Breast reconstuction and RT after mastectomy
Question
What is the strategy on immediate breast reconstruction and adjuvant radiation therapy (RT) following mastectomy?
Recommendation
For a patient with an indication for adjuvant radiation therapy:
- It is preferable not to perform immediate breast reconstruction with an implant.
- If immediate reconstruction is desired by the patient, autologous reconstruction is preferred.
Considerations
Immediate implant reconstruction followed by radiation therapy versus immediate implant reconstruction not followed by radiation therapy:
After completing the systematic search and analysis, a meta-analysis of the effects of adjuvant radiation therapy after immediate tissue expander-prosthesis reconstruction on reconstruction failure and capsular contraction was published (Lam et al, 2013). Twelve studies with a total of 1853 patients were used for the meta-analysis, of whom 715 had received radiation therapy and 1138 had not. Radiation therapy was mostly administered after full expansion of the tissue expander, but radiation therapy was only given after placement of the definite prosthesis in three of the studies.
The meta-analysis reveals the risk of prosthesis loss was 5.14 times higher if immediate implant reconstruction was followed by adjuvant radiation therapy compared with no RT (18.6% after RT versus 3.1% without RT; p<0.00001). The risk of prosthesis loss was 5.87 times higher if RT was given after full expansion of the tissue expander compared with no RT (29.7% after RT after tissue expander versus 5% without RT; p<0.00001). The risk of prosthesis loss was 5.32 times higher if RT was given after placement of the definite prosthesis compared with no RT (7.7% after RT after placement of a definite prosthesis versus 1.5% without RT; p=0.0003). The risk of prosthesis loss was 87% lower (OR 0.13) if RT was given after placement of the definite prosthesis instead of after full expansion of the tissue expander (5.6% versus 22.9%, respectively; p<0.0001).
The chance of severe capsular contracture was also significantly higher after immediate implant reconstruction followed by adjuvant radiation therapy: 8.9% after expansion of the tissue expander and RT versus 0.5% without RT (p=0.01), and 7.9% after placement of the definite prosthesis and RT versus 0.2% without RT (p=0.002).
Despite the fact the studies from the meta-analysis by Lam et al (2013) have several methodological limitations (see level of evidence for the literature), this meta-analysis clearly shows that adjuvant radiation therapy after immediate implant reconstruction results in an increased risk of reconstruction failure and capsular contracture. Furthermore, there are indications that the timing of radiation therapy has an effect on the chances of complications. Radiation therapy after placement of the definite prosthesis is associated with a lower risk of complications than radiation therapy after placement of the tissue expander (Lam et al, 2013). However, the question remains whether giving radiation therapy after placement of the definite prosthesis is practically feasible for the Dutch clinical practice. Adjuvant radiation therapy usually begins within six weeks after surgery, while filling the expander beings after about 2 weeks. This means there is only about one month for further filling of the expander and re-operation to place a definite prosthesis. Both the logistics and medical aspects of this represent major challenges. Additionally, the metal integrated valves of the tissue expanders may negatively impact the quality of radiation therapy.
Another prospective study in 141 patients who only underwent immediate tissue expander-prosthesis reconstruction, followed by adjuvant radiation therapy, examined the effect of various risk factors on reconstruction failure (Cowen et al, 2010). Three risk factors were identified: T3 or T4 tumor, smoking and positive axillary lymph nodes. The chance of reconstruction failure was 7%, 15.7%, 48.3% and 100% if 0, 1, 2 or 3 risk factors were present, respectively (Cowen et al, 2010).
Immediate autologous reconstruction followed by radiation therapy versus immediate autologous reconstruction not followed by radiation therapy:
Other than the elevated risk of developing fat necrosis, the risk of developing other complications after immediate autologous breast reconstruction does not appear to be increased by adjuvant radiation therapy according to the meta-analysis by Schaverien et al (2013). The chance of revision of the microsurgical anastomosis was not included in this meta-analysis, as this was generally not reported in the original studies. The aesthetic result of immediate autologous reconstruction, however, does not appear to be negatively affected by adjuvant radiation therapy.
Immediate autologous reconstruction followed by radiation therapy versus immediate implant reconstruction followed by radiation therapy:
If immediate autologous reconstruction is followed by adjuvant radiation therapy, the risk of re-operation, infection and moderate to poor aesthetic result appears to be lower compared with immediate implant reconstruction followed by radiation therapy (Anderson et al, 2004; Jhaveri et al, 2008;Wong et al, 2008; Strålman et al, 2008).
A prospective follow-up study in 73 patients with a latissimus dorsi + implant (LDI) or full autologous latissimus dorsi (ALD) breast reconstruction studied the effect of postoperative radiation therapy on complications and cosmetic result after three, six and twelve months, and then annually (Thompson et al, 2008). A LDI reconstruction followed by radiation therapy resulted in significantly more cases of capsular contracture (33%) compared with no need for radiation therapy (11%, p=0.048). During follow-up, progressive worsening of cosmetic result was seen after radiation therapy (p=0.0002), breast shape (p=0.0003), breast size (p=0.0003), cleavage (p=0.0009), scarring (p<0.0001) and skin discoloration (p<0.0001). ALD followed by radiation therapy led to fewer problems than LDI followed by radiation therapy (p=0.02), as did latissimus dorsi reconstructions (LDI and ALD) not followed by radiation therapy (p=0.02). Over time, the type of operation did not significantly affect overall cosmetic effect, size and shape of the reconstruction (p=0.02). The cosmetic result worsened between two and three years of follow-up, regardless of the surgical technique used or the use of radiation therapy.
Immediate autologous reconstruction followed by radiation therapy versus delayed autologous reconstruction with radiation therapy prior to surgery:
Schaverien et al (2013) also conducted a meta-analysis of the direct comparison between immediate autologous reconstruction followed by RT versus delayed autologous reconstruction after previous RT. In total, 16 studies with 2351 patients were included, including 891 patients with an immediate reconstruction followed by RT and 1460 patients with delayed reconstruction after previous RT. The meta-analysis showed that the risk of re-operation was 85% lower (OR=0.15) if a delayed autologous reconstruction was performed following previous RT compared with an immediate autologous reconstruction followed by RT (1.4% versus 15.1%, respectively; p=0.001). No difference was found in total number of complications, including fat necrosis (OR=1.13; 32.6% versus 32.5%; p=0.53), or fat necrosis (OR=0.63, 22.2% after immediate reconstruction versus 14.9% after delayed reconstruction; p=0.25). The elevated risk of re-operation after immediate autologous reconstruction and RT is very likely related more to the timing of the reconstruction (immediate versus delayed) than to RT, since the percentage of re-operations in the immediate reconstruction group with and without RT was similar (14.3% and 16.1%, respectively; Schaverien et al, 2013).
Aesthetic result was examined in six studies form the meta-analysis. Two studies reported a better aesthetic result after autologous reconstruction following previous RT compared with immediate autologous reconstruction followed by RT and four studies showed no difference (Schaverien et al, 2013).
In summary, the risk of immediate and late postoperative complications after immediate implant reconstruction followed by radiation therapy is elevated. After immediate autologous reconstruction, the negative effect of adjuvant radiation therapy is variable and less clear-cut. Most studies with immediate autologous reconstruction and radiation therapy found acceptable results. One meta-analysis of retrospective studies showed no difference in the incidence of postoperative complications or the number of repeat interventions. In conclusion, immediate autologous breast reconstruction is justifiable if there is an indication for adjuvant radiation therapy.
Evidence
Background
If it becomes clear during the preoperative multidisciplinary meeting that adjuvant radiation therapy (RT) of the breast/chest wall will likely or certainly be required, it is insufficiently clear whether immediate breast reconstruction is contraindicated.
Conclusions / Summary of Findings
1. Comparison of immediate implant reconstruction followed by radiation therapy versus immediate implant reconstruction without radiation therapy
|
- |
Local recurrence
No comparative research examined the effect of immediate implant reconstruction followed by radiation therapy versus immediate implant reconstruction not followed by radiation therapy on local recurrence. |
|
Very low (GRADE) |
Postoperative complications - capsular contracture
The risk of capsular contracture appears to be higher for immediate implant reconstruction followed by radiation therapy compared with immediate implant reconstruction without radiation therapy.
Sources (Tallet et al, 2003; Krueger et al, 2001; Cordeiro et al, 2004; Whitfield et al, 2009) |
|
Very low (GRADE) |
Postoperative complications - infection
The risk of infection appears to be higher for immediate implant reconstruction followed by radiation therapy compared with immediate implant reconstruction without radiation therapy.
Sources (Tallet et al, 2003; Krueger et al, 2001; Cordeiro et al, 2004) |
|
Very low (GRADE) |
Postoperative complications - re-operation
The risk of re-operation appears to be higher for immediate implant reconstruction followed by radiation therapy compared with immediate implant reconstruction without radiation therapy.
Sources (Tallet et al, 2003; Krueger et al, 2001; Cordeiro et al, 2004) |
|
Very low (GRADE) |
Aesthetic result
There appears to be a better aesthetic result for immediate implant reconstruction without radiation therapy compared with immediate implant reconstruction with postoperative radiation therapy.
Sources (Tallet et al, 2003; Krueger et al, 2001; Cordeiro et al, 2004; Nava et al, 2011) |
|
Very low (GRADE) |
Patient satisfaction
Patients who undergo immediate implant reconstruction appear to be more satisfied with the result if they do not receive adjuvant radiation therapy.
Sources (Nava et al, 2011) |
2. Comparison of immediate autologous reconstruction followed by radiation therapy versus immediate implant reconstruction without radiation therapy
|
Very low (GRADE) |
Postoperative complications - general
The risk of (general) complications appears to be similar if immediate autologous breast reconstruction is followed by radiation therapy compared with immediate autologous breast reconstruction not followed by radiation therapy.
Sources (Berry et al, 2010; Lee et a., 2010; Carlson et al, 2008; Spear et al, 2005; Williams et al, 1997) |
|
Very low (GRADE) |
Postoperative complications - fat necrosis
The risk of fat necrosis appears to be higher if immediate autologous breast reconstruction is followed by radiation therapy compared with immediate autologous breast reconstruction not followed by radiation therapy.
Sources (Lee et al, 2010; Carlson et al, 2008; Spear et al, 2005; Rogers and Allen, 2002; Tran et al, 2000; Williams et al, 1997) |
|
Very low (GRADE) |
Postoperative complications - re-operation
The risk of re-operation appears to be similar if immediate autologous breast reconstruction is followed by radiation therapy compared with immediate autologous breast reconstruction not followed by radiation therapy.
Sources (Carlson et al, 2008; Chatterjee et al, 2009; Rogers and Allen, 2002) |
|
Very low (GRADE) |
Aesthetic result
Aesthetic result appears to be worse if immediate autologous breast reconstruction is followed by radiation therapy compared with immediate autologous breast reconstruction not followed by radiation therapy.
Sources (Lee et al, 2010; Leonardi et al, 2010; Carlson et al, 2008; Thomson et al, 2008; Spear et al, 2005; Rogers and Allen, 2002; Tran et al, 2000) |
|
- (GRADE) |
Local recurrence, patient satisfaction
Comparative studies do not provide any information about differences in the incidence of local recurrence and patient satisfaction between immediate autologous breast reconstruction followed by radiation therapy and immediate autologous breast reconstruction not followed by radiation therapy. |
3. Comparison of immediate autologous reconstruction followed by radiation therapy versus immediate implant reconstruction followed by radiation therapy
|
Very low (GRADE) |
Postoperative complications - re-operation
The risk of re-operation appears to be higher if immediate implant reconstruction followed by radiation therapy is performed instead of immediate autologous reconstruction followed by radiation therapy.
Sources (Anderson et al, 2004; Christante et al, 2010; Jhaveri et al, 2008; Strålman et al, 2008; Wong et al, 2008) |
|
Very low (GRADE) |
Postoperative complications - infection
Patients with immediate implant reconstruction followed by radiation therapy appear to have a higher risk of infection than patients with immediate autologous reconstruction followed by radiation therapy.
Sources (Anderson et al, 2004; Wong et al, 2008) |
|
Very low (GRADE) |
Aesthetic result
Immediate autologous reconstruction followed by radiation therapy appears to yield a better aesthetic result than direct implant reconstruction followed by radiation therapy.
Sources ( Anderson et al, 2004; Jhaveri et al, 2008) |
|
- |
Patient satisfaction, local recurrence
No comparative research examined the effect of immediate autologous reconstruction followed by radiation therapy versus immediate implant reconstruction not followed by radiation therapy on patient satisfaction. |
Literature summary
1. Comparison of immediate implant reconstruction followed by radiation therapy versus immediate implant reconstruction without radiation therapy
One systematic review (Barry et al, 2011) of four comparative retrospective studies compared patients undergoing immediate breast reconstruction with a definite prosthesis or tissue expander followed by postoperative radiation therapy with patients who did not receive postoperative radiation therapy (Tallet et al, 2003; Krueger et al, 2001; Cordeiro et al, 2004; Whitfield et al, 2009). Average follow-up varied from 25 months (Tallet et al, 2003) to 51 months (Whitfield et al, 2009). As the review by Barry et al did not report the correct data from the individual studies, the data for the outcome measures aesthetic result and postoperative complications were extracted from the individual studies.
After the search date for the review by Barry, one additional study (Nava et al, 2011) was published and included in the literature analysis. This study compared three groups: a) 109 patients with an immediate reconstruction using a tissue expander and prosthesis who underwent radiation therapy after placement of the definite prosthesis, b) 50 patients who underwent immediate reconstruction with a tissue expander and prosthesis followed by radiation therapy after expansion of the tissue expander and c) 98 patients who underwent immediate reconstruction with a tissue expander and prosthesis without subsequent radiation therapy. Average follow-up was 50 months.
Local recurrence
The outcome measure local recurrence was not studied.
Capsular contracture
The pooled result (N=411) shows that the chances of capsular contracture are three times higher if immediate implant reconstruction with radiation therapy is performed (statistically significant, see figure 1). Cordeiro et al (2004) followed patients for at least one year after adjuvant radiation therapy.
Figure 1. Capsular contracture
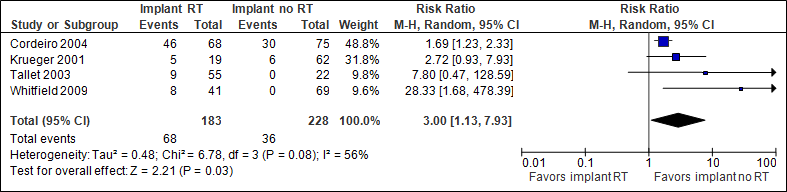
In the implant with RT group, the total number of capsular contractures was 372/1000, in the implant without RT group this was 158/1000.
Infection
The pooled result (N=781) shows that the chance of infection is twice as high if immediate implant reconstruction with radiation therapy is performed (statistically significant, see figure 2). Cordeiro et al (2004) followed patients for less than one year after adjuvant radiation therapy (reason for different N in the outcome measures).
Figure 2. Infection
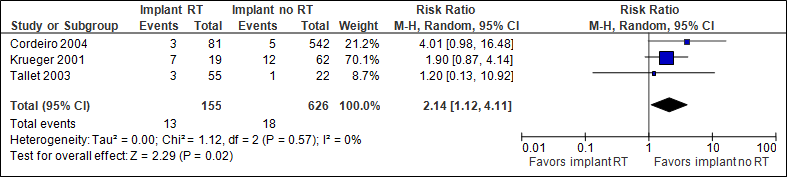
In the implant with RT group, the number of infections was 84/1000, in the implant without RT group this was 29/1000.
Re-operation
The pooled result (N=781) shows that the chance of re-operations is two and a half as high if immediate implant reconstruction with radiation therapy is performed (statistically significant, see figure 3). Cordeiro et al (2004) followed patients for less than one year after adjuvant radiation therapy (reason for different N in the outcome measures).
Figure 3. Re-operation
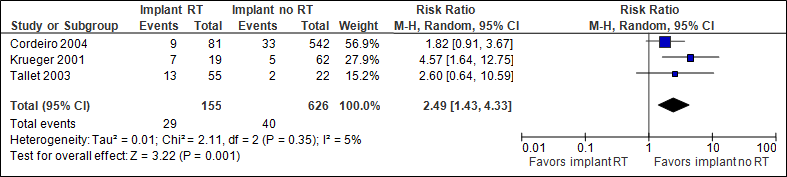
In the implant with RT group, the number of re-operations was 187/1000, in the implant without RT group this was 64/1000.
Fibrosis
The outcome measure fibrosis was not studied.
Aesthetic result
Four studies included aesthetic result as an outcome measure (Tallet et al, 2003; Krueger et al, 2001; Cordeiro et al, 2004; Nava et al, 2011). Aesthetic result was measured in a number of different ways.
In the study by Tallet et al (n=77), the aesthetic result was assessed by a physician based on five criteria: shape of the breast without a bra, contralateral symmetry, definition of the inframammary fold, mobility and consistency of the breast. A figure showed that 43% of patients with an implant reconstruction followed by adjuvant radiation therapy were graded as ‘good’. In the implant group without radiation therapy, this was 80% (the authors did not provide absolute numbers).
In the study by Kruger et al, the aesthetic result was assessed using a questionnaire. The questionnaire used a 5-point Likert scale, where 1 indicated high satisfaction and 5 low satisfaction. Only questionnaires with scores of 1 or 2 on all items were scored as ‘satisfied’. In the group that had received postoperative radiation therapy, 29% (4/14) of patients were satisfied, while 23% (10/43) of the group without postoperative radiation therapy was satisfied.
The study by Cordeiro et al does not describe how aesthetic result was measured. A distinction was made between excellent, good or poor to fair. Of all patients with an implant reconstruction followed by radiation therapy, 38% (25/66) were assessed as ‘excellent’. In the implant group without radiation therapy, this was 69% (52/75).
In the study by Nava et al (N=257), breast shape and symmetry were assessed by the surgeon and classified as good, moderate or poor. The outcome was generally scored as ‘good’ in the group that had not received postoperative radiation therapy (72.2% for shape and 46.1% for symmetry). In the group that had received immediate reconstruction using a tissue expander and prosthesis and had undergone radiation therapy after placement of the definite prosthesis, this was 58.7% and 28.8%, respectively. In the group that had undergone immediate reconstruction with a tissue expander and prosthesis followed by radiation therapy after expansion of the tissue expander, this was 30.8% and 15.4%, respectively (p=0.0009).
Comment: studies may have been missed for this outcome measure, because this literature analysis is an update of the review by Barry et al, and he did not include this outcome measure. The data were extracted from the individual studies.
Patient satisfaction
One study examined the outcome measure patient satisfaction (Nava et al 2011). Patients were asked to rate their reconstruction as good, moderate or poor. In the group that had not received postoperative radiation therapy, 68.1% of patients scored the result as good, while this was 52.2% in the group who had undergone immediate reconstruction using a tissue expander and prosthesis and had received RT after placement of the definite prosthesis, and 46.2% for reconstruction with a tissue expander and prosthesis followed by radiation therapy after expansion of the tissue expander (p=0.04).
Level of evidence of the literature
The level of evidence for the outcome measures postoperative complications (capsular contracture, infection, re-operation and fibrosis), aesthetic result and patient satisfaction is very low, as there are very serious limitations in study design (non-randomized studies) and due to the low number of patients (imprecision). Furthermore, statistical heterogeneity is present for the outcome measure capsular contracture (I2=56%). Furthermore, it is unclear whether the applied fractioning schedules and radiation therapy techniques are comparable to those used in The Netherlands, and whether the incidence of adverse events after radiation therapy can be extrapolated to the Dutch situation.
The level of evidence for the outcome measure local recurrence was not assessed, as there are no comparative studies with these outcome measures.
2. Comparison of immediate autologous reconstruction followed by radiation therapy versus immediate implant reconstruction without radiation therapy
One systematic review (Schaverien et al, 2013) compared patients with an immediate autologous breast reconstruction followed by postoperative radiation therapy with patients who had not received postoperative radiation therapy. Ten retrospective comparative studies were included (Berry et al, 2010; Lee et al, 2010; Leonardi et al, 2010; Chatterjee et al, 2009; Carlson et al, 2008; Thomson et al, 2008; Spear et al, 2005; Rogers and Allen, 2002; Tran et al, 2000; Williams et al, 1997). Follow-up was unknown for four studies, and ranged from 1 to 5 years for patients who had received postoperative radiation therapy in the other studies.
Local recurrence
The outcome measure local recurrence was not studied.
“Overall” complications
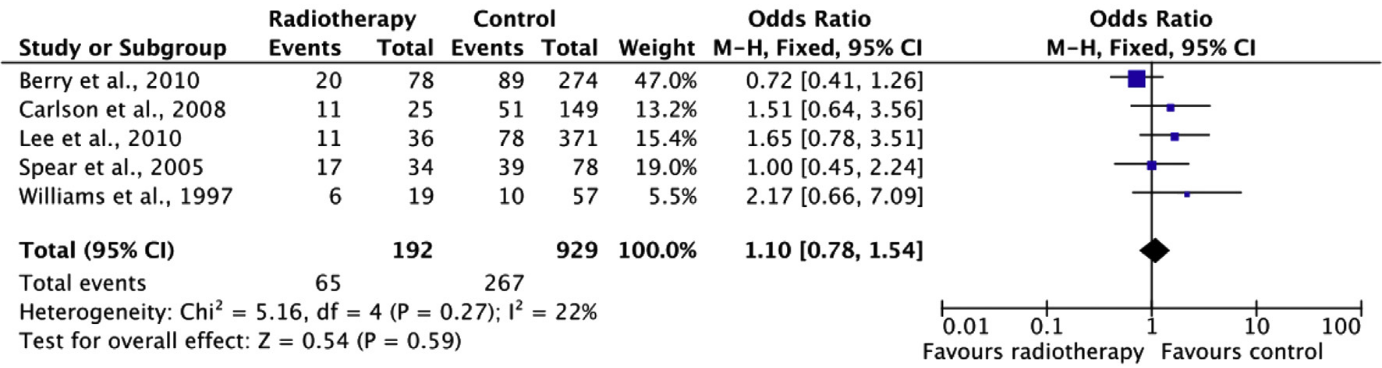
For the outcome measure complications, only those complications reported in all studies were included. Which complications were included specifically could not be determined based on the systematic review. The review did report that the complications ‘anastomosis revision’ and ‘abdominal weakness and bulging’ were excluded. The pooled result (N=1121) shows that the risk of complications is about the same when postoperative radiation therapy is performed compared to when no postoperative radiation therapy is performed (see figure 4).
Figure 4. “Overall” complications
Fat necrosis
The pooled result (N=2313) shows that the chance of fat necrosis is 2.8 times higher if postoperative radiation therapy is performed than if no postoperative radiation therapy is performed (statistically significant, see figure 5).
Figure 5. Fat necrosis
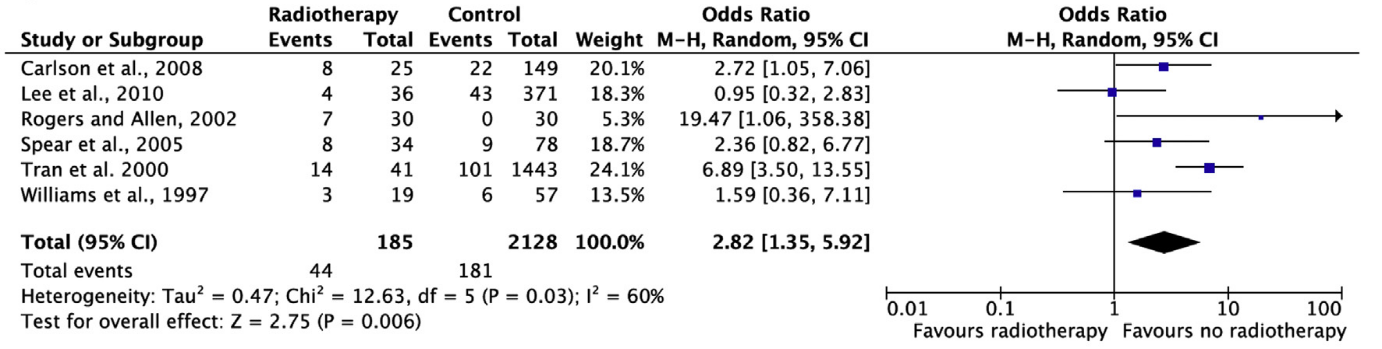
Re-operation
Re-operation is defined as a follow-up operation for reconstruction of the ipsilateral breast. The pooled result (N=272) shows that the chance of re-operation is lower if postoperative radiation therapy is performed than if no postoperative radiation therapy is performed (not statistically significant, see figure 6).
Figure 6. Re-operation
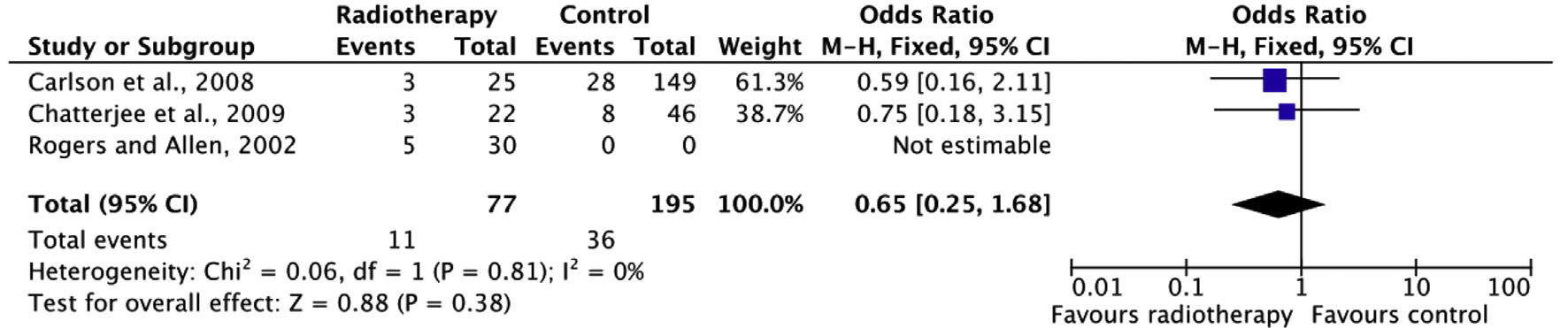
Aesthetic result
Seven studies included aesthetic result as an outcome measure (Lee et al, 2010; Leonardi et al, 2010; Carlson et al, 2008; Thomson et al, 2008; Spear et al, 2005; Rogers and Allen, 2002; Tran et al, 2000). Aesthetic result was measured in a number of different ways.
In the study by Lee et al (N=407), the aesthetic result was assessed based on patient satisfaction. Patient satisfaction was assessed with a questionnaire that used a 5-point Likert scale. In the group that had received postoperative radiation therapy, 66.7% of patients were satisfied, while 75.7% of the group without postoperative radiation therapy was satisfied.
In the study by Leonardi et al (N=33), 21 physicians evaluated the aesthetic result based on photographs of women who had undergone autologous breast reconstruction. Four items were scored: symmetry, volume, position of the inframammary fold and ptosis. Each item was scored between 0 (worst result) and 10 (best result) based on which the aesthetic result was assessed as ‘excellent’ (treated breast practically identical to the other breast), ‘good’ (treated breast only slightly different from the other breast), ‘fair’ (treated breast clearly different from the previous breast, but not deformed) or ‘poor’ (treated breast seriously deformed) as described by Harris et al (1979). In the group receiving postoperative radiation therapy, 47.6% was evaluated as ‘good’ or ‘excellent’. In the group that had not received postoperative radiation therapy this was 68.6% (statistically significant, p<0.0001).
In a study by Carlson et al (174 flaps), photographs of women were evaluated by four independent evaluators. They scored the breast reconstruction based on volume, breast shape, location of the breast and position of the inframammary fold. As was the case for the study by Leonardi et al, the total aesthetic result was assessed as ‘excellent, good, fair or poor’, which were given 4, 3, 2 or 1 points, respectively. On average, the postoperative radiation therapy group scored 2.56 points, and the group without postoperative radiation therapy 3.02 points.
In the study by Thompson et al (N=20), the aesthetic result was assessed twice, once based on patient satisfaction, and once based on the judgment of three medical professionals. Patient satisfaction was determined using a questionnaire covering 13 items identified based on the literature, scored with 5-point Likert scales. The medical professionals also gave their score on a 5-point Likert scale, based among other things on symmetry, shape, size, skin color, scars and nipple reconstruction. The medical professionals gave a mean score of 3.3 on the Likert scale to the postoperative radiation therapy group, and 4.5 points to the group without postoperative radiation therapy. Patients gave a mean score of 3 on the Likert scale when receiving postoperative radiation therapy, and a mean of 4 when not treated with postoperative radiation therapy.
In the study by Spear et al (N=112), photographs of women who underwent autologous breast reconstruction were assessed by 16 blinded judges. They scored the aesthetic result as ‘excellent, good, fair or poor’, which were given 4, 3, 2 or 1 points, respectively. The mean score for the postoperative radiation therapy group was 2.76, the mean score for the group without postoperative radiation therapy was 3.77 (statistically significant, p=0.0001).
In the study by Rogers and Allen (N=60), photographs of women who underwent autologous breast reconstruction were assessed by eight judges, including medical specialists and medical students. Photographs were made immediately after breast reconstruction, and new photographs were taken again later, potentially after radiation therapy. The photographs were assessed for symmetry, position and overall aesthetic proportion. Scores between 1 (worst result) and 5 (best result) were given on these three items, and an average was calculated based on the three scores. In the group treated with postoperative radiation therapy, photographs were scored 0.56 lower than photographs before radiation therapy. In the group not treated with postoperative radiation therapy, photographs were scored 0.50 higher than earlier photographs (statistically significant, p=0.006).
The study by Tran et al (N=1484) examined whether breast symmetry remained intact if radiation therapy was given after breast reconstruction. Data were collected retrospectively in a number of different ways, such as personal communications, physical examination and photographic review. Only seven of the 41 patients (17%) achieved and retained symmetry.
Patient satisfaction
The outcome measure patient satisfaction was not studied.
Level of evidence of the literature
The level of evidence for the outcome measures “overall” complications, fat necrosis and aesthetic result is very low, as there are very serious limitations in study design (non-randomized studies) and because studies were not powered for these outcome measures (imprecision). In addition, statistical heterogeneity is present for the outcome measure fat necrosis (I2=56%). Furthermore, it is unclear whether the applied fractioning schedules and radiation therapy techniques are comparable to those used in The Netherlands. Thus, the question remains whether the results from the study can be extrapolated to Dutch clinical practice.
The level of evidence for the outcome measure re-operation is very low for the same reasons listed above. A clinically relevant effect is present. However, this effect is highly uncertain due to the wide confidence intervals (imprecision).
The level of evidence for the outcome measures local recurrence and patient satisfaction was not assessed, as there are no comparative studies with these outcome measures.
3. Comparison of immediate autologous reconstruction followed by radiation therapy versus immediate implant reconstruction followed by radiation therapy
One systematic review (Barry et al, 2011) of four comparative retrospective studies compared patients undergoing immediate autologous reconstruction with an immediate implant reconstruction, both followed by radiation therapy (Anderson et al, 2004; Jhaveri et al, 2008;Wong et al, 2008; Strålman et al, 2008). After the search date for the review by Barry, one additional study (Christante et al, 2010) was published and included in the literature analysis. Follow-up varied from 10 months (Wong et al, 2008) to 34 months (Strålman et al, 2008).
Re-operation
The pooled result (N=441) shows that the chance of re-operation is more than twice as high if immediate implant reconstruction followed by radiation therapy is performed (statistically significant, see figure 7).
Figure 7. Re-operation
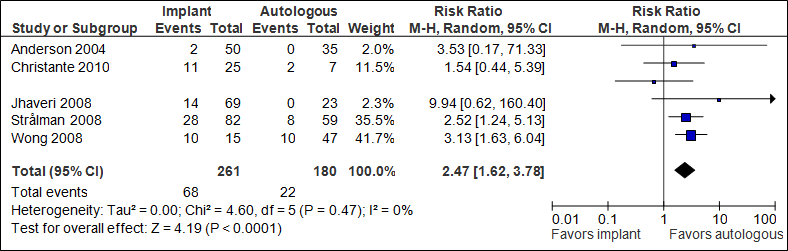
In the implant group + RT, the number of re-operations was 261/1000, in the autologous group +RT this was 122/1000.
Infection
Two studies examined the outcome measure infection (Anderson et al, 2004; Wong et al, 2008). Wong only looked at serious infections that required additional intervention, and found 13.3% (2/15) in the implant group versus 8.5% (4/47) in the autologous group.
In the study by Anderson et al, 4% (2/50) of patients with an implant reconstruction developed an infection, compared to 0% (0/35) in the autologous reconstruction group.
Aesthetic result
Two studies included aesthetic result as an outcome measure (Anderson et al, 2004; Jhaveri et al, 2008). Aesthetic result was measured in a number of different ways. In the study by Anderson et al, 82% (41/50) of patients with an implant reconstruction rated the result as ‘excellent/good’, compared to 90% (31.5/35) in the autologous reconstruction group. In the study by Jhaveri et al, 51% (35/69) of patients with an implant reconstruction rated the result as ‘acceptable’, compared to 83% (19/23) in the autologous reconstruction group. In summary, the two studies found better aesthetic results if, in case of postoperative radiation therapy an immediate autologous reconstruction was performed.
Patient satisfaction
The outcome measure patient satisfaction was not studied.
Level of evidence of the literature
The level of evidence for the outcome measure local recurrence, postoperative complications (re-operation, infection, fibrosis and fat necrosis) and aesthetic result is very low, as the study is not randomized (highly significant limitations in study design) and the number of patients with complications is low (imprecision). Furthermore, statistical heterogeneity is present for the outcome measure capsular contracture (I2=88%).
The level of evidence for the outcome measures local recurrence and patient satisfaction was not assessed, as they were not studied.
Search and select
Three systematic literature analyses were performed in order to answer the primary question, examining the following questions:
- What is the difference between the effects of immediate implant reconstruction followed by radiation therapy compared with immediate implant reconstruction not followed by radiation therapy on local recurrence, postoperative complications, aesthetic result or patient satisfaction?
- What is the effect of immediate autologous reconstruction followed by radiation therapy compared with immediate autologous reconstruction not followed by radiation therapy on local recurrence, postoperative complications, aesthetic result or patient satisfaction?
- What is the effect of immediate autologous reconstruction followed by radiation therapy compared with immediate implant reconstruction followed by radiation therapy on local recurrence, postoperative complications, aesthetic result or patient satisfaction?
Medline (OVID), Embase and Cochrane databases were searched for immediate reconstruction. The search justification is listed at the end of this chapter. The literature search yielded 136 results. Studies that met the following selection criteria were included in the literature summary: original studies; comparative studies; systematic review of comparative studies; comparison of immediate breast reconstruction followed by postoperative radiation therapy to immediate breast reconstruction not followed by radiation therapy, and at least one of the following outcome measures: local recurrence, postoperative complications, aesthetic result or patient satisfaction.
Of the 136 references identified, three studies met the selection criteria for the literature analysis (Barry et al, 2011; Nava et al, 2011; Christante et al, 2010), including one systematic review (Barry et al, 2011). The evidence tables for these studies may be found at the end of this chapter.
After the search date, Schaverien et al (2013) published an additional systematic review that also met the selection criteria and was therefore included in the literature analysis.
Methods
Authorization date and validity
Last review : 01-03-2015
The Board of the Dutch Society for Plastic and Reconstructive Surgery (NVPC) will assess whether this guideline is still up-to-date in 2018 at the latest. If necessary, a new working group will be appointed to revise the guideline. The guideline’s validity may lapse earlier if new developments demand revision at an earlier date.
As the holder of this guideline, the NVPC is chiefly responsible for keeping the guideline up to date. Other scientific organizations participating in the guideline or users of the guideline share the responsibility to inform the chiefly responsible party about relevant developments within their fields.
General details
Guideline development was funded by the Quality Fund for Medical Specialists (SKMS) and The Netherlands Organization for Health Research and Development (ZonMw).
Scope and target group
Guideline goal
To develop a multidisciplinary, evidence-based guideline for breast reconstruction in women undergoing breast conserving therapy or mastectomy for breast cancer, or following prophylactic mastectomy.
Guideline scope
The guideline focuses on all patients with an indication for breast reconstruction following breast conserving therapy or mastectomy. Additionally, the guideline may be applied to breast reconstruction in patients who have undergone surgical treatment for a benign breast condition. The guideline does not comment on the treatment of breast cancer. We refer the reader to the NABON guideline for the treatment of breast cancer (www.richtlijnendatabase.nl), which this guideline complements.
Unfortunately, circumstances did not permit a medical oncologist representing the NVMO to participate in the working group. Thus, the current version lacks a module on chemotherapy and breast reconstruction. The working group strives to create such a module for this guideline in the near future.
Intended audience for the guideline
The guideline aims to provide practical guidance for plastic surgeons and members of the multidisciplinary breast cancer team (surgical oncologist, medical oncologist, radiation oncologist, radiologist, pathologist, psychologist, breast care nurse specialist). A version for patients has recently been developed (https://www.b-bewust.nl/pif_borstreconstructie).
Samenstelling werkgroep
A multidisciplinary working group was appointed to develop the guideline in October 2011, consisting of representatives from all relevant specialties involved in the care for patients with breast reconstruction (see above for working group membership). Working group members were mandated by their professional organizations. The working group worked on developing the guideline for 2 years. The working group is responsible for the full text of this guideline.
- Dr. M.A.M. Mureau (President), MD, PhD, plastic surgeon, Erasmus MC Cancer Institute, Erasmus University Medical Center Rotterdam
- Professor Dr. R.R.W.J. van der Hulst, MD, PhD, plastic surgeon, Maastricht University Medical Center/Orbis Medical Center/Viecuri Medical Center, Maastricht
- Dr. L. A.E. Woerdeman, MD, PhD, plastic surgeon, Antoni van Leeuwenhoek / Netherlands Cancer Institute, Amsterdam
- Drs. A.A.W.M van Turnhout, MD, plastic surgeon, Tergooi Hospital, Hilversum Site
- N.A.S. Posch, MD, plastic surgeon, Haga Hospital, The Hague
- Dr. M.B.E. Menke-Pluijmers, MD, PhD, oncologic surgeon, Albert Schweitzer Hospital, Dordrecht
- Dr. E.J.T. Luiten, MD, PhD, oncologic surgeon, Amphia Hospital, Breda
- Drs. A.H. Westenberg, MD, radiotherapist/oncologist, Arnhem Radiotherapy Institute, Arnhem
- Dr. J.P. Gopie, PhD, psychologist, Leiden University Medical Center, Leiden
- Dr. H.M. Zonderland, MD, PhD, radiologist, Academic Medical Center, Amsterdam
- Drs. M. Westerhof, MSc, Netherlands Breast Cancer Association, Utrecht
- E.M.M. Krol-Warmerdam MA, V&VN Nurse Specialists, Leiden University Medical Center, Leiden
With support from
- Drs. B.S. Niël-Weise, MD, microbiologist / epidemiologist, senior advisor, Knowledge Institute for Medical Specialists
Declaration of interest
Working group members declared any (financial) ties with commercial companies, organizations or institutions involved in the field covered by the guideline for the past five years in writing. An overview is available on request from the office of the Knowledge Institute for Medical Specialists (KIMS).
Patient involvement
Patients are represented by a delegate from the Netherlands Breast Cancer Association in this guideline.
Method of development
Implementation
Guideline implementation and practical applicability of the recommendations was taken into consideration during various stages of guideline development. Factors that may promote or hinder implementation of the guideline in daily practice were given specific attention.
The guideline is distributed digitally among all relevant professional groups. The guideline can also be downloaded from the Dutch Society for Plastic and Reconstructive Surgery website: www.nvpc.nl, the guideline website: www.richtlijnendatabase.nl and the Quality Organization for Medical Specialists.
Methods and proces
AGREE
The guideline has been drafted in accordance with the requirements outlined in the ‘Guidelines 2.0’ report of the Guideline Advisory Committee of the Council on Science, Education and Quality (WOK). This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (www.agreecollaboration.org), an instrument designed to assess the quality of guidelines with broad international support.
Primary questions and outcome measures
Based on the outcomes of the bottleneck analysis, the president and advisor formulated draft primary questions. These were discussed and defined together with the working group. Subsequently, the working group determined which outcome measures were relevant for the patient for each primary question, examining both desired and undesirable effects. The working group valuated these outcomes based on their relative importance as crucial, important and unimportant.
Literature search and selection strategy
Specific search terms were used to identify published scientific studies related to each individual primary question in Medline, Cochrane and, where necessary, Embase. Additionally, the references of the selected articles were screened for additional relevant studies. Studies offering the highest level of evidence were sought out first. Working group members selected articles identified by the search based on predetermined criteria. The selected articles were used to answer the primary question. The searched databases, the search string or terms used during the search and selection criteria applied are listed in the chapter for each individual primary question.
Quality assessment of individual studies
Individual studies were assessed systematically based on predefined methodological quality criteria in order to assess the risk of biased study results. These assessments may be found in the column ‘Study quality assessment’ in an evidence table.
Literature summary
The relevant study results from all selected articles were presented clearly in evidence tables. The key findings from the literature are described in the literature summary. If studies were sufficiently similar in design, data were also summarized quantitatively (meta-analysis) using Review Manager 5.
Assessment of the level of scientific evidence
A) With regard to intervention questions:
The level of scientific evidence was determined using the GRADE method. GRADE is short for ‘Grading Recommendations Assessment, Development and Evaluation’ (see http://www.gradeworkinggroup.org/) (Atkins et al, 2004).
B) With regard to questions about the value of diagnostic tests, harm or adverse effects, etiology and prognosis:
GRADE cannot be used (yet) for these types of questions. The level of evidence of the conclusion was determined based on the accepted EBRO method (van Everdingen et al, 2004).
Formulation of conclusions
With regard to questions about the value of diagnostic tests, harm or adverse effects, etiology and prognosis, the scientific evidence is summarized in one or more conclusions, listing the level of evidence for the most relevant data.
For interventions, the conclusion does not refer to one or more articles, but is drawn based on the body of evidence. The working group looked at the net benefits of each intervention. This was done by determining the balance between favorable and unfavorable effects for the patient.
Considerations
When making recommendations, scientific evidence was considered together with other key aspects, such as working group member expertise, patient preferences, costs, availability of facilities and/or organizational aspects. Insofar as they are not part of the systematic literature review, these aspects are listed under ‘Considerations’.
Formulation of recommendations
Recommendations provide an answer to the primary question, and are based on the best scientific evidence available and the most important considerations. The level of scientific evidence and the importance given to considerations by the working group jointly determine the strength of the recommendation. In accordance with the GRADE method, a low level of evidence for conclusions in the systematic literature review does not rule out a strong recommendation, while a high level of evidence may be accompanied by weak recommendations. The strength of the recommendation is always determined by weighing all relevant arguments.
Development of indicators
Along with developing a draft guideline, internal quality indicators were developed to allow monitoring of the implementation of the guideline in daily practice. More information about the method for indicator development may be requested from KIMS.
Knowledge gaps
During the development of this guideline, systematic searches were conducted for research contributing to answering the primary questions. For each primary question, the working group determined whether (additional) scientific research is desirable.
Commentary and authorization phase
The draft guideline was submitted to the (scientific) organizations involved for comment. The guideline was also submitted to the following organizations for comment: Netherlands Breast Cancer Association (BVN), Netherlands Society for Medical Oncology (NVMO), Dutch College of General Practitioners (NHG), Healthcare Insurers Netherlands (ZN), The Dutch Healthcare Authority (NZA), Health Care Insurance Board (CvZ), the Health Care Inspectorate (IGZ), Achmea, CZ, Menzis and VGZ. Comments were collected and discussed with the working group. The draft guideline was updated and finalized by the working group based on the comments. The final guideline was submitted for authorization to the (scientific) organizations involved and authorized by them.
Legal standing of guidelines
Guidelines are not legal prescriptions, but contain evidence-based insights and recommendations that care providers must meet in order to provide high quality care. As these recommendations are primarily based on ‘general evidence for optimal care for the average patient’, care providers may deviate from the guideline based on their professional autonomy when they deem it necessary for individual cases. Deviating from the guideline may even be necessary in some situations. If care providers choose to deviate from the guideline, this should be done in consultation with the patient, where relevant. Deviation from the guideline must always be justified and documented.
