|
Tak 2017 |
review, limited information methods and included studies |
|
Thyagarajan 2012 |
letter |
|
Udechuku 2010 |
review, limited information methods and included studies |
|
Van Gelder 2014 |
review, limited information methods and included studies |
|
VanBlerk 1980 |
narrative review |
|
Ververs 2009 |
not relevant for PICO |
|
Viktorin 2018 |
not relevant for PICO |
|
Wen 2004 |
not fulltext available |
|
Wieck 2006 |
narrative review |
|
Wilton 1998 |
not relevant for PICO |
|
Wisner 1999 |
review, limited information methods and included studies |
|
Yaris 2004 |
not relevant for PICO |
|
Yonkers 2009 |
clinical guideline; narrative review |
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 23-07-2021
Bij het opstellen van de modules heeft de werkgroep een inschatting gemaakt over de maximale termijn waarop herbeoordeling moet plaatsvinden. De geldigheid van de richtlijnmodules komt eerder te vervallen indien nieuwe ontwikkelingen aanleiding zijn een herzieningstraject te starten.
|
Module1 |
Regiehouder (s)i |
Jaar van autorisatie |
Eerstvolgende beoordeling actualiteit richtlijn2 |
Frequentie van beoordeling op actualiteit3 |
Wie houdt er toezicht op actualiteit4 |
Relevante factoren voor wijzigingen in aanbeveling5 |
|
Niet-SSRI antidepressiva en aangeboren afwijkingen |
NVOG |
2021 |
2026 |
Eens in vijf jaar |
NVOG |
Nieuw onderzoek |
1 Naam van de module
i Regiehouder van de module (deze kan verschillen per module en kan ook verdeeld zijn over meerdere regiehouders)
2 Maximaal na vijf jaar
3 (Half)Jaarlijks, eens in twee jaar, eens in vijf jaar
4 Regievoerende vereniging, gedeelde regievoerende verenigingen, of (multidisciplinaire) werkgroep die in stand blijft
5Lopend onderzoek, wijzigingen in vergoeding/organisatie, beschikbaarheid nieuwe middelen
Algemene gegevens
De ontwikkeling van deze richtlijn werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodules.
De richtlijn is ontwikkeld in samenwerking met:
- Patiëntenfederatie Nederland
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijn is in 2018 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen die betrokken zijn bij de zorg voor zwangere patiënten die ‘niet-SSRI’ antidepressiva en/of antipsychotica gebruiken. De werkgroepleden zijn door hun beroepsverenigingen gemandateerd voor deelname. De werkgroep is verantwoordelijk voor de integrale tekst van deze richtlijn.
Werkgroep
- Dr. A. Coumans, gynaecoloog-perinatoloog, Maastricht UMC+, Maastricht, NVOG
- Dr. H.H. Bijma, gynaecoloog, Erasmus Medisch Centrum, Rotterdam, NVOG
- Drs. R.C. Dullemond, gynaecoloog, Jeroen Bosch Ziekenhuis, Den Bosch, NVOG
- Drs. S. Meijer, gynaecoloog, Gelre Ziekenhuis, Apeldoorn, NVOG
- Dr. M.G. van Pampus, gynaecoloog, OLVG, Amsterdam, NVOG
- Drs. M.E.N. van den Heuvel, neonatoloog, OLVG Amsterdam, NVK
- Drs. E.G.J. Rijntjes-Jacobs, neonatoloog, Leids Universitair Medisch Centrum, Leiden, NVK
- Dr. K.M. Burgerhout, psychiater, Amphia Ziekenhuis, Breda, NVvP
- Dr. E.M. Knijff, psychiater, Erasmus Medisch Centrum, Rotterdam, NVvP
Meelezers
- Dr. A.J. Risselada, klinisch farmacoloog, Wilhelmina Ziekenhuis Assen
Met ondersteuning van
- Dr. E. van Dorp-Baranova, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. M. Moret-Hartman, senior-adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- N. Verheijen, senior-adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- M. Wessels, literatuurspecialist, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Dr. A. Coumans |
gynaecoloog-perinatoloog Maastricht UMC+ |
geen |
geen |
geen |
|
Dr. H. H. Bijma |
Gynaecoloog, Erasmus MC, afdeling verloskunde en gynaecologie, subafdeling verloskunde en prenatale geneeskunde |
lid werkgroep wetenschap LKPZ, onbetaald |
geen |
geen |
|
Drs. S. Meijer |
gynaecoloog Gelre Ziekenhuis, Apeldoorn |
geen |
geen |
geen |
|
Drs. R. C. Dullemond |
gynaecoloog- perinatoloog, Jeroen Bosch Ziekenhuis |
LKPZ bestuur - voorzitter, onbetaald mind 2 care - raad van toezicht, onbetaald dagelijks bestuur @verlosdenbosch (integrale geboortezorg organisatie) als gynaecoloog uit het JBZ, onbetaald danwel deels in werktijd |
geen |
geen |
|
Drs. E. G. J. Rijntjes-Jacobs |
kinderarts-neonatoloog, afdeling neonatologie, Leids Universitair Medisch Centrum |
LKPZ bestuur – secretaris, onbetaald |
geen |
geen |
|
Dr. M. G. van Pampus |
Gynaecoloog, OLVG Amsterdam |
onbetaalde nevenfuncties |
geen |
geen |
|
Dr. E. M. Knijff |
Psychiater, Erasmus MC polikliniek psychiatrie & zwangerschap medisch coördinator polikliniek Erasmus MC |
geen |
geen |
geen |
|
Drs. M. E. N. van den Heuvel |
kinderarts-neonatoloog, OLVG Amsterdam |
geen |
geen |
geen |
|
Dr. K. Burgerhout |
Psychiater, Reinier van Arkel, POP-poli |
geen |
geen |
geen |
Inbreng patiëntenperspectief
Er is een aantal acties uitgevoerd om het patiëntperspectief mee te nemen bij het ontwikkelen van deze richtlijn. Allereerst is contact gezocht met het MIND Platform voor afvaardiging van een patiëntvertegenwoordiger in de werkgroep. Zij hebben ons in contact gebracht met de Stichting Me Mam. Het bleek niet mogelijk een patiëntvertegenwoordiger voor de werkgroep te vinden. Daarna is een focusgroepbijeenkomst voor patiënten georganiseerd, maar deze is geannuleerd vanwege onvoldoende aanmeldingen. Tot slot is een schriftelijke enquête voor patiënten in samenwerking met de Patiëntenfederatie Nederland opgesteld en uitgezet. Dit heeft helaas nauwelijks reactie opgeleverd, de enquête is door twee patiënten ingevuld. Voor de ontwikkeling van het product voor patiënten (informatie op de website www.Thuisarts.nl) is een ervaringsdeskundige van de patiëntenvereniging ‘Plusminus-leven met bipolariteit’ afgevaardigd. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland en het MIND Platform.
Methode ontwikkeling
Evidence based
Implementatie
In de verschillende fasen van de richtlijnontwikkeling is rekening gehouden met de implementatie van de richtlijnmodules en de praktische uitvoerbaarheid van de aanbevelingen. Daarbij is uitdrukkelijk gelet op factoren die de invoering van de richtlijn in de praktijk kunnen bevorderen of belemmeren. Het implementatieplan is te vinden in de bijlagen.
Werkwijze
AGREE
Deze richtlijn is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor vrouwen die antipsychotica of niet-SSRI antidepressiva tijdens zwangerschap en lactatie gebruiken. Tevens zijn er knelpunten aangedragen door IGJ, NHG, V&VN, Zorginstituut Nederland, Lareb, KNOV, NVvP, NVOG en NVK via een Invitational conference. Een verslag hiervan is opgenomen in de bijlagen. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijn is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module 'Organisatie van zorg'.
Commentaar- en autorisatiefase
De conceptrichtlijn werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijn aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijn werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. https://richtlijnendatabase.nl/over_deze_site/richtlijnontwikkeling.html.
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008 May 24;336(7654). doi: 10.1136/bmj.a139.
Schünemann, A Holger J (corrected to Schünemann, Holger J). PubMed PMID: 18483053; PubMed Central PMCID: PMC2386626. BMJ. 2008 May 24;336(7654). doi: 10.1136/bmj.a139. Schünemann, A Holger J [corrected to Schünemann, Holger J]
Wessels M, Hielkema L, van der Weijden T. How to identify existing literature on patients' knowledge, views, and values: the development of a validated search filter. J Med Libr Assoc. 2016 Oct;104(4):320-324. PubMed PMID: 27822157; PubMed Central PMCID: PMC5079497.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
