Bloeddrukmeting
Uitgangsvraag
Hoe dient bloeddruk te worden gemeten bij volwassen patiënten met obesitas die een chirurgische ingreep ondergaan?
De uitgangsvraag omvat de volgende deelvragen:
- Wat is de plaats van een bloeddrukmeting met een band om de vinger?
- Wat is de plaats van een bloeddrukmeting met een band om de onderarm?
- Wat is de plaats van een bloeddrukmeting met een band om de bovenarm?
Aanbeveling
Gebruik een bloeddrukband waarvan de lengte van het opblaasgedeelte van de bloeddrukband een lengte heeft van minimaal 80% van de bovenarmomtrek.
Verricht peroperatieve non-invasieve bloeddrukmetingen aan de onderarm in plaats van de bovenarm indien de bovenarmtrek >37,5 cm is (doorgaans bij een BMI van ≥40 kg/m2).
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is een systematische review uitgevoerd naar effecten van het gebruik van verschillende methoden (‘finger cuff’, boven arm ‘cuff’ en onderarm ‘cuff’) van bloeddrukmeting op complicaties en keuze van behandeling bij volwassen patiënten met obesitas die een chirurgische behandeling ondergaan onder algehele, regionale of spinale anesthesie. Daarnaast zijn de klinimetrische eigenschappen van de verschillende methoden van bloeddrukmeting onderzocht.
Voor de cruciale uitkomstmaten complicaties en therapeutische interventies naar aanleiding van verschillen in gemeten bloeddruk werd geen literatuur gevonden. Er bestaat een kennislacune over het effect van verschillende type meetinstrumenten om de bloeddruk te meten op klinische uitkomstmaten.
De belangrijke uitkomstmaten konden wel enige richting geven aan de klinische besluitvorming. Er werd geen literatuur gevonden ten aanzien van het effect van het gebruik van verschillende meetinstrumenten op kosten en ten aanzien van de betrouwbaarheid (test-hertest overeenkomst en meetfout), maar wel ten aanzien van de validiteit (tabel 7) en responsiviteit (tabel 8) wanneer non-invasieve methoden van bloeddrukmeting werden vergeleken met een meting middels intra-arteriële lijn. De bewijskracht werd voornamelijk verlaagd vanwege het relatief kleine aantal patiënten waarover de meeteigenschappen bepaald werden. In enkele gevallen kon de bewijskracht niet bepaald worden vanwege inconsistente resultaten, of werd aanvullend afgewaardeerd voor risk of bias vanwege gebrek aan studies met hoge kwaliteit die geschikte uitkomstmaten rapporteren.
Tabel 7: Validiteit meetinstrumenten
|
Type meetinstrument |
Type bloeddruk |
Conclusie |
Bewijskracht |
|
‘Finger cuff’ |
Systolisch |
Waarschijnlijk toereikend |
Redelijk |
|
|
Diastolisch |
Waarschijnlijk niet toereikend |
Redelijk |
|
|
Gemiddeld arterieel |
Waarschijnlijk toereikend |
Redelijk |
|
Lower arm ‘cuff’ |
Systolisch |
Toereikend |
Hoog |
|
|
Diastolisch |
Geen conclusie |
Geen |
|
|
Gemiddeld arterieel |
Geen conclusie |
Geen |
|
Upper arm ‘cuff’ |
Systolisch |
Geen conclusie |
Geen |
|
|
Diastolisch |
Waarschijnlijk niet toereikend |
Redelijk |
|
|
Gemiddeld arterieel |
Geen conclusie |
Geen |
Tabel 8: Responsiviteit meetinstrumenten
|
Type meetinstrument |
Type bloeddruk |
Conclusie |
Bewijskracht |
|
‘Finger cuff’ |
Systolisch |
Geen conclusie |
Zeer laag |
|
|
Diastolisch |
Geen conclusie |
Zeer laag |
|
|
Gemiddeld arterieel |
Geen conclusie |
Zeer laag |
|
Lower arm ‘cuff’ |
Systolisch |
Mogelijk toereikend |
Laag |
|
|
Diastolisch |
Mogelijk toereikend |
Laag |
|
|
Gemiddeld arterieel |
Mogelijk toereikend |
Laag |
|
Upper arm ‘cuff’ |
Systolisch |
Mogelijk toereikend |
Laag |
|
|
Diastolisch |
Mogelijk niet toereikend |
Laag |
|
|
Gemiddeld arterieel |
Mogelijk toereikend |
Laag |
Bloeddrukmeting tijdens de perioperatieve periode is van vitaal belang. Afhankelijk van de soort ingreep en bepaalde co-morbiditeit van de patiënt zal het anesthesiologisch team de afweging maken welke vorm van bloeddrukmonitoring noodzakelijk zal zijn.
Indien invasieve meting niet nodig is wordt er in de praktijk momenteel vaak gekozen voor een oscillometrische meting aan de bovenarm. De bovenarmomtrek moet gebruikt worden om de correcte bloeddrukband te selecteren. De lengte van het opblaasgedeelte van de bloeddrukband moet 80% zijn van de bovenarmomtrek, het wordt aangeraden om te kijken naar de markeringen op de band zelf en indien nodig de bovenarmomtrek te meten (Pickering, 2005). Bij patiënten met obesitas die een bovenarmomtrek van >37,5 cm hebben wordt er door een cilindrische bloeddrukband, zoals in de praktijk gebruikt wordt, bij 15% van de patiënten een onterechte hypertensie gemeten (Palatini, 2012). De bovenarmomtrek neemt toe naarmate het gewicht toeneemt, in de eerdergenoemde studie was het gemiddelde BMI 41,4 voor de patiënten met een bovenarmomtrek van 38,8 cm.
Studies kijken in toenemende maat naar oscillometrische bloeddrukmetingen om de onderarm (Leblanc, 2013; Leblanc, 2019; Mostafa, 2020; Schumann, 2021). In het algemeen wordt een onderarm meting verricht twee cm distaal van de elleboog of drie cm proximaal van de processus styloideus ulnae. Voor een onderarm meting volstaat een standaard bloeddrukband vaak (25-35 cm lengte), een kleinere band (18-26 cm) kan ook nodig zijn (Mostafa, 2020; Schumann, 2021).
De bovenstaande GRADE conclusies kunnen voor de bovenarm metingen bij patiënten met obesitas die een operatie ondergaan enkel concluderen dat de diastolische bloeddruk waarschijnlijk geen goede overeenkomst heeft met de gouden standaard (criterion validity). Veranderingen worden mogelijk toereikend gemeten voor systolische en gemiddelde arteriële bloeddruk, maar mogelijk niet voor diastolische bloeddruk (responsivity). Over de onderarm kan met zekerheid geconcludeerd worden dat de systolische overeenkomst goed is (criterion validity) en mogelijk toereikend voor alle drie de typen bloeddrukmeting ten aanzien van het meten van veranderingen in de bloeddruk (responsivity).
De studies van Schumann en Mostafa rapporteren in de meeste gevallen dat onderarm metingen een hogere overeenkomst (criterion validity en responsivity) hebben met de arterielijn genomen als gouden standaard ten opzichte van bovenarm metingen. De patiënten in deze studies hadden een gemiddeld BMI boven de 40 kg/m2. Metingen aan de onderarm waren ook sneller dan aan de bovenarm met een mediaan tijd van 30s (IQR 27-34) bij de onderarm vs. 63s (IQR 49-76) bij de bovenarm (Mostafa, 2020). Dit en de GRADE conclusies suggereren dat metingen aan de onderarm beter zijn dan metingen aan de bovenarm bij patiënten met een BMI ≥40 kg/m2.
Een fingercuff systeem van bijvoorbeeld ClearSight (Edwards Lifesciences, USA), voorheen ccNexfin (BMEYE B.V., The Netherlands) meet continu op non-invasieve wijze de bloeddruk middels een infrarood plethysmografie systeem in combinatie met een Volume Clamp-methode. Continue monitoring van de hemodynamiek kan van grote meerwaarde zijn gezien de associatie van obesitas en co-morbiditeit zoals coronair lijden en cerebrovasculaire aandoening (Ortiz, 2015). De overeenkomst met de gouden standaard voor de systolische bloeddruk en de mean arterial pressure (MAP) is waarschijnlijk voldoende, maar niet voor de diastolische bloeddruk (GRADE redelijk). Of veranderingen in de bloeddruk goed gemeten kunnen worden is nog onduidelijk (GRADE zeer laag).
Het gebruik van de ClearSight is een veelbelovende methode in patiënten waarbij oscillometrie nog gebruikt wordt als standaardmethode van bloeddrukmeting en eventueel na verdere validatie als vervanging van een invasieve bloeddrukmeting. Hij is echter niet gevalideerd bij atriumfibrilleren of ritmestoornissen, deze patiënten werden in alle studies geëxcludeerd. Tevens zijn er patiënt karakteristieken die de ClearSight ook onbetrouwbaar maken zoals een verlengde capillary refill tijd, een slechte perfusie index of oedeem van de hand (Lakhal, 2023). Daarom lijkt de ClearSight goed te gebruiken bij cardiaal stabiele patiënten, echter zodra het een cardiaal belast iemand betreft is de ClearSight niet te adviseren. Gebruik hiervan kan mogelijk leiden tot een hoger risico op onjuiste waarden.
Aangezien alle studies verricht waren in bariatrische populaties is het tot dusver ook nog onduidelijk of de resultaten generaliseerbaar zijn over de hele populatie met obesitas.
Aangezien er geen data waren ten aanzien van klinische complicaties ten gevolge van de gebruikte bloeddrukmeting kunnen andere methodes gebruikt worden om te kijken of er klinisch relevante verschillen zijn tussen de vergeleken bloeddrukken.
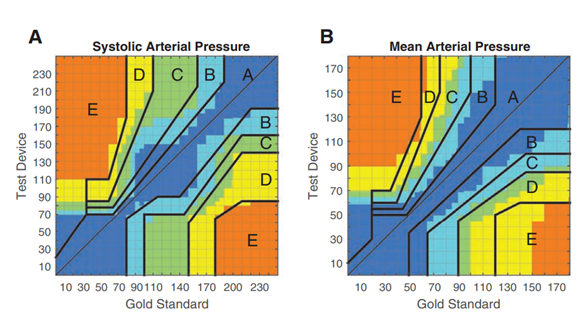
Hiervoor wordt in enkele studies (Eley, 2021; Rogge, 2019; Schumann, 2021) gebruik gemaakt van error grid analyses volgens Saugel (2018), zie figuur 1. Een error grid analyse vergelijkt een nieuwe meetmethode met de gouden standaard en deelt de concordante metingen op in verschillende zones op basis van het risico op complicaties naar aanleiding van het gebruik van de meetmethode en volgende behandelbeslissingen dat zou bestaan in die desbetreffende zone.
Figuur 1: Error grid analyse (Saugel, 2018)
De risico’s zijn gedefinieerd als:
- Geen risico (geen verschil in klinische actie tussen test en referentie methode)
- Laag risico (deviatie van testmethode en referentie, waarschijnlijk onschadelijk gevolg)
- Matig risico (testmethode wijkt af van referentie met onnodige behandeling met matige, niet-levensbedreigende gevolgen voor patiënt).
- Significant risico (testmethode wijkt af van referentie en zou leiden tot onnodige behandeling met ernstige niet-levensbedreigende gevolgen voor de patiënt)
- Gevaarlijk risico (testmethode wijkt af van referentie en zou leiden tot onnodige behandeling met levensbedreigende gevolgen voor de patiënt)
Tabel 9: Error grid analyses
|
Studie, Meetlocatie |
Bloeddruk |
Zone A % (n) |
Zone B % (n) |
Zone C % (n) |
Zone D % (n [N]) |
Zone E % (n) |
|
Schumann, 2021 Fingercuff |
SBD MAP |
89.5 (481) 77.1 (415) |
9.8 (53) 21.6 (116) |
0.2 (1) 0.9 (5) |
0.4 (2 [1]) 0.4 (2 [1]) |
0.2 (1) 0.0 (0) |
|
Schumann, 2021 Bovenarm |
SBD MAP |
85.1 (377) 65.9 (294) |
10.0 (44) 28.3 (126) |
3.8 (17) 4.0 (18) |
1.1 (5 [5]) 1.6 (7 [6]) |
0.0 (0) 0.2 (1) |
|
Schumann, 2021 Onderarm |
SBD MAP |
85.8 (459) 74.5 (400) |
11.2 (60) 22.2 (119) |
2.8 (15) 3.0 (16) |
0.2 (1 [1]) 0.4 (2 [2]) |
0.0 (0) 0.0 (0) |
|
Eley, 2021 Fingercuff |
SBD MAP |
90.8 (336) 91.4 (338) |
6.5 (24) 4.3 (16) |
2.7 (10) 4.3 (16) |
0.0 (0) 0.0 (0) |
0.0 (0) 0.0 (0) |
|
Rogge, 2019 Fingercuff |
SBD MAP |
93.7 89.5 |
6.0 10.0 |
0.3 0.5 |
0.0 (0) 0.0 (0) |
0.0 (0) 0.0 (0) |
n, aantal meetparen in de risicozone; N, aantal patiënten dat bijdraagt aan de meetparen n in de risicozone.
Door afrondingen kan het zijn dat de percentages niet optellen tot 100%
SBD: systolische bloeddruk, MAP: mean arterial pressure
Uit tabel 9 zou men kunnen concluderen dat de fingercuff en onderarm metingen veelal in risicozone A-C zitten met een enkele meting in zone D en E. Het grootste aandeel van de metingen in risicozone D zijn metingen verricht met de bloeddrukband om de bovenarm. Dit past bij de reeds beschreven problemen van de bloeddrukmetingen aan de bovenarm.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
De patiënt is gebaat bij nauwkeurige bloeddrukmonitoring in de perioperatieve fase om complicaties te kunnen voorkomen zoals CVA’s en myocardinfarcten. Invasieve metingen hebben het risico op complicaties en kunnen ook als pijnlijk worden ervaren. Bovendien is een invasieve meting niet in elke situatie vereist. De locatie van de non-invasieve bloeddrukmeting maakt doorgaans niet veel uit voor de patiënt zolang er maar gekozen wordt voor een correct passende bloeddrukband.
Kosten (middelenbeslag)
Methodes om een invasieve bloeddrukmeting te verrichten en non-invasieve oscillometrische bloeddrukbanden zijn beschikbaar in elk ziekenhuis.
De ClearSight (Edwards Lifesciences, USA) daarentegen wordt niet standaard in elk ziekenhuis gebruikt en zou moeten worden aangeschaft. De disposable fingercuffs zijn doorgaans 150 euro per stuk (Imedsales.com, 2023).
Aanvaardbaarheid, haalbaarheid en implementatie
Een andere locatie gebruiken om een non-invasieve bloeddruk te meten zal vermoedelijk geen problemen geven. Implementatie van ClearSight zou beperkt kunnen zijn door de kosten en bij gebrek aan bewijs buiten bariatrische studies.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Non-invasieve bloeddrukmetingen worden in de praktijk meestal verricht om de bovenarm. Bij patiënten met obesitas neemt niet alleen de bovenarmomtrek toe maar krijgen de bovenarmen ook een conischer verloop. Hierdoor ontstaat er een verhoogd risico op foutieve metingen, waarbij de bloeddruk met name overschat wordt (Palatini, 2012).
Bij 15% van de patiënten met een bovenarmomtrek van >37,5 cm wordt er hierdoor een onterechte hypertensie gemeten. Bij een BMI van 40 kg/m2 zal de bovenarmomtrek vaak >37,5 cm zijn (Palatini, 2012, Schumann 2021, Eley 2021).
Non-invasieve metingen aan de onderarm lijken beter overeen te komen met de gouden standaard en worden om die reden door de werkgroep aanbevolen indien de bovenarmomtrek >37,5cm is.
Aangezien de gevonden studies enkel peroperatieve bloeddrukwaarden vergeleken, kan de werkgroep deze aanbeveling niet extrapoleren naar andere situaties zoals bijvoorbeeld poliklinische metingen.
Te allen tijde dient er een correct passende bloeddrukband gebruikt te worden, de lengte van de band dient minimaal 80% te bedragen van de bovenarmomtrek (Pickering, 2005). Het wordt aanbevolen om de gebruiksaanwijzingen van de fabrikant op te volgen. Veelal worden er afmetingen aangegeven op de bloeddrukbanden die in Nederland gebruikt worden.
Er is nog onvoldoende bewijs om de ClearSight fingercuff aan te raden. Alle onderzoeken zijn verricht in de bariatrische populatie en het is onduidelijk of resultaten generaliseerbaar zijn over de gehele populatie met obesitas. Hier dient nog meer onderzoek naar verricht te worden.
Onderbouwing
Achtergrond
Een accurate vorm van bloeddrukmeting gedurende de perioperatieve periode is van groot belang gezien het reeds verhoogde risico op cardiale complicaties bij patiënten met obesitas (Bamgbade, 2007; Benalcazar, 2022; Poirier, 2009). Patiënten met obesitas hebben vaker een conisch verloop van de bovenarm waardoor het non-invasief meten van de bloeddruk minder betrouwbaar en soms onmogelijk is (Palatini, 2011; Palatini, 2012). Als alternatief wordt de bloeddruk in sommige gevallen gemeten op alternatieve locaties (non-invasief op de onderarm), middels invasieve methoden (arterielijnen) of middels een continue vingerbloeddruk meter (productnamen: Nexfin/ClearSight). Invasieve monitoring is de gouden standaard, echter zijn hier wel risico’s aan verbonden zoals ischemie, bloedingen en lokale infecties (Scheer, 2002; Nuttall, 2016). In deze module vergelijken we diverse methoden van non-invasieve bloeddrukmetingen t.o.v. de arterielijn.
Conclusies / Summary of Findings
PICO 1: What is the effect of non-invasive blood pressure measurements (upper arm vs. lower arm vs. finger) on clinical outcome?
Finger cuff blood pressure versus arterial line blood pressure
|
- GRADE |
No evidence was found regarding the effect of blood pressure measurement by finger cuff or lower arm cuff on complications when compared with blood pressure measurement by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
|
- GRADE |
No evidence was found regarding the effect of blood pressure measurement by finger cuff or lower arm cuff on therapeutic reactions to measured blood pressure when compared with blood pressure measurement by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
|
- GRADE |
No evidence was found regarding the effect of blood pressure measurement by finger cuff or lower arm cuff on costs when compared with blood pressure measurement by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
PICO 2: What are the clinimetric properties of non-invasive blood pressure measurements (upper arm, lower arm, finger) compared to an arterial line (gold standard)?
Finger cuff blood pressure versus arterial line blood pressure
Reliability and measurement error
|
- GRADE |
No evidence was found regarding reliability and measurement error of systolic blood pressure measurement by finger cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
|
- GRADE |
No evidence was found regarding reliability and measurement error of diastolic blood pressure measurement by finger cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
|
- GRADE |
No evidence was found regarding reliability and measurement error of mean arterial blood pressure measurement by finger cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
Criterion validity
|
Moderate GRADE |
Criterion validity is probably sufficient for systolic blood pressure measurements by finger cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Schumann, 2021 |
|
Moderate GRADE |
Criterion validity is probably insufficient for diastolic blood pressure measurements by finger cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Schumann, 2021 |
|
Moderate GRADE |
Criterion validity is probably sufficient for mean arterial blood pressure measurements by finger cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Schumann, 2021 |
Responsiveness
|
Very low GRADE |
The evidence is very uncertain about responsiveness for systolic blood pressure measurements by finger cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Eley, 2021; Hansen, 2022; Rogge, 2019; Schumann, 2021 |
|
Very low GRADE |
The evidence is very uncertain about responsiveness for diastolic blood pressure measurements by finger cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Eley, 2021; Hansen, 2022; Rogge, 2019; Schumann, 2021 |
|
Very low GRADE |
The evidence is very uncertain about responsiveness for mean arterial blood pressure measurements by finger cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Eley, 2021; Hansen, 2022; Rogge, 2019; Schumann, 2021 |
Lower arm blood pressure versus arterial line blood pressure
Reliability and measurement error
|
- GRADE |
No evidence was found regarding reliability and measurement error of systolic blood pressure measurement by lower arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
|
- GRADE |
No evidence was found regarding reliability and measurement error of diastolic blood pressure measurement by lower arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
|
- GRADE |
No evidence was found regarding reliability and measurement error of mean arterial blood pressure measurement by lower arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
Criterion validity
|
High GRADE |
Criterion validity is sufficient for systolic blood pressure measurements by lower arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Mostafa, 2020; Schumann, 2021 |
|
- GRADE |
Criterion validity for diastolic blood pressure measurements by lower arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia could not be graded as reported results were inconclusive.
Source: Source: Mostafa, 2020; Schumann, 2021 |
|
- GRADE |
Criterion validity for mean arterial blood pressure measurements by lower arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia could not be graded as reported results were inconclusive.
Source: Source: Mostafa, 2020; Schumann, 2021 |
Responsiveness
|
Low GRADE |
Responsiveness may be sufficient for systolic blood pressure measurements by lower arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Mostafa, 2020 |
|
Low GRADE |
Responsiveness may be sufficient for diastolic blood pressure measurements by lower arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Mostafa, 2020 |
|
Low GRADE |
Responsiveness may be sufficient for mean arterial blood pressure measurements by lower arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Mostafa, 2020 |
Upper arm blood pressure versus arterial line blood pressure
Reliability and measurement error
|
- GRADE |
No evidence was found regarding reliability and measurement error of systolic blood pressure measurement by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
|
- GRADE |
No evidence was found regarding reliability and measurement error of diastolic blood pressure measurement by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
|
- GRADE |
No evidence was found regarding reliability and measurement error of mean arterial blood pressure measurement by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia. |
Criterion validity
|
- GRADE |
Criterion validity for systolic blood pressure measurements by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia could not be graded as reported results were inconclusive.
Source: Hager, 2009; Mostafa, 2020 |
|
Moderate GRADE |
Criterion validity is probably insufficient for diastolic blood pressure measurements by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Hager, 2009; Mostafa, 2020 |
|
- GRADE |
Criterion validity for mean arterial blood pressure measurements by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia could not be graded as reported results were inconclusive.
Source: Hager, 2009; Mostafa, 2020 |
Responsiveness
|
Low GRADE |
Responsiveness may be sufficient for systolic blood pressure measurements by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Mostafa, 2020 |
|
Low GRADE |
Responsiveness may be insufficient for diastolic blood pressure measurements by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Mostafa, 2020 |
|
Low GRADE |
Responsiveness may be sufficient for mean arterial blood pressure measurements by upper arm cuff in patients with obesity undergoing surgery under general, regional, or spinal anesthesia.
Source: Mostafa, 2020 |
Samenvatting literatuur
PICO 1: What is the effect of non-invasive blood pressure measurements (upper arm, lower arm, finger vs. arterial line) on clinical outcome?
Description of studies
No studies were included in the analysis of the literature.
Results
Complications
No studies were included in the analysis of the literature.
Therapeutic reactions to measured blood pressure
No studies were included in the analysis of the literature.
Costs
No studies were included in the analysis of the literature.
Level of evidence of the literature
No studies were included in the analysis of the literature.
PICO 2: What are the clinimetric properties of non-invasive blood pressure measurements (upper arm, lower arm, finger) compared to an arterial line (golden standard)?
Description of studies
Eley (2021) investigated criterion validity and responsiveness in a prospective comparison study. Blood pressure was measured in 30 adult patients scheduled for elective laparoscopic bariatric surgery (mean age 45 years, SD 11.7; male 71%; median BMI 50.2 kg/m2, 25-75th percentile 48.3 to 55.3) by a ClearSight EV1000 Clinical Platform (Edwards Lifesciences
Corp, Irvine, CA). Measurement properties were compared to blood pressure measurements by a radial arterial catheter. For criterion validity and responsiveness high risk of bias was assessed (inadequate quality) as no correlations or AUC was reported. Reliability was not reported.
Hager (2009) investigated criterion validity in a prospective comparison study. Blood pressure was measured in 22 patients undergoing bariatric surgery (mean age 44.3 years, SD 9.5; male not reported%; median BMI 66.7 kg/m2, SD 13.8) by non-invasive blood pressure measurements for the upper arm (Datex-Ohmeda, Madison, WI, USA). Measurement properties were compared to blood pressure measurements by a radial arterial catheter. For criterion validity low risk of bias was assessed (very good quality. Reliability and responsiveness were not reported.
Hansen (2022) investigated criterion validity and responsiveness in a prospective observational cohort study. Blood pressure was measured in 56 adult patients scheduled for elective laparoscopic bariatric surgery (mean age 46.5 years, SD 12.1; male 27%; mean BMI 49.2 kg/m2, SD 5.7) by a
- Nexfin® system (BMEYE, Amsterdam, The Netherlands, now licensed as ClearSight® system by Edwards Lifesciences, Irvine, CA, USA;
- forearm cuff (DURA-CUF™ GE, Boston, MA, USA). Measurement properties were compared to blood pressure measurements by an arterial catheter.
There may be risk of bias for this study as 4 potential participants were not included while no clear reason (i.e., insufficient data acquisition) was given (doubtful quality for criterion validity outcome regarding upper arm and finger cuff). There was high risk of bias for responsiveness as no correlation or AUC was reported regarding upper arm and finger cuff.
Mostafa (2020) investigated criterion validity and responsiveness in consecutive patients in a prospective observational cohort study. Blood pressure was measured in 40 adult patients scheduled for elective laparoscopic bariatric surgery (mean age 38 years, SD 11; male 12.5%; mean BMI 46 kg/m2, SD 5) by a
- Nexfin® system (BMEYE, Amsterdam, The Netherlands, now licensed as ClearSight® system;
- forearm cuff (Mindray non-invasive blood pressure measurement cuff);
- upper arm cuff (Mindray non-invasive blood pressure measurement cuff).
Measurement properties were compared to blood pressure measurements by an 20g arterial catheter. No risk of bias (very good quality) was assessed for criterion validity and responsiveness. Reliability was not reported.
Rogge (2019) investigated criterion validity and responsiveness in a prospective comparison study. Blood pressure was measured in 35 adult patients scheduled for elective laparoscopic bariatric surgery (median age 53 years, 25-75th percentile 41-53; male 71%; median BMI 47 kg/m2, 25-75th percentile 42-53) by a ClearSight monitor (Dräger Infinity Delta; Dräger, Lübeck, Germany). Measurement properties were compared to blood pressure measurements by an arterial catheter. For criterion validity and responsiveness high risk of bias was assessed (inadequate quality) as no correlations or AUC was reported. Reliability was not reported.
Schumann (2021) investigated criterion validity and responsiveness in a prospective comparison study. Non-invasive blood pressure measurements were performed in 90 (108 included, but 18 lost to follow-up) adult patients scheduled for elective laparoscopic bariatric surgery (mean age 47 years, SD 13; male 29%; mean BMI 47 kg/m2, SD 7) by
- continuous finger cuff measurements (ccNexfin (BMEYE B.V., The Netherlands); now ClearSight (Edwards Lifesciences, USA)) that was fitted and placed on the middle phalanx of the third or fourth finger according to the manufacturer’s specification;
- oscillometric lower arm measurements with standard cuff (Criticon; GE Healthcare, USA);
- oscillometric upper arm measurements with large cuff (Criticon; GE Healthcare, USA).
Measurement instruments were compared with measurement by a 20-gauge radial artery catheter. A pressure transducer was leveled to the level of the right atrium. There may be risk of bias (doubtful quality) for the reported criterion validity of the upper arm measurements due to a relatively large number of missing values. No risk of bias (very good quality) was assessed for finger cuff and lower arm criterion validity. For responsiveness there was risk of bias assessed (inadequate quality) as no correlation or AUC was reported. Reliability was not reported.
Results
Finger cuff blood pressure versus arterial line blood pressure
Reliability/measurement error
No studies reporting reliability and measurement errors were included.
Criterion validity
Schumann (2021) reported criterion validity by reporting a correlation coefficient and by reporting the mean of the differences including limits of agreement for systolic blood pressure, diastolic blood pressure and mean arterial blood pressure between cuff measurements and arterial line (see table 1). For systolic and mean arterial blood pressure, sufficient criterion validity (compared to arterial line) was reported for finger cuff measurements. For diastolic blood pressure, insufficient criterion validity (compared to arterial line) was reported.
Table 1: Criterion validity finger blood pressure measurements (compared to arterial line).
|
Study |
Correlation coefficient (95%CI) |
Mean of the difference (SD) |
95% Limits of agreement |
Criterion validity |
|
Systolic blood pressure measurements |
||||
|
Schumann, 2021 |
0.75 (NR) |
-7 (14) |
-35 to 20 |
Sufficient |
|
Diastolic blood pressure measurements |
||||
|
Schumann, 2021 |
0.63 (NR) |
0 (11) |
-22 to 22 |
Insufficient |
|
Mean arterial blood pressure measurements |
||||
|
Schumann, 2021 |
0.75 (NR) |
-1 (11) |
-23 to 21 |
Sufficient |
NR: Not reported
Responsiveness
Schumann (2021), Rogge (2019), Hansen (2022) and Eley (2021) reported responsiveness by reporting the concordance rate (i.e., percentage of concordant pairs in a 4-quadrant grid plot) for systolic blood pressure, diastolic blood pressure and mean arterial blood pressure when comparing cuff and arterial line measurements (see table 2). For systolic, diastolic, and mean arterial blood pressure, sufficient responsiveness was reported for finger cuff measurements.
Table 2: Responsiveness of finger blood pressure measurements (compared to arterial line).
|
Type of measurement |
Concordance rate (95%CI) |
Responsiveness |
|
Systolic blood pressure |
||
|
Schumann, 2021 |
0.85 (NR) |
Unknown |
|
Rogge, 2019 |
0.93 (0.89 to 0.97) |
Unknown |
|
Hansen, 2022 |
0.90 (NR) |
Unknown |
|
Eley, 2021 |
0.93 (NR) |
Unknown |
|
Diastolic blood pressure |
||
|
Schumann, 2021 |
0.81 (NR) |
Unknown |
|
Rogge, 2019 |
0.88 (0.84 to 0.92) |
Unknown |
|
Hansen, 2022 |
0.86 (NR) |
Unknown |
|
Eley, 2021 |
0.92 (NR) |
Unknown |
|
Mean arterial blood pressure |
||
|
Schumann, 2021 |
0.88 (NR) |
Unknown |
|
Rogge, 2019 |
0.93 (0.89 to 0.96) |
Unknown |
|
Hansen, 2022 |
0.91 (NR) |
Unknown |
|
Eley, 2021 |
0.93 (NR) |
Unknown |
NR: Not reported
Lower arm blood pressure versus arterial line blood pressure
Reliability/measurement error
No studies reporting reliability and measurement errors were included.
Criterion validity
Schumann (2021) and Mostafa (2020) reported criterion validity by reporting a correlation coefficient and by reporting the mean of the differences including limits of agreement for systolic blood pressure, diastolic blood pressure and mean arterial blood pressure between cuff measurements and arterial line (see table 3). For systolic blood pressure, sufficient criterion validity (compared to arterial line) was reported for lower arm measurements. For diastolic and mean arterial blood pressure, inconsistent results were reported, so results were inconclusive.
Table 3: Criterion validity lower arm blood pressure measurements (compared to arterial line)
|
Study |
Correlation coefficient (95%CI) |
Mean of the difference (SD) |
95% Limits of agreement |
Criterion validity |
|
Systolic blood pressure measurements |
||||
|
Schumann, 2021 |
0.71 (NR) |
-4 (15) |
-33 to 26 |
Sufficient |
|
Mostafa, 2020 |
0.71 (0.64 to 0.76) |
4.3 (16) |
-27.2 to 35.8 |
Sufficient |
|
Diastolic blood pressure measurements |
||||
|
Schumann, 2021 |
0.61 (NR) |
2 (12) |
-22 to 26 |
Insufficient |
|
Mostafa, 2020 |
0.74 (0.68 to 0.80 |
2.5 (10) |
-17.2 (22.2) |
Sufficient |
|
Mean arterial blood pressure measurements |
||||
|
Schumann, 2021 |
0.67 (NR) |
-5 (13) |
-29 to 20 |
Insufficient |
|
Mostafa, 2020 |
0.79 (0.74 to 0.83) |
6.2 (8.4) |
-10.1 to 22.6 |
Sufficient |
NR: Not reported
Responsiveness
Mostafa, 2020) reported responsiveness by reporting a correlation coefficient and by reporting the mean of the difference’s values including limits of agreement for Dsystolic blood pressure, Ddiastolic blood pressure and Dmean arterial blood pressure between lower arm measurements and arterial line (see table 4). For systolic, diastolic, and mean arterial blood pressure, sufficient criterion validity (compared to arterial line) was reported for lower arm measurements.
Table 4: Responsiveness lower arm blood pressure measurements (compared to arterial line)
|
Study |
Correlation coefficient (95%CI) |
Mean of the difference (SD) |
95% Limits of agreement |
Responsiveness |
|
Systolic blood pressure measurements |
||||
|
Mostafa, 2020 |
0.76 (0.69 to 0.8) |
0.3 (10.3) |
020 to 20.7 |
Sufficient |
|
Diastolic blood pressure measurements |
||||
|
Mostafa, 2020 |
0.74 (0.67 to 0.79) |
0.3 (6.3) |
-12 to 12.7 |
Sufficient |
|
Mean arterial blood pressure measurements |
||||
|
Mostafa, 2020 |
0.74 (0.67 to 0.79) |
0.6 (12.3) |
-23.6 to 24.9 |
Sufficient |
NR: Not reported
Upper arm blood pressure versus arterial line blood pressure
Reliability/measurement error
No studies reporting reliability and measurement errors were included.
Criterion validity
Mostafa (2020) and Hager (2009) reported criterion validity by reporting a correlation coefficient and by reporting the mean of the differences including limits of agreement for systolic blood pressure, diastolic blood pressure and mean arterial blood pressure between cuff measurements and arterial line (see table 5). For systolic and mean arterial blood pressure, inconclusive results were reported for criterion validity (compared to arterial line) and no overall conclusions could be drawn for upper arm measurements. For diastolic blood pressure, insufficient criterion validity was reported.
Table 5: Criterion validity upper arm blood pressure measurements (compared to arterial line)
|
Study |
Correlation coefficient (95%CI) |
Mean of the difference (SD) |
95% Limits of agreement |
Criterion validity |
|
Systolic blood pressure measurements |
||||
|
Mostafa, 2020 |
0.74 (0.68 to 0.79) |
14.2 (13.6) |
-12.5 to 40.9 |
Sufficient |
|
Hager, 2009 |
0.63 (0.47 to 0.57) |
-3.6 (NR) |
-46.7 to 39.6 |
Insufficient |
|
Diastolic blood pressure measurements |
||||
|
Mostafa, 2020 |
0.67 (0.60 to 0.73 |
9.9 (10.7) |
-10.9 to 30.8 |
Insufficient |
|
Hager, 2009 |
0.47 (0.24 to 0.70) |
-7.6 (NR) |
-38.1 to 23 |
Insufficient |
|
Mean arterial blood pressure measurements |
||||
|
Mostafa, 2020 |
0.78 (0.73 to 0.83) |
12.8 (9) |
-4.9 to 30.6 |
Sufficient |
|
Hager, 2009 |
0.63 (0.48 to 0.74) |
1.7 (NR) |
-28.8 to 32.1 |
Insufficient |
NR: Not reported
Responsiveness
Mostafa, 2020) reported responsiveness by reporting a correlation coefficient and by reporting the mean of the difference’s values including limits of agreement for Dsystolic blood pressure, Ddiastolic blood pressure and Dmean arterial blood pressure between upper arm measurements and arterial line (see table 6). For systolic and mean arterial blood pressure, sufficient criterion validity (compared to arterial line) was reported for lower arm measurements. For diastolic blood pressure, insufficient criterion validity (compared to arterial line) was reported for lower arm measurements.
Table 6: Responsiveness upper arm blood pressure measurements (compared to arterial line)
|
Study |
Correlation coefficient (95%CI) |
Mean of the difference (SD) |
95% Limits of agreement |
Responsiveness |
|
Systolic blood pressure measurements |
||||
|
Mostafa, 2020 |
0.74 (0.67 to 0.79) |
-0.2 (9.9) |
-19 to 19.4 |
Sufficient |
|
Diastolic blood pressure measurements |
||||
|
Mostafa, 2020 |
0.60 (0.50 to 0.68) |
-0.3 (9.1) |
-18.2 to 17.7 |
Insufficient |
|
Mean arterial blood pressure measurements |
||||
|
Mostafa, 2020 |
0.78 (0.72 to 0.82) |
0.4 (7.3) |
-13.9 to 14.8 |
Sufficient |
NR: Not reported
Level of evidence of the literature
To determine the level of evidence, the COSMIN approach (Mokkink, 2018) was used for each individual outcome measure.
Finger cuff blood pressure versus arterial line blood pressure
Reliability and measurement errors
The level of evidence for reliability and measurement errors for systolic blood pressure could not be assessed as none of the included studies reported on these measurement properties.
The level of evidence for reliability and measurement errors for diastolic blood pressure could not be assessed as none of the included studies reported on these measurement properties.
The level of evidence for reliability and measurement errors for mean arterial blood pressure could not be assessed as none of the included studies reported on these measurement properties.
Criterion validity
The level of evidence of criterion validity for systolic blood pressure started at high and was downgraded to moderate because of imprecision (-1, less than 100, but more than 50 participants).
The level of evidence of criterion validity for diastolic blood pressure started at high and was downgraded to moderate because of imprecision (-1, less than 100, but more than 50 participants).
The level of evidence of criterion validity for mean arterial blood pressure started at high and was downgraded to moderate because of imprecision (-1, less than 100, but more than 50 participants).
Responsiveness
The level of evidence of responsiveness for systolic blood pressure started at high and was downgraded to very low because of risk of bias (-2, only multiple studies of inadequate quality available), imprecision (-1, unclear confidence intervals).
The level of evidence of responsiveness for diastolic blood pressure started at high and was downgraded to very low because of risk of bias (-2, only multiple studies of inadequate quality available), imprecision (-1, unclear confidence intervals).
The level of evidence of responsiveness for mean arterial blood pressure started at high and was downgraded to very low because of risk of bias (-2, only multiple studies of inadequate quality available), imprecision (-1, less unclear confidence intervals).
Lower arm blood pressure versus arterial line blood pressure
Reliability and measurement errors
The level of evidence for reliability and measurement errors for systolic blood pressure could not be assessed as none of the included studies reported on these measurement properties.
The level of evidence for reliability and measurement errors for diastolic blood pressure could not be assessed as none of the included studies reported on these measurement properties.
The level of evidence for reliability and measurement errors for mean arterial blood pressure could not be assessed as none of the included studies reported on these measurement properties.
Criterion validity
The level of evidence of criterion validity for systolic blood pressure started at high and was not further downgraded.
The level of evidence of criterion validity for diastolic blood pressure could not be graded as study results were inconclusive and no conclusions could be drawn.
The level of evidence of criterion validity for mean arterial blood pressure could not be graded as study results were inconclusive and no conclusions could be drawn.
Responsiveness
The level of evidence of responsiveness for systolic blood pressure started at high and was downgraded to low because of imprecision (-2, less than 50 participants included).
The level of evidence of responsiveness for diastolic blood pressure started at high and was downgraded to low because of imprecision (-2, less than 50 participants included).
The level of evidence of responsiveness for mean arterial blood pressure started at high and was downgraded to low because of imprecision (-2, less than 50 participants included).
Upper arm blood pressure versus arterial line blood pressure
Reliability and measurement errors
The level of evidence for reliability and measurement errors for systolic blood pressure could not be assessed as none of the included studies reported on these measurement properties.
The level of evidence for reliability and measurement errors for diastolic blood pressure could not be assessed as none of the included studies reported on these measurement properties.
The level of evidence for reliability and measurement errors for mean arterial blood pressure could not be assessed as none of the included studies reported on these measurement properties.
Criterion validity
The level of evidence of criterion validity for systolic blood pressure could not be graded as study results were inconclusive and no conclusions could be drawn.
The level of evidence of criterion validity for diastolic blood pressure started at high and was downgraded to moderate because of imprecision (-1, less than 100 participants, but more than 50 participants included).
The level of evidence of criterion validity for mean arterial blood pressure could not be graded as study results were inconclusive and no conclusions could be drawn.
Responsiveness
The level of evidence of responsiveness for systolic blood pressure started at high and was downgraded to low because of imprecision (-2, less than 50 participants included).
The level of evidence of responsiveness for diastolic blood pressure started at high and was downgraded to low because of imprecision (-2, less than 50 participants included).
The level of evidence of responsiveness for mean arterial blood pressure started at high and was downgraded to low because of imprecision (-2, less than 50 participants included).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following questions:
1) What is the effect of non-invasive blood pressure measurements (upper arm, lower arm, finger) compared to an arterial line in adult patients with obesity undergoing surgery under general, regional, or spinal anesthesia on clinical outcome?
| P: | Patients (age ≥18 years) with obesity (BMI ≥30 kg/m2) undergoing surgery under general, regional, or spinal anesthesia) |
| I1: | Non-invasive blood pressure measurement by finger cuff |
| I2: | Non-invasive blood pressure measurement by lower arm cuff |
| I3: | Non-invasive blood pressure measurement by upper arm cuff |
| C: | Blood pressure measurement by arterial line |
| O: | Complications, therapeutic reactions to measured blood pressure, costs |
2) What are the clinimetric properties of non-invasive blood pressure measurements (upper arm vs. lower arm vs. finger) compared to an arterial line (gold standard) in adult patients with obesity undergoing surgery under general, regional, or spinal anesthesia?
| P: | patients (age ≥18 years) with obesity (BMI ≥30 kg/m2) undergoing surgery under general, regional, or spinal anesthesia |
| I1: | non-invasive blood pressure measurement by finger cuff during surgical procedure at single time points |
| I2: | non-invasive blood pressure measurement by lower arm cuff during surgical procedure at single time points |
| I3: | non-invasive blood pressure measurement by upper arm cuff during surgical procedure at single time points |
| C: | blood pressure measurement by arterial line at single time points |
| O: | reliability (reliability, measurement error), validity (criterion validity), responsiveness (criterion approach) |
Relevant outcome measures
The guideline development group considered ‘clinical complications’ and ’therapeutic reactions to measured blood pressure’ as a critical outcome measures for decision making; and costs, reliability, validity, and responsivity as important outcome measures for decision making.
The working group defined the outcome measures as follows:
Complications:
Myocardial ischemia (peri-operative, 30d/in-hospital)
Kidney function disorder (peri-operative, in-hospital, 30d)
Cerebrovascular accident (peri-operative/in-hospital/30d)
Nerve damage (per-operative)
Local bleeding by measurement (peri-operative)
Local ischemia by measurement (peri-operative)
Therapeutic reactions to measured blood pressure:
As defined in the selected studies
Costs: Total costs during hospital stay
Reliability (according to COSMIN, see Mokkink, 2018):
Reliability: The proportion of the total variance in the measurements which is due to ‘true’ differences between patients
Measurement error: The systematic and random error of a patient’s score that is not attributed to true changes in the construct to be measured
Validity (according to COSMIN, see Mokkink, 2018):
Criterion validity: The degree to which the scores of a PROM are an adequate reflection of a ‘gold standard’
Responsiveness (according to COSMIN, see Mokkink, 2018):
The ability of a PROM to detect change over time in the construct to be measured
The working group defined the following values as a minimal clinically (patient) important difference:
| Myocardial ischemia: | 0.95≥RR≥1.05 |
| Kidney function disorder: | 0.95≥RR≥1.05 |
| Cerebrovascular accident: | 0.95≥RR≥1.05 |
| Nerve damage: | 0.95≥RR≥1.10 |
| Local bleeding by measurement: | 0.95≥RR≥1.05 |
| Local ischemia by measurement: | 0.95≥RR≥1.10 |
| Therapeutic reactions to measured blood pressure: | 10% difference compared to control group or 0.91≥RR≥1.10 |
| Costs: | 10% difference compared to control group |
The working group defined the following values as sufficient measurement properties (according to COSMIN, see Mokkink, 2018):
| Reliability: |
Intraclass correlation coefficient or weighted Kappa≥ 0.70 |
| Measurement error: |
Smallest detectable change (SDC) or limits of agreement (LoA) < minimal important change (MIC systolic = 5 mmHg or less (SD 8 mmHg or less) MIC diastolic = 5 mmHg or less (SD 8 mmHg or less) MIC mean arterial = 5 mmHg or less (SD 8 mmHg or less)) |
| Criterion validity: |
Correlation (r) with golden standard or area under the curve (AUC)≥ 0.70 |
| Responsiveness: |
The result is in accordance with the hypothesis (criterion approach: concordance correlation coefficient) OR AUC ≥ 0.70 |
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 03-03-2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 555 hits. Studies were selected based on the following criteria:
- Systematic reviews (searched in at least two databases, and detailed search strategy, risk of bias assessment and results of individual studies available), RCTs or observational peer reviewed studies in English or Dutch language;
- Investigating patients (age ³18 years) with obesity (BMI ≥30 kg/m2) undergoing surgery under general, regional or spinal anesthesia;
- Comparing the impact of blood pressure measurements by finger cuff and/or lower arm cuff to blood pressure measurements by upper arm cuff, or;
- Studies investigating the clinimetric characteristics by comparing single time point measurement properties of blood pressure measurements by finger cuff, lower arm cuff and/or upper arm to properties of an arterial line;
- At least one of the defined outcome measures was reported;
- Studies reporting construct validity and studies reporting responsiveness for upper arm and lower arm measurements had to be assessed with low risk of bias. Studies reporting responsiveness for finger cuff measurements or reporting reliability outcomes were also included if high risk of bias was assessed
Eleven studies were initially selected based on title and abstract screening. After reading the full text, five studies were excluded (see the table with reasons for exclusion under the tab Methods), and six studies were included. These studies met the criteria of PICO 2 (clinimetric properties).
Results
No studies were included for the analysis of the literature regarding clinical effects of different types of blood pressure measurements. Six studies were included in the analysis of the literature regarding clinimetric properties. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Bamgbade OA, Rutter TW, Naflu OO, Dorje P. Postoperative Complications in Obese and Nonobese Patients. World Journal of Surgery. 2007 April; 31(3):556-60.
- Benalcazar DA, Cascella M. Obesity Surgery Pre-Op Assessment and Preparation. Stat Pearls. 2022 July.
- Eley V, Christensen R, Guy L, Wyssusek K, Pelecanos A, Dodd B, Stowasser M, van Zundert A. ClearSight™ finger cuff versus invasive arterial pressure measurement in patients with body mass index above 45 kg/m2. BMC Anesthesiol. 2021 May 18;21(1):152. doi: 10.1186/s12871-021-01374-x. Erratum in: BMC Anesthesiol. 2023 Mar 10;23(1):75. PMID: 34006231; PMCID: PMC8130355.
- Hager H, Mandadi G, Pulley D, Eagon JC, Mascha E, Nutter B, Kurz A. A comparison of noninvasive blood pressure measurement on the wrist with invasive arterial blood pressure monitoring in patients undergoing bariatric surgery. Obes Surg. 2009 Jun;19(6):717-24. doi: 10.1007/s11695-008-9607-7. Epub 2008 Jul 10. PMID: 18618207.
- Hansen J, Pohlmann M, Beckmann JH, Klose P, Gruenewald M, Renner J, Lorenzen U, Elke G. Comparison of oscillometric, non-invasive and invasive arterial pressure monitoring in patients undergoing laparoscopic bariatric surgery - a secondary analysis of a prospective observational study. BMC Anesthesiol. 2022 Mar 28;22(1):83. doi: 10.1186/s12871-022-01619-3. PMID: 35346046; PMCID: PMC8962134.
- Imedsales.com Edwards Lifesciences CSCM ClearSight Finger Cuff Medium - Box of 5. Beschikbaar op: https://imedsales.com/edwards-lifesciences-cscm-clearsight-finger-cuff-medium-4, geraadpleegd op 19-12-2023
- Lakhal K, Dauvergne JE, Messet-Charriere H, Nay MA, Kamel T, Muller G, Robert-Edan V, Rozec B, Ehrmann S, Jacquier S, Boulain T. Risk Factors for Poor Performance in Finger Cuff Non-Invasive Monitoring of Arterial Pressure: A Prospective Multicenter Study. Anaesth Crit Care Pain Med. 2023 Dec 2:101333.
- Leblanc MÈ, Auclair A, Leclerc J, Bussières J, Agharazii M, Hould FS, Marceau S, Brassard P, Godbout C, Grenier A, Cloutier L, Poirier P. Blood Pressure Measurement in Severely Obese Patients: Validation of the Forearm Approach in Different Arm Positions. Am J Hypertens. 2019 Jan 15;32(2):175-185. doi: 10.1093/ajh/hpy152. PMID: 30312368.
- Leblanc MÉ, Croteau S, Ferland A, Bussières J, Cloutier L, Hould FS, Biertho L, Moustarah F, Marceau S, Poirier P. Blood pressure assessment in severe obesity: validation of a forearm approach. Obesity (Silver Spring). 2013 Dec;21(12):E533-41. doi: 10.1002/oby.20458. Epub 2013 Jun 22. PMID: 23512945.
- Mokkink, L. B., Prinsen, C. A., Patrick, D. L., Alonso, J., Bouter, L. M., de Vet, H.C., Terwee C. B. (2018). COSMIN methodology for systematic reviews of patient-reported outcome measures (PROMs). User manual. 78:1. Beschikbaar op: https://www.cosmin.nl/wp-content/uploads/COSMIN-syst-review-for-PROMs-manual_version-1_feb-2018-1.pdf.
- Mostafa MMA, Hasanin AM, Alhamade F, Abdelhamid B, Safina AG, Kasem SM, Hosny O, Mahmoud M, Fouad E, Rady A, Amin SM. Accuracy and trending of non-invasive oscillometric blood pressure monitoring at the wrist in obese patients. Anaesth Crit Care Pain Med. 2020 Apr;39(2):221-227. doi: 10.1016/j.accpm.2020.01.006. Epub 2020 Feb 14. PMID: 32068134.
- Nuttall G, Burckhardt J, Hadley A, Kane S, Kor D, Marienau MS, Schroeder DR, Handlogten K, Wilson G, Oliver WC: Surgical and patient risk factors for severe arterial line complications in adults. Anesthesiology. 2016; 124:590–7
- Ortiz, V.E., Kwo, J. Obesity: physiologic changes and implications for preoperative management. BMC Anesthesiol 15, 97 (2015).
- Palatini P, Parati G. Blood pressure measurement in very obese patients: a challenging problem. J Hypertens. 2011 Mar;29(3):425-9.
- Palatini P, Benetti E, Fania C, Malipiero G, Saladini F. Rectangular cuffs may overestimate blood pressure in individuals with large conical arms. J Hypertens. 2012 Mar;30(3):530-6.
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005 Feb 8;111(5):697-716. doi: 10.1161/01.CIR.0000154900.76284.F6. PMID: 15699287.
- Poirier et al. American Heart Association. Cardiovascular Evaluation and Management of Severely Obese Patients Undergoing Surgery. Circulation. 2009; 120:86-95.
- Rogge DE, Nicklas JY, Schön G, Grothe O, Haas SA, Reuter DA, Saugel B. Continuous Noninvasive Arterial Pressure Monitoring in Obese Patients During Bariatric Surgery: An Evaluation of the Vascular Unloading Technique (Clearsight system). Anesth Analg. 2019 Mar;128(3):477-483. doi: 10.1213/ANE.0000000000003943. PMID: 30649073.
- Saugel B, Grothe O, Nicklas JY. Error Grid Analysis for Arterial Pressure Method Comparison Studies. Anesth Analg. 2018 Apr;126(4):1177-1185.
- Scheer B, Perel A, Pfeiffer UJ: Clinical review: Complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002; 6:199–204
- Schumann R, Meidert AS, Bonney I, Koutentis C, Wesselink W, Kouz K, Saugel B. Intraoperative Blood Pressure Monitoring in Obese Patients. Anesthesiology. 2021 Feb 1;134(2):179-188. doi: 10.1097/ALN.0000000000003636. PMID: 33326001.
Evidence tabellen
|
Study |
Study characteristics |
Patient characteristics |
Measurement instrument (I) |
Measurement instrument (C; golden standard; reference method) |
Follow-up/Interpretability |
Measurement properties |
Comments |
|
Eley, 2021 |
Instrument assessed: Continuous non-invasive blood pressure via finger cuff
Setting and Country: The royal Brisbane and Women’s Hospital, Brisbane, Australia
Funding and conflicts of interest: Funding: ANZCA Research Foundation, Australian and New Zealand Colelge of Anaesthetists, Melbourne, Australia; Robert and Janelle Bird Post-Doctoral Fellowship awared by the Royal Brisbane and Women\s Hospital Foundation, Brisbane, Australia;
|
Inclusion criteria:
Exclusion criteria:
Sample size: N=67
Age in years (mean (SD; range)): 45 (11.7; 24 to 65)
Gender (% female):
BMI in kg/m2 (medan (IQR; range)): 50.2 (48.3 to 55.3; 45.1 to 69.2)
Disease: NA Disease duration (mean (SD) in years: NA Disease severity: NA |
Name: Continuous non-invasive blood pressure measurement using the vascular unloading technique via a finger cuff
Version (including language if applicable): Clearsight EV1000 Clincal Platform (Edwads Lifesciences Corp, Irvine, CA
Construct: Non-invasive blood pressure (SBP), mean arterial pressure (MAP) and diastolic blood pressure (DBP)
Specification of investigated component: Following standard positioning of the patient (one pillow under the head, table in the reverse Trendelenburg position) the first blood pressure measurements were obtained
Specification of time, occasion, user: anesthesia
Hypothesis (construct validity hypothesis testing): Not reported |
Name: Radial arterial catheter
Version (including language if applicable): invasive transducer (Edwards Lifesciences Tru- Wave™, Edwards Lifesciences Corp, Irvine, CA, USA); D19KT™ monitor with a E-PSMP Carescape Module™ (GE Healthcare, Chicago, IL, USA).
Construct: invasive radial arterial blood pressure monitoring
Specification of investigated component: Following standard positioning of the patient (one pillow under the head, table in the reverse Trendelenburg position) the first blood pressure measurements were obtained
Specification of time, occasion, user: Blood pressure was recorded digitally at 5 min intervals for each patient up to 1 hour, or until completion of anesthesia
|
Length of follow-up: Up to 1 hour, or until completion of anesthesia
Distribution of scores: I: NR
Loss to follow-up: Percentage of missing items/total scores/outcome: measurements up to 15 min, after which the number of measurements per method ranged between 26 and 30, due to variations in the duration of surgery
Floor effects (% of sample with the lowest score possible): Ceiling effects (% of sample with the highest score possible): I: NR
Minimally important change/difference:
|
Criterion validity: Mean arterial blood pressure, bland altman bias: Upper LOA:
Systolic blood pressure Bias: 14.3 mmHG
Diastolic blood pressure Bias: 2.6 mmHG
Responsiveness Disconcordant pairs (n of N (%)):
Mean arterial blood pressure:
18 of 251 (7%) Diastolic blood pressure:
Clinical impact outcome measures: Grid analyses (n (%)) The levels are based on whether or not the difference between the readings would trigger a therapeutic intervention and the potential consequences of that intervention (A: no risk; B: low risk; C: moderate risk; D: significant risk; E: dangerous risk)
Mean arterial pressure: Zone A: 338 (91.4%) Zone D: 0 (0%) Zone E: 0 (0%)
Systolic blood pressure: Zone A: 336 (90.8%) Zone D: 0 (0%) Zone E: 0 (0%)
Diastolic blood pressure: NR |
Remark: “In 4 (13%) participants the circumference of the finger was larger than the manufacturer’s recommendation for the largest finger cuff.”
Author’s conclusion: “ In this pilot study, the vascular unloading technique did not provide accurate blood pressure measurements when assessed over time, with the FC tending to provide lower values. The clinical consequences of these errors would have led to inappropriate interventions of moderate risk in a small but arguably significant fraction of readings, in this population with a high burden of comorbidities.” |
|
Hager, 2009 |
Instrument assessed: Blood pressure via Oscillometric cuff on the upper arm
Setting and Country: Washington University, St. Louis, MO, USA
Funding and conflicts of interest: This study was financially supported by the Clinical Research Division of the Department of Anesthesiology, Washington University, St. Louis, MO. None of the authors have personal financial interest related to this research.
|
Inclusion criteria:
Exclusion criteria:
Sample size: 22 patients
Age in years (mean (SD): 44.3 (9.5) years
Gender (% female):
BMI (SD):
Arm Circumference (SD):
Disease:
Disease duration (mean (SD) in years: NA
Disease severity: NA |
Name: Oscillometric noninvasive blood pressure upper arm
Version (including language if applicable): Oscillometric noninvasive blood pressure (Datex-Ohmeda, Madison, WI, USA)
Construct: systolic, diastolic, and mean blood pressure Specification of investigated component: NR
Specification of time, occasion, user:
Hypothesis (construct validity hypothesis testing): NR |
Name: Invasive arterial blood pressure monitoring
Version (including language if applicable): The catheters were 20-gauge and 4.45 cm long (Arrow International, Reading, CA, USA). The pressure monitoring tubing was 210 cm long; pressure transducers (both from Edwards Lifescience, Irvine, CA, USA)
Construct: systolic, diastolic, and mean blood pressure
Specification of investigated component: NR
Specification of time, occasion, user: Blood pressure measurement were collected every 5s.
|
Length of follow-up: More than 3 hours (mean 3.8 SD 1.1)
Distribution of scores: I: NR
Loss to follow-up: Percentage of missing items/total scores/outcome:
Floor effects (% of sample with the lowest score possible): Ceiling effects (% of sample with the highest score possible): I: NR
Minimally important change/difference:
|
Criterion validity: 1) Bland altman analysis (mean bias (lower to upper limit of agreement)
Systolic blood pressure:
Diastolic blood pressure: -7.6 (-38.1 to 23.0)
Mean arterial blood pressure 1.7 (-28.8 to 32.1)
2) Lin’s concordance correlation coefficient (2.5th percentile to 97th percentile)) Systolic blood pressure: 0.63 (0.47 to 0.75)
Diastolic blood pressure: 0.47 (0.24 to 0.70)
Mean arterial blood pressure: 0.63 (0.48 to 0.74)
|
|
|
Hansen, 2022 |
Instrument assessed: Non invasive blood pressure uisng a forearm cuff
Setting and Country: Department of Anaesthesiology and Intensive Care Medicine and General Surgery, University Medical Center, Schleswig- Holstein, Campus Kiel, Germany
Funding and conflicts of interest: Open Access funding enabled and organized by Projekt DEAL; no external funding, only institutional resources
|
Inclusion criteria:
Exclusion criteria:
Sample size: N=60 (56 included in final analysis)
Age in years (mean (SD; range)): 46.5 (12.1)
BMI in kg/m2 (mean (SD; range)): 49.2 (5.7)
Gender (% female): Female: 73%
Disease: NA
Disease duration (mean (SD) in years: NA
Disease severity: NA |
I1:
Name: Blood pressure using fingercuff (Nexfin)
Version (including language if applicable): Nexfin® system (BMEYE, Amsterdam, The Netherlands, now licensed as Clearsight ® system by Edwards Lifesciences, Irvine, CA, USA)
Construct: Systolic arterial pressure, Diastolic arterial pressure, Mean arterial pressure
Specification of investigated component: In the pre-, intra- and postoperative phase, the arterial pressure was measured at 16 predefined measurement time points at which hemodynamic changes were likely expected. (mainly under general anesthesia)
Specification of time, occasion, user:
Hypothesis (construct validity hypothesis testing): NR
I2: Name: Noninvasive blood pressure measurement using forearm cuff (NBP)
Version (including language if applicable): DURA-CUF™ GE, Boston, MA, USA
Construct: Systolic arterial pressure, Diastolic arterial pressure, Mean arterial pressure
Specification of investigated component: In the pre-, intra- and postoperative phase, the arterial pressure was measured at 16 predefined measurement time points at which hemodynamic changes were likely expected. (mainly under general anesthesia)
Specification of time, occasion, user:
Hypothesis (construct validity hypothesis testing): NR
|
Name: Blood pressure using arterial catheter (IAP)
Version (including language if applicable): Arrow R Intl., Reading, PA, USA; Transducer: DPT-6000, CODAN pvb Critical Care GmbH, Forstinning, Germany
Construct: Systolic arterial pressure, Diastolic arterial pressure, Mean arterial pressure
Specification of investigated component: In the pre-, intra- and postoperative phase, the arterial pressure was measured at 16 predefined measurement time points at which hemodynamic changes were likely expected. (mainly under general anesthesia)
Specification of time, occasion, user: See specification of investigated component
|
Length of follow-up: Until discharge from PACU
Distribution of scores: NR
Loss to follow-up: Percentage of missing items/total scores/outcome:
Floor effects (% of sample with the lowest score possible): Ceiling effects (% of sample with the highest score possible): I:NR
Minimally important change/difference:
|
Criterion validity: Correlation coefficient:
Lower arm (NBP)
Mean arterial pressure: 0.77 Systolic arterial pressure: 0.63 Diastolic arterial pressure: 0.72
Fingercuff
Mean arterial pressure: 0.88 Systolic arterial pressure: 0.87 Diastolic arterial pressure: 0.80
Bias (bland altman):
Lower arm (NBP) Mean arterial pressure: 3.94mmHg Diastolic pressure: 4mmHg
Finger cuff Lower arm (NBP) Mean arterial pressure: 1.43mmHg Diastolic pressure: 0.7mmHg
Responsiveness definition: concordance rate:
lower arm: Mean arterial pressure: 90% Systolic arterial pressure: 85% Diastolic arterial pressure: 89%
Fingercuff: Mean arterial pressure: 91% Systolic arterial pressure: 90% Diastolic arterial pressure: 86%
|
Author’s conclusions: In the perioperative management of patients undergoing laparoscopic bariatric surgery, our study indicates that NIBP and Nexfin® derived absolute arterial pressure recordings were not interchangeable with IAP, but Nexfin® was more precise than NIBP. However, a good trending ability even under different hemodynamic stresses was found. Thus, Nexfin® may serve clinically useful to detect arterial pressure changes and render perioperative hemodynamic treatment, particularly in those individuals where NIBP cannot be reliably established.
|
|
Mostafa, 2020 |
Instrument assessed: Noninvasive blood pressure cuff
Setting and Country: University Hospital, Egypt
Funding and conflicts of interest: None
|
Inclusion criteria:
Exclusion criteria:
Sample size: N=60 (40 included in the protocol)
Age in years (mean (SD; range)): 38 (11)
Gender (% female): BMI mean (SD) kg/m2: 46(5)
Disease: NA Disease duration (mean (SD) in years: NA Disease severity: NA |
Forearm:
Name: Noninvasive blood pressure cuff forearm
Version (including language if applicable): adult non-invasive blood pressure cuff (Mindray Adult NIBP cuff CM1203), sized 25–35 cm
Construct: Mean arterial pressure, Systolic arterial pressure, Diastolic arterial pressure Specification of investigated component: NR
Specification of time, occasion, user:
Hypothesis (construct validity hypothesis testing): NR
Upperarm:
Name: Noninvasive blood pressure cuff upperarm
Version (including language if applicable): a large adult non-invasive blood pressure cuff (Mindray Large Adult NIBP cuff CM1204), sized 33–47 cm
Construct: Mean arterial pressure, Systolic arterial pressure, Diastolic arterial pressure
Specification of investigated component: NR
Specification of time, occasion, user: 3 minute interval Hypothesis (construct validity hypothesis testing): NR |
Name: Radial arterial catheter Version (including language if applicable): 20-G radial arterial using a Progetti (PG M9500, Italy) monitor.
Construct: Mean arterial pressure, Systolic arterial pressure, Diastolic arterial pressure
Specification of investigated component: NA
Specification of time, occasion, user: 3 minute intervals
|
Length of follow-up: NR
Distribution of scores: I:NR
Loss to follow-up: Percentage of missing items/total scores/outcome: In the 40 included patients, 262, 259, and 264 pairs of non-invasive readings of blood pressure were obtained from the arm, forearm, and wrist with the corresponding invasive blood pressure readings.
Floor effects (% of sample with the lowest score possible): Ceiling effects (% of sample with the highest score possible): I:NR
Minimally important change/difference:
|
Criterion validity: Correlation coefficient (95%CI):
Forearm: Systolic arterial pressure: 0.71 (0.64 to 0.76) Diastolic arterial pressure: 0.74 (0.68 to 0.80) Mean arterial pressure: 0.79 (0.74 to 0.83)
Upper arm: Systolic arterial pressure: 0.74 (0.68 to 0.79) Mean arterial pressure: 0.79 (0.73 to 0.83)
Mean bias (SD) – bland altman
Forearm: Systolic arterial pressure: 4.3 (16) Diastolic arterial pressure: 2.5 (10) Mean arterial pressure: 6.2 (8.4)
Upper arm: Systolic arterial pressure: 14.2 (13.6) Diastolic arterial pressure: 9.9 (10.7) Mean arterial pressure: 12.8 (9)
Responsiveness: definition: Correlation coefficient (95%CI)
Systolic arterial pressure: 0.76 (0.69 to 0.80) Diastolic arterial pressure: 0.74 (0.67 to 0.79) Mean arterial pressure: 0.74 (0.67 to 0.79)
Upper arm: Systolic arterial pressure: 0.74 (0.67 to 0.79) Diastolic arterial pressure: 0.6 (0.5 to 0.68) Mean arterial pressure: 0.78 (0.72 to 0.89)
Mean bias (SD, 95% limits of agreement)
Forearm: Systolic arterial pressure: 0.3 ( 10.3, -20 to 20.7) Diastolic arterial pressure: 0.3 ( 6.3, -12 to 12.7) Mean arterial pressure: 0.6 ( 12.3, -23.6 to 24.9)
Upper arm: Systolic arterial pressure: -0.2 ( 9.8, -19 to 19.4) Diastolic arterial pressure: -0.3 ( 9.1, -18.2 to 17.7) Mean arterial pressure: 0.4 ( 7.3, -13.9 to 14.8)
|
|
|
Rogge, 2019 |
Instrument assessed: Clearsight system
Setting and Country: Department of Anesthesiology, Center of Anesthesiology and Intensive Care Medicine, University, Medical Center Hamburg-Eppendorf, Hamburg, Germany
Funding and conflicts of interest: Funding: Edwards Lifesciences Corp (Irvine, CA) No conlficts of interest
|
Inclusion criteria:
Exclusion criteria:
Sample size: N=35 (included in the final protocol)
Age in years (median (range)): 53 (41-59)
Gender (% female):
BMI in kg/m2 (median (range)) 47 (42-53)
Disease: NA
Disease duration (mean (SD) in years: NA
Disease severity: NA |
Name: Clearsight monitor with an interface cable to the patient monitor
Version (including language if applicable): Dräger Infinity Delta; Dräger, Lübeck, Germany
Construct: Mean arterial pressure, Systolic arterial pressure, Diastolic arterial pressure
Specification of investigated component: NR
Specification of time, occasion, user: and reference method in parallel for about 45 minutes
Hypothesis (construct validity hypothesis testing): NR |
Describe the assessed instrument and used procedures.
Name: Arterial catheter
Version (including language if applicable): NR
Construct: Mean arterial pressure, Systolic arterial pressure, Diastolic arterial pressure
Specification of investigated component: NR
Specification of time, occasion, user: arterial pressure measurements of the test and reference method in parallel for about 45 minutes
|
Length of follow-up: 45 minutes Distribution of scores: I: NR
Loss to follow-up:
Percentage of missing items/total scores/outcome: artifacts or technical problems during signal recording with the vascular unloading technology or the arterial catheter (not further specified)
Floor effects (% of sample with the lowest score possible): Ceiling effects (% of sample with the highest score possible): I: NR
Minimally important change/difference:
|
Criterion validity: Bland altman, mean of the difference (SD, 95%CI)
Mean arterial pressure: (7.4, -13.5 to 15.6) Systolic arterial pressure: 6.8 mm Hg (10.3, -14.4 to 27.9) Diastolic arterial pressure: 0.8 mm Hg (6.9, -12.9 to 14.4)
Responsiveness: definition: Concordance rate (95%CI)
Mean arterial pressure:
Clinical impact outcome measures: definition: grid error analysis: be assigned to each pair of measured arterial pressure value (test method) and “true” arterial pressure value (reference method) for systolic arterial pressure and MAP (A: no risk; B: low risk; C: moderate risk; D: significant risk; E: dangerous risk) Mean arterial pressure: Zone A: 89.5% Zone B: 10.0% Zone C: 0.5% Zone D: 0% Zone E: 0%
Systolic arterial pressure: Zone A: 93.7% Zone B: 6.0% Zone C: 0.3% Zone D: 0% Zone E: 0%
Diastolic arterial pressure: Not reported |
Author’s conclusion: In conclusion, the accuracy and precision of the vascular unloading technology (Clearsight system) was good for MAP and diastolic arterial pressure, but only moderate for systolic arterial pressure during laparoscopic bariatric surgery. The system showed good trending capabilities. According to error grid analysis, >99% of Clearsight arterial pressure measurements were categorized in no- or low-risk zones. |
|
Schumann, 2021 |
Instrument assessed:
Setting and Country: Not reported: authors are from Boston, Massachusetts; Munich, German; New York, New York; Irvine, California; Hamburg, Germany; Cleveland, Ohio
Funding and conflicts of interest: Funding: Edwards Lifesciences (Irvine, California)—provided the technical equipment for the study. Conflicts of interest: for giving lectures, and refunds of travel expenses from Edwards Lifesciences; honoraria for consulting, institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from Pulsion Medical Systems SE (Feldkirchen, Germany); institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from CNSystems Medizintechnik GmbH (Graz, Austria); institutional restricted research grants from Retia Medical LLC (Valhalla, New York); honoraria for giving lectures from Philips Medizin Systeme B.blingen GmbH (B.blingen, Germany); and honoraria for consulting, institutional restricted research grants, and refunds of travel expenses from Tensys Medical Inc. (San Diego, California). The other authors declare no competing interests.
|
Inclusion criteria:
Exclusion criteria:
Sample size: N=108
Age in years (mean (SD; range)): 47 (13)
Gender (n,%):
BMI (mean, SD) in kg/m2: 48 (7) kg/m2
Disease: NA Disease duration (mean (SD) in years: NA
Disease severity: NA |
Describe the assessed instrument and used procedures.
I1: Name: Continuous noninvasive finger cuff blood pressure: position; 3, 15, 30, and 45 min after the beginning of insufflation in the 30Åã reverse Trendelenburg position; and 3 min after desufflation in the horizontal position)
Version (including language if applicable): ccNexfin (BMEYE B.V., The Netherlands); now ClearSight (Edwards Lifesciences, USA)
Construct: Non-invasive blood pressure
Specification of investigated component/ Specification of time, occasion, user: finger cuff with an infrared plethysmograph to measure the blood volume in the finger arteries, keep the blood volume constant throughout the cardiac cycle based on an automated feedback system that inflates and deflates the finger cuff, and indirectly reconstruct the blood pressure waveform based on the required cuff pressure. The finger cuff was fitted and placed on the middle phalanx of the third or fourth finger according to the manufacturer’s specification for proper fit. The heart reference system that compensates for hydrostatic pressure differences between the level of the heart and the finger cuff was positioned at the level of the right atrium during the entire study period. Finger cuff blood pressure measurements were displayed on and extracted from the proprietary monitor of the ccNexfin system.
Hypothesis (construct validity hypothesis testing):
I2: Name: Intermittent noninvasive oscillometric upper arm
Version (including language if applicable): Criticon; GE Healthcare, USA
Construct: Nonivasive blood pressure: position; 3, 15, 30, and 45 min after the beginning of insufflation in the 30Åã reverse Trendelenburg position; and 3 min after desufflation in the horizontal position)
Specification of investigated component/ Specification of time, occasion, user: daily clinical practice, cuff fit was accepted when blood pressure measurements could be obtained. A cuff that would not allow for circumferential closure or that would spontaneously come undone during an inflation cycle despite the most feasible limb alignment was considered a cuff failure for that limb site, and no aids such as tape were used to rescue potential measurements. Oscillometric lower leg blood pressure measurements obtained during steep reverse Trendelenburg positioning were not included in the analysis because hydrostatic pressure differences would have influenced the difference between lower leg and intraarterial blood pressure measurements. Blood pressure measurements were displayed on the patient monitor (Philips Intellivue MP 90 anesthesia monitor; Philips Healthcare, USA).
Hypothesis (construct validity hypothesis testing): Not reported
I3: Name: Intermittent noninvasive oscillometric lower arm
Version (including language if applicable): Criticon; GE Healthcare, USA
Construct: Nonivasive blood pressure: : position; 3, 15, 30, and 45 min after the beginning of insufflation in the 30o reverse Trendelenburg position; and 3 min after desufflation in the horizontal position)
Specification of investigated component/ Specification of time, occasion, user: daily clinical practice, cuff fit was accepted when blood pressure measurements could be obtained. A cuff that would not allow for circumferential closure or that would spontaneously come undone during an inflation cycle despite the most feasible limb alignment was considered a cuff failure for that limb site, and no aids such as tape were used to rescue potential measurements. Oscillometric lower leg blood pressure measurements obtained during steep reverse Trendelenburg positioning were not included in the analysis because hydrostatic pressure differences would have influenced the difference between lower leg and intraarterial blood pressure measurements. Blood pressure measurements were displayed on the patient monitor (Philips Intellivue MP 90 anesthesia monitor; Philips Healthcare, USA). Hypothesis (construct validity hypothesis testing): Not reported
|
Describe the assessed instrument and used procedures.
Name: Radial artery catheter
Version (including language if applicable): 20-gauge arterial catheter
Construct: Intraarterial blood pressure: position; 3, 15, 30, and 45 min after the beginning of insufflation in the 30Åã reverse Trendelenburg position; and 3 min after desufflation in the horizontal position)
Specification of investigated component/ Specification of time, occasion, user: a 20-gauge arterial catheter was inserted in the radial artery after induction of general anesthesia and connected it to a disposable pressure transducer. The pressure transducer was leveled to the level of the right atrium throughout the study. The transducer system was examined for its damping properties with a fast flush test, and, if necessary, actions were taken to correct abnormal damping. Blood pressure measurements were displayed on the patient monitor (Philips Intellivue MP 90 anesthesia monitor; Philips Healthcare, USA).
|
Length of follow-up: NA
Distribution of scores: Systolic blood pressure mean(SD) min to max, nmeasurements: I1: 109 (21) 46 to 187, 538 I2: 110 (18) 60 to 167, 443 I3: 113 (18) 61 to 200, 535 C: 117 (21), 55 to 202, 538
Diastolic blood pressure mean(SD), min to max, nmeasurements: I1: 67(13) 30 to 106, 538 I2: 75 (15) 41 to 129, 443 I3: 69 (14) 11 to 443, 537
Mean arterial pressure (SD) min to max, nmeasurements: I2: 124 (24) 78 to 195, 446 I3: 79 (14) 44 to 126, 537
Loss to follow-up: failures with blood pressure monitoring equipment during the procedure (n = 3); change of the surgical procedure (n = 1); patient’s withdrawal from participation (n = 1); discovery of missed exclusion criterion (n = 1); and case canceled (n = 1).
Percentage of missing items/total scores/outcome: I2: Nparticipants=8, cuff failure Nparticipants, cuff to small I3: Missing values: 5 Nparticipants, cuff failure C:
Floor effects (% of sample with the lowest score possible): Ceiling effects (% of sample with the highest score possible): Not reported
Minimally important change/difference:
|
Criterion validity: Definition: mean of the difference (SD; 95% limits of agreement): I1 vs C: I2 vs C:
definition: pearson correlation coefficient I3 vs C: systolic blood pressure: 0.71
Responsiveness: I3 vs C: systolic blood pressure: 0.78
Clinical impact outcome measures: definition: Error grid analysis (%(n)) differences between the investigated method and the reference method D: significant risk; E: dangerous risk B: 9.8% (53) C: 0.2% (1) B: 21.6% (116) C: 0.9% (5) I2 vs C: systolic blood pressure: B: 10.0% (44) C: 3.8% (17) B: 28.3% (126) C: 4.8% (18) I3 vs C: systolic blood pressure: B: 10.2% (60) C: 2.8% (15) B: 22.2% (119) C: 3.0% (16)
|
Hypothesis: Measurements (C) is better than the agreement between oscillometric (I2+I3) and intraarterial measurements (C)“
“Patients in our study reflect a typical bariatric surgical population, rather than obese patients across an extended age range, with possibly advanced cardiovascular comorbid conditions presenting for a broader range of general surgical procedures. Such a relatively selected study population may limit the generalizability of our findings.” “In conclusion, the agreement between finger cuff and intraarterial measurements was better than the agreement between oscillometric and intraarterial measurements for mean arterial pressure and diastolic blood pressure in obese patients during surgery. In these patients, forearm oscillometry exhibits better measurement performance than upper arm or lower leg oscillometry.” |
|
Risk or bias: Criterion validity |
||||
|
|
For continuous scores: Were correlations, or the area under the receiver operating curve calculated? Very good: Correlations or AUC calculated Inadequate: Correlations or AUC not calculated Not applicable (fill box dichotomous scores) |
For dichotomous scores: Were sensitivity and specificity determined?
Very good: Sensitivity and specificity calculated Inadequate: Sensitivity and specificity not calculated Not applicable (fill box continuous scores) |
Were there any other important flaws in the design or statistical methods of the study? Very good: No other important methodological flaws Doubtful: Other minor methodological flaws Inadequate: Other important methodological flaws |
Overall rating (lowest score) |
|
Eley, 2021 (I1 vs C) |
Inadequate (No correlation or AUC reported) |
NA |
Very good |
Inadequate |
|
Hager, 2009 |
Very good |
NA |
Very good |
Very good |
|
Hansen, 2022 (i1 vs C) |
Very good |
NA |
Doubtful |
Doubtful |
|
Hansen, 2022 (I2 vs C) |
Very good |
NA |
Doubtful (relatively large number of missing values; four participants excluded without reported reasons) |
Doubtful |
|
Mostafa, 2020 (I2 vs C) |
Very good |
NA |
Very good |
Very good |
|
Mostafa, 2020 (I3 vs C) |
Very good |
NA |
Very good |
Very good |
|
Rogge, 2019 (I1 vs C) |
Inadequate |
NA |
Very good |
Inadequate |
|
Schuman, 2021 (I1 vs C) |
Very good |
NA |
Very good |
Very good |
|
Schuman, 2021 (I2 vs C) |
Very good |
NA |
Doubtful (relatively large number of missing values) |
Doubtful |
|
Schuman, 2021 (I3 vs C) |
Very good |
NA |
Very good |
Very good |
|
Risk or bias: Responsiveness (criterion approach (i.e. comparison to a gold standard) |
||||
|
|
For continuous scores: Were correlations between change scores, or the area under the Receiver Operator Curve (ROC) curve calculated? Very good: Correlations or AUC calculated Inadequate: Correlations or AUC not calculated Not applicable (fill box dichotomous scores) |
For dichotomous scales: Were sensitivity and specificity (changed versus not changed) determined?
Very good: Sensitivity and specificity calculated Inadequate: Sensitivity and specificity not calculated Not applicable (fill box continuous scores) |
Were there any other important flaws in the design or statistical methods of the study? Very good: No other important methodological flaws Doubtful: Other minor methodological flaws Inadequate: Other important methodological flaws |
Overall rating (lowest score) |
|
Eley, 2021 (i1 vs C) |
Inadequate |
NA |
Very good |
Inadequate |
|
Hansen, 2022 (I1 vs C) |
Inadequate |
NA |
Very good |
Inadequate |
|
Hansen, 2022 (I2 vs C) |
Inadequate |
NA |
Doubtful (relatively large number of missing values) |
Inadequate |
|
Mostafa, 2020 (I2 vs C) |
Very good |
NA |
Very good |
Very good |
|
Mostafa, 2020 (I3 vs C) |
Very good |
NA |
Very good |
Very good |
|
Rogge, 2019 (I1 vs C) |
Inadequate |
NA |
Very good |
Inadequate |
|
Schuman, 2021 (I1 vs C) |
Inadequate |
NA |
Very good |
Inadequate |
|
Schuman, 2021 (I2 vs C) |
Inadequate |
NA |
Doubtful (relatively large number of missing values) |
Inadequate |
|
Schuman, 2021 (I3 vs C) |
Inadequate |
NA |
Very good |
Inadequate |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Anast N, Olejniczak M, Ingrande J, Brock-Utne J. The impact of blood pressure cuff location on the accuracy of noninvasive blood pressure measurements in obese patients: an observational study. Can J Anaesth. 2016 Mar;63(3):298-306. doi: 10.1007/s12630-015-0509-6. Epub 2015 Oct 16. PMID: 26475165. |
High risk of bias due to type of outcome for construct validity. Responsiveness and reliability not reported. |
|
Leblanc MÈ, Auclair A, Leclerc J, Bussières J, Agharazii M, Hould FS, Marceau S, Brassard P, Godbout C, Grenier A, Cloutier L, Poirier P. Blood Pressure Measurement in Severely Obese Patients: Validation of the Forearm Approach in Different Arm Positions. Am J Hypertens. 2019 Jan 15;32(2):175-185. doi: 10.1093/ajh/hpy152. PMID: 30312368. |
Wrong timing of measurement (not during surgery, but in recovery room) |
|
Rogge DE, Nicklas JY, Haas SA, Reuter DA, Saugel B. Continuous Noninvasive Arterial Pressure Monitoring Using the Vascular Unloading Technique (CNAP System) in Obese Patients During Laparoscopic Bariatric Operations. Anesth Analg. 2018 Feb;126(2):454-463. doi: 10.1213/ANE.0000000000002660. PMID: 29261549. |
Wrong construct: mean blood pressure over period of time. |
|
Tobias JD, McKee C, Herz D, Teich S, Sohner P, Rice J, Barry N, Michalsky M. Accuracy of the CNAP™ monitor, a noninvasive continuous blood pressure device, in providing beat-to-beat blood pressure measurements during bariatric surgery in severely obese adolescents and young adults. J Anesth. 2014 Dec;28(6):861-5. doi: 10.1007/s00540-014-1835-5. Epub 2014 May 1. PMID: 24789660. |
No individual results for fingercuff, upper and lower arm reported |
|
Verkhovsky, A., Smit, M., Levin, A., & Coetzee, J. F. (2018). Blood pressure measurement in obese patients: non-invasive proximal forearm versus direct intra-arterial measurements. Southern African Journal of Anaesthesia and Analgesia, 24(3), 70-74. |
High risk of bias due to type of outcome for construct validity. Responsiveness and reliability not reported. |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 15-04-2025
Beoordeeld op geldigheid : 09-04-2025
Algemene gegevens
De ontwikkeling van deze richtlijn werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS).
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijn.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijn is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de anesthesiologische zorg voor patiënten met obesitas.
Werkgroep
Dr. T.A. Brouwer, anesthesioloog, NVA
Drs. M.A.M. Siepel, anesthesioloog, NVA
Drs. A.D. Pot, anesthesioloog, NVA
Drs. S.D.X. Oei, AIOS anesthesiologie, NVA (vanaf september 2022)
Dr. B. Torensma, PhD, anesthesiemedewerker, NVAM
Drs. J.A. Apers, chirurg, NVvH
Dr. L. Freeman, gynaecoloog, NVOG (tot oktober 2022)
Dr. D.D.C.A. Henriquez, gynaecoloog, NVOG (vanaf oktober 2022)
Prof. dr. C. A. J. Knibbe, ziekenhuisapotheker, NVZA
Drs. M.A. Damhof, ziekenhuisapotheker, NVZA (vanaf december 2023)
Dr. H.J. Reesink, longarts, NVALT
Drs. H.L. Lutgers, internist-endocrinoloog, NIV (vanaf april 2023)
N.G. Cnossen, patiëntvertegenwoordiger, Nederlandse Stichting Over Gewicht
Klankbordgroep
Dr. M. Klemt-Kropp, MDL-arts, NVMDL
Drs. Ö. Engin, oogarts, NOG
Dr. H. Buter, internist-intensivist, NVIC
N.J.C. Raeijmaekers, BSc, diëtist, NVD/NDBC
Met ondersteuning van
Drs. I. van Dusseldorp, literatuurspecialist, Kennisinstituut van de Federatie van Medisch Specialisten
Dr. J.C. Maas, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
Drs. I. van Dijk, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Tammo Brouwer (voorzitter) |
Anesthesioloog, vrij ondernemer. |
Geen |
Geen |
Geen restricties |
|
Alice Dorien Pot |
Anesthesioloog in Meander Medisch Centrum Amersfoort |
Co-auteur boek 'klinische kinderanesthesiologie in de praktijk', initiatief vanuit NVA, uitgeverij Prelum (onbetaald). |
Geen |
Geen restricties |
|
Bart Torensma |
Klinisch epidemioloog en data scientist via eigen bedrijf Torensma Research Consultancy BV. |
"Dagelijks bestuurslid (penningmeester) Stichting COREON Algemeen bestuurslid Vereniging voor epidemiologen. Lesgeven aan de research verpleegkundige Breederode hogeschool Rotterdam
Masterclass gemaakt samen met Medtronic AUE over ERABS en team samenwerkingen." |
Voor PhD (<3 jaar geleden) onderzoek gedaan naar deep block verslapping bij patiënten met obesitas. Deze studie is destijds door MSD gesponsord in de vorm van gratis ampullen sugammadex.
|
Geen restricties |
|
Catherijne A.J. Knibbe |
Ziekenhuisapotheker-klinisch farmacoloog, St. Antonius ziekenhuis Nieuwegein en Utrecht, afdeling Klinische farmacie |
Lid CCMO, Lid Board ACCP |
Diverse studies gefinancierd door ZonMw of Antonius Onderzoeksfonds. De onderzoeken zijn niet gerelateerd aan de richtlijn. |
Geen restricties |
|
Dacia Henriquez |
Gynaecoloog, Amphia Ziekenhuis |
Geen |
Geen |
Geen restricties |
|
Helen Lutgers |
Internist in Ommelander Ziekenhuis Groningen |
Geen |
Geen |
Geen restricties |
|
Herre Reesink |
Longarts, OLVG Amsterdam |
Geen |
Geen |
Geen restricties |
|
Jan Apers |
Chirurg Franciscus Gasthuis & Vlietland |
NVGIC bestuur Dutch Obesity Academy |
Geen |
Geen restricties |
|
Liv Freeman |
Gynaecoloog Ikazia Ziekenhuis Rotterdam |
Voorzitter samenwerking obstetrie anesthesie (onbetaald)
|
Geen |
Geen restricties |
|
Michiel Damhof
|
Ziekenhuisapotheker Medisch Centrum Leeuwarden |
Geen |
Geen |
Geen restricties |
|
Muriel Arianne Michelle Siepel |
Anesthesioloog OLVG Amsterdam |
Geen |
Geen |
Geen restricties |
|
Nienke Cnossen |
Patiëntvertegenwoordiger |
Geen |
Geen |
Geen restricties |
|
Sander Oei |
Aios anesthesiologie. ErasmusMC |
SITdiensten Park Medisch Centrum, betaald. 1-2x/mnd ANWdiensten verpleeghuizen, betaald. 1x/mnd |
Geen |
Geen restricties |
|
Hanneke Buter |
Intensivist |
bestuurslid NVIC |
Geen |
Geen restricties |
|
Michael Klemt-Kropp |
MDL-arts |
Geen |
Geen |
Geen restricties |
|
Natascha Raeijmaekers |
Diëtist Obesitas Centrum, ETZ. Betaald. |
Bestuur Netwerk Diëtisten Bariatrische chirurgie, onbetaald. |
Geen |
Geen restricties |
|
Ozlem Engin |
Oogarts |
Geen |
Geen |
Geen restricties |
|
Irma van Dijk |
Adviseur kennisinstituut van de Federatie Medisch Specialisten |
Geen |
Geen |
Geen restricties |
|
José Maas |
Adviseur kennisinstituut van de Federatie Medisch specialisten |
Geen |
Geen |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door het uitnodigen van een afvaardiging van de Nederlandse ‘Stichting ‘Overgewicht’ in de werkgroep. Het verslag van de knelpunteninventarisatie [zie aanverwante producten] is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Stichting en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijn op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Module |
Uitkomst raming |
Toelichting |
|
Module Bloeddruk meten |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijn is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de anesthesiologische zorg voor patiënten met obesitas. Tevens zijn er knelpunten aangedragen door relevante partijen via een schriftelijke knelpunteninventarisatie. Een verslag hiervan is opgenomen onder aanverwante producten.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effect model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijn is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijn werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijn aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijn werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy
|
Richtlijn: NVA anesthesie bij obesitas |
|
|
Uitgangsvraag: Hoe dient de bloeddruk gemeten te worden bij patiënten met obesitas die een chirurgisch ingreep ondergaan |
|
|
Database(s): Ovid/Medline, Embase |
Datum: 3-3-2023 |
|
Periode: 1990- |
Talen: nvt |
|
Literatuurspecialist: Ingeborg van Dusseldorp |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: Voor deze vraag is gezocht met de volgende concepten: Obesitas EN anesthesie of chirurgie EN bloeddruk meten EN volwassenen Het sleutelartikel wordt gevonden. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
29 |
7 |
32 |
|
RCTs, clinical trials |
163 |
38 |
180 |
|
Observationele studies |
271 |
97 |
304 |
|
Klinimetrisch |
21 |
30 |
39 |
|
Totaal |
|
|
555 |
Zoekstrategie
Embase
|
No. |
Query |
Results |
|
#21 |
#11 AND #20 sleutelartikel gevonden |
1 |
|
#20 |
#13 OR #14 OR #15 OR #16 |
484 |
|
#19 |
#16 NOT #15 NOT #14 NOT #13 klinimetrie |
21 |
|
#18 |
#15 NOT #14 NOT #13 OBS |
271 |
|
#17 |
#14 NOT #13 Clinical trials, RCT |
163 |
|
#16 |
#9 AND #12 |
159 |
|
#15 |
(#7 OR #8) AND #12 |
428 |
|
#14 |
#6 AND #12 |
173 |
|
#13 |
#5 AND #12 SR |
29 |
|
#12 |
#10 NOT (('adolescent'/exp OR 'child'/exp OR adolescent*:ti,ab,kw OR child*:ti,ab,kw OR schoolchild*:ti,ab,kw OR infant*:ti,ab,kw OR girl*:ti,ab,kw OR boy*:ti,ab,kw OR teen:ti,ab,kw OR teens:ti,ab,kw OR teenager*:ti,ab,kw OR youth*:ti,ab,kw OR pediatr*:ti,ab,kw OR paediatr*:ti,ab,kw OR puber*:ti,ab,kw) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp OR adult*:ti,ab,kw OR man:ti,ab,kw OR men:ti,ab,kw OR woman:ti,ab,kw OR women:ti,ab,kw)) |
586 |
|
#11 |
'intraoperative blood pressure monitoring in obese patients' |
1 |
|
#10 |
#4 AND [1-1-1990]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
622 |
|
#9 |
'intermethod comparison'/exp OR 'data collection method'/exp OR 'validation study'/exp OR 'feasibility study'/exp OR 'pilot study'/exp OR 'psychometry'/exp OR 'reproducibility'/exp OR reproducib*:ab,ti OR 'audit':ab,ti OR psychometr*:ab,ti OR clinimetr*:ab,ti OR clinometr*:ab,ti OR 'observer variation'/exp OR 'observer variation':ab,ti OR 'discriminant analysis'/exp OR 'validity'/exp OR reliab*:ab,ti OR valid*:ab,ti OR 'coefficient':ab,ti OR 'internal consistency':ab,ti OR (cronbach*:ab,ti AND ('alpha':ab,ti OR 'alphas':ab,ti)) OR 'item correlation':ab,ti OR 'item correlations':ab,ti OR 'item selection':ab,ti OR 'item selections':ab,ti OR 'item reduction':ab,ti OR 'item reductions':ab,ti OR 'agreement':ab,ti OR 'precision':ab,ti OR 'imprecision':ab,ti OR 'precise values':ab,ti OR 'test-retest':ab,ti OR ('test':ab,ti AND 'retest':ab,ti) OR (reliab*:ab,ti AND ('test':ab,ti OR 'retest':ab,ti)) OR 'stability':ab,ti OR 'interrater':ab,ti OR 'inter-rater':ab,ti OR 'intrarater':ab,ti OR 'intra-rater':ab,ti OR 'intertester':ab,ti OR 'inter-tester':ab,ti OR 'intratester':ab,ti OR 'interobeserver':ab,ti OR 'inter-observer':ab,ti OR 'intraobserver':ab,ti OR 'intertechnician':ab,ti OR 'inter-technician':ab,ti OR 'intratechnician':ab,ti OR 'interexaminer':ab,ti OR 'inter-examiner':ab,ti OR 'intraexaminer':ab,ti OR 'interassay':ab,ti OR 'inter-assay':ab,ti OR 'intraassay':ab,ti OR 'intra-assay':ab,ti OR 'interindividual':ab,ti OR 'inter-individual':ab,ti OR 'intraindividual':ab,ti OR 'intra-individual':ab,ti OR 'interparticipant':ab,ti OR 'inter-participant':ab,ti OR 'intraparticipant':ab,ti OR 'kappa':ab,ti OR 'kappas':ab,ti OR 'coefficient of variation':ab,ti OR repeatab*:ab,ti OR ((replicab*:ab,ti OR 'repeated':ab,ti) AND ('measure':ab,ti OR 'measures':ab,ti OR 'findings':ab,ti OR 'result':ab,ti OR 'results':ab,ti OR 'test':ab,ti OR 'tests':ab,ti)) OR generaliza*:ab,ti OR generalisa*:ab,ti OR 'concordance':ab,ti OR ('intraclass':ab,ti AND correlation*:ab,ti) OR 'discriminative':ab,ti OR 'known group':ab,ti OR 'factor analysis':ab,ti OR 'factor analyses':ab,ti OR 'factor structure':ab,ti OR 'factor structures':ab,ti OR 'dimensionality':ab,ti OR subscale*:ab,ti OR 'multitrait scaling analysis':ab,ti OR 'multitrait scaling analyses':ab,ti OR 'item discriminant':ab,ti OR 'interscale correlation':ab,ti OR 'interscale correlations':ab,ti OR (('error':ab,ti OR 'errors':ab,ti) AND (measure*:ab,ti OR correlat*:ab,ti OR evaluat*:ab,ti OR 'accuracy':ab,ti OR 'accurate':ab,ti OR 'precision':ab,ti OR 'mean':ab,ti)) OR 'individual variability':ab,ti OR 'interval variability':ab,ti OR 'rate variability':ab,ti OR 'variability analysis':ab,ti OR ('uncertainty':ab,ti AND ('measurement':ab,ti OR 'measuring':ab,ti)) OR 'standard error of measurement':ab,ti OR sensitiv*:ab,ti OR responsive*:ab,ti OR ('limit':ab,ti AND 'detection':ab,ti) OR 'minimal detectable concentration':ab,ti OR interpretab*:ab,ti OR (small*:ab,ti AND ('real':ab,ti OR 'detectable':ab,ti) AND ('change':ab,ti OR 'difference':ab,ti)) OR 'meaningful change':ab,ti OR 'minimal important change':ab,ti OR 'minimal important difference':ab,ti OR 'minimally important change':ab,ti OR 'minimally important difference':ab,ti OR 'minimal detectable change':ab,ti OR 'minimal detectable difference':ab,ti OR 'minimally detectable change':ab,ti OR 'minimally detectable difference':ab,ti OR 'minimal real change':ab,ti OR 'minimal real difference':ab,ti OR 'minimally real change':ab,ti OR 'minimally real difference':ab,ti OR 'ceiling effect':ab,ti OR 'floor effect':ab,ti OR 'item response model':ab,ti OR 'irt':ab,ti OR 'rasch':ab,ti OR 'differential item functioning':ab,ti OR 'dif':ab,ti OR 'computer adaptive testing':ab,ti OR 'item bank':ab,ti OR 'cross-cultural equivalence':ab,ti |
7587026 |
|
#8 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
13881736 |
|
#7 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6767914 |
|
#6 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
3302394 |
|
#5 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#4 |
#1 AND #2 AND #3 |
1279 |
|
#3 |
'blood pressure monitoring'/exp OR 'blood pressure measurement'/exp OR (('blood pressure' NEAR/3 (monitor* OR measur* OR record* OR determinat*)):ti,ab,kw) |
144750 |
|
#2 |
'anesthesia'/exp OR 'anesthetic agent'/exp OR (((general OR inhalat* OR rectal) NEAR/3 (anesthe* OR anesthe*)):ti,ab,kw) OR 'surgery'/exp OR 'surgical patient'/exp OR 'surgical risk'/exp OR 'perioperative period'/exp OR surgic*:ti,kw OR surger*:ti,ab,kw OR operation*:ti,ab,kw OR operative:ti,ab,kw OR perisurg*:ti,ab,kw OR perioperati*:ti,ab,kw OR intraoperati*:ti,ab,kw |
7517730 |
|
#1 |
'obesity'/exp/mj OR 'obese patient'/exp OR obes*:ti,ab,kw OR obesit*:ti,ab,kw OR overweight*:ti,ab,kw OR 'over weight*':ti,ab,kw OR 'adipositas':ti,ab,kw OR 'adiposity':ti,ab,kw OR 'excess body weight':ti,ab,kw OR 'corpulen*':ti,ab,kw OR 'fat overload syndrome':ti,ab,kw OR 'increased bmi':ti,ab,kw OR bariatr*:ti,ab,kw |
671322 |
Ovid/Medline
|
# |
Searches |
Results |
|
19 |
16 not 15 not 14 not 13 Klinimetrie |
30 |
|
18 |
15 not 14 not 13 OBS |
97 |
|
17 |
14 not 13 RCT, clinical trials |
38 |
|
16 |
11 and 12 |
123 |
|
15 |
(9 or 10) and 12 |
133 |
|
14 |
8 and 12 |
41 |
|
13 |
7 and 12 SR |
7 |
|
12 |
6 not ((Adolescent/ or Child/ or Infant/ or adolescen*.ti,ab,kf. or child*.ti,ab,kf. or schoolchild*.ti,ab,kf. or infant*.ti,ab,kf. or girl*.ti,ab,kf. or boy*.ti,ab,kf. or teen.ti,ab,kf. or teens.ti,ab,kf. or teenager*.ti,ab,kf. or youth*.ti,ab,kf. or pediatr*.ti,ab,kf. or paediatr*.ti,ab,kf. or puber*.ti,ab,kf.) not (Adult/ or adult*.ti,ab,kf. or man.ti,ab,kf. or men.ti,ab,kf. or woman.ti,ab,kf. or women.ti,ab,kf.)) |
202 |
|
11 |
(instrumentation or methods).fs. or Validation Study/ or Comparative Study/ or exp Psychometrics/ or psychometr*.ti,ab,kf. or clinimetr*.mp. or clinometr*.mp. or exp Outcome Assessment, Health Care/ or outcome assessment.ti,ab,kf. or outcome measure*.mp. or exp Observer Variation/ or observer variation.ti,ab,kf. or exp Health Status Indicators/ or exp "reproducibility of results"/ or reproducib*.ti,ab,kf. or exp Discriminant Analysis/ or reliab*.ti,ab,kf. or unreliab*.ti,ab,kf. or valid*.ti,ab,kf. or coefficient.ti,ab,kf. or homogeneity.ti,ab,kf. or homogeneous.ti,ab,kf. or internal consistency.ti,ab,kf. or (cronbach* and (alpha or alphas)).ti,ab,kf. or (item and (correlation* or selection* or reduction*)).ti,ab,kf. or agreement.mp. or precision.mp. or imprecision.mp. or precise values.mp. or test-retest.ti,ab,kf. or (test and retest).ti,ab,kf. or (reliab* and (test or retest)).ti,ab,kf. or stability.ti,ab,kf. or interrater.ti,ab,kf. or inter-rater.ti,ab,kf. or intrarater.ti,ab,kf. or intra-rater.ti,ab,kf. or intertester.ti,ab,kf. or inter-tester.ti,ab,kf. or intratester.ti,ab,kf. or intra-tester.ti,ab,kf. or interobserver.ti,ab,kf. or inter-observer.ti,ab,kf. or intraobserver.ti,ab,kf. or intra-observer.ti,ab,kf. or intertechnician.ti,ab,kf. or inter-technician.ti,ab,kf. or intratechnician.ti,ab,kf. or intra-technician.ti,ab,kf. or interexaminer.ti,ab,kf. or inter-examiner.ti,ab,kf. or intraexaminer.ti,ab,kf. or intra-examiner.ti,ab,kf. or interassay.ti,ab,kf. or inter-assay.ti,ab,kf. or intraassay.ti,ab,kf. or intra-assay.ti,ab,kf. or interindividual.ti,ab,kf. or inter-individual.ti,ab,kf. or intraindividual.ti,ab,kf. or intra-individual.ti,ab,kf. or interparticipant.ti,ab,kf. or inter-participant.ti,ab,kf. or intraparticipant.ti,ab,kf. or intra-participant.ti,ab,kf. or kappa.ti,ab,kf. or kappa's.ti,ab,kf. or kappas.ti,ab,kf. or repeatab*.mp. or ((replicab* or repeated) and (measure or measures or findings or result or results or test or tests)).mp. or generaliza*.ti,ab,kf. or generalisa*.ti,ab,kf. or concordance.ti,ab,kf. or (intraclass and correlation*).ti,ab,kf. or discriminative.ti,ab,kf. or known group.ti,ab,kf. or factor analysis.ti,ab,kf. or factor analyses.ti,ab,kf. or factor structure.ti,ab,kf. or factor structures.ti,ab,kf. or dimension*.ti,ab,kf. or subscale*.ti,ab,kf. or (multitrait and scaling and (analysis or analyses)).ti,ab,kf. or item discriminant.ti,ab,kf. or interscale correlation*.ti,ab,kf. or error.ti,ab,kf. or errors.ti,ab,kf. or individual variability.ti,ab,kf. or (variability and (analysis or values)).ti,ab,kf. or (uncertainty and (measurement or measuring)).ti,ab,kf. or standard error of measurement.ti,ab,kf. or sensitiv*.ti,ab,kf. or responsive*.ti,ab,kf. or (limit and detection).ti,ab,kf. or minimal detectable concentration.ti,ab,kf. or interpretab*.ti,ab,kf. or ((minimal or minimally or clinical or clinically) and (important or significant or detectable) and (change or difference)).ti,ab,kf. or (small* and (real or detectable) and (change or difference)).ti,ab,kf. or meaningful change.ti,ab,kf. or ceiling effect.ti,ab,kf. or floor effect.ti,ab,kf. or item response model.ti,ab,kf. or irt.ti,ab,kf. or rasch.ti,ab,kf. or differential item functioning.ti,ab,kf. or dif.ti,ab,kf. or computer adaptive testing.ti,ab,kf. or item bank.ti,ab,kf. or cross-cultural equivalence.ti,ab,kf. |
11145406 |
|
10 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5370146 |
|
9 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4379916 |
|
8 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2560797 |
|
7 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
652795 |
|
6 |
5 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
220 |
|
5 |
limit 4 to yr="1990 -Current" |
253 |
|
4 |
1 and 2 and 3 |
263 |
|
3 |
exp Blood Pressure Determination/ or (blood pressure adj3 (monitor* or measur* or record* or determinat*)).ti,ab,kf. |
74708 |
|
2 |
Anesthetics/ or Anesthetics, Combined/ or Anesthetics, General/ or anesthe*.ti,ab,kf. or anaesthe*.ti,ab,kf. or Anesthesia/ or Anesthesia, Conduction/ or Anesthesia, General/ or Anesthesia, Intravenous/ or Anesthesia, Obstetrical/ or Anesthesia, Cardiac Procedures/ or exp Surgical Procedures, Operative/ or exp Specialties, Surgical/ or su.fs. or exp Perioperative Period/ or surgic*.ti,ab,kf. or surger*.ti,ab,kf. or operation*.ti,ab,kf. or operative.ti,ab,kf. or presurg*.ti,ab,kf. or preoperati*.ti,ab,kf. or pre-surg*.ti,ab,kf. or pre-operati*.ti,ab,kf. or perisurg*.ti,ab,kf. or perioperati*.ti,ab,kf. or peri-surg*.ti,ab,kf. or peri-operati*.ti,ab,kf. or postsurg*.ti,ab,kf. or postoperati*.ti,ab,kf. or post-surg*.ti,ab,kf. or post-operati*.ti,ab,kf. or nonsurg*.ti,ab,kf. or nonoperatic*.ti,ab,kf. or non-surg*.ti,ab,kf. or non-operati*.ti,ab,kf. or laparoscop*.ti,ab,kf. |
5696229 |
|
1 |
exp Obesity/ or obese*.ti,ab,kf. or obesit*.ti,ab,kf. or overweight*.ti,ab,kf. or 'over weight*'.ti,ab,kf. |
442687 |