Delayed breast reconstruction after RT
Question
What is ideal timing for delayed breast reconstruction after previous radiation therapy?
Recommendation
For delayed breast reconstruction after previous radiation therapy:
- Wait until the acute side-effects of radiation therapy have resolved before performing breast reconstruction.
- It is preferable not to perform reconstruction with an implant only.
- Add non-irradiated tissue to cover the implant (e.g. an LD flap) if full autologous reconstruction is not chosen.
Considerations
It is possible that the risk of postoperative wound healing problems and microvascular complications is greater if delayed autologous reconstruction is performed within one year of previous radiation therapy. There are no randomized studies examining the effect of timing of radiation therapy in relation to delayed breast reconstruction. Based on the studies mentioned above (Momoh et al, 2012; Baumann et al, 2011), no firm conclusions may be drawn regarding the optimal time period between radiation therapy and delayed autologous breast reconstruction. The recommendation is to wait until any acute side-effects of previous radiation therapy have fully resolved before reconstructive surgery is performed. The time to healing depends on a number of factors, including patient related factors such as smoking and genetics, and non-patient related factors such as fractional and total dose, the use of bolus material, radiation volume and concurrent chemotherapy (Kraus-Tiefenbacher et al, 2012; Fiets et al, 2003).
Breast reconstruction using autologous tissue is primarily used to improve the quality of the tissue after previous radiation therapy, and thus improve chances of successful reconstruction (Lindegren et al, 2012). The chance of postoperative complications after a delayed implant reconstruction after previous radiation therapy is greater than after delayed autologous or combined implant-autologous reconstruction (such as latissimus dorsi with implant). Two retrospective studies examined the effect of previous radiation therapy on the type of breast reconstruction; using an implant, a latissimus dorsi flap with implant and an autologous breast reconstruction. Common outcome measures were capsular contracture and implant loss (Chang et al, 2008; Spear et al, 2007). Chang et al (2008) compared 776 implant reconstructions with 146 latissimus dorsi with implant and 78 free TRAM flap with implant reconstructions (follow-up 7-216 months) and analyzed the effect of radiation therapy before and after reconstruction. They found a clearly elevated risk of implant complications and failure for the implant reconstruction if preceded by radiation therapy (42.4% vs. 10.9%, p<0.001). If a latissimus dorsi with implant reconstruction was performed after previous radiation therapy, the chances of reconstruction failure were 15.2% compared with 12.8% without radiation therapy (p=NS). If a free TRAM flap with implant reconstruction was performed after previous radiation therapy, the chances of reconstruction failure were 10.0% compared with 5.3% without radiation therapy (p=NS). A latissimus dorsi with implant reconstruction after prior radiation therapy failed less often (15.2%) compared with an implant alone after prior radiation therapy (42.2%; p=0.028). This also held true for a TRAM flap with implant reconstruction (10.0% versus 42.2%, p=0.015). The conclusion was that after prior radiation therapy, the addition of autologous tissue to an implant reconstruction can reduce the chances of complications and failure of the reconstruction (Chang et al, 2008).
In a smaller, retrospective study, a latissimus dorsi with implant reconstruction was performed in 26 patients, and all patients received radiation therapy. Twenty-three patients underwent a delayed reconstruction and three an immediate reconstruction. Two patients suffered partial fat necrosis, one patient developed a hematoma, and three seroma formation. The study concluded that a latissimus dorsi with implant reconstruction after previous radiation therapy carries an acceptable risk of postoperative complications (Spear et al, 2007).
Levine et al (2012) evaluated the risks of postoperative complications in 75 patients with a delayed autologous reconstruction (average follow-up 22.7 months) versus 56 patients with a latissimus dorsi and implant reconstruction (average follow-up 32 months) after previous radiation therapy. Fewer complications were seen in the group with autologous reconstruction, but the difference was not statistically significant: failure of reconstruction due to total flap necrosis or infected prosthesis in 2.7% (2/75) versus 5.4% (3/56), partial flap necrosis in 4% (3/75) versus 7.1% (13/56), and minor complications (seroma and hematoma) in 22.7% (17/75) versus 23.2% (13/56), respectively (Levine et al, 2012).
Three retrospective studies reported on cosmetic results after delayed reconstruction with a latissimus dorsi with implant (Spear et al, 2007; Lindegren et al, 2012; McKeown et al, 2009). McKeown et al (2009) performed a retrospective follow-up study (37-81 months) in 24 women with ‘extended’ latissimus dorsi (ELD) breast reconstructions, 13 of whom had an immediate reconstruction followed by radiation therapy, and 11 a delayed reconstruction after previous radiation therapy. The cosmetic result was determined by panel assessments of patient photographs. The cosmetic result of a delayed ELD reconstruction preceded by radiation therapy was better than for immediate ELD reconstruction followed by radiation therapy (p=0.051). This was primarily determined by a better upper pole (p=0.017) and volume (p=0.036) of the breast (McKeown et al, 2009). In the study by Spear et al (2007), patients were presented with a score form with a self-assigned score for satisfaction with the cosmetic result (score from 1-10, 10 being the highest). An average score of 8.5 (range 7-10) was given at an average follow-up of 28.8 months. Lindegren et al (2012) studied the difference in satisfaction in 24 patients with a DIEP flap reconstruction versus 21 patients with a latissimus dorsi with implant after previous radiation therapy, in both patients and a panel of independent plastic surgeons. A validated survey (36 item short form health survey) and a patient satisfaction questionnaire were used. No significant difference was seen in the SF-36 outcomes or patient satisfaction between the two reconstruction groups, although differences were seen among the plastic surgeons. They scored the DIEP flap reconstruction group better on size (p=0.0024) and shape (p=0.039) of the reconstructed breast, while patients were more satisfied with the size (p=0.046) and shape (p=0.017) after latissimus dorsi with implant reconstruction. Regarding donor site scarring, the latissimus dorsi flap was scored higher than the DIEP flap by both patients (p=0.036) and surgeons (p=0.001).
Based on the literature above, delayed reconstruction using an implant without the addition of autologous tissue following prior radiation therapy is not the preferred approach, considering the high complication rates reported. In such a situation, a latissimus dorsi or free TRAM flap with implant reconstruction may have a lower complication rate and an acceptable cosmetic result (Spear et al, 2007; Lindegren et al, 2012; McKeown et al, 2009; Chang et al, 2008). The effect of the timing of a latissimus dorsi reconstruction following previous radiation therapy on wound problems and complications is unclear based on the literature, but based on general wound healing principles, there is no reason to assume that wound problems and infections after a latissimus dorsi flap reconstruction and radiation therapy would differ significantly from microsurgical reconstruction. This is a knowledge gap that warrants further research.
Evidence
Background
It is unclear whether the time period between last radiation therapy and delayed reconstruction is related to the risk of complications. If this relationship is present, there is a need to define the minimum waiting period before performing a delayed reconstruction. Currently, the timing of delayed reconstruction after previous radiation therapy is determined based on personal preferences.
Conclusions / Summary of Findings
|
very low (GRADE) |
Total flap necrosis
It is unclear whether there is a difference in the risk of total flap necrosis if a delayed autologous breast reconstruction is performed within one year after radiation therapy or at least one year after radiation therapy.
Sources (Momoh et al, 2012; Baumann et al, 2011) |
|
very low (GRADE) |
Partial flap necrosis
There appears to be no difference in the risk of partial flap necrosis if a delayed autologous breast reconstruction is performed within one year after radiation therapy or at least one year after radiation therapy.
Sources (Momoh et al, 2012; Baumann et al, 2011) |
|
very low (GRADE) |
Microvascular thrombosis resulting in repeat intervention
The risk of microvascular thrombosis resulting in repeat intervention appears to be higher if a delayed autologous breast reconstruction is performed within one year after radiation therapy and not at least one year after radiation therapy.
Sources (Momoh et al, 2012; Baumann et al, 2011) |
|
very low (GRADE) |
Wound healing problems (wound dehiscence)
There appears to be no difference in the risk of wound healing problems if a delayed autologous breast reconstruction is performed within one year after radiation therapy compared to at least one year after radiation therapy.
Sources (Momoh et al, 2012; Baumann et al, 2011) |
|
very low (GRADE) |
Fat necrosis
There may be a lower risk of fat necrosis if a delayed autologous reconstruction is performed within one year and not at least one year after radiation therapy.
Sources (Momoh et al, 2012; Baumann et al, 2011) |
|
very low (GRADE) |
Postoperative wound infection
The risk of infection may double if a delayed autologous breast reconstruction is performed within one year and not at least one year after radiation therapy.
Sources (Momoh et al, 2012; Baumann et al, 2011) |
|
- |
Aesthetic result
There is no evidence from comparative studies on the effect of delayed autologous breast reconstruction within one year after radiation therapy compared with delayed reconstruction at least one year after radiation therapy on aesthetic result. |
Literature summary
Two retrospective cohort studies (Momoh et al, 2012; Baumann et al, 2011) compared the negative effects of delayed autologous breast reconstruction within one year after radiation therapy to those of waiting at least one year after radiation therapy. A total of 289 patients were examined. The patients in the ‘at least one year after radiation therapy’ group had a mean follow-up of 28 months (Momoh et al, 2012) and 25 months (Baumann et al, 2011). The patients in the ‘within one year after radiation therapy’ group had a mean follow-up of 39 months (Momoh et al, 2012) and 31 months (Baumann et al, 2011).
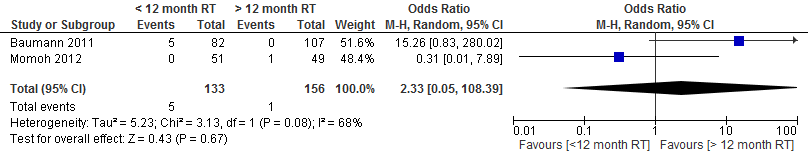
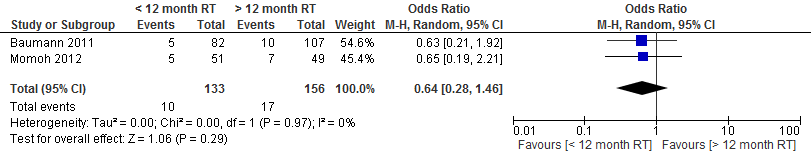
The pooled result at a cut-off point of 1 year shows that the risk of total flap necrosis was more than twice as high if delayed autologous breast reconstruction was performed within one year after radiation therapy (not statistically significant, see figure 1).
Figure 1. Total flap necrosis
Momoh et al studied the outcome measure partial flap necrosis. Of the patients who underwent a reconstruction within one year after radiation therapy, 3.9% (2/51) had partial flap necrosis versus none (0.49) of the patients who underwent reconstruction at least one year after radiation therapy (p=1.00).
The pooled result at a cut-off point of 1 year showed no difference in the incidence of partial flap necrosis if a delayed autologous breast reconstruction is performed within one year or at least one year after radiation therapy (see figure 2).
Figure 2. Partial flap necrosis
Momoh et al studied the outcome measure microvascular thrombosis. Of the patients who underwent a reconstruction within one year after radiation therapy, 5.8% (3/51) had microvascular thrombosis versus 2% (1/83) of the patients who underwent reconstruction at least one year after radiation therapy (p=0.53).
The pooled result showed that the chance of microvascular thrombosis resulting in a repeat intervention is two and a half times higher if a delayed autologous breast reconstruction is performed within one year after radiation therapy (not statistically significant, see figure 3).
Figure 3. Microvascular thrombosis
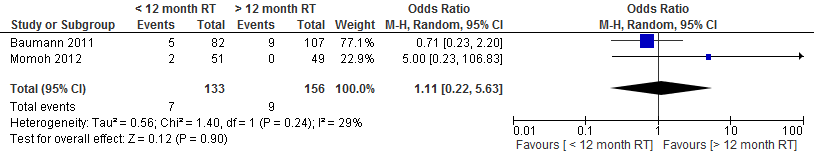
Momoh et al studied the outcome measure wound dehiscence. Of the patients who underwent a reconstruction within one year after radiation therapy, 13.7% (7/51) had wound dehiscence versus 8.2% (4/83) of the patients who underwent reconstruction at least one year after radiation therapy (p=1.00).
The pooled result showed no difference in the incidence of wound dehiscence if a delayed autologous breast reconstruction is performed within one year or at least one year after radiation therapy (see figure 4).
Figure 4. Wound dehiscence
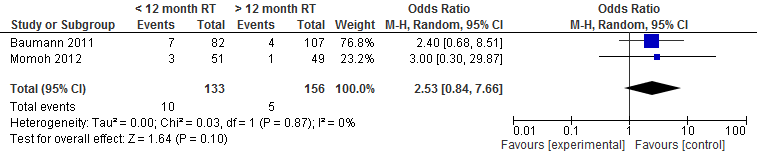
Momoh et al studied the outcome measure fat necrosis. Of the patients who underwent a reconstruction within one year after radiation therapy, 9.8% (5/51) had fat necrosis versus 14.3% (7/83) of the patients who underwent reconstruction at least one year after radiation therapy (p=0.89).
The pooled result showed that the chance of fat necrosis almost halves if a delayed autologous breast reconstruction is performed within one year after radiation therapy (not statistically significant, see figure 5).
Figure 5. Fat necrosis
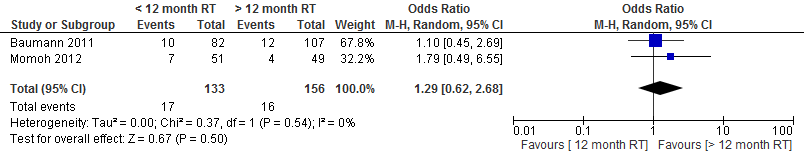
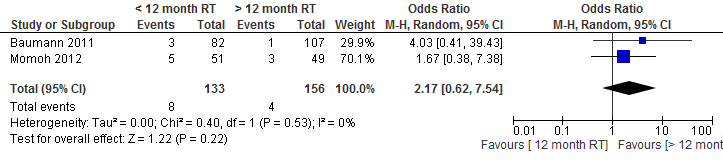
Momoh et al studied the outcome measure wound infection. Of the patients who underwent a reconstruction within one year after radiation therapy, 9.8% (5/17) had a wound infection versus 6.1% (3/83) of the patients who underwent reconstruction at least one year after radiation therapy (p=0.72).
The pooled result showed that the chance of postoperative wound infection doubles if a delayed autologous breast reconstruction is performed within one year after radiation therapy (not statistically significant, see figure 6).
Figure 6. Wound infection
Aesthetic result was not a studied outcome measure.
Level of evidence of the literature
The level of evidence for the outcome measure flap necrosis is very low, as the studies were not randomized (highly significant limitations in study design), the results were conflicting (I2 = 68%; heterogeneity) and the number of patients is low (imprecision).
The level of evidence for the outcome measure partial flap necrosis, microvascular thrombosis, wound healing problems (wound dehiscence), fat necrosis and postoperative wound infections is very low, as the studies are not randomized (highly significant limitations in study design) and the number of patients and complications is low (imprecision).
The level of evidence for the outcome measure aesthetic result was not assessed, as there are no comparative studies with this outcome measure.
Search and select
In order to answer the primary question, a systematic literature review was performed for the following question:
What are the effects of delayed breast reconstruction within one year after radiation therapy on aesthetic result and the incidence of postoperative complications, compared with delayed reconstruction at least one year after radiation therapy?
Medline (OVID) and Embase databases were searched for delayed breast reconstructions after radiation therapy. The search justification is listed in the appendix. The literature search yielded 58 results. Studies that met the following selection criteria were included in the literature summary: original studies; comparative studies; systematic review of comparative studies; comparison of delayed breast reconstruction within one year after radiation therapy to delayed breast reconstruction at least one year after radiation therapy, and at least one of the following outcome measures: aesthetic result or postoperative complications.
Two studies were included in the literature analysis (Momoh et al, 2012 en Baumann et al, 2011). The evidence tables for these studies are appended.
Methods
Authorization date and validity
Last review : 01-03-2015
The Board of the Dutch Society for Plastic and Reconstructive Surgery (NVPC) will assess whether this guideline is still up-to-date in 2018 at the latest. If necessary, a new working group will be appointed to revise the guideline. The guideline’s validity may lapse earlier if new developments demand revision at an earlier date.
As the holder of this guideline, the NVPC is chiefly responsible for keeping the guideline up to date. Other scientific organizations participating in the guideline or users of the guideline share the responsibility to inform the chiefly responsible party about relevant developments within their fields.
General details
Guideline development was funded by the Quality Fund for Medical Specialists (SKMS) and The Netherlands Organization for Health Research and Development (ZonMw).
Scope and target group
Guideline goal
To develop a multidisciplinary, evidence-based guideline for breast reconstruction in women undergoing breast conserving therapy or mastectomy for breast cancer, or following prophylactic mastectomy.
Guideline scope
The guideline focuses on all patients with an indication for breast reconstruction following breast conserving therapy or mastectomy. Additionally, the guideline may be applied to breast reconstruction in patients who have undergone surgical treatment for a benign breast condition. The guideline does not comment on the treatment of breast cancer. We refer the reader to the NABON guideline for the treatment of breast cancer (www.richtlijnendatabase.nl), which this guideline complements.
Unfortunately, circumstances did not permit a medical oncologist representing the NVMO to participate in the working group. Thus, the current version lacks a module on chemotherapy and breast reconstruction. The working group strives to create such a module for this guideline in the near future.
Intended audience for the guideline
The guideline aims to provide practical guidance for plastic surgeons and members of the multidisciplinary breast cancer team (surgical oncologist, medical oncologist, radiation oncologist, radiologist, pathologist, psychologist, breast care nurse specialist). A version for patients has recently been developed (https://www.b-bewust.nl/pif_borstreconstructie).
Samenstelling werkgroep
A multidisciplinary working group was appointed to develop the guideline in October 2011, consisting of representatives from all relevant specialties involved in the care for patients with breast reconstruction (see above for working group membership). Working group members were mandated by their professional organizations. The working group worked on developing the guideline for 2 years. The working group is responsible for the full text of this guideline.
- Dr. M.A.M. Mureau (President), MD, PhD, plastic surgeon, Erasmus MC Cancer Institute, Erasmus University Medical Center Rotterdam
- Professor Dr. R.R.W.J. van der Hulst, MD, PhD, plastic surgeon, Maastricht University Medical Center/Orbis Medical Center/Viecuri Medical Center, Maastricht
- Dr. L. A.E. Woerdeman, MD, PhD, plastic surgeon, Antoni van Leeuwenhoek / Netherlands Cancer Institute, Amsterdam
- Drs. A.A.W.M van Turnhout, MD, plastic surgeon, Tergooi Hospital, Hilversum Site
- N.A.S. Posch, MD, plastic surgeon, Haga Hospital, The Hague
- Dr. M.B.E. Menke-Pluijmers, MD, PhD, oncologic surgeon, Albert Schweitzer Hospital, Dordrecht
- Dr. E.J.T. Luiten, MD, PhD, oncologic surgeon, Amphia Hospital, Breda
- Drs. A.H. Westenberg, MD, radiotherapist/oncologist, Arnhem Radiotherapy Institute, Arnhem
- Dr. J.P. Gopie, PhD, psychologist, Leiden University Medical Center, Leiden
- Dr. H.M. Zonderland, MD, PhD, radiologist, Academic Medical Center, Amsterdam
- Drs. M. Westerhof, MSc, Netherlands Breast Cancer Association, Utrecht
- E.M.M. Krol-Warmerdam MA, V&VN Nurse Specialists, Leiden University Medical Center, Leiden
With support from
- Drs. B.S. Niël-Weise, MD, microbiologist / epidemiologist, senior advisor, Knowledge Institute for Medical Specialists
Declaration of interest
Working group members declared any (financial) ties with commercial companies, organizations or institutions involved in the field covered by the guideline for the past five years in writing. An overview is available on request from the office of the Knowledge Institute for Medical Specialists (KIMS).
Patient involvement
Patients are represented by a delegate from the Netherlands Breast Cancer Association in this guideline.
Method of development
Implementation
Guideline implementation and practical applicability of the recommendations was taken into consideration during various stages of guideline development. Factors that may promote or hinder implementation of the guideline in daily practice were given specific attention.
The guideline is distributed digitally among all relevant professional groups. The guideline can also be downloaded from the Dutch Society for Plastic and Reconstructive Surgery website: www.nvpc.nl, the guideline website: www.richtlijnendatabase.nl and the Quality Organization for Medical Specialists.
Methods and proces
AGREE
The guideline has been drafted in accordance with the requirements outlined in the ‘Guidelines 2.0’ report of the Guideline Advisory Committee of the Council on Science, Education and Quality (WOK). This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (www.agreecollaboration.org), an instrument designed to assess the quality of guidelines with broad international support.
Primary questions and outcome measures
Based on the outcomes of the bottleneck analysis, the president and advisor formulated draft primary questions. These were discussed and defined together with the working group. Subsequently, the working group determined which outcome measures were relevant for the patient for each primary question, examining both desired and undesirable effects. The working group valuated these outcomes based on their relative importance as crucial, important and unimportant.
Literature search and selection strategy
Specific search terms were used to identify published scientific studies related to each individual primary question in Medline, Cochrane and, where necessary, Embase. Additionally, the references of the selected articles were screened for additional relevant studies. Studies offering the highest level of evidence were sought out first. Working group members selected articles identified by the search based on predetermined criteria. The selected articles were used to answer the primary question. The searched databases, the search string or terms used during the search and selection criteria applied are listed in the chapter for each individual primary question.
Quality assessment of individual studies
Individual studies were assessed systematically based on predefined methodological quality criteria in order to assess the risk of biased study results. These assessments may be found in the column ‘Study quality assessment’ in an evidence table.
Literature summary
The relevant study results from all selected articles were presented clearly in evidence tables. The key findings from the literature are described in the literature summary. If studies were sufficiently similar in design, data were also summarized quantitatively (meta-analysis) using Review Manager 5.
Assessment of the level of scientific evidence
A) With regard to intervention questions:
The level of scientific evidence was determined using the GRADE method. GRADE is short for ‘Grading Recommendations Assessment, Development and Evaluation’ (see http://www.gradeworkinggroup.org/) (Atkins et al, 2004).
B) With regard to questions about the value of diagnostic tests, harm or adverse effects, etiology and prognosis:
GRADE cannot be used (yet) for these types of questions. The level of evidence of the conclusion was determined based on the accepted EBRO method (van Everdingen et al, 2004).
Formulation of conclusions
With regard to questions about the value of diagnostic tests, harm or adverse effects, etiology and prognosis, the scientific evidence is summarized in one or more conclusions, listing the level of evidence for the most relevant data.
For interventions, the conclusion does not refer to one or more articles, but is drawn based on the body of evidence. The working group looked at the net benefits of each intervention. This was done by determining the balance between favorable and unfavorable effects for the patient.
Considerations
When making recommendations, scientific evidence was considered together with other key aspects, such as working group member expertise, patient preferences, costs, availability of facilities and/or organizational aspects. Insofar as they are not part of the systematic literature review, these aspects are listed under ‘Considerations’.
Formulation of recommendations
Recommendations provide an answer to the primary question, and are based on the best scientific evidence available and the most important considerations. The level of scientific evidence and the importance given to considerations by the working group jointly determine the strength of the recommendation. In accordance with the GRADE method, a low level of evidence for conclusions in the systematic literature review does not rule out a strong recommendation, while a high level of evidence may be accompanied by weak recommendations. The strength of the recommendation is always determined by weighing all relevant arguments.
Development of indicators
Along with developing a draft guideline, internal quality indicators were developed to allow monitoring of the implementation of the guideline in daily practice. More information about the method for indicator development may be requested from KIMS.
Knowledge gaps
During the development of this guideline, systematic searches were conducted for research contributing to answering the primary questions. For each primary question, the working group determined whether (additional) scientific research is desirable.
Commentary and authorization phase
The draft guideline was submitted to the (scientific) organizations involved for comment. The guideline was also submitted to the following organizations for comment: Netherlands Breast Cancer Association (BVN), Netherlands Society for Medical Oncology (NVMO), Dutch College of General Practitioners (NHG), Healthcare Insurers Netherlands (ZN), The Dutch Healthcare Authority (NZA), Health Care Insurance Board (CvZ), the Health Care Inspectorate (IGZ), Achmea, CZ, Menzis and VGZ. Comments were collected and discussed with the working group. The draft guideline was updated and finalized by the working group based on the comments. The final guideline was submitted for authorization to the (scientific) organizations involved and authorized by them.
Legal standing of guidelines
Guidelines are not legal prescriptions, but contain evidence-based insights and recommendations that care providers must meet in order to provide high quality care. As these recommendations are primarily based on ‘general evidence for optimal care for the average patient’, care providers may deviate from the guideline based on their professional autonomy when they deem it necessary for individual cases. Deviating from the guideline may even be necessary in some situations. If care providers choose to deviate from the guideline, this should be done in consultation with the patient, where relevant. Deviation from the guideline must always be justified and documented.
