Bariatrische chirurgie
Uitgangsvraag
Wat is de plaats van bariatrische chirurgie in de behandeling van patiënten met MASLD, en in het bijzonder van patiënten met MASH met ernstige leverfibrose?
Aanbeveling
Overweeg bariatrische chirurgie bij patiënten met MASLD/MASH en gevorderde of ernstige fibrose met een BMI > 35 kg/m2 en bij wie leefstijlinterventies onvoldoende effect hebben gehad.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek verricht naar het effect van bariatrische chirurgie bij patiënten met MASLD. Resolutie van MASH en ≥ 1 fibrosestadium afname en lever-gerelateerde complicaties waren gedefinieerd als cruciale uitkomstmaten. Er zijn voor deze vraag uitsluitend observationele studies gevonden, waarbij sprake is van heterogeniteit. Op basis van de beschikbare literatuur is de bewijskracht voor deze cruciale uitkomstmaten daarom beoordeeld als zeer laag, waardoor geen richting gegeven kan worden aan de besluitvorming.
Voor patiënten met obesitas en MASLD/MASH is momenteel alleen van leefstijlinterventies een positief effect op de lever en geassocieerde cardiometabole complicaties beschreven. Hoewel dieet en beweging leiden tot gewichtsreductie, is bekend dat minder dan 15% van de patiënten het gewichtsverlies kan vasthouden. Daarnaast leidt een gewichtsverlies van 3-5% tot afname van steatose, maar is gewichtsverlies van minstens 7-10% nodig om een afname in inflammatie en fibrose te realiseren. Daarom is leefstijlinterventie alleen waarschijnlijk onvoldoende om patiënten met progressieve MASLD/MASH met fibrose te behandelen. In aanvulling op leefstijlinterventies zijn er momenteel geen bewezen farmacotherapeutische behandelingsopties beschikbaar. Vanuit observationele studies is bekend dat bariatrische chirurgie aanhoudend klinisch effect heeft op het gewicht en het histologische spectrum van MASLD (steatose, inflammatie, levercelverval en fibrose). Daarom zou bariatrische chirurgie overwogen kunnen worden voor patiënten met obesitas en MASLD/MASH met gevorderde fibrose die at risk zijn voor het ontwikkelen van cirrose.
Er dient rekening gehouden te worden met eventuele complicaties van bariatrische chirurgie: directe complicaties zoals naadlekkage en wondinfectie, maar ook complicaties op de lange termijn, zoals malabsorptie, dumping en nesidioblastose. De risico’s op beide vormen complicaties zijn laag (Gilmore, 1995; Seef, 2010). Er bestaat ook een MASLD-gerelateerde lange termijn complicatie, namelijk paradoxale progressie van MASLD/MASH na bariatrische chirurgie. In de meta-analyse van Lee (2019) werd dit tot in 12% van de patiënten beschreven. In de controleconditie, namelijk geen bariatrische chirurgie ondanks progressieve MASLD-stadia met fibrose, kan levercirrose, hepatocellulair carcinoom en lever-gerelateerde mortaliteit optreden. Dit zijn relatief zeldzame eindstadia maar ernstig en toenemend in incidentie door de toename van MASLD en progressieve/ernstige stadia van dit ziektebeeld.
Een gevorderde leverziekte met portale hypertensie wordt gezien als een relatieve contra-indicatie voor bariatrische chirurgie. Recente studies laten zien dat in een geselecteerde populatie patiënten met obesitas en gecompenseerde cirrose bariatrische chirurgie haalbaar lijkt (Ahmed, 2021). Een gedecompenseerde cirrose is een absolute contra-indicatie voor bariatrische chirurgie vanwege het verhoogd aantal postoperatieve complicaties, die gepaard gaan met een toegenomen mortaliteit. Patiënten met obesitas, cirrose en portale hypertensie bij wie bariatrische chirurgie wordt overwogen, dienen bij voorkeur verwezen te worden naar een bariatrisch centrum die een samenwerkingsverband heeft met een levertransplantatiecentrum.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
De belangrijkste doelen van bariatrische chirurgie voor de patiënt zijn genezing dan wel reductie van MASLD/MASH en het voorkomen van aan MASLD/MASH gerelateerde complicaties op de langere termijn: levercirrose, hepatocellulair carcinoom en lever-gerelateerde mortaliteit. Dit zijn voordelen die de patiënt ziet, en hiernaast zijn gewichtsreductie en zich gezonder en energieker voelen belangrijke voordelen. Nadelen die patiënten zien, kunnen ook zwaar wegen: een ingrijpende en invasieve procedure aan intacte organen, met een kans op complicaties, een kans op geen effect en zelfs een kleine kans op progressie van de ziekte van de ingreep. De waarde die individuele patiënten hechten aan deze voor- en nadelen, is overigens niet systematisch onderzocht.
Patiënten met fibrotische MASH hebben naast obesitas vaak co-morbiditeiten zoals DM2, hypertensie en obstructief slaapapneu syndroom. Deze factoren zijn naast de BMI bestaande meewegende factoren voor de indicatiestelling bariatrische chirurgie. De aan- dan wel afwezigheid van een of meerdere van deze factoren kan de voorkeur van de patiënt met MASLD/MASH voor bariatrische chirurgie beïnvloeden.
Kosten (middelenbeslag)
Er zijn geen publicaties waarbij specifiek voor patiënten met MASH leverfibrose (stadium F1 of hoger) met een BMI > 35 kg/m2 is gekeken naar kosteneffectiviteit (definitie: wanneer een interventie vergelijkbaar effectief is met lagere kosten in vergelijking met de controle interventie of hetzelfde kost maar effectiever is) of kostenbesparing (definitie: wanneer een interventie minder kost maar effectiever is dan de controle interventie). De studies die gedaan zijn naar kosteneffectiviteit van bariatrische chirurgie zijn heterogeen en hebben geen eenduidige uitkomst (o.a. Klebanoff, 2017 en Maciejewski, 2013).
Aanvaardbaarheid, haalbaarheid en implementatie
Door de obesitas pandemie met de hierbij groeiende aantallen patiënten met MASLD is er in aanvulling op leefstijlinterventie een sterke behoefte aan alternatieve behandelingen om progressieve leverziekte en cardiometabole complicaties te voorkomen. In Nederland zijn er 18 bariatrische centra die zodanig zijn verdeeld over Nederland dat deze zorg voor iedere Nederlander in de eigen regio beschikbaar is. Er zijn daarnaast ook duidelijke richtlijnen beschikbaar over wanneer patiënten verwezen kunnen worden voor bariatrische chirurgie en er vergoeding mogelijk is. Argumenten waardoor men terughoudend zou kunnen zijn om bariatrische chirurgie te verrichten bij patiënten met MASLD is het ontbreken van grote vergelijkende studies met MASLD als eindpunt. Verder vraagt een patiënt met MASLD met gevorderde fibrose dan wel cirrose extra expertise die niet in elk centrum beschikbaar is. Om voor iedere patiënt met MASLD en een verhoogd risico op een eindstadium leverziekte gepaste zorg te bieden, is het noodzakelijk om te investeren in het besef bij zorgverleners en patiënten dat obesitas kan leiden tot een chronische leveraandoening. Daarnaast is er behoefte aan een duidelijk (diagnostisch) zorgpad gericht op diagnostiek van MASLD bij obesitas en een behandelingsalgoritme voor deze categorie patiënten.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
MASLD/MASH is een progressieve leverziekte met mogelijk ernstige lever-gerelateerde complicaties en mortaliteit tot gevolg. Leefstijlinterventies zijn waarschijnlijk niet krachtig genoeg om patiënten met progressieve en ernstige stadia te kunnen stabiliseren dan wel te genezen. Er is nog geen bewezen of effectieve farmacotherapie voor ernstige, progressieve MASLD/MASH. Relatief kleine observationele studies met bariatrische chirurgie voor patiënten met MASLD/MASH tonen positieve behandeleffecten effecten op histologisch niveau voor alle drie de ziektecomponent van MASLD/MASH: steatose, MASH-activiteit en fibrose. Deze laatste hangt sterk samen met levergerelateerde mortaliteit en all cause mortality. De complicatiesrisico’s van bariatrische ingrepen zijn laag. Ondanks de nog lage bewijskracht van de onderliggende evidence, geven wij daarom een aanbeveling om bij een patiënt met BMI > 35 kg/m2 met ≥F2 fibrose ten gevolge van MASLD/MASH bij wie progressieve leverschade optreedt ondanks goed uitgevoerde leefstijlinterventies en bij wie farmacotherapeutische behandeling in studieverband geen optie is, om bariatrische chirurgie te overwegen als behandeling van het ernstige stadium van MASLD/MASH.
Onderbouwing
Achtergrond
Voor patiënten met MASH en ernstige leverfibrose die niet verbeterd op leefstijlinterventies is er momenteel nog geen bewezen effectieve of geregistreerde farmacotherapie beschikbaar. Bariatrische chirurgie kan bij patiënten die aan de selectiecriteria voldoen, wel regressie en zelfs resolutie van MASH en fibrose induceren. Dit potentieel gunstige effect is mechanistisch plausibel: bariatrische ingrepen leiden tot snellere verzadiging en vermindering van insulineresistentie door toegenomen secretie van GLP-1 en PYY vanuit de dunne darm, tot toegenomen galzout-activatie van FXR waardoor lipogenese in de lever afneemt en afname van de inflammatie van perifeer vetweefsel waarvoor de vetzuurflux naar de lever afneemt (Lefere, 2021).
Mogelijk wordt deze behandeloptie voor MASH met fibrose nog te weinig toegepast. Er zijn echter ook data over ‘paradoxale’ progressie van MASH na bariatrische chirurgie. Daarnaast lijken de verschillende bariatrische procedures onvoldoende met elkaar vergeleken te zijn met betrekking tot het effect op MASH en MASH-fibrose.
Conclusies / Summary of Findings
Reduction of hepatic steatosis
|
very low GRADE |
The evidence is very uncertain on the effect of bariatric surgery on biopsy proven reduction of steatosis, in patients with MASH and liver fibrosis.
Agarwal, 2021; Cabré, 2019; Garg, 2018; Salman, 2020; Salman, 2021a and Lee, 2019 |
Resolution of MASH
|
very low GRADE |
The evidence is very uncertain on the effect of bariatric surgery on biopsy-proven complete resolution of MASH features including lobular inflammation and ballooning degeneration, in patients with MASH and liver fibrosis.
Agarwal, 2021; Esquivel, 2018; Cabré, 2019; Garg, 2018; Lassailly, 2020; Salman, 2020; Salman, 2021a; Salman, 2021b and Lee, 2019. |
Resolution of MASLD-related liver fibrosis
|
very low GRADE |
The evidence is very uncertain on the effect of bariatric surgery on biopsy-proven complete resolution of MASLD-related liver fibrosis, in patients with MASH and liver fibrosis.
Agarwal, 2021; Esquivel, 2018; Cabré, 2019; Garg, 2018; Lassailly, 2020; Salman, 2020; Salman, 2021a; Salman, 2021b and Lee, 2019. |
Liver-related (serious) adverse events
|
very low GRADE |
The evidence is very uncertain on the effect of bariatric surgery on (serious) liver-related adverse events, in patients with MASH and liver fibrosis.
Agarwal, 2021; Salman, 2020; Salman, 2021a; Salman, 2021b and Lee, 2019. |
Samenvatting literatuur
Description of studies
Lee (2019) conducted a systemic review and meta-analysis evaluating the effects of bariatric surgery on MASLD in obese patients. Lee (2019) covers the literature until May 2018 and literature searches were conducted in MEDLINE, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials. Lee (2019) included only randomized controlled trials (RCTs) or observational studies examining the effect of bariatric surgery on MASLD. They included both single-arm studies (effect of bariatric surgery on MASLD status before and after surgery) or double-arm studies (bariatric surgery vs placebo or medical therapy) However, there were no double-arm studies identified. In total, 32 studies were included: 15 retrospective and 17 prospective cohort studies. In total, there is information on 3093 liver biopsies (the total number of included patients was not specified). There were no RCTs identified. All studies were single-arm studies examining the effect of bariatric surgery on MASLD before and after surgery with no comparators. Article quality of individual studies was assessed using the Methodological Index for Non-Randomized Studies (MINORS) tool. Quality of evidence for estimates derived from meta-analyses were assessed by Grading of Recommendations, Assessment, Development and Evaluation (GRADE). Outcomes were liver biopsy proven complete resolution of MASLD features (defined as the absence of histologic (biopsy) features of MASLD such as steatosis, inflammation, ballooning degeneration, and
fibrosis after bariatric surgery) and NAFLD activity score (NAS), which is a sum of the degree of steatosis (0-3), lobular inflammation (0-3) and hepatocyte ballooning (0-2). If a histopathologic grading system had a scale of 0 to 4, then 0 was considered to be complete resolution and 1 to 4 were categorized as disease.
Agarwal (2021) conducted a prospective cohort study assessing the impact of bariatric surgery on the course of MASLD. MASLD was assessed using paired liver biopsy (intra-operative and post-bariatric surgery at 1-year follow-up). Included were all consecutive patients undergoing bariatric surgery, as per the standard National Institute of Health (NIH) criteria. In total, 58 patients (70.7% females, mean age 39.9 ± 11.2 years, mean BMI …) underwent paired liver biopsy. Laparoscopic sleeve gastrectomy (LSG), laparoscopic Roux-en-Y gastric bypass (LRYGB), and laparoscopic one anastomosis gastric bypass (OAGB) were the bariatric procedures performed. During bariatric surgery, a liver biopsy was taken from the left lobe of the liver under laparoscopic vision. A follow-up liver biopsy was taken 1 year post bariatric surgery. Liver stiffness measurement (LSM) and controlled attenuation parameter (CAP) measurements were performed with FibroScanÒ both pre- and post-bariatric surgery. Fibrosis was graded based on the absence of fibrosis (F0); mild fibrosis (F1); moderate fibrosis (F2); bridging fibrosis (F3); and cirrhosis (F4). Steatosis was graded based on the percentage of hepatocytes containing lipid droplets as S0: <5%; S1: 5%–33%; S2: 34%–66%; and S3 >66%. Lobular inflammation was graded as L0: none; L1: <2 foci/200 fields; L2: 2–4 foci/200 fields; and L3: >4 foci/ 200 fields. Hepatocyte ballooning was graded as B0: none; B1: few; and B2: prominent ballooning. The outcome was the assessment of change in grade of steatosis and stage of fibrosis 1-year post-bariatric surgery using both liver biopsy and FibroScanÒ elastography.
Cabré (2019) conducted a prospective cohort study assessing the modulation of hepatic indices of oxidative stress and inflammation in obese patients undergoing laparoscopic sleeve gastrectomy (LSG). Included were 436 patients who attended an obesity clinic and underwent LSG for weight loss. A diagnostic intraoperative liver biopsy was obtained in a subcohort of 120 patients who agreed to a 1-year follow-up that included donation of blood samples and additional liver biopsies. Biopsies were performed by ultrasound-guided, percutaneous needle puncture. Patients were classified according to the NAS system. Values assigned were ≤2 for non-MASH, >2 and ≤4 for probable MASH, and ≥5 for definite MASH. Fibrosis was staged as the absence of fibrosis (F0), mild to moderate fibrosis (F1 and F2), bridging fibrosis (F3) or cirrhosis (F4). Outcomes were change in steatosis grade, lobular inflammation, hepatocellular ballooning, fibrosis and cirrhosis pre- and post-bariatric surgery.
Esquivel (2018) conducted a prospective cohort study evaluating the evolution of MASLD in patients with obesity after 1 year of sleeve gastrectomy (SG). Included were patients between 18 and 70 years old with a BMI >40 kg/m2 or a BMI >35 kg/m2 with comorbidities. In total, 63 patients were included (mean age was 40 ± 10 years; 65% of the patients were female; mean initial weight was 129 ± 23 kg). Intraoperative liver biopsy was performed in 63 obese patients who underwent SG. Forty-three patients were again biopsied 1 year after surgery. The first hepatic biopsy was carried out during the SG, and the second one after 1 year by using percutaneous CT scan technique. Demographics, body mass index, percentage of excess weight loss, liver function tests, lipid panel, glucose panel, and histological changes of liver were prospectively analysed. Histological grades and stages of liver fibrosis were categorized by using the Brunt classification. Outcomes were changes in grade of steatosis, grade of steatohepatitis and grade of fibrosis. Patients were classified according to classified
according to Brunt classification.
Garg (2018) conducted a prospective cohort study assessing the utility of CAP for assessment of hepatic steatosis in morbidly obese individuals and evaluated the effect of bariatric surgery on hepatic steatosis and fibrosis. Included were consecutive patients with body mass index (BMI) >40 kg/m2 or patients with BMI >35 kg/m2 and associated co-morbidities undergoing bariatric surgery between October 2014 and June 2016. Baseline details of anthropometric data, laboratory parameters, FibroScanÒ (XL probe), and liver biopsy were collected. Follow-up liver biopsy was done at 1 year. MASLD was graded using the NAFLD activity score (NAS). NAS ≥5 with or without fibrosis was considered as metabolic dysfunction-associated steatohepatitis (MASH). Fibrosis was staged from 0 to 4: F0—the absence of fibrosis; F1— perisinusoidal or portal; F2—perisinusoidal and portal/ periportal; F3—septal or bridging fibrosis; and F4—cirrhosis. Fibrosis with stage 2 or above was considered as significant fibrosis and fibrosis with stage 3 or above was considered advanced fibrosis. Outcomes were change in liver histology pre- and post-bariatric surgery.
Lassailly (2020) conducted a prospective study evaluating sequential liver samples, collected the time of bariatric surgery and 1 and 5 years later, to assess the long-term effects of bariatric surgery in patients with MASH. Included were patients >18 years of age with body mass index (BMI) >40 kg/m2 or patients with BMI >35 kg/m2 and associated co-morbidities undergoing bariatric surgery with biopsy-proven MASH, defined by the MASH clinical research network histologic scores. In total, 180 patients were included (mean age 46.7 ± 10.6 years; 66% female). The patients underwent bariatric surgery at a single centre in France and were followed for 5 years. Liver biopsies were systematically planned during the surgical procedure and approximately 1 and 5 years after surgery. The pathologists first diagnosed MASH, then determined the NAFLD Activity Score (NAS) and graded the severity of necroinflammatory activity using the Brunt’s score. Liver fibrosis was assessed using Kleiner’s fibrosis score, defined as follows: F0, normal; F1 stage is divided into 3 subclasses: 1a, mild pericellular fibrosis in zone 3, 1b, moderate pericellular fibrosis in zone 3, and 1c, portal fibrosis; F2, perivenular and pericellular fibrosis confined to zones 2 and 3, with or without portal or periportal fibrosis; F3, bridging or extensive fibrosis with architectural distortion and no clear-cut cirrhosis; and F4, cirrhosis The primary endpoint was the resolution of MASH without worsening of fibrosis at 5 years. Secondary end points were improvement in fibrosis (reduction of >1 stage) at 5 years and regression of fibrosis and MASH at 1 and 5 years.
Salman (2020) conducted a prospective cohort study investigating the histological changes in MASLD that occur in obese patients 1-year post-laparoscopic sleeve gastrectomy (LSG) based on standardized NAS. Included were patients >18 years of age with body mass index (BMI) >40 kg/m2 or patients with BMI >35 kg/m2 and associated co-morbidities undergoing LSG. In total, 94 patients underwent a second liver biopsy (mean age 41.39 + 7.64 years; 60% male). Intraoperative wedge liver biopsy was taken from all patients, with a follow-up liver biopsy at 12 months after the operation. NAS is a validated score that is used to grade disease activity in patients with MASLD. The NAS is the sum of the biopsy’s individual scores for steatosis (0 to 3), lobular inflammation (0 to 3), and hepatocellular ballooning (0 to 2). Fibrosis is not included in the NAS. For statistical analyses, patients were grouped into the three different NAS groups (group 1 = NAS 0–2: probable no MASH; group 2 = NAS 3–4: borderline MASH; group 3 = NAS 5–8: probable MASH). Outcome was histological change (steatosis grade, hepatocyte ballooning, lobular inflammation, fibrosis stage) pre- and post-LSG.
Salman (2021a) conducted a prospective study evaluating the safety of laparoscopic sleeve gastrectomy (LSG) in cases that have compensated metabolic dysfunction-associated steatohepatitis (MASH)-related cirrhosis and its impact on fibrosis stage. Included were Child-A cirrhotic patients between 18 and 60 years of age with body mass index (BMI) >40 kg/m2 or patients with BMI >35 kg/m2 and associated co-morbidities undergoing laparoscopic sleeve gastrectomy (LSG). In total, 70 patients completed follow-up (mean age 44.4 ± 5.6 years; 54% male). Patients underwent intraoperative wedge liver biopsy with F4 stage, and had a follow-up after 30 months with ultrasound-guided true-cut liver biopsy. NAFLD Activity Score (NAS) was determined using these biopsies. For statistical purposes, subjects were divided according to NAS into three subgroups (1: NAS 0–2, probable no MASH; 2: NAS 3–4, borderline MASH; 3: NAS 5–8, probable MASH). Importantly, fibrosis stage after 30 months was also staged (F0 = absent fibrosis; F1 = perisinusoidal or portal fibrosis; F2 = perisinusoidal and portal/ periportal fibrosis; F3 = septal or bridging fibrosis; and F4 = cirrhosis). The needle biopsies were taken at the same time point for each patient participating in the study. The primary outcome measure was the impact of LSG on fibrosis stage. Secondary outcome measures were the improvement of other features of steatohepatitis in addition to operation-related morbidity.
Salman (2021b) conducted a prospective study evaluating the effect of one-anastomosis gastric bypass (OAGB) on pathological liver changes in severely obese cases with MASLD. Included were patients between 18 and 60 years of age with body mass index (BMI) >40 kg/m2 or patients with BMI >35 kg/m2 and associated co-morbidities scheduled for OAGB. In total, 67 patients were included (mean age 44.4 ± 5.7; 52% male). Intraoperative liver biopsy was performed and follow-up biopsy was performed at 15 months. A single expert physician examined the liver biopsies (at first and 15 months postoperatively) using a light microscope or histological assessment guided by the NAS scoring system, which is a well-established method employed to describe the severity in MASLD cases. Cases were divided into the three NAS categories (Category 1: NAS = 0–2: probable no MASH; Category 2: NAS = 3–4: borderline MASH; and Category 3: NAS = 5–8: probable MASH). Outcomes were the effect of OAGB on MASH status and fibrosis severity.
Results
Reduction of hepatic steatosis
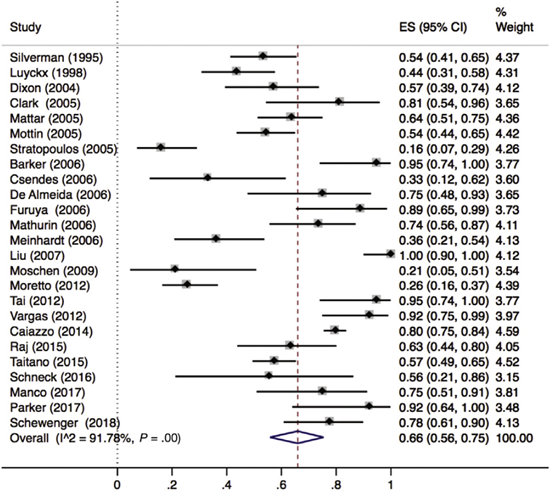
Lee (2019) included 32 observational studies with histologically characterized MASLD in patients undergoing bariatric surgery for obesity, of which, 25 reported hepatic steatosis (n=1329 patients). Lee 2019 found that 66% (95% CI 56% to 75%) of the patients had complete resolution of hepatic steatosis after bariatric surgery (Figure 1).
Figure 1. Proportion meta-analysis forest plot of biopsy-proven complete resolution of steatosis (adapted from Lee, 2019). ES, effect size.
Agarwal (2021) compared the proportion of steatosis pre- and post-bariatric surgery found that 42/58 (72.4%) patients had steatosis >1 pre-bariatric surgery compared to 11/58 (19.0%) patients at 12 months post-bariatric surgery. The RR was 0.26 (95% CI 0.15 to 0.46) in favor of bariatric surgery. Overall, 36/58 (60.2%) patients showed improvement in hepatic steatosis at 12 months post-bariatric surgery.
Cabré (2019) compared the proportion of steatosis pre- and post-LSG found that 95/12 (79.2%) patients had steatosis >1 pre-LSG compared to 4/120 (3.3%) patients at 12 months post-LSG. The RR was 0.04 (95% CI 0.02 to 0.11) in favor of LSG.
Garg (2018) compared steatosis pre- and post-bariatric surgery and found that 2/32 (6.2%) patients showed worsening steatosis while 30/32 (93.8%) patients showed improvement or no change in steatosis. The RR was 0.07 (95% CI 0.02 to 0.26) in favor of bariatric surgery.
Salman (2020) compared the proportion of steatosis pre- and post-LSG found that 93/94 (98.9%) patients had steatosis >1 pre-LSG compared to 84/94 (89.4%) patients at 12 months post-LSG. The RR was 0.90 (95% CI 0.84 to 0.97) in favor of LSG.
Salman (2021a) compared the proportion of steatosis pre- and post-LSG found that 70/71 (98.6%) patients had steatosis >1 pre-LSG compared to 62/71 (87.3%) patients at 12 months post-LSG. The RR was 0.89 (95% CI 0.81 to 0.97) in favor of LSG.
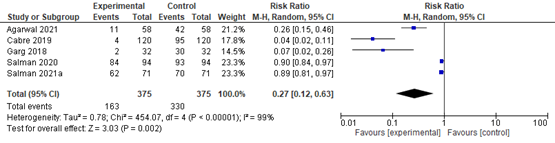
In summary, there were 5 additional observational studies that reported on steatosis before and after bariatric surgery. The pooled RR was 0.27 (95% CI 0.12 to 0.63; Figure 2) in favor of bariatric surgery. This is a clinically relevant difference.
Figure 2. Forest plot showing the proportion of patients experiencing steatosis pre-bariatric surgery compared to post-bariatric surgery. Pooled risk ratio, random effects model.
Reduction of MASH
Lobular inflammation
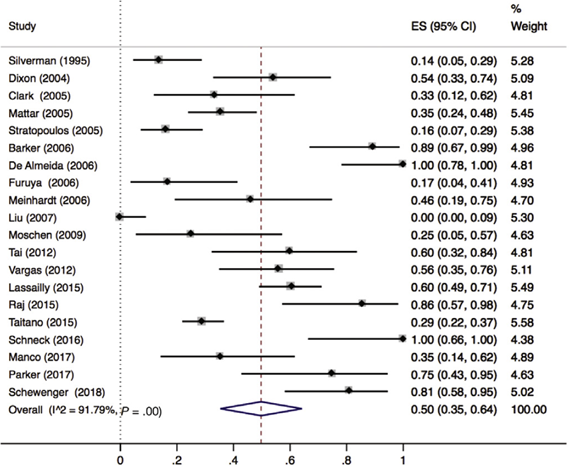
Lee (2019) included 32 observational cohort studies (n=2925 patients) that reported on resolution of MASH. Of these 32 studies, 21 reported lobular inflammation (n=657 patients). Lee 2019 found that 50% (95% CI 35% to 64%) of the patients had complete resolution of lobular inflammation (Figure 3).
Figure 3. Proportion meta-analysis forest plot of biopsy-proven complete resolution of lobular inflammation (adapted from Lee, 2019). ES, effect size.
Agarwal (2021) compared the proportion of lobular inflammation pre- and post-bariatric surgery found that 47/58 (81.0%) patients had lobular inflammation >1 pre-bariatric surgery compared to 25/58 (43.1%) patients at 12 months post-bariatric surgery. The RR 0.53 (95% CI 0.39 to 0.73) in favour of bariatric surgery. Overall, 24/58 (41.4%) patients showed improvement in lobular inflammation at 12 months post-bariatric surgery.
Cabré (2019) compared the proportion of lobular inflammation pre- and post-LSG found that 95/120 (79.2%) patients had lobular inflammation >1 pre-LSG surgery compared to 22/120 (18.3%) patients at 12 months post-LSG. The RR was 0.23 (95% CI 0.16 to 0.34) in favour of LSG.
Garg (2018) compared lobular inflammation pre- and post-bariatric surgery and found that 2/32 (6.2%) patients showed worsening lobular inflammation while 30/32 (93.8%) patients showed improvement or no change in lobular inflammation. The RR was 0.07 (95% CI 0.02 to 0.26) in favour of bariatric surgery.
Lassailly (2020) compared the proportion of lobular inflammation pre- and post-bariatric surgery found that 59/64 (92.2%) patients had lobular inflammation >1 pre-bariatric surgery compared to 26/64 (40.6%) patients at 5 years post-bariatric surgery. The RR was 0.44 (95% CI 0.32 to 0.60) in favour of bariatric surgery.
Salman (2020) compared the proportion of lobular inflammation pre- and post-LSG found that 83/94 (88.3%) patients had lobular inflammation >1 pre-LSG surgery compared to 50/94 (53.2%) patients at 12 months post-LSG. The RR was 0.60 (95% CI 0.49 to 0.74) in favour of LSG.
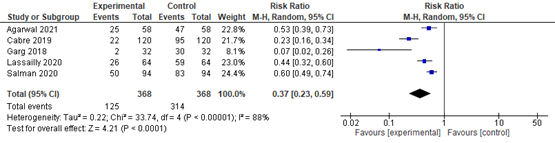
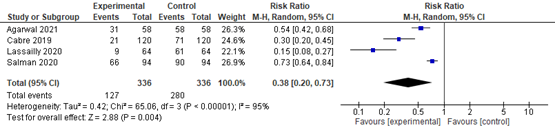
In summary, there were 5 additional observational studies that reported on lobular inflammation before and after bariatric surgery. The pooled RR was 0.37 (95% CI 0.23 to 0.59; Figure 4) in favour of bariatric surgery. This is a clinically relevant difference.
Figure 4. Forest plot showing the proportion of patients experiencing lobular inflammation pre-bariatric surgery compared to post-bariatric surgery. Pooled risk ratio, random effects model.
Ballooning degeneration of hepatocytes
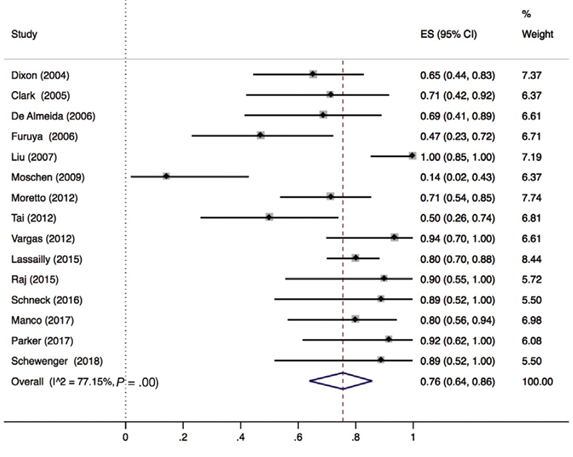
Lee (2019) included 32 observational studies of which 15 studies reported ballooning degeneration of hepatocytes (n=320 patients). Lee 2019 found that 76% (95% CI 64% to 86%) of the patients had complete resolution of ballooning degeneration after bariatric surgery (Figure 5).
Figure 5. Proportion meta-analysis forest plot of biopsy-proven complete resolution of ballooning degeneration (adapted from Lee, 2019). ES, effect size.
Agarwal (2021) compared the proportion of ballooning pre- and post-bariatric surgery and found that 58/58 (100%) patients had ballooning >1 pre-bariatric surgery compared to 31/58 (53.4%) patients at 12 months post-bariatric surgery. The RR was 0.54 (95% CI 0.42 to 0.68) in favour of bariatric surgery. Overall, 30/58 (51.7%) patients showed improvement in ballooning at 12 months post-bariatric surgery.
Cabré (2019) compared the proportion of ballooning pre- and post-LSG found that 71/120 (59.2%) patients had ballooning >1 pre-LSG compared to 21/120 (17.5%) patients at 12 months post-LSG. The RR was 0.30 (95% CI 0.20 to 0.45) in favour of LSG.
Garg (2018) compared ballooning pre- and post-bariatric surgery and found that 0/32 (0%) patients showed worsening ballooning while 32/32 (100%) patients showed improvement or no change in ballooning.
Lassailly (2021) compared the proportion of ballooning pre- and post-bariatric surgery found that 61/64 (95.3%) patients had ballooning >1 pre-bariatric surgery compared to 9/64 (14.1%) patients at 12 months post-bariatric surgery. The RR was 0.15 (95% CI 0.08 to 0.27) in favour of bariatric surgery.
Salman (2020) compared the proportion of ballooning pre- and post-LSG found that 90/94 (95.7%) patients had ballooning >1 pre-LSG compared to 66/94 (70.2%) patients at 12 months post-LSG. The RR was 0.73 (95% CI 0.64 to 0.84) in favour of LSG.
In summary, there were 5 additional observational studies that reported on ballooning degeneration before and after bariatric surgery. One study was excluded from pooled analyses because no RR could be calculated. The pooled RR of the remaining 4 observational studies was 0.38 (95% CI 0.20 to 0.72; Figure 6) in favour of bariatric surgery. This is a clinically relevant difference.
Figure 6. Forest plot showing the proportion of patients experiencing ballooning degeneration pre-bariatric surgery compared to post-bariatric surgery. Pooled risk ratio, random effects model.
MASH
Agarwal (2021) compared the proportion of MASH pre- and post-bariatric surgery and found that 3/58 (5.2%) patients showed MASH pre-bariatric surgery while 2/58 (3.4%) patients showed MASH post-bariatric surgery. The RR was 0.67 (95%CI 0.12 to 3.84) in favour of bariatric surgery.
Esquivel (2018) compared the proportion of MASH pre- and 12 months post-LSG and found that 3/ 43 (48.8%) patients showed MASH pre-LSG surgery while 0/43 (0%) patients showed MASH at 12 months post-LSG surgery meaning that in all patients, MASH resolved completely.
Garg (2018) compared the proportion of MASH pre- and post-bariatric surgery and found that 4/32 (1.25%) patients showed MASH pre-bariatric surgery while 1/32 (0.03%) patients showed MASH post-bariatric surgery. The RR was 0.25 (0.03 to 2.12) in favour of bariatric surgery.
Lassailly (2020) found that resolution of MASH with no worsening of fibrosis
occurred in 54/64 patients with MASH (84.4%; 95% CI, 73.1%–92.2%) 5 years after bariatric surgery.
Salman (2020) compared the proportion of MASH pre- and post-LSG and found that 83/94 (88.3%) patients showed borderline or definite MASH pre-LSG while 49/94 (52.1%) patients showed borderline or definite MASH at 12 months post-LSG. The RR was 0.59 (95% CI 0.48 to 0.73) in favour of LSG.
Salman (2021a) compared the proportion of MASH pre- and post-LSG and found that 64/71 (90.1%) patients showed borderline or definite MASH pre-LSG while 7/71 (9.8%) patients showed borderline or definite MASH at 30 months post-LSG. The RR was 0.11 (95% CI 0.05 to 0.22) in favour of LSG.
Salman (2021b) compared the proportion of MASH pre- and at 15 months post-LSG and found that 62/71 (92.5%) patients showed borderline or definite MASH pre-LSG surgery while 34/67 (50.8%) patients showed borderline or definite MASH post-LSG surgery. The RR was 0.55 (0.43 to 0.70) in favour of LSG.
In summary, there were 6 observational studies that reported on MASH before and after bariatric surgery. One study was excluded from pooled analyses because no RR could be calculated. The pooled RR of the remaining 5 observational studies was 0.43 (95% CI 0.36 to 0.51; Figure 7) in favour of bariatric surgery. This is a clinically relevant difference.
Figure 7. Forest plot showing the proportion of MASH pre-bariatric surgery compared to post-bariatric surgery. Pooled risk ratio, random effects model.
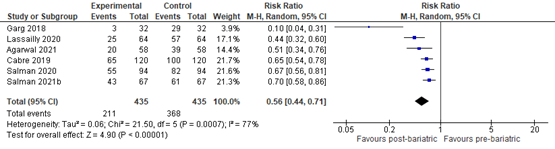
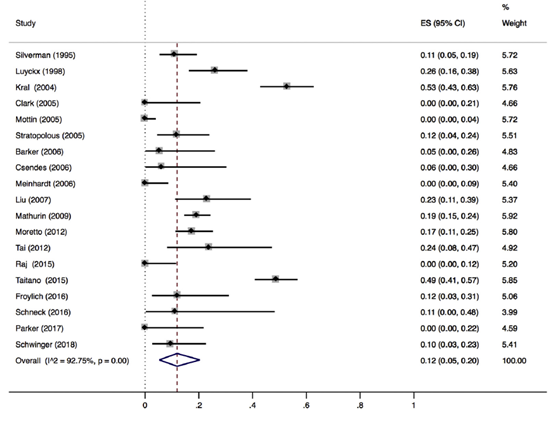
Reduction of liver fibrosis
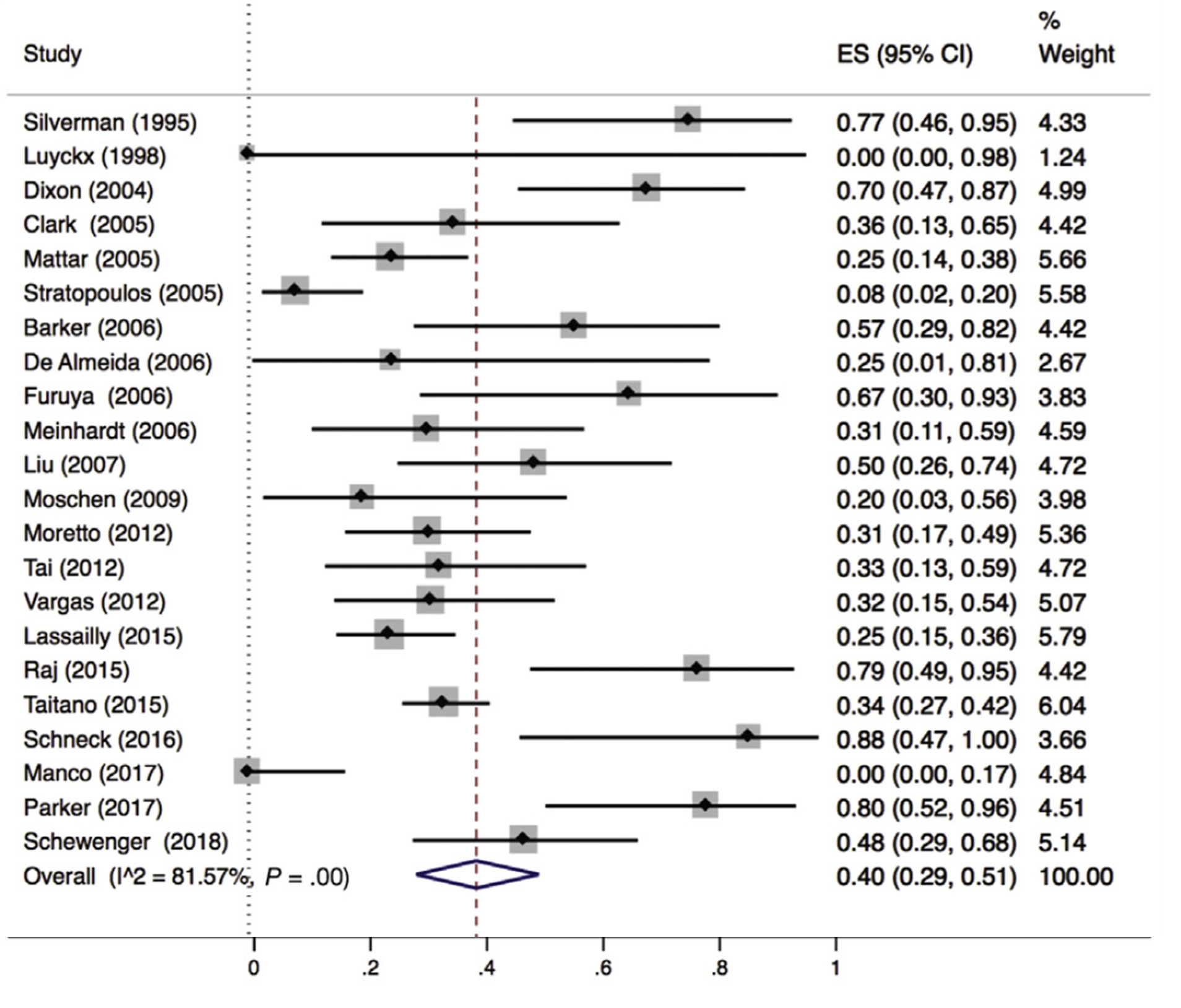
Lee (2019) included 32 observational studies of which 22 studies reported liver fibrosis (n=619 patients). Lee 2019 found that 40% of patients (95% CI 29% to 51%) of the patients had complete resolution of fibrosis upon bariatric surgery (Figure 8).
Figure 8. Proportion meta-analysis forest plot of biopsy-proven complete resolution of fibrosis (adapted from Lee, 2019). ES, effect size.
Agarwal (2021) compared the proportion of MASLD-related liver fibrosis pre- and post-bariatric surgery and found that 39/58 (67.2%) patients had fibrosis stage >1 pre-bariatric surgery compared to 20/58 (34.5%) patients at 12 months post-bariatric surgery. The RR was 0.51 (95% CI 0.34 to 0.76) in favour of bariatric surgery. Overall, 10/58 (17.2%) patients showed worsening fibrosis while 48/58 patients showed improvement or no change in fibrosis at 12 months post-bariatric surgery. The RR was 0.21 (95% CI 0.12 to 0.37) in favour of bariatric surgery.
Cabré (2019) compared the proportion of MASLD-related liver fibrosis pre- and post-LSG found that 100/120 (80.0%) patients had fibrosis >1 pre-LSG compared to 65/120 (54.2%) patients at 12 months post-LSG. The RR was 0.65 (95% CI 0.54 to 0.78) in favour of LSG.
Esquivel (2018) compared MASLD-related liver fibrosis stages pre- and 12 months post-LSG. A second liver biopsy at 12 months was performed in only one of the patients with previous diagnosis of liver fibrosis, and showed fibrosis was resolved at 12 months after LSG.
Garg (2018) compared MASLD-related liver fibrosis stages pre- and post-bariatric surgery and found that 3/32 (10%) patients showed worsening fibrosis while 29/32 (90%) patients showed improvement or no change in fibrosis. The RR was 0.10 (95% CI 0.04 to 0.31) in favour of bariatric surgery.
Lassailly (2020) compared MASLD-related liver fibrosis stages pre- and post-bariatric surgery and found that 57/64 (89.1%) patients had fibrosis at baseline compared to 25/64 (39.1%) patients 5-years post-bariatric surgery. The RR was 0.44 (95% CI 0.32 to 0.60) in favour of bariatric surgery. Overall, 62.9% of patients with F1-F2 fibrosis showed resolution at 5 years post bariatric surgery compared to 45.5% with F3-F4 fibrosis at baseline.
Salman (2020) compared MASLD-related liver fibrosis stages pre- and post-LSG and found that 82/94 (89.1%) patients had fibrosis > 1 at baseline compared to 55/94 (58.5%) patients 12 months post-LSG. The RR was 0.67 (95% CI 0.56 to 0.81) in favour of LSG.
Salman (2021b) compared MASLD-related liver fibrosis stages pre- and post-OAGB and found that 61/67 (91%) patients showed worsening fibrosis while 43/67 (64.2%) patients showed improvement in fibrosis at 15 months post-OAGB. The RR was 0.70 (95% CI 0.58 to 0.86) in favour of OAGB.
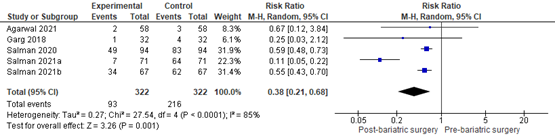
In summary, there were 7 additional observational studies that reported on MASLD-related liver fibrosis before and after bariatric surgery. One study was excluded from pooled analyses because no RR could be calculated. The pooled RR of the remaining 6 observational studies was 0.56 (95% CI 0.44 to 0.71; Figure 9) in favour of bariatric surgery. This is a clinically relevant difference.
Figure 9. Forest plot showing the proportion of patients experiencing fibrosis pre-bariatric surgery compared to post-bariatric surgery. Pooled risk ratio, random effects model.
Liver-related (serious) adverse events
Lee (2019) included 32 observational studies of which 19 studies reported on postoperative worsening of MASLD (n=1231 biopsies). Lee (2019) concluded that development or worsening of MASLD-characteristics occurred in 12% (95CI: 5% to 20%) of the patients (Figure 10).
Figure 10. Proportion meta-analysis forest plot of postoperative histologic worsening of MASLD (adapted from Lee, 2019). ES, effect size.
Agarwal (2021) reported on postoperative worsening of MASLD-characteristics, including steatosis, lobular inflammation, ballooning and MASH. In total, 10/58 (17.2%) patients had worsening of MASLD-characteristics. Four (7%) patients had worsening of steatosis, five (8.6%) patients had worsening of lobular inflammation, one (1.7%) patient had worsening of ballooning, five (8.6%) patients had worsening of MASH and ten (17.2%) patients had worsening of fibrosis.
Salman (2020) reported on postoperative worsening of MASLD-characteristics. One patient of the 94 patients had worsening of steatohepatitis. At first, this patient had non-MASH and turned into borderline MASH at 1-year follow-up. None of the patients showed worsening of fibrosis stage.
Salman (2021a) reported on (serious) adverse events, including postoperative worsening of MASLD-characteristics. In total 3/71 (4.2%) patients had postoperative worsening of steatosis.
Salman (2021b) reported on postoperative worsening of MASLD-characteristics. None of the 67 patients had worsening of MASH grade.
Level of evidence of the literature
Reduction of hepatic steatosis, resolution of MASH features, resolution of MASLD-related fibrosis and (serious) adverse events
The level of evidence regarding the outcome measures reduction of hepatic steatosis, resolution of MASH features, resolution of MASLD-related fibrosis and liver-related (serious) adverse events came from observational studies and started as low. The level of evidence was downgraded by one level because of inconsistency (heterogeneity; downgraded one level) resulting in a very low level of evidence.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the effect of bariatric surgery on MASLD in obese patients, particularly on MASH with advanced fibrosis?
P (population): Patients with MASH and liver fibrosis (stage F1 or higher) and a BMI > 35 kg/m2
I (intervention): Bariatric surgery
C (control): (combined) lifestyle intervention, placebo or no treatment.
O (outcomes): Reduction of steatosis on follow-up liver biopsy, resolution of MASH on follow-up liver biopsy, or reduction of ≥1 stage liver fibrosis on follow-up liver biopsy, liver-related (serious) adverse events
Relevant outcome measures
The guideline development group considered resolution of MASH on follow-up liver biopsy, reduction of ≥1 stage liver fibrosis on follow-up liver biopsy and liver-related (serious) adverse events as critical outcome measure for decision making. Reduction of steatosis was considered as an important outcome measure for decision making.
A priori, the working group defined the outcome measures as listed above but in the literature analysis they used the definitions of the outcome defined in the included studies.
The working group defined the GRADE standard limit of 25% difference for dichotomous outcomes (RR <0.8 or >1.25) and 0.5 SD for continuous outcomes as a minimal clinically (patient) important difference.
Search and select (Methods)
Two different literature searches were performed. At first we searched for systematic reviews and after this we performed an additional search to supplement the selected review(s) with observational studies that were published after the search date of the selected review(s).
Search 1: Systematic reviews (SR)
- The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 25 Augustus 2021. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 121 hits. First, systematic reviews (SR) and randomized controlled trials (RCTs) were selected evaluating the effects of bariatric surgery on MASLD in obese patients compared to a lifestyle intervention, placebo, or no treatment. Studies measuring outcomes ≥ 3 months follow-up were included. 22 studies were initially selected based on title and abstract screening. After reading the full text, 21 studies were excluded (see the table with reasons for exclusion under the tab Methods), and one SR study (Lee, 2019) was included.
Search 2: Observational studies
- The search strategy of the systematic review (Lee, 2019) was completed on May 2019. Therefore, we performed an additional search on observational studies in the databases Medline (via OVID) and Embase (via Embase.com) with relevant search terms between 1st of January 2019 until the 1st of November 2021. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 265 hits. Studies measuring outcomes for periods of ≥ 3 months post bariatric surgery were included. 45 studies were initially selected based on title and abstract screening. After reading the full text, 37 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 8 studies were included.
In total, 1 SR (search 1) and 8 observational studies (search 2) were included.
Results
Nine studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Agarwal, L., Aggarwal, S., Yadav, R. et al (2021). Bariatric surgery in nonalcoholic fatty liver disease (NAFLD): impact assessment using paired liver biopsy and fibroscan. Obesity surgery, 31(2), 617-626.
- Ahmed, S., Pouwels, S., Parmar, C. et al (2021). Outcomes of bariatric surgery in patients with liver cirrhosis: a systematic review. Obesity Surgery, 31(5), 2255-2267.
- Cabré, N., Luciano-Mateo, F., Fernández-Arroyo, S. et al (2019). Laparoscopic sleeve gastrectomy reverses non-alcoholic fatty liver disease modulating oxidative stress and inflammation. Metabolism, 99, 81-89.
- Esquivel, C. M., Garcia, M., Armando, L. et al (2018). Laparoscopic sleeve gastrectomy resolves NAFLD: another formal indication for bariatric surgery?. Obesity surgery, 28(12), 4022-4033.
- Garg, H., Aggarwal, S., Yadav, R. et al (2018). Utility of transient elastography (fibroscan) and impact of bariatric surgery on nonalcoholic fatty liver disease (NAFLD) in morbidly obese patients. Surgery for Obesity and Related Diseases, 14(1), 81-91.
- Gilmore, I. T., Burroughs, A., Murray-Lyon, I. M. et al (1995). Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut, 36(3), 437-441.
- Klebanoff, M. J., Corey, K. E., Samur, S. et al (2019). Cost-effectiveness analysis of bariatric surgery for patients with nonalcoholic steatohepatitis cirrhosis. JAMA network open, 2(2), e190047-e190047.
- Lassailly, G., Caiazzo, R., Ntandja-Wandji, L. C. et al (2020). Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology, 159(4), 1290-1301.
- Lee, Y., Doumouras, A. G., Yu, J. et al (2019). Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clinical gastroenterology and Hepatology, 17(6), 1040-1060.
- Maciejewski, M. L., & Arterburn, D. E. (2013). Cost-effectiveness of bariatric surgery. JAMA, 310(7), 742-743.
- Salman, M. A., Salman, A. A., Abdelsalam, A. et al (2020). Laparoscopic sleeve gastrectomy on the horizon as a promising treatment modality for NAFLD. Obesity Surgery, 30(1), 87-95.
- Salman, M. A., Mikhail, H. M. S., Nafea, M. A. et al (2021). Impact of laparoscopic sleeve gastrectomy on fibrosis stage in patients with child-A NASH-related cirrhosis. Surgical Endoscopy, 35(3), 1269-1277.
- Salman, M. A., Salman, A. A., Omar, H. S. et al (2021). Long-term effects of one-anastomosis gastric bypass on liver histopathology in NAFLD cases: A prospective study. Surgical endoscopy, 35(4), 1889-1894.
- Seeff, L. B., Everson, G. T., Morgan, T. R. et al & HALTC Trial Group. (2010). Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clinical gastroenterology and hepatology, 8(10), 877-883.
Evidence tabellen
Research question: What is the effect of bariatric surgery on MASLD in obese patients, particularly on MASH with advanced fibrosis?
Evidence table for systematic reviews
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control I
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Lee, 2019
|
SR and met cohort studies
Literature search up to may, 2018
See table 1 (Lee, 2019) for study characteristics of the included studies.
Source of funding and conflicts of interest: The authors of report no conflict of interest but conflict of interests for the included studies were not reported.
No funding was reported but included studies were funded private or by public funds. |
Inclusion criteria SR: Articles were eligible for inclusion if the studies examined the effect of bariatric surgery on MASLD. We included both single-arm studies (effect of bariatric surgery on MASLD status before and after surgery) or double-arm studies (bariatric surgery vs placebo or medical therapy).
Exclusion criteria SR:
32 studies included (1) case-series/reports, expert opinions, basic science, and review articles; (2) nonhuman studies; (3) studies with fewer than 10 eligible patients; and (4) patients with cirrhosis or a history of liver transplants.
Important patient characteristics at baseline: See table 1 (Lee, 2019) for study characteristics of the included studies.
Groups comparable at baseline? Yes |
Describe intervention:
MASLD features pre-bariatric surgery
|
Describe control:
MASLD features post-bariatric surgery
|
End-point of follow-up:
1 month to 5 years. (See table 1, Lee, 2019 for study characteristics of the included studies.)
….
For how many participants were no complete outcome data available? (intervention/control) Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Resolution of inflammation; % (95% CI) 21 studies/657 patients
50% (95% CI 35% to 64%) of the patients had complete resolution of inflammation
Resolution of ballooning degeneration; % (95% CI) 15 studies /320 patients
76% (95% CI 64% to 86%) of the patients had complete resolution of ballooning degeneration after bariatric surgery
Resolution of steatosis; % (95% CI) 25 studies/1329 patients 66% (95% CI 56% to 75%) of the patients had complete resolution of steatosis after bariatric surgery
Resolution of fibrosis; % (95% CI) 22 studies/619 patients
40% of patients (95% CI 29% to 51%) of the patients had complete resolution of fibrosis after bariatric surgery |
Facultative:
The authors conclude that bariatric surgery leads to complete resolution in histologic features of MASLD as well as a significant reduction of NAS in a substantial proportion of patients. However, the certainty of evidence is very low, warrants further high-quality studies, preferably RCTs, to recommend bariatric surgery as a therapy for MASLD remission.
|
Evidence table for intervention studies
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control I
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Agarwal 2021 |
Type of study: prospective observational cohort study
Setting and country: Hospital India
Funding and conflicts of interest: No funding source had been reported.
The authors have no conflict of interest. |
Inclusion criteria: Included were all consecutive patients undergoing bariatric surgery, as per the standard National Institute of Health (NIH) criteria
Exclusion criteria: Alcohol consumption > 21 drinks/week for men and > 14 drinks per week for women; Patients with hepatitis B virus or hepatitis C virus infection; Associated other causes of liver diseases such as autoimmune disease or any other metabolic storage disorders; Consumption of medicines known to induce fatty liver or insulin sensitization such as estrogens, tamoxifen, methotrexate, and glitazones
N total at baseline: N=179
Important prognostic factors2: MASH: 78.2% (140/179) Any grade of fibrosis (≥ F1): 117 (65.4%) |
MASH features pre-bariatric surgery
|
MASH features post-bariatric surgery
|
Length of follow-up: 12 months
Loss-to-follow-up: 12 patients did not have valid Transient Elastography(TE) 83 patients did not give consent for post bariatric 1-year follow-up biopsy
Incomplete outcome data: None
|
Outcome measures and effect size (include 95%CI and p-value if available):
Resolution of MASH; RR (95% CI) Pre-bariatric surgery: 3/58 (5.2%) Post-bariatric surgery: 2/58 (3.4%) RR= 0.67 (95%CI 0.12 to 3.84)
Resolution of inflammation; RR (95% CI) Pre-bariatric surgery: 47/58 (81.0%) Post-bariatric surgery: 25/58 (43.1%) RR= 0.53 (95% CI 0.39 to 0.73)
Resolution of ballooning degeneration; RR (95% CI) Pre-bariatric surgery: 58/58 (100%) Post-bariatric surgery: 31/58 (53.4%) RR= 0.54 (95% CI 0.42 to 0.68)
Resolution of steatosis; RR (95% CI) Pre-bariatric surgery: 42/58 (72.4%) Post-bariatric surgery: 11/58 (19.0%) RR= 0.26 (95% CI 0.15 to 0.46)
Resolution of fibrosis; RR (95% CI) Pre-bariatric surgery: 39/58 (67.2%) Post-bariatric surgery: 20/58 (34.5%) RR= 0.51 (95% CI 0.34 to 0.76) |
The authors conclude that Bariatric surgery significantly improves MASLD histology, including steatosis, ballooning, inflammation, and fibrosis. |
|
Cabré, 2019 |
Type of study: prospective observational cohort study
Setting and country: Hospital spain
Funding and conflicts of interest: This study was funded by Instituto de Salud Carlos III, the Fondo Europeo de Desarrollo Regional (FEDER), and by Fundació La Marató de TV3
The authors have no conflict of interest. |
Inclusion criteria: Included were all consecutive patients with severe obesity who underwent laparoscopic sleeve gastrectomy (LSG)
Exclusion criteria: age <25 years, alcohol abuse, infectious diseases, primary sclerosing cholangitis, autoimmune diseases, and cancer.
N total at baseline: N=436
Important prognostic factors2: Male: 24.5% (107/436)
MASH: 56.2% (245/436)
Any grade of fibrosis (≥ F1): 71.3% (311/436)
Any grade of steatosis: 63.6% (277/436)
Any grade of inflammation: 63.3% (363/436)
Any grade of Ballooning : 43.8% (191/436) |
MASH features pre-LSG
|
MASH features post-LSG
|
Length of follow-up: 12 months
Loss-to-follow-up: 316 patients did not have a post bariatric 1-year follow-up biopsy
Incomplete outcome data: None
|
Outcome measures and effect size (include 95%CI and p-value if available):
Resolution of inflammation; RR (95% CI) Pre-bariatric surgery: 95/120 (79.2%) Post-bariatric surgery: 22/120 (18.3%) RR= 0.23 (95% CI 0.16 to 0.34)
Resolution of ballooning degeneration; RR (95% CI) Pre-bariatric surgery: 71/120 (59.2%) Post-bariatric surgery: 21/120 (17.5%) RR= 0.30 (95% CI 0.20 to 0.45)
Resolution of steatosis; RR (95% CI) Pre-bariatric surgery: that 95/12 (79.2%) Post-bariatric surgery: 4/120 (3.3%) RR= 0.07 (95% CI 0.02 to 0.26)
Resolution of fibrosis; RR (95% CI) Pre-bariatric surgery: 100/120 (80.0%) Post-bariatric surgery: 65/120 (54.2%) RR= 0.65 (CI 0.54 to 0.78) |
The authors conclude that that LSG improves the histology and liver function of patients with morbid obesity. |
|
Esquivel, 2018 |
Type of study: prospective observational cohort study
Setting and country: Hospital Argentina
Funding and conflicts of interest: No funding source had been reported.
The authors have no conflict of interest. |
Inclusion criteria: Included were all consecutive patients aged >18 and <70 years with a BMI >40 without comorbidities or a BMI >35 with comorbidities who underwent laparoscopic sleeve gastrectomy (LSG)
Exclusion criteria: Patients with major psychiatric disorders or major depression. Patients with severe mental retardation. Patients who suffer from alcohol or drug abuse.
N total at baseline: N=63
Important prognostic factors2: mean age 40 ± 10 years;
female 65% (n = 41). Mean initial weight 129 ± 23 kg |
MASH features pre-LSG
|
MASH features post-LSG
|
Length of follow-up: 12 months
Loss-to-follow-up: 20 patients did not have a post bariatric 1-year follow-up biopsy
Incomplete outcome data: None
|
Outcome measures and effect size (include 95%CI and p-value if available):
Resolution of MASH; RR (95% CI) Pre-bariatric surgery: 3/ 43 (48.8%) Post-bariatric surgery: 0/43 (0%)
|
The authors conclude that LSG significantly improves MASLD after 12 months of follow-up, showing even a total regression in some of the patients. Neither of the patients presented worsening of the disease. |
|
Garg, 2018 |
Type of study: prospective observational cohort study
Setting and country: Hospital India
Funding and conflicts of interest: No funding source had been reported.
The authors have no conflict of interest. |
Inclusion criteria: Included were all consecutive patients with a BMI >40 without comorbidities or a BMI >35 with comorbidities who underwent bariatric surgery.
Exclusion criteria: Exclusion criteria were patients undergoing revision surgery; patients with alcohol consumption >21 drinks/week for men and >14 drinks per week for women; patients with hepatitis B or hepatitis C infection; patients with liver diseases due to other etiologies–autoimmune liver disease or metabolic storage disorders; patients on drugs known to induce fatty liver or insulin sensitization, such as estrogens, tamoxifen, amiodarone, methotrexate, and glitazones.
N total at baseline: N=124
Important prognostic factors2: mean age 39.3 ± 10.9 years
female 76.6% (n = 95)
mean initial weight 117.9 ± 20.2 kg |
MASH features pre-bariatric surgery
|
MASH features post-bariatric surgery
|
Length of follow-up: 12 months
Loss-to-follow-up: 15 patients did not have valid TE measurements 33 patients did not undergo liver biopsy 1 patient died due to cellulitis of leg and septic shock 6 months a er surgery
7 patients lost to follow-up 10 patients did not give consent for follow-up liver Biopsy
Incomplete outcome data: None
|
Outcome measures and effect size (include 95%CI and p-value if available):
Resolution of MASH; RR (95% CI) Worsened: 4/32 (1.25%) Improved: 4/32 (1.25%) RR= 0.25 (0.03 to 2.12)
Resolution of inflammation; RR (95% CI) Worsened: 2/32 (6.2%) Improved: 30/32 (93.8%) RR= 0.07 (95% CI 0.02 to 0.26)
Resolution of ballooning degeneration; RR (95% CI) Worsened: 0/32 (0%) Improved: 32/32 (100%)
Resolution of steatosis; RR (95% CI) Worsened: 2/32 (6.2%) Improved: 30/32 (93.8%) RR= 0.90 (95% CI 0.84 to 0.97)
Resolution of fibrosis; RR (95% CI) Worsened: 3/32 (10%) Improved: 29/32 (90%) RR= 0.10 (95% CI 0.04 to 0.31)
|
The authors conclude that bariatric surgery is associated with significant improvement in metabolic parameters, LSM, CAP, steatohepatitis, and fibrosis on liver biopsy postsurgery |
|
Lassailly, 2020 |
Type of study: prospective observational cohort study
Setting and country: Hospital France
Funding and conflicts of interest: This study was funded the French Ministry of Health, the Conseil Régional Nord-Pas de Calais, Agence National de la Recherche and the European commission.
The authors have no conflict of interest. |
Inclusion criteria: Included were patients over 18 years, with a BMI >40 without comorbidities or a BMI >35 with comorbidities who underwent bariatric surgery.
Exclusion criteria: Exclusion criteria: medical or psychologic contraindications to bariatric surgery; drink excessively, defined as an average daily consumption of alcohol of 20 g/d for women and 30 g/d for men, and had no history of excessive drinking for a period longer than 2 years at any time in the past 20 years; history of long-term consumption of hepatotoxic drugs; positive for chronic liver disease
N total at baseline: N=94
Important prognostic factors2: mean age 48.1 ± 7.9 years
female 66% (n = 116)
mean initial weight 117.9 ± 20.2 kg |
MASH features pre-bariatric surgery
|
MASH features post-bariatric surgery
|
Length of follow-up: 5 years
Loss-to-follow-up: 30 did not have a liver biopsy at 5 years follow-up
Incomplete outcome data: None
|
Outcome measures and effect size (include 95%CI and p-value if available):
Resolution of MASH; % (95% CI) resolution of MASH with no worsening of fibrosis occurred in 54/64 patients with MASH (84.4%; 95% CI, 73.1%–92.2%) 5 years after bariatric surgery
Resolution of inflammation; RR (95% CI) Pre-bariatric surgery: 59/64 (92.2%) Post-bariatric surgery: 26/64 (40.6%) RR= 0.44 (95% CI 0.32 to 0.60)
Resolution of ballooning degeneration; RR (95% CI) Pre-bariatric surgery: 61/64 (95.3%) Post-bariatric surgery: 9/64 (14.1%) RR= 0.30 (95% CI 0.20 to 0.45)
Resolution of fibrosis; RR (95% CI) Pre-bariatric surgery: 57/64 (89.1%) Post-bariatric surgery: 25/64 (39.1%) RR= 0.44 (95% CI 0.32 to 0.60 |
The authors conclude that the 5-year follow-up of obese MASH patients showed that the beneficial effects of bariatric surgery on the resolution of MASH were durable and led to a sustained reduction in fibrosis over 5 years |
|
Salman, 2020 |
Type of study: prospective observational cohort study
Setting and country: Hospital Egypt
Funding and conflicts of interest: No funding was received.
The authors have no conflict of interest. |
Inclusion criteria: Included were all consecutive patients aged >18 and <60 years with a BMI >40 without comorbidities or a BMI >35 with comorbidities who underwent laparoscopic sleeve gastrectomy (LSG)
Exclusion criteria: Severe medical diseases making anesthesia risky, unwilling to change lifestyle after surgery, psychologically unstable, those with unsupportive home environment, history of bariatric surgery, pregnancy, or lactation at screening or surgery
N total at baseline: N=94
Important prognostic factors2: mean age 41.39 + 7.64 years;
male 60.6% (n = 57).
Mean initial weight 123.56 ± 13.96 kg |
MASH features pre-LSG
|
MASH features post-LSG
|
Length of follow-up: 12 months
Loss-to-follow-up: none
Incomplete outcome data: None
|
Outcome measures and effect size (include 95%CI and p-value if available):
Resolution of MASH; RR (95% CI) Pre-bariatric surgery: 83/94 (88.3%) Post-bariatric surgery: 49/94 (52.1%) RR= 0.59 (95% CI 0.48 to 0.73)
Resolution of inflammation; RR (95% CI) Pre-bariatric surgery: 83/94 (88.3%) Post-bariatric surgery: 50/94 (53.2%) RR= 0.60 (95% CI 0.49 to 0.74)
Resolution of ballooning degeneration; RR (95% CI) Pre-bariatric surgery: 90/94 (95.7%) Post-bariatric surgery: 66/94 (70.2%) RR= 0.73 (95% CI 0.64 to 0.84)
Resolution of steatosis; RR (95% CI) Pre-bariatric surgery: 93/94 (98.9%) Post-bariatric surgery: 84/94 (89.4%) RR= was 0.90 (95% CI 0.84 to 0.97)
Resolution of fibrosis; RR (95% CI) Pre-bariatric surgery: 82/94 (89.1%) Post-bariatric surgery: 55/94 (58.5%) RR= was 0.67 (95% CI 0.56 to 0.81) |
The authors conclude that LSG significantly improves steatosis, steatohepatitis, and fibrosis at a 1-year follow-up. LSG can lead to resolution of MASLD. |
|
Salman, 2021a |
Type of study: prospective observational cohort study
Setting and country: Hospital Egypt
Funding and conflicts of interest: No funding was received.
The authors have no conflict of interest. |
Inclusion criteria: Included were all consecutive patients aged >18 and <60 years with a BMI >40 without comorbidities or a BMI >35 with comorbidities who underwent laparoscopic sleeve gastrectomy (LSG)
Exclusion criteria: dangerous medical conditions preventing anesthesia, not intending to modify their habits after the procedure; psychological issues; underwent previous metabolic surgery; gestation or lactation at initial nrolment or operation. Those who proved to have other etiologies of hepatic disorders, including viral, alcohol, immune-related, drug-elicited, cholestatic, genetical, or metabolic disorders
N total at baseline: N=71
Important prognostic factors2: mean age 44.4 ± 5.6 years;
male 53.5% (n = 38).
Mean initial weight 122.5 ± 14.5 kg |
MASH features pre-LSG
|
MASH features post-LSG
|
Length of follow-up: 30 months
Loss-to-follow-up: 61 patients did not undergo follow-up biopsy
Incomplete outcome data: None
|
Outcome measures and effect size (include 95%CI and p-value if available):
Resolution of MASH; RR (95% CI) Pre-bariatric surgery: 64/71 (90.1%) Post-bariatric surgery: 7/71 (9.8%) RR= .11 (95% CI 0.05 to 0.22)
Resolution of steatosis; RR (95% CI) Pre-bariatric surgery: 70/71 (98.6%) Post-bariatric surgery: 62/71 (87.3%) RR= 0.89 (95% CI 0.81 to 0.97)
|
The authors conclude that in patients with MASH-related liver cirrhosis of Child class A, LSG may be a secure approach for the management of morbid obesity. It has a long-term benefit for both obesity and liver condition. It assured a considerable weight reduction and resolution of comorbidities. Steatosis, steatohepatitis, and degree of liver fibrosis showed a significant resolution in more than two-thirds of patients. |
|
Salman 2021b |
Type of study: prospective observational cohort study
Setting and country: Hospital Egypt
Funding and conflicts of interest: No funding was received.
The authors have no conflict of interest. |
Inclusion criteria: Included were all consecutive patients aged >18 and <60 years with a BMI >40 without comorbidities or a BMI >35 with comorbidities who underwent laparoscopic sleeve gastrectomy (LSG)
Exclusion criteria: dangerous medical conditions preventing anesthesia, not intending to modify their habits after the procedure; psychological issues; underwent previous metabolic surgery; gestation or lactation at initial nrolment or operation. Those who proved to have other etiologies of hepatic disorders, including viral, alcohol, immune-related, drug-elicited, cholestatic, genetical, or metabolic disorders
N total at baseline: N=67
Important prognostic factors2: mean age 44.4 ± 5.7 years;
male 52.2% (n = 35).
Mean initial weight 122.1 ± 14.0 kg |
MASH features pre-one‑anastomosis gastric bypass (OAGB)
|
MASH features post-OAGB
|
Length of follow-up: 15 months
Loss-to-follow-up: No loss to follow-up reported
Incomplete outcome data: None
|
Outcome measures and effect size (include 95%CI and p-value if available):
Resolution of MASH; RR (95% CI) Pre-bariatric surgery: 62/71 (92.5%) Post-bariatric surgery: 34/67 (50.8%) RR= 0.55 (0.43 to 0.70)
Resolution of fibrosis; RR (95% CI) Pre-bariatric surgery: 61/67 (91%) Post-bariatric surgery: 43/67 (64.2%) RR= 0.70 (95% CI 0.58 to 0.86) |
The authors conclude that OAGB resolved MASH from nearly 42% of patients and reduced the histological features of MASLD 15 months after surgery.
|
Risk of bias tables
Risk of bias table for systematic reviews
|
|
Appropriate and clearly focused question?
Yes/no/unclear |
Comprehensive and systematic literature search?
Yes/no/unclear |
Description of included and excluded studies?
Yes/no/unclear |
Description of relevant characteristics of included studies? Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies? Yes/no/unclear |
Enough similarities between studies to make combining them reasonable? Yes/no/unclear |
Potential risk of publication bias taken into account? Yes/no/unclear |
Potential conflicts of interest reported?
Yes/no/unclear |
|
Lee, 2019 |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Unclear1 |
1 Potential conflicts of interest are not reported for the individual studies.
Risk of bias table for interventions studies
|
Author, year |
Selection of participants
Was selection of exposed and non-exposed cohorts drawn from the same population?
|
Exposure
Can we be confident in the assessment of exposure?
|
Outcome of interest
Can we be confident that the outcome of interest was not present at start of study?
|
Confounding-assessment
Can we be confident in the assessment of confounding factors?
|
Confounding-analysis
Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables? |
Assessment of outcome
Can we be confident in the assessment of outcome?
|
Follow up
Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed?
|
Co-interventions
Were co-interventions similar between groups?
|
Overall Risk of bias
|
|
|
|
|
|
|
|
|
|
|
|
|
Agarwal 2021 |
Definitely yes
Reason: Consecutive patients were follow-up pre and post bariatric surgery |
Probably yes
Reason: Exposure was based on histological biopsy |
Definitely yes
Reason: participants were followed up pre-and post-bariatric surgery to determine improvement on MAFLD features present at baseline. |
Probably yes
Reason: confounding variables were part of the exclusion criteria. |
Probably yes
Reason: confounding variables were part of the exclusion criteria |
Definitely yes
Reason: There was no missing data. |
Definitely yes
Reason: There was no incomplete data. Only patients with adequate follow-up were included |
Definitely yes
Reason: There were no co-interventions |
Low
|
|
Cabré, 2019 |
Definitely yes
Reason: Consecutive patients were follow-up pre and post LSG |
Probably yes
Reason: Exposure was based on histological biopsy |
Definitely yes
Reason: participants were followed up pre-and post-LSG to determine improvement on MAFLD features present at baseline. |
Probably yes
Reason: confounding variables were part of the exclusion criteria. |
Probably yes
Reason: confounding variables were part of the exclusion criteria |
Definitely yes
Reason: There was no missing data. |
Definitely yes
Reason: There was no incomplete data. Only patients with adequate follow-up were included |
Definitely yes
Reason: There were no co-interventions |
Low
|
|
Esquivel, 2018 |
Definitely yes
Reason: Consecutive patients were follow-up pre and post LSG |
Probably yes
Reason: Exposure was based on histological biopsy |
Definitely yes
Reason: participants were followed up pre-and post-LSG to determine improvement on MAFLD features present at baseline. |
Probably yes
Reason: confounding variables were part of the exclusion criteria. |
Probably yes
Reason: confounding variables were part of the exclusion criteria |
Definitely yes
Reason: There was no missing data. |
Definitely yes
Reason: There was no incomplete data. Only patients with adequate follow-up were included |
Definitely yes
Reason: There were no co-interventions |
Low
|
|
Garg, 2018 |
Definitely yes
Reason: Consecutive patients were follow-up pre and post bariatric surgery |
Probably yes
Reason: Exposure was based on histological biopsy |
Definitely yes
Reason: participants were followed up pre-and post-bariatric surgery to determine improvement on MAFLD features present at baseline. |
Probably yes
Reason: confounding variables were part of the exclusion criteria. |
Probably yes
Reason: confounding variables were part of the exclusion criteria |
Definitely yes
Reason: There was no missing data. |
Definitely yes
Reason: There was no incomplete data. Only patients with adequate follow-up were included |
Definitely yes
Reason: There were no co-interventions |
Low
|
|
Lassailly, 2020 |
Definitely yes
Reason: Consecutive patients were follow-up pre and post bariatric surgery |
Probably yes
Reason: Exposure was based on histological biopsy |
Definitely yes
Reason: participants were followed up pre-and post-bariatric surgery to determine improvement on MAFLD features present at baseline. |
Probably yes
Reason: confounding variables were part of the exclusion criteria. |
Probably yes
Reason: confounding variables were part of the exclusion criteria |
Definitely yes
Reason: There was no missing data. |
Definitely yes
Reason: There was no incomplete data. Only patients with adequate follow-up were included |
Definitely yes
Reason: There were no co-interventions |
Low
|
|
Salman, 2020 |
Definitely yes
Reason: Consecutive patients were follow-up pre and post LSG |
Probably yes
Reason: Exposure was based on histological biopsy |
Definitely yes
Reason: participants were followed up pre-and post-LSG to determine improvement on MAFLD features present at baseline. |
Probably yes
Reason: confounding variables were part of the exclusion criteria. |
Probably yes
Reason: confounding variables were part of the exclusion criteria |
Definitely yes
Reason: There was no missing data. |
Definitely yes
Reason: There was no incomplete data. Only patients with adequate follow-up were included |
Definitely yes
Reason: There were no co-interventions |
Low
|
|
Salman, 2021a |
Definitely yes
Reason: Consecutive patients were follow-up pre and post LSG |
Probably yes
Reason: Exposure was based on histological biopsy |
Definitely yes
Reason: participants were followed up pre-and post-LSG to determine improvement on MAFLD features present at baseline. |
Probably yes
Reason: confounding variables were part of the exclusion criteria. |
Probably yes
Reason: confounding variables were part of the exclusion criteria |
Definitely yes
Reason: There was no missing data. |
Definitely yes
Reason: There was no incomplete data. Only patients with adequate follow-up were included |
Definitely yes
Reason: There were no co-interventions |
Low
|
|
Salman 2021b |
Definitely yes
Reason: Consecutive patients were follow-up pre and post-OAGB |
Probably yes
Reason: Exposure was based on histological biopsy |
Definitely yes
Reason: participants were followed up pre-and post-OAGB to determine improvement on MAFLD features present at baseline. |
Probably yes
Reason: confounding variables were part of the exclusion criteria. |
Probably yes
Reason: confounding variables were part of the exclusion criteria |
Definitely yes
Reason: There was no missing data. |
Definitely yes
Reason: There was no incomplete data. Only patients with adequate follow-up were included |
Definitely yes
Reason: There were no co-interventions |
Low
|
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Search 1: systematic reviews and RCTs |
|
|
Froylich D, Corcelles R, Daigle C, Boules M, Brethauer S, Schauer P. Effect of Roux-en-Y gastric bypass and sleeve gastrectomy on nonalcoholic fatty liver disease: a comparative study. Surg Obes Relat Dis. 2016 Jan;12(1):127-31. doi: 10.1016/j.soard.2015.04.004. Epub 2015 Apr 8. PMID: 26077701. |
Included in Systematic Review Lee (2019) |
|
Jamialahmadi T, Jangjoo A, Rezvani R, Goshayeshi L, Tasbandi A, Nooghabi MJ, Rajabzadeh F, Ghaffarzadegan K, Mishamandani ZJ, Nematy M. Hepatic Function and Fibrosis Assessment Via 2D-Shear Wave Elastography and Related Biochemical Markers Pre- and Post-Gastric Bypass Surgery. Obes Surg. 2020 Jun;30(6):2251-2258. doi: 10.1007/s11695-020-04452-0. PMID: 32198617. |
C does not meet PICO |
|
Ahmed S, Pouwels S, Parmar C, Kassir R, de Luca M, Graham Y, Mahawar K; Global Bariatric Research Collaborative. Outcomes of Bariatric Surgery in Patients with Liver Cirrhosis: a Systematic Review. Obes Surg. 2021 May;31(5):2255-2267. doi: 10.1007/s11695-021-05289-x. Epub 2021 Feb 17. PMID: 33595790. |
P does not meet PICO |
|
Baldwin D, Chennakesavalu M, Gangemi A. Systematic review and meta-analysis of Roux-en-Y gastric bypass against laparoscopic sleeve gastrectomy for amelioration of NAFLD using four criteria. Surg Obes Relat Dis. 2019 Dec;15(12):2123-2130. doi: 10.1016/j.soard.2019.09.060. Epub 2019 Sep 18. PMID: 31711944. |
P does not meet PICO |
|
Bower G, Toma T, Harling L, Jiao LR, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. Bariatric Surgery and Non-Alcoholic Fatty Liver Disease: a Systematic Review of Liver Biochemistry and Histology. Obes Surg. 2015 Dec;25(12):2280-9. doi: 10.1007/s11695-015-1691-x. PMID: 25917981. |
O does not meet PICO |
|
Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD007340. doi: 10.1002/14651858.CD007340.pub2. PMID: 20091629; PMCID: PMC7208314. |
Lee (2019) is a more recent systematic review and of better quality |
|
de Brito E Silva MB, Tustumi F, de Miranda Neto AA, Dantas ACB, Santo MA, Cecconello I. Gastric Bypass Compared with Sleeve Gastrectomy for Nonalcoholic Fatty Liver Disease: a Systematic Review and Meta-analysis. Obes Surg. 2021 Jun;31(6):2762-2772. doi: 10.1007/s11695-021-05412-y. Epub 2021 Apr 13. PMID: 33846949. |
Lee (2019) includeds the same references but is of better quality |
|
Fakhry TK, Mhaskar R, Schwitalla T, Muradova E, Gonzalvo JP, Murr MM. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. 2019 Mar;15(3):502-511. doi: 10.1016/j.soard.2018.12.002. Epub 2018 Dec 6. PMID: 30683512. |
Lee (2019) is a more recent systematic review and of better quality |
|
Jan A, Narwaria M, Mahawar KK. A Systematic Review of Bariatric Surgery in Patients with Liver Cirrhosis. Obes Surg. 2015 Aug;25(8):1518-26. doi: 10.1007/s11695-015-1727-2. PMID: 25982807. |
P does not meet PICO |
|
Keleidari B, Mahmoudieh M, Gorgi K, Sheikhbahaei E, & Shahabi S. Hepatic failure after bariatric surgery: a systematic review. Hepatitis Monthly. 2019; 19(1). |
Wrong publication type (no meta analysis) |
|
Laursen TL, Hagemann CA, Wei C, Kazankov K, Thomsen KL, Knop FK, Grønbæk H. Bariatric surgery in patients with non-alcoholic fatty liver disease - from pathophysiology to clinical effects. World J Hepatol. 2019 Feb 27;11(2):138-149. doi: 10.4254/wjh.v11.i2.138. PMID: 30820265; PMCID: PMC6393715. |
Wrong publication type (no meta analysis) |
|
Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008 Dec;6(12):1396-402. doi: 10.1016/j.cgh.2008.08.012. Epub 2008 Aug 19. PMID: 18986848. |
Lee (2019) is a more recent systematic review and of better quality |
|
Panunzi S, Maltese S, Verrastro O, Labbate L, De Gaetano A, Pompili M, Capristo E, Bornstein SR, Mingrone G. Pioglitazone and bariatric surgery are the most effective treatments for non-alcoholic steatohepatitis: A hierarchical network meta-analysis. Diabetes Obes Metab. 2021 Apr;23(4):980-990. doi: 10.1111/dom.14304. Epub 2021 Jan 15. PMID: 33368954. |
C does not meet PICO |
|
Baltasar A, Serra C, Pérez N, Bou R, Bengochea M. Clinical hepatic impairment after the duodenal switch. Obes Surg. 2004 Jan;14(1):77-83. doi: 10.1381/096089204772787338. PMID: 14980038. |
P does not meet PICO |
|
Billeter AT, Senft J, Gotthardt D, Knefeli P, Nickel F, Schulte T, Fischer L, Nawroth PP, Büchler MW, Müller-Stich BP. Combined Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Sleeve Gastrectomy or Gastric Bypass?-a Controlled Matched Pair Study of 34 Patients. Obes Surg. 2016 Aug;26(8):1867-74. doi: 10.1007/s11695-015-2006-y. PMID: 26660688. |
P does not meet PICO |
|
Sherf-Dagan S, Zelber-Sagi S, Zilberman-Schapira G, Webb M, Buch A, Keidar A, Raziel A, Sakran N, Goitein D, Goldenberg N, Mahdi JA, Pevsner-Fischer M, Zmora N, Dori-Bachash M, Segal E, Elinav E, Shibolet O. Probiotics administration following sleeve gastrectomy surgery: a randomized double-blind trial. Int J Obes (Lond). 2018 Feb;42(2):147-155. doi: 10.1038/ijo.2017.210. Epub 2017 Aug 30. PMID: 28852205. |
I does not meet PICO |
|
Jirapinyo P, McCarty TR, Dolan RD, Shah R, Thompson CC. Effect of Endoscopic Bariatric and Metabolic Therapies on Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022 Mar;20(3):511-524.e1. doi: 10.1016/j.cgh.2021.03.017. Epub 2021 Mar 13. PMID: 33727164. |
O does not meet PICO |
|
Popov VB, Thompson CC, Kumar N, Ciarleglio MM, Deng Y, Laine L. Effect of Intragastric Balloons on Liver Enzymes: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016 Sep;61(9):2477-87. doi: 10.1007/s10620-016-4178-2. Epub 2016 May 20. PMID: 27207181. |
O does not meet PICO |
|
Ramai D, Singh J, Lester J, Khan SR, Chandan S, Tartaglia N, Ambrosi A, Serviddio G, Facciorusso A. Systematic review with meta-analysis: bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021 May;53(9):977-984. doi: 10.1111/apt.16335. Epub 2021 Mar 15. PMID: 33721336. |
P does not meet PICO |
|
Tsochatzis E, Coilly A, Nadalin S, Levistky J, Tokat Y, Ghobrial M, Klinck J, Berenguer M. International Liver Transplantation Consensus Statement on End-stage Liver Disease Due to Nonalcoholic Steatohepatitis and Liver Transplantation. Transplantation. 2019 Jan;103(1):45-56. doi: 10.1097/TP.0000000000002433. PMID: 30153225. |
Wrong publication type |
|
Search 2: observational studies |
|
|
Schwenger KJP, Fischer SE, Jackson T, Okrainec A, Allard JP. In nonalcoholic fatty liver disease, Roux-en-Y gastric bypass improves liver histology while persistent disease is associated with lower improvements in waist circumference and glycemic control. Surg Obes Relat Dis. 2018 Sep;14(9):1233-1239. doi: 10.1016/j.soard.2018.06.007. Epub 2018 Jul 12. PMID: 30049593. |
Included in systematic review Lee (2019) |
|
von Schönfels W, Beckmann JH, Ahrens M, Hendricks A, Röcken C, Szymczak S, Hampe J, Schafmayer C. Histologic improvement of NAFLD in patients with obesity after bariatric surgery based on standardized NAS (NAFLD activity score). Surg Obes Relat Dis. 2018 Oct;14(10):1607-1616. doi: 10.1016/j.soard.2018.07.012. Epub 2018 Jul 24. PMID: 30146425. |
O does not meet PICO |
|
Chaim FDM, Pascoal LB, Chaim FHM, Palma BB, Damázio TA, da Costa LBE, Carvalho R, Cazzo E, Gestic MA, Utrini MP, Milanski M, Chaim EA, Leal RF. Histological grading evaluation of non-alcoholic fatty liver disease after bariatric surgery: a retrospective and longitudinal observational cohort study. Sci Rep. 2020 May 22;10(1):8496. doi: 10.1038/s41598-020-65556-2. PMID: 32444690; PMCID: PMC7244764. |
P does not meet PICO |
|
Berry MA, Urrutia L, Lamoza P, Molina A, Luna E, Parra F, Domínguez MJ, Alonso R. Sleeve Gastrectomy Outcomes in Patients with BMI Between 30 and 35-3 Years of Follow-Up. Obes Surg. 2018 Mar;28(3):649-655. doi: 10.1007/s11695-017-2897-x. PMID: 28975492; PMCID: PMC5803286. |
P does not meet PICO |
|
Eilenberg M, Langer FB, Beer A, Trauner M, Prager G, Staufer K. Significant Liver-Related Morbidity After Bariatric Surgery and Its Reversal-a Case Series. Obes Surg. 2018 Mar;28(3):812-819. doi: 10.1007/s11695-017-2925-x. PMID: 28965313; PMCID: PMC5803276. |
Wrong publication type (case series) |
|
Jimenez LS, Mendonça Chaim FH, Mendonça Chaim FD, Utrini MP, Gestic MA, Chaim EA, Cazzo E. Impact of Weight Regain on the Evolution of Non-alcoholic Fatty Liver Disease After Roux-en-Y Gastric Bypass: a 3-Year Follow-up. Obes Surg. 2018 Oct;28(10):3131-3135. doi: 10.1007/s11695-018-3286-9. PMID: 29725976. |
P does not meet PICO |
|
Luo RB, Suzuki T, Hooker JC, Covarrubias Y, Schlein A, Liu S, Schwimmer JB, Reeder SB, Funk LM, Greenberg JA, Campos GM, Sandler BJ, Horgan S, Sirlin CB, Jacobsen GR. How bariatric surgery affects liver volume and fat density in NAFLD patients. Surg Endosc. 2018 Apr;32(4):1675-1682. doi: 10.1007/s00464-017-5846-9. Epub 2017 Dec 7. PMID: 29218660; PMCID: PMC6690434. |
P does not meet PICO |
|
McCarty TR, Echouffo-Tcheugui JB, Lange A, Haque L, Njei B. Impact of bariatric surgery on outcomes of patients with nonalcoholic fatty liver disease: a nationwide inpatient sample analysis, 2004-2012. Surg Obes Relat Dis. 2018 Jan;14(1):74-80. doi: 10.1016/j.soard.2017.09.511. Epub 2017 Sep 14. PMID: 29055669. |
O does not meet PICO |
|
Nickel F, Tapking C, Benner L, Sollors J, Billeter AT, Kenngott HG, Bokhary L, Schmid M, von Frankenberg M, Fischer L, Mueller S, Müller-Stich BP. Bariatric Surgery as an Efficient Treatment for Non-Alcoholic Fatty Liver Disease in a Prospective Study with 1-Year Follow-up : BariScan Study. Obes Surg. 2018 May;28(5):1342-1350. doi: 10.1007/s11695-017-3012-z. PMID: 29119336. |
P does not meet PICO |
|
Nobili V, Carpino G, De Peppo F, Caccamo R, Mosca A, Romito I, Overi D, Franchitto A, Onori P, Alisi A, Gaudio E. Laparoscopic Sleeve Gastrectomy Improves Nonalcoholic Fatty Liver Disease-Related Liver Damage in Adolescents by Reshaping Cellular Interactions and Hepatic Adipocytokine Production. J Pediatr. 2018 Mar;194:100-108.e3. doi: 10.1016/j.jpeds.2017.10.036. Epub 2017 Dec 1. PMID: 29198531. |
O does not meet PICO |
|
Tan CH, Al-Kalifah N, Ser KH, Lee YC, Chen JC, Lee WJ. Long-term effect of bariatric surgery on resolution of nonalcoholic steatohepatitis (NASH): An external validation and application of a clinical NASH score. Surg Obes Relat Dis. 2018 Oct;14(10):1600-1606. doi: 10.1016/j.soard.2018.05.024. Epub 2018 Jun 6. PMID: 30077664. |
O does not meet PICO |
|
Batman B, Altun H, Simsek B, Aslan E, Koc SN. The Effect of Laparoscopic Sleeve Gastrectomy on Nonalcoholic Fatty Liver Disease. Surg Laparosc Endosc Percutan Tech. 2019 Dec;29(6):548-549. doi: 10.1097/SLE.0000000000000713. PMID: 31574021. |
P does not meet PICO |
|
Endo Y, Ohta M, Tada K, Nakanuma H, Saga K, Masuda T, Hirashita T, Iwashita Y, Ozeki Y, Masaki T, Inomata M. Improvement of non-alcoholic fatty liver disease after laparoscopic sleeve gastrectomy in Japanese obese patients. Ann Gastroenterol Surg. 2019 Jan 29;3(3):285-290. doi: 10.1002/ags3.12234. PMID: 31131357; PMCID: PMC6524101. |
P does not meet PICO |
|
Fazeli Dehkordy S, Fowler KJ, Mamidipalli A, Wolfson T, Hong CW, Covarrubias Y, Hooker JC, Sy EZ, Schlein AN, Cui JY, Gamst AC, Hamilton G, Reeder SB, Sirlin CB. Hepatic steatosis and reduction in steatosis following bariatric weight loss surgery differs between segments and lobes. Eur Radiol. 2019 May;29(5):2474-2480. doi: 10.1007/s00330-018-5894-0. Epub 2018 Dec 13. PMID: 30547206; PMCID: PMC6483076. |
P does not meet PICO |
|
Feng W, Yin T, Chu X, Shan X, Jiang C, Wang Y, Qian Y, Zhu D, Sun X, Bi Y. Metabolic effects and safety of Roux-en-Y gastric bypass surgery vs. conventional medication in obese Chinese patients with type 2 diabetes. Diabetes Metab Res Rev. 2019 Jul;35(5):e3138. doi: 10.1002/dmrr.3138. Epub 2019 Mar 1. PMID: 30740871. |
P does not meet PICO |
|
Kahramanoğlu Aksoy E, Göktaş Z, Albuz Ö, Akpınar M Y, Öztürk D, Buluş H & Uzman M. Effects of sleeve gastrectomy on liver enzymes, non-alcoholic fatty liver disease-related fibrosis and steatosis scores in morbidly obese patients: first year follow-up. Journal of Laboratory Medicine, 2019; 43(2), 115-122. |
P does not meet PICO |
|
Kashihara H, Shimada M, Yoshikawa K, Higashijima J, Miyatani T, Tokunaga T, Nishi M, Takasu C, Hamada Y. The Effect of Laparoscopic Sleeve Gastrectomy on Obesity and Obesity-related Disease : the Results of 10 Initial Cases. J Med Invest. 2019;66(3.4):289-292. doi: 10.2152/jmi.66.289. PMID: 31656291. |
P does not meet PICO |
|
Ledoux S, Sami O, Calabrese D, Le Gall M, Flamant M, Coupaye M. Gastric bypass specifically impairs liver parameters as compared with sleeve gastrectomy, independently of evolution of metabolic disorders. Surg Obes Relat Dis. 2019 Feb;15(2):220-226. doi: 10.1016/j.soard.2018.10.035. Epub 2018 Nov 15. PMID: 30598254. |
P does not meet PICO |
|
Lyo V, Schafer AL, Stewart L. Roux-en-Y gastric bypass is a safe and effective option that improves major Co-Morbidities associated with obesity in an older, veteran population. Am J Surg. 2019 Oct;218(4):684-688. doi: 10.1016/j.amjsurg.2019.07.027. Epub 2019 Jul 20. PMID: 31399194; PMCID: PMC6996506. |
P does not meet PICO |
|
Motamedi MAK, Khalaj A, Mahdavi M, Valizadeh M, Hosseinpanah F, Barzin M. Longitudinal Comparison of the Effect of Gastric Bypass to Sleeve Gastrectomy on Liver Function in a Bariatric Cohort: Tehran Obesity Treatment Study (TOTS). Obes Surg. 2019 Feb;29(2):511-518. doi: 10.1007/s11695-018-3537-9. PMID: 30298459. |
P does not meet PICO |
|
Pooler BD, Wiens CN, McMillan A, Artz NS, Schlein A, Covarrubias Y, Hooker J, Schwimmer JB, Funk LM, Campos GM, Greenberg JA, Jacobsen G, Horgan S, Wolfson T, Gamst AC, Sirlin CB, Reeder SB. Monitoring Fatty Liver Disease with MRI Following Bariatric Surgery: A Prospective, Dual-Center Study. Radiology. 2019 Mar;290(3):682-690. doi: 10.1148/radiol.2018181134. Epub 2018 Dec 18. PMID: 30561273; PMCID: PMC6394737. |
P does not meet PICO |
|
Uehara D, Seki Y, Kakizaki S, Horiguchi N, Tojima H, Yamazaki Y, Sato K, Yamada M, Uraoka T, Kasama K. Long-term Results of Bariatric Surgery for Non-alcoholic Fatty Liver Disease/Non-alcoholic Steatohepatitis Treatment in Morbidly Obese Japanese Patients. Obes Surg. 2019 Apr;29(4):1195-1201. doi: 10.1007/s11695-018-03641-2. PMID: 30542827. |
P does not meet PICO |
|
Allen AM, Shah VH, Therneau TM, Venkatesh SK, Mounajjed T, Larson JJ, Mara KC, Kellogg TA, Kendrick ML, McKenzie TJ, Greiner SM, Li J, Glaser KJ, Wells ML, Gunneson TJ, Ehman RL, Yin M. Multiparametric Magnetic Resonance Elastography Improves the Detection of NASH Regression Following Bariatric Surgery. Hepatol Commun. 2019 Nov 5;4(2):185-192. doi: 10.1002/hep4.1446. PMID: 32025604; PMCID: PMC6996337. |
P does not meet PICO |
|
Kaul A, Singla V, Baksi A, Aggarwal S, Bhambri A, Shalimar D, Yadav R. Safety and Efficacy of Bariatric Surgery in Advanced Liver Fibrosis. Obes Surg. 2020 Nov;30(11):4359-4365. doi: 10.1007/s11695-020-04827-3. Epub 2020 Jul 3. PMID: 33900587. |
P does not meet PICO |
|
Kwak M, Mehaffey JH, Hawkins RB, Hsu A, Schirmer B, Hallowell PT. Bariatric surgery is associated with reduction in non-alcoholic steatohepatitis and hepatocellular carcinoma: A propensity matched analysis. Am J Surg. 2020 Mar;219(3):504-507. doi: 10.1016/j.amjsurg.2019.09.006. Epub 2019 Sep 16. PMID: 31575419. |
P does not meet PICO |
|
Pereira PF, Von Diemen V, Trindade EN, Michalczuk MT, Cerski CTS, Mussi AC, Aldabe DF, Branchi RN, Knijnik PG, Brum PW, Alvares-da-Silva MR, Trindade MRM. Are Noninvasive Methods Comparable to Liver Biopsy in Postoperative Patients After Roux-en-Y Gastric Bypass? Obes Surg. 2020 Jul;30(7):2566-2571. doi: 10.1007/s11695-020-04513-4. PMID: 32124221. |
P does not meet PICO |
|
Teixeira J, Marroni CA, Zubiaurre PR, Henz A, Faina L, Pinheiro LK, Mottin CC, Fernandes SA. Phase angle and non-alcoholic fatty liver disease before and after bariatric surgery. World J Hepatol. 2020 Nov 27;12(11):1004-1019. doi: 10.4254/wjh.v12.i11.1004. PMID: 33312425; PMCID: PMC7701974. |
P does not meet PICO |
|
Alkharaiji M, Anyanwagu U, Crabtree T, Idris I. The metabolic and liver-related outcomes of bariatric surgery in adult patients with insulin-treated type 2 diabetes and nonalcoholic fatty liver disease at high risk of liver fibrosis. Surg Obes Relat Dis. 2021 Apr;17(4):792-798. doi: 10.1016/j.soard.2020.11.015. Epub 2020 Nov 20. PMID: 33676874. |
P does not meet PICO |
|
Codjia T, Rebibo L, François A, Lagnel C, Huet E, Bekri S, Pattou F, Régimbeau JM, Schwarz L. Evolution of Non-alcoholic Fatty Liver Disease (NAFLD) Biomarkers in Response to Weight Loss 1 Year After Bariatric Surgery-a Post Hoc Analysis of the FibroTest Prospective Study. Obes Surg. 2021 Aug;31(8):3548-3556. doi: 10.1007/s11695-021-05402-0. Epub 2021 Apr 12. PMID: 33844174. |
P does not meet PICO |
|
Elyasinia F, Jalali SM, Zarini S, Sadeghian E, Sorush A, Pirouz A. The Effect of Laparoscopic Sleeve Gastrectomy and Gastric Bypass Surgery on Non-Alcoholic Steatohepatitis in Iranian Patients with Obesity. Middle East J Dig Dis. 2021 Jul;13(3):200-207. doi: 10.34172/mejdd.2021.226. Epub 2021 May 11. PMID: 36606220; PMCID: PMC9489465. |
C does not meet PICO |
|
Goday A, Julià H, de Vargas-Machuca A, Pedro-Botet J, Benavente S, Ramon JM, Pera M, Casajoana A, Villatoro M, Fontané L, Bisbe M, Climent E, Castañer O, Flores Le Roux JA, Benaiges D. Bariatric surgery improves metabolic and nonalcoholic fatty liver disease markers in metabolically healthy patients with morbid obesity at 5 years. Surg Obes Relat Dis. 2021 Dec;17(12):2047-2053. doi: 10.1016/j.soard.2021.07.021. Epub 2021 Aug 12. PMID: 34509375. |
O does not meet PICO |
|
Hajifathalian K, Mehta A, Ang B, Skaf D, Shah SL, Saumoy M, Dawod Q, Dawod E, Shukla A, Aronne L, Brown RS, Cohen DE, Dannenberg AJ, Fortune B, Kumar S, Sharaiha RZ. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointest Endosc. 2021 May;93(5):1110-1118. doi: 10.1016/j.gie.2020.08.023. Epub 2020 Aug 27. PMID: 32861753. |
P does not meet PICO |
|
Izzy M, Angirekula M, Abu Dayyeh BK, Bazerbachi F, Watt KD. Bariatric surgery proves long-term benefit in patients with cirrhosis. Gastroenterol Rep (Oxf). 2020 Nov 24;9(3):252-256. doi: 10.1093/gastro/goaa057. PMID: 34316375; PMCID: PMC8309677. |
Wrong publication type (Case description) |
|
LiuLiu SY, Wong VW, Wong SK, Wong GL, Lai CM, Lam CC, Shu SS, Chan HL, Ng EK. A prospective 5-year study on the use of transient elastography to monitor the improvement of non-alcoholic fatty liver disease following bariatric surgery. Sci Rep. 2021 Mar 8;11(1):5416. doi: 10.1038/s41598-021-83782-0. PMID: 33686111; PMCID: PMC7940649. |
P does not meet PICO |
|
Pedersen JS, Rygg MO, Serizawa RR, Kristiansen VB, Albrechtsen NJW, Gluud LL, Madsbad S, Bendtsen F. Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Non-Alcoholic Fatty Liver Disease: A 12-Month Follow-Up Study with Paired Liver Biopsies. J Clin Med. 2021 Aug 24;10(17):3783. doi: 10.3390/jcm10173783. PMID: 34501231; PMCID: PMC8432029. |
C does not meet PICO |
|
Russo MF, Lembo E, Mari A, Angelini G, Verrastro O, Nanni G, Pompili M, Raffaelli M, Vecchio FM, Bornstein SR, Mingrone G. Insulin Resistance Is Central to Long-Term Reversal of Histologic Nonalcoholic Steatohepatitis After Metabolic Surgery. J Clin Endocrinol Metab. 2021 Mar 8;106(3):750-761. doi: 10.1210/clinem/dgaa892. PMID: 33248441. |
O does not meet PICO |
|
Yang A, Nguyen M, Ju I, Brancatisano A, Ryan B, van der Poorten D. Utility of Fibroscan XL to assess the severity of non-alcoholic fatty liver disease in patients undergoing bariatric surgery. Sci Rep. 2021 Jul 7;11(1):14006. doi: 10.1038/s41598-021-93294-6. PMID: 34234198; PMCID: PMC8263818. |
P does not meet PICO |
|
de Barros F, Fonseca ABM. Bariatric surgery during the evolution of fatty liver-A randomized clinical trial comparing gastric bypass and sleeve gastrectomy based on transient elastography. Clin Obes. 2020 Dec;10(6):e12393. doi: 10.1111/cob.12393. Epub 2020 Sep 4. PMID: 32885600. |
P does not meet PICO |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 04-04-2024
Beoordeeld op geldigheid : 19-03-2024
De NVMDL is regiehouder van deze richtlijn en eerstverantwoordelijke op het gebied van de actualiteitsbeoordeling van de richtlijn. De andere aan deze richtlijn deelnemende wetenschappelijke verenigingen of gebruikers van de richtlijn delen de verantwoordelijkheid en informeren de regiehouder over relevante ontwikkelingen binnen hun vakgebied. De werkgroep heeft per module een inschatting gemaakt van de periode waarbinnen de modules beoordeeld zouden moeten worden voor eventuele herziening.
|
Module |
Uiterlijk jaar voor beoordeling |
|
Identificatie MASLD-fibrose |
2027 |
|
Leefstijlinterventies |
2027 |
|
Medicamenteuze behandeling |
2025 |
|
Bariatrische chirurgie |
2027 |
|
Cardiovasculair risicomanagement |
2027 |
|
Monitoring progressie van leverfibrose |
2027 |
|
HCC-surveillance |
2025 |
|
Organisatie van zorg |
2025 |
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). Patiëntenparticipatie bij deze richtlijn werd medegefinancierd uit de Kwaliteitsgelden Patiënten Consumenten (SKPC) binnen het programma KIDZ.
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2020 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met MASLD/MASH.
Werkgroep
- Dr. M.E. Tushuizen (voorzitter), MDL-arts, Leids Universitair Medisch Centrum, NVMDL
- Dr. A.G. Holleboom, internist-endocrinoloog en vasculaire geneeskunde, Amsterdam UMC, NIV
- Dr. J.G.P. Reijnders (vice-voorzitter), MDL-arts, HagaZiekenhuis, NVMDL
- Dr. M.M.J. Guichelaar, MDL-arts, Medisch Spectrum Twente, NVMDL
- Dr. J. Blokzijl, MDL-arts, Universitair Medisch Centrum Groningen, NVMDL
- Dr. G. H. Koek, MDL-arts en leefstijlcoach, Maastricht UMC+, NVMDL
- Dr. S. Simsek, internist-endocrinoloog en vasculaire geneeskunde, Noordwest Ziekenhuisgroep, NIV
- Prof. dr. M.E. Numans, hoogleraar huisartsengeneeskunde/huisarts, NHG
- T.A. Korpershoek, Verpleegkundig specialist MDL, Albert Schweitzer ziekenhuis, V&VN MDL
- Drs. L.C. te Nijenhuis-Noort, Diëtist, NVD
- Drs. J.A. Willemse, Directeur/patiëntvertegenwoordiger, NLV
Klankbordgroep
- Prof. dr. J. Verheij, patholoog, Amsterdam UMC, NVVP
Met ondersteuning van
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Dr. M.E. Tushuizen (voorzitter) |
MDL-arts, Leids Universitair Medisch Centrum |
Geen |
Ontwikkeling screening NAFLD bij DM2, projectleider, financier: Novo Nordisk; Ontwikkeling zorgpad NAFLD/NASH, geen projectleider, financier: Gilead; Ontwikkeling zorgpad NAFLD/NASH, projectleider, financier MLDS; Internationaal onderwijsprogramma, projectleider, financier: Pfizer. |
Vicevoorzitter aangesteld, voor module over medicamenteuze behandeling en module over CVRM. Daarmee restricties ten aanzien van besluitvorming bij de betreffende modules. |
|
Dr. J.G.P. Reijnders (vice-voorzitter) |
MDL-arts, HagaZiekenhuis, Erasmus MC (vanaf sept 2023) |
Lid Raad Kwaliteit NVMDL (onbetaald); Lid clinical board DGEA/DRCE/PRISMA netwerk |
Geen |
Geen restricties |
|
Dr. M.M.J. Guichelaar |
MDL-arts, Medisch Spectrum Twente |
Lid Kwaliteitsvisitatie commissie-audits; Lid Auto-immuun hepatitis werkgroep (onbetaald); Secretaris NAFLD/ NASH werkgroep (onbetaald) |
Ontwikkeling Serious game voor NAFLD, financier: MLDS |
Geen restricties |
|
Dr. J. Blokzijl |
MDL-arts, Universitair Medisch Centrum Groningen |
Geen |
Extracellular vesicles: towards personalized monitoring NAFLD, geen projectleider, financier MLDS |
Geen restricties |
|
Dr. G. Koek |
MDL-arts, Maastricht UMC+, onderzoeker NUTRIM Universiteit Maastricht |
Lid adviesraad NLV |
Nieuwe medicatie bij NASH, projectleider, financier: Boehringer Ingelheim; Genfit Resolve-it trial, projectleider, financier Convance/Genfit; Onderzoek semaglutide, projectleider, financier: Novo Nordisk; ontwikkeling Happi Lever App, rol als projectleider, financier MLDS |
Restricties ten aanzien van besluitvorming bij de module over medicamenteuze behandeling. |
|
Dr. A.G. Holleboom |
Internist-endocrinoloog en vasculaire geneeskunde en assistant professor Amsterdam UMC; staflid vasculaire geneeskunde |
Betaald adviseurschap Novo Nordisk (neergelegd) |
Studie naar autophagic turnover of lipid droplets in NASH, projectleider, financier: Gilead; Ontwikkeling zorgpad NAFLD/NASH, geen projectleider, financier: Gilead; onderzoek naar non-invasieve proxies en Fibroscan in NAFLD in Helius study population, financier: Novo Nordisk. |
Restricties ten aanzien van besluitvorming bij de module over medicamenteuze behandeling. |
|
Dr. S. Simsek |
Internist-endocrinoloog Noordwest Ziekenhuisgroep en Amsterdam UMC |
Voorzitter wetenschapscommissie NWZ; Begeleider promovendi |
Onderzoek naar semaglutide, participatie, financier: Novo Nordisk |
Restricties ten aanzien van besluitvorming bij de module over medicamenteuze behandeling. |
|
Prof. dr. M.E. Numans |
hoogleraar huisartsengeneeskunde LUMC |
Huisarts in Utrecht (betaald); voorzitter Autorisatiecommissie Standaarden NHG (vacatiegelden); Bestuurslid stichting SALTRO2 Diagnostic Living Lab (vacatiegelden); Adviesraad Leefstijl Norgine (onbetaald) |
Geen |
Geen restricties |
|
T.A. Korpershoek |
Verpleegkundig specialist MDL, Albert Schweitzer ziekenhuis; flex-docent HAN-MDL opleiding |
Bestuurslid V&VN MDL; Voorzitter netwerk VS MDL V&VN MDL (vacatiegelden) |
Geen |
Geen restricties |
|
Drs. L.C. te Nijenhuis-Noort |
Diëtist/Klinisch epidemioloog |
Lid Netwerk MDL diëtisten (onbetaald); Lid Commissie Voeding NVMDL (onbetaald) |
Geen |
Geen restricties |
|
Drs. J.A. Willemse |
Directeur NLV |
Board member Liver Patients International (onbetaald); Member management board ERN RARE LIVER (patient advocate, (onbetaald); lid diverse internationale expert groups NAFLD/NASH (onbetaald) |
|
Geen restricties |
|
Klankbordgroep lid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Prof. dr. J. Verheij |
Patholoog, Amsterdam UMC |
Geen |
Geen |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door afvaardiging van de Nederlandse Leverpatiënten Vereniging in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen (zie kopje waarden en voorkeuren van patiënten). De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Nederlandse Leverpatiënten Vereniging en de aangeleverde commentaren zijn bekeken en verwerkt.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
Module |
Uitkomst raming |
Toelichting |
|
Bariatrische chirurgie |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met MASLD/MASH. Tevens zijn er (aanvullende) knelpunten aangedragen door de Nederlandse Vereniging van Maag-Darm-Leverartsen, Nederlandse Internisten Vereniging, Nederlandse Vereniging voor Klinische Geriatrie en de Nederlandse Vereniging van Ziekenhuizen via een schriftelijke knelpuntenanalyse. Een verslag hiervan is opgenomen onder aanverwante producten. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy
Algemene informatie
|
Richtlijn: Non-alcoholische leververvetting (NAFLD/NASH) |
|
|
Uitgangsvraag: Wat is het effect van bariatrische chirurgie op NAFLD, in het bijzonder van NASH met ernstige fibrose? |
|
|
Database(s): Ovid/Medline, Embase |
Datum: 25-08-2021, 1-11-2021 |
|
Periode: 2000 - heden |
Talen: Engels, Nederlands |
|
Literatuurspecialist: Miriam van der Maten |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting en opmerkingen:
|
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase.com en Ovid/Medline is op 25-8 met relevante zoektermen gezocht naar systematische reviews en randomized controlled trials over het effect van bariatrische chirurgie op NAFLD. De literatuurzoekactie leverde 121 unieke treffers op. |
|
Zoekopbrengst
|
|
Embase |
OVID/MEDLINE
|
Ontdubbeld
|
|
SR |
64 |
48 |
69 |
|
RCT |
44 |
52 |
52 |
|
Observationeel vanaf mei 2018 |
262 |
196 |
265 |
|
Totaal |
370 |
296 |
386 |
Zoekstrategie
Embase
|
No. |
Query |
Results |
|
#9 |
#4 AND #6 NOT #8 = RCT |
44 |
|
#8 |
#4 AND #5 = SR |
64 |
|
#6 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1812605 |
|
#5 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
754729 |
|
#4 |
#1 AND #2 AND #3 AND ([english]/lim OR [dutch]/lim) AND [2000-2021]/py NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) |
855 |
|
#3 |
'bariatric surgery'/exp/mj OR 'gastrectomy'/exp/mj OR 'gastroplasty'/exp/mj OR 'gastric band'/exp/mj OR 'roux-en-y gastric bypass'/exp/mj OR bariatric*:ti,ab,kw OR (((gastric OR jejunoileal OR 'jejuno-ilea' OR ileojejunal OR 'ileo jejunal' OR gastroileal OR 'roux-en-y' OR intestinal) NEAR/2 bypass*):ti,ab,kw) OR gastroplast*:ti,ab,kw OR gastrectom*:ti,ab,kw OR gastrojejunostom*:ti,ab,kw OR 'gastric band*':ti,ab,kw OR 'stomach band*':ti,ab,kw OR 'duodenal switch':ti,ab,kw OR 'biliopancreatic bypass':ti,ab,kw OR 'biliopancreatic diversion':ti,ab,kw OR 'biliointestinal bypass':ti,ab,kw OR 'biliointestinal diversion':ti,ab,kw OR 'bilio-pancreatic bypass':ti,ab,kw OR 'bilio-pancreatic diversion':ti,ab,kw OR 'bilio-intestinal bypass':ti,ab,kw OR 'bilio-intestinal diversion':ti,ab,kw |
106059 |
|
#2 |
'obese patient'/exp/mj OR 'obesity'/exp/mj OR obese*:ti,ab,kw OR obesity:ti,ab,kw OR overweight:ti,ab,kw OR 'body mass'/exp OR 'body mass index':ti,ab,kw OR bmi:ti,ab,kw |
989695 |
|
#1 |
'nonalcoholic fatty liver'/exp/mj OR (((nonalcoholic OR 'non-alcoholic') NEAR/3 (steato* OR 'fatty liver*')):ti,ab,kw) OR nash*:ti,ab,kw OR nafld:ti,ab,kw |
57849 |
Ovid/Medline
|
# |
Searches |
Results |
|
9 |
(4 and 6) not 8 = RCT |
52 |
|
8 |
4 and 5 = SR |
48 |
|
6 |
exp randomized controlled trial/ or random*.ti,ab,kf. or ((pragmatic or practical) adj clinical trial*).ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. or rct.ti,ab,kf. |
1383464 |
|
5 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
539713 |
|
4 |
1 and 2 and 3 |
899 |
|
3 |
exp bariatric surgery/ or exp Gastrectomy/ or exp Biliopancreatic Diversion/ or exp biliopancreatic bypass/ or bariatric*.ti,ab,kf. or ((gastric or jejunoileal or 'jejuno-ilea' or ileojejunal or 'ileo jejunal' or gastroileal or 'roux-en-y' or intestinal) adj2 bypass*).ti,ab,kf. or Gastroplast*.ti,ab,kf. or Gastrectom*.ti,ab,kf. or Gastrojejunostom*.ti,ab,kf. or 'gastric band*'.ti,ab,kf. or 'stomach band*'.ti,ab,kf. or 'duodenal switch'.ti,ab,kf. or 'biliopancreatic bypass'.ti,ab,kf. or 'biliopancreatic diversion'.ti,ab,kf. or 'biliointestinal bypass'.ti,ab,kf. or 'biliointestinal diversion'.ti,ab,kf. or 'bilio-pancreatic bypass'.ti,ab,kf. or 'bilio-pancreatic diversion'.ti,ab,kf. or 'bilio-intestinal bypass'.ti,ab,kf. or 'bilio-intestinal diversion'.ti,ab,kf. |
84343 |
|
2 |
Overweight/ or obese*.ti,ab,kf. or obesity.ti,ab,kf. or overweight.ti,ab,kf. or exp Body Mass Index/ or 'body mass index'.ti,ab,kf. or bmi.ti,ab,kf. |
548940 |
|
1 |
exp Non-alcoholic Fatty Liver Disease/ or ((nonalcoholic or 'non-alcoholic') adj3 (steato* or 'fatty liver*')).ti,ab,kf. or nash*.ti,ab,kf. or nafld.ti,ab,kf. |
34607 |
Gebruikt observationeel filter EMBASE:
'controlled study'/de OR 'pretest posttest design'/exp OR 'pretest posttest control group design'/exp OR 'controlled clinical trial'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 study):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR 'single blind procedure'/exp OR 'triple blind procedure'/exp OR 'double blind procedure'/exp OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi experimental study'/de OR 'quasi-experiment*':ti,ab,kw OR 'crossover procedure'/de OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'control group'/de OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/exp OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/exp OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) OR versus:ti OR vs:ti OR compar*:ti OR 'comparative study'/exp OR ((compar* NEAR/1 study):ti,ab,kw) OR 'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti)
Gebruikt observationeel filter MEDLINE
controlled clinical trial/ or Control groups/ or Cross-Over Studies/ or Double-Blind Method/ or Single-Blind Method/ or Controlled Before-After Studies/ or (((control or controlled) adj6 trial) or ((control or controlled) adj6 study) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or crossover or "cross over" or "parallel group*" or "factorial trial").ti,ab,kf. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or (phase adj5 (study or trial)).ti,ab,kf. or (Case-Control Studies/ or Matched-Pair Analysis/ or ((case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*))).ti,ab,kf. or (propensity adj6 (scor* or match*)).ti,ab,kf.) or ((exp cohort studies/ or observational study/ or cross-sectional studies/ or multicenter study/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or cross sectional* or cross?ectional* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) or (versus or vs or compar*).ti. or Comparative study/ or (compar* adj study).ti,ab,kf. or historically controlled study/ or Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort*.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/