Risicostratificatie en stratificatietools
Uitgangsvraag
Hoe kunnen patiënten met een hoog risico op post-contrast acute nierschade (PC-AKI) bij toediening van intravasculair jodiumhoudend contrastmedium (CM) worden geïdentificeerd?
Subvragen
- Wat is het risico op PC-AKI bij patiënten die jodiumhoudend contrast toegediend krijgen, vergeleken bij patienten die geen contrast krijgen toegediend?
- Welke risicofactoren voor PC-AKI kunnen worden geïdentificeerd bij patiënten die een beeldvormend onderzoek met jodiumhoudend contrast ondergaan?
- Hoe dient er rekening te worden gehouden met een niertransplantatie bij het inschatten van het risico op PC-AKI?
- Hoe dient er rekening te worden gehouden met een solitaire nier bij het inschatten van het risico op PC-AKI?
- Hoe dient er rekening te worden gehouden met de osmolaliteit van het jodiumhoudend contrastmiddel bij het inschatten van het risico op PC-AKI?
- Wat is de rol van vragenlijsten en voorspellingsmodellen bij het inschatten van het risico op PC-AKI?
Aanbeveling
Voor patiënten die intravasculaire jodiumhoudend CM-toediening ondergaan:
Beschouw patiënten met een eGFR <30 ml/min/1,73m2 behorende tot een hoog-risico groep voor PC-AKI.
Consulteer een internist/nefroloog voor patiënten met een eGFR <30 ml/min/1,73m2.
Pas dezelfde aanbevelingen toe bij patiënten met een niertransplantatie of een mononier als bij patiënten met bilaterale nieren die jodiumhoudend CM krijgen toegediend.
Beschouw het risico van PC-AKI vergelijkbaar bij laagosmolaire jodiumhoudend CM en iso-osmolaire jodiumhoudend CM wanneer deze intravasculair worden geïnjecteerd.
Optimale nefrologische zorg dient het primaire doel te zijn bij alle patiënten met chronische nierziekten, met specifieke aandacht voor hydratietoestand en medicatiegebruik.
Overweeg alternatieve beeldvorming zonder jodiumhoudend CM bij alle patiënten met een verhoogd risico op PC-AKI.
Streef naar klinische euvolemie voorafgaand aan een onderzoek met intravasculair jodiumhoudend CM.
Gebruik geen vragenlijsten en predictiemodellen om het risico van PC-AKI te schatten, omdat de validiteit en het effect hiervan op de klinische uitkomst onduidelijk is.
Overwegingen
1 Risk factors for PC-AKI
Exposure of intravascular iodine-containing contrast media has been associated with the development of PC-AKI. Low- or iso-osmolar contrast medium (LOCM or IOCM) is used for all intravascular CM administration. There is controversy regarding the causal relation between intravascular CM and PC-AKI, since prospective controlled trials are lacking. Moreover, most prospective studies of PC-AKI included patients undergoing coronary angiography or percutaneous coronary intervention. There are several important differences that separate procedures with IA from IV CM administration. First, athero-emboli and hemodynamic instability during cardiac angiography may cause procedure-related AKI. Second, the cardiac angiography studies thus far lacked a matched control group, and can therefore not discriminate between AKI and PC-AKI. Third, the effect of the concentrated intra-arterial CM bolus given via a catheter may not be generalized to typical IV injections.
In our literature summary we have chosen not to focus on the identification of risk factors that are associated with an increased risk of PC-AKI on top of impaired kidney function, but rather on factors that are associated with a reduction of PC-AKI risk when these patient groups receive hydration. Studies that have described risk factors for PC-AKI have been extracted from the first literature search. Although many factors have been shown to be associated with risk of PC-AKI, it is unclear whether hydration of patients will actually reduce their PC-AKI risk.
2 to 4 Risk stratification for PC-AKI
The most important methodological limitations regarding observational studies with IV CM is that these studies are not controlled by randomization. For this reason, two large observational studies used PS-matching to compare contrast-enhanced computed tomographic (CT) scan recipients and clinically similar patients who underwent an unenhanced CT scan. Davenport et al showed in a 10-year propensity score-matched retrospective study, including 20,242 hospitalised patients with a stable kidney function, that patients with an eGFR <30 ml/min/1.73m2 had a 3-fold increased risk of PC-AKI compared to patients without LOCM enhanced CT (Davenport, 2013b). A limitation of this study is that the risk of PC-AKI was assessed solely in inpatients and that the initial PS-model did not include hydration status. Inpatients are probably older, have a lower eGFR and are at higher risk for AKI than the general population. McDonald, 2015 showed in a 10-year PS-matched retrospective study, including about 12,500 predominantly hospitalised patients with an eGFR ≥30 ml/min/1.73m2, no evidence of risk of PC-AKI (McDonald, 2014). The risk of AKI following CT examinations, with or without LOCM, was increased in patients with an eGFR <30 ml/min/1.73m2. In addition, IV LOCM was not related to excess risk of dialysis or death (McDonald, 2014; McDonald, 2015). In contrast to the study of Davenport, where a single PS model was applied to the entire cohort, the findings of McDonald were derived from propensity scores generated for each distinct CKD group. AKI rates ranged from 1% in the group with eGFR >90 ml/min/1.73m2 to 14% in the group with eGFR <30 ml/min/1.73m2. A limitation of the studies of McDonald’s is that due to the non-randomized design only known confounders were included in their PS-model and unmeasured confounders may have affected the results. In particular, patients who received CM are more likely to have received intravenous hydration or other preventive measures compared with patients who underwent unenhanced CT. In addition, patients who were administered potentially nephrotoxic medications at the time of scanning or who had severe renal impairment may have been less likely to receive CM.
In the Saliña-trial, Kooiman showed in 570 CKD patients that ultra-short hydration with sodium bicarbonate prior to IV CM enhanced CT was non-inferior to peri-procedural saline hydration with respect to risk of PC-AKI. This outcome may result in healthcare savings in The Netherlands (Kooiman, 2014a). Kooiman also studied the risk of PC-AKI in another RCT (Nefros-trial): no hydration vs. sodium bicarbonate hydration (250 ml 1h before CT) in 139 patients with eGFR <60 ml/min/1.73m2) undergoing CT-pulmonary angiography. The Nefros-trial showed no difference in risk of PC-AKI and need of dialysis between both groups. These results suggest that pre-hydration can be safely withheld in CKD patients exposed to IV CM for CT (Kooiman, 2014b).
Apart from preventive hydration, patients should receive adequate volume replacement therapy (with normal saline or Ringer’s lactate) if they have clinical signs of hypovolemia, i.e. hypotension, tachycardia, oliguria and / or loss of renal function.
5 Risk models or tools for stratification of patient risk
Prediction models which give an accurate estimated risk of developing PC-AKI are of great value and benefit in clinical decision making (Davenport, 2013a). The development of risk prediction models cumulating in prediction models is not a new phenomenon (Davenport, 2013b). The continuing need for these models comes from need of clinicians for easy targeting patients who have a high risk for developing PC-AKI and thus zeroing of preventive measures for those patients not at risk.
A risk prediction model should undergo three analytical phases before putting it in use:
First phase: The risk score or algorithm should be derived from a study that clearly defined its endpoint of interest and that was conducted in a well-defined population.
Second phase: External validation, this should take place in several independent populations.
Third phase: Verification whether the prediction model improves clinical outcome.
The questionnaires that are nowadays in use outside the Netherlands cannot be considered highly valid, since these tools perform poorly when validated externally, and studies verifying whether the application of the prediction model improved clinical outcome are lacking. Web-based tools and apps derived from these questionnaires have the same low level of evidence.
A promising novel tool has been advocated by Gurm (Lenhard, 2013). This web-based and easy to use risk prediction algorithm may prove useful for both bedside clinical decision making. (Link: https://bmc2.org/calculators/cin) A limitation of this tool is that it is primarily focused on patients undergoing PCI procedures, since it was derived from this specific patient population.
Considering all these factors, the Working Group recommends the future development of an easy to use robust tool, which can be used in all cases where iodine-containing contrast is used in patients. Such a tool must be preferably usable in a bedside manner; therefore a web-based or app solution would be optimal.
Patients with a kidney transplantation and risk of PC-AKI
Given the limited information available in literature, it is unclear whether kidney transplantation patients have an increased risk of PC-AKI and whether hydration of these patients will decrease this risk. Therefore, the Working Group advises to apply the same preventive measures to reduce the risk of PC-AKI in kidney transplantation patient.
Solitary kidney and risk of PC-AKI
According to the Working Group, patients with a solitary kidney do not have an increased risk of PC-AKI and thus recommends that this patient group should be evaluated for PC-AKI in a similar way as patients with bilateral kidneys.
Dialysis patients with residual-diuresis of at least 100 ml/24h
There is no literature available with regard to protection of residual-diuresis in dialysis patients after exposure with iodine-containing CM. Since a residual-diuresis of >100 ml/24h is important for the quality of life, the Working Group recommends to strive for euvolemia before performing any CM-enhanced radiographic investigation in dialysis patients.
Contrast medium dose and risk of PC-AKI
For intravenous iodine-containing CM administration there is no upper dose limit above which the risk of PC-AKI is increased. Nevertheless, the CM dose should be as low as reasonable achievable for a diagnostic study. In modern CT imaging at 70-100 kVp may be used effectively to lower the CM volume (compared to 120 kVp, a reduction of 20-25% at 100 kVp, and 40-50% at 70-80 kVp is feasible).
For intra-arterial iodine-containing CM administration, and especially for interventional procedures, the CM dose with regard to PC-AKI is critical above a certain level. It has been advocated by Nyman et al. to use the absolute eGFR that is corrected for body surface area (see also chapter 5) and that the risk of PC-AKI is limited when the administered iodine dose (in gram iodine) to eGFR ratio remains below 1.1 (Nyman, 2008). In the cardiology literature Gurm et al. indicate that the risk of PC-AKI is increased above a CM volume to creatinine clearance (or eGFR) ratio of 3.0. This corresponds at a cut-off level of eGFR 45 ml/min/1.73m2 to a CM volume of 135ml.
The Working Group suggests considering the use of these ratios, especially in intra-arterial CM administration with first pass renal exposure. See for explanation Table 1 in Appendix below.
According to the Working Group expert opinion hydration is not indicated in hemodynamic stable or euvolemic patients when a low (<30 ml) volume of intra-arterial iodine-containing CM is administered, e.g. for shunt angiography in patients on haemodialysis.
Iodine-containing CM osmolality and risk of PC-AKI
The literature contains conflicting reports about whether IOCM is associated with less risk for AKI than LOCM. The available studies have several limitations. About 7 different LOCM are considered as a group in comparison with one IOCM. Studies generally provided little detail about clinical indications for the diagnostic or therapeutic procedures or other clinical details, such as the severity of the renal impairment, comorbidity, total contrast volume, length of procedure, and contrast injection rates. Studies had to report the incidence of AKI based on serum creatinine levels at baseline and within 72 hours of contrast injection. A more objective picture will be obtained if secondary end points would be evaluated. Relevant secondary end points are the proportion of patients who required specific treatment for acute renal failure, who required dialysis, or who died of acute renal failure at 1 month.
IOCM is isotonic to plasma, but with a much higher viscosity than the LOCM. In animal studies it has been shown that renal iodine-containing CM concentration was increased for IOCM and retention was prolonged 24 hours post injection compared with LOCM injection. Also, enhanced expression of kidney injury markers was found after IOCM injection. These effects were strengthened by severely impaired renal function. Liss et al described in 2006 a higher risk of PC-AKI in patients after IOCM injection in comparison with LOCM injection (Liss, 2006).
The data are further confirmed by a recent propensity score study by McDonald et al. in which 5,758 patients (1538 with stage 1-2 CKD, 2899 with stage 3 CKD, and 1321 with stage 4-5 CKD) were included. After propensity score adjustment, rates of AKI, dialysis, and mortality were not significantly higher in the IOCM group compared with the non-contrast group for all CKD subgroups (AKI odds ratios [ORs], 0.74-0.91, P = .16-0.69; dialysis ORs, 0.74-2.00, P = .42-.76; mortality ORs, 0.98-1.24, P = .39-.88). Sensitivity analyses yielded similar results (McDonald, 2017).
Risks and costs of preventive hydration
From the patients’ perspective it is important to notice that hydration with 1L saline pre- and post-iodine-containing CM can harm an individual patient and cause acute heart failure.
Finally, the annual healthcare costs for preventive hydration defined by the CBO 2007 guideline are estimated to be 60 million euros. These costs are substantial, especially when considering that the clinical relevance of PC-AKI is still under debate.
In summary, IV administered iodine-containing CM is most likely a weak independent nephrotoxic risk factor in patients with stable eGFR of less than 30 ml/min/1.73m2, for which hydration might be needed to prevent PC-AKI. Intravenous CM does not appear to be a risk factor in patients with stable eGFR between 30 and 60 ml/min/1.73m2.
When iodine-containing CM is administrated intra-arterially, it is most likely an independent risk factor for PC-AKI in patients with stable eGFR of less than 30 ml/min/1.73m2, therefore hydration is needed to prevent PC-AKI.
Appendix: A little help for interpretation of contrast enhanced CT studies
The most relevant CM injection parameter for enhancement in CT of solid organs (e.g. liver) is usually the CM Dose (in mgI) which is equivalent to CM volume x CM concentration. Typical values range from 30,000-60,000 mgI, depending on body weight for CT at 120 kVp.
The most relevant parameter for enhancement in CT angiography or for arterial enhancement in CT of organs (e.g. liver, pancreas, adrenal glands) is the CM Iodine Delivery Rate or Iodine Flux (in mg Iodine/s), which is equivalent to CM injection rate x CM concentration. For large vessels typical values range from 1200-1500 mgI/s and for smaller vessels 1600-2000 mgI/s for CT at 120 kVp.
As noted above, because of increased signal of iodine-containing CM at lower tube voltages, a voltage of 70-100 kVp may be used effectively to lower the iodine-containing CM dose. In comparison to 120 kVp a reduction in CM volume of 20-25% at 100 kVp and 40-50% at 70-80 kVp is feasible. For the same reason low kVp imaging is also an effective way to reduce iodine loads in patients with renal impairment (Nyman, 2011).
A range of iodine-containing CM concentrations of various agents are in clinical use and Table 1 provides a help for conversion of iodine dose (in mg Iodine) to CM volume (in ml) and vice versa.
Table 1 Conversion of CM dose (in mgI) to CM volume (in ml) for CM concentrations @ 120 kVp
|
CM Dose in mgI |
CM concentration in mgI/ml |
|||||
|
|
270 |
300 |
320 |
350 |
370 |
400 |
|
5,000 |
19 |
17 |
16 |
15 |
14 |
13 |
|
10,000 |
37 |
33 |
31 |
29 |
27 |
25 |
|
20,000 |
74 |
67 |
63 |
58 |
54 |
50 |
|
30,000 |
111 |
100 |
94 |
86 |
81 |
75 |
|
45,000 |
166 |
150 |
141 |
128 |
122 |
113 |
|
60,000 |
222 |
200 |
188 |
171 |
162 |
150 |
Onderbouwing
Achtergrond
Post-contrast acute kidney injury (PC-AKI) is acute kidney injury after exposure to iodine-containing contrast medium. The Dutch Centraal Begeleidings Orgaan (CBO) 2007 guideline defined CIN (PC-AKI in this guideline) as an increase of serum creatinine of >25% or >44µmol/L within 3 to 5 days after exposure to iodine-containing contrast medium. In the CBO 2007 guideline the prediction of the risk for PC-AKI and dialysis was based on the Mehran risk-score. A risk-score of >1% for dialysis treatment was considered “high risk of PC-AKI” for which pre-hydration and post-hydration with 1L NaCl 0.9% are indicated. The CBO 2007 guideline has been implemented in the Safety-Management-System of the Hospitals in The Netherlands.
Recent studies show a much lower risk of PC-AKI and need for dialysis treatment after exposure to iodine-containing contrast media. Most likely, incidence and severity of PC-AKI have been overestimated by previous uncontrolled studies. All instances of AKI after iodine-containing contrast media administration were ascribed to PC-AKI, even though there are many other causes of AKI. Therefore, we explored from recent studies the risk of PC-AKI in patients scheduled for intravenous or intra-arterial iodine-containing CM-enhanced procedures.
Optimal Nephrology Care
In addition to prevention of PC-AKI, optimal nephrology care is important to prevent AKI in patients with impaired renal function. Currently, end stage renal disease (ESRD) is most often caused by atherosclerotic vascular disease, hypertension and type 2 diabetes. The goal in patients with chronic kidney disease (CKD) stage 3 to 5 (non-dialysis) is to slow down deterioration of renal function and prevent or postpone cardiovascular morbidity and mortality. According to the guideline Care of the Patient with Chronic Renal Damage (2009) of the Dutch Federation of Nephrology (NFN), the following advices for optimal nephrology care are relevant for the present guideline: avoid nephrotoxic medications, avoid dehydration and hypovolemia, and refer patients with eGFR <30 ml/min/1.73m2 to a nephrologist.
Conclusies
Risk Factor analysis
|
|
There are no studies that identified risk factors for PC-AKI that can reliably discriminate between risk of AKI and PC-AKI. |
|
Low GRADE |
There is a low level of evidence that the risk of PC-AKI was similar in patients who underwent CT-scans with intravenous iodine-containing contrast and those who underwent CT-scans without intravenous contrast.
(Bruce, 2009; McDonald, 2013) |
|
Low GRADE |
The following risk factors for the development of PC-AKI were consistently identified in multiple studies in patients who underwent a CT-scan and intravenous iodine-containing contrast medium administration: chronic heart failure, diabetes and eGFR<60 mL/min/1.73m2. |
|
Low GRADE |
The following risk factors for the development of PC-AKI were consistently identified in multiple studies in patients who underwent CAG and intra-arterial iodine-containing contrast medium administration: chronic kidney disease, multivessel coronary artery disease, older age, heart failure, diabetes, overweight, peripheral vascular disease, metabolic syndrome, and eGFR<60 mL/min/1.73m2, anaemia, albumin, hyperuricemia, proteinuria, use of an intra-aortic balloon pump, contrast volume and emergency PCI. |
|
Very low GRADE |
We are uncertain what the risk is of PC-AKI after iodinated CM in patients with a kidney transplant. |
|
Very low GRADE |
We are uncertain what risk is of PC-AKI after iodinated CM in patients with a solitary kidney. |
Type of iodine-containing CM administration
|
Low GRADE |
There is a low level of evidence that iso-osmolar CM administration has a lower risk of PC-AKI than low osmolar CM administration in patients undergoing intra-arterial contrast administration.
(Eng, 2016) |
|
Low GRADE |
There is a low level of evidence that iso-osmolar contrast administration has a similar risk of PC-AKI when compared with low osmolar contrast medium administration in patients with undergoing intra-venous contrast administration.
(Eng, 2016) |
Tools for estimation of risk for PC-AKI
|
B EBRO |
It is unclear whether one measurement tool for the prediction of PC-AKI risk in patients undergoing intra-arterial contrast administration is superior to another measurement tool to accurately predict this risk in clinical practice.
(Aykan, 2013; Bartholomew, 2004; Chen, 2014; Fu, 2012; Ghani, 2009; Gao, 2004; Gurm, 2014; Inohara, 2014; Ivanes, 2014; Jin, 2013; Kul, 2015; Ling, 2015; Maioli, 2012; Marenzi, 2004; Mehran, 2004; Mizuno, 2014; Raposeiras-Roubin, 2014; Sguro, 2010; Tziakas 2013; Tziakas, 2014; Victor, 2014) |
|
|
No studies have been found that study prediction tools for PC-AKI risk in patients undergoing intra-venous iodine-containing contrast administration. |
Samenvatting literatuur
1. Studies comparing iodine-containing contrast administration to no contrast administration
Description of studies
There are no RCTs that compared risk of AKI after a radiological procedure with or without iodine-containing CM. Moreover, most identified risk factors for PC-AKI are also risk factors for AKI. As a consequence, we can only summarize risk factors for PC-AKI from observational studies. Since these risk factors cannot reliably discriminate between risk of AKI or PC-AKI, we could not use these specific risk factors for the present guideline to identify patients who are at increased risk for PC-AKI.
Study results
There are no prospective randomized controlled trials (RCTs) that compared the risk of AKI in patients undergoing CT scans with or without low osmolar (LO) CM. Three retrospective observational studies compared the incidence of AKI in patients who underwent CT-scans either with or without intravenous contrast administration (Bruce, 2009; McDonald RJ, 2013; Davenport 2013a). Bruce, 2009 matched contrast and non-contrast patients by eGFR, while McDonald and Davenport used Propensity Score matching.
Both Bruce (2009) and McDonald (2013) reported in respectively 11,588 and 53,439 patients that risk of post CT-scan AKI was similar in patients who underwent CT-scans with intravenous contrast and those who underwent CT-scans without intravenous contrast.
Bruce (2009) reported that 525/5,328 (10%) of patients receiving iohexol CM developed PC-AKI compared to 45/462 (10%) patients receiving iodixanol CM and 658/7,484 (9%) patients receiving no CM (p>0.05).
McDonald (2013) reported that AKI risk was not significantly different between "contrast" and "non-contrast" groups in any risk subgroup after propensity score (PS) matching by using reported risk factors of CIN (low risk: odds ratio [OR], 0.93; 95%CI: 0.76, 1.13; p=0.47; medium risk: OR, 0.97; 95% CI: 0.81, 1.16; p=0.76; high risk: OR, 0.91; 95% CI: 0.66, 1.24; p=0.58). Counterfactual analysis revealed no significant difference in AKI incidence between enhanced and unenhanced CT scans in the same patient (McNemar test: χ(2) = 0.63, p=0.43) (OR = 0.92; 95% CI: 0.75, 1.13; p=0.46).
In contrast, Davenport (2013) showed in a 10-year 1:1 propensity score-matched retrospective study, including 17,652 patients with a stable kidney function, that inpatients with an eGFR <30 ml/min/1.73m2 had a 3-fold increased risk of PC-AKI compared to patients without LOCM enhanced CT (OR 2.96 (95%CI: 1.22-7.17) (Davenport 2013a), with a trend toward significance in patients with an eGFR 30-44 ml/min/1.73m2. IV LOCM did not appear to be associated with PC-AKI in patients with an eGFR >45 ml/min/1.73m2.
2. Risk Factor Analysis (Which risk factors for PC-AKI can be identified in patients scheduled for an imaging procedure with iodine-containing CM?)
Description of studies
A total of 54 observational studies that examined the determinants of PC-AKI risk in a multivariable model were included in this literature analysis.
Ten studies examined PC-AKI risk in patients undergoing Computed Tomography scans with intravenous iodine-containing contrast. The study populations of these studies ranged from 189 to 17,672 patients. The multivariable models contained 4 to 14 parameters. (Balemans, 2012; Davenport, 2013a; Diogo, 2014; Ho, 2015; Kwasa, 2014; Matsushima, 2011; Moos, 2014; Selistre, 2015; Sonhaye, 2015; Yazici, 2016)
Forty-four studies examined PC-AKI risk in patients undergoing coronary angiography (CAG) and/or percutaneous coronary intervention (PCI) with intra-arterial iodine-containing contrast medium. The study populations of these studies ranged from 102 to 8357. The multivariable models contained 2 to 12 parameters. (Aguiar-Souto, 2010; Barbieri, 2014; Chong, 2009; Chong, 2010; Chong, 2010_1; Chong, 2015; Cicek, 2015; Cirit, 2006; Dangas, 2005; Ding, 2013; Diogo, 2010; Ebisawa, 2016; Farhan, 2016; Fu, 2012; Gao, 2014; Guo, 2015; Gurm, 2013; Ivanes, 2014; Kiski, 2010; Kolte, 2016; Lin, 2014; Liu, 2012; Liu, 2012_1; Lucrezziotti, 2014; Mager, 2011; Maioli, 2011; Medalion, 2010; Mehran, 2004; Nikolsky, 2005; Ozcan, 2015; Ozturk, 2016; Pakfertat, 2010; Ranucci, 2013; Sahin, 2014; Saito, 2015; Taniguchi, 2013; Toprak, 2006; Toprak, 2006_1; Toprak, 2007; Uçar, 2014; Watanabe, 2016; Zhu, 2016; Zuo, 2016)
Study results
1. PC-AKI risk for CT with: intravenous iodine-containing contrast administration
As shown in tables 1, 2 and 3 (Appendix) the following risk factors for the development of PC-AKI were identified in patients who underwent a CT-scan and intravenous iodine-containing contrast medium administration:
Patient factors:
- chronic heart failure (risk factor in 5 out of 7 studies);
- diabetes (risk factor in 5 out of 7 studies);
- older age (risk factor in 3 out of 7 studies);
- sex (male) (risk factor in 2 out of 6 studies);
- chronic kidney disease (risk factor in 2 out of 4 studies);
- inflammation (clinical sepsis or high C-reactive protein) (risk factor in 1 study);
- medication: use of hydrochlorothiazide, diuretics or concurrent use of 4 nephrotoxic agents (all reported in 1 study);
- hypotension (risk factor in 1 study);
- Injury Severity Score in trauma CT (risk factor in 1 study);
- African American race (risk factor in 1 study);
Laboratory parameters:
- risk of PC-AKI is increased for patients if eGFR<60 mL/min/1.73m2 (risk factor in 3 out of 3 studies);
- risk of PC-AKI is inversely associated with kidney function (risk factor in 1 out of 2 studies);
- Haemoglobin level (<9.3 g/dl) (risk factor in 1 out of 3 studies)
Treatment-related parameters:
- emergency CT-scan (decrease of risk in 1 study);
- length of hospital stay (risk factor in 1 study);
- blood transfusion (risk factor in 1 study).
2. PC-AKI risk for CAG and PCI with intra-arterial iodine-containing contrast administration
As shown in tables 4, 5 and 6 (Appendix) the following risk factors for the development of PC-AKI were identified in patients who underwent a CAG and/or PCI and intra-arterial contrast administration:
Patient factors:
- chronic kidney disease (risk factor in 4 out of 4 studies);
- multivessel coronary artery disease (risk factor in 3 out of 3 studies).
- older age (risk factor in 16 out of 22 studies);
- history of heart failure (risk factor in 12 out of 19 studies);
- history of diabetes (risk factor in 16 out of 23 studies);
- body mass index (BMI), either overweight (>25 kg/m2, risk factor in 2 out of 3 studies) or underweight (<18.5 kg/m2, risk factor in 1 out of 3 studies);
- peripheral vascular disease (risk factor in 2 out of 3 studies);
- metabolic syndrome (risk factor in 2 out of 3 studies);
- sex (women) (risk factor in 6 out of 13 studies);
- hypertension (risk factor in 2 out of 13 studies) or hypotension at admission (risk factor in 2 out of 13 studies);
- risk score (SYNTAX) (risk factor in 1 study);
- medication: statins (decrease of risk in 1 study), diuretics, calcium antagonists, insulin, angiotensin converting enzyme (ACE) inhibitors or angiotensin-II receptor blockers (ARB) (no consistent risk factors);
- ST-elevation myocardial infarction (risk factor in 1 study)
- cardiogenic shock (risk factor in 1 study);
- pulmonary oedema at presentation (risk factor in 1 study);
Laboratory parameters:
- eGFR (lower) (risk factor in 18 out of 27 studies);
- serum creatinine (risk factor in 6 out of 9 studies)
- low haemoglobin / anaemia (risk factor in 10 out of 15 studies);
- low albumin (risk factor in 3 out of 3 studies)
- hyperuricemia (risk factor based on meta-analysis);
- proteinuria (risk factor in 2 out of 3 studies);
- cysteine-C (risk factor in 2 out of 2 studies)
- hypercholesterolemia (risk factor in 1 out of 2 studies);
- myoglobin (risk factor in 1 study);
- serum glucose (risk factor in 1 study)
- increased C-reactive protein (risk factor in 1 study);
- serum ferritin (risk factor in 1 study);
Treatment-related parameters:
- intra-aortic balloon pump (risk factor in 7 out of 7 studies);
- contrast volume: sometimes reported as ratio between administered contrast volume and eGFR, ratio between contrast volume and body surface area or maximal estimated contrast dose (risk factor in 16 out of 22 studies);
- emergency PCI (risk factor in 2 out of 3 studies);
- surgical procedure on the same day (risk factor in 1 study);
- duration of cardiac bypass (CABG) (risk factor in 1 study);
- nadir haematocrit during CABG (risk factor in 1 study);
- prehydration with saline or non-normal saline hydration (both risk factor in 1 study);
- multivessel intervention (risk factor in 1 study);
- periprocedural hypotension (risk factor in 1 study).
2. How should a history of kidney transplantation be taken into account when assessing a patient for PC-AKI risk?
Description of studies
Only a limited number of studies reported about kidney transplant recipients that received intravascular iodine-containing contrast. We found no prospective studies of PC-AKI in kidney transplant recipients. We included three retrospective studies with a limited number of patients. No studies were found about kidney transplant recipients with more advanced CKD (eGFR <45 ml/min/1.73m2) and risk of PC-AKI.
Study results
Haider, 2015 conducted a retrospective study to evaluate the incidence of PC-AKI in kidney transplant recipients. Patients received intravascular iodine-containing contrast for a CT scan, pulmonary angiogram, or cardiac catheterization. PC-AKI was defined as a rise in serum creatinine of ≥0.5 mg/dl or a ≥25% decrease in eGFR from baseline value at 48 to 72 hours following the exposure of iodine-containing contrast media. Patients were only included if they had a stable kidney function before contrast administration. 124 patients were included. At baseline all patients had a high baseline eGFR (mean eGFR 74 ml/min/1.73m2). Seven patients developed PC-AKI (5.6%). Patients who developed PC-AKI had a mean age of 47 years, mean eGFR 78 ml/min/1.73m2, and received a mean volume of iodine-containing contrast of 109 ml. Acute dialysis was not required in any patient. The authors concluded that in kidney transplant recipients with a baseline eGFR >70 ml/min/1.73m2, the incidence of PC-AKI is low (Haider, 2015).
Agrawal, 2009 conducted a retrospective study to evaluate the incidence of PC-AKI in kidney transplant recipients. They included 57 patients for an elective or emergent cardiac catheterization procedure. Two definitions for PC-AKI were used: 1) rise in serum creatinine of 25% or 0.5 mg/dl within 72 hours post-iodine-containing contrast medium exposure, and 2) rise in serum creatinine of 50% or 0,3 mg/dl within 48 days post iodine-containing contrast medium exposure. All patients received peri-procedural hydration with intravenous saline or sodium bicarbonate. The mean age was 58 years. The median baseline eGFR was 52 ml/min/1.73m2 (33-90 ml/min/1.73m2). Diabetes was present in 35 patients. The incidence of PC-AKI using the primary definition was 15.5%. This included 1 patient requiring temporary dialysis. The incidence of PC-AKI using the secondary definition was 12.5%. No information was given about the volumes of iodine-containing contrast media used. The authors concluded that PC-AKI is common in kidney transplant recipients (Agrawal, 2009).
Fananapazir, 2016 conducted a retrospective study in kidney transplant recipients. One hundred patients underwent a renal graft arteriography. PC-AKI was defined as an increase in serum creatinine of 0.5 mg/dl or more compared to the creatinine value before arteriography. PC-AKI could be assessed in 37 patients. The mean age was 57 years. Diabetes was present in 48% and hypertension in 100% of patients. All patients received peri-procedural hydration with intravenous saline or sodium bicarbonate. Three patients (8%) met the criteria for PC-AKI. At 30 days after the procedure, none of the patients required dialysis or had graft failure. In a subgroup analysis, patients who had an arteriography without angioplasty or stenting, there was a statistically significant higher rate of PC-AKI (Fananapazir, 2016).
3. How should a solitary kidney be taken into account when assessing a patient for PC-AKI risk?
Description of studies
There is no evidence that in patients with a solitary kidney the risk of PC-AKI is higher than in patients with bilateral kidneys. No data on intravascular contrast administration are available.
Study results
McDonald (2016) conducted a retrospective study evaluating differences in clinical characteristics and outcomes between the solitary and bilateral kidney groups after intravenous iodine-containing contrast administration. Propensity score matching yielded a cohort of 247 patients with solitary kidneys and 691 patients with bilateral kidneys. Patients were included if they were 18 years or older and underwent contrast-enhanced CT. PC-AKI was defined as an increase in serum creatinine level of either (a) at least 0,5 mg/dl or (b) at least 0.3 mg/dl or 50% over baseline in the 24-72 hours after the CT scan. The mean age of the group of solitary kidney patients was 67 years, of whom 25% had diabetes mellitus. 51% had an eGFR >60 ml/min/1.73m2, 49% an eGFR 30-59 ml/min/1.73m2, and 0.4% an eGFR <30 ml/min/1.73m2. All patients received intravascular hydration with saline (pre-hydration and post-hydration). The study did not demonstrate any significant differences in the rate of PC-AKI, dialysis, or death attributable to contrast-enhanced CT in patients with a solitary kidney versus bilateral kidneys (McDonald, 2016).
In summary, it is unclear whether patients with a solitary kidney have an increased risk of PC-AKI and whether hydration in these patients will decrease this risk.
4. How should the osmolality of iodine-containing contrast medium be taken into account when assessing PC-AKI risk?
Description of studies
A meta-analysis by Eng, 2016 including a total of 17 studies with 4,518 patients who underwent intra-arterial contrast administration, and in whom the risk of PC-AKI was compared between iso-osmolar contrast (IOCM) and low-osmolar contrast medium (LOCM), was included in this analysis. Furthermore, the meta-analysis described a total of 6 studies with 1,405 patients who underwent intra-venous contrast administration, and in whom the risk of PC-AKI was compared between IOCM and LOCM, were also analysed.
Study results
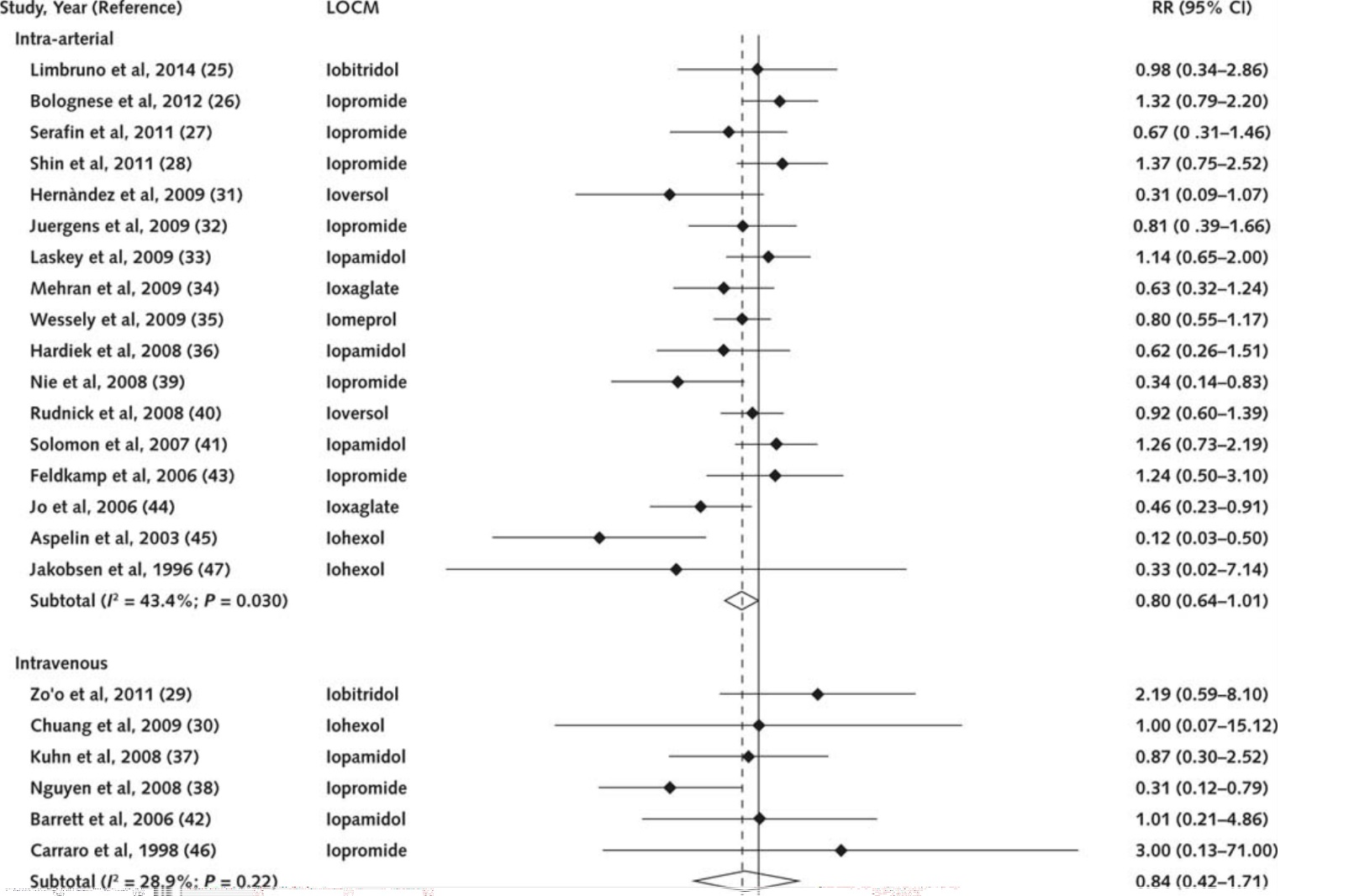
A pooled analysis of the systematic review by Eng, 2016 is shown below in Figure 1. Pooled results of 17 studies in 4,518 patients who underwent intravascular contrast administration showed a barely significant difference in risk of PC-AKI between iso-osmolar contrast media and low osmolar contrast media (RR: 0.80, 95% CI: 0.64 to 1.01, p=0.03), in favour of iso-osmolar contrast media. However, this difference is not clinically relevant if a minimal clinically relevant difference of 10% is applied. Pooled results of 6 studies in 1,405 patients who underwent intra-venous contrast administration find no significant difference in risk of PC-AKI between iso-osmolar contrast media and low osmolar contrast media (RR: 0.84, 95% CI: 0.72 to 1.71, p=0.22).
Figure 1 Pooled analysis of studies comparing different types of iodine-containing contrast medium. Reference for figure: Eng, 2016
5. Tools for Risk Estimation of PC-AKI
Description of studies
A total of 28 studies with 93,668 patients were identified that developed or validated a model to predict the risk of PC-AKI in patients undergoing either CAG or PCI (intra-arterial contrast administration) (Abellas-Sequeiros, 2016; Araujo, 2016; Aykan, 2013; Bartholomew, 2004; Chen, 2014; Chou, 2016; Duan, 2017; Fu, 2013; Ghani, 2009; Gao, 2014; Gurm, 2013; Inohara, 2015; Ivanes, 2014; Ji, 2015; Kul, 2015; Lazaros, 2016; Lian, 2017; Lin, 2017; Liu, 2016; Maioli, 2010; Marenzi, 2004; Mehran, 2004; Mizuno, 2015; Raposeiras-Roubin, 2013; Sguro, 2010; Tziakas 2013; Tziakas, 2014; Victor, 2014).
Thirteen studies reported on the Mehran Risk score (Abellas-Sequeiros, 2016; Araujo, 2016; Aykan, 2013; Chou, 2016; Gao, 2004; Ivanes, 2014; Jin, 2013; Kul, 2015; Liu, 2016; Maioli, 2010; Mehran, 2004; Mizuno, 2014; Sgura, 2010), this was the most frequently reported risk score. External validation of the Mehran score was performed in 2 studies in 6,852 patients (Maioli, 2010; Mehran, 2004).
No studies were found to design or validate risk stratifications tools for patients undergoing intra-venous contrast administration.
Study results
The summaries of the results of these studies are described in Table 10 (Appendix). In most studies only internal validation of the risk model was performed. When external validation of a model was performed, the predictive ability of the model was not strong (AUC <0.8 in most cases). Furthermore, from the information provided in the included studies it was not possible to conclude whether one type of risk model was superior to the other prediction models.
The concordance statistic (c-statistic) or area under a ROC curve (AUC) of the risk model was calculated in numerous studies. These were interpreted as follows:
- A value of 0.5 means that the model is no better than predicting an outcome than random chance;
- Values over 0.7 indicate a good model;
- Values over 0.8 indicate a strong model;
- A value of 1 means that the model perfectly predicts those who will experience a certain outcome and those who will not.
The following risk scores showed a c-statistic or AUC higher than 0.7, indicating that the models were ‘good’ in predicting PC-AKI: the Mehran score (Abellas-Sequeiros, 2016; Araujo, 2016; Kul, 2015; Lin, 2014; Liu, 2016), the New Preprocedure Risk Score by Duan (2017), the Athens CIN Score (Lazaros, 2016), the risk scores by Chen, Gao, the ACEF, the AGEF, GRACE (Liu, 2016; Gao, 2014)), the risk score by Gurm (2014), the Zwolle risk score (Kul, 2015), the risk score by Lin (2014), the Bartholomew model (Lin, 2014) and the National Cardiovascular Data Register (NCDR) Risk Model of Acute Kidney Injury (Tsai, 2014).
The sensitivity of the tools for risk estimation varied from 42% (CHADS2 score, Chou, 2016) to 94% of the simple risk score of Victor (2014). Based on an external data set Victor (2014) found 92% sensitivity for this risk score. The Mehran score showed up to 79% sensitivity in an acute STEMI patient population (Aykan, 2014).
Specificity was highest for the Athens CIN Score (Lazaros, 2016), and this was accompanied with a positive predictive value of 77% and a negative predictive value of 87%. Highest reported specificity of the Mehran score was 89% (Aykan, 2013). Specificity of the simple risk score of Victor (2014) was found to be 82% based on an external data set.
The utility of patient questionnaires that can predict impaired kidney function and guide which patients need eGFR evaluation will be discussed briefly in chapter 5 on eGFR evaluation. However, in NL it has been common practice to determine eGFR in all patients receiving intravascular iodine-containing CM and therefore their use is not commonplace.
Quality of evidence
1 Risk Factor Analysis for PC-AKI
A summary of risk factors for PC-AKI was made from observational studies with, unfortunately, very low to low quality of evidence.
2 to 4 Risk Stratification of PC-AKI
Studies comparing contrast administration to no contrast administration
The level of evidence has been graded as low due to the observational nature of the included studies.
For the patients receiving iodine-containing contrast for CT-scan the level of evidence has been graded low, due to downgrading by 2 points: 1 for imprecision and 1 for heterogeneity of included studies.
For the patients receiving iodine-containing contrast media for CAG and/or PCI the level of evidence has been graded low, due to downgrading by 2 points for imprecision (wide confidence interval, surpassing borders of clinical relevance.
5 Tools for risk evaluation of PC-AKI
Grading of evidence by using the GRADE method was not possible, since this was a diagnostic question. Thus the EBRO methodology was applied (van Everdingen, 2004). The included studies were graded as EBRO B quality.
Zoeken en selecteren
To answer our clinical question a systematic literature analysis was performed for the sub questions 1-5. We formulated the following research questions and accompanying PICOs:
Which risk factors have the best value in identification of patients with increased risk of PC-AKI?
PICO 1
P (patient category) adult (≥18 years) patients receiving intravascular contrast
I (intervention) risk factors: patient-related, treatment-related, contrast administration related
C (comparison) absence of these risk factors
O (outcome) PC-AKI, complications of PC-AKI (hospitalization, start of dialysis, mortality)
PICO 2
P (patient category) adult (≥18 years) patients receiving intravascular contrast;
I (intervention) iodine-containing contrast medium administration;
C (comparison) no iodine-containing contrast medium administration;
O (outcome) PC-AKI, complications of PC-AKI (hospitalization, start of dialysis, mortality).
PICO 3
P (patient category) adult (≥18 years) patients receiving intravascular contrast;
I (intervention) iodine-containing contrast medium administration with hydration;
C (comparison) iodine-containing contrast medium administration with no hydration;
O (outcome) PC-AKI, complications of PC-AKI (hospitalization, start of dialysis, mortality).
PICO 4
P (patient category) adult (≥18 years) patients receiving intravascular contrast;
I (intervention) administration with iso-osmolar iodine-containing contrast medium;
C (comparison) administration with low osmolar iodine-containing contrast medium;
O (outcome) PC-AKI, complications of PC-AKI (hospitalization, start of dialysis, mortality).
Which clinical tools or questionnaires have the best diagnostic value in identification of patients with increased risk of PC-AKI?
PICO 5
P (patient category) adult (≥18 years) patients receiving intravascular iodine-containing contrast medium;
I (intervention) questionnaires or other clinical tools to estimate risk of PC-AKI;
C (comparison) other questionnaires or other clinical tools to estimate risk of PC-AKI;
Reference test development of PC-AKI after intravascular contrast administration;
O (outcome) sensitivity, specificity, area under curve (AUC), validity, reliability.
Relevant outcome measures
The working group considered sensitivity, specificity, AUC, validity, reliability critical outcome measures for the decision making process. The working group defined PC-AKI as described in the chapter Terminology.
Search and select (method)
A separate search strategy was developed for the first four research sub questions (PICO 1 – 4) and the fifth sub question (PICO 5).
For the sub questions 1 – 4, the databases Medline (OVID), Embase and the Cochrane Library were searched from 1st of January 2000 up to 19th of August 2015 using relevant search terms for systematic reviews (SRs), randomized controlled trials (RCTs) and observational studies (OBS). This search was updated on April 14th 2017. A total of 1058 studies were found. The initial literature search procured 868 hits and the update retrieved an additional 190 studies.
Studies were selected based on the following criteria:
- adult patients who underwent radiological examination using intravascular iodine-containing contrast media (including radiological examination during percutaneous angiography);
- potential risk factors related either to patient characteristics and/or treatment characteristics and/or iodine-containing contrast medium characteristics were studied in how they influenced the risk of PC-AKI;
- risk factors were corrected for confounders in multivariable models;
- at least one of the outcome measures was described: PC-AKI, complications of PC-AKI (hospitalization, start of dialysis, mortality).
For sub question 1, the working group selected the studies in which the risk of PC-AKI was compared for patients receiving intravascular contrast to patients receiving no intravascular contrast.
For the fifth sub question, the databases Medline (OVID), Embase and the Cochrane Library were searched from 1st of January 1995 up to 24th of September 2015 using relevant search terms for systematic reviews (SRs), randomized controlled trials (RCTs) and observational studies (OBS). This search was updated on April 14th, 2017. A total of 393 studies were found. The initial literature search procured 311 hits and the update retrieved an additional 82 studies.
Studies were selected based on the following criteria:
- adult patients who underwent radiological examination using intravascular iodine-containing contrast media (including radiological examination during percutaneous angiography);
- a measurement instrument that has been validated and estimates the risk of PC-AKI;
- if patients had to fill in the measurement instrument, we applied an additional criterion that the instrument had to be validated in Dutch and available in the Netherlands;
- at least one of the outcome measures was described: sensitivity, specificity, AUC, validity, reliability.
PICO 1
Based on title and abstract a total of 385 studies were initially selected (325 in the initial search and 60 in the updated search). After examination of full text a total of 331 studies were excluded and 54 studies definitely included in the literature summary.
PICO 2-4
Based on title and abstract a total of 210 studies were selected. After examination of full text a total of 186 studies were excluded and 24 studies definitely included in the literature summary. A total of two studies were added after the update of the search: one was regarding patients with a history of kidney transplantation and one regarding patients with a solitary kidney.
PICO 5
Based on title and abstract a total of 91 studies were selected (56 in the initial search and 35 in the updated search). One more study was added through cross-referencing. After examination of full text a total of 73 studies were excluded and 19 studies definitely included in the literature summary.
Results
PICO 1
54 studies were included in the literature analysis, the most important study characteristics and results were included in the evidence tables. The evidence tables and assessment of individual study quality are included.
PICO 2-4
26 studies were included in the literature analysis, the most important study characteristics and results were included in the evidence tables. The evidence tables and assessment of individual study quality are included.
PICO 5
19 studies were included in the literature analysis, the most important study characteristics and results were included in the evidence tables. The evidence tables and assessment of individual study quality are included.
Referenties
- Abellás-Sequeiros RA, Raposeiras-Roubín S, Abu-Assi E, et al. Mehran contrast nephropathy risk score: is it still useful 10 years later? J Cardiol. 2016; 67(3), 262-267.
- Agrawal V, Swami A, Kosuri R, et al. Contrast-induced acute kidney injury in renal transplant recipients after cardiac catheterization. Clin Nephrol. 2009;Jun;71(6):687-96.
- Aguiar-Souto P, Ferrante G, Del Furia F, et al. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol. 2010;Feb 18;139(1):68-74
- Araujo GN, Wainstein MV, McCabe JM, et al. Comparison of two risk models in predicting the incidence of contrast-induced nephropathy after percutaneous coronary intervention. J Intervent Cardiol. 2016; 29(5), 447-453.
- Aykan AÇ, Gül I, Gökdeniz T, et al. Is coronary artery disease complexity valuable in the prediction of contrast induced nephropathy besides Mehran risk score, in patients with ST elevation myocardial infarction treated with primary percutaneous coronary intervention? Heart Lung Circ. 2013;22(10):836-43.
- Balemans CE, Reichert LJ, van Schelven BI, et al. Epidemiology of contrast material-induced nephropathy in the era of hydration. Radiology. 2012;Jun;263(3):706-13
- Barbieri L, Verdoia M, Schaffer A, et al. Pre-diabetes and the risk of contrast induced nephropathy in patients undergoing coronary angiography or percutaneous intervention. Diabetes Res Clin Pract. 2014 Dec;106(3):458-64
- Bartholomew BA, Harjai KJ, Dukkipati S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93(12):1515-9.
- Bruce RJ, Djamali A, Shinki K, et al. Background fluctuation of kidney function versus contrast-induced nephrotoxicity. AJR Am J Roentgenol. 2009;192(3):711-8.
- Chen SL, Zhang J, Yei F, et al. Clinical outcomes of contrast-induced nephropathy in patients undergoing percutaneous coronary intervention: a prospective, multicenter, randomized study to analyze the effect of hydration and acetylcysteine. Int J Cardiol. 2008;126(3):407-13.
- Chen YL, Fu NK, Xu J, et al. A simple preprocedural score for risk of contrast-induced acute kidney injury after percutaneous coronary intervention. Cathet Cardiovasc Intervent. 2014;83(1):E8-16.
- Chong E, Poh KK, Lu Q, et al. Comparison of combination therapy of high-dose oral N-acetylcysteine and intravenous sodium bicarbonate hydration with individual therapies in the reduction of Contrast-induced Nephropathy during Cardiac Catheterisation and Percutaneous Coronary Intervention (CONTRAST): A multi-centre, randomised, controlled trial. Int J Cardiol. 2015; 201:237-42.
- Chong E, Poh KK, Shen L, et al. Diabetic patients with normal baseline renal function are at increased risk of developing contrast-induced nephropathy post-percutaneous coronary intervention. Singapore Med J. 2009 Mar;50(3):250-4
- Chong E, Poh KK, Liang S, et al. Risk factors and clinical outcomes for contrast-induced nephropathy after percutaneous coronary intervention in patients with normal serum creatinine. Ann Acad Med Singapore. 2010 May;39(5):374-80
- Chong E, Poh KK, Liang S, et al. Comparison of risks and clinical predictors of contrast-induced nephropathy in patients undergoing emergency versus nonemergency percutaneous coronary interventions. J Interv Cardiol. 2010 Oct;23(5):451-9
- Chou, RH, Huang, PH, Hsu, CY, et al. CHADS 2 score predicts risk of contrast-induced nephropathy in stable coronary artery disease patients undergoing percutaneous coronary interventions. J Formos Med Assoc. 2016; 115(7), 501-509.
- Cicek G, Bozbay M, Acikgoz SK, et al. The ratio of contrast volume to glomerular filtration rate predicts in-hospital and six-month mortality in patients undergoing primary angioplasty for ST-elevation myocardial infarction. Cardiol J. 2015;22(1):101-7.
- Cirit M, Toprak O, Yesil M, et al. Angiotensin-converting enzyme inhibitors as a risk factor for contrast-induced nephropathy. Nephron Clin Pract. 2006;104(1):c20-7
- Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005 Jan 1;95(1):13-9
- Davenport MS, Khalatbari S, Cohan RH, et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28.
- Davenport MS, Khalatbari S, Dillman JR, et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94-105.
- Ding FH, Lu L, Zhang RY, Zhu TQ, et al. Impact of elevated serum glycated albumin levels on contrast-induced acute kidney injury in diabetic patients with moderate to severe renal insufficiency undergoing coronary angiography. Int J Cardiol. 2013 Jul 31;167(2):369-73.
- Diogo LP, Saitovitch D, Biehl M, et al. Is there an association between non-steroidal anti-inflammatory drugs and contrast nephropathy?. Arq Bras Cardiol, 2010; 95(6), 726-731.
- Diogo LP, Bahlis LF, Carvalhal GF. Computerized tomography contrast induced nephropathy (CIN) among adult inpatients. J Bras Nefrol. 2014 Oct-Dec;36(4):446-50.
- Duan C, Cao Y, Liu Y, et al. A new preprocedure risk score for predicting contrast-induced acute kidney injury. Cardiol. 2017;33(6), 714-723.
- Ebisawa S, Kurita T, Tanaka N, et al. Impact of minimum contrast media volumes during elective percutaneous coronary intervention for prevention of contrast-induced nephropathy in patients with stable coronary artery disease. Cardiovasc Interv Ther. 2016;31(1):13-20.
- Eng J, Wilson RF, Subramaniam RM, et al. Comparative Effect of Contrast Media Type on the Incidence of Contrast-Induced Nephropathy. Ann Intern Med. 2016;164(6):417-24.
- Fananapazir G, Troppmann C, Corwin MT, et al. Incidence of Contrast-Induced Nephropathy After Renal Graft Catheter Arteriography Using Iodine-Based Contrast Medium. AJR Am J Roentgenol. 2016 Apr;206(4):783-6.
- Fu N, Li X, Yang S, et al. Risk score for the prediction of contrast-induced nephropathy in elderly patients undergoing percutaneous coronary intervention. Angiology. 2013;64(3):188-94.
- Farhan S, Vogel B, Tentzeris I, et al. Contrast induced acute kidney injury in acute coronary syndrome patients: A single centre experience. Eur Heart J Acute Cardiovasc Care. 2016;5(1):55-61.
- Gao YM, Li D, Cheng H, et al. Derivation and validation of a risk score for contrast-induced nephropathy after cardiac catheterization in Chinese patients. Clin Expl Nephrol. 2014;18(6):892-8.
- Ghani AA, Tohamy KY. Risk score for contrast induced nephropathy following percutaneous coronary intervention. Saudi J Kidney Dis Transplant.2009;20(2):240-5.
- Guo W, Liu Y, Chen JY, et al. Hyperuricemia Is an Independent Predictor of Contrast-Induced Acute Kidney Injury and Mortality in Patients Undergoing Percutaneous Coronary Intervention. Angiology. 2015;66(8):721-6.
- Gurm HS, Seth M, Kooiman J, et al. A novel tool for reliable and accurate prediction of renal complications in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2013;61(22):2242-8.
- Haider M, Yessayan L, Venkat KK, et al. Incidence of contrast-induced nephropathy in kidney transplant recipients. Transplant Proc. 2015 Mar;47(2):379-83.
- Ho YF, Hsieh KL, Kung FL, et al. Nephrotoxic polypharmacy and risk of contrast medium-induced nephropathy in hospitalized patients undergoing contrast-enhanced CT. AJR Am J Roentgenol. 2015;205(4):703-8.
- Hsieh M-S, Chiu C-S, How C-K, et al. Contrast medium exposure during computed tomography and risk of development of end-stage renal disease in patients with chronic kidney disease: A national population based propensity score-matched, longitudinal follow-up study. Medicine. 2016;95:16.
- Inohara T, Kohsaka S, Abe T, et al. Development and validation of a pre-percutaneous coronary intervention risk model of contrast-induced acute kidney injury with an integer scoring system. Am J Cardiol. 2015;115(12):1636-42.
- Ivanes F, Isorni MA, Halimi JM, et al. Predictive factors of contrast-induced nephropathy in patients undergoing primary coronary angioplasty. Arch Cardiovasc Dis.. 2014;107(8-9)424-32.
- Ji L, Su X, Qin W, et al. Novel risk score of contrast-induced nephropathy after percutaneous coronary intervention. Nephrology. 2015;20(8):544-51.
- Jin R, Grunkemeier GL, Brown JR, et al. Estimated glomerular filtration rate and renal function. Ann Thorac Surg. 2008 Jul;86(1):1-3
- Jurado-Román A, Hernández-Hernández F, García-Tejada J, et al. Role of hydration in contrast-induced nephropathy in patients who underwent primary percutaneous coronary intervention. Am J Cardiol. 2015;115(9):1174-8.
- Kiski D, Stepper W, Breithardt G, et al. Impact of female gender on frequency of contrast medium-induced nephropathy: post hoc analysis of dialysis versus diuresis trial. J Womens Health (Larchmt). 2010 Jul;19(7):1363-8
- Kolte D, Spence N, Puthawala M, et al. Association of radial versus femoral access with contrast-induced acute kidney injury in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction. Cardiovasc Revasc Med. 2016;17(8):546-51.
- Kooiman J, Sijpkens YW, de Vries JP, et al. A randomized comparison of 1-h sodium bicarbonate hydration versus standard peri-procedural saline hydration in patients with chronic kidney disease undergoing intravenous contrast-enhanced computerized tomography. Nephrol Dial Transplant. 2014;29(5):1029-36.
- Kooiman J, Sijpkens YW, van Buren M, et al. Randomised trial of no hydration vs. sodium bicarbonate hydration in patients with chronic kidney disease undergoing acute computed tomography-pulmonary angiography. J Thromb Haemost. 2014;12(10):1658-66.
- Kul S, Uyarel H, Kucukdagli OT, et al. Zwolle risk score predicts contrast-induced acute kidney injury in STEMI patients undergoing PCI. Herz. 2015;40(1):109-15.
- Kwasa EA, Vinayak S, Armstrong R. The role of inflammation in contrast-induced nephropathy. Br J Radiol. 2014 Sep;87(1041):20130738
- Lazaros G, Zografos T, Oikonomou E, et al. Usefulness of C-Reactive Protein as a Predictor of Contrast-Induced Nephropathy After Percutaneous Coronary Interventions in Patients With Acute Myocardial Infarction and Presentation of a New Risk Score (Athens CIN Score) Am J Cardiol. 2016;118(9). 1329-1333.
- Lenhard DC, Frisk AL, Lengsfeld P, et al. The effect of iodinated contrast agent properties on kinetics and oxygenation. Invest Radiol. 2013;48(4):175-82.
- Lenhard DC, Pietsch H, Sieber MA, et al. The osmolality of nonionic, iodinated contrast agents as an important factor for renal safety. Invest Radiol. 2012;47(9):503-10.
- Lian D, Liu Y, Liu YH, et al. Pre-Procedural Risk Score of Contrast-Induced Nephropathy in Elderly Patients Undergoing Elective Coronary Angiography. Internat Heart J. 2017;58(2). 197-204.
- Lin YS, Fang HY, Hussein H, et al. Predictors of contrast-induced nephropathy in chronic total occlusion percutaneous coronary intervention. EuroIntervention. 2014;9(10)1173-80.
- Lin KY, Zheng WP, Bei WJ, et al. A novel risk score model for prediction of contrast-induced nephropathy after emergent percutaneous coronary intervention. Internat J Cardiol. 2017;230. 402-412.
- Liss P, Persson PB, Hansell P, et al. Renal failure in 57,925 patients undergoing coronary procedures using iso-osmolar or low-osmolar contrast media. Kidney Int. 2006;70(10):1811-7.
- Liu Y, Tan N, Zhou YL, et al. The contrast medium volume to estimated glomerular filtration rate ratio as a predictor of contrast-induced nephropathy after primary percutaneous coronary intervention. Int Urol Nephrol. 2012 Feb;44(1):221-9
- Liu Y, Tan N, Chen J, et al. The relationship between hyperuricemia and the risk of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with relatively normal serum creatinine. Clinics (Sao Paulo). 2013 Jan;68(1):19-25
- Lucreziotti S, Centola M, Salerno-Uriarte D, et al.. Female gender and contrast-induced nephropathy in primary percutaneous intervention for ST-segment elevation myocardial infarction. Int J Cardiol. 2014 Jun 1;174(1):37-42
- Luo Y, Wang X, Ye Z, et al. Remedial hydration reduces the incidence of contrast-induced nephropathy and short-term adverse events in patients with ST-segment elevation myocardial infarction: a single-center, randomized trial. Intern Med. 2014;53(20):2265-72
- Mager, A., Vaknin Assa, H., Lev, E. I., et al. The ratio of contrast volume to glomerular filtration rate predicts outcomes after percutaneous coronary intervention for ST-segment elevation acute myocardial infarction. Catheter Cardiovasc Intervent. 2011. 78(2), 198-201.
- Maioli M, Toso A, Gallopin M, et al. Preprocedural score for risk of contrast-induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med. 2010;11(6):444-9.
- Maioli M, Toso A, Leoncini M, et al. Effects of hydration in contrast-induced acute kidney injury after primary angioplasty a randomized, controlled trial. Circ Cardiovasc Interv. 2011;4(5):456-62.
- Marenzi G, Bartorelli AL. Hemofiltration in the prevention of radiocontrast agent induced nephropathy. Minerva Anestesiol. 2004 Apr;70(4):189-91
- Matsushima K, Peng M, Schaefer EW, et al. Posttraumatic contrast-induced acute kidney injury: minimal consequences or significant threat? J Trauma. 2011 Feb;70(2):415-9
- McDonald JS, Katzberg RW, McDonald RJ, et al. Is the presence of a solitary kidney an independent risk factor for for acute kidney injury after contrast-enhanced CT? Radiology. 2016;278:74-81
- McDonald JS, McDonald RJ, Carter RE, et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score-matched study stratified by baseline estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.
- McDonald JS, McDonald RJ, Lieske JC, et al. Risk of acute kidney injury, dialysis, and mortality in patients with chronic kidney disease after intravenous contrast material exposure. Mayo Clin Proc. 2015;90(8):1046-53.
- McDonald RJ, McDonald JS, Bida JP, et al. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology. 2013;267:106-118.
- McDonald RJ, McDonald JS, Carter RE, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology. 2014;273(3):714-25.
- McDonald JS, McDonald RJ, Lieske JC, et al. Risk of acute kidney injury, dialysis, and mortality in patients with chronic kidney disease after intravenous contrast material exposure. In Mayo Clinic Proceedings 2016;90(8),1046-1053. Elsevier.
- Medalion B, Cohen H, Assali A, et al. The effect of cardiac angiography timing, contrast media dose, and preoperative renal function on acute renal failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2010 Jun;139(6):1539-44
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393-9.
- Mizuno A, Ohde S, Nishizaki Y, et al. Additional value of the red blood cell distribution width to the Mehran risk score for predicting contrast-induced acute kidney injury in patients with ST-elevation acute myocardial infarction. J Cardiol. 2015;66(1):41-5.
- Moos SI, Stoker J, Nagan G, et al. Prediction of presence of kidney disease in a general patient population undergoing intravenous iodinated contrast enhanced computed tomography. Eur Radiol. 2014; 24(6):1266-75.
- Nikolsky E, Mehran R, Lasic Z, et al. Low hematocrit predicts contrast-induced nephropathy after percutaneous coronary interventions. Kidney Int. 2005 Feb;67(2):706-13
- Nyman U, Björk J, Aspelin P, et al. Contrast medium dose-to-GFR ratio: a measure of systemic exposure to predict contrast-induced nephropathy after percutaneous coronary intervention. Acta Radiol. 2008;49(6):658-67.
- Nyman U, Elmståhl B, Geijer H, et al. Iodine contrast iso-attenuating with diagnostic gadolinium doses in CTA and angiography results in ultra-low iodine doses. A way to avoid both CIN and NSF in azotemic patients? Eur Radiol. 2011;21(2):326-36.
- Ozcan OU, Adanir EH, Gulec S, et al. Impact of metabolic syndrome on development of contrast-induced nephropathy after elective percutaneous coronary intervention among nondiabetic patients. Clin Cardiol. 2015;38(3):150-6.
- Ozturk D, Celik O, Erturk M, et al. Utility of the Logistic Clinical Syntax Score in the Prediction of Contrast-Induced Nephropathy After Primary Percutaneous Coronary Intervention. Can J Cardiol. 2016;32(2):240-6.
- Pakfetrat M, Nikoo MH, Malekmakan L, et al. Risk Factors for contrast-related acute kidney injury according to risk, injury, failure, loss, and end-stage criteria in patients with coronary interventions. Iran J Kidney Dis. 2010 Apr;4(2):116-22
- Ranucci M, Ballotta A, Agnelli B, et al; Surgical and Clinical Outcome Research (SCORE) Group. Acute kidney injury in patients undergoing cardiac surgery and coronary angiography on the same day. Ann Thorac Surg. 2013 Feb;95(2):513-9
- Raposeiras-Roubín S, Aguiar-Souto P, Barreiro-Pardal C, et al. GRACE risk score predicts contrast-induced nephropathy in patients with acute coronary syndrome and normal renal function. Angiology. 2013;64(1):31-9.
- Sahin I, Gungor B, Can MM, et al. Lower blood vitamin D levels are associated with an increased incidence of contrast-induced nephropathy in patients undergoing coronary angiography. Can J Cardiol. 2014 Apr;30(4):428-33
- Saito Y, Watanabe M, Aonuma K, et al. CINC-J study investigators. Proteinuria and reduced estimated glomerular filtration rate are independent risk factors for contrast-induced nephropathy after cardiac catheterization. Circ J. 2015;79(7):1624-30
- Selistre Lda S, Souza VC, Dubourg L, et al. Contrast-induced nephropathy after computed tomography. J Bras Nefrol. 2015;37(1):27-31.
- Sgura FA, Bertelli L, Monopoli D, et al. Mehran contrast-induced nephropathy risk score predicts short-and long-term clinical outcomes in patients with ST-elevationmyocardial infarction. Circ Cardiovasc Interv. 2010;3(5)491-8.
- Sonhaye L, Kolou B, Tchaou M, et al. Intravenous contrast medium administration for computed tomography scan in emergency: a possible cause of contrast-induced nephropathy. Radiol Res Pract. 2015;2015:805786.
- Taniguchi Y, Sakakura K, Wada H, et al. Contrast induced exacerbation of renal dysfunction in the advanced chronic kidney disease. Cardiovasc Interv Ther. 2013 Apr;28(2):157-61
- Toprak O, Cirit M, Yesil M, et al. Metabolic syndrome as a risk factor for contrast-induced nephropathy in non-diabetic elderly patients with renal impairment. Kidney Blood Press Res. 2006;29(1):2-9
- Toprak O, Cirit M, Esi E, et al. Hyperuricemia as a risk factor for contrast-induced nephropathy in patients with chronic kidney disease. Catheter Cardiovasc Interv. 2006 Feb;67(2):227-35
- Toprak O, Cirit M, Yesil M, et al. Impact of diabetic and pre-diabetic state on development of contrast-induced nephropathy in patients with chronic kidney disease. Nephrol Dial Transplant. 2007 Mar;22(3):819-26
- Trivedi HS, Moore H, Nasr S, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003 Jan;93(1):C29-34
- Tziakas D, Chalikias G, Stakos D, et al. Development of an easily applicable risk score model for contrast-induced nephropathy prediction after percutaneous coronary intervention: a novel approach tailored to current practice. Internat J Cardiol. 2013;163(1):46-55.
- Tziakas D, Chalikias G, Stakos D, et al. Validation of a new risk score to predict contrast-induced nephropathy after percutaneous coronary intervention. Am J Cardiol. 2014;113(9)1487-93.
- Uçar H, Gür M, Yildirim A, et al. Increased aortic stiffness predicts contrast-induced nephropathy in patients with stable coronary artery disease undergoing percutaneous coronary intervention. Angiology. 2014 Oct;65(9):806-11
- Victor SM, Gnanaraj A, S V, et al. Risk scoring system to predict contrast induced nephropathy following percutaneous coronary intervention. Ind Heart J. 2014;66(5):517-24.
- Yazici S, Kiris T, Emre A, et al. Relation of contrast nephropathy to adverse events in pulmonary emboli patients diagnosed with contrast CT. Am J Emerg Med. 2016;34(7):1247-50.
- Watanabe M, Saito Y, Aonuma K, et al. Prediction of contrast-induced nephropathy by the serum creatinine level on the day following cardiac catheterization. J Cardiol. 2016;68(5):412-8.
- Zhu B, Hou J, Gong Y, et al. Association between serum ferritin and contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Biomed Res Int. 2016;2016:5420345.
- Zuo T, Jiang L, Mao S, et al. Hyperuricemia and contrast-induced acute kidney injury: A systematic review and meta-analysis. Int J Cardiol. 2016;224:286-94.
Evidence tabellen
Exclusion after examination of full text (initial search): Risk factors for PC-AKI
|
Author and year |
Reasons to exclude |
|
Abe, 2011 |
Does not meet selection criteria |
|
Abujudeh, 2008 |
Examines risk of PC-AKI in patients who underwent 2 CT-scans within 24 hours, not applicable for overall recommendations |
|
Acosta, 2010 |
Does not meet selection criteria |
|
Agrawal, 2009 |
Does not meet selection criteria |
|
Aguiar-Suato, 2010 |
Does not meet selection criteria |
|
Ahuja, 2010 |
Does not meet selection criteria |
|
Akgullu, 2015 |
Does not meet selection criteria |
|
Akrawinthawong, 2015 |
Does not meet selection criteria |
|
Alharazy, 2013 |
Does not meet selection criteria |
|
Bachorzewska-Gajewska, 2006 |
Does not meet selection criteria |
|
Balemans, 2012 |
Does not meet selection criteria |
|
Band, 2007 |
Does not meet selection criteria |
|
Barbieri, 2014 |
Does not meet selection criteria |
|
Becker, 2006 |
Does not meet selection criteria |
|
Canyigit, 2013 |
Does not meet selection criteria |
|
Caruso, 2011 |
Does not meet selection criteria |
|
Cely, 2012 |
Does not meet selection criteria |
|
Chang, 2013 |
Studies gene polymorphisms and their relation to PC-AKI risk; not applicable in common Dutch clinical practice. |
|
Chavakula, 2013 |
Does not meet selection criteria |
|
Chen, 2014 |
Does not meet selection criteria |
|
Cho, 2011 |
Does not meet selection criteria |
|
Chong, 2009 |
Does not meet selection criteria |
|
Chong, 2010_1 |
Does not meet selection criteria |
|
Chong, 2010_2 |
Does not meet selection criteria |
|
Chong, 2012 |
Does not meet selection criteria |
|
Cheruvu, 2007 |
Does not meet selection criteria |
|
Crit, 2006 |
Does not meet selection criteria |
|
Clark, 2011 |
Does not meet selection criteria |
|
Clec'h, 2013 |
Does not meet selection criteria |
|
Colling, 2014 |
Does not meet selection criteria |
|
Conen, 2006 |
Does not meet selection criteria |
|
Cowburn, 2005 |
Does not meet selection criteria |
|
Dangas, 2005 |
Does not meet selection criteria |
|
Davidson, 2008 |
Does not meet selection criteria |
|
Ding, 2013 |
Does not meet selection criteria |
|
Diogo, 2010 |
Does not meet selection criteria |
|
Diogo, 2014 |
Does not meet selection criteria |
|
Dittrich, 2006 |
Does not meet selection criteria |
|
Dittrich, 2007 |
Does not meet selection criteria |
|
Durukan, 2012 |
Does not meet selection criteria |
|
Elias, 2005 |
Does not meet selection criteria |
|
Erdogan, 2003 |
Does not meet selection criteria |
|
Erselcan, 2012 |
Does not meet selection criteria |
|
Friedewald, 2013 |
Does not meet selection criteria |
|
From, 2008 |
Does not meet selection criteria |
|
Fu, 2013 |
Does not meet selection criteria |
|
Gao, 2011 |
Does not meet selection criteria |
|
Gao, 2014 |
Does not meet selection criteria |
|
Garcia, 2014 |
Does not meet selection criteria |
|
Garcia-Ruiz, 2003 |
Does not show multivariate model that predicts risk factors of PC-AKI |
|
Goldenberg, 2005 |
Does not meet selection criteria |
|
Golshahi, 2014 |
Does not meet selection criteria |
|
Goo, 2014 |
Does not meet selection criteria |
|
Guevara, 2004 |
Does not meet selection criteria |
|
Gurm, 2011 |
Does not meet selection criteria |
|
Grum, 2013 |
Does not meet selection criteria |
|
Hassen, 2014 |
Does not meet selection criteria |
|
Haveman, 2006 |
Does not meet selection criteria |
|
Hayakawa, 2014 |
Patient population: patients with hepatocellular carcinoma undergoing trans-arterial chemo-embolization. Article too specific to draw overall conclusions over intra-arterial contrast administration and risk of PC-AKI. |
|
Hernández, 2009 |
Already included in systematic review Bondi-Zoccai, 2014 |
|
Hipp, 2008 |
Does not meet selection criteria |
|
Holscher, 2008 |
Does not meet selection criteria |
|
Hoste, 2011 |
Does not meet selection criteria |
|
Huang, 2013 |
Does not meet selection criteria |
|
Huggins, 2014 |
Does not meet selection criteria |
|
Ivanes, 2014 |
Does not meet selection criteria |
|
Jaipaul, 2010 |
Does not meet selection criteria |
|
Jarai, 2012 |
Does not meet selection criteria |
|
Ji, 2015 |
Does not meet selection criteria |
|
Jochheim, 2014 |
Does not meet selection criteria |
|
Jo, 2015 |
Does not meet selection criteria |
|
Kato, 2008 |
Does not meet selection criteria |
|
Kian, 2006 |
Does not meet selection criteria |
|
Kim, 2011 |
Does not meet selection criteria |
|
Kim, 2012 |
Does not meet selection criteria |
|
Kim, 2015 |
Does not meet selection criteria |
|
Kiski, 2009 |
Does not meet selection criteria |
|
Kiski, 2010 |
Does not meet selection criteria |
|
Koo, 2013 |
Does not meet selection criteria |
|
Kougias, 2014 |
Does not meet selection criteria |
|
Kuhn, 2008 |
Does not meet selection criteria |
|
Kwasa, 2014 |
Does not meet selection criteria |
|
Lameire, 2006 |
Does not meet selection criteria |
|
Laskey,2009 |
Does not meet selection criteria |
|
Lee, 2014 |
Does not meet selection criteria |
|
Lencioni, 2010 |
Does not meet selection criteria |
|
Leung, 2014 |
Model predicts use of cardiac medication after development of PC-AKI, but does not predict risk of PC-AKI |
|
Li, 2013 |
Does not meet selection criteria |
|
Li, 2014 |
Does not meet selection criteria |
|
Liebetrau, 2014 |
Does not meet selection criteria |
|
Limbruno, 2014 |
Does not meet selection criteria |
|
Lin, 2014 |
Does not meet selection criteria |
|
Liu, 2012_1 |
Does not meet selection criteria |
|
Liu, 2012_2 |
Does not meet selection criteria |
|
Liu, 2013 |
Does not meet selection criteria |
|
Liu, 2014 |
Does not meet selection criteria |
|
Lodhia, 2009 |
Does not meet selection criteria |
|
Lucreziotti, 2014 |
Does not meet selection criteria |
|
Lui, 2012 |
Does not meet selection criteria |
|
Macaulay, 2015 |
Does not answer research question, no multivariate analysis performed (n=7) |
|
Madershahian, 2012 |
Does not meet selection criteria |
|
Madershahian, 2012 |
Does not meet selection criteria |
|
Madsen, 2009 |
Does not meet selection criteria |
|
Mager, 2011 |
Does not meet selection criteria |
|
Maioli, 2010 |
Does not meet selection criteria |
|
Maioli, 2012 |
Does not meet selection criteria |
|
Malyszko, 2009 |
Does not meet selection criteria |
|
Marenzi, 2004_1 |
Does not meet selection criteria |
|
Marenzi, 2004_2 |
Does not meet selection criteria |
|
Matsushima, 2011 |
Does not meet selection criteria |
|
McCullough, 2006_1 |
Does not meet selection criteria |
|
McCullough, 2006_2 |
Does not meet selection criteria |
|
McDonald, 2014_1 |
Does not meet selection criteria |
|
McDonald, 2014_2 |
Does not meet selection criteria |
|
Medalion, 2010 |
Does not meet selection criteria |
|
Mehran, 2004 |
Does not meet selection criteria |
|
Mehran, 2009 |
Does not meet selection criteria |
|
Mehta, 2004 |
Does not meet selection criteria |
|
Mekan, 2004 |
Does not meet selection criteria |
|
Moos, 2013 |
Does not meet selection criteria |
|
Moos, 2014 |
Does not show multivariate model that predicts risk factors of PC-AKI (but tests existing models) |
|
Morabito, 2012 |
Does not meet selection criteria |
|
Morcos, 2012 |
Does not meet selection criteria |
|
Murakami, 2013 |
Does not meet selection criteria |
|
Najjar (ea) 2002 |
Does not meet selection criteria |
|
Naruse, 2012 |
Does not meet selection criteria |
|
Ng, 2010 |
Does not meet selection criteria |
|
Nikolsky, 2004 |
Does not meet selection criteria |
|
Nikolsky, 2005 |
Does not meet selection criteria |
|
Nozue, 2009 |
Does not meet selection criteria |
|
Nyman, 2005 |
Does not meet selection criteria |
|
Onuigbo, 2008 |
Does not meet selection criteria |
|
Osman, 2014 |
Does not meet selection criteria |
|
Owen, 2014 |
Does not meet selection criteria |
|
Padhy, 2014 |
Does not meet selection criteria |
|
Pahade, 2011 |
Does not meet selection criteria |
|
Pakfetrat, 2010_1 |
Does not meet selection criteria |
|
Pakfetrat, 2010_2 |
Does not meet selection criteria |
|
Parra, 2004 |
Does not meet selection criteria |
|
Patel, 2010 |
Review, not systematic and does not answer research question |
|
Peguero, 2014 |
Does not meet selection criteria |
|
Peng, 2015 |
Does not meet selection criteria |
|
Piskinpasa, 2013 |
Combination of CAG and CT-scan patients (n=70), not analysed separately. |
|
Polena, 2005 |
Does not meet selection criteria |
|
Prasad, 2014 |
No multivariate analysis of risk factors for PC-AKI was performed |
|
Rahman, 2005 |
Does not meet selection criteria |
|
Raingruber, 2011 |
Does not meet selection criteria |
|
Ranucci, 2013 |
Does not meet selection criteria |
|
Raposeiras, 2015 |
Does not meet selection criteria |
|
Raposeiras, 2015 |
Does not meet selection criteria |
|
Ray, 2013 |
Does not meet selection criteria |
|
Reuter, 2014 |
No multivariate analysis of risk factors for PC-AKI was performed |
|
Sahin, 2014 |
Does not meet selection criteria |
|
Saito, 2015 |
Does not meet selection criteria |
|
Saritemur, 2014 |
Does not meet selection criteria |
|
Sendur, 2013 |
Does not meet selection criteria |
|
Sharma, 2013 |
Does not meet selection criteria |
|
Shema, 2009 |
Does not meet selection criteria |
|
Sidhu, 2008 |
Does not meet selection criteria |
|
Skelding, 2007 |
Does not answer research question, validation of risk score |
|
Spatz, 2012 |
Does not meet selection criteria |
|
Spini, 2013 |
Does not meet selection criteria |
|
Standstede, 2007 |
Does not meet selection criteria |
|
Stermer, 2001 |
Does not meet selection criteria |
|
Subedi, 2011 |
Does not meet selection criteria |
|
Tan, 2013 |
Does not meet selection criteria |
|
Taniguchi, 2013 |
Does not meet selection criteria |
|
Thomsen, 2003 |
Does not meet selection criteria |
|
Thomsen, 2009 |
Does not meet selection criteria |
|
Toprak, 2006_1 |
Does not meet selection criteria |
|
Toprak, 2006_2 |
Does not meet selection criteria |
|
Toprak, 2007 |
Does not meet selection criteria |
|
Trivedi, 2010 |
Does not meet selection criteria |
|
Tziakas, 2014 |
Does not meet selection criteria |
|
Ucar, 2014 |
Does not meet selection criteria |
|
Ugur, 2014 |
Does not meet selection criteria |
|
Umruddin, 2012 |
Does not meet selection criteria |
|
Utsunomiyama, 2011 |
Studies risk factors for kidney insufficiency, not risk factors for development of PC-AKI after CT-scan |
|
Victor, 2014 |
Does not meet selection criteria |
|
Wacker-Gusmann, 2014 |
Does not meet selection criteria |
|
Wang, 2011 |
Does not meet selection criteria |
|
Weisbord, 2006 |
Does not meet selection criteria |
|
Wessely, 2009 |
Does not meet selection criteria |
|
Wi, 2013 |
Does not meet selection criteria |
|
Yamamoto, 2013 |
Does not meet selection criteria |
|
Zaytseva, 2009 |
Does not meet selection criteria |
Exclusion after examination of full text (update 2017): Risk factors for PC-AKI
|
Author and year |
Redenen van exclusie |
|
Kanda, 2016 |
Does not meet selection criteria |
|
Prasad, 2016. |
Does not meet selection criteria |
|
Abouzeid, 2016 |
Does not meet selection criteria |
|
Agarwal, 201 |
Does not meet selection criteria |
|
Azzalini, 2016 |
Does not meet selection criteria |
|
Cernigliaro, 2016 |
Does not meet selection criteria |
|
Briguori, 2016 |
Does not meet selection criteria |
|
Chong, 2015 |
Does not meet selection criteria |
|
de Francesco, 2015 |
Does not meet selection criteria |
|
Dong, 2016 |
Does not meet selection criteria |
|
Filomia 2016 |
Does not meet selection criteria |
|
Guneyli, 2015 |
Does not meet selection criteria |
|
Gurm, 2016. |
Does not meet selection criteria |
|
Subramaniam, 2016 |
Does not meet selection criteria |
|
Ye, 2016 / Ye, 2017 |
Does not meet selection criteria |
|
Zapata-Chica, 2015 |
Does not meet selection criteria |
|
Hinson, 2017 |
Does not meet selection criteria |
|
Hong, 2016 |
Does not meet selection criteria |
|
Hsieh, 2016 |
Does not meet selection criteria |
|
Huber, 2016 |
Does not meet selection criteria |
|
Kanbay, 2017, |
Does not meet selection criteria |
|
Khaledifar, 2015 |
Does not meet selection criteria |
|
Kim, 2015 |
Does not meet selection criteria |
|
Komiyama, 2017 |
Does not meet selection criteria |
|
Liu 2015 |
Does not meet selection criteria |
|
McDonald 2015 |
Does not meet selection criteria |
|
Nijssen, 2017 |
Does not meet selection criteria |
|
Nyman, 2015 |
Does not meet selection criteria |
|
Ortega, 2015 |
Does not meet selection criteria |
|
Park, 2016 |
Does not meet selection criteria |
|
Sato, 2015 |
Does not meet selection criteria |
|
Shema, 2016 |
Does not meet selection criteria |
|
Sigterman, 2016 |
Does not meet selection criteria |
|
Salomon, 2015 |
Does not meet selection criteria |
|
Tong, 2016, |
Does not meet selection criteria |
|
Turedi, 2016 |
Does not meet selection criteria |
|
Usmiani, 2016 |
Does not meet selection criteria |
|
Valette, 2017 |
Does not meet selection criteria |
|
Vontobel, 2015 |
Does not meet selection criteria |
|
Winther, 2016 |
Does not meet selection criteria |
|
Xu, 2016 |
Does not meet selection criteria |
|
Yang, 2014 |
Does not meet selection criteria |
|
Zeller, 2016 |
Does not meet selection criteria |
Exclusion after examination of full tekst: Measurement instruments for PC-AKI risk
|
Author and year |
Reasons for exclusion |
|
Aguiar, 2008 |
Letter to the editor |
|
Akgullu, 2015 |
Does not fulfill selection criteria, no risk score is validated/developed |
|
Balemans, 2012 |
Does not fulfill selection criteria, no risk score is validated/developed |
|
Bartholemew, 2004 |
Already included in systematic review Silver, 2015 |
|
Benko, 2007 |
Not an original article (guideline) |
|
Celik, 2015 |
The diagnostic properties of a laboratory analysis (contrast media volume toe GFR ratio) to predict PC-AKI are examined, not of a non-invasive method. |
|
Chen, 2014 |
Already included in systematic review Silver, 2015 |
|
Chong, 2012 |
Does not fulfill selection criteria, no risk score is validated/developed |
|
Crit, 2006 |
Does not fulfill selection criteria, no risk score is validated/developed |
|
Davenport, 2013 |
The diagnostic properties of a laboratory analysis (different eGFR cut-off values) to predict PC-AKI are examined, not of a non-invasive method. |
|
Davenport, 2013_1 |
The diagnostic properties of a laboratory analysis (different eGFR cut-off values) to predict PC-AKI are examined, not of a non-invasive method |
|
Erselcan, 2009 |
The diagnostic properties of a laboratory analysis (eGFR by MDRD formula) to predict PC-AKI are examined, not of a non-invasive method. |
|
Feldkamp, 2008 |
Narrative review |
|
Fu, 2013 |
Already included in systematic review Silver, 2015 |
|
Gao, 2014 |
Already included in systematic review Silver, 2015 |
|
Ghani, 2009 |
Already included in systematic review Silver, 2015 |
|
Gurm, 2013 |
Already included in systematic review Silver, 2015 |
|
Holscher, 2008 |
Does not fulfill selection criteria, no risk score is validated/developed |
|
Kim, 2011 |
Does not fulfill selection criteria, no risk score is validated/developed |
|
Kooiman, 2010 |
Does not fulfill selection criteria, no risk score is validated/developed |
|
Kowalczyk, 2007 |
Does not fulfill selection criteria, no risk score is validated/developed |
|
Lepanto, 2011 |
Narrative review |
|
Li, 2013 |
The diagnostic properties of a laboratory analysis (anemia) to predict PC-AKI are examined, not of a non-invasive method. |
|
Liu, 2014 |
Already included in systematic review Silver, 2015 |
|
Maioli, 2011 |
Already included in systematic review Silver, 2015 |
|
Marenzi, 2004 |
Already included in systematic review Silver, 2015 |
|
Martainez – Lomakin, 2014 |
The diagnostic properties of a laboratory analysis (point of care creatinin test) to predict PC-AKI are examined, not of a non-invasive method. |
|
McCullough, 2001 |
Narrative review |
|
McCullough, 2007 |
Narrative review |
|
McDonald, 2014 |
Does not fulfill selection criteria, no risk score is validated/developed |
|
Mehran, 2004 |
Already included in systematic review Silver, 2015 |
|
Owen, 2014 |
Not an original article (guideline) |
|
Pakfetrat, 2010 |
Does not fulfill selection criteria, no risk score is validated/developed |
|
Rainburger, 2011 |
PC-AKI is not an outcome measure. |
|
Saito, 2015 |
The diagnostic properties of a laboratory analysis (proteinuria and to predict PC-AKI are examined, not of a non-invasive method. |
|
Sany, 2013 |
Does not meet selection criteria, no risk score is validated/developed |
|
Skelding, 2007 |
Does not fulfill selection criteria, pre-defined outcome variables not reported |
|
Skluzacek, 2003 |
The diagnostic properties of a laboratory analysis (eGFR) to predict PC-AKI are examined, not of a non-invasive method. |
|
Tong, 1996 |
The diagnostic properties of a laboratory analysis (neutrophil gelatinase associated lipoprotein) to predict PC-AKI are examined, not of a non-invasive method. |
|
Too, 2015 |
PC-AKI is not an outcome measure. The questionnaire’s ability to predict eGFR is examined. |
|
Tziakas, 2013 |
Already included in systematic review Silver, 2015 |
|
Wackecker-Guβmann, 2014 |
The diagnostic properties of a laboratory analysis (cystatin C) to predict PC-AKI are examined, not of a non-invasive method. |
|
Wang, 2011 |
The diagnostic properties of a laboratory analysis (contrast media volume toe GFR ratio) to predict PC-AKI are examined, not of a non-invasive method. |
|
Worasuwannarack, 2011 |
Article not found (Taiwanese journal) |
|
Zahringer, 2014 |
PC-AKI is not an outcome measure. The questionnaire’s ability to predict eGFR is examined. |
Exclusion after examination of full text (update 2017): Measurement instruments for PC-AKI risk
|
Author and year |
Reasons for exclusion |
|
Akrawinthawong, 2015 |
Does not meet selection criteria |
|
Ando, 2013 |
Does not meet selection criteria |
|
Anonymous, 2015 |
Erratum |
|
Balli, 2016 |
Does not meet selection criteria |
|
Barbieri, 2016 |
Does not meet selection criteria |
|
Chatterjee, 2017 |
Does not meet selection criteria |
|
Garfinkle, 2015 |
Does not meet selection criteria |
|
Goussot, 2015 |
Does not meet selection criteria |
|
Grossman, 2017 |
Does not meet selection criteria |
|
Gurm, 2016 |
Does not meet selection criteria |
|
Hsieh, 2016 |
Does not meet selection criteria |
|
Kim, 2015 |
Does not meet selection criteria |
|
Li, 2016 |
Does not meet selection criteria |
|
Liu, 2015 |
Does not meet selection criteria |
|
Oksuz, 2015 |
Does not meet selection criteria |
|
Osugi, 2016 |
Does not meet selection criteria |
|
Ozturk, 2016 |
Does not meet selection criteria |
|
Park, 2017 |
Does not meet selection criteria |
|
Prasad, 2016 |
Does not meet selection criteria |
|
Raposeiras-Roubin, 2013 |
Does not meet selection criteria |
|
Sato, 2015 |
Does not meet selection criteria |
|
Tao, 2016 |
Does not meet selection criteria |
|
Victor, 2014 |
Does not meet selection criteria |
|
Watanabe, 2016 |
Does not meet selection criteria |
|
Xu, 2016 |
Does not meet selection criteria |
|
Yin, 2017 |
Does not meet selection criteria |
|
Yuan, 2017 |
Does not meet selection criteria |
|
Brown, 2015 |
Does not meet selection criteria |
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Eng, 2016 |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
No |
No |
- Research question (PICO) and inclusion criteria should be appropriate and predefined
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs)
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table etc.)
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (e.g. Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (e.g., funnel plot, other available tests) and/or statistical tests (e.g., Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias table for intervention studies (randomized controlled trials)
Research question:
|
Study reference
(first author, publication year) |
Describe method of randomisation1 |
Bias due to inadequate concealment of allocation?2
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?4
(unlikely/likely/unclear) |
Bias due to loss to follow-up?5
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?6
(unlikely/likely/unclear) |
|
Chen, 2007 |
Not described “patients were randomly allocated” |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
|
Jurado-Roman, 2014 |
Not described “patients were randomly assigned” |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
|
Kooiman, 2014 |
Computer generated allocation sequence |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
|
Maioli, 2011 |
Computer generated, open-label randomization block |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
- Randomisation: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomisation process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomisation (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomisation procedures or open allocation schedules..
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the risk of bias is unclear
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the risk of bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Risk of bias table for intervention studies (observational: non-randomized clinical trials, cohort and case-control studies)
Research question:
|
Study reference
(first author, year of publication) |
Bias due to a non-representative or ill-defined sample of patients?1
(unlikely/likely/unclear) |
Bias due to insufficiently long, or incomplete follow-up, or differences in follow-up between treatment groups?2
(unlikely/likely/unclear) |
Bias due to ill-defined or inadequately measured outcome ?3
(unlikely/likely/unclear) |
Bias due to inadequate adjustment for all important prognostic factors?4
(unlikely/likely/unclear) |
|
Bruce, 2009 |
Unlikely |
Unclear |
Unlikely |
Likely |
|
Davenport, 2013 |
Unlikely |
Unclear |
Unlikely |
Likely |
|
McDonald, 2013 |
Unlikely |
Unclear |
Unlikely |
Likely |
- Failure to develop and apply appropriate eligibility criteria: a) case-control study: under- or over-matching in case-control studies; b) cohort study: selection of exposed and unexposed from different populations.
- 2 Bias is likely if: the percentage of patients lost to follow-up is large; or differs between treatment groups; or the reasons for loss to follow-up differ between treatment groups; or length of follow-up differs between treatment groups or is too short. The risk of bias is unclear if: the number of patients lost to follow-up; or the reasons why, are not reported.
- Flawed measurement, or differences in measurement of outcome in treatment and control group; bias may also result from a lack of blinding of those assessing outcomes (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Failure to adequately measure all known prognostic factors and/or failure to adequately adjust for these factors in multivariate statistical analysis.
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question:
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Eng, 2016
[individual study characteristics deduced from [1st author, year of publication]
PS., study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs
Literature search up to June 2015
Study design: RCT [parallel]
Setting and Country: United States of America
Source of funding: non-commercial
|
Inclusion criteria SR: 1) RCTs that compared LOCM to IOCM with CIn incidence as the main outcome as the main outcome in patients having diagnostic imaging or image-based therapeutic procedures 2) CIN incidence is based on sCr or eGFR at baseline and within 72 hours of injection
Exclusion criteria SR: 1) language other than English 2) mixed route of contrast administration
29 studies included
Groups comparable at baseline? Unclear |
Describe intervention:
LOCM contrast administration
Both ia and iv
|
Describe control:
Iodixanol contrast administration
Both ia and iv
|
End-point of follow-up: 72 hours
For how many participants were no complete outcome data available? (intervention/control) Not described
|
Outcome measure-1 Defined as CIN
Intra-arterial contrast administration Favors iodixanol: Relative risk (RR): 0.80 (0.64 – 1.01) I2=43%, p=0.03)
Intra-venous contrast administration Favors iodixanol: Relative risk (RR): 0.84 (0.42 – 1.71) I2=29%, p=0.22)
|
Facultative:
Brief description of author’s conclusion
No differences were found in CIN risk among types of LOCM. Iodixanol had a slightly lower risk for CIN than LOCM, but the lower risk did not exceed the criterium for clinical importance.
Level of evidence: GRADE (per comparison and outcome measure) including reasons for down/upgrading
Most of the included studies GRADEd as Low (due to imprecision) |
AKI: acute kidney injury; CI-AKI: contrast induced acute kidney injury; CIN: contrast induced nephropathy; CT: Computed Tomography; eGFR: estimated glomerular filtration ration; ia: intra-arterial; IOCM: iso-osmolar contrast medium; iv: intravenous; LOCM: low osmolair contrast medium; RCT: randomized controlled trial; sCr: serum creatinine;
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])1
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy – otherwise the evidence table for studies of diagnostic test accuracy should be used.
Research question:
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Contrast administration versus no contrast administration for Computed Tomography |
|||||||
|
Bruce, 2009 |
Type of study: retrospective observational
Setting: in- and outpatients, multicentre study
Country: United States of America
Source of funding: not reported |
Inclusion criteria: 1) age at least 18 years, 2) measurement of serum creatinine concentration within 30 days before CT, and creatinine measurement with result available within 3 days after the CT examination
Exclusion criteria: 1) patient received iodinated contrast material as part of another procedure (e.g., cardiac catheterization) within 30 days before or 3 days after the reference CT examination. 2) patients with a preexisting status of undergoing long-term Dialysis 3) any record of dialysis within 30 days before or on the day of the CT examination
N total at baseline: Intervention: 337 Control: 6815
Important prognostic factors2: For example age ± SD: I: 63 ± 16 C: 59 ± 19
Sex: I: 65% M C: 53% M
Groups comparable at baseline? Yes
|
Describe intervention (treatment/procedure/test):
administration of isoosmolarcontrast medium (IOCM) (iodixanol) prior to Computed Tomography (CT)
|
Describe control (treatment/procedure/test):
Unenchanced Computed Tomography |
Length of follow-up: 3 days
Loss-to-follow-up: Unclear, only patients that had a creatinine measurement at baseline and after 3 days were included in this retrospective study.
Incomplete outcome data: As above
|
Outcome measures and effect size (include 95%CI and p-value if available):
Acute kidney injury (=a 0.5 mg/dL increase in serum creatinine concentration or a 25% or greater decrease in estimated glomerular filtration rate within 3 days after CT)
In all groups, the incidence of acute kidney injury increased with increasing baseline creatinine concentration. No significant difference in incidence of presumed contrast- induced kidney injury was identified between the isoosmolar contrast medium and the control groups. The incidence of acute kidney injury in the low-osmolar contrast medium cohort paralleled that of the control cohort up to a creatinine level of 1.8 mg/dL, but increases above this level were associated with a higher incidence of acute kidney injury. |
Authors’ conclusion:
We identified a high incidence of acute kidney injury among control subjects undergoing unenhanced CT. The incidence of creatinine elevation in this group was statistically similar to that in the isoosmolar contrast medium group for all baseline creatinine values and all stages of chronic kidney disease. These findings suggest that the additional risk of acute kidney injury accompanying administration of contrast medium (contrast-induced nephrotoxicity) may be overstated and that much of the creatinine elevation in these patients is attributable to background fluctuation, underlying disease, or treatment.
Only patients that had a creatinine measurement at baseline and after 3 days were included in this retrospective study.
IV administration of low-osmolar contrast medium (LOCM) (iohexol) to patients with a documented serum creatinine concentration of 2.0mg/dL or less if they did not have diabetes and to patients with a serum creatinine concentration of 1.5 mg/dL if they did have diabetes. We added a high-risk tier, allowing administration of iso-osmolar contrast medium (IOCM) (iodixanol) to nondiabetic patients with baseline creatinine values up to a maximum of 2.5 mg/dL and to diabetic patients with values up to a maximum of 2.0 mg/dL. Estimated GFR values are currently computed for all outpatients but have not supplanted serum creatinine concentration for contrast administration decisions. |
|
Davenport, 2013 |
Type of study: retrospective observational
Setting: in- and outpatients, multicentre study
Country: United States of America
Source of funding: not reported |
Inclusion criteria: 1) CT studies performed in patients who had never undergone renal replacement therapy (eg, dialysis, renal transplantation), 2) patients had available data to permit calculation of the four-variable Modification of Diet in Renal Disease formula for eGFR, 3) patients had all of the following SCr measurements available: (a) baseline SCr (the most recent SCr obtained more than 5 days before the index CT); (b) pre-CT SCr (the most recent SCr obtained between the time of the index CT and 5 days before); (c) at least one of three early post-CT SCr values (the first SCr obtained in each 24-hour period for the first 72 hours after the index CT).
Exclusion criteria: 1) CT performed in a patient who had an earlier CT examination that met the inclusion criteria 2) missing data regarding contrast material administration 3) unstable renal function before the CT study 4) calculated eGFR was greater than 200 mL/min/1.73 m2 5) patients lacked a 1:1 propensity-matched control
N total at baseline: Intervention: 8826 Control: 8826
Important prognostic factors2: For example age ± SD: I: 59 ± 17 C: 59 ± 18
Sex: I: 48% M C: 48% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
contrast-enhanced CT examinations with LOCM |
Describe control (treatment/procedure/test):
CT examinations without contrast enhancement |
Length of follow-up: 72 hours
Loss-to-follow-up: Early post- CT SCr data were available for 1) 15 724 of 17 652 patients (89.1%) 0–24 hours after CT (7882 nonenhanced, 7842 contrast-enhanced), 2) 12 941 of 17 652 patients (73.3%) 25–48 hours after CT (6450 nonenhanced, 6491 contrast-enhanced), 3) 10 213 of 17 652 patients (57.9%) 49–72 hours after CT (5091 nonenhanced, 5122 contrast-enhanced).
Incomplete outcome data: As described above
|
Outcome measures and effect size (include 95%CI and p-value if available):
Post CT-AKI (= difference between baseline and pre-CT SCr within 0.3 mg/dL and 50% of baseline) IV LOCM had a significant effect on the development of post-CT AKI (P = .04).
This risk increased with decreases in pre-CT eGFR (>60 mL/ min/1.73 m2: odds ratio, 1.00; 95% confidence interval: 0.86, 1.16; 45–59 mL/min/1.73 m2: odds ratio, 1.06; 95% confidence interval: 0.82, 1.38; 30–44 mL/min/1.73 m2: odds ratio, 1.40; 95% confidence interval: 1.00, 1.97; <30 mL/min/1.73 m2: odds ratio, 2.96; 95% confidence interval: 1.22, 7.17) |
Authors’ conclusion:
Intravenous LOCM is a nephrotoxic risk factor in patients with a stable eGFR less than 30 mL/min/1.73 m2, with a trend Toward significance at 30–44 mL/min/1.73 m2. IV LOCM does not appear to be a nephrotoxic risk factor in patients with a pre-CT eGFR of 45 mL/min/1.73 m2 or greater. |
|
McDonald, 2014 |
Type of study: retrospective observational
Setting: in- and outpatients, multicentre study
Country: United States of America
Source of funding: not reported |
Inclusion criteria: 1) all patients who underwent an unenhanced (noncontrast group) or intravenous contrastenhanced (contrast group) abdominal, pelvic, and/or thoracic CT scan from January 1, 2000, to December 31, 2010, at our institution; 2) who had one or more postscan SCr results during the time period of expected development of CIN (24–72 hours after CT-scanning) 3) who also had at least one baseline SCr result in the 24-hour window prior to scanning
Exclusion criteria: 1) patients who had preexisting renal dialysis requirements; 2) did not have sufficient SCr data to permit detection of AKI; 3) patients who underwent multiple distinct CT-scans or percutaneous cardiac interventions with iodinated contrast material within a 14-day period
N total at baseline: Intervention: 10686 Control: 10686
Important prognostic factors2: For example age (range): I: Low risk: 62 (49-74) Medium risk: 71 (59-79) High risk: 69 (58-77) C: Low risk: 63 (48-74) Medium risk: 71 (59-80) High risk: 68 (56-77)
Sex: I: % M Low risk: 48% Medium risk: 65% High risk: 63%
C: % M Low risk: 49% Medium risk: 64% High risk: 64%
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
contrast-enhanced CT examinations
Scan recipients were stratified with respect to their presumptive risk for AKI by baseline SCr level as follows: 1) low risk, SCr ,<1.5 mg/dL; 2) medium risk, SCr 1.5–2.0 mg/dL; 3) high risk, SCr > 2.0 mg/dL. |
Describe control (treatment/procedure/test):
CT examinations without contrast enhancement
Scan recipients were stratified with respect to their presumptive risk for AKI by baseline SCr level as follows: 1) low risk, SCr ,<1.5 mg/dL; 2) medium risk, SCr 1.5–2.0 mg/dL; 3) high risk, SCr > 2.0 mg/dL. |
Length of follow-up: 72 hours
Loss-to-follow-up: Unclear, only patients that had a creatinine measurement at baseline and after 3 days were included in this retrospective study.
Incomplete outcome data: As above
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=SCr ≥0.5 mg/dL above baseline)
AKI risk was not significantly different between contrast and noncontrast groups in any risk subgroup after propensity score adjustment by using reported risk factors of CIN 1) low risk: odds ratio [OR], 0.93; 95% confidence interval [CI]: 0.76,1.13; P = .47; 2) medium risk: odds ratio, 0.97; 95% CI: 0.81, 1.16; P = .76; 3) high risk: OR, 0.91; 95% CI: 0.66, 1.24; P = .58).
Counterfactual analysis revealed no significant difference in AKI incidence between enhanced and unenhanced CT scans in the same patient (McNemar test: x2 =0.63, P = 0.43) (OR = 0.92; 95% CI: 0.75, 1.13; P = .46).
|
Authors’ conclusion:
Following adjustment for presumed risk factors, the incidence of CIN was not significantly different from contrast material–independent AKI. These two phenomena were clinically indistinguishable with established SCr-defined criteria, suggesting that intravenous iodinated contrast media may not be the causative agent in diminished renal function after contrast material administration. |
|
Hydration versus no hydration at contrast administration |
|||||||
|
Chen, 2008 |
Type of study: RCT
Setting: in- and outpatients, multicentre study
Country: China
Source of funding: not reported |
Inclusion criteria: Patients with myocardial ischemia (angina or positive exercise treadmill) scheduled for percutaneous coronary intervention (PCI) in one of the three participating centers
Exclusion criteria: (1) the coronary anatomy not suitable for PCI; (2) emergency coronary artery bypassgrafting (CABG) being required; (3) patients in chronic peritoneal or hemodialytic treatment; (4) acute myocardial infarction (AMI) at admission; (5) no written formal consent from patients
N total at baseline: sCr<1.5mg/dL Intervention: 330 Control: 330 sCr ≥1.5mg/dL Intervention: 188 Control: 188
Important prognostic factors2: For example age ± SD: not reported
Sex: %M sCr<1.5mg/dL 85% sCr ≥1.5mg/dL 82%
Groups comparable at baseline? Unclear (patient data not reported for intervention and control group separately) |
Describe intervention (treatment/procedure/test):
sCr<1.5mg/dL: 0.45% saline given intravenously at a rate of 1 ml/kg/h starting from 12 h before scheduled time for coronary angiogram
sCr ≥1.5mg/dL: 1) 0.45% saline given intravenously at a rate of 1 ml/kg/h starting from 12 h before scheduled time for coronary angiogram 2) twice orally loading dose of 1200 mg NAC at 12 h before scheduled time for coronary angiogram and immediately after procedure |
Describe control (treatment/procedure/test):
sCr<1.5mg/dL: No hydration
sCr ≥1.5mg/dL: twice orally loading dose of 1200 mg NAC at 12 h before scheduled time for coronary angiogram and immediately after procedure |
Length of follow-up: 6 months
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=increase in SCrN0.5 mg/dl at 48 h after PCI)
sCr<1.5mg/dL: I: 6.7% C: 7.0% p>0.05
sCr ≥1.5mg/dL: I: 21.3% C: 34.0% P<0.001 |
Author’s conclusion:
Patients with CIN and preexisting renal insufficiency had worse clinical outcomes. Hydration with 0.45% sodium chloride alone had no potential effect on the occurrence of CIN in patients with normal renal function. Combination of hydration with ATLS could reduce the incidence of CIN in patients at high risk.
Groups comparable at baseline? Unclear (patient data not reported for intervention and control group separately)
|
|
Jurado-Roman, 2014 |
Type of study: RCT
Setting: in- and outpatients, single centre study
Country: Spain
Source of funding: not reported |
Inclusion criteria: patients who were admitted for STEMI and underwent a PPCI from July 2012 to November 2013 at our institution.
Exclusion criteria: 1) end-stage renal failure requiring dialysis, 2) cardiac arrest, 3) severe heart failure (Killip III to IV)
N total at baseline: Intervention: 204 Control: 204
Important prognostic factors2: For example age ± SD: I:62 ± 14 C: 64 ± 12
Sex: I: 72% M C: 75% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
Hydration: isotonic saline at an infusion rate of 1 ml/kg/h since the beginning of the procedure and during the following 24 hours.
Prior to PPCI |
Describe control (treatment/procedure/test):
No hydration Prior to PPCI |
Length of follow-up: 3 days
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
Crossover between study arms: 28% How this was handled in the data analysis is not reported. 74 patients changed from no hydration to hydration group because of sever hypotension 42 patients were changed from hydration to no hydration group because they developed heart failure |
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=a ≥25% or ≥0.5 mg/dl increase in serum a _25% or _0.5 mg/dl increase in serum)
CIN was observed in 14% of patients: I: 11% C: 21% (p=0.016).
In multivariate analysis, the only predictors of CIN were: 1) hydration (OR=0.29 [0.14 to 0.66]; p=0.003) 2) hemoglobin before the procedure (OR=0.69 [0.59 to 0.88]; p <0.0001) |
Authors’ conclusion:
In conclusion, intravenous saline hydration during PPCIreduced the risk of CIN to 48%. Given the higher incidence of CIN in emergentprocedures, and its morbidity and mortality, preventive hydration should be mandatory in them unless contraindicated.
Crossover between study arms: 28% How this was handled in the data analysis is not reported. |
|
Kooiman, 2014 |
Type of study: RCT
Setting:in- and outpatients, single centre
Country: the Netherlands
Source of funding: non-commercial |
Inclusion criteria: 1) Inpatients and outpatients with high clinical suspicion of acute PE requiring CTPA (i.e. Wells score ≥ 4 or D-dimer levels > 500 ng mL_1). 2) at least 18 years old 3) CKD (estimated glomerular filtration rate [eGFR] < 60 mL min _1/1.73 m2 estimated by using the Modification of Diet in Renal Disease formula
Exclusion criteria: 1) pregnancy, 2) previous contrast administration within the past 7 days, 3) documented allergy for iodinated contrast media, 4) hemodynamic instability (systolic blood pressure < 100 mm Hg) 5) participation in another trial
N total at baseline: Intervention: 71 Control: 67
Important prognostic factors2: For example age ± SD: I: 71 ± 13 C: 70 ± 12
Sex: I: 48% M C: 52% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
Sodium bicarbonate hydration prior to CTPA
250 mL intravenous 1.4% sodium bicarbonate 1 h before CTPA without hydration after CTPA.
|
Describe control (treatment/procedure/test):
No hydration prior to CTPA |
Length of follow-up: 96 hours for laboratory parameters 2 months for clinical outcomes
Loss-to-follow-up: Intervention: 2/71 (3%) 1 withdrew informed consent 1 died 24 hours after CTPA
Control: 2/67 (3%) Lost to follow-up
Incomplete outcome data: As above
|
Outcome measures and effect size (include 95%CI and p-value if available):
CI-AKI > 25%/> 0.5 mg dL_1) I: 5/71 (7%) C: 6/67 (9%) RR: 1.29, 95% confidence interval 0.41–4.03
None of the CI-AKI patients developed a need for dialysis. |
Authors’ conclusion:
Our results suggest that preventive hydration could be safely withheld in CKD patients undergoing CTPA for suspected acute pulmonary embolism. This will facilitate management of these patients and prevents delay in diagnosis as well as unnecessary start of anticoagulant treatment while receiving volume expansion. |
|
Maioli, 2011 |
Type of study: RCT
Setting: in- and outpatients, single centre
Country: Italy
Source of funding: not reported |
Inclusion criteria: 1) patients with STEMI who were candidates for primary PCI
Exclusion criteria: 1) contrast medium administration within the previous 10 days, 2) end-stage renal failure requiring dialysis, 3) refusal to give informed consent
N total at baseline: Intervention: 154 Control: 153
Important prognostic factors2: For example age ± SD: I:65 ± 13 C: 64 ± 12
Sex: I: 77% M C: 73% M
Groups comparable at baseline? Unclear |
Describe intervention (treatment/procedure/test):
Patients assigned to early hydration were administered a bolus of 3 mL/kg of sodium bicarbonate solution (154 mEq/L in dextrose and water) in 1 hour, starting in the emergency room, followed by infusion of 1 mL/kg per hour for 12 hours after PCI.
Hydration rate was reduced to 0.5 mL/kg per hour in patients with left ventricular ejection fraction (EF) <40% or New York Heart Association class III–IV in both groups.
|
Describe control (treatment/procedure/test):
No hydration prior to PCI. |
Length of follow-up: 3 days
Loss-to-follow-up: Intervention: 4/150 (3%) 1 had emergency procedure 3 no PCI
Control: 3/153 (2%) 1 had emergency procedure 2 no PCI
Incomplete outcome data: As above
|
Outcome measures and effect size (include 95%CI and p-value if available):
CI-AKI
I: 12% C: 27% P<0.001
Death I: 3 (2%) C: 8 (5%) p>0.05
Hemofiltration I: 2 (1%) C: 1 (1%) p>0.05 |
Authors’ conclusion:
Adequate intravenous volume expansion may prevent CI-AKI in patients undergoing primary PCI. A regimen of preprocedure and postprocedure hydration therapy with sodium bicarbonate appears to be more efficacious than postprocedure hydration only with isotonic saline. |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors [(potential) confounders]
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
AKI: acute kidney injury; CI-AKI: contrast induced acute kidney injury; CIN: contrast induced nephropathy; CT: Computed Tomography; CTPA: Computed Tomogrpahy of the pulmonary artery; eGFR: estimated glomerular filtration ration; ia: intra-arterial; IOCM: iso-osmolar contrast medium; iv: intravenous; LOCM: low osmolair contrast medium; OR: odds ratio; PCI: Percutaneous Coronary Intervention; PE: pulmonary embolism; PPCI: primary Percutaneous Coronary Intervention; RCT: randomized controlled trial; RR: relative risk; sCr: serum creatinine; STEMI: ST-elevation myocardial infarction
Risk of bias assessment diagnostic accuracy studies (QUADAS II, 2011)
Research question:
|
Study reference |
Patient selection |
Index test |
Reference standard |
Flow and timing |
Comments with respect to applicability |
|
Duan, 2017 |
Was a consecutive or random sample of patients enrolled? Yes, consecutive
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
Lian, 2017 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
Abellas-Sequeiros, 2016 |
Was a consecutive or random sample of patients enrolled? Yes, consecutive
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
Araujo, 2016 |
Was a consecutive or random sample of patients enrolled? Yes, consecutive
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Chou, 2016 |
Was a consecutive or random sample of patients enrolled? Unclear
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
Lazaros, 2016 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
Liu, 2016 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? Yes
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Unclear
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
Aykan, 2013 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
Bartholomew, 2004 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
Chen, 2014 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Fu, 2012 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Gao, 2013 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Gurm, 2013 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Inohara, 2015 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Ivanes, 2014 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Ji, 2015 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Kul, 2014 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Maioli, 2010 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Mehran, 2004 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Mizuno, 2015 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Raposeiras-Roubín, 2013 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
Sgura, 2010 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Tziakas, 2013 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Tziakas, 2014 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Victor, 2014 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
|
|
Lin, 2014 |
Was a consecutive or random sample of patients enrolled? Yes
Was a case-control design avoided? Yes
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Yes
If a threshold was used, was it pre-specified? Unclear
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes
|
Was there an appropriate interval between index test(s) and reference standard? Unclear
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes |
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No |
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: LOW |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: LOW |
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: LOW |
|
Judgments on risk of bias are dependent on the research question: some items are more likely to introduce bias than others, and may be given more weight in the final conclusion on the overall risk of bias per domain:
Patient selection:
- Consecutive or random sample has a low risk to introduce bias.
- A case control design is very likely to overestimate accuracy and thus introduce bias.
- Inappropriate exclusion is likely to introduce bias.
Index test:
- This item is similar to “blinding” in intervention studies. The potential for bias is related to the subjectivity of index test interpretation and the order of testing.
- Selecting the test threshold to optimise sensitivity and/or specificity may lead to overoptimistic estimates of test performance and introduce bias.
Reference standard:
- When the reference standard is not 100% sensitive and 100% specific, disagreements between the index test and reference standard may be incorrect, which increases the risk of bias.
- This item is similar to “blinding” in intervention studies. The potential for bias is related to the subjectivity of index test interpretation and the order of testing.
Flow and timing:
- If there is a delay or if treatment is started between index test and reference standard, misclassification may occur due to recovery or deterioration of the condition, which increases the risk of bias.
- If the results of the index test influence the decision on whether to perform the reference standard or which reference standard is used, estimated diagnostic accuracy may be biased.
- All patients who were recruited into the study should be included in the analysis, if not, the risk of bias is increased.
Judgement on applicability:
Patient selection: there may be concerns regarding applicability if patients included in the study differ from those targeted by the review question, in terms of severity of the target condition, demographic features, presence of differential diagnosis or co-morbidity, setting of the study and previous testing protocols.
Index test: if index tests methods differ from those specified in the review question there may be concerns regarding applicability.
Reference standard: the reference standard may be free of bias but the target condition that it defines may differ from the target condition specified in the review question.
Evidence table for diagnostic test accuracy studies
Research question:
|
Study reference |
Study characteristics |
Patient characteristics |
Index test (test of interest) |
Reference test
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Aykan, 2013 |
Type of study[1]: cohort study
Setting: in- and outpatients
Country: Turkey
Conflicts of interest: not reported
|
Inclusion criteria: Acute STEMI patients within 12 hours of symptom onset
Exclusion criteria: Patients with previous coronary artery bypass
N= 402
Prevalence: 32%
Mean age ± SD: 63 ± 13
Sex: 76 % M |
Describe index test: SYNTAX score
Comparator test[2]: Mehran score |
Describe reference test[3]: ≥25% increase of serum creatinine concentrations form baseline within 72 hours after PCI
|
Time between the index test en reference test: 72 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available)4:
Mehran: Sens: 73% Spec: 89%
SYNTAX: Sens: 79% Spec: 89%
Mehran: Cut-off value: 12.5 AUC: 0.68 (95% CI: 0.63 – 0.74, p<0.001)
SYNTAX: Cut-off value: 31.5 AUC: 0.66 (95% CI: 0.60 – 0.71, p<0.001) |
Internal validation only
Patients with previous coronary artery bypass were excluded |
|
Bartholomew, 2004 |
Type of study: cohort
Setting: in- and outpatients
Country: United States of America
Conflicts of interest: commercial
|
Inclusion criteria: Coronary interventional procedures (single center)
Exclusion criteria: -
N= 10 481
Incidence of events: Derivation cohort: 2.8% Validation cohort: 1.2%
Mean age ± SD: 65 ± 12
Sex: 67% M |
Describe index test: RCIN risk score
|
Describe reference test: ≥1.0mg/dL increase in serum creatinine from baseline within 48 hours of PCI
|
Time between the index test en reference test: 48 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
External validation Cohort 1: patients admitted for elective PCI N=2689 Discrimination: 0.59 Calibration: NR
Cohort 2: patients admitted for elective or emergency PCI N=488 Discrimination: 0.58 Calibration: NR |
|
|
Chen, 2014 |
Type of study[4]: cohort study
Setting: in- and outpatients
Country: China
Conflicts of interest: not reported
|
Inclusion criteria: patients receiving PCI, single center
Exclusion criteria: -
N=1500
ncidence of events: Derivation cohort: 16% Validation cohort: 17%
Mean age ± SD: 64 ± 10
Sex:68 % M |
Describe index test: “preprocedural risk scoring system” |
Describe reference test: >0.5 mg/dL (44.2µmol/L) or 25% increase in serum creat8inine within 5 days of PCI
|
Time between the index test en reference test: 5 days
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
Discrimination/calibration: 0.82 P=0.89
Risk score range associated with PC-AKI risk: Low: 5.3% Moderate: 19.9% High: 32.5% Very high: 59.5%
|
Internal validation only |
|
Fu, 2012 |
Type of study[5]: cohort study
Setting: in- and outpatients
Country: China
Conflicts of interest: not reported
|
Inclusion criteria: patients undergoing PCI, single center
Exclusion criteria: -
N= 668
Prevalence: 16%
Mean age ± SD: 70 ± 6
Sex: 48% M |
Describe index test: “risk score for contrast induced nephropathy in elderly patients”
|
Describe reference test: >0.5 mg/dL (44.2µmol/L) or 25% increase in serum creatinine within 48-72 hours of PCI
|
Time between the index test en reference test: 72 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
External validation Elderly patients at same institution N=277 Discrimination: 0.79 Calibration: p>0.05 |
|
|
Gao, 2004 |
Type of study[6]: cohort study
Setting: in- and outpatients
Country: China
Conflicts of interest: not reported
|
Inclusion criteria: Coronary angiography or PCI, single center
Exclusion criteria: -
N=2764
Incidence of events: Derivation cohort: 5.5% Validation cohort: 5.0%
Mean age ± SD: 60 ± 11
Sex: 71% M |
Describe index test: “simple risk score for prediction of CIN”
Comparator test: Mehran risk score |
Describe reference test: >0.5 mg/dL or 25% increase in serum creatinine within 72 hours of PCI |
Time between the index test en reference test: 72 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
Discrimination / calibration: 0.76 p>0.05
AUC: 1) “simple risk score”: 0.75 (95% CI: 0.71 – 0.78) 2) Mehran: 0.57 (95%CI:0.54 – 0.60)
Incidence of events: Derivation cohort: 4.6% Validation cohort: 4.2% |
Internal validation only |
|
Ghani, 2009 |
Type of study[7]: cohort study
Setting: in- and outpatients
Country: Kuwait
Conflicts of interest: not reported
|
Inclusion criteria: patients undergoing PCI, single center
Exclusion criteria:-
N= 247
Incidence of events: Derivation cohort: 5.5% Validation cohort: 5.0%
Mean age ± SD: 63 ± 10
Sex: 68% M |
Describe index test: “simple risk score for CIN” |
Describe reference test: >0.5 mg/dL increase in serum creatinine within 48 hours of PCI
|
Time between the index test en reference test: 48 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
Risk score range associated with PC-AKI: <4: 9.2% 5-8: 32% 9-12: 54% >12: 84%
|
Internal validation only |
|
Gurm, 2014 |
Type of study[8]: cohort study
Setting: in- and outpatients
Country: United States of America / the Netherlands
Conflicts of interest: not reported
|
Inclusion criteria: patients undergoing PCI, multiple center
Exclusion criteria: 1) patients on dialysis 2) patients with missing serum creatinine values
N= 48001
Prevalence: 3%
Mean age ± SD: 65 ± 12
Sex: NR |
Describe index test: “novel easy-to-use computational tool” |
Describe reference test: >0.5 mg/dL increase in serum creatinine within 7 days of PCI
|
Time between the index test en reference test: 7 days
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
AUC: 0.88
Risk score range associated with PC-AKI: Low: 0.5% Medium: 2.8% High: 13%
Incidence of events: Derivation cohort: 2.6% Validation cohort: 2.5% |
Internal validation only |
|
Inohara, 2014 |
Type of study[9]: cohort study
Setting: in- and outpatients
Country: Japan
Conflicts of interest: not reported |
Inclusion criteria:
Exclusion criteria:
N= 3957
Prevalence: 9%
Mean age ± SD: 69 ± 11
Sex: 79% M |
Describe index test: “pre-percutaneous cornary intervention risk model” |
Describe reference test: An increase in serum creatinine of 50% or 0.3mg/dL compared with baseline
|
Time between the index test en reference test: 30 days
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
External validation: N=1979 Discrimination: c-statistic 0.79 |
|
|
Ivanes, 2014 |
Type of study[10]: cohort study
Setting: in- and outpatients
Country: France
Conflicts of interest: not reported |
Inclusion criteria: PCI, single center
Exclusion criteria: -
N=322
Prevalence:9%
Mean age ± SD: 64 ± 14
Sex: 66% M |
Describe index test: Mehran risk score
|
Describe reference test: ≥25% or 44.2µmol/L increase in serum creatinine following contrast administration
|
Time between the index test en reference test: 48 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
AUC: 0.59 CIN incidence: 9% |
Internal validation only |
|
Jin, 2013 |
Type of study[11]: cohort study
Setting: in- and outpatients
Country: China
Conflicts of interest: not reported
|
Inclusion criteria: Acute myocardial infarction patients undergoing PCI
Exclusion criteria: -
N= 1041
Prevalence: 14%
Mean age ± SD: 68 ± 12
Sex: 52% M |
Describe index test: Mehran risk score |
Describe reference test: >0.5 mg/dL (44.2µmol/L) or 25% increase in serum creatinine within 48 hours of PCI
|
Time between the index test en reference test: 48 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
Risk score range associated with PC-AKI: Low: 12% Medium: 35% High: 36%
|
Internal validation only |
|
Kul, 2015 |
Type of study[12]: cohort study
Setting: in- and outpatients
Country: Turkey
Conflicts of interest: not reported
|
Inclusion criteria: patients with acute STEMI and undergoing emergency PCI
Exclusion criteria: -
N= 314
Prevalence: 12%
Mean age ± SD: 56 ± 11
Sex: 81% M |
Describe index test: Zwolle risk score
Comparator test: Mehran risk score |
Describe reference test: >0.5 mg/dL or 25% increase in serum creatinine within 72 hours of PCI
|
Time between the index test en reference test: 72 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
1) Zwolle score >2 Sens: 76% Spec: 75% AUC: 0.85
2) Mehran score > 5 Sens: 71% Spec: 74% AUC:0.79 |
Internal validation only |
|
Lin, 2015 |
Type of study[13]: cohort study
Setting: in- and outpatients
Country: Taiwan / Egypt
Conflicts of interest: not reported
|
Inclusion criteria: PCI, single center (including emergency PCI)
Exclusion criteria: -
N= 516
Prevalence: 12%
Mean age ± SD: 64 ± 11
Sex: 83% M
|
Describe index test: 1) “comprehensive risk score model”, WHC model 2) Bartholomew model 3) Mehran model 4) Tziakas model 5) Ghain model
|
Describe reference test: >0.5 mg/dL (44.2µmol/L) or 25% increase in serum creatinine within 72 hours of PCI
|
Time between the index test en reference test: 72 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
AUC: 1) own model: 0.92 (95%CI: 0.88 – 0.96) 2) Bartholomew model 0.91 (95%CI: 0.87 – 0.95) 3) Mehran model: 0.90 (95%CI: 0.86 – 0.94) 4) Tziakas model: 0.70 (95%CI: 0.58 – 0.83) 5) Ghain model: 0.65 (95% CI: 0.53 – 0.78)
External validation: n=241 Discrimination and calibration NR |
|
|
Maioli, 2010 |
Type of study[14]: cohort study
Setting: in- and outpatients
Country: Italy
Conflicts of interest: not reported
|
Inclusion criteria: patients with an indication for coronary angiography or PCI, single center
Exclusion criteria: -
N=1281
Prevalence: 3%
Mean age ± SD: 69 ± 10
Sex: 67% M
|
Describe index test: Global Registry for Acute Coronary Events (GRACE) risk score
Comparator test: Mehran risk score |
Describe reference test: >0.5 mg/dL (44.2µmol/L) or 25% increase in serum creatinine within 5 days of PCI
|
Time between the index test en reference test: 5 days
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
GRACE Cut-off 160 Sens: 79% Spec: 61%
Mehran NR
Incidence of events: Derivation cohort: 3.0% Validation cohort: NR
AUC: 1) GRACE: 0.72 (0.3) and 0.69 (0.5) 2) Mehran: 0.78 (0.3) and 0.84 (0.5)
External validation N=502 Discrimination and calibration NR |
Risk score range associated with PC-AKI risk: 0-1: 0% 2-3: 1% 4: 2% 5: 6% 6: 12% 7: 19% 8: 24% 9: 36% 10: 50%
|
|
Marenzi, 2004 |
Type of study[15]: cohort study
Setting: in- and outpatients
Country: Italy
Conflicts of interest: not reported
|
Inclusion criteria: patients referred for PCI for STEMI, single center
Exclusion criteria:
N= 218
Incidence of events: Derivation cohort: 19% Validation cohort: 14% M |
Describe index test: Marenzi risk score |
Describe reference test: >0.5 mg/dL increase in serum creatinine within 5 days of PCI
|
Time between the index test en reference test: 5 days
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
External validation N=891 Discrimination 0.57 and calibration NR
|
|
|
Mehran, 2004 |
Type of study[16]: cohort study
Setting: in- and outpatients
Country: United States of America
Conflicts of interest: not reported
|
Inclusion criteria: patients referred for PCI, single center
Exclusion criteria: -
N= 5571
Prevalence: 14%
Mean age ± SD: 64 ± 11
Sex: 71% M
|
Describe index test: Mehran risk score |
Describe reference test: >0.5 mg/dL or 25% increase in serum creatinine within 48 hours of PCI
|
Time between the index test en reference test: 48 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
For Creatinine: Discrimination: 0.69 Validation: p=0.43
For eGFR: Discrimination: 0.70 Validation: p=0.42
External validation Cohort 1: patients undergoing cardiac catheterization or PCI, single center N=3945 Discrimination: 0.57 Calibration: NR
Cohort 2: patients admitted for elective or emergency PCI, single center N=5571 Discrimination: 0.59 Calibration: NR |
|
|
Mizuno, 2014 |
Type of study[17]: cohort study
Setting: in- and outpatients
Country: Japan
Conflicts of interest: not reported
|
Inclusion criteria: patients undergoing a PCI for STEMI, single center
Exclusion criteria: -
N= 102
Prevalence: 10%
Mean age ± SD: 62 ± 14
Sex: 78 % M |
Describe index test: Mehran Risk score
(and red cell distribution width) |
Describe reference test: >0.5 mg/dL or 25% increase in serum creatinine within 3 days of PCI
|
Time between the index test en reference test: 3 days
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
AUC Mehran: 0.72 (0.54 – 0.90)
|
Internal validation only |
|
Raposeiras-Roubín, 2013 |
Type of study[18]: cohort study
Setting: in- and outpatients
Country: Spain
Conflicts of interest: not reported
|
Inclusion criteria: Patients with myocardial infarction after corronary angiography
Exclusion criteria: -
N=202
Prevalence: 28%
Mean age ± SD: 63 ± 13
Sex: 75% M |
Describe index test: GRACE risk score |
Describe reference test: ≥25% or ≥0.3mg/dL (or 0.5) rise in serum creatinine levels after 72 hours
|
Time between the index test en reference test: 72 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
GRACE risk score >140 was an independent predictor of CIN |
Internal validation only |
|
Sgura, 2010 |
Type of study[19]: cohort study
Setting: in- and outpatients
Country: Italy
Conflicts of interest: not reported
|
Inclusion criteria: patients undergoing PCI for STEMI, single center
Exclusion criteria: -
N= 891
Prevalence: 14%
Mean age ± SD: 64 ± 13
Sex: 78% M |
Describe index test: Mehran risk score
Comparator test: Marenzi risk score |
Describe reference test: >0.5 mg/dL (44.2µmol/L) or 25% increase in serum creatinine within 48 hours of PCI
|
Time between the index test en reference test: 48 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
AUC Mehran: 0.57 (95% CI 0.52 – 0.62) Marenzi: 0.57 (95% CI 0.51 – 0.62) |
Internal validation only |
|
Tziakas, 2013 |
Type of study[20]: cohort study
Setting: in- and outpatients
Country: Greece
Conflicts of interest: not reported
|
Inclusion criteria: Elective or emergency PCI, single center
Exclusion criteria: -
N= 688
Incidence of events: Derivation cohort: 10% Validation cohort: 14%
Mean age ± SD: 64 ± 11
Sex: 74% M |
Describe index test: Tziakas score |
Describe reference test: >0.5 mg/dL or 25% increase in serum creatinine within 48 hours of PCI
|
Time between the index test en reference test: 48 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
Calibration / discrimination: 0.76 p>0.05
External validation Cohort 1: PCI patient same single center N=200 Discrimination: 0.86 Calibration: NR
Cohort 2: patients admitted for elective or emergency PCI, multiple centers (tertiary care) N=2689 Discrimination: 0.70 Calibration: p=0.18 |
|
|
Tziakas, 2014 |
Type of study[21]: cohort study
Setting: in- and outpatients
Country: Greece
Conflicts of interest: not reported
|
Inclusion criteria: PCI, elective or urgent, multiple centers
Exclusion criteria: -
N=2882
Prevalence: 16%
Mean age ± SD: 61 ± 12
Sex: 70% M |
Describe index test: Tziakas score |
Describe reference test: >0.5 mg/dL or 25% increase in serum creatinine within 48 hours of PCI
|
Time between the index test en reference test: 48 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
AUC: 0.70
Risk score range associated with PC-AKI risk: ≤3: <20% >3: ≥20%
|
Internal validation only
|
|
Victor, 2014 |
Type of study[22]: cohort study
Setting: in- and outpatients
Country: India
Conflicts of interest: not reported
|
Inclusion criteria: patients with an indication for PCI, single center
Exclusion criteria: -
N=900
Incidence of events: Derivation cohort: 9.7% Validation cohort: 8.7%
Mean age ± SD: 57 v 10
Sex: 84% M |
Describe index test: “simple risk score for CIN” |
Describe reference test: >0.5 mg/dL or 25% increase in serum creatinine within 48 hours of PCI
|
Time between the index test en reference test: 48 hours
For how many participants were no complete outcome data available? NR
Reasons for incomplete outcome data described? NR |
Outcome measures and effect size (include 95%CI and p-value if available):
Sens: 94% Spec: 90%
External validation N=300 Sens: 92% Spec: 82% |
|
[1] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[2] Comparator test is vergelijkbaar met de C uit de PICO van een interventievraag. Er kunnen ook meerdere tests worden vergeleken. Voeg die toe als comparator test 2 etc. Let op: de comparator test kan nooit de referentiestandaard zijn.
[3] De referentiestandaard is de test waarmee definitief wordt aangetoond of iemand al dan niet ziek is. Idealiter is de referentiestandaard de Gouden standaard (100% sensitief en 100% specifiek). Let op! dit is niet de “comparison test/index 2”.
4 Beschrijf de statistische parameters voor de vergelijking van de indextest(en) met de referentietest, en voor de vergelijking tussen de indextesten onderling (als er twee of meer indextesten worden vergeleken).
[4] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[5] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[6] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[7] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[8] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[9] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[10] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[11] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[12] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[13] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[14] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[15] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[16] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[17] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[18] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[19] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[20] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[21] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[22] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
Verantwoording
Autorisatiedatum en geldigheid
Laatst beoordeeld : 01-11-2017
Laatst geautoriseerd : 01-11-2017
Geplande herbeoordeling : 01-12-2023
Validity
The board of the Radiological Society of the Netherlands will determine at the latest in 2023 if this guideline (per module) is still valid and applicable. If necessary, a new working group will be formed to revise the guideline. The validity of a guideline can be shorter than 5 years, if new scientific or healthcare structure developments arise, that could be seen as a reason to commence revisions. The Radiological Society of the Netherlands is considered the keeper of this guideline and thus primarily responsible for the actuality of the guideline. The other scientific societies that have participated in the guideline development share the responsibility to inform the primarily responsible scientific society about relevant developments in their field.
Initiative
Radiological Society of the Netherlands
Authorization
The guideline is submitted for authorization to:
- Association of Surgeons of the Netherlands
- Dutch Association of Urology
- Dutch Federation of Nephrology
- Dutch Society Medical Imaging and Radiotherapy
- Dutch Society of Intensive Care
- Netherlands Association of Internal Medicine
- Netherlands Society for Clinical Chemistry and Laboratory Medicine
- Netherlands Society of Cardiology
- Netherlands Society of Emergency Physicians
- Radiological Society of the Netherlands
Algemene gegevens
General Information
The guideline development was assisted by the Knowledge Institute of Medical Specialists (https://www.kennisinstituut.nl) and was financed by the Quality Funds for Medical Specialists (Kwaliteitsgelden Medisch Specialisten: SKMS).
Doel en doelgroep
Goal of the current guideline
The aim of the Part 1 of Safe Use of Iodine-containing Contrast Media guidelines is to critically review the present recent evidence with the above trend in mind, and try to formulate new practical guidelines for all hospital physicians to provide the safe use of contrast media in diagnostic and interventional studies. The ultimate goal of this guideline is to increase the quality of care, by providing efficient and expedient healthcare to the specific patient populations that may benefit from this healthcare and simultaneously guard patients from ineffective care. Furthermore, such a guideline should ideally be able to save money and reduce day-hospital waiting lists.
Users of this guideline
This guideline is intended for all hospital physicians that request or perform diagnostic or interventional radiologic or cardiologic studies for their patients in which CM are involved.
Samenstelling werkgroep
Working group members
A multidisciplinary working group was formed for the development of the guideline in 2014. The working group consisted of representatives from all relevant medical specialization fields that are involved with intravascular contrast administration.
All working group members have been officially delegated for participation in the working group by their scientific societies. The working group has developed a guideline in the period from October 2014 until July 2017.
The working group is responsible for the complete text of this guideline.
Working group
Cobbaert C., clinical chemist, Leiden University Medical Centre (member of advisory board from September 2015)
Danse P., interventional cardiologist, Rijnstate Hospital, Arnhem
Dekker H.M., radiologist, Radboud University Medical Centre, Nijmegen
Geenen R.W.F., radiologist, Noordwest Ziekenhuisgroep (NWZ), Alkmaar/Den Helder
Hoogeveen E.K., nephrologist, Jeroen Bosch Hospital, ‘s-Hertogenbosch
Kooiman J., research physician, Leiden University Medical Centre, Leiden
Oudemans - van Straaten H.M., internist-intensive care specialist, Free University Medical Centre, Amsterdam
Pels Rijcken T.H., interventional radiologist, Tergooi, Hilversum
Sijpkens Y.W.J., nephrologist, Haaglanden Medical Centre, The Hague
Vainas T., vascular surgeon, University Medical Centre Groningen (until September 2015)
van den Meiracker A.H., internist-vascular medicine, Erasmus Medical Centre, Rotterdam
van der Molen A.J., radiologist, Leiden University Medical Centre, Leiden (chairman)
Wikkeling O.R.M., vascular surgeon, Heelkunde Friesland Groep, location: Nij Smellinghe Hospital, Drachten (from September 2015)
Advisory board
Demir A.Y., clinical chemist, Meander Medical Center, Amersfoort, (member of working group until September 2015)
Hubbers R., patient representative, Dutch Kidney Patient Association
Mazel J., urologist, Spaarne Gasthuis, Haarlem
Moos S., resident in Radiology, HAGA Hospital, The Hague
Prantl K., Coordinator Quality & Research, Dutch Kidney Patient Association
van den Wijngaard J., resident in Clinical Chemistry, Leiden University Medical Center
Methodological support
Boschman J., advisor, Knowledge Institute of Medical Specialists (from May 2017)
Burger K., senior advisor, Knowledge Institute of Medical Specialists (until March 2015)
Harmsen W., advisor, Knowledge Institute of Medical Specialists (from May 2017)
Mostovaya I.M., advisor, Knowledge Institute of Medical Specialists
Persoon S., advisor, Knowledge Institute of Medical Specialists (March 2016 – September 2016)
van Enst A., senior advisor, Knowledge Institute of Medical Specialists (from January 2017)
Belangenverklaringen
Conflicts of interest
The working group members have provided written statements about (financially supported) relations with commercial companies, organisations or institutions that are related to the subject matter of the guideline. Furthermore, inquiries have been made regarding personal financial interests, interests due to personal relationships, interests related to reputation management, interest related to externally financed research and interests related to knowledge valorisation. The statements on conflict of interest can be requested at the administrative office of the Knowledge Institute of Medical Specialists and are summarised below.
|
Member |
Function |
Other offices |
Personal financial interests |
Personal relationships |
Reputation management |
Externally financed research |
Knowledge-valorisation |
Other potential conflicts of interest |
Signed |
|
Workgroup |
|||||||||
|
Burger |
Advisor, Knowledge Institute of Medical Specialists |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Cobbaert |
Member, physician clinical chemistry |
Head of clinical chemistry department in Leiden LUMC. Tutor for post-academic training of clinical chemists, coordinator/host for the Leiden region Member of several working groups within the Dutch Society for Clinical Chemistry and member of several international working groups for clinical chemistry |
None |
None |
Member of several working groups within the Dutch Society for Clinical Chemistry and member of several international working groups for clinical chemistry |
None |
None |
None |
Yes |
|
Danse |
Member, cardiologist |
Board member committee of Quality, Dutch society for Cardiology (unpaid) Board member Conference committee DRES (unpaid) |
None |
None |
None |
None |
None |
None |
Yes |
|
Dekker |
Member, radiologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Geenen |
Member, radiologist |
Member Contrast Media Safety Committee of the European Society of Urogenital Radiology (unpaid, meetings are partially funded by CM industry))) |
None |
None |
None |
None |
None |
Has been a public speaker during symposia organised by GE Healthcare about contrast agents (most recently in June 2014) |
Yes |
|
Hoogeveen |
Member, nephrologist |
Member of Guideline Committee of Dutch Federation of Nephrology |
None |
None |
Member of Guideline Committee of Dutch Society for Nephrology |
Grant from the Dutch Kidney Foundation to study effect of fish oil on kidney function in post-MI patients |
None |
None |
Yes |
|
Kooiman |
Member, research physician |
Resident in department of gynaecology & obstetrics |
None |
None |
None |
None |
None |
None |
Yes |
|
Mostovaya |
Advisor, Knowledge Institute of Medical Specialists |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Oudemans – van Straaten |
Member, intensive care medical specialist Professor Intensive Care |
none |
None |
None |
None |
None |
None |
None |
Yes |
|
Pels Rijcken |
Member, interventional radiologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Sijpkens |
Member, nephrologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Vainas |
Member, vascular surgeon |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Van den Meiracker |
Member, internist vascular medicine |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Van der Molen |
Chairman, radiologist |
Member Contrast Media Safety Committee of the European Society of Urogenital Radiology (unpaid,CMSC meetings are partially funded by CM industry)) |
None |
None |
Secretary section of Abdominal Radiology; Radiological Society of the Netherlands (until spring of 2015) |
None |
None |
Receives Royalties for books: Contrast Media Safety, ESUR guidelines, 3rd ed. Springer, 2015 Received speaker fees for lectures on CM safety by GE Healthcare, Guerbet, Bayer Healthcare and Bracco Imaging (2015-2016) |
Yes |
|
Wikkeling |
Member, vascular surgeon |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Advisory Board |
|||||||||
|
Demir |
Member, physician clinical chemistry |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Hubbers |
Member, patient’s representative, Dutch Society of Kidney Patients |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Mazel |
Member, urologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Prantl |
Member, policy maker, Dutch Society of Kidney Patients |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Van den Wijngaard |
Member, resident clinical chemistry |
Reviewer for several journals (such as American Journal of Physiology) |
None |
None |
None |
None |
None |
None |
Yes |
Inbreng patiëntenperspectief
Patients’ perspective was represented, firstly by membership and involvement in the advisory board of a policy maker and a patients’ representative from the Dutch Kidney Patient Association. Furthermore, an online survey was organized by the Dutch Kidney Patient Association about the subject matter of the guideline. A summary of the results of this survey has been discussed during a working group meeting at the beginning of the guideline development process. Subjects that were deemed relevant by patients were included in the outline of the guideline. The concept guideline has also been submitted for feedback during the comment process to the Dutch Patient and Consumer Federation, who have reported their feedback through the Dutch Kidney Patient Association.
Methode ontwikkeling
Evidence based
Implementatie
In the different phases of guideline development, the implementation of the guideline and the practical enforceability of the guideline were taken into account. The factors that could facilitate or hinder the introduction of the guideline in clinical practice have been explicitly considered. The implementation plan can be found with the Related Products. Furthermore, quality indicators were developed to enhance the implementation of the guideline. The indicators can also be found with the Related Products.
Werkwijze
AGREE
This guideline has been developed conforming to the requirements of the report of Guidelines for Medical Specialists 2.0; the advisory committee of the Quality Counsel. This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (www.agreetrust.org), a broadly accepted instrument in the international community and on the national quality standards for guidelines: “Guidelines for guidelines” (www.zorginstituutnederland.nl).
Identification of subject matter
During the initial phase of the guideline development, the chairman, working group and the advisor inventory the relevant subject matter for the guideline. Furthermore, an Invitational Conference was organized, where additional relevant subjects were suggested by the Dutch Kidney Patient Association, Dutch Society for Emergency Physicians, and Dutch Society for Urology. A report of this meeting can be found in Related Products.
Clinical questions and outcomes
During the initial phase of guideline development, the chairman, working group and advisor identified relevant subject matter for the guideline. Furthermore, input was acquired for the outline of the guideline during an Invitational Conference. The working group then formulated definitive clinical questions and defined relevant outcome measures (both beneficial land harmful effects). The working group rated the outcome measures as critical, important and not important. Furthermore, where applicable, the working group defined relevant clinical differences.
Strategy for search and selection of literature
For the separate clinical questions, specific search terms were formulated and published scientific articles were sought after in (several) electronic databases. Furthermore, studies were looked for by cross-referencing other included studies. The studies with potentially the highest quality of research were looked for first. The working group members selected literature in pairs (independently of each other) based on title and abstract. A second selection was performed based on full text. The databases search terms and selection criteria are described in the modules containing the clinical questions.
Quality assessment of individual studies
Individual studies were systematically assessed, based on methodological quality criteria that were determined prior to the search, so that risk of bias could be estimated. This is described in the “risk of bias” tables.
Summary of literature
The relevant research findings of all selected articles are shown in evidence tables. The most important findings in literature are described in literature summaries. When there were enough similarities between studies, the study data were pooled.
Grading the strength of scientific evidence
A) For intervention questions
The strength of the conclusions of the scientific publications was determined using the GRADE-method. GRADE stands for Grading Recommendations Assessment, Development and Evaluation (see http://www.gradeworkinggroup.org/) (Atkins, 2004).
GRADE defines four gradations for the quality of scientific evidence: high, moderate, low or very low. These gradations provide information about the amount of certainty about the literature conclusions. (http://www.guidelinedevelopment.org/handbook/).
B) For diagnostic, etiological, prognostic or adverse effect questions, the GRADE-methodology cannot (yet) be applied. The quality of evidence of the conclusion is determined by the EBRO method (van Everdingen, 2004)
Formulating conclusion
For diagnostic, etiological, prognostic or adverse effect questions, the evidence was summarized in one or more conclusions, and the level of the most relevant evidence was reported. For intervention questions, the conclusion was drawn based on the body of evidence (not one or several articles). The working groups weighed the beneficial and harmful effects of the intervention.
Considerations
Aspects such as expertise of working group members, patient preferences, costs, availability of facilities, and organization of healthcare aspects are important to consider when formulating a recommendation. These aspects were discussed in the paragraph Considerations.
Formulating recommendations
The recommendations answer the clinical question and were based on the available scientific evidence and the most relevant considerations.
Constraints (organization of healthcare)
During the development of the outline of the guideline and the rest of the guideline development process, the organization of healthcare was explicitly taken into account. Constraints that were relevant for certain clinical questions were discussed in the Consideration paragraphs of those clinical questions. The comprehensive and additional aspects of the organization of healthcare were discussed in a separate chapter.
Development of quality indicators
Internal (meant for use by scientific society or its members) quality indicators are developed simultaneously with the guideline. Furthermore, existing indicators on this subject were critically appraised; and the working group produces an advice about such indicators. Additional information on the development of quality indicators is available by contacting the Knowledge Institute for Medical Specialists. (secretariaat@kennisinstituut.nl).
Knowledge Gaps
During the development of the guideline, a systematic literature search was performed the results of which help to answer the clinical questions. For each clinical question the working group determined if additional scientific research on this subject was desirable. An overview of recommendations for further research is available in the appendix Knowledge Gaps.
Comment- and authorisation phase
The concept guideline was subjected to commentaries by the involved scientific societies. The commentaries were collected and discussed with the working group. The feedback was used to improve the guideline; afterwards the working group made the guideline definitive. The final version of the guideline was offered for authorization to the involved scientific societies, and was authorized.
References
Atkins D, Eccles M, Flottorp S, et al. GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004 Dec 22;4(1):38.
Van Everdingen JJE, Burgers JS, Assendelft WJJ, et al. Evidence-based richtlijnontwikkeling. Bohn Stafleu van Loghum. Houten, 2004
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
